The reaction of 4-amino 3-nitrobenzoic acid and manganese dichloride tetrahydrate in an ethanol–water mixture yielded the title complex[Mn(C7H5N2O4)2(C7H6N2O4)2(H2O)2]·2H2O. In the crystal, molecules are linked by N—H⋯O, O—H⋯O and C—H⋯O hydrogen bonds.
Keywords: 4-amino 3-nitrobenzoic acid, MnII , crystal structure, hydrogen bond
Abstract
The manganese title complex, [Mn(C7H5N2O4)2(C7H6N2O4)2(H2O)2]·2H2O, is one of the first 4-amino 3-nitrobenzoic acid (4 A3NBA) monoligand metal complexes to be synthesized. It crystallizes in the centrosymmetric monoclinic space group P21/n with the complex molecules located on inversion centers. Four 4 A3NBA ligand molecules are monodentately coordinated by the Mn2+ ion through the carboxylic oxygen atoms while the other two positions of the inner coordination sphere are occupied by water molecules, giving rise to a distorted octahedron, and two water molecules are in the outer coordination sphere. There are two intramolecular hydrogen bonds in the complex molecule. The first is of the common N—H⋯O=N type, while the second is a rarely occurring very strong hydrogen bond in which a common proton is shared by two uncoordinated oxygen atoms of neighboring carboxylate groups. In the crystal, an intricate system of intermolecular hydrogen bonds links the complex molecules into a three-dimensional-network.
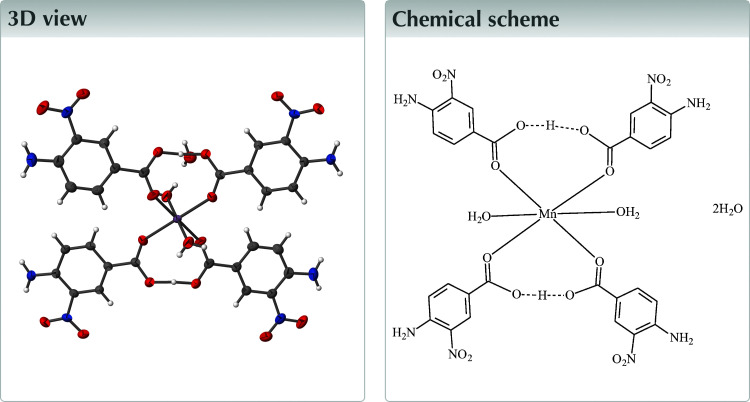
Structure description
The molecular structure of the title complex is shown in Fig. 1 ▸. It crystallizes in the centrosymmetric monoclinic space group P21/n with the complex molecules located on inversion centers. Four 4-amino 3-nitrobenzoic acid (4 A3NBA) ligands are monodentately coordinated by the Mn2+ ion through the oxygen atoms of carboxylic groups while two other positions of the inner coordination sphere are occupied by water molecules. The outer coordination sphere contains two water molecules, i.e. the complex is crystal hydrate. The length of the Mn—O1 bond is 2.1575 (12) Å while Mn—O5 is 2.1600 (13) Å and Mn—O1W = 2.1630 (14) Å and bond angles are in the range 84.29 (5) to 95.71 (5)°. The geometry of the manganese atom is therefore a slightly distorted octahedron. The carboxylate groups C7,O2,O1 and C14,O6,O5 are practically coplanar with the aromatic rings to which they are attached, forming dihedral angles of 4.1 (1) and 11.9 (1)°, respectively. The analogous angles for nitro groups N1,O3,O4 and N3,O7,O8 are 2.82 (9) and 8.6 (1)°. Thus in the ligand with the C8–C13 aromatic ring, the functional groups are more inclined relatively to the benzene ring.
Figure 1.
The molecular structure of the title compound, showing the atom-labeling scheme and displacement ellipsoids drawn at the 50% probability level. H atoms are shown as small spheres of arbitrary radius and hydrogen bonds are shown as dashed lines. Symmetry code: (i) 1 − x, 1 − y, −z.
