Abstract
Cell migration is a complex and intricate network of physical, chemical, and molecular events that ultimately leads to cell motility. This phenomenon is involved in both physiological and pathological processes, such as proper immune and inflammatory responses. Dysregulation of cell migration machinery in immune cells can have a tremendous impact in the trajectory of inflammation, infection, and resolution. The small vertebrate, the Zebrafish, has a remarkable capacity for genetic and pharmacological manipulation aligned to transparency that enables modulation and visualization of cell migration in vivo non-invasively. Such characteristics revolutionized the field of leukocyte biology, particularly neutrophils. In this review we will focus on leukocyte migration and highlight findings made in the Zebrafish that demonstrate how this small vertebrate system is a unique model to perform in vivo imaging and study mechanisms that regulate the dynamic behavior of immune cells in their native environment under homeostasis or upon challenge.
Keywords: Cell Migration, Zebrafish, Non-invasive Imaging
Zebrafish model – an unparallel system to study in vivo cell migration.
Zebrafish, danio rerio, are a small vertebrate model that first gained traction in the 1980s for their fast external development and genetic tractability [1]. Zebrafish has over 70% of gene homology with humans and 84% of disease-causing genes have at least one zebrafish orthologue [2]. The community has developed a wide number of disease models, and transgenic lines readily available (ZFIN; zfin.org) [3] to study cell dynamics in real time in their environemnt. Moreover, the zebrafish optical transparency and small size have proven to be an ideal model to follow cells in real time in a whole-animal context. The development of transgenic lines with fluorescently tagged neutrophils [4–6] macrophages [7,8] and T cells [9–12] provided access to visualize immune cell migration behaviors and dynamics in vivo under homeostasis or upon challenges (e.g., wounding, infection, or malignancy). The use of this system has revolutionized the study cell morphology, migration, and dynamics both in health and disease [5,9,13–15].
Revolutionizing the study of neutrophil migration
Directed cell migration, towards or away from diverse environmental cues, relies on four events which consist of generating, sensing, transmitting, and executing the signal, recently termed the “four pillars of directed migration (reviewed in [16]).” Leukocyte motility is often classified as an ameboid type of migration, which is characterized as an adhesion-independent movement observed for example in neutrophils [17]. However, macrophages can also display a mesenchymal migration pattern, which is protease- and podosome-dependent [17] (Figure 1).
Figure 1: Amoeboid and mesenchymal migration - Similarities and differences.
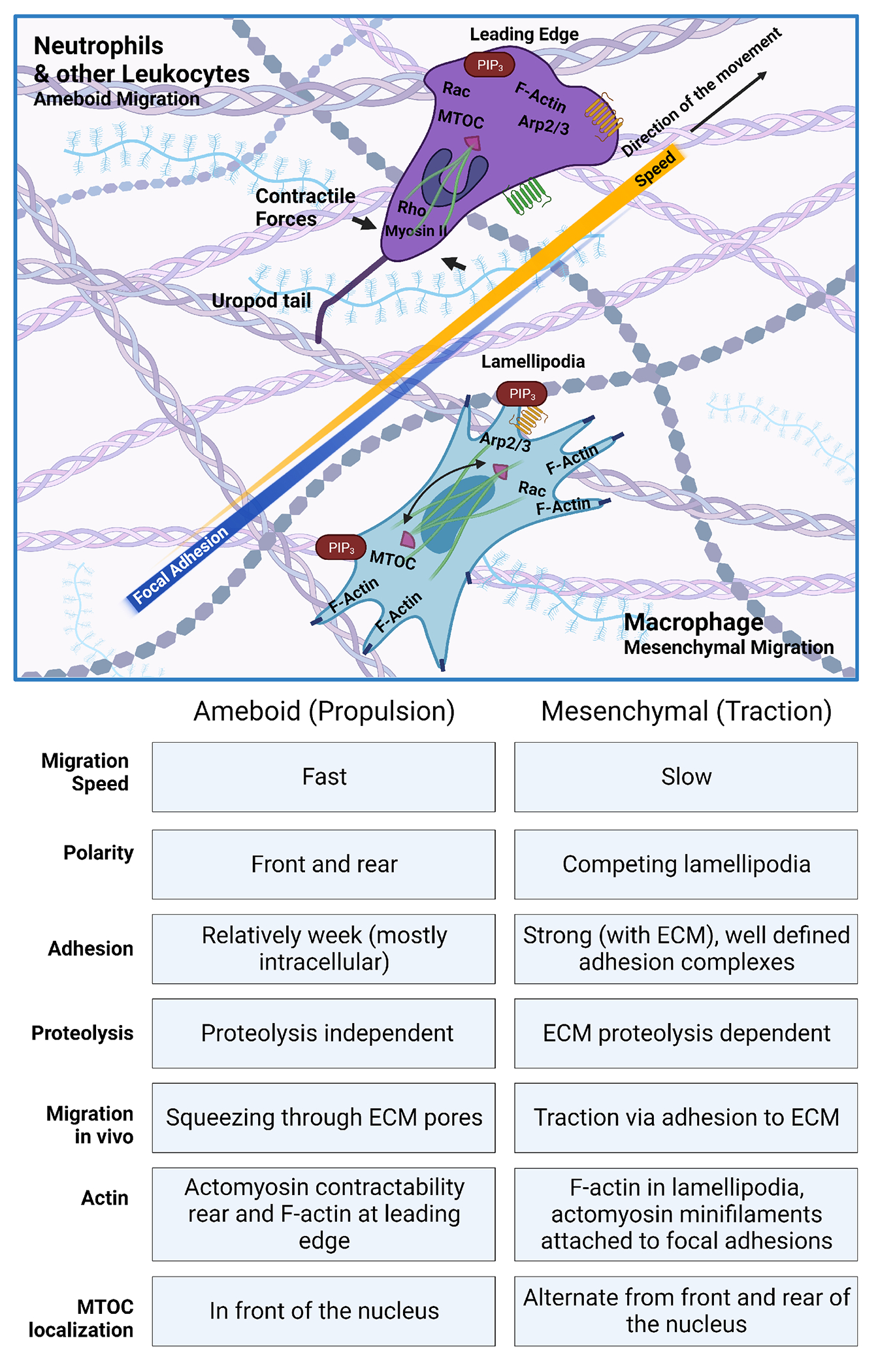
Overall, all leukocytes, including neutrophils use an ameboid migration pattern. This type of migration does not rely on proteolytic degradation of the ECM nor strong focal adhesion therefore this type of migration is fast. Establishment of a clear cell polarity is crucial for proper migration with F-actin localizing at the leading edge together with PIP3 gradient. Rho activation and Myosin II generate contractile forces that are responsible for the propulsion of neutrophils. Recently, using LLSM it has been shown that neutrophils display a long Uropod trail that eventually loses while migrating. Interestingly MTOC localizes in the front of the nucleus. Macrophages are known by their morphological plasticity and can also acquire mesenchymal phenotypes with a slow migration speed. This type of migration uses lamellipodia that competes to guide cells through the interstitial tissue. F-actin is localized in lamellipodia, and small myosin filaments attach to focal adhesion allowing cell to interact strongly with ECM. Mesenchymal migration relies heavily on traction. Interestingly, contrary to ameboid cells MTOC is located both at the rear and front of macrophage nucleus.
