Abstract
Rates of opioid-related deaths and overdoses in the United States are at record-high levels. Thus, novel neurobiological targets for the treatment of OUD are greatly needed. Given the close interaction between the endogenous opioid system and the endocannabinoid system (ECS), targeting the ECS may have therapeutic potential in OUD. The various components of the ECS, including cannabinoid receptors, their lipid-derived endogenous ligands (endocannabinoids [eCBs]), and the related enzymes, present potential targets for developing new medications in OUD treatment. The purpose of this paper is to review the clinical and preclinical literature on the dysregulation of the ECS after exposure to opioids. We review the evidence of ECS dysregulation across various study types, exposure protocols, and measurement protocols and summarize the evidence for dysregulation of ECS components at specific brain regions. Preclinical research has shown that opioids disrupt various ECS components that are region-specific. However, the results in the literature are highly heterogenous and sometimes contradictory, possibly due to variety of different methods used. Further research is needed before a confident conclusion could be made on how exposure to opioids can affect ECS components in various brain regions.
Keywords: Opioid Use Disorder, Opioids, Endocannabinoids, Endocannabinoid system, Pharmacologic Actions, Animal Models, Addiction
1. Introduction
Over the last decades, the prevalence of opioid use disorder (OUD) has risen, and opioid-related deaths and overdoses are at previously unseen levels. Currently, at least three million individuals in the United States (US), either currently meet the criteria for OUD, or have a lifetime history of the disorder (Azadfard et al., 2021). Opioid-related overdose deaths have also climbed by approximately 10-fold in recent years (Volkow and Blanco, 2021). Between April 2020-April 2021, the US National Center for Health Statistics reported overdose-related deaths increased to a new peak of over 100,000 annual deaths; with root cause analysis estimating that upwards of 75% of these deaths were opioid-related overdoses (Ahmad et al., 2021; Volkow and Blanco, 2021). The three FDA-approved medications for opioid use disorder (OUD) all act primarily on opioid receptors (Kampman and Jarvis, 2015). These medications currently have an essential role in the management of OUD, as up to 80% of individuals with OUD relapse within 1-2 years after detoxification without treatment (Calabria et al., 2010; Vaillant, 1973). However, even with these available treatments there remain high rates of treatment discontinuation and relapse (Hser et al., 2014; Lo et al., 2018; Nosyk et al., 2010; Smyth et al., 2010). In addition, many patients do not completely abstain from illicit opioids even when on maintenance treatments. Some studies report up to two-thirds of patients receiving treatment also continue illicit opioid use, which places them at continued risk of overdose and other undesired consequences (Strain et al., 2021). Thus, alternate treatment options that function in conjunction with, in parallel to, or separate from the opioid receptor systems are greatly needed (Lee et al., 2022; Strain et al., 2021).
There is growing evidence that the endocannabinoid system (ECS) may present as a possible target in the treatment of OUD (Chye et al., 2019; Sloan et al., 2017; Wiese and Wilson-Poe, 2018). The ECS is closely linked to the endogenous opioid system and dopaminergic/reward systems. Cannabinoid receptor type 1 (CB1R) and mu-opioid receptor (MOR) are both Gi/o-coupled receptors, which are colocalized in several brain areas (Rodriguez et al., 2001; Scavone et al., 2010). There is bidirectional modulation of the rewarding and reinforcing properties of opioids and cannabinoids in different brain areas(Ahmad et al., 2013). Moreover, ECS functions as a major stress regulatory system (Katzman et al., 2016; Micale and Drago, 2018), and may have an important role in the stress-induced relapse in OUD (Parsons and Hurd, 2015), which is one of the main risk factors of treatment discontinuation (Moitra et al., 2013; Panlilio et al., 2019; Sinha, 2008).
To date, the potential ECS system alterations in OUD have not been extensively investigated in humans. However, increasing evidence from preclinical research suggests that different components of ECS are dysregulated in animal models of opioid exposure. Here we provide an updated review of the evidence of ECS dysregulation in animal models and human studies of OUD with specific attention to the amount of opioid exposure and the studied brain regions. This research provides the foundation for targeting the ECS in the treatment of OUD by developing novel ECS modulators.
2. The endocannabinoid system (ECS)
The ECS functions as one of the body’s key stress regulatory systems (Figure 1). It is comprised of (1) cannabinoid receptor type 1 (CB1R) and type 2 (CB2R), (2) their lipid-derived endogenous ligands (eCBs): N-arachidonoylethanolamine or anandamide (AEA) and 2-Arachidonoylglycerol (2-AG), (3) the main enzymes responsible for the eCB synthesis: N-acyl-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) for AEA and diacyl glycerol lipase (DAGL) α or β for 2-AG, and (4) the main eCB catabolic enzymes: fatty acid amide hydrolase (FAAH) for AEA and monoacylglycerol lipase (MAGL), α/β-hydrolase domain-containing 6 (ABHD6), and α/β-hydrolase domain-containing 12 (ABHD12) for 2-AG (Bassir Nia et al., 2019; Cao et al., 2019; Lu and Mackie, 2021; Piomelli and Mabou Tagne, 2022).
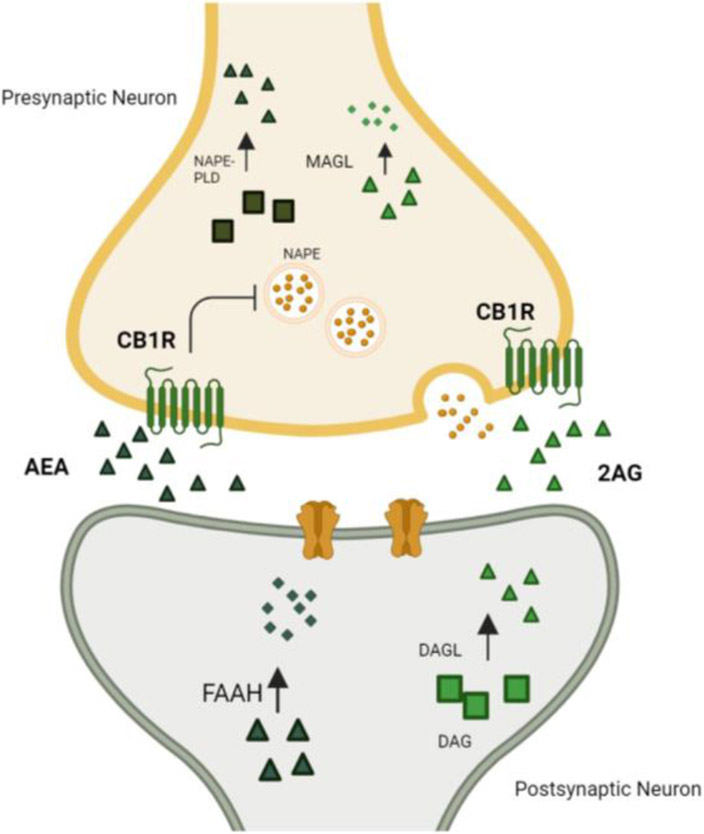
Figure 1: The ECS components.
CB1R receptors are located predominantly in the presynaptic neurons. Upon activation of the postsynaptic neuron, the endocannabinoid 2-AG is made on demand by the DAGL enzyme in the postsynaptic neuron. Given its lipophilic properties, 2-AG crosses the neuron postsynaptic neuron membrane and binds to presynaptic CB 1R. The 2-AG is degraded by the MAGL enzyme in the presynaptic neuron. The AEA is synthesized mostly by the NAPE-PLD enzyme which is located predominantly in the presynaptic neuron and degrades by the FAHH enzyme in the postsynaptic neuron.
Abbreviations: 2-AG, 2-Arachidonoylglycerol; AEA, Anandamide; CB1R, Cannabinoid Receptor type 1; DAG, Diacylglycerol; DAGL, Diacylglycerol Lipase; FAAH, Fatty Acid Amide Hydrolase; MAGL, Monoacylglycerol Lipase; NAPE, N-acyl-phosphatidylethanolamine; NAPE-PLD, N-acyl-phosphatidylethanolamine-specific Phospholipase D.
2.1. Cannabinoid receptors
There are two main cannabinoid receptors: CB1R and CB2R. Both CB1R and CB2R are Gi/o protein-coupled receptors (GPCR) (Atwood and Mackie, 2010; Manzanares et al., 2018). However, their distribution differs. While CB1R is thought to be primarily expressed in the brain, CB2Rs seem to be predominantly located on immune cells, spleen, and macrophage-derived cells. Studies of CB1R emphasize a preference for clustering on GABAergic synaptic terminals (Manzanares et al., 2018). Less CB1R has been identified on glutamatergic synaptic terminals, and even fewer on astrocytes. Interestingly, CB1R also has been identified in the mitochondria and lysosomes of neurons and astrocytes (Lu and Mackie, 2016). The intracellular CB1Rs are a distinct subgroup of CB1Rs from surface receptors and have different mechanisms of action. While surface CB1R inhibits cyclic adenosine monophosphate (cAMP) production and consequently influx of calcium ions into the cytoplasm after activation by extracellular ligands, the intracellular ligand activates the lysosomal CB1Rs which results in the internal release of calcium ions from internal organelles and elevated intracellular calcium level. Upon activation by intracellular agonists, the mitochondrial CB1Rs result in decreased cAMP concentration in mitochondria and decreased mitochondrial respiration (Zou and Kumar, 2018). Although primarily it was believed that CB2R is absent in the CNS, recent findings provide strong evidence for the presence of CB2R in the CNS neurons, however, in considerably lower concentrations compared to CB1R. In contrast to CB1Rs, the CB2Rs are located in the postsynaptic cells in various brain regions, where their activation results in reduced excitability of the postsynaptic neurons. Additionally, the CB2R is expressed in CNS glial cells where its activation plays a role in CNS immunologic and inflammatory processes. (Chen et al., 2017)
2.2. Endocannabinoids and their metabolism
Cannabinoid receptors are activated by the eCBs 2-AG and AEA. Despite their shared functionality and origin, they differ significantly in their affinity for cannabinoid receptors. While AEA binds with a high affinity to CB1R as a partial agonist, it has a low affinity to CB2R. Unlike AEA, 2-AG has agonistic qualities for both CB1R and CB2R, with moderate to low affinity for both receptors (Zou and Kumar, 2018). Of note, although AEA has a higher affinity for CB1R, its efficacy is lower than 2-AG, due to AEA’s partial agonist activity compared to the full-agonist activity of 2-AG.(Savinainen et al., 2001)
AEA and 2-AG are synthesized on demand. AEA is mainly synthesized from N-arachidonoyl phosphatidyl ethanol (NAPE) (Maccarrone, 2017), and 2-AG is synthesized from 2-arachidonoyl-containing phospholipids, primarily phosphatidyl inositol bis-phosphate (PIP2) (Baggelaar et al., 2018). Once inside neurons, eCBs are rapidly degraded. AEA is predominantly metabolized by FAAH (Maccarrone, 2017). Likewise, MAGL, ABHD6, and ABHD 12 hydrolyze most of 2-AG (Cao et al., 2019; Lu and Mackie, 2021; Piomelli and Mabou Tagne, 2022).
2.3. The ECS and the opioid system
The ECS, endogenous opioid system, and dopamine-related reward pathways appear to have significant crosstalk at various levels, and thus gaining a better understanding of this relationship provides opportunities for interventions to modulate stress, pain, and addiction-related behaviors (Scavone et al., 2013; Vigano et al., 2005). The agonistic activation of CB1R and mu-opioid receptors (MOR), results in similar behavioral outcomes such as sedation, analgesia, and perception of reward, which suggests a closely linked relationship between these sets of regulatory systems (Lopez-Moreno et al., 2010; Wenzel and Cheer, 2018).
