Abstract
Previous studies have shown the central role of interleukin 12 (IL-12) in the development of resistance to Leishmania major infection in C3H mice. We now show that during the innate immune response the lymph node cells of L. major-infected C3H mice upregulate the IL-12 receptor on CD4+, CD8+, and B220+ cells. An increase in the ability of the lymph node cells to bind IL-12 correlates with 9.3- and 4.6-fold increases in the mRNA expression levels of the IL-12Rβ1 and -β2 subunits, respectively. In contrast, BALB/c mice, which are susceptible to L. major infection, have no increase in the ability of the lymph node cells to bind IL-12 and correspondingly smaller increases in the mRNA expression levels of the IL-12Rβ1 and -β2 subunits of 2- and 1.5-fold, respectively. Neutralizing IL-4 and the administration of exogenous IL-12 upregulate IL-12R expression in BALB/c mice, while the neutralization of IL-12 in C3H mice blocks increased IL-12 receptor expression. These experiments reveal an important role for the regulation of the IL-12 receptor during the innate immune response after infection of mice with a pathogen.
It is now widely accepted that cytokines produced during the innate immune response influence the type of adaptive immune response that will dominate following infection (19, 25). One of the most important of these cytokines is interleukin 12 (IL-12), a heterodimer produced by phagocytic cells that initiates the development of Th1 cells both in vitro and in vivo (1, 10, 15, 24). We have investigated the role of IL-12 in T-cell subset development by studying mice infected with the protozoan parasite Leishmania major. In this model, infection of C3H mice leads to a dominant Th1 type response and healing, while infection of BALB/c mice results in the development of a Th2 response and an ultimately fatal infection (18, 23). We found that IL-12 is produced soon after infection of C3H mice, that it induces an NK-cell response, and that it is required for the development of Th1 cells and resistance to L. major (21, 22). In addition, the administration of IL-12 to BALB/c mice early during the infection promotes resistance to L. major (9, 27). These data are consistent with a paradigm of Th1 cell development proposed for several intracellular pathogens in which early IL-12 production by macrophages activates NK cells, which then produce gamma interferon (IFN-γ), leading to a dominant Th1 response (25). While certain aspects of this model have yet to be established, it is apparent that in the absence of IL-12 the development of cell-mediated immunity to intracellular pathogens is severely compromised (16, 22).
Critical to understanding the role of IL-12 in the development of the Th1 cell phenotype is understanding how the IL-12 receptor is regulated. Early studies indicated that the receptor was upregulated on human phytohemagglutinin-activated lymphocytes (2, 4), although the molecular mechanisms involved in this upregulation have not been defined. More recently, it has been shown that Th1 and Th2 cells differ in their expression of a functional IL-12 receptor (28, 29), and it has been proposed that the inability to efficiently activate IL-12 signaling pathways is responsible for the development of a Th2 phenotype in BALB/c mice infected with L. major (7). The IL-12 receptor is now known to be composed of at least two chains, designated IL-12Rβ1 and IL-12Rβ2 (3, 17). It would appear that the presence of both subunits of the IL-12 receptor is required for functional activity (17, 28) and that the inability of Th2 cells to transduce an IL-12 signal is due to the lack of expression of the IL-12Rβ2 chain, although expression of the IL-12Rβ1 chain is upregulated after antigen stimulation (28). In vitro studies with isolated T-cell receptor (TCR) transgenic cells have shown that IL-12 responsiveness develops during T-cell activation in the presence of IL-12 or IFN-γ (or both) and that IL-4 can inhibit the development of IL-12 responsiveness in the absence of IFN-γ (28).
In this study, we investigated the expression of the IL-12 receptor in vivo during the innate immune response to L. major. We found that there was an increase in the ability of lymph node (LN) cells to bind IL-12 at two days after infection in C3H mice and that cells from the draining LN show an upregulation of the mRNA for both the IL-12Rβ1 and the -β2 subunits. In contrast, cells from BALB/c mice exhibited little or no increase in IL-12 binding activity and a smaller increase in IL-12Rβ1 and -β2 subunit mRNA. We also found that the IL-12 receptor is upregulated by promoting a resistant phenotype in normally susceptible BALB/c mice through the administration of either anti-IL-4 antibodies or recombinant IL-12, suggesting both an inhibitory activity for IL-4 and a positive feedback loop for IL-12. These data suggest that expression of the IL-12 receptor is induced by L. major infection during the innate immune response in resistant C3H mice and that differential expression of both IL-12R subunits, β2 and β1, contributes to the development of responsiveness to IL-12 in L. major infections.
