Abstract
Background:
Consumption of fish yields many nutritional benefits, but also results in exposure to methylmercury (MeHg). The developing brain is known to be particularly susceptible to MeHg toxicity in high doses. However, the potential impact of low-level environmental exposure from fish consumption on children’s neurodevelopment remains unclear.
Methods:
We investigated postnatal MeHg exposure at 7 years and its association with a battery of 17 neurodevelopmental outcomes in a subset of children (n=376) from 1535 enrolled mother-child pairs in Nutrition Cohort 2 of the Seychelles Child Development Study (SCDS NC2). Each outcome was modeled in relation to postnatal MeHg exposure using linear regression, adjusting for prenatal MeHg exposure, levels of maternal polyunsaturated fatty acids (PUFA), and several other covariates known to be associated with neurodevelopmental outcomes.
Results:
Median postnatal MeHg exposure at 7 years was 2.5 ppm, while the median prenatal MeHg exposure was 3.5 ppm. We found no statistically significant associations between postnatal MeHg exposure and any of the 17 neurodevelopmental outcomes after adjusting for prenatal MeHg exposure and other covariates.
Conclusions:
These findings are consistent with previous cross-sectional analyses of the SCDS Main Cohort. Continued follow-up of the entire NC2 cohort at later ages with repeated exposure measures is needed to further confirm these findings.
Keywords: Mercury, neurodevelopment, Seychelles, postnatal exposure
1. Introduction
Methylmercury (MeHg) is produced naturally by environmental bacteria and is present in all fish. At high levels of exposure MeHg is known to have detrimental effects on children’s neurodevelopment (Bakir et al., 1973; Harada, 1995). However, the potential impact of low-level environmental exposure from fish consumption on children’s neurodevelopment remains unclear (Strain et al., 2015; Vejrup et al., 2016; van Wijngaarden et al., 2017). Previous studies have focused primarily on prenatal MeHg exposure because the developing brain is known to be the most vulnerable during gestation (Spyker et al., 1972; Choi, 1989). Current fish consumption guidelines primarily rely on studies examining prenatal exposure (FAO/WHO, 2011; FDA/EPA, 2022). However, neurodevelopment is an ongoing process that extends throughout childhood, adolescence, and early adulthood (Shonkoff et al., 2000; Tau & Peterson, 2010), and ongoing MeHg exposure during postnatal development may impact neurodevelopmental and neurobehavioral outcomes.
MeHg is known to readily cross the blood-brain barrier and accumulate in the brain, and experimental studies have shown that MeHg can disrupt key neurodevelopmental processes such as neuronal migration, synaptogenesis, and myelination (Clarkson & Magos, 2006). These processes are essential for the establishment of normal neural circuitry, and they continue well into adolescence (Shonkoff et al., 2000; Tau & Peterson, 2010). Thus, the brain is susceptible to environmental exposures during postnatal development. However, the epidemiologic literature on postnatal MeHg exposure and neurodevelopment appears inconclusive (Murata et al., 1999; Murata et al., 2004; Despres et al., 2005; Debes et al., 2006; Saint-Amour et al., 2006; Ha et al., 2009; Boucher et al., 2010; Cao et al., 2010; Freire et al., 2010; Plusquellec et al., 2010; Dorea et al., 2012; Deroma et al., 2013; Grandjean et al., 2014; Wang et al., 2014; Jacobson et al., 2015; Marques et al., 2015; Boucher et al., 2016; Llop et al., 2020; Lee et al., 2021; Lozano et al., 2021).
In the Seychelles Child Development Study, we have not reported consistent adverse associations between prenatal MeHg exposure and neurodevelopmental outcomes across analyses of our cohorts (Strain et al., 2012; Strain et al., 2015; van Wijngaarden et al., 2017; Strain et al., 2021). In our Main Cohort, where children were followed until 24 years of age, we observed some adverse associations with postnatal exposure (Myers et al., 2009; van Wijngaarden et al., 2017; Thurston, Myers, et al., 2022). In the present study we sought to further clarify postnatal MeHg associations in early childhood data from the more recent Nutrition Cohort 2.
2. Methods
2.1. Study Population
The Seychelles Child Development Study (SCDS) Nutrition Cohort 2 (NC2) was originally designed to assess the association between prenatal MeHg exposure from fish consumption and child neurodevelopmental outcomes, while considering the potential influence of nutrition (Strain et al., 2015). Between 2008 and 2011, a total of 1535 mother-child pairs were recruited at their first prenatal visit on Mahé, the main island of the Seychelles. Inclusion criteria were native Seychellois, age ≥ 16 years, and no known health concerns.
For this analysis, we selected a subset of the NC2 cohort that included the first 389 participants evaluated at age 7 years with measured postnatal MeHg data available. Of these, 11 were missing data on maternal polyunsaturated fatty acid (PUFA) status using the omega-6:omega-3 (n-6:n-3) ratio, 1 was missing both prenatal MeHg exposure and maternal PUFA levels, and 1 was missing a maternal Kaufman Brief Intelligence Test (KBIT-2) score, resulting in 376 mother-child pairs with complete covariate data.
2.2. Neurodevelopmental and Neurobehavioral Outcomes
This study focused on the 17 primary endpoints derived from a battery of 14 neurodevelopmental tests administered to the children in the cohort, and three questionnaires completed by their parents. The neurodevelopmental tests included the Clinical Evaluation of Language Fundamentals (CELF-5), consisting of six endpoints: Total, Follow Directions, Linguistic Concepts, Recalling Sentences, Sentence Comprehension, and Understand Spoken Paragraphs. Additionally, the KBIT-2 contributed two endpoints: Word Knowledge and Matrices. Other tests included the Boston Naming Test (BNT; total score), Trailmaking A (TM-A), Finger Tapping (FT) for dominant and non-dominant hands; and the Woodcock-Johnson Test for Achievement (WJ-III), with endpoints for letter word identification and applied math. The remaining three instruments used as primary endpoints were the parent-reported Child Behavior Checklist (CBCL Total), Social Responsiveness Scale 2 (SRS-2), and Social Communication Questionnaire (SCQ). Altogether, these 14 neurodevelopmental tests and three parent-reported instruments constituted the 17 primary endpoints evaluated for all children in the NC2 cohort. For more detailed information about these tests and the specific neurodevelopmental domains they assess, we refer the reader to Strain, et al. (2021).