The use of the zebrafish has drastically changed the way biologists study cell migration through interstitial tissues, particularly neutrophil migration(reviewed in [18]). Through the generation of the first transgenic lines that enabled the visualization and tracking of neutrophils in their native microenvironment, the dogma that all neutrophils were doomed to die at injury sites was challenged. In 2006, Huttenlocher et al reported for the first time that neutrophils have the capacity to migrate away from the inflammatory site back into the vasculature [4]. With this discovery, and later support in a mammalian model more than a decade later [19], a new area of research emerged with exceptional potential for therapeutical target to promote resolution [20]. The specific mechanism that neutrophils use to undergo reverse migration is still unclear (reviewed in [21–23]). Up to now some of the main findings in the zebrafish suggest that macrophages control neutrophil reverse migration through redox and Src family kinase signals, via macrophage-neutrophil contact [24]. Zebrafish Cxcl8a and Cxcr2 are required for neutrophil reverse migration and resolution in vivo [25], as well as lipid mediators such as, prostaglandin E2 (PGE2) and lipoxin A4 (LXA4) [26]. In addition to signals that drive reverse migration, there are also signals that retain neutrophils at sites of injury such as the activation of the oxygen-sensing transcription factor hypoxia-inducible factor-1α (HIF-1α) or CXCL12 / CXCR4 signal axis, inhibition of these signals promotes reverse migration of neutrophils and accelerates resolution [27,28] (Figure 2). Interestingly, neutrophils that undergo reverse migration have a unique phenotype, compared to those in circulation or tissue resident neutrophils, which seems to be associated with dissipating inflammation throughout the body [29]. However, zebrafish studies from Ellet et al, showed that neutrophils’ response and antibacterial effect after reverse migration upon secondary injury were not significantly different from those at the recruitment stage [30]. Not surprisingly, the zebrafish are being used as a main high throughput screening tool to identify new drugs to modulate this phenomenon, as a way to promote resolution [20,31,32].
Figure 2: Myeloid migration becomes clearer with zebrafish.
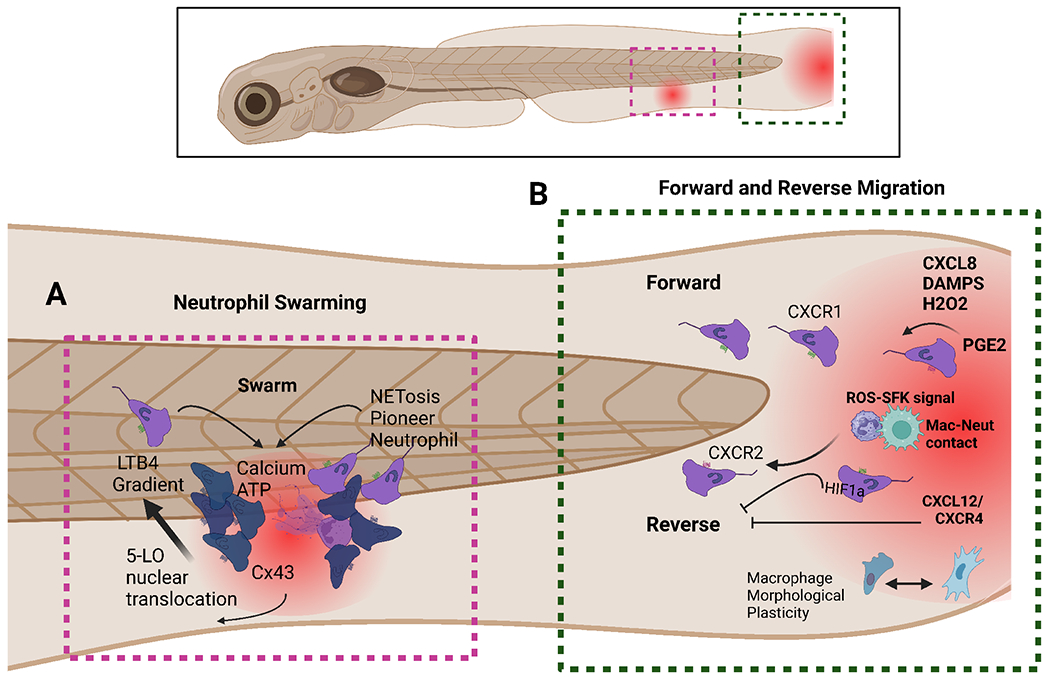
In the last decade and a half, the neutrophil community has been engaged in unravelling the mechanism and pathological role of reverse migration. A. Calcium alarm signals in neutrophil clusters locally promote attractant synthesis and are dependent on ATP sensing and contact with necrotic tissue. Clustering neutrophils initiate and propagate calcium alarm signals via Cx43 channels Neutrophil swarms and Cx43 restrict wound colonization by opportunistic bacteria. B. CXCL8 and other chemoattractants regulate neutrophil migration to injuries via CXCR1. CXCR2 on the other hand is needed for forward migration. Additionally, several mediators coordinate neutrophil reverse migration such as PGE2. Some other molecules such as ROS-SFK signal in macrophages and cell-cell contact are also vital for proper neutrophil reverse migration. Cxcl12/CXCR4 signal has been identified as a druggable retention signal for neutrophils at tissue damage that can be blocked using AMD3100.
