Summary
Classic galactosemia (CG) and clinical variant galactosemia (CVG) are allelic inborn errors of metabolism that result from profound deficiency, and near-profound deficiency, respectively, of galactose-1-P uridylyltransferase (GALT). Despite early detection and lifelong dietary restriction of galactose, which is the current standard of care, most patients with CG/CVG grow to experience a range of long term developmental and other complications. One of the less well-understood complications of CG/CVG is decreased hand grip strength, reported by Potter and colleagues in 2013. Here we confirm this phenotype in an independent cohort of 36 cases (4-18y) and 19 controls (4-17y), and further demonstrate that the grip strength deficit observed in cases may be secondary to growth delay. Specifically, we found that when grip strength of cases and controls in a new cohort recruited in 2022 was plotted by weight, rather than age, the difference between cases and controls for both sexes disappeared. Reanalyzing data from the original 2013 cohort, we found that differences in weight accounted for grip strength differences between cases and controls in girls and young women, but not in boys and young men. Finally, we tested whether a GALT-null rat model of CG also showed a grip strength deficit – it did – and again the difference between GALT-null and wild-type rats associated with differences in body mass. Combined, these results confirm that GALT deficiency is associated with a grip strength deficit in both young patients with CG/CVG and GALT-null rats, and further demonstrate that this phenotype may be secondary to growth delay, and therefore not evidence of a muscle abnormality.
Keywords: galactosemia, grip strength, growth delay, rat model
Introduction
Classic galactosemia (CG) and clinical variant galactosemia (CVG) are allelic inborn errors of metabolism that result from profound, or near profound deficiency, respectively, of galactose-1-phosphate uridylyltransferase (GALT), the middle enzyme in the Leloir pathway of galactose metabolism (Berry 2020). Early detection of CG/CVG, often by newborn screening (NBS), enables rapid dietary restriction of galactose, typically initiated by switching the baby from milk to a low galactose formula (Welling et al 2017). This dietetic intervention together with any needed medical treatment can be lifesaving for infants with CG/CVG, as it prevents or resolves the potentially lethal acute symptoms of disease. However, even life-long dietary restriction of galactose fails to prevent the many long-term developmental and other complications that often occur. These can include deficits in growth, speech, cognitive function, motor, psychosocial, ovarian, and other outcome domains (Rubio-Gozalbo et al 2019).
In 2013, Potter and colleagues (Potter et al 2013) reported an additional complication – hand grip strength deficit -- detected in a cohort of children with CG who also experienced speech disorders. Specifically, these authors reported finding hand grip strength weakness co-occurring with speech and other motor problems in children with CG and hypothesized that the combined problems might arise from a diffuse cerebellar abnormality. This was a reasonable hypothesis especially considering that the 3 most common motor problems reported among a cohort of patients in a European/UK study (Rubio-Agusti et al 2013) were tremor, dystonia, and “cerebellar signs.” Since 2013, no other reports have addressed the phenotype of hand grip strength deficit in CG/CVG, leaving it one of the more poorly understood outcomes in galactosemia.
Here we explored the phenotype of hand grip strength deficit in CG/CVG by asking 3 specific questions: (1) Does an independent cohort of cases and controls replicate the hand grip strength phenotype first described by Potter and colleagues, and if so, might the growth delay also seen in many young people with CG/CVG at least partially explain the grip strength deficit?, (2) Does adjusting for subject weight also resolve most of the grip strength deficit seen in the original Potter study cohort?, and finally, (3) Do GALT-null rats mimic the hand grip strength deficit seen in CG patients, and if so, does the paw grip strength deficit in GALT-null rats also associate with differences in body mass? Combined, our results confirm the existence of, and offer a potential explanation for, the hand grip strength deficit previously reported in classic galactosemia.
