The sequencing of the human genome has been heralded as a major milestone in biological science that will, without doubt, provide fundamentally new ways of studying the human condition (Lander et al., 2001; Subramanian et al., 2001a; Venter et al., 2001). Yet, for a substantial portion of the world’s population, the human condition is defined by diseases such as malaria, tuberculosis, cholera, filariasis, soil-transmitted helminthiasis, trypanosomiais, schistosomiasis, onchocerciasis, yellow fever, dengue, measles and rotaviral gastroenteritis. And, of course, the pandemic of HIV/AIDS looms as an ever-increasing threat over the Third World. It is in the developing countries where genome science could make the biggest impact. Indeed, the World Health Organization, in its report on ‘Genomics and World Health’ published this April, sees it the same way. ‘Within the next few years, new diagnostic agents, vaccines and therapeutic agents will likely be available for communicable diseases,’ the report’s authors expect, and state that ‘the time has come to plan how this technology and its potential clinical advances can be distributed fairly among the world’s population. Otherwise, this new field will simply widen the gap in health care between the rich and poor countries of the world’ (WHO, 2002).
Moreover, the diseases of the developing world—sometimes referred to as tropical diseases—can no longer be viewed in a purely medical or public health context. There is an emerging body of evidence that suggests that infectious diseases pose a major risk to the economic survival of many Third World nations. Even more striking, recent data suggest that some of these diseases may have wider implications for geopolitical stability or the probability that a nation will experience armed conflict.
Clearly, genomics is already advancing our knowledge of infectious diseases (Subramanian et al., 2001b). Automated DNA analysers and powerful software have made it possible to establish the complete genomic sequence of many pathogens responsible for morbidity and mortality in the developing world, including cholera, trachoma and tuberculosis (www.tigr.org, www.niaid.nih.gov). The new tools in comparative genomics, computational biology and informatics have already yielded promising results in the study of invertebrate parasites. But ironically, even though the source for some of this research is the endoparasitic hookworm, a major parasite in many developing countries, it is being conducted for the benefit of patients in the First World. Through the process of evolution, hookworms have completed successful ‘research’ programs to produce and secrete a battery of pharmacologically active peptides that anticoagulate blood, inhibit platelet aggregation and downregulate the host’s immune response (Stanssens et al., 1996; Zhan et al., 2002). These peptides, or their derivatives, make potentially interesting new biopharmaceutical candidates—one of the hookworm VIIa/tissue factor inhibitor anticoagulants is now in clinical trials for thromboembolic disease (Stanssens et al., 1996), and a glycoprotein that binds to CD11/CD18 on leukocytes is in trials for the treatment of stroke (Moyle et al., 1994). Also, three gene products were discovered in Brugia filarial nematodes with similarities to mammalian cytokines, including macrophage migration inhibitory factor (MIF), transforming growth factor β (TGF-β) and translationally controlled tumor protein (TCTP) (Maizels et al., 2001). And we are only just beginning to analyse >200 000 expressed sequence tags from parasitic nematodes (www.nematodes.org) (Parkinson et al., 2001). Potential sources for new drugs also include blood-feeding insects, such as Anopheles mosquitoes, Reduviid kissing bugs and Phlebotomus sandflies, which produce a large and diverse array of salivary anticlotting, antiplatelet, vasodilatory and other physiologically active substances (Ribeiro, 1995).
Ideally, the recognition that pathogenic invertebrates can yield useful products for the pharmaceutical industry may also revive and extend governmental and philanthropic support for research into developing world diseases, as some of these proteins could also be reformulated into vaccine targets (Hotez, 2002a). In the case of hookworm, a vaccine initiative is underway, which relies heavily on eliciting host antibodies against the major secreted hookworm proteins—an idea that was first put forward >50 years ago by Chandler and Thorson (Chandler, 1936; Thorson, 1956). Researchers have also shown that a family of cysteine-rich proteins, known as the Ancylostoma secreted proteins, are promising vaccine antigens for many parasitic nematode infections, including hookworm, ruminant gastrointestinal parasites, lymphatic filariasis and onchocerciasis (Sharp and Wagland, 1998; Sen et al., 2000). Similarly, SP15, a 15 kDa protein secreted by Phlebotomus sandflies, is now being developed as a vaccine to interrupt the transmission of leishmaniasis through the insect (Valenzuela et al., 2001).
