Abstract
Growing evidence suggests that maternal experiences of stress shape children’s functional brain activity in the first years of life. Individuals living in poverty are more likely to experience stress from a variety of sources. However, it is unclear how stress is related to resting brain activity among children born into poverty. The present study examines whether infants born into households experiencing poverty show differences in brain activity associated with maternal reports of experiencing stress. The analytic sample comprised 247 mother-infant dyads who completed maternal questionnaires characterizing stress, and for whom recordings of infant resting brain activity were obtained at 1 year of age (M=12.93 months, SD=1.66; 50% female). Mothers (40% Black, non-Hispanic, 40% Hispanic, 12% White, non-Hispanic) who reported higher stress had infants who showed more resting brain activity in the lower end of the frequency spectrum (relative theta power) and less resting brain activity in the middle range of the frequency spectrum (relative alpha power). While statistically detectable at the whole-brain level, follow-up exploratory analyses revealed that these effects were most apparent in electrodes over frontal and parietal regions of the brain. These findings held after adjusting for a variety of potentially confounding variables. Altogether, the present study suggests that, among families experiencing low economic resources, maternal reports of stress are associated with differences in patterns of infant resting brain activity during the first year of life.
Keywords: Poverty, Maternal Stress, EEG, Resting Brain Activity, Theta, Alpha, Beta, Gamma, SES
Introduction
Growing evidence suggests that high levels of maternal stress shape resting functional brain activity in the first years of life (Brandes-Aitken et al., 2023; Pierce et al., 2019; Troller-Renfree et al., 2020). While the type and degree of stressor that mothers experience can vary widely, there may be similarities in the downstream influence on child development. Examples of stressors include neighborhood violence, chaos in the home, parenting a new child, and/or limited availability of economic resources – all of which may have different psychological impacts on an individual. In addition, individual differences exist in how people perceive and respond to different stressors. Mothers living in poverty are more likely to experience stress from a variety of sources as compared with individuals who have economic advantage (Algren et al., 2018; Attar et al., 2010; Blair & Raver, 2016; Evans, 2004; Evans & English, 2002; Evans & Kim, 2010; Hackman et al., 2010; McLoyd, 1990; Senn et al., 2014). Together, both the presence of stressors as well as the psychological or cognitive appraisals of stressors likely contribute to overall maternal stress. In the present manuscript, we will discuss and investigate stress broadly, using a multivariate approach that encompasses both the objective presence of stressors as well as the subjective appraisals of stress (see S1 for further discussion). Theory suggests that experiences of stress likely shape the brain development of children born into poverty (Brito & Noble, 2014). However, to date, there have been no large-scale investigations of how maternal stress relates to resting brain activity during very early childhood for infants living in poverty. Understanding how maternal stress shapes children’s brains early in life is of particular interest, since early patterns of resting brain activity have been shown to predict later language (Benasich et al., 2008; Gou et al., 2011), cognitive (Brito et al., 2016; Corning et al., 1986; Williams et al., 2012), and socioemotional functioning (Brito et al., 2019).
To measure resting functional brain activity early in life, electroencephalography (EEG) is commonly employed (Marshall et al., 2002). EEG is a direct, non-invasive measure of electrical brain activity, which is recorded by placing small recording devices called electrodes on the scalp. Resting EEG measures brain activity while the brain is not executing any particular task of interest. When examining resting EEG, researchers typically consider two measures: frequency and power. “Frequency” refers to the oscillatory speed of brain activity, and is reported in Hz (e.g., 3 Hz is a signal that oscillates three times a second). When reporting resting EEG, individual frequencies are typically divided into bands. Some of these bands represent lower-frequency (slower) oscillations (e.g., the “theta” band), and some represent higher-frequency (faster) brain activity (e.g., the “alpha,” “beta,” and “gamma” bands). All individuals exhibit brain activity in each frequency band. “Power” refers to the amount or amplitude of brain activity in a particular frequency band. More EEG power reflects that the brain is generating more electrical activity. “Absolute power” refers to the amount of brain activity (power) within a particular frequency band. “Relative power” expresses absolute power within a band as a fraction of power summed across all frequency bands.
Resting EEG bands are, by convention, denoted by Greek letters, and reflect brain oscillations in specific ranges. In infancy, oscillations between approximately 3 and 5 Hz encompass the theta band, 6 and 8 Hz encompass the alpha band, 13 and 19 Hz encompass the beta band, and oscillations beginning at 21 Hz and extending to higher frequencies encompass the gamma band (Tomalski et al., 2013; Troller-Renfree et al., 2020; Troller-Renfree, Costanzo, et al., 2022). Importantly, these bands are not uniformly defined across all research studies (Anderson & Perone, 2018). Power in EEG bands can be measured across the whole scalp (e.g., whole-brain theta) or over regions of the scalp (e.g., frontal theta). Resting EEG bands are thought to be functionally distinct, and regional oscillations are thought to further refine the functional definition within a band.
Developmental electrophysiology research has associated EEG power with a variety of cognitive and social outcomes, in an effort to understand what resting EEG power means for long-term development. To briefly review band-related functional definitions, a few studies have linked infant resting theta activity to expressions of emotion, feeding, and attention (for review, see Saby & Marshall, 2012). Higher theta power, particularly when coupled with lower power in the higher-frequency beta band, has been linked to ADHD, risk-taking behaviors, less inhibition in response to fearful faces, and poorer top-down control (for review Anderson & Perone, 2018). In contrast, higher resting alpha power in infancy, particularly in frontal regions, has been linked with better executive functioning (Kraybill & Bell, 2013). Higher resting beta activity in frontal, temporal, and parietal regions has been related to higher socioemotional functioning (Brito et al., 2019). Higher frontal beta power has also been related to higher cognitive skills (Williams et al., 2012). Higher resting gamma activity in frontal, temporal, and parietal regions has been related to higher socioemotional functioning (Brito et al., 2019). Higher frontal gamma power has also been associated with higher scores on tests of cognition (Benasich et al., 2008; Brito et al., 2016) and language (Benasich et al., 2008; Gou et al., 2011). Higher parietal gamma power has also been associated with higher language scores (Brito et al., 2016). However, it is important to note that resting EEG bands in infancy can vary in their boundaries from study to study, and the specificity of regional power is not well understood.
Among typically developing children, studies using electroencephalography (EEG) have shown that the relative contribution of lower-frequency (e.g., theta) resting EEG power decreases with age, whereas the relative contribution of higher-frequency resting EEG power (e.g., alpha, beta, gamma) increases with age (Anderson & Perone, 2018; Clarke et al., 2001; Marshall et al., 2002). A growing body of research suggests that early-life environmental and maternal stress may alter the distribution of lower- and higher-frequency resting brain activity. Indeed, exposure to early adversity, such as poverty or psychosocial deprivation (e.g., institutional rearing or neglect), has been linked to differences in resting brain activity, compared to individuals without such exposures (Debnath, Tang, et al., 2020; Marshall et al., 2004; Otero et al., 2003; Tomalski et al., 2013; Vanderwert et al., 2010). Accumulating evidence also suggests that maternal stress – both perceived and physiological – is associated with differences in infants’ resting brain activity (Brandes-Aitken et al., 2023; Pierce et al., 2019; Troller-Renfree et al., 2020). Across these studies, a pattern is emerging whereby children who experience early life stress (indexed by either the objective presence of a stressor in the environment or by maternal report of perceived stress) tend to exhibit more lower-frequency (e.g., theta) brain power and less higher-frequency (e.g., alpha, beta, and gamma) power, compared to their peers raised in lower-stress environments (though see Jensen et al., 2021 for findings that are inconsistent with this pattern).
