Abstract
Purpose of review
Electrical stimulation of the peripheral and central vestibular system using non-invasive (galvanic vestibular stimulation, GVS) or invasive (intracranial electrical brain stimulation, iEBS) approaches have a long history of use in studying self-motion perception and balance control. The aim of this review is to summarize recent electrophysiological studies of the effects of GVS, and functional mapping of the central vestibular system using iEBS in awake patients.
Recent findings
The use of GVS has become increasingly common in the assessment and treatment of a wide range of clinical disorders including vestibulopathy and Parkinson’s disease. The results of recent single unit recording studies have provided new insight into the neural mechanisms underlying GVS-evoked improvements in perceptual and motor responses. Furthermore, the application of iEBS in patients with epilepsy or during awake brain surgery has provided causal evidence of vestibular information processing in mostly the middle cingulate cortex, posterior insula, inferior parietal lobule, amygdala, precuneus and superior temporal gyrus.
Summary
Recent studies have established that GVS evokes robust and parallel activation of both canal and otolith afferents that is significantly different from that evoked by natural head motion stimulation. Furthermore, there is evidence that GVS can induce beneficial neural plasticity in the central pathways of patients with vestibular loss. In addition, iEBS studies highlighted an underestimated contribution of areas in the medial part of the cerebral hemispheres to the cortical vestibular network.
Keywords: Galvanic vestibular stimulation, Electrical brain stimulation, Epilepsy, Vestibular cortex, Vestibular system
INTRODUCTION
Non-invasive (galvanic vestibular stimulation, GVS) and invasive (intracranial electrical brain stimulation, iEBS) electrical stimulation of the peripheral and central vestibular system have a long history of use in studying self-motion perception and balance control [1,2].
Over the past two decades, GVS has increasingly been used to probe the vestibular contributions to several components of perception, cognition and emotions (reviewed in [3,4]). However, until recently there was a paucity of data about the neural mechanisms of GVS and its oculomotor, postural and perceptual effects in non-human primates and humans.
As GVS is compatible with fMRI and PET, it has been used to describe the human equivalent of non-human primate’s cortical vestibular network in neurotypical participants [5–7]. A complementary way to delineate central vestibular pathways and structures is iEBS, a gold standard for mapping brain functions in neurology [8]. Pioneering work in patients with epilepsy or during brain tumor resection in awake patients [9] has demonstrated that iEBS can provide new insights into the neural substrate of vestibular representations within the cerebral cortex.
Electrical stimulation of both the peripheral and central vestibular systems are now common and their underlying mechanisms need to be understood, especially, given the growing evidence of potential therapeutic effects of various forms of GVS and iEBS [10–12].
This review summarizes a selection of recent studies that have used GVS and iEBS. We focus on single unit recordings to understand the effects of GVS on vestibular afferents and central structures, and the effects of iEBS to identify the cortical and subcortical structures processing vestibular signals.
GALVANIC VESTIBULAR STIMULATION
GVS is commonly used to non-invasively probe and perturb vestibular function in humans. The use of GVS involves stimulating the peripheral vestibular system via the application of current to external electrodes placed on the mastoid processes. In addition to its use in basic research, GVS is also now increasingly used in the assessment and treatment of a wide range of clinical disorders including vestibulopathy and Parkinson’s disease (reviewed in [13,14]).
Method of activation, and basic/clinical findings
In contrast to natural motion, GVS bypasses the mechanotransduction of both canal and otolith vestibular sensory organs to directly activate the vestibular afferents of the VIII nerve (and potentially hair cells themselves). The resulting afferent activation in turn, evokes eye movements via central VOR pathways (reviewed in [13]), postural response via central vestibulo-spinal pathways (reviewed in [15]), as well as virtual sensations of self-motion (e.g., [16–18]).
GVS is typically applied in a binaural manner that activates afferents from all 5 vestibular sensory end organs on one side with concurrent inhibition of those on the contralateral side. The resulting activation is thus unnatural since the pattern of combined otolith and semicircular canal afferent activation has no physiological motion equivalent. At the population level, the net effect of GVS has been modeled as a vector of the summed canal activation with an overall net cancellation of the otolithic signals [1,19], where asymmetries in afferent responses induces a directional bias in the net population response [20].
To date, three primary classes of wave forms have been utilized in basic and clinical GVS human studies: currents steps, sinusoids, and band-limited noise (also termed stochastic GVS or noisy GVS (reviewed in [13]). Most current work is focused on the use of stochastic GVS due to its efficiency of application and improved subject comfort. Suprathreshold noisy GVS has been applied to probe vestibulo-motor responses of both the eyes and different axial and appendicular muscles. Leg and trunk muscles show response over a limited frequency range (<15−25 Hz) that modulate with standing posture [21,22], postural transitions [23], and gait cycle [24,25]. In contrast, neck muscles show GVS responses for frequencies up to 150 Hz [26]) that are task independent [1]. This latter result suggests that the vestibulocollic reflex is largely hardwired (reviewed by [1]).
Moreover, there has been much recent discussion regarding the use of subthreshold noisy GVS to improve balance function. While a number of studies have reported positive outcomes (healthy [11,27] and patients [27–29]), the mechanisms by which improvements occur remain unknown. On the one hand, it has been proposed that subthreshold induces stochastic resonance (see for example, [30,31]). However, several recent investigations have failed to provide consistent support for this view (e.g., [32,33], reviewed in [34–36]). Importantly, an open question is whether the stimuli used in studies demonstrating positive outcomes have employed supra- rather than subthreshold stimuli.
Effects of GVS on the peripheral vestibular system
Single unit recording studies are required to understand how GVS actually activates the central neural pathways underlying perception and behavior. In turn, this fundamental knowledge has significant implications for advancing the development of more accurate stimulation techniques to selectively probe and perturb vestibular function. A series of recent experiments conducted on macaque monkeys used a setup comparable to that commonly used in humans to explicitly establish how GVS activates vestibular afferents [20,26,37]. GVS was applied to surface electrodes placed behind the monkeys’ ears (Figure 1A) and induced torsional eye movements similar to those observed in humans [37]. Single unit recording experiments then established that GVS 1) evokes robust and comparable responses in canal and otolith afferents and 2) that these responses differ from those evoked by natural head motion stimulation. This latter finding is not unexpected given that GVS activates afferents by bypassing the biomechanics of both the semicircular canals and otolith organs, which contribute to the dynamics of responses to rotation and translation, respectively.
Figure 1. Effects of GVS on the peripheral vestibular system.