Subjects and Methods
Human Subjects:
Both cases and controls presented in Supplemental Table 1 of this study, referred to here as the Galactosemia Foundation Conference 2022 (GFC2022) cohort, were recruited from among participants at the 2022 Galactosemia Foundation Conference in Orlando, Florida (July 28-30, 2022). Cases were children and teens ages 4 to 18 years with a diagnosis of classic or clinical variant galactosemia (CG/CVG), and controls were the unaffected siblings of cases in the same age range who were also in attendance at the conference. We did not exclude any cases or controls from this cohort who were in the appropriate age range (4-18y), present at the conference, and agreed to participate. All participants completed informed consent to enroll in Emory protocol IRB00024933 (PI: JL Fridovich-Keil) prior to participating. Table 1 presents demographic information about the GFC2022 cohort. More details and raw data are presented in Supplemental Table 1, including GALT genotype, if known, and whether or not the combination of alleles in a given case is predicted to have clearly detectable residual GALT activity (>0.4%), if known. The cut-off of 0.4% predicted residual GALT activity is one we have used previously, finding that patients with >0.4% predicted residual GALT activity show milder ovarian and scholastic outcomes than their counterparts with essentially no detectable residual GALT activity (<0.4%) (Ryan et al 2013, Spencer et al 2013). Of note, where a patient carried a very rare allele of GALT that might be considered identifying, for privacy we referred to that allele here as “G”.
Table 1:
Demographic characteristics of participants from the GFC2022 and Potter 2013 cohorts described here.
| Demographic Category | GFC2022 Cases (n=36) | GFC2022 Controls (n=19) | Potter 2013 Cases (n=18) | Potter 2013 Controls (n=92) |
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Female | 22 (61%) | 10 (53%) | 7 (39%) | 36 (39%) |
| Male | 14 (39%) | 9 (47%) | 11 (61%) | 56 (61%) |
|
| ||||
| Age: mean (range) | 11 (4-18y) | 11 (4-17y) | 9.14 (4-16y) | 10.1 (4-16y) |
|
| ||||
| Race/Ethnicity | ||||
| White Not Hispanic/Latino | 29 (81%) | 17 (89%) | 14 (78%) | 87 (95%) |
| Black Not Hispanic/Latino | 1 (3%) | 0 (0%) | 1 (6%) | 0 (0%) |
| White Hispanic/Latino | 0 (0%) | 0 (0%) | 3 (17%) | 2 (2%) |
| Asian Not Hispanic/Latino | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Asian Hispanic/Latino | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nat Amer Not Hispanic/Latino | 1 (3%) | 0 (0%) | 0 (0%) | 2 (2%) |
| Mixed Race Not Hispanic/Latino | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Unknown | 3 (8%) | 2 (11%) | 0 (0%) | 0 (0%) |
Once enrolled, each participant (case or control) was weighed using a digital scale and assessed for peak hand grip strength, reported in pounds of force, using the Jamar Hydraulic Hand Dynamometer (Part# 081028935, Serial# 20210511565). All assessments were conducted by the same study team member in the same study room with cases and controls interspersed. Each participant was given the same instructions, specifically to sit comfortably in the chair provided, place both feet flat on the ground, with knees facing forward, feet positioned directly below the knees. Participants were instructed to drop their “test” arm down and bend it at the elbow so the hand to be tested was pointing straight forward. The tester modeled the correct body and hand positions, as needed, and adjusted the grip dimensions of the Jamar instrument to match the size of the participant’s hand. Parents were allowed to sit or stand next to their child, but not touch the child or the testing device during the assessment.
Participants were first handed the Jamar device in their right hand and, when they were ready, instructed to squeeze the grip as hard as they could while the tester timed 3 seconds. At the 3 second mark, the participant released the grip and rested for 15 seconds while the tester recorded the result from that trial. The tester then switched the device to the participant’s left hand for the next trial. A complete test set included 3 cycles with alternating hands, for a total of 6 trials interspersed with short rest periods. In accordance with prior studies of hand grip strength (Häger-Ross and Rösblad 2002), when analyzing data, we used only the peak grip strength recorded for each participant.
