Abstract
Trophoblast injury is central to clinically relevant placenta dysfunction. We hypothesized that the mRNA of primary human trophoblasts, exposed to distinct injuries in vitro, capture transcriptome patterns of placental biopsies obtained from common obstetrical syndromes. We deployed a CIBERSORTx deconvolution method to correlate trophoblastic RNAseq-based expression matrices with the transcriptome of omics-defined placental dysfunction patterns in vivo. We found distinct trophoblast injury patterns in placental biopsies from women with fetal growth restriction and a hypertensive disorder, or in biopsies clustered by their omics analysis. Our RNAseq data are useful for defining the contribution of trophoblast injuries to placental dysfunction syndromes.
Keywords: Placenta, trophoblast, apoptosis, ferroptosis, CIBERSORTx
Introduction
Placental dysfunction is a leading cause of common obstetrical syndromes, such as preeclampsia (PE), fetal growth restriction (FGR), and preterm birth (PTB). Such dysfunction impacts villous trophoblasts, which govern placental gas and nutrient exchange, hormone production, immune and mechanical defense [1]. Whereas trophoblast hypoxia is typical early in pregnancy [2], fluctuating or persistent hypoxia later in pregnancy has been implicated in placental pathophysiology [2-4]. Further, inadequate trophoblast differentiation, apoptosis, and ferroptosis, are detectable in trophoblasts from placentas of women diagnosed with PE, FGR, or spontaneous PTB [5-9].
Cultured primary human trophoblasts (PHT cells) are commonly used for studying placental cell injury [10, 11]. Considering the heterogeneity of obstetrical syndromes, defining placental injury-related molecular patterns may provide insight into the underlying pathologies [12 , 13]. Moreover, our recent study implied that multiomics-based placental clusters (including transcriptomics, proteomics, and metabolomics) faithfully recapitulate histopathological changes [14]. We therefore hypothesized that mRNA from PHT cells, exposed to distinct injuries in vitro, would capture transcriptome patterns of placental biopsies obtained from common obstetrical syndromes.
Methods
All placentas used for PHT cell isolation in our studies were obtained from uncomplicated term pregnancies and deliveries at Magee-Womens Hospital in Pittsburgh, under an approved protocol (#19120076, University of Pittsburgh). The PHT cells were dispersed and cultured for three days as we previously detailed [15, 16]. The timeline of exposure to altered O2 concentration or to ligands is shown in Fig. 1A. Exposure included Hams/Waymouth’s medium (H/W 50/50, Thermo Fisher, Waltham, MA), staurosporine (100 nM, Cell Signaling, Danvers, MA), or RSL3 (200 nM, Selleck Chemicals, Houston, TX) [8, 13, 16-19]. RNA extraction, library preparation, and sequencing processes were performed as we described [13]. Our RNAseq analysis of placental biopsies was recently detailed [14]. RNA count data were transformed to approximate log scale normal distribution through variance-stabilizing transformation using DESeq2 (v1.36.0), conditioned on trophoblast injury [20, 21].
