Abstract
Repetitive or persistent cellular stimulation in vivo has been associated with the development of a heterogeneous B cellular population which exhibit a distinctive phenotype and, in addition to classical B cell markers, often express the transcription factor T-bet and myeloid markers CD11c. Research suggests that this atypical population consists of B cells with distinct B cell receptor specificities capable of binding the antigens responsible for their development. The expansion of this population occurs in the presence of chronic inflammatory conditions and autoimmune diseases where different nomenclatures have been used to describe them. However, due to the diverse contexts in which they have been investigated, these cells have remained largely enigmatic, with much ambiguity regarding their phenotype and function in humoral immune response, as well as their role in autoimmunity. Atypical B cells have garnered considerable interest due to their ability to produce specific antibodies/autoantibodies and with their association with key disease manifestations. Although they have been widely described in the adult context little is present for the pediatric side. Therefore, the aim of this narrative review is to describe the characteristics of this population, suggest their function in pediatric immune related diseases and chronic infections and explore their potential therapeutic avenues.
Keywords: Atypical B cells, B cells, CD11c+, T-bet+, Double Negative B cells, CD21low, pediatric diseases
Introduction
B cells constitute a critical arm of the immune system and are responsible for the short-term and long-term generation of humoral antibody responses. B cells also perform antibody-independent functions including antigen-presentation, modulation of T cell differentiation and survival, and production of both regulatory and pro-inflammatory cytokines1,2,3. The B cell lineage undergoes a maturation process resulting in considerable plasticity of the antibody response. The differentiation process results in the generation of two types of affinity-matured B-cells: memory B-cells (MBCs) and antibody- secreting plasma cells (PCs)4,5,6. Although the steps that underlie the activation and differentiation of antigen-engaged B cells have been extensively characterized, studies have revealed additional complexities to these responses, especially in the context of chronic immune stimulation. Indeed, over the past decade it has become increasingly evident that many chronic human infectious diseases as well as immune system disorders are associated with alterations in the composition of MBCs compartment. A common feature of these diseases appears to be a large expansion of a unique B cell subset, often denoted as Age-associated B Cells (ABCs), atypical, or pro-inflammatory 7,8,9,10. Since their initial discovery, the downregulation of both CD21 and CD27 and the expression of the Th1 master transcription factor T-bet and the integrin CD11c have become a well-known feature of this population, and hence, these cells are also now known as T-bet+ CD11c+ B cells 7 ,11,12. This novel population was classified as a memory cell due to their negligible expression of BCL6 and BLIMP1, which are hallmark transcription factors of germinal center (GC) B cells and PC, respectively13.Moreover, these cells were identified as MBCs based on the presence of additional markers, such as CD95 and CD62L, similar to classical MBC cells 14.
Atypical B cells (atBCs) proliferate following the exposure to innate and adaptive signals, in particular activation of the endosomal Toll Like Receptor (TLR)-7 and TLR-9 and cytokines like IFN-γ and IL-2112. This cell population displays a wide variety of functional abilities 8. They are potent antigen-presenting cells (APCs), can develop into plasma blasts (PBs), and generate antibodies in addition to having a greater propensity than other B cell subsets to produce proinflammatory cytokines and chemokines 7,15,16. It has been proven that this peculiar population has a significant role in various pathological conditions, ranging from immune system disorders, transplant rejection and inappropriate responses to chronic infections 17,18,19,20. Additionally, a recent study utilizing singlecell RNA sequencing revealed that a distinct group of atBCs are part of an alternative B cell lineage that participates in normal responses to vaccination and infections 21. As a result, researchers are giving more attention to this population. In this narrative review, we further discuss these cells in the context of various pediatric diseases and emphasize what is known about their genesis, differentiation, migration, and any potential roles they could play in the immune responses.
The many names of atypical B cells
Although this review focuses on the role of atBCs in pediatric conditions, it is necessary to specify that much information on their role, development, and functions is drawn from studies conducted on adult patients suffering from several diseases. Moreover, due to the broad range of pathological conditions in which these cells have been studied, it is not surprising that a wide range of terminology has been used to define this population. Initially described as ABCs based on their prominence in aging mice 22, these cells were then identified in young lupus-prone mice and demonstrated to be critical for viral clearance 23,24. Similar populations have been reported in humans and often lumped together as ABCs despite growing evidence for the presence of high degree of heterogeneity within these populations. Indeed, in many studies this population has often been defined based on the expression of one or more ABCs markers (preferably CD21low/−, CD11c+ or T-bet+) in MBCs. However, ABC-like populations have also been reported based on the expression of additional markers, in the contest of chronic infectious diseases such as FCRL4 and FCRL5 25,26,27. Lack of CD27 and CD21 has been frequently used to identify human ABCs during chronic or repeated Plasmodium infection, referred in this scenario as atypical B cells 28,29,30. Human immunodeficiency virus (HIV)-associated atBCs were first identified in 2008 by Moir et al as Tissue-like memory B cells (TLM), which resulted abnormally expanded in the blood of HIV-viremic patients 31. TLM have a similar CD21-CD27- phenotype as malaria atBCs, but also express high levels of several inhibitory receptors, including FCRL4, CD22 and CD85j. Due to the evidence of a profile of trafficking receptors (CXCR3, and CCR6) similar to those described for antigen-specific T cell exhaustion, they have also defined exhausted MBCs 31,32. In the contest of autoimmune diseases and immune system disorders other names have been used. Early studies in common variable immunodeficiency (CVID) detected a population of CD19hiCD21low CD11c+ cells that was aberrantly expanded in the subgroup of patients who develop autoimmune complications, especially autoimmune cytopenia 33,34. Reports in systemic lupus erythematosus (SLE) patients, identified atBCs population within a specific subset of Double Negative (DN) B cells, the so-called “DN2” (CD27−,IgD−,CD21−,T-bet+,CD11c+,CXCR5−). These cells have shown to be correlated with disease activity, autoantibodies production and renal manifestations 35. It is now generally considered that atBCs are a heterogeneous population, which might partly account for the lack of a uniform definition and the various phenotyping criteria applied among different groups, and because they represent different maturational stages differentiation according to the BCR isotype and the expression of CD27 36. Notably, despite their different maturation stages, a recent study demonstrated a similar global transcriptomic profile between circulating atBCs induced by infections (HIV and malaria) and autoimmune diseases (SLE, Rheumatoid arthritis) and immune system disorders (CVID)37. Common features characterizing atBCs generally encompass the downregulation of CD21, increased expression of CD11c, the presence of inhibitory receptors such as CD95, FCRL4, and FCRL5, the expression of the transcription factor T-bet as well as downregulation of receptors involved in B-cell survival and homeostasis (BAFF-R, CXCR4, CXCR5, and CCR7) 36, 38. Flow cytometric analysis of T-bet+CD21low B cells from individuals with autoimmune disorders and infections, not only supported the shared phenotype but also revealed a notable impairment in their signaling cascade following B Cell Receptor (BCR) activation as an additional shared attribute 39. Interestingly, a recent study, utilizing an in vitro B cell culture system highlighted a notable overlap in the regulation of CD11c and FcRL5 in response to BCR and TLR-9 activation. In contrast, T-bet expression demonstrated a strong dependency on IFN-γ signaling 40. These findings suggest that CD11c, FcRL5, and T-bet expression represent various stages of activation and underscore the importance of employing multiple markers when assessing the atBCs differentiation. Due to the inconsistency of T-bet+CD21low B cells nomenclature across studies (Table 1) and the limited evaluation in the pediatric setting, we defined this population as atBCs based on the shared markers most frequently found in the scientific literature. Altogether, based upon current knowledge, we suggest considering atBCs as identified by the following markers: CD19+,CD27−, IgD−,CD21lo, CD11c+,T-bet+.
Table 1.
Numerous designations for atBCs. Selected examples of the diverse terms employed to characterize cells exhibiting atBCs-like features in healthy and pathological conditions.