Revisiting data from the Potter 2013 cohort:
Participants in the Potter 2013 study were recruited and tested as described previously (Potter et al 2013). Specifically, grip strength was measured using an Iowa Oral Performance Instrument (IOPI 2005) fitted with an air-and-silicone-filled hand bulb so that hand grip strength was reported in kilopascals (kPa). Weight was obtained with a digital scale. For the purposes of comparison with our GFC2022 cohort, any 2013 participants for whom either peak grip strength or weight were not available were excluded, and any controls outside the age range of cases were also excluded. Finally, we excluded any participant whose weight fell either below the 5th percentile or above the 95th percentile of weight for age and sex, as defined by the CDC clinical growth charts (CDC 2022). This cutoff excluded 18 controls and no cases from the 2013 cohort, and no controls or cases from the GFC2022 cohort. Table 1 presents demographic information about the Potter 2013 cohort. Further details are presented in Supplemental Table 2. We did not have GALT genotypes, predicted residual GALT activity levels, or heights for participants in this cohort. Further, because cases in the 2013 cohort were described as having CG, and not CG/CVG (Potter et al 2013), that is how we also refer to them here.
Rat studies:
All rat studies were conducted in Division of Animal Resources space at Emory University with prior approval of the Emory Institutional Animal Care and Use Committee (IACUC) under protocol PROTO201700095 (PI: JL Fridovich-Keil). Rat mass was measured in grams using an Etekcity scale model EK4150. Paw grip strength was measured initially using a custom device and later using a Columbus Instruments Grip Strength Meter (950 North Hague Ave, Columbus, OH 43204). In accordance with prior studies of grip strength in rats (Conte 2017, Maurissen et al 2003), when analyzing rat data for this study we used average rather than peak grip strength recorded for each animal.
Our custom grip strength meter included a 10 x 11.5-inch grid supported on roller ball bearings and attached to a newton meter spring scale at one end. Once the rat gripped the grid with all 4 paws, the tester gently pulled back from the base of the rat’s tail, applying increasing force until the rat’s hold on the grid began to slip, at which point maximum force on the spring scale was recorded and the tester released their grip on the rat. This test was repeated 4 separate times for each rat, spaced approximately 24 hours apart.
We also used a Columbus Instruments Grip Strength Meter to measure rat front paw grip strength as described by the manufacturer (Columbus Instruments, manual 0167-007M) with minor modifications. Specifically, we coated the metal grid with a thin layer of black “Plasti Dip” to protect the rat from minor sharp imperfections in the grid, and we mounted the grid with the horizontal bars facing down, so that when the rat’s paw would start to slip its claws would not catch on other horizontal bars. Key differences in testing with the Columbus Instruments device versus the custom device were (i) the rat was only able to grip the Columbus Instruments’ grid with its 2 front paws, not all 4 paws simultaneously, and (ii) although each rat tested on the Columbus Instruments device was also tested a total of 4 times, these trials were spaced across 2 rather than 4 days, with 2 sequential tests on each day separated by at least three hours. Details about the rats and their grip strength measurements are presented in Supplemental Table 3.
Data analysis:
Cross-sectional peak grip strength data from cases and controls in both the GFC2022 and Potter 2013 cohorts were graphed as scatterplots, first with age and then with weight on the x-axis, and with the participant’s peak hand grip strength on the y-axis (Figure 1). We added lines of best fit (R2 for each line presented on each graph) and shaded 95% confidence intervals for each group. To test the significance of differences between cases and controls in each graph, we applied a linear mixed effects model adjusting for potential covariates, with fixed covariates of age and then weight, and with patient ID as a random covariate in each model.
Figure 1: Grip strength of cases and controls in the GFC2022 and Potter 2013 cohorts by age and weight.
