Abstract
Aim:
Bacterial vaginosis (BV) is a common vaginal dysbiosis associated with adverse clinical sequelae, most notably, increased risk of sexually transmitted infections (STIs). The aims of this study were to estimate the frequency of BV recurrence, treatment patterns, other gynecological (GYN) conditions, and the associated healthcare resource utilization (HCRU) and costs among commercially insured patients in the USA.
Patients & methods:
Female patients aged 12–49 years with an incident vaginitis diagnosis and ≥1 pharmacy claim for a BV medication (fungal treatment only excluded) were selected from the Merative™ MarketScan commercial database (2017–2020). During a minimum 12-month follow-up, additional treatment courses, treatment patterns, frequency of other GYN conditions, and HCRU and costs were assessed. Generalized linear models were used to identify baseline predictors of total all-cause healthcare costs and number of treatment courses.
Results:
The study population included 140,826 patients (mean age: 31.5 years) with an incident vaginitis diagnosis and ≥1 BV medication claim. During the follow-up, 64.2% had 1 treatment course, 22.0% had 2, 8.1% had 3, and 5.8% had ≥4; 35.8% had a BV recurrence (≥2 BV medication claims). The most commonly prescribed BV medication was oral metronidazole (73.6%). Approximately 12% (n = 16,619) of patients had a new diagnosis of another GYN condition in the follow-up; 8.2% had a new STI, which were more common among patients with ≥4 treatment courses (12.9%). During follow-up, total all-cause healthcare costs averaged $8987 per patient per year (PPPY) of which $470 was BV-related. BV-related healthcare costs increased from $403 PPPY among those with 1 treatment course to $806 PPPY among those with ≥4 with nearly half the costs attributed to outpatient office visits.
Conclusion:
BV recurrence among this population represented a substantial clinical and healthcare economic burden warranting improvements in women's healthcare.
Keywords: bacterial vaginosis, commercially insured population, gynecological complications, healthcare costs, healthcare resource utilization, treatment
Plain language summary
What was the aim of this research?
The aims of this study were to estimate the frequency of bacterial vaginosis (BV) recurrence, treatment patterns, rates of other gynecological (GYN) conditions, and the associated healthcare resource utilization (HCRU) and costs among commercially insured patients in the USA.
How was the research carried out?
Female patients aged 12–49 years with an incident vaginitis diagnosis and at least 1 pharmacy claim for a BV medication were selected from the MarketScan commercial database (2017–2020). During a minimum 12-month follow-up, additional treatment courses, treatment patterns, frequency of other GYN conditions and HCRU and costs were assessed.
What were the results?
Among our study population of 140,826 commercially insured patients 12–49 years of age who were diagnosed with incident vaginitis and were prescribed at least one BV medication, 36% had BV recurrence during the follow-up period (mean: 28.5 months). Approximately 12% of patients had a new diagnosis of a GYN condition in the follow-up; 8.2% had a new sexually transmitted infection, which were more common among patients with ≥4 treatment courses (12.9%). During follow-up, total all-cause healthcare costs averaged $8987 per patient per year (PPPY) of which $470 was BV-related. BV-related healthcare costs increased from $403 PPPY among those with 1 treatment course to $806 PPPY among those with ≥4 with nearly half the costs attributed to outpatient office visits.
What do the results of the study mean?
BV recurrence among this population represented a substantial clinical and healthcare economic burden warranting improvements in women's healthcare.
Bacterial vaginosis (BV) is a vaginal dysbiosis characterized by the replacement of Lactobacillus bacterial populations with facultative and anaerobic bacteria and is the most common cause of vaginitis and abnormal vaginal discharge in people of reproductive age in the USA [1–3]. Approximately, 50% of vaginitis cases are attributed to BV, with vulvovaginal candidiasis accounting for 20% to 25% of cases, and trichomoniasis for 15% to 20% [3]. Noninfectious causes (e.g., irritant, allergen, inflammatory agent) of vaginitis account for 5% to 10% of cases [3]. Approximately a third (29%) of women aged 14–49 years in the USA are estimated to have symptomatic or asymptomatic BV [4]; although the proportion of women affected varies by race and ethnicity among a number of other factors [4–7]. In addition to the physical and psychosocial discomfort people can experience with BV, it increases susceptibility to sexually transmitted infections (STIs) and has been associated with pelvic inflammatory disease, endometritis, preterm birth, other complications of pregnancy and complications following gynecological surgery [2,8,9].
CDC guideline-recommended treatments and US FDA-approved alternative treatment options for symptomatic BV include orally or vaginally administered metronidazole, orally or vaginally administered clindamycin, oral secnidazole and oral tinidazole [2,8]. Such treatments have approximately an 80% cure rate of incident BV [8–10]. However, within 6–12 months of treatment of incident BV, more than 50% of women may have a BV recurrence that may result from reinfection, failure to eradicate the pathogens responsible for the incident case of BV, or failure to restore the protective vaginal Lactobacillus populations [8–11]. For the treatment of recurrent BV, healthcare professionals may choose to repeat the same treatment regimen that was used with the incident infection or select a different treatment option [2,12]. More effective treatment and prevention strategies for BV are widely needed, especially considering the limitations of current treatment options, including increasing resistance rates [8,13,14]; however, limited data exist on optimal treatment strategies.
Two commercial administrative claims database analyses (2016–2018 dataset) conducted in USA populations have reported on the healthcare costs of women 18–64 years of age who were diagnosed with BV according to different diagnostic methods, with all-cause healthcare costs ranging $7305 to $8232 per patient during 12 months of follow-up [15,16]. Lacking in the scientific literature is a comprehensive analysis of the treatment patterns of BV and its recurrence and the associated healthcare resource utilization (HCRU) and costs, specifically that which is BV-related (i.e., claims related to a BV diagnosis and usage of BV medications) and associated with other gynecological (GYN) conditions potentially related to BV among patients of reproductive age in the USA. To address the research gaps, the primary aims of this study were to evaluate the frequency of BV recurrence, treatment patterns, rates of other gynecological (GYN) conditions and the associated HCRU and costs among commercially insured people (12–49 years of age) in the USA. Secondary objectives of this study were to identify baseline predictive demographic and clinical characteristics of total all-cause healthcare costs and number of treatment courses following an incident BV diagnosis.
Patients & methods
Study design & data source
This study was a retrospective cohort study that utilized the Merative™ MarketScan® commercial database. The MarketScan commercial database contains patient-level, de-identified healthcare claims information from employers, health plans and hospitals with >215 million individuals with commercial, non-publicly funded insurance (i.e., not Medicaid) coverage represented. The data provide evidence of real-world treatment patterns, HCRU and costs by tracking millions of patients as they travel through the healthcare system, offering detailed information about all aspects of care. Used primarily for research, the MarketScan commercial database is fully compliant with USA privacy laws and regulations (i.e., Health Insurance Portability and Accountability Act [HIPAA]). Ethical approval was not required as this was a retrospective analysis of administrative claims data.