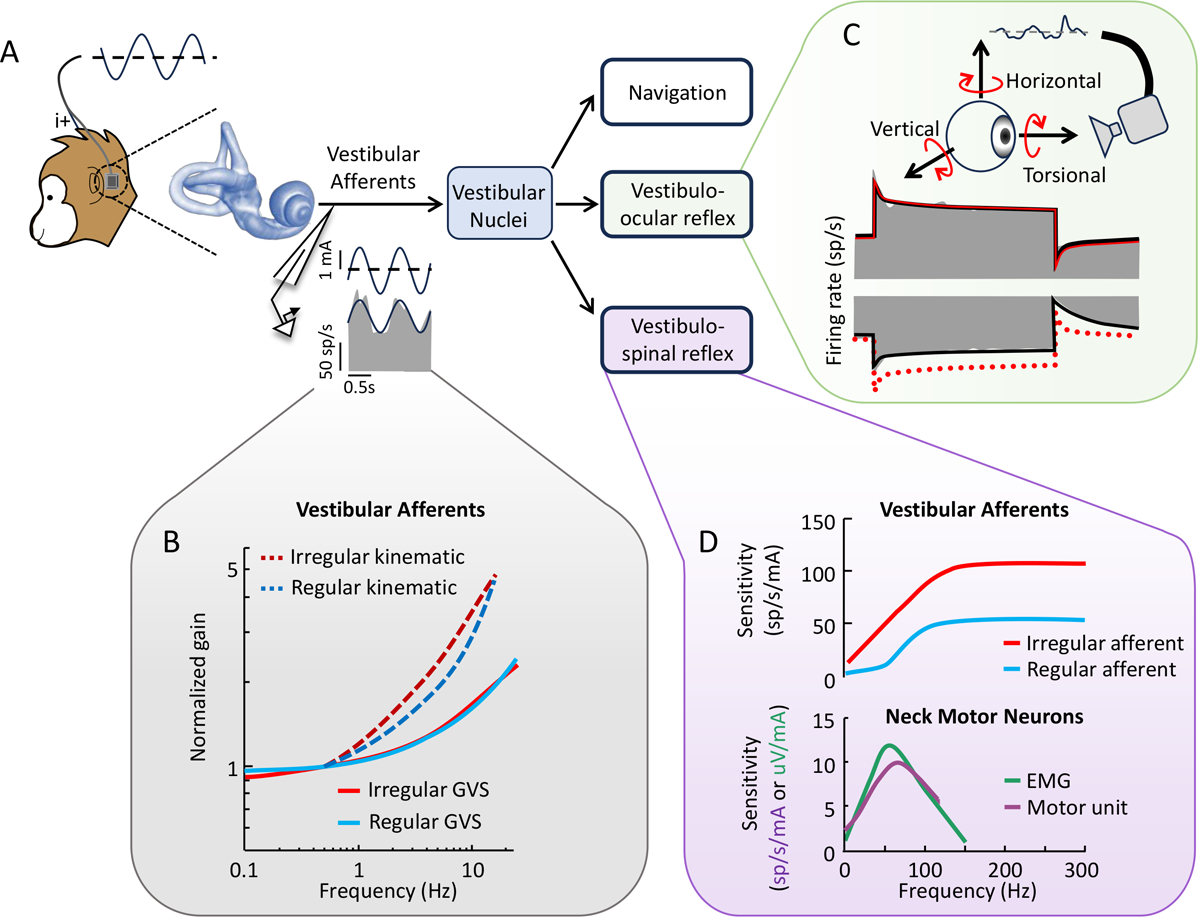
(A) Schematic of the setup used to apply GVS in the nonhuman primate model. Stimulation is applied between surface electrodes placed on the mastoid processes behind the ears, while the animal’s eye movements and neural activity are recorded. (B) Response dynamics of vestibular canal afferents to sinusoidal GVS where the population averaged gain is shown for regular and irregular (blue and red, respectively) canal afferents. Dashed lines illustrating the corresponding responses to actual rotational motion are shown for comparison. (C) Constant current GVS evokes asymmetric changes in afferent firing rates during stimuli of opposing polarity, primarily for irregular afferents. Schematic of the asymmetric responses (gray) of an irregular afferent is shown, respectively. The red traces show the response fit to cathodal stimulation (solid), and the mirrored fit superimposed on the anodal response. (D) Response dynamics of monkey vestibular afferents and neck motor neurons to high-frequency (0–300 Hz) GVS. Population responses are shown for irregular versus regular canal afferents (top, red and blue respectively) versus neck single unit versus EMG activity (bottom, purple and green respectively). From Kwan et al. [85], Forbes et al. [26] and [20], with permission from the authors.
More specifically, in response to GVS canal and otolith afferents both demonstrate a similar increase in gain with increasing stimulation frequency. Further, more irregular afferents demonstrate larger firing sensitivity compared to their regular counterparts. However, this gain increase is less than that observed for natural head motion (Figure 1B). GVS evoked-afferent responses also became more phasically dynamic with increasing frequency (Figure 1B), contrasting with reports based on internal stimulation of the inner ear [38,39]. Overall, the dynamics of GVS-induced afferent responses cannot be predicted by a simple stochastic model of repetitive afferent activity [40], suggesting that other key factors (e.g., hair-cell mediated activity, nonquantal transmission, and the dynamics of vestibular afferent conductances) also shape afferent responses to GVS. Future work focused on the development of a mechanistic GVS model (e.g., [41]) is an important direction for future work.
Recent experimental findings in monkey have also challenged the prevailing view that GVS linearly activates the vestibular system. In response to cathodal versus anodal GVS currents, canal and otolith afferents both display significant response asymmetries, which also manifest in asymmetric eye movement responses via vestibulo-ocular pathways (Figure 1C). For example, the onset of a cathodal versus anodal current step induces more pronounced changes in afferent responses [20]. These nonlinearities are more pronounced in irregular afferents relative to their regular counterparts. Simulations combining these experimental results with a well-established computational model of the net effect of GVS [1], has led to the unexpected (and not yet tested) prediction that GVS induces directional biases in centrally integrated head motion signals [20].
Finally, it is noteworthy that recent GVS studies in macaque monkeys have directly compared the activation of afferents versus the neck musculature via vestibulo-spinal pathways, specifically the vestibulocollic reflex. The vestibulocollic reflex stabilizes head position in space by activating the neck musculature in response to unexpected self-motion across the natural range of head motion ([42]; Figure 1D). Strikingly, both neck motoneurons and primary vestibular afferents respond to sinusoidal GVS stimulation up to 300 Hz, peaking around 70−80 Hz ([26]; Figure 1D). Taken together, these findings suggest that that the high-frequency information encoded by afferents is indeed transmitted through the vestibular system to stabilize the head during unexpected head transients.
Effects of GVS on the central vestibular system
Despite the recent progress made in understanding GVS’s impact on individual vestibular afferents, our understanding of its effect on central vestibular pathways remains quite limited. Vestibular afferents directly target central neurons in the vestibular nuclei (reviewed in [43]). In-vitro studies using vestibular nuclei slices have long been used to investigate electrophysiological properties of individual neurons (reviewed in [44]). More recent experiments (reviewed in [34]) have further shown that stochastic versus sinusoidal current waveforms – comparable to those used in the clinic – induce differential changes in neuronal membrane potential, neuronal regularity, and response gain across neurons. To date, however, only a handful of in-vivo studies have investigated how GVS alters signal transmission in central vestibular pathways. One 2022 study assessing how vestibular nuclei neurons encode combined GVS and motion stimulation [45] reported that sub additive responses similar to those reported for the integration of semicircular canal and otolith afferents in these cells [46]. This same group also reported that repeated GVS reduces vestibular nuclei neuronal potentials [45]. Notably, this reduction was accompanied by a decrease in AMPA and NMDA receptors, leading to proposal that GVS induces a reduction in the number of glutamate receptors that in turn modifies neuronal potentials in the vestibular nucleus.
Vestibular stimulation also activates higher level areas of central vestibular processing. The vestibular input to the hippocampus is thought to play an essential role in spatial navigation and for updating brain representations of spatial information (reviewed in [43]). A recent study in rats demonstrated that GVS altered hippocampal cell proliferation and neurogenesis; high amplitude GVS causes a marked decrease in cell proliferation, and corresponding decrease in neurogenesis [47]. Surprisingly, however, these changes were not linked to functional impairments in spatial memory. Human imaging studies have likewise shown that GVS activates the hippocampus (e.g., [48,49]). In addition, imaging studies have shown that GVS activates regions of the cerebellum, thalamus, and cortical areas (i.e., PIVC, 3aV, and 2v) associated with self-motion processing [7,50–53]. Interestingly, bilateral vestibulopathy patients demonstrate reduced resting state brain activity in several of these core cortical vestibular regions, that can be increased via GVS (Figure 2A; [54,55]). Moreover, these GVS-induced changes were linked to better patient outcomes, suggesting that GVS might induce beneficial neural plasticity. A recent comparison of noisy versus conventional (current step) GVS indicated that the former resulted in greater increase brain activity in vestibular cortical areas [56]. While this result led the authors to suggest that noisy GVS evokes stochastic resonance, further investigation will be required to i) understand the actual neural mechanisms responsible for the observed enhancements and to ii) further optimize the application of GVS as a non-invasive therapeutic approach to improve patient outcomes. Correspondingly, the development of new methods for internal peripheral stimulation using a vestibular implant (Figure 2B, see “Future Directions” below) provides another promising approach for improving patient outcomes.
Figure 2. Effects of GVS and vestibular implant stimulation on the central vestibular system.
