Abstract
Despite the thousands of lives lost during the ongoing opioid crisis, a scarcity of new and effective clinical treatments for opioid use disorder (OUD) remains. To address this unmet need, some researchers have turned to dissociative and psychedelic drugs to treat multiple psychiatric conditions. In particular, low doses of ketamine have been shown to attenuate opioid withdrawal and drug use in clinical and preclinical studies. However, ketamine has abuse liability and dissociative side effects that may limit its widespread application as a treatment for OUD. More recently, (2R,6R)-hydroxynorketamine (HNK), a ketamine metabolite that lacks abuse potential, has gained attention for its effectiveness in depression and stress models. To uncover its role in OUD, we tested the time-dependent effects of (2R,6R)-HNK on oxycodone withdrawal and reinstatement of oxycodone conditioned place preference (CPP). In male and female oxycodone-dependent mice, we found that 24 h pretreatment with (2R,6R)-HNK (10 or 30 mg/kg, s.c.) reduced the frequency of withdrawal-like behaviors and global withdrawal scores during naloxone-precipitated withdrawal, whereas 1 h pretreatment with (2R,6R)-HNK only reduced paw tremors and the sum of global withdrawal scores but not GWS Z-scores. In other experiments, both 1 h and 24 h pretreatment with (2R,6R)-HNK (30 mg/kg, s.c.) blocked drug-induced reinstatement of oxycodone CPP. Finally, we found (2R,6R)-HNK (30 mg/kg, sc) had no effect on locomotor activity and thigmotaxis. Together, these results indicate that acute (2R,6R)-HNK has efficacy in some preclinical models of OUD without producing locomotor or anxiety-like side effects.
Keywords: opioid use disorder, oxycodone, hydroxynorketamine, HNK, precipitated withdrawal
Graphical Abstract
Introduction
Misuse and abuse of prescription opioids has played a significant role in the ongoing opioid crisis and opioid-related overdose deaths. (Centers for Disease Control and Prevention 2023; U.S. Department of Health and Human Services 2015). For example, between 1991 and 2007, prescriptions for oxycodone, a semi-synthetic mu-opioid receptor agonist, increased by 850%, which correlated to a significant increase in opioid-related deaths (Dhalla et al., 2009). Despite interventions to restrict prescription opioid access, illicit opioid use is projected to rise by 61% between 2015 and 2025 (Chen et al., 2019). Although FDA-approved treatments for opioid use disorder (OUD) are available, the majority of patients seeking treatment for OUD relapse within the first six months of abstinence (Gandhi et al., 2003; Sinha, 2011). Thus, more effective treatments are clearly needed to blunt the opioid crisis and reduce relapse and overdose mortalities.
Growing evidence supports a potential role of ketamine, a dissociative anesthetic, in the treatment of multiple psychiatric disorders, including treatment-resistant depression (McIntyre et al., 2021; Hashimoto 2019; Spyridi et al., 2021), post-traumatic stress disorder (PTSD) (Albott et al., 2018; Feder et al., 2014; Hartberg et al., 2018), and substance use disorders (SUD) (Krupitsky et al., 2002; Krupitsky et al., 2007; Jovaisa et al., 2006; Dakwar et al., 2019). Although ketamine is classically defined as a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, it has multiple downstream effects on neuroplasticity-associated targets including brain-derived neurotrophic factor (BDNF), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and mammalian target of rapamycin (mTOR) (Sleigh et al., 2014; Ivan Ezquerra-Romano et al., 2018), all of which likely play key roles in its therapeutic response. As a potential treatment for OUD, low-dose ketamine has been shown to reduce opioid-seeking behaviors and withdrawal symptoms in clinical and preclinical studies (Krupitsky et al., 2002; Jovaisa et al., 2006; Witkin et al., 2020; McKendrick et al., 2020; Hailozian et al., 2022). Despite these promising results, ketamine therapy for OUD may have some limitations due to its abuse liability and other undesirable side effects (Liu et al., 2016; Trujillo et al., 2011; Sassano-Higgins et al., 2016). (2R,6R)-hydroxynorketamine (HNK) is a secondary metabolite of ketamine, and new evidence indicates that it plays a critical role in the long-lasting therapeutic response of ketamine (Pham et al., 2018; Zanos et al., 2016). When directly administered in rodents, (2R,6R)-HNK was found to reduce depression- and PTSD-like behaviors in preclinical models (Elmer et al., 2020; Zanos et al., 2019; Chen et al., 2020), but unlike ketamine, (2R,6R)-HNK did not have side effects or abuse liability (Zanos et al., 2019; Pham et al., 2018; Bonaventura et al., 2022). Despite having little to no binding at the NMDA receptor, (Bonaventura et al., 2022; Zanos et al., 2016), (2R,6R)-HNK produces similar downstream effects as sub-anesthetic doses of ketamine. For instance, several studies have demonstrated that (2R,6R)-HNK enhances neurogenesis, neuroplasticity, and synaptogenesis through stimulatory effects on BDNF-TrkB signaling, mTOR signaling, and/or AMPA receptor activity (Ju et al., 2022; Zanos et al., 2016; Collo et al., 2018; Lumsden et al., 2019). Given that (2R,6R)-HNK has similar efficacy as ketamine in some preclinical models, (2R,6R)-HNK may be an alternative, safer treatment approach for psychiatric disorders, including OUD.
In the current experiments, we examined time-dependent effects of (2R,6R)-HNK on opioid withdrawal and seeking behavior. To investigate (2R,6R)-HNK’s effects on withdrawal, we utilized an escalating dose model of oxycodone administration followed by naloxone-precipitated withdrawal that has previously been demonstrated to reliably induce physical dependence and withdrawal-like symptoms (Enga et al., 2016; Carper et al., 2021). In other experiments, we investigated the effects of (2R,6R)-HNK on reinstatement of oxycodone conditioned place preference and open field behavior. Together, these initial experiments demonstrate the efficacy of (2R,6R)-HNK in animal models of OUD and open the door for future experiments to investigate the behavioral and molecular effects of (2R,6R)-HNK in advanced models of OUD, including drug self-administration procedures.