Study population
From the MarketScan commercial database between 1 January 2017 and 30 September 2020, patients 12–49 years of age with an incident vaginitis diagnosis, defined by ICD-10 code N76.0 or N76.1 at any diagnosis position were selected. The index date was defined as the first inpatient or outpatient diagnosis date of vaginitis during the index identification period. Patients were required to have ≥1 pharmacy claim for a CDC guideline recommended or FDA-approved alternative BV medication (i.e., oral or vaginal metronidazole, oral or vaginal clindamycin, oral secnidazole, or oral tinidazole) [2,8] within 30 days following their vaginitis diagnosis, which takes into consideration typical treatment delays, or 7 days prior to their diagnosis, since there could have been a documentation error of the index date. In the absence of a specific BV ICD-10 code, vaginitis with a pharmacy claim for the above mentioned medications was used as a proxy for BV. Patients were additionally required to have continuous medical and pharmacy insurance enrollment during the 12 months prior to index date (baseline period) and ≥12 months after and including index date (follow-up period). The patient follow-up period ended either at the end of the study period or the date of insurance disenrollment, whichever occurred earliest. Patients were excluded from the study population if they had no BV treatment, a pharmacy claim for a fungal treatment only, missing age or sex information, an error in their claims data (e.g., no data on the route of administration to determine BV medication), or participation in a clinical trial at any time during the study period.
Demographic & clinical characteristics
Demographic and clinical characteristics including age, USA geographic region, location (urban or rural), health plan type, Charlson comorbidity index (CCI) score and CCI comorbidities were determined on patients' index dates or during the baseline period. Pregnancy status was also documented during the baseline period and anytime during the entire study period.
Treatment patterns
During the follow-up period, additional treatment courses were defined as each new pharmacy claim for a BV medication. We used ≥2 treatment courses as a proxy for BV recurrence. Among the patients with ≥2 treatment courses, the duration in days between treatment courses was measured. Also, the proportions who switched BV antibiotic (e.g., metronidazole to clindamycin), route of medication administration (e.g., vaginal to oral), or both antibiotic type and route of administration were evaluated.
Other GYN conditions
The proportions of patients with other GYN conditions [17], including STIs (chlamydia, gonorrhea, viral hepatitis, genital herpes, human papillomavirus infection), pelvic inflammatory disease (PID) with a diagnosis of gonorrhea and/or chlamydia and without a diagnosis of gonorrhea and/or chlamydia, postprocedural gynecological infections, post hysterectomy cuff cellulitis, female infertility, preterm labor/delivery, postpartum fever, postpartum endometritis and post abortion endometritis among the study population were measured during the baseline period. During the follow-up period, the incidence of any new cases of other GYN conditions (i.e., diagnosis in the follow-up but not in the baseline period) was additionally determined for the overall population and also stratified by the number of treatment courses. All other GYN conditions were identified based on ICD-10 codes indicating the condition (Supplementary Table 1).
HCRU & associated costs
All-cause HCRU, including hospitalizations (i.e., inpatient claims and length of stay [LOS]), outpatient claims (office visits and emergency room [ER] visits) and outpatient pharmacy claims, were evaluated for each patient in the study population and reported per patient per year (PPPY) among the overall study population and by the number of treatment courses. BV-related HCRU, defined as any inpatient or outpatient medical service claim containing an ICD-10 code indicating vaginitis (in any position) or a pharmacy claim for a BV medication was additionally evaluated, alongside other GYN condition-associated HCRU, which included any inpatient or outpatient medical service claim that contained an ICD-10 code (in any position) for any of the other GYN conditions. Due to the small number of other GYN conditions, pharmacy claims were not assessed. Associated costs were determined for all HCRU categories, in addition to for other outpatient visit costs (i.e., not office or ER visit). All costs were inflation adjusted to the 2021 cost level and reported PPPY.
Statistical analyses
We utilized descriptive statistics to summarize patient demographic and clinical characteristics, treatment patterns, BV-related complications and HCRU and the associated costs. Counts and percentages were reported for categorical variables and means, standard deviations and medians were reported for continuous variables. A generalized linear model (GLM) regression analysis with gamma distribution with log link function used for direct cost modelling was conducted to evaluate the predictive baseline demographic and clinical characteristics of total all-cause healthcare costs during the follow-up period. In another GLM regression analysis with Poisson distribution used for number of treatment course modelling we evaluated the predictive baseline demographic and clinical characteristics of receiving >1 treatment courses during the follow-up period. All statistical analyses were carried out using SAS software 9.4 (SAS Institute Inc., NC, USA).
Results
Demographic & clinical characteristics of study population
The overall study population included 140,826 patients with an incident BV diagnosis who had ≥1 pharmacy claims for a BV medication between January 2017 and September 2020 recorded in the data source. Table 1 shows the demographic and clinical characteristics of the overall study population. Mean age was 31.5 years, 55.4% resided in the South, and 48.8% had a preferred provider organization health plan. In the 12 months prior to index BV diagnosis, 3.2% of patients had a pregnancy; 5.5% were pregnant during anytime of the entire study period. Comorbidity was low with most (89.3%) patients having a score of 0. The most prevalent CCI conditions were chronic pulmonary disease (4.4%) and diabetes without complications (4.3%). The mean (median) follow-up duration across the study population was 28.5 (26) months.
Table 1. . Demographic and clinical characteristics of overall study population (n = 140,826).
| Variable | n (%) |
|---|---|
| Age (on index date) | |
| Age† | 31.5 ± 9.6 (31) |
| Age group | |
| 12–17 years | 5136 (3.6) |
| 18–29 years | 58,429 (41.5) |
| 30–39 years | 41,438 (29.4) |
| 40–49 years | 35,823 (25.4) |
| USA geographic region | |
| South | 78,002 (55.4) |
| North Central | 25,699 (18.2) |
| Northeast | 18,877 (13.4) |
| West | 17,851 (12.7) |
| Missing/unknown | 397 (0.3) |
| Location | |
| Urban | 114,934 (81.6) |
| Rural | 13,464 (9.6) |
| Missing/unknown | 12,428 (8.8) |
| Health plan | |
| Preferred provider organization | 68,736 (48.8) |
| Consumer-driven/high deductible | 39,087 (27.8) |
| Health maintenance organization | 18,449 (13.1) |
| Point of service | 9374 (6.7) |
| Comprehensive | 1473 (1.0) |
| Exclusive provider organization | 979 (0.7) |
| Missing/unknown | 1397 (1.0) |
| Other† | 1331 (0.9) |
| Pregnancy status | |
| Any time during the baseline period | |
| Pregnant | 4555 (3.2) |
| Not pregnant | 136,271 (96.8) |
| Any time during the entire study period | |
| Pregnant | 7714 (5.5) |
| Not pregnant | 133,112 (94.5) |
| Charlson Comorbidity Index (CCI) | |
| CCI score‡ | 0.1 ± 0.5 (0) |
| CCI score group | |
| 0 | 125,741 (89.3) |
| 1–2 | 14,523 (10.3) |
| 3–4 | 304 (0.2) |
| ≥5 | 258 (0.2) |
| CCI comorbidities | |
| Myocardial infarction | 144 (0.1) |
| Congestive heart failure | 431 (0.3) |
| Peripheral vascular disease | 422 (0.3) |
| Cerebrovascular disease | 0 (0) |
| Dementia | 0 (0) |
| Chronic pulmonary disease | 6134 (4.4) |
| Connective tissue/rheumatic disease | 140 (0.1) |
| Peptic ulcer disease | 0 (0) |
| Mild liver disease | 2706 (1.9) |
| Diabetes without complications | 6106 (4.3) |
| Diabetes with complications | 0 (0) |
| Paraplegia and hemiplegia | 49 (0.03) |
| Renal disease | 192 (0.1) |
| Cancer | 415 (0.3) |
| Moderate or severe liver disease | 25 (0.02) |
| Metastatic carcinoma | 0 (0) |
| Acquired immunodeficiency syndrome/ human immunodeficiency syndrome | 236 (0.2) |
| Follow-up period (months) † | 28.5 ± 12.4 (26) |
Other includes point of service with capitation and base/major medical.