Myeloid cells migrate towards signal gradients, called chemoattractants that trigger actin polymerization at one end and depolymerization at the other [3]. Actin formation and assembly are important features for leukocyte motility. As actin is polymerized and protrusions form, global inhibition will generate contractile force, providing the cell polarity and movement towards the chemoattractant. In neutrophils, contractile tension is more important than protrusions when interpreting chemoattractant gradients [33]. Neutrophil motility (random and upon challenge) relies on the persistent polarization of stable F-actin via Actin-related protein 2/3 complex (Arp2/3). For such PI(3)K activation at the leading edge through the modulation of both Rac-mediated protrusion and F-actin polarity is needed. PI(3)K activation resulted in accumulation of dynamics of its products PI(3,4,5)P3-PI(3,4)P2 during neutrophil migration, as visualized in living zebrafish using radiometric imaging [34]. In vivo visualization of microtubule dynamics during neutrophil migration in intact tissues showed a strong polarity toward the rear of the cell, with microtubule-organizing center (MTOC) localized in front of the nucleus[17,35]. Furthermore the microtubule cytoskeleton suppresses neutrophil polarity and motility through negative regulation of both Rho and Rac in a PI(3)K-independent manner [36]. In response to wounding, the heterotrimeric G protein subunit Gβ1 signaling controls neutrophil migration by activating PI(3)K and modulates actin dynamics [37]. Recently, lattice lightsheet microscopy (LLSM) has been used to visualize neutrophils in vivo, revealing that migrating neutrophils commonly carry thin trailing extensions at the uropod, with surprising length that are left behind as cell moves away [38]. Uropod extension dynamics and detachment are regulated by myosin II [38]. Additionally, intravascular behaviors like leukocyte rolling and transmigration were visualized using non-invasive intravital microscopy, allowing detailed visualization of rolling neutrophils extending cytoplasmic tethers or lengthier slings that attached and detached as neutrophils moved along the inner endothelium [38].
Temporally and spatially coordinated gradients of chemoattractants and chemorepellents are crucial in mediating neutrophil directional motility in interstitial tissues, characterized by distinct phases including scouting, amplification, and stabilization (reviewed in [21,39]). Under normal conditions most neutrophils reside in the hematopoietic tissue and upon stimulus of the promigratory pathway and the CXCR2 receptor, neutrophils are released into circulation. The primary ligand of CXCR2, CXCL8, is not found in the mouse genome. However, zebrafish have two homologs of Cxcl8 (Cxcl8a and Cxcl8b), which make it a unique and suitable vertebrate model to study migration and the circulation of neutrophils into the bloodstream [40]. Upon mild and severe tailfin wounding in zebrafish, Cxcl8b controls the release of neutrophils into the bloodstream and Cxcl8a recruits neutrophils in circulation to the wound [41]. In response to injury, neutrophils (and macrophages) are recruited to injury from distal sites and also mobilize into peripheral blood, using abluminal endothelial surfaces of lymphatic and blood vessels as migration highways [42]. After leaving the blood vessels neutrophils are guided by chemotactic signals, through G-coupled protein receptors (GPCRs) such as BLT1, CXCR1, and CXCR2 [43]. CXCR2 activation causes intracellular calcium to rise [44]. This influx in calcium concentration comes from cytosolic storage and permeable ion channels like transient receptor potential channels (TRP) [44]. Using an extended mathematical model to study the trajectory of neutrophil migration in vivo and in vitro towards chemotactic signals, it was found that chemotaxis results in anomalous migration, a Brownian motion, moving towards the signal [44]. Interestingly, CXCR2 inhibition and a TRPC6 knockout (KO), resulted in tempered migration, also defined as a lapse in the memory of migration [44].
One of the most complex phenomena that involves neutrophil coordinated motility is swarming behavior. Upon tissue damage neutrophil migration changes from passive surveillance to coordinated swarming in response to chemoattractant signals, such as leukotriene B4 (LTB4) that is sensed by BLT1 [43]. While external cues drive neutrophil activation, recruitment is largely sustained by local and self-organized cues [45]. Zebrafish have emerged as a unique system capable of studying swarming behavior, at a single cell level. Combining new biosensors and live imaging in zebrafish, it has been shown that pioneer neutrophils sense the damage signal ATP, which leads to LTB4 synthesis that is triggered by calcium flux upon neutrophil contact with the necrotic tissue [43]. The subsequent neutrophil coordinated motion and swarming is contact-dependent via connexin-43 (Cx43) hemichannels, which enhance chemoattractant biosynthesis and sustain cluster growth [43]. Inhibition of neutrophil Cx43 impairs clearance of wound-colonizing bacteria, supporting a crucial role in the sensing and propagation of alarm signals in the formation of the dense antimicrobial cell masses that block opportunistic pathogens to breach tissue barriers [43]. In addition, pioneer neutrophils adopt distinct phenotypes from other migrating neutrophils, developing a rounded morphology which is coupled with a reduction in speed and displacement [46]. The release of extracellular traps (NETs) that contain chromatin and myeloperoxidase from pioneering neutrophils promotes neutrophil swarming [46] supporting that pioneer neutrophil and the mechanisms that are triggered upon sensing alarm signals in damaged tissues, are crucial in regulating swarming formation [43].
Neutrophils are recruited to sites of injury and infection through the detection of endogenous signals called damage associated molecule patterns (DAMPs) or by exogenous molecules, pathogen associated molecular patterns (PAMPs) [21]. DAMPs that drive early neutrophil recruitment, such as ATP, Calcium and hydrogen peroxide [47,48] increase chemokine production, specifically Cxcl8 [49], which further activate neutrophil recruitment and neutrophil release into circulation [21]. While neutrophils respond to both tissue injury and infection, it remains unclear what signals differentiate these two responses. Comparing transcriptomic profiles from zebrafish neutrophils the myeloid derived growth factor (MYDGF) emerged as a damage signal that regulates neutrophil interstitial motility and inflammation through a HIF-1α pathway towards damaged tissues, but not infected [50].
New regulators of neutrophil migration are emerging. Interestingly, mitochondrial outer membrane protein Mitofusin 2 (MFN2) regulates cell migration and the actin cytoskeleton in vivo [51]. In addition, micro-RNAs (miRs), which are known as powerful regulators of neutrophil biology, have emerged also as key modulators of neutrophil chemotaxia in vivo [52]. miR-99 for example, directly targets the transcriptional factor RAR-related orphan receptor alpha (roraa), which has important functions in other immune cells, but is necessary for neutrophil recruitment and receptor activation [53]. The therapeutical potential of selectively targeted miRs to manipulate neutrophil migration, at different inflammatory and disease contexts, is a largely unexplored field that zebrafish can serve as a fast large-scale screening resource.
Cell migratory plasticity of macrophages.
Neutrophils and macrophages display distinct modes of motility [17]. While neutrophils display ameboid cell migration characteristics, in vivo live imaging of macrophages in a sterile wound context revealed that macrophages display behavioral plasticity during inflammation and change their shape from amoeboid to elongated during healing [54]. Macrophages are therefore reprogramed at injury sites based on environmental cues that regulate their polarization and behavior switching, such as 15-lipoxygenase (LOX) [54]. Additionally, morphometric analysis identified four different cell shapes that macrophages adopt, consisting of amoeboid, star-like, elongated, and rounded shapes [55].