Understanding the impact of maternal stress on the developing brain in the first years of life is essential for several reasons. First, as the brain grows and changes most rapidly in the first few years of life, identifying the experiences that shape the brain during this period is integral for understanding how to support long-term child development (for review Sakai, 2020). Second, evidence suggests that adversity-related changes in patterns of EEG activity can persist throughout childhood into adolescence (Debnath, Tang, et al., 2020; Otero, 1994, 1997; Otero et al., 2003; Vanderwert et al., 2010). Third, differences in early patterns of resting brain activity predict later neurocognitive functioning, perhaps suggesting a mechanism by which stress impacts later language (Benasich et al., 2008; Gou et al., 2011), cognitive skills (Brito et al., 2016; Corning et al., 1986; Williams et al., 2012), and socioemotional functioning (Brito et al., 2019). Finally, emerging evidence suggests that interventions designed to reduce early adversity may change patterns of resting brain activity (Debnath, Tang, et al., 2020; Troller-Renfree, Costanzo, et al., 2022). A better understanding of how stress shapes brain development may therefore have implications for the development of prevention and intervention strategies.
The present study investigates whether families with limited economic resources show associations between maternal stress and infant resting brain activity. Specifically, we examined how maternal reports of stress are associated with infant EEG power in four frequency bands – theta, alpha, beta, and gamma power. We hypothesized that infants of mothers who reported higher experiences of stress would show proportionally more lower-frequency power (i.e., relative theta) and proportionally less mid-to-high-frequency power (i.e., relative alpha, beta, and gamma) when compared to infants of mothers who reported lower levels of stress (See EEG Collection and Processing for more information on absolute vs. relative power, and see Supplement S2 for investigation of absolute power). We also conduct exploratory analyses examining whether associations between maternal stress and resting brain activity are stronger over certain regions of the scalp (frontal, central, parietal, occipital). Finally, in supplemental analyses, we examined whether maternal physiological stress (measured via maternal hair cortisol collection) relates to infant brain function (See Supplement S3).
Methods
Participants
Participants were drawn from the 600 mother-infant dyads in the comparison group of Baby’s First Years, the first randomized control trial of poverty reduction in early childhood in the United States (see Table 1 for comparison group characteristics). Briefly, in the larger study, mothers were recruited in hospital postpartum wards in four U.S. metropolitan areas (New York City, the greater New Orleans metropolitan area, the greater Omaha metropolitan area, and the Twin Cities of Minneapolis and St. Paul). Shortly after giving birth, mothers were randomized to receive a monthly unconditional cash gift of either $333/month (“high-cash gift group,” i.e., the intervention group) or $20/month (“low-cash gift group,” i.e., the comparison group) for the first several years of their children’s lives. (For more information concerning the larger study design, see www.babysfirstyears.com and Noble et al., 2021).
Table 1.
Comparison group descriptive statistics at baseline data collection for EEG subsample (N=251).
| Categorical | Percentage | N |
|---|---|---|
| Child is female | 50% | N/A |
| Mother education | ||
| Less than high school | 23% | N/A |
| High school | 49% | N/A |
| Some college | 20% | N/A |
| Associates | 3% | N/A |
| Bachelors | 5% | N/A |
| Unknown | 0.4% | N/A |
| Mother race/ethnicity | ||
| White, non-Hispanic | 12% | N/A |
| Black, non-Hispanic | 39% | N/A |
| Multiple, non-Hispanic | 6% | N/A |
| Other | 4% | N/A |
| Hispanic | 40% | N/A |
| Continuous | Mean | SD |
| Child weight at birth (pounds) | 7.07 | 1.03 |
| Mother age at birth (years) | 26.85 | 6.06 |
| Child gestational age (weeks) | 39.06 | 1.33 |
To be eligible, mothers’ self-reported income in the prior calendar year had to fall below the federal poverty threshold for their family size. Additional study inclusion criteria were: (1) the mother was of legal age for informed consent (age 18 or older in NY, MN, and LA; 19 or older in NE); (2) the infant was admitted to the newborn nursery (not the neonatal intensive care unit); (3) the mother was residing in the state of recruitment; (4) the mother indicated that she was not "highly likely" to move to a different state or country in the next 12 months; (5) the infant was discharged in the custody of the mother; and (6) the mother spoke English or Spanish.
Following screening for eligibility, mothers completed a baseline interview and then were informed about the opportunity to receive a cash gift. Following consent to receive the cash gift, families were randomized to the high-cash or low-cash gift group in a 40/60 ratio. Here we limit analyses to mothers in the low-cash gift group, who were randomized to receive the $20 monthly cash gift ($240 annually) for the first several years of their infants’ lives (N=600). As a part of the baseline interview, mothers reported on demographic factors including maternal education, race, ethnicity, and infant sex.
At approximately the time of the infants’ first birthday (Mlow-cash EEG Subsample=12.93 months, SD=1.66), 548 mother-infant dyads completed an age-1 interview (91% response rate; complete survey instruments available at www.babysfirstyears.com). As described below, here we examine associations between age-1 maternal reports of stress and infant resting brain activity in the low-cash gift group. Interviews were initially conducted in-person, with questions read to the participant by an interviewer (n=343). After the onset of the COVID-19 pandemic, data collection was conducted remotely, with surveys administered over the phone (n=205). As remote EEG data collection is not possible, the present investigation is limited to those families who completed in-person data collection. Informed consent was obtained by interviewers for both the baseline and age-1 interviews before data collection began.
Measures of Maternal Stress
Perceived Stress
Perceived maternal stress was assessed using the perceived stress scale (PSS; Cohen et al., 1994; Cohen & Williamson, 1988). The PSS questionnaire assesses the degree to which the respondent has perceived situations as stressful within the last month. Erroneously, one item was omitted from the survey, leaving a total of 9 items drawn from the larger 10-question questionnaire (see Troller-Renfree, Hart, et al., 2022 for items administered). The items were summed, with higher scores indicating greater perceived stress. The measure showed acceptable internal consistency (αEEG Subsample = 0.790). Mothers needed to complete at least six of the nine items for their score to be considered valid.
Household Chaos
Household stress was measured through the Confusion, Hubbub, and Order Scale (CHAOS; Matheny et al., 1995). The CHAOS is designed to measure the order, routine, and disorganization of the home environment. Consistent with past work (e.g., Evans et al., 2005), we added items to increase coverage of routines and rituals in the home such as, “We have a regular morning routine at home,” and “We eat together as a family once a day.” Mothers responded to each item as true or false of their home most of the time. Positively stated items were reverse-coded before being summed, and higher scores indicated greater household chaos. Overall, the CHAOS showed acceptable internal consistency after this recode (αEEG Subsample = 0.724), which is consistent with past studies (Evans et al., 2005). Mothers needed to answer at least 11 items to have a valid CHAOS score.
Parenting Stress
Parenting stress was assessed through a 7-item index, which had been pre-registered as part of the larger Baby’s First Years project. Of the 7 items, three were drawn from the Aggravation in Parenting Scale (PSID-Child Development Supplement), and four were drawn from the Cleminshaw—Guidubaldi Parent Satisfaction Scale (Guidubaldi & Cleminshaw, 2010). The scale included seven statements related to the rewards and stresses of parenting (e.g., “When it comes to raising kids, I have a lot of confidence in my abilities,” “I feel trapped by my responsibilities as a parent”). For each item, mothers indicated the extent to which they agreed or disagreed with the statement using a five-point Likert scale ranging from “strongly disagree” to “strongly agree”. Items were summed, and possible scores ranged from 7 to 35, with higher scores indicating more parenting stress. Mothers needed to answer at least 50% of the items to have a valid score.
