Reports of insufficient sleep have steadily increased over the past decade. Sleep fragmentation (SF) promotes hypocretin-dependent myelopoiesis and atherogenesis.1 While these studies identified a neuro-immune link between sleep and atherosclerosis, it is unknown whether SF may affect plaque stability. Therefore, we tested the effects of SF on plaque stability and its underlying pathological mechanisms.
A motorized slow-moving bar sweeping across the cage floor every 2 minutes was used during the light period to achieve sleep fragmentation (SF)2 and during the dark period to control for forced activity and stress associated with the motorized bar (AC-activity control). We included a group of home cage (HC) Apoe−/− female mice.
12 weeks of SF promoted atherogenesis, resulting in increased brachiocephalic arterial lesions (Grade 2–3) in a high fat diet (HFD) fed Apoe−/− female mice when compared with AC- or HC-Apoe−/− mice (Fig.[A]). Sleep disturbances are associated with increased CVD mortality due to myocardial infarction or stroke. Within advanced plaques, necrotic core size is a key determinant of plaque vulnerability.3 SF promoted enlargement of necrotic cores, a reduction in total collagen and thick collagen fibers, and increased the thin fibers in aortic valves, suggesting that SF is associated with vulnerable plaques (Fig.[B]). The plaques from SF-Apoe−/− compared to AC- or HC-Apoe−/− females also demonstrated a reduced cap to core ratio, reflecting an attenuated fibrous cap thickness, a characteristic of an unstable plaque (Fig.[B]). Overall, SF promoted plaque growth characterized by increased necrotic areas and attenuated formation of collagen fibers and fibrotic cap.
Sleep fragmentation increases neutrophil activation and promotes atherosclerosis and plaque necrosis.
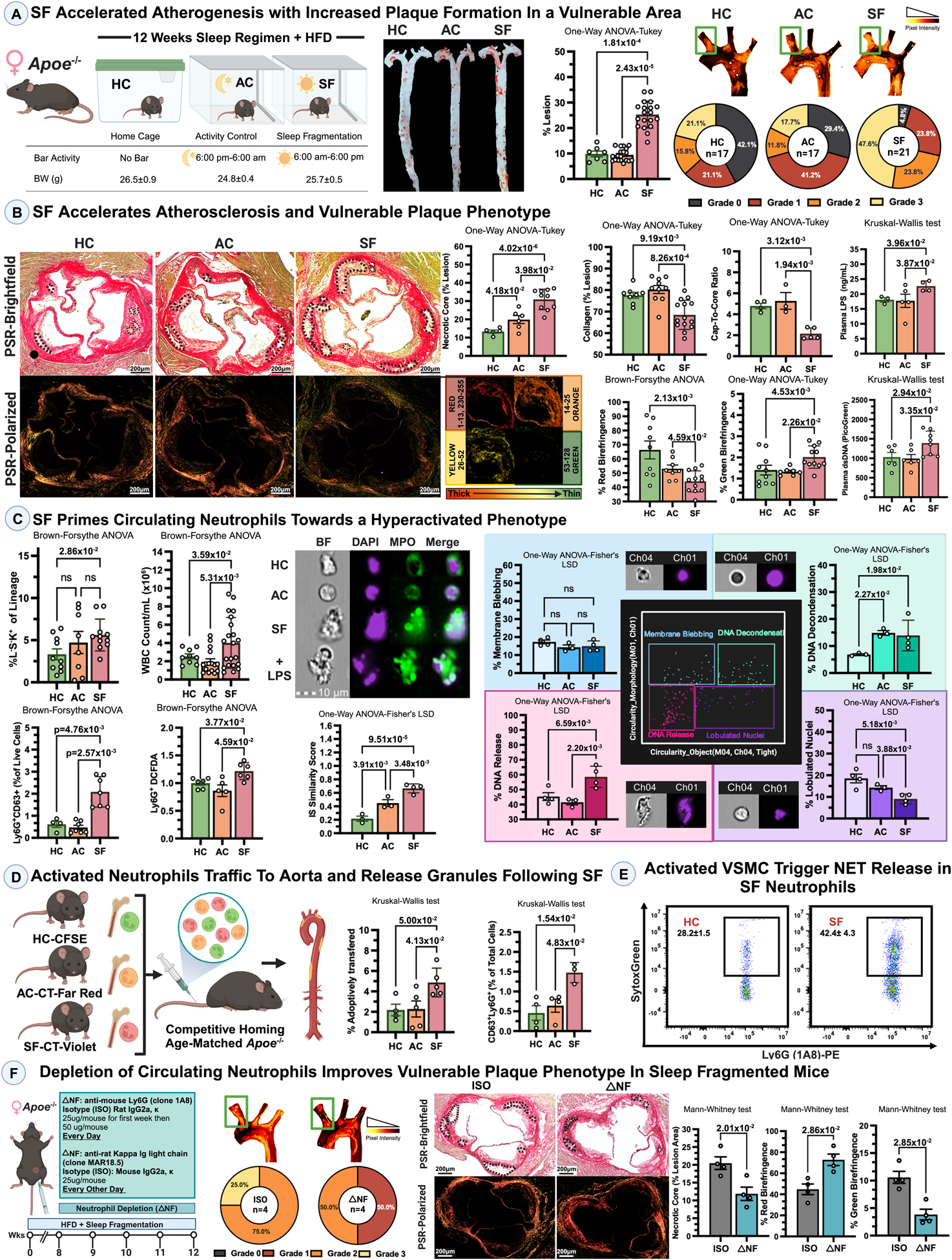
SF accelerated atherogenesis with increased plaque formation in a vulnerable area. 8–10 weeks-old Apoe−/− females were placed either in sleep fragmented (SF) or activity control (AC) or regular home cages (HC) and fed a High Fat Diet (Envigo, TD.88137) for 12 weeks. Histological images underwent double-blind analysis, followed by data processing and the selection of representative images reflecting the main conclusion. Representative images and lesion quantification of Oil Red O-stained aortas (n=7–21/per group, One-Way ANOVA-Turkey) and aortic arches with lesion grading (from Grade 0 – as no lesions; (n=17–21 per group). Here and below, each data point represents an animal in a figure. Normality of data was assessed using the Shapiro-Wilk test and visual inspection using a QQ plot in GraphPad Prism.