MATERIALS AND METHODS
L. major infections.
Female BALB/cByJ and C3HeB/FeJ mice at between 5 and 6 weeks of age were obtained from the Jackson Laboratory (Bar Harbor, Maine) and maintained in a specific pathogen-free facility. L. major parasites (MHOM/IL/80/Friedlin) were grown to stationary phase in Grace’s insect cell culture medium with 20% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Metacyclic promastigote parasites were isolated by negative selection with Arachis hypogaea agglutinin (20). Mice were infected in the hind footpads with 2 × 106 metacyclic parasites.
Antibodies and cytokines and treatment regimens.
IL-12 was a generous gift from Stanley Wolf (Genetics Institute, Cambridge, Mass.) and was used in vivo at 0.2 μg per foot injected simultaneously with the metacyclic parasites in a volume of 50 μl. In vivo IL-4 depletion studies used 2.5 mg of anti-IL-4 monoclonal antibody (MAb) 11B11 (Biological Response Modifiers Program, Frederick, Md.) administered intraperitoneally the day before and the day of infection. In vivo IL-12 depletion studies used 1 mg of anti-IL-12 MAb C17.8 administered intraperitoneally the day before and the day of infection as described previously (22). Rat immunoglobulin (Ig; Sigma) was administered as a control antibody.
RPA.
LNs were removed and frozen in liquid nitrogen, and total RNA was extracted with Ultraspec RNA extraction buffer (Biotecx Laboratories, Houston, Tex.) according to the manufacturer’s recommendation. LNs from individual mice were processed and analyzed separately. The RNA was resuspended in diethyl pyrocarbonate-treated water and stored at −20°C. The RNase protection assay (RPA) was carried out essentially as described by Gilman (6). Briefly, antisense RNA probes were prepared with the T7 promoter in Bluescript (Stratagene, La Jolla, Calif.) or the Sp6 promoter in PCRII (Invitrogen, San Diego, Calif.), labelled with [32P]UTP or [32P]CTP (400 to 800 Ci/mM), purified from a 6% acrylamide sequencing gel, and used the same day. Depending on availability, 5 to 30 μg of total RNA was hybridized with a 5 × 105-CPM antisense probe in 80% formamide buffer at 60°C. The IL-12Rβ1 probe was generated from a mouse cDNA clone (26) and covers nucleotides 1301 to 1621; the IL-12Rβ2 probe covers nucleotides 1607 to 1870 of the cDNA (15). The IFN-γ probe covers nucleotides 375 to 530 of the cDNA. A mouse cyclophilin antisense probe (Ambion, Austin, Tex.) was used as an internal control for standardization of expression levels between samples. Samples were processed as described previously (6) and fractionated on 6% sequencing gels. The gels were dried and exposed to a phosphorimager screen for 1 to 4 days, and the relative signals were quantitated with Image Quant software (Molecular Dynamics, Sunnyvale, Calif.). IFN-γ, IL-12Rβ1, and IL-12Rβ2 levels were determined by comparing the signal intensities of the protected fragments to the signal intensity of the cyclophilin control after standardizing the values for C or U content. Increases are expressed as β1/cyclophilin or β2/cyclophilin ratios.
Flow cytometry.
Surface IL-12R expression was examined by the detection of IL-12 binding to cell populations by a modification of methods previously described (4). Briefly, single-cell suspensions obtained from the draining LNs of four or five mice were pooled, washed in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline containing 0.1% bovine serum albumin and 0.08% sodium azide), and incubated with 0.5 μg of recombinant IL-12 (diluted in FACS buffer) for 1 h on ice. Cells were washed in 2 ml of FACS buffer and then incubated for 15 min on ice with 10 μg of anti-FcγIII/IIR antibody (2.4G2) plus 10 μg of rat IgG (Sigma) to block nonspecific binding of antibodies to FcR. Cells were then incubated with biotinylated anti-IL-12 MAb (C17.8) for 30 min on ice. After being washed, cells were stained with streptavidin-phycoerythrin (PE) (Pharmingen, San Diego, Calif.) for the detection of bound IL-12. Control samples were incubated on ice in the absence of IL-12, followed by an incubation with biotinylated anti-IL-12 MAb and staining with streptavidin-PE. Similar patterns of staining were observed when control samples were incubated with a biotinylated isotype control MAb (data not shown). To detect IL-12 binding to specific cell populations, cells were surface stained with fluorescein isothiocyanate-labeled anti-CD4, -CD8, or -B220 or rat IgG isotype control MAb (Pharmingen) for an additional 30 min. Propidium iodide was added to cells to allow the exclusion of dead cells during acquisition on a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.). A minimum of 10,000 live events were acquired from each sample. Analysis of surface IL-12 binding to LN cell populations was performed with CELLQUEST (Becton Dickinson) software.
Measurement of IL-12 responsiveness of LN cells.
