Abstract
Aim:
There are limited data on the clinical and economic burden of exacerbations in patients with myasthenia gravis (MG). We assessed patient clinical characteristics, treatments and healthcare resource utilization (HCRU) associated with MG exacerbation.
Patients & methods:
This was a retrospective analysis of adult patients with MG identified by commercial, Medicare or Medicaid insurance claims from the IBM® MarketScan® database. Eligible patients had two or more MG diagnosis codes, without evidence of exacerbation or crisis in the baseline period (12 months prior to index [first eligible MG diagnosis]). Clinical characteristics were evaluated at baseline and 12 weeks before each exacerbation. Number of exacerbations, MG treatments and HCRU costs associated with exacerbation were described during a 2-year follow-up period.
Results:
Among 9352 prevalent MG patients, 34.4% (n = 3218) experienced ≥1 exacerbation after index: commercial, 53.0% (n = 1706); Medicare, 39.4% (n = 1269); and Medicaid, 7.6% (n = 243). During follow-up, the mean (standard deviation) number of exacerbations per commercial and Medicare patient was 3.7 (7.0) and 2.7 (4.1), respectively. At least two exacerbations were experienced by approximately half of commercial and Medicare patients with ≥1 exacerbation. Mean total MG-related healthcare costs per exacerbation ranged from $26,078 to $51,120, and from $19,903 to $49,967 for commercial and Medicare patients, respectively. AChEI use decreased in patients with multiple exacerbations, while intravenous immunoglobulin use increased with multiple exacerbations.
Conclusion:
Despite utilization of current treatments for MG, MG exacerbations are associated with a high clinical and economic burden in both commercial and Medicare patients. Additional treatment options and improved disease management may help to reduce exacerbations and disease burden.
Keywords: commercial, database, disease management, healthcare costs, healthcare resource utilization, IBM® MarketScan®, Medicaid, Medicare, real-world data, treatment, United States claims database
Myasthenia gravis (MG) is a rare, chronic, heterogeneous, autoimmune disease, in which patients present with fluctuating weakness and fatigue of certain muscular groups [1–3]. Approximately two-thirds of people who receive an MG diagnosis by 40 years of age are female, whereas people with late-onset MG are more likely to be male [4]. The estimated prevalence of MG in the US is 14 to 20 cases per 100,000 people, although it is likely that MG is underdiagnosed owing to the fluctuating symptoms that people with MG experience [5].
Treatment guidance by international experts has been primarily based on real-world evidence owing to limited clinical trials with small patient populations [6]. For most patients, acetylcholinesterase inhibitors (AChEIs) are used as initial symptomatic treatment, followed by steroidal and non-steroidal immunosuppressive therapies (NSISTs) when sufficient treatment response is not achieved with AChEIs [7]. While corticosteroids (CSs) are effective for some patients, they are associated with adverse side effects such as weight gain, diabetes and osteoporosis [8,9]. NSISTs can also be effective, but are associated with a slow onset of action and several adverse effects [9]. Targeted immunotherapies, such as eculizumab, efgartigimod, or ravulizumab, and in some cases, rituximab, can be offered to those who do not respond to AChEIs or NSISTs [9–11]. However, owing to the fluctuating nature of MG, and limitations of current treatments that leave some patients with inadequately controlled symptoms, patients may experience exacerbations, during which MG symptoms increase in frequency or become more severe and require urgent treatment [12]. Additionally, patients can experience MG crisis, where respiratory support is required for life-threatening, disease-related muscle weakness [12]. Exacerbations may require MG-related hospitalization, or rescue treatments such as intravenous immunoglobulin (IVIg) or plasma exchange (PLEX) [13,14]. Use of IVIg or PLEX is dependent on patient comorbidities and risk of hemodynamic and venous access complications [7], so their use may be limited to certain patient profiles.
A US retrospective claims analysis found that the likelihood of hospitalization and admission to an intensive care unit (ICU) was 2.6-times and 4.5-times greater, respectively, for patients with MG compared with healthy controls [15]. In addition, the early years following MG diagnosis were found to be a period of particularly high healthcare resource utilization (HCRU) [15]. Another study of US insurance claims found that patients with MG who had >1 MG exacerbation in the first year after diagnosis had mean MG-related HCRU costs 8.3-fold higher than patients with one or no exacerbations [16]. However, there is a paucity of data on how single or multiple exacerbation episodes following an initial exacerbation may influence treatment patterns and HCRU.
In the US, it is particularly important to understand the impact that repeated MG exacerbations can have on clinical and economic burden as, depending on insurance type, patients may have variable access to treatment and HCRU. Of patients with MG in the US with medical insurance from January 2018 to December 2019, 35% were estimated to have employer-based commercial insurance, while 59% and 6% were estimated to have government-sponsored Medicare and Medicaid insurance, respectively [17]. Eligibility for Medicare insurance is determined by age [18], while Medicaid eligibility is primarily focused on low income families and individuals but varies by US state [19]. The age threshold for Medicare eligibility is 65 years [18], closer to the peak incidence of newly diagnosed MG in males than that of early-onset MG, which is more likely to be associated with females than males [20]. There is an unmet need for improved, accessible treatment options for MG in the US, especially treatment options that can reduce the likelihood of MG exacerbation and MG-related hospitalization, and subsequently improve patient quality of life.
Here, we conducted a retrospective analysis of a US medical claims database to describe the clinical and economic burden of MG exacerbation using claims data from patients in the US with commercial or Medicare insurance, stratified by the presence or absence of repeat exacerbation.
Methodology
Study design & data source
This study was a retrospective analysis of IBM® MarketScan® claims data from 1 January 2008 to 30 September 2019 of prevalent MG patients aged ≥18 years (Figure 1). The IBM® MarketScan® database is Health Insurance Portability and Accountability Act-compliant, whereby patient data were de-identified before delivery to the study team. People with MG were identified using International Classification of Diseases, Ninth and Tenth revision (ICD-9 and ICD-10) MG diagnosis codes (ICD-9: 358.0, 358.00, 358.01; ICD-10: G70.0, G70.00, G70.01). The study required patients to have 3 years of continuous medical and pharmacy enrollment including a baseline period of 12 months prior to index (first eligible MG diagnosis) and a follow-up period of 2 years post-index. Because ICD-10 codes are often used interchangeably, in addition to the MG diagnosis an MG exacerbation was defined as any MG-related hospitalization, PLEX use, or acute IVIg use (five cycles or fewer in a 12-month period; chronic IVIg use was not included in the criteria). MG exacerbation was considered to begin on the date of the qualifying treatment start date or hospital admission, whichever occurred first. MG exacerbation was considered to end on the end date of the treatment or hospital admission. MG crisis was defined as one primary diagnosis of acute respiratory failure with inpatient hospitalization, or at least one event of regulated mechanical ventilation within 3 days following the hospital admission date, with at least one acute respiratory failure diagnosis and acute IVIg treatment or PLEX within 7 days after the hospital admission date. Exacerbations that occurred within 14 days of each other were considered as one episode. In order to understand the impact of single and multiple exacerbations, patients who experienced ≥1 exacerbation during follow-up were grouped according to whether they experienced a subsequent exacerbation (‘repeat exacerbation’) or whether they did not experience a subsequent exacerbation (‘no repeat exacerbation’) following a given exacerbation. The ‘repeat’ or ‘no repeat’ categorization was made for each of the first, second and third exacerbations during follow-up. For example, a patient who experienced two exacerbations during follow-up would be in the ‘repeat’ group for the first exacerbation, but in the ‘no repeat’ group for the second exacerbation.
Figure 1. . Study design*.