Mean ± standard deviation (median).
Treatment patterns
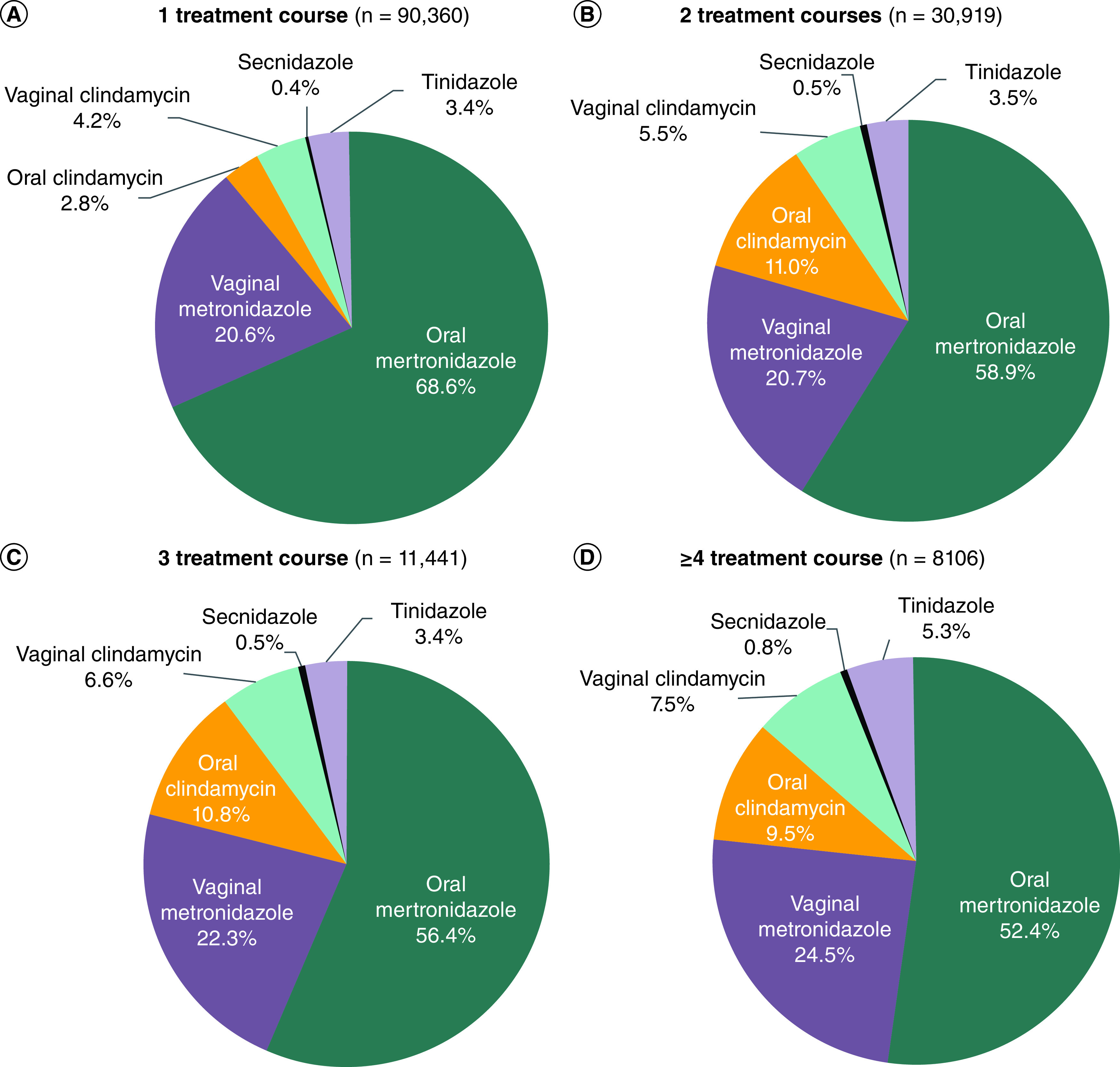
During the follow-up period, 64.2% of patients had 1 treatment course for BV, 22.0% had 2, 8.1% had 3 and 5.8% had ≥4. Using ≥2 treatment courses as a proxy for BV recurrence, 35.8% (n = 50,466) of patients had a BV recurrence during the follow-up period. Most (73.6%) patients were prescribed oral metronidazole; however, its use declined among patients with a greater number of treatment courses (1: 68.6%, 2: 58.9%, 3: 56.4%, ≥4: 52.4%, Figure 1). The use of vaginal metronidazole increased slightly among patients with more treatment courses (1: 20.6%, 2: 20.7%, 3: 22.3%, ≥4: 24.5%). Use of oral clindamycin increased among patients with a 2nd treatment course but then its use slightly declined (1: 2.8%, 2: 11.0%, 3: 10.8%, ≥4: 9.5%), while use of vaginal clindamycin increased in each subsequent treatment course (1: 4.2%, 2: 5.5%, 3: 6.6%, ≥4: 7.5%). Use of secnidazole and tinidazole also increased among patients with an increasing number of treatment courses, although their use remained relatively low (<1%).
Figure 1. . Bacterial vaginosis medications by treatment course.
Among patients with ≥2 treatment courses, the duration between treatment courses declined with more treatment courses (mean duration between 1st and 2nd course: 132 days, between 2nd and 3rd course: 99 days, between 3rd and 4th course: 78 days). Among patients with 2 treatment courses, 20.8% switched antibiotics, 26.2% switched route of administration, and 7.5% switched both. Among the patients with 3 treatment courses, 41.5% switched antibiotics, 53.4% switched route of administration, and 16.1% switched both. Among patients with ≥4 treatment courses, 75.8% switched antibiotics, 93.0% switched route of administration, and 31.6% switched both.
Other GYN conditions
During the baseline period, 13.5% (n = 18,990) of the overall study population were diagnosed with any of the evaluated other GYN conditions; 7.9% had an STI and 3.1% had PID mostly without a concurrent diagnosis of gonorrhea or chlamydia (Table 2). A new diagnosis of another GYN condition during the follow-up period (i.e., not in the baseline period) occurred in 11.8% (n = 16,619) of the overall study population and 8.2% had a new STI diagnosis, representing 69.6% of the evaluated other GYN conditions (Table 2). The proportion of patients with any new GYN condition (1: 10.3%, 2: 13.6%, 3: 15.8%, ≥4: 17.8%) and new STIs (1: 7.0%, 2: 9.5%, 3: 11.2%, ≥4: 12.9%) increased among those who had an increasing number of treatment courses (Table 2). This trend was also observed for some of the other evaluated GYN conditions (e.g., PID, postprocedural gynecological infections, post hysterectomy cuff cellulitis, Table 2).