2.3. Methylmercury Assessment
Determinations of prenatal MeHg exposure were performed by analyzing total Hg in maternal hair samples collected at delivery (Strain et al., 2015). Mercury in hair that best recapitulated growth during pregnancy was analyzed using the longest hair segment available emanating from the scalp. Hair was assumed to grow at a rate of 1.1 cm per month. Mercury deposited in hair is more than 80% MeHg and is known to correlate with mercury deposited in the infant brain (Cernichiari et al., 1995). Determination of MeHg in prenatal hair samples were performed using cold vapor atomic absorption spectroscopy with previously-described quality control procedures (Cernichiari et al., 1995).
We measured total Hg concentrations in child’s hair collected at 7 years using inductively coupled plasma mass spectrometry (ICP-MS) (NexION2000, Perkin-Elmer, Waltham, MA). Consistent with previous assessments (Thurston, Myers, et al., 2022), concurrent postnatal MeHg exposure in the children was measured as total Hg in the 1 cm of hair closest to the scalp at the time of testing. Hair samples were prepared with a standard microwave-concentrated acid digestion (3:1 HNO3:HCl, ultra trace grade) followed by dilution prior to ICP-MS determinations in a 1400W power standard mode, measuring the Hg202 isotope using Bi209 as an internal standard. The limit of detection (LOD) for was 0.1 ng/mL (0.1 ppb), and the limit of quantification is 10 times the LOD, 1 ng/mL (1 ppb). The ICP-MS was calibrated using National Institute of Standards and Technology (NIST) certified reference material containing Hg. Duplicate analyses were performed on all sample batches unless sample material was limited.
2.4. Covariates
The statistical analysis in this study followed a similar approach to prior analyses of children in the NC2 cohort at 20 months (Strain et al., 2015) and 7 years (Strain et al., 2021). The primary analysis was determined a priori and consisted of linear regression with adjustment for covariates known to be associated with child neurodevelopment. Maternal-related covariates included mother’s age at delivery, her IQ as measured by the Kaufmann Brief Intelligence Test (KBIT-2 Matrices), prenatal MeHg from maternal hair collected at delivery, and the n-6:n-3 ratio using maternal serum PUFA from a blood sample collected at 28 weeks of gestation. Child-related covariates included the age at the time of testing, sex, socioeconomic status (Hollingshead SES), and family status (i.e. whether both parents were living with the child, represented by a binary variable). Additionally, for models of all six CELF-5 endpoints, the interviewer's identity (a categorical variable with four levels) was included as a covariate since interviewer was a significant predictor of outcomes for this assessment in prior analyses (Strain et al., 2021).
2.5. Statistical Analysis
The goal of this analysis was to assess the main effect of postnatal MeHg exposure at 7 years, while accounting for prenatal MeHg exposure and the previously outlined maternal biomarkers and covariates. Several endpoints were transformed to align with our previous report (Strain et al., 2021), including the BNT, CBCL, CELF-5 understanding spoken paragraphs, SCQ and SRS (square root), CELF-5 linguistic concepts and WJ-III applied problems (squared), and TM-A (log10). A separate linear regression model was fit for each transformed endpoint. Participants who had missing values for any of the specified covariates were excluded from this analysis. If a participant had missing data for an endpoint but had records available for other endpoints, they were included in the models for which data were available.
All statistical analyses were performed using R version 4.2.1 on RStudio Server (R Core Team, 2022). The assumptions of the linear regression model were assessed visually using residual versus fitted value plots to examine linearity and constant variance. Normality was checked using QQ plots, and Cook's distance was utilized to identify potentially influential observations. No significant deviations from linearity or constant variance were observed. Although a few models exhibited minor deviations from the normality of residuals, results from the linear model are typically robust to this assumption (Seber & Lee, 2003). The transformations applied to the outcomes were re-evaluated using Box-Cox criteria on the subset of the NC2 cohort included in this study, and the recommended transformations were consistent with those used in our previous report (Strain et al., 2021).
For each of the 17 transformed primary endpoints, we report the regression coefficients (slopes), standard errors, and p-values regarding the main effect of postnatal MeHg exposure at 7 years. The statistical significance of this main effect was assessed using a nominal type I error rate (α) of 0.05 in each model.
3. Results
Summary statistics for covariates, primary endpoints, and postnatal MeHg are presented in Table 1. Of the 389 participants for whom postnatal MeHg was measured, all had measurements for at least one of the 17 primary endpoints. Most participants had measurements for all or nearly all the endpoints. Forty-nine participants did not have a measurement for Trailmaking A. The median postnatal MeHg concentration was 2.5 ppm, while the median prenatal MeHg concentration (in maternal hair) was 3.5 ppm. Measures of postnatal and prenatal MeHg concentration in hair were weakly correlated, with a Pearson correlation of 0.05. The summary statistics for this subset of the NC2 cohort were similar to those reported in a previous analysis of prenatal MeHg exposure in the overall NC2 cohort (Strain et al., 2021).