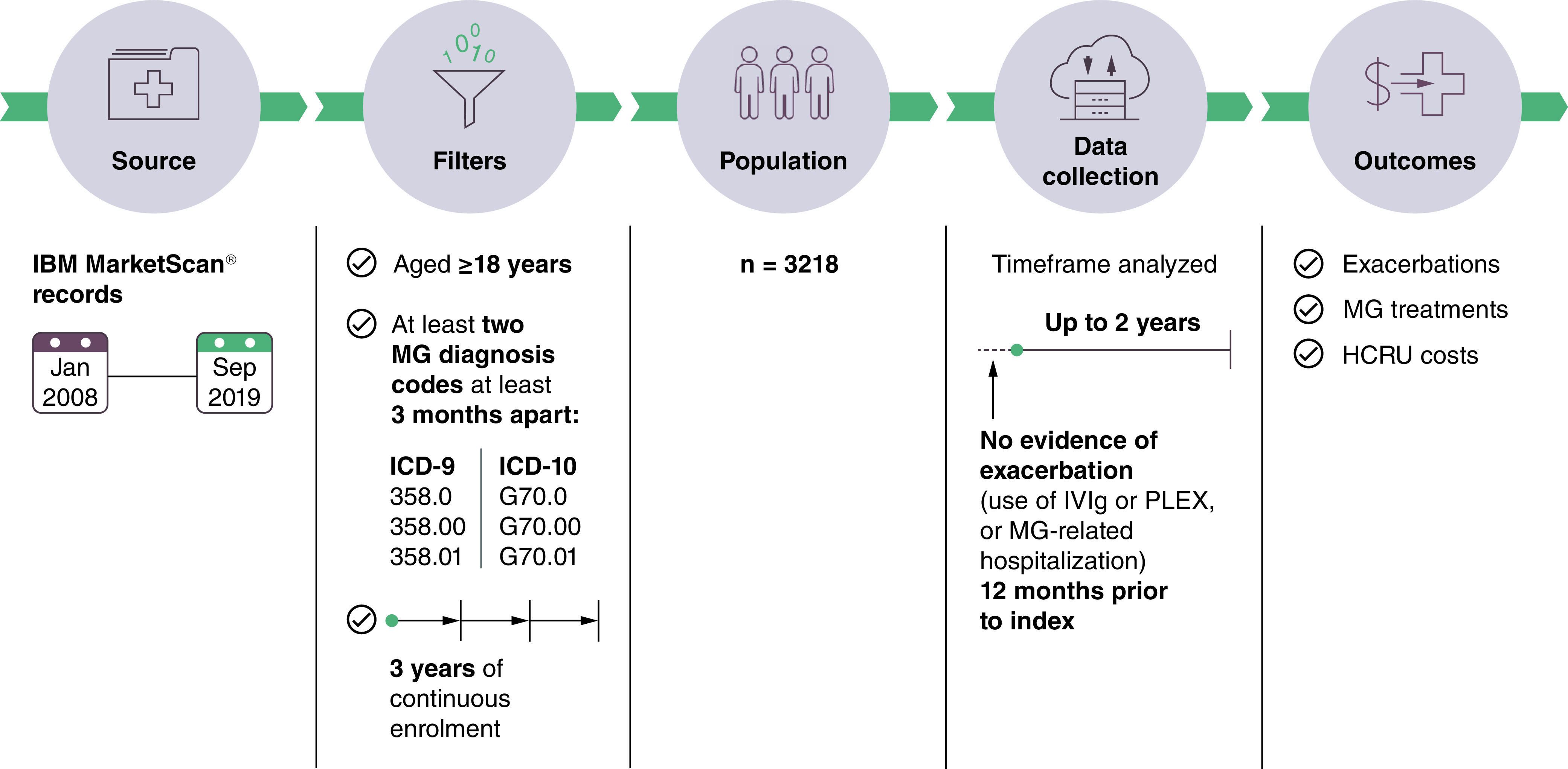
*The study also included a population of patients who did not experience exacerbation or crisis during follow-up.
HCRU: Healthcare resource utilization; ICD: International Classification of Diseases; IVIg: Intravenous immunoglobulin; MG: Myasthenia gravis; PLEX: Plasma exchange.
Study population
Commercial and Medicare patients were identified from the IBM® MarketScan® Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database.
The study population consisted of adults (aged ≥18 years) who received at least two ICD-9 and ICD-10 MG diagnoses at least 3 months apart and had 3 years of continuous enrolment. Eligible patients had no exacerbation in the baseline period, or crisis in the baseline and follow-up periods.
Study outcomes & data analysis
All results are descriptive, and no statistical comparisons were made. Patient characteristics (n [%]) including age, gender assigned at birth, region, payer type, MG treatments and comorbidities were assessed at baseline, and MG treatments and comorbidities were assessed again 12 weeks prior to each exacerbation. Comorbidities were assessed using ICD-9 and ICD-10 codes and reported as n (%). Use of medical services was assessed at each exacerbation (between the start and end dates of qualifying treatment or hospital admission) and in the 12-week period after the end of the exacerbation. Subsequent exacerbations could overlap during this time period. MG treatments received (n [%]) during the 2-year follow-up (Supplementary Table 1) were described between index and first exacerbation, first and second exacerbation, and second and third exacerbation, as applicable. Mean (standard deviation [SD]) time between index and first, second and third exacerbation and time between first, second and third exacerbations were assessed. Costs incurred during exacerbations were described by the mean (SD) cost per exacerbation episode, stratified by the absence or presence of repeat exacerbation. Total all-cause costs associated with exacerbation episodes are the sum of emergency department (ED), inpatient, outpatient and pharmacy costs. Total MG-related costs associated with exacerbation episodes represent costs for claims with MG diagnoses in the ED, inpatient and outpatient setting, as well as MG-related pharmacy costs. MG-related inpatient and outpatient costs represent costs associated with an occurrence of an inpatient or outpatient claim, respectively, when the place of service was not an ED. MG-related ED costs are inpatient or outpatient claims when the place of service was the ED. MG-related ICU costs include those associated with the facility, professional encounters and services of the ICU service component of inpatient stay. Costs were adjusted to 2020 USD using the medical care components of the Consumer Price Index (CPI) [21]. Total all-cause and MG-related costs were stratified based on the presence or absence of repeat exacerbation, relative to the first, second and third exacerbation.
Results
Patient demographics & baseline comorbidities
A total of 9352 patients with MG aged ≥18 years who satisfied the baseline and follow-up inclusion criteria were identified from the database (Figure 2). Of 3218 patients with MG with ≥1 exacerbation, 53.0% (n = 1706), 39.4% (n = 1269) and 7.6% (n = 243) had commercial, Medicare and Medicaid insurance, respectively. Data from patients with Medicaid insurance are not reported further owing to the low patient number. Of patients with ≥1 exacerbation, the South and North Central US were the regions with the highest proportion of commercial patients and Medicare patients, respectively (Table 1). Of patients with ≥1 exacerbation, the mean (SD) age at index was 49.1 (11.55) years for commercial patients and 75.9 (7.43) years for Medicare patients. A higher proportion of commercial patients than Medicare patients were female (Table 1). For both commercial and Medicare patients, anxiety disorders, depression, diabetes type I or II, infection, and obesity at baseline were reported in a higher proportion of patients who had ≥1 exacerbation than for those patients who never had an MG exacerbation or crisis during follow-up (Table 1). Among patients with ≥1 exacerbation, compared with Medicare patients at baseline, a higher proportion of commercial patients had anxiety disorders, depression and obesity, whereas a higher proportion of Medicare versus commercial patients had diabetes type I or II (Table 1).
Figure 2. . Study cohort of commercial and Medicare patients identified from IBM® MarketScan® records.