Draining LN cells obtained from mice at 2 days postinfection (and from uninfected mice) were plated in complete tissue culture medium as described previously (22) in the presence or absence of 1 ng of recombinant IL-12 per ml. Supernatants were harvested after 24 h, and IFN-γ levels were measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS
Surface expression of the IL-12 receptor following infection of mice with L. major.
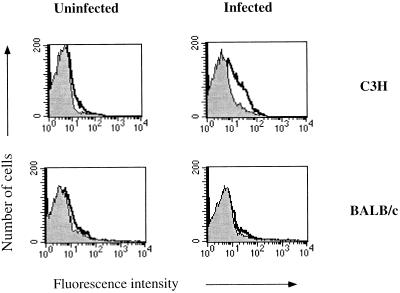
To determine if the observed differences in IFN-γ production between C3H and BALB/c mice during the innate immune response might reflect differences in the expression of the IL-12 receptor during this same time period, the levels of IL-12 binding to LN cells for normal and infected mice were examined by flow cytometry. We found that the level of IL-12 binding was low to undetectable in LN cells taken from uninfected C3H and BALB/c mice. However, IL-12 binding increased on LN cells taken from C3H mice infected with L. major for 2 days, while there was little detectable change in IL-12 binding on LN cells from infected BALB/c mice either at 2 days postinfection (Fig. 1) or any time points examined up to 14 days postinfection (data not shown).
FIG. 1.
IL-12 binding to LN cells from L. major-infected C3H and BALB/c mice. The results of a flow-cytometric analysis of IL-12 binding to lymphocytes isolated from the draining LNs of uninfected mice and mice infected 2 days previously are displayed. The data from one representative mouse from each group (n = 3 to 5) are shown. Peaks outlined by dark solid lines represent cells incubated with 0.5 μg of IL-12 and then stained with the anti-IL-12 MAb; these peaks are compared to those for control samples stained with the anti-IL-12 MAb alone (shaded peaks). These results are representative of four experiments.
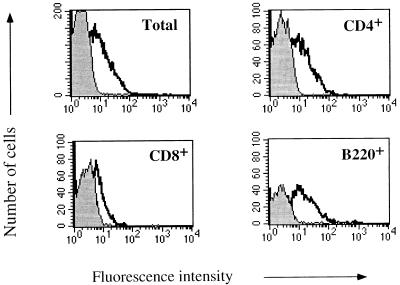
The shift in the fluorescence intensity of the LN cells from infected C3H mice suggests that the increase in expression of the IL-12 receptor reflects changes in the population as a whole, rather than the appearance of a specific subpopulation that upregulates the IL-12 receptor. To more fully explore IL-12 receptor expression, we examined which cell types expressed the receptor in C3H mice at 2 days postinfection with L. major and found upregulation of the receptor on CD4+, B220+, and CD8+ cells (Fig. 2). These cells constitute 95% of the LN population. By 7 and 14 days postinfection C3H mice had reduced binding of IL-12 to LN cells (data not shown), again suggesting that the increase in the IL-12 receptor at 2 days postinfection is a characteristic of the LN population as a whole and reflects a response by both antigen-specific and nonspecific cells. We presume that if sufficient numbers of antigen-specific cells from C3H mice could be identified at 14 days postinfection they would be expressing the IL-12 receptor.
FIG. 2.
IL-12 binding to LN cells and cell subsets from L. major-infected C3H mice. A two-color flow-cytometric analysis of IL-12 binding to lymphocyte subsets was performed with LN cells from C3H mice infected 2 days previously that were surface stained for CD4, CD8, or B220. Results show IL-12 binding by the total and gated populations. The peaks outlined by the dark solid lines and the shaded peaks are as defined in the legend for Fig. 1. These results are representative of four experiments.
IL-12 regulates the expression of the IL-12 receptor.
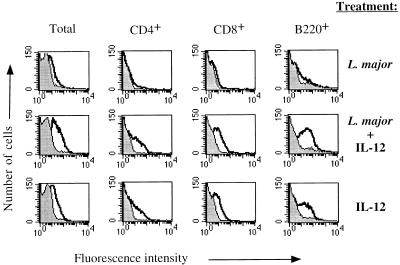
We were interested in determining if exogenous IL-12 administered to BALB/c mice might upregulate IL-12 receptor expression. An increase in the IL-12 receptor in response to IL-12 would be consistent with the ability of IL-12 administration to BALB/c mice to promote Th1 cell development and resistance to L. major (9, 27) and with an increase in the ability of LN cells from IL-12-treated mice to produce IFN-γ (1, 14). As seen in Fig. 3, the inclusion of IL-12 in the parasite inoculum given to BALB/c mice led to an increase in the IL-12 binding activity on CD4+, CD8+, and B220+ cells in the draining LN. In order to determine if the parasite was stimulating a cofactor that might be required for upregulation of the receptor, we tested the ability of IL-12 to enhance IL-12 receptor expression in the absence of L. major. The results were almost identical to those seen when mice received IL-12 and L. major; there was a dramatic increase in IL-12 receptor binding on CD4+, CD8+, and B220+ populations within 2 days of the administration of IL-12 alone (Fig. 3). LN cells from BALB/c mice treated with phosphate-buffered saline as a control and analyzed 2 days later showed no increase in IL-12 binding (data not shown), a finding identical to the results for BALB/c mice infected with L. major alone. These results are similar to those of Galbiati et al., who demonstrated that IL-12 administration enhanced IL-12 responsiveness and receptor expression in vivo (5).