| Condition | Name | Location | Phenotype | Additional markers |
T bet | Proposed functional role |
Disease association | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Adult | |||||||||
| Healthy subjects | Tissue-resident | Tonsil | CD19+IgD− CD27−CD38− |
FcRL4+ CD11c+ | − | 13 | |||
| Immune system disorders | Systemic Lupus Erythematosus | DN2 | PB/Kidney | CD19+IgD− CD27−CD21− |
CXCR5−FcRL5+ | + | Precursor of extrafollicular ASCs | Autoantibodies, disease activity, Lupus nephritis |
35
64 131 |
| Rheumatoid Arthritis | PB/SF | CD19+IgD− CD27− CD21low |
NA | Joint destruction in ACPA+/RF+ patients | 117 | ||||
| Primary Sjogren Syndrome | PB | CD19+CD27− CD21low CD38low |
CD11c+ | + | Anergic autoreactive memory cells | Associated with lymphoproliferation | 118 | ||
| Systemic Sclerosis | PB | CD19+CD27− CD21lowCD38low |
CD11c+ | NA | Disease activity, vascular complication | 119 | |||
| Multiple sclerosis | CD21low | PB/CSL | CD19+IgD−CD27− CD21low |
CD11c+ | Switched memory | Correlated with the presence of brain inflammatory lesions. |
120
121 |
||
| CVID | CD21low | PB/BAL | CD19+IgD+CD27− CD38lowCD11c+ |
FcRL4+ | + | Splenomegaly and autoimmune manifestations |
33
142 |
||
| Infectious diseases | Malaria | Atypical | PB | CD19+CD27−CD21− | CD11c+ CXCR5− FcRL5+ |
+/− | Precursor of antigen-specific ab, auto-abs to red blood cell | Associated with anaemia |
99
100 |
| HIV | Exhausted, tissue-like | PB | CD27− CD21− | CD11c+ | + | Exhausted memory cells | HIV-specific Ig |
31
60 |
|
| COVID-19 | Atypical | PB | CD27− CD21− | CD11c+ | + | Morbidity | 90 | ||
| Other conditions | Obesity | Aged-Adipose B Cells | Adipose tissue | CD19+IgD−CD27− CD21− |
CD11c+ | + | Precursor of extrafollicular ASCs | Autoantibodies production Exacerbates metabolic disorder in obesity |
151-153 |
| Vaccination | Atypical | PB | CD19+CD20lowIgD− CD27− |
CD11c+ CXCR3+ |
NA | primary response to antigen vaccine and respond to booster immunization | Induced after vaccination against different pathogens |
21 73, 115 |
|
| Children | |||||||||
| Healthy children | PB | CD19+CD27−CD21− | NA | 55 | |||||
| Immune system disorders | CVID | CD21low | PB | CD19+CD27− CD21low |
NA | enteropathy and autoimmune symptoms | 143,144 | ||
| Systemic Lupus Erythematosus | PB | CD19+CD27− | CD11c+ | + |
135
136 |
||||
| Juvenile idiopathic Arthritis | PB/SF | CD19+IgD−CD27− CD21− |
CD11c+ | NA |
140
141 |
||||
| Infectious diseases | Malaria | Atypical | PB | CD19+CD27−CD21− | CXCR3+ CD86+ FcRL5+ |
+ | Precursors of ASCs | May contribute to humoral immunity to malaria | 15 |
| HIV | DN | PB | CD19+IgD− CD27− | NA | Exhausted memory cells | Negative correlation with immune response after seasonal influenza vaccination. Negative correlation with time under antiretroviral therapy |
109
110 112 |
||
| Respiratory Syncytial virus | Atypical | PB/Adenoid | CD19+IgA−IgG− CD27− |
NA | Produce RSV neutralizing antibodies in adenoid tissue | 85 | |||
| Other conditions | Trisomy 21 | PB | CD19+CD27−CD21− | CD11c+CXCR5− CXCR3+ |
+ | More likely to have self-reactive features | Correlated with cytokine levels, plasma IgG and pCs | 161 |
Abbreviations. ACPA: Anti-citrullinated protein antibodies; ASCs: Antibody-secreting cells; BAL: bronchoalveolar lavage; CSF: Cerebrospinal fluid; DN: Double Negative; SF: synovial fluid; ND: not available; PB: peripheral blood; RF: Rheumatoid Factor
B cells subsets and atBCs modification during childhood
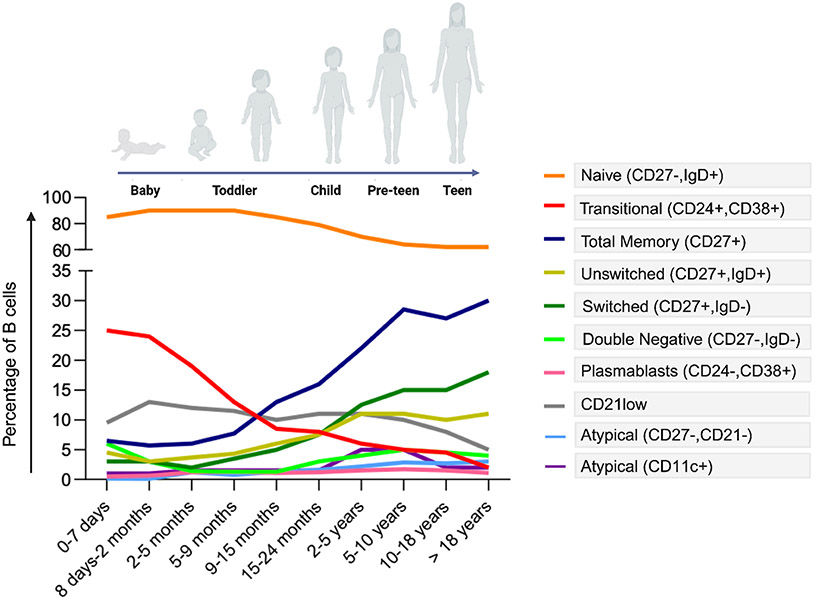
Changes in the composition of the peripheral B-cell pool occur in the first 5 years of life when children encounter a multitude of different antigens 41,42. A recent meta-analysis encompassing 28 studies has reported significant fluctuations in B cells whitin the first year of life 43. According to this report, the changes in B cell levels can be summarized as follow: 1) initially, B cell levels decrease from cord blood to the first week of life, 2) subsequently, there is a rapid increase over the next two months; 3) B cell levels continue to expand until they peak at approximately six months of age; 4) after reaching their peak, B cell levels gradually decline and 5) and may plateau at around 10-12 months of age. Literature evidence suggest that this plateau might extends to the second year of life, followed by a gradual decrease until adulthood, where levels remain relatively stable 44,45,46. The initial decline in B cell numbers from cord blood to the first week of life is believed to be linked to significant phenotypic changes in B cells during the initial days of life. This is marked by a temporary reduction in transitional and naive B cells, without a corresponding expansion in other B-cell subsets 44,47,48 (Figure 1). Then, B cell levels rise until 6 months of life, when immature/transitional and naive B-cell subsets reach their highest levels ever. Thereafter, following exposure to foreign antigens, the size of MBCs and PCs increases, while the proportion of naïve B cells gradually decreases, starting around 18 months of age 41,44,45,48. In the first weeks of life, CD27+IgD+ unswitched MBCs constitute the largest subgroup within the MBCs compartment. However, continuous exposure to foreign antigens leads to a reduction in the size of this subgroup during childhood, stabilizing in young adults. Conversely, as children age, the number of switched MBCs slowly increases, progressing from around 0.3% in early life to approximately 12% of total B cells at 3 years of age 41,45,49,50.
Figure 1.
Variations in B Cell Populations, encompassing atypical B cell subsets, during childhood and adolescence (adapted from references 42, 46, 48, 55)
Moving to atBCs, it has been observed that during the first year of life there is an increase of proportion of MBCs which lacked CD21 (C3d receptor) 48. Although CD21− B cells are considered by many authors as part of atBCs scenario, it should be noted that the CD21 downregulation by might be not associated to chronic inflammatory process but rather to a limited availability of C3d and C3d-antigen complexes 51. Indeed, the reduced serum levels of C3 in infants less than one year may contribute to less signaling for the expression of the CD21 receptor during antigen recognition 52. Blanco and colleagues reported that the proportion of atBCs (identified here as CD27−CD21−) increases and peaks during the first five months of life reaching about 10% of total MBCs. Interestingly, the majority of this population was IgG3+, aligning with previous research indicating that the expression of the transcription factor T-bet regulates the immunoglobulin isotype switching to IgG3 in humans 53,54. After the first year of life, Jalali et al. observed a gradually decrease of CD11c+ atBCs to ~1.4% in children aged 3-4 years 50, which then raises again at 5-9 years of life reaching approximately 5% of B cells in the peripheral blood of healthy children. This proportion decrease in adults to ~1.0%. Considering DN B cells, this study revealed that they constituted approximately 3% of total B cells during the first 4 years of life, increasing to around 12% in the 5–9-year age group, and maintaining elevated levels over time (>10%). This is in contrast with previous studies reporting that this percentage was around 5% 7,48,55. An extensive analysis on a large pediatric cohort revealed that the percentage of atBCs in healthy children ranges between 0.1% and 5.2%. This analysis also highlighted that within the entire cohort, the most abundant subsets in atBCs were IgM+IgD+ and IgM+ 55.
Atypical B cells ontogenesis and route of differentiation
Atypical B cells primarily represent antigen-experienced MBCs, characterized by isotype switching and expression of BCRs that have undergone somatic hypermutation (SHM), but the precise origin of this population in humans is still uncertain (Figure 2). Indeed, a significant number of CD21lowT-bet+ or CD11c+ B cells exhibit an unswitched BCR 56, suggesting they may originate from naïve B cells. This is further supported by BCR sequencing, which reveals some shared repertoire and gene characteristics between naïve B cells and unswitched CD21low B cells 57. Moreover, this idea is supported by the observation that atBCs in Plasmodium-exposed Malian children could be separated into IgD−IgG+, IgD+IgM+ and IgD+IgMlow subsets with SHM rates equivalent, respectively, to classical MBCs (suggesting GC and classical MBC origin), naïve B cells (suggesting a naïve B cell origin) and intermediate between naïve and classical MBC (suggesting T-B border origin) 37.
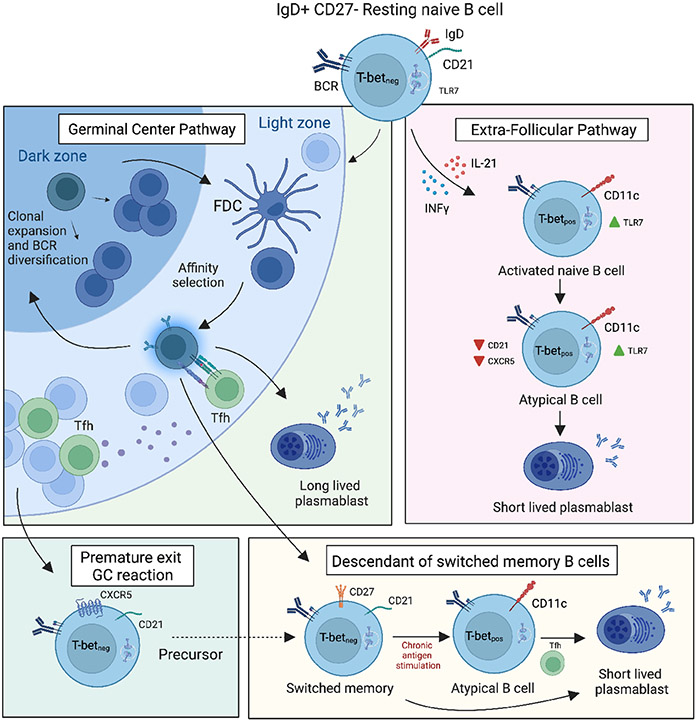
Figure 2.
Possible origins of atBCs during persistent antigen stimulation. Multiple pathways by which atypical B cells arise have been proposed: (1) via an extrafollicular differentiation pathway; (2) via premature exit from the germinal center reaction; (3) via an altered lineage pathway compared with classical memory B cells. Dashed lines indicate potential developmental pathways that need further investigation.
Most atBCs cells show signs of a GC reaction, namely in their Ig isotype-switched phenotype and somatically mutated Ig genes 58,59. The presence of SHM in atBCs does not prove their origin in the GC, although it may suggest it. In the case of HIV, these cells were found to have a clonal relationship with GC B cells, but with fewer SHM and reduced neutralization capacity 36,60. This suggests that they may either originate from common progenitors that follow distinct differentiation pathways or that atBCs may exit the GC response at an earlier stage. After influenza as well as SARS-CoV-2 vaccination, SHM rates were similar to classical memory and clonal relation was interpreted as post GC B cells 36,61,62. Although it could potentially develop within GCs, extrafollicular pathways (EF) have been suggested in some pathological conditions, such as SLE 35. Indeed, both EF and GC B cells can undergo class-switch recombination and SHM. Jenks et al. identify various characteristics of atBCs, observed in SLE patients, which are indicative of EF differentiation. These features include the absence of CXCR5 and CD62L, a chemokine receptor responsible for migration to secondary lymphoid organs, and crucial for lymph node trafficking, respectively 35. Notably, the EF pathway has been proposed also for CVID, HIV and toxoplasmosis, where atBCs were observed to accumulate outside GC 60,63. Of note, atBCs could also be GC independent, but carry high levels of SHM if they arose from GC-experienced classical MBCs. This idea was supported by the observation that secondary vaccination or infection can induce stronger CD11c+ atBCs production than the primary response 21,56.
While it may be tempting to claim that CD11c+, T-bet+ atBCs originate from a single source and follow a singular differentiation pathway, the EF pathways and GC development are not mutually exclusive. Moreover, it is plausible that the inflammatory conditions largely dictate the specific pathway chosen. Elsner and Schlomchik have further elaborated on this matter, proposing that elevated levels of IFN-γ hinder Tfh development and subsequent GC responses, leading to differentiation via the extrafollicular route 64. Conversely, lower levels of IFN-γ may permit Tfh-mediated differentiation of T-bet+ GC B cells.