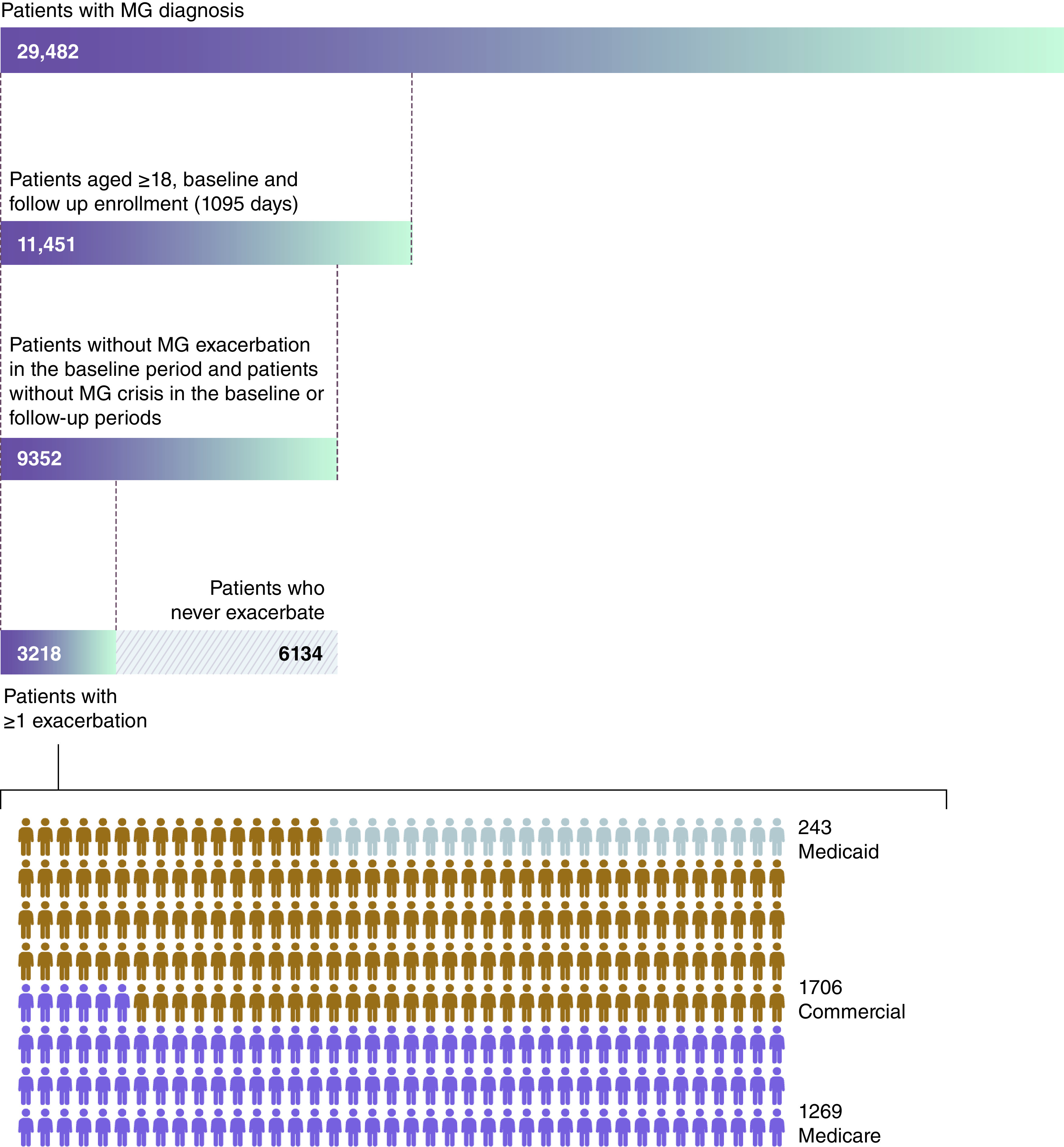
MG: Myasthenia gravis.
Table 1. . Baseline demographics of study cohort: Commercial and Medicare patients grouped by experience of MG exacerbation.
| Commercial population (n = 5340) | Medicare population (n = 3423) | ||||
|---|---|---|---|---|---|
| Never exacerbation/crisis patients (n = 3634) | Patients with ≥1 exacerbation (n = 1706) | Never exacerbation/crisis patients (n = 2154) | Patients with ≥1 exacerbation (n = 1269) | ||
| Age, years | Mean (SD) | 50.09 (10.89) | 49.09 (11.55) | 74.89 (7.08) | 75.89 (7.43) |
| Median (IQR) | 53 (44–59) | 52 (42–59) | 75 (69–80) | 76 (70–81) | |
| Sex, n (%) | Female | 2096 (57.7) | 1014 (59.4) | 886 (41.1) | 534 (42.1) |
| Region, n (%) | Northeast | 729 (20.1) | 328 (19.2) | 516 (24.0) | 298 (23.5) |
| North Central | 758 (20.9) | 389 (22.8) | 643 (29.9) | 443 (34.9) | |
| South | 1516 (41.7) | 723 (42.4) | 677 (31.4) | 333 (26.2) | |
| West | 588 (16.2) | 247 (14.5) | 308 (14.3) | 190 (15.0) | |
| Unknown | 43 (1.2) | 19 (1.1) | 10 (0.5) | 5 (0.4) | |
| Comorbidities, n (%) | Anxiety disorders | 348 (9.6) | 228 (13.4) | 107 (5.0) | 102 (8.0) |
| Depression | 375 (10.3) | 217 (12.7) | 118 (5.5) | 119 (9.4) | |
| Diabetes (types I and II) | 548 (15.1) | 357 (20.9) | 633 (29.4) | 419 (33.0) | |
| Infection | 1344 (37.0) | 774 (45.4) | 943 (43.8) | 606 (47.8) | |
| Obesity | 218 (6.0) | 176 (10.3) | 87 (4.0) | 72 (5.7) | |
IQR: Interquartile range; MG: Myasthenia gravis; SD: Standard deviation.
MG exacerbations during follow-up
Of commercial and Medicare patients eligible for inclusion in the study, 31.9% (n = 1706/5340) and 37.1% (n = 1269/3423), respectively, had ≥1 exacerbation during follow-up. Of these patients, the mean (SD) number of exacerbations per commercial patient during follow-up was 3.7 (7.0; median, 2.0; interquartile range [IQR] 1.0–5.0), while Medicare patients experienced a mean (SD) of 2.68 (4.1) exacerbations (median, 1.0; IQR, 1.0–3.0). At least two exacerbations during follow-up were experienced by 52.5% (n = 895/1706) of commercial patients and 44.8% (n = 569/1269) of Medicare patients; 36.5% (n = 623/1706) and 27.3% (n = 347/1269) of commercial and Medicare patients, respectively, experienced at least three exacerbations (Figure 3). For patients who experienced ≥1 exacerbation during follow-up, the mean (median) time from index to the first exacerbation was 203 (134) days and 225 (162) days for commercial and Medicare patients, respectively. The mean (median) time between exacerbations shortened from 141 (78) days between first and second exacerbation to 87 (41) days between the second and third exacerbation for commercial patients, and from 185 (122) days to 126 (69) days for Medicare patients for the same time points (Figure 4). Among patients with ≥1 exacerbation, rates of depression and anxiety disorders increased incrementally from the first to the third exacerbation for both commercial and Medicare patients (Table 2). Throughout these exacerbations, a higher proportion of commercial than Medicare patients experienced anxiety disorders, depression and obesity, while diabetes type I or II were reported by a larger proportion of Medicare than commercial patients.
Figure 3. . Number of myasthenia gravis exacerbations during 2-year follow-up for patients with commercial and Medicare insurance.