FIG. 3.
IL-12 binding to LN cells from BALB/c mice given exogenous IL-12. Shown is a two-color flow-cytometric analysis of IL-12 binding to lymphocyte subsets using LN cells from BALB/c mice infected 2 days previously, BALB/c mice infected 2 days previously and treated in vivo with 0.2 μg of IL-12 at the time of infection, and BALB/c mice treated with 0.2 μg of IL-12 alone 2 days prior to analysis. The peaks outlined by the dark solid line and the shaded peaks are as defined in the legend for Fig. 1. These results are representative of three experiments.
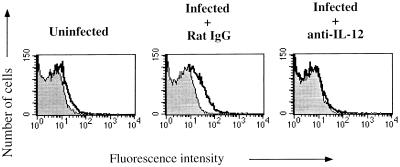
We next assessed the role of endogenously produced IL-12 in the upregulation of the IL-12 receptor. Previous work has shown that IL-12 plays a central role in the development of an effective cell-mediated immune response to L. major infection in C3H mice. C3H mice treated with anti-IL-12 antibodies and then infected with L. major have decreased levels of IFN-γ-producing cells during the innate immune response and develop large lesions, with the accompanying development of CD4+ Th2 cells (21, 22). We found that treatment of L. major-infected C3H mice with anti-IL-12 antibodies prevented the increase of the IL-12 receptor normally seen on CD4+ cells (Fig. 4).
FIG. 4.
Inhibition of IL-12 receptor upregulation in C3H mice treated with anti-IL-12. Shown is a two-color flow-cytometric analysis of IL-12 binding to LN cells from C3H mice infected 2 days previously that were surface stained for CD4. Control rat IgG and anti-IL-12 antibodies were administered the day before and the day of infection.
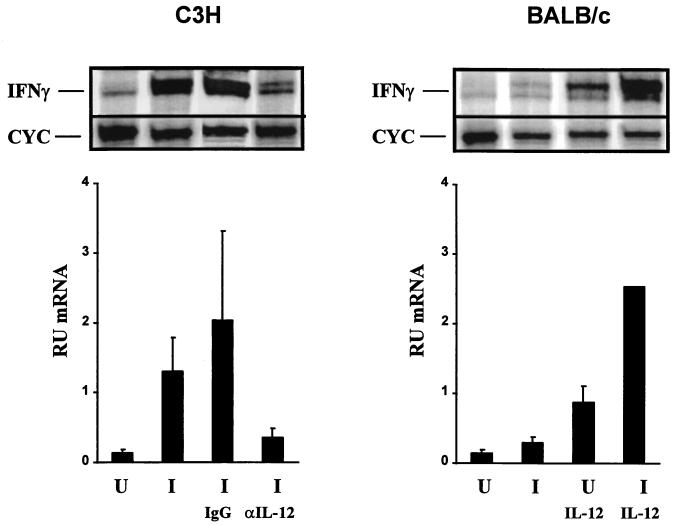
Early IFN-γ production in the BALB/c-C3H L. major infection model serves as a marker for eventual Th1 development as well as a functional marker of IL-12 responsiveness. Therefore, we analyzed IFN-γ mRNA levels in uninfected and L. major-infected mice to correlate IFN-γ upregulation and the expression of the IL-12 receptor. RPA of total LN cells from infected C3H mice and IL-12-treated BALB/c mice demonstrates increases in IFN-γ mRNA levels compared to uninfected animals (Fig. 5). These IFN-γ mRNA increases correlated with increases in IL-12 receptor expression. Anti-IL-12 treatment of L. major-infected C3H mice resulted in a decrease in the induction of IFN-γ mRNA in the total LN cell population (Fig. 5) compared to that for infected animals treated with a control antibody. This inhibition of IFN-γ mRNA upregulation also correlates with the inhibition of IL-12 receptor expression seen with anti-IL-12 treatment (Fig. 4).
FIG. 5.