The development and persistence of atBCs rely on T cells and IL21R, where these two pathways are likely not mutually exclusive and may have varying impacts across different disorders. Although a stronger involvement of TLR 7/8 and 9 signals has been suggested in the context of SLE 35,65, investigations on patients with monogenic inborn errors of immunity have revealed the critical importance of IFNγR and NF-κB signaling for the differentiation of human atBCs cells, both in vitro and in vivo 66. IFN-γ is a T helper type 1 (Th1) cytokine, which upon binding to the IFNγR on B cells activates the JAK-STAT signaling pathway, resulting in up- regulation of the transcription factor T-bet67. These findings strongly suggest a unique role of T cell assistance, as evidenced by the reduced presence of atBCs in patients with deficiencies in IL21R, CD40, or CD40L 66. For these reasons both T peripheral helper cells in inflamed tissues and Tfh cells with a Th1 profile in secondary lymphoid tissues emerge as excellent candidates for delivering the necessary factors for their alternative B cell differentiation. Interestingly, it was observed that atBCs exhibited normal development in patients lacking MyD88 and IRAK4 which suggests that the classical TLR 7/8/9 signaling pathway is not essential for the formation of atBCs in humans 66. In addition, the evaluation of the first reported T-bet deficient patient highlighted the crucial role of the T-bet for the differentiation of atBCs even if other transcription factors play a critical role in influencing the gene expression of additional characteristic markers 68.
Transcriptional profile of atBCs
T-bet, encoded by TBX21 gene, is often considered a key transcription factor for atBCs formation. Perhaps the best described role of T-bet in the humoral immune system is to regulate antibody class switching to IgG2a/c in mice and IgG1 or IgG3 in humans9. But other roles have also been highlighted. In infection models, expression of B cell T-bet had a greater impact on the control of chronic than acute viral infections and, while not necessary for the initial phase, was required for optimal protective humoral responses 69. Moreover, T-bet in B cells was linked to the expression of CXCR3 or S1pr5, which control the migration and tissue residency of immune cells 70,71. Thus, the expression of T-bet may be particularly important in regulating the trafficking and homing patterns of atBCs and ensuring their proper colocalization with other effector cells. In addition, compared to other B cell subsets, atBCs usually upregulates the integrin CD11c (encoded by ITGAX) as well as other myeloid markers like FcγRs, CD14, CD68, CD163 hinting that these cells employ unique trafficking patterns and can target distinct microenvironmental niches 12,72,73. Notably, CD11c emerges as a valuable marker for identifying atBCs, as highlighted in a recent cytometry study by CyToF examining 351 surface molecules on human circulating B cells 74. Moreover, these cells exhibited elevated expression of inhibitory markers (CD95, FCRL4, and FCRL5), activation-related molecules (TACI, CD80, and CD86), as well as downregulation of receptors involved in B-cell survival and homeostasis (BAFF-R, CXCR4, CXCR5, and CCR7) 17,38.
As mentioned above, human T-bet governed atBCs differentiation by controlling chromatin accessibility of lineage-defining genes in these cells: FAS, IL21R, SEC61B, DUSP4, DAPP1, SOX5, CD79B and CXCR4 68. IRF5 and ZEB2 had also been reported to be required for atBCs formation in the SWAP-70 and DEF6 double knockout lupus model72 75, as excellently reported in the ref12. The functional outcomes regulated by T-bet become more intricate as atBCs differentiate into various effector progeny, including GC B cells and PBs/PCs, due to the interplay between T-bet, BCL6, and BLIMP1 76. Research by Pernis' group indicates that certain CD11c+ effector progeny, like PBs, can exhibit a core atBCs transcriptional profile even when T-bet expression is downregulated. This finding supports the notion that an "atypical signature" can persist in the absence of this transcription factor 12,77. Indeed, using RNA sequencing, Wang et al. noted that CD11c+ B cells in SLE had upregulated genes associated with antibody-secreting cells (ASCs) differentiation, such as PRDM1, AICDA, XBP1, BMP6 78. Similarly, Golinski and colleagues discovered that a greater proportion of CD11c+ B cells underwent differentiation into ASC after 7-day culture with BCR ligation, TLR-9-ligand, and IL-21 79. Characterization of in vitro responses of human CD11c+ B-cell subsets by Steuten et al revealed that different CD11c+ B cells yielded ASCs as well as CD138+ PCs in response to stimulation with CD40L/IL-21 16. The capacity of distinct CD11c+ B-cell subsets to produce ASCs in vitro aligns with previous observations indicating that CD21low B cells possess a transcriptional profile indicative of pre-plasma cells, characterized by elevated expressions of BLIMP1, XBP1, IGJ, IL6R, and TNFRSF17 (BCMA), along with diminished levels of BACH2 73.
These findings contrast to the initial reports conducted in patients with chronic diseases, which did not demonstrate the capacity of this cell population to differentiate PBs/PCs. This could be attributed to intrinsic differences between autoimmune and infectious diseases. Additionally, it is important to consider the influence of experimental conditions and the specific cell types (according to the phenotype) studied, as these factors can contribute to the observed discrepancies.
Moreover, a recent study provides additional insights into a poorly investigated role of atBCs as APC, a function previously observed in mice where these cells exhibited superior antigen presentation to T cells compared to FO B cells 80. Kleberg et al. demonstrated that atBCs could enhance CD4+ T cell survival and proliferation through IL-6 production. Surprisingly, this capacity was not clearly associated with T-bet levels but rather to the BCR. However, this connection was not clearly dependent on specific levels of other atBCs markers, such as CD11c or FcRL5. Therefore, additional research is required to delve deeper into this topic.
Atypical B cells in infectious diseases.
Atypical B cells has been identified in the contest of several infections such as: HIV 31,54, malaria 15,37, HBV81, HCV 82, tuberculosis 83, SARS-CoV-2 84, Respiratory Syncytial Virus 85, Dengue 86, Influenza 87, but its role changes depending on whether the infection is acute or chronic. For instance, an expansion of atBCs during natural acute infections and vaccinations, has been linked to various useful functionalities. Eccles et al. demonstrate that the acute phase of human Rhinovirus infection coincided with local rapid expansion of T-bet+ B cells and with their secretion of cross-reactive IgG 88. On the other hand, excessive expansion of atBCs during acute infections has been correlated with pathogenic responses. Indeed, in severe COVID-19 patients atBCs have been associated with poor outcomes, high mortality rates, and with the production of autoantibodies 89,90,91 65. In contrast, in chronic infections like HIV, HCV, and malaria, atBCs expression of many inhibitory receptors (FcRL4, FcRL5, CD85j and CD22) and the refractoriness to stimulation through their BCR, TLRs, CD40 and cytokine receptors have been suggested to be a critical aspect of the ineffective immune responses known to accompany these infections 8,92. Alternatively, the anergic nature of these B cells may also be beneficial to protect against a potentially damaging immune response.
Malaria
Children generally mount short-lived antibody responses to Plasmodium falciparum (Pf) infection, leaving them susceptible to repeated bouts of malaria 93. As a result, most cases of malaria occur in children under the age of 10, while adults with life-long exposure have asymptomatic infections. atBCs can represent up to 20% of the circulating B cells in children living in malaria-endemic areas and in children persistently exposed to malaria 27,94. A longitudinal analysis of Pf infected children has suggested a positive correlation between the incidence of febrile malaria and the expansion of T-bet B cells via Th1 cytokines 95. Malaria may potentially impact the B cell compartment also by affecting the B cell repertoire, although this area of research has not yet been thoroughly explored. One study examined the V gene repertoires of naïve B cells, atBCs, and MBCs and found that the VH and VL repertoires of classical MBCs and atBCs had similar V gene usage, SHM rates, VH CDR3 length and composition 96. Using an accurate and high- coverage Ig sequencing method, the same researchers group found unexpected high levels of SHM in infants as young as 3 months 97. Antibody lineage analysis showed that SHM also increased in both infants and young children upon febrile malaria.
Atypical B cells have been hypothesized to be exhausted or dysfunctional based on their increased expression of inhibitory receptors, such as CD22, CD85j, and FcgRIIB, and homing receptors, such as CD11c, CCR6, CXCR4, and CXCR3 95. In addition, these cells have reduced responsiveness to restimulation of sorted human CD21lo FcRL5+ or FcRL4+ B cells 98. Works by Crompton’s group highlighted FcRL5 as an inhibitory indicator on atBCs as FcRL5hi expressing B cells were less responsive to BCR stimulation and showed a key role of T-bet, which correlates inversely with BCR signaling and skews toward IgG3 class switching 28,95. Muellenbeck et al. showed that these cells were enriched for self- or polyreactive BCR specificities, suggesting that they could be anergy in order to safeguard the host from autoimmune reactions 99. Indeed, in some patients (including children) with acute malaria, the expansion of atBCs cells correlates with the production of autoantibodies against phosphatidylserine, contributing to the development of anemia 100.
Although atBCs cells can appear dysfunctional, one report provided evidence of Pf-specific Ig transcripts produced by atBCs in vivo and showed that broadly neutralizing Pf-specific antibodies can be cloned from atBCs 99. In addition, atBCs expand in response to Pf sporozoite vaccination 21 . According to Ambegaonkar et al., atBCs can still contribute to the production of protective antibodies 101. The authors proposed that inhibitory receptors, particularly FcgRIIB, were responsible for restricting the responsiveness of CD21lowCD27lo B cells to soluble antigen. However, when the BCR ligand or antigen was presented to the cells while fixed in a lipid bilayer, FcgRIIB was removed from the immunological synapse, making it possible for CD19 to engage with the BCR 101 (Figure 3).
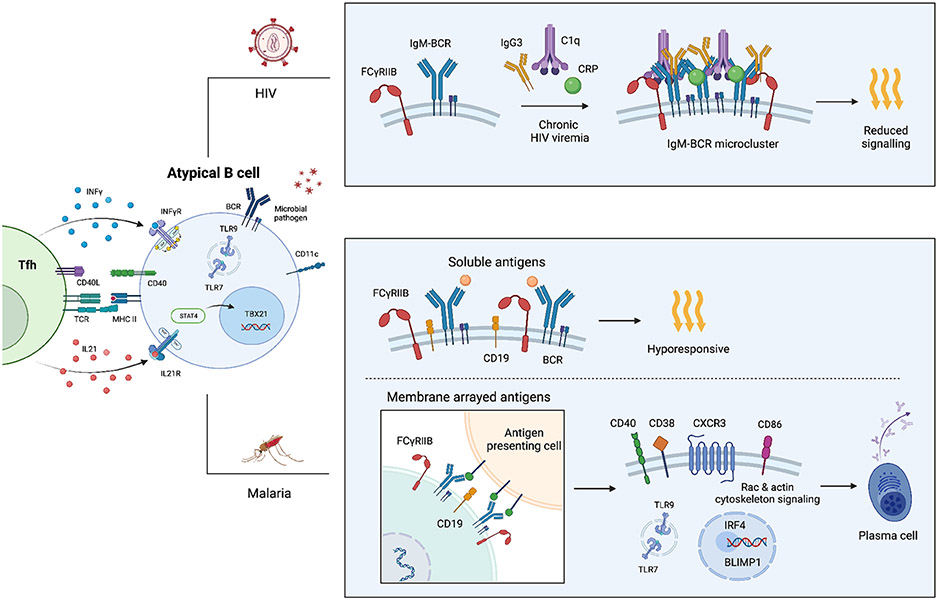
Figure 3.
