Abstract
Aim:
To compare all-cause and acute lymphoblastic leukemia (ALL)-related healthcare resource utilization (HCRU) and costs among patients receiving inotuzumab ozogamicin (InO) and blinatumomab (Blina) for ALL in the first relapsed/refractory (R/R) setting.
Patients & methods:
We studied retrospective claims for adult commercial and Medicare Advantage enrollees with ALL receiving InO (n = 29) or Blina (n = 23) from 1 January 2015 to 16 February 2021. Mean per-patient-per-month (PPPM) HCRU and total costs were described and multivariable-adjusted PPPM total all-cause and ALL-related predicted costs were calculated.
Results:
Mean monthly ALL-related hospitalizations were the same for patients receiving InO and Blina (PPPM = 0.8 stays); however, the length of ALL-related hospital stay was almost twice as long among patients receiving Blina versus InO (ALL-related: InO = 7.6 days; Blina = 14.1 days; p = 0.346). In multivariable models, total ALL-related costs were 43% lower for InO compared with Blina (PPPM costs: InO = $93,767; Blina = $163,470; p = 0.021).
Conclusion:
In the first R/R setting, patients who used InO had significantly lower all-cause and ALL-related costs compared with patients who used Blina, in part driven by hospitalization patterns.
Keywords: acute lymphoblastic leukemia, costs, health care resource utilization, medications, relapse/refractory
Plain language summary
What was this article about?
We studied adults with acute lymphoblastic leukemia, which is a rare type of blood cancer. This cancer frequently returns (relapses) or does not completely go away (refractory), even after cancer treatment. We studied patients who were experiencing relapsed or refractory blood cancer for the first time. Death from this cancer is high. Two newer medications for treating this cancer are inotuzumab ozogamicin and blinatumomab, also known as InO and Blina. Patients who receive these medications have lower death rates. We studied how much patients with this cancer used healthcare services and the costs of these services. We looked at whether there were differences in costs for patients receiving InO compared with Blina, after accounting for differences in characteristics between these two patient groups.
What were the results?
Patients who received InO had shorter stays in the hospital to treat their blood cancer than patients receiving Blina. Patients receiving InO had lower medical costs to treat the blood cancer than patients receiving Blina.
What do the results of the study mean?
Patients who received Blina may have had higher medical costs than patients who received InO because hospitalizations are costly. The results of our study are important for healthcare decision makers who decide which medications have the best value for money. We studied a relatively small number of patients. We do not know if the results of this study apply to all patients with this blood cancer in the same situation. This is one of the first studies to examine these questions. The patterns we found are like earlier studies, which used different scientific approaches. This increases our confidence in the results of this study.
Acute lymphoblastic leukemia (ALL) is a life-threatening malignancy of lymphocyte precursors that originates in the bone marrow (lymphoblasts) and disseminates rapidly through the blood stream when left untreated [1]. ALL develops from uncontrolled cell division of lymphocytes that are immature and fail to function normally. These cells proliferate in the bone marrow diminishing normal hematopoiesis and leading to pancytopenia with secondary constitutional symptoms, including infection, bleeding and death [1]. Acute lymphoblastic leukemia of B-cell lineage represents 75% of cases and includes Philadelphia (Ph) chromosome positive ALL, a distinct subtype of B-cell ALL. T-cell ALL accounts for 25% of ALL cases [1,2]. Through hematogenous spread, the malignant lymphoblasts typically spread to organs including the liver, spleen, and lymph nodes but do not commonly generate solid tumors like other cancers [1]. Although relatively rare, an estimated 107,620 people were living with ALL in the USA in 2019. In 2023, 6540 new cases and 1390 deaths are estimated, with adults representing four of every 10 cases [3].
Standard chemotherapy for newly diagnosed ALL is administered in a range of combinations and treatment schedules, typically in three phases: induction of remission, post-remission consolidation/intensification of therapy, and remission maintenance therapy [4]. ALL patients may also receive allogeneic hematopoietic stem cell transplantation (HSCT) after achieving remission, depending on the risk of relapse [5]. Outcomes using standard of care (SoC) salvage chemotherapy for adults with relapsed or refractory (R/R) ALL are poor and have been associated with a median survival of only 4.5 to 6 months, and a 5-year overall survival rate below 10% [4,6].
In R/R ALL, the use of targeted therapies, InO and Blina, have been separately shown to improve outcomes and induce longer and deeper remissions, relative to SoC chemotherapy [7–9]. Inotuzumab ozogamicin is a CD22-directed humanized monoclonal antibody drug conjugate [9] that targets and binds to CD22 proteins on the surface of ALL cells and delivers calicheamicin, killing ALL cells [10]. Blinatumomab, a CD19-CD3 bispecific antibody approved for the treatment of R/R B-cell precursor ALL [11], enables CD3-positive T-cells to find and terminate CD19-positive ALL lymphoblast cells [7].
Inotuzumab ozogamicin received US FDA approval in 2017 for use in adult patients with R/R B-cell precursor ALL, supported by results from the INO-VATE ALL randomized, open-label phase III trial [8,10]. The INO-VATE ALL study comprised 326 adults with Ph-negative or Ph-positive R/R B-cell precursor ALL with at least 5% bone marrow blasts. Study patients had received one or no prior salvage chemotherapy regimen for ALL [8–10]. The rate of complete remission/complete remission with incomplete hematologic recovery (CR/CRi) was greater in patients treated with InO relative to those receiving SoC (73.8% vs 30.9%, p < 0.001) [8]. During long-term follow-up, median overall survival for InO was 7.7 months versus 6.2 months for SoC with two-year overall survival rates of 22.8% and 10.0%, respectively (HR: 0.75; 97.5% CI 0.57–0.99; p = 0.01) [8].
Blina was granted accelerated approval by the FDA in 2014 for the treatment of patients with Ph chromosome-negative R/R B-cell precursor ALL, later expanding to patients who are in remission but still have minimal residual disease and patients who are Ph chromosome positive [11]. The efficacy and safety of Blina were evaluated in Phase III of the TOWER study, which compared outcomes in adult patients with Ph-negative R/R B-cell precursor ALL. The study included 271 Blina patients and 134 patients who received SoC chemotherapy [7]. Compared with SoC, Blina patients had a longer median overall survival (7.7 vs 4.0 months, respectively; HR: 0.71; 95% CI 0.55–0.93; p = 0.01) and a greater percentage of complete remission with full, partial, or incomplete hematologic recovery (CR, CRh or CRi) 12 weeks after treatment initiation (44% vs 25%, respectively; p < 0.001) [7].