At the level of receptors, CB1R and MOR share several anatomical and functional similarities. Both receptors are GPCRs and both colocalize within brain regions implicated in drug-seeking behaviors such as the Ventral Tegmental area (VTA), Nucleus Accumbens (NAc), and basal ganglia (Maldonado and Rodriguez de Fonseca, 2002; Molaei et al., 2016; Rodriguez et al., 2001; Scavone et al., 2010; Valverde et al., 2001). The MOR and CB1R both interact with the Gi/o protein and some evidence suggests that they can form heterodimers and start the intracellular signaling pathway together, leading to synergistic effects such as inhibition of GABAergic neurons in the NAc (Hojo et al., 2008; Schoffelmeer et al., 2006). Both receptors exert their function through similar intracellular signaling pathway, which includes the inhibition of cAMP production and voltage-gated calcium channels, and activation of potassium channels and the signaling pathway of mitogen-activated protein kinase (Wenzel and Cheer, 2018). Chronic exposure to cannabinoids and opioids will result in cross-tolerance (Newman et al., 1974). Moreover, the intraperitoneal administration of CB1R agonists was found to increase endogenous opioid release in NAc of rodents (Valverde et al., 2001), and MOR density was observed to be increased in various brain regions such as NAc, Caudate-Putamen, amygdala, pre-frontal cortex, and hippocampus in rodents after a cannabinoid self-administration experiment (Fattore et al., 2007). Conversely, exposure to exogenous opioids leads to alterations in the ECS. The alterations in ECS following exposure to exogenous opioids will be reviewed in this paper in detail.
The bi-directional relationship between the endogenous opioid and ECS suggests there may be therapeutic potential in modulating the ECS in the treatment of OUD (Scavone et al., 2013; Strain et al., 2021).
2.4. Common laboratory methods for studying the endocannabinoid system after opioid exposure
2.4.1. Opioid exposure
Different methodologies were used in the preclinical studies of the effects of opioids on the ECS. In non-cue-conditioned approaches, the animals receive opioids without any contextual stimuli and conditioning (Smith, 2020). Cue-conditioned strategies involve a significant process of memory formation. Conditioned-place preference (CPP) model uses classical conditioning process, in which rodents receive opioids while confined in one cage and receive saline injections while confined in another cage with different colors and structures (contextual stimuli). (Kuhn et al., 2019) In some CPP studies, investigators also include a “morphine-unpaired” group in which the rodents receive opioids, but without pairing the injection with contextual stimuli (Huston et al., 2013). The other cue-conditioned models are contingent models, in which the delivery of the drug is contingent upon specific behaviors (Kuhn et al., 2019). Self-administration (SA) models utilize an operant conditioning process, in which the rodents are provided with two levers, interaction by one lever will result in an opioid injection, and interaction with the other will result in a saline injection. The rodents that show significantly more interactions with the opioid-paired lever, will be considered as those who successfully developed SA behavior and will be compared to other groups (Lynch et al., 2010). In both cue-conditioned models, the daily opioid injection or the amount of opioid injected per lever interaction could be either constant or escalating along the experiment (Kuhn et al., 2019).
2.4.2. Assessment of the ECS
Moreover, several methodologies have been used to examine the different components of the ECS. CB1R receptor density is typically measured using autoradiography with CB1R radioligands such as CP-55,940 and WIN 55,212-2a on both tissue sections and homogenates (Dean et al., 2001; McPartland et al., 2007). However, the density of a receptor does not always reflect its function. In another autoradiographic technique aimed to measure the GPCRs’ functionality in coupling and activating G-proteins, the tissue of interest is treated with a specific GPCR agonist and a nonhydrolyzable, radio-labeled analog of GTP, the [35S]-GTPγS. The level of the Gα-[35S]-GTPγS couples after the process can provide an estimation of the number of G-proteins coupled with and activated by the GPCR of interest (Harrison and Traynor, 2003). The [35S]-GTPγS assay, assesses the GPCR activation proximal to receptor activation, which makes the assay less amenable to change by other intracellular pathways or amplification in the intracellular signaling cascade. However, the filtering step needed to wash out the unbound GTPγS limits the overall throughput and the essay usually has a low signal-to-background ratio (DeLapp et al., 2012; Thomsen et al., 2005).
The level of expression of ECS proteins can be evaluated at the mRNA level by in-situ hybridization techniques or real-time reverse polymerase changing reactions (RT-PCR) (Ransick, 2004). RT-PCR is a quantitative measure with a high sensitivity and specificity that can be used even if the genetic material is available in low concentrations, in-situ hybridization on the other hand, is semi-quantitative and more time-consuming, but allows the detection of multiple mRNAs in one sample (Böhm-Hofstätter et al., 2010). At the protein level, the expression is evaluated by the Western blot technique or proteomic analysis (Dutt and Lee, 2000; Taylor and Posch, 2014). Western blot is a semi-quantitative, targeted method that can only detect the proteins that their targeting antibodies are used. Also, the specificity and sensitivity of western blot is dependent on the antibodies used. Proteomic analysis using mass-spectrometry offers the identification of many proteins simultaneously with high sensitivity and specificity and can provide a holistic picture of changes in proteins (Mann, 2008). The endocannabinoids concentrations can be measured both in vitro and in vivo. In the in vitro techniques, the cellular extract of the sacrificed rodents’ brains is examined by mass spectrometry, usually at least a couple of hours after the last drug administration session (Zoerner et al., 2011). On the other hand, in the microdialysis technique, investigators implant a cannula in different parts of a alive rodent and can take real-time CSF samples during a drug administration session (Torregrossa and Kalivas, 2008). However, microdialysis has a limited time resolution, and temporal resolution is limited by the diameter of the catheter. Moreover, catheter placement is a relatively invasive procedure that might alter the baseline biochemical and anatomical structure of the studied region and can limit the animal’s movement to some degree (Chefer et al., 2009).
A recently developed promising technique to study real-time eCB dynamics is using a genetically coded fluorescent GPCR activation-based (GRAB) eCB sensor. GRABeCB2.0 is a sensor that was developed recently by combining the CB1R genetic code with a circular-permutated fluorescent protein. The resultant protein is a CB1R which produces fluorescent light when attached to an agoinst. The sensor genetic code can be transferred to cultured neurons, acute brain slices, and living animals’ brains. Using standard fluorescent microscopy, endocannabinoid system dynamics can be detected with high spatiotemporal resolution after different exposures.(Dong et al., 2022; Dudok and Soltesz, 2022) To the best of our knowledge, this method has not been used to investigate the effect of opioid exposure on the eCB system.
3. Alterations of ECS Following a Single Opioid Exposure
We did not find any clinical study investigating the effects of a single dose of opioids on ECS in humans. However, there were a couple of animal studies that were summarized below.
3.1. Cannabinoid receptor 1
The acute effects of opioids on CB1R have been investigated in a few studies. A single dose of 5 mg/kg morphine did not change CB1R density or its G protein coupling capacity in any of the studied brain regions in an autoradiographic study (Vigano et al., 2003). However, a single dose of 10 mg/kg morphine injection, in another study in which animals were sacrificed 24 hours after the injection, resulted in an increase in the CB1R mRNA expression in the cortex and a decrease of the same mRNA expression in the cerebellum. The only detected change at the protein level was an increase in the expression of CB1R protein in the hippocampus (Jin et al., 2014).
3.2. Endocannabinoids and their metabolic enzymes
While a single dose of 5 mg/kg morphine did not alter AEA and 2-AG levels in the whole-brain samples (Vigano et al., 2003), it increased AEA levels in the Nucleus accumbens (NAc), caudate-putamen (CP), and the hippocampus, and decreased 2-AG levels in the NAc and hippocampus (Vigano et al., 2004). Consistent with increased AEA levels, there was a decline in FAAH activity observed in the CP and hippocampus (Vigano et al., 2004). A microdialysis study reported no changes in the AEA or 2-AG levels in the VTA after a 5 mg/kg morphine injection (Zhang et al., 2021).
4. Alterations of The ECS in Response to Repeated Opioid Exposure
The only available human study is a postmortem study that found no difference in the CB1R protein expression in the PFC of people who used heroin and/or methadone for six to 24 months before their death compared to those who did not use opioids (Alvaro-Bartolome and Garcia-Sevilla, 2013).
We will now appraise preclinical studies on the ECS alterations after exposure to multiple doses of opioids and across various components of the ECS.
4.1. Cannabinoid receptor 1
Several studies suggest that opioid-induced alterations of the CB1R are “region-specific” within the CNS. Thus, here we summarize the findings for these brain regions as they pertain to CB1R changes.
4.1.1. Cortex
Five studies used autoradiographic techniques to investigate the CB1R in the whole-cortex samples. Four studies found that the non-cue-conditioned regimens of morphine did not change cortical CB1R density. Two of these studies used the [35S]-GTPγS assays and reported the same unchanged pattern in the G protein coupling capacity (Gonzalez et al., 2002; Romero et al., 1998; Rubino et al., 1997; Vigano et al., 2003). However, a non-cue-conditioned escalating dose of morphine for six days resulted in a decreased CB1R density and an increased G protein coupling capacity in the whole-cortex sample (Gonzalez et al., 2003). Furthermore, non-cue-conditioned escalating doses of morphine for six days induced no changes in CB1R mRNA expression and protein-level expression in the whole cortex (Alvaro-Bartolome and Garcia-Sevilla, 2013; Gonzalez et al., 2002). However, another mRNA and protein expression study using a twice-daily non-cue-conditioned regimen of 10 mg/kg of morphine for 12 days found a significant increase in both mRNA and protein expressions of CB1R in the cortex of male rats (Jin et al., 2014).
Two studies used the opioids SA model to evaluate the CB1R attributes in the cortex, with one reporting unchanged density of CB1R in the Pre-frontal cortex (PFC), but an increased G protein coupling capacity (Fattore et al., 2007), and the other reporting no significant change in the CB1R G protein coupling capacity in the PFC and Cingulate cortex. (Sim-Selley et al., 2000).
4.1.2. Hippocampus
Of the five autoradiographic studies that investigated the effect of different regimens of non-cue-conditioned morphine injections for four to six days in male rats’ hippocampus, three reported no changes in the density of CB1R (two that included [35S]-GTPγS assay also reported unchanged G protein coupling capacity), one reported a lower density only in the dentate gyrus (but unchanged in the cornu ammonis 1 [CA1], CA2 and CA3) and one reported a lower density across all hippocampus, but with no accompanied change in the G protein coupling capacity (Gonzalez et al., 2002; Gonzalez et al., 2003; Romero et al., 1998; Rubino et al., 1997; Vigano et al., 2003). Another study that investigated expression, concluded that treating male rats with a twice-daily regimen of 10 mg/kg morphine for 12 days, results in an increased expression of CB1R in both mRNA and protein levels in the hippocampus (Jin et al., 2014). Expression studies have provided some evidence that a six-day escalating regimen of morphine in rats will result in an increased mRNA expression of CB1R only in CA2 but unchanged levels in CA1, CA3, or the dentate gyrus (Gonzalez et al., 2002).
Two studies investigated the effect of morphine administration in CPP models in rats on the expression of CB1R on a protein level. Both studies reported an increased expression of CB1R protein in the hippocampus of morphine-paired rats (Zhang et al., 2016a; Zhao et al., 2017). One study continued the investigation further and found that the expression level changes back to the previous levels after the extinction period and will increase again with a reinstatement dose (Zhao et al., 2017). Another study that investigated the mRNA level expression using the same model, reported a decrease in the CB1R mRNA level in the dorsal hippocampus after the conditioning period that returns to the baseline after the extinction training and increases after a single reinstatement injection (Li et al., 2017). Two other studies used autoradiographic methods to investigate the heroin self-administration model. While one reported an unchanged density and increased G protein coupling capacity of CB1R in the hippocampus (Fattore et al., 2007), the other reported no change in the G protein coupling of CB1R in the hippocampus (Sim-Selley et al., 2000).