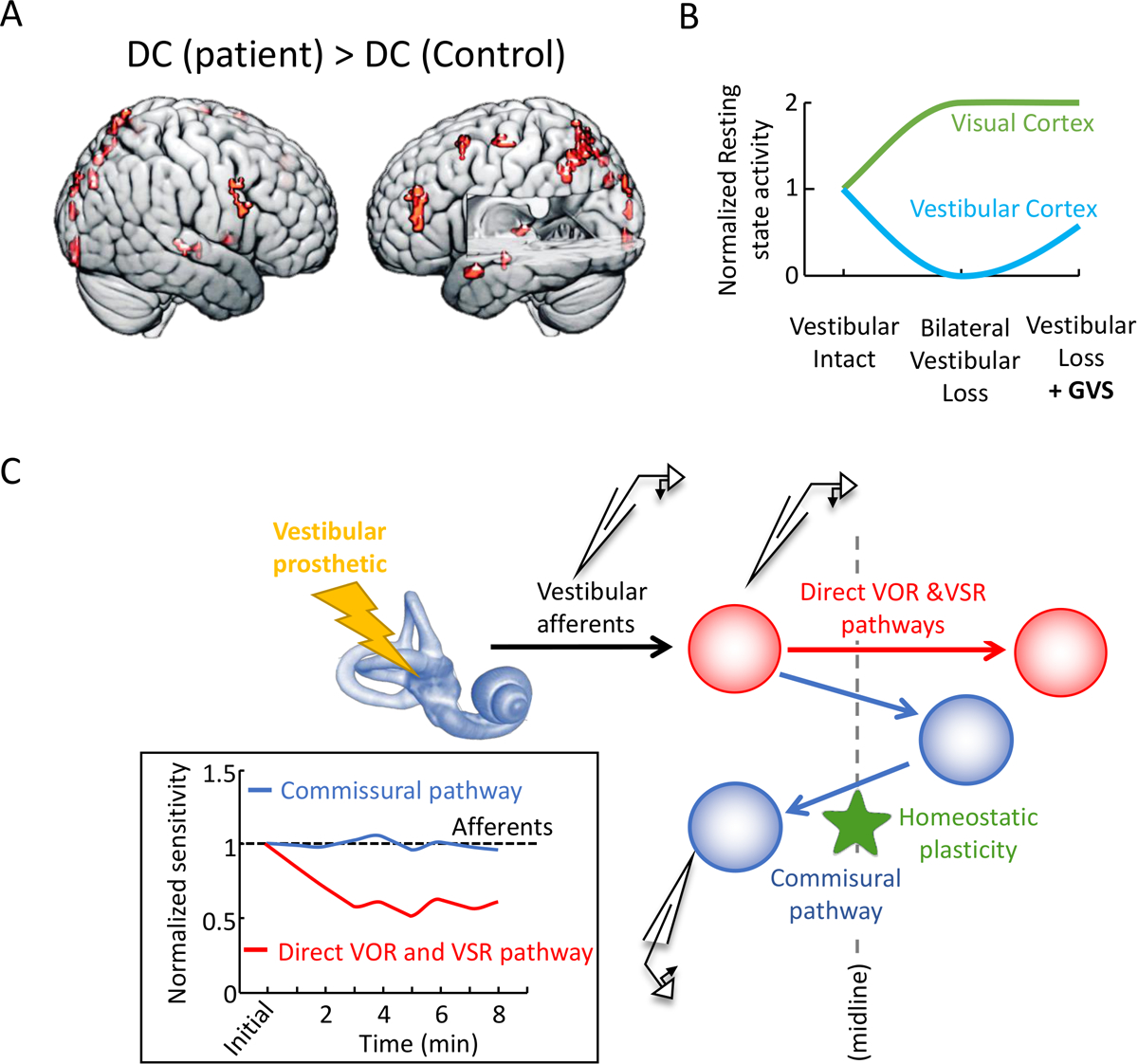
(A) Compared to healthy control subjects, resting state brain activity of bilateral loss patients was smaller in vestibular core regions but larger in several fronto-parietal and occipital brain areas associated with visual processing. (B) GVS increased resting state in vestibular core regions in both groups, as well as in visual regions in patients that was associated with lower dizziness. Helmchen et al. [55]. (C) Schematic diagram of the direct versus commissural pathways mediating the VOR and vestibulo-spinal reflexes. Inset: Normalized sensitivity of direct versus commissural pathway neurons in the vestibular nuclei over a 10-min period following activation of the vestibular nerve. The black dashed line is showing, for comparison, the lack of adaption in vestibular afferents. Adapted from Mitchell et al. [98] with permission from the authors.
INTRACRANIAL ELECTRICAL BRAIN STIMULATION
The central vestibular network described above [7,50–53] has been revealed by neuroimaging studies contrasting BOLD signal during GVS with various control conditions. Complementary to this approach, iEBS can disrupt neural activity within specific nodes of the central vestibular pathways. iEBS can provide causal evidence [8] of the specific contribution of cortical and subcortical structures in the neural network processing vestibular information. iEBS consists of delivering an electrical current through electrodes directly at the surface of the cortex, in deep structures, or subcortical fibres. iEBS can evoke from simple perceptions, such as vestibular illusions, to complex hallucinations and behaviours.
Cortical networks underpinning vestibular representations as revealed by iEBS
One part of the cortex that has long been related to vestibular information processing in non-human primates is the posterior insula (with adjacent parietal operculum and retroinsular cortex) [57–59]. Surprisingly, Penfield [60] had originally reported that iEBS in the insula evoked no vestibular sensation during surgery for focal epilepsy. However, a retrospective analysis of 219 patients with epilepsy revealed that vestibular sensations (illusory body rotation or translation, vertigo, dizziness) in fact represents ~8% of all responses evoked by iEBS through implanted electrodes in the insula [61]. Notably, vestibular sensations were mostly elicited by iEBS in the posterior insula, and rotatory and translational self-motion illusions were evoked by iEBS more posterior than vertigo and dizziness [61]. Following up on this, Yu et al. [62] analysed the effect of operculo-insular iEBS in patients with epilepsy, and found that dizziness was evoked by stimulation of the superior part of the left posterior long gyrus (i.e., posterior insula). Other functional mapping of the insula with iEBS revealed that its posterior part, while eliciting some vestibular sensations, was more involved in somatosensory and pain perception, whereas the anterior insula was more involved in visceral functions [63].
The parietal cortex has also long been associated with vestibular information processing in animal models, as evidenced by vestibular-sensitive neurons in the primary somatosensory cortex, intraparietal sulcus, retroinsular cortex and parietal operculum (reviewed in [64]). Consistent with these findings, iEBS in epileptic patients demonstrated vestibular sensations in both the lateral and medial parts of the parietal cortex [65]. Operculo-insular stimulation in patients with epilepsy revealed that vertigo and the feeling of body elevation or movement to one side were evoked by iEBS of the bilateral parietal operculum, and by no other opercular region [62]. A recent retrospective analysis in a large patient cohort with epilepsy (n=165) revealed that ~21% of iEBS evoking vertigo were in the posterior insula or parietal operculum (area OP2) [66]. This data provides causal evidence that stimulation in this core area within the vestibular cortical network [7,67–69] produces a vestibular sign. Balestrini et al. [70] retrospectively analyzed data from iEBS in the parietal cortex in 172 patients with epilepsy. Vertigo was observed during iEBS in the precuneus, inferior parietal lobule, posterior cingulum, superior parietal lobule, intraparietal sulcus, and postcentral gyrus. In contrast, no such responses were evoked by iEBS in the primary somatosensory cortex in two retrospective studies [71,72], despite evidence of vestibular projections to the primary somatosensory cortex in several animal species [73,74].
Interestingly, the precuneus, which was until recently not considered a main vestibular area, is the parietal area where most vestibular responses have been elicited [70]. A recent study emphasized vestibular illusions in the anterior precuneus [75], with iEBS in the left precuneus evoking mostly sensations of dropping, slipping, falling and dizziness, whereas iEBS in the right precuneus evoked mostly sensations of floating and elevation. This finding is consistent with those of recent fMRI studies identifying an egomotion selective area in the anterior precuneus, referred to as Pcm (precuneus motion area), that responded to both GVS and optic flow [7]. Yet, other studies described area Pcm as only sensitive to optic flow [76].
Taken together, these studies suggest that the medial part of the cerebral hemispheres may play a more substantive role in shaping vestibular representations than has been assumed based on early studies in non-human primates [57,77]. In fact, in the last few years, evidence of vestibular responses in the cingulate cortex (CC) were provided by electrophysiological recordings in the macaque posterior CC [78], fMRI in rats during optogenetic activation of the vestibular nuclei [79], and fMRI in humans using GVS or caloric vestibular stimulation [7,76,80].
Consistent with these findings, three recent retrospective analyses in patients with epilepsy reported vestibular responses during iEBS in the CC [81–83]. Vestibular responses represented 4.3% [83] to 8.3% [81] of all responses evoked by CC stimulation. The two studies with the largest samples (n=329 [81] and n=124 [83]) identified most vestibular responses in the middle CC (right posterior middle CC, as well as to a smaller extent the ventral anterior middle CC) and the posterior CC (dorsal part [81]). By contrast, the study with the smallest sample (n=47 [82]) reported that vestibular sensations were evoked by iEBS in the left anterior and posterior CC. Altogether, the data suggest predominant vestibular representations at the junction between the middle and posterior CC [83]. Although data in animals are rare, it was also in the posterior CC that neurons responded to whole-body translations and rotations, and to a lesser extent to optic flow [78]. Altogether, results from recent iEBS studies confirm the existence of a vestibular and visual area, referred to as the cingulate sulcus visual area (CSv), in the posterior part of the middle CC [7,76,80,84].
