Abstract
The term extracellular vesicles (EVs) refers to a variety of heterogeneous nanovesicles secreted by almost all cell types, primarily for intercellular communication and maintaining cellular homeostasis. The role of EVs has been widely reported in the genesis and progression of multiple pathological conditions, and these vesicles are suggested to serve as ‘liquid biopsies’. In addition to their use as biomarkers, EVs secreted by specific cell types, especially with stem cell properties, have shown promise as cell-free nanotherapeutics. Stem cell-derived EVs (SC-EVs) have been increasingly used as an attractive alternative to stem cell therapies and have been reported to promote regeneration of aging-associated tissue loss and function. SC-EVs treatment ameliorates brain and peripheral aging, reproductive dysfunctions and inhibits cellular senescence, thereby reversing several aging-related disorders and dysfunctions. The anti-aging therapeutic potential of SC-EVs depends on multiple factors, including the type of stem cells, the age of the source stem cells, and their physiological state. In this review, we briefly describe studies related to the promising effects of SC-EVs against various aging-related pathologies, and then we focus in-depth on the therapeutic benefits of SC-EVs against Alzheimer’s disease, one of the most devastating neurodegenerative diseases in elderly individuals. Numerous studies in transgenic mouse models have reported the usefulness of SC-EVs in targeting the pathological hallmarks of Alzheimer’s disease, including amyloid plaques, neurofibrillary tangles, and neuroinflammation, leading to improved neuronal protection, synaptic plasticity, and cognitive measures. Cell culture studies have further identified the underlying molecular mechanisms through which SC-EVs reduce amyloid beta (Aβ) levels or shift microglia phenotype from pro-inflammatory to anti-inflammatory state. Interestingly, multiple routes of administration, including nasal delivery, have confirmed that SC-EVs could cross the blood-brain barrier. Due to this, SC-EVs have also been tested to deliver specific therapeutic cargo molecule/s (e.g., neprilysin) to the brain. Despite these promises, several challenges related to quality control, scalability, and biodistribution remain, hindering the realization of the vast clinical promise of SC-EVs.
Keywords: Extracellular vesicles, Stem cell, Mesenchymal stem cell, Aging, Alzheimer’s disease
1. Introduction
Extracellular vesicles (EVs), once thought to be cell debris, are now well known for their role in intercellular communication, maintaining cellular homeostasis, stem cell regulation, and tissue regeneration (Ma, Z. et al., 2020; Raposo and Stahl, 2019). EVs are heterogeneous membrane-bound vesicles containing metabolites, proteins, and various nucleic acids. EVs are secreted by all cell types and are found in all bodily fluids and secretions, for instance, plasma, urine, cerebrospinal fluid (CSF), synovial fluid, amniotic fluid, saliva, ascitic fluid, milk, etc. EVs have been broadly categorized into subtypes based on their size and origin, such as exosomes, microvesicles (MVs), and apoptotic bodies (Rani et al., 2015); however, new subtypes are constantly emerging (Ikegami and Ijaz, 2021; Melentijevic et al., 2017; Nishimura et al., 2021).
Exosomes are endocytic in origin and are among the smaller-sized EVs (sEV) with a diameter ranging from ~30 to less than 200 nm. Their biogenesis involves the endosomal system. These small vesicles are formed by the inward budding of the endosomes, which leads to the formation of a multivesicular body (MVB) in the lumen of endosomes (Hessvik and Llorente, 2018). The MVBs are either degraded by fusion with lysosomes or fuse with the plasma membrane of the cell to facilitate the release of exosomes in the extracellular milieu by exocytosis (Colombo et al., 2014). Exosome-mediated intercellular communication plays a critical role in both normal and pathological conditions (Donoso-Quezada et al., 2021; Salido-Guadarrama et al., 2014; Smalheiser, 2007; Yates et al., 2022a, b). Exosomes contain a lipid bilayer membrane structure, proteins, metabolites, and nucleic acids (noncoding RNAs, mRNA, and DNA), and the level of these cargo biomolecules depends upon the cellular and environmental context of the parent cell (Panigrahi et al., 2018; Ramteke et al., 2015; Schlaepfer et al., 2015; Valadi et al., 2007). As a result, exosomes have been widely studied for the development of molecular biomarkers and have been proposed as ‘liquid biopsies’ for a spectrum of diseases (Gao, Z. et al., 2021; Kumar et al., 2021; Kumar et al., 2022; Kumar et al., 2023; Zhao et al., 2022). Besides biomarkers, exosomes are also being studied for cargo delivery and therapeutic purposes. For example, exosomes secreted by various stem cells especially mesenchymal stem cells (MSC), have been extensively studied for their therapeutic benefits as discussed in detail below. In fact, MSC-derived exosomes are suggested as a preferred choice because of their safety profile, relatively low immunogenicity, ability to cross biological barriers, and low risk for tumorigenicity (Gowen et al., 2020). Furthermore, unlike stem cells, exosomes do not need to be cultured before transplantation.
MVs originate by outward budding of the plasma membrane into the extracellular milieu and range from ~100 to ≥1000 nm in diameter. The origin of MVs is still not well known; however, certain reports suggest the involvement of cytoskeletal components (Muralidharan-Chari et al., 2009; Ratajczak and Ratajczak, 2020). It is reported that the MVs are released in tumor cells via actomyosin-based membrane abscission, which is regulated by nucleotide (GTP/GDP) cycling on GTP-binding protein ADP-ribosylation factor 6 (Muralidharan-Chari et al., 2009). Nabhan et al., have discovered that the direct budding of the plasma membrane to generate MVs involves specific interaction of tumor susceptibility gene 101 (TSG101) protein with a tetrapeptide Prosaposin motif of an accessory protein, arrestin domain-containing protein 1 (ARRDC1) localized to the plasma membrane (Nabhan et al., 2012). Like exosomes, MVs’ cargo is also dependent upon the cellular and environmental context of the cell.
Apoptotic bodies originate from the blebbing of the plasma membrane in cells undergoing apoptotic stress. They have an approximate size range of ~500 to 4,000 nm in diameter. Annexin V, thrombospondin, and complement component C3b are well-accepted markers of apoptotic bodies (Akers et al., 2013). Cargo of apoptotic bodies could be distinguished from the exosomes and MVs as they contain intra-cytoplasmic components and various organelles, such as mitochondria, as a part of their cargo (Dieudé et al., 2015; Pallet et al., 2013).
Currently, based on the existing knowledge and tools, it is challenging to definitively determine whether the vesicles isolated from biofluids originated from the endosomal pathway or released from the outward budding or blebbing of the plasma membrane. Additionally, the overlapping size and absence of specific biomarkers for various subtypes further complicate the task of achieving 100% purity when separating these vesicles. For these reasons, a common term, ‘extracellular vesicle’ (EV), has been recommended to encompass these heterogeneous vesicles whenever purity or site of origin cannot be definitively determined. In this review, we have used the terms ‘exosomes’ and ‘EVs’ interchangeably. However, wherever the source article specifically referred to exosomes, we attempted to use the exact terminology. Initially, we briefly touched upon the therapeutic potential of stem cells but subsequently focused primarily on the role of EVs isolated from stem cells (SC-EVs) in the therapeutic management of aging-related disorders. We then delved deeply into the therapeutic utility of SC-EVs in combating Alzheimer’s disease, exploring the underlying mechanisms, their utility as biological nanoparticles for cargo delivery, and finally, the potential challenges associated with their clinical translation.
2. Therapeutic efficacy of stem cells
The self-renewal and differentiation potential of stem cells to any of the cell lineages has gained them a top choice for the development and regeneration of various tissues. These cells can be isolated from almost all tissue types and manipulated for their differentiation potential into a particular lineage using appropriate physio-chemical factors. Four main stem cell types that have been widely used include embryonic stem cells, fetal stem cells, adult tissue stem cells, and induced pluripotent stem cells (iPSCs). Stem cell-based therapies have been applied for the treatment of numerous disease models and medical conditions (Aboody et al., 2013; Honmou et al., 2021; Lee et al., 2009; Leng et al., 2020; Pers et al., 2016). For instance, in a preclinical study, Aboody et al., found that neural stem cells harboring genes specific for cytosine deaminase display selective tropism to the brain and convert the prodrug fluorocytosine (5-FC) to 5-fluorouracil (5-FU); and this combined treatment of neural stem cells and 5-FU reduced the growth of orthotopic gliomas without toxicity (Aboody et al., 2013). Further, haploidentical stem cell transplantation is considered a feasible treatment option for pediatric patients with refractory or relapsed metastatic neuroblastoma (Illhardt et al., 2018). Allogenic human MSCs have also been used to treat E. coli endotoxin-induced acute lung injury in ex vivo perfused human lungs, reducing extravascular lung water, improving lung endothelial barrier permeability, and restoring alveolar fluid clearance (Lee et al., 2009). Recently, MSCs were utilized in patients with Covid 19 pneumonia. Within two days of MSCs transplantation, patients’ pulmonary functions showed significant improvement associated with higher IL (interleukin)-10 and lower tumor necrosis factor-alpha (TNF-α) levels in the serum (Leng et al., 2020). Notably, patient-derived MSCs have been used to treat spinal cord injury without any serious adverse events (Honmou et al., 2021). The intra-articular injection of autologous adipose-derived stromal cells in patients with knee osteoarthritis demonstrated significant functional improvement without any serious adverse events (Pers et al., 2016). These are only a few examples, as stem cells have been extensively studied for their therapeutic potential to treat various disorders, including those associated with aging (Brody et al., 2023; Chen et al., 2022; Demurtas et al., 2021; Karimian et al., 2023; Li, T.T. et al., 2022; Moreira et al., 2017; Naji et al., 2019; Semsarzadeh and Khetarpal, 2022; Sivandzade and Cucullo, 2021; Soebadi et al., 2017; Sun et al., 2019; Tran et al., 2023; Zakrzewski et al., 2019). As the focus of this review is on EVs, next, we have only briefly highlighted the usefulness of stem cells in addressing disorders associated with aging and neurodegeneration.
2.1. Stem cell therapy in aging and neurodegeneration
2.1.1. Stem cell therapy in combating aging
Since stem cells can self-renew and have the potential to differentiate into any cell type, these cells could be useful for rejuvenating aging-related damaged tissue and improving loss of functions. Few such studies are described here.
Human umbilical cord Wharton’s Jelly-derived MSCs were tested to treat sarcopenia in aged (24-month-old C57BL/6) mice. Whole body muscle strength and endurance, as well as gastrocnemius muscle mass and cross-sectional area, were significantly increased in MSC-transplanted mice compared to control mice (Wang, Q.Q. et al., 2018). Human umbilical cord-derived MSCs treatment improved muscle strength and restored skeletal muscle morphology in D-galactose-induced aged C57BL/6 mice and SAMP8 mice- a senescence-accelerated mouse model, commonly used as the age-associated sarcopenia model (Wang, C. et al., 2023). Interestingly, MSC treatment noticeably increased the extracellular matrix proteins- dystrophin and laminin, suggesting the restoration of muscle cells (Wang, C. et al., 2023). Notably, clinical studies demonstrated the safety of intravenously injected human bone marrow allogenic MSCs in aged humans with frailty (Golpanian et al., 2017; Tompkins et al., 2017). The treatment showed no adverse events and resulted in remarkable improvement in physical performance measures and inflammation biomarkers (Golpanian et al., 2017; Tompkins et al., 2017).
Intra-myocardial injection of preconditioned human umbilical cord-derived MSCs with hormone ghrelin and nicotinamide-mononucleotide reduced infarct size in aged Sprague Dawley rats (20–22 months old) against induced myocardial ischemia-reperfusion injury (Sun and Zhang, 2021). Bone marrow MSCs isolated from old human donors (72–80 years age) transfected with anti-microRNA (miR)-155–5p improved cardiac functions by inhibiting apoptosis in cardiomyocytes and enhancing angiogenesis in the hearts of infarcted mouse (C57/B6J, 12 months of age) (Hong et al., 2020). In a clinical study, human embryonic SCs were utilized to generate cardiovascular progenitor cells for the cardioprotective application in aged patients (60.5 to 74.7 years) with severe ischemic left ventricular dysfunction. SC-derived cardiovascular progenitor cells were embedded in a fibrin patch that was epicardially delivered during a coronary artery bypass procedure. No severe adverse effects, such as tumor formation, were detected for 18 months follow-up, and most patients symptomatically improved with better systolic motion of the cell/patch-treated infarcted area (Menasché et al., 2018).
Recently, the anti-aging effect of human adipocyte-derived MSC conditioned media in combination with niacinamide (a form of vitamin B3) was evaluated after laser therapy in a double-blind randomized controlled study. The results indicated that the wrinkle index, melanin index, patient satisfaction score, and the investigator’s global esthetic improvement scale were significantly higher with this combination compared to the vehicle cream (Lee, Y.I. et al., 2021). Furthermore, in vitro UVB irradiation assays with human keratinocytes showed lower levels of pro-inflammatory cytokines and a higher expression of collagen type I with this combination (Lee, Y.I. et al., 2021).
2.1.2. Stem cell therapy to counter neurodegeneration
The generation of terminally differentiated neuronal cells from stem cells has opened the way forward for their use in neurodegenerative diseases. Stem cell therapies have been used for Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, etc (Cecerska-Heryc et al., 2023; Hernandez and Garcia, 2021; Sugaya and Vaidya, 2018). For Parkinson’s disease therapy, dopaminergic neurons were produced from the embryonic stem cells by using a specific combination of growth factors, mainly fibroblast growth factor 8 (FGF8) and sonic hedgehog (SHH) (Yan et al., 2005). In another study, human iPSCs were differentiated into functional dopaminergic neurons in scalable numbers and used for modeling or treating Parkinson’s disease (Swistowski et al., 2010). Kikuchi et al., derived cells from Parkinson’s disease patients and induced those into iPSCs. These iPSCs were then differentiated into dopaminergic neuron progenitors and transplanted into monkeys with Parkinson’s disease. These iPSC-derived dopaminergic neuron progenitor cells survived and functioned as midbrain dopaminergic neurons, and monkeys displayed increased spontaneous movement, demonstrating the potential clinical applicability of iPSCs for treating Parkinson’s disease (Kikuchi et al., 2017). Intravenous transplantation of Wharton’s Jelly-derived MSCs into APP/PS1 transgenic mice improved their spatial learning by reducing the amyloid beta (Aβ) deposition and soluble Aβ levels. Furthermore, the expression of anti-inflammatory cytokine, IL-10 was increased; whereas, expressions of pro-inflammatory cytokines, IL-1β and TNF-α, were significantly down-regulated in the brain (Xie et al., 2016). In another study, 5XFAD mice overexpressing the mutant human amyloid precursor protein (APP) and presenilin 1 (PS1) were transplanted with human umbilical cord blood-derived MSCs (Kim et al., 2018). The paracrine action of MSCs protected the mice from synaptic density loss in the hippocampus. Thrombospondin-1, a protein secreted by these MSCs, was identified to regulate the neuroprotective effect of these cells (Kim et al., 2018). The phase I testing of Lomecel-B (MSCs) in a double-blind, randomized, placebo-controlled trial in mild Alzheimer’s disease patients showed the safety as well as the potential effectiveness of this therapeutic approach against Alzheimer’s disease(Brody et al., 2023).
3. Therapeutic potential of SC-EVs
Despite the tremendous therapeutic promise of stem cell-based therapies, as briefly outlined above, several potential concerns remain, including reaction at the site of administration, the possibility of the stem cells moving from the placement site and changing into inappropriate cell types and then multiplying, failure of cells to work as expected, side effects, potential for neoplastic growth and adverse immune response (Nauta et al., 2006; Zangi et al., 2009) (2019; Sivandzade and Cucullo, 2021). Furthermore, embryonic stem cell research remains controversial due to ethical concerns. Another limitation is that adult stem cells have limited ability to grow in culture for longer periods, and the number of adult stem cells in each tissue is very small, making the generation of large quantities of adult stem cells for therapies challenging (2016). Numerous studies have now established that the therapeutic efficacy of stem cells is largely mediated through their secretome in a paracrine manner (Han et al., 2022; Paquet et al., 2015). This has led to the testing of EVs isolated from stem cell conditioned media (SC-EVs) as an alternative for therapeutic application in a wide variety of pathological conditions.