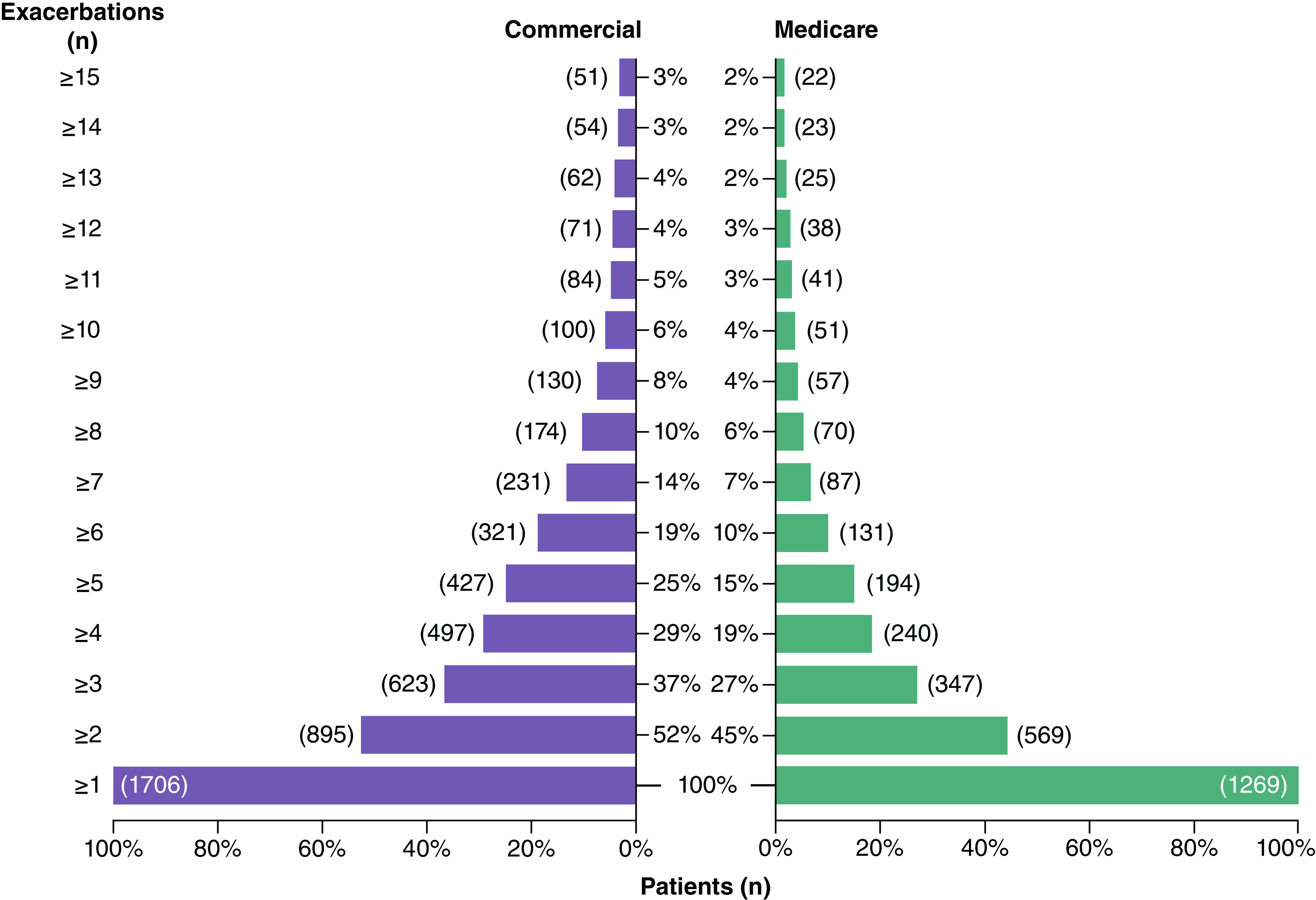
Figure 4. . Mean time to myasthenia gravis exacerbations for patients with commercial and Medicare insurance*.
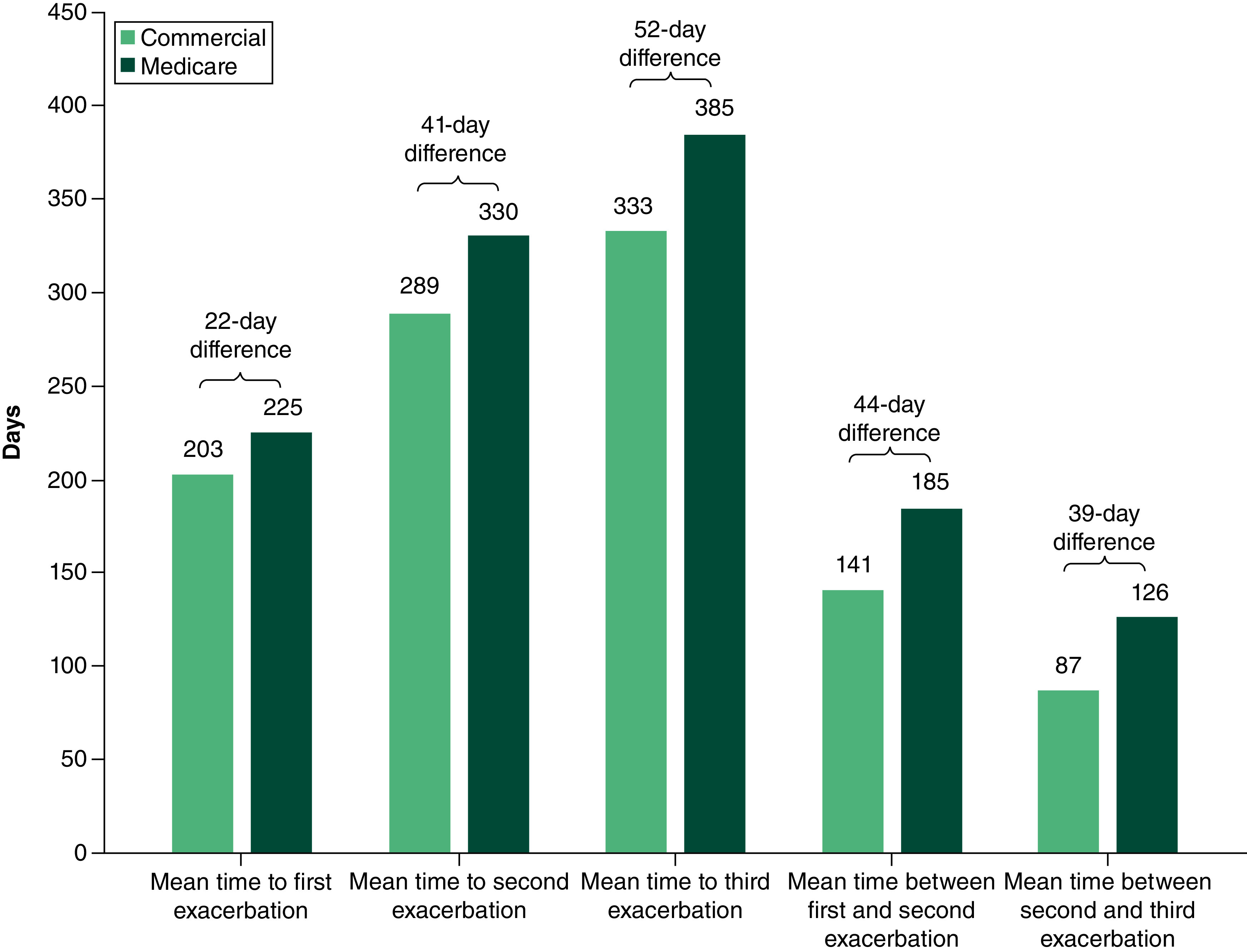
*Mean time to and between exacerbations may not align owing to variation in patient number between time points.