Table 2. . Other GYN conditions, overall and by treatment course.
| Other GYN conditions – n (%)§ | Overall study population n = 140, 826 |
New cases of other GYN conditions by TC | ||||
|---|---|---|---|---|---|---|
| Baseline† | New cases‡ | 1 TC n = 90,360 (64.2%) |
2 TCs n = 30,919 (22.0%) |
3 TCs n = 11,441 (8.1%) |
≥4 TCs n = 8106 (5.8%) |
|
| Unique patients with any other GYN conditions§ | 18,990 (13.5) | 16,619 (11.8) | 9154 (10.3) | 4216 (13.6) | 1805 (15.8) | 1444 (17.8) |
| Any sexually transmitted infection(s)¶ | 11,115 (7.9) | 11,571 (8.2) | 6307 (7.0) | 2930 (9.5) | 1287 (11.2) | 1047 (12.9) |
| Chlamydia | 3623 (2.6) | 3763 (2.7) | 1883 (2.1) | 1023 (3.3) | 457 (4.0) | 400 (4.9) |
| Gonorrhea | 834 (0.6) | 1420 (1.0) | 638 (0.7) | 372 (1.2) | 208 (1.8) | 202 (2.5) |
| Viral hepatitis | 373 (0.3) | 244 (0.2) | 139 (0.2) | 64 (0.2) | 22 (0.2) | 19 (0.2) |
| Genital herpes | 1596 (1.1) | 2024 (1.4) | 1051 (1.2) | 252 (0.8) | 259 (2.3) | 189 (2.3) |
| Human papillomavirus infection | 5498 (3.9) | 6105 (4.3) | 3481 (3.9) | 1497 (4.8) | 630 (5.5) | 497 (6.1) |
| Pelvic inflammatory disease (PID) | 4421 (3.1) | 3978 (2.8) | 2083 (2.3) | 1050 (3.4) | 460 (4.0) | 385 (4.7) |
| PID w/o diagnosis of gonorrhea and chlamydia | 11 (0.01) | 27 (0.02) | 12 (0.0) | 6 (0.0) | 4 (0.0) | 5 (0.1) |
| PID w/o diagnosis of gonorrhea or chlamydia | 205 (0.1) | 224 (0.2) | 86 (0.1) | 72 (0.2) | 34 (0.3) | 32 (0.4) |
| PID w/o diagnosis of gonorrhea and chlamydia | 4216 (3.0) | 3754 (2.7) | 1997 (2.2) | 978 (3.2) | 426 (3.7) | 353 (4.4) |
| PID w/o diagnosis of gonorrhea or chlamydia | 4410 (3.1) | 3951 (2.8) | 2071 (2.3) | 1044 (3.4) | 456 (4.0) | 380 (4.7) |
| Postprocedural gynecological infection | 1444 (1.0) | 1280 (0.9) | 517 (0.6) | 395 (1.3) | 205 (1.8) | 163 (2.0) |
| Post hysterectomy cuff cellulitis | 411 (0.3) | 475 (0.3) | 173 (0.2) | 147 (0.5) | 86 (0.8) | 69 (0.9) |
| Female infertility | 2888 (2.1) | 2300 (1.6) | 1389 (1.5) | 541 (1.7) | 222 (1.9) | 148 (1.8) |
| Preterm labor/delivery | 890 (0.6) | 1110 (0.8) | 690 (0.8) | 264 (0.9) | 94 (0.8) | 62 (0.8) |
| Postpartum fever | 70 (0.05) | 100 (0.1) | 58 (0.1) | 32 (0.1) | 8 (0.1) | 2 (0.0) |
| Postpartum endometritis | 87 (0.1) | 142 (0.1) | 77 (0.1) | 37 (0.1) | 18 (0.2) | 10 (0.1) |
| Post abortion endometritis | 16 (0.01) | 7 (0.0) | 2 (0.0) | 4 (0.0) | 0 (0) | 1 (0.0) |
Number and percentage of patients with other GYN conditions during the baseline period.
Number and percentage of patients with new other GYN condition-associated claims that were in the ≥12-month follow-up period but not in the baseline period.
This row represents the number of unique individuals for each respective stratum. A patient may have had more than 1 other GYN condition, thus the number of patients from all sub-categories of other GYN conditions may not add up to the number of unique patients with any other GYN conditions.
This row represents the number of unique patients with any sexually transmitted infection for each respective stratum.
GYN: Gynecological; TC: Treatment course.
HCRU & associated costs
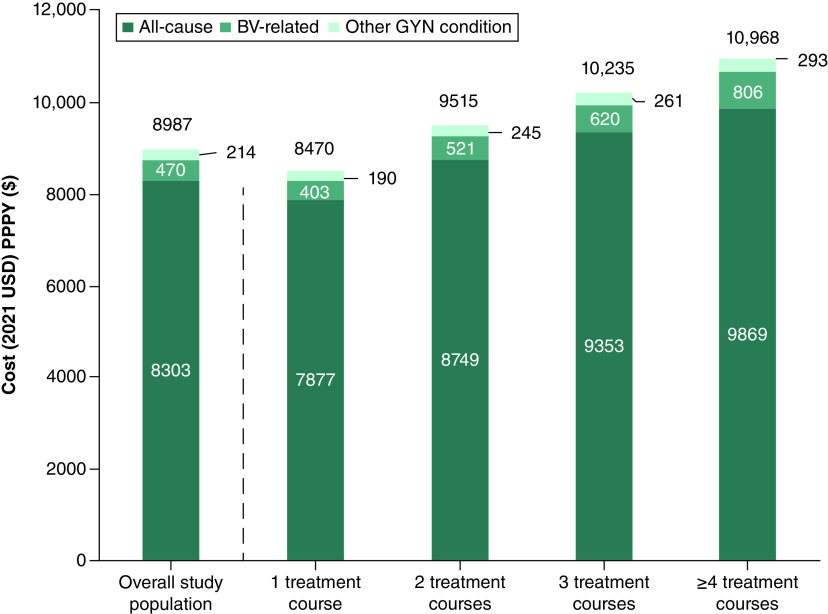
In Table 3, all-cause HCRU and the associated direct costs during the follow-up period are summarized. Among the overall study population, total all-cause healthcare costs averaged $8987 PPPY during the follow-up period and were 29% higher than the average total all-cause healthcare costs during the baseline period, which were $6966 PPPY. Patients with an increasing number of treatment courses used more healthcare resources for any cause, with the mean number of office visits increasing from 9.9 PPPY among those with 1 treatment course to 13.0 PPPY among those with ≥4 treatment courses, ER visits increasing from 0.8 to 1.3 PPPY, respectively, and pharmacy claims increasing from 29.4 to 40.0 PPPY. Expectedly, total mean all-cause healthcare costs PPPY increased among patients with more treatment courses (1: $8470, 2: $9515, 3: $10,235, ≥4: $10,968, Figure 2).
Table 3. . All-cause HCRU and direct costs (2021 USD) per patient per year during the follow-up period, overall and by treatment course.
| Total n = 140,826 |
1 TC n = 90,360 (64.2%) |
2 TCs n = 30,919 (22.0%) |
3 TCs n = 11,441 (8.1%) |
≥4 TCs n = 8106 (5.8%) |
|
|---|---|---|---|---|---|
| All-cause HCRU † | |||||
| Hospitalizations, n (%) | 14,724 (10.5) | 9298 (10.3) | 3324 (10.8) | 1215 (10.6) | 887 (10.9) |
| Length of stay (days)† | 3.6 ± 5.1 (2) | 3.6 ± 5.4 (2) | 3.6 ± 4.5 (2) | 3.6 ± 5.0 (3) | 4.0 ± 5.0 (3) |
| Office visit claims | 10.6 ± 11.1 (7) | 9.9 ± 10.9 (7) | 11.2 ± 11.2 (8) | 12.1 ± 11.8 (9) | 13.0 ± 12.0 (10) |
| ER visit claims | 0.9 ± 1.7 (0) | 0.8 ± 1.5 (0) | 1.1 ± 1.8 (0) | 1.2 ± 2.1 (0) | 1.3 ± 2.1 (1) |
| Pharmacy claims | 31.2 ± 31.4 (22) | 29.4 ± 30.6 (20) | 32.7 ± 31.8 (23) | 35.5 ± 32.5 (26) | 40.0 ± 35.6 (29) |
| All-cause HCRU costs † | |||||
| Total cost | $8987 ± $21,586 ($3151) | $8470 ± $21,458 ($2868) | $9515 ± $20,885 ($3474) | $10,235 ± $21,458 ($3848) | $10,968 ± $25,274 ($4284) |
| Inpatient | $2032 ± $12,284 ($0) | $2028 ± $12,980 ($0) | $2049 ± $11,042 ($0) | $1950 ± $9506 ($0) | $2117 ± $12,276 ($0) |
| Outpatient office visits | $1995 ± $4725 ($1187) | $1878 ± $4965 ($1092) | $2124 ± $4352 ($1295) | $2275 ± $4023 ($1434) | $2407 ± $4182 ($1525) |
| Outpatient ER visits | $782 ± $2275 ($0) | $695 ± $2050 ($0) | $890 ± $2620 ($0) | $994 ± $2686 ($0) | $1048 ± $2544 ($0) |
| Other outpatient visits | $2480 ± $9929 ($114) | $2302 ± $9499 ($74) | $2684 ± $10,695 ($165) | $2905 ± $10,534 ($240) | $3085 ± $10,628 ($280) |
| Pharmacy | $1698 ± $8302 ($287) | $1567 ± $7753 ($241) | $1769 ± $8092 ($325) | $2111 ± $10,880 ($391) | $2311 ± $10,480 ($526) |
Mean ± standard deviation (median).
