Abstract
Breast cancer chemoprevention with selective estrogen receptor modulators (SERMs) or aromatase inhibitors (AIs) remains underutilized among high-risk women. A potential barrier to chemoprevention is competing comorbidities such as atherosclerotic cardiovascular disease (ASCVD), due to concern for additional medication side effects. We conducted a retrospective cohort study among women with atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS), an important target population for chemoprevention. We compared risks for breast cancer and ASCVD, as well as use of SERMs/AIs vs. statins among high-risk women (defined as a 5-year invasive breast cancer risk ≥1.67% and 10-year ASCVD risk ≥7.5%, respectively). We used clinical data extracted from the electronic health record (EHR) to calculate breast cancer risk according to the Breast Cancer Surveillance Consortium (BCSC) model and ASCVD risk according to the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) risk calculator. Among 298 evaluable women, mean age was 58.2 years (SD, 8.34), with 33% non-Hispanic White, 41% Hispanic, 9% non-Hispanic Black, 6% Asian, and 11% other/unknown race/ethnicity. About 98% of women met high-risk criteria for breast cancer, whereas 30% were high-risk for ASCVD. Mean 10-year risk of breast cancer was higher than mean 10-year risk of ASCVD (9.14% vs. 6.69%, p<0.001). Among women who met high-risk criteria for both diseases, use of statins was higher compared to SERMs/AIs (58% vs. 21%, p<0.001). Among women with AH or LCIS, statin use was higher compared to breast cancer chemoprevention among eligible women, despite having a higher mean risk of breast cancer than ASCVD.
INTRODUCTION
Women with high-risk breast lesions, such as atypical hyperplasia (AH) and lobular carcinoma in situ (LCIS), have a 4- to 10-fold increased risk of invasive breast cancer compared to those with non-proliferative breast disease [1]. An estimated 10 million U.S. women, age 35 to 79 years, are eligible for breast cancer chemoprevention with selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene, and aromatase inhibitors (AIs), such as exemestane and anastrozole, for 5 years [2]. In randomized controlled trials, chemoprevention has been shown to reduce breast cancer incidence by 50-65% among high-risk women, with relative risk reduction of 70-80% among women with AH or LCIS [3]. Recently, low-dose tamoxifen (5 mg daily) taken for 3 years has been shown to reduce breast cancer events by 52% in women with AH or LCIS [4]. Despite this evidence, chemoprevention use among high-risk women remains low, at under 15% [5]. Reasons include lack of routine breast cancer risk assessment in the primary care setting, lack of patient and physician knowledge about SERMs and AIs, insufficient time for counseling, and concerns about side effects [2, 6].
Atherosclerotic cardiovascular disease (ASCVD) is one of the most common chronic diseases [7]. HMG CoA reductase inhibitors, or “statins,” have been used for the primary prevention of ASCVD, with a relative risk reduction of ~25% for major vascular events, such as myocardial infarction, stroke, and coronary revascularization [8]. About 50% of eligible U.S. patients report statin use for the primary prevention of ASCVD [9]. Another barrier for chemoprevention use is competing comorbidities [2, 6]. Patients who are at increased risk for both ASCVD and breast cancer, and are on a statin, may not see breast cancer chemoprevention as a priority and may also be concerned about increased toxicities.
To place chemoprevention use in the context of medications used to prevent other chronic diseases, we compared breast cancer and ASCVD risks among women with AH or LCIS, an important target population for chemoprevention, as well as use of SERMs and AIs vs. statins among women at high-risk for both conditions. We hypothesized that women with high-risk breast lesions would have a higher mean 10-year risk of invasive breast cancer compared to ASCVD, but eligible women would have lower use of SERMs or AIs compared to statins.