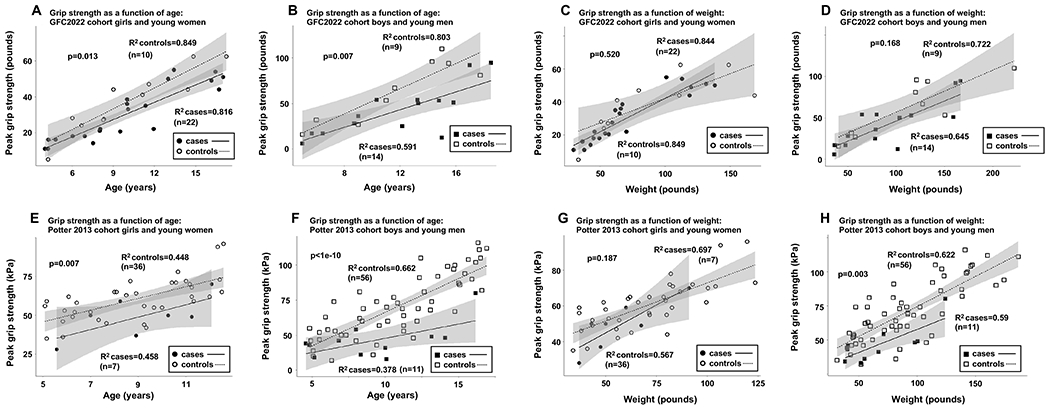
(A) Girls and young women with CG in the GFC2022 cohort showed significantly diminished peak hand grip strength as a function of age relative to controls. (B) Boys and young men with CG in the GFC2022 cohort showed significantly diminished peak hand grip strength as a function of age relative to controls. (C) Peak hand grip strength as a function of weight was not statistically lower in girls and young women with CG in the GFC2022 cohort compared to controls. (D) Peak hand grip strength as a function of weight was not statistically lower in boys and young men with CG in the GFC2022 cohort compared to controls. (E) Girls and young women with CG in the Potter 2013 cohort showed significantly diminished peak hand grip strength as a function of age relative to controls. (F) Boys and young men with CG in the Potter 2013 cohort showed significantly diminished peak hand grip strength as a function of age relative to controls. (G) Peak hand grip strength as a function of weight was not statistically lower in girls and young women with CG in the Potter 2013 cohort compared to controls. (H) Peak hand grip strength as a function of weight remained statistically lower in boys and young men with CG in the Potter 2013 cohort compared to controls. P-values were calculated using non-linear mixed effects models. In panels A, B, E, and F age was a fixed covariate in the model, and in panels C, D, G, and H weight was a fixed covariate in the model. In all analyses, an individual’s ID number was included as a random variable.
The results of grip strength tests using a GALT-null rat model of CG at ages 1-month and 4-months were visualized using box-and-whisker plots with grip strength plotted either directly or normalized for rat body mass on the final day of testing (Figure 2). The significance of cross-sectional comparisons among same-sex, same-age groups of animals, stratified by genotype, was tested using parametric t-tests if the data to be compared passed the Shapiro-Wilk test for normality, and the Wilcoxon rank-sum test if they did not or were too sparse to test for normality, or appeared clearly skewed. When assessing whether mass could account for the grip strength differences seen between comparison groups, we compared as the response variable the average of a rat’s grip strength divided by its mass on the final day of testing.
Figure 2: Grip strength deficit in GALT-null rats correlates with decreased body mass.
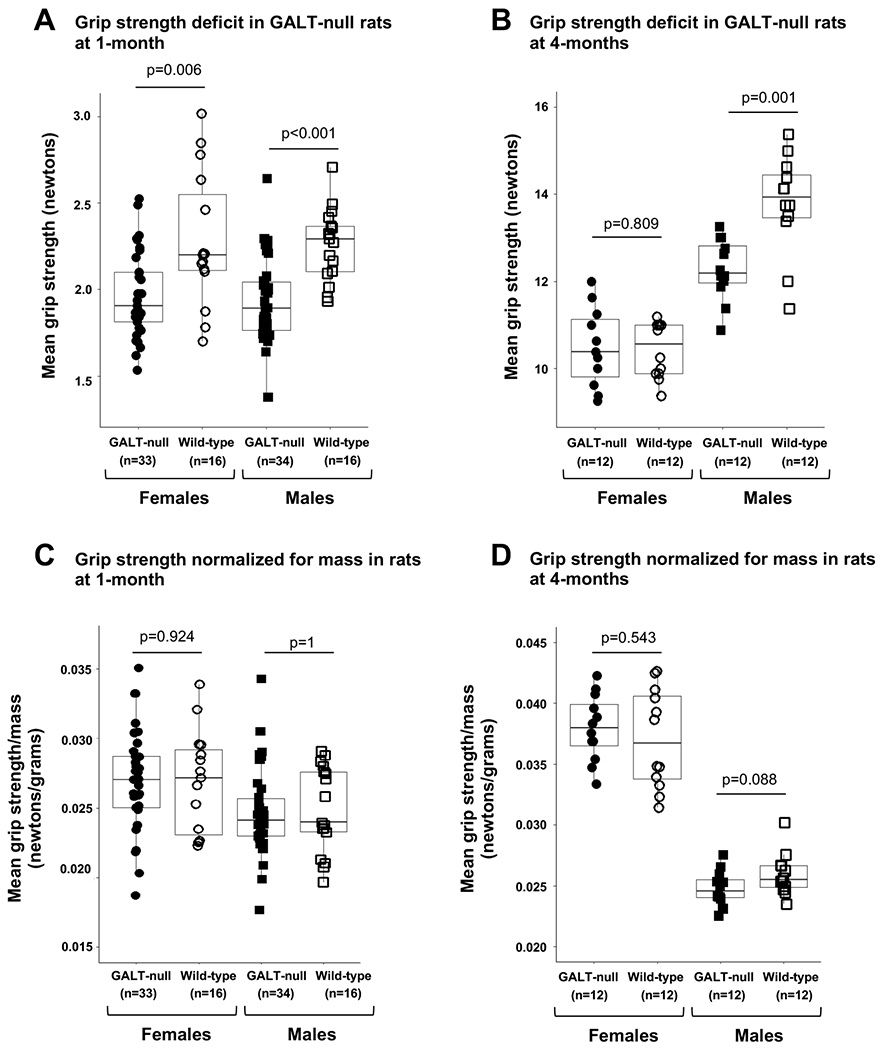
(A) Both male and female GALT-null rats show significantly diminished mean front paw grip strength at 1-month of age when both sexes show growth delay (Supplemental Figure 1, panel E). (B) At 4-months of age, when male but not female GALT-null rats show continued growth delay (Supplemental Figure 1, panel F), only male GALT-null rats show significantly diminished mean front paw grip strength. (C) Normalizing mean front paw grip strength by body mass eliminates any apparent grip strength deficit in 1-month old GALT-null rats. (D) Normalizing mean front paw grip strength by body mass renders grip strength in 4-month-old GALT-null and control rats no longer statistically different. P-values in panels A and C were calculated using Wilcoxon rank sum tests, and in panels B and D, p-values were calculated using t- tests.