SC-EVs have demonstrated beneficial effects against several pathological and physiological conditions (de Castro et al., 2017; Li, Y. et al., 2022; Sengupta et al., 2020; Zhang et al., 2021). A systemic review of 206 studies by Tieu et al., demonstrated that MSC-EVs have been explored in various preclinical studies and the majority of the studies reported beneficial effects of these EVs (Tieu et al., 2020). Zhang et al., demonstrated the use of exosomes from human umbilical cord blood MSCs in stimulating wound healing and preventing scar formation in part via miR-21–5p and miR-125b-5p mediated inhibition of type I and type II transforming growth factor β receptor (TGFβR) in rats (Zhang et al., 2021). Recently, allogeneic bone marrow MSC-exosomes were utilized for the treatment of severe COVID-19. Patients were treated intravenously with commercially available EVs isolated from human bone marrow MSCs (ExoFlo™). Following MSC-exosome treatment, 71% of patients (17/24) recovered entirely and were discharged after a mean of 5.6 days, while 13% of patients (3/24) remained critically ill though stable, and 16% of the patients (4/24) expired for reasons unrelated to treatment (Sengupta et al., 2020). Furthermore, patients’ oxygenation capacity was restored, and cytokine storm was downregulated to reconstitute immunity (Sengupta et al., 2020). MSC-EVs have also been used as a therapeutic tool for inflammatory lung diseases. Human adipose tissue MSCs and their EVs reduced eosinophil counts in lung tissue and affected airway remodeling in an immunocompetent mouse model of allergic asthma (de Castro et al., 2017). Hypoxic human umbilical cord MSC-EVs attenuate allergic airway inflammation in mice with chronic asthma (ovalbumin-sensitized and challenged BALB/c female mice) (Dong, L. et al., 2021). MSC-EVs attenuated influenza virus-induced acute lung injury in a preclinical large animal model (Khatri et al., 2018). Exosomes secreted from MSCs overexpressing a zinc finger transcriptional factor GATA-4 served as a reservoir of anti-apoptotic miR-19a for cardioprotection (Yu et al., 2015). Direct intramyocardial transplantation of MSC-exosomes in rats at the border of an ischemic region significantly restored cardiac contractile function and reduced infarct size (Yu et al., 2015). In other studies, exosomes derived from neural progenitor cells delayed photoreceptor degeneration, preserved visual function, prevented thinning of the outer nuclear layer, and decreased apoptosis of photoreceptors in a retinal degradation rat model. These exosomes suppressed inflammatory signal pathways by targeting TNF-α, IL-1β, and cyclooxygenase-2 (COX-2) in retinal microglia (Bian et al., 2020). Importantly, MSC-EVs have reached clinical assessment for chronic kidney diseases. Nassar et al., demonstrated that administration of umbilical cord MSC-EVs was safe, ameliorated the inflammatory immune reaction, and improved the overall kidney function in grade III-IV chronic kidney disease patients (Nassar et al., 2016).
Injection of MSC-exosomes promoted bone fracture healing in CD9−/− mice, a strain characterized by diminished exosome production (Furuta et al., 2016). Exosomes derived from miR-92a-3p overexpressing human bone marrow MSCs enhanced chondrogenesis and suppressed cartilage degradation (Mao et al., 2018). Naïve and interferon-γ (IFN-γ)-primed adipose-derived SC-EVs promoted the repair of Achilles tendon injury by suppressing the inflammatory response and facilitating the collagen formation at the injury site (Shen et al., 2020). Adipose tissue-derived SC-EVs attenuated bone loss in osteoporosis mice by inhibiting the osteoclast differentiation of macrophages by virtue of osteoprotegerin and miR-21–5p loaded in these EVs (Lee, K.S. et al., 2021). Human umbilical cord blood EVs attenuated bone loss in senile osteoporotic mice (Hu et al., 2019). The EV treatment increased trabecular and cortical bone mass, enhanced osteoblast formation, and reduced osteoclast formation compared to the control mice (Hu et al., 2019). These EVs were highly enriched with miR-3960 and its inhibition reversed the stimulatory effect of EVs on osteoblastic differentiation of bone marrow MSCs (Hu et al., 2019).
A study by Ono et al., demonstrated that the exosomes from human bone marrow MSCs contain miR-23b that promotes dormancy in human metastatic breast cancer cells (Ono et al., 2014). Mir-23b caused suppression of target gene MARCKS (Myristoylated Alanine Rich C-Kinase Substrate), which encodes a protein that promotes cell cycling and motility (Ono et al., 2014). However, dependent upon their source, MSCs-EVs could be pretty heterogeneous, loaded with different bioactive cargo, and so likely to have diverse biological effects (Lai et al., 2016). Fattore et al., reported that MSC-EVs could play a dual role in cancer and can either promote or suppress cancer progression (Del Fattore et al., 2015). For example, bone marrow MSC-EVs and umbilical cord MSC-EVs decreased proliferation and induced apoptosis in glioblastoma cells, whereas adipose-derived MSC-EVs enhanced tumor cell growth and had no effect on their apoptosis (Del Fattore et al., 2015), suggesting a contrasting effect based upon the source of MSC-EVs.
4. SC-EVs in combating aging
Aging can be defined as the loss or decline of physiological functions over time, which increases the likelihood of death (Panagiotou et al., 2018). It is associated with a high prevalence of chronic degenerative diseases. With increasing life span, aging is emerging as a key challenge for the global healthcare system. It affects the quality of life and impacts the financial security system. Therefore, understanding the driving force of aging and the development of therapeutic strategies to alleviate aging-related disorders are of prime concern. EVs play a key role in mediating cellular homeostasis by removing excess and damaged biomolecules such as misfolded proteins, cytoplasmic DNA, and oxidized lipids (Desdin-Mico and Mittelbrunn, 2017; Takahashi et al., 2017). Therefore, EVs could be associated with age-related dyshomeostasis, such as loss of proteostasis related to multiple neurodegenerative disorders. Due to age-associated stress, the secretion of EVs may be altered, affecting the clearance of damaged cellular molecules, disturbing cellular homeostasis, and thus accelerating aging and associated disorders. Furthermore, EVs mediate paracrine signaling in both physiological and pathological states. The altered EV secretion could affect the channel through which cells communicate at multiple levels, including metabolic interdependence, as well as transmitting any distress signals, enabling neighboring cells to prepare in response to any stressful stimulus (Ramteke et al., 2015; Urbanelli et al., 2016). The treatment of SC-EVs could restore a few of these traditional EVs’ functions through their specific cargo molecules. Further, SC-EVs could deliver bioactive cargo from stem cells to areas needing regeneration to maintain tissue viability in age-related complications. Numerous studies have reported SC-EVs as a therapeutic option for multiple aging-related disorders(Boulestreau et al., 2021; Boulestreau et al., 2020; Cha et al., 2020; Li, J. et al., 2023; Liu, Y.R. et al., 2021; Mahindran et al., 2023; Ruiz et al., 2016; Sanz-Ros et al., 2022a; Shen et al., 2021; Ullah et al., 2020; Wu et al., 2022; Yao et al., 2019) (summarized in Table 1 and briefly outlined below).
Table 1:
Therapeutic effects of stem cell-derived extracellular vesicles in different aging-related pathological conditions.
4.1. SC-EVs application against brain aging
The nervous system has a clear role in aging, particularly the hypothalamus (Dacks et al., 2013; Zhang et al., 2013). Adult neural stem cells are reported to mediate local neurogenesis and brain functioning (Merkle et al., 2014), and recently, it was reported that adult neural stem cells present in the hypothalamus play a crucial role in the neuroendocrine regulation and physiological homeostasis of the body (Li et al., 2012; Maggi et al., 2015). With the onset of aging, hypothalamic activity is retarded because of the reduced activity of the hypothalamic stem cells. These cells contribute significantly to miRNAs in the CSF, which are linked to stem cell function and regulation of brain aging (Li and Gregory, 2008; Shi et al., 2010). During aging, the level of these cells declines, resulting in reduced exosomal miRNAs and accelerated aging. The study performed by Zhang et al., demonstrated that the treatment of hypothalamic neural stem cells-derived exosomes could control whole-body aging through the release of miRNAs, including miR-106a-5p, miR-20a-5p and miR-466m-5p as cargo (Zhang et al., 2017). Human embryonic SC (H9) derived EVs treatment also alleviated hippocampal neural stem cell senescence, recovered compromised self-renewal and neurogenesis capacities, and reversed cognitive impairment in mice. At a mechanistic level, SC-EVs treatment rejuvenated senescent neural stem cells through transferring SMAD4 (Suppressor of Mothers against Decapentaplegic 4) and SMAD5 proteins to activate MYT1 (Myelin transcription factor 1), which subsequently led to downregulation of egl-9 family hypoxia-inducible factor 3 (Egln3), and activation of hypoxia-inducible factor 2α (HIF-2α), nicotinamide phosphoribosyl transferase (NAMPT), and sirtuin 1 (Sirt1)(Hu et al., 2021). Ultimately, this study suggested that the senescence of hippocampal neural stem cells can be reversed by SC-EVs. Exosomes from young neural stem progenitor cells rescued insulin receptor substrate-1 (IRS-1)/ Forkhead box O (FoxO) activation and counteracted the reduced proliferation and senescence in neural stem progenitor cells (Natale et al., 2022). Also, exosome treatment counteracted the high-fat diet-dependent impairment of adult hippocampal neurogenesis in mice by restoring the balance between proliferating and senescent neural stem cells in the hippocampus (Natale et al., 2022).
Myelin damage and oligodendrocyte dysfunction exacerbate in the aged brain and likely contribute to age-related susceptibility to brain injury and subsequent neuronal dysfunction (Bowley et al., 2010). Oligodendrocytes found in aged brains have reduced proliferation (Miyamoto et al., 2013) and increased oxidative DNA damage (Tse and Herrup, 2017), resulting in diminished myelin production. Go et al., have demonstrated the effect of MSC-EVs treatment on changes in oligodendrocyte maturation and associated myelin markers in the sublesional white matter of aged rhesus monkeys (Go et al., 2021). MSC-EVs treated monkeys showed a reduction in the density of damaged oligodendrocytes and subsequent enhanced myelin maintenance (Go et al., 2021).
Aging-related ischemic events in the brain could trigger inflammatory responses contributing to neurological deficits. Therefore, therapeutics that modulate neuroinflammation in the aging brain have the potential to reduce neurological damage. In this regard, iPSC-EVs treatment shifted microglia to an anti-inflammatory phenotype, which reduced the apoptosis of neurons (Niu et al., 2023). Mechanistically, iPSC-EVs reversed the senescent characteristic of microglia in aged brains after stroke via delivering TGF-β1 to upregulate Rictor and p-AKT (Niu et al., 2023). Furthermore, iPSC-EVs treatment activated the endothelial nitric oxide synthase (eNOS) and upregulated Sirt1 in senescent endothelial cells to rejuvenate the blood-brain barrier in aged mice and protected against ischemic stroke partially, through delivering AKT1 and calmodulin to activate eNOS–Sirt1 axis (Li, Q. et al., 2023). Further, MSC-EVs reduced neurological deficits, infarct volume, brain edema, and neuronal injury in young and aged mice of both sexes (Wang et al., 2022). MSC-EVs also decreased leukocyte and, specifically, polymorphonuclear neutrophil, monocyte, and macrophage infiltrates in ischemic brains of aged mice (Wang et al., 2022). MSC-EVs prevented body weight loss and promoted functional neurological recovery and brain tissue remodeling in aged rats post-stroke (Dumbrava et al., 2022). MSC-EVs have been explored as therapeutic agents in aged rhesus monkeys after cortical injury. The treatment of MSC-EVs resulted in greater densities of ramified, homeostatic microglia, along with reduced pro-inflammatory microglial markers in the monkey brain (Go et al., 2020). Overall, SC-EVs have showcased the neuroprotective features against age-related brain aging.
4.2. SC-EVs role in combating peripheral aging
Besides overcoming brain aging, several studies have shown the therapeutic benefits of SC-EVs against aging-related disorders in other organs. A study by Sanz-Rios et al., demonstrated the anti-aging effect of adipose MSC-EVs isolated from young mice (3–6 months old) on the aged mice (20–24 months old). The old mice treated with adipose MSC-EVs from young mice showed improvement in several parameters, such as motor coordination, grip strength, fatigue resistance, fur regeneration, renal function, and frailty (Sanz-Ros et al., 2022b). Also, MSC-EVs induced regenerative effects and decreased oxidative stress, inflammation, and senescence markers in muscle and kidney tissues. Moreover, predicted epigenetic age was lower in tissues of old mice treated with MSC-EVs, and their metabolome also changed to a youth-like pattern (Sanz-Ros et al., 2022b). Exosomes secreted from young bone marrow MSCs promoted new bone formation during distraction osteogenesis in older rats (Jia et al., 2020). Dorronsoro et al., demonstrated that EVs derived from young bone marrow MSCs extend the life span of the aged mice, similar to the injection of young MSCs (Dorronsoro et al., 2021). Thus, MSC-EVs present an effective and safe approach for conferring the therapeutic effects of adult stem cells, avoiding the potential for neoplastic growth and rejection by the donor. Exosomes secreted by young stem cells from human exfoliated deciduous teeth modulated histone methylation and inhibited nuclear factor kappa B (NF-κB) to alleviate the aging phenotypes of aged tendon stem/progenitor cells and maintain their tenogenic capacity (Jin et al., 2023). Systemic administration of these exosomes retarded tendon degeneration, and their local delivery aided by microspheres reduced senescent cells and decreased ectopic bone formation, thereby functionally and structurally rescuing endogenous tendon regeneration capacity in aged rats (Jin et al., 2023). Antler SC-exosomes reduced senescence of MSCs in vitro and alleviated anterior cruciate ligament transection (ACLT)-induced osteoarthritis in mice (Lei et al., 2022). Hypoxia preconditioned glutaredoxin3 (GLRX3) positive MSC-EVs prevented cellular reactive oxygen species (ROS) accumulation and senescence cascade expansion in vitro in nucleus pulposus cells. Furthermore, these EVs loaded with supramolecular hydrogel attenuated mitochondrial damage, restored extracellular matrix deposition by modulating the redox homeostasis, and thus attenuated disc degeneration in a rat model (Liu et al., 2023).
MSC-EVs also reduce photoaging effects on skin by reducing apoptosis and senescence, increasing collagen type I expression, and reducing matrix metalloproteinase expression in photo-aged cells (Liu, S.J. et al., 2021; Yan et al., 2023). MSC-exosomes elicited antioxidant and anti-inflammatory effects, 14–3-3ζ protein was abundantly expressed in exosomes, which exerted a cytoprotective function via the modulation of a SIRT1-dependent antioxidant pathway and alleviated ultraviolet (UV) radiation-induced photodamage (Wu et al., 2021). EVs isolated from human adipose-derived SCs overexpressing circ_0011129 attenuated the cell photoaging by UVA radiation, as well as in the H2O2-induced oxidative stress model (Zhang et al., 2022). Human adipose SC-exosomes have also shown the anti-aging potential in UVB-induced photoaged skin in Sprague-Dawley rats, decreasing epidermal thickness and increasing dermal thickness via modulating the gene expression of extracellular matrix proteins (type I collagen and type III collagen) and metalloproteinases (MM-1 and MMP-3) (Liang et al., 2020). Adipose SC-EVs decreased skin wrinkles in mice while promoting epidermal cell proliferation and attenuating immune cell infiltration and ROS production in UVB-induced photoaged mice (Cao et al., 2021; Xu et al., 2020).
Adipose-derived MSC-EVs loaded with quercetin and vitamin A enhanced their therapeutic efficacy by reducing the acute senescence-like response and targeted delivery to acute liver injury (Fang and Liang, 2021). Treatment with human placental MSC-exosomes delayed the aging progress and reduced the levels of senescence-associated secretory phenotypic (SASP) components in an in vitro H2O2-induced aging model of cholangioid (Chen, W. et al., 2021). Umbilical cord MSC-exosomes also decreased lipotoxicity, inflammation, structural disorder, senescence markers, and genome instability in aging livers (Ling et al., 2023). MSC-EVs promoted wound closure and new blood vessel formation in natural aging and type-2 diabetes mouse models. Mechanically, miR-146a was highly expressed in MSC-EVs, which could suppress Src phosphorylation and downstream targets VE-cadherin and Caveolin-1 in senescent cells (Xiao et al., 2021). iPSC-derived MSC-EVs attenuated aging-related arterial stiffness and hypertension while enhancing endothelium-dependent vascular relaxation and arterial compliance in the old C57BL/6 mice. Furthermore, these EVs rescued the downregulation of Sirt1 and eNOS protein expression in the aortas of the older mice (Feng, R. et al., 2020). Intravenous injection of iPSC-exosomes for three months significantly decreased p53 and p16 expression levels in the kidney, skin, muscle, and lung tissues of aged mice (Li, X. et al., 2023). Urine-derived SC-exosomes promoted cell viability and proliferation of D-galactose-induced aging retinal ganglion cells (Dan et al., 2023). Overall, SC-EVs have pleiotropic beneficial effects in multiple disease models.