Table 2. . Comorbidities at the first, second and third MG exacerbation during 2-year follow-up for commercial and Medicare patients.
| First exacerbation | Second exacerbation | Third exacerbation | ||||
|---|---|---|---|---|---|---|
| Commercial (n = 1706) | Medicare (n = 1269) | Commercial (n = 801) | Medicare (n = 541) | Commercial (n = 504) | Medicare (n = 282) | |
| Infection, n (%) | 336 (19.7) | 327 (25.8) | 210 (26.2) | 168 (31.1) | 134 (26.6) | 94 (33.3) |
| Diabetes type I or II, n (%) | 261 (15.3) | 303 (23.9) | 164 (20.5) | 159 (29.4) | 112 (22.2) | 94 (33.3) |
| Anxiety disorders, n (%) | 119 (7.0) | 51 (4.0) | 70 (8.7) | 28 (5.2) | 57 (11.3) | 15 (5.3) |
| Depression, n (%) | 119 (7.0) | 71 (5.6) | 84 (10.5) | 33 (6.1) | 65 (12.9) | 23 (8.2) |
| Obesity, n (%) | 105 (6.2) | 34 (2.7) | 71 (8.9) | 17 (3.1) | 38 (7.5) | 10 (3.5) |
MG: Myasthenia gravis.
Treatments received during follow-up
For patients who experienced ≥1 exacerbation during follow-up, AChEIs were the most common treatment used by both commercial and Medicare patients between index and first exacerbation. AChEI use in both patient groups decreased with subsequent exacerbations (Figure 5). CSs were the second most common treatment used from index to first exacerbation for both commercial and Medicare patients. While CS use decreased with subsequent exacerbation for commercial patients, CS use was highest for Medicare patients between the first and second exacerbation. For commercial patients and Medicare patients, IVIg use increased with repeated exacerbations: a higher proportion of commercial patients than Medicare patients used IVIg from the first to the third exacerbation (Figure 5). Less than 20% of commercial or Medicare patients used NSISTs from index to third exacerbation (Figure 5). The highest proportion of patients using NSISTs occurred between the first and second exacerbation for both commercial and Medicare patients. PLEX use was low overall: the proportion of patients that used PLEX from index to third exacerbation ranged between 4.9–5.6% and 2.7–6.3% for commercial and Medicare patients, respectively. From index to third exacerbation, less than 1% of commercial or Medicare patients used biologics.
Figure 5. . Treatments received by commercial and Medicare patients from index to first myasthenia gravis exacerbation and between exacerbations during 2-year follow-up.
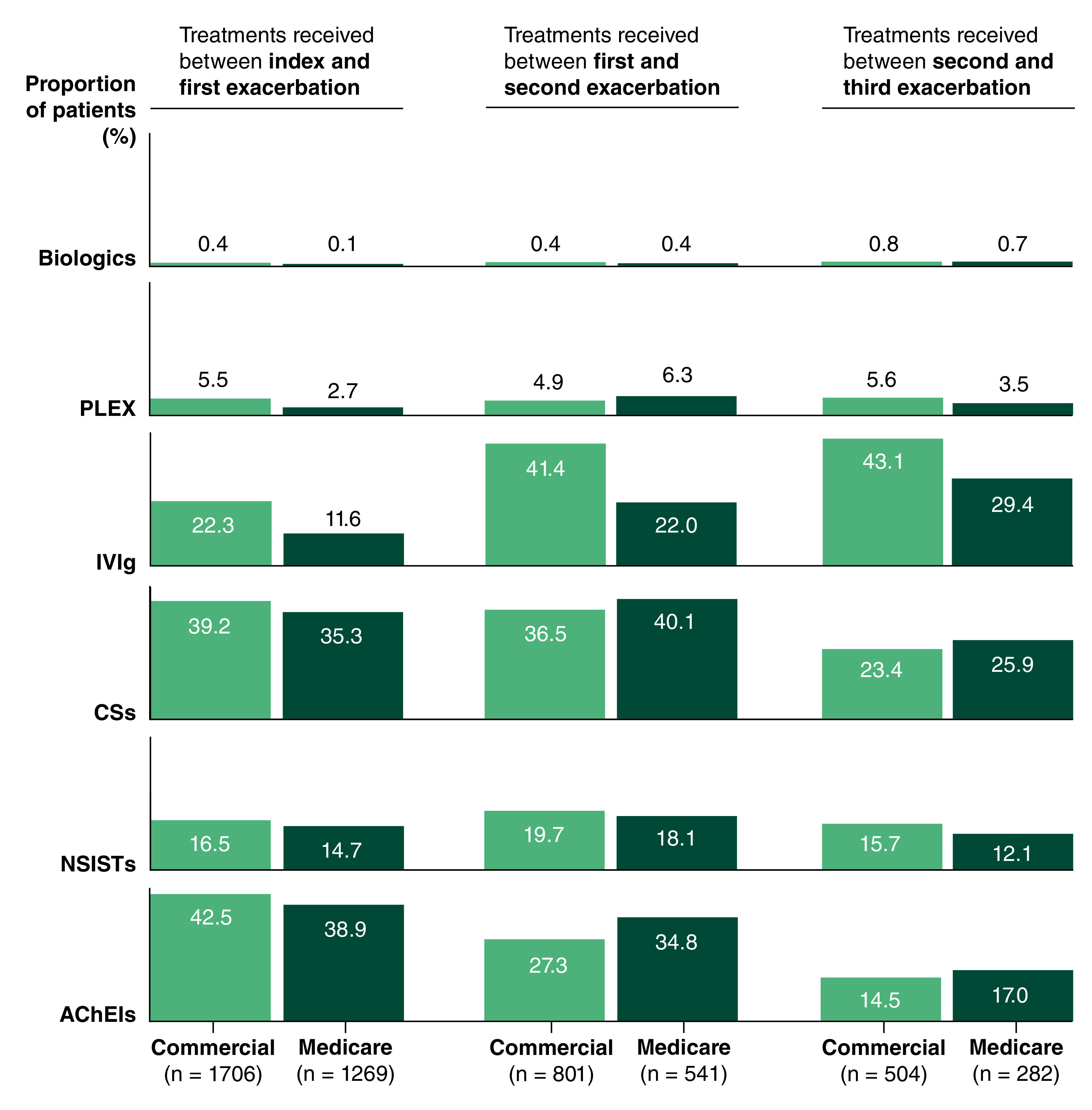
‘Biologics’ includes rituximab and eculizumab.
AChEI: Acetylcholinesterase inhibitor; CS: Corticosteroid; IVIg: Intravenous immunoglobulin; NSIST: Non-steroidal immunosuppressive therapy; PLEX: Plasma exchange.