There are two intramolecular hydrogen bonds in the complex molecule (Table 1 ▸). The first bond is of the usual N—H⋯O=N type, closing a six-membered ring with an
 (6) graph-set motif (Etter 1990 ▸; Ibragimov et al., 2017 ▸; Ruzmetov et al., 2022 ▸). The second is a rarely occurring very strong hydrogen bond closing a nine-membered ring where a common proton, H20, is shared by two uncoordinated oxygen atoms O2 and O6 of neighboring carboxylate groups. The atom H20, situated between the two oxygen atoms, is located closer to atom O2 at a distance of 1.270 (2) Å [and 1.198 (2) Å from O6]. Despite this, it is impossible to indicate which of the four carboxylic groups present are deprotonated. The total negative charge of the carboxylic groups is 2 and it compensates the +2 charge of the Mn2+ ion.
(6) graph-set motif (Etter 1990 ▸; Ibragimov et al., 2017 ▸; Ruzmetov et al., 2022 ▸). The second is a rarely occurring very strong hydrogen bond closing a nine-membered ring where a common proton, H20, is shared by two uncoordinated oxygen atoms O2 and O6 of neighboring carboxylate groups. The atom H20, situated between the two oxygen atoms, is located closer to atom O2 at a distance of 1.270 (2) Å [and 1.198 (2) Å from O6]. Despite this, it is impossible to indicate which of the four carboxylic groups present are deprotonated. The total negative charge of the carboxylic groups is 2 and it compensates the +2 charge of the Mn2+ ion.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1W—H1WA⋯O3i | 0.83 (2) | 2.06 (2) | 2.8850 (19) | 176 (2) |
| O1W—H1WA⋯O4i | 0.83 (2) | 2.55 (2) | 3.1418 (19) | 130 (2) |
| O1W—H1WA⋯N1i | 0.83 (2) | 2.63 (2) | 3.4029 (19) | 157 (2) |
| O1W—H1WB⋯O2W | 0.81 (2) | 1.98 (2) | 2.784 (2) | 170 (2) |
| O2—H2O⋯O5 | 1.27 (4) | 2.57 (4) | 3.448 (2) | 124 (2) |
| O6—H2O⋯O2 | 1.20 (4) | 1.27 (4) | 2.4541 (18) | 168 (4) |
| N2—H2A⋯O6ii | 0.86 | 2.19 | 3.0160 (19) | 162 |
| N2—H2B⋯O4 | 0.85 | 1.99 | 2.629 (2) | 131 |
| N4—H4A⋯O8 | 0.87 | 2.03 | 2.648 (2) | 128 |
| N4—H4B⋯O2W i | 0.87 | 2.16 | 3.021 (2) | 173 |
| C5—H5⋯O2ii | 0.93 | 2.57 | 3.489 (2) | 170 |
| C9—H9⋯N2iii | 0.93 | 2.66 | 3.544 (2) | 160 |
| C12—H12⋯O8iv | 0.93 | 2.42 | 3.178 (2) | 138 |
| O2W—H2WA⋯O7v | 0.86 | 2.14 | 2.995 (2) | 173 |
| O2W—H2WB⋯O1W vi | 0.85 | 2.39 | 3.127 (2) | 145 |
| O2W—H2WB⋯O5vii | 0.85 | 2.65 | 3.341 (2) | 139 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 ; (v)
; (v)
 ; (vi)
; (vi)
 ; (vii)
; (vii)
 .
.
There are 17 proton-acceptor oxygen atoms, 4 proton-donor nitrogen atoms and 2 water molecules in the title complex. These atoms are involved in a complex system of intermolecular hydrogen bonds (Table 1 ▸). Moreover, three weak C—H⋯O hydrogen bonds are also observed in the structure (Table 1 ▸, Fig. 2 ▸). Together these hydrogen bonds link the complex molecules into a three-dimensional network (Fig. 2 ▸.).
Figure 2.
The crystal packing viewed along [100] showing the O—H⋯O, N—H⋯O and C—H⋯O hydrogen bonds (dashed red lines) in the crystal structure.