4.3. SC-EVs as therapy for aging-related reproductive health conditions
In men, erectile dysfunction is associated with the advanced age. It is more prevalent in men above 40 years old. A study by Feldman et al., demonstrated that the prevalence of complete impotence tripled from 5 to 15% between subjects ages 40 and 70 years (Feldman et al., 1994). Several studies have shown the potential of SC-EVs to reverse erectile dysfunction in preclinical animal models (Liang et al., 2021; Ouyang et al., 2018; Wang et al., 2020). Ouyang et al., demonstrated that MSC-exosomes could recover erectile dysfunction in a rat model by alleviating the apoptosis of corpus cavernous smooth muscle cells (Ouyang et al., 2018). MSC-exosome therapy in a rat model of internal iliac artery injury-induced erectile dysfunction promoted cavernous sinus endothelial formation, reduced the organization of oxidative stress damage, and improved the nitric oxide synthase and smooth muscle content in the corpus cavernosum (Liu, Y. et al., 2019). Exosomes derived from adipose-derived MSCs overexpressing miR-301a-3p mimic reversed erectile dysfunction by reducing the apoptosis of corpus cavernous smooth muscle cells (Liang et al., 2021).
In women, aging has been associated with menopause as the primordial follicles deplete at approximately 50 years of age (Macklon and Fauser, 1999). It is also reported that ovarian aging starts even before menopause. A woman’s fertility begins to decline in her early 30s, with a further decrease after the age of 35 (Broekmans et al., 2009). The aging in females is associated with the gradual decrease in quantity as well as the quality of the oocytes within the primordial follicles of the ovary (Broekmans et al., 2009; Qiao et al., 2014). Studies have demonstrated that MSC-EVs are effective in recovering ovarian functions. Recent reports have demonstrated that MSC-exosomes can repair ovarian damage in mouse models by regulating the proliferation and apoptosis of granulosa cells, which are vital for the growth of follicles (Huang et al., 2018; Xiao et al., 2016). Amniotic fluid SC-exosomes recapitulate the anti-apoptotic effect on chemotherapy-damaged granulosa cells via delivery of miR-146a and miR-10a (Xiao et al., 2016). Human adipose SC-exosomes also improved ovarian function in premature ovarian insufficiency disease via regulating the SMAD signaling pathway (Huang et al., 2018). Yang et al., demonstrated the effects of human umbilical MSC-exosomes on primordial follicles through the activation of the PI3K/mTOR signaling pathway in oocytes. When human umbilical MSC-exosomes were injected into aged female mice, a significant improvement in oocyte production and quality was observed. The stimulatory effects of MSC-exosomes were through carrying functional miRNAs, such as miR-146a-5p or miR-21–5p (Yang, W. et al., 2020). Supplementation of endometrial human MSC-EVs to in vitro fertilized zygote formed from 24-week-old B6D2 eggs and young male sperms (8–12 weeks) improved developmental competence of embryos as well as total blastomere count (Marinaro et al., 2019). Hence, MSC-EVs may represent a novel approach to address aging-associated declined fertility in men with erectile dysfunction and women with diminished ovarian reserve.
4.4. Anti-senescence effects of SC-EVs
During aging, senescent cells accumulate in various tissues, impairing homeostasis and resulting in a decline of their regenerative potential (van Deursen, 2014). Notably, in aged tissue, the number of senescent endothelial cells increases, and their function is impaired (Mistriotis and Andreadis, 2017; Valcarcel-Ares et al., 2012). The increase in the number of senescent endothelial cells compromises angiogenesis, which otherwise acts as an endogenous repair mechanism and plays a key role in tissue regeneration by restoring blood supply and delivering nutrients to the regenerating site (Li et al., 2005). A study by Chen et al., demonstrated that embryonic SC-exosomes enhanced angiogenesis and increased the number of matured blood vessels in aged mice. These results indicated that embryonic SC-exosomes could reduce endothelial cells’ senescence and recover aging-related angiogenic dysfunction in aged mice. These exosomes were highly enriched with miR-200a, which resulted in the rejuvenation of senescent endothelial cells and activation of nuclear factor erythroid-derived 2-like 2 (Nrf2) signaling, which is one of the important pathways involved in anti-aging (Chen et al., 2019). Similarly, human adipose SC-exosomes reduced the induced premature senescence in endothelial progenitor cells and accelerated vascularisation in diabetic rats (Li et al., 2018).
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a long noncoding RNA associated with cellular senescence and is considered one of the anti-aging candidates. It is reported that the MALAT1 expression is reduced in aged mice, and the treatment of human umbilical cord MSC-exosomes containing MALAT1 prevented aging in mice and senescence in cardiomyocytes. It prevented aging-induced cardiac dysfunction by releasing MALAT1, which in turn inhibited the NF-κB/TNF-α signaling pathway (Zhu et al., 2019). Exosomes isolated from umbilical cord derived-MSCs were also used to deliver miR-675 mimic, which reduced senescence-associated (SA)-β-galactosidase expression and downregulated the levels of p21 and TGF-β1 proteins in H2O2-induced senescent H9C2 cardiac myocytes (Han et al., 2019). Delivery of miR-675 by exosomes encapsulated in silk fibroin hydrogel also prevented aging-induced vascular dysfunction in mouse hind limb (Han et al., 2019). Human adipose SC-EVs were also reported to reduce IL-1β-induced senescence in osteoblasts from osteoarthritis patients by reducing oxidative stress and the levels of SA-β-galactosidase, γH2AX, IL-6, and prostaglandin E2 (Tofiño-Vian et al., 2017). Human gingiva-derived MSC-EVs abrogated oxidative stress-induced cellular senescence in human endothelial cells and skin fibroblasts by downregulating the expression of cellular senescence-related genes (Shi et al., 2021). Furthermore, systemic administration of these EVs attenuated the aging-associated elevation in the expression levels of p21, mTOR/pS6, IL6, and TNF-α in the skin and heart tissues of aged mice (Shi et al., 2021). Adipose SC-exosomes also alleviated human dermal fibroblast senescence and stimulated their migration in vitro (Guo et al., 2022). Yu et al., found that miR-15b-5p and miR-290a-5p were highly enriched in embryonic SC-EVs, which rejuvenated senescent mouse embryonic fibroblasts by silencing the Ccn2-mediated AKT/mTOR pathway (Yu et al., 2023). The embryonic SC-EV treatment further ameliorated the senescence status of several aged organs, including the kidney, liver, and spleen (Yu et al., 2023). MSC-exosomes antagonized senescence in murine kidney primary tubular epithelial cells (Liao et al., 2021). Human dental pulp SC-EVs reduced senescence-related gene and SASP factors expression in ductal epithelial cells in an irradiated-submandibular gland mouse model (Dong, J. et al., 2021). Hemin pretreatment enriched the level of miR-183–5p in MSC-exosomes, and these exosomes inhibited the serum deprivation and hypoxia-induced senescence in cardiomyocytes via regulation of the high mobility group box-1 (HMGB1) / extracellular signal-regulated kinase (ERK) pathway (Zheng et al., 2021).
SC-EVs have also been reported to rejuvenate senescent stem cells to prevent several aging-related disorders. Zhang et al., demonstrated that the mouse embryonic stem cell (D3 ES cell line) derived EVs significantly rejuvenated the senescent MSCs in vitro and improved the therapeutic effects of MSCs in a mouse cutaneous wound model. They also identified that the IGF1/PI3K/AKT pathway mediated the anti-senescence effects of SC-EVs on MSCs (Zhang et al., 2019). Lei et al., found that human neonatal umbilical cord MSC-EVs could rejuvenate senescent adult MSCs derived from bone marrow by transfer of proliferating cell nuclear antigen (PCNA) (Lei et al., 2021). EVs alleviated aging phenotypes in bone marrow MSCs and increased self-renewal capacity and telomere length. These EVs also improved skin wound repair, decreased oxidative stress, and reduced aging-related markers in different organs of mice (Lei et al., 2021). Mas-Bargues et al., reported that human dental pulp MSC-EVs reduced the cellular senescence in MSCs (Mas-Bargues et al., 2020). The EVs collected from the MSCs grown under hypoxia (3% oxygen) when cultured with prematurely senescent MSCs, reduced the SA-β-galactosidase activity and increased the expression of pluripotency factors: octamer-binding transcription factor 4 (OCT4), sex-determining region Y-box 2 (SOX2), Krüppel-like factor 4 (KLF4), and cellular myelocytomatosis (cMYC). Moreover, they identified that dental pulp MSC-EVs upregulated miR-302b, which triggered HIF-1α upregulation, leading to the activation of different pathways to delay premature senescence, improve stemness, and switch bioenergetic metabolism towards glycolysis (Mas-Bargues et al., 2020). In another study, Gong et al., showed that the chronic application of human embryonic SC-EVs rescued the function of senescent bone marrow MSCs by upregulating the expression of genes involved in anti-aging, stem cell proliferation, and osteogenic differentiation and prevented age-related bone loss in aging mice (Gong et al., 2020).
iPSCs-derived EVs have also been used to counter cellular senescence (Lee et al., 2020). The study by Oh et al., demonstrated the role of human iPSC-exosomes in ameliorating the aging of skin fibroblasts. They showed that exosomes derived from iPSC reduced the expression level of SA-β-galactosidase. The exosome treatment also restored the expression of Collagen type I in senescent human fibroblasts (Oh et al., 2018). EVs secreted from both iPSCs and young MSCs alleviated senescence-associated cellular phenotypes of aged MSCs by reducing intracellular ROS levels via transferring peroxiredoxin (Liu, S. et al., 2019). Intradiscally injected iPSC-derived MSC-EVs improved senescence in the nucleus pulposus and attenuated the development of intravertebral disc degeneration (Sun et al., 2021). Overall, the anti-senescent properties of SC-EVs could be due to multiple biological effects, including rejuvenating cells undergoing senescence, improving the stemness of stem cells, reducing oxidative stress, and increasing angiogenesis.
4.5. The anti-aging therapeutic potential of SC-EVs is dependent on the age of their source
Available literature supports that the therapeutic potential of SC-EVs declines with the age of the sourced individual (Abbasi Sourki et al., 2023; Ahmadi and Rezaie, 2021). Human MSC-EVs isolated from young (median age: 22 years) and aged (median age: 69 years) showed differences in the amount of distinct miRNAs such as miR-29a and miR-34a, which were significantly higher in aged MSC-EVs. Hematopoietic stem and progenitor cells incubated with young EVs showed a significant increase in cell number and higher viability. The expression of the tumor suppressors genes phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a known target of mir-29a, and cyclin-dependent kinase inhibitor 2A (CDKN2A) was increased in hematopoietic stem and progenitor cells (HSPCs) incubated with young EVs (Fichtel et al., 2022). Huang et al., compared the anti-inflammatory and protective effects of EVs against lipopolysaccharide (LPS)-induced acute lung injury (Huang et al., 2019). They isolated EVs from adipose-MSCs, which were isolated from healthy donors of 25 years (young MSC-EVs) and 72 years (aged MSC-EVs) of age, respectively. Their results demonstrated that both young and aged MSC-EVs had similar physical and phenotypical properties. However, the internalization of young MSC-EVs by macrophages was significantly higher than the aged MSC-EVs. Interestingly, young MSC-EVs exhibited anti-inflammatory and protective effects, while aged MSC-EVs did not show these protective effects (Huang et al., 2019). Su et al., showed that bone marrow MSC-exosomes of aged mice (18 months) could be taken up by adipocytes, myocytes, and hepatocytes, resulting in insulin resistance both in vitro and in young mice (2 months). These exosomes were enriched with miR-29b-3p with Sirt1 as the downstream target in regulating insulin resistance. The downregulation of miR-29b-3p in bone marrow MSC-derived exosomes using an aptamer-mediated nanocomplex delivery system significantly ameliorated the insulin resistance of aged mice (Su et al., 2019). Davis et al., isolated EVs from bone marrow interstitial fluid of young (3–4 months) and aged (24–28 months) mice. Their results demonstrated that the concentration and size distribution of bone marrow EVs were similar between the young and aged mice; however, their miRNA profile differed significantly. Specifically, the miR-183 cluster (miR-96/miR-182/miR-183) was highly expressed in EVs from aged mice. Furthermore, these EVs from aged mice were readily endocytosed by young primary bone marrow stromal cells and inhibited their osteogenic differentiation potential (Colleen Davis, 2017). Similarly, another study reported that EVs isolated from the plasma of healthy donors, either younger than 25 years or older than 55 years, had a differential influence on the osteogenic differentiation potential of the adipose-derived MSCs (Weilner et al., 2016). EVs isolated from the older individuals inhibited the osteogenesis in adipose-derived MSCs. The effect was found to be associated with the reduced intravesicular galectin-3 levels in EVs from aged individuals compared to EVs from young individuals (Weilner et al., 2016). Human umbilical cord MSC-exosomes renewed biological activities and reduced senescence phenotypes of old MSCs (from > 65 years old humans). Exosomes collected from old MSC pretreated with umbilical cord MSC-exosomes also resulted in better cardiac function, less fibrosis, and more angiogenesis. Umbilical cord MSC-exosome pretreatment enriched miR-136 expression in old MSC-exosomes, which regulated apoptotic peptidase activating factor (Apaf1), which negatively affects cell aging (Zhang, N. et al., 2020).
Overall, the above-described studies suggest that the anti-aging therapeutic properties of SC-EVs could be due to a multitude of biological effects shown in Figure 1.
Figure 1: Schematic showing the release of SC-EVs and their biological effects.
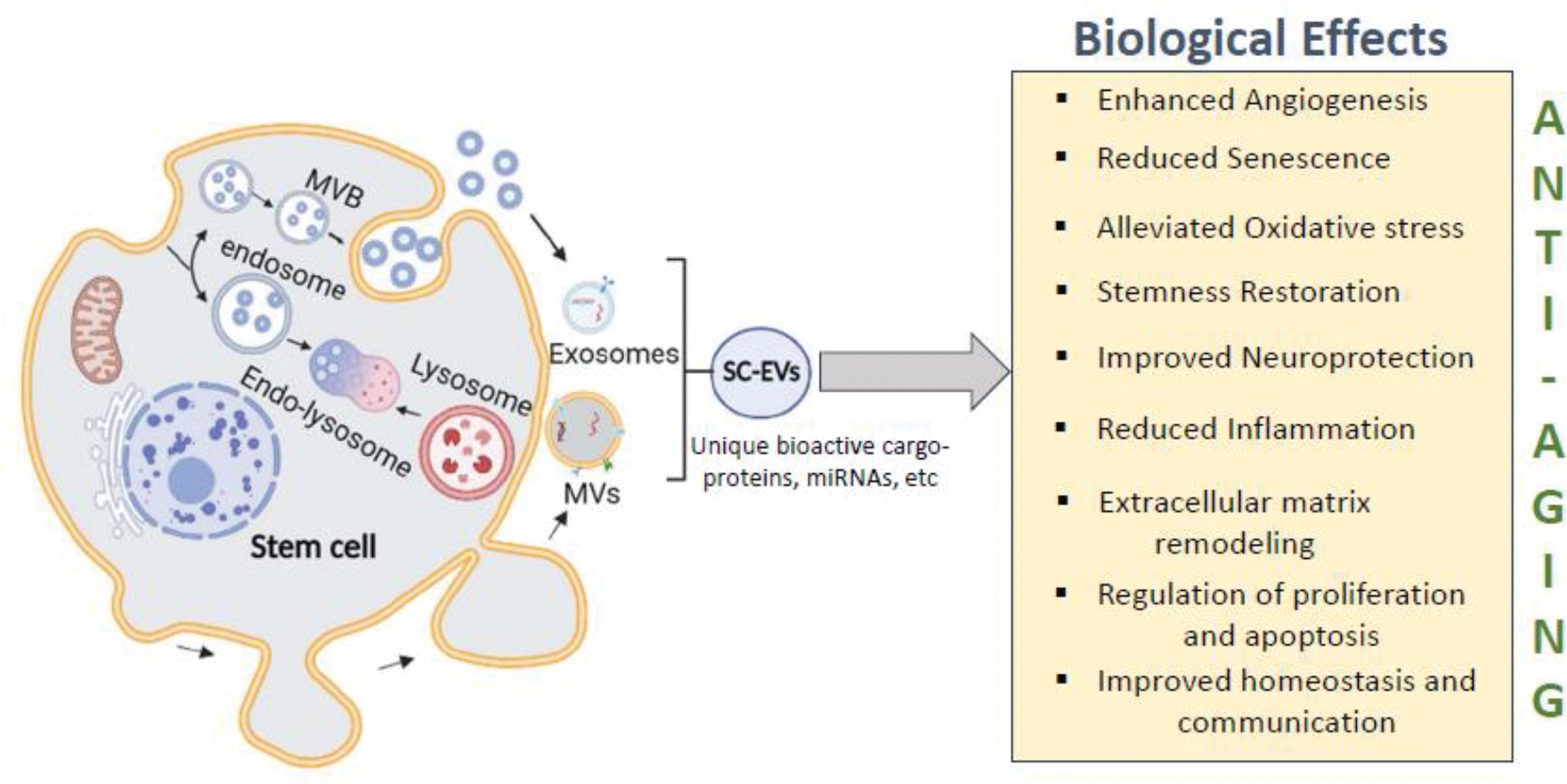
SC-EVs demonstrate anti-aging properties through multitude of listed biological effects. Image was created in BioRender software.