4.1.3. Amygdala
Three studies reported results of non-cue-conditioned administration of morphine in rats on the density or expression of the CB1R in the amygdala, with most reporting no significant difference. One study investigated the effect of a stable 5 mg/kg daily morphine dose on the density and G protein coupling capacity of CB1R in the anterior amygdala with no significant change (Vigano et al., 2003). Another investigated the effect of a 5-day escalating dose of morphine on the density and G protein coupling capacity in the amygdaloid nucleus of rats and reported no significant change (Romero et al., 1998). The third studied the density and mRNA expression level after an escalating morphine regimen in the basolateral amygdala and reported decreased density but unchanged mRNA expression (Gonzalez et al., 2002). Two studies that investigated the contingent opioid use effect on the amygdala, found contradictory results in heroin SA models. While one showed a significant increase in both density and G protein coupling capacity of the CB1R in the amygdala (Fattore et al., 2007), the other one found no difference in the G protein coupling capacity of CBRs in the amygdala (Sim-Selley et al., 2000).
4.1.4. Nucleus accumbens (NAc)
Three studies investigated the effects of non-cue-conditioned chronic morphine administration on the CB1R in the NAc. While 4.5 days of a 5 mg/kg stable dose of morphine resulted in no significant change in CB1R density and decreased G protein coupling capacity (Vigano et al., 2003), five days of escalating morphine doses resulted in insignificant changes both in the density of CB1R and the G protein coupling capacity (Romero et al., 1998). In another study, a 6-day escalating twice-daily dose of 10-100 mg/kg was associated with a significant increase in the density of CB1R (Gonzalez et al., 2002). Similarly, unchanged density, but increased G protein coupling capacity of CB1R in NAc were reported in a heroin self-administration model in mice (Fattore et al., 2007). Two other studies investigated CB1R protein expression in rats after the conditioning phase in a CPP model, and both reported an increased expression (Yuan et al., 2013; Zhang et al., 2016a). One of the studies had also a non-morphine paired comparison, which could not find the same change in this non-cue-conditioned group (Zhang et al., 2016a).
4.1.5. Other parts of the limbic system
In two autoradiographic studies, authors concluded that non-cue-conditioned stable or escalating regimens of morphine, have no significant effect on the CB1R density and its G protein coupling capacity in the septum nucleus (Romero et al., 1998; Vigano et al., 2003). On the contrary, a six-day non-cue-conditioned escalating twice-daily regimen of morphine started at 10 mg/kg and ended at 100 mg/kg resulted in an increase of both CB1R density and its mRNA expression in septum nucleus (Gonzalez et al., 2002), though no significant changes in the CB1R density and its G protein coupling capacity in whole-limbic-forebrain samples were reported in a subsequent study using the same methodology (Gonzalez et al., 2003).
4.1.6. Basal ganglia
A study with a stable 5 mg/kg regimen of non-cue-conditioned morphine for 4.5 days found no significant change in the CB1R density and G protein coupling capacity in the CP and globus pallidus (GP) (Vigano et al., 2003). Another autoradiographic study used a non-cue-conditioned escalating dose of morphine for 5 days and found no difference in the density and G protein coupling capacity of CB1R in medial and lateral CP, and entopeduncular nucleus (EPN), regarding the GP, an increased CB1R density and an unchanged G protein coupling capacity was reported (Romero et al., 1998). A third study with a non-cue-conditioned escalating dose of twice daily morphine from 10 mg/kg to 100 mg/kg found no significant changes in the density and G protein coupling capacity of CB1R in a sample of whole-striatum in rats.(Gonzalez et al., 2003) However, in another study by the same group that used the same escalating dosing schedule, when they collected more specific samples of medial CP, lateral CP, and GP, they found that the same regimen resulted in an increased CB1R density in the medial CP; but unchanged in lateral CP, GP, and EPN; and a decrease in CB1R mRNA expression in both medial and lateral CP (Gonzalez et al., 2002). On the contrary, another study that used non-cue-conditioned opioid administration found an increase in both density and CB1R mRNA expression in the CP after five days (Rubino et al., 1997).
Three studies also investigated the effect of cue-conditioned models of opioid use on basal ganglia. In a heroin SA model, an increase in the CB1R G protein coupling capacity in CP was reported, however, the CB1R density was not changed (Fattore et al., 2007). In another study investigating the CB1R G protein coupling capacity, no significant change in the CP was observed (Sim-Selley et al., 2000). Similarly, CPP training with 10 mg/kg morphine was reported to have no effect on the CB1R protein expression in the rats’ striatum (Yuan et al., 2013).
4.1.7. Diencephalon
Among four studies that used different regimens of stable and escalating non-cue-conditioned morphine, and contingent heroin SA models to study the effects of opioids on diencephalon CB1R properties, none reported any significant difference. Their outcomes included the CB1R density, CB1R G protein coupling capacity, and CB1R mRNA expression in the rodents’ whole-diencephalon sample or more specific samples of the diencephalon subregions (Fattore et al., 2007; Gonzalez et al., 2002; Gonzalez et al., 2003; Vigano et al., 2003).
4.1.8. Midbrain
Five studies investigated the effects of non-cue-conditioned regimens of opioids on CB1R properties in midbrain samples. Three studies using stable or escalating doses of morphine reported unchanged CB1R density in substantia nigra (SN), with two also finding unchanged G protein coupling capacity (Gonzalez et al., 2002; Romero et al., 1998; Vigano et al., 2003). However, another autoradiographic study with an escalating dose of non-cue-conditioned morphine for five days found a decreased density of CB1R receptors in a whole-midbrain sample, though the G protein coupling capacity remained unchanged (Gonzalez et al., 2003). In another study, the mRNA and protein-level expressions of CB1R were found to be unchanged in VTA after a stable dose of 5 mg/kg morphine in a non-cue-conditioned model (Zhang et al., 2021). Only one study utilized an SA model to look into changes in CB1R in the VTA and found that after obtaining the SA behavior, the rats showed an increased density of the CB1R in the VTA, however, the G protein coupling capacity remained unchanged (Fattore et al., 2007).
4.1.9. Hindbrain
An escalating twice-daily of non-cue-conditioned morphine regimen from 10 mg/kg to 100 mg/kg did not change the density of CB1R in either the brainstem or the cerebellum. Also, the G protein coupling capacity of the cerebellum remained unchanged, however, a decrease in the G protein coupling capacity of CB1R was observed in the brainstem (Gonzalez et al., 2002; Gonzalez et al., 2003). In another study, a dose of 5 mg/kg daily regimen of morphine for 4.5 days resulted in a decline in the CB1R density in the cerebellum, however, its G protein coupling capacity remained unchanged (Vigano et al., 2003). Moreover, the CB1R mRNA expression in the cerebellum decreased after an escalating dose of non-cue-conditioned morphine for five days (Gonzalez et al., 2002). However, in another study, CB1R mRNA and protein expressions increased in the cerebellum after a 10 mg/kg morphine twice-daily regimen for 12 days (Jin et al., 2014).
4.2. Cannabinoid receptor 2
Though the research on the presence of CB2Rs in the brain is limited, two studies explored the effects of non-cue-conditioned opioid exposure in rodents’ CNS CB2R expression. One found that chronic exposure to 10 mg/kg of morphine does not change the CB2R mRNA expression in the mice whole-brain samples (Onaivi et al., 2008), while the other reported that a twice-daily stable dose of 5mg/kg of morphine decreased CB2R mRNA expression in the VTA of rats (Zhang et al., 2021).
Regarding CPP models, increased CB2R mRNA expression in the cortex, decreased CB2R mRNA expression in the brainstem, and decreased CB2R protein expression in both sites are reported after a 4-day CPP training with 10 mg/kg morphine (Zhang et al., 2012), and increased CB2R mRNA expression was reported in the dorsal hippocampus after a 4-day successful CPP training with escalating doses of morphine, which returned to the baseline after the extinction period and did not increase again by reinstatement (Li et al., 2017).
4.3. Endocannabinoids and their metabolic enzymes
All identified studies that measured the levels of endocannabinoids after chronic exposure to opioids, regardless of their dose or method, consistently reported unchanged levels of AEA compared to the control rodents in different regions of the brain, at least two hours after the last injection, i.e., not in the acute intoxication phase (Caille et al., 2007; Gonzalez et al., 2003; Vigano et al., 2003; Vigano et al., 2004; Zhang et al., 2021). Moreover, a non-cue-conditioned twice-daily dose of 5 mg/kg morphine in rats for 5 days resulted in no significant changes in the protein-level expression of FAAH and NAPE-PLD in VTA (Zhang et al., 2021). However, escalating non-cue-conditioned morphine administration was reported to result in a decreased FAAH activity in the CP and hippocampus, and escalating dose in a CPP model study resulted in increased FAAH mRNA expression, but not NAPE-PLD expression, in the dorsal hippocampus at the end of the conditioning phase (Li et al., 2017; Vigano et al., 2004).
A decreased 2-AG level was reported in the striatum, cortex, hippocampus, limbic area, and hypothalamus after a stable twice-daily dose of 5 mg/kg morphine for 4.5 days (Vigano et al., 2003). Moreover, a decreased 2-AG level was reported in NAc, but not in PFC, CP, and hippocampus, in an escalating twice-daily dosage of morphine for 3 days (10 to 40 mg/kg) (Vigano et al., 2004). However, these changes were not found in another study using a non-cue-conditioned model in VTA after a twice-daily 5 mg/kg dose of morphine for 5 days (Zhang et al., 2021). Similarly, a heroin SA study found no significant change in the levels of 2-AG in NAc shell (Caille et al., 2007). Consistently, in a study on non-cue-conditioned morphine dosage for 5 days, no change in the protein-level expression of MAGL and DAGLα was observed in VTA (Zhang et al., 2021). However, a CPP model study reported that after their conditioning phase, the mRNA expression of MAGL was increased in the dorsal hippocampus, but there was no change in the level of DAGLα/β (Li et al., 2017).
Two studies measured the endocannabinoid levels in opioid-treated rats, during a session of active opioid use, using the microdialysis technique. One study reported that after five days of a non-cue-conditioned twice-daily regimen of morphine, a 5 mg/kg morphine injection resulted in no change of AEA and 2-AG levels in VTA (Li et al., 2017). However, another study that used an SA method found a dose-dependent increase in AEA and a decrease in 2-AG levels during a self-administration session, in their NAc shell (Caille et al., 2007).
We were able to find only one study that followed the endocannabinoid levels in different parts of the brain after a drug-free period and then following a challenge dose. The investigators exposed male rats to three days of escalating twice-daily SQ morphine, and then following a two-week drug-free period, they exposed the animals to another single morphine dose. The study reported some interesting, counter-intuitive results. The investigators found increased AEA levels in NAc, CP, PFC, and hippocampus, and decreased 2-AG levels in NAc, CP, and hippocampus were reported after the two-week abstinence period. Interestingly, a significant decrease in the AEA levels in the CP and hippocampus (but not NAc and PFC) and a significant increase in the 2-AG levels in the hippocampus (but not CP, NAc, and PFC) were reported after the administration of the reinstatement dose of morphine, changes that are in the reverse direction compared to the effects of morphine on morphine-naïve rodents (Vigano et al., 2004).
5. Discussion
Throughout this review, we aimed to provide a summary of preclinical and clinical studies that investigated the alterations in the ECS following single or multiple doses of exogenous opioids; in order to provide a translational model to guide future clinical studies and pharmacological interventions for the treatment of OUD. However, our most important finding is the highly heterogeneous methodologies and contradictory findings, which makes it complicated to build a clear translational model based on available evidence. Many of the included studies were designed to address more basic neuroscience questions and did not aim to model OUD as it occurs in humans or to provide generalizable results for translational purposes. Hence, most of the included studies utilized non-cue-conditioned models of opioid exposure, with many having a fixed-dosing schedule. Currently, many preclinical investigators argue that the non-cue-conditioned models of substance use, especially with a fixed-dosing schedule, would not have high face validity for translational purposes (Kuhn et al., 2019; Smith, 2020). The complex transition of humans from recreational drug use into compulsive addictive behaviors incorporates processes other than the direct effect of a drug on the brain, such as memory formation, incentive salience, and changes in motivation (Koob and Volkow, 2016; Volkow et al., 2019); To date, preclinical researchers have a notable list of contingent drug use animal models with various drug delivery schedules to investigate different processes involved in OUD, such as models utilizing long access and intermittent access schedules in a context that animals have a choice between the drugs and other rewarding activities (Kuhn et al., 2019). Incorporating assessment of real-time ECS dynamics using more recent techniques such as the GRABeCB2.0 sensor in future studies on rodents using the more translationally valid animal substance use disorder models can pave the way for the utilization of animal model investigation results in clinical settings.