METHODS
We conducted a retrospective cohort study among women, age 40-74 years, who were diagnosed with AH or LCIS from 2007-2015 at Columbia University Irving Medical Center (CUIMC) in New York City. We excluded women who had a prior history of invasive breast cancer or ductal carcinoma in situ (DCIS), bilateral mastectomies or breast augmentation, or incomplete data in the electronic health record (EHR) to calculate breast cancer and ASCVD risk. We restricted to women, age 40-74 years, because these are the ages that overlap for use of the Breast Cancer Surveillance Consortium (BCSC) model and 2013 American College of Cardiology (ACC)/American Heart Association (AHA) ASCVD risk calculator. This study was approved by the Institutional Review Board at CUIMC and conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
We extracted clinical data from the EHR to calculate women’s 5- and 10-year invasive breast cancer risk according to the BCSC risk calculator [10] and 10-year ASCVD risk using the 2013 ACC/AHA ASCVD risk calculator (https://www.mdcalc.com/calc/3398/ascvd-atherosclerotic-cardiovascular-disease-2013-risk-calculator-aha-acc). Breast cancer risk factors included: 1) age; 2) race/ethnicity (White, Black, Hispanic, Asian, Native American, Other/Multiple Races, Unknown); 3) first-degree family history of breast cancer (yes, no, unknown); 4) breast biopsy results based upon previous breast pathology reports and diagnostic codes (ICD-9/10 diagnostic codes of 610.9/N60.99 and 233.0/D05.90 for AH and LCIS, respectively), defined as the most advanced lesion (LCIS>AH); 6) mammographic density according to the Breast Imaging and Reporting Data System (BI-RADS) 4-category system on the mammogram report (almost entirely fatty; scattered fibroglandular densities; heterogeneously dense; extremely dense). Additional ASCVD risk factors included most recent EHR data on: 1) systolic blood pressure; 2) total cholesterol (100-333 mg/dL); 3) HDL cholesterol (17-124 mg/dL); 4) history of diabetes (yes/no, based upon ICD-9/10 diagnostic codes 250, E08, E09, E10, E11, E12, E13); 5) current treatment for hypertension (yes/no, based upon inclusion of any antihypertensive in the medication list); 6) current smoking status (yes/no). High-risk criteria for breast cancer and ASCVD to determine eligibility for SERMs/AIs and statins were defined as a 5-year invasive breast cancer risk ≥1.67% and a 10-year ASCVD risk ≥7.5%, respectively. We also assessed use of statins and chemoprevention (tamoxifen, raloxifene, anastrozole, exemestane) among eligible women based upon medication list in the EHR with follow-up until April 2021.