Comparisons of the heights and weights of same-sex groups of cases versus controls in the GFC2022 cohort were graphed as scatterplots (Supplemental Figure 1, panels A-D) with age on the x-axis and the participant’s height (panels A and C) or weight (panels B and D) on the y-axis. We added lines of best fit (R2 for each line presented on each graph) for each group. To test the significance of differences between cases and controls in each graph, we applied a linear mixed effects model with age as a fixed covariate and an individual’s ID as a random covariate.
Comparisons of mass across same-sex, age-matched groups of rats, stratified by genotype, were graphed as box-and-whisker plots (Supplemental Figure 1, panels E-F) and tested for significance using parametric t-tests if the data to be compared passed the Shapiro-Wilk test for normality, and the non-parametric Wilcoxon rank-sum test if they did not, or if they appeared clearly skewed.
Results
An independent cohort confirms that young people with CG/CVG show a deficit in hand grip strength that associates with body weight.
Our motivation for repeating hand grip strength measurements in an independent cohort of CG/CVG cases and controls was to test generalizability of the result originally reported in 2013 by Potter and colleagues (Potter et al 2013). Specifically, we were worried about 2 potential confounding factors in the original study. First, all cases in the 2013 cohort also demonstrated speech difficulties, which though common among CG/CVG patients, are not universal, meaning there was a potential selection bias in the cohort. Second, cases and controls in the Potter 2013 study were matched by age and sex, but not by other factors and were recruited separately and tested under different conditions. Specifically, cases lived in 17 different states and were tested individually during home visits. Controls were recruited from preschools and public schools in Washington State and tested in a school setting (Potter et al 2013).
To avoid these potential confounders, cases in our GFC2022 cohort included both CG/CVG patients with and without reported speech problems, and the controls in our study were unaffected siblings from the same set of families. All cases and controls in our cohort were tested over a 3-day period in the same study room at the 2022 Galactosemia Foundation Conference in Orlando, FL, with cases and controls interspersed. That cases in our cohort also showed a significant grip strength deficit relative to controls (Figure 1 panels A and B) therefore supports the conclusion that the Potter 2013 result is generalizable and not an artifact of confounding factors.