ER: Emergency room; HCRU: Healthcare resource utilization; TC: Treatment course.
Figure 2. . Average all-cause, bacterial vaginosis-related and other gynecological condition-associated healthcare resource utilization costs per patient per year.
BV: Bacterial vaginosis; HCRU: Healthcare resource utilization; PPPY: Per patient per year.
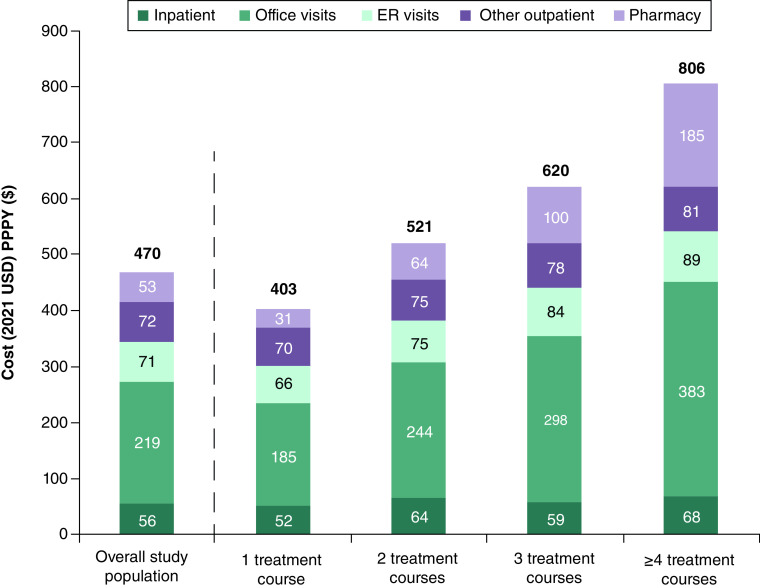
In Table 4, BV-related HCRU and the associated costs during the follow-up period are summarized. Among the overall study population, 0.2% had a BV-related hospitalization with an average LOS of 4.5 days. The average number of claims PPPY for a BV-related outpatient office visit, ER visit, and pharmacy use were 1.1, 0.1, and 2.1, respectively. Total BV-related healthcare costs averaged $470 PPPY. Patients with an increasing number of treatment courses used healthcare resources more; most notably, the mean number of BV-related office visits increased from 0.9 PPPY among those with 1 treatment course to 2.3 PPPY among those with ≥4 treatment courses. Total mean BV-related healthcare costs PPPY increased as well among patients with more treatment courses (1: $403, 2: $521, 3: $620, ≥4: $806), and the increases in costs were observed across all HCRU categories (Figure 3).
Table 4. . BV-related HCRU and direct costs (2021 USD) per patient per year during the follow-up period, overall and by treatment course.
| Total n = 140,826 |
1 TC n = 90,360 (64.2%) |
2 TCs n = 30,919 (22.0%) |
3 TCs n = 11,441 (8.1%) |
≥4 TCs n = 8106 (5.8%) |
|
|---|---|---|---|---|---|
| BV-related HCRU † | |||||
| Hospitalizations, n (%) | 280 (0.2) | 156 (0.2) | 64 (0.2) | 32 (0.3) | 28 (0.3) |
| Length of stay (days)† | 4.5 ± 6.1 (3) | 4.9 ± 7.0 (3) | 4.1 ± 3.8 (3) | 3.7 ± 5.8 (3) | 4.8 ± 5.7 (3) |
| Office visit claims | 1.1 ± 0.9 (1) | 0.9 ± 0.6 (1) | 1.3 ± 0.9 (1) | 1.7 ± 1.1 (1) | 2.3 ± 1.6 (2) |
| ER visit claims | 0.1 ± 0.4 (0) | 0.1 ± 0.3 (0) | 0.1 ± 0.4 (0) | 0.2 ± 0.5 (0) | 0.2 ± 0.6 (0) |
| Pharmacy claims | 2.1 ± 1.7 (2) | 1.4 ± 0.9 (1) | 2.6 ± 1.1 (2) | 3.8 ± 1.4 (3) | 6.2 ± 2.9 (5) |
| BV-related HCRU costs † | |||||
| Total cost | $470 ± $1911 ($258) | $403 ± $1797 ($219) | $521 ± $2353 ($309) | $620 ± $1573 ($400) | $806 ± $1620 ($558) |
| Inpatient | $56 ± $1757 ($0) | $52 ± $1642 ($0) | $64 ± $2227 ($0) | $59 ± $1390 ($0) | $68 ± $1391 ($0) |
| Outpatient office visits | $219 ± $248 ($166) | $185 ± $207 ($148) | $244 ± $255 ($192) | $298 ± $316 ($233) | $383 ± $393 ($292) |
| Outpatient ER visits | $71 ± $384 ($0) | $66 ± $377 ($0) | $75 ± $397 ($0) | $84 ± $413 ($0) | $89 ± $377 ($0) |
| Other outpatient visits | $72 ± $523 ($0) | $70 ± $546 ($0) | $75 ± $497 ($0) | $78 ± $465 ($0) | $81 ± $425 ($0) |
| Pharmacy claims | $53 ± $87 ($10) | $31 ± $48 ($7) | $64 ± $78 ($18) | $100 ± $107 ($66) | $185 ± $203 ($127) |
Mean ± standard deviation (median).
BV: Bacterial vaginosis; ER: Emergency room; HCRU: Healthcare resource utilization; TC: Treatment course.
Figure 3. . Average bacterial vaginosis-related costs per patient per year by healthcare resource utilization category.
BV: Bacterial vaginosis; ER: Emergency room; HCRU: Healthcare resource utilization; PPPY: Per patient per year.
The proportion of total all-cause healthcare costs PPPY that were BV-related increased stepwise for patients with an increasing number of treatment courses, ranging from 4.8% among patients with 1 treatment course to 7.3% among those with ≥4 (Figure 2). Among the overall study population, 11.9% of total BV-related costs were attributed to inpatient costs, 46.5% to outpatient office visit costs, 15.0% to outpatient ER visit costs, 15.4% to other outpatient costs, and 11.2% to pharmacy costs (Figure 3). Compared with patients with only 1 treatment course, those with ≥4 treatment courses had BV-related inpatient costs that were 1.3-fold higher, office visit costs that were 2.1-fold higher, ER visit costs that were 1.3-fold higher, other outpatient visit costs that were 1.2-fold higher, and pharmacy costs that were sixfold higher (Figure 3).