Materials and methods
Drugs:
Oxycodone hydrochloride (MilliporeSigma) was dissolved in 0.9% sterile saline and administered subcutaneously (s.c.) at the concentrations described below. Naloxone hydrochloride (Tocris) was dissolved in 0.9% sterile saline and injected subcutaneously (10 mg/kg, s.c.). (2R,6R)-hydroxynorketamine (MilliporeSigma) was dissolved in 0.9% sterile saline and injected subcutaneously (10 or 30 mg/kg, s.c.). Injection volumes for all treatments were approximately 0.1 ml.
Animals:
Male and female C57BL6 mice (8–10 weeks old; Charles River Laboratories) were housed 4 mice per cage under a reverse 12 h/12 h light/dark cycle and given ad libitum access to food and water. An equal number of male and female mice were used in every experiment. Different mice were used for each experiment. Mice were housed in a temperature-controlled AAALAC-accredited animal facility at the University of Connecticut and allowed to acclimate to the facility for at least one week prior to experimentation. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Connecticut and performed in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Drug administration:
Oxycodone was administered using a dosing regimen previously described to induce oxycodone dependence in mice (Enga et al., 2016; Carper et al., 2021). Briefly, male and female mice (equal number of males and females per group) were injected for eight days with increasing doses of oxycodone (9, 17.8, 23,7, and 33 mg/kg, s.c. twice daily on days 1–2, 3–4, 5–6, and 7–8, respectively). During the 8 days of injections, oxycodone or saline was administered at 9 AM and 3 PM each day. One hour or 24 hours before the withdrawal test (described below), mice received a subcutaneous injection of saline (n = 16), 10 mg/kg, s.c. of (2R,6R)-HNK (n = 16), or 30 mg/kg, s.c. of (2R,6R)-HNK (n = 16). The dose range of (2R,6R)-HNK (10–30 mg/kg) was chosen based on previous experiments that have demonstrated efficacy at these doses (Yost et al., 2022; Elmer et al., 2020; Yao et al., 2018; Bonaventura et al., 2022; Zanos et al., 2019). The 1 h and 24 h timepoints were chosen based on previous behavioral studies that have observed therapeutic effects of (2R,6R)-HNK within this timeframe (Zanos et al., 2016; Zanos et al., 2019; Elmer et al., 2020).
Naloxone-induced precipitated withdrawal:
To precipitate withdrawal, mice were injected with naloxone (10 mg/kg, s.c.) on day 10, 1 h or 24 h after the vehicle or (2R,6R)-HNK treatment. Immediately following the naloxone injection, mice were placed individually in a clear plexiglass box, and their behavior was video recorded for 30 minutes. Videos of the mice were scored individually for somatic symptoms of withdrawal including the number of paw tremors, rearing, grooming, jumps, writhing, and hind limb scratching using methods previously described (Mori et al., 2013; Towers et al., 2019, Iyer et al., 2022). The sum of the somatic signs of withdrawal was used to calculate the global withdrawal score. Also, to account for the varying frequencies of each withdrawal-like behavior, Z-scoring was used as a method of standardization global withdrawal scores, as previously described (Bravo et al., 2021). Behavioral scoring was conducted by an experienced and trained researcher that was blinded to treatment conditions.
Conditioned place preference (CPP):
The CPP apparatus (Med Associates Inc.) consisted of two compartments with black walls and grid rod-style floors on one side and white walls and mesh-style floors on the other side. The sides were separated by a divider containing a small door that could be opened or closed during testing and conditioning, respectively. In the drug-free pre-test, the partition door was opened, and mice were allowed to freely explore both sides of the chamber for 15 minutes. The time spent on each side of the CPP chamber was automatically recorded by infrared photobeam detectors. Mice that exhibited a strong bias for one side of the chamber during the pretest (>65% of time spent on one side) were excluded from further testing. For the next three consecutive days, mice were conditioned as previously described (Babigian et al., 2021; Sartor et al., 2016; Sartor et al., 2015). During the 30-minute conditioning sessions, one side of the chamber was paired with a subcutaneous saline injection and the other side was paired with an oxycodone injection (3 mg/kg, s.c.). Conditioning occurred in morning and afternoon sessions in a counterbalanced design (at least 6 h apart). One day after the last conditioning session, mice received a drug-free CPP test where they had free access to each side of the chamber. The preference score was calculated by subtracting the time spent on the oxycodone-paired side during the post-test from the time spent on the same side during the pre-test. One female mouse failed to exhibit a preference for the oxycodone-paired side on test day and was excluded from further testing (Figure 5). For extinction, mice were passively extinguished by giving a drug-free CPP test each day until preference scores were reduced by at least 50% (relative to their initial preference) for two consecutive days, as previously described (Sartor et al., 2016). Treatment groups were assigned such that there was no difference in initial preference score and number of extinction days between treatment groups. In oxycodone-primed reinstatement tests, (2R,6R)-HNK (30 mg/kg, s.c.) or saline was administered 1 h or 24 h before the oxycodone-primed reinstatement test. To reinstate the place preference, oxycodone (1 mg/kg, s.c.) was injected immediately before the reinstatement CPP test.
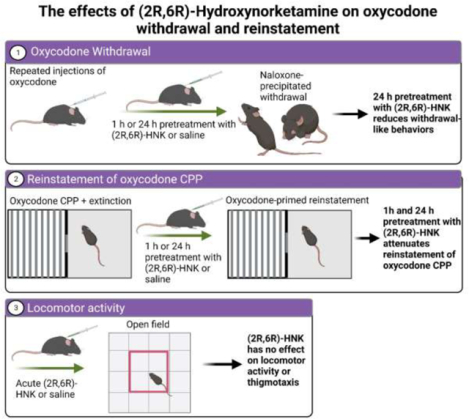
Figure 5. 1-hour pretreatment with (2R,6R)-HNK significantly attenuated oxycodone-induced reinstatement.