To date, InO and Blina have not been compared directly in a randomized clinical trial. One study using respective trial data to indirectly compare the effectiveness of InO and Blina found similar short-term survival rates, but higher rates of CR/CRi and subsequent HSCT for InO versus Blina [12]. An alternative indirect comparison found no significant differences between InO and Blina with respect to CR (when excluding CRi), median overall survival, and restricted mean survival time at 20.7 months; however, restricted mean survival time at 12 months favored Blina [12,13]. A real-world, multicenter, retrospective study found similar rates of CR/CRi, duration of response, and overall survival among R/R ALL patients treated with InO versus Blina [14].
Real-world economic evidence comparing InO and Blina is limited. A single study conducted in 2022 among Medicare beneficiaries reported that all-cause costs for treatment of R/R ALL were 14% lower for InO (n = 55) versus Blina (n = 209) [15]. Payers are increasingly seeking cost–effectiveness data to support their decisions on access and reimbursements for these novel treatments [16]. Cognizant of the limited evidence available, this study sought to examine all-cause and ALL-related healthcare resource utilization (HCRU) and costs among patients receiving InO and Blina for R/R ALL using claims data from a large national US health insurer.
Methods
Data source & study design
This observational study comprised commercial and Medicare Advantage (MAPD) enrollees in the Optum Research Database (ORD) from 1 January 2015 to 28 February 2021 (study period). In the USA, all adults aged ≥65 years are eligible for Medicare, a government-administered health plan. Some commercial insurers are approved to offer Medicare Advantage coverage. Medicare Advantage can include coverage for a wider range of services than government-administered Medicare, but typically has restrictions on the choice of provider [17]. Optum's proprietary administrative claims research database (ORD) is one of the largest and most complete in the USA [18]. The ORD is fully de-identified and contains medical and pharmacy claims data (including linked enrollment) from 1993 to the present on more than 73 million lives. The ORD represents approximately 8% of the commercially-insured and 18% of MAPD population in the USA. ALL diagnoses were identified using International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM) diagnosis codes and chemotherapy was identified via codes from the Healthcare Common Procedure Coding System (HCPCS) and National Drug Code (NDC). Administrative claims data without personal identifiers were used, therefore institutional review board approval, waiver, or informed consent procedures were not required. Patient privacy was strictly preserved, and compliance with relevant US Health Insurance Portability and Accountability Act (HIPAA) data procedures was observed throughout.
Study sample
The study sample comprised patients with ≥2 non-diagnostic medical or pharmacy claims with codes for ALL (ICD-9-CM 204.0X or ICD-10-CM C91.0X) on separate days, in any position on the claim from 1 July 2015 to 31 August 2020 (identification period) and ≥1 claim for chemotherapy on or after the ALL diagnosis date (date of the first claim for ALL). The date of the first chemotherapy claim was the index date. Patients were also required to be ≥18 years of age on the index date and have continuous health plan enrollment six months before the index date (baseline period) and six months following and including the index date (follow-up period).
Patients were excluded from the study if they were pregnant, had participated in a clinical trial during the study period, had evidence of another cancer (i.e., ≥2 claims, on different days, for the same cancer), or had previous treatment for ALL (i.e., ≥1 claim for an oncology therapy).
Cohorts
A line of therapy (LOT) algorithm based on claims for oncology therapy was run among all patients meeting the inclusion and exclusion criteria. A LOT was defined as receipt of oncology therapy until the earliest of one of the following criteria was met: discontinuation of all agents in the regimen (i.e., treatment gap of ≥60 days), start of a new LOT (additional or change to a new agent, including HSCT), death, end of the study period, or health plan disenrollment. The algorithm was used to identify patients in each of the following settings: frontline, maintenance, and R/R.
Patients were classified as receiving InO or Blina if they had at least one claim for the respective medication at any time during the follow-up period. Rules for classifying patients receiving InO or Blina in the R/R setting, also known as first salvage, were developed in accordance with National Comprehensive Care Network (NCCN) Guidelines and consultation with clinical experts. Specifically, patients were classified as receiving InO or Blina in the R/R setting if they received it after frontline and maintenance therapy (identified using the LOT algorithm). Among patients with no previous LOTs, InO was assumed to be in the R/R setting if patients received it, regardless of other medications, before or during 2019 or as a monotherapy after 2019. Patients with Blina monotherapy as their only LOT during the follow-up were excluded from the study as we could not distinguish the setting in which it was used (i.e., R/R, front-line). Among patients who received both InO and Blina during the R/R period, cohort assignment was based on the first medication received; patients who received both in the same LOT were excluded from the analysis.
Baseline characteristics
Baseline characteristics studied were age (18–44, 45–64, ≥65 years), sex, health insurance plan (commercially insured or Medicare Advantage), US census region (Northeast, Midwest, South, West) and National Cancer Institute Comorbidity Index (NCI CI) score [19]. The NCI CI is similar to the Charlson Comorbidity Index except that it does not include either primary or metastatic cancer [20].
Outcomes
All-cause and ALL-related HCRU and cost outcomes were examined during the first R/R setting and reported as mean per-patient-per month (PPPM) values to allow for varying lengths of follow-up due to disenrollment or death. Costs and utilization were considered ALL-related if the diagnosis code for ALL was in the first position on an inpatient claim or in any position on other types of claims or if there was a claim for a cancer medication. Due to the LOT definition used in the study, HSCT occurred outside the first R/R setting and was not included in utilization and cost measures.
Healthcare resource utilization captured office, outpatient hospital, and emergency room visits, inpatient stays, and intensive care unit stays and was calculated among patients with ≥1 visit for the specific utilization category. Healthcare costs were a combination of health plan and actual patient paid amounts in the follow-up period. Total healthcare costs were analyzed using both pharmacy and medical costs. Medical costs encompassed ambulatory care office visits, outpatient hospital visits, emergency room visits, inpatient stays, intensive care unit (ICU) stays, and other medical costs (including services such as laboratory tests, ambulance, and home healthcare). Infusions like InO and Blina are administered in a medical setting, and therefore, costs for those medications were captured as medical costs. Costs were adjusted to 2020 USD based on the medical care component of the Consumer Price Index (CPI), which was the most recent CPI available at the time of the study [21].
Statistical analysis
Descriptive analysis
A complete case analysis (i.e., a sample comprising patients with complete data for all variables) was conducted. Demographics, baseline characteristics, and outcomes for patients receiving InO or Blina were compared. Sample sizes and percentages were calculated for dichotomous and categorical measures. Mean, standard deviation (SD), and median were presented by cohort for continuous measures. T-tests were conducted for statistical testing of differences between the InO and Blina cohorts.
Inverse probability of treatment weighting
Inverse probability treatment weighting (IPTW) analyses were performed to standardize comparisons of InO versus Blina in the descriptive analysis. Specifically, the weights were created to balance the distribution of potential confounders of receipt of InO and Blina and were used as an alternative to propensity score matching to maximize retention of the full sample [22]. The IPTWs were calculated using data from the baseline period with the following characteristics: age (18–64 and ≥65 years); region (South vs Midwest, Northeast and West); comorbidities, based on NCI CI (0 and ≥1 comorbidities); and prior treatment (0, 1 and ≥2 LOTs).