Interestingly the dynamic morphological changes of macrophages during migration regulate their speed and motility [55]. Mesenchymal migration of macrophages depends on proteases to degrade the extracellular matrix (ECM) [17]. Inhibition of macrophage proteolysis, using an MMP-9 inhibitor, causes macrophages to adopt an ameboid like shape impacting their migration patterns [55]. Mesenchymal migration is also a Rac dependent process, as Rac inhibition dampens macrophage migration [56] and degradation of the ECM [55]. Macrophage’s ability to degrade and remodel the ECM is extremely important during development, cancer metastasis, and cell intravasation [55]. Consequently, Rac inhibition of macrophages in the aorta-gonad-mesonephros (AGM) leads to ineffective hematopoietic stem cell colonization and organization in the caudal hematopoietic tissue (CHT) [55]. Additionally, the ADP ribosylation factor (ARF) GTPase activation protein ASAP1 that regulates cytoskeletal dynamics, small GTP-binding protein receptor recycling, and intracellular vesicle trafficking has been shown to regulate macrophage migration. Zebrafish larvae deficient in Asap1 displayed impaired macrophage migration to tail injury or upon infection with M. marinum, impacting susceptibility to infection [57].
Focal adhesions are fundamental molecular structures that link the cell cytoskeleton and ECM and are responsible for proper cell migration [58]. They are dynamically assembled and disassembled at the leading and trailing edge of the cell, generating mechanical forces causing the cell cytoskeleton to contract, facilitating cell movement [58]. Paxillin a core protein in focal adhesions that signals downstream to activate cell migration, is regulated by several phosphorylation events that recruit adaptor proteins, such as CRKII that activate guanine exchange factors (GEFs) [58]. Neutrophils and macrophages rely differently on paxillin during migration through interstitial tissues in vivo [17]. Indeed, although neutrophils express the cytoskeletal proteins vinculin and paxillin, they do not form mature focal adhesions contrary to macrophages [59]. Using zebrafish, the visualization of focal adhesions became possible by overexpressing fluorescently tagged paxillin both in macrophages and neutrophils. Elongated ameboid macrophages form paxillin-containing puncta display reduced speed and increased directional persistence, while the ameboid morphology of migrating neutrophils lacks defined paxillin puncta allowing these cells to migrate fast [17].
Macrophage random migration is also dependent on Arp2/3; however, upon the activation of directional migration this mechanism is less reliant on Arp2/3, suggesting other actin nucleators are involved [17]. Additionally, as in neutrophils radiometric live imaging of PIP3 using PHAKT-GFP biosensor in macrophages allowed visualization of PIP3 localization at the leading edge, however projections at the rearward side of the cell during both protrusion elongation and retraction also localize PIP3 [17]. Microtubules network, which are essential in promoting turnover of adhesion complexes and regulating the actin cytoskeleton, also present a distinct localization in macrophages and neutrophils. In vivo visualization of microtubules with ensconsin microtubule-binding domain fluorescent probe (EMTB-3X GFP), showed that microtubules localize both at the rear and the front in macrophages, while neutrophil microtubules localize toward the rear of the polarized cell [17].
Dendritic Cell and Lymphocyte migration – un unexplored field in zebrafish model.
As in mammal, dendritic cells (DCs) form a link between the adaptive and innate immune systems through their ability to stimulate T-cell activation and proliferation serving as antigen presenting cells (APCs) [60,61]. A population of dendritic like cells have been identified in the kidney marrow of adult zebrafish using differential lectin binding assays [60]. More recently, using cross-organ single cell transcriptomic analysis Zhou et. al., described two major classes of DCs, conventional DCs (cDCs) and plasmacytoid DCS (pDCs), found widely distributed in the non-lymphoid organs of zebrafish and emerging at larval stages. Functional studies on DCs and their migration patterns and behavior are now possible due to the generation of specific reporter lines that allow visualization and tracking of diferent subsets of DCs and distinction from macrophage subsets[62] (Table 1). Like the innate immune system, the adaptive immune system between fish and mammals is remarkably conserved, reviewed [63]. Zebrafish have B-cells that express Ig proteins, as well as T-cells with receptor components that are mediated by Rag1 and Rag2 protein rearrangements [9]. Additionally, although zebrafish have many lymphocyte receptor homologs, they lack antibodies for these populations, relying heavily on transgenic lines to visualize cell dynamics, including lymphocyte migration [9]. For many years lymphocyte migration has not been properly explored in the zebrafish model, even though specific transgenic reporter lines for B and T cells have been developed in the last two decades (Table 1). Recent advancements and important contributions from the Huttenlocher lab might change this trend [64]. In mammals naïve T-cells migrate from the lymph nodes, located throughout the body, to scan for antigens. With a combination of scRNA-seq and microscopy, a lymphoid network, devoid of lymph nodes, that performs whole-body antigen surveillance was identified in zebrafish [64]. This tessellated lymphoid network (TLN) maintains many characteristics of motile lymphocytes, including morphology and directed and coordinated movement [64]. Using scRNA-seq revealed three major APC populations and naïve like T-cells that express ccr7, both indicative hallmarks of lymph nodes [64]. T-cells within this network collectively and directionally migrate, but in the context of infection transition to single cell Brownian walk, allowing these cells to interact with APCs, activate, and differentiate [64].This groundbreaking study from Robertson et. al. [64] will for sure push the field to make use of the tremendous characteristics of the zebrafish that associated with the increasing knowledge on fish immunology and evolution of the immune system, will open opportunities for new research programs be established focused on lymphocyte cell dynamics and migration.
Table 1:
Transgenic reporter zebrafish lines targeting Lymphocytes and Dendritic cells.
| Transgenic line | Immune cell | Allele (Ref.) |
|---|---|---|
| Tg(rag1:GFP) | Lymphocytes | la5Tg [10] |
| Tg(rag2:GFP) | Lymphocytes | zdf8Tg & 1a6Tg [11,80] |
| Tg2(rag2:mCherry) | Lymphocytes | bu3Tg [81] |
| Tg(lck:lck-EGFP) | T Cells | cz1Tg [9] |
| TgBAC(foxp3a:TagRFP,cryaa:EGFP) | Treg | vcc3Tg [82] |
| TgBAC(cd4-1:mCherry) | CD4 T Cells | umc13Tg [83] |
| Tg(-14cd79a:EGFP) | B Cells | fcc97Tg [84] |
| Tg(-5cd79b:EGFP) | B Cells | fcc90Tg [84] |
| Tg(Cau.Ighm:EGFP) | B Cells | sd19Tg [85] |
| TgBAC(ccl35.1:EGFP) | Dendritic cells | n.a. [62] |
Boosting research in rare genetic diseases and discovery of novel therapeutics.