Table 1:
Descriptive statistics for outcomes, exposures, and covariates.
| Variable | n | Mean | SD | Min | Median | Max |
|---|---|---|---|---|---|---|
| Outcomes | ||||||
| BNT total | 388 | 22.14 | 4.97 | 10.00 | 22.00 | 39.00 |
| CBCL total | 381 | 42.89 | 23.96 | 0.00 | 40.00 | 148.00 |
| CELF-5 total | 382 | 79.55 | 16.48 | 6.00 | 80.00 | 132.00 |
| CELF-5 FD | 389 | 11.31 | 4.06 | 0.00 | 11.00 | 23.00 |
| CELF-5 LC | 389 | 17.62 | 2.68 | 1.00 | 18.00 | 24.00 |
| CELF-5 RS | 387 | 25.78 | 7.42 | 4.00 | 25.00 | 49.00 |
| CELF-5 SC | 385 | 19.31 | 4.24 | 1.00 | 20.00 | 26.00 |
| CELF-5 USP | 387 | 5.54 | 3.43 | 0.00 | 5.00 | 16.00 |
| FT dominant hand | 389 | 30.51 | 4.83 | 16.20 | 30.40 | 47.40 |
| FT non-dominant hand | 389 | 26.69 | 4.53 | 14.60 | 26.60 | 40.60 |
| KBIT-2 word knowledge | 389 | 19.79 | 7.61 | 5.00 | 21.00 | 41.00 |
| KBIT-2 matrices | 389 | 16.89 | 4.98 | 0.00 | 16.00 | 32.00 |
| SCQ total | 381 | 8.52 | 4.14 | 0.00 | 8.00 | 21.00 |
| SRS total | 388 | 47.81 | 17.82 | 6.00 | 47.00 | 121.00 |
| Trailmaking time A | 350 | 100.73 | 46.44 | 37.00 | 89.50 | 300.00 |
| WJ-III applied problems | 388 | 23.16 | 3.59 | 7.00 | 23.50 | 32.00 |
| WJ-III letter word | 387 | 50.16 | 22.54 | 3.00 | 58.00 | 76.00 |
| Exposure | ||||||
| Child’s 7 year hair MeHg, ppm | 389 | 3.10 | 2.12 | 0.16 | 2.50 | 18.25 |
| Prenatal biomarkers | ||||||
| Mother’s age at delivery | 389 | 26.57 | 5.93 | 16.27 | 25.65 | 44.84 |
| Maternal KBIT-2 matrices | 388 | 29.18 | 6.85 | 13.00 | 30.00 | 43.00 |
| Mother’s hair MeHg, ppm | 388 | 4.72 | 4.23 | 0.12 | 3.54 | 31.66 |
| n-6:n-3 ratio | 377 | 4.10 | 1.63 | 1.56 | 3.78 | 15.81 |
| Other Covariates | ||||||
| Child’s age at 7 year testing | 389 | 7.18 | 0.08 | 7.02 | 7.16 | 7.50 |
| Child sex, female | 389 | 0.45 | - | - | - | - |
| Family status at 7 years, both parents with child | 389 | 0.52 | - | - | - | - |
| SES at 7 years | 389 | 32.89 | 10.78 | 14.00 | 32.00 | 63.00 |
The primary results of regressing the transformed endpoints on postnatal MeHg and covariates are reported in Table 2. At a significance level of 0.05, postnatal MeHg at 7 years did not demonstrate a significant association with any of the 17 primary endpoints. The estimated slopes for MeHg exposure were generally small and negative for square root CBCL, FT dominant hand, FT non-dominant hand, and square root SCQ total. Positive slopes were estimated for all CELF-5, KBIT-2, and WJ-III outcomes, as well as log10 TM-A and square root SRS total. A slope of zero was estimated for square root BNT total.
Table 2:
Regression model results for the main effect of postnatal MeHg exposure for all 17 transformed endpoints
| Outcome | n | Slope | SE | Lower | Upper | p-value |
|---|---|---|---|---|---|---|
| square root, BNT total | 375 | 0.000 | 0.012 | −0.024 | 0.024 | 1.00 |
| square root, CBCL total | 369 | −0.041 | 0.045 | −0.129 | 0.046 | 0.35 |
| CELF-5 total | 369 | 0.651 | 0.381 | −0.099 | 1.401 | 0.09 |
| CELF-5 FD | 376 | 0.186 | 0.100 | −0.010 | 0.382 | 0.06 |
| squared, CELF-5 LC | 376 | 3.747 | 1.951 | −0.090 | 7.584 | 0.06 |
| CELF-5 RS | 374 | 0.170 | 0.179 | −0.183 | 0.522 | 0.35 |
| squared, CELF-5 SC | 372 | 5.517 | 3.235 | −0.845 | 11.879 | 0.09 |
| square root, CELF-5 USP | 374 | 0.012 | 0.020 | −0.027 | 0.051 | 0.55 |
| FT dominant hand | 376 | −0.084 | 0.117 | −0.315 | 0.146 | 0.47 |
| FT non-dominant hand | 376 | −0.005 | 0.108 | −0.217 | 0.207 | 0.96 |
| KBIT-2 word knowledge | 376 | 0.204 | 0.191 | −0.172 | 0.580 | 0.29 |
| KBIT-2 matrices | 376 | 0.178 | 0.121 | −0.061 | 0.416 | 0.14 |
| square root, SCQ total | 369 | −0.001 | 0.020 | −0.040 | 0.039 | 0.98 |
| square root, SRS total | 375 | 0.004 | 0.030 | −0.055 | 0.062 | 0.90 |
| log10, trailmaking time A | 337 | 0.003 | 0.005 | −0.006 | 0.012 | 0.46 |
| squared, WJ-III applied problems | 375 | 4.642 | 3.805 | −2.840 | 12.124 | 0.22 |
| WJ-III letter word | 374 | 0.942 | 0.545 | −0.129 | 2.014 | 0.08 |
Note: n = sample size, slope = regression coefficient for MeHg, SE = standard error of slope, Lower = 2.5% lower confidence bound for slope, Upper = 97.5% upper confidence bound for slope. All outcomes were regressed on child’s 7 year hair MeHg (ppm) and the following covariates: mother’s age at delivery, maternal KBIT-2 matrices, prenatal MeHg from maternal hair at delivery (ppm), n-6:n-3 ratio, child’s age at 7 year testing, child sex (female = 1), family status at 7 years (both parents with child = 1), Hollingshead SES at 7 years.