Another missing piece of the puzzle to reach a clear translational explanation is the lack of studies on non-human primates and the paucity of human studies. Compared to rodents, non-human primates share more similarities with humans in terms of CNS, and their longer life and more complex behavioral repertoire make them better candidates for studies that aim to generalize their findings to humans (Banks et al., 2017). Moreover, we are currently equipped with some sophisticated non-invasive techniques to conduct in-vivo human studies to investigate ECS, such as studying the density of cannabinoid receptors using radiolabeled ligand of cannabinoid receptors in Positron Emission Tomography (PET) imaging studies, and using peripheral endocannabinoid levels to obtain some information about the endocannabinoid levels in CNS.(Centonze et al., 2008; Sloan et al., 2019) Using these techniques to investigate the ECS condition in the context of opioid exposure and OUD in non-human primates and humans can help bridge the preclinical findings to clinical settings.
We categorized the animal models into three groups: non-cue-conditioned models, conditioned place preference (CPP) models, and self-administration (SA) models (Figures 2 and 3). As mentioned previously, compared to non-contingent models, SA has higher face validity for translational purposes, followed by CPP (Kuhn et al., 2019). It is also important to note that the ECS alterations are region-specific in the brain. We also need to pay specific attention to different components of the ECS. For example, though AEA and 2AG are both ECS ligands and bind to the same receptors, they seem to have different functions and need to be assessed separately.
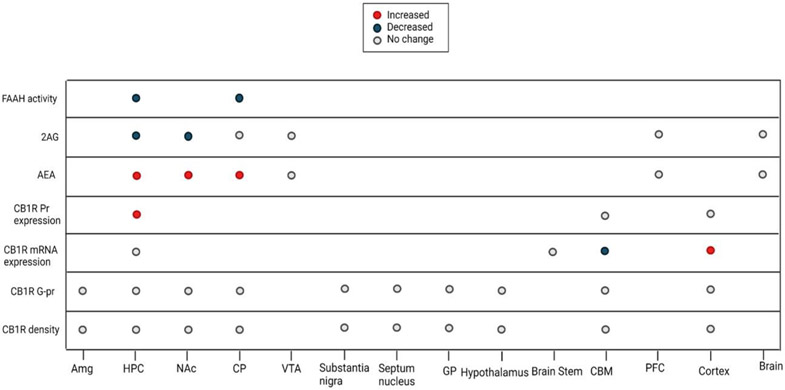
Figure 2: The alterations of ECS Following a Single Day of Opioid Exposure.
Abbreviations: Amg, Amygdala; CBM, Cerebellum; CP, Caudate-Putamen; GP, Globus pallidus; HPC, Hippocampus; NAc, Nucleus Accumbens; PFC, PreFrontal Cortex; VTA, Ventral Tegmental Area.
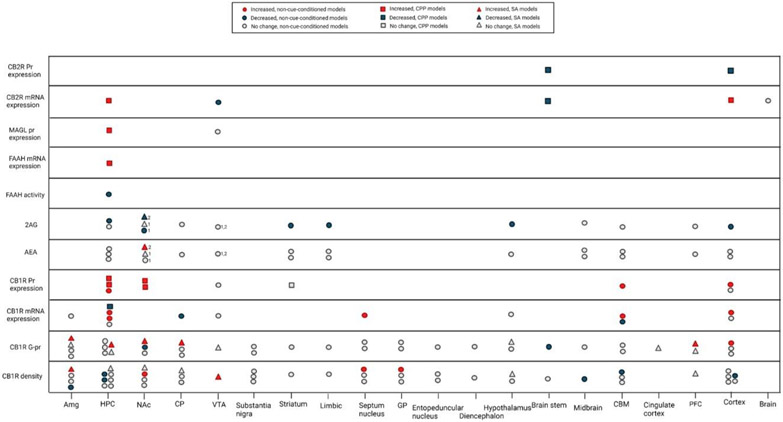
Figure 3: The alterations in the endocannabinoid system components after repeated opioid exposures.
1 between exposures, 2 during an exposure
Abbreviations: Amg, Amygdala; CBM, Cerebellum; CP, Caudate-Putamen; GP, Globus pallidus; HPC, Hippocampus; NAc, Nucleus Accumbens; PFC, PreFrontal Cortex; VTA, Ventral Tegmental Area.
Considering the few studies that addressed the effect of a single dose of opioids, we observe a pattern of increased AEA levels and decreased 2-AG levels in the NAc, hippocampus, and CP (Figure 2). Consistent with the increased levels of AEA, FAAH activity is decreased in the hippocampus and CP (Figure 2). These findings could be explained in light of the brain reward neurocircuitry. The MOR and CB1R are co-located in the presynaptic GABAergic neurons innervating the mesolimbic dopamine pathway, and upon activation, they will result in a surge of dopamine by suppressing the inhibitory effect of presynaptic GABAergic neurons on the mesolimbic pathway (Cheer et al., 2007; Volkow et al., 2019). In this context of a similar function of CB1R and MOR, the decrease in the 2-AG levels can be viewed as negative feedback, trying to counterbalance the effects of excessive exogenous opioid agonists. Since the 2-AG is about 1000 times more available in the brain and is considered the main endocannabinoid involved in synaptic neurotransmission, a decrease in the 2-AG levels following exogenous opioid exposure is expected (Zou and Kumar, 2018). AEA on the other hand is present in less concentration in the brain, but there has been a suggested role for AEA as a negative feedback agent to control 2-AG levels, explaining the increase in its levels (Maccarrone et al., 2008). However, the evidence about AEA's role in controlling 2-AG levels is specific to the stratum and has not been replicated since. Hence, the increase in AEA could be interpreted in other ways. This model can also explain the synergism of cannabinoids and opioids in producing euphoria (Schoffelmeer et al., 2006). However, these findings are mostly relevant to acute rewarding effects of drugs which is not considered as a criterion for diagnosing substance use disorders based on the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5.
Similarly, acute exposure of rats acquired SA behavior to morphine -i.e., intoxication phase- in an SA model seems to result in an increase in AEA levels and a decrease in 2-AG levels in the NAc, which both normalize after the termination of opioid exposure (Caille et al., 2007). Considering that the other studies that sacrificed rodents after two hours of the last morphine exposure did not detect endocannabinoid change (Vigano et al., 2004), this return to normal levels may occur during the first two hours after exposure, which suggests the development of a counterregulatory mechanism to maintain the level of the endocannabinoid in presence of exogenous opioids. However, the only study that studied the level of endocannabinoids after a two-week period of abstinence, reported an increased level of AEA and decreased level of 2-AG -as opposed to the intuitive expectation that the change will be in the reverse direction while the counterregulatory mechanism works in the absence of the exogenous opioid. The authors also observed that the re-introduction of the opioid after the abstinence period can increase the 2-AG level in the hippocampus and decrease the AEA in the hippocampus and CP and explained these findings suggesting that the effect of the opioids might change from inhibitory to excitatory after repeated exposure and sensitization (Vigano et al., 2004). This suggestion can also explain the findings that FAAH inhibitor and AEA transporter inhibitor were successful in the attenuation of opioid withdrawal symptoms in rats (Del Arco et al., 2002; Ramesh et al., 2011). These findings are of special interest since withdrawal is one of the DSM-5 criteria for substance use disorders. The replication of this phenomenon in the more generalizable animal models and understanding its exact chronology is imperative for guiding clinical interventions. While some manipulations of ECS might provide therapeutic effects at early abstinence or withdrawal periods, the same manipulation can have no effect or hazardous effects in late abstinence phases, when the brain is returned to its baseline homeostasis.
The results pertaining to CB1R density and function following repeated opioid exposure are highly inconsistent. These inconsistencies are most probably due to the variety of models and drug schedules used and the differences between levels of expression, density, and the function of the CB1R. However, as the models incorporated more translationally valid approaches, some changes were observed. For example, while the non-cue-conditioned models mostly failed to detect any changes in CB1R density and function in NAc and hippocampus, when the CPP models were used they showed an increase in the expression of the CB1R in the NAc and hippocampus. Similarly, although non-cue-conditioned strategies failed to induce any change in CB1R in the cortex, amygdala, and basal ganglia regions, some contingent models suggest an increase in the density or function of CB1R in these regions, although contradictory results for both regions exist. Observation of changes only with the cue-conditioned models, suggests that the observed changes are not due to direct drug effect, but to a kind of learning process that might result in changes in incentive salience. These changes could roughly be interpreted as DSM criteria like using a substance in the presence of negative consequences, which show an alteration in the priority of incentives of the individual.
It appears that the strongest level of evidence suggests that CB1R protein expression increases in the hippocampus and NAc of cue-conditioned, morphine-dependent rodents. Also, the result of few available studies using an SA model, suggest that either CB1R density or its G protein coupling capacity increases in different parts of the limbic system (hippocampus, NAc, and amygdala), basal ganglia (caudate-putamen) and the VTA (Figure 3). This increase in CB1R protein expression, density, or G protein coupling capacity can be a counter-regulatory response to the decreased levels of 2-AG in these regions. These ECS alterations may explain the successful attenuation of the opioids rewarding effects and tolerance development by CB1R antagonists in animal studies and support their role as promising pharmacological targets for OUD treatment (Altun et al., 2015; Caille and Parsons, 2003; De Vries et al., 2003; Le Foll and Goldberg, 2005). However, the first CB1R antagonist on the market (Rimonabant) resulted in elevated anxiety, depression, and suicidality (Christensen et al., 2007). Some investigators speculate that the negative psychiatric side effects of Rimonabant are due to its inverse agonism effects and have pursued a neutral antagonist formulation with some preclinical success in decreasing self-administration of opioids and opioid withdrawal (He et al., 2019; Wills et al., 2014). Nevertheless, investigation of any CB1R antagonist component in the clinical setting should proceed with caution given the side effects of Rimonabant.
Studies documenting opioids’ influence on CB2R (versus CB1R) are scarce and there are inconsistencies within the limited number of reports that do exist. However, preclinical data suggest a role for CB2R agonists in attenuating opioid rewards (Grenald et al., 2017; Iyer et al., 2020; Zhang et al., 2021), delaying the development tolerance (Li et al., 2019; Zhang et al., 2018; Zhang et al., 2016b), and reducing naloxone-induced opioid withdrawal (Iyer et al., 2020; Li et al., 2019; Zhang et al., 2018). Taken together, pursuing CB2R agonists as a candidate for OUD treatment seems a plausible line of research.
Notably, we must proceed with caution in eliciting translational conclusions based on current findings. Most of our data is based on non-cue-conditioned models, some are based on CPP models, and a few on contingent self-administration models with simple drug delivery schedules. Hence, the available data does not offer a high face validity for translational purposes (Kuhn et al., 2019; Smith, 2020). The transition from recreational drug use to a compulsive addictive disorder in humans happens in a complex environment and is not only due to the euphorigenic results of the drug, and some changes happen after a long time of repeated exposure to the drug. When drugs are delivered in a context of environmental stimuli, and contingent upon certain behaviors from the organism, the previously neutral stimuli transform into drug cues via conditional memory formation. These drug cues can stimulate the ventral striatum dopaminergic pathways independently and motivate the organism for drug-seeking behavior (Peciña and Berridge, 2013; Uhl et al., 2019). As the drug-seeking behaviors result in pleasurable outcomes repeatedly, the memory will become more consolidated in the dorsal striatum and the behavior will change from a goal-directed deliberate behavior to an automated habitual behavior (Koob and Volkow, 2016; Singer et al., 2018). As these repetitions weaken some connections between PFC and the dorsal striatum, the control over these habits attenuates and the compulsions will become less controllable (Tang et al., 2015; Volkow et al., 2019; Winstanley et al., 2010). Animal studies that use more sophisticated contingent modeling, while the drug availability is intermittent, a choice for alternative rewards is present, or the organism is exposed to cues that make them susceptible to relapse after abstinence, promise more validity in producing more generalizable results. Given the wide presence of ECS in different brain regions and its responsibility in higher functions such as emotion regulation, memory, learning, and reward sensation, it is plausible to assume a role for ECS in these processes (Zou and Kumar, 2018). However, these methods are underutilized in the study of ECS changes in opioid use models. It is plausible to assume as we move toward studying these mechanisms, the observed changes might not be attributable to a specific drug class, but rather to the transdiagnostic changes that underlie any addictive disorder. However, this does not undermine the clinical implication of such findings.