Zebrafish has emerged as a powerful in vivo model to investigate “untouched” human rare diseases that present as severe, chronic, progressive, and mostly with a genetic cause. Zebrafish has been pivotal in offering an affordable system to increase knowledge about the pathophysiological mechanism of disease in rare conditions including blood, skeletal and neurological rare diseases (reviewed in [65–68]). The zebrafish is currently a main choice to confirm gene discovery, to perform genetic analysis of human variants, or to study orthologs to mouse lethal allele. A group of rare blood diseases named primary immunodeficiencies (PIDs) represent a heterogeneous group of over 200 genetic disorders characterized by the partial or complete absence of the immune system or its improper activity (i.e., function and migration) [67]. Patients with PIDs present increased vulnerability to germs such as bacteria, viruses, fungi, and protozoa. There are over 230 identified PID-causing genes, but many more for sure to be discovered. In the last two decades, zebrafish have been used to model different PIDs and determine how genes associated with different diseases impact production, function and migration of immune cells. Some of the available zebrafish PIDs models are: Wiskott-Aldrich syndrome (WAS) [69] warts, hypo-gammaglobulinemia, infections, and myelokathexis (WHIM) syndrome [70], chronic granulomatous disease (CGD) [71], leukocyte adhesion deficiency (LAD) [72,73] REF, activated PI3K delta syndrome (APDS)[74]. Most of these models were used to explore mechanisms that impact myeloid cells but the impact in dendritic cells, T and B cells is still to be explored. Additionally, the use of PID models to design therapeutics to restore proper immune response in patients with PIDs is still vastly unexplored and deserves further attention since it could provide valuable approaches for patients. Access to next-generation sequencing techniques that quickly identify gene defects in small populations (or single patients) with inherited diseases aligned to the latest genome-editing techniques such as CRIPR/Cas9, and the numerous unique features of the zebrafish model, are offering an unprecedent opportunity to model and understand the physiopathology of PIDs and other rare genetic human diseases. Interestingly, even though many human disease models have been developed and fully characterized in zebrafish, their use in designing and developing new therapeutics is far from reaching its full potential. In the years to come private sector investment needs to be stimulated and encouraged so that they can transition from in vitro to in vivo drug screening pipelines with zebrafish models.
Future areas to explore leukocyte dynamics.
Evaluating leukocyte migration within their in vivo environment is crucial to better understand their physiological behavior in the context of homeostasis and disease. As the scientific community becomes more elucidated and open to non-mammalian animal systems, they will transition from conventional 2D models to more complex and physiological scenarios, such as the zebrafish. The combination of an optimized in vitro system with in vivo approaches where cells are being exposed to multiple migration-inducing cues, simultaneously, is key to understanding leukocyte migration in the context of disease. A big question that zebrafish will continue to assess is how cells switch their migration direction, particularly neutrophils. Are neutrophils reprogrammed at the injury site and disseminate inflammation upon reverse migration, or is this a specific subset of neutrophils? Our most recent work shows that the presence of metainflammation drastically impacts neutrophil recruitment. We found that neutrophils are activated at the nonalcoholic fatty liver and able to reverse migrate displaying a fast and exacerbated migratory behavior towards an injury. In vivo time lapse microscopy showed that neutrophil exposed to metainflammation move faster than control larvae and seem to be unable to slow down at the wound suggesting defects in migration machinery [75]. So, how is neutrophil polarization and subsequent forward and reverse migration impacted by the presence of systemic inflammation (e.g., metainflammation or inflammaging) Clarification in this area is key to properly develop and use therapies that target neutrophil migration in scenarios of neutrophilic inflammation that are sustaining chronic or hyperactive inflammation (autoimmune diseases, cytokine storm, chronic lung disease, cancer early progression, etc.) and contributing to poor outcomes. Another important topic to expand our knowledge is how leukocytes use different migration modes (amoeboid versus mesenchymal) to migrate in response to various environmental cues that are present in different disease states. Are macrophages the only leukocytes that display morphological plasticity in vivo? Do dendritic cells behavior similarly? And B and T cells, what are the cell dynamics and the mechanisms these cells use to migrate in tissues and organs? New emergent techniques such as spatial-transcriptomics [76], single cell proteomics [77], or behavioral immune landscapes of inflammation [78] will play important roles in answering this and many other questions. Finally, studying immune cell trafficking through non-invasive intravital imaging, and establishing consistency of analysis across different laboratories is crucial for proper advancement of the field of cell migration [79].
Acknowledgments:
The authors thank Joaquin Canton Sandoval for useful feedback on the manuscript.