During chronic HIV-1 viremia, the inhibitory Fc receptor FcgRIIB (CD32b) rises and becomes linked to micro clusters of IgG3−IgM−BCR, in addition to C1q and c-reactive protein (CRP). This clustering predominantly takes place in atBCs and involves direct engagements between IgG3 and the IgM− BCR, leading to a reduced intracellular signaling (106). In the context of malaria, the responsiveness of atBCs relies on the way the antigen is presented. atBCs that express CD11c, T-bet, and FCRL5 also exhibit heightened expression of inhibitory receptors, such as FcgRIIB. When the BCR is bound, FcgRIIB diminishes the interaction between CD19 and the BCR, thus impeding downstream signaling and resulting in reduced responsiveness. Conversely, atBCs cells that bind to antigens arrayed on the cell membrane establish an immunological synapse that excludes FcgRIIB. This exclusion enables CD19 to effectively engage the BCR and facilitate downstream signaling, which subsequently triggers the transcription of IRF4 and BLIMP1. This process promotes differentiation into antibody-secreting cells. In addition, atBCs may capture membrane bound antigen for presentation to Tfh cells. Acute malaria may also prime atBCs to respond to TLR-7/9 which together with IFNγ may contribute to T-bet expression (15).
Recent research has indicated that atBCs may actively contribute to humoral immunity to infectious pathogens. Hopp and colleagues found that in response to acute malaria, Pf-specific atBCs of Malian children are activated, with increased frequency and up-regulation of molecules (CXCR3, CD86) that mediate B-T cell interactions 15. Consistent with this ex vivo findings, the authors found that atBCs upregulated PRDM1 and the activation PCs marker CD38 when co cultured with autologous Tfh cells from malaria-exposed individuals, suggesting that atBCs may actively contribute to humoral immunity to infectious pathogens. Recently, Reyes et al, showed that CXCR3 and CD95 atBCs expression was higher in adults than children suggesting that this marker is acquired as a result of chronic antigen exposure and should probably be considered a marker of activation102. Moreover, the study unraveled through a single cell sequencing and BCR analysis that atBCs cells, in malaria setting, contribute to a productive and antigen specific immune response against infection.
HIV
HIV infection exerts a significant impact on the B cell compartment, resulting in marked changes in cell phenotype and functionality 32, 103,104. B cells lacking CD21 and CD27, but expressing CD11c and FcRL4, appear in association with HIV viraemia, are more frequent in viremic compared with non-viremic patients, and decreased with antiretroviral treatment (ART) 31, 32, 103. A recent study analyzing lymph nodes has shown that HIV-specific B cells in infected individuals were enriched among CD19+Tbethi B cells and that this population was not present in healthy individuals60. This subset exhibits a weak response to BCR stimulation and expresses inhibitory receptors, resulting in decreased capacity for proliferation, affinity maturation, and secretion of cytokines or antibodies 60 105. However, Knox and colleagues revealed that during HIV infections in adult patients the specific HIV gp140 response is dominated by expanded atBCs 25. Atypical B cells dysfunction is seemed to be associated with the binding of soluble IgG3 to IgM-expressing B cells, along with C1q and, the inhibitory Fc receptor CD32b (also known as FcRγIIB), which led to an increased clustering of the IgM BCR and decreased response to stimulation 106. In line with this “exhausted” status, our group showed a positive association between atBCs, and plasma complement cascade proteins in children 107. Additionally, our group's studies have indicated that the atBCs expansion in HIV infected pupils is associated with a decreased ability to respond to childhood influenza and MMR vaccinations 107,108,109. Recently, we investigated the evolution and maturation of the B cell compartment over the first two years of life in children with perinatal HIV infection. We observed an expansion of atBCs at 40 days of life in these children, which may contribute to B-cell exhaustion 110. Indeed, in our study, children with perinatal HIV infection and uncontrolled viral replication exhibited a diminished capacity to sustain protective tetanus antibody titers over time.
Notably, in pediatric HIV infected children, a longer time on ART is related to lower atBCs while an earlier start is associated with lower frequencies of mature activated B cells (CD19+CD10−CD21−)111,112,111.
The poor response to BCR stimulation had led to the original designation that atBCs was anergic or exhausted cells. Recent discoveries, especially in the malaria field, suggest that current in vitro investigations may not have adequately replicated the in vivo functionality of this population. To better mimic their natural function, it is crucial to consider additional factors such as cytokines, B cell activating factor (BAFF), TLR-ligands, and various forms of costimulation.
Atypical B cells in vaccine induced responses
Recent evidence suggests that atBCs play a significant role in the adaptive response triggered by vaccines in healthy adult individuals 21, 114. Steuten and colleagues undertook a dedicated endeavor to provide a more comprehensive understanding of these cells in the context of immunization using SARS-CoV-2 mRNA vaccines 16. Their investigation unveiled a substantial increase of atBCs, exhibiting a remarkable 20- to 40-fold increase post SARS-CoV-2 vaccination. Interestingly, their study highlighted variations across distinct CD11c+Tbet+ B cell subsets. The expansion of spike-specific CD11c+ B cells was primarily orchestrated by the DN2 (CD11c+, IgD−,CD27−) and ABCs (CD11c+) subsets, which exhibited robust expansion shortly after the second vaccination, followed by subsequent contraction 16. These findings on SARS-CoV-2 immunization align with those documented in studies about seasonal influenza 73,115 and tetanus vaccinations 116. In their study Lau et al, demonstrated that atBCs emerged as the predominant subset among hemagglutinin-specific B cells, maintaining their dominance for an extended 60 days period following vaccine boost 73. Furthermore, Sutton and colleagues established that B cells with an atypical transcriptional profile emerge during the primary immune response to vaccination and can be reactivated upon subsequent exposure, as evidenced through influenza vaccine challenges and sporozoite immunizations 21. However, investigation of atBCs transcriptome have unveiled that these cells do not exhibit spontaneous antibody secretion and were primed for PC differentiation while exhibiting resistance to further differentiation in the GC 73.
Despite these results which highlight that atBCs are part of a normal B cell antigen-response 21,27,73,115, 116, the relevance of antibody production by this population in the infection control is less clear. A fundamental issue that remains unresolved is whether atBCs yields antibodies with distinct qualitative properties compared to those generated through the conventional GC- pathway.
Of note, in the first T-bet-deficient patient, the vaccination response against the investigate bacterial antigens seemed normal but respiratory viruses (Influenza, SARS-CoV-2) response was not investigated in this patient 68.
Additionally, the consequences of accumulated atBCs in vaccine-induced immunity in children with chronic inflammation remains unclear. This uncertainty primarily stems from the lack of dedicated research on healthy children and reports in HIV-infected children. Indeed, HIV infected children with an expansion of atBCs, demonstrate a compromised vaccine response 108. Thus, it is plausible that the inflammatory context makes them anergic or exhausted as previously suggested 32.
These disparate observations may also stem from the existence of various subsets of atBCs with differing effector functions, stages of maturation or attributable to differences in context in which have been investigated. Further investigations into the role, breadth, and dynamics of atypical B cells in the context of vaccination among healthy children and adults are warranted.
Atypical B cells in systemic immune disorders
The connection between atBCs and autoimmunity has been firmly established and widely acknowledged. This population has been found elevated in adults patients affected by Rheumatoid Arthritis 117, SLE 35,78, primary Sjogren Syndrome 118, Systemic Sclerosis 119, ANCA-associated vasculitis 39, Multiple Sclerosis 58,120,121, Crohn’s disease 122, Graves’ Disease 123, Hashimoto’s thyroiditis 124, Myasthenia Gravis 125 and Guillain-Barrè syndrome 125. Furthermore, research linking atBCs to autoimmune and inflammatory diseases indicates that TLR-7/9, IFN-γ, and IL-21 play crucial roles in enabling differentiation into PBs 7,59. Moreover, atBCs have been linked also to several immunodeficiency disorders especially CVID33,34, Ataxia-Telangiectasia126, Wiskott-Aldrich syndrome 127, IgA deficiency 128, Chronic granulomatous disease 129 and partial RAG deficiency 130 but their role in this conditions, is still controversial.
Autoimmune diseases
Systemic Lupus Erythematosus
SLE is a chronic autoimmune disease characterized by the production of autoantibodies and a wide spectrum of clinical manifestations. In this scenario, atBCs can contribute more than 50% of all B cells in active SLE and may become the largest circulating population of isotype switched IgD− cells, also in young children with active disease 35. In this scenario, atBCs have been identified as DN2 cells (CD27−, IgD−,CD38low,CD11c+,CXCR5−,FcRL5+,FcRL4−,T-bet+) or as CD27−,CD38low, CD11c+, FRL5+;FcRL4+,T-bet + and have been shown to be major producers of autoantibodies (anti-Sm, anti-RNP), and their accumulation has been demonstrated to correlate with disease activity and severe clinical manifestations, such as lupus nephritis 35,78. Moreover, these cells have been identified not just in the peripheral blood but also in areas of organ injury, such as the kidneys 131,132,133. The transcriptional profile found higher expression of IRF4 and lower expression of IFR8 compared to other B cell subsets, indicating the tendency towards differentiation into PBs/PCs 134. Although atBCs have been extensively studied in SLE mouse models and adult patients there is very few data on pediatric populations. Corrente and colleagues reported an increase of atBCs (CD21low CD11c+ B cells) in children with immune system disorders, including SLE 55. A recent study revealed a notable increase in T-bet-expressing naïve B cells and DN B (CD21−,CD11c+) cells in patients with childhood SLE, as opposed to healthy children 135. Approximately half of T-bet+ B cells displayed an activated phenotype, characterized by CD21 negativity and CD11c positivity. The expression of T-bet is induced specifically by IFN-γ and not by IFN-α and define a patient population with higher disease severity, higher frequency of ENA and anti-dsDNA positivity and higher proportion of proliferative lupus nephritis 135. In another study, a multi omics approach combined with an unsupervised hierarchical clustering analysis was performed on children with SLE and resulted in the identification of clusters of patients with distinct biological phenotypes associated with disease activity states 136. In this regard, atBCs were increased in the group of patients with high cytokine profile and high gene expression.