RPA of IFN-γ mRNA from total LNs of individual C3H mice and BALB/c mice 2 days postinfection with L. major. Representative RPAs for a single C3H mouse and BALB/c mouse are shown on the left and right, respectively. Cyc, cyclophilin. The averages and standard deviations for IFN-γ mRNA quantitation in relative units are shown for one experiment, which included three C3H mice. U, uninfected; I, infected; I IgG, infected plus treatment with control antibody; I αIL-12, infected plus treatment with anti-IL-12 antibodies. These results are representative of three experiments. IFN-γ mRNA quantitation for BALB/c mice is identical to that for C3H mice. U IL-12, uninfected plus treatment with IL-12; I IL-12, infected plus treatment with IL-12. The IFN-γ mRNA relative-unit value for infected mice treated with IL-12 is the average of results for two individual mice. Infection and treatment are described in Materials and Methods.
Regulation of IL-12 receptor expression in BALB/c mice by IL-4.
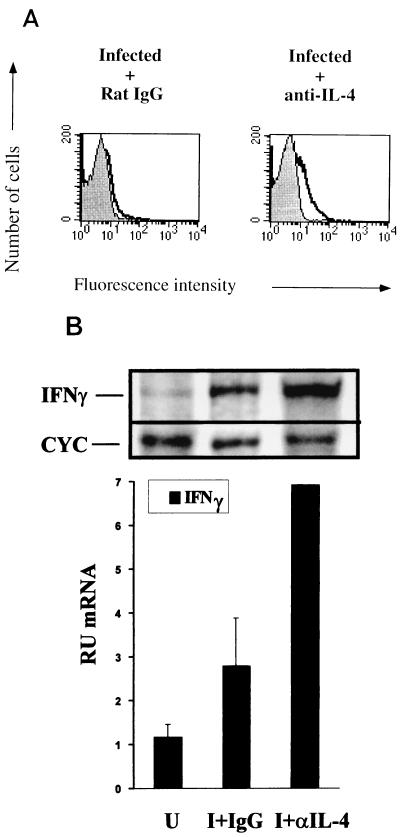
IL-4 is rapidly produced by BALB/c mice following infection with L. major, and neutralization of this early IL-4 burst with MAbs promotes Th1 cell development and resistance to infection (8). In addition, in vitro analysis of IL-12 receptor expression has demonstrated that IL-4 can decrease the expression of the functional IL-12 receptor (28). The production of IL-4 during the innate immune response has been shown to influence the ability of CD4+ T cells to respond to IL-12 as measured by the production of IFN-γ (14). Thus, CD4+ cells from BALB/c mice treated with anti-IL-4 antibodies prior to infection were responsive to IL-12, whereas cells from untreated counterparts showed an inability to produce IFN-γ in response to IL-12 (14). Since our data show a correlation between the upregulation of the IL-12 receptor within the LN cell population and an ability of that same population to respond to IL-12, we hypothesized that CD4+ cells from anti-IL-4-treated BALB/c mice would demonstrate an increase in IL-12 receptor. Indeed, we found that neutralization of IL-4 in BALB/c mice led to an increase in IL-12 receptor binding on CD4+ cells at 2 days postinfection (Fig. 6A). The IL-12 receptor increase detected was comparable to that seen on CD4+ cells in L. major-infected C3H mice (Fig. 2). The increase also correlated with an increase in IFN-γ mRNA expression (Fig. 6B).
FIG. 6.
Upregulation of the IL-12 receptor in BALB/c mice treated with anti-IL-4. (A) Anti-IL-4 MAb or control antibody was administered to BALB/c mice the day before and at the time of infection, and LN cells were harvested 2 days later for flow-cytometric analysis like that shown in Fig. 2. (B) A representative RPA of an individual mouse, and IFN-γ mRNA quantitation of total LN cells of three BALB/c mice treated as described for panel A. U, uninfected; I+IgG, infected plus treatment with control IgG; I+αIL-4, infected plus treatment with anti-IL-4 antibodies. Infection and treatment are described in Materials and Methods. The value for infected mice treated with anti-IL-4 antibodies is the average of results for two mice.
Expression of IL-12Rβ1 and -β2 subunits following L. major infection.