We performed descriptive statistics for all baseline breast cancer and ASCVD risk factors, stratified by race (White vs Non-White). For continuous variables, we compared means (standard deviations) using 2-sample t-tests, and we performed chi-squared tests for categorical variables. For the entire cohort, we compared mean 10-year invasive breast cancer and 10-year ASCVD risk using a 2-sample t-test. Among women who met high-risk criteria for both breast cancer and ASCVD, we used McNemar’s test to compare use of SERMs/AIs vs. statins. All statistical analyses were performed using SAS studio.
DATA AVAILABILITY STATEMENT:
Data were generated by the authors and available on request.
RESULTS
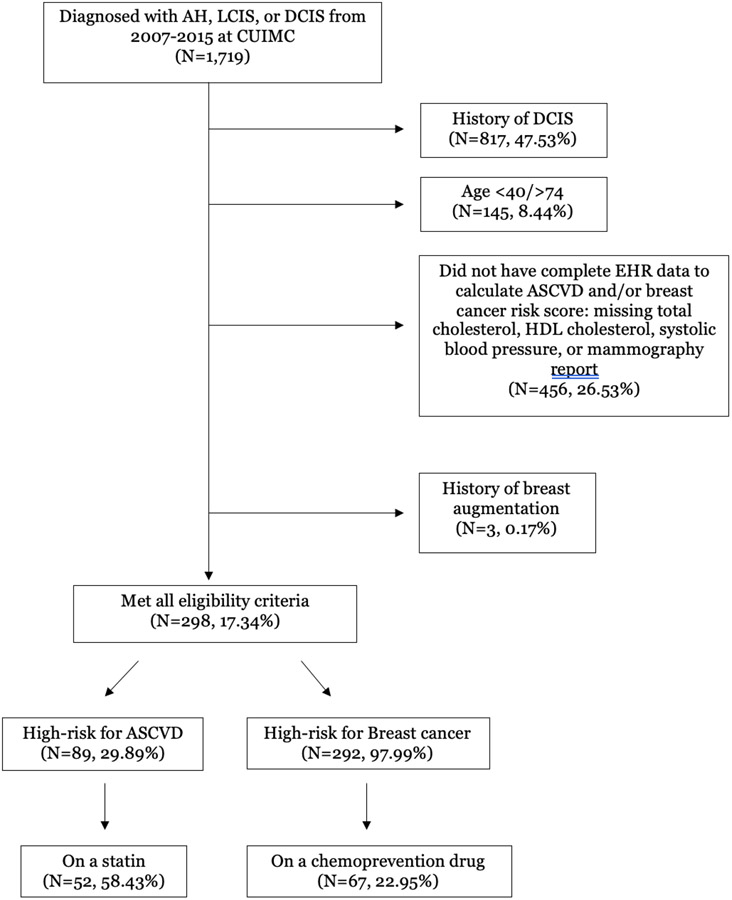
Of 1,719 women diagnosed with AH, LCIS, or DCIS at CUIMC from 2007-2015 [11], we excluded those with DCIS, age <40 or >74 years, prior breast augmentation, or incomplete clinical data in the EHR to calculate breast cancer and ASCVD risk (Figure 1). Among 298 evaluable women (Table 1), mean age was 58.2 years (SD, 8.34). Our study population included 40.9% Hispanic, 32.6% non-Hispanic White, 8.7% non-Hispanic Black, 6.4% Asian, and 11.4% other/unknown race/ethnicity. In terms of breast cancer risk factors, 22% had a first-degree relative with breast cancer, 57% had heterogeneously or extremely dense breasts, 64% had AH and 36% had LCIS. In terms of ASCVD risk factors, 29% had a history of diabetes, 48% were being treated with antihypertensives, and 3% were current smokers.
Figure 1.
CONSORT diagram depicting eligibility criteria and characteristics of women in final cohort
Table 1.
Baseline characteristics of women, age 40-74 years, with a history of AH or LCIS, undergoing screening mammography at Columbia University Irving Medical Center, New York, NY, stratified by Non-Hispanic White vs Racial/Ethnic Minority
| Characteristic | White (N=97, 32.6%) |
Non-White (N=201, 67.5%) |
Total (N=298) |
P-value |
|---|---|---|---|---|
| Mean age (SD) | 60.5 (8.4) | 57.1 (8.2) | 58.2 (8.3) | 0.001 |
| Mean systolic blood pressure (SD), mm Hg | 120.6 (16.3) | 127.8 (15.4) | 125.4 (16.0) | <.001 |
| Mean total cholesterol (SD), mg/dL | 202.7 (39.7) | 195.6 (43.4) | 197.9 (42.3) | 0.174 |
| Mean HDL cholesterol (SD), mg/dL | 69.7 (19.7) | 60.1 (17.2) | 63.2 (18.6) | <.001 |
| History of diabetes, N (%) | ||||
| Yes | 15 (15.5) | 71 (35.3) | 86 (28.9) | <.001 |
| No | 82 (84.5) | 130 (64.7) | 212 (71.1) | |
| Treatment for hypertension, N (%) | ||||
| Yes | 37 (38.1) | 107 (53.2) | 144 (48.3) | 0.015 |
| No | 60 (61.9) | 94 (46.8) | 154 (51.7) | |
| Current smoker, N (%) | ||||
| Yes | 2 (2.1) | 6 (3.0) | 8 (2.7) | 0.644 |
| No | 95 (97.9) | 195 (97.0) | 290 (97.3) | |