Synthesis and crystallization
All reagents and solvents were purchased from Sigma-Aldrich (Darmstadt, Germany) and they were used as received. MnCl2·H2O (0.198 g, 1.0 mmol) was dissolved in a small amount of water. 4-Amino 3-nitrobenzoic acid (0.364 g, 2 mmol) was dissolved in a mixed solvent of 3 ml of absolute alcohol and 3 ml of distilled water. After dropwise addition of the 4 A3NBA solution to the manganese salt solution, the resultant solution was stirred for 2 h with a magnetic stirrer at 55°C. The solution was allowed to stand at 30°C in a beaker with small holes in the cover for evaporation. After about eight days, block-shaped single crystals ofthe title compound appeared. Analysis calculated: C28H30MnN8O20: C, 39.40%; H, 3.54; N, 13.13%. Found: C, 39.32%; H, 3.47%; N, 13.08%.
Refinement
Crystal data, data collection and structure refinement details for the structure of the synthesized compound are summarized in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Mn(C7H5MnN2O4)2(C7H6MnN2O4)2(H2O)2]·2H2O |
| M r | 853.54 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 293 |
| a, b, c (Å) | 7.0419 (1), 19.2513 (3), 12.7175 (2) |
| β (°) | 100.513 (2) |
| V (Å3) | 1695.12 (5) |
| Z | 2 |
| Radiation type | Cu Kα |
| μ (mm−1) | 4.08 |
| Crystal size (mm) | 0.28 × 0.22 × 0.14 |
| Data collection | |
| Diffractometer | XtaLAB Synergy, Single source at home/near, HyPix3000 |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2023 ▸) |
| T min, T max | 0.523, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 3291, 3291, 2966 |
| R int | 0.037 |
| (sin θ/λ)max (Å−1) | 0.615 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.035, 0.097, 1.06 |
| No. of reflections | 3291 |
| No. of parameters | 269 |
| No. of restraints | 3 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.27, −0.44 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624000403/bv4050sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624000403/bv4050Isup2.hkl
CCDC reference: 2324651
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| [Mn(C7H5MnN2O4)2(C7H6MnN2O4)2(H2O)2]·2H2O | F(000) = 878 |
| Mr = 853.54 | Dx = 1.672 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| a = 7.0419 (1) Å | Cell parameters from 11377 reflections |
| b = 19.2513 (3) Å | θ = 2.3–71.4° |
| c = 12.7175 (2) Å | µ = 4.08 mm−1 |
| β = 100.513 (2)° | T = 293 K |
| V = 1695.12 (5) Å3 | Block, light pink |
| Z = 2 | 0.28 × 0.22 × 0.14 mm |
Data collection
| XtaLAB Synergy, Single source at home/near, HyPix3000 diffractometer | 3291 independent reflections |