Conclusion and Future Perspectives
Numerous studies have investigated ECS dysregulation following a single or repeated exposure to opioids and there is a wide range of publications on the levels of endocannabinoids as well as the density, functionality, and expression of key receptors and metabolic enzymes. But the similarities between these experiments seem to end there. The opioid used, dosing protocols, mode of administration, measuring techniques, and even indicators of dependence or addiction used in their experiments vary widely. Most importantly, the majority of the data come from studies that were not designed for translational purposes which limits their utility in reaching conclusions that are generalizable to clinical settings. These factors contribute to the difficulty associated with identifying clear patterns of ECS dysregulation within the existing literature.
All the results we presented in this study should be interpreted with caution. There is a need for investigations with more translationally valid animal models and studies on human subjects with OUD to better understand the alterations in the ECS in different stages of the OUD. Meanwhile, given the available evidence -mainly from using exogenous ECS modulators in OUD animal models- some ECS modulator molecules seem to be promising pharmaceutical agents for the treatment of OUD. AEA enhancer molecules, such as FAAH inhibitors are candidates for suppression of opioid withdrawal symptoms and are currently being tested in human trials for anxiety disorders (Schmidt et al., 2021; Sloan et al., 2017). MAGL inhibitors are another candidate that has been shown to reduce opioid withdrawal symptoms in animal models. (Ramesh et al., 2011; Sloan et al., 2017). We are aware of at least one MAGL inhibitor that reached clinical trail stage, however for other indications than substance use disorders (Müller-Vahl et al., 2022). Neutral CB1R antagonists, that lack the inverse agonistic properties of Rimonabant, have shown promising results in decreasing the self-administration of drugs in animal models without the adverse effect of Rimonabant (Gueye et al., 2016). Finally, CB2R agonists have also shown efficacy in decreasing self-administration of drugs in animal studies (Gueye et al., 2016; Zhang et al., 2015). Although CB2R agonists reached clinical trial stages for other indications, to the best of our knowledge, they have not been studied for substance use disorders in humans until today (Ostenfeld et al., 2011; Sloan et al., 2017).
Table 1:
The alterations of endocannabinoid system components after acute opioid exposure
| Source | Species | Opioid Compound (Route) |
Addiction Model/Exposure Details |
Endocannabinoid outcome measures and method |
Results |
|---|---|---|---|---|---|
| Vigano et.al., 2003 [54] | Male Rats | Morphine (SQ) | Non-cue-conditioned model. Single 5 mg/kg injection. Animals were sacrificed two hours after the injection. |
CB1R Density ([3H]-CP-55,940 binding) CB1R G protein coupling capacity (CP-55,940-induced [35S]-GTPγS binding) AEA and 2-AG levels (GC/MS) |
CB1R Density |
| → CP, Cortex, NAc, Septum nucleus, anterior amygdala, Hypothalamus, SN, GP, Hippocampus, Cerebellum | |||||
| CB1R G protein coupling capacity | |||||
| → CP, Cortex, NAc, Septum nucleus, anterior amygdala, Hypothalamus, SN, GP, Hippocampus, Cerebellum | |||||
| AEA level | |||||
| →Whole brain | |||||
| 2-AG level | |||||
| →Whole brain | |||||
| Vigano et al., 2004 [56] | Male Rats | Morphine (SQ) | Non-cue-conditioned model. Single 5 mg/kg injection. Animals were sacrificed 30 minutes after the injection. |
AEA and 2-AG levels (LC/MS) FAAH activity (in vitro arachidonoyl-[14C]ethanolamide metabolism) |
AEA level |
| ↑ NAc, CP, Hippocampus → PFC | |||||
| 2-AG level | |||||
| ↓NAc, Hippocampus → PFC, CP | |||||
| FAAH activity | |||||
| ↓ CP, Hippocampus | |||||
| Jin et al., 2014 [55] | Male Rats | Morphine (IP or SC?) | Non-cue-conditioned model. Single 10 mg/kg injection. Animals were sacrificed 24 hours after the last injection |
CB1R mRNA expression (RT-PCR) CB1R Protein expression (Western blot) |
CB1R mRNA expression |
| ↑ Cortex ↓ Cerebellum → Brain stem, Hippocampus | |||||
| CB1R Protein expression | |||||
| ↑ Hippocampus → Cerebellum, Cortex | |||||
| Zhang et.al. 2021 [57] | Male Rats | Morphine (IP) | Non-cue-conditioned model. Injection of 5 mg/kg and then obtaining in vivo microdialysis samples from VTA. In vivo, micro-dialysis samples were obtained every 30 minutes until 6 hours after the injection. |
AEA and 2-AG levels (MS) | AEA level |
| → VTA | |||||
| 2-AG level | |||||
| → VTA |
Abbreviations: AEA: Anandamide; CB1R: Cannabinoid receptor 1; CP: Caudate-putamen; FAAH: Fatty acid amide hydrolase; GC/MS: Gas chromatography/ Mass spectrometry; GP: Globus palidus; IP: Intra-peritoneal; LC/MS: Liquid chromatography/ Mass spectrometry; NAc: Nucleus accumbens; PFC: Pre-frontal cortex; SN: Substantia nigra; SQ: Subcutaneous; RT-PCR: Reverse transcriptase polymerase chain reaction; 2-AG: 2-Arachidonoylglycerol.
Definitions: ↑: Increased significantly; ↓: Decreased significantly; →: Not changed/insignificant change.
Table 2:
CB1R alterations after chronic opioid exposure
| Source | Species | Opioid Compound (Route) |
Addiction Model/Exposure Details |
CB1R outcome measures and method |
Results |
|---|---|---|---|---|---|
| Rubino et.al., 1997 [61] | Male Rats | Morphine (SQ pellets) | Non-cue-conditioned model. One SQ pellet of 75 mg morphine each day for 5 days. Tolerance to the analgesic effects of morphine was proven by the tail-flick test. Animals were sacrificed one day after the insertion of the last pellet. |
mRNA expression (In situ hybridization) Density ([3H]-CP-55,940 binding) |
mRNA expression |
| ↑ CP | |||||
| Density | |||||
| ↑ CP → Cortex, Hippocampus | |||||
| Romero et al., 1998 [60] | Male Mice | Morphine (SQ) | Non-cue-conditioned model. Twice-daily escalating dose of 5 mg/kg to 45 mg/kg for 5 days. The existence of opioid dependence was assessed by the naloxone-induced jumping behavior test. Animals were sacrificed two hours after the last injection. |
Density ([3H]-CP-55,940 binding) G protein coupling capacity (WIN 55,212-21-induced [35S]-GTPγS binding) |
Density |
| ↑ GP → Medial CP, lateral CP, SN, EPN, CA, Dentate gyrus, NAc, Septum nucleus, Amygdaloid nucleus, Superficial and deep layers of the cortex, Central gray substance | |||||
| G protein coupling capacity 2 | |||||
| → GP, Medial CP, lateral CP, SN, EPN, CA, Dentate gyrus, NAc, Septum nucleus, Amygdaloid nucleus, Superficial and deep layers of the cortex, Central gray substance | |||||
| Sim-Selley et al., 2000 [64] | Male Rats | Heroin (IV) | SA model. The animals were engaged in daily 4-hour self-administration sessions on a fixed-ratio 10 schedule of reinforcement for 29-39 days Each lever interaction resulted in escalating heroin injection, from 0.06 mg/kg at the beginning to 6 mg/kg per infusion at the end of training. At the end of the training, the maximum daily heroin intake reached 366 mg/kg. |
G protein coupling capacity (WIN 55,212-2a-induced [35S]-GTPγS binding) | G protein coupling capacity |
| → PFC, Cingulate cortex, CP, Amygdala, Hippocampus | |||||
| Gonzalez et al., 2002 [59] | Male Rats | Morphine (IP) | Non-cue-conditioned model. Escalating dose every day for 6 days. Day 1: 10 and 10 mg/kg weight; day 2: 20 and 20 mg/kg; day 3: 40 and 40 mg/kg; day 4: 60 and 60 mg/kg; day 5: 80 and 80 mg/kg; and day 6: 100 mg/kg. Animals sacrificed two hours after the last injection |
mRNA expression (In situ hybridization) Density ([3H]-CP-55,940 binding) |
mRNA expression |
| ↑ in CA2, Septum nucleus ↓ in Medial and lateral CP, Cerebellum → in Superficial and deep layers of the cortex, ventromedial hypothalamic nucleus, CA1, CA3, dentate gyrus, basolateral amygdala | |||||
| Density | |||||
| ↑ in medial CP, NAc, septum nucleus ↓ in the dentate gyrus and basolateral amygdala → in superficial and deep layers of the cortex, lateral CP, GP, EPN, SN, Cerebellum, CA1, CA2, CA3 | |||||
| Gonzalez et al., 2003 [62] | Male Rats | Morphine (SQ) | Non-cue-conditioned model. Escalating dose every day for 6 days. Day 1: 10 and 10 mg/kg weight; day 2: 20 and 20 mg/kg; day 3: 40 and 40 mg/kg; day 4: 60 and 60 mg/kg; day 5: 80 and 80 mg/kg; and day 6: 100 mg/kg. Animals were sacrificed two hours after the last injection |
Density ([3H]-CP-55,940 binding) G protein coupling capacity (CP-55,940-induced [35S]-GTPγS binding) |
Density |
| ↓ Cerebral cortex, Midbrain → Striatum, Limbic forebrain, Hippocampus, Diencephalon, Cerebellum, Brainstem | |||||
| G protein coupling capacity | |||||
| ↑ Cerebral cortex ↓ Brainstem → Striatum, Limbic forebrain, Hippocampus, Midbrain, Diencephalon, Cerebellum | |||||
| Vigano et.al., 2003 [54] | Male Rats | Morphine (SQ) | Non-cue-conditioned model. Stable 5mg/kg dose, twice daily for 4.5 days. Animals were sacrificed two hours after the last injection |
Density ([3H]-CP-55,940 binding) G protein coupling capacity (CP-55,940-induced [35S]-GTPγS binding) |
Density |
| ↓ Hippocampus, Cerebellum → CP, Cortex, NAc, Septum nucleus, anterior amygdala, Hypothalamus, SN, GP | |||||
| G protein coupling capacity | |||||
| ↓ NAc → CP, Cortex, Hippocampus, Septum nucleus, anterior amygdala, Hypothalamus, SN, GP, Cerebellum | |||||
| Fattore et al., 2007 [63] | Male rats | Heroin (IV) | SA model. Each lever depression resulted in a 0.03 mg/kg heroin injection, after stabilization of SA behavior the training continued for another week. Animals were sacrificed one week after acquiring SA behavior |
Density ([3H]-CP-55,940 binding) G protein coupling capacity (CP-55,940-induced [35S]-GTPγS binding) |
Density |
| ↑ Amygdala, VTA → PFC, NAc, CP, Hippocampus, Hypothalamus | |||||
| G Protein coupling capacity | |||||
| ↑ PFC, NAc, CP, Hippocampus, Amygdala →Hypothalamus, VTA | |||||
| Álvaro-Bartolomé and García-Sevilla, 2013 [58] | Male and Female Humans (postmortem) | Heroin and/or Methadone | Taking these opioids for the last 6-24 months All died due to opioid overdose |
Protein expression (Western Blot) | Protein expression |
| → PFC | |||||
| Male Rats | Morphine (IP) | Non-cue-conditioned model. 10-100 mg/kg three times a day for 6 days. |
Protein expression (Western Blot) | Protein expression | |
| → Cerebral Cortex | |||||
| Yuan et al., 2013 [68] | Male Rats | Morphine (SQ) | CPP model. 10mg/kg daily injections for 5 days accompanied by place conditioning. Then the morphine injections stopped. No morphine unpaired control group. Animals were sacrificed on days 2, 4, and 22 after the last injection. |
Protein expression (Western blot) | Protein expression |
| ↑ NAc → Striatum Note: The same changes were observed in all three withdrawal groups with no significant difference between them. | |||||
| Jin et al., 2014 [55] | Male Rats | Morphine (IP or SC?) | Non-cue-conditioned model. 10 mg/kg twice a day for 12 days Animals were sacrificed 24 hours after the last injection |
mRNA expression (RT-PCR) Protein expression (Western blot) |
mRNA expression |
| ↑ in Cortex, Hippocampus, Cerebellum → Brain stem | |||||
| Protein expression | |||||
| ↑ in Cortex, Hippocampus, Cerebellum | |||||
| Zhang et al., 2016 [65] | Male Mice | Morphine (IP) | CPP model. 10 mg/kg daily injections, every other day for 8 days with (morphine CPP) or without (morphine no-CPP) place conditioning procedure. Animals were sacrificed 24 hours after the last injection |
Protein expression (Western blot) | Protein expression |
| ↑ NAc, Hippocampus Note: significant changes were observed only in the morphine CPP group but not in the morphine no-CPP group. | |||||
| Zhao et al., 2017 [66] | Male Rats | Morphine (SQ) | CPP model. Conditioning phase: Escalating daily dose for 4 days (5, 8, 10, and 15 mg/kg) with (morphine-paired group) and without (morphine-unpaired group) place conditioning procedure. Extinction phase: five days without morphine. Reinstatement phase: Single 5 mg/Kg morphine injection. Animals were sacrificed 24 hours after each phase. |