Upon calculating the IPTWs, the distributions of unweighted and weighted characteristics were compared to assess the impact of weighting. The performance of the IPTWs was assessed by comparing the standardized differences in unweighted and weighted baseline variables for the InO and Blina groups. All descriptive results, unless otherwise noted, were weighted using IPTWs.
Multivariable-adjusted regression analysis
The type of medication that a patient receives can be determined by characteristics like age, number of comorbidities, and previous LOTs. Relatedly, outcomes may be determined by these characteristics. Therefore, multivariable modeling was conducted to identify the independent effects of medication after controlling for the influence of potential confounding characteristics. Per patient per month all-cause total costs and PPPM ALL-related total costs during the first R/R setting were examined with multivariable models using a generalized linear model with a gamma distribution and log link. This model was selected based on the distribution of costs. Regression diagnostics were performed for each model to assess the goodness of fit and violations of model assumptions (e.g., multicollinearity). The multivariable model was adjusted for the four baseline characteristics in the IPTWs (age, region, NCI CI, and number of prior LOTs), and sex. Cost ratios and corresponding 95% CIs and p-values were reported. Predicted costs were calculated by setting the index chemotherapy as InO for all patients and then calculating the average predicted cost. This procedure was then repeated for Blina, providing a comparison of predicted costs that was adjusted for covariate imbalance.
All statistical analyses were performed with SAS v 9.4 (SAS Institute, Cary, NC). A significance level of α = 0.05 was set a priori.
Results
Study sample
A total of 175 patients received oncology therapy in the first R/R setting (Figure 1). Four of those patients were subsequently excluded because they either received both InO and Blina during the same LOT (n = 1) or their only LOT during the follow-up period was Blina monotherapy (n = 3), thus it was not possible to differentiate whether Blina was used in the R/R or front-line setting. After exclusion, 171 patients were included in the study analyses (InO: n = 29; Blina: n = 23; other oncology therapies: n = 119). All results were weighted with IPTWs unless otherwise noted.
Figure 1. . Patient sample selection.
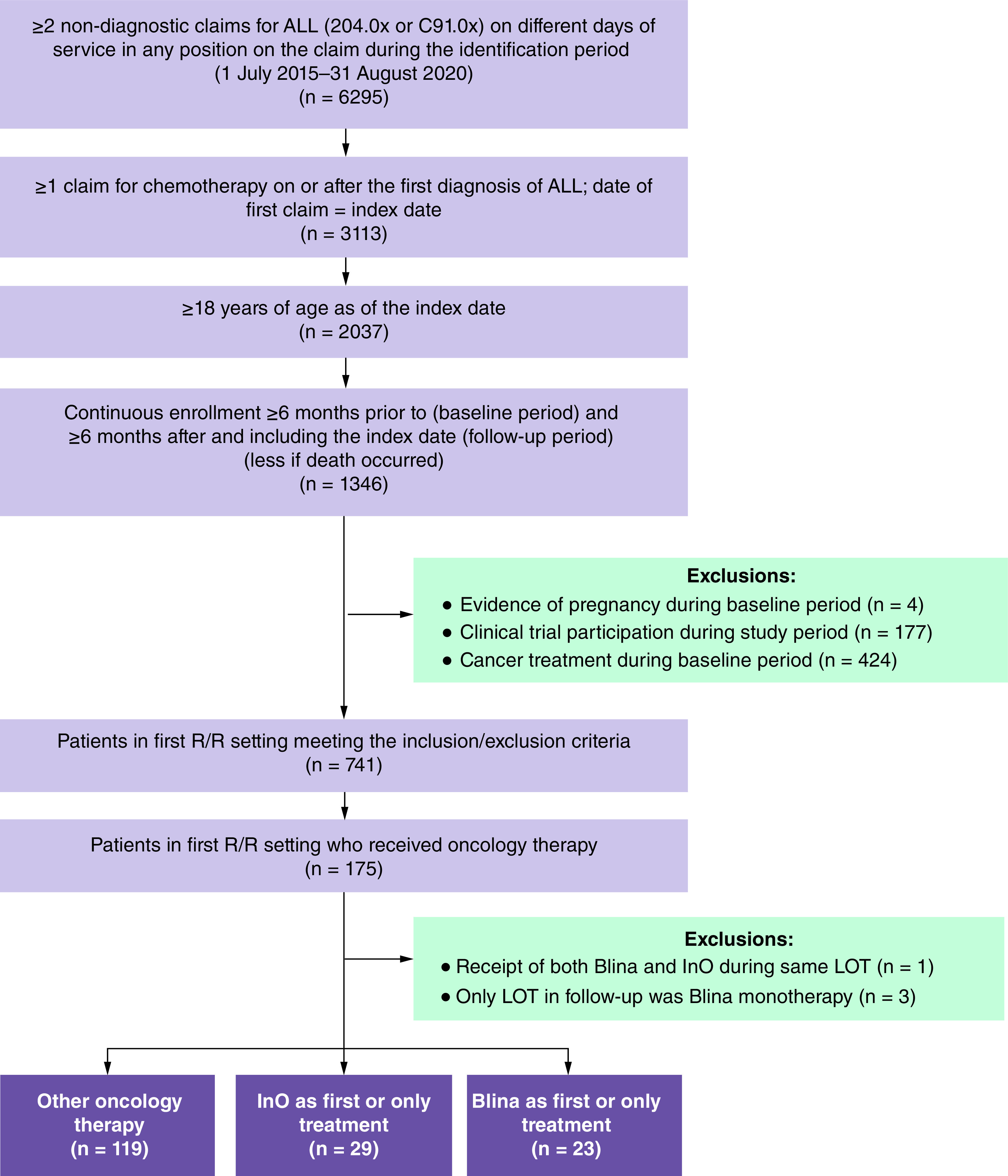
ALL: Acute lymphoblastic leukemia; Blina: Blinatumomab; InO: Inotuzumab ozogamicin; LOT: Line of therapy; R/R: Relapse/refractory.
Baseline demographics
Table 1 shows that patients who received Blina during the first R/R setting were younger than those receiving InO (mean [SD] = 49 [17] and 54 [19] years). Almost half of the patients who received Blina (49.3%) were aged 18–44 years compared with a third (32.7%) of InO patients. The percentage of patients who were female was similar between Blina and InO (56.2% vs 49.9%, respectively). Consistent with their younger age, most Blina patients (89.4%) were commercially insured, whereas just over half (54.1%) of InO patients were Medicare beneficiaries. Approximately half (51.6%) of the patients in the Blina cohort were from the South, whereas the highest percentage of InO patients were from the Midwest (36.0%).