To address the issue of transformed endpoints, which can result in models with less interpretable coefficients, we refit the models for the 17 primary endpoints using either untransformed or log10 transformed values of the endpoints. This approach altered the coefficients and their standard errors but did not change our conclusions. The details of the results of these secondary analyses can be found in the supplementary materials (Appendix 1).
The covariate slopes with 95% confidence intervals most commonly excluding zero (indicating an association) across the models for all 17 endpoints were Hollingshead SES, mother's KBIT-2 matrices score, and child sex. The direction of associations between these three covariates and endpoints were generally consistent within groups of tests (e.g., all CELF-5 endpoints). Prenatal MeHg exposure was not significantly associated with any of the endpoints. A summary of the covariate slopes across all 17 endpoints is available in supplementary materials (Appendix 2).
4. Discussion
This cross-sectional study of postnatal exposure found no evidence to support the hypothesis that concurrent postnatal MeHg exposure influences neurodevelopmental and neurobehavioral outcomes in childhood. The slopes of postnatal MeHg exposure were small in magnitude, and not statistically significant for any of the 17 neurodevelopmental outcomes. Previous cross-sectional analyses of the NC2 cohort found no significant associations between neurodevelopmental outcomes and prenatal MeHg exposure at either 20 months or 7 years of age (Strain et al., 2015; Strain et al., 2021). This study demonstrates a similar result for postnatal MeHg exposure while controlling for prenatal MeHg exposure and other covariates (Appendix 3).
More generally, the literature on postnatal MeHg exposure is mainly cross-sectional and associations with neurodevelopment are inconsistent. Some longitudinal birth cohort studies have reported associations between postnatal MeHg exposure and neurodevelopmental outcomes at differing ages throughout childhood, including the Faroese birth cohort (Murata et al., 2004; Debes et al., 2006; Grandjean et al., 2014), the Canadian Nunavik study of school-age Inuit children (Despres et al., 2005; Saint-Amour et al., 2006; Boucher et al., 2016), and the Spanish INMA birth cohort (Freire et al., 2010; Lozano et al., 2021). However, other studies have been unable to confirm such associations, sometimes in the same cohorts listed above that reported some adverse associations (Murata et al., 1999; Ha et al., 2009; Boucher et al., 2010; Cao et al., 2010; Plusquellec et al., 2010; Dorea et al., 2012; Deroma et al., 2013; Wang et al., 2014; Jacobson et al., 2015; Marques et al., 2015; Llop et al., 2020; Lee et al., 2021). Differences in study findings may be explained by methodological variability in exposure and outcome assessment across the different studies. Furthermore, some of the cohorts studied (e.g. Faroese and Nunavik cohorts) consumed sea mammals, resulting in co-exposure to multiple toxicants. While the brain is susceptible to postnatal MeHg exposure, these studies do not provide strong support for an association between postnatal MeHg exposure and neurodevelopment during childhood and adolescence.
Prior analysis of the SCDS Main Cohort using different postnatal exposure metrics that accounted for repeated measures of exposure over time did find some adverse associations with neurodevelopment outcomes. However, there was considerable uncertainty about the nature and implications of these findings (Davidson et al., 1998; Myers et al., 2009). A recent analysis of the Main Cohort using more refined time-weighted metrics of postnatal MeHg exposure (Thurston, Harrington, et al., 2022) also found some adverse associations (Thurston, Myers, et al., 2022). The time-weighted metrics accounted for the additional years of exposure data from following this cohort up to 24 years of age. Among the 85 neurodevelopmental outcomes measured in children from ages 9-24 years, there were 13 adverse associations and one beneficial association with a time-weighted postnatal MeHg exposure metric (Thurston, Myers, et al., 2022). None of the remaining associations with postnatal MeHg were statistically significant. Strengths of this prior analysis includes the use of a time-weighted metric that is potentially representative of a long-term postnatal exposure, and follow up of a relatively large cohort with ~80% success at 24 years. We noted, however, that the potential clinical significance of these findings was unclear and required follow-up in future cohorts. The present NC2 cohort also had lower average postnatal exposure levels of 2.5 ppm, as compared to 5.0-8.0 ppm in the Main Cohort (Thurston, Myers, et al., 2022). This lower exposure along with the lack of a longitudinal component may explain some of the differences in findings between these analyses. In addition, other than the Boston Naming Test, we did not measure the same neurodevelopmental outcomes in executive function, attention, and achievement domains that were associated with postnatal exposure in our previous paper.
Strengths of this study include excellent participant retention, the comprehensive nature of the neurodevelopmental examination administered at 7 years, and the fact that the environmental exposure to MeHg among the Seychellois population is from fish and thus not potentially confounded by co-exposures to other toxicants typically found at elevated levels in ocean mammals.
Limitations of the study include the relatively small number of participants, and the possibility that we did not collect or include some important covariates. We also view the cross-sectional nature of the current analysis as limitation, since an observation at a single point in time provides less information about exposure and how it relates to neurodevelopmental outcomes than repeated measures of exposure and outcomes throughout childhood and adolescence.
In conclusion, this analysis did not identify any associations between concurrent postnatal MeHg exposure at 7 years and a battery of 17 neurodevelopmental outcomes. Continued follow-up of the NC2 cohort into adolescence and adulthood, measuring postnatal exposure repeatedly in the entire cohort, and focusing on outcomes previously reported to be associated with MeHg exposure may help clarify any potential associations.