| High-risk for ASCVD, N (%) | ||||
| Yes | 33 (34.0) | 56 (24.9) | 89 (29.9) | 0.276 |
| No | 64 (66.0) | 145 (72.1) | 209 (70.1) | |
| Statin use, N (%) | ||||
| Yes | 29 (29.9) | 76 (37.8) | 105 (35.2) | 0.180 |
| No | 68 (70.1) | 125 (62.2) | 193 (64.8) | |
| Mean 10-year ASCVD risk, % (SD) | 6.3 (7.2) | 6.9 (8.4) | 6.7 (8.0) | 0.526 |
| First-degree relatives with breast cancer, N (%) | ||||
| Yes | 25 (25.8) | 40 (19.9) | 65 (21.8) | 0.469 |
| No | 70 (72.2) | 158 (78.6) | 228 (76.5) | |
| Unknown | 2 (2.1) | 3 (1.5) | 5 (1.7) | |
| Prior breast biopsy result, N (%) | ||||
| AH | 47 (48.5) | 145 (72.1) | 192 (64.4) | <.001 |
| LCIS | 50 (51.6) | 56 (27.9) | 106 (35.6) | |
| BI-RADS breast density, N (%) | ||||
| Almost entirely fatty | 2 (2.1) | 4 (2.0) | 6 (2.0) | 0.191 |
| Scattered fibroglandular densities | 32 (33.0) | 89 (44.3) | 121 (40.6) | |
| Heterogeneously dense | 60 (61.9) | 106 (52.7) | 166 (55.7) | |
| Extremely dense | 3 (3.1) | 2 (1.0) | 5 (1.7) | |
| High-risk for breast cancer, N (%) | ||||
| Yes | 97 (100.0) | 195 (97.0) | 292 (98.0) | 0.086 |
| No | 0 (0.0) | 6 (3.0) | 6 (2.0) | |
| Chemoprevention use, N (%) | ||||
| Yes | 23 (23.7) | 45 (22.4) | 68 (22.8) | 0.799 |
| No | 74 (76.3) | 156 (77.6) | 230 (77.2) | |
| Mean 10-year breast cancer risk, % (SD) | 12.1 (5.3) | 7.7 (3.2) | 9.1 (4.5) | <.001 |
- Abbreviations:
- ASCVD: Atherosclerotic Cardiovascular Disease
- AH: Atypical hyperplasia
- LCIS: Lobular carcinoma in situ
- BI-RADS: Breast Imaging and Reporting Data System
- Non-White includes Hispanic, non-Hispanic Black, Asian, and other/unknown race/ethnicity
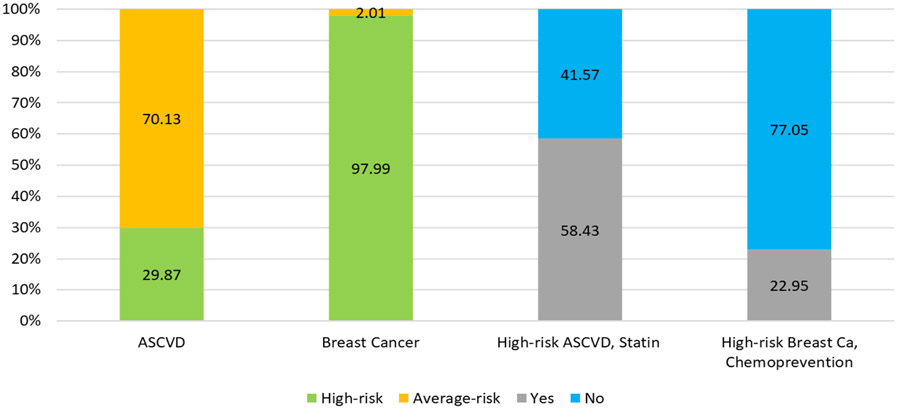
Majority (98%) met high-risk criteria for breast cancer, whereas 30% met high-risk criteria for ASCVD (Figure 2). In the entire cohort, mean 10-year risk of breast cancer was higher than ASCVD (9.14% vs. 6.69%, p<0.001). Among women who met high-risk criteria for both conditions, use of statins was 58% vs. 21% for SERMs/AIs (p<0.001).
Figure 2:
Proportion of the total population which is high-risk for ASCVD or high-risk for breast cancer and subsequently on a statin or taking chemoprevention.
Compared to White women (Table 1), non-White women were younger, had higher mean systolic blood pressure and lower mean HDL cholesterol, as well as a higher prevalence of diabetes and use of antihypertensives, however, there was no difference in mean 10-year ASCVD risk or statin use. In terms of breast cancer risk, Whites had a higher mean 10-year invasive breast cancer risk compared to non-Whites (12.12% vs. 7.71%, p<0.001), however, there was no significant difference in chemoprevention use (23.71% vs. 22.39%, respectively).