Table 1. . Inverse probability treatment weighted baseline patient demographics and clinical characteristics for patients who received inotuzumab ozogamicin and blinatumomab in the first relapsed/refractory setting.
| Characteristic |
InO and Blina combined (n = 52) |
InO (n = 29) |
Blina (n = 23) |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean (SD) |
51.6 (18.0) |
53.6 (18.9) |
48.8 (16.8) |
| Median |
56.0 |
61.0 |
50.0 |
| Age group (years), n (%) | |||
| 18–44 |
19 (39.6) |
9 (32.7) |
10 (49.3) |
| 45–64 |
16 (33.3) |
9 (32.4) |
7 (34.5) |
| ≥65 |
13 (27.1) |
10 (34.9) |
3 (16.3) |
| Female sex, n (%) |
26 (52.5) |
14 (49.9) |
11 (56.2) |
| Insurance coverage, n (%) | |||
| Commercial |
31 (64.0) |
13 (45.9) |
18 (89.4) |
| Medicare Advantage |
18 (36.0) |
15 (54.1) |
2 (10.7) |
| Geographic region, n (%) | |||
| Northeast |
8 (15.6) |
4 (15.7) |
3 (15.6) |
| Midwest |
14 (29.1) |
10 (36.0) |
4 (19.4) |
| South |
18 (37.6) |
8 (27.6) |
11 (51.6) |
| West | 9 (17.7) | 6 (20.7) | 3 (13.5) |
| Clinical characteristics | |||
|---|---|---|---|
| NCI CI, mean (SD) |
1.6 (1.8) |
1.9 (2.0) |
1.3 (1.6) |
| NCI CI, n (%) | |||
| 0 |
19 (38.7) |
11 (39.6) |
8 (37.4) |
| 1–2 |
16 (33.4) |
8 (26.6) |
9 (42.9) |
| 3–4 |
9 (17.4) |
6 (20.3) |
3 (13.4) |
| ≥5 |
5 (10.5) |
4 (13.6) |
1 (6.3) |
| Index year, n (%) | |||
| 2015 |
4 (7.9) |
1 (2.8) |
3 (15.1) |
| 2016 |
5 (11.2) |
3 (9.3) |
3 (13.8) |
| 2017 |
4 (8.1) |
3 (11.1) |
1 (3.8) |
| 2018 |
18 (37.8) |
13 (44.4) |
6 (28.5) |
| 2019 |
12 (23.7) |
6 (20.2) |
6 (28.7) |
| 2020 |
6 (11.3) |
3 (12.2) |
2 (10.1) |
| 2021 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Blina: Blinatumomab; InO: Inotuzumab ozogamicin; NCI CI: National Cancer Institute Comorbidity Index; SD: Standard deviation.
Unweighted baseline demographic characteristics are shown in Supplementary Table 1 for all patients with ALL in the first R/R setting (n = 171) and for patients who received InO (n = 29) and Blina (n = 23). An IPTW comparison of comorbid conditions between the InO and Blina cohorts is shown in Supplementary Table 2.
All-cause PPPM healthcare resource utilization
The mean (median) follow-up time for patients receiving InO and Blina in the first R/R setting was 528 (445) days and 588 (456) days, respectively. The mean (SD) number of office visits were 3.5 (3.1) and 4.2 (2.8) (p = 0.430) and the mean number of hospital outpatient visits were 11.2 (5.7) and 10.5 (7.4) (p = 0.719), for patients receiving InO and Blina, respectively (Table 2). The mean (SD) emergency room visits were 0.8 (0.7) for InO patients and 0.6 (0.5) for Blina patients (p = 0.490). Patients receiving InO had numerically more inpatient stays relative to Blina patients (1.0 vs 0.8 stays, p = 0.175); however, among patients with an inpatient visit, the mean length of inpatient stay was almost double for Blina patients versus InO patients (14.1 vs 7.2 days, p = 0.346). Among those with an inpatient stay, patients receiving InO had more ICU visits than those receiving Blina (0.6 and 0.3 visits, p = 0.160).
Table 2. . Inverse probability treatment weighted all-cause and acute lymphoblastic leukemia-related healthcare resource utilization during the first relapsed/refractory in follow-up period.
| Healthcare resource | All-cause HCRU, PPPM | ALL-related HCRU, PPPM | ||
|---|---|---|---|---|
| InO (n = 29) | Blina (n = 23) | InO (n = 29) | Blina (n = 23) | |
| Office visits | ||||
| With visit, n | 26 | 20 | 20 | 20 |
| Mean (SD) | 3.5 (3.1) | 4.2 (2.8) | 3.7 (2.9) | 3.2 (2.5) |
| Median | 2.0 | 3.4 | 2.7 | 2.1 |
| Hospital outpatient visits | ||||
| With visit, n | 29 | 23 | 28 | 22 |
| Mean (SD) | 11.2 (5.7) | 10.5 (7.4) | 8.5 (5.1) | 9.0 (5.9) |
| Median | 10.4 | 9.2 | 6.7 | 8.6 |
| Emergency room visits | ||||
| With visit, n | 14 | 7 | <5 | <5 |
| Mean (SD) | 0.8 (0.7) | 0.6 (0.5) | –† | –† |
| Median | 0.5 | 0.3 | –† | –† |
| Inpatient stays | ||||
| With visit, n | 19 | 16 | 16 | 16 |
| Mean (SD) | 1.0 (0.6) | 0.8 (0.3) | 0.8 (0.4) | 0.8 (0.3) |
| Median | 0.8 | 0.7 | 0.8 | 0.7 |
| Length of stay (days), mean (SD) | 7.2 (8.5) | 14.1 (25.9) | 7.6 (8.9) | 14.1 (25.9) |
| Intensive care unit stays | ||||
| With stay, n | 16 | 16 | 16 | 16 |
| Mean (SD) | 0.6 (0.5) | 0.3 (0.4) | 0.2 (0.4) | 0.1 (0.2) |
| Median | 0.7 | 0.0 | 0.0 | 0.0 |
Values suppressed due to small sample size (<5 patients).
Blina: Blinatumomab; HCRU: Healthcare resource utilization; InO: Inotuzumab ozogamicin; PPPM: Per-patient-per-month; n: Number of patients; SD: Standard deviation.
ALL-related PPPM healthcare resource utilization
Most all-cause HCRU was ALL-related. (Table 2) Similar to all-cause HCRU, in both cohorts, hospital outpatient visits (InO = 8.5; Blina = 9.0, p = 0.768) were the most frequent healthcare utilization. The mean number of office visits was numerically greater for InO versus Blina (3.7 vs 3.2, p = 0.529) patients. The two groups had a comparable mean number of inpatient stays (0.8 for both groups, p = 0.555) but patients receiving Blina spent a numerically higher mean number of days as inpatients (14.1 vs 7.6 days, p = 0.381). ALL-related ICU stays were numerically greater for patients who received InO than those who received Blina (0.2 vs 0.1, p = 0.257).