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood affecting not only joints but also extra-articular structures such as eyes, skin, and internal organs. Although the pathogenesis is still unexplained, the occurrence of autoantibodies (e.g., Antinuclear antibodies, ANA) in a significant proportion of patients suggests the involvement of autoreactive B cells 137,138. A recent study investigated the differences in B cells among ANA+ JIA patients by analyzing the distribution of B cell subpopulations in peripheral blood and synovial fluid (SF). Increased frequencies of atBCs (CD21lo/−CD27− IgM DN2 B cells) were observed in the SF of ANA+ JIA patients, suggesting that DN B cells might be involved in the development of disease and could be a characteristic subset in ANA+ JIA patients 139. A previous study showed that atBCs accumulated in the joints of JIA patients and displayed features of APCs, with expression of costimulatory molecules (CD80/CD86) and a polarized pattern of cytokine secretion capable of inducing T cell activation and Th1 differentiation 140. Fischer and colleagues reported that synovial CD4+ T cells promote aberrant B cell activation in ANA+ JIA by promoting the differentiation of B cells toward the CD21low/− CD11c+ phenotype through the secretion of cytokines like IL-21 and IFN-γ 141. These findings suggest that in children's inflammatory arthritis settings, expanded Tfh cells in the synovium might promote B cell differentiation into atBCs through the secretion of cytokines like IL-21 and IFN-γ.
Immunodeficiency disorders
Common Variable Immunodeficiency
CVID is a heterogeneous disease characterized by hypogammaglobulinemia, defective antibody responses and recurrent infections. atBCs have been extensively studied in CVID adult patients where they have been linked to splenomegaly and autoimmune cytopenia33, and subsequently to granulomatous disease142. In pediatric patients, a study revealed that the increase in atBCs (referred here as CD21low), was linked to the development of enteropathy and autoimmune symptoms but, was not found to be associated with the development of splenomegaly 143. On the other hand, granuloma formation was not confirmed in another single-center pediatric cohort study 144. In CVID patients, atBCs were CD21−/low,CD27−,CD38low,CD11c+,FcRL4+,FcRL5+ and expressed unmutated IgM and IgD, although this may reflect an inability to class-switch or form functional GCs 145. Recently, it has been discovered that this population expresses T-bet. Interestingly, these cells have been observed not only in secondary lymphoid organs and the spleen but also in bronchoalveolar lavage samples obtained from patients who have developed interstitial lung disease 66.
Other immunodeficiency syndromes
A recent study involving 1180 pediatric patients demonstrated significant variability in the percentage of atBCs depending on the underlying medical condition 55. Among these patients, ~16% exhibited an elevated population of this cell population (>5% of total B cells). Notably, patients with primary immunodeficiency accounted for approximately half of those with a moderate (10-20%) or high (>20%) increase in atBCs. The authors reported a high increase of atBCs in children with combined immunodeficiencies and severe combined immunodeficiencies, as well as Wiskott-Aldrich syndrome and ataxia-telangiectasia, and a low increase (5-9%) in patients with Di George syndrome and IgA deficiency. Recently, it has been reported that children with impaired RAG function had impaired primary BCR repertoire formation with remarkable alterations in the composition of B cell subsets, along with widespread, promiscuous activation that favors extrafollicular pathway and expansion of T-bet+ B cells and poly/ autoreactive B cell clones in the periphery130. These alterations are likely to be caused by environmental triggers (such as chronic infections and microbiota translocation) along with intrinsic factors (such as elevated BAFF, reduced Treg/Tfh cell ratio and inflammatory cytokine milieu. In addition, heightened levels of this population of atBCs have been observed in pediatric cases of Fisher-Evans syndrome, immune thrombocytopenia, and autoimmune hemolytic anemia. These findings align with previous observations of increased atBCs in children affected by these conditions 146,147. Nevertheless, the function of these cells in these diseases is still unknown although an association with the development of autoimmune cytopenia has been suggested 147 .
According to this finding several authors suggest Rituximab as an effective second or third line off-label treatment for autoimmune cytopenia in children with autoimmune cytopenia associated with an expansion of these subsets 148. Further studies are needed to better characterize the function of these cells in patients with immunodeficiency syndromes.
Obesity and metabolic diseases
Obesity generated low-grade chronic inflammation that led multiple metabolic diseases such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease 149. Atypical B cells have gained attention in recent years due to their potential involvement in obesity-related inflammation and metabolic dysfunction.
Research from Blomberg’ group initially identified a connection between CD21−T−Bet+ B cells and obesity. Their findings revealed an accumulation of this B cell subset in white adipose tissue of obese patients and demonstrated a correlation with body mass index and weight 150,151. Subsequently, Frasca et al. reported that atBCs (CD21−,CD27−,IgD−, T+bet+,CD11c+) in obese patients resulted associated with increased secretion of IgG with autoimmune specificity 152. Recently, Hagglof and colleagues have deepened this topic, showing that T-bet+, CD11c+ B cells were causally related to the onset and exacerbation of metabolic disease in obese patients 153.
The authors demonstrated that adipose tissue-resident atBCs were regulated by invariant natural killer T (iNKT) cells and that this atypical B cell population could be expanded by stimulation of TLR-7, in an iNKT cell-dependent manner 153,154. These interactions result in the production of chemokines and antibody mediators (IgG2c) that amplify the initiation and severity of metabolic disorders. By employing murine model with a B cell-specific knockout of T-bet the researchers demonstrate that the lack of atBCs diminishes the prevalence and onset of metabolic disease. Furthermore, they established that glucose intolerance can be restored by transferring either whole serum or purified IgG obtained from obese mice, a process that recruits pro-inflammatory macrophages. This approach unveils pathologic immunoglobulins as the central mechanism driving atBCs inflammation in obesity, thereby highlighting the potential of targeting atBCs in future therapeutic strategies to limit metabolic disorders. However, for this specific topic, there is no data available in the pediatric population. Therefore, additional studies are required to gain a better understanding of the role and functions of these cells in children who suffer from obesity and metabolic disorders.
Future perspectives
Sex difference and potential therapeutic approaches for atBCs in autoimmune diseases
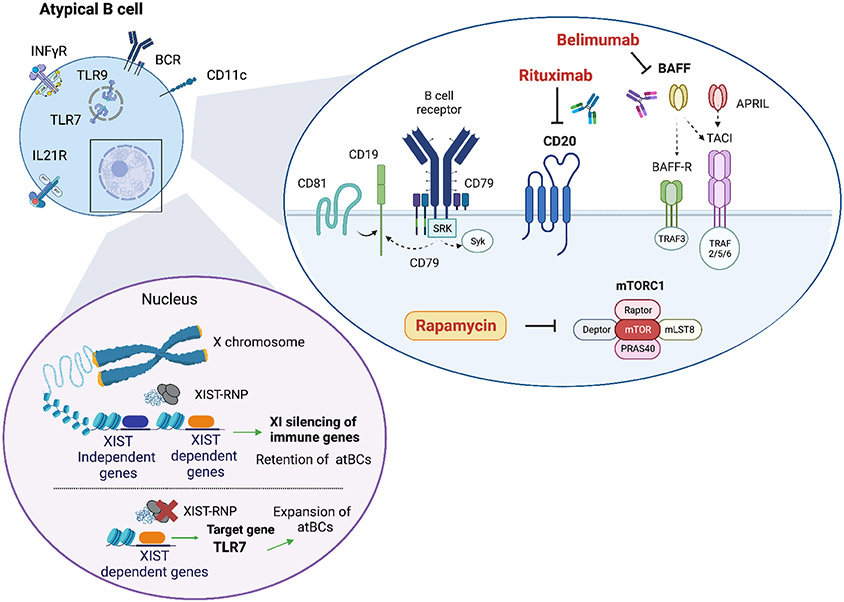
One of the most striking aspects of the atBCs population is its potential to be controlled in a sex-specific manner with a greater degree in females than males. The expansion of this compartment in females suggests their role in autoimmune disease development and potentially contributes to the documented sex-based differences in immune responses during viral infections and vaccinations 155. However, sex differences extend beyond the mere accumulation of atBCs, encompassing various aspects within the atBCs compartment. Research on lupus murine models have revealed that atBCs from females but not male, express an “interferon signature” and were more prone to differentiate in CD11c+ effector population 77. Moreover, the duplication of TLR-7 in male mice lacking SWEF proteins overrode the sex-related bias and intensified the pathogenic effects of atBCs 77. Recent work has provided interesting insights into the mechanisms that might contribute to incomplete X chromosome inactivation (XCI), particularly in atBCs. These investigations have unveiled that the long non-coding RNA XIST, responsible XCI in female cells during development, plays a crucial role in preserving XCI for a specific group of X-linked genes in B cells, including TLR-7 and CXorf21/TASL (an adaptor that regulates IRF5 activity) 156. Interestingly, escape of XIST-dependent genes, coupled with TLR-7 activation, facilitate the development of CD11c+ B cells in autoimmune settings 12,156,157, (Figure 4). Further exploration of the atBCs population in males versus females during infections and vaccinations is necessary to ascertain whether the sex bias extends beyond frequency and leads in distinct functional capacities in the atBCs population between sexes.
Figure 4.
Rituximab (anti-CD20 monoclonal antibodies) and Belimumab [anti-BLyS (B-Lymphocyte Stimulator) monoclonal antibodies] can reduce the level of atBCs cells in SLE patients (158, 159). Moreover, mTORC1 hyperactivation has been linked to the dysfunction of atBCs in SLE (133). Thus, the inhibition of this pathway could reduce the levels of this B cell population. In the cell nucleus is shown the model proposed of XCI maintenance in human B cells (156). In detail, XIST loss and TLR-7 stimulation promotes CD11c+ atypical B cell formation.
Delving into the mechanisms underlying the differentiation of these cells offers promising therapeutic perspectives. Little is known about the effectiveness of drugs on this cell population. B depleting drugs have demonstrated the ability to reduce atBCs in SLE 158,159 (Figure 4). There is significant evidence indicating a connection between the process of reconstitution of B cell subsets following B cell depletion and the clinical progression in autoimmune diseases. Therefore, it is necessary to analyze the reconstitution pattern of atBCs, including both the percentage of reappearing atBCs and their distinct phenotypic and functional traits. Unraveling these mechanisms could prove in the development of tailored drugs for these cells.
Concluding remarks
In various pediatric chronic inflammatory conditions, there is a consistent observation of an expanded population of atBCs with different physiological and pathogenetic roles, although these different functions may be context dependent. According to scientific literature160, potential roles for atBCs emerge: they could display exhaustion and functional deficits akin to CD8+ exhausted memory T cells; they demonstrate a capacity for differentiation with reduced dependence on antigens compared to classical MBCs, and potentially specialize in antigen presentation, primarily aimed at activating T cells.
In the autoimmunity field, this cell populations often correlate with disease-specific manifestations and autoantibodies production, warranting consideration for elimination. However, the exact function of atBCs during immunodeficiencies and chronic infections remains unclear. Conflicting results on anergy versus hyperresponsiveness are likely context dependent, with refractory phenotype in chronic exposure and hyperresponsiveness in acute antigenic exposure. In murine acute infection, atBCs have been postulated to participate directly in the anti-pathogen antibody response while in human the relevance of antibody production by this population in the infection control is less clear.
Notably, in the case of Malaria, atBCs exhibit PCs genes during the convalescent phase but not during the acute phase, implying different functions at different stages of the disease. While the expression of PCs genes has not been reported in most other infectious conditions, it could be due to either the lack of testing or undetectable expression. Hence, in other conditions, atBCs are unlikely to serve as precursors to PCs, indicating the presence of unknown functions.