(A) Conditioned mice exhibited oxycodone CPP, and the oxycodone preference was reduced following extinction testing. Saline or (2R,6R)-HNK (30 mg/kg, s.c.) was administered 1 h prior to the oxycodone-induced reinstatement test. Reinstatement of CPP was significantly attenuated in (2R,6R)-HNK-treated mice compared to saline-treated mice (n = 11–12 per group). (B) No differences in expression, extinction, and reinstatement were observed when comparing male and female mice. Data were analyzed via two-way ANOVA followed by Bonferroni post hoc tests. **** P < 0.0001. Data are expressed as means (±SEM).
Open Field:
The open field apparatus consists of a 46 cm × 46 cm chamber with opaque gray walls (40 cm high). One hour after a saline or (2R,6R)-HNK (30 mg/kg, s.c.) injection, each mouse was placed in the middle of the chamber and allowed to freely explore the environment for 30 minutes while being recorded by an overhead camera, as previously described (Singh et al., 2022) (n = 6 males and 6 females per treatment group). Here, we focus on 1 h pretreatment with (2R,6R)-HNK as a previous study found that 24 h pretreatment had no effect on locomotor activity (Bonaventura et al., 2022). EthoVision video tracking software was used to quantify time spent in the inner and outer zones, as well as distance traveled. Between each trial, the apparatus was thoroughly cleaned with 70% ethanol.
Data analysis:
Statistical analyses were performed with GraphPad Prism 7.0 software. A Student’s t-test was used to compare locomotor activity between saline and (2R,6R)-HNK treated mice. One-way analysis of variance (ANOVA) was used to evaluate differences between treatment conditions for withdrawal scores. Two-way ANOVA was used to evaluate sex-dependent effects for withdrawal scores, CPP scores, and open field behavior. For ANOVA tests, when a significant F value was obtained, post hoc comparisons were performed using Bonferroni correction. All data are expressed as means ± SEM and the level of significance was set to P < 0.05.
Results
The effects of (2R,6R)-HNK on naloxone-induced precipitated withdrawal
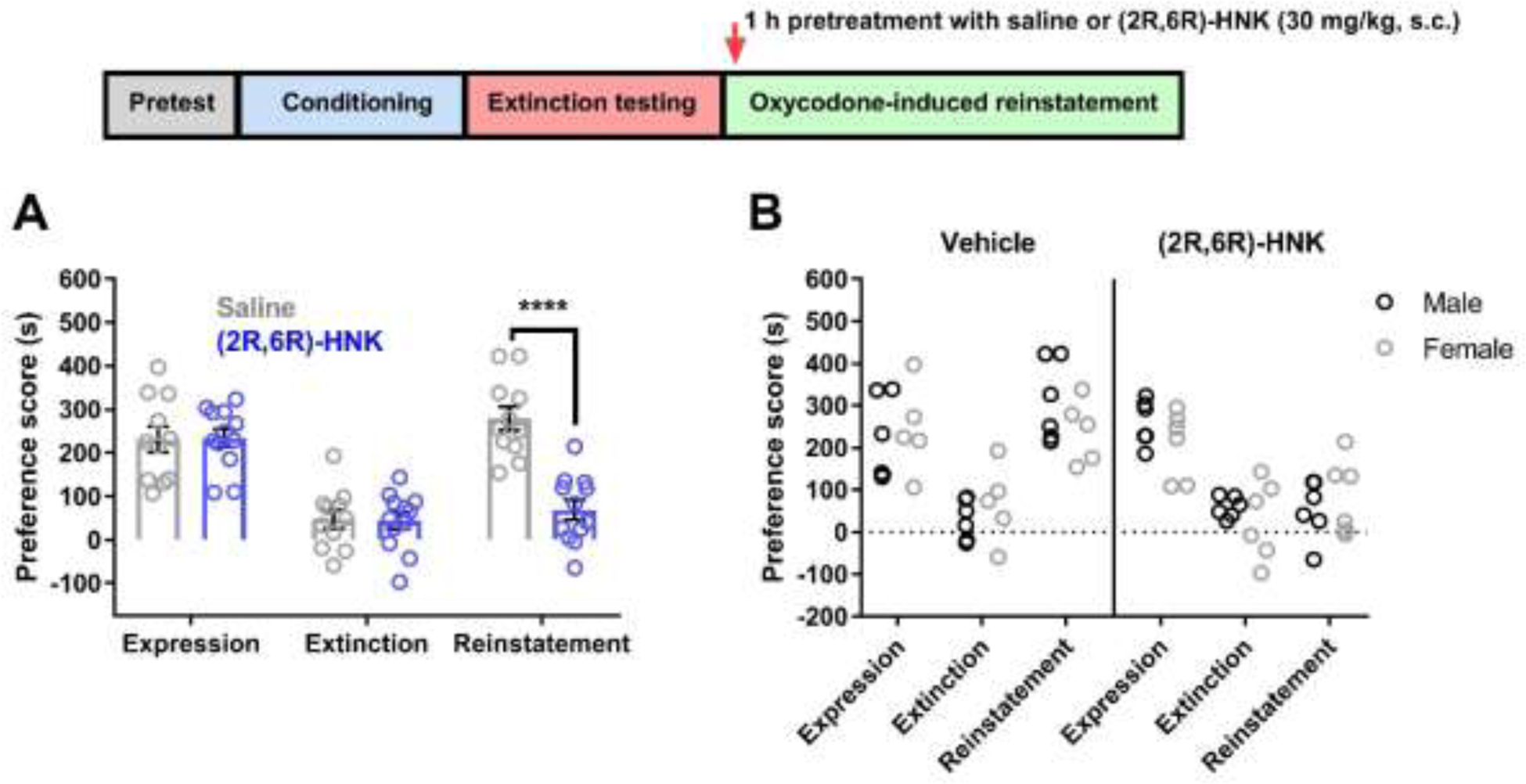
In Figure 1, oxycodone-dependent mice were treated with saline or (2R,6R)-HNK (10 or 30 mg/kg, s.c.) 1 h before the naloxone-induced withdrawal test. A one-way ANOVA showed a significant main effect in paw tremors (F(2, 45) = 13.16, P < 0.0001) (Figure 1A). Bonferroni post hoc analysis revealed that (2R,6R)-HNK significantly decreased frequency of paw tremors at 10 mg/kg, s.c. (P = 0.0001) and 30 mg/kg, s.c. (P = 0.0002) when compared to saline. In contrast, 1 h pretreatment with (2R,6R)-HNK did not alter rearing (F(2, 45) = 0.2952, P = 0.7458, Figure 1B), grooming (F(2, 45) = 1.875, P = 0.1651, Figure 1C), jumps (F(2, 45) = 0.331, P = 0.7199, Figure 1D), hind limb scratching (F(2, 45) = 2.721, P = 0.0767, Figure 1E), or writhing (F(2, 45) = 1.568, P = 0.2197, Figure 1F) when compared to saline. Additionally, no sex-dependent effects in paw tremors (F(1, 42) = 1.548, P = 0.2203), jumps (F(1, 42) = 2.735, P = 0.1056), rearing (F(1, 42) = 3.542, P = 0.0668), hind-limb scratching (F(1, 42) = 2.040, P = 0.1606), writhing (F(1, 42) = 0.6512, P = 0.4242), or grooming (F(1, 42) = 1.438, P = 0.2372) were observed (data not shown).