All-cause PPPM healthcare costs
The mean (SD) total PPPM all-cause costs were $122,461 ($104,348) for InO and $176,306 ($201,727) for Blina, as shown in Table 3. Mean (SD) all-cause costs were numerically higher for InO for office visits ($9344 [$21,313] vs $3132 [$11,312]) and hospital outpatient visits ($90,718 [$105,269] vs $69,459 [$83,529]), but lower for inpatient stays ($34,375 [$51,849] vs $110,364 [$215,119]) compared with Blina; however, these differences were not statistically significant. The only healthcare resource with a significant difference between the InO versus Blina cohorts was other medical costs ($894 [$3764] vs $19,589 [$26,580], p < 0.001).
Table 3. . Inverse probability treatment weighted all-cause and acute lymphoblastic leukemia-related costs during first relapse/refractory in the follow-up period.
| Cost category† | All-cause costs, PPPM, mean (SD) | ALL-related costs, PPPM, mean (SD) | ||
|---|---|---|---|---|
| InO (n = 29) | Blina (n = 23) | InO (n = 29) | Blina (n = 23) | |
| Total healthcare costs | 122,461 (104,348) | 176,306 (201,727) | 116,774 (105,327) | 171,093 (198,208) |
| Medical costs | 121,344 (104,549) | 175,788 (201,625) | 116,641 (105,388) | 171,093 (198,208) |
| Office visits | 9344 (21,313) | 3132 (11,312) | 11,646 (23,403) | 2898 (11,353) |
| Outpatient visits | 90,718 (105,269) | 69,459 (83,529) | 90,308 (106,078) | 71,071 (77,453) |
| Emergency room visits | 360 (439) | 264 (298) | –‡ | –‡ |
| Inpatient stays | 34,375 (51,849) | 110,364 (215,119) | 37,636 (54,316) | 110,364 (215,119) |
| ICU stays | 27,649 (53,552) | 22,582 (32,996) | 25,176 (47,625) | 5479 (15,652) |
| Other medical costs | 894 (3,764) | 19,589 (26,580) | 240 (836) | 19,483 (26,498) |
| Pharmacy costs | 1117 (1471) | 519 (774) | 133 (714) | 0 (0) |
Bolded numbers are statistically significant (p < 0.001).
Total costs calculated among all patients; costs for specific utilization categories calculated among patients with ≥1 claim for the specific utilization type. Costs were adjusted to 2020 US dollars using the Consumer Price Index.
Values suppressed due to small sample size (<5 patients).
Blina: Blinatumomab; InO: Inotuzumab ozogamicin; ICU: Intensive care unit; PPPM: Per-patient-per-month; SD: Standard deviation.
ALL-related PPPM healthcare costs
Total mean (SD) PPPM ALL-related costs were $116,774 ($105,327) for InO patients and $171,093 ($198,208) for Blina patients, as shown in Table 3. Compared with patients who received Blina, patients who received InO had numerically higher mean (SD) ALL-related costs for office visits ($11,646 [$23,403] vs $2,898 [$11,353]) and hospital outpatient visits ($90,308 [$106,078] vs $71,071 [$77,453]), but numerically lower costs for inpatient stays ($37,636 [$54,316] vs $110,364 [$215,119]). None of these differences were statistically significant.
Multivariable-adjusted regression
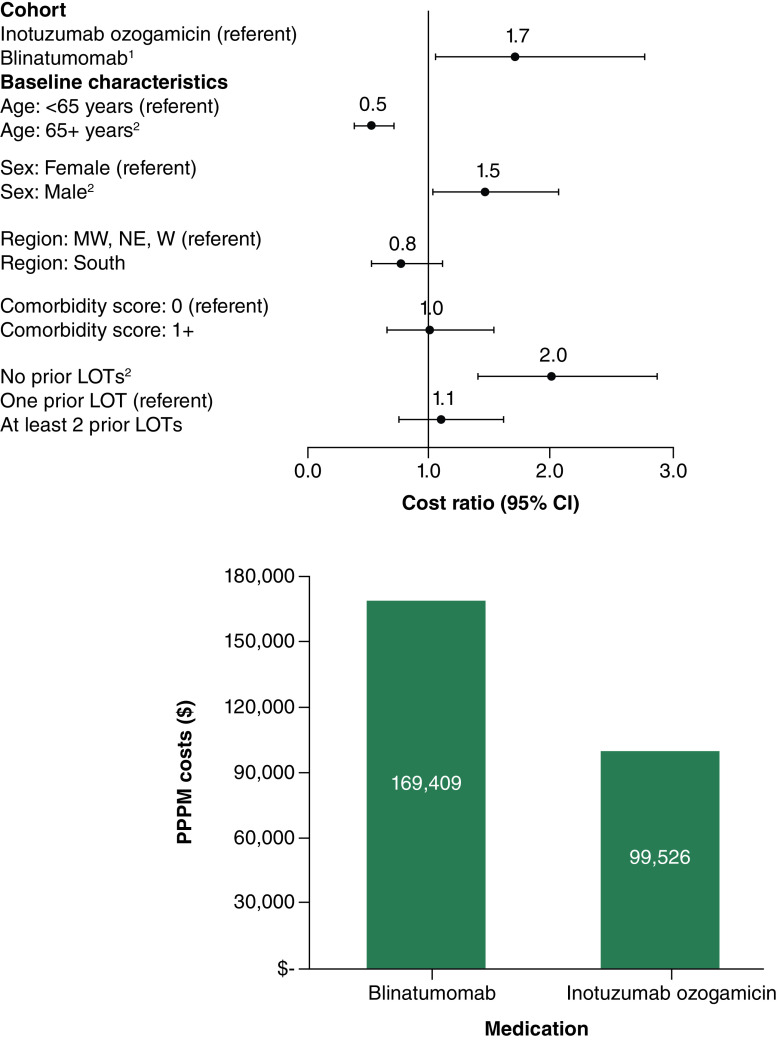
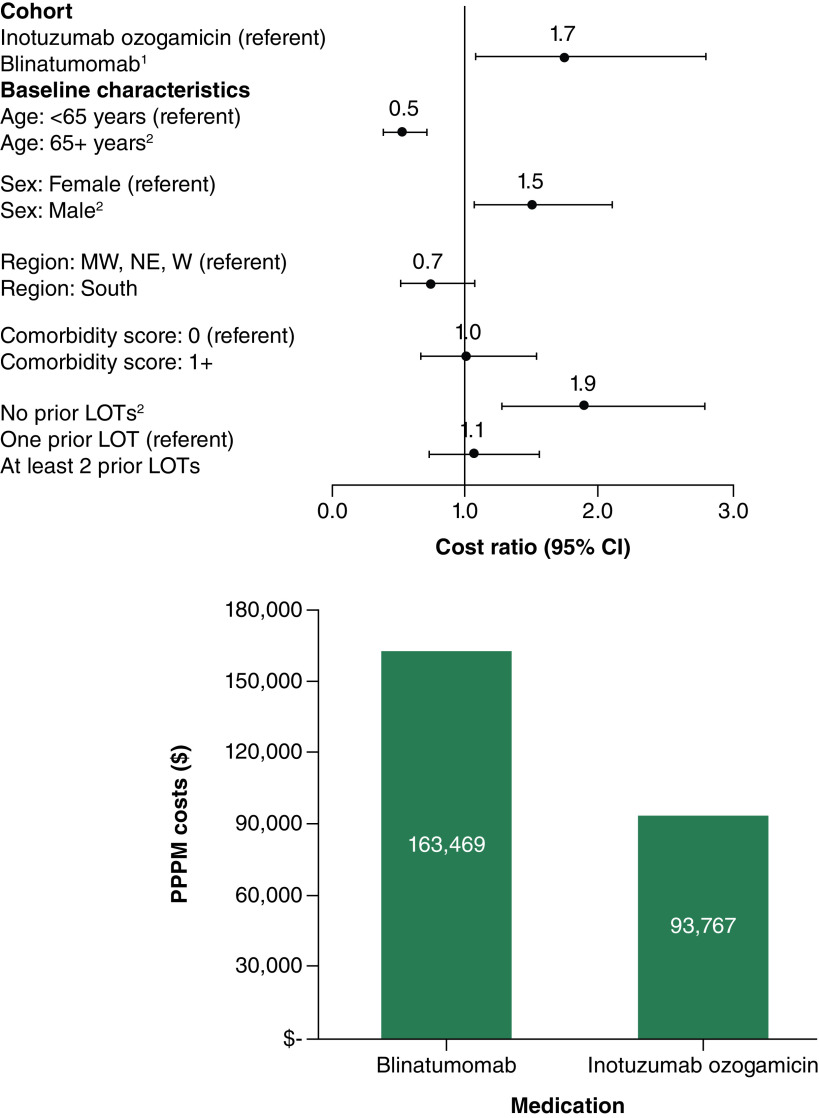
In the first R/R setting, multivariable-adjusted all-cause PPPM total costs were significantly higher, on average, among Blina patients compared with InO patients (cost ratio = 1.7, 95% confidence interval (CI) = 1.0–2.8; p = 0.031; predicted costs = $169,409 for Blina; $99,526 for InO) (Figure 2). Similarly, ALL-related PPPM total costs were significantly greater in the Blina cohort compared with the InO cohort (cost ratio = 1.7; 95% CI = 1.1–2.8; p = 0.021; predicted costs = $163,469 for Blina; $93,767 for InO) (Figure 3).
Figure 2. . Multivariable-adjusted cost ratios and predicted per-patient-per-month total all-cause healthcare costs in the first relapse/refractory setting for blinatumomab (n = 29) and inotuzumab ozogamicin (n = 23).
1Differences in predicted costs for InO and Blina were statistically significant (p = 0.031).
2Cost ratio was statistically significant at alpha = 0.01.
Pearson chi-square = 19.395, degrees of freedom = 44; Specification link test p-value = 0.199; Park test estimate = 2.869, gamma distribution p-value = 0.154; Robust standard errors were calculated.
LOT: Line of therapy; MW: Midwest; NE: Northeast; PPPM: Per-patient-per-month; W: West.