Thus, in infectious scenarios, particularly those involving chronic infectious diseases, further research is imperative to elucidate their precise function.
This review encompasses recent data derived from human samples across various research fields. Although the inconsistent use of names and markers to identify these cells often hinders direct comparisons, several studies indicate significant overlap in the phenotypic and transcriptional characteristics as well as homing patterns of atBCs. However, it should be noted that there is substantial heterogeneity in marker expression among these cells, both between different diseases and over time. Standardizing cell nomenclature, definition and clear cut-off of abnormal expansion is crucial for driving immunomodulatory treatments and facilitating comparisons across various models and research findings offering insight into their role in immune responses and autoimmunity.
Acknowledgments
This work was supported by U.S. NIH/NIAID immune development in early life (IDEAL) award (U19AI168643)
Abbreviations used.
- ABCs
Age-associated B cells
- ANA
Antinuclear antibodies
- APCs
Antigen-presenting cells
- ART
antiretroviral treatment
- ASCs
Antibody-secreting cells
- atBCs
Atypical B cells
- BAFF
B-cell activating factor
- BAL
Bronchoalveolar lavage
- BCR
B Cell Receptor
- CD
Cluster of Differentiation
- CDR3
Complementarity determining region 3
- CSF
Cerebrospinal fluid
- CVID
common variable immunodeficiency
- DN
Double Negative
- EF
Extrafollicular
- ENA
Extractable Nuclear Antigen
- GC
Germinal Center
- HIV
Human immunodeficiency virus
- IFN
Interferon
- iNKT
invariant natural killer T cells
- JIA
Juvenile idiopathic arthritis
- MBCs
Memory B-cells
- PB
Peripheral blood
- PBs
Plasma blasts
- PCs
Plasma cells
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SF
Synovial fluid
- SHM
Somatic hypermutation
- SLE
Systemic Lupus Erythematosus
- Tfh
T follicular helper cells
- TLM
Tissue-like memory B cells
- TLR
Toll Like Receptor
- Treg
Regulatory T cells
- VH
Variable heavy chain
- VL
Variable light chain
- XCI
X chromosome inactivation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Wang Y, Liu J, Burrows PD, Wang JY. B Cell Development and Maturation. Adv Exp Med Biol. 2020;1254:1–22. [DOI] [PubMed] [Google Scholar]
- 2.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. [DOI] [PubMed] [Google Scholar]
- 3.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–51. [DOI] [PubMed] [Google Scholar]
- 4.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesin L, Ersching J, Victora GD. Germinal Center B Cell Dynamics. Immunity. 2016;45:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247:52–63. [DOI] [PubMed] [Google Scholar]
- 7.Cancro MP. Age-Associated B Cells. Annu Rev Immunol. 2020;38:315–40. [DOI] [PubMed] [Google Scholar]
- 8.Courey-Ghaouzi AD, Kleberg L, Sundling C. Alternative B Cell Differentiation During Infection and Inflammation. Front Immunol. 2022;13:908034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knox JJ, Myles A, Cancro MP. T-bet+ memory B cells: Generation, function, and fate. Immunol Rev. 2019;288:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper L, Good-Jacobson KL. Dysregulation of humoral immunity in chronic infection. Immunol Cell Biol. 2020;98:456–66. [DOI] [PubMed] [Google Scholar]
- 11.Myles A, Sanz I, Cancro MP. T-bet+ B cells: A common denominator in protective and autoreactive antibody responses? Curr Opin Immunol. 2019;57:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phalke S, Rivera-Correa J, Jenkins D, Flores Castro D, Giannopoulou E, Pernis AB. Molecular mechanisms controlling age-associated B cells in autoimmunity. Immunol Rev. 2022;307:79–100. [DOI] [PubMed] [Google Scholar]
- 13.Ehrhardt GRA, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorarinsdottir K, Camponeschi A, Cavallini N, Grimsholm O, Jacobsson L, Gjertsson I, et al. CD21(−/low) B cells in human blood are memory cells. Clin Exp Immunol. 2016;185:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopp CS, Skinner J, Anzick SL, Tipton CM, Peterson ME, Li S, et al. Atypical B cells upregulate costimulatory molecules during malaria and secrete antibodies with T follicular helper cell support. Sci Immunol. 2022;7:eabn1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steuten J, Bos AV, Kuijper LH, Claireaux M, Olijhoek W, Elias G, et al. Distinct dynamics of antigen-specific induction and differentiation of different CD11c+Tbet+ B-cell subsets. J Allergy Clin Immunol. 2023;S0091-6749(23)00234–8. [DOI] [PubMed] [Google Scholar]
- 17.Gjertsson I, McGrath S, Grimstad K, Jonsson CA, Camponeschi A, Thorarinsdottir K, et al. A close-up on the expanding landscape of CD21−/low B cells in humans. Clin Exp Immunol. 2022;210:217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouat IC, Goldberg E, Horwitz MS. Age-associated B cells in autoimmune diseases. Cell Mol Life Sci. 2022;79:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambegaonkar AA, Holla P, Dizon BL, Sohn H, Pierce SK. Atypical B cells in chronic infectious diseases and systemic autoimmunity: puzzles with many missing pieces. Curr Opin Immunol. 2022;77:102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis K, Bailly E, Macedo C, Lau L, Ramaswami B, Chang A, et al. T-bet+CD27+CD21− B cells poised for plasma cell differentiation during antibody-mediated rejection of kidney transplants. JCI Insight. 2021;6:e148881, 148881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton HJ, Aye R, Idris AH, Vistein R, Nduati E, Kai O, et al. Atypical B cells are part of an alternative lineage of B cells that participates in responses to vaccination and infection in humans. Cell Rep. 2021;34:108684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouat IC, Horwitz MS. Age-associated B cells in viral infection. PLoS Pathog. 2022;18:e1010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, et al. T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI Insight. 2017;2:e92943, 92943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK. Atypical memory B cells in human chronic infectious diseases: An interim report. Cell Immunol. 2017;321:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portugal S, Tipton CM, Sohn H, Kone Y, Wang J, Li S, et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife. 2015;4:e07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss GE, Clark EH, Li S, Traore B, Kayentao K, Ongoiba A, et al. A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PLoS One. 2011;6:e15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochayoo P, Thawornpan P, Wangriatisak K, Changrob S, Leepiyasakulchai C, Khowawisetsut L, et al. Interferon-γ signal drives differentiation of T-bethi atypical memory B cells into plasma cells following Plasmodium vivax infection. Sci Rep. 2022;12:4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warnatz K, Wehr C, Dräger R, Schmidt S, Eibel H, Schlesier M, et al. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology. 2002;206:502–13. [DOI] [PubMed] [Google Scholar]
- 34.Guffroy A, Mourot-Cottet R, Gérard L, Gies V, Lagresle C, Pouliet A, et al. Neutropenia in Patients with Common Variable Immunodeficiency: a Rare Event Associated with Severe Outcome. J Clin Immunol. 2017;37:715–26. [DOI] [PubMed] [Google Scholar]
- 35.Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49:725–739.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller B, Warnatz K. T-bethighCD21low B cells: the need to unify our understanding of a distinct B cell population in health and disease. Curr Opin Immunol. 2023;82:102300. [DOI] [PubMed] [Google Scholar]
- 37.Holla P, Dizon B, Ambegaonkar AA, Rogel N, Goldschmidt E, Boddapati AK, et al. Shared transcriptional profiles of atypical B cells suggest common drivers of expansion and function in malaria, HIV, and autoimmunity. Sci Adv. 2021;7:eabg8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tangye SG. Do multiple subsets of CD11c+ B cells exist? You (T)-Bet! J Allergy Clin Immunol. 2023;S0091-6749(23)00924–7. [DOI] [PubMed] [Google Scholar]
- 39.Freudenhammer M, Voll RE, Binder SC, Keller B, Warnatz K. Naive- and Memory-like CD21low B Cell Subsets Share Core Phenotypic and Signaling Characteristics in Systemic Autoimmune Disorders. J Immunol. 2020;205:2016–25. [DOI] [PubMed] [Google Scholar]
- 40.Kleberg L, Courey-Ghaouzi AD, Lautenbach MJ, Färnert A, Sundling C. Regulation of B cell function and expression of CD11c, T-bet, and FcRL5 in response to different activation signals [Internet]. Immunology; 2023. Mar [cited 2023 Oct 12]. Available from: http://biorxiv.org/lookup/doi/10.1101/2023.03.08.531830 [DOI] [PubMed] [Google Scholar]
- 41.Duchamp M, Sterlin D, Diabate A, Uring-Lambert B, Guérin-El Khourouj V, Le Mauff B, et al. B-cell subpopulations in children: National reference values. Immun Inflamm Dis. 2014;2:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol. 2010;162:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borriello F, Pasquarelli N, Law L, Rand K, Raposo C, Wei W, et al. Normal B-cell ranges in infants: A systematic review and meta-analysis. J Allergy Clin Immunol. 2022;150:1216–24. [DOI] [PubMed] [Google Scholar]
- 44.Berrón-Ruíz L, López-Herrera G, Ávalos-Martínez CE, Valenzuela-Ponce C, Ramírez-SanJuan E, Santoyo-Sánchez G, et al. Variations of B cell subpopulations in peripheral blood of healthy Mexican population according to age: Relevance for diagnosis of primary immunodeficiencies. Allergol Immunopathol (Madr). 2016;44:571–9. [DOI] [PubMed] [Google Scholar]
- 45.Piątosa B, Wolska-Kuśnierz B, Pac M, Siewiera K, Gałkowska E, Bernatowska E. B cell subsets in healthy children: reference values for evaluation of B cell maturation process in peripheral blood. Cytometry B Clin Cytom. 2010;78:372–81. [DOI] [PubMed] [Google Scholar]
- 46.Schatorjé EJH, Gemen EFA, Driessen GJA, Leuvenink J, van Hout RWNM, van der Burg M, et al. Age-matched reference values for B-lymphocyte subpopulations and CVID classifications in children. Scand J Immunol. 2011;74:502–10. [DOI] [PubMed] [Google Scholar]
- 47.Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, et al. Stereotypic Immune System Development in Newborn Children. Cell. 2018;174:1277–1292.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanco E, Pérez-Andrés M, Arriba-Méndez S, Contreras-Sanfeliciano T, Criado I, Pelak O, et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J Allergy Clin Immunol. 2018;141:2208–2219.e16. [DOI] [PubMed] [Google Scholar]
- 49.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133:95–107. [DOI] [PubMed] [Google Scholar]
- 50.Jalali S, Harpur CM, Piers AT, Auladell M, Perriman L, Li S, et al. A high-dimensional cytometry atlas of peripheral blood over the human life span. Immunol Cell Biol. 2022;100:805–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedraz C, Lorente F, Pedraz MJ, Salazar Villalobos V. [Development of the serum levels of complement during the first year of life]. An Esp Pediatr. 1980;13:571–6. [PubMed] [Google Scholar]
- 52.Johnston RB, Altenburger KM, Atkinson AW, Curry RH. Complement in the newborn infant. Pediatrics. 1979;64:781–6. [PubMed] [Google Scholar]