| Radiation source: micro-focus sealed X-ray tube | 2966 reflections with I > 2σ(I) |
| Detector resolution: 10.0000 pixels mm-1 | Rint = 0.037 |
| ω scans | θmax = 71.5°, θmin = 4.2° |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2023) | h = −8→8 |
| Tmin = 0.523, Tmax = 1.000 | k = −23→22 |
| 3291 measured reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.035 | Hydrogen site location: mixed |
| wR(F2) = 0.097 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0608P)2 + 0.2227P] where P = (Fo2 + 2Fc2)/3 |
| 3291 reflections | (Δ/σ)max < 0.001 |
| 269 parameters | Δρmax = 0.27 e Å−3 |
| 3 restraints | Δρmin = −0.44 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. The hydrogen atoms of water molecules and amino groups were located in difference-Fourier maps and refined freely. The H atoms of the benzene ring were calculated geometrically with C—H = 0.93 A° and Uiso(H) = 1.2Ueq(C). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Mn1 | 0.500000 | 0.500000 | 0.000000 | 0.02947 (13) | |
| O1 | 0.4809 (2) | 0.39058 (6) | 0.03322 (11) | 0.0489 (4) | |
| O1W | 0.8075 (2) | 0.50832 (7) | 0.05836 (12) | 0.0464 (3) | |
| H1WA | 0.848 (4) | 0.5418 (9) | 0.0964 (19) | 0.070* | |
| H1WB | 0.879 (4) | 0.4760 (9) | 0.078 (2) | 0.070* | |
| O2 | 0.6022 (2) | 0.36153 (7) | 0.20147 (10) | 0.0515 (4) | |
| H2O | 0.619 (6) | 0.422 (2) | 0.242 (3) | 0.155 (15)* | |
| O3 | 0.5562 (2) | 0.13009 (7) | 0.31842 (10) | 0.0560 (4) | |
| O4 | 0.4652 (2) | 0.04466 (7) | 0.21207 (11) | 0.0545 (4) | |
| O5 | 0.4355 (2) | 0.52907 (8) | 0.15374 (10) | 0.0457 (3) | |
| O6 | 0.6122 (2) | 0.47449 (7) | 0.29174 (10) | 0.0483 (4) | |
| O7 | 0.7667 (2) | 0.59190 (8) | 0.62838 (11) | 0.0590 (4) | |
| O8 | 0.6914 (2) | 0.69874 (8) | 0.65088 (11) | 0.0612 (4) | |
| N1 | 0.4964 (2) | 0.10712 (7) | 0.22733 (11) | 0.0363 (3) | |
| N2 | 0.3472 (2) | 0.06488 (7) | 0.00606 (12) | 0.0431 (4) | |
| H2A | 0.302809 | 0.057204 | −0.060340 | 0.052* | |
| H2B | 0.365807 | 0.034904 | 0.056060 | 0.052* | |
| N3 | 0.6948 (2) | 0.64740 (8) | 0.59270 (12) | 0.0410 (4) | |
| N4 | 0.5654 (3) | 0.77954 (8) | 0.48424 (14) | 0.0481 (4) | |
| H4A | 0.587279 | 0.778868 | 0.553450 | 0.058* | |
| H4B | 0.516269 | 0.814368 | 0.445160 | 0.058* | |
| C1 | 0.4755 (2) | 0.27328 (8) | 0.08203 (13) | 0.0297 (3) | |
| C2 | 0.5071 (2) | 0.22409 (8) | 0.16193 (13) | 0.0294 (3) | |
| H2 | 0.557893 | 0.237429 | 0.231674 | 0.035* | |
| C3 | 0.4636 (2) | 0.15439 (8) | 0.13907 (12) | 0.0291 (3) | |