Figure 1: Withdrawal-like behaviors in mice treated with saline or (2R,6R)-HNK 1-hour before naloxone-induced withdrawal.
Oxycodone-dependent mice were treated with saline or (2R,6R)-HNK (10 or 30 mg/kg, sc), and the number of (A) paw tremor, (B) rearing, (C) grooming, (D) jumps, (E) hind limb scratching, and (F) writhing events were measured during naloxone-induced withdrawal (n = 16 per group). Data were analyzed via one-way ANOVA followed by Bonferroni post hoc tests. ***P < 0.001. Data are expressed as means (±SEM).
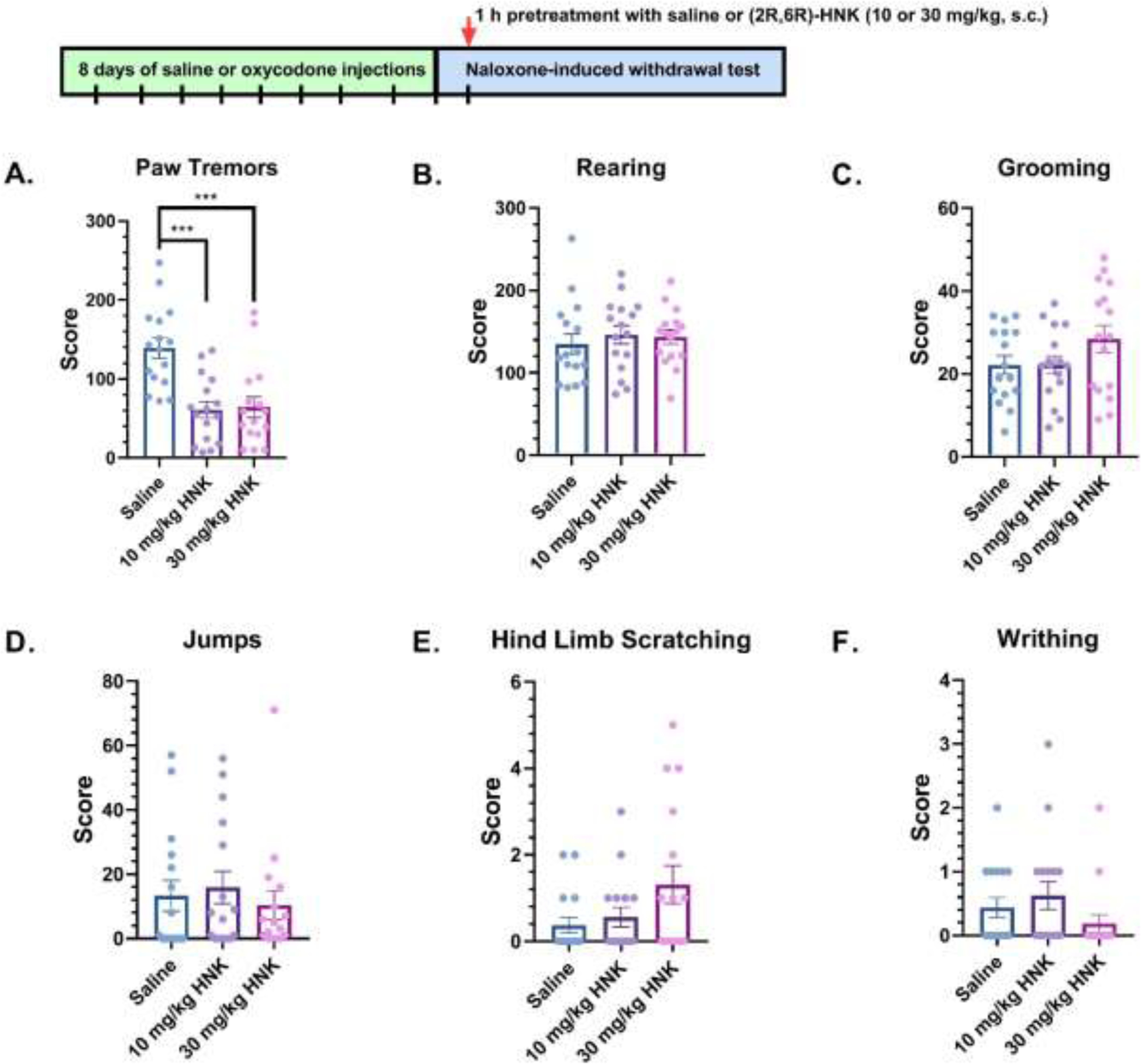
In Figure 2, global withdrawal scores (GWS) were calculated in the mice treated with saline or (2R,6R)-HNK (10 or 30 mg/kg, s.c.) 1 h before the naloxone-induced withdrawal test. A one-way ANOVA revealed a significant main effect of GWS (F(2, 45) = 5.13, P = 0.0098, Figure 2A). Bonferroni post hoc analysis indicated that GWS was significantly reduced by 10 mg/kg, s.c. (P = 0.0208) and 30 mg/kg, s.c. of (2R,6R)-HNK (P = 0.0281) compared to the saline group. No significant difference in GWS was observed between 10 and 30 mg/kg, s.c. of (2R,6R)-HNK (P > 0.05). To account for the varying frequencies of each individual behavior, Z-scoring was used as a method of standardization. Z-score reports values as a number of standard deviations each individual animal’s score falls from a mean of observed scores for each behavior (Bravo et al., 2021). One-way ANOVA revealed that (2R,6R)-HNK, when administered 1 h prior to naloxone-precipitated withdrawal, had no significant effect on GWS Z-scores (F(2, 45) = 0.639, P = 0.5325, Figure 2B). A two-way ANOVA showed no sex-dependent treatment effects in global withdrawal scores (P values > 0.05, data not shown).