Economic Stress
Economic stress was assessed through mothers’ self-report on nine questions (Kling et al., 2007). For seven of the questions, mothers responded “yes” or “no” (e.g., “In the past 12 months, have you ever missed a rent or mortgage payment?”). “Yes” responses were scored as 0, and “no” responses were scored as 1. For one question, mothers also rated the frequency with which they worried about being able to meet monthly living expenses (i.e., “all of the time,” “very frequently,” “occasionally,” “rarely,” “very rarely,” “never”). Responses of “occasionally,” “rarely,” or “very rarely” were scored 0, and responses of “very frequently” or “all of the time” were scored 1. Finally, mothers responded to the question, “In the past 12 months, would you say that your household has spent more, less, or about as much as all of your sources of income combined?” Responses of “more” or “about the same” were scored 1 and “less” was scored 0. A total score was created by summing the scores for each of the nine questions. Higher scores indicated higher economic stress. Mothers needed to complete at least five of the nine questions for their score to be considered valid.
Neighborhood Safety
Mothers responded to two questions about the perceived safety of their neighborhood. Using a Likert scale (0=very unsafe to 3=very safe), mothers responded with their perceived safety of the streets near their home both during the day and at night. These scores were then summed to create one neighborhood safety variable with a range of 0 to 6, with higher scores indicating greater perceived neighborhood safety, MEEG Subsample = 4.339, SD = 1.393. Maternal reports of neighborhood safety have been used in previous research (e.g., Giurgescu et al., 2015), and maternal reports of neighborhood safety have been linked to perceptions of stress (e.g., Henderson et al., 2016).
Maternal Stress Composite
To examine whether our five stress measures (perceived, household, parenting, economic, neighborhood safety) may be reduced into one or more stress factors, the five stress measures were analyzed in an exploratory factor analysis using principal factor analysis with Varimax rotation. This approach is consistent with the approach used previously with this project (Troller-Renfree, Hart, et al., 2022). The analysis yielded a one-factor solution with an Eigenvalue greater than 1 (eigenvalue = 1.506). This factor was labeled ‘Maternal Stress Composite’ due to the loadings by the following items: perceived stress (loading = 0.718), household chaos (loading = 0.565), parenting stress (loading = 0.598), economic stress (loading = 0.397)1, and neighborhood safety (loading = −0.395). This Maternal Stress Composite factor was extracted for further analysis from a total of 247 mothers in the low-cash gift group who were not missing data for any stress measures. Higher scores on the Maternal Stress Composite indicate higher reports of maternal stress.
EEG Collection and Processing
To assess resting brain activity, EEG data were collected using a mobile system in the home. The utility, feasibility, and cultural appropriateness of mobile EEG were evaluated prior to the commencement of data collection through a series of pilot visits and focus groups (see Troller-Renfree et al., 2021 for full details of piloting and interviewer training). Following this piloting process, a team of interviewers was trained to collect in-home EEG.
EEG was recorded using a 20-channel Neuroelectrics cap with an Enobio 20 amplifier (Neuroelectrics, Barcelona, Spain) in families’ homes. However, owing to the COVID-19 pandemic and concerns for participant and interviewer safety, only 343 infants in the low-cash gift group had the opportunity to complete EEG data collection. Of the 343 in-person visits, 326 mother-infant dyads consented to EEG data collection (95%). During the EEG recording, infants sat on their caregivers’ laps while watching infant-friendly wordless videos or observing bubbles or infant toys. Recordings lasted a maximum of 7 minutes with a goal of recording at least 5 minutes of artifact-free data. Data were analyzed off-line by data processors who were blind to participant group.
EEG was analyzed using the EEGLAB toolbox (Delorme & Makeig, 2004), MATLAB (The MathWorks, Natick, MA), and a low-density version of the MADE pipeline (Debnath, Buzzell, et al., 2020) known as the miniMADE pipeline (Troller-Renfree et al., 2021). Data were high-pass filtered at 0.3 Hz and low-pass filtered at 50 Hz. Then, data were segmented into epochs of 1 s with 50% overlap between epochs. Epochs were baseline corrected to the mean voltage of each epoch. To remove ocular artifact, a voltage threshold rejection (+/− 250 μV) was applied to two frontal channels (FP1, FP2). If both frontal electrodes exceeded the voltage threshold of +/− 250 μV in an epoch, that epoch was removed from processing. For the remaining channels, those channels containing artifact in each epoch were identified using three criteria: a voltage threshold (+/− 250 μV), a flat channel threshold (range < 1 microvolt for at least half of the epoch), and a jump channel threshold (increases greater than 50 microvolts from sample to sample). Finally, data were re-referenced to an average of T7 and T8.
Following preprocessing, thresholds were applied to ensure adequate artifact-free data remained for each participant prior to power decomposition. First, consistent with previous studies (e.g., Troller-Renfree et al., 2020), at least 80% (16 out of 20) of electrodes were required to contribute usable data for any given epoch. Second, split-half reliabilities were computed and examined and a cutoff of 20 epochs was selected so that each band had at least good (>.8) split-half reliability (for more information, see Troller-Renfree et al., 2021). Epochs with fewer than 16 artifact-free electrodes and participants with fewer than 20 artifact-free epochs were excluded from further analysis. After data cleaning was completed, the mean number of epochs per participant was 288.2 (SD = 183.7).
A Fast Fourier Transformation (FFT) was applied to the epoched data. Consistent with other infant studies (Tomalski et al., 2013; Troller-Renfree et al., 2020), spectral power (μV2) was computed for the theta (3-5 Hz), alpha (6-9 Hz), beta (13-19 Hz), and gamma frequency ranges (21-45 Hz). Relative power was computed by dividing the absolute power within one frequency band (e.g., theta) by total absolute power from all frequency bands (theta, alpha, beta, and gamma). Analysis code is available at https://github.com/ChildDevLab.
In total, 251 children provided sufficient usable EEG data. Given that differences in the magnitude of absolute power can be due to functionally irrelevant attributes (e.g., skull thickness), relative EEG power is a preferable measure for correlational investigations. Therefore, our hypotheses are formed around relative power and relative power results are reported below. Absolute power results are reported in S2.
Participant Inclusion
The analyses in this paper center around children in the low-cash gift comparison group with valid, usable EEG data (N=251). Of these children, 247 also had valid scores for maternal report of stress measures. As such, our final analyses examined 247 mother-infant dyads.
Analytic Plan and Hypotheses
The present manuscript aims to examine whether maternal stress is associated with infant resting brain activity at the end of the first year of life. Specifically, consistent with prior work (Brandes-Aitken et al., 2023; Pierce et al., 2019; Troller-Renfree et al., 2020), we hypothesized that higher reports of maternal stress would be associated with more lower-frequency power (theta) and less higher-frequency power (alpha, beta, gamma). We expected that these effects would be detectable both at the whole brain and regional level, although we did not have strong hypotheses concerning which regions would maximally show these associations, and thus consider these follow-up analyses exploratory. To investigate these hypotheses, data analysis took place in two steps.
First, the Maternal Stress Composite was entered as the predictor of each of the four whole-brain relative power bands (theta, alpha, beta, gamma). Covariates included maternal education (dummy variables for high school graduate, some college, associate’s degree, bachelor’s degree, and unknown), maternal race and ethnicity (dummy variables for Black, multiple races, race other, and Hispanic), child age, child sex, and number of EEG epochs retained.