But on the whole, as malaria, African trypanosomiasis, lymphatic filariasis, hookworm and leishmaniasis occur among the world’s poorest people, there has been little or no commercial interest in developing treatments against these diseases. It is difficult for large pharmaceutical companies to persuade their shareholders to develop such products without affecting their own requirements to succeed in the highly competitive and unforgiving markets closer to home. In a few instances, drug companies have donated drugs that were originally developed for veterinary use when it became apparent that they were effective against Third World diseases—ivermectin for the treatment of human onchocerciasis or benzimidazoles to treat lymphatic filariasis. And although organisations such as The MacArthur Foundation, The Rockefeller Foundation, The Wellcome Trust and the National Institutes of Health (NIH) have previously devoted some of their resources to tropical medicine, these funds were sometimes not adequate to take the first significant steps towards vaccine and drug development (Hotez, 2001).
However, some improvement in research support has taken place over the past decade, mainly with the infusion of >$1 billion from The Bill and Melinda Gates Foundation to develop an HIV vaccine. This also included support for either new or improved vaccines to combat malaria, tuberculosis, leishmaniasis, African trypanosomiasis hookworm, meningococcal meningitis, shigellosis, cholera and measles. Perhaps one of the most important goals for the future of research on tropical diseases will be the linking of these funds to parallel efforts in parasite genomics and proteomics, many of which are being funded by the NIH or The Wellcome Trust (Table I).
Table I. New genome projects (completed or in progress) and Gates Foundation-funded orphan drug and vaccine initiatives for developing countries.
| Infectious disease | Genome project | Genomics funding | Drug | Vaccine initiative | Amount ($ million) |
|---|---|---|---|---|---|
| African trypanosomiasis | TIGR/Sanger | NIAID/Beowolf | Drug development | University of North Carolina | 15 |
| Children’s Vaccine Fund | GAVI (Geneva) | 750 | |||
| Cholera/Typhoid/Shigella | TIGR/Sanger/University of Wisconsin | NIAID/Wellcome | International Vaccine Institute (Seoul) | 40 | |
| HIV/AIDS | International AIDS Vaccine Initiative | 125 | |||
| Hookworm | Washington University/Sanger | NIAID/Beowolf | Hookworm Vaccine | Sabin Institute | 18 |
| Leishmaniasis | SBRI/Sanger | Beowolf/EC/NIAID | Leishmaniasis Vaccine | IDRI | 15 |
| Lymphatic filariasis | Sanger/Smith College | Beowolf/NIAID | Drug control | World Bank | 20 |
| Malaria | TIGR/NMRI/Sanger | NIAID/BWF/Wellcome | Malaria Vaccine Initiative | PATH | 50 |
| SAIC | Malaria Vaccine | NIH | 40 | ||
| London School of Hygiene & Tropical Medicine | 40 | ||||
| Measles | Improved vaccine | Johns Hopkins University/UMD | 20 | ||
| Meningococcal meningitis | TIGR/Sanger | Chiron/Wellcome | Meningitis Vaccine | PATH–WHO | 70 |
| Trachoma | TIGR | NIAID | Drug control | International Trachoma Initiative | 20 |
| Tuberculosis | Sanger/TIGR | Wellcome/NIAID | TB Vaccine Initiative | Sequella | 25 |
| MDR | TB | Control | Harvard | 45 | |
| TB Diagnosis Initiative/TDR | WHO | 10 |
Sources: www.gatesfoundation.org, www.niaid.nih.gov, www.tigr.org, Hotez (2001).
Malaria is a prime example of how these initiatives could dramatically improve the quality of life for millions of people in the developing world. The enormous suffering caused by the sporozoite Plasmodium falciparum is astonishing in its own right, and, in many ways, it is the classic emblem for the public health burden of poor countries. The disease kills up to 2.7 million people every year, the vast majority of them children under the age of five. In Africa alone, at least 300 million clinical attacks of malaria occur annually, although this figure may well be an underestimate, as many take place in rural dispensaries or at home and may not be reported. Malaria is particularly dangerous during pregnancy, with a substantial risk of severe anaemia in the mother and intrauterine growth retardation, as well as other acutely life-threatening and chronic complications for the baby. Even harder to quantify are the hardships and economic damage suffered by survivors due to permanent neurologic sequelae (Holding and Snow, 2001).