DISCUSSION
We demonstrated that among high-risk women with AH or LCIS, use of statins was higher than chemoprevention with SERMs or AIs among eligible women, despite having a greater risk for breast cancer compared to ASCVD. We also showed that there was no difference in mean 10-year ASCVD risk or statin use among Whites and non-Whites, but White women had a higher mean 10-year invasive breast cancer risk compared, although rates of chemoprevention use were similar. From the initial cohort of 1719 women, one-third of women with AH/LCIS and DCIS were seen by a medical oncologist, which was associated with a >6-fold increase in chemoprevention use with a SERM or AI compared to those not referred [11].
ASCVD and breast cancer are two of the most common chronic diseases affecting women in the U.S. and they share several risk factors, such as obesity, dyslipidemia, hypertension, and diabetes [7]. Although the use of statins for ASCVD prevention is widely adopted, the same is not the case with breast cancer chemoprevention, and our findings are consistent with this. In 2011-2012, approximately 1 in 4 adults (23.2%) over the age of 40 reported statin use within the past 30 days [12]. Additionally, about half of eligible U.S. patients are currently on a statin for primary prevention of ASCVD [9]. This contrasts with the less than 15% of eligible high-risk women taking chemoprevention [5]. We specifically focused on women with AH or LCIS, since they derive the greatest benefit from SERMs and AIs [3], but even among this high-risk group, only about 29.4% received chemoprevention [11].
By comparing low chemoprevention to statin use, we demonstrate that there is still much room for the promotion of chemoprevention use in the primary care setting. Major reasons for low chemoprevention use include lack of routine breast cancer risk assessment in the primary care setting, lack of patient and physician knowledge about chemoprevention, fears of side effects from chemoprevention, costs, and competing comorbidities [2, 6].
One contributing factor to the large difference in use of statins versus chemoprevention could be their differences in mortality benefits. Statin therapy for primary prevention of CVD is associated with reduced risk of all-cause mortality and CVD events [8, 13], with a risk ratio [RR]=0.92, 95% confidence interval [CI]=0.87-0.98), stroke (RR=0.78, 95% CI=0.68-0.90), and myocardial infarction (RR=0.67, 95% CI=0.60-0.75) [13]. However, data on the absolute benefit of statins in terms of all-cause mortality, myocardial infarction, and stroke are modest, at 0.8%, 1.3%, and 0.4%, respectively [14]. By comparison, although chemoprevention has been shown to reduce breast cancer incidence, randomized controlled trials have not demonstrated an overall or breast cancer-specific mortality benefit in the prevention setting [2, 15]. This is largely due to low numbers of breast cancer events, limited statistical power of trials, and the potential for crossover in the placebo arms once the trial results were reported [15].
Moreover, among women who are high risk for both ASCVD and breast cancer, chemoprevention use may be low due to concerns about the effects of chemoprevention on ASCVD. Although most research has found that SERMs have a protective effect on the heart by lowering LDL cholesterol and blood pressure, there is conflicting evidence regarding the effects of AIs on ASCVD [16, 17]. Some studies have shown AIs to be associated with increased risk of myocardial infarction and angina, but it is possible that these results arise from comparing AI to SERM users [17]. Other research suggests that AIs, when taken for a duration of more than four years, appear to be associated with increased risk of ischemic heart disease and arrhythmias [17]. Given the potentially negative effects of AIs on ASCVD, the decision to begin these medications should be based on a shared decision-making process that accounts for each patient’s other chronic diseases. Interestingly, among women with breast cancer, use of statins along with AI therapy has a favorable outcome on breast cancer recurrence and mortality, mitigating the negative effects of AIs on ASCVD [16]. This information is useful in discussing concerns about medication interactions with patients, and in explaining that chemoprevention and statin use do not have to be mutually exclusive.
Our results are largely consistent with those of a 57-study meta-analysis in which race/ethnicity was not found to be strongly associated with chemoprevention use [18]. However, other studies have had conflicting results, with Trivedi et al. [11] reporting a trend toward increased chemoprevention use in Hispanic women (p=0.075) and Kaplan et al. reporting decreased use by Hispanic women compared to Whites, Blacks, and Asians [19]. These racial/ethnic disparities may be due to a lack of knowledge about these risk-reducing strategies [20]. Additionally, White women tend to have a higher risk of developing breast cancer compared to non-White women, and this might influence chemoprevention use [2].