Protein expression (Western blot) | Protein expression |
| ↑ Dorsal hippocampus after conditioning and reinstatement phases. → Dorsal hippocampus after extinction phase. Note: significant changes were observed only in the morphine-paired group but not in the morphine-unpaired group. | |||||
| Li et.al, 2017 [67] | Male Rats | Morphine | CPP model. Conditioning phase: Escalating daily dose for 4 days (5, 8, 10, and 15 mg/kg) with (morphine-paired group) and without (morphine-unpaired group) place conditioning procedure. Extinction phase: five days without morphine. Reinstatement phase: Single 5 mg/Kg morphine injection. The control group received saline injections throughout the investigation except for the reinstatement phase, in which they also received the morphine injection. Animals were sacrificed 24 hours after each phase. |
mRNA expression (RT-PCR) | mRNA expression |
| ↑ Dorsal hippocampus after reinstatement phase vs. saline pretreated controls that received one morphine injection. ↓ Dorsal hippocampus after conditioning phase vs. saline-treated controls. → Dorsal hippocampus after extinction phase vs. saline-treated controls. Note: significant changes were observed only in the morphine-paired group but not in the morphine-unpaired group. | |||||
| Zhang et.al. 2021 [57] | Male Rats | Morphine (IP) | Non-cue-conditioned model. Stable dose of 5 mg/kg, twice daily for 5 days. Animals were sacrificed one hour after the last injection. |
mRNA expression (RT-PCR) Protein expression (Western blot & proteomic analysis) |
mRNA expression |
| → VTA | |||||
| Protein expression | |||||
| → VTA |
Abbreviations: CA1-2-3: Cornu Ammonis 1-2-3; CP: Caudate-putamen; CPP: Conditioned place preference; EPN: Entopeduncular nucleus, GP: Globus palidus; IV: Intra-venous; IP: Intra-peritoneal; NAc: Nucleus accumbens; PFC: Pre-frontal cortex; SA: Self-administration; SN: Substantia Nigra; SQ: Subcutaneous; VTA: Ventral tegmental area; RT-PCR: Real-time polymerase chain reactions.
Definitions: ↑: Increased significantly; ↓: Decreased significantly; →: Not changed/insignificant change.
WIN 55,212-2 is a non-specific CB1R and CB2R agonist, hence in these studies, the G protein coupling capacity results are not specific to CB1R. However, because CB1R is considerably more abundant in the CNS compared to CB2R, we considered these results as pertaining to CB1R.
The source paper calculated the significance of the G protein coupling differences of before vs. after agonist stimulation in the morphine and saline-treated mice. We presented the significance of the difference in agonist-induced activation of saline vs. morphine-treated mice using the crude results presented in Table 3 of the report.
Table 3:
CB2R alterations following chronic opioid exposure.
| Source | Species | Opioid Compound (Route) |
Addiction Model/Exposure Details |
CB2R outcome measures and method |
Results |
|---|---|---|---|---|---|
| Onaivi et al., 2008 [69] | Male and Female Mice | Heroin (NR) | Non-cue-conditioned model. Chronic exposure to 10 mg/kg heroin, (daily frequency and duration: NR) |
mRNA expression (RT-PCR) | mRNA expression |
| → Whole brain | |||||
| Zhang et al., 2012 [70] | Male Rats | Morphine (SQ) | CPP model. Conditioning phase: 4 days of stable 10 mg/kg daily morphine injections alongside place conditioning procedure. No morphine-unpaired group. Animals were sacrificed the day after the injection |
mRNA expression (RT-PCR) Protein expression (Western blot) Note: the western blot is semi-quantified, and it is not clear whether the changes are statistically significant or not. |
mRNA expression |
| ↑ Cortex ↓ Brain stem → Cerebellum | |||||
| Protein expression | |||||
| ↓ Cortex, Brain stem → Cerebellum | |||||
| Li et.al, 2017 [67] | Male Rats | Morphine (SQ) | CPP model. Conditioning phase: Escalating daily dose for 4 days (5, 8, 10, and 15 mg/kg) with (morphine-paired group) and without (morphine-unpaired group) place conditioning procedure. Extinction phase: five days without morphine. Reinstatement phase: Single 5 mg/Kg morphine injection. The control group received saline injections throughout the investigation except for the reinstatement phase, in which they also received the morphine injection. Animals were sacrificed 24 hours after each phase |
mRNA expression (RT-PCR) | mRNA expression |
| ↑ Dorsal hippocampus after conditioning phase vs. saline-treated controls. → Dorsal hippocampus after extinction vs. saline-treated controls and in reinstatement phases compared to saline-pretreated controls that had a single morphine injection. Note: significant changes were observed only in the morphine-paired group but not in the morphine-unpaired group. | |||||
|
Zhang et.al. 2021 [57] |
Male Rats | Morphine (IP) | Non-cue-conditioned model. Stable dose of 5 mg/kg, twice daily for 5 days Animals were sacrificed one hour after the last injection. |
mRNA expression (RT-PCR) | mRNA expression |
| ↓ VTA |
Abbreviation: CPP: Conditioned place preference IP: Intra-peritoneal; RT-PCR: Reverse transcriptase polymerase chain reactions; SQ: Subcutaneous; VTA: Ventral tegmental area.
Table 4:
Alterations in the level of endocannabinoids and their metabolic enzymes following opioid chronic exposure.
| Source | Species | Opioid Compound (Route) |
Addiction Model/Exposure Details |
Endocannabinoid outcome measures and method |
Results |
|---|---|---|---|---|---|
| Gonzalez et al., 2003 [59] | Male Rats | Morphine (SQ) | Non-cue-conditioned model. Escalating dose every day for 6 days Day 1: 10 and 10 mg/kg weight; day 2: 20 and 20 mg/kg; day 3: 40 and 40 mg/kg; day 4: 60 and 60 mg/kg; day 5: 80 and 80 mg/kg; and day 6: 100 mg/kg. Animals were sacrificed two hours after the last injection |
AEA level (GC/MS) | AEA level |
| → Cerebral Cortex, Striatum, Limbic forebrain, Hippocampus, Midbrain, Diencephalon, Cerebellum, Brain stem | |||||
| Vigano et.al, 2003 [54] | Male Rats | Morphine (SQ) | Non-cue-conditioned model. Stable 5mg/kg dose, twice daily for 4.5 days Animals were sacrificed two hours after the last injection |
AEA and 2-AG levels (GC/MS) | AEA level |
| → Striatum, Cortex, Hippocampus, Limbic area1, Hypothalamus, Cerebellum, Mesencephalon. | |||||
| 2-AG level | |||||
| ↓ Striatum, Cortex, Hippocampus, Limbic area1, Hypothalamus. → Cerebellum, Mesencephalon. | |||||
| Vigano et.al., 2004 [56] | Male Rats | Morphine (SQ) | Non-cue-conditioned model. Chronic morphine phase: escalating dose for 3 days (10, 20, 40 mg/kg) twice daily, Withdrawal phase: 2 weeks without drug, Expression phase: a single dose of 5mg/kg morphine after withdrawal Animals were sacrificed 30 minutes after the last injection or after the withdrawal phase |
AEA and 2-AG levels (LC-APCI-MS) FAAH activity (in vitro arachidonoyl-[14C]ethanolamide metabolism) | AEA level |
| ↑ In NAc, PFC, CP, and Hippocampus at the end of withdrawal and reinstatement phases → In NAc, PFC, CP, and Hippocampus at the end of the chronic morphine phase | |||||
| 2-AG level | |||||
| ↓ in NAc at the end of the chronic morphine phase. In NAc, CP and hippocampus at the end of withdrawal and expression phases → in CP and hippocampus after chronic phase. In PFC after chronic morphine, withdrawal, and reinstatement phases. | |||||
| FAAH activity | |||||
| ↓ in CP and hippocampus at the end of the chronic phase and reinstatement phases. | |||||
|
Note: The above-mentioned significant changes at the end of the reinstatement phase were compared to saline control rats. However, there are some significant changes in the morphine-treated animal at the end of the withdrawal phase and after receiving the expression dose. ↓ AEA level in CP and Hippocampus → AEA level in NAc and PFC ↑ 2-AG in the Hippocampus → 2-AG in the CP, NAc and PFC | |||||
| Caille´ et.al., 2007 [71] | Male Rats | Heroin (IV) | SA model. After the rats acquired a stable SA behavior, a 2-hour session in the SA chamber alongside In vivo micro-dialysis sampling was started. |
AEA and 2-AG levels (LC/MS) | AEA level |
| ↑ in NAc shell (directly correlated with heroin dose) during the self-administration session vs. before the session. → in NAc shell before self-administration session vs. controls | |||||
| Mean self-administered heroin during the 2-hour sampling session administration: 443 ± 61 μg/kg | 2-AG level | ||||
| ↓ in NAc shell (Inversely correlated with heroin dose) during the self-administration session vs. before the session. → in NAc shell before self-administration session vs. controls | |||||
| Li et.al, 2017 [67] | Male Rats | Morphine SQ) | CPP model. Conditioning phase: Escalating daily dose for 4 days (5, 8, 10, and 15 mg/kg) with (morphine-paired group) and without (morphine-unpaired group) place conditioning procedure. Extinction phase: five days without morphine. Reinstatement phase: Single 5 mg/Kg morphine injection. The control group received saline injections throughout the investigation except for the reinstatement phase, in which they also received the morphine injection. Animals were sacrificed 24 hours after each phase |
NAPE-PLD, DAGLα/β, FAAH, and MAGL mRNA expression (RT-PCR) | NAPE-PLD expression |
| → in the dorsal hippocampus after conditioning, extinction, and reinstatement phases. | |||||
| DAGLα/β expression | |||||
| → in the dorsal hippocampus after conditioning, extinction, and reinstatement phases. | |||||
| FAAH expression | |||||
| ↑ in dorsal hippocampus after conditioning phase. → in the dorsal hippocampus after extinction and reinstatement phases. | |||||
| MAGL expression | |||||
| ↑ in dorsal hippocampus after conditioning phase. ↓ in the dorsal hippocampus after the reinstatement phase. → in the dorsal hippocampus after the extinction phase. | |||||
| Note: significant changes were observed only in the morphine-paired group but not in the morphine-unpaired group. The comparisons in the conditioning and extinction phases are between morphine-treated and saline-treated rats. However, in the reinstatement phase, the comparison is between morphine-pretreated and saline-pretreated rats that both received a morphine injection. | |||||
| Zhang et al., 2021 [57] | Male Rats | Morphine (IP) | Non-cue-conditioned model. Stable dose of 5 mg/kg, twice daily for 5 days. Animals were sacrificed one hour after the last injection. |
FAAH and NAPE-PLD Protein expression (proteome analysis) MAGL and DAGLα protein expression (proteome analysis and Western blot) |
FAAH expression |
| → VTA | |||||
| NAPE-PLD expression | |||||
| → VTA | |||||
| MAGL expression | |||||
| → VTA | |||||
| DAGLα expression | |||||
| → VTA | |||||
| Non-cue-conditioned model. Stable dose of 5 mg/kg, twice daily for 5 days, then an additional 5 mg/kg on the next day (challenge injection) and measuring endocannabinoid trends. In vivo, micro-dialysis samples were obtained every 30 minutes until 6 hours after the challenge injection. |
AEA and 2-AG levels (Mass spectrometry) | AEA level | |||
| → In VTA before challenge injection vs. saline-treated control group. In VTA before vs. after challenge injection. | |||||
| 2-AG level | |||||
| → In VTA before challenge injection vs. saline-treated control group. In VTA before vs. after challenge injection |
Abbreviations: AEA: Anandamide; DAGLα: diacylglycerol lipase α; CP: Caudate-putamen; FAAH: Fatty acid amide hydrolase; GC/MS: Gas chromatography, mass spectrometry; IP: Intra-peritoneal; IV: Intra-venous; MAGL: Monoacylglycerol lipase; NAc: Nucleus accumbens; NAPE-PLD: N-Acyl-Phosphatidylethanolamine-Hydrolyzing Phospholipase D; RT-PCR: Reverse transcriptase polymerase chain reaction; SA: Self-administration; PFC: Pre-frontal cortex; VTA: Ventral tegmental area; 2-AG: 2-Arachidonoylglycerol. Definitions: ↑: Increased significantly; ↓: Decreased significantly; →: Not changed/insignificant change.