| C4 | 0.3884 (2) | 0.13131 (8) | 0.03426 (12) | 0.0298 (3) | |
| C5 | 0.3551 (3) | 0.18352 (9) | −0.04571 (13) | 0.0351 (4) | |
| H5 | 0.303735 | 0.170966 | −0.115751 | 0.042* | |
| C6 | 0.3963 (2) | 0.25158 (9) | −0.02257 (13) | 0.0333 (4) | |
| H6 | 0.371609 | 0.284412 | −0.076992 | 0.040* | |
| C7 | 0.5212 (2) | 0.34754 (8) | 0.10562 (13) | 0.0335 (4) | |
| C8 | 0.5352 (2) | 0.59261 (9) | 0.31259 (13) | 0.0330 (4) | |
| C9 | 0.6043 (2) | 0.59219 (9) | 0.42108 (13) | 0.0338 (4) | |
| H9 | 0.643858 | 0.550494 | 0.455105 | 0.041* | |
| C10 | 0.6160 (2) | 0.65326 (9) | 0.48065 (13) | 0.0333 (4) | |
| C11 | 0.5581 (2) | 0.71818 (9) | 0.43305 (15) | 0.0358 (4) | |
| C12 | 0.4899 (3) | 0.71660 (9) | 0.32115 (15) | 0.0409 (4) | |
| H12 | 0.453293 | 0.758055 | 0.285614 | 0.049* | |
| C13 | 0.4760 (3) | 0.65655 (10) | 0.26390 (14) | 0.0384 (4) | |
| H13 | 0.426366 | 0.657803 | 0.190964 | 0.046* | |
| C14 | 0.5253 (3) | 0.52789 (9) | 0.24730 (13) | 0.0352 (4) | |
| O2W | 1.0872 (2) | 0.40951 (8) | 0.13520 (11) | 0.0542 (4) | |
| H2WA | 1.119577 | 0.406931 | 0.203299 | 0.081* | |
| H2WB | 1.161577 | 0.433931 | 0.103999 | 0.081* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mn1 | 0.0436 (2) | 0.02138 (19) | 0.01972 (19) | −0.00214 (14) | −0.00417 (15) | 0.00137 (12) |
| O1 | 0.0807 (10) | 0.0235 (6) | 0.0358 (7) | −0.0052 (6) | −0.0070 (6) | 0.0050 (5) |
| O1W | 0.0456 (7) | 0.0419 (8) | 0.0444 (8) | 0.0030 (6) | −0.0114 (6) | −0.0121 (6) |
| O2 | 0.0808 (10) | 0.0297 (7) | 0.0340 (7) | −0.0014 (6) | −0.0157 (6) | −0.0019 (5) |
| O3 | 0.0926 (11) | 0.0393 (7) | 0.0283 (7) | −0.0025 (7) | −0.0099 (7) | 0.0063 (5) |
| O4 | 0.0851 (10) | 0.0251 (6) | 0.0454 (8) | −0.0059 (6) | −0.0086 (7) | 0.0091 (5) |
| O5 | 0.0584 (8) | 0.0528 (8) | 0.0221 (6) | 0.0066 (6) | −0.0030 (5) | −0.0078 (5) |
| O6 | 0.0748 (9) | 0.0322 (7) | 0.0303 (7) | 0.0027 (6) | −0.0106 (6) | −0.0053 (5) |
| O7 | 0.0864 (11) | 0.0519 (9) | 0.0318 (7) | 0.0122 (8) | −0.0079 (7) | 0.0002 (6) |
| O8 | 0.0824 (11) | 0.0605 (9) | 0.0366 (8) | 0.0046 (8) | −0.0006 (7) | −0.0225 (7) |
| N1 | 0.0459 (8) | 0.0285 (7) | 0.0305 (7) | 0.0019 (6) | −0.0038 (6) | 0.0066 (5) |
| N2 | 0.0643 (10) | 0.0265 (7) | 0.0336 (8) | −0.0027 (7) | −0.0044 (7) | −0.0028 (6) |
| N3 | 0.0479 (9) | 0.0443 (9) | 0.0287 (7) | −0.0013 (7) | 0.0012 (6) | −0.0084 (6) |
| N4 | 0.0589 (10) | 0.0334 (8) | 0.0510 (10) | 0.0015 (7) | 0.0072 (8) | −0.0093 (7) |
| C1 | 0.0353 (8) | 0.0235 (8) | 0.0280 (8) | 0.0022 (6) | 0.0001 (6) | 0.0019 (6) |
| C2 | 0.0360 (8) | 0.0249 (8) | 0.0247 (7) | 0.0012 (6) | −0.0010 (6) | −0.0008 (6) |