Picrosirius Red-stained aortic roots were quantified for necrotic core (n=5–10/per group), fibrotic cap (cap-to-core ratio, n=3–5/per group, One-Way ANOVA), collagen fibers (n=8–14/per group, One-Way ANOVA or Brown-Forsythe ANOVA). Peripheral blood dsDNA and LPS levels were analyzed using PicoGreen and ELISA (n=4–8/per group, Kruskal-Wallis test).
SF primes neutrophils towards a hyperactivated phenotype. Bone marrow Lin−Sca1−Kit+ cells (n=8–11/per group); WBC Count (n=8–11/per group), CD63+Ly6G+ and DCFDA+ROS+ neutrophils (n=4–7/per group) in peripheral blood of Apoe−/− mice; Brown-Forsyth-ANOVA. Representative images of blood neutrophils: BF: bright-field. IS Similarity Score quantifying the degree of nuclear translocation of MPO (~100cells/mouse, n=3–4/per group; One-way ANOVA-Fisher’s LSD). Gated neutrophils are analyzed for nuclear vs cell circularity. The images were obtained from events in each quadrant. Some neutrophils were stimulated with LPS.
Activated neutrophils traffic to the aorta following SF. Enzymatically-digested aortas were analyzed for CD63+Ly6G+CD45+ neutrophils by FACS. Competitive homing: 10×106 of fluorescent dye-labeled Leukocytes from SF-, AC-, and HC-Apoe−/− mice were injected IV/tail vein into aged Apoe−/− recipients (n=4–5/per group; Kruskal-Wallis test). 14 hrs later, aortas were analyzed for Dye+CD45+Ly6G+ neutrophils. Data were normalized to the start population of injected cells.
Activated VMSCs trigger NETosis in SF neutrophils. Collected supernatants from VSMCs treated with PDGF-BB (10 ng/mL/6 hrs) were incubated with neutrophils from SF- or HC- Apoe−/− mice - and stained with SytoxGreen/Ly6G (n=5/per group). Representative images and averages are shown.
Neutrophil depletion improves plaque phenotype of SF Apoe−/− mice. Scheme of neutrophil depletion, representative images of aortic arches with lesion grading (from Grade 0– as no lesions). Picrosirius Red-stained aortic roots were quantified for necrotic core and collagen fibers (n=4/per group; Matt-Whitney test).
Neutrophils accumulate in atherosclerotic plaques and are associated with rupture-prone lesions.3 We therefore evaluated how SF affects neutrophil numbers and functions in SF-Apoe−/− mice. We corroborated findings of McAlpine and colleagues1 by demonstrating SF increased the leukocyte numbers due to augmented hematopoiesis (Fig.[C]). Interestingly, GMP+ and LSK+ populations were unchanged in AC-Apoe−/− mice, indicating that forced activity and/or stress associated with a moving bar during the dark period, does not affect myelopoiesis (Fig.[C]).