Second, the Maternal Stress Composite was entered as a predictor of each of the four relative power bands (theta, alpha, beta, gamma) across four regions of the head (frontal, central, parietal, occipital). As above, maternal education (dummy variables for the achievement of bachelors, some college, associates, high school graduate, and unknown), maternal race and ethnicity (dummy variables for Black, multiple races, other race, and Hispanic), child age, child sex, and number of EEG epochs retained were entered as covariates.
Results
Sample Characteristics
Sample demographics are presented in Table 1. At baseline, mothers self-identified as 12% White, non-Hispanic, 39% Black, non-Hispanic, 6% multiracial, non-Hispanic, and 40% Hispanic. Mothers were an average of 26.9 (SD = 6.1) years old when they gave birth to the target child. 50% of the infants were female. At age 1, the average reported household income was $25,059.
Maternal Stress is Associated with Differences in Whole-brain Resting Activity
Relative Theta
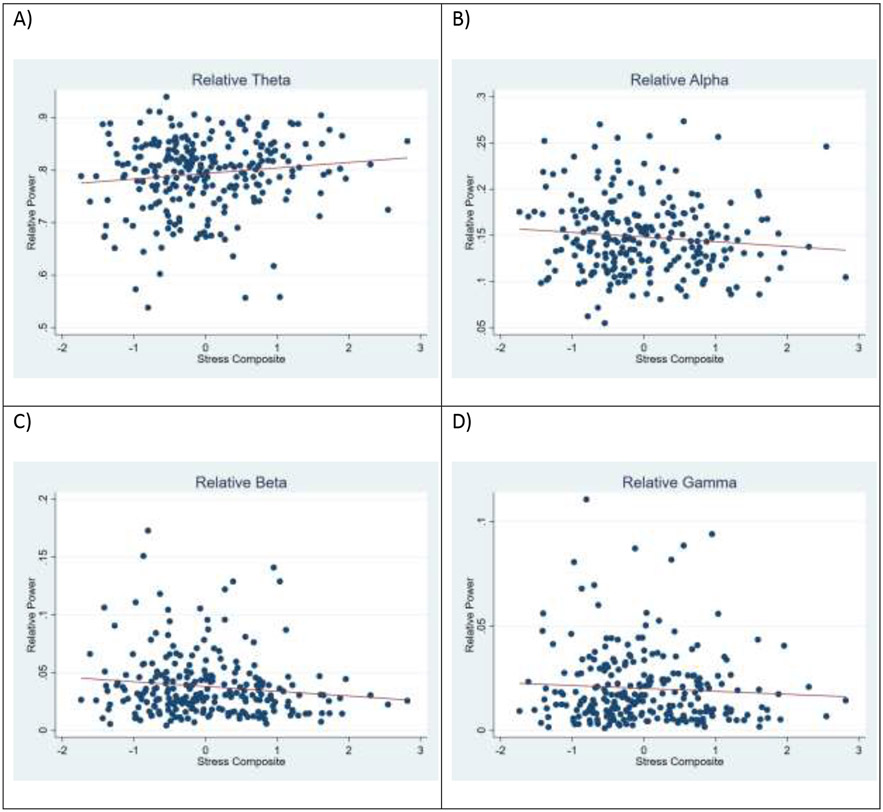
Regression analyses indicated that higher maternal reports of stress were associated with significantly greater whole-brain relative theta power (β=.012, p=.037, partialη2=.019).
Relative Alpha
Regression analyses indicated that higher maternal reports of stress were associated with significantly less whole-brain relative alpha power (β=−.006, p=.047, partialη2=.017).
Relative Beta
Regression analyses indicated that higher maternal reports of stress were not significantly associated with less whole-brain relative beta power (β=−.004, p=.078, partialη2=.013).
Relative Gamma
Regression analyses indicated that maternal reports of stress were not significantly associated with relative gamma power (β=−.002, p=.267, partialη2=.005).
Maternal Stress is Associated with Differences in Regional Resting Brain Activity
Frontal Brain Region
Regression analyses indicated that higher maternal reports of stress were associated with significantly more frontal relative theta power (β=.015, p=.009, partialη2=.029) and less frontal relative alpha power (β=−.010, p=.005, partialη2=.034). Maternal reports of stress were not significantly associated with frontal relative beta (β=−.004, p=.092, partialη2=.012) or gamma power (β=−.001, p=.417, partialη2=.003).
Parietal Brain Region
Regression analyses indicated that higher maternal reports of stress were associated with significantly more parietal relative theta power (β=.013, p=.036, partialη2=.019). Stress was not significantly associated with parietal relative alpha power (β=−.005, p=.067, partialη2=.014), relative beta power (β=−.005, p=.073, partialη2=.014), or frontal relative gamma power (β=−.003, p=.105, partialη2=.011).
Central Brain Region
Maternal reports of stress were not significantly associated with central relative theta (β=.008, p=.293, partialη2=.005), alpha (β=−.003, p=.437, partialη2=.003), beta (β=−.004, p=.182, partialη2=.008), or gamma power (β=−.001, p=.719, partialη2=.001).
Occipital Brain Region
Maternal reports of stress were not significantly associated with occipital relative theta (β=.005, p=.400, partialη2=.003), alpha (β=−.002, p=.607, partialη2=.001), beta (β=−.002, p=.255, partialη2=.006), or gamma power (β=−.001, p=.598, partialη2=.001).
As discussed in the supplemental materials, links between dimensional measures of maternal stress (S1) and whole-brain relative EEG, maternal stress and absolute EEG power (S2), as well as links between maternal hair cortisol concentration and infant EEG power (S3), were not statistically significant.
Discussion
Here we offer evidence that maternal reports of stress are associated with patterns of resting brain activity in infants born into poverty at the end of the first year of life. Specifically, mothers who report higher stress have infants who show more resting brain activity in the lower end of the frequency spectrum (relative theta power), less resting brain activity in the middle range of the frequency spectrum (relative alpha power), and some evidence of less brain activity in the higher end of the frequency spectrum (beta power; marginal significance). While statistically detectable at the whole-brain level, our follow-up -exploratory analyses revealed that these effects were most apparent in electrodes over the frontal and parietal regions of the brain. These findings held after adjusting for a variety of potentially confounding variables including race, ethnicity, maternal education, child age, child sex, and the amount of usable EEG data available for each child.
The present study is the largest investigation to date linking maternal reports of stress to infant resting brain activity among families experiencing low economic resources. Mothers living in poverty are more likely to experience stress when compared to individuals with economic advantage (Algren et al., 2018; Attar et al., 2010; Blair & Raver, 2016; Evans, 2004; Evans & English, 2002; Evans & Kim, 2010; Hackman et al., 2010; McLoyd, 1990; Senn et al., 2014). As such, the present findings suggest that maternal stress may be one pathway by which poverty may influence the developing brain.
While the present study shows that an additive maternal stress composite is associated with infant brain function in both lower- and higher-frequency bands, the mechanisms by which maternal stress gets “under the skin” of infants are largely unknown. For instance, it is plausible that environmental stressors such as neighborhood safety, economic stress, noise, pollution, or crowding may impact mothers and infants similarly, such that maternal stress is a proxy for infant stress (e.g., Ursache et al., 2017). Alternatively, work has suggested that higher maternal stress may lead to reductions in warm, contingent caregiving, which in turn can impact infant neurodevelopment (Twardosz & Lutzker, 2010; Ursache et al., 2017). It is also possible that maternal stress as measured in infancy is a proxy for prenatal stress exposure, and that stress perceptions and experiences during pregnancy shape development (Graignic-Philippe et al., 2014). While linking maternal reports of stress to infant brain function among mother-infant dyads experiencing low economic resources is an important advancement, future research should aim to further identify the particular pathways and mechanisms through which maternal stress impacts the child. Furthermore, it is important to note that all reported findings were small in magnitude (as determined by the reported magnitudes of partialη2), highlighting that maternal stress is likely one of many factors that shape infant resting brain activity.