Next, motivated by the knowledge that growth delay is a common finding among children with CG/CVG (Panis et al 2007) and seeing that the children with CG/CVG in our GFC2022 cohort did tend to be smaller than their unaffected counterparts (Supplemental Figure 1, panels A-D) we tested whether plotting hand grip strength as a function of participant weight, rather than age, would close the gap in grip strength between cases and controls; it did (Figure 1, panels C and D). This result suggests that the grip strength deficit seen among young people with CG/CVG may be secondary to growth delay, and not due to abnormal muscle weakness.
Re-evaluation of data from the Potter 2013 cohort.
To test whether growth delay in CG/CVG might also account for the hand grip strength deficit seen in the 2013 Potter cohort, we replotted data collected from that cohort first as a function of participant age, and then as a function of participant weight (Figure 1, panels E-H). As previously reported (Potter et al 2013), when the data were plotted as a function of age, cases of both sexes showed significantly decreased hand grip strength relative to controls (Figure 1, panels E and F). When grip strength was plotted as a function of weight, however, girls and young women with CG no longer showed a significant hand grip strength deficit (Figure 1, panel G). In contrast, boys and young men with CG continued to show a deficit (Figure 1, panel H).
A grip strength deficit in GALT-null rats.
Finally, we tested grip strength in a GALT-null rat model of CG versus controls, testing animals at both 1- and 4-months. To be clear, rats at 1-month are post-wean but still pre-pubertal, and both GALT-null male and female rats show a significant growth delay at this age (Supplemental Figure 1, panel E). At 4-months, rats are adults and while GALT-null males continue to show a significant growth delay relative to their wild-type counterparts, GALT-null females have closed the size gap (Supplemental Figure 1, panel F).
We tested grip strength in our GALT-null and control rats using each of two devices, one custom-made that allowed the rat to grip a large grid with all 4 paws, and the other a commercial Grip Strength Meter purchased from Columbus Instruments (Serial# 220070-KP-01) that allowed the rat to grip a small grid with just its front paws. Grip strength data from rats tested using both instruments (Supplemental Figure 2, panel A) confirmed correlation of the results. Further, grip strength data collected from the same rats tested on the same (custom) device at different adult ages (4- and 6-months) confirmed reproducibility of the results over time (Supplemental Figure 2, panel B).
As in young patients, we observed a clear grip strength deficit in GALT null male and female rats tested at age 1-month (Figure 2, panel A). At 4-months, GALT-null female rats no longer showed a grip strength deficit, but GALT-null males did (Figure 2, panel B). Adjusting grip strength by mass of the rat on the last day of testing resolved all apparent grip strength deficits (Figure 2, panels C and D). Combined, these results in GALT-null rats replicated what we saw in our GFC2022 cohort of CG/CVG patients and controls.
Discussion
The results of this study demonstrate 2 key points. First, both young patients with CG/CVG and young GALT-null rats exhibit weaker hand grip strength than age- and sex-matched controls. Second, body size, measured as weight in humans, and as mass in rats, negates the grip strength deficit seen in most but not all cohorts.
This was an important study to complete because the initial Potter 2013 report of diminished hand grip strength in children and teens with CG raised the troubling possibility of a generalized muscle weakness phenotype not otherwise appreciated in CG/CVG. That smaller body size largely accounted for the diminished grip strength seen in both sexes of CG/CVG patients in our GFC2022 cohort reported here, and in girls and young women from the Potter 2013 cohort, and in both sexes of GALT-null rats reported here, strongly suggests that diminished grip strength in GALT deficiency is secondary to growth delay, meaning smaller muscles, not abnormally weak muscles.
One point we observed but cannot fully explain is why adjusting for weight eliminated the apparent grip strength deficit among cases of both sexes in our GFC2022 cohort, but only among girls and young women, but not boys and young men, in the Potter 2013 cohort. We suspect a mismatch between how cases and controls were tested in the 2013 study may underlie the disparity. Specifically, because controls in the 2013 cohort were tested in a school setting, whereas cases were tested individually at home, a sense of competition among boys tested at school, that was not present for the boys tested individually at home, may have motivated some controls to squeeze the grip meter harder. That we did not see the same pattern among girls in the 2013 cohort may be explained by prior studies that suggest girls in school may be less impacted than boys by competitive pressure relating to physical displays of strength (Soares et al 2013). All cases and controls from the GFC2022 cohort were tested in the same setting – a study room at the 2022 Galactosemia Foundation Conference.