A recent systematic review of the literature by Dary et al. [85] summarized cases of illusory whole-body translations and rotations (excluding vertigo/dizziness) evoked by iEBS. Across the different studies (Figure 3A–C), most iEBS evoking vestibular sensations were in the middle CC, posterior insula, inferior parietal lobule, amygdala, precuneus and superior temporal gyrus, whereas vestibular sensations were very rare after occipital and frontal cortex stimulation (Figure 3B) [85]. In addition, the proportion of vestibular sensations after iEBS in the right hemisphere was significantly higher than it was in the left hemisphere, a finding coherent with the right-sided dominance of vestibular pathways to the cerebral cortex in neurotypical individuals [86].
Figure 3. Localization of iEBS evoking vestibular illusions reported in a systematic review of the literature.

The review identified 131 cases of illusory self-motion perception (excluding vertigo and dizziness) evoked by iEBS reported in the literature between 1937 and 2022. Most of the electrode contacts where vestibular illusions were evoked by iEBS could be located retrospectively according to published electrode coordinates, or with the best approximation possible considering the published MRI, implantation schema, or the original description of iEBS in terms of gyrus, sulcus, and Brodmann area. The number of illusory self-motion perception evoked by iEBS is showed for different brain areas (A), and as a function of iEBS in the different lobes and cingulate cortex (B). (C) iEBS sites evoking illusory self-motion perception are displayed on 3D views of a right cerebral hemisphere. The rightmost part shows iEBS in the insula and mesio-temporal region. IPL, inferior parietal lobule; SPL, superior parietal lobule. Adapted from Dary et al. [85], with permission from the authors.
Effects of iEBS on subcortical structures
Evidence for vestibular representations in subcortical structures has recently been investigated during presurgical evaluation of epilepsy [87,88] and in Parkinson’s disease. Here we focus on the former, since the latter has been reviewed in detail elsewhere [2,12,89].
Recently, Qi et al. [87] reviewed the effects of iEBS in the basal ganglia in 35 patients with epilepsy. Vestibular sensations were the second most frequent responses after sensorimotor responses. They were elicited by iEBS in the putamen and external globus pallidus, mostly on the right side (Figure 4A). No vestibular response was found in the dorsal caudate nucleus, internal globus pallidus and subthalamic nucleus. This causal demonstration of vestibular representations in the basal ganglia helps disambiguate heterogenous results about the basal ganglia involvement in vestibular networks [90]. Indeed, recent studies in rats identified very few striatal neurons responding to electrical stimulation applied to the round window [91]. However, in response to the same type of stimulation, local field potentials were recorded in the tail of the rat striatum [92] and neurochemical changes were found in the striatum [93]. Vestibular pathways to the striatum may involve the perifascicular nucleus in the thalamus and direct projections from the cortex [90] (Figure 4B).
Figure 4. Vestibular representations in the basal ganglia.
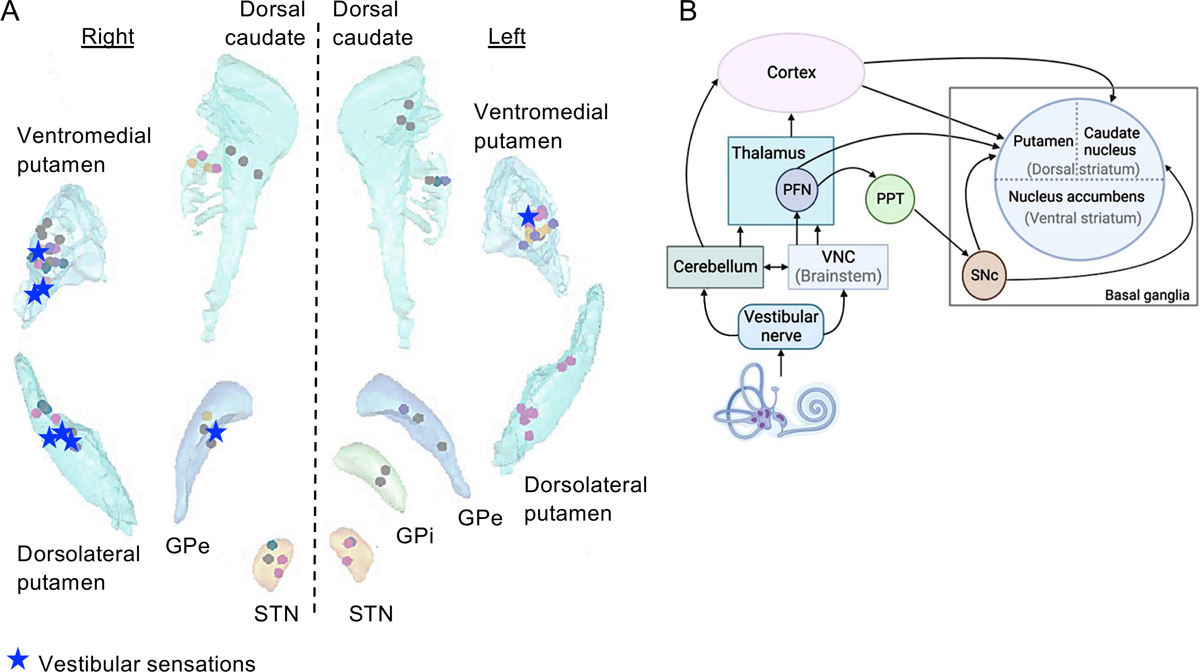
(A) Location of electrode contacts evoking vestibular sensations during iEBS. After sensorimotor responses (n = 23), vestibular sensations were the second most frequent responses evoked by iEBS (n = 8; dizziness, spinning, floating). Vestibular sensations were evoked by iEBS in the ventromedial putamen (n = 4), dorsolateral putamen (n = 3) and external globus pallidus (n = 1). Modified from Qi et al. [87] (images under Creative Commons Attribution 4.0 International License). (B) Probable pathways from the vestibular apparatus to the basal ganglia. PFN, parafascicular nucleus; PPT, pedunculopontine tegmental nucleus; SNc, substantia nigra pars compacta. Reproduced from Sabzevar et al. [92] (images under Creative Commons Attribution 4.0 International License).
Furthermore, vestibular responses evoked by iEBS of the amygdala have recently been described in patients with epilepsy [88]. Out of 250 responses evoked by iEBS in the amygdala, vestibular sensations represented ~6% of all reports. Sites at which iEBS evoked vestibular responses were in the laterobasal and superficial groups of the amygdala. It is unclear how vestibular information reaches the amygdala. Yet, the parabrachial nucleus, which responds to vestibular stimulation, is reciprocally connected with the vestibular nuclei, amygdala, hypothalamus and prefrontal cortex [94].
Causal demonstration of vestibular representations in subcortical structures should motivate to study their contribution to spatially-oriented behaviors and spatial cognition.
FUTURE DIRECTIONS
Above we have reviewed how the electrical stimulation of the peripheral and central vestibular systems has advanced our understanding of the neural mechanisms and networks underpinning vestibular processing and self-motion perception. These lines of research offer opportunities for developing novel therapeutic strategies in patients with vestibular disorders and have clinical relevance for neurology.
Internal peripheral stimulation and vestibular implants
Pulsatile stimulation of chronically implanted electrodes in animals has been an essential tool for probing the circuitry of vestibular pathways. Recent work based on this approach has led to the development of vestibular implants for patients with bilateral peripheral loss. These implants typically comprise electrodes implanted into or near the ampulla of the semicircular canals. Head motion information, sensed by gyroscopes, is first projected into the three canal planes, and then converted into a sequence of electrical pulses for each implanted electrode (reviewed in [10,13]). Ongoing clinical trials have reported improvements in postural control [95] and to a lesser extent gaze stability [96,97]. Significant challenges must still be overcome, including the reduction of central vestibular pathway efficacy due to stimulation induced afferent synchrony (Figure 2C; [98,99]) and expanding vestibular implants to restore otolith as well as canal function.