It may be tempting to interpret the exploratory resting regional power differences in terms of the functional significance of bands established in the broader literature. Although we did not explicitly examine a theta/beta or theta/alpha ratio, the pattern of elevated theta and reductions in alpha and beta among children of mothers experiencing more stress may suggest risk for attentional problems, risk-taking behaviors, and/or lower cognitive control (Anderson & Perone, 2018; McLaughlin et al., 2010). Lower frontal alpha power has been associated with lower executive functioning (Kraybill & Bell, 2013) and lower frontal and parietal beta power have been associated with later risk for socioemotional problems (Brito et al., 2019). However, it is important to note that the associations between resting brain power and these various outcomes have not been investigated in the present sample. Furthermore, it is important to consider that much of the correlational literature linking resting EEG to cognitive and socioemotional skills has been conducted in much less diverse and more advantaged samples than in the present manuscript. Additionally, although not commonly discussed, it is important to note that a byproduct of how relative power is computed (a proportion with all band power in the denominator) makes it likely that differences in one band (e.g., higher theta) will be accompanied by corresponding differences in other bands (e.g., lower alpha).
While increasing evidence suggests that maternal stress is associated with differences in children’s resting brain activity, what this means for child development is not well understood. Dating to the 1980s, the pattern we have described here – a relative increase of lower-frequency resting brain activity and a relative reduction of high-frequency activity, observed in the context of a stressful environment – has been described as a “maturational lag” in neural development (Coming et al., 1982) which may persist over many years (Otero, 1994, 1997; Otero et al., 2003; Vanderwert et al., 2010). The concept of a “maturational lag” has been used to frame correlational work linking this pattern of resting brain activity to lower scores on subsequent language (Benasich et al., 2008; Gou et al., 2011), cognitive (Brito et al., 2016; Williams et al., 2012), and socioemotional outcomes (Brito et al., 2019). However, the deficit-based “maturational lag” theory has come under scrutiny in recent years, as it has become increasingly clear that children’s brain development reflects an adaptation to their lived experiences (Johnson et al., 2015; Nketia et al., 2021). Importantly, different patterns of resting brain activity are likely to be adaptive in different contexts, and a typically developing brain will adapt to the environment it experiences (Ellis et al., 2017). In some cases, such malleability may confer obvious benefits, whereas in other cases, it may lead to the development of adaptive but costly learning or decision-making strategies (Ellis et al., 2020). In the latter case, adaptation does not necessarily represent dysfunction or dysregulation, but rather an expected and appropriate response that equips children to function within their environment (Ellis & Del Giudice, 2019), albeit as a response to adverse circumstances. It is imperative for future research to connect patterns of resting brain activity with lived experiences and consequential, real-world outcomes.
Three supplemental analyses – examining links between dimensional measures of maternal stress and EEG power (S1), maternal stress and absolute EEG power (S2), as well as links between maternal hair cortisol concentration and infant EEG power (S3) – were not statistically significant, as discussed in the supplemental materials. The findings of S1 and S3 suggest that the observed associations may have been driven by the cumulative experience of perceived stress and stressor exposure, and not uniquely by either of these factors, or by physiological stress. Given that measures of absolute EEG power tend to be biased by extraneous factors such as skull thickness, null associations observed between absolute power and brain function are not entirely unexpected.
Several limitations should be noted when considering these results. First, the extent to which individual differences in infant resting brain activity are stable over time is not well established in infancy (Anderson & Perone, 2018; Begus & Bonawitz, 2020), and the longevity versus malleability of associations between maternal reports of stress and infant brain function is unknown. Second, because of the pandemic, EEG data could not be collected on the full set of mother-infant dyads intended for this analysis (N=600). This inability to sample the entire comparison group slightly limited our statistical power, and also limited the representativeness of the findings with respect to the entire comparison group. Third, while associations between infant resting brain activity and subsequent cognitive, linguistic, and/or social-emotional functioning have been observed in other samples (Brito et al., 2016, 2019; Gou et al., 2011; Williams et al., 2012), some studies do not find that infant resting brain activity predicts subsequent skills (Brito et al., 2019; Gou et al., 2011). Given that the current study did not assess associations between resting brain activity and behavioral or cognitive development, the functional significance of the observed stress-related differences in brain activity remains unknown in our sample. Fourth, the present research is cross-sectional and correlational, and therefore cannot determine causation. Indeed, an alternate explanation for our findings could be that the observed pattern of infant resting brain activity had behavioral correlates which led to higher stress for mothers. Additionally, it is important to note that the stress composite used in the present investigation conflates different aspects of the stress response (e.g., the presence of a stressor and appraisal of a stressor [for further discussion, see S1]. This rather blunt conceptualization of stress, while useful for understanding whether stress is associated with brain activity, needs more specificity for intervention. Future research should aim to increase specificity in understanding the types and appraisals of stressors that impact infant neurodevelopment most. Finally, as the current mother-infant dyads were part of the active control group of a larger intervention with a hypothesized possible impact on stress, one may wonder whether stress was impacted by the unconditional cash transfer intervention. Early evidence suggests that the cash gift intervention did not significantly impact stress levels (Magnuson et al., 2022), although it is impossible to discern with the data available whether stress levels were changed in both groups similarly (e.g., stress changed similarly for the control and treatment groups).
Altogether, the present study finds that, among families experiencing low economic resources, stress reported by mothers is associated with differences in patterns of infant resting brain activity during the first year of life. These findings are important given that resting brain activity differences can be long-lasting (Debnath, Tang, et al., 2020; Otero et al., 2003) and have been shown to predict later language, neurocognitive, and socioemotional functioning in some previous work (Brito et al., 2016, 2019; Gou et al., 2011; Tan et al., 2023; Williams et al., 2012). In light of this past work, the present findings suggest the possibility that reducing maternal stress in the earliest years could be important for subsequent development and well-being.
Supplementary Material
Figure 1.
Scatterplot with linear trend line for the Stress Composite (x-axis; higher numbers, more stress) and relative EEG power in the Theta (A), Alpha (B), Beta (C), and Gamma (D) bands.
Table 2.