Highlights.
Fish consumption guidelines rely primarily on studies of prenatal exposure.
The literature on exposure to MeHg from fish and neurodevelopment is inconclusive.
We examined 17 neurodevelopmental outcomes in relation to postnatal MeHg exposure.
We found no association between postnatal MeHg and neurodevelopment at 7 years.
Strengths are the prospective design and comprehensive neurodevelopmental testing.
Acknowledgements
We acknowledge the participation of the nurses and laboratory personnel in Seychelles in the collection of data and biological samples.
This work was supported by the National Institutes of Health (grants R01-ES010219, P30-ES001247, T32-ES007271, and R24-ES029466-01) and in-kind support from the government of Seychelles. The content is the responsibility of the authors and does not represent the official views of the National Institutes of Health or any other federal agency.
Appendix
Appendix 1:
Regression model results for the main effect of MeHg exposure when endpoints were either log10 transformed or untransformed.
| Outcome | n | Slope | SE | Lower | Upper | p-value |
|---|---|---|---|---|---|---|
| log10, BNT total | 375 | 0.000 | 0.002 | −0.004 | 0.005 | 0.95 |
| CBCL total | 369 | −0.608 | 0.560 | −1.710 | 0.494 | 0.28 |
| CELF-5 total | 369 | 0.651 | 0.381 | −0.099 | 1.401 | 0.09 |
| CELF-5 FD | 376 | 0.186 | 0.100 | −0.010 | 0.382 | 0.06 |
| CELF-5 LC | 376 | 0.124 | 0.065 | −0.004 | 0.253 | 0.06 |
| CELF-5 RS | 374 | 0.170 | 0.179 | −0.183 | 0.522 | 0.35 |
| CELF-5 SC | 372 | 0.117 | 0.098 | −0.075 | 0.309 | 0.23 |
| CELF-5 USP | 374 | 0.027 | 0.080 | −0.130 | 0.185 | 0.73 |
| FT dominant hand | 376 | −0.084 | 0.117 | −0.315 | 0.146 | 0.47 |
| FT non-dominant hand | 376 | −0.005 | 0.108 | −0.217 | 0.207 | 0.96 |
| KBIT-2 word knowledge | 376 | 0.204 | 0.191 | −0.172 | 0.580 | 0.29 |
| KBIT-2 matrices | 376 | 0.178 | 0.121 | −0.061 | 0.416 | 0.14 |
| SCQ total | 369 | −0.042 | 0.101 | −0.241 | 0.157 | 0.68 |
| SRS total | 375 | 0.068 | 0.407 | −0.733 | 0.868 | 0.87 |
| log10, trailmaking time A | 337 | 0.003 | 0.005 | −0.006 | 0.012 | 0.46 |
| log10, WJ-III applied problems | 375 | 0.002 | 0.002 | −0.001 | 0.006 | 0.20 |
| WJ-III letter word | 374 | 0.942 | 0.545 | −0.129 | 2.014 | 0.08 |
Note: n = sample size, slope = regression coefficient for MeHg, SE = standard error of slope, Lower = 2.5% lower confidence bound for slope, Upper = 97.5% upper confidence bound for slope. All outcomes were regressed on child’s 7 year hair MeHg (ppm) and the following covariates: mother’s age at delivery, maternal KBIT-2 matrices, prenatal MeHg from maternal hair at delivery (ppm), n-6:n-3 ratio, child’s age at 7 year testing, child sex (female = 1), family status at 7 years (both parents with child = 1), Hollingshead SES at 7 years.
Appendix 2:
Coefficient values and 95% confidence intervals for covariates from models for all 17 transformed endpoints.
| Mom Age | Mom KBIT | Prenatal Hg | n-6:n-3 | Child Age (yrs) | Child Sex (F) | Family Status | SES | |
|---|---|---|---|---|---|---|---|---|
| square root, BNT total | 0 (−0.01, 0.01) | 0.01 (0, 0.02) | 0 (−0.01, 0.01) | 0.02 (−0.01, 0.05) | −0.11 (−0.7, 0.49) | 0 (−0.1, 0.1) | 0.15 (0.04, 0.25) | 0.01 (0.01, 0.02) |
| square root, CBCL total | −0.05 (−0.08, −0.01) | −0.03 (−0.06, 0) | 0 (−0.04, 0.05) | 0.05 (−0.06, 0.17) | 2.11 (−0.08, 4.29) | −0.87 (−1.23, −0.51) | 0.06 (−0.32, 0.44) | −0.05 (−0.07, −0.03) |