Figure 3. . Multivariable-adjusted cost ratios and predicted per-patient-per-month total acute lymphoblastic leukemia-related healthcare costs in the first relapse/refractory setting for blinatumomab (n = 29) and inotuzumab ozogamicin (n = 23).
1Differences in predicted costs for InO and Blina were statistically significant (p = 0.021).
2Cost ratio was statistically significant at alpha = 0.01.
Pearson chi-square = 18.628, degrees of freedom = 44; Specification link test p-value = 0.159; Park test estimate = 2.824, gamma distribution p-value = 0.163; Robust standard errors were calculated.
LOT: Line of therapy; MW: Midwest; NE: Northeast; PPPM: Per-patient-per-month; W: West.
Discussion
This observational study compared HCRU and costs for two relatively small cohorts of patients with ALL who received Blina or InO during the first R/R setting. Descriptive analyses, which were standardized with IPTWs, found that the largest difference in HCRU between InO and Blina patients was for ALL-related inpatient stays. Hospitalized patients treated with Blina had ALL-related inpatient stays almost twice as long as hospitalized patients treated with InO. Inpatient stays are typically associated with higher costs [23,24], which was seen with numerically higher PPPM costs for patients treated with Blina compared with those treated with InO. The lower inpatient costs offset the higher costs of ambulatory care among patients treated with InO. None of the differences in the descriptive analyses above were statistically significant; however, in multivariable-adjusted regression analyses that controlled for differences in key characteristics, both total mean all-cause and ALL-related PPPM costs were significantly higher for patients treated with Blina relative to those treated with InO. Additionally, ALL-related multivariable-adjusted total predicted costs accounted for most of the all-cause total predicted costs for both InO (94%) and Blina (96%) during the first R/R setting. For the treatment of R/R ALL with Blina, hospitalization is recommended during infusion for the first nine days of the first cycle and the first two days of the second cycle [25]. Conversely, InO has no recommendations for inpatient hospitalization [26] and is typically administered in an outpatient setting. This may explain why patients with Blina had numerically higher costs for inpatient stays compared with InO. An additional driver of higher inpatient costs and longer length of inpatient stays for patients on Blina may have been cytokine release syndrome, a potentially fatal overactivation of immune effector cells, particularly during the earlier years following drug approval [27,28].
These results are directionally consistent with findings from a recent abstract among Medicare beneficiaries with R/R ALL that estimated all-cause resource utilization and costs [15]. Patients who received Blina had higher PPPM rates of inpatient stays and greater mean total costs ($65,437 vs $55,995) compared with patients receiving InO. The larger absolute PPPM costs reported in our study for both InO and Blina may be due to the medical complexity of patients in our study sample, however the conclusion that Blina is associated with higher costs than InO remains the same across the studies. A cost–effectiveness model published in 2019 by Delea et al. simulated the treatment of R/R ALL patients with either Blina or InO, finding that treatment with Blina was associated with $7023–$36,244 higher costs than InO [29]. The higher modeled costs for Blina are consistent with the real-world cost data we report in this study. One notable difference is that the Delea et al. model includes the cost of HSCT and our study does not. Delea et al. attributed a higher cost per patient to InO than Blina due to the higher number of patients reaching subsequent HSCT and still found Blina to be associated with a greater overall expense. If transplantation costs were excluded from their model, as they were in our real-world study, the model would show an even greater variance in costs between InO and Blina cohorts.