Malaria is also a prototype for understanding the complex interplay between science, medicine, economics and international security. It puts substantial stress on the medical systems of affected nations, to say nothing of their productivity and capacity to compete in global markets. Indeed, there is an emerging recognition that malaria is a formidable cause of poverty in sub-Saharan Africa, rather than vice versa (Gallup and Sachs, 2001). The disease is an intractable force retarding the economic growth of affected nations or overtly contracting their per capita gross domestic product. Poverty, in turn, reverberates throughout society and has independent health and geopolitical consequences. Moreover, the biology of P. falciparum, coupled with climate, ecology and the biology of its vector, the Anopheles gambiae mosquito, often defeats malaria control measures.
It is therefore unlikely that existing solutions or strategies will eliminate this disease in the foreseeable future. We need novel approaches to control mosquito populations and interrupt human/mosquito contact. We need creative approaches to produce safe and effective insecticides and to reverse the resistance to established insecticides, including exploring genetic strategies against A. gambiae, whose genome sequence was recently completed (Holt et al., 2002). We need to develop new generations of therapeutic agents to target Plasmodium and overcome multi-drug resistance. And most importantly, we need a practical antimalarial vaccine (Doolan and Hoffman, 2001). With variations on a theme, each of these measures also apply to controlling tropical infectious diseases in general.
The possibility of studying whole genomes offers new opportunities—and an antidote to a sense of futility—in each of these areas. We will soon have in hand the trio of complete genome sequences relevant for malaria: the protozoan pathogen, its arthropod vector and the human. However, in some instances, the technology of parasite genome science has outpaced the capacity of the scientific community to assimilate the information (Coppel, 2001). This is particularly true for the field of tropical medicine, where many scientists have received little or no formal training in bioinformatics and also lack adequate access to sequence data. Efforts, such as the PlasmoDB database (www.plasmodb.org), may help to stimulate the nascent field of malaria functional genomics.
Indeed, based on this approach, researchers are already devising novel strategies for developing antimalaria vaccines (Hoffman, 2000; Doolan and Hoffman, 2001; Hoffman et al., 2002a). There is a proof-of-principle for a successful vaccine by using radiation-attenuated sporozoites (Hoffman et al., 2002b), but it is not a practical option for a clinical vaccine. Another field under intense study is acquired immune protection. In areas of Africa with the most intense malaria transmission, children who survive to the age of 7–10 rarely develop life-threatening P. falciparum infections, because their immune system is able to control subsequent infections. Probably central to this ‘naturally acquired’ protective immunity are antibodies against parasite proteins expressed on erythrocytes that prevent sequestration in the microcirculation and antibodies against surface proteins of the merozoites that prevent invasion of erythrocytes.
But all efforts to develop subunit vaccines comparable to irradiated sporozoites and naturally acquired immunity have shown little success. It is quite likely that immunisation with only a few parasite proteins cannot duplicate the immunity elicited by exposure to a parasite expressing thousands of proteins. In the case of naturally acquired immunity, this ‘breadth’ would be expanded by exposure to many polymorphic strains of P. falciparum. If so, the malaria genome and various projects to define single nucleotide polymorphisms in Plasmodium and human host surface proteins (Broder and Venter, 2000; Subramanian et al., 2001b; Hoffman et al., 2002b) may provide the essential foundation for duplicating this aspect of immunity and thereby developing effective malaria vaccines. However, genomics alone will not be enough. Immune responses are generally directed against proteins or carbohydrates, not genes per se, and the parasite expresses different proteins at different stages of its life cycle. We anticipate that gene expression and proteomics studies will lead to the generation of comprehensive databases that catalogue stage-specific expression of all genes and proteins in the P. falciparum genome and the polymorphisms in the DNA sequences of these genes in field isolates. Concurrent efforts to establish the functional importance of each gene, and the significance of protein–protein interactions, will take time, but will form the foundations on which to build effective vaccines.
Such application of genomics and other biotechnologies has implications that extend beyond simply fighting infectious diseases. Since the end of the Cold War, an increasing body of evidence has suggested that the public health of developing nations is a global economic and security issue (Moodie and Taylor, 2000; Kassalow, 2001; Hotez, 2002b). One way in which endemic infectious disease can contribute to the destabilisation of a nation or region is to disrupt its economy. It is worth remembering that malaria was an important contributor to the economic underdevelopment of Italy, Greece and Spain prior to World War II, with dramatic catch-up growth following the eradication of Anopheles mosquitoes through aggressive use of DDT in the post-war period (Gallup and Sachs, 2001). Today, malaria is slowing the economic development in Africa, as discussed above, and the same may be true of hookworm in China (Hotez, 2002c).