| C3 | 0.0347 (8) | 0.0242 (8) | 0.0267 (8) | 0.0035 (6) | 0.0011 (6) | 0.0042 (6) |
| C4 | 0.0333 (8) | 0.0243 (7) | 0.0299 (8) | 0.0013 (6) | 0.0003 (6) | −0.0020 (6) |
| C5 | 0.0468 (9) | 0.0304 (9) | 0.0244 (8) | −0.0012 (7) | −0.0036 (7) | −0.0015 (6) |
| C6 | 0.0422 (9) | 0.0282 (8) | 0.0268 (8) | 0.0010 (7) | −0.0013 (7) | 0.0045 (6) |
| C7 | 0.0421 (9) | 0.0248 (8) | 0.0307 (8) | 0.0003 (7) | −0.0012 (7) | 0.0030 (6) |
| C8 | 0.0391 (9) | 0.0335 (9) | 0.0252 (8) | −0.0017 (7) | 0.0030 (6) | −0.0033 (6) |
| C9 | 0.0394 (9) | 0.0312 (8) | 0.0286 (8) | 0.0011 (7) | 0.0004 (7) | −0.0015 (6) |
| C10 | 0.0362 (8) | 0.0354 (9) | 0.0264 (8) | −0.0002 (7) | 0.0012 (7) | −0.0027 (6) |
| C11 | 0.0331 (8) | 0.0337 (9) | 0.0402 (9) | −0.0015 (7) | 0.0058 (7) | −0.0043 (7) |
| C12 | 0.0484 (10) | 0.0324 (9) | 0.0401 (10) | 0.0039 (8) | 0.0032 (8) | 0.0050 (7) |
| C13 | 0.0437 (9) | 0.0418 (10) | 0.0271 (8) | 0.0016 (8) | −0.0005 (7) | 0.0029 (7) |
| C14 | 0.0432 (9) | 0.0372 (9) | 0.0237 (8) | −0.0032 (7) | 0.0021 (7) | −0.0037 (7) |
| O2W | 0.0659 (9) | 0.0496 (8) | 0.0448 (8) | 0.0015 (7) | 0.0040 (7) | 0.0036 (6) |
Geometric parameters (Å, º)
| Mn1—O1i | 2.1575 (12) | N4—H4A | 0.8655 |
| Mn1—O1 | 2.1575 (12) | N4—H4B | 0.8680 |
| Mn1—O5i | 2.1600 (13) | C1—C2 | 1.377 (2) |
| Mn1—O5 | 2.1600 (13) | C1—C6 | 1.409 (2) |
| Mn1—O1W | 2.1630 (14) | C1—C7 | 1.484 (2) |
| Mn1—O1Wi | 2.1630 (14) | C2—C3 | 1.395 (2) |
| O1—C7 | 1.233 (2) | C2—H2 | 0.9300 |
| O1W—H1WA | 0.827 (16) | C3—C4 | 1.413 (2) |
| O1W—H1WB | 0.813 (16) | C4—C5 | 1.419 (2) |
| O2—C7 | 1.277 (2) | C5—C6 | 1.363 (2) |
| O2—H2O | 1.27 (4) | C5—H5 | 0.9300 |
| O3—N1 | 1.2398 (19) | C6—H6 | 0.9300 |
| O4—N1 | 1.2314 (19) | C8—C9 | 1.377 (2) |
| O5—C14 | 1.242 (2) | C8—C13 | 1.406 (2) |
| O6—C14 | 1.275 (2) | C8—C14 | 1.492 (2) |
| O6—H2O | 1.20 (4) | C9—C10 | 1.393 (2) |
| O7—N3 | 1.233 (2) | C9—H9 | 0.9300 |
| O8—N3 | 1.237 (2) | C10—C11 | 1.416 (2) |
| N1—C3 | 1.4307 (19) | C11—C12 | 1.417 (3) |
| N2—C4 | 1.345 (2) | C12—C13 | 1.360 (3) |
| N2—H2A | 0.8581 | C12—H12 | 0.9300 |
| N2—H2B | 0.8510 | C13—H13 | 0.9300 |
| N3—C10 | 1.436 (2) | O2W—H2WA | 0.8558 |
| N4—C11 | 1.345 (2) | O2W—H2WB | 0.8537 |
| O1i—Mn1—O1 | 180.0 | C3—C2—H2 | 119.7 |
| O1i—Mn1—O5i | 92.57 (6) | C2—C3—C4 | 121.96 (14) |
| O1—Mn1—O5i | 87.43 (6) | C2—C3—N1 | 116.77 (14) |
| O1i—Mn1—O5 | 87.43 (6) | C4—C3—N1 | 121.27 (14) |
| O1—Mn1—O5 | 92.57 (6) | N2—C4—C3 | 125.16 (15) |
| O5i—Mn1—O5 | 180.0 | N2—C4—C5 | 118.87 (14) |