In the current study, the Blina cohort had higher NCI CI scores, which may be implicated in their longer, costlier inpatient hospitalizations. Comorbidities are generally associated with greater use of concomitant medications, which could likely increase treatment costs [30–32]. We used IPTWs and multivariable analysis to account for differences between the InO and Blina patient groups including comorbidities; however, these methods do not account for unobserved characteristics that may be associated with utilization and costs, such as provider preference and practice behaviors, which could lead to residual confounding [22,33]. All the patients in this study had health insurance coverage, thus these results may not be generalizable to other populations, including the uninsured, patients with traditional Medicare or Medicaid insurance coverage, and people outside of the USA.
Currently, at least three previously conducted studies have used different methods and proxies to indirectly compare the performance of InO and Blina [12,13,29], including comparisons of cost–effectiveness, CR rate and overall survival, and rates of HSCT [12,13,29]. While these studies have provided valuable data to the literature base, none have directly compared HCRU and costs for patients treated with InO and Blina. Our study results do not include HCRU and costs associated with HSCTs because this procedure was classified as another, subsequent, line of therapy. Because the timing of HSCTs vary across patients, including HSCT-related HCRU and costs for the subset of patients who have the procedure immediately after the first R/R may introduce bias because the costs for HSCT and costs for patients who underwent the procedure before the first R/R or after a subsequent R/R would not be captured.
The limited available data with which to directly compare our results illustrates the gap in real-world HRCU and costs for patients receiving InO and Blina in the first R/R setting. However, the conclusion that InO is associated with lower costs than Blina is consistent between the few studies, both real-world and modeled, that we have identified. The results of our study are particularly relevant because, at this time of rapid growth in health expenditures and shrinking healthcare budgets, healthcare stakeholders are assessing treatment performance more closely. When evaluating agents with comparable efficacy profiles, there is a greater emphasis on cost effectiveness and on obtaining value for money. Data on the cost effectiveness of different therapies, especially novel cancer agents, are in demand by decision makers to guide their access and reimbursement decisions [34].
Strengths & limitations
This study was based on administrative claims data, which are effective and efficient for examining HCRU and costs in the real-world setting. The analyses conducted in this study were comprehensive, using both IPTW in descriptive analysis and multivariable regression to control for confounding factors, allowing for better direct comparisons. Even though the sample size in this study was small, we were able to show a significant difference between patients who received InO versus Blina. Additionally, the costs used in this study were not estimated by modelling, nor by assigning hypothetical dollar values to resource use, but rather through direct costs paid by the patient and health plan identified in the claims data. Lastly, the real-world nature of this study for a varied population provides insight into how medications work outside of randomized controlled trials and portrays a better reflection of clinical practice. Despite these strengths, claims data are subject to multiple limitations including coding errors and other inconsistencies linked to the management of claims data. Second, LOTs were identified from claims data using an algorithm and manually verified by clinical experts; nevertheless, patient R/R status may have been misclassified. Third, HCRU and costs were interpreted as ALL-related if there was an ICD code for ALL on the claim; however, some of the HCRU and costs calculated as ALL-related may have been for the treatment of comorbidities and thus misclassified. Fourth, although the study data were from one of the largest US health insurers, the sample size was small, and few statistically significant differences were observed in the descriptive analyses. The small sample size may limit the generalizability of the study findings. Fifth, we did not examine the impact of survival on HCRU and medical costs. At least two studies found similar survival among patients receiving InO and Blina [12,14] and therefore it is unlikely that HCRU and cost differences in our study are attributable to survival. Last, this study did not separate drug acquisition costs from total medical costs. This is because InO and Blina are infused medications administered in a clinical setting. The utilization and costs for these medications are billed on medical claims that also include costs for all services associated with medication administration. Therefore, it is not possible to reliably identify drug acquisition costs on medical claims.
Conclusion
We believe that this is the first published manuscript using real-world data from Medicare Advantage and commercially insured enrollees that compares HCRU and costs for InO and Blina in the first R/R setting. The results, calculated from patients in the first R/R setting, are not a lifetime economic evaluation. The results indicate that while both all-cause and ALL-related ambulatory care costs were higher among patients receiving InO, substantially higher costs for inpatient stays among patients receiving Blina offset the cost of higher outpatient care among patients who received InO, resulting in significantly higher costs for patients treated with Blina. The results appear consistent with other studies we reviewed and provide payers with information to quantify the budgetary impact of these drugs. Based on the available utilization and economic data in the studied dataset, patients with InO in the first R/R setting had lower observed costs than those receiving Blina. Additional studies would help in corroborating these findings.
Summary points.
The use of targeted immunotherapies inotuzumab ozogamicin (InO) and blinatumomab (Blina), has been shown to improve outcomes and induce longer and deeper remissions, relative to standard of care chemotherapy, in patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL).
Payers are increasingly seeking cost–effectiveness data to support their decisions on access and reimbursement for novel treatments such as targeted immunotherapy.
This retrospective database study comprised commercial and Medicare Advantage patients within the Optum Research database receiving inotuzumab ozogamicin (InO) and blinatumomab (Blina) for R/R ALL.
A total of 29 patients who received InO and 23 patients who received Blina for ALL in the first R/R setting met the study inclusion criteria and were examined in cost analyses.
Inverse probability of treatment weights (IPTW) were applied in the descriptive analysis to adjust for differences in the socio-demographic and comorbidity characteristics of the InO and Blina patients.
Patients receiving Blina and InO had the same ALL-related mean monthly inpatient stays (per patient per month [PPPM] stays = 0.8); however, the mean length of ALL-related inpatient stay was almost double for patients on Blina versus InO (14.1 vs 7.6 days; p = 0.346).
In the first R/R setting, multivariable-adjusted all-cause PPPM total costs were significantly higher among Blina patients compared with InO patients (cost ratio = 1.7; 95% confidence interval (CI) = 1.0–2.8; p = 0.031; predicted costs = $169,409 for Blina, $99,526 for InO).
ALL-related PPPM total costs were significantly greater in the Blina cohort compared with the InO cohort (cost ratio = 1.7; 95% CI = 1.1–2.8; p = 0.021; predicted costs = $163,469 for Blina; $93,767 for InO).
In the first R/R setting, patients receiving InO had significantly lower all-cause and ALL-related costs compared with patients who used Blina, in part driven by hospitalization patterns. The small sample size of this study may limit its generalizability.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the contribution of Bernard Tulsi and Deja Scott-Shemon for medical writing and editorial support and Stephanie Gallagher for Project Manager support.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2023-0142
Author contributions
A Russell-Smith, L Murphy, A Nguyen and R Shah were responsible for study conception and design. All authors were responsible for data analysis and drafting and revision of the manuscript. C Blauer-Peterson, F Cao and S Li were responsible for acquisition of the retrospective claims data.