HCRU costs incurred during MG exacerbation episodes
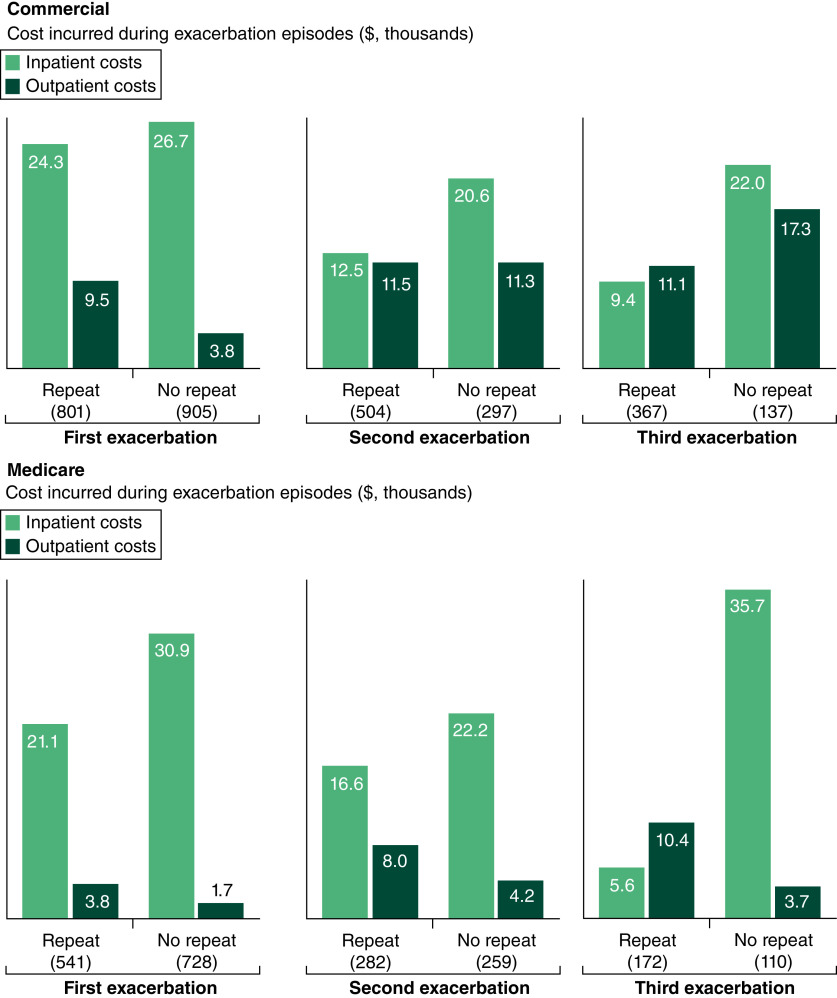
Healthcare costs incurred per exacerbation episode varied considerably but were on average higher for commercial patients than Medicare, for both all-cause (mean $41,194 vs $34,802 per exacerbation) and MG-related costs (mean $39,079 vs $33,446 per exacerbation; Table 3). The mean MG-related ED costs per exacerbation episode were higher for commercial patients than Medicare patients (mean $8143 vs $5179 per exacerbation; Table 3). The highest mean MG-related ED costs per exacerbation episode were incurred by commercial and Medicare patients at the first exacerbation (repeat and no repeat exacerbation costs combined). The mean MG-related ICU costs per exacerbation episode were higher for commercial than Medicare patients (mean $2174 vs $1624 per exacerbation), and the mean MG-related ICU costs per exacerbation episode were highest at the first exacerbation for both commercial and Medicare patients (Table 3). The mean MG-related inpatient costs per exacerbation episode were lower for commercial patients than Medicare patients (mean $19,257 vs $22,009 per exacerbation; Table 3). Per exacerbation episode during follow-up, MG-related inpatient costs for commercial patients decreased relative to the first exacerbation, whereas for Medicare patients, the highest mean MG-related inpatient cost per exacerbation episode occurred during the third exacerbation (Figure 6). The mean MG-related outpatient costs per exacerbation episode were higher for commercial patients than Medicare patients (mean $10,752 vs $5304 per exacerbation; Table 3). MG-related outpatient costs per exacerbation episode during follow-up increased from the first to the third exacerbation for commercial patients and were lowest during the first exacerbation for Medicare patients (Figure 6).
Table 3. . HCRU costs incurred during exacerbation episodes by commercial and Medicare patients grouped according to last recorded MG exacerbation during follow-up†.
| First exacerbation | Second exacerbation | Third exacerbation | ||||
|---|---|---|---|---|---|---|
| Repeat exacerbation | No repeat exacerbation | Repeat exacerbation | No repeat exacerbation | Repeat exacerbation | No repeat exacerbation | |
| Commercial patients, n | 801 | 905 | 504 | 297 | 367 | 137 |
| Total all-cause costs ($), mean (SD) | 45,164 (105,736) | 44,700 (120,855) | 33,425 (49,133) | 41,907 (100,668) | 27,749 (46,562) | 54,217 (94,295) |
| Total MG-related costs ($), mean (SD) | 43,043 (103,223) | 43,140 (120,249) | 31,787 (47,438) | 39,304 (100,271) | 26,078 (45,431) | 51,120 (87,818) |
| MG-related ED cost ($), mean (SD) | 8361 (29,928) | 12,420 (106,377) | 6472 (27,367) | 6829 (20,927) | 3778 (20,160) | 10,995 (44,729) |
| MG-related ICU cost ($), mean (SD) | 3634 (18,189) | 4155 (26,886) | 1825 (14,716) | 1484 (5202) | 1729 (7606) | 3458 (17,289) |
| MG-related IP cost ($), mean (SD) | 24,280 (96,530) | 26,732 (57,595) | 12,510 (33,359) | 20,622 (38,298) | 9354 (33,775) | 22,045 (42,475) |
| MG-related OP cost ($), mean (SD) | 9470 (23,673) | 3822 (11,503) | 11,507 (22,738) | 11,287 (91,920) | 11,095 (23,730) | 17,328 (54,945) |
| Medicare patients, n | 541 | 728 | 282 | 259 | 172 | 110 |
| Total all-cause costs ($), mean (SD) | 32,625 (50,928) | 39,743 (74,427) | 30,127 (57,423) | 33,265 (45,754) | 21,024 (27,787) | 52,029 (177,860) |
| Total-MG-related costs ($), mean (SD) | 31,625 (50,283) | 38,529 (74,154) | 29,274 (57,076) | 31,378 (43,511) | 19,903 (26,362) | 49,967 (173,860) |
| MG-related ED cost ($), mean (SD) | 6033 (21,063) | 5652 (17,010) | 3493 (15,128) | 4335 (11741) | 2245 (9488) | 9318 (35,104) |
| MG-related ICU cost ($), mean (SD) | 3361 (19,104) | 2358 (12,024) | 811 (4674) | 1537 (7060) | 821 (6380) | 854 (2285) |
| MG-related IP cost ($), mean (SD) | 21,082 (48,296) | 30,925 (73,667) | 16,579 (49,513) | 22,221 (39,096) | 5591 (16,696) | 35,656 (167,388) |
| MG-related OP costs ($), mean (SD) | 3805 (11,068) | 1723 (9843) | 7976 (22,467) | 4229 (20,777) | 10,366 (20,179) | 3725 (10,219) |
Patients who experienced ≥1 exacerbation during follow-up were grouped according to whether they experienced a subsequent exacerbation – ‘repeat exacerbation’ or whether they did not experience a subsequent exacerbation – ‘no repeat exacerbation’. The ‘repeat’ or ‘no repeat’ categorization was made for each of the first, second and third exacerbation.
ED: Emergency department; HCRU: Healthcare resource utilization; ICU: Intensive care unit; IP: Inpatient; MG: Myasthenia gravis; OP: Outpatient; SD: Standard deviation.
Figure 6. . Mean myasthenia gravis-related inpatient and outpatient costs by exacerbation number for commercial and Medicare patients grouped according to last recorded exacerbation during follow-up*.
*Patients who experienced ≥1 exacerbation during follow-up were grouped according to whether they experienced a subsequent exacerbation (‘repeat exacerbation’) or whether they did not experience a subsequent exacerbation (‘no repeat exacerbation’). The ‘repeat’ or ‘no repeat’ categorization was made for each of the first, second and third exacerbations.
When grouped by the last recorded exacerbation episode, multiple exacerbations during follow-up were associated with lower MG-related costs per exacerbation than single exacerbations episodes for commercial patients. For Medicare patients, single or multiple exacerbation episodes during follow-up were associated with high mean MG-related costs.
Discussion
In this study, we used the IBM® MarketScan® database to capture longitudinal, individual-level administrative claims data from patients in the US with employer-based commercial or government-sponsored Medicare insurance. Our analysis shows that baseline comorbidities at index were more common in patients with MG who went on to experience at least one exacerbation during the 2-year follow-up. Compared with patients who never had an exacerbation or crisis during follow-up, a higher proportion of patients who experienced ≥1 exacerbation during follow-up had baseline comorbidities, including anxiety disorders, depression, diabetes type I or II, and obesity. Among patients with at least one exacerbation during follow-up, the proportion of patients with metabolic conditions at baseline was higher in the older Medicare population than in the younger commercial population. This finding is consistent with another study that showed patients with late-onset MG are more likely to have diabetes type I or II and metabolic syndrome than patients with early-onset MG [22].