HCRU and costs for other GYN conditions during the follow-up period are reported in Supplementary Table 2. Among the overall study population, 0.4% had a GYN condition-associated hospitalization. The proportion hospitalized for a GYN condition increased from 0.3% among patients with 1 treatment course to 0.6% among those with ≥4 treatment courses. Average total other GYN condition-associated healthcare costs were $214 PPPY, which also trended higher for those patients with more treatment courses (Figure 2). Patients who had ≥4 treatment courses also had greater utilization of outpatient healthcare resources that were associated with other GYN conditions compared with those with fewer treatment courses.
Baseline patient characteristics predictive of total all-cause healthcare costs & number of treatment courses
In the regression analysis, significant baseline predictive patient characteristics of higher total all-cause healthcare costs during the follow-up period included older age, residing in a US geographic region other than the South, pregnancy, presence of comorbidity as measured by CCI score group (≥1 vs 0), and a diagnosis of postprocedural gynecological infection or female infertility (Table 5).
Table 5. . Baseline patient characteristics predictive of total all-cause healthcare costs during the follow-up period.
| Baseline characteristic | Mean rate ratio† | Mean confidence limits | p-value | |
|---|---|---|---|---|
| Age group vs 12–17 years | ||||
| 18–29 years | 1.05 | 1.02 | 1.09 | .004 |
| 30–39 years | 1.28 | 1.24 | 1.33 | <.0001 |
| 40–49 years | 1.41 | 1.36 | 1.46 | <.0001 |
| USA geographic region: other vs South | 1.05 | 1.04 | 1.07 | <.0001 |
| Pregnancy any time during the baseline period: yes vs no | 1.48 | 1.43 | 1.54 | <.0001 |
| Baseline CCI score group: ≥1 vs 0 | 1.61 | 1.57 | 1.64 | <.0001 |
| Baseline other GYN conditions: yes vs no | ||||
| Any sexually transmitted infection | 0.99 | 0.97 | 1.02 | .66 |
| Pelvic inflammatory disease | 0.96 | 0.93 | 1.00 | .046 |
| Postprocedural gynecological infection | 1.48 | 1.39 | 1.58 | <.0001 |
| Preterm labor/delivery | 0.98 | 0.91 | 1.06 | .62 |
| Female infertility | 1.56 | 1.50 | 1.63 | <.0001 |
Mean rate ratio interpretation: ratio of 1.41 for women 40–49 vs 12–17 means the mean healthcare cost for women 40–49 was 41% higher than that for the 12–17 group.
BV: Bacterial vaginosis; GYN: Gynecological; CCI: Charlson Comorbidity Index.
Significant baseline predictive patient characteristics of having >1 treatment courses of BV medications during the follow-up period included older age and having a diagnosis of any STI or a postprocedural gynecological infection (Table 6). Residence in US geographic regions other than the South and pregnancy during the baseline period were predictive of having a lower number of treatment courses.
Table 6. . Baseline patient characteristics predictive of having >1 treatment courses during the follow-up period.
| Baseline characteristic | Mean estimate† | Mean confidence limits | p-value | |
|---|---|---|---|---|
| Age group vs 12–17 years | ||||
| 18–29 years | 1.06 | 1.04 | 1.08 | <.0001 |
| 30–39 years | 1.06 | 1.03 | 1.08 | <.0001 |
| 40–49 years | 1.04 | 1.01 | 1.06 | 0.002 |
| USA geographic region: other vs South | 0.98 | 0.97 | 0.99 | <.0001 |
| Pregnancy any time during the baseline period: yes vs no | 0.95 | 0.93 | 0.98 | <.0001 |
| Baseline CCI score group: ≥1 vs 0 | 1.01 | 1.00 | 1.03 | .055 |
| Baseline other GYN conditions: yes vs no | ||||
| Any sexually transmitted infection | 1.06 | 1.05 | 1.08 | <.0001 |
| Pelvic inflammatory disease | 1.01 | 0.99 | 1.03 | .41 |
| Postprocedural gynecological infection | 1.05 | 1.00 | 1.09 | .03 |
| Preterm labor/delivery | 0.99 | 0.94 | 1.04 | .59 |
| Female infertility | 0.97 | 0.95 | 1.00 | .09 |
Mean estimate >1 indicates more treatment courses and <1 indicates lower number of treatment courses compared with the reference group.
BV: Bacterial vaginosis; CCI: Charlson Comorbidity Index; GYN: Gynecological.
Discussion
The current study represents the first large-scale analysis of a commercially insured population in the USA that has comprehensively evaluated BV recurrence rates, its treatment patterns, and impact on rates of other GYN conditions, HCRU and costs. Among our study population of 140,826 commercially insured patients 12–49 years of age who were diagnosed with incident BV and were prescribed at least one BV medication, 36% had BV recurrence during the follow-up period (mean: 28.5 months) based on having at least one subsequent pharmacy claim for a BV medication. Although BV recurrence is significantly understudied in the USA, a few studies of small populations of patients (<100 patients) have reported recurrence or non-response to treatment rates ranging 35% to 42%; however, the timeframes of measurement of BV recurrence (90 days up to 8 months) and methods of clinical evaluation (vaginitis symptoms, symptoms of BV and ≥3 Amsel criteria, microbial and immune predictors of treatment success) differed across the studies [18–21]. The findings of this study indicate that BV recurrence is prevalent and is linked to higher rates of STIs and other adverse GYN conditions in addition to greater HCRU and higher healthcare costs, all of which underscore the shortcomings in the preventive healthcare of patients with BV.
Following an incident BV diagnosis, a new case of another GYN condition, of which a majority (70%) were a new STI, occurred in 12% of the overall study population. Emphasizing the association of BV with contracting an STI, the incidence of new cases of any STI rose stepwise among patients with an increasing number of treatment courses. In 2018 in the USA, the CDC estimated that approximately 20% of the population had an STI (>90% were chlamydia, trichomoniasis, genital herpes and human papilloma virus infection), with those aged 15–24 years the most prominently affected age group (i.e., comprised 45.5% of all incident infections) [22]. The prevalence and incidence of STIs have been increasing for several years in the USA [23], and compared with men, women are at higher risk for most STIs, due to higher efficiency of male-to-female transmission of STIs and greater exposure and vulnerability of their urogenital anatomy, and are more severely impacted by the long-term consequences of STIs [22,24]. Several studies have associated BV with higher risk for developing STIs [8,25–27], which we again demonstrate with the findings of this study. Reducing the incidence of BV and its recurrence will likely assist in achieving the goals of the USA national STI strategic plan, which include preventing STIs and improving population health by reducing harmful outcomes of STIs [28], as well as lower the healthcare economic burden associated with STIs and long-term sequelae.