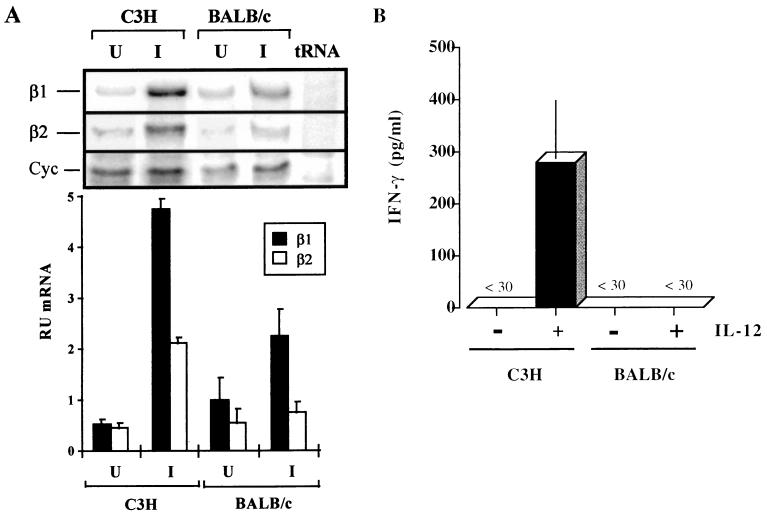
Since the IL-12 binding data show a shift in the entire cell population and since the upregulation appears as a synchronous event during the early immune response, with no subpopulation becoming uniquely positive, we analyzed the regulation of the β1 and β2 subunits of the IL-12 receptor from the total LN cell population by RPA. We found that C3H mice upregulated the β1 subunit from 0.513 relative units (RU) of mRNA in uninfected animals to 4.76 RU of mRNA in infected animals (a 9.3-fold increase). In addition, there was a concomitant increase in the β2 subunit from 0.463 RU of mRNA in uninfected animals to 2.11 RU of mRNA in infected animals (a 4.6-fold increase). In contrast to the C3H mice, BALB/c mice exhibited smaller changes in the expression of both IL-12Rβ1 and -β2, resulting in approximately a 2-fold increase of these subunits over uninfected levels (Fig. 7A). In addition, a semiquantitative PCR analysis of LN cells from BALB/c mice on days 1, 2, and 3 following infection demonstrated that there was no significant increase in IL-12Rβ1 mRNA expression at any of these early time points while LN cells from C3H mice showed maximal IL-12Rβ1 expression on day 2 (data not shown).
FIG. 7.
IL-12 receptor β1 and β2 subunit mRNA expression and IL-12 responsiveness of LN cells during the early immune response to L. major infection in C3H and BALB/c mice. (A) Representative RPA of IL-12Rβ1 and -β2 mRNA from LNs of individual C3H or BALB/c mice 2 days after infection with L. major. The RPA shown is representative of samples from five or six individual mice per group. U, uninfected; I, infected. Also shown are the relative units of β1 or β2 mRNA at 2 days postinfection in C3H and BALB/c mice. Each column shows the means and standard deviations for five or six mice. (B) Lymphocytes isolated from the LNs of C3H or BALB/c mice infected 2 days previously were incubated with IL-12 (1 ng/ml) for 24 h. The supernatants were then harvested, and IFN-γ levels were measured by ELISA to assess IL-12 responsiveness. The results are representative of two experiments with three individual mice each.
We next determined if this early increase in IL-12 receptor expression corresponded to an increase in the ability of LN cells to respond to IL-12. For these experiments LN cells from mice infected 2 days earlier were incubated with IL-12 for 24 h and the levels of IFN-γ in the supernatant were measured by ELISA. We found that IL-12-stimulated cells from C3H mice produced significant levels of IFN-γ, while little to no IFN-γ was produced by cells from BALB/c mice (Fig. 7B). LN cells from uninfected animals of either strain did not produce detectable levels of IFN-γ at this time point. The lack of IL-12 responsiveness seen in BALB/c mice was similar to that reported by Launois et al. (14), where antigen-stimulated cells also failed to respond to IL-12 in vitro.
DISCUSSION
In this study, we found that L. major infection rapidly increases the expression of the IL-12 receptor in C3H mice. This increase can be detected as changes in IL-12 binding on CD4+, CD8+, and B220+ cells, as well as an increase in IL-12Rβ1 and -β2 mRNA expression in the total LN population. In contrast, very little change was detected in the expression of the receptor on cells from L. major-infected BALB/c mice. These results suggest that the lack of expression of the IL-12 receptor in BALB/c mice during the innate immune response to L. major may contribute to their inability to develop a Th1 response.