Correlations between covariates and measures of interest.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Mother Education: Grade school | 1 | ||||||||||||||||
| 2. Mother Education: Some College | −0.484*** | 1 | |||||||||||||||
| 3. Mother Education: Associates | −0.178** | −0.0910 | 1 | ||||||||||||||
| 4. Mother Education: Bachelors | −0.220*** | −0.112 | −0.0413 | 1 | |||||||||||||
| 5. Mother Education: Unknown | −0.0620 | −0.0317 | −0.0117 | −0.0144 | 1 | ||||||||||||
| 6. Mother race/ethnicity: Black | 0.122 | −0.0554 | 0.0921 | 0.0168 | 0.0813 | 1 | |||||||||||
| 7. Mother race/ethnicity: Multiple | −0.0281 | −0.0341 | −0.0448 | −0.0554 | −0.0156 | −0.192** | 1 | ||||||||||
| 8. Mother race/ethnicity: Other | −0.131* | 0.188** | −0.0395 | 0.0425 | −0.0138 | −0.169** | −0.0529 | 1 | |||||||||
| 9. Mother race/ethnicity: Hispanic | 0.00688 | −0.0587 | −0.0577 | −0.0329 | −0.0526 | −0.646*** | −0.202** | −0.178** | 1 | ||||||||
| 10. Age at Interview | −0.0290 | 0.137* | −0.0650 | 0.0471 | −0.0512 | −0.0102 | −0.0143 | 0.140* | −0.0719 | 1 | |||||||
| 11. Child is Female | 0.0363 | −0.0691 | 0.0465 | 0.114 | 0.0640 | −0.0635 | −0.0690 | 0.0598 | 0.0528 | 0.0467 | 1 | ||||||
| 12. Number of Epochs | −0.0874 | −0.0611 | −0.0199 | −0.0219 | 0.0142 | 0.0689 | −0.0486 | 0.0453 | 0.00794 | 0.153* | 0.0902 | 1 | |||||
| 13.Maternal Stress Factor | 0.0154 | 0.0705 | −0.166** | −0.0646 | −0.0644 | −0.0152 | −0.0138 | 0.0724 | −0.0207 | 0.0257 | −0.0536 | 0.0237 | 1 | ||||
| 14. Relative Theta (whole brain) | −0.0539 | 0.0287 | −0.00786 | 0.0975 | 0.0500 | 0.0531 | −0.0454 | 0.0304 | −0.00792 | −0.203** | 0.00300 | −0.0644 | 0.124 | 1 | |||
| 15. Relative Alpha (whole brain) | 0.0520 | 0.0565 | −0.0162 | −0.110 | −0.0777 | −0.0349 | 0.0617 | −0.0692 | 0.0383 | 0.238*** | −0.0243 | 0.126* | −0.105 | −0.824*** | 1 | ||
| 16. Relative Beta (whole brain) | 0.00552 | −0.0918 | 0.0466 | −0.0487 | −0.00462 | −0.0604 | 0.0136 | 0.0317 | −0.0123 | 0.118 | 0.0457 | 0.00641 | −0.124 | −0.839*** | 0.401*** | 1 | |
| 17. Relative Gamma (whole brain) | 0.0891 | −0.0984 | −0.00481 | −0.0645 | −0.0172 | −0.0390 | 0.0212 | −0.0148 | −0.0354 | 0.0907 | −0.0283 | −0.0360 | −0.0654 | −0.826*** | 0.409*** | 0.881*** | 1 |
"* p<0.05, ** p<0.01, *** p<0.001"
Table 3.
Regression coefficients for relative, whole-brain Theta, Alpha, Beta, and Gamma power.
| Relative, whole-brain power | (1) Theta |
(2) Alpha |
(3) Beta |
(4) Gamma |
|---|---|---|---|---|
| Maternal Stress Factor | 0.012** (0.006) |
−0.006** (0.003) |
−0.004* (0.002) |
−0.002 (0.001) |
| Mother Education: High School | −0.001 (0.011) |
0.006 (0.006) |
−0.005 (0.005) |
0.000 (0.003) |
| Mother Education: Some College | 0.007 (0.014) |
0.009 (0.008) |
−0.011** (0.006) |
−0.005 (0.004) |
| Mother Education: Associates | −0.006 (0.027) |
0.003 (0.015) |
0.003 (0.011) |
−0.000 (0.007) |
| Mother Education: Bachelors | 0.037 (0.022) |
−0.015 (0.013) |
−0.014 (0.009) |
−0.007 (0.006) |
| Mother Education: Unknown | 0.052 (0.070) |
−0.041 (0.039) |
−0.006 (0.028) |
−0.005 (0.018) |
| Mother race/ethnicity: Black | 0.010 (0.016) |
−0.002 (0.009) |
−0.004 (0.006) |
−0.005 (0.004) |
| Mother race/ethnicity: Multiple | −0.004 (0.023) |
0.011 (0.013) |
−0.003 (0.009) |
−0.004 (0.006) |
| Mother race/ethnicity: Other | 0.020 (0.025) |
−0.017 (0.014) |
0.001 (0.010) |
−0.004 (0.006) |
| Mother race/ethnicity: Hispanic | −0.007 (0.017) |
0.008 (0.009) |
−0.000 (0.007) |
−0.001 (0.004) |
| Age at Interview | −0.009*** (0.003) |
0.006*** (0.002) |
0.002* (0.001) |
0.001 (0.001) |
| Child is Female | 0.000 (0.009) |
−0.002 (0.005) |
0.003 (0.004) |
−0.001 (0.002) |
| Number of Epochs | −0.000 (0.000) |
0.000 (0.000) |
−0.000 (0.000) |
−0.000 (0.000) |
| Site 2 | 0.006 (0.015) |
−0.003 (0.009) |
−0.000 (0.006) |
−0.002 (0.004) |
| Site 3 | −0.010 (0.013) |
−0.000 (0.007) |
0.008 (0.005) |
0.002 (0.003) |
| Site 4 | 0.024 (0.015) |
−0.011 (0.009) |
−0.006 (0.006) |
−0.007* (0.004) |
| Constant | 0.901*** (0.040) |
0.067*** (0.023) |
0.017 (0.016) |
0.014 (0.010) |
| Observations | 247 | 247 | 247 | 247 |
| R-squared | 0.113 | 0.128 | 0.095 | 0.084 |
Standard errors in parentheses. *** p<0.01, ** p<0.05, * p<0.1
Highlights:
Mothers experiencing poverty are likely to report experiencing stress
Maternal stress is postulated to be associated with infant resting brain activity
Our data suggest that maternal stress is associated with infant brain activity
Stress is associated with more lower-frequency and less higher-frequency activity
Acknowledgments:
The research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Numbers K99HD10492 and R01HD087384. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was additionally supported by the US Department of Health and Human Services, Administration for Children and Families, Office of Planning, Research and Evaluation; Andrew and Julie Klingenstein Family Fund; Annie E. Casey Foundation; Arnold Ventures; Arrow Impact; BCBS of Louisiana Foundation; Bezos Family Foundation, Bill and Melinda Gates Foundation; Bill Hammack and Janice Parmelee, Brady Education Fund; Chan Zuckerberg Initiative (Silicon Valley Community Foundation); Charles and Lynn Schusterman Family Philanthropies; Child Welfare Fund; Esther A. and Joseph Klingenstein Fund; Ford Foundation; Greater New Orleans Foundation; Heising-Simons Foundation; Holland Foundation; Jacobs Foundation; JPB Foundation; J-PAL North America; Lozier Foundation; New York City Mayor’s Office for Economic Opportunity; Perigee Fund; Robert Wood Johnson Foundation; Robin Hood; Sherwood Foundation; Valhalla Foundation; Weitz Family Foundation; W.K. Kellogg Foundation; and three anonymous donors. This work is independently initiated work, and builds on the parent grant, which is a multidisciplinary collaboration of seven Principal Investigators: Kimberly Noble (lead PI, neuroscience), Katherine Magnuson (lead PI, social science), Greg Duncan, Lisa Gennetian, Hirokazu Yoshikawa, Nathan Fox, and Sarah Halpern-Meekin. We thank the University of Michigan Survey Research Center, our recruitment and data collection partners.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing Interests: Authors have no competing interests to declare.
One may worry that household income may be related to economic stress, thus conflating the relations between income and stress. In our sample, there was a weak correlation between household income and economic stress (r=−.178) at age 1. As a robustness check, we added household income as a covariate to our whole-brain EEG analyses, and there was no meaningful change in the pattern or significance of results.