Advances in diagnosis and treatment of focal epilepsy
Epileptic seizures with prominent vertigo and dizziness have been related to epileptic foci in the temporal cortex and temporo-parieto-occipital junction [100]. Recent evidence of vestibular representations in the medial part of the cerebral hemispheres should help understand the complex semiology of seizures in patients with cingulate [101] or precuneal epilepsy [102], which are difficult to characterize by surface EEG. To better characterize seizures associated with vertigo and dizziness, it would be important to delineate the exact connectivity within the cortical vestibular network, using effective connectivity (e.g., cortico-cortical evoked potentials) and functional connectivity (e.g., non-linear correlation of EEG signals) from iEBS and stereo-electroencephalography in patients with epilepsy (for similar approaches, see [75,82]).
CONCLUSION
Studies summarized in this review help understand long-described effects of electrical stimulation of the peripheral and central vestibular system, which commonly evoke illusory self-motion perception and modulate multisensory integration properties to ensure balance and gaze stability. By modulating the functioning of large networks from the brainstem to the cerebral cortex, basal ganglia, and other subcortical structures, GVS offer avenues for the neuromodulation of vestibular functions using safe, accessible, and inexpensive procedures.
KEY POINTS.
GVS produces robust and parallel activation of both canal and otolith afferents that significantly differ from those evoked by natural head motion stimulation
GVS evokes highly asymmetric responses in irregular afferents for cathodal versus anodal currents, leading to a directional bias in the net population central response
Recent studies suggest that GVS induces beneficial neural plasticity in the central pathways of patients with vestibular loss
iEBS has provided novel causal evidence of vestibular representations in medial brain areas (middle cingulate cortex and precuneus) and subcortical structures (basal ganglia, amygdala)
GVS can contribute to the assessment and treatment of vestibulopathy and Parkinson’s disease, while iEBS can help understand cingulate and precuneal epilepsies
Acknowledgements
We thank Jerome Carriot for helping to prepare Figures 1 and 2, and Zoé Dary for helping to prepare Figure 3.
Financial support and sponsorship
This work was supported by the ANR VESTISELF project, grant ANR-19-CE37-0027 of the French Agence Nationale de la Recherche to C. Lopez and by NIH grants (Grants R01-DC002390 and R01-DC013069) to K.E. Cullen.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–16. [DOI] [PubMed] [Google Scholar]
- 2.Tarnutzer AA, Ward BK, Shaikh AG. Novel ways to modulate the vestibular system: Magnetic vestibular stimulation, deep brain stimulation and transcranial magnetic stimulation / transcranial direct current stimulation. J Neurol Sci. 2023. Feb 15;445:120544. [DOI] [PubMed] [Google Scholar]
- 3.Lopez C The vestibular system: balancing more than just the body. Curr Opin Neurol. 2016. Feb;29(1):74–83. [DOI] [PubMed] [Google Scholar]
- 4.Utz KS, Dimova V, Oppenländer K, Kerkhoff G. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology--a review of current data and future implications. Neuropsychologia. 2010. Aug;48(10):2789–810. [DOI] [PubMed] [Google Scholar]
- 5.Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998;80:2699–709. [DOI] [PubMed] [Google Scholar]
- 6.Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol. 2001;85:886–99. [DOI] [PubMed] [Google Scholar]
- 7.Ruehl RM, Flanagin VL, Ophey L, Raiser TM, Seiderer K, Ertl M, et al. The human egomotion network. NeuroImage. 2022. Dec 1;264:119715. [DOI] [PubMed] [Google Scholar]; ■■ A functional neuroimaging study that used binaural GVS in the to date largest cohort of right-handed participants (n = 131). Participants received local anaesthesia of the postauricular region to control for unspecific effects of GVS. Some participants were shown an optic flow that was compatible or incompatible with egomotion (i.e., coherent/incoherent motion of dots). The conjunction of BOLD responses to vestibular stimulation and to egomotion-compatible visual stimulation was particularly evident in the uvula, cingulate sulcus (CSv [cingulate sulcus visual area] and Pcm/pCi [precuneus motion area]) and in the temporo-parietal cortex (newly identified “area h7a” in the supramarginal gyrus and area VPS [visual posterior Sylvian area]).
- 8.Siddiqi SH, Kording KP, Parvizi J, Fox MD. Causal mapping of human brain function. Nat Rev Neurosci. 2022. Jun;23(6):361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penfield W Vestibular sensation and the cerebral cortex. Ann Otol Rhinol Laryngol. 1957;66:691–698. [DOI] [PubMed] [Google Scholar]
- 10.Sluydts M, Curthoys I, Vanspauwen R, Papsin BC, Cushing SL, Ramos A, et al. Electrical Vestibular Stimulation in Humans: A Narrative Review. Audiol Neurootol. 2020;25(1–2):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wuehr M, Boerner JC, Pradhan C, Decker J, Jahn K, Brandt T, et al. Stochastic resonance in the human vestibular system - Noise-induced facilitation of vestibulospinal reflexes. Brain Stimulat. 2018;11(2):261–3. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh AG, Antoniades C, Fitzgerald J, Ghasia FF. Effects of Deep Brain Stimulation on Eye Movements and Vestibular Function. Front Neurol. 2018. Jun 12;9:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dlugaiczyk J, Gensberger KD, Straka H. Galvanic vestibular stimulation: from basic concepts to clinical applications. J Neurophysiol. 2019. Jun 1;121(6):2237–55. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Liu A, McKeown MJ. Current perspectives on galvanic vestibular stimulation in the treatment of Parkinson’s disease. Expert Rev Neurother. 2021. Apr;21(4):405–18. [DOI] [PubMed] [Google Scholar]
- 15.Forbes PA, Siegmund GP, Schouten AC, Blouin JS. Task, muscle and frequency dependent vestibular control of posture. Front Integr Neurosci. 2014;8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosravi-Hashemi N, Forbes PA, Dakin CJ, Blouin JS. Virtual signals of head rotation induce gravity-dependent inferences of linear acceleration. J Physiol. 2019. Nov;597(21):5231–46. [DOI] [PubMed] [Google Scholar]; ■ This study recorded participants’ perceptions of head motion during GVS. The finding that subjects perceive both linear acceleration and rotation motion, when electrical stimulation-induced rotational vector with a component orthogonal to gravity, has important implications for the use of GVS in human biomedical or sensory augmentation applications.
- 17.St George RJ, Day BL, Fitzpatrick RC. Adaptation of vestibular signals for self-motion perception. J Physiol. 2011. Feb 15;589(Pt 4):843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters RM, Rasman BG, Inglis JT, Blouin JS. Gain and phase of perceived virtual rotation evoked by electrical vestibular stimuli. J Neurophysiol. 2015. Jul;114(1):264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider E, Glasauer S, Dieterich M. Comparison of human ocular torsion patterns during natural and galvanic vestibular stimulation. J Neurophysiol. 2002. Apr;87(4):2064–73. [DOI] [PubMed] [Google Scholar]
- 20.Forbes PA, Kwan A, Mitchell DE, Blouin JS, Cullen KE. The Neural Basis for Biased Behavioral Responses Evoked by Galvanic Vestibular Stimulation in Primates. J Neurosci. 2023. Mar 15;43(11):1905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ GVS current steps were revealed to evoke asymmetries in vestibular afferent responses recorded in macaque monkeys. These response asymmetries to cathodal versus anodal stimulation, which were more marked for irregular canal and otolith afferents, have implications regarding the optimization of GVS stimuli to recreate physiologically plausible sensations of motion.
- 21.Forbes PA, Luu BL, Van der Loos HFM, Croft EA, Inglis JT, Blouin JS. Transformation of Vestibular Signals for the Control of Standing in Humans. J Neurosci. 2016. Nov 9;36(45):11510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ The results of this GVS study in humans showed that vestibular-evoked postural muscle responses are directionally specific. Specifically, responses are aligned with the balance direction and thus relevant to the balance task, not to the cumulative afferent activity, as might be expected for vestibulospinal reflex loops.
- 22.Mian OS, Day BL. Violation of the craniocentricity principle for vestibularly evoked balance responses under conditions of anisotropic stability. J Neurosci. 2014. May 28;34(22):7696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tisserand R, Dakin CJ, Van der Loos MH, Croft EA, Inglis TJ, Blouin JS. Down regulation of vestibular balance stabilizing mechanisms to enable transition between motor states. eLife. 2018. Jul 10;7:e36123. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ GVS was applied as healthy volunteers transitioned between standing still and walking. Evoked stabilizing balance responses were suppressed just prior to walking to enable transition between motor states. This finding has implications for understanding movement disorders such as Parkinson’s disease.