Several studies, including a large 2013 meta-analysis, have found that statin use and adherence tend to be lower in non-White patients [21]. In our results, we found no statistically significant difference in statin use between White and non-White women with high-risk breast lesions. Unlike prior studies which showed that racial minorities (especially Blacks) tend to have higher rates of ASCVD [22], we found no difference in mean 10-year ASCVD risk score between White and non-White women at high risk for breast cancer. However, the meta-analysis by Smith et al. and the other studies on chemoprevention use by race/ethnicity were not restricted to women with high-risk breast lesions [18].
Our study has several strengths, including having a racially/ethnically diverse population of women who are understudied in cancer prevention studies, and the well-annotated clinical data from the EHR for calculating breast cancer and ASCVD risk. Limitations include the relatively modest sample size. Additionally, our study was conducted at a single institution, at an urban academic setting, therefore the results may not be generalizable to all women, such as those in community practices or more rural settings. We also excluded several women seen at the CUIMC breast clinic for management of AH or LCIS who had missing data for calculating ASCVD risk, which may have caused selection bias. Due to inconsistent documentation in the medical record, we did not conduct a manual chart review to ascertain whether eligible patients were offered statins or anti-estrogen therapy for primary prevention. In addition, the timing of breast cancer and ASCVD risk assessment did not coincide with the timing of initiation of chemoprevention and statins, respectively. Also, we did not document duration of therapy or long-term adherence. Our main focus was on the initiation of statins and chemoprevention. Long-term persistence of statins and antiestrogen therapy are critical to derive the clinical benefits of these drugs. However, chemoprevention with antiestrogen therapy is limited to 5 years, whereas statins typically require life-long therapy.
In summary, our study provides evidence of the low use of chemoprevention among women at high-risk for breast cancer. By comparing it to the use of statins for ASCVD, we hope to raise awareness that emphasizes medications used to prevent multiple chronic diseases. Ultimately, increasing chemoprevention use will require addressing all the aforementioned barriers. Given that several studies have found that physician recommendation for chemoprevention and referral to a medical oncologist have been associated with increased chemoprevention use [11, 23], further research should measure the impact of increased physician knowledge on chemoprevention use. Additionally, primary care providers (PCPs) need to be better equipped with tools that make routinely calculating breast cancer risk easier and more time efficient [2]. An informed approach about the concurrent use of statins and chemoprevention is necessary for the successful management of multiple comorbidities. Other potential reasons for greater statin use may include: a) few subjective symptoms for low to moderate dose statins, b) low-cost, c) improved survival and d) inexpensive means to monitor probable treatment response with LDL cholesterol. Reducing side effects of chemoprevention, for example with low-dose tamoxifen, will be crucial in improving use.
We recently published a randomized controlled trial using a web-based decision aid among high-risk women, which resulted in significant improvements during short-term follow-up on accurate breast cancer risk perceptions, adequate chemoprevention knowledge, reduced decision conflict and increased informed choice about chemoprevention [24]. Future studies, such as the ongoing SWOG 1904 cluster-randomized controlled trial (clinicaltrials.gov identifier, NCT04496739), should focus on increasing chemoprevention informed choice and use among high-risk women with AH or LCIS, as these groups derive the most benefit from chemoprevention with SERMs and AIs.
Prevention Relevance:
Among women with high-risk breast lesions, mean absolute risk of breast cancer was higher compared to cardiovascular disease; however, statin use was significantly higher than chemoprevention. To address underutilization of breast cancer chemoprevention, these drugs should be placed in the context of medications used to prevent other chronic diseases.