- 53.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013;110:E3216–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kardava L, Moir S. B-cell abnormalities in HIV-1 infection: roles for IgG3 and T-bet. Curr Opin HIV AIDS. 2019;14:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corrente F, Terreri S, Palomba P, Capponi C, Mirabella M, Perno CF, et al. CD21− CD27− Atypical B Cells in a Pediatric Cohort Study: An Extensive Single Center Flow Cytometric Analysis. Front Pediatr. 2022; 10:822400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sundling C, Rönnberg C, Yman V, Asghar M, Jahnmatz P, Lakshmikanth T, et al. B cell profiling in malaria reveals expansion and remodelling of CD11c+ B cell subsets. JCI Insight. 2019;5:e126492, 126492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzales SJ, Bol S, Braddom AE, Sullivan R, Reyes RA, Ssewanyana I, et al. Longitudinal analysis of FcRL5 expression and clonal relationships among classical and atypical memory B cells following malaria. Malar J. 2021;20:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fraussen J, Marquez S, Takata K, Beckers L, Montes Diaz G, Zografou C, et al. Phenotypic and Ig Repertoire Analyses Indicate a Common Origin of IgD−CD27− Double Negative B Cells in Healthy Individuals and Multiple Sclerosis Patients. J Immunol. 2019;203:1650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckers L, Somers V, Fraussen J. IgD−CD27− double negative (DN) B cells: Origins and functions in health and disease. Immunol Lett. 2023;255:67–76. [DOI] [PubMed] [Google Scholar]
- 60.Austin JW, Buckner CM, Kardava L, Wang W, Zhang X, Melson VA, et al. Overexpression of T-bet in HIV infection is associated with accumulation of B cells outside germinal centers and poor affinity maturation. Sci Transl Med. 2019;11:eaax0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellebedy AH, Jackson KJL, Kissick HT, Nakaya HI, Davis CW, Roskin KM, et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol. 2016;17:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zurbuchen Y, Michler J, Taeschler P, Adamo S, Cervia C, Raeber ME, et al. Human memory B cells show plasticity and adopt multiple fates upon recall response to SARS-CoV-2. Nat Immunol. 2023;24:955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jöhrens K, Moos V, Schneider T, Stein H, Anagnostopoulos I. Interferon-gamma and T-bet expression in a patient with toxoplasmic lymphadenopathy. Hum Immunol. 2010;71:366–71. [DOI] [PubMed] [Google Scholar]
- 64.Elsner RA, Shlomchik MJ. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity. 2020;53:1136–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, et al. IFNγ induces epigenetic programming of human T-bethi B cells and promotes TLR7/8 and IL-21 induced differentiation. Elife. 2019;8:e41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keller B, Strohmeier V, Harder I, Unger S, Payne KJ, Andrieux G, et al. The expansion of human T-bethighCD21low B cells is T cell dependent. Sci Immunol. 2021;6:eabh0891. [DOI] [PubMed] [Google Scholar]
- 67.Myles A, Gearhart PJ, Cancro MP. Signals that drive T-bet expression in B cells. Cell Immunol. 2017;321:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang R, Avery DT, Jackson KJL, Ogishi M, Benhsaien I, Du L, et al. Human T-bet governs the generation of a distinct subset of CD11chighCD21low B cells. Sci Immunol. 2022;7:eabq3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnett BE, Staupe RP, Odorizzi PM, Palko O, Tomov VT, Mahan AE, et al. Cutting Edge: B Cell-Intrinsic T-bet Expression Is Required To Control Chronic Viral Infection. J Immunol. 2016;197:1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evrard M, Wynne-Jones E, Peng C, Kato Y, Christo SN, Fonseca R, et al. Sphingosine 1-phosphate receptor 5 (S1PR5) regulates the peripheral retention of tissue-resident lymphocytes. J Exp Med. 2022;219:e20210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ly A, Liao Y, Pietrzak H, Ioannidis LJ, Sidwell T, Gloury R, et al. Transcription Factor T-bet in B Cells Modulates Germinal Center Polarization and Antibody Affinity Maturation in Response to Malaria. Cell Rep. 2019;29:2257–2269.e6. [DOI] [PubMed] [Google Scholar]
- 72.Manni M, Gupta S, Ricker E, Chinenov Y, Park SH, Shi M, et al. Regulation of age-associated B cells by IRF5 in systemic autoimmunity. Nat Immunol. 2018;19:407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau D, Lan LYL, Andrews SF, Henry C, Rojas KT, Neu KE, et al. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol. 2017;2:eaai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glass DR, Tsai AG, Oliveria JP, Hartmann FJ, Kimmey SC, Calderon AA, et al. An Integrated Multi-omic Single-Cell Atlas of Human B Cell Identity. Immunity. 2020;53:217–232.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao X, Cockburn IA. The development and function of CD11c+ atypical B cells - insights from single cell analysis. Front Immunol. 2022;13:979060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheikh AA, Groom JR. Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment. Cell Mol Immunol. 2021;18:528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ricker E, Manni M, Flores-Castro D, Jenkins D, Gupta S, Rivera-Correa J, et al. Altered function and differentiation of age-associated B cells contribute to the female bias in lupus mice. Nat Commun. 2021;12:4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat Commun. 2018;9:1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Golinski ML, Demeules M, Derambure C, Riou G, Maho-Vaillant M, Boyer O, et al. CD11c+ B Cells Are Mainly Memory Cells, Precursors of Antibody Secreting Cells in Healthy Donors. Front Immunol. 2020;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c− Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol. 2015;195:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest. 2018;128:4588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang LY, Li Y, Kaplan DE. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J Viral Hepat. 2017;24:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joosten SA, van Meijgaarden KE, Del Nonno F, Baiocchini A, Petrone L, Vanini V, et al. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog. 2016;12:e1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wildner NH, Ahmadi P, Schulte S, Brauneck F, Kohsar M, Lütgehetmann M, et al. B cell analysis in SARS-CoV-2 versus malaria: Increased frequencies of plasmablasts and atypical memory B cells in COVID-19. J Leukoc Biol. 2021;109:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shehata L, Wieland-Alter WF, Maurer DP, Chen E, Connor RI, Wright PF, et al. Systematic comparison of respiratory syncytial virus-induced memory B cell responses in two anatomical compartments. Nat Commun. 2019;10:1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rouers A, Chng MHY, Lee B, Rajapakse MP, Kaur K, Toh YX, et al. Immune cell phenotypes associated with disease severity and long-term neutralizing antibody titers after natural dengue virus infection. Cell Rep Med. 2021;2:100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nipper AJ, Smithey MJ, Shah RC, Canaday DH, Landay AL. Diminished antibody response to influenza vaccination is characterized by expansion of an age-associated B-cell population with low PAX5. Clin Immunol. 2018;193:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eccles JD, Turner RB, Kirk NA, Muehling LM, Borish L, Steinke JW, et al. T-bet+ Memory B Cells Link to Local Cross-Reactive IgG upon Human Rhinovirus Infection. Cell Rep. 2020;30:351–366.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knight JS, Caricchio R, Casanova JL, Combes AJ, Diamond B, Fox SE, et al. The intersection of COVID-19 and autoimmunity. J Clin Invest. 2021;131:e154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020;21:1506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pape KA, Dileepan T, Kabage AJ, Kozysa D, Batres R, Evert C, et al. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep. 2021;37:109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knox JJ, Kaplan DE, Betts MR. T-bet-expressing B cells during HIV and HCV infections. Cell Immunol. 2017;321:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol. 2013;190:3039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Obeng-Adjei N, Portugal S, Holla P, Li S, Sohn H, Ambegaonkar A, et al. Malaria-induced interferon-γ drives the expansion of Tbethi atypical memory B cells. PLoS Pathog. 2017;13:e1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zinöcker S, Schindler CE, Skinner J, Rogosch T, Waisberg M, Schickel JN, et al. The V gene repertoires of classical and atypical memory B cells in malaria-susceptible West African children. J Immunol. 2015;194:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wendel BS, He C, Qu M, Wu D, Hernandez SM, Ma KY, et al. Accurate immune repertoire sequencing reveals malaria infection driven antibody lineage diversification in young children. Nat Commun. 2017;8:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sullivan RT, Kim CC, Fontana MF, Feeney ME, Jagannathan P, Boyle MJ, et al. FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Plasmodium falciparum Exposure. PLoS Pathog. 2015;11:e1004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, et al. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med. 2013;210:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rivera-Correa J, Yasnot-Acosta MF, Tovar NC, Velasco-Pareja MC, Easton A, Rodriguez A. Atypical memory B-cells and autoantibodies correlate with anemia during Plasmodium vivax complicated infections. PLoS Negl Trop Dis. 2020;14:e0008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ambegaonkar AA, Kwak K, Sohn H, Manzella-Lapeira J, Brzostowski J, Pierce SK. Expression of inhibitory receptors by B cells in chronic human infectious diseases restricts responses to membrane-associated antigens. Sci Adv. 2020;6:eaba6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reyes RA, Batugedara G, Dutta P, Reers AB, Garza R, Ssewanyana I, et al. Atypical B cells consist of subsets with distinct effector functions [Internet]. Immunology; 2022. Sep [cited 2023 Jul 22]. Available from: http://biorxiv.org/lookup/doi/10.1101/2022.09.28.509955 [Google Scholar]
- 103.Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev. 2013;254:207–24. [DOI] [PubMed] [Google Scholar]
- 104.Palma P, Rinaldi S, Cotugno N, Santilli V, Pahwa S, Rossi P, et al. Premature B-cell senescence as a consequence of chronic immune activation. Hum Vaccin Immunother. 2014;10:2083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meffre E, Louie A, Bannock J, Kim LJY, Ho J, Frear CC, et al. Maturational characteristics of HIV-specific antibodies in viremic individuals. JCI Insight. 2016;1:e84610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kardava L, Sohn H, Youn C, Austin JW, Wang W, Buckner CM, et al. IgG3 regulates tissue-like memory B cells in HIV-infected individuals. Nat Immunol. 2018;19:1001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruggiero A, Pascucci GR, Cotugno N, Domínguez-Rodríguez S, Rinaldi S, Tagarro A, et al. Determinants of B-Cell Compartment Hyperactivation in European Adolescents Living With Perinatally Acquired HIV-1 After Over 10 Years of Suppressive Therapy. Front Immunol. 2022;13:860418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cotugno N, De Armas L, Pallikkuth S, Rinaldi S, Issac B, Cagigi A, et al. Perturbation of B Cell Gene Expression Persists in HIV-Infected Children Despite Effective Antiretroviral Therapy and Predicts H1N1 Response. Front Immunol. 2017;8:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rinaldi S, Pallikkuth S, George VK, de Armas LR, Pahwa R, Sanchez CM, et al. Paradoxical aging in HIV: immune senescence of B Cells is most prominent in young age. Aging (Albany NY). 2017;9:1307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cotugno N, Pallikkuth S, Sanna M, Dinh V, De Armas L, Rinaldi S, et al. B-cell immunity and vaccine induced antibody protection reveal the inefficacy of current vaccination schedule in infants with perinatal HIV-infection in Mozambique, Africa. eBioMedicine. 2023;93:104666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cagigi A, Rinaldi S, Cotugno N, Manno EC, Santilli V, Mora N, et al. Early highly active antiretroviral therapy enhances B-cell longevity: a 5 year follow up. Pediatr Infect Dis J. 2014;33:e126–131. [DOI] [PubMed] [Google Scholar]