- 24.Dakin CJ, Inglis JT, Chua R, Blouin JS. Muscle-specific modulation of vestibular reflexes with increased locomotor velocity and cadence. J Neurophysiol. 2013. Jul;110(1):86–94. [DOI] [PubMed] [Google Scholar]
- 25.Forbes PA, Vlutters M, Dakin CJ, van der Kooij H, Blouin JS, Schouten AC. Rapid limb-specific modulation of vestibular contributions to ankle muscle activity during locomotion. J Physiol. 2017. Mar 15;595(6):2175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes PA, Kwan A, Rasman BG, Mitchell DE, Cullen KE, Blouin JS. Neural Mechanisms Underlying High-Frequency Vestibulocollic Reflexes In Humans And Monkeys. J Neurosci. 2020. Feb 26;40(9):1874–87. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This study combined neurophysiology and GVS to provide insight into the neural dynamics producing vestibulocollic reflexes. GVS-evoked vestibular afferent and neck muscle responses were recorded in rhesus monkeys. Vestibular primary afferents encoded high-frequency stimulation (up to 300 Hz) via frequency-dependent increases in sensitivity and phase-locking that then underwent low-pass filtering when transmitted to neck.
- 27.Keywan A, Jahn K, Wuehr M. Noisy Galvanic Vestibular Stimulation Primarily Affects Otolith-Mediated Motion Perception. Neuroscience. 2019. Feb 10;399:161–6. [DOI] [PubMed] [Google Scholar]
- 28.Wuehr M, Eder J, Keywan A, Jahn K. Noisy galvanic vestibular stimulation improves vestibular perception in bilateral vestibulopathy. J Neurol. 2023. Feb;270(2):938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schniepp R, Boerner JC, Decker J, Jahn K, Brandt T, Wuehr M. Noisy vestibular stimulation improves vestibulospinal function in patients with bilateral vestibulopathy. J Neurol. 2018. Oct;265(Suppl 1):57–62. [DOI] [PubMed] [Google Scholar]
- 30.Gensberger KD, Kaufmann AK, Dietrich H, Branoner F, Banchi R, Chagnaud BP, et al. Galvanic Vestibular Stimulation: Cellular Substrates and Response Patterns of Neurons in the Vestibulo-Ocular Network. J Neurosci. 2016. Aug 31;36(35):9097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lajoie K, Marigold DS, Valdés BA, Menon C. The potential of noisy galvanic vestibular stimulation for optimizing and assisting human performance. Neuropsychologia. 2021. Feb 12;152:107751. [DOI] [PubMed] [Google Scholar]
- 32.Assländer L, Giboin LS, Gruber M, Schniepp R, Wuehr M. No evidence for stochastic resonance effects on standing balance when applying noisy galvanic vestibular stimulation in young healthy adults. Sci Rep. 2021. Jun 10;11(1):12327. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This study tested the effects of subthreshold stochastic GVS on body sway in young healthy participants. Reductions in body sway did not consistently follow the expected SR-like bell-shaped performance curve for stochastic resonance, leading to the conclusions that other factors contributed to the observed postural effects.
- 33.Piccolo C, Bakkum A, Marigold DS. Subthreshold stochastic vestibular stimulation affects balance-challenged standing and walking. PloS One. 2020;15(4):e0231334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefani SP, Serrador JM, Breen PP, Camp AJ. Impact of galvanic vestibular stimulation-induced stochastic resonance on the output of the vestibular system: A systematic review. Brain Stimulat. 2020;13(3):533–5. [DOI] [PubMed] [Google Scholar]
- 35.McLaren R, Smith PF, Taylor RL, Ravindran S, Rashid U, Taylor D. Efficacy of nGVS to improve postural stability in people with bilateral vestibulopathy: A systematic review and meta-analysis. Front Neurosci. 2022;16:1010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaren R, Smith PF, Taylor RL, Niazi IK, Taylor D. Scoping out noisy galvanic vestibular stimulation: a review of the parameters used to improve postural control. Front Neurosci. 2023;17:1156796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwan A, Forbes PA, Mitchell DE, Blouin JS, Cullen KE. Neural substrates, dynamics and thresholds of galvanic vestibular stimulation in the behaving primate. Nat Commun. 2019. Apr 23;10(1):1904. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This study established for the first time how GVS activates single vestibular afferents in the alert behaving macaque monkeys. Neural dynamics and thresholds to GVS were quantified and found to be comparable for canal and otolith afferents. Moreover, afferent tuning to GVS differed significantly as compared to natural self-motion stimulation – a finding that has important implications for optimizing the use of GVS in research as well as clinical applications.
- 38.Kim KS, Minor LB, Della Santina CC, Lasker DM. Variation in response dynamics of regular and irregular vestibular-nerve afferents during sinusoidal head rotations and currents in the chinchilla. Exp Brain Res. 2011. May;210(3–4):643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg JM, Fernández C, Smith CE. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res. 1982. Dec 2;252(1):156–60. [DOI] [PubMed] [Google Scholar]
- 40.Smith CE, Goldberg JM. A stochastic afterhyperpolarization model of repetitive activity in vestibular afferents. Biol Cybern. 1986;54:41–51. [DOI] [PubMed] [Google Scholar]
- 41.Govindaraju AC, Quraishi IH, Lysakowski A, Eatock RA, Raphael RM. Nonquantal transmission at the vestibular hair cell-calyx synapse: KLV currents modulate fast electrical and slow K+ potentials. Proc Natl Acad Sci U S A. 2023. Jan 10;120(2):e2207466120. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This paper presents a physiologically realistic computational model of the vestibular hair cell-calyx synapse, which demonstrates how nonquantal transmission functions to facilitate the speed of mechanosensory transduction to expedite reflex control.
- 42.Mildren RL, Cullen KE. Vestibular Contributions to Primate Neck Postural Muscle Activity during Natural Motion. J Neurosci. 2023. Mar 29;43(13):2326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cullen KE. Vestibular processing during natural self-motion: implications for perception and action. Nat Rev Neurosci. 2019. Jun;20(6):346–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eugène D, Idoux E, Beraneck M, Moore LE, Vidal PP. Intrinsic membrane properties of central vestibular neurons in rodents. Exp Brain Res. 2011. May;210(3–4):423–36. [DOI] [PubMed] [Google Scholar]
- 45.Kim G, Lee S, Kim KS. Repeated Galvanic Vestibular Stimulation Modified the Neuronal Potential in the Vestibular Nucleus. Neural Plast. 2020;2020:5743972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carriot J, Jamali M, Brooks JX, Cullen KE. Integration of canal and otolith inputs by central vestibular neurons is subadditive for both active and passive self-motion: implication for perception. J Neurosci. 2015. Feb 25;35(8):3555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y, Geddes L, Sato G, Stiles L, Darlington CL, Smith PF. Galvanic vestibular stimulation impairs cell proliferation and neurogenesis in the rat hippocampus but not spatial memory. Hippocampus. 2014. May;24(5):541–52. [DOI] [PubMed] [Google Scholar]
- 48.Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, et al. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005;26:721–32. [DOI] [PubMed] [Google Scholar]
- 49.Stephan T, Hüfner K, Brandt T. Stimulus profile and modeling of continuous galvanic vestibular stimulation in functional magnetic resonance imaging. Ann N Y Acad Sci. 2009. May;1164:472–5. [DOI] [PubMed] [Google Scholar]
- 50.Rühl M, Kimmel R, Ertl M, Conrad J, Zu Eulenburg P. In Vivo Localization of the Human Velocity Storage Mechanism and Its Core Cerebellar Networks by Means of Galvanic-Vestibular Afternystagmus and fMRI. Cerebellum. 2023. Apr;22(2):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study used high-resolution fMRI and GVS to understand the neural basis if the human velocity storage mechanism. The authors conclude that loops between the vestibular nuclei and cerebellum (e.g., uvula and nodulus) serve an essential role.
- 51.Hernández-Román J, Montero-Hernández S, Vega R, Orihuela-Espina F, Soto E. Galvanic vestibular stimulation activates the parietal and temporal cortex in humans: A functional near-infrared spectroscopy (fNIRS) study. Eur J Neurosci. 2023. May 10; [DOI] [PubMed] [Google Scholar]
- 52.Habig K, Krämer HH, Lautenschläger G, Walter B, Best C. Processing of sensory, painful and vestibular stimuli in the thalamus. Brain Struct Funct. 2023. Mar;228(2):433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Brain activity in the thalamic nuclei evoked by GVS versus touch and mechanical pain stimulation were measured using fMRI. Overlap in activations patterns lead to the conclusion that sensory, painful and vestibular stimuli are processed in thalamic network rather than a distinct nucleus.