There is also evidence that infectious diseases may have consequences for global security and play a role in the ‘pathophysiology’—or possibly even the ‘pathogenesis’—of armed conflict in the developing world (Hotez, 2001). Relatively recent analyses suggest a relationship between infectious disease-related childhood mortality rates or tuberculosis incidence and the probability of a nation becoming engaged in armed conflict. We do not claim that a cause-and-effect relationship has been proven, nor would it be surprising if cause and effect sometimes become one in this context. However, there is clearly a complex interplay among medical, economic and political factors linking infection rates and the risk of armed conflict. Poverty and various forms of political oppression and social or economic inequalities have direct ramifications for both public health and a predisposition towards conflict (Murray et al., 2002; Stewart, 2002), and wars disrupt the food supply and medical infrastructure.
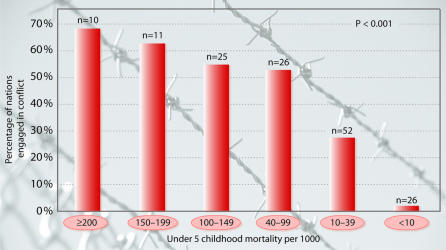
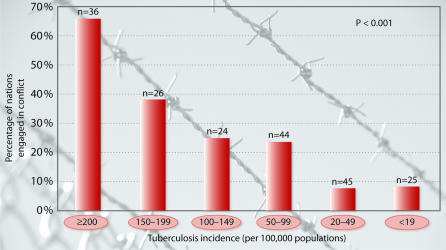
Moreover, a high infant mortality rate was recently shown to be a significant predictor of nation-state collapse through coups d’etat, civil strife and other means (Esty et al., 1999). It is generally thought that infant mortality is an exceedingly powerful indicator, or ‘surrogate marker’, for overall quality of material life. The percentage of countries at war sharply increases when childhood mortality rates exceed 100 deaths per 1000 (Figure 1) and the incidence of tuberculosis exceeds 200 per 100 000 (Figure 2) (World Bank, 1997). Nations such as Niger, Sierra Leone and Afghanistan, which suffer from the world’s highest childhood mortality rates, are thus dramatically more likely to be engaged in armed conflict than nations with low childhood mortality rates.
Fig. 1. Relationship between under-5 childhood mortality (per 1000) and armed conflict during the 1990s. Reproduced from Hotez (2001).
Fig. 2. Relationship between tuberculosis incidence in 1995 (per 100 000) and armed conflict during the 1990s.
Alexander the Great was probably among the first to experience infectious diseases altering the outcome of wars, but he was certainly not the last. ‘In 1943, for every man evacuated with wounds, we had 120 evacuated sick. The annual malaria rate alone was 84% per annum of the total strength of the army and still higher among the forward troops [...]. A simple calculation showed me that in a matter of months at this rate my army would have melted away. Indeed, it was doing so under my eyes,’ wrote Field Marshall General Slim, commander of the British Army in Burma (Slim, 1956). We also believe it is worth considering that, in effect, infectious diseases might also influence the onset or ‘pathogenesis’ of modern wars in the developing world. And the terrorist attacks of September 11 have shown that these wars have a global effect when terrorists use nations sunk in civil war or anarchy as training grounds and hideouts.
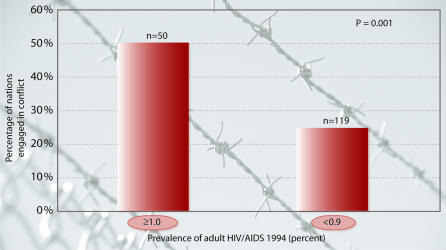
The problem of HIV/AIDS requires a special discussion. In parts of sub-Saharan Africa and, more recently, in Asia, AIDS threatens to destabilise almost a dozen nations where, in some cases, the infection will eventually kill one in four adults. Approximately 40 million people worldwide are HIV positive and most will die from the disease (Dixon et al., 2002) (www.unaids.org/epidemic_update). The economic effects include reduced labour supply and reduced labour productivity, causing reduced exports and increased imports. A new report published by the International Crisis Group in Washington, DC, and Brussels links the AIDS pandemic to deteriorations in national and even global security by promoting human migration, creating orphans, threatening social and economic progress and affecting police and civil service capability. A South African military analyst warned that AIDS is interfering with peacekeeping operations in some parts of sub-Saharan Africa (ICG, 2001). As shown in Figure 3, there is also a tentative correlation between HIV prevalence and armed conflict during the 1990s. As we are still in the early stages of the AIDS pandemic, it unfortunately—in many senses—remains to be seen whether HIV prevalence rates will correlate with conflict in the coming decade.