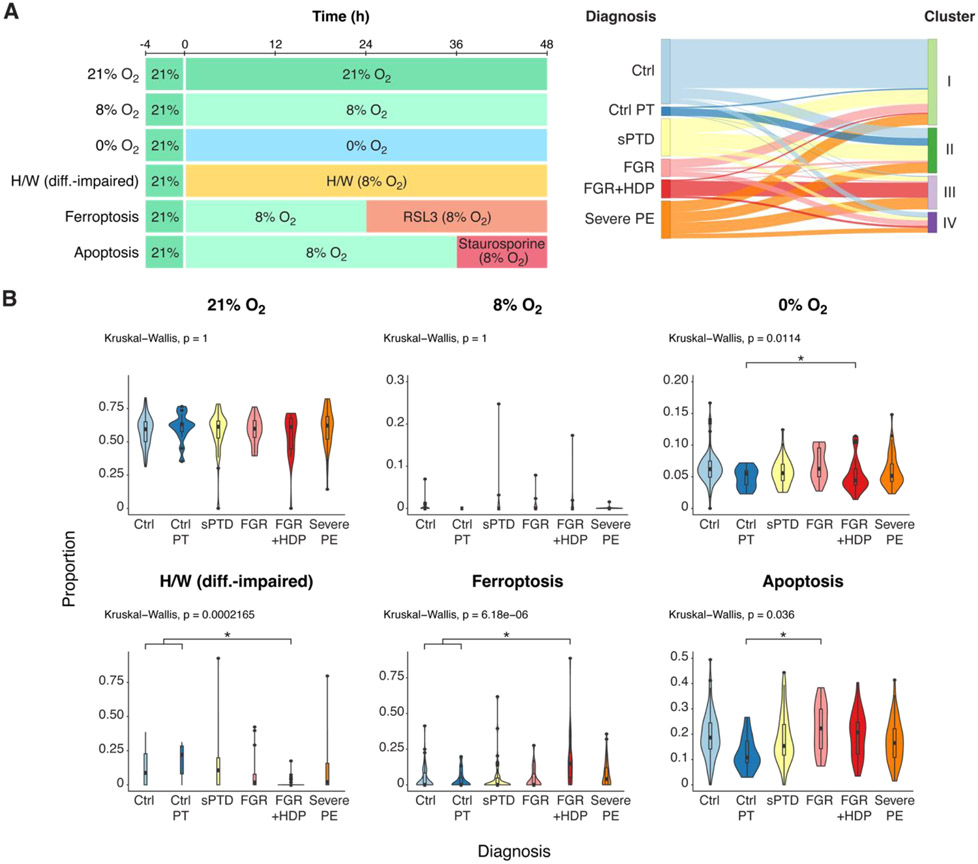
Figure 1. The effect of culture conditions on the representation of PHT cell transcripts among relevant clinical conditions .
(A) An overview of the datasets and experiments used in this study. Left panel: an illustration of the experimental design for PHT cell exposure. PHT cells, isolated from placentas of five uncomplicated term pregnancies, were initially cultured in 20% O2 for 4 h and subsequently exposed to varying oxygen conditions—8% O2, 20% O2, hypoxia at near 0% O2, or to H/W (differentiation-impaired) medium—for 48 h. Cells were exposed to RSL3 induction of ferroptosis (24 h) or staurosporine induction of apoptosis (12 h). At 48 h the cells were harvested for total RNA sequencing. Right panel: a Sankey plot depicting the allocation of placental biopsies from six clinical conditions into four molecular-based clusters (Barak et al, ref. 14), created on the basis of transcriptomic, proteomic, and metabolomic analyses, with Similarity Network Fusion analysis applied to identify clusters (Ctrl, Control; Control-PT, control preterm; sPTD, spontaneous preterm delivery; FGR, fetal growth restriction; FGR+HDP, fetal growth restriction with hypertensive disorder of pregnancy; PE, preeclampsia. (B) A violin plot depicting the effect of culture conditions on the representation of PHT cell transcripts among relevant clinical conditions. The x-axis defines the clinical diagnosis, and the y-axis delineates the proportion of transcripts defining each condition. P-values were calculated by the nonparametric Kruskal-Wallis test, with FDR controlling for multiple comparisons using the Benjamini-Hochberg method. For significant variables the Dunn’s post hoc test was performed. Ctrl, control; Control-PT, control preterm; sPTD, spontaneous preterm delivery; FGR, fetal growth restriction; FGR+HDP, fetal growth restriction with hypertensive disorder of pregnancy; PE, preeclampsia.