- 53.Huber J, Ruehl M, Flanagin V, Zu Eulenburg P. Delineating neural responses and functional connectivity changes during vestibular and nociceptive stimulation reveal the uniqueness of cortical vestibular processing. Brain Struct Funct. 2022. Apr;227(3):779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helmchen C, Rother M, Spliethoff P, Sprenger A. Increased brain responsivity to galvanic vestibular stimulation in bilateral vestibular failure. NeuroImage Clin. 2019;24:101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helmchen C, Machner B, Rother M, Spliethoff P, Göttlich M, Sprenger A. Effects of galvanic vestibular stimulation on resting state brain activity in patients with bilateral vestibulopathy. Hum Brain Mapp. 2020. Jun 15;41(9):2527–47. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ The study used fMRI to explore GVS induced changes in patients with bilateral vestibulopathy. GVS increased functional connectivity in regions processing vestibular signals and improved visual-vestibular interaction and also induced positive changes in the cerebellum and visual cortex, suggesting that these compensatory changes contributed to improved self-motion perception in patients.
- 56.Mitsutake T, Sakamoto M, Horikawa E. Comparing activated brain regions between noisy and conventional galvanic vestibular stimulation using functional magnetic resonance imaging. Neuroreport. 2021. May 5;32(7):583–7. [DOI] [PubMed] [Google Scholar]
- 57.Guldin WO, Grüsser OJ. Is there a vestibular cortex? Trends Neurosci. 1998;21:254–9. [DOI] [PubMed] [Google Scholar]
- 58.Liu S, Dickman JD, Angelaki DE. Response Dynamics and Tilt versus Translation Discrimination in Parietoinsular Vestibular Cortex. Cereb Cortex. 2011;21:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen A, DeAngelis GC, Angelaki DE. Macaque parieto-insular vestibular cortex: responses to self-motion and optic flow. J Neurosci. 2010. Feb 24;30(8):3022–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penfield W, Faulk MEJ. The insula: further observations on its functions. Brain. 1955;78:445–470. [DOI] [PubMed] [Google Scholar]
- 61.Mazzola L, Lopez C, Faillenot I, Chouchou F, Mauguière F, Isnard J. Vestibular responses to direct stimulation of the human insular cortex. Ann Neurol. 2014. Aug 20; [DOI] [PubMed] [Google Scholar]
- 62.Yu K, Yu T, Qiao L, Liu C, Wang X, Zhou X, et al. Electrical stimulation of the insulo-opercular region: visual phenomena and altered body-ownership symptoms. Epilepsy Res. 2018;148:96–106. [DOI] [PubMed] [Google Scholar]
- 63.Mazzola L, Mauguière F, Isnard J. Functional mapping of the human insula: Data from electrical stimulations. Rev Neurol (Paris). 2019. Mar;175(3):150–6. [DOI] [PubMed] [Google Scholar]
- 64.Lopez C, Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev. 2011;67:119–46. [DOI] [PubMed] [Google Scholar]
- 65.Kahane P, Hoffmann D, Minotti L, Berthoz A. Reappraisal of the human vestibular cortex by cortical electrical stimulation study. Ann Neurol. 2003;54:615–24. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Qi L, Schaper FLWVJ, Wu D, Friedrich M, Du J, et al. A vertigo network derived from human brain lesions and brain stimulation. Brain Commun. 2023;5(2):fcad071. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This study provides causal evidence of a network underpinning vertigo by merging (1) data from 23 published cases of brain lesions associated with vertigo and (2) data from 42 iEBS in 17 patients with epilepsy evoking vertigo. Both approaches identified a common brain network underpinning vertigo.
- 67.Eickhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K. Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp. 2006;27:611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibitoye RT, Mallas EJ, Bourke NJ, Kaski D, Bronstein AM, Sharp DJ. The human vestibular cortex: functional anatomy of OP2, its connectivity and the effect of vestibular disease. Cereb Cortex. 2023. Jan 5;33(3):567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ The study reports the functional connectivity of area OP2 and adjacent area OP2+ (sensitive to visual motion) using data from the Human Connectome Project.
- 69.Raiser TM, Flanagin VL, Duering M, van Ombergen A, Ruehl RM, Zu Eulenburg P. The human corticocortical vestibular network. NeuroImage 2020. Dec;223:117362. [DOI] [PubMed] [Google Scholar]
- 70.Balestrini S, Francione S, Mai R, Castana L, Casaceli G, Marino D, et al. Multimodal responses induced by cortical stimulation of the parietal lobe: a stereo-electroencephalography study. Brain. 2015. Sep;138(Pt 9):2596–607. [DOI] [PubMed] [Google Scholar]
- 71.Sun F, Zhang G, Yu T, Zhang X, Wang X, Yan X, et al. Functional characteristics of the human primary somatosensory cortex: An electrostimulation study. Epilepsy Behav. 2021. May;118:107920. [DOI] [PubMed] [Google Scholar]
- 72.Sun F, Zhang G, Ren L, Yu T, Ren Z, Gao R, et al. Functional organization of the human primary somatosensory cortex: A stereo-electroencephalography study. Clin Neurophysiol. 2021. Feb;132(2):487–97. [DOI] [PubMed] [Google Scholar]
- 73.Schwarz DWF, Fredrickson JM. Rhesus monkey vestibular cortex: a bimodal primary projection field. Science. 1971;172:280–281. [DOI] [PubMed] [Google Scholar]
- 74.Rancz EA, Moya J, Drawitsch F, Brichta AM, Canals S, Margrie TW. Widespread vestibular activation of the rodent cortex. J Neurosci. 2015. Apr 15;35(15):5926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyu D, Stieger JR, Xin C, Ma E, Lusk Z, Aparicio MK, et al. Causal evidence for the processing of bodily self in the anterior precuneus. Neuron. 2023. Jun 6;S0896–6273(23)00386–0. [DOI] [PubMed] [Google Scholar]; ■■ This is a multimodal imaging study of the anterior precuneus in patients with epilepsy. iEBS in the anterior precuneus evoked vestibular sensations, distortions of the body image and self-dissociation. The association between vestibular responses and sensations of depersonalization supports a vestibular contribution to the bodily self.
- 76.Beer AL, Becker M, Frank SM, Greenlee MW. Vestibular and visual brain areas in the medial cortex of the human brain. J Neurophysiol. 2023. Apr 1;129(4):948–62. [DOI] [PubMed] [Google Scholar]; ■■ This fMRI study investigates the response of several areas in the medial cortex to caloric vestibular stimulation, thermal tactile stimulation and optic flow (laminar of radial motion) in 36 healthy participants. The cingulate sulcus visual area (CSv) responded to vestibular and visual stimulation, whereas the V6 complex and the precuneus motion area (PcM) responded mainly to optic flow. Two new areas were identified: (1) the “vestibular pericallosal sulcus area” (vPCS), located ventrally to CSv within the pericallosal sulcus and close to anterior retrosplenial, which responded to vestibular stimulation but not to optic flow; and (2) the “motion sensitive retrosplenial complex” (mRSC), which responded to optic flow but not to vestibular stimulation.
- 77.Guldin WO, Akbarian S, Grüsser OJ. Cortico-cortical connections and cytoarchitectonics of the primate vestibular cortex: a study in squirrel monkeys (Saimiri sciureus). J Comp Neurol. 1992;326:375–401. [DOI] [PubMed] [Google Scholar]
- 78.Liu B, Tian Q, Gu Y. Robust vestibular self-motion signals in macaque posterior cingulate region. eLife. 2021. Apr 8;10:e64569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leong ATL, Gu Y, Chan YS, Zheng H, Dong CM, Chan RW, et al. Optogenetic fMRI interrogation of brain-wide central vestibular pathways. Proc Natl Acad Sci U S A. 2019. May 14;116(20):10122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith AT, Wall MB, Thilo KV. Vestibular inputs to human motion-sensitive visual cortex. Cereb Cortex N Y N 1991. 2012. May;22(5):1068–77. [DOI] [PubMed] [Google Scholar]
- 81.Caruana F, Gerbella M, Avanzini P, Gozzo F, Pelliccia V, Mai R, et al. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain. 2018. Aug 13; [DOI] [PubMed] [Google Scholar]
- 82.Oane I, Barborica A, Chetan F, Donos C, Maliia MD, Arbune AA, et al. Cingulate cortex function and multi-modal connectivity mapped using intracranial stimulation. NeuroImage. 2020. Oct 15;220:117059. [DOI] [PubMed] [Google Scholar]
- 83.Xue Y, Yan H, Hao G, Gao Y, Wang X, Ni D, et al. Symptomatic responses elicited by electrical stimulation of the cingulate cortex: Study of a cohort of epileptic patients and literature review. Hum Brain Mapp. 2023. Jun 15; [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ A large study of iEBS in 124 epileptic patients with electrodes implanted in the cingulate cortex for drug-refractory partial epilepsy. iEBS in the cingulate cortex evoked 196 responses, including sensory, affective, autonomic, language, visual, vestibular, and motor responses. Vestibular and visual responses were found in the middle and posterior cingulate cortex.