We next assessed the effects of SF on neutrophil phenotypes. Peripheral blood neutrophils from SF- vs. AC- and HC-Apoe−/− mice exhibited increased levels of CD63 (Fig.[C]), CD40, CD1d, and CD11b, (not shown), reflecting an activation phenotype. No differences in CD62L or CXCR2, were found (not shown). Functionally, SF-Apoe−/− neutrophils showed elevated basal levels of reactive oxygen specifies (ROS) vs AC- and HC-Apoe−/− groups (Fig.[C]). Interestingly, higher levels of dsDNA were also detected in the blood of SF-Apoe−/− mice, suggesting either an ongoing release of dsDNA as a consequence of cell death or active NETosis (Fig.[C]). HFD feeding and SF can independently alter gut immunity, resulting in the leaking of LPS into the circulation. In concert with neutrophil activation, we detected increased LPS levels in the blood of SF- vs AC- and HC-Apoe−/− controls (Fig.[B]).
NETosis plays a crucial role in innate immunity but also supports chronic inflammation. SF induced NETosis with a higher Similarity Score/higher colocalization of DAPI+/DNA/MPO+, a feature of suicidal NETosis4 (Fig.[C]). We also detected signs of “vital” NETosis (condensed nuclei/elongated cell shape/polarized bleb) in HC- and AC-, but not in SF-Apoe−/− neutrophils. Based on the morphology/circularity parameters of cells, we analyzed 4 neutrophil groups (Fig.[C]). While lobulated nuclei were decreased, DNA release and DNA decondensation were elevated in SF-Apoe−/− neutrophils, suggesting that these cells were undergoing active NETosis.
Increased neutrophil numbers are found within plaques.3 Due to the functionally activated SF-Apoe−/− neutrophils, we next questioned how SF affects neutrophil content in the aorta. We found increased numbers of activated aortic CD63+Ly6G+ neutrophils in SF- vs. AC- and HC-groups (Fig.[D]). To test whether SF-activated leukocytes can be recruited into the aorta, we performed competitive homing experiments with leukocytes from SF-, AC-, and HC-Apoe−/− donor mice into aged Apoe−/− recipients (Fig.[D]). Neutrophils from SF Apoe−/− mice exhibited a preferential homing into aortas of Apoe−/− recipients.
Vascular smooth muscle cells (VSMCs) play a key role in neutrophil activation and induction of NETs, supporting neutrophil-dependent plaque rupture/erosion.3 We next sought to determine how SF supports the unstable plaque phenotype via promotion of NETosis, induced by activated VSMCs. Neutrophils from SF-Apoe−/− mice co-cultured with supernatant from PDGF-activated VSMCs were more responsive to a VSMCs-dependent activation and showed increased levels of NETosis compared to HC-Apoe−/− neutrophils (Fig.[E]).
Next, we depleted neutrophils5 in SF HFD-fed Apoe−/− mice for last 4 weeks of 12 wks of SF regimen (Fig.F). Neutrophil depletion resulted in reduced plaque formation in BCA and an improved plaque phenotype reflected by increased total collagen content, a smaller necrotic core, and thicker collagen fibers (2.34±0.63 vs 0.96±0.19, p<0.01) in neutrophil-depleted vs isotype control SF-Apoe−/− mice. These data suggests that neutrophils play a key role in driving vulnerable plaque phenotype upon SF.
The present study demonstrates a crucial role of SF in promoting atherosclerosis and particularly plaque destabilization. Importantly, our results indicate that SF supports neutrophil functions such as ROS and NET production. We detected increased circulating levels of LPS that might serve as a neutrophil activator during SF. Competitive homing experiments clearly demonstrate that SF directs neutrophil recruitment into the aorta. Importantly, neutrophil depletion improves plaque phenotype of SF mice. Thus, our findings support a model in which SF induces atherosclerosis with unstable plaque phenotype via a neutrophil-dependent mechanism. Our data are consistent with clinical data, which largely implicate SF as a risk factor in promoting CVD with increased mortality.
Sources of Funding
Supplementary Material
Acknowledgements.
We thank the EVMS Flow Cytometry Core for the excellent technical assistance.
This work was supported by the National Institutes of Health grants R01HL142129, (EVG), AHA pre-doctoral fellowship 20PRE35180156 (AKM), and AHA Innovative grant (EVG).
Footnotes
Disclosures.
None.
Animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals (NIH) and approved by EVMS IACUC.
References
- 1.McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, et a. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramesh V, Nair D, Zhang SX, Hakim F, Kaushal N, Kayali F, Wang Y, Li RC, Carreras A and Gozal D. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. Journal of neuroinflammation. 2012;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrpouya-Bahrami P, Moriarty AK, De Melo P, Keeter WC, Alakhras NS, Nelson AS, Hoover M, Barrios MS, Nadler JL, Serezani CH. et al. STAT4 is expressed in neutrophils and promotes antimicrobial immunity. JCI insight. 2021; 22;6(14):e141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boivin G, Faget J, Ancey PB, Gkasti A, Mussard J, Engblom C, Pfirschke C, Contat C, Pascual J, Vazquez J, et al. Durable and controlled depletion of neutrophils in mice. Nature communications. 2020;11:2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