To correlate injury in PHT cells with placental tissue, we used CIBERSORTx [22] for bulk RNAseq deconvolution (Fig. S1). A signature matrix was constructed to represent unique gene expression patterns for each trophoblast injury. We performed differential expression analysis, and the first 194 differentially expressed genes (DEG) for each comparison, optimized on the basis of the most robust estimation of PHT injury signatures, were used to construct a signature matrix. The resulting matrix was used to estimate cell type proportions from the bulk placental RNAseq samples. We previously used a similarity network fusion (SNF) method to interrogate multiomics-based analysis of placental biopsies from different diseases [14].
Statistics and data availability
Differential expression analysis was performed with the lmerSeq package [23], using a linear mixed model. False discovery rate (FDR) was controlled using the Benjamini-Hochberg method. Differential expression with respect to SNF clusters was analyzed with the DESeq2 package [20], with FDR control as above. The significance of associations of trophoblast injury signatures, estimated by CIBERSORTx with clinical diagnoses or SNF clusters was assessed using the Kruskal-Wallis test and Dunn’s post hoc test. The family-wise error rate was controlled using the Holm-Bonferroni method. Our RNAseq data from PHT cells have been deposited in the NCBI Sequence Read Archive, Bio Project ID PRJNA995610.
Results and Discussion
To identify injury patterns in PHT cells, we cultured them in six conditions, including standard conditions in 21% O2 and 8% O2 as controls, 0% O2, H/W medium for hindering differentiation, staurosporine for induction of apoptosis, and RSL3 for ferroptosis [8, 17, 18, 24, 25]. Key DEG comparisons are shown in Table S1 and in Fig. S2.
We constructed a signature matrix to capture unique gene expression patterns that characterize each PHT injury (Table S2 and Fig. S1). Applying CIBERSORTx to the mRNA data from the six clinically defined placental biopsy (n=271) groups, the 21% O2 signature accounted for 55-60% of the transcripts across all disease paradigms (Fig. 1B and Table S3). The ferroptosis signature was particularly prominent in the FGR+HDP group. In contrast, placentas from pregnancies with isolated FGR correlated with apoptotic signature, consistent with previous results of higher apoptotic signaling in FGR placentas [26].
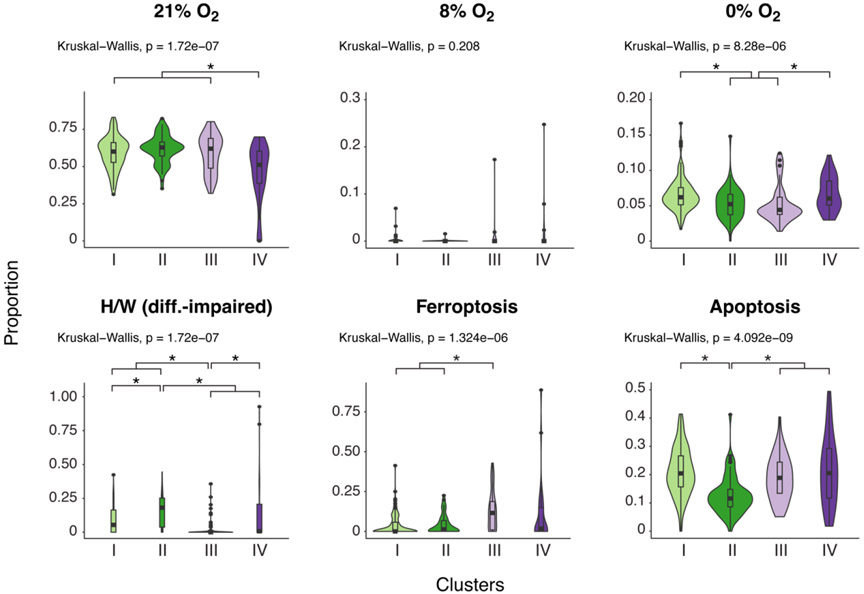
Our previous investigation harnessed SNF to reclassify the six clinically defined placental groups into four multiomics-defined clusters (Fig. 1A). The SNF clusters better correlated with the histopathology and were more distinctive of placental injuries [14]. We therefore compared the PHT injury signatures to placental biopsies that were analyzed using the unsupervised, multiomics-based SNF method (Fig. 2 and Table S4) and performed Bayesian model selection to compare the association of gene expression signatures in the six PHT culture conditions with clinical diagnoses vs. with SNF clusters. The Bayesian Information Criterion score was used to evaluate the posterior likelihood for each multiple linear regression model under a uniform prior. PHT injury signatures were better explained by the SNF clusters than by the clinical diagnoses (Bayes factor: 1.07 x1031). Notably, the differentiation-impaired (H/W) signature was highest in cluster II and lowest in Cluster III, effectively differentiating these from other clusters. Ferroptosis was prominent in cluster III, and 0% O2 mainly differentiated clusters I and IV from clusters II and III.