References
- Algren MH, Ekholm O, Nielsen L, Ersbøll AK, Bak CK, & Andersen PT (2018). Associations between perceived stress, socioeconomic status, and health-risk behaviour in deprived neighbourhoods in Denmark: A cross-sectional study. BMC Public Health, 18(1), 1–12. 10.1186/S12889-018-5170-X/TABLES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AJ, & Perone S (2018). Developmental change in the resting state electroencephalogram: Insights into cognition and the brain. Brain and Cognition, 126, 40–52. 10.1016/j.bandc.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Attar BK, Guerra NG, & Tolan PH (2010). Neighborhood disadvantage, stressful life events and adjustments in urban elementary-school children. 10.1207/S15374424jccp2304_5, 23(4), 391–400. [DOI] [Google Scholar]
- Baan EJ, van den Akker ELT, Engelkes M, de Rijke YB, de Jongste JC, Sturkenboom MCJM, Verhamme KM, & Janssens HM (2020). Hair cortisol and inhaled corticosteroid use in asthmatic children. Pediatric Pulmonology, 55(2), 316–321. 10.1002/ppul.24551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begus K, & Bonawitz E (2020). The rhythm of learning: Theta oscillations as an index of active learning in infancy. Developmental Cognitive Neuroscience, 45, 100810. 10.1016/J.DCN.2020.100810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, & Harris KD (2008). Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behavioural Brain Research, 195(2), 215–222. 10.1016/j.bbr.2008.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2016). Poverty, Stress, and Brain Development: New Directions for Prevention and Intervention. Academic Pediatrics, 16(3), S30–S36. 10.1016/J.ACAP.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes-Aitken A, Pini N, Weatherhead M, & Brito NH (2023). Maternal hair cortisol predicts periodic and aperiodic infant frontal EEG activity longitudinally across infancy. Developmental Psychobiology, 65(5), e22393. 10.1002/dev.22393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Elliott AJ, Isler JR, Rodriguez C, Friedrich C, Shuffrey LC, & Fifer WP (2019). Neonatal EEG linked to individual differences in socioemotional outcomes and autism risk in toddlers. Developmental Psychobiology, 61(8), 1110–1119. 10.1002/dev.21870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. 10.1016/j.dcn.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 0(SEP), 276. 10.3389/FNINS.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo Vázquez M, Johnson-Ferguson L, Zimmermann J, Baumgartner MR, Binz TM, Beuschlein F, Ribeaud D, Shanahan L, & Quednow BB (2022). Associations of different hormonal contraceptive methods with hair concentrations of cortisol, cortisone, and testosterone in young women. Comprehensive Psychoneuroendocrinology, 12, 100161. 10.1016/j.cpnec.2022.100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, & Selikowitz M (2001). Age and sex effects in the EEG: Development of the normal child. Clinical Neurophysiology, 112(5), 806–814. 10.1016/S1388-2457(01)00488-6 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1994). Perceived stress scale. Measuring Stress: A Guide for Health and Social Scientists, 235–283. [Google Scholar]
- Cohen S, & Williamson G (1988). Perceived Stress in a Probability Sample of the United States. In Spacapan S & Oskamp S (Eds.), The Social Psychology of Health. Sage Publishers. [Google Scholar]
- Coming WC, Steffy RA, & Chaprin IC (1982). EEG Slow Frequency and WISC-R Correlates. Journal of Abnormal Child Psychology, 10(4), 511–530. [DOI] [PubMed] [Google Scholar]
- Corning WC, Steffy RA, Anderson E, & Bowers P (1986). EEG “maturational lag” profiles: Follow-up analyses. Journal of Abnormal Child Psychology, 14(2), 235–249. 10.1007/BF00915443 [DOI] [PubMed] [Google Scholar]
- Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, & Fox NA (2020). The Maryland Analysis of Developmental EEG (MADE) Pipeline. Psychophysiology, 57(6), e13580. 10.1111/psyp.13580 [DOI] [PubMed] [Google Scholar]
- Debnath R, Tang A, Zeanah CH, Nelson CA, & Fox NA (2020). The long-term effects of institutional rearing, foster care intervention and disruptions in care on brain electrical activity in adolescence. Developmental Science, 23(1), e12872. 10.1111/desc.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Abrams LS, Masten AS, Sternberg RJ, Tottenham N, & Frankenhuis WE (2020). Hidden talents in harsh environments. Development and Psychopathology, 1–19. 10.1017/S0954579420000887 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Bianchi J, Griskevicius V, & Frankenhuis WE (2017). Beyond Risk and Protective Factors: An Adaptation-Based Approach to Resilience: 10.1177/1745691617693054, 12(4), 561–587. 10.1177/1745691617693054 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, & Del Giudice M (2019). Developmental Adaptation to Stress: An Evolutionary Perspective. 10.1146/Annurev-Psych-122216-011732, 70, 111–139. [DOI] [PubMed] [Google Scholar]
- Evans GW (2004). The Environment of Childhood Poverty. American Psychologist, 59(2), 77–92. 10.1037/0003-066X.59.2.77 [DOI] [PubMed] [Google Scholar]
- Evans GW, & English K (2002). The Environment of Poverty: Multiple Stressor Exposure, Psychophysiological Stress, and Socioemotional Adjustment. Child Development, 73(4), 1238–1248. 10.1111/1467-8624.00469 [DOI] [PubMed] [Google Scholar]
- Evans GW, Gonnella C, Marcynyszyn LA, Gentile L, & Salpekar N (2005). The Role of Chaos in Poverty and Children’s Socioemotional Adjustment: Psychological Science, 16(7), 560–565. 10.1111/J.0956-7976.2005.01575.X [DOI] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2010). Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Annals of the New York Academy of Sciences, 1186, 174–189. 10.1111/j.1749-6632.2009.05336.x [DOI] [PubMed] [Google Scholar]
- Flom M, St. John AM, Meyer JS, & Tarullo AR (2017). Infant hair cortisol: Associations with salivary cortisol and environmental context. Developmental Psychobiology, 59(1), 26–38. 10.1002/dev.21449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgescu C, Misra DP, Sealy-Jefferson S, Caldwell CH, Templin TN, Slaughter-Acey JC, & Osypuk TL (2015). The impact of neighborhood quality, perceived stress, and social support on depressive symptoms during pregnancy in African American women. Social Science & Medicine, 130, 172–180. 10.1016/J.SOCSCIMED.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, & Benasich AA (2011). Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research, 220(2), 263–270. 10.1016/j.bbr.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graignic-Philippe R, Dayan J, Chokron S, Jacquet A-Y, & Tordjman S (2014). Effects of prenatal stress on fetal and child development: A critical literature review. Neuroscience & Biobehavioral Reviews, 43, 137–162. 10.1016/j.neubiorev.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Guidubaldi J, & Cleminshaw HK (2010). The Development of the Cleminshaw—Guidubaldi Parent Satisfaction Scale. 10.1207/S15374424jccp1404_4, 14(4), 293–298. [DOI] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience 2010 11:9, 11(9), 651–659. 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson H, Child S, Moore S, Moore JB, & Kaczynski AT (2016). The Influence of Neighborhood Aesthetics, Safety, and Social Cohesion on Perceived Stress in Disadvantaged Communities. American Journal of Community Psychology, 58(1–2), 80–88. 10.1002/AJCP.12081 [DOI] [PubMed] [Google Scholar]