Figure 2: Global withdrawal scores in mice treated with saline or (2R,6R)-HNK 1-hour before naloxone-induced withdrawal.
(A) Oxycodone-dependent mice were injected with saline or (2R,6R)-HNK (10 or 30 mg/kg, sc) and the sum of all withdrawal-like behaviors during naloxone-precipitated withdrawal was calculated as the global withdrawal score (n = 16 per group). (B) Global withdrawal scores reported as Z-scores during naloxone-precipitated withdrawal. Data were analyzed via one-way ANOVA followed by Bonferroni post hoc tests. *P < 0.05. Data are expressed as means (±SEM).
In Figure 3, a different group of oxycodone-dependent mice were treated with saline or (2R,6R)-HNK (10 or 30 mg/kg, s.c.) 24 h before the naloxone-induced withdrawal test. A one-way ANOVA showed a significant main effect in paw tremors (F(2, 45) = 8.304; P = 0.0009) (Figure 3A), rearing (F(2, 45) = 5.239; P = 0.0090) (Figure 3B), grooming (F(2, 45) = 9.332; P = 0.0004) (Figure 3C), and jumps (F(2, 45) = 3.449; P = 0.0404) (Figure 3D). Bonferroni post hoc analysis revealed that (2R,6R)-HNK significantly decreased frequency of paw tremors at 30 mg/kg, s.c. (P = 0.0006) (Figure 3A) and grooming at 10 mg/kg, s.c. (P = 0.0002) and 30 mg/kg, s.c. (P = 0.0004) (Figure 3C), but no significant effect between groups for jumps was observed in post hoc analysis (Figure 3D). Compared to saline, rearing was significantly reduced in mice receiving a 10 mg/kg, s.c. dose of (2R,6R)-HNK (P = 0.0299) but not 30 mg/kg, s.c. (P > 0.05) (Figure 3B). Although there was a decreasing trend in (2R,6R)-HNK treated mice, no significant effects were observed for hind limb scratching (F(2, 45) = 1.0, P = 0.3758, Figure 3E) or writhing (F(2, 45) = 3.155, P = 0.0522, Figure 3F). One saline mouse under writhing (Figure 3F) was identified as a significant outlier (Z-score = 3.11, P < 0.05). With this mouse removed, a one-way ANOVA indicated a significant main effect in writhing (F(2, 44) = 8.768, P = 0.0006), and Bonferroni post-hoc test revealed significant differences between 10 mg/kg, s.c. and 30 mg/kg, s.c. of (2R,6R)-HNK (P = 0.002) when compared to saline. In other analyses, a two-way ANOVA did not reveal sex-dependent effects in paw tremors (F(1, 42) = 0.0314; P = 0.8600), jumps (F(1, 42) = 1.852; P = 0.1808), rearing (F(1, 42) = 0.0137; P = 0.9071), hind-limb scratching (F(1, 42) = 0.8469; P = 0.3627), writhing (F(1, 42) = 1.032; P = 0.3154), or grooming (F(1, 42) = 0.7504; P = 0.4784) (data not shown).
Figure 3: Withdrawal-like behaviors in mice treated with saline or (2R,6R)-HNK 24-hours before naloxone-induced withdrawal.