5. Therapeutic role of SC-EVs in Alzheimer’s disease
Alzheimer’s disease is a progressive neurodegenerative disease. It is the most common form of dementia in elderly people and is emerging as a devastating public health concern (2022). Alzheimer’s disease is associated with loss of cognitive functioning, leading to a high disability rate, which causes a heavier burden to society than any other aging-related disorder (2022; Agüero-Torres et al., 1998). Alzheimer’s disease is characterized by neuropathological hallmarks, including Aβ aggregation forming extracellular amyloid plaques and abnormally hyperphosphorylated tau resulting in neurofibrillary tangles (NFTs), leading to progressive neuronal loss in the brain (Hickman et al., 2016; Murphy and LeVine, 2010). The development of novel therapeutic approaches to reduce Alzheimer’s disease burden is an area of prime interest. A burgeoning body of research has suggested that SC-EVs application holds promise as a viable therapeutic option against Alzheimer’s disease (Chen, Y.A. et al., 2021; Elia, C. A. et al., 2019; Goncalves et al., 2023; Guo et al., 2020; Jeyaraman et al., 2023; Liew et al., 2017; Meldolesi, 2022; Yang, Y. et al., 2020; Yin et al., 2023). Based upon the existing literature, the therapeutic utility of SC-EVs against Alzheimer’s disease could be broadly divided into two categories (Figure 2): a) SC-EVs acting as the therapeutic agent, and b) SC-EVs serving as a vehicle to deliver specific therapeutic cargo molecule/s. In the subsequent sections, we delve into various studies falling within these two categories, providing comprehensive insights into their potential roles in combating Alzheimer’s disease.
Figure 2: Schematic showing the therapeutic effects of SC-EVs.
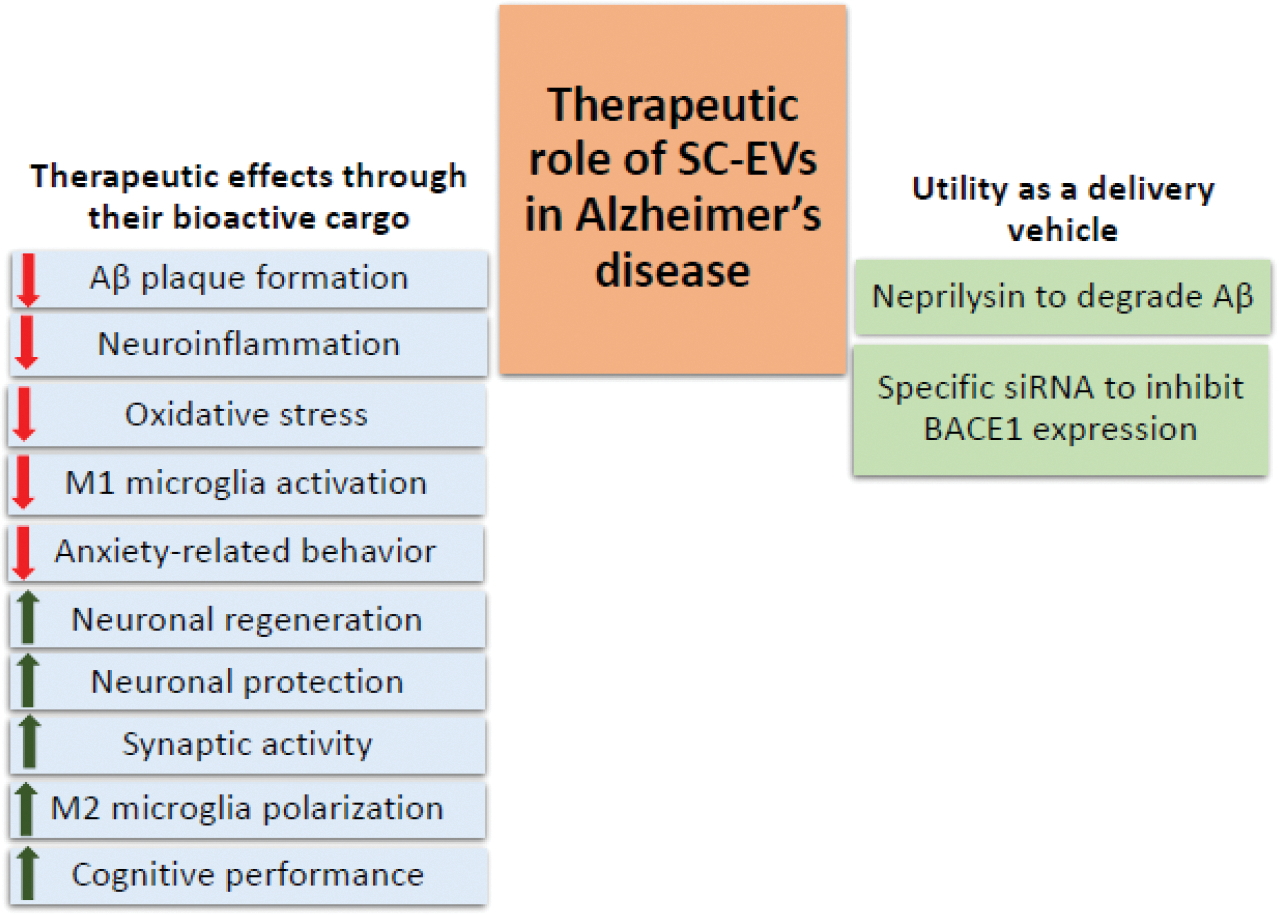
Key mechanisms underlying SC-EVs therapeutic effects are shown including their direct therapeutic effects as well as their use as delivery vehicle. Red downward arrows demonstrate decrease/reduced effect and green upward arrows demonstrate increase/improved effect of SC-EV.
5.1. SC-EVs as a therapeutic agent for Alzheimer’s disease
As mentioned above, EVs play a key role in maintaining cellular homeostasis via the removal of excess, unwanted, or damaged biomolecules (protein, nucleotides, and lipids). This function of EVs is a double-edged sword in Alzheimer’s disease pathogenesis as secretion of these vesicles could not only clear misfolded proteins (such as Aβ and tau) as cargo but could also result in the dissemination of toxic proteins to neighboring neurons, amplifying the effects of toxic proteins loaded in EVs (Liang et al., 2023). There are several studies supporting both protective and disease-promoting aspects of EVs’ function (Cai et al., 2018; Liang et al., 2023). For example, Yuyama et al., reported that neuron-derived exosomes could drive conformational changes in Aβ to form non-toxic amyloid fibrils and subsequent clearance by microglia through lysosomal-mediated degradation (Yuyama et al., 2012). Glycan-enriched glycosphingolipids on the surface of these exosomes play a critical role in Aβ binding and assembly on exosomes for subsequent clearance (Yuyama et al., 2014). On the other hand, there are several studies showing that exosomes play a critical role in the production and dissemination of Aβ, as well as suggesting their role in Aβ aggregation and plaque formation (Cai et al., 2018; Liang et al., 2023). For example, Rajendran et al., described the role of exosomes in Aβ production and secretion (Rajendran et al., 2006). Interestingly, this study also reported that neuritic plaques in the hippocampal section of Alzheimer’s disease patients were enriched in exosomal protein Alix, suggesting the role of exosomes in plaque formation (Rajendran et al., 2006).
Due to the closed anatomic structure of the brain, it is evident that different brain cells communicate with each other, and EVs are well known to play a critical role in maintaining intercellular communication, including between various brain cells, coordinating complex neurological activities (Frühbeis et al., 2013; Men et al., 2019). For example, Men et al., found that the neuron-specific exosomes contain the subset of miRNAs that are distinct from the miRNA profile of neurons. These exosomes were potentially internalized into astrocytes and upregulated the glutamate transporter GLT1 expression by suppressing the GLT1-inhibiting miRNAs (Men et al., 2019). Similarly, Frühbeis et al., demonstrated the reciprocal oligodendrocyte–neuron communication where transfer of exosomes from oligodendrocytes to neurons improved the neuronal viability under oxidative stress and starvation conditions (Frühbeis et al., 2013). A breakdown or abnormality in communication between brain cells could contribute to the onset or progression of neurodegenerative diseases. Therefore, EVs-mediated intercellular communication in the brain could play a critical role in the pathogenesis of Alzheimer’s disease. This notion is further supported by recent studies where neuron- and other brain cells (astrocytes, microglia, oligodendrocytes, pericytes, and endothelial)-derived EVs were extensively characterized as potential biomarkers for Alzheimer’s disease, highlighting the role of these cells as well as communication between them in the neurodegeneration process (Kumar et al., 2022; Kumar et al., 2023; Winston et al., 2016).
Literature suggests that SC-EVs from healthy cells, through multiple molecular routes, target Aβ production and removal of Aβ plaques, reduce neuroinflammation and oxidative stress, restore communication among brain cells, improve neuronal survival and growth, and, lastly, alleviate cognitive dysfunction. Since most of these effects are connected or interrelated, e.g., Aβ reduction could reduce oxidative stress as well as microglia activation and neuroinflammation and improve neuronal survival. Therefore, all studies describing SC-EVs’ therapeutic effects against Alzheimer’s disease are presented together below.
Numerous studies in Alzheimer’s disease animal models have shown the efficacy of SC-EVs in preventing neurodegeneration, neuroinflammation and alleviating cognitive decline. Wang et al., reported that treatment with MSC-EVs reduced hippocampal Aβ aggregation, neuronal loss, and improved cognition through repairing neuronal morphology, restoring neuronal excitability and mitochondrial changes in APP/PS1 mice (Wang et al., 2021). This study also suggested the role of the Nrf2 system in the therapeutic effects of MSC-EVs(Wang et al., 2021). Further, bone marrow MSC-EVs treatment improved cognition and repressed the levels of Aβ1–40, Aβ1–42, beta-secretase 1 (BACE1), and presenilin 1 (PS1), and promoted the expression of neprilysin in APP/PS1 mice, and these effects were mainly dependent upon the increased expression of sphingosine kinase (SphK) and sphingosine-1-phosphate (S1P)(Wang and Yang, 2021). Apodaca et al., demonstrated that the intra-venous injection of human neuronal SC-EVs in 2- and 6-months old 5xFAD mice restored fear extinction memory consolidation, reduced anxiety-related behaviors, and protected against synaptic loss as well as reduced Aβ plaque accumulation and microglial activation (Apodaca et al., 2021). Elia et al., reported that intra-cerebral injection of mouse bone marrow MSC-EVs into the neocortex of APP/PS1 mice not only promoted the disaggregation of pre-existing Aβ deposits but also prevented the formation of new plaques (Elia, Chiara A. et al., 2019). Furthermore, MSC-EVs reduced the formation of dystrophic neurites surrounding the Aβ plaques in APP/PS1 mice (Elia, Chiara A. et al., 2019). Furthermore, this study also reported that mouse bone marrow MSC express 100 times more neprilysin mRNA compared to fibroblasts and confirmed neprilysin expression in MSC-EVs, potentially contributing to the observed therapeutic effects of these vesicles (Elia, Chiara A. et al., 2019). Wang et al., suggested that the beneficial effects of MSC-EVs on cognitive behavior and hippocampal plasticity of APP/PS1 mice could probably be through suppressing inducible nitric oxide synthase (iNOS) expression (Wang, S.S. et al., 2018). Li et al., reported that treatment of neural SC-EVs in APP/PS1 mice enhanced the mitochondrial function, SIRT1 activation, synaptic activity, and decreased oxidative stress and inflammation, and rescued cognitive deficits without altering the Aβ level (Li et al., 2020). Bone marrow-derived MSC-EVs were reported to carry miR-29c-3p to the hippocampal neurons, inhibit BACE1 expression, and activate the Wnt/β-catenin pathway, thereby playing a therapeutic role in Alzheimer’s disease rat model (Sprague-Dawley rats injected with oligomer Aβ1–42) (Sha et al., 2021). MSC-EVs treatment also reduced the Aβ deposition area and plaques, decreased soluble Aβ1–42 levels in the cerebral cortex and hippocampus, and lowered the levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) (Sha et al., 2021).
While characterizing the therapeutic effects of SC-EVs, besides neurons, several studies have characterized the molecular changes in other brain cells, including astrocytes and microglia. Exosomes derived from human umbilical cord MSCs injection in APP/PS1 mice increased the spatial learning and memory function, reduced the Aβ deposition by increasing the levels of Aβ-degrading enzymes (neprilysin and insulin-degrading enzyme), activating M2-type microglia, and exerted an anti-inflammatory effect (Ding et al., 2018). Liu et al., demonstrated that in a sporadic Alzheimer’s disease mouse model, lateral ventricle administration but not the caudal vein injection of bone marrow-derived MSC exosomes improved the behavioral performance in streptozotocin (STZ)-treated mice (Liu et al., 2022). The therapeutic effects of MSC exosomes involved the regulation of glial activation and associated neuroinflammation and brain-derived neurotrophic factor (BDNF)-related neuropathological changes in the hippocampus (Liu et al., 2022). In a rat model of vascular dementia, treatment with human umbilical cord MSC-EVs activated the PI3K/AKT/NRF2 pathways and inhibited microglial M1 polarization, inflammation, and oxidative stress, thereby protecting the nerve functions (Wang, P. et al., 2023). Feng et al., showed that the administration of MSC-EVs alleviated the trained-immunity-induced increased load of Aβ in APP/PS1 mice (Feng, Y. et al., 2020). Interestingly, this study also found that MSC-EVs have comparable effects to those of MSCs in the mitigation of trained immunity in the brain (Feng, Y. et al., 2020). Adipose-derived MSC-EVs entered into the brain quickly and efficiently after intranasal administration and majorly accumulated in neurons as compared to microglia or astrocytes within the central nervous system (Ma, X. et al., 2020). Furthermore, MSC-EVs increased the number of newly formed neurons and rescued memory deficits in APP/PS1 transgenic mice. These MSC-EVs also reduced Aβ deposition and decreased microglia activation (Ma, X. et al., 2020). This study also characterized the cargo proteins of these EVs by proteomics analyses and identified several neuroprotective proteins such as neprilysin, neuroplastin, and eukaryotic translation initiation factor 5A (eIF5A) (Ma, X. et al., 2020).
Cell culture studies have provided a greater understanding of molecular mechanisms underlying the biological effects of SC-EVs outlined above. Katsuda et al., showed that adipose-derived MSCs and their exosomes express enzymatically active neprilysin that could reduce the Aβ levels in N2a cells, genetically modified to overproduce human Aβ (Katsuda et al., 2013). Adipose-derived MSC-exosomes reduced the Aβ42 and Aβ40 levels, reduced apoptosis, and improved neurite growth in differentiated neuronal stem cells isolated from TG2576 transgenic mice (Lee et al., 2018). Bone marrow MSC-exosomes treatment upregulated the expression of neprilysin and insulin-degrading enzyme through AKT/GSK-3β/β-catenin pathway, relieving the cellular damage caused by Aβ42 treatment in SH-SY5Y cells. These protective effects were attributed to the growth differentiation factor-15 (GDF-15) loaded in these exosomes (Xiong, W.P. et al., 2021). Calcium imbalance induced by Aβ could also affect synaptic plasticity and neuronal loss in Alzheimer’s disease (Pannaccione et al., 2020), and MSC-EVs treatment ameliorated the calcium transients in Aβ-stimulated primary hippocampal neurons(Wang et al., 2021). Similarly, Aβ-iNOS (inducible nitric oxide synthase) nexus is known to promote Alzheimer’s disease pathogenesis (Akama et al., 1998; Nathan et al., 2005), and MSC-EVs treatment inhibited iNOS mRNA and protein expression induced by Aβ or the higher iNOS expression in neurons derived from APP/PS1 mice (Wang, S.S. et al., 2018). Adipose-derived MSC-EVs also exerted a neuroprotective effect against the Aβ1–42 oligomer or glutamate-induced neuronal toxicity (Ma, X. et al., 2020). This study showed that following treatment of neurons with these MSC-EVs, several genes involved in synaptic function and memory were upregulated, while few genes related to cell death were downregulated (Ma, X. et al., 2020). MSC-EVs have also been reported to protect hippocampal neurons against oxidative stress and synapse damage induced by Aβ oligomers in cell culture (Bodart-Santos et al., 2019; de Godoy et al., 2018). Neuroprotection by MSC-EVs was mainly mediated by active catalase as cargo since their efficacy was abolished in the presence of catalase inhibitor aminotriazole (Bodart-Santos et al., 2019). MSC-EVs treatment also inhibited the LPS-induced activation of pro-inflammatory microglia phenotype (Garcia-Contreras and Thakor, 2021; Zavatti et al., 2022). Similarly, MSC-EVs suppressed the pro-inflammatory effects of Aβ on microglia cells (Kaniowska et al., 2022). This effect of MSC-EVs seems to be partially mediated by restoration of the expression of cell surface receptor CD36, a class B scavenger receptor expressed on microglia. Exosomes isolated from bone marrow MSCs transfected with miR-146a were taken up by astrocytes, resulting in an increased level of miR-146a and a decreased level of tumor necrosis factor receptor-associated factor 6 (TRAF6) and NF-κB (Nakano et al., 2020). These results, along with others, suggested that restoration of astrocytic function leads to synaptogenesis and correction of cognitive impairment (Nakano et al., 2020).