Approximately half of the cohort experienced at least two exacerbations during the 2-year follow-up, highlighting the need for new and effective treatment approaches that can reduce the clinical symptoms of patients with MG. During follow-up, the average count of exacerbations per patient was lower among Medicare patients than commercial patients. This observation could be a consequence of the smaller proportion of female patients in the Medicare population, compared with the commercial population, as evidence in the literature suggests that MG may be more severe in women [24–26], including a previous study which reported a higher MG exacerbation rate for females than males [27]. Mechanisms for the greater disease severity in women are unknown, however chromosomal inactivation and hormonal fluctuations have been suggested as potential explanations [28–30]. The increased ratio of female to male patients in the commercial population compared with the Medicare population may be further reflective of gender differences in MG, whereby female patients tend to be diagnosed younger than male patients [2]. Additionally, the mean time to first, second, or third exacerbation was consistently longer for Medicare than commercial patients. This may be due to the older age and thus a higher proportion of patients with late-onset MG present in the Medicare population, as late-onset MG has a favorable prognosis [23]. There was a small proportion of commercial and Medicare patients who experienced ≥15 exacerbations, who may represent a subset who do not respond to currently available treatments, or who have comorbidities that prevent a wider disease management approach.
Consistent with current treatment guidance [6,7], AChEIs were the most commonly used treatment from index to first exacerbation by commercial and Medicare patients. The proportion of patients receiving NSISTs was generally similar from index to third exacerbation, suggesting that NSISTs were not a common add-on therapy for either commercial or Medicare patients. IVIg use increased with each recorded exacerbation after index for both commercial and Medicare patients, which is expected as IVIg is used as an acute treatment for exacerbation [7]. PLEX use was low overall for both commercial and Medicare patients. Targeted treatments, such as FcRn inhibitors or complement inhibitors, have shown statistically significant reductions in MG-ADL score, which has a correlation to risk of exacerbations. Therefore, targeted treatments may help to reduce the risk of MG exacerbation [14,31–34].
Prior studies in the literature have shown increased HCRU and HCRU costs for patients with MG compared with a general non-MG population or patients with non-MG chronic neurological disease [15,35,36]. A systematic literature review of economic costs across eight countries revealed the mean per-patient costs per hospitalization of patients with MG ranged from $2550 to $164,630 (2018 USD) [37]. In our study, mean MG-related healthcare costs per exacerbation episode were in this range, at $39,079 and $33,446 for commercial and Medicare patients, respectively. Inpatient costs were typically the main driver of MG-related costs at each exacerbation during follow-up for both commercial and Medicare patients. Relative to the first exacerbation episode, inpatient costs decreased, and outpatient costs increased per subsequent exacerbation episodes for commercial patients during follow-up. This may be the result of commercial patients opting to manage subsequent MG exacerbations in an outpatient setting, perhaps owing to employment and other commitments associated with younger age. Relatedly, a reduction in the proportion of commercial patients requiring MG-related ED and ICU services after the first exacerbation may suggest that subsequent exacerbations were less severe or were more manageable in the outpatient setting. For Medicare patients, MG-related inpatient costs per exacerbation episode generally remained higher than MG-related outpatient costs per exacerbation episode throughout follow-up. This observation suggests that some Medicare patients may have required an inpatient stay to manage MG exacerbations, perhaps to some extent owing to age or comorbidities such as diabetes type I or II.
This study had several limitations, many of which are inherent to medical claim database analyses. Administrative real-world claims data are subject to reliability of ICD coding. Diagnosis codes may have been incorrectly coded or included as rule-out criteria, rather than actual confirmed diagnoses; inclusion criteria required two diagnoses of MG at least 3 months apart to lower the risk of patients not having MG being wrongly included. The study population comprised patients in the US with commercial or Medicare insurance. Therefore, treatment and cost data may not be generalizable to patients with MG in the US who do not have health insurance coverage, or patients with MG in other countries. Further, we adjusted costs using the 2020 CPI; if more recent CPI data had been used it is likely that the costs associated with exacerbations would be even higher. Another limitation is that the database did not capture data on race or ethnicity. A retrospective claims study of patients with MG in the US reported racial differences in treatment utilization and health outcomes associated with hospitalization [38]. The definition of an MG exacerbation used in this study may have skewed toward identifying only severe exacerbations, as an exacerbation episode was captured from interventions (HCRU and rescue treatments) recorded in the claims database. Therefore, it is possible that patients experienced mild exacerbations for which patients did not receive treatment or use additional healthcare resources. A short duration following an exacerbation was selected to attempt to identify HCRU and costs that were related to the exacerbation. However, it is possible that some of the costs recorded in this period were associated with non-exacerbation related conditions. In addition, there is no standard recovery time for an MG exacerbation, so exacerbations that occurred within 14 days were considered as one event, and patients could have experienced another exacerbation during the 12-week post-exacerbation period. If patients had their third exacerbation close to the end of the continuous enrollment period, the potential for repeat exacerbation may not have been recorded. If the actual number of exacerbations were underestimated, treatment and HCRU costs associated with a single per exacerbation episode would be inflated. Our analysis did not capture symptom worsening or shifts in treatment practice that may have resulted from the 2020 update [6] of the international consensus guidance for MG management, previously published in 2016 [7]. Lastly, the presence of a prescription claim did not necessarily mean that the treatment was taken at all or according to the indicated regimen. For example, while CSs are a common treatment for MG, they have a broad indication and could have been prescribed for conditions not associated with MG.
Conclusion
MG exacerbations are associated with a high clinical and economic burden in both Medicare and commercial patients, despite treatment with conventional therapies. Disease management approaches that reduce the occurrence of MG exacerbation will be crucial to lower the economic burden of MG. In addition to the need for novel therapies to reduce the likelihood of experiencing an exacerbation, patients with MG may benefit from personalized disease management approaches that incorporate exacerbation risk. Mitigating exacerbations is likely to improve patient quality of life and help to reduce the clinical and economic burden of MG.
Summary points.
Despite conventional treatment, some patients with myasthenia gravis (MG) continue to experience disease exacerbations, suggesting an inconsistent clinical response to current treatment options.
This retrospective study used IBM® MarketScan® claims data from 2008 to 2019 of adults with MG and who had commercial, Medicare or Medicaid insurance to understand the economic and clinical burden associated with single or multiple exacerbation episodes.
Around 50% of patients in our cohort, comprising patients who experienced at least one exacerbation, experienced at least two exacerbations during the 2-year follow-up. Those who experienced exacerbations were more likely to have comorbidities, including anxiety disorders, depression, diabetes type I or II, infection and obesity.
The mean MG-related healthcare costs incurred during exacerbation episodes ranged from $26,078 to $51,120 (mean per exacerbation, $39,079) for commercial patients and from $19,903 to $49,967 (mean per exacerbation, $33,446) for Medicare patients, mainly driven by inpatient costs.
Novel therapies and personalized treatment approaches that may decrease MG exacerbations will be crucial to lower the clinical and economic burden of MG and increase patient quality of life.
Supplementary Material
Acknowledgments
The authors acknowledge V Porkess of UCB Pharma for publication coordination.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2023-0108
Author contributions
All authors conceptualized the study and study design. JP performed data collection and statistical analysis. All authors contributed to interpretation of the results and critical revision of the manuscript.
Financial disclosure
This study was funded by UCB Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
J Pisc is an employee and option holder of Alpha Medical Group and was an employee and is a stockholder of Aetion Inc. A Ting, O Sinno and E Lee are employees and stockholders of UCB Pharma. M Skornicki is an employee and option holder of Aetion Inc. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
The authors acknowledge R Carney and R Price of Ogilvy Health, London, UK, for medical writing support, which was funded by UCB, in accordance with Good Publication Practice 2022 guidelines (www.ismpp.org/gpp-2022).