These experiments extend a growing body of knowledge that describes events involved in the development of cell-mediated immunity and show for the first time how the IL-12 receptor and the β1 and β2 subunits may be regulated in vivo during an infection. An in vitro analysis of the IL-12 receptor with cells from transgenic mice having an ovalbumin-specific TCR has defined a developmental scenario in which IL-12Rβ1 is present on both Th1 and Th2 cells, yet Th2 cells fail to exhibit a functional receptor due to the lack of the IL-12Rβ2 chain (28, 29). Loss of the ability of T cells to respond to IL-12 may be a critical event in their differentiation towards mature Th2 cells. In our study, we addressed the issue of IL-12 receptor expression during the innate immune response to L. major, prior to or during the development of antigen-specific Th1 or Th2 cells. Previous studies have indicated that early after infection, cells from BALB/c mice lose their ability to respond to IL-12, as indicated by IFN-γ production, and that this loss is related to the production of IL-4 by a Vα8, Vβ4 CD4+ T-cell population (12–14). In the present study we show that after infection with L. major, the increase in the ability of the LN cells to bind IL-12 as well as produce IFN-γ in response to IL-12 differs dramatically between BALB/c and C3H mice. We also show that differences in the ability of these mouse strains to upregulate IL-12Rβ1 and -β2 mRNA by 2 days postinfection correlate with the ability of the LN cells to bind to IL-12. These data demonstrate a differential regulation of the IL-12 receptor during the innate immune response and suggest that regulation of the expression of both IL-12 receptor subunits may play a critical role in vivo during the development of cell-mediated immunity.
In vitro studies using DO11.10 CD4+ T cells from TCR transgenic mice have shown that during the first few days after activation, the expression of the IL-12 receptor is critical for Th1 cell development, even though at this time point these cells are producing only low levels of IFN-γ (10). Similarly, in C3H mice infected with L. major, we show that the IL-12 receptor is rapidly upregulated on CD4+ T cells, although at this time point the IFN-γ associated with the infection is primarily coming from NK cells (21). However, we do see some differences in the expression of the IL-12 receptor subunits when our results are compared with that seen with the DO11.10 TCR transgenic system. DO11.10 CD4+ T cells respond to antigen in vitro by upregulating the IL-12Rβ1 chain regardless of whether they will ultimately become Th1 or Th2 cells, but only the Th1 cells upregulate the β2 subunit (28). After infection of C3H mice with L. major, we see upregulation of both chains of IL-12R in the whole LN cell population; in contrast, there is little increase in either the β1 or β2 subunit in infected BALB/c mice. Although we describe the expression of the β1 and β2 IL-12 receptor subunits in the whole LN cell population rather than a specific cell population, we believe that our data demonstrate that CD4+ T cells fail to upregulate the β1 chain because we see the lack of β1 mRNA upregulation in the whole population and the lack of IL-12 binding to the CD4+ T cell population. One difference between our study and those with TCR transgenic mice is that most of the CD4+ T cells expressing the IL-12 receptor during the innate immune response are probably not antigen specific, while the TCR transgenic model creates a system where all the cells are antigen specific. Alternatively, under in vivo conditions other cytokines produced in BALB/c mice during the innate immune response, such as IL-10 and transforming growth factor β (22), may downregulate expression of the IL-12Rβ1 subunit (31). However, our results confirm a central role for IL-4 in the expression of IL-12R, since the blocking of endogenous IL-4 at the time of infection results in an increase in the ability of CD4+ LN cells to bind IL-12. This increase in IL-12 binding correlates with both an ability of these cells to respond to IL-12 (14) and the development of an L. major-resistant phenotype (9).
The differences observed between BALB/c and C3H mice are probably not due to differences in the magnitude of the inflammatory response occurring in the draining LN since, following infection of BALB/c and C3H mice with L. major, similar numbers of cells infiltrate the LN (data not shown). We have previously shown that IL-12 is transiently produced in the LN following L. major infection of BALB/c mice, in spite of their lack of a strong IFN-γ or NK cell response (22). Our studies and those of others (5) demonstrate that IL-12 can enhance the expression of its own receptor, which suggests that there are sufficient functional IL-12 receptors present in a naive LN cell population to mediate this effect. However, it would appear that the endogenous levels of IL-12 seen in the BALB/c mice are unable to effectively upregulate the IL-12 receptor, which may be due to the concomitant production of inhibitory cytokines as discussed above.
The increase in IL-12 binding to LN B cells early after infection in C3H mice is intriguing. In vitro studies have shown that unstimulated splenic B cells do not bind IL-12 but rather increase IL-12 binding after stimulation (30). The increased IL-12 binding detected on the B cells after L. major infection is probably not associated with significant IFN-γ production from this cell population (21), although IL-12 has been shown to have effects on B-cell growth and differentiation independent of IFN-γ (11, 30).
The upregulation of the IL-12 receptor that we observed in C3H mice is part of the early immune events that influence T-cell development in response to a pathogen. Consequently, the early inflammatory response in the LN draining the site of infection may serve not only to select T cells that have specificity for leishmanial antigens but also to prime those cells to become Th1 cells by increasing IL-12R expression on the CD4+ population as a whole, including antigen-specific and non-antigen-specific cells. However, in the absence of IL-12R upregulation, observed following L. major infection of BALB/c mice, T cells will be unable to differentiate into Th1 cells. These findings define an important mechanism by which the innate immune response influences the development of adaptive immunity in vivo.