Fig. 3. Relationship between adult HIV/AIDS prevalence in 1994 and armed conflict during the 1990s.
Western nations have been able to quickly develop therapeutic interventions to halt the progression of the HIV virus. However, antiretrovirals are very expensive and have in large measure been beneficial only for patients in the First World, as developing countries simply cannot afford to pay for these drugs. A recent report in a prestigious medical journal has explored the ethically sensitive possibility that ‘to maintain economic stability it may be necessary to target expensive antiretroviral drugs at highly productive socioeconomic groups in specific industries on the basis of their contribution to economic output rather than healthcare needs’ (Dixon et al., 2002). We do not endorse this view. Sir Richard Sykes, chairman of GlaxoSmithKline, has noted that ‘it is easy although misguided to assume the costs of drugs used to treat HIV and AIDS is the primary barrier to people in poor countries having general access to such drugs’ (Sykes, 2002). In any event, the overall scientific, medical and ethical dilemmas are not likely to disappear in the near future. There are also substantial programmes to develop an antiretroviral vaccine (Nabel, 2001), although none is as yet available. With the support of the World Bank, UN Secretary General Kofi Annan has called for a global fund, which would commit $10 billion per year in order to provide antiretroviral therapy for 3 million of the world’s poorest AIDS patients, treatment for opportunistic microbial and parasitic agents in 6 million individuals and preventative measures.
In a sense, the HIV/AIDS pandemic illustrates the limits of what genomics and basic science can accomplish. Unlike malaria, HIV may be controllable using cost-effective measures for primary prevention that are within reach in the developing world. Explicit public education is thus an absolute priority, but these programmes cannot succeed without considerable resources from both the public and private sector. Global efforts to stem the HIV/AIDS pandemic are therefore absolutely essential, otherwise any gains made in transferring genomics technology to the developing world would be eroded.
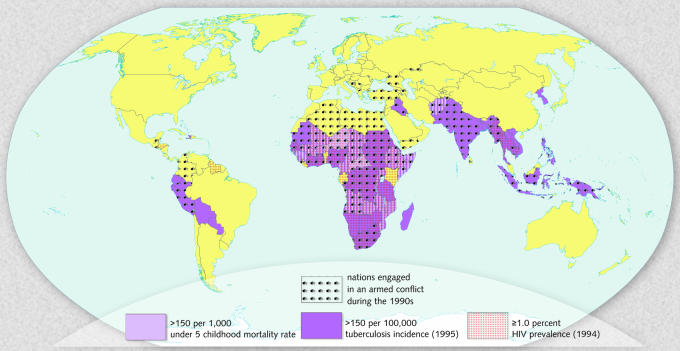
As pointed out above, there is clear evidence that infection rates reflect a nation’s likelihood of engaging in armed conflict (Figure 4). The urgency to develop new tools for preventing the conditions that promote conflict is apparent, given new estimates indicating that 160 million people perished in violent conflicts over the last 100 years (McNamara and Blight, 2001). It is an irony of our time that medical technology cannot be effectively transferred to the developing world, whereas military technology can be transferred with great speed and efficiency. Because it is unlikely that such technology will disappear or abate, some have argued for the need to develop innovative counter-technologies.
Fig. 4. Overview of countries that experience armed conflict and suffer from high rates of endemic infection.
Perhaps biotechnology coupled with genomics could be one such foil. The number of lives saved from childhood vaccines in the 20th Century approximates to the numbers of individuals killed in warfare (McNamara and Blight, 2001). Genomics might emerge as the new key counter-technology of the 21st Century. With calls for a new-generation Marshall Plan to aid the poorest in developing nations (Newman, 2001), it will be essential to look to emerging biotechnologies as part of the solution. Reducing the intolerable health and economic burdens of infectious/parasitic diseases should be an urgent priority for biology and medicine. But this should also be an urgent priority for nations pursuing global stability. The new tools provided by genomic science makes the task more urgent, and the failure to act, more tragic.