ACKNOWLEDGEMENTS
Financial support: This work was supported by the National Institutes of Health, National Cancer Institute R01CA226060 (K.D. Crew, R. Kukafka), P30 CA013696 (All authors received); National Center for Advancing Translational Sciences UL1 TR000040 (All authors received). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Dupont WD, Page DL: Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985, 312(3):146–151. [DOI] [PubMed] [Google Scholar]
- 2.Crew KD: Addressing barriers to uptake of breast cancer chemoprevention for patients and providers. Am Soc Clin Oncol Educ Book 2015:e50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. : Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005, 97(22):1652–1662. [DOI] [PubMed] [Google Scholar]
- 4.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, et al. : Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol 2019, 37(19):1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ropka ME, Keim J, Philbrick JT: Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol 2010, 28(18):3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravdin PM: The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila) 2010, 3(6):686–688. [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR: Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007, 50(15):1435–1441. [DOI] [PubMed] [Google Scholar]
- 8.Brown F, Singer A, Katz A, Konrad G: Statin-prescribing trends for primary and secondary prevention of cardiovascular disease. Can Fam Physician 2017, 63(11):e495–e503. [PMC free article] [PubMed] [Google Scholar]
- 9.Sparrow RT, Khan AM, Ferreira-Legere LE, Ko DT, Jackevicius CA, Goodman SG, et al. : Effectiveness of Interventions Aimed at Increasing Statin-Prescribing Rates in Primary Cardiovascular Disease Prevention: A Systematic Review of Randomized Clinical Trials. JAMA Cardiol 2019, 4(11):1160–1169. [DOI] [PubMed] [Google Scholar]
- 10.Ballard-Barbash R, Taplin SH, Yankaskas BC, Ernster VL, Rosenberg RD, Carney PA, et al. : Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol 1997, 169(4):1001–1008. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi MS, Coe AM, Vanegas A, Kukafka R, Crew KD: Chemoprevention Uptake among Women with Atypical Hyperplasia and Lobular and Ductal Carcinoma In Situ. Cancer Prev Res (Phila) 2017, 10(8):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Q, Paulose-Ram R, Burt VL, Kit BK: Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS Data Brief 2014(177):1–8. [PubMed] [Google Scholar]
- 13.Chou R, Cantor A, Dana T, Wagner J, Ahmed AY, Fu R, et al. : Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328(8):754–771. [DOI] [PubMed] [Google Scholar]
- 14.Byrne P, Demasi M, Jones M, Smith SM, O'Brien KK, DuBroff R: Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment: A Systematic Review and Meta-analysis. JAMA Intern Med 2022, 182(5):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, et al. : Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol 2019, 37(33):3152–3165. [DOI] [PubMed] [Google Scholar]
- 16.Harborg S, Heide-Jorgensen U, Ahern TP, Ewertz M, Cronin-Fenton D, Borgquist S: Statin use and breast cancer recurrence in postmenopausal women treated with adjuvant aromatase inhibitors: a Danish population-based cohort study. Breast Cancer Res Treat 2020, 183(1):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sund M, Garcia-Argibay M, Garmo H, Ahlgren J, Wennstig AK, Fredriksson I, et al. : Aromatase inhibitors use and risk for cardiovascular disease in breast cancer patients: A population-based cohort study. Breast 2021, 59:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. : Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol 2016, 27(4):575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan CP, Kim SE, Wong ST, Sawaya GF, Walsh JM, Perez-Stable EJ: Willingness to use tamoxifen to prevent breast cancer among diverse women. Breast Cancer Res Treat 2012, 133(1):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padamsee TJ, Meadows R, Hils M: Layers of information: interacting constraints on breast cancer risk-management by high-risk African American women. Ethn Health 2021, 26(6):787–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewey J, Shrank WH, Bowry AD, Kilabuk E, Brennan TA, Choudhry NK: Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J 2013, 165(5):665–678, 678 e661. [DOI] [PubMed] [Google Scholar]
- 22.American Heart Association. Cardiovascular Disease: A Costly Burden for America. Projections Through 2035. [cited 2023 Oct 20] Available From: https://www.heart.org/-/media/Files/Get-Involved/Advocacy/Burden-Report-Consumer-Report.pdf [Google Scholar]
- 23.Reimers LL, Sivasubramanian PS, Hershman D, Terry MB, Greenlee H, Campbell J, et al. : Breast Cancer Chemoprevention among High-risk Women and those with Ductal Carcinoma In Situ. Breast J 2015, 21(4):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crew KD, Bhatkhande G, Silverman T, Amenta J, Jones T, McGuinness JE, et al. : Patient and Provider Web-Based Decision Support for Breast Cancer Chemoprevention: A Randomized Controlled Trial. Cancer Prev Res (Phila) 2022, 15(10):689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were generated by the authors and available on request.