Grant Support:
CM supported by T32 GM145438 and SdO supported by R35 GM118027.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Streisinger G, Walker C, Dower N, Knauber D, Singer F: Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291:293–296. [DOI] [PubMed] [Google Scholar]
- 2.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al. : The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Slyke CE, Bradford YM, Howe DG, Fashena DS, Ramachandran S, Ruzicka L: Using ZFIN:Data Types, Organization, and Retrieval. Methods Mol Biol 2018, 1757:307–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A: Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of leukocyte biology 2006, 80:1281–1288. [DOI] [PubMed] [Google Scholar]
- 5.Renshaw SA, Loynes CA, Trushell DMI, Elworthy S, Ingham PW, Whyte MKB: A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108:3976–3978. [DOI] [PubMed] [Google Scholar]
- 6.Hall C, Flores MV, Storm T, Crosier K, Crosier P: The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Developmental Biology 2007, 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ: mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117:e49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walton EM, Cronan MR, Beerman RW, Tobin DM: The Macrophage-Specific Promoter mfap4 Allows Live, Long-Term Analysis of Macrophage Behavior during Mycobacterial Infection in Zebrafish. PLoS One 2015, 10:e0138949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langenau DM, Ferrando AA, Traver D, Kutok JL, Hezel JP, Kanki JP, Zon LI, Look AT, Trede NS: In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci U S A 2004, 101:7369–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jessen JR, Willett CE, Lin S: Artificial chromosome transgenesis reveals long-distance negative regulation of rag1 in zebrafish. Nat Genet 1999, 23:15–16. [DOI] [PubMed] [Google Scholar]
- 11.Jessen JR, Jessen TN, Vogel SS, Lin S: Concurrent expression of recombination activating genes 1 and 2 in zebrafish olfactory sensory neurons. Genesis 2001, 29:156–162. [DOI] [PubMed] [Google Scholar]
- 12.Dee CT, Nagaraju RT, Athanasiadis EI, Gray C, Fernandez del Ama L, Johnston SA, Secombes CJ, Cvejic A, Hurlstone AFL: CD4-Transgenic Zebrafish Reveal Tissue-Resident Th2- and Regulatory T Cell-like Populations and Diverse Mononuclear Phagocytes. The Journal of Immunology 2016, 197:3520–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathias JR, Perrin BJ, Liu T-X, Kanki J, Look AT, Huttenlocher A: Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of Leukocyte Biology 2006, 80:1281–1288. [DOI] [PubMed] [Google Scholar]
- 14.Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, Lin S, Prochownik E, Trede NS, Zon LI, Look AT: Myc-Induced T Cell Leukemia in Transgenic Zebrafish. Science 2003, 299:887–890. [DOI] [PubMed] [Google Scholar]
- 15.Huemer K, Squirrell JM, Swader R, Pelkey K, LeBert DC, Huttenlocher A, Eliceiri KW: Long-term Live Imaging Device for Improved Experimental Manipulation of Zebrafish Larvae. J Vis Exp 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SenGupta S, Parent CA, Bear JE: The principles of directed cell migration. Nature Reviews Molecular Cell Biology 2021, 22:529–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barros-Becker F, Lam P-Y, Fisher R, Huttenlocher A: Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. Journal of Cell Science 2017, 130:3801–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam PY, Huttenlocher A: Interstitial leukocyte migration in vivo. Curr Opin Cell Biol 2013, 25:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P: Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358:111–116. [DOI] [PubMed] [Google Scholar]
- 20.Robertson AL, Holmes GR, Bojarczuk AN, Burgon J, Loynes CA, Chimen M, Sawtell AK, Hamza B, Willson J, Walmsley SR, et al. : A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med 2014, 6:225ra229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Oliveira S, Rosowski EE, Huttenlocher A: Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 2016, 16:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Q, Zhao W, Yan M, Mei H: Neutrophil reverse migration. Journal of Inflammation 2022, 19:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nourshargh S, Renshaw SA, Imhof BA: Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol 2016, 37:273–286. [DOI] [PubMed] [Google Scholar]
- 24.Tauzin S, Starnes TW, Becker FB, Lam PY, Huttenlocher A: Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J Cell Biol 2014, 207:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, Huttenlocher A: Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Rep 2017, 19:1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loynes CA, Lee JA, Robertson AL, Steel MJ, Ellett F, Feng Y, Levy BD, Whyte MKB, Renshaw SA: PGE(2) production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci Adv 2018, 4:eaar8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isles HM, Herman KD, Robertson AL, Loynes CA, Prince LR, Elks PM, Renshaw SA: The CXCL12/CXCR4 Signaling Axis Retains Neutrophils at Inflammatory Sites in Zebrafish. Front Immunol 2019, 10:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, Whyte MK, Walmsley SR, Renshaw SA: Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011, 118:712–722. [DOI] [PubMed] [Google Scholar]
- 29.Li B, Han X, Ye X, Ni J, Wu J, Dai J, Wu Z, Chen C, Wan R, Wang X, Hu G: Substance P-regulated leukotriene B4 production promotes acute pancreatitis-associated lung injury through neutrophil reverse migration. Int Immunopharmacol 2018, 57:147–156. [DOI] [PubMed] [Google Scholar]
- 30.Ellett F, Elks PM, Robertson AL, Ogryzko NV, Renshaw SA: Defining the phenotype of neutrophils following reverse migration in zebrafish. J Leukoc Biol 2015, 98:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson AL, Ogryzko NV, Henry KM, Loynes CA, Foulkes MJ, Meloni MM, Wang X, Ford C, Jackson M, Ingham PW, et al. : Identification of benzopyrone as a common structural feature in compounds with anti-inflammatory activity in a zebrafish phenotypic screen. Dis Model Mech 2016, 9:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson JD, Ward JR, Avila-Olias M, Battaglia G, Renshaw SA: Targeting Neutrophilic Inflammation Using Polymersome-Mediated Cellular Delivery. J Immunol 2017, 198:3596–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgantzoglou A, Poplimont H, Walker HA, Lämmermann T, Sarris M: A two-step search and run response to gradients shapes leukocyte navigation in vivo. Journal of Cell Biology 2022, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using a laser wound assay to generate gradients and quantitative analysis, Sarris et al identify a two stage process in which neutrophils use to interepret and respond to gradients, first a “search” phase, which is then followed by a “run” phase.
- 34.Yoo SK, Deng Q, Cavnar PJ, WU YI, Hahn KM, Huttenlocher A: Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell 2010, 18:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo SK, Lam PY, Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A: The role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci 2012, 125:5702–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo SK, Lam P-y, Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A: The role of microtubules in neutrophil polarity and migration in live zebrafish. Journal of Cell Science 2012, 125:5702–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ke W, Ye D, Mersch K, Xu H, Chen S, Lin F: Gβ1 is required for neutrophil migration in zebrafish. Dev Biol 2017, 428:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manley HR, Potter DL, Heddleston JM, Chew TL, Keightley MC, Lieschke GJ: Frontline Science: Dynamic cellular and subcellular features of migrating leukocytes revealed by in vivo lattice lightsheet microscopy. J Leukoc Biol 2020, 108:455–468. [DOI] [PubMed] [Google Scholar]; * Making use of the remarkable transparency of zebrafish larvae the authors reporte how LLSM is a porwerful tool to perfome intravital leukocyte imaging and can be used to study cellular and subcellular complexities of phagocyte biology.