| O1i—Mn1—O1W | 84.29 (5) | C3—C4—C5 | 115.97 (14) |
| O1—Mn1—O1W | 95.71 (5) | C6—C5—C4 | 121.70 (15) |
| O5i—Mn1—O1W | 88.12 (5) | C6—C5—H5 | 119.1 |
| O5—Mn1—O1W | 91.88 (5) | C4—C5—H5 | 119.1 |
| O1i—Mn1—O1Wi | 95.71 (5) | C5—C6—C1 | 121.42 (15) |
| O1—Mn1—O1Wi | 84.29 (5) | C5—C6—H6 | 119.3 |
| O5i—Mn1—O1Wi | 91.88 (5) | C1—C6—H6 | 119.3 |
| O5—Mn1—O1Wi | 88.12 (5) | O1—C7—O2 | 124.97 (16) |
| O1W—Mn1—O1Wi | 180.0 | O1—C7—C1 | 118.99 (15) |
| C7—O1—Mn1 | 142.04 (12) | O2—C7—C1 | 116.03 (14) |
| Mn1—O1W—H1WA | 118.6 (17) | C9—C8—C13 | 117.94 (15) |
| Mn1—O1W—H1WB | 125.3 (18) | C9—C8—C14 | 121.66 (15) |
| H1WA—O1W—H1WB | 106 (2) | C13—C8—C14 | 120.40 (15) |
| C7—O2—H2O | 124.8 (18) | C8—C9—C10 | 121.00 (16) |
| C14—O5—Mn1 | 135.11 (13) | C8—C9—H9 | 119.5 |
| C14—O6—H2O | 120.4 (19) | C10—C9—H9 | 119.5 |
| O4—N1—O3 | 121.07 (14) | C9—C10—C11 | 121.91 (15) |
| O4—N1—C3 | 119.93 (14) | C9—C10—N3 | 116.57 (15) |
| O3—N1—C3 | 119.00 (14) | C11—C10—N3 | 121.50 (15) |
| C4—N2—H2A | 116.6 | N4—C11—C10 | 125.85 (17) |
| C4—N2—H2B | 116.7 | N4—C11—C12 | 118.74 (17) |
| H2A—N2—H2B | 126.7 | C10—C11—C12 | 115.41 (15) |
| O7—N3—O8 | 121.58 (15) | C13—C12—C11 | 122.26 (16) |
| O7—N3—C10 | 119.44 (14) | C13—C12—H12 | 118.9 |
| O8—N3—C10 | 118.98 (16) | C11—C12—H12 | 118.9 |
| C11—N4—H4A | 117.6 | C12—C13—C8 | 121.45 (16) |
| C11—N4—H4B | 115.2 | C12—C13—H13 | 119.3 |
| H4A—N4—H4B | 124.9 | C8—C13—H13 | 119.3 |
| C2—C1—C6 | 118.40 (14) | O5—C14—O6 | 123.99 (16) |
| C2—C1—C7 | 120.90 (14) | O5—C14—C8 | 118.82 (16) |
| C6—C1—C7 | 120.70 (14) | O6—C14—C8 | 117.19 (15) |
| C1—C2—C3 | 120.53 (15) | H2WA—O2W—H2WB | 115.4 |
| C1—C2—H2 | 119.7 | ||
| C6—C1—C2—C3 | 0.7 (2) | C13—C8—C9—C10 | −0.2 (3) |
| C7—C1—C2—C3 | 179.94 (15) | C14—C8—C9—C10 | 178.81 (16) |
| C1—C2—C3—C4 | 0.8 (3) | C8—C9—C10—C11 | −0.1 (3) |
| C1—C2—C3—N1 | −178.46 (15) | C8—C9—C10—N3 | −178.47 (16) |
| O4—N1—C3—C2 | −178.27 (16) | O7—N3—C10—C9 | 7.9 (3) |
| O3—N1—C3—C2 | 1.7 (2) | O8—N3—C10—C9 | −173.23 (17) |
| O4—N1—C3—C4 | 2.5 (2) | O7—N3—C10—C11 | −170.52 (17) |
| O3—N1—C3—C4 | −177.55 (16) | O8—N3—C10—C11 | 8.4 (3) |
| C2—C3—C4—N2 | 178.46 (16) | C9—C10—C11—N4 | −179.64 (18) |
| N1—C3—C4—N2 | −2.3 (3) | N3—C10—C11—N4 | −1.3 (3) |
| C2—C3—C4—C5 | −1.7 (2) | C9—C10—C11—C12 | −0.6 (3) |
| N1—C3—C4—C5 | 177.57 (15) | N3—C10—C11—C12 | 177.70 (16) |
| N2—C4—C5—C6 | −179.08 (17) | N4—C11—C12—C13 | −179.19 (18) |
| C3—C4—C5—C6 | 1.0 (3) | C10—C11—C12—C13 | 1.7 (3) |
| C4—C5—C6—C1 | 0.5 (3) | C11—C12—C13—C8 | −2.1 (3) |