Strengths and limitations:
Strengths of this study included matching of cases and controls in our GFC2022 cohort across multiple factors, replication of results in 2 independent patient cohorts recruited 9 years apart (2013 and 2022), and replication in a GALT-null rat model of CG. Limitations included relatively small cohort sizes and potential bias in the case and control cohorts because of how and where participants were recruited and tested. For example, we are aware that the racial and ethnic distribution of cases in our study did not fully reflect the racial and ethnic distribution of CG/CVG patients across the US (Stettner et al 2023). Another limitation is that total body weight is not a perfect proxy for muscle mass, and grip strength may vary among people for a variety of reasons – not only body size.
Conclusions
Young people with classic or clinical variant galactosemia may demonstrate a deficit in hand grip strength, but this outcome is likely secondary to growth delay and not evidence of a muscle abnormality.
Supplementary Material
Supplemental Table 1: Raw data for study participants in the GFC2022 cohort.
Supplemental Figure 2: Comparison of results collected using a custom grip strength meter and a commercial grip strength meter. (A) Correlation of rat mean grip strength measured using a custom device versus a grip strength meter purchased from Columbus Instruments. Gray shading indicates the 95% confidence interval. (B) Correlation of rat mean grip strength measured longitudinally using a custom device in rats at age 4-months and again at 6-months. Gray shading indicates the 95% confidence interval.
Supplemental Figure 1: Growth delay in patients with CG/CVG and in GALT-null rats. (A, B) Girls and young women with CG/CVG in the GFC2022 cohort show diminished weight relative to controls. (C, D) Boys and young men with CG/CVG in the GFC2022 cohort show diminished height and weight relative to controls. Even where not statistically significant in this small cohort, these results were consistent with prior reports (Panis et al 2007). (E) At age 1-month, both male and female GALT-null rats are significantly smaller than their wild-type counterparts. (F) At age 4-months, male but not female GALT-null rats are significantly smaller than their wild-type counterparts.
Supplemental Table 2: Raw data for study participants in the Potter 2013 cohort.
Supplemental Table 3: Raw data concerning rats included in this study.
1-sentence take-home message:
The grip strength deficiency apparent in young people with classic or clinical variant galactosemia, and in a GALT-null rat model of classic galactosemia, associates with delayed growth in most cohorts and therefore may not be evidence of a muscle abnormality.
Acknowledgments
We are especially grateful to the many young people, with and without galactosemia, who participated in this study at the 2022 Galactosemia Foundation Conference. Without their involvement, this study would not have been possible. We also thank Dr. Shauna Rasmussen for her hard work maintaining the rat colony, and Nathan Paull for contributions in the early stages of this project. Finally, we gratefully acknowledge funding from NIH R21HD092785 (to JLFK) that supported early work on this project, and funding from Jaguar Gene Therapy, LLC (to JLFK) that also supported some of the rat studies.
Funding:
We gratefully acknowledge funding from NIH R21HD092785 (to JLFK) that supported early work on this project, and funding from Jaguar Gene Therapy, LLC (to JLFK) that also supported some of the rat studies described here.
Footnotes
Conflict of Interest
Jared Druss, Josephine Rudd Zhong Manis, Nancy Potter, and Judith Fridovich-Keil all declare that they have no competing interests with this work (ICMJE forms from all co-authors uploaded).
Ethics Approval
The human subjects studies described here were conducted following approval of the Emory University Institutional Review Board (Emory IRB protocol 00024933, PI: JL Fridovich-Keil).
Informed Consent
All volunteers who participated in this study did so only after appropriate informed consent under Emory IRB protocol 00024933 (GFC 2022 cohort, PI: JL Fridovich-Keil). Participants from the Potter 2013 cohort, whose de-identified data were used here, were consented at the time of the 2013 study under Washington State University protocol 0009003 (PI: NL Potter).