ACKNOWLEDGMENTS
D. Jones, M. M. Elloso, and L. Showe contributed equally to the work presented.
We thank M. Kopf, C. Hunter, and J. Farrell for helpful discussions.
This work was supported by National Institutes of Health grants AI35914, AI07278-12, and AI01366-02. L. Showe was supported by grants from the Elsa Pardee and Ruth Estrin-Goldberg memorial foundations. P. Scott is the recipient of a Burroughs Wellcome Award in Molecular Parasitology.
REFERENCES
- 1.Afonso L C C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Chizzonite R, Truitt T, Desai B B, Nunes P, Podlaski F J, Stern A S, Gately M K. IL-12 receptor. I. Characterization of the receptor on phytohemagglutinin-activated human lymphoblasts. J Immunol. 1992;148:3117–3124. [PubMed] [Google Scholar]
- 3.Chua A O, Wilkinson V L, Presky D H, Gubler U. Cloning and characterization of a mouse IL-12 receptor-β component. J Immunol. 1995;155:4286–4294. [PubMed] [Google Scholar]
- 4.Desai B B, Quinn P M, Wolitzky A G, Mongini P K, Chizzonite R, Gately M K. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–3132. [PubMed] [Google Scholar]
- 5.Galbiati F, Rogge L, Guery J, Smiroldo S, Adorini L. Regulation of the IL-12 receptor β2 subunit by soluble antigen and IL-12 in vivo. Eur J Immunol. 1998;28:209–220. doi: 10.1002/(SICI)1521-4141(199801)28:01<209::AID-IMMU209>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Gilman M. Ribonuclease protection assay. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1996. pp. 4.7.1–4.7.8. [Google Scholar]
- 7.Guler M L, Gorham J D, Hsieh C-S, Mackey A J, Steen R G, Dietrich W F, Murphy K M. Genetic susceptibility to Leishmania: IL-12 responsiveness in Th1 cell development. Science. 1996;271:984–986. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 8.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or IL4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 11.Jelinek D F, Braaten J K. Role of IL-12 in human B cell proliferation and differentiation. J Immunol. 1995;154:1606–1615. [PubMed] [Google Scholar]
- 12.Launois P, Maillard I, Pingel S, Swihart K G, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley R M, MacDonald H R, Louis J A. IL-4 rapidly produced by Vα8 Vβ4 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 13.Launois P, Ohteki T, Swihart K, MacDonald H R, Louis J A. In susceptible mice, Leishmania major induces very rapid interleukin-4 production by CD4+ T cells which are NK1.1+ Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 14.Launois P, Swihart K G, Milon G, Louis J A. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 15.Manetti R, Parronchi P, Giudizi M G, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (NKSF/IL-12) induces Th1-type specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattner F, Magram J, Ferrante J, Launois P, DiPadova K, Behin R, Gately M K, Louis J A, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 17.Presky D H, Yang H, Minetti L J, Chua A O, Nabavi N, Wu C-Y, Gately M K, Gubler U. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 19.Romagnani S. Induction of Th1 and Th2 responses: a key role for the ‘natural’ immune response? Immunol Today. 1992;13:379–383. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 20.Sacks D H, Perkins P V. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 21.Scharton T M, Scott P. Natural killer cells are a source of IFN-γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharton-Kersten T, Afonso L C C, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 23.Scott P. T helper cell development and regulation in experimental cutaneous leishmaniasis. Chem Immunol. 1996;63:98–114. [PubMed] [Google Scholar]
- 24.Seder R A, Gazzinelli R, Sher A, Paul W E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sher A, Coffman R L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 26.Showe L C, Wysocka M, Wang B, Lineman-Williams D, Peritt D, Showe M K, Trinchieri G. Structure of the mouse IL-12Rβ1 chain and regulation of its expression in BCG/LPS treated mice. Ann N Y Acad Sci. 1996;795:413–415. doi: 10.1111/j.1749-6632.1996.tb52708.x. [DOI] [PubMed] [Google Scholar]
- 27.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective Th1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin-12 receptor β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo S J, Jacobson N G, Dighe A S, Gubler U, Murphy K M. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 30.Vogel L A, Showe L C, Lester T L, McNutt R M, Van Cleave V H, Metzger D W. Direct binding of IL-12 to human and murine B lymphocytes. Int Immunol. 1996;8:1955–1962. doi: 10.1093/intimm/8.12.1955. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Warrier R R, Wang X, Presky D H, Gately M K. Regulation of interleukin-12 receptor beta 1 chain expression and interleukin-12 binding by human peripheral blood mononuclear cells. Eur J Immunol. 1997;27:147–154. doi: 10.1002/eji.1830270122. [DOI] [PubMed] [Google Scholar]