Acknowledgments
Acknowledgements
S.B. and S.L.H. express their gratitude to their co-workers at Celera for their contribution to genomic sequencing, proteomics and computational biology. We thank Azra Dobardzic for performing the statistical analysis of the data used in this study, and Jeffrey Sachs for his helpful comments. P.J.H. is supported by the Human Hookworm Vaccine Initiative of the Sabin Vaccine Institute and The Bill and Melinda Gates Foundation, and grants from the NIH, March of Dimes and The China Medical Board of New York.
REFERENCES
- Broder S. and Venter, J.C. (2000) Sequencing the entire genomes of free-living organisms: the foundation of pharmacology in the new millennium. Annu. Rev. Pharmacol. Toxicol., 40, 97–132. [DOI] [PubMed] [Google Scholar]
- Chandler A.C. (1936) Studies on the nature of immunity to intestinal helminthis. III. Renewal of growth and egg production in Nippostrongylus after transfer from immune to non-immune rats. Am. J. Hyg., 23, 36–54. [Google Scholar]
- Coppel R.L. (2001) Bioinformatics and the malaria genome: facilitating access and exploitation of sequence information. Mol. Biochem. Parasitol., 118, 139–145. [DOI] [PubMed] [Google Scholar]
- Dixon S., McDonald, S. and Roberts, J. (2002) The impact of HIV and AIDS on Africa’s economic development. Br. Med. J., 324, 232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan D.L. and Hoffman, S.L. (2001) DNA-based vaccines against malaria: status and promise of the multi-stage malaria DNA vaccine operation. Int. J. Parasitol., 31, 753–762. [DOI] [PubMed] [Google Scholar]
- Esty D.C., Goldstone, J.A., Gurr, T.R., Harff, B., Levy, M., Dabelko, G.D., Surko, P.T. and Ungar, A.N. (1999) State failure task report: Phase II findings. Environ. Change Security Rep., 5, 49–68. [Google Scholar]
- Gallup J.L. and Sachs, J.D. (2001) The economic burden of malaria. Am. J. Trop. Med. Hyg., 64, 85–96. [DOI] [PubMed] [Google Scholar]
- Hoffman S.L. (2000) Infectious disease. Research (genomics) is crucial to attacking malaria. Science, 290, 1509. [DOI] [PubMed] [Google Scholar]
- Hoffman S.L., Subramanian, G.M., Collins, F.H. and Venter, J.C. (2002a) Plasmodium, human and Anopheles genomics and malaria. Nature, 415, 702–709. [DOI] [PubMed] [Google Scholar]
- Hoffman S.L. et al. (2002b) Protection of humans against malaria by immunization with radiation attenuated Plasmodium sp. sporozoites. J. Infect. Dis., 185, 1155–1164. [DOI] [PubMed] [Google Scholar]
- Holding P.A. and Snow, R.W. (2001) Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. Am. J. Trop. Med. Hyg., 64, 68–75. [DOI] [PubMed] [Google Scholar]
- Holt R.A. et al. (2002) The genome sequence of the malaria mosquito Anopheles gambiae. Science, in press. [DOI] [PubMed] [Google Scholar]
- Hotez P.J. (2001) Vaccines as instruments of foreign policy. The new vaccines for tropical infectious diseases may have unanticipated uses beyond fighting diseases. EMBO rep., 2, 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J. (2002a) Vaccine against human hookworm disease. In Levine, M., Kaper, J.B., Rappuoli, R., Liu, M. and Good, M.F. (eds), New Generation Vaccines, 3rd edn. Marcel Dekker, New York, NY, in press.
- Hotez P.J. (2002b) Appeasing Wilson’s ghost: the expanded role of the new vaccines in international diplomacy. CBACI Health and Security Series. Chemical and Biological Arms Control Institute, Occasional Paper 3, pp. 1–16.
- Hotez P.J. (2002c) China’s hookworms. China Quart., in press [Google Scholar]
- ICG (2001) HIV/AIDS as a security issue. Interantional Crisis Group, Washington, DC (www.crisisweb.org).
- Kassalow J.S. (2001) Why health is important to U.S. foreign policy. Council on Foreign Relations and Milbank Memorial Fund, New York, NY.