| CELF-5 total | 0.04 (−0.24, 0.32) | 0.3 (0.04, 0.55) | −0.25 (−0.63, 0.14) | 0.84 (−0.12, 1.81) | −4.15 (−24.6, 16.3) | 4.67 (1.53, 7.81) | −1.56 (−4.83, 1.71) | 0.28 (0.12, 0.44) |
| CELF-5 FD | 0.05 (−0.02, 0.13) | 0.05 (−0.02, 0.11) | −0.09 (−0.2, 0.01) | 0.2 (−0.06, 0.45) | −1.27 (−6.58, 4.03) | 0.71 (−0.1, 1.52) | −0.17 (−1.02, 0.68) | 0.04 (0, 0.09) |
| squared, CELF-5 LC | 1.08 (−0.36, 2.53) | 1.03 (−0.25, 2.32) | −1.69 (−3.67, 0.29) | 6.18 (1.23, 11.12) | −22.08 (−126.05, 81.89) | 16.12 (0.16, 32.09) | −8.96 (−25.63, 7.7) | 1 (0.18, 1.81) |
| CELF-5 RS | −0.03 (−0.16, 0.1) | 0.05 (−0.07, 0.17) | −0.07 (−0.25, 0.11) | 0.3 (−0.16, 0.75) | −1.67 (−11.24, 7.91) | 1.33 (−0.14, 2.8) | −0.41 (−1.94, 1.13) | 0.08 (0, 0.16) |
| squared, CELF-5 SC | −1.1 (−3.49, 1.29) | 3.21 (1.07, 5.36) | −1.38 (−4.66, 1.9) | 1.84 (−6.38, 10.06) | 11.57 (−161.47, 184.61) | 31.32 (4.72, 57.93) | −6.22 (−33.97, 21.53) | 3.04 (1.69, 4.39) |
| square root, CELF-5 USP | 0.01 (−0.01, 0.02) | 0.01 (0, 0.03) | 0 (−0.02, 0.02) | 0.03 (−0.02, 0.08) | −0.22 (−1.29, 0.85) | 0.3 (0.14, 0.47) | −0.06 (−0.23, 0.12) | 0.01 (0, 0.02) |
| FT dominant hand | 0.01 (−0.07, 0.1) | −0.02 (−0.09, 0.06) | −0.05 (−0.17, 0.07) | −0.08 (−0.38, 0.22) | 3.7 (−2.04, 9.44) | −0.79 (−1.75, 0.17) | 0.22 (−0.79, 1.22) | 0.1 (0.05, 0.15) |
| FT non-dominant hand | 0.1 (0.02, 0.18) | 0.01 (−0.07, 0.08) | −0.12 (−0.23, −0.01) | −0.1 (−0.38, 0.17) | 5.59 (0.31, 10.86) | −1.64 (−2.52, −0.75) | −0.21 (−1.13, 0.72) | 0.07 (0.03, 0.12) |
| KBIT-2 word knowledge | −0.04 (−0.18, 0.1) | 0.06 (−0.07, 0.18) | −0.11 (−0.3, 0.08) | 0.39 (−0.09, 0.88) | −5.42 (−14.77, 3.94) | 0.45 (−1.12, 2.02) | 0.58 (−1.06, 2.21) | 0.03 (−0.05, 0.11) |
| KBIT-2 matrices | 0.02 (−0.07, 0.11) | 0.1 (0.02, 0.18) | 0.04 (−0.08, 0.16) | 0.14 (−0.17, 0.44) | −1.25 (−7.19, 4.69) | 0.46 (−0.54, 1.45) | −0.43 (−1.47, 0.61) | 0.08 (0.03, 0.13) |
| square root, SCQ total | −0.01 (−0.02, 0.01) | −0.02 (−0.03, −0.01) | −0.01 (−0.03, 0.01) | 0 (−0.05, 0.05) | 0.16 (−0.81, 1.12) | −0.28 (−0.44, −0.12) | −0.11 (−0.28, 0.06) | −0.01 (−0.02, 0) |
| square root, SRS total | −0.02 (−0.04, 0) | −0.03 (−0.05, −0.01) | 0.01 (−0.02, 0.04) | −0.01 (−0.09, 0.06) | −0.31 (−1.76, 1.14) | −0.35 (−0.6, −0.11) | −0.19 (−0.44, 0.07) | −0.04 (−0.05, −0.03) |
| log10, trailmaking time A | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.01) | −0.01 (−0.02, 0) | 0.01 (−0.22, 0.24) | −0.04 (−0.08, −0.01) | −0.01 (−0.05, 0.03) | 0 (0, 0) |
| squared, WJ-III applied | 2.97 (0.15, 5.8) | 2.41 (−0.11, 4.93) | −1.41 (−5.25, 2.44) | −0.67 (−10.31, 8.98) | 66.44 (−119.78, 252.66) | 37.73 (6.5, 68.96) | −14.35 (−46.97, 18.27) | 3.02 (1.42, 4.62) |
| WJ-III letter word | 0.26 (−0.15, 0.66) | 0.36 (0, 0.72) | −0.35 (−0.9, 0.2) | 0.44 (−0.94, 1.83) | −12.6 (−39.32, 14.12) | 3.53 (−0.95, 8) | −0.9 (−5.57, 3.78) | 0.39 (0.16, 0.62) |
Appendix 3:
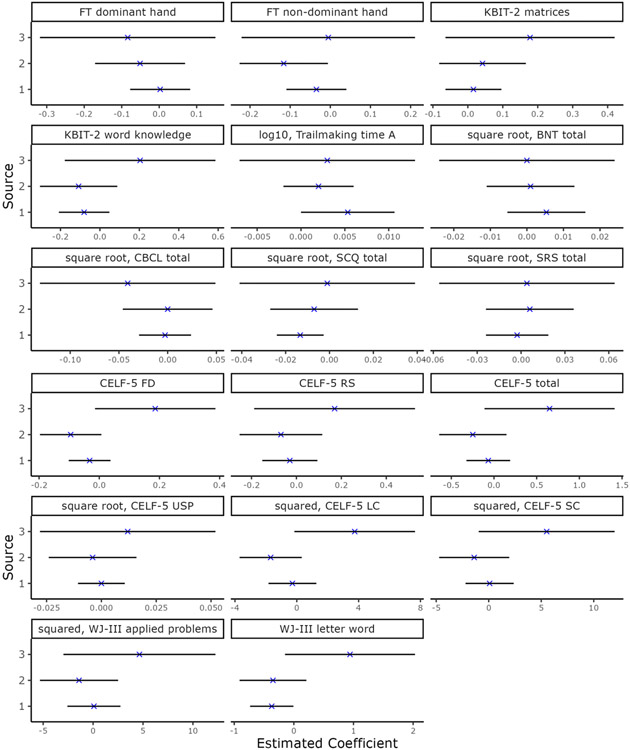
Comparison of regression coefficients of (1) prenatal MeHg from Strain, et al. (2021)*, (2) prenatal MeHg from the present analysis, (3) postnatal MeHg at 7 years from the present analysis. Each plot denotes the point estimate and 95% confidence interval (Coef. ± 2*SE) for the regression coefficient. Strain, et al. accounted for prenatal exposure only (n=1237), while the present analysis included prenatal and postnatal MeHg exposure in the same model (n=376).