Animal Studies:
All animal work described here was conducted with approval of the Emory University Institutional Animal Care and Use Committee (IACUC; Protocol PROTO201700095 (PI: JL Fridovich-Keil).
Data Sharing Statement:
All data analyzed for this study are included in Supplemental Tables 1–3.
References
- Berry G (2020) Classic Galactosemia and Clinical Variant Galactosemia. In Adam ED M P, Mirzaa GM, et al. , eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, https://www.ncbi.nlm.nih.gov/books/NBK1518/. [PubMed] [Google Scholar]
- Conte E, Camerino GM, Mele A, De Bellis M, Pierno S, et al. (2017) Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia. Journal of Cachexia, Sarcopenia and Muscle 8: 386–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hӓger-Ross C, Rösblad B (2002) Norms for grip strength in children aged 4-16 years. Acta Pædiatrica 91: 617–625. [DOI] [PubMed] [Google Scholar]
- Maurissen JP, Marable BR, Andrus AK, Stebbins KE (2003) Factors affecting grip strength testing. Neurotoxicol Teratol 25: 543–53. [DOI] [PubMed] [Google Scholar]
- Panis B, Gerver WJ, Rubio-Gozalbo ME (2007) Growth in treated classical galactosemia patients. Eur J Pediatr 166: 443–6. [DOI] [PubMed] [Google Scholar]
- Potter NL, Nievergelt Y, Shriberg LD (2013) Motor and speech disorders in classic galactosemia. JIMD Rep 11: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Agusti I, Carecchio M, Bhatia KP et al. (2013) Movement disorders in adult patients with classical galactosemia. Mov Disord 28: 804–10. [DOI] [PubMed] [Google Scholar]
- Rubio-Gozalbo ME, Haskovic M, Bosch AM et al. (2019) The natural history of classic galactosemia: lessons from the GalNet registry. Orphanet J Rare Dis 14: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EL, Lynch ME, Taddeo E, Gleason TJ, Epstein MP, Fridovich-Keil JL (2013) Cryptic residual GALT activity is a potential modifier of scholastic outcome in school age children with classic galactosemia. J Inherit Metab Dis 36: 1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Antunnes H, Van Den Tillaar R (2013) A comparison between boys and girls about the motives for the participation in school sport. Journal of Physical Education and Sport 13: 303–307. [Google Scholar]
- Spencer JB, Badik JR, Ryan EL et al. (2013) Modifiers of ovarian function in girls and women with classic galactosemia. J Clin Endocrinol Metab 98: E1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner NM, Cutler DJ, Fridovich-Keil JL (2023) Racial and ethnic diversity of classic and clinical variant galactosemia in the United States. Mol Genet Metab 138: 107542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling L, Bernstein LE, Berry GT et al. (2017) International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. J Inherit Metab Dis 40: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Raw data for study participants in the GFC2022 cohort.
Supplemental Figure 2: Comparison of results collected using a custom grip strength meter and a commercial grip strength meter. (A) Correlation of rat mean grip strength measured using a custom device versus a grip strength meter purchased from Columbus Instruments. Gray shading indicates the 95% confidence interval. (B) Correlation of rat mean grip strength measured longitudinally using a custom device in rats at age 4-months and again at 6-months. Gray shading indicates the 95% confidence interval.
Supplemental Figure 1: Growth delay in patients with CG/CVG and in GALT-null rats. (A, B) Girls and young women with CG/CVG in the GFC2022 cohort show diminished weight relative to controls. (C, D) Boys and young men with CG/CVG in the GFC2022 cohort show diminished height and weight relative to controls. Even where not statistically significant in this small cohort, these results were consistent with prior reports (Panis et al 2007). (E) At age 1-month, both male and female GALT-null rats are significantly smaller than their wild-type counterparts. (F) At age 4-months, male but not female GALT-null rats are significantly smaller than their wild-type counterparts.
Supplemental Table 2: Raw data for study participants in the Potter 2013 cohort.
Supplemental Table 3: Raw data concerning rats included in this study.
Data Availability Statement
All data analyzed for this study are included in Supplemental Tables 1–3.