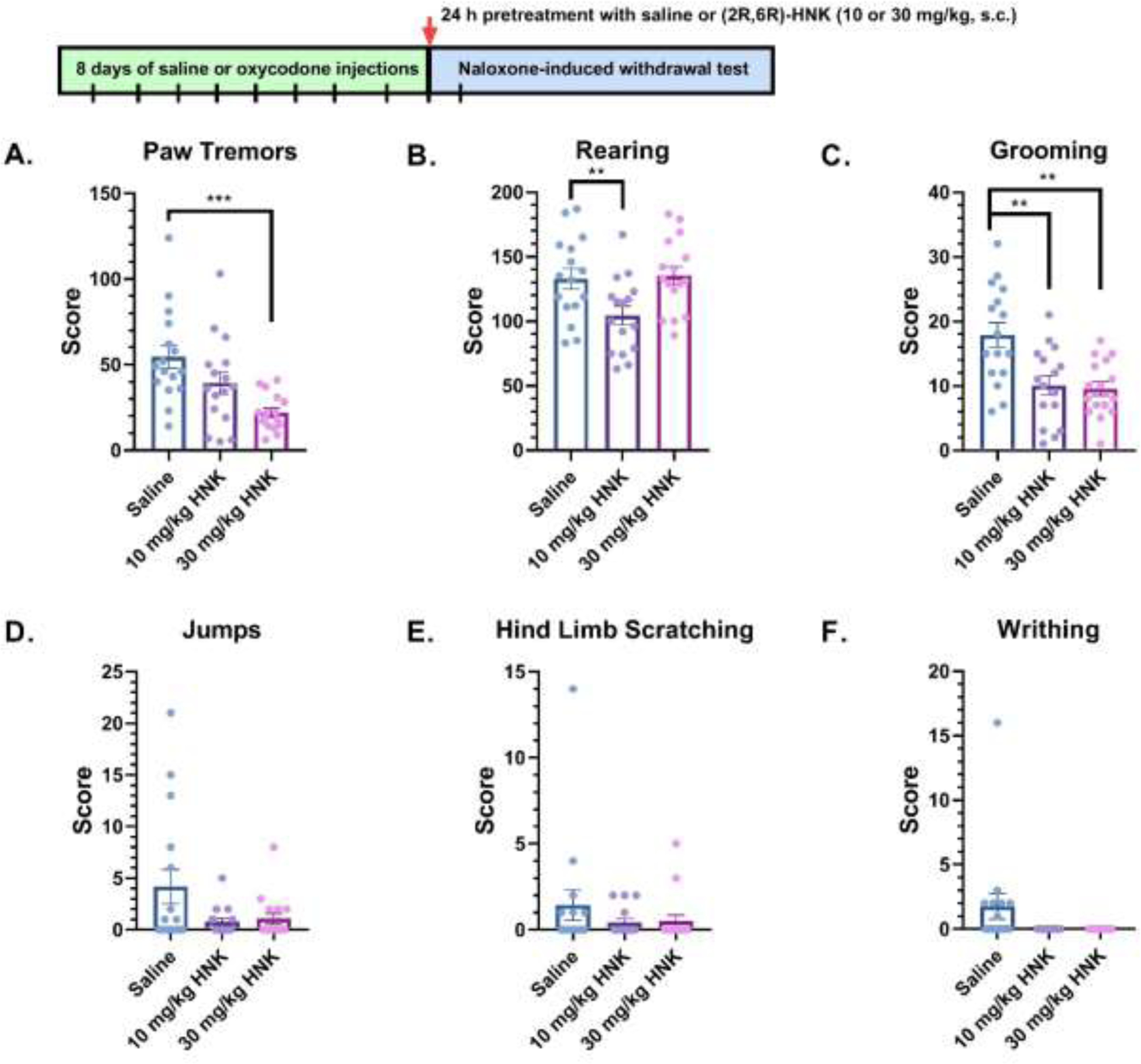
Oxycodone-dependent mice were treated with saline or (2R,6R)-HNK (10 or 30 mg/kg, sc), and the number of (A) paw tremor, (B) rearing, (C) grooming, (D) jumps, (E) hind limb scratching, and (F) writhing events were measured during naloxone-induced withdrawal (n = 16 per group). Data were analyzed via one-way ANOVA followed by Bonferroni post hoc tests. **P < 0.01, ***P < 0.001. Data are expressed as means (±SEM).
In Figure 4, global withdrawal scores were calculated in mice treated with saline or (2R,6R)-HNK (10 or 30 mg/kg, s.c.) 24 h before the naloxone-induced withdrawal test. A one-way ANOVA revealed a significant main effect in GWS (F(2, 45) = 10.98; P = 0.0001). Bonferroni post hoc analysis showed that GWS was significantly reduced by 10 mg/kg, s.c. (P = 0.0002) and 30 mg/kg, s.c. of (2R,6R)-HNK (P = 0.0031) compared to the saline group (Figure 4A). No significant difference in GWS was observed between 10 and 30 mg/kg, s.c. of (2R,6R)-HNK (P > 0.05). For GWS Z-scores, a one-way ANOVA revealed a significant main effect (F(2, 45) = 20.74; P < 0.0001), and in Bonferroni post hoc analysis, GWS Z-scores were found to be significantly attenuated by 10 mg/kg, s.c. (2R,6R)-HNK (P < 0.0001) and 30 mg/kg, s.c. of (2R,6R)-HNK (P < 0.0001) compared to the saline group (Figure 4B). No significant difference was observed for GWS Z-scores between 10 and 30 mg/kg, s.c. of (2R,6R)-HNK (P > 0.05). A two-way ANOVA showed no sex-dependent treatment effects in global withdrawal scores (P values > 0.05, data not shown).
Figure 4: Global withdrawal scores in mice treated with saline or (2R,6R)-HNK 24-hours before naloxone-induced withdrawal.