- 39.Weninger W, Biro M, Jain R: Leukocyte migration in the interstitial space of non-lymphoid organs. Nature Reviews Immunology 2014, 14:232–246. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira S, Reyes-Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado A: Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol 2013, 190:4349–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuñiga-Traslaviña C, Bravo K, Reyes AE, Feijóo CG: Cxcl8b and Cxcr2 Regulate Neutrophil Migration through Bloodstream in Zebrafish. J Immunol Res 2017, 2017:6530531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaveh A, Bruton FA, Buckley C, Oremek MEM, Tucker CS, Mullins JJ, Taylor JM, Rossi AG, Denvir MA: Live Imaging of Heart Injury in Larval Zebrafish Reveals a Multi-Stage Model of Neutrophil and Macrophage Migration. Front Cell Dev Biol 2020, 8:579943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poplimont H, Georgantzoglou A, Boulch M, Walker HA, Coombs C, Papaleonidopoulou F, Sarris M: Neutrophil Swarming in Damaged Tissue Is Orchestrated by Connexins and Cooperative Calcium Alarm Signals. Curr Biol 2020, 30:2761–2776.e2767. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Through the use of biosensors and intravital microscopy in zebrafish Sarris et al reveal that neutrophil chemoattractant signals, such as LTB4, are triggered and sustained by calcium through contact with damaged tissues. This calcium flux, is triggered by contact dependent connexin-43 hemichannels (Cx43) in neutrophil clusters, which further enhances chemoattractant biosynthesis and cluster growth.
- 44.Dieterich P, Lindemann O, Moskopp ML, Tauzin S, Huttenlocher A, Klages R, Chechkin A, Schwab A: Anomalous diffusion and asymmetric tempering memory in neutrophil chemotaxis. PLOS Computational Biology 2022, 18:e1010089. [DOI] [PMC free article] [PubMed] [Google Scholar]; Making use powerful stochastic mathematical models the authors analyzed data sets obtained in vitro (murine neutrophils) and in vivo (zebrafish neutrophils). They found that spatially differential regulation of anomalous dynamics appears to play a central role in guiding efficient chemotactic behavior .
- 45.Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN: Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isles HM, Loynes CA, Alasmari S, Kon FC, Henry KM, Kadochnikova A, Hales J, Muir CF, Keightley MC, Kadirkamanathan V, et al. : Pioneer neutrophils release chromatin within in vivo swarms. Elife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using intravital microscopy, Elks et al show that pioneer neutrophils are the focus for collective neutrophil migration and swarming behavior. Pioneer neutrophils adopt distinct morphologies, such as rounded shape, reduction in speed, and displacement, but they do not undergo apoptosis. Additionally, using high resolution microscopy these pioneers release cellular fragments, later discovered to be extracellular DNA (including histones and myeloperoxidase) around developing swarms, similar to mammalian neutrophil extraceullar traps (NETs).
- 47.Niethammer P, Grabher C, Look AT, Mitchison TJ: A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459:996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Oliveira S, Lopez-Munoz A, Candel S, Pelegrin P, Calado A, Mulero V: ATP modulates acute inflammation in vivo through dual oxidase 1-derived H2O2 production and NF-kappaB activation. J Immunol 2014, 192:5710–5719. [DOI] [PubMed] [Google Scholar]
- 49.de Oliveira S, Boudinot P, Calado A, Mulero V: Duox1-derived H2O2 modulates Cxcl8 expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J Immunol 2015, 194:1523–1533. [DOI] [PubMed] [Google Scholar]
- 50.Houseright RA, Miskolci V, Mulvaney O, Bortnov V, Mosher DF, Rindy J, Bennin DA, Huttenlocher A: Myeloid-derived growth factor regulates neutrophil motility in interstitial tissue damage. J Cell Biol 2021, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Authors reported for the first time that the myeloid-derived growth factor (MYDGF) regulates neutrophil motility to tissue damage but not infected tissues.
- 51.Zhou W, Hsu AY, Wang Y, Syahirah R, Wang T, Jeffries J, Wang X, Mohammad H, Seleem MN, Umulis D, Deng Q: Mitofusin 2 regulates neutrophil adhesive migration and the actin cytoskeleton. J Cell Sci 2020, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gurol T, Zhou W, Deng Q: MicroRNAs in neutrophils: potential next generation therapeutics for inflammatory ailments. Immunol Rev 2016, 273:29–47. [DOI] [PubMed] [Google Scholar]
- 53.Hsu AY, Wang T, Syahirah R, Liu S, Li K, Zhang W, Wang J, Cao Z, Tian S, Matosevic S, et al. : Rora Regulates Neutrophil Migration and Activation in Zebrafish. Front Immunol 2022, 13:756034. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Using microRNAs, specfically miR-99, the authors discovered a unique role for RAR-related orphan receptor (RORa) in regulating neutrophil migration. Additionaly they show that microRNAs specifcally miR-99, can function as therapeutic targets to modulate neutrophil recruitment during acute inflammation.
- 54.Sipka T, Park SA, Ozbilgic R, Balas L, Durand T, Mikula K, Lutfalla G, Nguyen-Chi M: Macrophages undergo a behavioural switch during wound healing in zebrafish. Free Radical Biology and Medicine 2022, 192:200–212. [DOI] [PubMed] [Google Scholar]
- 55.Travnickova J, Nhim S, Abdellaoui N, Djouad F, Nguyen-Chi M, Parmeggiani A, Kissa K: Macrophage morphological plasticity and migration is Rac signalling and MMP9 dependant. Sci Rep 2021, 11:10123. [DOI] [PMC free article] [PubMed] [Google Scholar]; * In vivo imaging of zebrafish, during hematopoiesis, showed that macrophages display morphological plasticity during migration. This plasticitiy is a Rac dependent process that allows for the remodeling of the cytoskeleton via MMP-9 proteolysis, an extremely important process for cellular migration in both development and cancer.
- 56.Rosowski EE, Deng Q, Keller NP, Huttenlocher A: Rac2 Functions in Both Neutrophils and Macrophages To Mediate Motility and Host Defense in Larval Zebrafish. J Immunol 2016, 197:4780–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui J, Chen G, Wen D, Wang Y, Zhao Z, Wu C: Asap1 Affects the Susceptibility of Zebrafish to Mycobacterium by Regulating Macrophage Migration. Frontiers in Cellular and Infection Microbiology 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue Q, Varady SRS, Waddell TQA, Roman MR, Carrington J, Roh-Johnson M: Lack of Paxillin phosphorylation promotes single-cell migration in vivo. J Cell Biol 2023, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson ZS, Witt H, Hazlett L, Harman M, Neumann BM, Whitman A, Patel M, Ross RS, Franck C, Reichner JS, Lefort CT: Context-Dependent Role of Vinculin in Neutrophil Adhesion, Motility and Trafficking. Scientific Reports 2020, 10:21421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lugo-Villarino G, Balla KM, Stachura DL, Bañuelos K, Werneck MB, Traver D: Identification of dendritic antigen-presenting cells in the zebrafish. Proc Natl Acad Sci U S A 2010, 107:15850–15855. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A novel in vivo model, using high resolution imaging to study lymphocyte migration and T-cell A survellience. Huttenlocher et al effectively idenitfy a lymphoid network, named tessellated lymphoid network (TLN), where T-cells, but not other leukocytes are organized into a regular repeating pattern. Important study that opens doors for community to engage in using zebrafish models to study lymphocyte migration in native environemnt.