- 84.Ertl M, Zu Eulenburg P, Woller M, Mayadali Ü, Boegle R, Dieterich M. Vestibular mapping of the naturalistic head-centered motion spectrum. J Vestib Res. 2023. Jul 13; [DOI] [PubMed] [Google Scholar]; ■ This EEG study in healthy participants measured vestibular-evoked potentials and event-related synchronization-desynchronization in response to natural whole-body rotations, translations and tilts on a motorized platform. Source localization analysis revealed that activity in the cingulate cortex (area CSv) reflecting vestibular-evoked potentials amplitude was related to the motion direction, whereas this was not the case of the posterior insula.
- 85.Dary Z, Lenggenhager B, Lagarde S, Medina Villalon S, Bartolomei F, Lopez C. Neural bases of the bodily self as revealed by electrical brain stimulation: A systematic review. Hum Brain Mapp. 2023. May;44(7):2936–59. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ A systematic review of the literature about the effects of iEBS on various phenomenal components of the bodily self (i.e., sense of self-location, agency, first-person perspective and body ownership) and the body image. The study analyzed illusory self-motion (vestibular responses) as a component of the bodily self. iEBS in the parietal cortex induced disturbances of all five components of the bodily self, including illusory self-location and motion. Vestibular responses were reported mostly during iEBS in the middle cingulate cortex, posterior insula, inferior parietal lobule and parietal operculum.
- 86.Dieterich M, Kirsch V, Brandt T. Right-sided dominance of the bilateral vestibular system in the upper brainstem and thalamus. J Neurol. 2017. Oct;264(Suppl 1):55–62. [DOI] [PubMed] [Google Scholar]
- 87.Qi L, Xu C, Wang X, Du J, He Q, Wu D, et al. Intracranial direct electrical mapping reveals the functional architecture of the human basal ganglia. Commun Biol. 2022. Oct 23;5(1):1123. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This is the first study to analyse systematically the effects of iEBS in the basal ganglia in 35 patients with drug-refractory partial epilepsy. An important finding is the high prevalence of vestibular responses after iEBS in the basal ganglia, which was the second highest after sensorimotor responses. Due to precise localization of electrode contacts in the basal ganglia, the study identified vestibular representations only in the putamen and external globus pallidus.
- 88.Zhang H, Wang D, Wei P, Fan X, Yang Y, An Y, et al. Integrative roles of human amygdala subdivisions: Insight from direct intracerebral stimulations via stereotactic EEG. Hum Brain Mapp. 2023. Jun 15;44(9):3610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This study describes the effects of iEBS in amygdala subnuclei in 48 patients with drug-resistant epilepsy. Various sensory illusions, including vestibular responses, were reported during iEBS in addition to the expected emotional, neurovegetative and olfactory responses. Vestibular responses were mostly elicited by iEBS in the laterobasal nuclei of the amygdala.
- 89.Beylergil SB, Noecker AM, Petersen M, Gupta P, Ozinga S, Walker MF, et al. Subthalamic deep brain stimulation affects heading perception in Parkinson’s disease. J Neurol. 2022. Jan;269(1):253–68. [DOI] [PubMed] [Google Scholar]; ■■ Fourteen patients with Parkinson’s disease were tested with a vestibular or a visual perception task (two-alternative forced-choice task about direction of linear motion) while they received or not electrical stimulation of the subthalamic nuclei. Patients had lower performance than healthy controls regarding response accuracy and threshold in the vestibular perception task, but not in the visual task. In addition, stimulation of the subthalamic nuclei improved both accuracy and threshold only in the vestibular perception task.
- 90.Stiles L, Smith PF. The vestibular-basal ganglia connection: balancing motor control. Brain Res. 2015. Feb 9;1597:180–8. [DOI] [PubMed] [Google Scholar]
- 91.Stiles L, Reynolds JN, Napper R, Zheng Y, Smith PF. Single neuron activity and c-Fos expression in the rat striatum following electrical stimulation of the peripheral vestibular system. Physiol Rep. 2018. Jul;6(13):e13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sabzevar FT, Vautrelle N, Zheng Y, Smith PF. Vestibular modulation of the tail of the rat striatum. Sci Rep. 2023. Mar 17;13(1):4443. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ First study to record local field potentials in the striatal tail of anesthetized rats receiving electrical stimulation of the peripheral vestibular system (in the round window). Vestibular nystagmus was recorded as a mean to confirm vestibular system activation. Waveforms with latency below 22 ms were recorded in the tail of the striatum. Cochlear lesions did not abolish responses in the striatum, suggesting vestibular projections to the striatum.
- 93.Stiles L, Zheng Y, Smith PF. The effects of electrical stimulation of the peripheral vestibular system on neurochemical release in the rat striatum. PloS One. 2018;13(10):e0205869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balaban CD. Projections from the parabrachial nucleus to the vestibular nuclei: potential substrates for autonomic and limbic influences on vestibular responses. Brain Res. 2004. Jan 16;996(1):126–37. [DOI] [PubMed] [Google Scholar]
- 95.Chow MR, Ayiotis AI, Schoo DP, Gimmon Y, Lane KE, Morris BJ, et al. Posture, Gait, Quality of Life, and Hearing with a Vestibular Implant. N Engl J Med. 2021. Feb 11;384(6):521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Patients with bilateral vestibular hypofunction were tested more than 1 year after unilateral implantation of a vestibular implant. Measures of posture, gait, and quality of life demonstrated improvement relative to baseline, but hearing was reduced in the ear with the implant in all but 1 participant.
- 96.Boutros PJ, Schoo DP, Rahman M, Valentin NS, Chow MR, Ayiotis AI, et al. Continuous vestibular implant stimulation partially restores eye-stabilizing reflexes. JCI Insight. 2019. Nov 14;4(22):e128397, 128397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiboonsaksakul KP, Roberts DC, Della Santina CC, Cullen KE. A prosthesis utilizing natural vestibular encoding strategies improves sensorimotor performance in monkeys. PLoS Biol. 2022. Sep;20(9):e3001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell DE, Della Santina CC, Cullen KE. Plasticity within non-cerebellar pathways rapidly shapes motor performance in vivo. Nat Commun. 2016. May 9;7:11238. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Behaviourally relevant activation patterns of vestibular nerve stimulation were applied using a chronically implanted vestibular implant in rhesus monkeys. Implant stimulation produces rapid attenuation of direct pathway VOR neurons, but not their nerve input. Correspondingly, indirect brainstem pathways display complementary nearly instantaneous homeostatic changes, that contributed to compensating for the reduced sensitivity of primary VOR pathway.
- 99.Mitchell DE, Della Santina CC, Cullen KE. Plasticity within excitatory and inhibitory pathways of the vestibulo-spinal circuitry guides changes in motor performance. Sci Rep. 2017. Apr 12;7(1):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tarnutzer AA, Lee SH, Robinson KA, Kaplan PW, Newman-Toker DE. Clinical and electrographic findings in epileptic vertigo and dizziness: a systematic review. Neurology. 2015. Apr 14;84(15):1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pelliccia V, Avanzini P, Rizzi M, Caruana F, Tassi L, Francione S, et al. Association Between Semiology and Anatomo-functional Localization in Patients With Cingulate Epilepsy: A Cohort Study. Neurology. 2022. May 31;98(22):e2211–23. [DOI] [PubMed] [Google Scholar]
- 102.Harroud A, Boucher O, Tran TPY, Harris L, Hall J, Dubeau F, et al. Precuneal epilepsy: Clinical features and surgical outcome. Epilepsy Behav EB. 2017. Aug;73:77–82. [DOI] [PubMed] [Google Scholar]