- 112.Cagigi A, Rinaldi S, Santilli V, Mora N, C Manno E, Cotugno N, et al. Premature ageing of the immune system relates to increased anti-lymphocyte antibodies (ALA) after an immunization in HIV-1-infected and kidney-transplanted patients. Clin Exp Immunol. 2013;174:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cagigi A, Palma P, Nilsson A, Di Cesare S, Pensieroso S, Kakoulidou M, et al. The impact of active HIV-1 replication on the physiological age-related decline of immature-transitional B-cells in HIV-1 infected children. AIDS. 2010;24:2075–80. [DOI] [PubMed] [Google Scholar]
- 114.Sanz I, Wei C, Jenks SA, Cashman KS, Tipton C, Woodruff MC, et al. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front Immunol. 2019;10:2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andrews SF, Chambers MJ, Schramm CA, Plyler J, Raab JE, Kanekiyo M, et al. Activation Dynamics and Immunoglobulin Evolution of Pre-existing and Newly Generated Human Memory B cell Responses to Influenza Hemagglutinin. Immunity. 2019;51:398–410.e5. [DOI] [PubMed] [Google Scholar]
- 116.Kim CC, Baccarella AM, Bayat A, Pepper M, Fontana MF. FCRL5+ Memory B Cells Exhibit Robust Recall Responses. Cell Rep. 2019;27:1446–1460.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thorarinsdottir K, Camponeschi A, Jonsson C, Granhagen Önnheim K, Nilsson J, Forslind K, et al. CD21−/low B cells associate with joint damage in rheumatoid arthritis patients. Scand J Immunol. 2019;90:e12792. [DOI] [PubMed] [Google Scholar]
- 118.Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, et al. Expansion of autoreactive unresponsive CD21−/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Visentini M, Pellicano C, Leodori G, Marrapodi R, Colantuono S, Gigante A, et al. CD21low B cells are predictive markers of new digital ulcers in systemic sclerosis. Clin Exp Immunol. 2021;205:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, et al. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J Immunol. 2016;197:4576–83. [DOI] [PubMed] [Google Scholar]
- 121.Palanichamy A, Apeltsin L, Kuo TC, Sirota M, Wang S, Pitts SJ, et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med. 2014;6:248ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Z, Wang Z, Wang J, Diao Y, Qian X, Zhu N. T-bet-Expressing B Cells Are Positively Associated with Crohn’s Disease Activity and Support Th1 Inflammation. DNA Cell Biol. 2016;35:628–35. [DOI] [PubMed] [Google Scholar]
- 123.Cao Y, Zhao X, You R, Zhang Y, Qu C, Huang Y, et al. CD11c+ B Cells Participate in the Pathogenesis of Graves’ Disease by Secreting Thyroid Autoantibodies and Cytokines. Front Immunol. 2022;13:836347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Y, Gong Y, Qu C, Zhang Y, You R, Yu N, et al. CD32b expression is down-regulated on double-negative memory B cells in patients with Hashimoto’s thyroiditis. Mol Cell Endocrinol. 2017;440:1–7. [DOI] [PubMed] [Google Scholar]
- 125.Ruschil C, Gabernet G, Lepennetier G, Heumos S, Kaminski M, Hracsko Z, et al. Specific Induction of Double Negative B Cells During Protective and Pathogenic Immune Responses. Front Immunol. 2020;11:606338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pereira CTM, Bichuetti-Silva DC, da Mota NVF, Salomão R, Brunialti MKC, Costa-Carvalho BT. B-cell subsets imbalance and reduced expression of CD40 in ataxia-telangiectasia patients. Allergol Immunopathol (Madr). 2018;46:438–46. [DOI] [PubMed] [Google Scholar]
- 127.Castiello MC, Bosticardo M, Pala F, Catucci M, Chamberlain N, van Zelm MC, et al. Wiskott-Aldrich Syndrome protein deficiency perturbs the homeostasis of B-cell compartment in humans. J Autoimmun. 2014;50:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grosserichter-Wagener C, Franco-Gallego A, Ahmadi F, Moncada-Vélez M, Dalm VA, Rojas JL, et al. Defective formation of IgA memory B cells, Th1 and Th17 cells in symptomatic patients with selective IgA deficiency. Clin Transl Immunology. 2020;9:e1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cotugno N, Finocchi A, Cagigi A, Di Matteo G, Chiriaco M, Di Cesare S, et al. Defective B-cell proliferation and maintenance of long-term memory in patients with chronic granulomatous disease. J Allergy Clin Immunol. 2015;135:753–761.e2. [DOI] [PubMed] [Google Scholar]
- 130.Csomos K, Ujhazi B, Blazso P, Herrera JL, Tipton CM, Kawai T, et al. Partial RAG deficiency in humans induces dysregulated peripheral lymphocyte development and humoral tolerance defect with accumulation of T-bet+ B cells. Nat Immunol. 2022;23:1256–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol. 2019;20:902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest. 2017;127:1392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu C, Fu Q, Guo Q, Chen S, Goswami S, Sun S, et al. Lupus-associated atypical memory B cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis. 2019;78:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dörner T, Szelinski F, Lino AC, Lipsky PE. Therapeutic implications of the anergic/postactivated status of B cells in systemic lupus erythematosus. RMD Open. 2020;6:e001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moneta GM, Bracaglia C, Caiello I, Farroni C, Pires Marafon D, Carlomagno R, et al. Persistently active interferon-γ pathway and expansion of T-bet+ B cells in a subset of patients with childhood-onset systemic lupus erythematosus. Eur J Immunol. 2023;53:e2250319. [DOI] [PubMed] [Google Scholar]
- 136.Wahadat MJ, van Tilburg SJ, Mueller YM, de Wit H, Van Helden-Meeuwsen CG, Langerak AW, et al. Targeted multiomics in childhood-onset SLE reveal distinct biological phenotypes associated with disease activity: results from an explorative study. Lupus Sci Med. 2023;10:e000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiegering V, Girschick HJ, Morbach H. B-cell pathology in juvenile idiopathic arthritis. Arthritis. 2010;2010:759868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li ZY, Cai ML, Qin Y, Chen Z. Age/autoimmunity-associated B cells in inflammatory arthritis: An emerging therapeutic target. Front Immunol. 2023; 14:1103307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dirks J, Fischer J, Haase G, Holl-Wieden A, Hofmann C, Girschick H, et al. CD21lo/−CD27−IgM− Double-Negative B Cells Accumulate in the Joints of Patients With Antinuclear Antibody-Positive Juvenile Idiopathic Arthritis. Front Pediatr. 2021;9:635815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morbach H, Wiegering V, Richl P, Schwarz T, Suffa N, Eichhorn EM, et al. Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:3458–66. [DOI] [PubMed] [Google Scholar]
- 141.Fischer J, Dirks J, Klaussner J, Haase G, Holl-Wieden A, Hofmann C, et al. Effect of Clonally Expanded PD-1high CXCR5−CD4+ Peripheral T Helper Cells on B Cell Differentiation in the Joints of Patients With Antinuclear Antibody-Positive Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2022;74:150–62. [DOI] [PubMed] [Google Scholar]
- 142.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. [DOI] [PubMed] [Google Scholar]
- 143.Piątosa B, Pac M, Siewiera K, Pietrucha B, Klaudel-Dreszler M, Heropolitańska-Pliszka E, et al. Common variable immune deficiency in children--clinical characteristics varies depending on defect in peripheral B cell maturation. J Clin Immunol. 2013;33:731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Szczawińska-Popłonyk A, Ta Polska-Jóźwiak K, Schwartzmann E, Popłonyk N. Immune Dysregulation in Pediatric Common Variable Immunodeficiency: Implications for the Diagnostic Approach. Front Pediatr. 2022;10:855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106:13451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stepensky P, Rensing-Ehl A, Gather R, Revel-Vilk S, Fischer U, Nabhani S, et al. Early-onset Evans syndrome, immunodeficiency, and premature immunosenescence associated with tripeptidyl-peptidase II deficiency. Blood. 2015;125:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zama D, Conti F, Moratti M, Cantarini ME, Facchini E, Rivalta B, et al. Immune cytopenias as a continuum in inborn errors of immunity: An in-depth clinical and immunological exploration. Immun Inflamm Dis. 2021;9:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pacillo L, Giardino G, Amodio D, Giancotta C, Rivalta B, Rotulo GA, et al. Targeted treatment of autoimmune cytopenias in primary immunodeficiencies. Front Immunol. 2022;13:911385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech Ageing Dev. 2017;162:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Metabolic requirements of human pro-inflammatory B cells in aging and obesity. PLoS One. 2019;14:e0219545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frasca D, Diaz A, Romero M, Blomberg BB. Phenotypic and Functional Characterization of Double Negative B Cells in the Blood of Individuals With Obesity. Front Immunol. 2021;12:616650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hägglöf T, Vanz C, Kumagai A, Dudley E, Ortega V, Siller M, et al. T-bet+ B cells accumulate in adipose tissue and exacerbate metabolic disorder during obesity. Cell Metabolism. 2022;34:1121–1136.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leadbetter EA, Karlsson MCI. Reading the room: iNKT cells influence B cell responses. Molecular Immunology. 2021;130:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu B, Qi Y, Li R, Shi Q, Satpathy AT, Chang HY. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell. 2021;184:1790–1803.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pyfrom S, Paneru B, Knox JJ, Cancro MP, Posso S, Buckner JH, et al. The dynamic epigenetic regulation of the inactive X chromosome in healthy human B cells is dysregulated in lupus patients. Proc Natl Acad Sci U S A. 2021;118:e2024624118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ramsköld D, Parodis I, Lakshmikanth T, Sippl N, Khademi M, Chen Y, et al. B cell alterations during BAFF inhibition with belimumab in SLE. EBioMedicine. 2019;40:517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Faustini F, Sippl N, Stålesen R, Chemin K, Dunn N, Fogdell-Hahn A, et al. Rituximab in Systemic Lupus Erythematosus: Transient Effects on Autoimmunity Associated Lymphocyte Phenotypes and Implications for Immunogenicity. Front Immunol. 2022;13:826152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Inoue T, Kurosaki T. Memory B cells. Nat Rev Immunol. 2023; [DOI] [PubMed] [Google Scholar]
- 161.Malle L, Patel RS, Martin-Fernandez M, Stewart OJ, Philippot Q, Buta S, et al. Autoimmunity in Down’s syndrome via cytokines, CD4 T cells and CD11c+ B cells. Nature. 2023;615:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]