Figure 2. A violin plot depicting the effect of culture conditions on the representation of PHT cell transcript SNF clusters.
The x-axis defines the SNF clusters, and the y-axis delineates the proportion of transcripts defining each cluster. P-values were calculated by the nonparametric Kruskal-Wallis test, with FDR controlling for multiple comparisons using the Benjamini-Hochberg method. For significant variables, Dunn’s post hoc test was performed.
Our study was based on PHT cells, cultured under various conditions. Naturally, the in vitro settings may not fully capture the placental environment in vivo. Moreover, while single-cell RNAseq could have been used to define cell injury in the placental biopsies, we relied on integrated multiomics data, which remain challenging at the single cell level. Nevertheless, our work represents a stride toward a comprehensive understanding of placental pathophysiology, providing insights into the contribution of PHT injuries to placental syndromes.
Supplementary Material
Highlights.
We compared RNAseq matrices in cultured trophoblasts and in placental samples.
CIBERSORTx deconvolution method correlated in vitro and in vivo injury patterns.
Correlations were strongest in preeclampsia and fetal growth restriction clusters.
Acknowledgements
We thank Lori Rideout for assistance in manuscript preparation, and Bruce Campbell for editing. We thank Heather Sorenson for technical assistance.
Funding
The project was supported by grants from the Richard King Mellon Foundation Grant and an anonymous foundation (to YS), and National Institutes of Health (NIH) grants R01HL159805, R01HL157879, and R01DK130294 (PVB), and F31LM013966 (TL).
Footnotes
Declaration of Interest Statement
Statement: The authors certify that there are no financial interests that influence the results and findings obtained through this study. As stated on the title page, Yoel Sadovsky is a consultant to Bio-Rad Laboratories, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sadovsky Y, Jansson T, Placenta and placental transport function, in: Plant TM, Zeleznik AJ (Eds.), Knobil and Neill's Physiology of Reproduction, Academic Press, San Diego, 2015. [Google Scholar]
- [2].Burton GJ, Cindrova-Davies T, Yung HW, Jauniaux E, Hypoxia and reproductive health: Oxygen and development of the human placenta, Reproduction 161(1) (2021) F53–F65. [DOI] [PubMed] [Google Scholar]
- [3].Colson A, Sonveaux P, Debieve F, Sferruzzi-Perri AN, Adaptations of the human placenta to hypoxia: Opportunities for interventions in fetal growth restriction, Hum Reprod Update 27(3) (2021) 531–569. [DOI] [PubMed] [Google Scholar]
- [4].Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F, Inflammation and pregnancy, Reprod Sci 16(2) (2009) 206–15. [DOI] [PubMed] [Google Scholar]
- [5].Smith SC, Baker PN, Symonds EM, Increased placental apoptosis in intrauterine growth restriction, Am J Obstet Gynecol 177(6) (1997) 1395–401. [DOI] [PubMed] [Google Scholar]
- [6].DiFederico E, Genbacev O, Fisher SJ, Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall, Am J Pathol 155(1) (1999) 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beharier O, Kajiwara K, Sadovsky Y, Ferroptosis, trophoblast lipotoxic damage, and adverse pregnancy outcome, Placenta 108 (2021) 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beharier O, Tyurin VA, Goff JP, Guerrero-Santoro J, Kajiwara K, Chu T, Tyurina YY, St Croix CM, Wallace CT, Parry S, Parks WT, Kagan VE, Sadovsky Y, PLA2G6 guards placental trophoblasts against ferroptotic injury, Proc Natl Acad Sci USA 117(44) (2020) 