HCRU and the associated costs, all-cause, BV-related and other GYN condition-associated all also rose in a stepwise manner among patients with an increasing number of treatment courses for BV. All-cause healthcare costs PPPY ranged from $8470 among patients with 1 treatment course to $10,968 among those with ≥4, BV-related healthcare costs PPPY ranged from $403 to $806, respectively, and other GYN condition-associated costs PPPY ranged from $190 to $293, respectively. Similar to the findings of our study, the administrative claims database studies of Kong et al. [15] and Troeger et al. [16] also found substantial 12-month healthcare costs among women diagnosed with BV (2016–2018 dataset); in these studies, average all-cause healthcare costs ranged between $7660 and $8232 per patient in the overall cohort and between $7305 and $7906 in the non-pregnant sub-cohort with costs depending on the diagnostic method used in both cohorts. Compared with the average all-cause healthcare costs found in this study, among the overall study population ($8987 PPPY), the all-cause healthcare costs among the cohort of women with BV diagnosed by clinical evaluation ($8232) of Kong et al. [15] are to a small extent lower (∼9%). However, in the study of Kong et al. [15], patients with recurrent BV were excluded from the study population. Thus, it would be more appropriate to compare the average all-cause healthcare costs of the patients with 1 treatment course in our study, which were $8470 PPPY. Other notable differences between our study population and that of Kong et al. [15] included the age groups represented (12–49 years vs 18–64 years, respectively), study years and that patients with significant comorbidities were excluded from the population in the study of Kong et al. [15].
It has been found that clinical evaluation and laboratory diagnosis of vaginitis can be very imprecise with approximately 50% of symptomatic patients evaluated for vaginitis being misdiagnosed [29,30]. Another main finding of the studies of Kong et al. [15] and Troeger et al. [16] was that use of molecular testing, in particular nucleic acid amplification testing (NAAT), which has high analytic performance to diagnose BV, was associated with a cost-savings among women. Furthermore, women who received NAAT, more frequently had STI testing, but still had lower all-cause healthcare costs [15,16]. Also noted in our study, patients who had recurrent BV used outpatient healthcare resources for BV management to a considerable extent and the increase in BV-related pharmacy costs among them was also substantial. Although further study is warranted, the findings of Kong et al. [15] and Troeger et al. [16], alongside the findings of our study, suggest advanced diagnostic testing providing a clearer treatment path may lead to a reduction in follow-up healthcare visits needed for further BV testing, BV recurrence and/or other GYN conditions and improved patient care [15,16,18]. Such a reduction in use of healthcare resources, as well as total medical expenditures, have been observed with advanced molecular testing and diagnosis of vulvovaginitis among a large commercially insured population of women in the USA (n = 46,810 symptomatic women with NAAT testing matched 1:1 to women with non-amplified molecular probe testing) [30]. Another advantage of using advanced molecular testing is the ability to simultaneously test for STIs.
We also recently conducted a similar study to the current one among a population of patients covered by Medicaid (2017–2020) [31]. In that study, BV recurrence affected 43% of the 114,313 patients with an incident BV diagnosis and ≥1 pharmacy claim for a BV medication. Following an incident BV diagnosis, occurrences of new GYN conditions affected 18%, most (77%) of which were STIs. Among those with an increasing number of treatment courses, other GYN conditions, including STIs, were more frequent. Total all-cause and BV-related healthcare costs PPPY averaged $5794 and $300, respectively, among the population of patients with Medicaid coverage and both costs increased among those with more treatment courses. Although we observed the same trends regarding rates of other GYN conditions and all-cause, BV-related and GYN condition-associated healthcare costs all rising among patients who received more treatment courses, among the Medicaid population, BV recurrence and rates of other GYN conditions were higher, by approximately 7% and 6%, respectively. Also, treatment patterns differed, with an even greater proportion of the overall Medicaid study population being treated with oral metronidazole (89% vs 74% among those commercially insured) and a smaller proportion receiving other BV medications in subsequent treatment courses. Other differences included that the Medicaid population (mean age: 28.4 years) was younger than the commercially insured population (31.5 years). We could not evaluate population differences in the proportions of race/ethnicity groups, since the MarketScan commercial database does not contain such information. Conversely, we could not evaluate differences in the proportions from USA geographic regions, since the MarketScan Medicaid database does not provide USA regional data.
The findings of this study should be interpreted in the context of its limitations. Firstly, inherent to all claims database analyses, claims may contain possible coding errors, coding for the purpose of rule-out, and under-/upper-coding. Furthermore, since there are no specific ICD-10 codes for BV, codes indicating vaginitis were used to identify BV. Thus, some patients with vaginitis caused by other non-bacterial mechanisms (e.g., vulvovaginal candidiasis, trichomoniasis, allergens) may have been included in the study population. However, treatment with a BV medication was a requirement for patients to be included in the study population and those with a vaginitis code but only anti-fungal treatment were excluded. The stringent criteria we applied to enhance the selection process of patients only with BV may have led to underreporting of BV cases in the study population. The frequency of other GYN conditions, especially STIs (e.g., lack of syphilis, trichomoniasis, spontaneous abortion and induced abortion) may also be underreported in this study, because some patients may seek care from providers not represented in the MarketScan commercial database (e.g., public health funded sexually transmitted infection clinics). Moreover, the other GYN conditions we evaluated are only potentially associated with BV and could have been caused by other factors (e.g., another STI, an untreated/unresolved STI, a change in sexual activity, a new sexual partner, etc.) and may or may not be indirectly or directly associated with the occurrence of BV. Regarding pharmacy claims, the data only reflected those filled by patients and not whether the patient adhered to or completed the treatment, a considerably understudied subject among patients treated for BV in the USA. Additionally, as a proxy for BV recurrence, subsequent pharmacy claims for BV medications during the follow-up were used, and recurrence was not clinically documented and verified nor was the type of recurrence (e.g., new BV infection or unresolved infection) known. As discussed, our study findings among commercially insured patients may not completely generalize to patients with other types of health insurance coverage, such as Medicaid.
Conclusion
This large-scale study of commercially insured patients is novel because the impact of BV recurrence and the occurrence of other GYN conditions, HCRU and costs have not been previously comprehensively studied nor have treatment patterns among patients with BV recurrence been widely examined in the USA. Given the high rates of BV recurrence and subsequent increases in burden of disease, these study findings underscore the necessity for more effective treatments to prevent recurrence and other improvements in women's reproductive healthcare, which may help to minimize the risk of adverse GYN condition outcomes and reduce the healthcare economic burden of BV. Additionally, study findings among commercially insured patients compared with patients with Medicaid coverage [31], particularly the higher burden of disease among Medicaid patients, call more attention to the socioeconomic disparities in women's healthcare.
Summary points.
Bacterial vaginosis (BV) is a common vaginal dysbiosis associated with adverse clinical sequelae, most notably, increased risk for sexually transmitted infections (STIs).
Among the patients with an incident BV diagnosis and ≥1 pharmacy claim for a BV medication, 64.2% had 1 treatment course, 22.0% had 2, 8.1% had 3, and 5.8% had ≥4 during the follow-up.
Using ≥2 treatment courses as a proxy for BV recurrence, 35.8% of patients had a BV recurrence during the follow-up period.
Most (73.6%) patients were prescribed oral metronidazole; however, its use declined among those with an increasing number of treatment courses.
Approximately 12% of patients had a new diagnosis of a GYN condition in the follow-up; 8.2% had a new STI, which were more common among patients with ≥4 treatment courses (12.9%).
During follow-up, total all-cause healthcare costs averaged $8987 per patient per year (PPPY) of which $470 was BV related.
BV-related healthcare costs increased from $403 PPPY among those with 1 treatment course to $806 PPPY among those with ≥4 with nearly half the costs attributed to outpatient office visits.
Given the high rates of BV recurrence and subsequent increases in burden of disease, these study findings underscore the necessity for more effective treatments to prevent recurrence and other improvements in women's reproductive healthcare.
Additionally, study findings among commercially insured patients compared with patients with Medicaid coverage, particularly the higher burden of disease among Medicaid patients, call more attention to the socioeconomic disparities in women's healthcare.