Ethical conduct of research
The IBM® MarketScan® database is Health Insurance Portability and Accountability Act-compliant. Only de-identified patient data were used in this study; therefore, Institutional Review Board approval was not required. Reporting guideline: STROBE.
Data sharing statement
Data from non-interventional studies are outside of UCB Pharma's data sharing policy and are unavailable for sharing.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat. Rev. Dis. Primers 5(1), 30 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Juel VC, Massey JM. Myasthenia gravis. Orphanet J. Rare Dis. 2, 44 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melzer N, Ruck T, Fuhr P et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the guidelines of the German Neurological Society. J. Neurol. 263(8), 1473–1494 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaminski HJ, Denk J. Corticosteroid treatment-resistance in myasthenia gravis. Front. Neurol. 13, 886625 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myasthenia Gravis Foundation of America. Clinical overview of MG. https://myasthenia.org/Professionals/Clinical-Overview-of-MG (2023).
- 6.Narayanaswami P, Sanders DB, Wolfe G et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology 96(3), 114–122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders DB, Wolfe GI, Benatar M et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology 87(4), 419–425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol. Clin. 36(2), 311–337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon D, Barnett C, Bril V. Novel treatments in myasthenia gravis. Front. Neurol. 11, 538 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalakas MC. Role of complement, anti-complement therapeutics, and other targeted immunotherapies in myasthenia gravis. Expert Rev. Clin. Immunol. 18(7), 691–701 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther. Adv. Neurol. Disord. 11, 1756285617749134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myasthenia Gravis Foundation of America. MG emergencies. https://myasthenia.org/MG-Community/-MG-Emergencies#:∼:text=MG%20Exacerbations%20%26%20Crisis&text=An%20exacerbation%2C%20or%20flare%2C%20is,and%2F%20or%20become%20more%20severe (2023).
- 13.Sieb JP. Myasthenia gravis: an update for the clinician. Clin. Exp. Immunol. 175(3), 408–418 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard JF Jr, Utsugisawa K, Benatar M et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 16(12), 976–986 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Mahic M, Bozorg A, Rudnik J, Zaremba P, Scowcroft A. Healthcare resource use in myasthenia gravis: a US health claims analysis. Ther. Adv. Neurol. Disord. 16, 17562864221150327 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This US retrospective claims analysis reports the high healthcare resource utilization incurred by patients with newly diagnosed MG compared with age-matched controls and identifies the early years following diagnosis as a period of particularly high healthcare burden.
- 16.Ting A, Story T, Lecomte C, Estrin A, Syed S, Lee E. A real-world analysis of factors associated with high healthcare resource utilization and costs in patients with myasthenia gravis receiving second-line treatment. J. Neurol. Sci. 445, 120531 (2023). [DOI] [PubMed] [Google Scholar]; •• This US retrospective claims analysis describes the high clinical burden and high healthcare resource utilization of patients with MG who initiated second-line therapy.
- 17.Klaisner JK, Schweitzer K, Ally AJ. Myasthenia gravis patient payer channel distribution. https://uk.milliman.com/en-GB/insight/myasthenia-gravis-patient-payer-channel-distribution (2022).
- 18.Card D, Dobkin C, Maestas N. Does Medicare save lives? Q. J. Econ. 124(2), 597–636 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center on Budget Policy Priorities. Policy basics: Introduction to Medicaid. https://www.cbpp.org/research/health/introduction-to-medicaid (2020).
- 20.Bubuioc AM, Kudebayeva A, Turuspekova S, Lisnic V, Leone MA. The epidemiology of myasthenia gravis. J. Med. Life 14(1), 7–16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review highlights the global increase in the incidence and prevalence of MG within the past several decades.
- 21.US Bureau of Labor Statistics. CPI for all Urban Consumers (CPI-U). https://www.bls.gov (2021).
- 22.Misra UK, Kalita J, Singh VK, Kumar S. A study of comorbidities in myasthenia gravis. Acta Neurol. Belg. 120(1), 59–64 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijayan J, Menon D, Barnett C, Katzberg H, Lovblom LE, Bril V. Clinical profile and impact of comorbidities in patients with very-late-onset myasthenia gravis. Muscle Nerve 64(4), 462–466 (2021). [DOI] [PubMed] [Google Scholar]; • This Canadian retrospective chart review describes the clinical profile of patients with late-onset MG and the impact of comorbidites on disease status and health outcomes.
- 24.Thomsen JLS, Vinge L, Harbo T, Andersen H. Gender differences in clinical outcomes in myasthenia gravis: a prospective cohort study. Muscle Nerve 64(5), 538–544 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Lee I, Leach JM, Aban I, McPherson T, Duda PW, Cutter G. One-year follow-up of disease burden and medication changes in patients with myasthenia gravis: from the MG Patient Registry. Muscle Nerve 66(4), 411–420 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong D, Chong MK-C, Wu Y, Kaminski H, Cutter G, Xu X et al. Gender differences in quality of life among patients with myasthenia gravis in China. Health Qual. Life Outcomes 18(1), 296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abuzinadah AR, Alanazy MH, Butt NS, Barohn RJ, Dimachkie MM. Exacerbation rate in generalized myasthenia gravis and its predictors. Eur. Neurol. 84(1), 43–48 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This retrospective chart review of a cohort of patients in the US with acetylcholine receptor antibody (AChR)-positive generalized MG estimates the incidence rate and predictors of MG exacerbations.
- 28.Nicoli V, Tabano SM, Colapietro P, Maestri M, Ricciardi R, Stoccoro A et al. Preferential X chromosome inactivation as a mechanism to explain female preponderance in myasthenia gravis. Genes 13(4), 696 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boldingh MI, Maniaol AH, Brunborg C, Weedon-Fekjær H, Verschuuren JJ, Tallaksen CM. Increased risk for clinical onset of myasthenia gravis during the postpartum period. Neurology 87(20), 2139–2145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leker RR, Karni A, Abramsky O. Exacerbation of myasthenia gravis during the menstrual period. J. Neurol. Sci. 156(1), 107–111 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Howard JF Jr, Bril V, Vu T et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 20(7), 526–536 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Vu T, Meisel A, Mantegazza R et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evidence 1, EVIDoa2100066 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Bril V, Druzdz A, Grosskreutz J et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 22(5), 383–394 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Howard JF Jr, Bresch S, Genge A et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 22(5), 395–406 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Guptill JT, Sharma BK, Marano A, Soucy A, Krueger A, Sanders DB. Estimated cost of treating myasthenia gravis in an insured U.S. population. Muscle Nerve 45(3), 363–366 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Omorodion JO, Pines JM, Kaminski HJ. Inpatient cost analysis for treatment of myasthenia gravis. Muscle Nerve 56(6), 1114–1118 (2017). [DOI] [PubMed] [Google Scholar]; • This cost analysis study revealed geographic differences in the US for inpatient costs associated with MG.
- 37.Landfeldt E, Pogoryelova O, Sejersen T, Zethraeus N, Breiner A, Lochmuller H. Economic costs of myasthenia gravis: a systematic review. Pharmacoeconomics 38(7), 715–728 (2020). [DOI] [PubMed] [Google Scholar]; •• This systematic literature review of economic costs associated with MG in eight countries highlights important regional variation in healthcare resource utlization, healthcare costs and disease management.
- 38.Syed MJ, Khawaja A, Lisak RP. Are there racial differences in inpatient outcomes and treatment utilization following hospitalization for myasthenia gravis exacerbation? Neuroepidemiology 56(5), 380–388 (2022). [DOI] [PubMed] [Google Scholar]; •• This US retrospective claims analysis of adult patients hospitalized with MG reports racial differences in health outcomes and inpatient treatment for MG.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.