- Lander E.S. et al. (2001) Initial sequencing and analysis of the human genome. Nature, 409, 860–921. [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Blaxter, M.L. and Scott, A.L. (2001) Immunological genomics of Brugia malayi: filarial genes implicated in immune evasion and protective immunity. Parasite Immunol., 23, 327–344. [DOI] [PubMed] [Google Scholar]
- McNamara R.S. and Blight, J.G. (2001) Wilson’s ghost: reducing the risk of conflict, killing, and catastrophe in the 21st century. Public Affairs, 218–229. [Google Scholar]
- Moodie M. and Taylor, W.J. (2000) Contagion and conflict: health as a global security challenge. A report of the Chemical and Biological Arms Control Institute and the CSIS International Security Program. Center for Strategic and International Studies, Washington, DC.
- Moyle M. et al. (1994) A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J. Biol. Chem., 269, 10008–10015. [PubMed] [Google Scholar]
- Murray C.J.L., King, G., Lopez, A.D., Tomijima, N. and Krug, E.G. (2002) Armed conflict as a public health problem. Br. Med. J., 324, 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G.J. (2001) Challenges and opportunities for development of an AIDS vaccine. Nature, 410, 1002–1007. [DOI] [PubMed] [Google Scholar]
- Newman C. (2001) Finance minister calls for new Marshall Plan to aid poor. Financial Times, December 17. [Google Scholar]
- Parkinson J., Whitton, C., Guilliano, D., Daub, J. and Blaxter, M. (2001) 200 000 nematode expressed sequence tags on the Net. Trends Parasitol., 17, 394–396. [DOI] [PubMed] [Google Scholar]
- Ribeiro J.M. (1995) Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis., 4, 143–152. [PubMed] [Google Scholar]
- Sen L., Ghosh, K., Bin, Z., Qiang, S., Thompson, M.G., Hawdon, J.M., Koski, R.A., Shuhua, X. and Hotez, P.J. (2000) Hookworm burden reductions in BALB/c mice vaccinated with recombinant Ancylostoma secreted protein 1 (ASPs) from Ancylostoma duodenale, Ancylostom caninum and Necator americanus. Vaccine, 18, 1096–1102. [DOI] [PubMed] [Google Scholar]
- Sharp P.J. and Wagland, B.M. (1998) Nematode vaccine. US Patent 5 734 035, March 31.
- Slim W. (1956) Defeat into Victory, 2nd edn. Cassell, London, UK, p. 177.
- Stanssens P. et al. (1996) Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc. Natl Acad. Sci. USA, 93, 2149–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart F. (2002) Root causes of violent conflict in developing countries. Br. Med. J., 324, 342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian G., Adams, M.D., Venter, J.C. and Broder, S. (2001a) Implications of the human genome for understanding human biology and medicine. J. Am. Med. Assoc., 286, 2296–2307. [DOI] [PubMed] [Google Scholar]
- Subramanian G., Mural, R., Hoffman, S.L., Venter, J.C. and Broder, S. (2001b) Microbial disease in humans: a genomic perspective. Mol. Diagn., 6, 243–252. [DOI] [PubMed] [Google Scholar]
- Sykes R. (2002) Commentary. The reality of treating HIV and AIDS in poor countries. Br. Med. J., 324, 216–217. [PubMed] [Google Scholar]
- Thorson R.E. (1956) The stimulation of acquired immunity in dogs by injection of extracts of the oesophagus of adult hookworms. J. Parasitol., 42, 501–504. [PubMed] [Google Scholar]
- Valenzuela J.G., Belkaid, Y., Garfield, M.K., Mendez, S., Kamhawi, S., Rowton, E.D., Sacks, D.L. and Ribeiro, J.M. (2001) Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J. Exp. Med., 194, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter J.C. et al. (2001) The sequence of the human genome. Science, 291, 1304–1351. [DOI] [PubMed] [Google Scholar]
- WHO (2002) Genomics and world health. World Health Organization, http://www3.who.int/whosis/genomics/genomics_report.cfm
- World Bank (1997) HNP at a glance: indicators by country. The Human Development Network, Health, Nutrition & Population series. The World Bank Group, Table A.6., pp. 48–51.
- Zhan B., Badamchian, M., Bo, M.H., Ashcom, J., Feng, J.J., Hawdon, J., Xiao, S.H. and Hotez, P.J. (2002) Molecular cloning and purification of Ac-TMP: a developmentally regulated putative tissue inhibitor of metalloprotease released in relative abundance by adult Ancylostoma hookworms. Am. J. Trop. Med. Hyg., 66, 233–244. [DOI] [PubMed] [Google Scholar]