- Jensen SKG, Xie W, Kumar S, Haque R, Petri WA, & Nelson CA (2021). Associations of socioeconomic and other environmental factors with early brain development in Bangladeshi infants and children. Developmental Cognitive Neuroscience, 50, 100981. 10.1016/j.dcn.2021.100981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Jones EJH, & Gliga T (2015). Brain adaptation and alternative developmental trajectories. Development and Psychopathology, 27(2), 425–442. 10.1017/S0954579415000073 [DOI] [PubMed] [Google Scholar]
- Kling JR, Liebman JB, & Katz LF (2007). Experimental Analysis of Neighborhood Effects. Econometrica, 75(1), 83–119. 10.1111/J.1468-0262.2007.00733.X [DOI] [Google Scholar]
- Kraybill JH, & Bell MA (2013). Infancy predictors of preschool and post-kindergarten executive function. Developmental Psychobiology, 55(5). https://onlinelibrary.wiley.com/doi/full/10.1002/dev.21057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson K, Yoo P, Duncan G, Yoshikawa H, Trang K, Gennetian LA, Halpern-Meekin S, Fox N, & Noble K (2022). Can a Poverty Reduction Intervention Reduce Family Stress Among Families with Infants? An Experimental Analysis. (SSRN Scholarly Paper 4188131). 10.2139/ssrn.4188131 [DOI] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA, & BEIP Core Group. (2004). A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience, 16(8), 1327–1338. [DOI] [PubMed] [Google Scholar]
- Matheny AP, Wachs TD, Ludwig JL, & Phillips K (1995). Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. Journal of Applied Developmental Psychology, 16(3), 429–444. 10.1016/0193-3973(95)90028-4 [DOI] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Sheridan M. a, Marshall P, & Nelson CA (2010). Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biological Psychiatry, 68(4), 329–336. 10.1016/j.biopsych.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC (1990). The Impact of Economic Hardship on Black Families and Children: Psychological Distress, Parenting, and Socioemotional Development. Child Development, 61(2), 311–346. 10.1111/J.1467-8624.1990.TB02781.X [DOI] [PubMed] [Google Scholar]
- Meyer J, Novak M, Hamel A, & Rosenberg K (2014). Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments : JoVE, 83, e50882. 10.3791/50882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nketia J, Amso D, & Brito NH (2021). Towards a more inclusive and equitable developmental cognitive neuroscience. Developmental Cognitive Neuroscience, 52, 101014. 10.1016/J.DCN.2021.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Magnuson K, Gennetian LA, Duncan GJ, Yoshikawa H, Fox NA, & Halpern-Meekin S (2021). Baby’s First Years: Design of a Randomized Controlled Trial of Poverty Reduction in the U.S. Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero GA (1994). Eeg spectral analysis in children with sociocultural handicaps. International Journal of Neuroscience, 79(3–4), 213–220. 10.3109/00207459408986082 [DOI] [PubMed] [Google Scholar]
- Otero GA (1997). Poverty, cultural disadvantage and brain development: A study of preschool children in Mexico. Electroencephalography and Clinical Neurophysiology, 102(6), 512–516. 10.1016/S0013-4694(97)95213-9 [DOI] [PubMed] [Google Scholar]
- Otero GA, Pliego-Rivero FB, Fernández T, & Ricardo J (2003). EEG development in children with sociocultural disadvantages: A follow-up study. Clinical Neurophysiology, 114(10), 1918–1925. 10.1016/S1388-2457(03)00173-1 [DOI] [PubMed] [Google Scholar]
- Pierce LJ, Thompson BL, Gharib A, Schlueter L, Reilly E, Valdes V, Roberts S, Conroy K, Levitt P, & Nelson CA (2019). Association of Perceived Maternal Stress During the Perinatal Period With Electroencephalography Patterns in 2-Month-Old Infants. JAMA Pediatrics, 173(6), 561–570. 10.1001/jamapediatrics.2019.0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby JN, & Marshall PJ (2012). The Utility of EEG Band Power Analysis in the Study of Infancy and Early Childhood. Developmental Neuropsychology, 37(3), 253–273. 10.1080/87565641.2011.614663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai J. (2020). How synaptic pruning shapes neural wiring during development and, possibly, in disease. Proceedings of the National Academy of Sciences, 117(28), 16096–16099. 10.1073/pnas.2010281117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, & Wüst S (2008). Covariance Between Psychological and Endocrine Responses to Pharmacological Challenge and Psychosocial Stress: A Question of Timing. Psychosomatic Medicine, 70(7), 787–796. 10.1097/PSY.0b013e3181810658 [DOI] [PubMed] [Google Scholar]
- Senn TE, Walsh JL, & Carey MP (2014). The Mediating Roles of Perceived Stress and Health Behaviors in the Relation Between Objective, Subjective, and Neighborhood Socioeconomic Status and Perceived Health. Annals of Behavioral Medicine, 48(2), 215–224. 10.1007/S12160-014-9591-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, Wadhwa PD, & Buss C (2016). Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology, 71, 12–18. 10.1016/j.psyneuen.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smy L, Shaw K, Amstutz U, Smith A, Berger H, Carleton B, & Koren G (2016). Hair cortisol as a hypothalamic-pituitary-adrenal axis biomarker in pregnant women with asthma: A retrospective observational study. BMC Pregnancy and Childbirth, 16(1), 176. 10.1186/s12884-016-0962-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Tang A, Debnath R, Humphreys KL, Zeanah CH, Nelson CA, & Fox NA (2023). Resting brain activity in early childhood predicts IQ at 18 years. Developmental Cognitive Neuroscience, 63, 101287. 10.1016/j.dcn.2023.101287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, Johnson MH, & Kushnerenko E (2013). Socioeconomic status and functional brain development—Associations in early infancy. Developmental Science, 16(5), 676–687. 10.1111/desc.12079 [DOI] [PubMed] [Google Scholar]
- Troller-Renfree SV, Brito NH, Desai PM, Leon-Santos AG, Wiltshire CA, Motton SN, Meyer JS, Fifer WP, & Noble KG (2020). Infants of mothers with higher physiological stress show alterations in brain function. Developmental Science, 23(6), e12976. 10.1111/desc.12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree SV, Costanzo MA, Duncan GJ, Magnuson K, Gennetian LA, Yoshikawa H, Halpern-Meekin S, Fox NA, & Noble KG (2022). The impact of a poverty reduction intervention on infant brain activity. Proceedings of the National Academy of Sciences, 119(5), e2115649119. 10.1073/pnas.2115649119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree SV, Hart ER, Sperber JF, Fox NA, & Noble KG (2022). Associations among stress and language and socioemotional development in a low-income sample. Development and Psychopathology, 34(2), 597–605. 10.1017/S0954579421001759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree SV, Morales S, Leach SC, Bowers ME, Debnath R, Fifer WP, Fox NA, & Noble KG (2021). Feasibility of Assessing Brain Activity using Mobile, In-home Collection of Electroencephalography: Methods and Analysis. Developmental Psychobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardosz S, & Lutzker JR (2010). Child maltreatment and the developing brain: A review of neuroscience perspectives. Aggression and Violent Behavior, 15(1), 59–68. 10.1016/J.AVB.2009.08.003 [DOI] [Google Scholar]
- Ursache A, Merz EC, Melvin S, Meyer J, & Noble KG (2017). Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology, 78, 142–150. 10.1016/J.PSYNEUEN.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Marshall PJ, Nelson CA, Zeanah CH, & Fox NA (2010). Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PloS One, 5(7), e11415. 10.1371/journal.pone.0011415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM, & Fifer WP (2012). Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound in Obstetrics and Gynecology, 40(3), 304–309. 10.1002/uog.11144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.