27319–27328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT, Beyond oxygen: Complex regulation and activity of hypoxia inducible factors in pregnancy, Hum Reprod Update 16(4) (2010) 415–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crocker IP, Cooper S, Ong SC, Baker PN, Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction, Am J Pathol 162(2) (2003) 637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fuenzalida B, Kallol S, Zaugg J, Mueller M, Mistry HD, Gutierrez J, Leiva A, Albrecht C, Primary human trophoblasts mimic the preeclampsia phenotype after acute hypoxia-reoxygenation insult, Cells 11(12) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leavey K, Bainbridge SA, Cox BJ, Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia, PLoS One 10(2) (2015) e0116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chu T, Mouillet JF, Cao Z, Barak O, Ouyang Y, Sadovsky Y, RNA network interactions during differentiation of human trophoblasts, Front Cell Dev Biol 9 (2021) 677981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barak O, Lovelace T, Piekos S, Chu T, Cao Z, Sadovsky E, Mouillet JF, Ouyang Y, Parks WT, Hood L, Price ND, Benos PV, Sadovsky Y, Integrated unbiased multiomics defines disease-independent placental clusters in common obstetrical syndromes, BMC Med 21(1) (2023) 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3rd, Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae, Endocrinology 118(4) (1986) 1567–82. [DOI] [PubMed] [Google Scholar]
- [16].Nelson DM, Johnson RD, Smith SD, Anteby EY, Sadovsky Y, Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta, Am J Obstet Gynecol 180(4) (1999) 896–902. [DOI] [PubMed] [Google Scholar]
- [17].Oh SY, Chu T, Sadovsky Y, The timing and duration of hypoxia determine gene expression patterns in cultured human trophoblasts, Placenta 32(12) (2011) 1004–9. [DOI] [PubMed] [Google Scholar]
- [18].Douglas GC, King BF, Differentiation of human trophoblast cells in vitro as revealed by immunocytochemical staining of desmoplakin and nuclei, J Cell Sci 96 ( Pt 1) (1990) 131–41. [DOI] [PubMed] [Google Scholar]
- [19].Kajiwara K, Beharier O, Chng CP, Goff JP, Ouyang Y, St Croix CM, Huang C, Kagan VE, Hsia KJ, Sadovsky Y, Ferroptosis induces membrane blebbing in placental trophoblasts, J Cell Sci 135(5) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2, Genome Biol 15(12) (2014) 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Anders S, Huber W, Differential expression analysis for sequence count data, Genome Biol 11(10) (2010) R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA, Determining cell type abundance and expression from bulk tissues with digital cytometry, Nat Biotechnol 37(7) (2019) 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vestal BE, Wynn E, Moore CM, lmerSeq: An R package for analyzing transformed RNA-Seq data with linear mixed effects models, BMC Bioinformatics 23(1) (2022) 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chang CW, Wakeland AK, Parast MM, Trophoblast lineage specification, differentiation and their regulation by oxygen tension, J Endocrinol 236(1) (2018) R43–R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Crocker IP, Barratt S, Kaur M, Baker PN, The in-vitro characterization of induced apoptosis in placental cytotrophoblasts and syncytiotrophoblasts, Placenta 22(10) (2001) 822–30. [DOI] [PubMed] [Google Scholar]
- [26].Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM, Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression, Am J Obstet Gynecol 186(5) (2002) 1056–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.