The composition of exosomal cargo is significantly affected by physiological or pathological conditions (Ramteke et al., 2015; Schlaepfer et al., 2015). The culture settings (e.g., hypoxic condition, cytokines or LPS priming or 3-dimensional [3D] growth conditions) for stem cell affects their cargo as well as their therapeutic effects(Saparov et al., 2016; Xin et al., 2012; Yang, L. et al., 2020). Yang et al reported that human umbilical cord MSCs secreted exosomes have better therapeutic properties when cells were cultured on a 3D graphene scaffold versus 2D graphene (Yang, L. et al., 2020). 3D-exosomes reduced the secreted and intracellular Aβ by increasing the expression of α-secretase while lowering the expression of β-secretase in both Alzheimer’s disease pathology cells and transgenic mice (Yang, L. et al., 2020). 3D-exosomes also exerted enhanced therapeutic effects on ameliorating memory and cognitive deficits as well as in mitigating the inflammation and oxidative stress in the brain of APP/PS1 mice (Yang, L. et al., 2020). This study also showed that these improved therapeutic effects were related to the cargo (miRNAs and proteins) of the exosomes secreted by cells when cultured over 3D scaffolds (Yang, L. et al., 2020). For example, 3D-exosomes showed enrichment of neprilysin, insulin-degrading enzyme, and heat shock protein 70 (HSP70) as compared to 2D-exosomes. Similarly, Cui et al., reported the enhanced therapeutic effects of exosomes derived from hypoxia-preconditioned MSCs (Cui et al., 2018). Learning and memory capabilities were improved, Aβ levels and plaque deposition were reduced by increasing the level of miR-21 in APP/PS1 mice treated with exosomes isolated from hypoxia-preconditioned MSCs (Cui et al., 2018). Furthermore, pro-inflammatory factors (TNF-α and IL-1β) were reduced, and anti-inflammatory factors (IL-4 and IL-10) were increased in the brain tissues of mice treated with exosomes from hypoxia-preconditioned MSCs (Cui et al., 2018). In another study, Markoutsa et al., first primed the MSCs with the secretome of LPS- or Aβ-activated microglia cells (Markoutsa et al., 2022). EVs from primed cells were more effective in inhibiting microglia and astrocyte activation, amyloid deposition, demyelination, memory loss, and motor and anxiety-like behavioral dysfunction compared to EVs from non-primed cells (Markoutsa et al., 2022). MicroRNA profiling identified the upregulation of at least 19 miRNAs in EVs from primed cells, offering a mechanistic understanding of their higher therapeutic efficacy (Markoutsa et al., 2022). Losurdo et al., reported that EVs derived from cytokines (TNF-α and IFN-γ) preconditioned MSCs induced immunomodulatory and neuroprotective effects (Losurdo et al., 2020). Treatment with these EVs polarized the murine primary microglia toward an anti-inflammatory phenotype in vitro, and their intranasal administration showed neuroprotective effects in a triple-transgenic 3xTg mice model of Alzheimer’s disease (Losurdo et al., 2020). Hou et al. reported an enhanced therapeutic efficacy of MSC-derived exosomes in 5xFAD mice after administering broad-spectrum antibiotics, which led to the depletion of gut microbiota and their associated metabolites, overcoming resistance (Hou et al., 2023). This study suggested that the efficacy of SC-EVs could also be affected by other variables, such as gut microbiome dysbiosis or antibiotics.
5.1. SC-EVs as a vehicle for targeted delivery of cargo against Alzheimer’s disease
EVs can cross the blood-brain barrier and deliver their cargo to the brain (Qu et al., 2018; Wang et al., 2019). Therefore, EVs have also been explored as a potential vehicle for transporting specific therapeutic molecules to the brain to treat Alzheimer’s disease (Iranifar et al., 2019). Moreover, exosomes improve the bioavailability of the drug across the blood-brain barrier (Wang et al., 2019). Perets et al., labeled bone marrow MSC-exosomes with gold nanoparticles and tracked their migration and homing patterns after intranasal administration in different brain pathologies, including stroke, autism, Parkinson’s disease, and Alzheimer’s disease. They found that MSC-exosomes specifically targeted and accumulated in pathologically relevant brain regions up to 96 hours post-administration, while in healthy controls, a diffuse migration pattern and clearance by 24 hours was observed. The MSC-exosomes accumulation was correlated with the neuro-inflammatory signal in pathological brains, suggesting that their homing mechanism was inflammation-driven (Perets et al., 2019).
EVs have been bioengineered for loading cargo or targeted delivery to brain cells. For instance, surface-engineered exosomes were utilized for specific delivery of siRNA for BACE1 (a therapeutic target in Alzheimer’s disease) to mouse brain (Alvarez-Erviti et al., 2011). These exosomes were derived from engineered dendritic cells to express Lamp2b, an exosomal membrane protein fused to the neuron-specific rabies viral glycoprotein (RVG) peptide. These exosomes could selectively bind to acetylcholine receptor because of the Rabies virus glycoprotein fused to Lamp2b on their surface (Alvarez-Erviti et al., 2011). In a similar manner, MSC-exosomes were tagged with RVG peptide for the targeted delivery in APP/PS1 mice. The targeted binding increased the concentration of intravenously administered exosomes in the hippocampus and cortex of mice (Cui et al., 2019). Targeted MSC-exosome delivery sharply decreased the plaque deposition as well as Aβ40 and Aβ42 levels in the hippocampus and cortex as compared to untargeted delivery. Furthermore, it improved the cognitive function in APP/PS1 mice, and reduced the expression of pro-inflammatory mediators TNF-α, IL-β, IL-6, and significantly raised the levels of anti-inflammatory mediators IL-10, IL-4, IL-13 (Cui et al., 2019). Engineered MSC-EVs with high expression of Src homology 2 domain-containing protein tyrosine phosphatase-2 (SHP2) facilitated delivery to the brain by crossing the blood-brain barrier in Aβ1–42 treated C57BL/6 mice. These EVs reduced the Aβ1–42 accumulation and induced mitophagy in neurons, which further diminished the neuronal cells’ apoptosis and neuroinflammation (Xu et al., 2022). These EVs also rescued synaptic loss and cognitive decline in Aβ1–42 treated mice (Xu et al., 2022). Adipose-derived MSC-EVs loaded with miRNA-22 enhanced motor ability in APP/PS1 mice and reduced the expression of inflammatory factors (Zhai et al., 2021).
As mentioned above, neprilysin is an Aβ degrading enzyme; thereby, it offers a potential therapeutic tool against Alzheimer’s disease via exosome-mediated delivery of neprilysin to the brain. Izadpanah et al., used the mouse bone marrow MSC-EVs for the delivery of neprilysin (Izadpanah et al., 2020). MSC-EVs were loaded with neprilysin by freeze-thaw cycle and then administrated intranasally in a rat model of Alzheimer’s disease. This study demonstrated that the MSC-EVs loaded with neprilysin decreased the expression of IL-1β and BCL2 associated X (BAX) but increased the B-cell leukemia 2 (Bcl2) expression in the rat brain. Furthermore, MSC-EVs loaded neprilysin improved the brain-related behavioral functions and memory improvement (Izadpanah et al., 2020).
The abovementioned studies suggest the promising role of SC-EVs as a novel therapeutic option against Alzheimer’s disease.
6. Challenges, Future Directions, and Conclusion
SC-EVs have displayed remarkable therapeutic potential in the delay of aging, overcoming aging-associated disorders, and the mitigation of Alzheimer’s disease. However, it is crucial to acknowledge that the majority of SC-EVs testing has occurred in preclinical models, particularly in the context of Alzheimer’s disease. For the promise of SC-EVs to be realized in clinical settings, substantial efforts are required to develop safe and effective strategies for their use in addressing aging-related complications.
One of the primary challenges lies in the production of clinical-grade SC-EVs with minimal batch-to-batch variation. As previously mentioned, the biological properties and effects of EVs can be influenced by various factors, including the type of stem cells, the age of the source individual (young versus old), physiological state, passage number of the cell, and culture condition. For instance, exosomes secreted by early passage rat bone marrow MSCs exhibited more efficient neuroprotection compared to later passage cells, and the neuroprotective effectiveness of exosome was dosage dependent (Venugopal et al., 2017). The inherent heterogeneity of EVs in biofluids introduces an additional layer of complexity. This complexity is further compounded by the use of various methods to isolate EVs, with some yielding a purer population but lower concentrations and others providing higher yields with relatively lower purity. Most importantly, the method of EV isolation can impact the final product and its therapeutic benefits significantly.
Another challenge related to SC-EVs’ quality is ensuring that cultured stem cells maintain their inherent properties, particularly after multiple passages. Culturing stem cells in three-dimensional (3D) conditions, which mimic native tissue architecture, has shown promise. EVs isolated from 3D MSC cultures, for example, reduced the amyloid plaque load in the hippocampus of 5XFAD mice following intranasal administration (Cone et al., 2021). Bioreactors have also been explored to grow cells in 3D physiological conditions for EV isolation, offering the potential for significant increases in EV production.
The biodistribution and bioavailability of SC-EVs present another potential challenge. Several studies have indicated the relatively quick clearance of EVs and varying uptake and accumulation in specific cell types and organs. However, new tools are emerging to improve bioavailability and targeted delivery of SC-EVs. For example, as stem cells are applied with the localized carrier agents to prolong their presence in the treated area, SC-EVs could be cross-linked with protective biocompatible and biodegradable biopolymer-based formulations to improve their bioavailability. Furthermore, SC-EVs surface could be appropriately modified for their targeted delivery and sustained release, further enhancing their regenerative and rejuvenating efficacy. For instance, Arg-Gly-Asp (RGD) hydrogel increased the retention and stability of MSC-EVs. Hydrogel functionalization also augmented MSC-EV efficacy in the treatment of acute kidney injury (Zhang, C. et al., 2020). Similarly, specific surface tags (e.g., aptamers and peptides) could be presented on EVs for delivery to specific cell or tissue types and to improve their efficiency. Zhang et al., showed that human umbilical cord MSC-exosomes could not readily penetrate through porcine skin ex vivo. However, when combined with sponge Haliclona sp. Spicules, the absorption of exosomes was strongly increased through creating microchannels. The combined therapy showed significant anti-photoaging effects in mice, including reducing micro-wrinkles, alleviating histopathological changes, and promoting the expression of extracellular matrix constituents (Zhang, K. et al., 2020).
Overall, SC-EVs have demonstrated a broad spectrum of efficacy in addressing aging-related complications, including Alzheimer’s disease, through a multitude of mechanisms. SC-EVs are also emerging as valuable biological nano-vehicles for specific cargo or drug delivery, particularly to the brain, offering a less invasive approach. There are ongoing efforts at multiple levels to overcome the challenges associated with the clinical translation of this promising nanotherapeutics option.
Highlights.
SC-EVs have demonstrated therapeutic efficacy in addressing aging and Alzheimer’s disease.
SC-EVs reduce the age-related senescence and tissue dysfunctions.
The therapeutic potential of SC-EVs is dependent on the source and culture conditions.
SC-EVs degrade amyloid beta via neprilysin-mediated mechanisms.
SC-EVs serve as a vehicle for targeted delivery of therapeutic cargo in Alzheimer’s disease.
Acknowledgments
Funding source
This work was supported by the National Institute of Health grant R01 AG061805, R21 AG075611, R01 AG061805 (to GD).
Footnotes
Conflict of interest
G.D. is the founder of LiBiCo, which has no influence or contribution to the work presented in this manuscript.
Declaration of generative artificial intelligence (AI) in scientific writing
In this manuscript, no AI or AI-assisted technologies were used in the writing process.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2016. NIH Stem Cell Information Home Page. In Stem Cell Information. National Institutes of Health, U.S. Department of Health and Human Services, 2016. Available at Clinical Trial. https://stemcells.nih.gov/info/basics/stc-basics. (Accessed 2/12/2023 2023).
- 2019. FDA Warns About Stem Cell Therapies. https://www.fda.gov/consumers/consumer-updates/fda-https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapieswarns-about-stem-cell-therapies. (Accessed 6/1/22 2022).