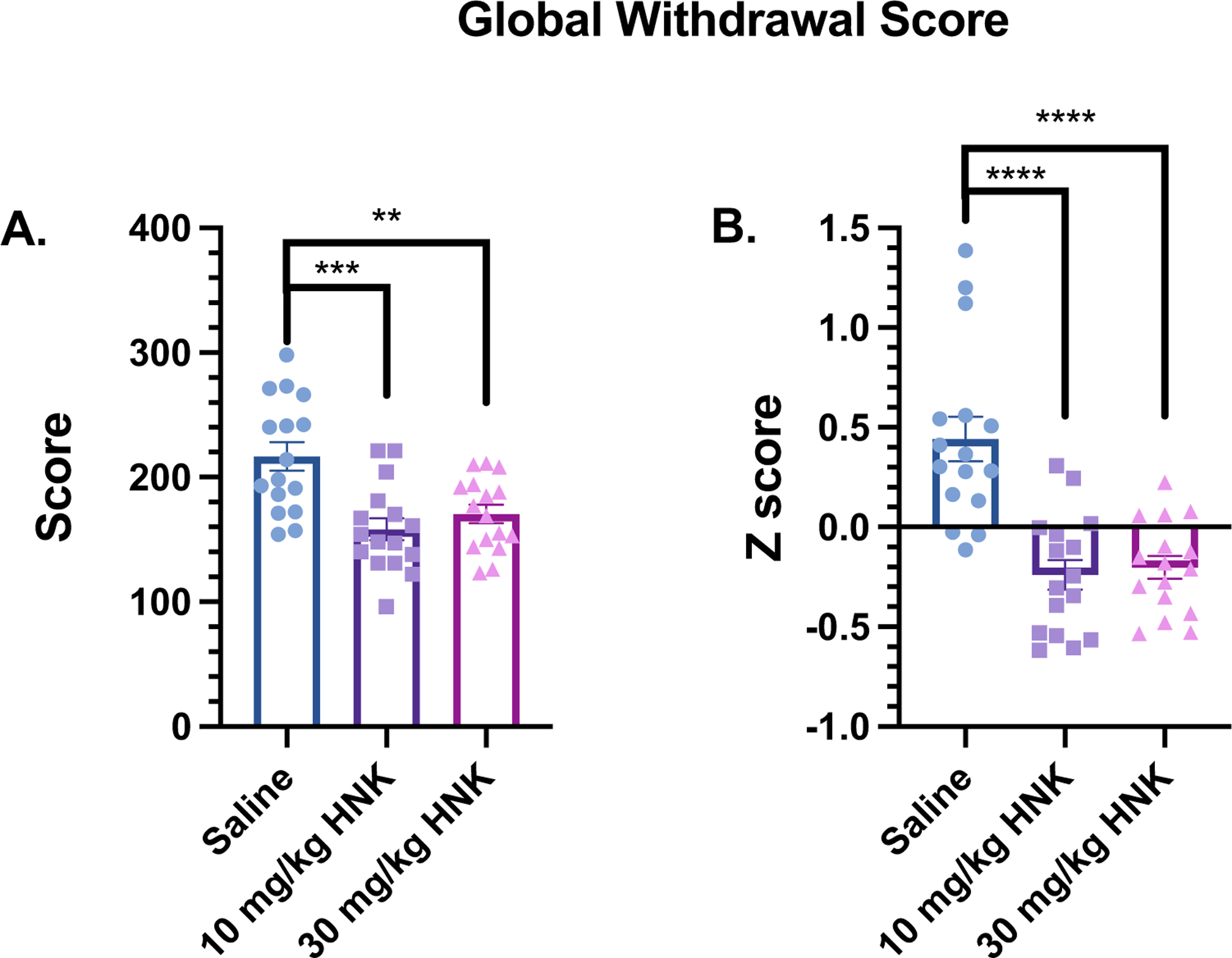
(A) Oxycodone-dependent mice were injected with saline or (2R,6R)-HNK (10 or 30 mg/kg, sc) and the sum of all withdrawal-like behaviors during naloxone-precipitated withdrawal was calculated as the global withdrawal score (n = 16 per group). (B) Global withdrawal scores reported as Z-scores during naloxone-precipitated withdrawal. Data were analyzed via one-way ANOVA followed by Bonferroni post hoc tests. **P < 0.01; ***P < 0.001; **** P < 0.0001. Data are expressed as means (±SEM).
Effects of (2R,6R)-HNK on reinstatement of oxycodone CPP
Because SUD is defined as a chronic relapsing condition, we sought to investigate the effects of (2R,6R)-HNK on the reinstatement of oxycodone CPP. After conditioning, mice showed robust expression of oxycodone CPP during the initial test (n = 11–12 per group) (Figure 5A). Next, mice were passively extinguished by daily, drug-free CPP testing, and the number of days to reach the extinction criteria was not different between treatment groups (7.91 ± 0.5793 for saline and 8.75 ± 0.9303 for HNK; t21 = 0.7509, P = 0.4611). Prior to reinstatement testing, there was no significant difference between groups during expression and extinction tests (P values > 0.05). Once extinguished, mice received an injection of saline or (2R,6R)-HNK (30 mg/kg, s.c.) 1 h prior to the oxycodone-primed reinstatement test. A two-way ANOVA showed a significant effect of treatment (F(1, 63) = 13.36, P = 0.0005), test (F(2, 63) = 33.44, P < 0.0001), and interaction (F(2, 63) = 13.44, P < 0.0001) (Figure 5A). Bonferroni post hoc test revealed that (2R,6R)-HNK significantly reduced reinstatement scores compared to saline-treated mice (P < 0.0001). A two-way ANOVA did not reveal any sex differences between groups during the expression, extinction, and reinstatement tests (P values < 0.05) (Figure 5B).
In the next experiment, different oxycodone conditioned mice were treated with saline or (2R,6R)-HNK (30 mg/kg, s.c., n = 12 per group) 24 h before the oxycodone-induced reinstatement test (Figure 6). Prior to reinstatement testing, there were no significant differences between groups during expression and extinction tests (P values > 0.05). Also, the number of days to reach the extinction criteria was not different between treatment groups (6.417 ± 0.7829 for saline and 6.25 ± 0.7295 HNK; t22 = 0.1557, P = 0.8777). A two-way ANOVA showed a significant effect of treatment (F(1, 66) = 10.95, P = 0.0015), test (F(2, 66) = 34.57, P < 0.0001), and interaction (F(2, 66) = 16.02, P < 0.0001) (Figure 6A). Bonferroni post hoc test revealed that (2R,6R)-HNK (30 mg/kg, s.c.) significantly reduced reinstatement scores compared to saline-treated mice (P < 0.0001). A two-way ANOVA did not reveal any sex differences between groups during the expression, extinction, and reinstatement tests (P values < 0.05) (Figure 6B).
Figure 6. 24-hour pretreatment with (2R,6R)-HNK significantly attenuated oxycodone-induced reinstatement.