Supplementary Material
Acknowledgments
Some aspects of the data contained within this manuscript were presented at the Professional Society for Health Economics and Outcomes Research (ISPOR) Annual Meeting, 7–10 May 2023, Boston, MA, USA.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2023-0079
Author contributions
E Watkins, C Chow, M Lingohr-Smith, J Lin, C Yong, K Tangirala, K Collins, J Li, R Brooks and J Amico were all responsible for study conception and design. Authors, C Chow and K Tangirala, were responsible for data acquisition and analysis. M Lingohr-Smith and J Lin were responsible for drafting the manuscript. E Watkins, C Chow, M Lingohr-Smith, J Lin, C Yong, K Tangirala, K Collins, J Li, R Brooks and J Amico contributed to data interpretation, critically revising the manuscript, and provided approval for the final version of the manuscript.
Financial disclosure
This study, manuscript preparation, and article processing charges were funded by Organon. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
E Watkins, C Yong, K Tangirala, K Collins and J Li are employees of Organon. CM Chow, R Brooks and J Amico are external consultants for Organon, which provided consulting fees in connection with this study and the development of this manuscript. M Lingohr-Smith and J Lin are employees of Novosys Health, which received financial support from Organon in connection with this study and the development of this manuscript. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study utilized the MarketScan commercial database, which is fully compliant with USA privacy laws and regulations (i.e., HIPAA). Ethical approval was not required as this was a retrospective analysis of administrative claims data.
Data sharing statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Morris M, Nicoll A, Simms I, Wilson J, Catchpole M. Bacterial vaginosis: a public health review. BJOG 108(5), 439–450 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Muzny CA, Balkus J, Mitchell C et al. Diagnosis and management of bacterial vaginosis: summary of evidence reviewed for the 2021 Centers for Disease Control and Prevention sexually transmitted infections treatment guidelines. Clin. Infect. Dis. 74(Suppl. 2), S144–S151 (2022). [DOI] [PubMed] [Google Scholar]; •• Presents Centers for Disease Control and Prevention (CDC) recommended bacterial vaginosis (BV) treatment guidelines.
- 3.Paladine HL, Desai U. Vaginitis: diagnosis and treatment. Am. Fam. Physician 97(5), 321–329 (2018). [PubMed] [Google Scholar]
- 4.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet. Gynecol. 109(1), 114–120 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Mollin A, Katta M, Sobel JD, Akins RA. Association of key species of vaginal bacteria of recurrent bacterial vaginosis patients before and after oral metronidazole therapy with short- and long-term clinical outcomes. PLOS ONE 17(7), e0272012 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar AS, Nogueira NF, Rodriguez VJ et al. A syndemic approach to explore factors associated with bacterial vaginosis. AIDS Behav. 26(9), 3110–3118 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muzny CA, Harbison HS, Austin EL et al. Sexually transmitted infection risk among women is not fully explained by partner numbers. South Med. J. 110(3), 161–167 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw CS, Sobel JD. Current treatment of bacterial vaginosis – limitations and need for innovation. J. Infect. Dis. 214(Suppl. 1), S14–S20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Review of current BV treatment.
- 9.Bradshaw CS, Morton AN, Hocking J et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 193(11), 1478–1486 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst. Rev. 8(3), CD006055 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Sobel JD, Ferris D, Schwebke J et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am. J. Obstet. Gynecol. 194(5), 1283–1289 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 64(RR-03), 1–137 (2015). [PMC free article] [PubMed] [Google Scholar]
- 13.Faught BM, Reyes. Characterization and treatment of recurrent bacterial vaginosis. J. Womens Health (Larchmt). 28(9), 1218–1226 (2019). [DOI] [PubMed] [Google Scholar]; •• Presents CDC recommended BV treatment guidelines.
- 14.Landlinger C, Oberbauer V, Tisakova LP et al. Preclinical data on the Gardnerella-specific endolysin PM-477 indicate its potential to improve the treatment of bacterial vaginosis through enhanced biofilm removal and avoidance of resistance. Antimicrob. Agents Chemother. 66(5), e0231921 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong AM, Jenkins D, Troeger K, Kim G, London RS. Diagnostic testing of vaginitis: improving the value of care. Popul. Health Manag. 24(4), 515–524 (2021). [DOI] [PubMed] [Google Scholar]; • Study of costs associated with BV.
- 16.Troeger KA, Thiel ER, London RS. Optimizing vaginitis diagnosis to reduce health care costs in nonpregnant women utilizing molecular diagnostics. Popul. Health Manag. 25(4), 449–454 (2022). [DOI] [PubMed] [Google Scholar]; • Study of costs associated with BV.
- 17.Centers for Disease Control and Prevention. Bacterial vaginosis – CDC fact sheet. (2023). www.cdc.gov/std/bv/stdfact-bacterial-vaginosis.htm
- 18.Hillier SL, Austin M, Macio I, Meyn LA, Badway D, Beigi R. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin. Infect. Dis. 72(9), 1538–1543 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobel JD, Kaur N, Woznicki NA et al. Conventional oral and secondary high dose vaginal metronidazole therapy for recurrent bacterial vaginosis: clinical outcomes, impacts of sex and menses. Infect. Drug Resist. 12, 2297–2307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobel JD, Kaur N, Woznicki NA et al. Prognostic indicators of recurrence of bacterial vaginosis. J. Clin. Microbiol. 57(5), e00227–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong E, Hemmerling A, Joag V et al. Treatment success following standard antibiotic treatment for bacterial vaginosis is not associated with pretreatment genital immune or microbial parameters. Open Forum Infect. Dis. 10(1), ofad007 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreisel K, Spicknall IH, Gargano JW et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex. Transm. Dis. 48(4), 208–214 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Reported STDs reach all-time high for 6th consecutive year. (2023). www.cdc.gov/media/releases/2022/p0412-STD-Increase.html
- 24.Van Gerwen OT, Muzny CA, Marrazzo JM. Sexually transmitted infections and female reproductive health. Nat. Microbiol. 7(8), 1116–1126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brotman RM, Klebanoff MA, Nansel TR et al. Bacterial vaginosis assessed by Gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J. Infect. Dis. 202(120), 1907–1915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shipitsyna E, Khusnutdinova T, Budilovskaya O et al. Bacterial vaginosis-associated vaginal microbiota is an age-independent risk factor for Chlamydia trachomatis, Mycoplasma genitalium and Trichomonas vaginalis infections in low-risk women, St. Petersburg, Russia. Eur. J. Clin. Microbiol. Infect. Dis. 39(7), 1221–1230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin. Infect. Dis. 36(5), 663–685 (2003). [DOI] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services. STI national strategic plan overview. (2023). www.hhs.gov/programs/topic-sites/sexually-transmitted-infections/plan-overview/index.html
- 29.Brown H, Drexler M. Improving the diagnosis of vulvovaginitis: perspectives to align practice, guidelines, and awareness. Popul. Health Manag. 23(Suppl. 1), S3–S12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Covers STI USA national strategic plan.
- 30.Ackerman SJ, Knight T, Wahl PM, Cartwright CP. Health care utilization and costs following amplified versus non-amplified molecular probe testing for symptomatic patients with suspected vulvovaginitis: a US commercial payer population. Clinicoecon. Outcomes Res. 11, 179–189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins E, Chow C, Lingohr-Smith M et al. Bacterial vaginosis treatment patterns, associated complications, and healthcare economic burden of women with Medicaid coverage in the United States. Ann. Pharmacother. (2023) (Epub ahead of Print). [DOI] [PubMed] [Google Scholar]; •• Comparative study among patients with Medicaid coverage.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.