Authors specimen of the limbic area contained nucleus accumbens, septum nuclei, and parts of the anterior amygdaloid nuclei.
Highlights.
Endogenous cannabinoid and opioid systems have multi-level cross-talks.
Exposure to exogenous opioids dysregulates the endocannabinoid system.
Alterations of the endocannabinoid system are region-specific in the brain.
Acknowledgment
The work described in this article (or chapter or book) was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
Funding:
This work was supported by the National Institutes of Health [grant number K12 DA000167]; National Institute of Health/ National Institute on Drug Abuse (NIDA) [R21 DA046030 and P30DA046345].
Conflict of interest
AMA: No conflicts of interest.
AS: No conflicts of interest.
MAC: No conflicts of interest.
JW: No conflicts of interest.
DCD: DCD receives research funding administered through Yale University from the US National Institute of Health, US Dept. of Veteran Affairs, Takeda, Biogen, Boehringer Ingelheim, Ceruvia, Heffter Institute and Wallace Foundation. DCD has served as a paid consultant to Jazz Pharmaceuticals, Biohaven and Abide.
GAA: No conflicts of interest. GAA received funding for this work from NIH/NIDA grants R21 DA046030 and P30DA046345.
ABN: ABN received funding for this work from the National Institute of Health K12 DA000167 grant. She is also a member of the Scientific Advisory Committee of Synendos Therapeutics AG, Switzerland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad FB, Rossen LM, Sutton P, 2021. Provisional drug overdose death counts. National Center for Health Statistics 12. [Google Scholar]
- Ahmad T, Lauzon NM, de Jaeger X, Laviolette SR, 2013. Cannabinoid transmission in the prelimbic cortex bidirectionally controls opiate reward and aversion signaling through dissociable kappa versus mu-opiate receptor dependent mechanisms. J Neurosci 33 (39), 15642–15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun A, Ozdemir E, Yildirim K, Gursoy S, Durmus N, Bagcivan I, 2015. The effects of endocannabinoid receptor agonist anandamide and antagonist rimonabant on opioid analgesia and tolerance in rats. Gen Physiol Biophys 34 (4), 433–440. [DOI] [PubMed] [Google Scholar]
- Alvaro-Bartolome M, Garcia-Sevilla JA, 2013. Dysregulation of cannabinoid CB1 receptor and associated signaling networks in brains of cocaine addicts and cocaine-treated rodents. Neuroscience 247, 294–308. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K, 2010. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160 (3), 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadfard M, Huecker M, JM L, 2021. Opioid Addiction, Treasure Island (FL). [Google Scholar]
- Baggelaar MP, Maccarrone M, van der Stelt M, 2018. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog Lipid Res 71, 1–17. [DOI] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Negus SS, 2017. Utility of nonhuman primates in substance use disorders research. ILAR journal 58 (2), 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassir Nia A, Bender R, Harpaz-Rotem I, 2019. Endocannabinoid System Alterations in Posttraumatic Stress Disorder: A Review of Developmental and Accumulative Effects of Trauma. Chronic Stress (Thousand Oaks) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm-Hofstätter H, Tschernutter M, Kunert R, 2010. Comparison of hybridization methods and real-time PCR: their value in animal cell line characterization. Applied microbiology and biotechnology 87, 419–425. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH, 2007. Specific Alterations of Extracellular Endocannabinoid Levels in the Nucleus Accumbens by Ethanol, Heroin, and Cocaine Self-Administration. Journal of Neuroscience 27 (14), 3695–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Parsons LH, 2003. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci 18 (11), 3145–3149. [DOI] [PubMed] [Google Scholar]
- Calabria B, Degenhardt L, Briegleb C, Vos T, Hall W, Lynskey M, Callaghan B, Rana U, McLaren J, 2010. Systematic review of prospective studies investigating "remission" from amphetamine, cannabis, cocaine or opioid dependence. Addict Behav 35 (8), 741–749. [DOI] [PubMed] [Google Scholar]
- Cao JK, Kaplan J, Stella N, 2019. ABHD6: its place in endocannabinoid signaling and beyond. Trends in pharmacological sciences 40 (4), 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Battistini L, Maccarrone M, 2008. The endocannabinoid system in peripheral lymphocytes as a mirror of neuroinflammatory diseases. Current pharmaceutical design 14 (23), 2370–2382. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien MLAV, Ariansen JL, Aragona BJ, Phillips PEM, Wightman RM, 2007. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. Journal of Neuroscience 27 (4), 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Thompson AC, Zapata A, Shippenberg TS, 2009. Overview of brain microdialysis. Current protocols in neuroscience 47 (1), 7–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D-J, Gao M, Gao F-F, Su Q-X, Wu J, 2017. Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacologica Sinica 38 (3), 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A, 2007. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. The Lancet 370 (9600), 1706–1713. [DOI] [PubMed] [Google Scholar]
- Chye Y, Christensen E, Solowij N, Yucel M, 2019. The Endocannabinoid System and Cannabidiol's Promise for the Treatment of Substance Use Disorder. Front Psychiatry 10, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raasø H, Schoffelmeer ANM, 2003. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology 168, 164–169. [DOI] [PubMed] [Google Scholar]
- Dean B, Sundram S, Bradbury R, Scarr E, Copolov D, 2001. Studies on [3H] CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 103 (1), 9–15. [DOI] [PubMed] [Google Scholar]
- Del Arco I, Navarro M, Bilbao A, Ferrer B, Piomelli D, De Fonseca F.R.g., 2002. Attenuation of spontaneous opiate withdrawal in mice by the anandamide transport inhibitor AM404. European journal of pharmacology 454 (1), 103–104. [DOI] [PubMed] [Google Scholar]
- DeLapp NW, Gough WH, Kahl SD, Porter AC, Wiernicki TR, 2012. GTPγS binding assays. Assay Guidance Manual [Internet]. [Google Scholar]
- Dong A, He K, Dudok B, Farrell JS, Guan W, Liput DJ, Puhl HL, Cai R, Wang H, Duan J, 2022. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nature biotechnology 40 (5), 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudok B, Soltesz I, 2022. Imaging the endocannabinoid signaling system. Journal of neuroscience methods 367, 109451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt MJ, Lee KH, 2000. Proteomic analysis. Current opinion in biotechnology 11 (2), 176–179. [DOI] [PubMed] [Google Scholar]
- Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D, 2007. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur J Neurosci 25 (7), 2191–2200. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA, 2002. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend 66 (1), 77–84. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Schmid PC, Fernandez-Ruiz J, Krebsbach R, Schmid HH, Ramos JA, 2003. Region-dependent changes in endocannabinoid transmission in the brain of morphine-dependent rats. Addict Biol 8 (2), 159–166. [DOI] [PubMed] [Google Scholar]
- Grenald SA, Young MA, Wang Y, Ossipov MH, Ibrahim MM, Largent-Milnes TM, Vanderah TW, 2017. Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology 116, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueye AB, Pryslawsky Y, Trigo JM, Poulia N, Delis F, Antoniou K, Loureiro M, Laviolette SR, Vemuri K, Makriyannis A, 2016. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. International Journal of Neuropsychopharmacology 19 (12), pyw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Traynor JR, 2003. The [35S] GTPγS binding assay: approaches and applications in pharmacology. Life sciences 74 (4), 489–508. [DOI] [PubMed] [Google Scholar]
- He XH, Jordan CJ, Vemuri K, Bi GH, Zhan J, Gardner EL, Makriyannis A, Wang YL, Xi ZX, 2019. Cannabinoid CB(1) receptor neutral antagonist AM4113 inhibits heroin self-administration without depressive side effects in rats. Acta Pharmacol Sin 40 (3), 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, Kanaide M, Taniyama K, Sumikawa K, Uezono Y, 2008. μ-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. Journal of pharmacological sciences 108 (3), 308–319. [DOI] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, Ling W, 2014. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction 109 (1), 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JP, de Souza Silva MA, Müller CP, 2013. What's conditioned in conditioned place preference? Trends in pharmacological sciences 34 (3), 162–166. [DOI] [PubMed] [Google Scholar]
- Iyer V, Slivicki RA, Thomaz AC, Crystal JD, Mackie K, Hohmann AG, 2020. The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. European journal of pharmacology 886, 173544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Pan L, Guo Y, Zheng Y, Nie Z, Zhu R, 2014. Expression and localization of cannabinoid receptor 1 in rats' brain treated with acute and repeated morphine. Acta Neurobiol Exp (Wars) 74 (3), 288–297. [DOI] [PubMed] [Google Scholar]
- Kampman K, Jarvis M, 2015. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. Journal of addiction medicine 9 (5), 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman MA, Furtado M, Anand L, 2016. Targeting the endocannabinoid system in psychiatric illness. Journal of clinical psychopharmacology 36 (6), 691–703. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry 3 (8), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn BN, Kalivas PW, Bobadilla A-C, 2019. Understanding addiction using animal models. Frontiers in behavioral neuroscience 13, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, 2005. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. Journal of Pharmacology and Experimental Therapeutics 312 (3), 875–883. [DOI] [PubMed] [Google Scholar]
- Lee YK, Gold MS, Fuehrlein BS, 2022. Looking beyond the opioid receptor: A desperate need for new treatments for opioid use disorder. J Neurol Sci 432 (1878-5883 (Electronic)), 120094. [DOI] [PubMed] [Google Scholar]
- Li AL, Lin X, Dhopeshwarkar AS, Thomaz AC, Carey LM, Liu Y, Nikas SP, Makriyannis A, Mackie K, Hohmann AG, 2019. Cannabinoid CB2 Agonist AM1710 Differentially Suppresses Distinct Pathological Pain States and Attenuates Morphine Tolerance and Withdrawal. Mol Pharmacol 95 (2), 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang CL, Qiu ZG, 2017. Differential expression of endocannabinoid system-related genes in the dorsal hippocampus following expression and reinstatement of morphine conditioned place preference in mice. Neurosci Lett 643, 38–44. [DOI] [PubMed] [Google Scholar]
- Lo A, Kerr T, Hayashi K, Milloy MJ, Nosova E, Liu Y, Fairbairn N, 2018. Factors associated with methadone maintenance therapy discontinuation among people who inject drugs. J Subst Abuse Treat 94, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Lopez-Jimenez A, Gorriti MA, de Fonseca FR, 2010. Functional interactions between endogenous cannabinoid and opioid systems: focus on alcohol, genetics and drug-addicted behaviors. Current drug targets 11 (4), 406–428. [DOI] [PubMed] [Google Scholar]
- Lu H-C, Mackie K, 2021. Review of the endocannabinoid system. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 6 (6), 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Mackie K, 2016. An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry 79 (7), 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL, 2010. Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comparative medicine 60 (3), 177–188. [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, 2017. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front Mol Neurosci 10 (166), 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, 2008. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nature neuroscience 11 (2), 152–159. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F, 2002. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci 22 (9), 3326–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M., 2008. Can proteomics retire the western blot? ACS Publications, pp. 3065–3065. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Cabanero D, Puente N, Garcia-Gutierrez MS, Grandes P, Maldonado R, 2018. Role of the endocannabinoid system in drug addiction. Biochem Pharmacol 157, 108–121. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG, 2007. Meta- analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. British journal of pharmacology 152 (5), 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale V, Drago F, 2018. Endocannabinoid system, stress and HPA axis. European journal of pharmacology 834, 230–239. [DOI] [PubMed] [Google Scholar]