*Strain, et al. (2021) reported prenatal MeHg exposure regression coefficients in terms of an IQR difference (3.75 ppm). The coefficients and corresponding standard errors from Strain in the figure above were rescaled so that they are comparable to those from the present analysis, i.e. they correspond to a 1 ppm difference in MeHg exposure.
6. References
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, & Doherty RA (1973). Methylmercury poisoning in Iraq. Science, 181(4096), 230–241. 10.1126/science.181.4096.230 [DOI] [PubMed] [Google Scholar]
- Boucher O, Bastien CH, Saint-Amour D, Dewailly E, Ayotte P, Jacobson JL, Jacobson SW, & Muckle G (2010). Prenatal exposure to methylmercury and PCBs affects distinct stages of information processing: an event-related potential study with Inuit children. Neurotoxicology, 31(4), 373–384. 10.1016/j.neuro.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Ayotte P, Dewailly E, Jacobson SW, & Jacobson JL (2016). Altered fine motor function at school age in Inuit children exposed to PCBs, methylmercury, and lead. Environ Int, 95, 144–151. 10.1016/j.envint.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Chen A, Jones RL, Radcliffe J, Caldwell KL, Dietrich KN, & Rogan WJ (2010). Does background postnatal methyl mercury exposure in toddlers affect cognition and behavior? Neurotoxicology, 31(1), 1–9. 10.1016/j.neuro.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, Shamlaye CF, Berlin M, Davidson PW, & Clarkson TW (1995). Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. Neurotoxicology, 16(4), 705–710. https://www.ncbi.nlm.nih.gov/pubmed/8714874 [PubMed] [Google Scholar]
- Choi BH (1989). The effects of methylmercury on the developing brain. Prog Neurobiol, 32(6), 447–470. 10.1016/0301-0082(89)90018-x [DOI] [PubMed] [Google Scholar]
- Clarkson TW, & Magos L (2006). The toxicology of mercury and its chemical compounds. Crit Rev Toxicol, 36(8), 609–662. 10.1080/10408440600845619 [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, & Clarkson TW (1998). Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA, 280(8), 701–707. 10.1001/jama.280.8.701 [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jorgensen E, Weihe P, White RF, & Grandjean P (2006). Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol, 28(5), 536–547. 10.1016/j.ntt.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Deroma L, Parpinel M, Tognin V, Channoufi L, Tratnik J, Horvat M, Valent F, & Barbone F (2013). Neuropsychological assessment at school-age and prenatal low-level exposure to mercury through fish consumption in an Italian birth cohort living near a contaminated site. Int J Hyg Environ Health, 216(4), 486–493. 10.1016/j.ijheh.2013.02.004 [DOI] [PubMed] [Google Scholar]
- Despres C, Beuter A, Richer F, Poitras K, Veilleux A, Ayotte P, Dewailly E, Saint-Amour D, & Muckle G (2005). Neuromotor functions in Inuit preschool children exposed to Pb, PCBs, and Hg. Neurotoxicol Teratol, 27(2), 245–257. 10.1016/j.ntt.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Dorea JG, Marques RC, & Isejima C (2012). Neurodevelopment of Amazonian infants: antenatal and postnatal exposure to methyl- and ethylmercury. J Biomed Biotechnol, 2012, 132876. 10.1155/2012/132876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO. (2011). Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption. W. H. Organization. [Google Scholar]
- FDA/EPA. (2022). Advice about Eating Fish. Retrieved July 7 from https://www.fda.gov/food/consumers/advice-about-eating-fish [Google Scholar]
- Freire C, Ramos R, Lopez-Espinosa MJ, Diez S, Vioque J, Ballester F, & Fernandez MF (2010). Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ Res, 110(1), 96–104. 10.1016/j.envres.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Debes F, Choi AL, & Budtz-Jorgensen E (2014). Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol Teratol, 43, 39–44. 10.1016/j.ntt.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Kwon HJ, Lim MH, Jee YK, Hong YC, Leem JH, Sakong J, Bae JM, Hong SJ, Roh YM, & Jo SJ (2009). Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: a report of the children's health and environment research (CHEER). Neurotoxicology, 30(1), 31–36. 10.1016/j.neuro.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Harada M (1995). Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol, 25(1), 1–24. 10.3109/10408449509089885 [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Muckle G, Ayotte P, Dewailly E, & Jacobson SW (2015). Relation of Prenatal Methylmercury Exposure from Environmental Sources to Childhood IQ. Environ Health Perspect, 123(8), 827–833. 10.1289/ehp.1408554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kim KN, Ahn YD, Choi YJ, Cho J, Jang Y, Lim YH, Kim JI, Shin CH, Lee YA, Kim BN, & Hong YC (2021). Prenatal and postnatal exposures to four metals mixture and IQ in 6-year-old children: A prospective cohort study in South Korea. Environ Int, 157, 106798. 10.1016/j.envint.2021.106798 [DOI] [PubMed] [Google Scholar]
- Llop S, Murcia M, Amoros R, Julvez J, Santa-Marina L, Soler-Blasco R, Rebagliato M, Iniguez C, Aguinagalde X, Iriarte G, Lopez-Espinosa MJ, Andiarena A, Gonzalez L, Vioque J, Sunyer J, & Ballester F (2020). Postnatal exposure to mercury and neuropsychological development among preschooler children. Eur J Epidemiol, 35(3), 259–271. 10.1007/s10654-020-00620-9 [DOI] [PubMed] [Google Scholar]
- Lozano M, Murcia M, Soler-Blasco R, Gonzalez L, Iriarte G, Rebagliato M, Lopez-Espinosa MJ, Esplugues A, Ballester F, & Llop S (2021). Exposure to mercury among 9-year-old children and neurobehavioural function. Environ Int, 146, 106173. 10.1016/j.envint.2020.106173 [DOI] [PubMed] [Google Scholar]