Financial disclosure
This study was sponsored by Pfizer Pharmaceuticals, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
A Russell-Smith, S Dorman and R Shah are employees of, and own stock in, Pfizer. L Murphy and T Bancroft are employees of Optum and own stock in UnitedHealth Group. A Nguyen, C Blauer-Peterson, F Cao, S Li and N Webb are employees of Optum. M Terpenning has received funds for consulting with Optum and Curio Sciences. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing and editorial support were provided by B Tulsi and D Scott-Shemon of Optum, and were funded by Pfizer Pharmaceuticals, Inc.
Ethical conduct of research
Administrative claims data without personal identifiers were used, therefore institutional review board approval, waiver, or informed consent procedures were not required. Patient privacy was strictly preserved, and compliance with relevant US Health Insurance Portability and Accountability Act (HIPAA) data procedures was observed throughout.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 7(6), e577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 119(1), 34–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 72(1), 7–33 (2022). [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Acute Lymphoblastic Leukemia. Version 2.2022. (2023). www.nccn.org/professionals/physician_gls/default.aspx#all
- 5.Gorin NC. Autologous stem cell transplantation in acute lymphocytic leukemia. Stem Cells 20(1), 3–10 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Gokbuget N, Dombret H, Ribera JM et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica 101(12), 1524–1533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarjian H, Stein A, Gokbuget N et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376(9), 836–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian HM, DeAngelo DJ, Stelljes M et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 125(14), 2474–2487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian HM, DeAngelo DJ, Stelljes M et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl. J. Med. 375(8), 740–753 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BESPONSA (inotuzumab ozogamicin) for injection, for intra venous use [full prescribing information]. Initial US Approval: 2017. Pfizer, NY, USA: (2023). http://labeling.pfzer.com/ShowLabeling.aspx?id=9503&format=PDF [Google Scholar]
- 11.Amgen. BLINCYTO® (blinatumomab) for injection, for intra venous use [full prescribing information]. Initial US Approval: 2014. (2023). www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-om/Blincyto/blincyto_pi_hcp_english.pdf
- 12.Proskorovsky I, Su Y, Fahrbach K et al. Indirect treatment comparison of inotuzumab ozogamicin versus blinatumomab for relapsed or refractory acute lymphoblastic leukemia. Adv. Ther. 36(8), 2147–2160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This indirect treatment comparison evaluated clinical endpoints among patients with relapsed/refractory acute lymphoblastic leukemia receiving each of inotuzumab ozogamicin (InO) and blinatumomab (Blina) and found that patients receiving InO had a statistically significant advantage in remission and HSCT rates compared with Blina.
- 13.Song J, Ma Q, Gao W et al. Matching-adjusted indirect comparison of blinatumomab vs. inotuzumab ozogamicin for adults with relapsed/refractory acute lymphoblastic leukemia. Adv. Ther. 36(4), 950–961 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badar T, Szabo A, Dinner S et al. Sequencing of novel agents in relapsed/refractory B-cell acute lymphoblastic leukemia: blinatumomab and inotuzumab ozogamicin may have comparable efficacy as first or second novel agent therapy in relapsed/refractory acute lymphoblastic leukemia. Cancer 127(7), 1039–1048 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Russell-Smith A, Shah R, Silverstein AR et al. Characteristics, healthcare utilization and costs associated with inotuzumab ozogamicin, blinatumomab, or other agents for the treatment of relapsed or refractory acute lymphoblastic leukemia. Presented at: European Hematology Association Congress. Vienna, Austria, 9–17 June 2022. [Google Scholar]
- 16.Delea TE, Amdahl J, Boyko D et al. cost–effectiveness of blinatumomab versus salvage chemotherapy in relapsed or refractory Philadelphia-chromosome-negative B-precursor acute lymphoblastic leukemia from a US payer perspective. J. Med. Econ. 20(9), 911–922 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Compare Original Medicare & Medicare Advantage. Centers for Medicare and Medicaid Services. (2023). www.medicare.gov/basics/get-started-with-medicare/get-more-coverage/your-coverage-options/compare-original-medicare-medicare-advantage
- 18.Optum. Addressing the need for real-world observational research solutions. (2023). www.optum.com/content/dam/optum3/optum/en/resources/white-papers/heor-observational-research-sol-wp.pdf
- 19.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann. Epidemiol. 17(8), 584–590 (2007). [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute (NCI). NCI Comorbidity Index Overview. (2023). https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html
- 21.US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Medical Care. Series ID: CUUR0000SAM. US Department of Labor, Bureau of Labor Statistics, WA, USA: (2023). http://data.bls.gov/cgi-bin/surveymost?cu [Google Scholar]
- 22.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 34(28), 3661–3679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomicki S, Dieguez G, DeStephano D, Chang M, Cockrum P. Costs by site of service for commercially-insured patients with metastatic pancreatic cancer receiving guideline-recommended chemotherapy: comparing community oncology and hospital outpatient settings. Clinicoecon. Outcomes Res. 14, 653 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winn AN, Keating NL, Trogdon JG, Basch EM, Dusetzina SB. Spending by commercial insurers on chemotherapy based on site of care, 2004–2014. JAMA Oncol. 4(4), 580–581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescribing information. (2023). www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/blincyto/blincyto_pi_hcp_english.pdf
- 26.Prescribing information. (2023). https://labeling.pfizer.com/ShowLabeling.aspx?id=9503&format=PDF
- 27.Teachey DT, Rheingold SR, Maude SL et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121(26), 5154–5157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimabukuro-Vornhagen A, Godel P, Subklewe M et al. Cytokine release syndrome. J. Immunother. Cancer 6(1), 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delea TE, Zhang X, Amdahl J et al. Cost effectiveness of blinatumomab versus inotuzumab ozogamicin in adult patients with relapsed or refractory b-cell precursor acute lymphoblastic leukemia in the United States. Pharmacoeconomics 37(9), 1177–1193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlson M, Charlson RE, Briggs W, Hollenberg J. Can disease management target patients most likely to generate high costs? The impact of comorbidity. J. Gen. Intern. Med. 22(4), 464–469 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortaredona S, Ventelou B. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med. 15(1), 216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams GR, Mackenzie A, Magnuson A et al. Comorbidity in older adults with cancer. J. Geriatr. Oncol. 7(4), 249–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz MH. Multivariable analysis: a primer for readers of medical research. Ann. Intern. Med. 138(8), 644–650 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Cherla A, Renwick M, Jha A, Mossialos E. cost–effectiveness of cancer drugs: comparative analysis of the United States and England. EClinicalMedicine 29–30, 100625 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.