| C2—C1—C6—C5 | −1.4 (3) | C9—C8—C13—C12 | 1.3 (3) |
| C7—C1—C6—C5 | 179.42 (17) | C14—C8—C13—C12 | −177.73 (17) |
| Mn1—O1—C7—O2 | 0.8 (3) | Mn1—O5—C14—O6 | −51.2 (3) |
| Mn1—O1—C7—C1 | −178.67 (15) | Mn1—O5—C14—C8 | 128.20 (16) |
| C2—C1—C7—O1 | −175.97 (17) | C9—C8—C14—O5 | 169.25 (17) |
| C6—C1—C7—O1 | 3.2 (3) | C13—C8—C14—O5 | −11.7 (3) |
| C2—C1—C7—O2 | 4.5 (3) | C9—C8—C14—O6 | −11.3 (3) |
| C6—C1—C7—O2 | −176.31 (16) | C13—C8—C14—O6 | 167.69 (17) |
Symmetry code: (i) −x+1, −y+1, −z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1W—H1WA···O3ii | 0.83 (2) | 2.06 (2) | 2.8850 (19) | 176 (2) |
| O1W—H1WA···O4ii | 0.83 (2) | 2.55 (2) | 3.1418 (19) | 130 (2) |
| O1W—H1WA···N1ii | 0.83 (2) | 2.63 (2) | 3.4029 (19) | 157 (2) |
| O1W—H1WB···O2W | 0.81 (2) | 1.98 (2) | 2.784 (2) | 170 (2) |
| O2—H2O···O5 | 1.27 (4) | 2.57 (4) | 3.448 (2) | 124 (2) |
| O6—H2O···O2 | 1.20 (4) | 1.27 (4) | 2.4541 (18) | 168 (4) |
| N2—H2A···O6iii | 0.86 | 2.19 | 3.0160 (19) | 162 |
| N2—H2B···O4 | 0.85 | 1.99 | 2.629 (2) | 131 |
| N4—H4A···O8 | 0.87 | 2.03 | 2.648 (2) | 128 |
| N4—H4B···O2Wii | 0.87 | 2.16 | 3.021 (2) | 173 |
| C5—H5···O2iii | 0.93 | 2.57 | 3.489 (2) | 170 |
| C9—H9···N2iv | 0.93 | 2.66 | 3.544 (2) | 160 |
| C12—H12···O8v | 0.93 | 2.42 | 3.178 (2) | 138 |
| O2W—H2WA···O7vi | 0.86 | 2.14 | 2.995 (2) | 173 |
| O2W—H2WB···O1Wvii | 0.85 | 2.39 | 3.127 (2) | 145 |
| O2W—H2WB···O5viii | 0.85 | 2.65 | 3.341 (2) | 139 |
Symmetry codes: (ii) −x+3/2, y+1/2, −z+1/2; (iii) x−1/2, −y+1/2, z−1/2; (iv) x+1/2, −y+1/2, z+1/2; (v) x−1/2, −y+3/2, z−1/2; (vi) −x+2, −y+1, −z+1; (vii) −x+2, −y+1, −z; (viii) x+1, y, z.
Funding Statement
The authors gratefully acknowledge the Ministry of Higher Education, Science and Innovation for financial support (project No. F3–20200929348) and would also like to thank the Uzbekistan government for direct financial support of this research.
References
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Etter, M. C. (1990). Acc. Chem. Res. 23, 120–126.
- Ibragimov, A. B., Ashurov, Z. M. & Zakirov, B. S. (2017). J. Struct. Chem. 58, 588–590.
- Rigaku OD (2023). CrysAlis PRO. Rigaku Oxford Diffraction Ltd, Yarnton, England.
- Ruzmetov, A., Ibragimov, A., Ashurov, J., Boltaeva, Z., Ibragimov, B. & Usmanov, S. (2022). Acta Cryst. E78, 660–664. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624000403/bv4050sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624000403/bv4050Isup2.hkl
CCDC reference: 2324651
Additional supporting information: crystallographic information; 3D view; checkCIF report