- Marques RC, Bernardi JV, Abreu L, & Dorea JG (2015). Neurodevelopment outcomes in children exposed to organic mercury from multiple sources in a tin-ore mine environment in Brazil. Arch Environ Contam Toxicol, 68(3), 432–441. 10.1007/s00244-014-0103-x [DOI] [PubMed] [Google Scholar]
- Murata K, Weihe P, Araki S, Budtz-Jorgensen E, & Grandjean P (1999). Evoked potentials in Faroese children prenatally exposed to methylmercury. Neurotoxicol Teratol, 21(4), 471–472. 10.1016/s0892-0362(99)00026-4 [DOI] [PubMed] [Google Scholar]
- Murata K, Weihe P, Budtz-Jorgensen E, Jorgensen PJ, & Grandjean P (2004). Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J Pediatr, 144(2), 177–183. 10.1016/j.jpeds.2003.10.059 [DOI] [PubMed] [Google Scholar]
- Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, Cernichiari E, & Clarkson TW (2009). Postnatal exposure to methyl mercury from fish consumption: a review and new data from the Seychelles Child Development Study. Neurotoxicology, 30(3), 338–349. 10.1016/j.neuro.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Begin G, Desrosiers C, Despres C, Saint-Amour D, & Poitras K (2010). The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology, 31(1), 17–25. 10.1016/j.neuro.2009.10.008 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A language and environment for statistical computing. In R Foundation for Statistical Computing. [Google Scholar]
- Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Despres C, Gingras S, & Muckle G (2006). Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology, 27(4), 567–578. 10.1016/j.neuro.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Seber G, & Lee A (2003). Linear Regression Analysis (2 ed.). John Wiley & Sons. [Google Scholar]
- Shonkoff J, Phillips D, & Council NR (2000). US Committee on Integrating the Science of Early Childhood Development. From neurons to neighborhoods: the science of early child development. In: Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- Spyker JM, Sparber SB, & Goldberg AM (1972). Subtle consequences of methylmercury exposure: behavioral deviations in offspring of treated mothers. Science, 177(4049), 621–623. 10.1126/science.177.4049.621 [DOI] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Thurston SW, Harrington D, Mulhern MS, McAfee AJ, van Wijngaarden E, Shamlaye CF, Henderson J, Watson GE, Zareba G, Cory-Slechta DA, Lynch M, Wallace JM, McSorley EM, Bonham MP, Stokes-Riner A, Sloane-Reeves J, Janciuras J, … Myers GJ (2012). Maternal PUFA status but not prenatal methylmercury exposure is associated with children's language functions at age five years in the Seychelles. J Nutr, 142(11), 1943–1949. 10.3945/jn.112.163493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Love TM, Yeates AJ, Weller D, Mulhern MS, McSorley EM, Thurston SW, Watson GE, Mruzek D, Broberg K, Rand MD, Henderson J, Shamlaye CF, Myers GJ, Davidson PW, & van Wijngaarden E (2021). Associations of prenatal methylmercury exposure and maternal polyunsaturated fatty acid status with neurodevelopmental outcomes at 7 years of age: results from the Seychelles Child Development Study Nutrition Cohort 2. Am J Clin Nutr, 113(2), 304–313. 10.1093/ajcn/nqaa338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Yeates AJ, van Wijngaarden E, Thurston SW, Mulhern MS, McSorley EM, Watson GE, Love TM, Smith TH, Yost K, Harrington D, Shamlaye CF, Henderson J, Myers GJ, & Davidson PW (2015). Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr, 101(3), 530–537. 10.3945/ajcn.114.100503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, & Peterson BS (2010). Normal development of brain circuits. Neuropsychopharmacology, 35(1), 147–168. 10.1038/npp.2009.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston SW, Harrington D, Mruzek DW, Shamlaye C, Myers GJ, & van Wijngaarden E (2022). Development of a long-term time-weighted exposure metric that accounts for missing data in the Seychelles Child Development Study. Neurotoxicology, 92, 49–60. 10.1016/j.neuro.2022.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston SW, Myers G, Mruzek D, Harrington D, Adams H, Shamlaye C, & van Wijngaarden E (2022). Associations between time-weighted postnatal methylmercury exposure from fish consumption and neurodevelopmental outcomes through 24 years of age in the Seychelles Child Development Study Main Cohort. Neurotoxicology, 91, 234–244. 10.1016/j.neuro.2022.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden E, Thurston SW, Myers GJ, Harrington D, Cory-Slechta DA, Strain JJ, Watson GE, Zareba G, Love T, Henderson J, Shamlaye CF, & Davidson PW (2017). Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24years. Neurotoxicol Teratol, 59, 35–42. 10.1016/j.ntt.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejrup K, Schjolberg S, Knutsen HK, Kvalem HE, Brantsaeter AL, Meltzer HM, Alexander J, Magnus P, & Haugen M (2016). Prenatal methylmercury exposure and language delay at three years of age in the Norwegian Mother and Child Cohort Study. Environ Int, 92-93, 63–69. 10.1016/j.envint.2016.03.029 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen A, Dietrich KN, Radcliffe J, Caldwell KL, & Rogan WJ (2014). Postnatal exposure to methyl mercury and neuropsychological development in 7-year-old urban inner-city children exposed to lead in the United States. Child Neuropsychol, 20(5), 527–538. 10.1080/09297049.2013.824955 [DOI] [PMC free article] [PubMed] [Google Scholar]