- 2022. 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 16(3), 391–460. [DOI] [PubMed] [Google Scholar]
- Abbasi Sourki P, Pourfathollah AA, Kaviani S, Soufi Zomorrod M, Ajami M, Wollenberg B, Multhoff G, Bashiri Dezfouli A, 2023. The profile of circulating extracellular vesicles depending on the age of the donor potentially drives the rejuvenation or senescence fate of hematopoietic stem cells. Exp Gerontol 175, 112142. [DOI] [PubMed] [Google Scholar]
- Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA, Blanchard S, Moats RA, Garcia E, Aramburo S, Valenzuela VV, Frank RT, Barish ME, Brown CE, Kim SU, Badie B, Portnow J, 2013. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med 5(184), 184ra159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B, 1998. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health 88(10), 1452–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M, Rezaie J, 2021. Ageing and mesenchymal stem cells derived exosomes: Molecular insight and challenges. Cell Biochem Funct 39(1), 60–66. [DOI] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ, 1998. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci U S A 95(10), 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers JC, Gonda D, Kim R, Carter BS, Chen CC, 2013. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA, 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology 29(4), 341–345. [DOI] [PubMed] [Google Scholar]
- Apodaca LA, Baddour AAD, Garcia C, Alikhani L, Giedzinski E, Ru N, Agrawal A, Acharya MM, Baulch JE, 2021. Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimer’s Research & Therapy 13(1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian B, Zhao C, He X, Gong Y, Ren C, Ge L, Zeng Y, Li Q, Chen M, Weng C, He J, Fang Y, Xu H, Yin ZQ, 2020. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. Journal of Extracellular Vesicles 9(1), 1748931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodart-Santos V, de Carvalho LRP, de Godoy MA, Batista AF, Saraiva LM, Lima LG, Abreu CA, De Felice FG, Galina A, Mendez-Otero R, Ferreira ST, 2019. Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. Stem Cell Res Ther 10(1), 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulestreau J, Maumus M, Jorgensen C, Noel D, 2021. Extracellular vesicles from mesenchymal stromal cells: Therapeutic perspectives for targeting senescence in osteoarthritis. Adv Drug Deliv Rev 175, 113836. [DOI] [PubMed] [Google Scholar]
- Boulestreau J, Maumus M, Rozier P, Jorgensen C, Noel D, 2020. Mesenchymal Stem Cell Derived Extracellular Vesicles in Aging. Front Cell Dev Biol 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowley MP, Cabral H, Rosene DL, Peters A, 2010. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. Journal of Comparative Neurology 518(15), 3046–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody M, Agronin M, Herskowitz BJ, Bookheimer SY, Small GW, Hitchinson B, Ramdas K, Wishard T, McInerney KF, Vellas B, Sierra F, Jiang Z, McClain-Moss L, Perez C, Fuquay A, Rodriguez S, Hare JM, Oliva AA Jr., Baumel B, 2023. Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer’s disease. Alzheimers Dement 19(1), 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC, 2009. Ovarian Aging: Mechanisms and Clinical Consequences. Endocrine Reviews 30(5), 465–493. [DOI] [PubMed] [Google Scholar]
- Cai Z-Y, Xiao M, Quazi SH, Ke Z-Y, 2018. Exosomes: a novel therapeutic target for Alzheimer’s disease? Neural regeneration research 13(5), 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Jin S, Wang P, He Q, Yang Y, Gao Z, Wang X, 2021. Microneedle based adipose derived stem cells-derived extracellular vesicles therapy ameliorates UV-induced photoaging in SKH-1 mice. J Biomed Mater Res A 109(10), 1849–1857. [DOI] [PubMed] [Google Scholar]
- Cecerska-Heryc E, Pekala M, Serwin N, Glizniewicz M, Grygorcewicz B, Michalczyk A, Heryc R, Budkowska M, Dolegowska B, 2023. The Use of Stem Cells as a Potential Treatment Method for Selected Neurodegenerative Diseases: Review. Cell Mol Neurobiol 43(6), 2643–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha H, Hong S, Park JH, Park HH, 2020. Stem Cell-Derived Exosomes and Nanovesicles: Promotion of Cell Proliferation, Migration, and Anti-Senescence for Treatment of Wound Damage and Skin Ageing. Pharmaceutics 12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Sun Y, Zhang J, Zhu Q, Yang Y, Niu X, Deng Z, Li Q, Wang Y, 2019. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Research & Therapy 10(1), 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-X, Liang F-C, Gu P, Xu B-L, Xu H-J, Wang W-T, Hou J-Y, Xie D-X, Chai X-Q, An S-J, 2020. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death & Disease 11(4), 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu J, Lin F, Xu Y, Feng B, Feng X, Sheng X, Shi X, Pan Q, Yang J, Yu J, Li L, Cao H, 2021. Human placenta mesenchymal stem cell-derived exosomes delay H(2)O(2)-induced aging in mouse cholangioids. Stem Cell Res Ther 12(1), 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Jiang Y, Duan Y, Zhang X, Li X, 2022. Mesenchymal-Stem-Cell-Based Strategies for Retinal Diseases. Genes (Basel) 13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Lu CH, Ke CC, Liu RS, 2021. Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapy for Alzheimer’s Disease: Progress and Opportunity. Membranes (Basel) 11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleen Davis AD, Drewry Michelle, Helwa Inas, Johnson Maribeth H, Isales Carlos M, Hill William D, Liu Yutao, Shi Xingming, Fulzele Sadanand, and Hamrick Mark W., 2017. MicroRNA-183–5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Engineering Part A 23(21–22), 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Théry C, 2014. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annual Review of Cell and Developmental Biology 30(1), 255–289. [DOI] [PubMed] [Google Scholar]
- Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, Kenyon SM, Carver SR, Benthem SD, Stimmell AC, Moseley SC, Hike D, Grant SC, Wilber AA, Olcese JM, Meckes DG Jr., 2021. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 11(17), 8129–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, Liu JS, Dong YR, Hou SX, Liu JR, 2019. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immunity & ageing : I & A 16, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD, 2018. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32(2), 654–668. [DOI] [PubMed] [Google Scholar]
- Dacks PA, Moreno CL, Kim ES, Marcellino BK, Mobbs CV, 2013. Role of the hypothalamus in mediating protective effects of dietary restriction during aging. Frontiers in Neuroendocrinology 34(2), 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan QQ, Chen L, Shi LL, Zhou X, Wang TH, Liu H, 2023. Urine-derived mesenchymal stem cells-derived exosomes enhances survival and proliferation of aging retinal ganglion cells. BMC Mol Cell Biol 24(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro LL, Xisto DG, Kitoko JZ, Cruz FF, Olsen PC, Redondo PAG, Ferreira TPT, Weiss DJ, Martins MA, Morales MM, Rocco PRM, 2017. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res Ther 8(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB, Silva LRP, Leal RB, Monteiro VHS, Braga CV, de Araujo-Silva CA, Sinis LC, Bodart-Santos V, Kasai-Brunswick TH, Alcantara CL, Lima A, da Cunha ESNL, Galina A, Vieyra A, De Felice FG, Mendez-Otero R, Ferreira ST, 2018. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem 293(6), 1957–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, Alessandri G, Pessina A, Perrotta A, Fierabracci A, Muraca M, 2015. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opinion on Biological Therapy 15(4), 495–504. [DOI] [PubMed] [Google Scholar]
- Demurtas J, Fanelli GN, Romano SL, Solari M, Yang L, Soysal P, Lopez Sanchez GF, Grabovac I, Smith L, Zorzi A, Luchini C, Veronese N, 2021. Stem cells for treatment of cardiovascular diseases: An umbrella review of randomized controlled trials. Ageing Res Rev 67, 101257. [DOI] [PubMed] [Google Scholar]
- Desdin-Mico G, Mittelbrunn M, 2017. Role of exosomes in the protection of cellular homeostasis. Cell Adh Migr 11(2), 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Béland C, Dhahri W, Cailhier JF, Rousseau M, Duchez AC, Lévesque T, Lau A, Rondeau C, Gingras D, Muruve D, Rivard A, Cardinal H, Perreault C, Desjardins M, Boilard É, Thibault P, Hébert MJ, 2015. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med 7(318), 318ra200. [DOI] [PubMed] [Google Scholar]
- Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H, 2018. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochemical research 43(11), 2165–2177. [DOI] [PubMed] [Google Scholar]
- Dong J, Sakai K, Koma Y, Watanabe J, Liu K, Maruyama H, Sakaguchi K, Hibi H, 2021. Dental pulp stem cell-derived small extracellular vesicle in irradiation-induced senescence. Biochem Biophys Res Commun 575, 28–35. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang Y, Zheng T, Pu Y, Ma Y, Qi X, Zhang W, Xue F, Shan Z, Liu J, Wang X, Mao C, 2021. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Research & Therapy 12(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso-Quezada J, Ayala-Mar S, González-Valdez J, 2021. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 22(7), 204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorronsoro A, Santiago FE, Grassi D, Zhang T, Lai RC, McGowan SJ, Angelini L, Lavasani M, Corbo L, Lu A, Brooks RW, Garcia-Contreras M, Stolz DB, Amelio A, Boregowda SV, Fallahi M, Reich A, Ricordi C, Phinney DG, Huard J, Lim SK, Niedernhofer LJ, Robbins PD, 2021. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 20(4), e13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbrava DA, Surugiu R, Borger V, Ruscu M, Tertel T, Giebel B, Hermann DM, Popa-Wagner A, 2022. Mesenchymal stromal cell-derived small extracellular vesicles promote neurological recovery and brain remodeling after distal middle cerebral artery occlusion in aged rats. Geroscience 44(1), 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia CA, Losurdo M, Malosio ML, Coco S, 2019. Extracellular Vesicles from Mesenchymal Stem Cells Exert Pleiotropic Effects on Amyloid-beta, Inflammation, and Regeneration: A Spark of Hope for Alzheimer’s Disease from Tiny Structures? Bioessays 41(4), e1800199. [DOI] [PubMed] [Google Scholar]
- Elia CA, Tamborini M, Rasile M, Desiato G, Marchetti S, Swuec P, Mazzitelli S, Clemente F, Anselmo A, Matteoli M, Malosio ML, Coco S, 2019. Intracerebral Injection of Extracellular Vesicles from Mesenchymal Stem Cells Exerts Reduced Aβ Plaque Burden in Early Stages of a Preclinical Model of Alzheimer’s Disease. Cells 8(9), 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Liang W, 2021. ASCs -derived exosomes loaded with vitamin A and quercetin inhibit rapid senescence-like response after acute liver injury. Biochem Biophys Res Commun 572, 125–130. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB, 1994. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 151(1), 54–61. [DOI] [PubMed] [Google Scholar]
- Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z, 2020. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. Journal of Extracellular Vesicles 9(1), 1783869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Guo M, Zhao H, Han S, Dong Q, Cui M, 2020. Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Mitigate Trained Immunity in the Brain. Front Bioeng Biotechnol 8, 599058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtel P, von Bonin M, Kuhnert R, Mobus K, Bornhauser M, Wobus M, 2022. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Modulate Hematopoietic Stem and Progenitor Cell Viability and the Expression of Cell Cycle Regulators in an Age-dependent Manner. Front Bioeng Biotechnol 10, 892661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave K-A, Schneider A, Simons M, Klugmann M, Trotter J, Krämer-Albers E-M, 2013. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte–Neuron Communication. PLOS Biology 11(7), e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi M, 2016. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med 5(12), 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Feng Q, Cui B, Mao Y, Zhao Z, Liu Z, Zhu H, 2023. Loading Nanoceria Improves Extracellular Vesicle Membrane Integrity and Therapy to Wounds in Aged Mice. ACS biomaterials science & engineering 9(2), 732–742. [DOI] [PubMed] [Google Scholar]
- Gao W, Wang X, Si Y, Pang J, Liu H, Li S, Ding Q, Wang Y, 2021. Exosome Derived from ADSCs Attenuates Ultraviolet B-mediated Photoaging in Human Dermal Fibroblasts. Photochemistry and photobiology 97(4), 795–804. [DOI] [PubMed] [Google Scholar]
- Gao Z, Pang B, Li J, Gao N, Fan T, Li Y, 2021. Emerging Role of Exosomes in Liquid Biopsy for Monitoring Prostate Cancer Invasion and Metastasis. Frontiers in Cell and Developmental Biology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Contreras M, Thakor AS, 2021. Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Discov 7(1), 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL, 2020. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. GeroScience 42(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go V, Sarikaya D, Zhou Y, Bowley BGE, Pessina MA, Rosene DL, Zhang ZG, Chopp M, Finklestein SP, Medalla M, Buller B, Moore TL, 2021. Extracellular vesicles derived from bone marrow mesenchymal stem cells enhance myelin maintenance after cortical injury in aged rhesus monkeys. Experimental Neurology 337, 113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpanian S, DiFede DL, Khan A, Schulman IH, Landin AM, Tompkins BA, Heldman AW, Miki R, Goldstein BJ, Mushtaq M, Levis-Dusseau S, Byrnes JJ, Lowery M, Natsumeda M, Delgado C, Saltzman R, Vidro-Casiano M, Pujol MV, Da Fonseca M, Oliva AA Jr, Green G., Premer C., Medina A., Valasaki K., Florea., Anderson E., El-Khorazaty J., Mendizabal A., Goldschmidt-Clermont PJ., Hare JM., 2017. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. The Journals of Gerontology: Series A 72(11), 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves RGJ, Vasques JF, da Silva-Junior AJ, Gubert F, Mendez-Otero R, 2023. Mesenchymal stem cell- and extracellular vesicle-based therapies for Alzheimer’s disease: progress, advantages, and challenges. Neural Regen Res 18(8), 1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Chen B, Zhang J, Sun Y, Yuan J, Niu X, Hu G, Chen Y, Xie Z, Deng Z, Li Q, Wang Y, 2020. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. Journal of Extracellular Vesicles 9(1), 1800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV, 2020. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Frontiers in Cell and Developmental Biology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JA, Yu PJ, Yang DQ, Chen W, 2022. The Antisenescence Effect of Exosomes from Human Adipose-Derived Stem Cells on Skin Fibroblasts. Biomed Res Int 2022, 1034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Yin Z, Chen F, Lei P, 2020. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res Ther 12(1), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Zhou J, Liu B, Liang C, Pan X, Zhang Y, Zhang Y, Wang Y, Shao L, Zhu B, Wang J, Yin Q, Yu X-Y, Li Y, 2019. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Materials Science and Engineering: C 99, 322–332. [DOI] [PubMed] [Google Scholar]
- Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C, Shi Y, 2022. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther 7(1), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE, Garcia E, 2021. Mesenchymal Stem Cell Therapy for Alzheimer’s Disease. Stem Cells Int 2021, 7834421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessvik NP, Llorente A, 2018. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75(2), 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman RA, Faustin A, Wisniewski T, 2016. Alzheimer Disease and Its Growing Epidemic: Risk Factors, Biomarkers, and the Urgent Need for Therapeutics. Neurologic clinics 34(4), 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, He H, Jiang G, Zhang H, Tao W, Ding Y, Yuan D, Liu J, Fan H, Lin F, Liang X, Li X, Zhang Y, 2020. miR-155–5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell 19(4), e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Yamashita T, Morita T, Oshigiri T, Hirota R, Iyama S, Kato J, Sasaki Y, Ishiai S, Ito YM, Namioka A, Namioka T, Nakazaki M, Kataoka-Sasaki Y, Onodera R, Oka S, Sasaki M, Waxman SG, Kocsis JD, 2021. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clinical Neurology and Neurosurgery 203, 106565. [DOI] [PubMed] [Google Scholar]
- Hou X, Jiang H, Liu T, Yan J, Zhang F, Zhang X, Zhao J, Mu X, Jiang J, 2023. Depletion of gut microbiota resistance in 5xFAD mice enhances the therapeutic effect of mesenchymal stem cell-derived exosomes. Biomed Pharmacother 161, 114455. [DOI] [PubMed] [Google Scholar]
- Hu G, Xia Y, Chen B, Zhang J, Gong L, Chen Y, Li Q, Wang Y, Deng Z, 2021. ESC-sEVs Rejuvenate Aging Hippocampal NSCs by Transferring SMADs to Regulate the MYT1-Egln3-Sirt1 Axis. Mol Ther 29(1), 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Xu R, Chen CY, Rao SS, Xia K, Huang J, Yin H, Wang ZX, Cao J, Liu ZZ, Tan YJ, Luo J, Xie H, 2019. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism 95, 93–101. [DOI] [PubMed] [Google Scholar]
- Huang B, Lu J, Ding C, Zou Q, Wang W, Li H, 2018. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther 9(1), 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Qin C, Wang J, Hu Y, Zheng G, Qiu G, Ge M, Tao H, Shu Q, Xu J, 2019. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY) 11(18), 7996–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Ijaz F, 2021. Current understandings of the relationship between extracellular vesicles and cilia. J Biochem 169(2), 139–145. [DOI] [PubMed] [Google Scholar]
- Illhardt T, Toporski J, Feuchtinger T, Turkiewicz D, Teltschik H-M, Ebinger M, Schwarze C-P, Holzer U, Lode HN, Albert MH, Gruhn B, Urban C, Dykes JH, Teuffel O, Schumm M, Handgretinger R, Lang P, 2018. Haploidentical Stem Cell Transplantation for Refractory/Relapsed Neuroblastoma. Biology of Blood and Marrow Transplantation 24(5), 1005–1012. [DOI] [PubMed] [Google Scholar]
- Iranifar E, Seresht BM, Momeni F, Fadaei E, Mehr MH, Ebrahimi Z, Rahmati M, Kharazinejad E, Mirzaei H, 2019. Exosomes and microRNAs: New potential therapeutic candidates in Alzheimer disease therapy. Journal of Cellular Physiology 234(3), 2296–2305. [DOI] [PubMed] [Google Scholar]
- Izadpanah M, Dargahi L, Ai J, Asgari Taei A, Ebrahimi Barough S, Mowla SJ, TavoosiDana G, Farahmandfar M, 2020. Extracellular Vesicles as a Neprilysin Delivery System Memory Improvement in Alzheimer’s Disease. Iran J Pharm Res 19(2), 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraman M, Rajendran RL, Muthu S, Jeyaraman N, Sharma S, Jha SK, Muthukanagaraj P, Hong CM, Furtado da Fonseca L, Santos Duarte Lana JF, Ahn BC, Gangadaran P, 2023. An update on stem cell and stem cell-derived extracellular vesicle-based therapy in the management of Alzheimer’s disease. Heliyon 9(7), e17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Qiu S, Xu J, Kang Q, Chai Y, 2020. Exosomes Secreted by Young Mesenchymal Stem Cells Promote New Bone Formation During Distraction Osteogenesis in Older Rats. Calcif Tissue Int 106(5), 509–517. [DOI] [PubMed] [Google Scholar]