- Moitra E, Anderson BJ, Stein MD, 2013. Perceived stress and substance use in methadone-maintained smokers. Drug Alcohol Depend 133 (2), 785–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei M, Fatahi Z, Zaringhalam J, Haghparast A, 2016. CB1 Cannabinoid Agonist (WIN55,212-2) Within the Basolateral Amygdala Induced Sensitization to Morphine and Increased the Level of mu-Opioid Receptor and c-fos in the Nucleus Accumbens. J Mol Neurosci 58 (4), 446–455. [DOI] [PubMed] [Google Scholar]
- Müller-Vahl KR, Fremer C, Beals C, Ivkovic J, Loft H, Schindler C, 2022. Endocannabinoid modulation using monoacylglycerol lipase inhibition in tourette syndrome: A phase 1 randomized, placebo-controlled study. Pharmacopsychiatry 55 (03), 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LM, Lutz MP, Domino EF, 1974. Delta9-tetrahydrocannabinol and some CNS depressants: evidence for cross-tolerance in the rat. Archives internationales de pharmacodynamie et de therapie 207 (2), 254–259. [PubMed] [Google Scholar]
- Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH, 2010. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996-2006. J Subst Abuse Treat 39 (1), 22–31. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Liu QR, Chirwa SS, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR, 2008. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci 1139, 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld T, Price J, Albanese M, Bullman J, Guillard F, Meyer I, Leeson R, Costantin C, Ziviani L, Nocini PF, 2011. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. The Clinical journal of pain 27 (8), 668–676. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Stull SW, Kowalczyk WJ, Phillips KA, Schroeder JR, Bertz JW, Vahabzadeh M, Lin JL, Mezghanni M, Nunes EV, Epstein DH, Preston KL, 2019. Stress, craving and mood as predictors of early dropout from opioid agonist therapy. Drug Alcohol Depend 202, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL, 2015. Endocannabinoid signalling in reward and addiction. Nature Reviews Neuroscience 16 (10), 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC, 2013. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue- triggered ‘wanting’for reward: entire core and medial shell mapped as substrates for PIT enhancement. European Journal of Neuroscience 37 (9), 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Mabou Tagne A, 2022. Endocannabinoid-based therapies. Annual Review of Pharmacology and Toxicology 62, 483–507. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, 2011. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. Journal of Pharmacology and Experimental Therapeutics 339 (1), 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransick A, 2004. Detection of mRNA by in situ hybridization and RT-PCR. Methods in cell biology 74, 601–620. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM, 2001. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci 21 (3), 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J, Fernández-Ruiz JJ, Vela G, Ruiz-Gayo M, Fuentes JA, Ramos JA, 1998. Autoradiographic analysis of cannabinoid receptor binding and cannabinoid agonist-stimulated [35S] GTPγS binding in morphine-dependent mice. Drug and Alcohol Dependence 50 (3), 241–249. [DOI] [PubMed] [Google Scholar]
- Rubino T, Tizzoni L, Vigano D, Massi P, Parolaro D, 1997. Modulation of rat brain cannabinoid receptors after chronic morphine treatment. Neuroreport 8 (15), 3219–3223. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Järvinen T, Laine K, Laitinen JT, 2001. Despite substantial degradation, 2- arachidonoylglycerol is a potent full efficacy agonist mediating CB1 receptor- dependent G- protein activation in rat cerebellar membranes. British journal of pharmacology 134 (3), 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ, 2010. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res 1312, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Sterling RC, Van Bockstaele EJ, 2013. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience 248, 637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ME, Liebowitz MR, Stein MB, Grunfeld J, Van Hove I, Simmons WK, Van Der Ark P, Palmer JA, Saad ZS, Pemberton DJ, 2021. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: a double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology 46 (5), 1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ, 2006. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology 51 (4), 773–781. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ, 2000. Chronic heroin self-administration desensitizes μ opioid receptor-activated G-proteins in specific regions of rat brain. Journal of Neuroscience 20 (12), 4555–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BF, Fadanelli M, Kawa AB, Robinson TE, 2018. Are cocaine-seeking “habits” necessary for the development of addiction-like behavior in rats? Journal of Neuroscience 38 (1), 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Gowin JL, Ramchandani VA, Hurd YL, Le Foll B, 2017. The endocannabinoid system as a target for addiction treatment: Trials and tribulations. Neuropharmacology 124, 73–83. [DOI] [PubMed] [Google Scholar]
- Sloan ME, Grant CW, Gowin JL, Ramchandani VA, Le Foll B, 2019. Endocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studies. Acta Pharmacologica Sinica 40 (3), 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, 2020. Nonhuman animal models of substance use disorders: Translational value and utility to basic science. Drug and Alcohol Dependence 206, 107733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth BP, Barry J, Keenan E, Ducray K, 2010. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J 103 (6), 176–179. [PubMed] [Google Scholar]
- Strain EC, Kampman KM, Weiss RD, 2021. Moving Beyond Medications That Act at the mu Receptor in the Treatment of Opioid Use Disorder. JAMA Psychiatry 78 (7), 701–702. [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Posner MI, Rothbart MK, Volkow ND, 2015. Circuitry of self-control and its role in reducing addiction. Trends in cognitive sciences 19 (8), 439–444. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Posch A, 2014. The design of a quantitative western blot experiment. BioMed research international 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen W, Frazer J, Unett D, 2005. Functional assays for screening GPCR targets. Current opinion in biotechnology 16 (6), 655–665. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW, 2008. Microdialysis and the neurochemistry of addiction. Pharmacology Biochemistry and Behavior 90 (2), 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Koob GF, Cable J, 2019. The neurobiology of addiction. Annals of the New York academy of sciences 1451 (1), 5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant GE, 1973. A 20-year follow-up of New York narcotic addicts. Arch Gen Psychiatry 29 (2), 237–241. [DOI] [PubMed] [Google Scholar]
- Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP, 2001. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur J Neurosci 13 (9), 1816–1824. [DOI] [PubMed] [Google Scholar]
- Vigano D, Grazia Cascio M, Rubino T, Fezza F, Vaccani A, Di Marzo V, Parolaro D, 2003. Chronic morphine modulates the contents of the endocannabinoid, 2-arachidonoyl glycerol, in rat brain. Neuropsychopharmacology 28 (6), 1160–1167. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D, 2005. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav 81 (2), 360–368. [DOI] [PubMed] [Google Scholar]
- Vigano D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T, 2004. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci 20 (7), 1849–1857. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Blanco C, 2021. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry 26 (1), 218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M, Baler R, 2019. The neuroscience of drug reward and addiction. Physiological reviews 99 (4), 2115–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Cheer JF, 2018. Endocannabinoid regulation of reward and reinforcement through interaction with dopamine and endogenous opioid signaling. Neuropsychopharmacology 43 (1), 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese B, Wilson-Poe AR, 2018. Emerging Evidence for Cannabis' Role in Opioid Use Disorder. Cannabis Cannabinoid Res 3 (1), 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills KL, Vemuri K, Kalmar A, Lee A, Limebeer CL, Makriyannis A, Parker LA, 2014. CB1 antagonism: interference with affective properties of acute naloxone-precipitated morphine withdrawal in rats. Psychopharmacology 231 (22), 4291–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD, 2010. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism: Clinical and Experimental Research 34 (8), 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan WX, Heng LJ, Ma J, Wang XQ, Qu LJ, Duan L, Kang JJ, Chen LW, Gao GD, 2013. Increased expression of cannabinoid receptor 1 in the nucleus accumbens core in a rat model with morphine withdrawal. Brain Res 1531, 102–112. [DOI] [PubMed] [Google Scholar]
- Zhang H-Y, Bi G-H, Li X, Li J, Qu H, Zhang S-J, Li C-Y, Onaivi ES, Gardner EL, Xi Z-X, 2015. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 40 (4), 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lipinski AA, Liktor-Busa E, Smith AF, Moutal A, Khanna R, Langlais PR, Largent-Milnes TM, Vanderah TW, 2021. The Effects of Repeated Morphine Treatment on the Endogenous Cannabinoid System in the Ventral Tegmental Area. Front Pharmacol 12, 632757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang N, Chen B, Wang Y, He J, Cai X, Zhang H, Wei S, Li S, 2016a. Blockade of Cannabinoid CB1 receptor attenuates the acquisition of morphine-induced conditioned place preference along with a downregulation of ERK, CREB phosphorylation, and BDNF expression in the nucleus accumbens and hippocampus. Neurosci Lett 630, 70–76. [DOI] [PubMed] [Google Scholar]
- Zhang M, Dong L, Zou H, Li J, Li Q, Wang G, Li H, 2018. Effects of Cannabinoid Type 2 Receptor Agonist AM1241 on Morphine-Induced Antinociception, Acute and Chronic Tolerance, and Dependence in Mice. J Pain 19 (10), 1113–1129. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang K, Ma M, Tian S, Wei N, Wang G, 2016b. Low-dose cannabinoid type 2 receptor agonist attenuates tolerance to repeated morphine administration via regulating μ-opioid receptor expression in walker 256 tumor-bearing rats. Anesthesia & Analgesia 122 (4), 1031–1037. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Zhang M, Cao Y, 2012. Exposure to morphine affects the expression of endocannabinoid receptors and immune functions. J Neuroimmunol 247 (1-2), 52–58. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yao L, Wang F, Zhang H, Wu L, 2017. Cannabinoid 1 receptor blockade in the dorsal hippocampus prevents the reinstatement but not acquisition of morphine-induced conditioned place preference in rats. Neuroreport 28 (10), 565–570. [DOI] [PubMed] [Google Scholar]
- Zoerner AA, Gutzki F-M, Batkai S, May M, Rakers C, Engeli S, Jordan J, Tsikas D, 2011. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1811 (11), 706–723. [DOI] [PubMed] [Google Scholar]
- Zou S, Kumar U, 2018. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. International journal of molecular sciences 19 (3), 833. [DOI] [PMC free article] [PubMed] [Google Scholar]