- 61.Dong F, Song X, Xing J, Tang X, Sheng X, Chi H, Zhan W: Immunological characteristics of dendritic cells marker CD83 in flounder (Paralichthys olivaceus). Fish and Shellfish Immunology Reports 2021, 2:100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Q, Zhao C, Yang Z, Qu R, Li Y, Fan Y, Tang J, Xie T, Wen Z: Cross-organ single-cell transcriptome profiling reveals macrophage and dendritic cell heterogeneity in zebrafish. Cell Rep 2023, 42:112793. [DOI] [PubMed] [Google Scholar]; ** This paper used transcriptomic analysi of cells from diferent tissues to characterized and develop lines to visualize dendritic cells.
- 63.Miao KZ, Kim GY, Meara GK, Qin X, Feng H: Tipping the Scales With Zebrafish to Understand Adaptive Tumor Immunity. Front Cell Dev Biol 2021, 9:660969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson TF, Hou Y, Schrope J, Shen S, Rindy J, Sauer JD, Dinh HQ, Huttenlocher A: A tessellated lymphoid network provides whole-body T cell surveillance in zebrafish. Proc Natl Acad Sci U S A 2023, 120:e2301137120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Son M, Kim DY, Kim C-H: Disease Modeling of Rare Neurological Disorders in Zebrafish. In International Journal of Molecular Sciences. Edited by; 2022. vol 23.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crouzier L, Richard EM, Sourbron J, Lagae L, Maurice T, Delprat B: Use of Zebrafish Models to Boost Research in Rare Genetic Diseases. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rissone A, Burgess SM: Rare Genetic Blood Disease Modeling in Zebrafish. Frontiers in Genetics 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marí-Beffa M, Mesa-Román AB, Duran I: Zebrafish Models for Human Skeletal Disorders. Frontiers in Genetics 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones RA, Feng Y, Worth AJ, Thrasher AJ, Burns SO, Martin P: Modelling of human Wiskott-Aldrich syndrome protein mutants in zebrafish larvae using in vivo live imaging. J Cell Sci 2013, 126:4077–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A: Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood 2010, 116:2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoen TJ, Rosowski EE, Knox BP, Bennin D, Keller NP, Huttenlocher A: Neutrophil phagocyte oxidase activity controls invasive fungal growth and inflammation in zebrafish. J Cell Sci 2019, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A: Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev Cell 2011, 21:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bader A, Gao J, Riviére T, Schmid B, Walzog B, Maier-Begandt D: Molecular Insights Into Neutrophil Biology From the Zebrafish Perspective: Lessons From CD18 Deficiency. Front Immunol 2021, 12:677994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elworthy S, Rutherford HA, Prajsnar TK, Hamilton NM, Vogt K, Renshaw SA, Condliffe AM: Activated PI3K delta syndrome 1 mutations cause neutrophilia in zebrafish larvae. Dis Model Mech 2023, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feliz-Norberto M, Michael C, Oliveira Sd: Neutrophil reverse migration from liver fuels neutrophilic inflammation to tissue injury in Nonalcoholic Steatohepatitis. bioRxiv 2021:2021.2010.2003.462893. [Google Scholar]; ** Metainflammation is a complex health situation that trigers systemic metabolic dysfunction associated with a systemic low grade of inflammation. This paper shows a deep in vivo analysis of the impact of metabolic syndrome ans associated systemic inflammation in zebrafish.
- 76.Mitamura Y, Reiger M, Kim J, Xiao Y, Zhakparov D, Tan G, Rückert B, Rinaldi AO, Baerenfaller K, Akdis M, et al. : Spatial transcriptomics combined with single-cell RNA-sequencing unravels the complex inflammatory cell network in atopic dermatitis. Allergy 2023. [DOI] [PubMed] [Google Scholar]
- 77.Bennett HM, Stephenson W, Rose CM, Darmanis S: Single-cell proteomics enabled by next-generation sequencing or mass spectrometry. Nature Methods 2023, 20:363–374. [DOI] [PubMed] [Google Scholar]
- 78.Crainiciuc G, Palomino-Segura M, Molina-Moreno M, Sicilia J, Aragones DG, Li JLY, Madurga R, Adrover JM, Aroca-Crevillén A, Martin-Salamanca S, et al. : Behavioural immune landscapes of inflammation. Nature 2022, 601:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Georgantzoglou A, Matthews J, Sarris M: Neutrophil motion in numbers: How to analyse complex migration patterns. Cells & Development 2021, 168:203734. [DOI] [PubMed] [Google Scholar]; ** Authors provide a summary of considerations and recommendations for quantitative analysis of neutrophil swarming and reverse migration. In addition, authors introduced relevant analysis tools to new researchers in the field with the goal of establishing common frameworks and standards on cell motion analysis.
- 80.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI: Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol 2003, 4:1238–1246. [DOI] [PubMed] [Google Scholar]
- 81.Harrold I, Carbonneau S, Moore BM, Nguyen G, Anderson NM, Saini AS, Kanki JP, Jette CA, Feng H: Efficient transgenesis mediated by pigmentation rescue in zebrafish. Biotechniques 2016, 60:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hui SP, Sheng DZ, Sugimoto K, Gonzalez-Rajal A, Nakagawa S, Hesselson D, Kikuchi K: Zebrafish Regulatory T Cells Mediate Organ-Specific Regenerative Programs. Dev Cell 2017, 43:659–672 e655. [DOI] [PubMed] [Google Scholar]
- 83.Dee CT, Nagaraju RT, Athanasiadis EI, Gray C, Fernandez Del Ama L, Johnston SA, Secombes CJ, Cvejic A, Hurlstone AF: CD4-Transgenic Zebrafish Reveal Tissue-Resident Th2- and Regulatory T Cell-like Populations and Diverse Mononuclear Phagocytes. J Immunol 2016, 197:3520–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu X, Li YS, Shinton SA, Rhodes J, Tang L, Feng H, Jette CA, Look AT, Hayakawa K, Hardy RR: Zebrafish B Cell Development without a Pre-B Cell Stage, Revealed by CD79 Fluorescence Reporter Transgenes. J Immunol 2017, 199:1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Page DM, Wittamer V, Bertrand JY, Lewis KL, Pratt DN, Delgado N, Schale SE, McGue C, Jacobsen BH, Doty A, et al. : An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood 2013, 122:e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