- Jin S, Wang Y, Wu X, Li Z, Zhu L, Niu Y, Zhou Y, Liu Y, 2023. Young Exosome Bio-Nanoparticles Restore Aging-Impaired Tendon Stem/Progenitor Cell Function and Reparative Capacity. Adv Mater 35(18), e2211602. [DOI] [PubMed] [Google Scholar]
- Kaniowska D, Wenk K, Rademacher P, Weiss R, Fabian C, Schulz I, Guthardt M, Lange F, Greiser S, Schmidt M, Braumann UD, Emmrich F, Koehl U, Jaimes Y, 2022. Extracellular Vesicles of Mesenchymal Stromal Cells Can be Taken Up by Microglial Cells and Partially Prevent the Stimulation Induced by beta-amyloid. Stem Cell Rev Rep 18(3), 1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian M, Nouri N, Ghasemi LV, Mohammadi AH, Behjati M, 2023. Administration of stem cells against cardiovascular diseases with a focus on molecular mechanisms: Current knowledge and prospects. Tissue Cell 81, 102030. [DOI] [PubMed] [Google Scholar]
- Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T, 2013. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri M, Richardson LA, Meulia T, 2018. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther 9(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, Yamamoto M, Okita K, Nakagawa M, Parmar M, Takahashi J, 2017. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 548(7669), 592–596. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lim H, Lee D, Choi SJ, Oh W, Yang YS, Oh JS, Hwang HH, Jeon HB, 2018. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer’s disease model. Scientific Reports 8(1), 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kim S, Su Y, Sharma M, Kumar P, Singh S, Lee J, Furdui CM, Singh R, Hsu FC, Kim J, Whitlow CT, Nader MA, Deep G, 2021. Brain cell-derived exosomes in plasma serve as neurodegeneration biomarkers in male cynomolgus monkeys self-administrating oxycodone. Ebiomedicine 63, 103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sharma M, Su Y, Singh S, Hsu FC, Neth BJ, Register TC, Blennow K, Zetterberg H, Craft S, Deep G, 2022. Small extracellular vesicles in plasma reveal molecular effects of modified Mediterranean-ketogenic diet in participants with mild cognitive impairment. Brain Commun 4(6), fcac262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Su Y, Sharma M, Singh S, Kim S, Peavey JJ, Suerken CK, Lockhart SN, Whitlow CT, Craft S, Hughes TM, Deep G, 2023. MicroRNA expression in extracellular vesicles as a novel blood-based biomarker for Alzheimer’s disease. Alzheimers Dement. [DOI] [PubMed] [Google Scholar]
- Lai RC, Tan SS, Yeo RWY, Choo ABH, Reiner AT, Su Y, Shen Y, Fu Z, Alexander L, Sze SK, Lim SK, 2016. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. Journal of Extracellular Vesicles 5(1), 29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Cha H, Park JH, 2020. Derivation of Cell-Engineered Nanovesicles from Human Induced Pluripotent Stem Cells and Their Protective Effect on the Senescence of Dermal Fibroblasts. Int J Mol Sci 21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Fang X, Gupta N, Serikov V, Matthay MA, 2009. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A 106(38), 16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Lee J, Kim HK, Yeom SH, Woo CH, Jung YJ, Yun YE, Park SY, Han J, Kim E, Sul JH, Jung JM, Park JH, Choi JS, Cho YW, Jo D-G, 2021. Extracellular vesicles from adipose tissue-derived stem cells alleviate osteoporosis through osteoprotegerin and miR-21–5p. Journal of Extracellular Vesicles 10(12), e12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Ban JJ, Yang S, Im W, Kim M, 2018. The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res 1691, 87–93. [DOI] [PubMed] [Google Scholar]
- Lee YI, Kim S, Kim J, Kim J, Chung KB, Lee JH, 2021. Randomized controlled study for the anti-aging effect of human adipocyte-derived mesenchymal stem cell media combined with niacinamide after laser therapy. Journal of Cosmetic Dermatology 20(6), 1774–1781. [DOI] [PubMed] [Google Scholar]
- Lei J, Jiang X, Li W, Ren J, Wang D, Ji Z, Wu Z, Cheng F, Cai Y, Yu ZR, Belmonte JCI, Li C, Liu GH, Zhang W, Qu J, Wang S, 2022. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell 13(3), 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Gao F, Liu T, Ren W, Chen L, Cao Y, Chen W, Guo S, Zhang Q, Chen W, Wang H, Chen Z, Li Q, Hu Y, Guo A-Y, 2021. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow–derived mesenchymal stem cells and slow age-related degeneration. Science Translational Medicine 13(578), eaaz8697. [DOI] [PubMed] [Google Scholar]
- Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC, 2020. Transplantation of ACE2(−) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis 11(2), 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Liu J, Gu G, Han X, Zhang Q, Zhang W, 2020. Impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. Journal of Neurochemistry 154(5), e15001. [DOI] [PubMed] [Google Scholar]
- Li J, Huang Y, Sun H, Yang L, 2023. Mechanism of mesenchymal stem cells and exosomes in the treatment of age-related diseases. Front Immunol 14, 1181308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang Y, Cai D, 2012. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nature cell biology 14(10), 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Gregory RI, 2008. MicroRNA Regulation of Stem Cell Fate. Cell Stem Cell 2(3), 195–196. [DOI] [PubMed] [Google Scholar]
- Li Q, Niu X, Yi Y, Chen Y, Yuan J, Zhang J, Li H, Xia Y, Wang Y, Deng Z, 2023. Inducible Pluripotent Stem Cell-Derived Small Extracellular Vesicles Rejuvenate Senescent Blood-Brain Barrier to Protect against Ischemic Stroke in Aged Mice. ACS Nano 17(1), 775–789. [DOI] [PubMed] [Google Scholar]
- Li TT, Wang ZR, Yao WQ, Linghu EQ, Wang FS, Shi L, 2022. Stem Cell Therapies for Chronic Liver Diseases: Progress and Challenges. Stem Cells Transl Med 11(9), 900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW, 2005. The role of therapeutic angiogenesis in tissue repair and regeneration. Advances in skin & wound care 18(9), 491–500; quiz 501–492. [DOI] [PubMed] [Google Scholar]
- Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M, 2018. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Experimental & Molecular Medicine 50(4), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang H, Wang X, Lu M, Ding Q, Chen AF, Xiang M, Chen S, 2023. iPSC-derived exosomes promote angiogenesis in naturally aged mice. Aging (Albany NY) 15(12), 5854–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang G, Wang Q, Zhang Y, Cui L, Huang X, 2022. Exosomes Secreted from Adipose-Derived Stem Cells Are a Potential Treatment Agent for Immune-Mediated Alopecia. J Immunol Res 2022, 7471246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JX, Liao X, Li SH, Jiang X, Li ZH, Wu YD, Xiao LL, Xie GH, Song JX, Liu HW, 2020. Antiaging Properties of Exosomes from Adipose-Derived Mesenchymal Stem Cells in Photoaged Rat Skin. Biomed Res Int 2020, 6406395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Zheng D, Lu C, Xi Q, Bao H, Li W, Gu Y, Mao Y, Xu B, Gu X, 2021. Exosomes derived from miR-301a-3p-overexpressing adipose-derived mesenchymal stem cells reverse hypoxia-induced erectile dysfunction in rat models. Stem Cell Res Ther 12(1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Wu Z, Li J, Wu S, Shi W, Wang L, 2023. The emerging double-edged sword role of exosomes in Alzheimer’s disease. Front Aging Neurosci 15, 1209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CM, Luo T, von der Ohe J, de Juan Mora B, Schmitt R, Hass R, 2021. Human MSC-Derived Exosomes Reduce Cellular Senescence in Renal Epithelial Cells. Int J Mol Sci 22(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew LC, Katsuda T, Gailhouste L, Nakagama H, Ochiya T, 2017. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer’s disease. Int Immunol 29(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Ling M, Tang C, Yang X, Yu N, Song Y, Ding W, Sun Y, Yan R, Wang S, Li X, Gao H, Zhang Z, Xing Y, 2023. Integrated metabolomics and phosphoproteomics reveal the protective role of exosomes from human umbilical cord mesenchymal stem cells in naturally aging mouse livers. Exp Cell Res 427(1), 113566. [DOI] [PubMed] [Google Scholar]
- Liu C, Fan L, Guan M, Zheng Q, Jin J, Kang X, Gao Z, Deng X, Shen Y, Chu G, Chen J, Yu Z, Zhou L, Wang Y, 2023. A Redox Homeostasis Modulatory Hydrogel with GLRX3(+) Extracellular Vesicles Attenuates Disc Degeneration by Suppressing Nucleus Pulposus Cell Senescence. ACS Nano 17(14), 13441–13460. [DOI] [PubMed] [Google Scholar]
- Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, Ge JF, 2022. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation 19(1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW, Cheng L, 2019. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. STEM CELLS 37(6), 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Meng MY, Han S, Gao H, Zhao YY, Yang Y, Lin ZY, Yang LR, Zhu K, Han R, Huang WW, Wang RQ, Yang LL, Wang WJ, Li L, Wang XD, Hou ZL, Liao LW, Yang L, 2021. Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Ameliorate HaCaT Cell Photo-Aging. Rejuvenation Res 24(4), 283–293. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao S, Luo L, Wang J, Zhu Z, Xiang Q, Deng Y, Zhao Z, 2019. Mesenchymal stem cell-derived exosomes ameliorate erection by reducing oxidative stress damage of corpus cavernosum in a rat model of artery injury. J Cell Mol Med 23(11), 7462–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YR, Cheng YQ, Wang SB, Su YR, Liu Y, Li CY, Jin L, Wan Q, Sang X, Wang ZC, 2021. Therapeutic effects and perspective of stem cell extracellular vesicles in aging and cancer. J Cell Physiol 236(7), 4783–4796. [DOI] [PubMed] [Google Scholar]
- Losurdo M, Pedrazzoli M, D’Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L, Molteni L, Bresciani E, Dander E, D’Amico G, Bulbarelli A, Torsello A, Matteoli M, Buffelli M, Coco S, 2020. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. STEM CELLS Translational Medicine 9(9), 1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Huang M, Zheng M, Dai C, Song Q, Zhang Q, Li Q, Gu X, Chen H, Jiang G, Yu Y, Liu X, Li S, Wang G, Chen H, Lu L, Gao X, 2020. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. Journal of Controlled Release 327, 688–702. [DOI] [PubMed] [Google Scholar]
- Ma Z, Wang Y, Li H, 2020. Applications of extracellular vesicles in tissue regeneration. Biomicrofluidics 14(1), 011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon NS, Fauser BCJM, 1999. Aspects of Ovarian Follicle Development throughout Life. Hormone Research in Paediatrics 52(4), 161–170. [DOI] [PubMed] [Google Scholar]
- Maggi R, Zasso J, Conti L, 2015. Neurodevelopmental origin and adult neurogenesis of the neuroendocrine hypothalamus. Frontiers in Cellular Neuroscience 8(440). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahindran E, Wan Kamarul Zaman WS, Ahmad Amin Noordin KB, Tan YF, Nordin F, 2023. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Hype or Hope for Skeletal Muscle Anti-Frailty. Int J Mol Sci 24(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y, 2018. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Research & Therapy 9(1), 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaro F, Macias-Garcia B, Sanchez-Margallo FM, Blazquez R, Alvarez V, Matilla E, Hernandez N, Gomez-Serrano M, Jorge I, Vazquez J, Gonzalez-Fernandez L, Pericuesta E, Gutierrez-Adan A, Casado JG, 2019. Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine modeldagger. Biol Reprod 100(5), 1180–1192. [DOI] [PubMed] [Google Scholar]
- Markoutsa E, Mayilsamy K, Gulick D, Mohapatra SS, Mohapatra S, 2022. Extracellular vesicles derived from inflammatory-educated stem cells reverse brain inflammation-implication of miRNAs. Mol Ther 30(2), 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Gimeno-Mallench L, Inglés M, Viña J, Borrás C, 2020. Extracellular Vesicles from Healthy Cells Improves Cell Function and Stemness in Premature Senescent Stem Cells by miR-302b and HIF-1α Activation. Biomolecules 10(6), 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J, 2022. News about Therapies of Alzheimer’s Disease: Extracellular Vesicles from Stem Cells Exhibit Advantages Compared to Other Treatments. Biomedicines 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, Hall DH, Driscoll M, 2017. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542(7641), 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, Brown E, Jarvis R, Yang Y, 2019. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nature Communications 10(1), 4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J, 2018. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol 71(4), 429–438. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A, 2014. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nature Neuroscience 17(2), 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistriotis P, Andreadis ST, 2017. Vascular aging: Molecular mechanisms and potential treatments for vascular rejuvenation. Ageing Research Reviews 37, 94–116. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Pham L-DD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim K-W, Boas D, Lo EH, Arai K, 2013. Age-Related Decline in Oligodendrogenesis Retards White Matter Repair in Mice. Stroke 44(9), 2573–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed AS, Abdel-Fattah DS, Abdel-Aleem GA, El-Sheikh TF, Elbatch MM, 2023. Biochemical study of the effect of mesenchymal stem cells-derived exosome versus L-Dopa in experimentally induced Parkinson’s disease in rats. Molecular and cellular biochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A, Kahlenberg S, Hornsby P, 2017. Therapeutic potential of mesenchymal stem cells for diabetes. J Mol Endocrinol 59(3), R109–R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C, 2009. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Current Biology 19(22), 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, LeVine H 3rd, 2010. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis 19(1), 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q, 2012. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proceedings of the National Academy of Sciences 109(11), 4146–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N, 2019. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci 76(17), 3323–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N, Fujimiya M, 2020. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Scientific Reports 10(1), 10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad A-N, Essa W, Adel H, 2016. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomaterials Research 20(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale F, Leone L, Rinaudo M, Sollazzo R, Barbati SA, La Greca F, Spinelli M, Fusco S, Grassi C, 2022. Neural Stem Cell-Derived Extracellular Vesicles Counteract Insulin Resistance-Induced Senescence of Neurogenic Niche. Stem Cells 40(3), 318–331. [DOI] [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF, 2005. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med 202(9), 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE, 2006. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108(6), 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Oyama T, Hu HT, Fujioka T, Hanawa-Suetsugu K, Ikeda K, Yamada S, Kawana H, Saigusa D, Ikeda H, Kurata R, Oono-Yakura K, Kitamata M, Kida K, Hikita T, Mizutani K, Yasuhara K, Mimori-Kiyosue Y, Oneyama C, Kurimoto K, Hosokawa Y, Aoki J, Takai Y, Arita M, Suetsugu S, 2021. Filopodium-derived vesicles produced by MIM enhance the migration of recipient cells. Dev Cell 56(6), 842–859 e848. [DOI] [PubMed] [Google Scholar]
- Niu X, Xia Y, Luo L, Chen Y, Yuan J, Zhang J, Zheng X, Li Q, Deng Z, Wang Y, 2023. iPSC-sEVs alleviate microglia senescence to protect against ischemic stroke in aged mice. Mater Today Bio 19, 100600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M, Lee J, Kim YJ, Rhee WJ, Park JH, 2018. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. International journal of molecular sciences 19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R. u., Yoshida M, Tsuda H, Tamura K, Ochiya T, 2014. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling 7(332), ra63–ra63. [DOI] [PubMed] [Google Scholar]
- Ouyang X, Han X, Chen Z, Fang J, Huang X, Wei H, 2018. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther 9(1), 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallet N, Sirois I, Bell C, Hanafi L-A, Hamelin K, Dieudé M, Rondeau C, Thibault P, Desjardins M, Hebert M-J, 2013. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 13(7), 1108–1120. [DOI] [PubMed] [Google Scholar]
- Panagiotou N, Neytchev O, Selman C, Shiels PG, 2018. Extracellular Vesicles, Ageing, and Therapeutic Interventions. Cells 7(8), 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi GK, Ramteke A, Birks D, Abouzeid Ali HE, Venkataraman S, Agarwal C, Vibhakar R, Miller LD, Agarwal R, Abd Elmageed ZY, Deep G, 2018. Exosomal microRNA profiling to identify hypoxia-related biomarkers in prostate cancer. Oncotarget 9(17), 13894–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannaccione A, Piccialli I, Secondo A, Ciccone R, Molinaro P, Boscia F, Annunziato L, 2020. The Na(+)/Ca(2+)exchanger in Alzheimer’s disease. Cell Calcium 87, 102190. [DOI] [PubMed] [Google Scholar]
- Paquet J, Deschepper M, Moya A, Logeart-Avramoglou D, Boisson-Vidal C, Petite H, 2015. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl Med 4(7), 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T, Ashery U, Popovtzer R, Offen D, 2019. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett 19(6), 3422–3431. [DOI] [PubMed] [Google Scholar]
- Pers Y-M, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noël D, Canovas F, Cyteval C, Lisignoli G, Schrauth J, Haddad D, Domergue S, Noeth U, Jorgensen C, Consortium, o.b.o.t.A., 2016. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. STEM CELLS Translational Medicine 5(7), 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang Z-B, Feng H-L, Miao Y-L, Wang Q, Yu Y, Wei Y-C, Yan J, Wang W-H, Shen W, Sun S-C, Schatten H, Sun Q-Y, 2014. The root of reduced fertility in aged women and possible therapentic options: Current status and future perspects. Molecular Aspects of Medicine 38, 54–85. [DOI] [PubMed] [Google Scholar]
- Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X, 2018. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. Journal of Controlled Release 287, 156–166. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K, 2006. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proceedings of the National Academy of Sciences 103(30), 11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, Graner M, Frederick B, Agarwal R, Deep G, 2015. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog 54(7), 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani S, Ryan AE, Griffin MD, Ritter T, 2015. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther 23(5), 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stahl PD, 2019. Extracellular vesicles: a new communication paradigm? Nature Reviews Molecular Cell Biology 20(9), 509–510. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Ratajczak J, 2020. Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia 34(12), 3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M, Cosenza S, Maumus M, Jorgensen C, Noel D, 2016. Therapeutic application of mesenchymal stem cells in osteoarthritis. Expert Opin Biol Ther 16(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Salido-Guadarrama I, Romero-Cordoba S, Peralta-Zaragoza O, Hidalgo-Miranda A, Rodríguez-Dorantes M, 2014. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther 7, 1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Ros J, Mas-Bargues C, Romero-Garcia N, Huete-Acevedo J, Dromant M, Borras C, 2022a. Therapeutic Potential of Extracellular Vesicles in Aging and Age-Related Diseases. Int J Mol Sci 23(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Ros J, Romero-Garcia N, Mas-Bargues C, Monleon D, Gordevicius J, Brooke RT, Dromant M, Diaz A, Derevyanko A, Guio-Carrion A, Roman-Dominguez A, Ingles M, Blasco MA, Horvath S, Vina J, Borras C, 2022b. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci Adv 8(42), eabq2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saparov A, Ogay V, Nurgozhin T, Jumabay M, Chen WC, 2016. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int 2016, 3924858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Nambiar DK, Ramteke A, Kumar R, Dhar D, Agarwal C, Bergman B, Graner M, Maroni P, Singh RP, Agarwal R, Deep G, 2015. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget 6(26), 22836–22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsarzadeh N, Khetarpal S, 2022. Rise of stem cell therapies in aesthetics. Clin Dermatol 40(1), 4956. [DOI] [PubMed] [Google Scholar]
- Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N, 2020. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev 29(12), 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha S, Shen X, Cao Y, Qu L, 2021. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging 13(11), 15285–15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Yoneda S, Abu-Amer Y, Guilak F, Gelberman RH, 2020. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. Journal of Orthopaedic Research 38(1), 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Song S, Chen N, Liao J, Zeng L, 2021. Stem cell-derived exosomes: A supernova in cosmetic dermatology. J Cosmet Dermatol 20(12), 3812–3817. [DOI] [PubMed] [Google Scholar]
- Shi HZ, Zeng JC, Shi SH, Giannakopoulos H, Zhang QZ, Le AD, 2021. Extracellular Vesicles of GMSCs Alleviate Aging-Related Cell Senescence. Journal of Dental Research 100(3), 283–292. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhao X, Hsieh J, Wichterle H, Impey S, Banerjee S, Neveu P, Kosik KS, 2010. MicroRNA Regulation of Neural Stem Cells and Neurogenesis. The Journal of Neuroscience 30(45), 14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F, Cucullo L, 2021. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. Int J Mol Sci 22(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, 2007. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct 2, 35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soebadi MA, Milenkovic U, Weyne E, Castiglione F, Albersen M, 2017. Stem Cells in Male Sexual Dysfunction: Are We Getting Somewhere? Sex Med Rev 5(2), 222–235. [DOI] [PubMed] [Google Scholar]
- Su T, Xiao Y, Xiao Y, Guo Q, Li C, Huang Y, Deng Q, Wen J, Zhou F, Luo XH, 2019. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano 13(2), 2450–2462. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Vaidya M, 2018. Stem Cell Therapies for Neurodegenerative Diseases. Adv Exp Med Biol 1056, 61–84. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang W, 2021. Preconditioning of mesenchymal stem cells with ghrelin exerts superior cardioprotection in aged heart through boosting mitochondrial function and autophagy flux. European Journal of Pharmacology 903, 174142. [DOI] [PubMed] [Google Scholar]
- Sun XL, Hao QK, Tang RJ, Xiao C, Ge ML, Dong BR, 2019. Frailty and Rejuvenation with Stem Cells: Therapeutic Opportunities and Clinical Challenges. Rejuvenation Res 22(6), 484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang W, Li X, 2021. Induced pluripotent stem cell-derived mesenchymal stem cells deliver exogenous miR-105–5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration. Stem Cell Res Ther 12(1), 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X, 2010. Efficient Generation of Functional Dopaminergic Neurons from Human Induced Pluripotent Stem Cells Under Defined Conditions. STEM CELLS 28(10), 1893–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, Hara E, 2017. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8, 15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu A, Lalu MM, Slobodian M, Gnyra C, Fergusson DA, Montroy J, Burger D, Stewart DJ, Allan DS, 2020. An Analysis of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Preclinical Use. ACS Nano 14(8), 9728–9743. [DOI] [PubMed] [Google Scholar]
- Tofiño-Vian M, Guillén MI, Pérez del Caz MD, Castejón MA, Alcaraz MJ, 2017. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxidative Medicine and Cellular Longevity 2017, 7197598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins BA, DiFede DL, Khan A, Landin AM, Schulman IH, Pujol MV, Heldman AW, Miki R, Goldschmidt-Clermont PJ, Goldstein BJ, Mushtaq M, Levis-Dusseau S, Byrnes JJ, Lowery M, Natsumeda M, Delgado C, Saltzman R, Vidro-Casiano M, Da Fonseca M, Golpanian S, Premer C, Medina A, Valasaki K, Florea V, Anderson E, El-Khorazaty J, Mendizabal A, Green G, Oliva AA, Hare JM, 2017. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci 72(11), 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DK, Phuong TNT, Bui NL, Singh V, Looi QH, Koh B, Zaman U, Foo JB, Wu CC, Show PL, Chu DT, 2023. Exploring the Potential of Stem Cell-Based Therapy for Aesthetic and Plastic Surgery. IEEE Rev Biomed Eng 16, 386–402. [DOI] [PubMed] [Google Scholar]
- Tse K-H, Herrup K, 2017. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mechanisms of Ageing and Development 161, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M, Ng NN, Concepcion W, Thakor AS, 2020. Emerging role of stem cell-derived extracellular microRNAs in age-associated human diseases and in different therapies of longevity. Ageing Res Rev 57, 100979. [DOI] [PubMed] [Google Scholar]
- Urbanelli L, Buratta S, Sagini K, Tancini B, Emiliani C, 2016. Extracellular Vesicles as New Players in Cellular Senescence. Int J Mol Sci 17(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology 9(6), 654–659. [DOI] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A, 2012. Disruption of Nrf2 Signaling Impairs Angiogenic Capacity of Endothelial Cells: Implications for Microvascular Aging. The Journals of Gerontology: Series A 67(8), 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen JM, 2014. The role of senescent cells in ageing. Nature 509(7501), 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal C, Shamir C, Senthilkumar S, Babu JV, Sonu PK, Nishtha KJ, Rai KS, K, S., Dhanushkodi A., 2017. Dosage and Passage Dependent Neuroprotective Effects of Exosomes Derived from Rat Bone Marrow Mesenchymal Stem Cells: An In Vitro Analysis. Curr Gene Ther 17(5), 379–390. [DOI] [PubMed] [Google Scholar]
- Wang C, Borger V, Mohamud Yusuf A, Tertel T, Stambouli O, Murke F, Freund N, Kleinschnitz C, Herz J, Gunzer M, Popa-Wagner A, Doeppner TR, Giebel B, Hermann DM, 2022. Postischemic Neuroprotection Associated With Anti-Inflammatory Effects by Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles in Aged Mice. Stroke 53(1), e14–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhao B, Zhai J, Wang A, Cao N, Liao T, Su R, He L, Li Y, Pei X, Jia Y, Yue W, 2023. Clinical-grade human umbilical cord-derived mesenchymal stem cells improved skeletal muscle dysfunction in age-associated sarcopenia mice. Cell Death Dis 14(5), 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu Y, Li J, Wang T, Hei Y, Li H, Wang X, Wang L, Zhao R, Liu W, Long Q, 2021. Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP / PS1 mice. Cell Death Discov 7(1), 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J, Zhao L, 2019. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 11(15), 7481–7496. [DOI] [PubMed] [Google Scholar]
- Wang J, Mi Y, Wu S, You X, Huang Y, Zhu J, Zhu L, 2020. Exosomes from adipose-derived stem cells protect against high glucose-induced erectile dysfunction by delivery of corin in a streptozotocin-induced diabetic rat model. Regen Ther 14, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yi T, Mao S, Li M, 2023. Neuroprotective mechanism of human umbilical cord mesenchymal stem cell-derived extracellular vesicles improving the phenotype polarization of microglia via the PI3K/AKT/Nrf2 pathway in vascular dementia. Synapse 77(4), e22268. [DOI] [PubMed] [Google Scholar]
- Wang QQ, Jing XM, Bi YZ, Cao XF, Wang YZ, Li YX, Qiao BJ, Chen Y, Hao YL, Hu J, 2018. Human Umbilical Cord Wharton’s Jelly Derived Mesenchymal Stromal Cells May Attenuate Sarcopenia in Aged Mice Induced by Hindlimb Suspension. Med Sci Monit 24, 9272–9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Jia J, Wang Z, 2018. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppresses iNOS Expression and Ameliorates Neural Impairment in Alzheimer’s Disease Mice. J Alzheimers Dis 61(3), 1005–1013. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang G, 2021. Bone marrow mesenchymal stem cells-derived exosomes reduce Abeta deposition and improve cognitive function recovery in mice with Alzheimer’s disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol Int 45(4), 775–784. [DOI] [PubMed] [Google Scholar]
- Weilner S, Keider V, Winter M, Harreither E, Salzer B, Weiss F, Schraml E, Messner P, Pietschmann P, Hildner F, Gabriel C, Redl H, Grillari-Voglauer R, Grillari J, 2016. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging (Albany NY) 8(1), 16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA, 2016. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 3, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Wu SN, Zhang LP, Zhao XS, Li Y, Yang QY, Yuan RY, Liu JL, Mao HJ, Zhu NW, 2022. Stem Cell-Derived Exosomes: A New Method for Reversing Skin Aging. Tissue Eng Regen Med 19(5), 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Zhang B, Han X, Sun Y, Sun Z, Li L, Zhou X, Jin Q, Fu P, Xu W, Qian H, 2021. HucMSC exosome-delivered 14–3-3zeta alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging (Albany NY) 13(8), 11542–11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G-Y, Cheng C-C, Chiang Y-S, Cheng WT-K, Liu IH, Wu S-C, 2016. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Scientific Reports 6(1), 23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Xu M, Yu H, Wang L, Li X, Rak J, Wang S, Zhao RC, 2021. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct Target Ther 6(1), 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z-H, Liu Z, Zhang X-R, Yang H, Wei L-F, Wang Y, Xu S-L, Sun L, Lai C, Bi J-Z, Wang X-Y, 2016. Wharton’s Jelly-derived mesenchymal stem cells alleviate memory deficits and reduce amyloid-β deposition in an APP/PS1 transgenic mouse model. Clinical and Experimental Medicine 16(1), 89–98. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M, 2012. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7), 1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WP, Yao WQ, Wang B, Liu K, 2021. BMSCs-exosomes containing GDF-15 alleviated SH-SY5Y cell injury model of Alzheimer’s disease via AKT/GSK-3beta/beta-catenin. Brain Res Bull 177, 92–102. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Xiong Y, Zhang H, Zhao Y, Han K, Zhang J, Zhao D, Yu Z, Geng Z, Wang L, Wang Y, Luan X, 2021. hPMSCs-Derived Exosomal miRNA-21 Protects Against Aging-Related Oxidative Damage of CD4(+) T Cells by Targeting the PTEN/PI3K-Nrf2 Axis. Front Immunol 12, 780897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Wu Y, Yang Q, Cheng Y, Xu J, Zhang Y, Dai H, Wang B, Ma Q, Chen Y, Lin F, Wang C, 2022. Engineered Extracellular Vesicles with SHP2 High Expression Promote Mitophagy for Alzheimer’s Disease Treatment. Adv Mater 34(49), e2207107. [DOI] [PubMed] [Google Scholar]
- Xu P, Xin Y, Zhang Z, Zou X, Xue K, Zhang H, Zhang W, Liu K, 2020. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther 11(1), 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, Li X, Ba L, Zhang M, Yang Y, Gao Y, Sun Z, Han Q, Zhao RC, 2021. MSC-Derived Exosomes can Enhance the Angiogenesis of Human Brain MECs and Show Therapeutic Potential in a Mouse Model of Parkinson’s Disease. Aging Dis 12(5), 1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Huang L, Yan Y, Zhong Y, Xie H, Wang X, 2023. Bone marrow mesenchymal stem cell-derived exosome miR-29b-3p alleviates UV irradiation-induced photoaging in skin fibroblast. Photodermatol Photoimmunol Photomed 39(3), 235–245. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC, 2005. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23(6), 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G, 2020. The Regulatory Functionality of Exosomes Derived from hUMSCs in 3D Culture for Alzheimer’s Disease Therapy. Small 16(3), 1906273. [DOI] [PubMed] [Google Scholar]
- Yang W, Zhang J, Xu B, He Y, Liu W, Li J, Zhang S, Lin X, Su D, Wu T, Li J, 2020. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol Ther 28(4), 1200–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhou J, Li J, 2020. Regulation of exosome for Alzheimer’s disease derived from mesenchymal stem cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban 45(2), 169–175. [DOI] [PubMed] [Google Scholar]
- Yao X, Wei W, Wang X, Chenglin L, Bjorklund M, Ouyang H, 2019. Stem cell derived exosomes: microRNA therapy for age-related musculoskeletal disorders. Biomaterials 224, 119492. [DOI] [PubMed] [Google Scholar]
- Yates AG, Pink RC, Erdbrügger U, Siljander PR, Dellar ER, Pantazi P, Akbar N, Cooke WR, Vatish M, Dias-Neto E, Anthony DC, Couch Y, 2022a. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology. J Extracell Vesicles 11(1), e12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AG, Pink RC, Erdbrügger U, Siljander PR, Dellar ER, Pantazi P, Akbar N, Cooke WR, Vatish M, Dias-Neto E, Anthony DC, Couch Y, 2022b. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part II: Pathology: Part II: Pathology. J Extracell Vesicles 11(1), e12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Liu Y, Ji W, Zhuang J, Chen X, Gong B, Chu J, Liang W, Gao J, Yin Y, 2023. Engineered mesenchymal stem cell-derived extracellular vesicles: A state-of-the-art multifunctional weapon against Alzheimer’s disease. Theranostics 13(4), 1264–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M, 2015. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 182, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wen H, Liu C, Wang C, Yu H, Zhang K, Han Q, Liu Y, Han Z, Li Z, Liu N, 2023. Embryonic stem cell-derived extracellular vesicles rejuvenate senescent cells and antagonize aging in mice. Bioact Mater 29, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Mitsutake S, Igarashi Y, 2012. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. The Journal of biological chemistry 287(14), 10977–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y, 2014. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem 289(35), 24488–24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z, 2019. Stem cells: past, present, and future. Stem Cell Res Ther 10(1), 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R, Reisner Y, 2009. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 27(11), 2865–2874. [DOI] [PubMed] [Google Scholar]
- Zavatti M, Gatti M, Beretti F, Palumbo C, Maraldi T, 2022. Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Preserve Microglia and Neuron Cells from Abeta. Int J Mol Sci 23(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Shen H, Sheng Y, Guan Q, 2021. ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer’s disease. J Cell Mol Med 25(15), 7513–7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Shang Y, Chen X, Midgley AC, Wang Z, Zhu D, Wu J, Chen P, Wu L, Wang X, Zhang K, Wang H, Kong D, Yang Z, Li Z, Chen X, 2020. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 14(9), 12133–12147. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D, 2013. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497(7448), 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lu T, Xiao J, Du C, Chen H, Li R, Sui X, Pan Z, Xiao C, Zhao X, Yao J, Liu Y, Lei Y, Ruan Y, Zhang J, Li H, Zhang Q, Zhang Y, Cai J, Yang Y, Zheng J, 2023. MSC-derived extracellular vesicles as nanotherapeutics for promoting aged liver regeneration. Journal of controlled release : official journal of the Controlled Release Society 356, 402–415. [DOI] [PubMed] [Google Scholar]
- Zhang K, Yu L, Li FR, Li X, Wang Z, Zou X, Zhang C, Lv K, Zhou B, Mitragotri S, Chen M, 2020. Topical Application of Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells in Combination with Sponge Spicules for Treatment of Photoaging. Int J Nanomedicine 15, 2859–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhu J, Ma Q, Zhao Y, Wang Y, Hu X, Chen J, Zhu W, Han Z, Yu H, 2020. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res Ther 11(1), 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D, 2017. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548(7665), 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pan Y, Liu Y, Li X, Tang L, Duan M, Li J, Zhang G, 2021. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Research & Therapy 12(1), 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu J, Liu S, Lim M, Zhao S, Cui K, Zhang K, Wang L, Ji Q, Han Z, Kong D, Li Z, Liu N, 2019. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics 9(23), 6976–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang M, Yao A, Xie Y, Lin J, Sharifullah F, Hong Y, Chen H, Cheng F, Lai W, 2022. Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging. Int J Mol Sci 23(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Xu L, Yang D, Tang H, Chen Y, Zhang X, Xu Y, Ou R, Li D, 2022. Exosome-driven liquid biopsy for breast cancer: Recent advances in isolation, biomarker identification and detection. Extracellular Vesicle 1, 100006. [Google Scholar]
- Zheng H, Liang X, Han Q, Shao Z, Zhang Y, Shi L, Hong Y, Li W, Mai C, Mo Q, Fu Q, Ma X, Lin F, Li M, Hu B, Li X, Zhang Y, 2021. Hemin enhances the cardioprotective effects of mesenchymal stem cell-derived exosomes against infarction via amelioration of cardiomyocyte senescence. J Nanobiotechnology 19(1), 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y, Zhang Y, Zhang Y, Xie W, Yan B, Liu F, Yip H-K, Yu X. y., Li Y, 2019. Stem Cell-Derived Exosomes Prevent Aging-Induced Cardiac Dysfunction through a Novel Exosome/lncRNA MALAT1/NF- κ B/TNF- α Signaling Pathway. Oxidative Medicine and Cellular Longevity 2019, 9739258. [DOI] [PMC free article] [PubMed] [Google Scholar]