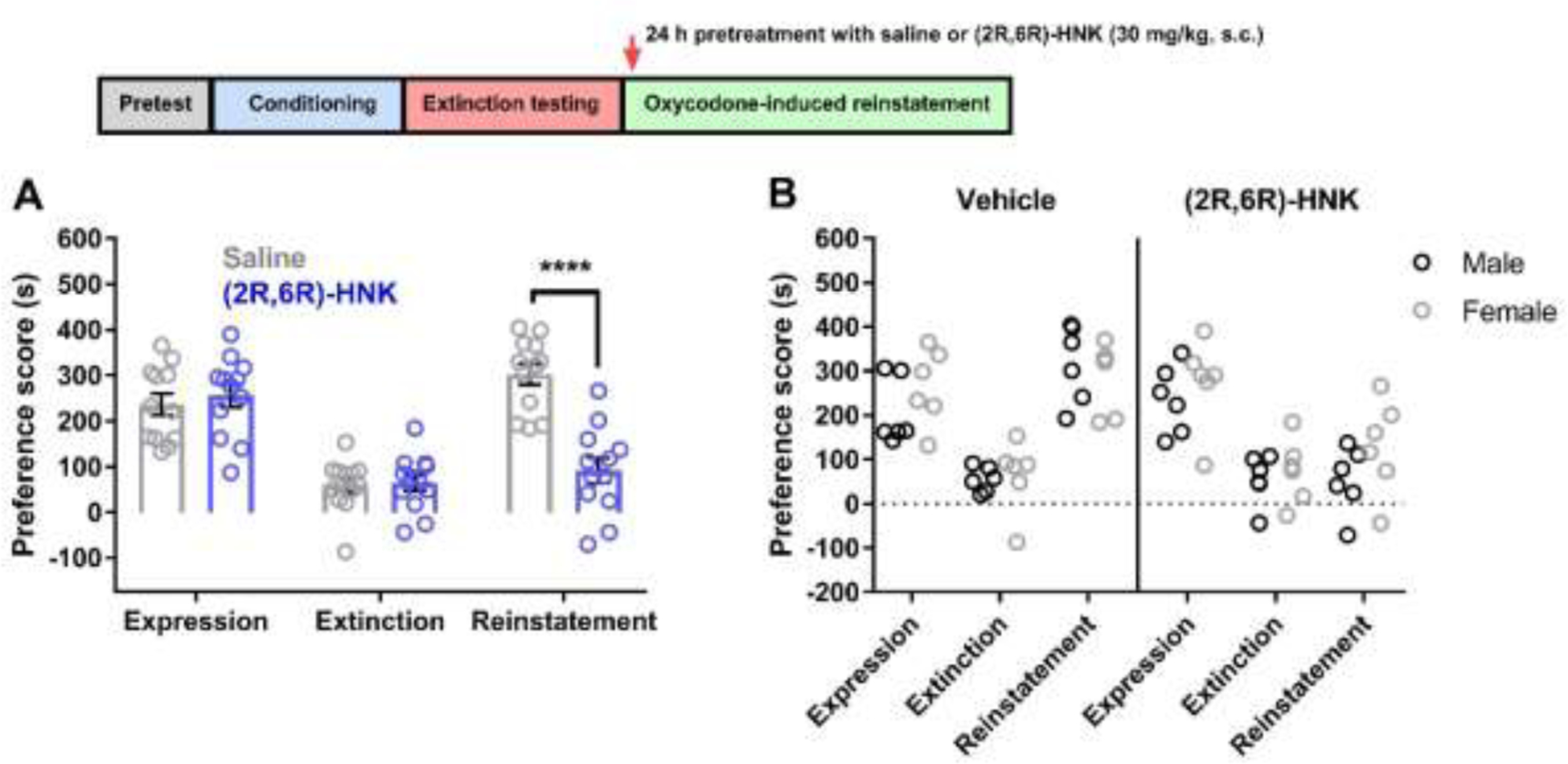
(A) Conditioned mice exhibited oxycodone CPP, and the oxycodone preference was reduced following extinction testing. Saline or (2R,6R)-HNK (30 mg/kg, s.c.) was administered 24 h prior to the oxycodone-induced reinstatement test. Reinstatement of CPP was significantly attenuated in (2R,6R)-HNK-treated mice compared to saline-treated mice (n = 12 per group). (B) No differences in expression, extinction, and reinstatement were observed when comparing male and female mice. Data were analyzed via two-way ANOVA followed by Bonferroni post hoc tests. **** P < 0.0001. Data are expressed as means (±SEM).
Effects of (2R,6R)-HNK on open field behavior
In previous studies, (2R,6R)-HNK did not alter locomotor activity when injected 24 h before testing (Bonaventura et al., 2022). However, it is unclear if (2R,6R)-HNK has effects on locomotor activity or thigmotaxis when measured 1 h post-injection, a timepoint used in the withdrawal and CPP reinstatement experiments described above. In Figure 7, a new group of male and female mice were injected with saline or (2R,6R)-HNK (30 mg/kg, s.c.) 1 h before being placed in an open field (n = 12 per group). Distance traveled was not altered between treatments (t22 = 0.6994; P = 0.7842) (Figure 7A), and no sex differences were observed between groups (treatment effect: F(1, 20) = 0.492, P = 0.4911; sex effect: F(1, 20) = 0.04466, P = 0.8348; interaction effect: F(1, 20) = 2.084, P = 0.1644) (Figure 7B). Time spent in the inner or outer zone did not differ between treatment groups (t22 = 1.162, P = 0.2577) (Figure 7C), and no sex differences in time spent in the inner and outer zones were observed between treatment groups (treatment effect: F(1, 20) = 1.379, P = 0.2540; sex effect: F(1, 20) = 2.152, P = 0.1580; interaction effect: F(1, 20) = 0.3222, P = 0.5766) (Figure 7D).
Figure 7. Effects of (2R,6R)-HNK on open field behavior.
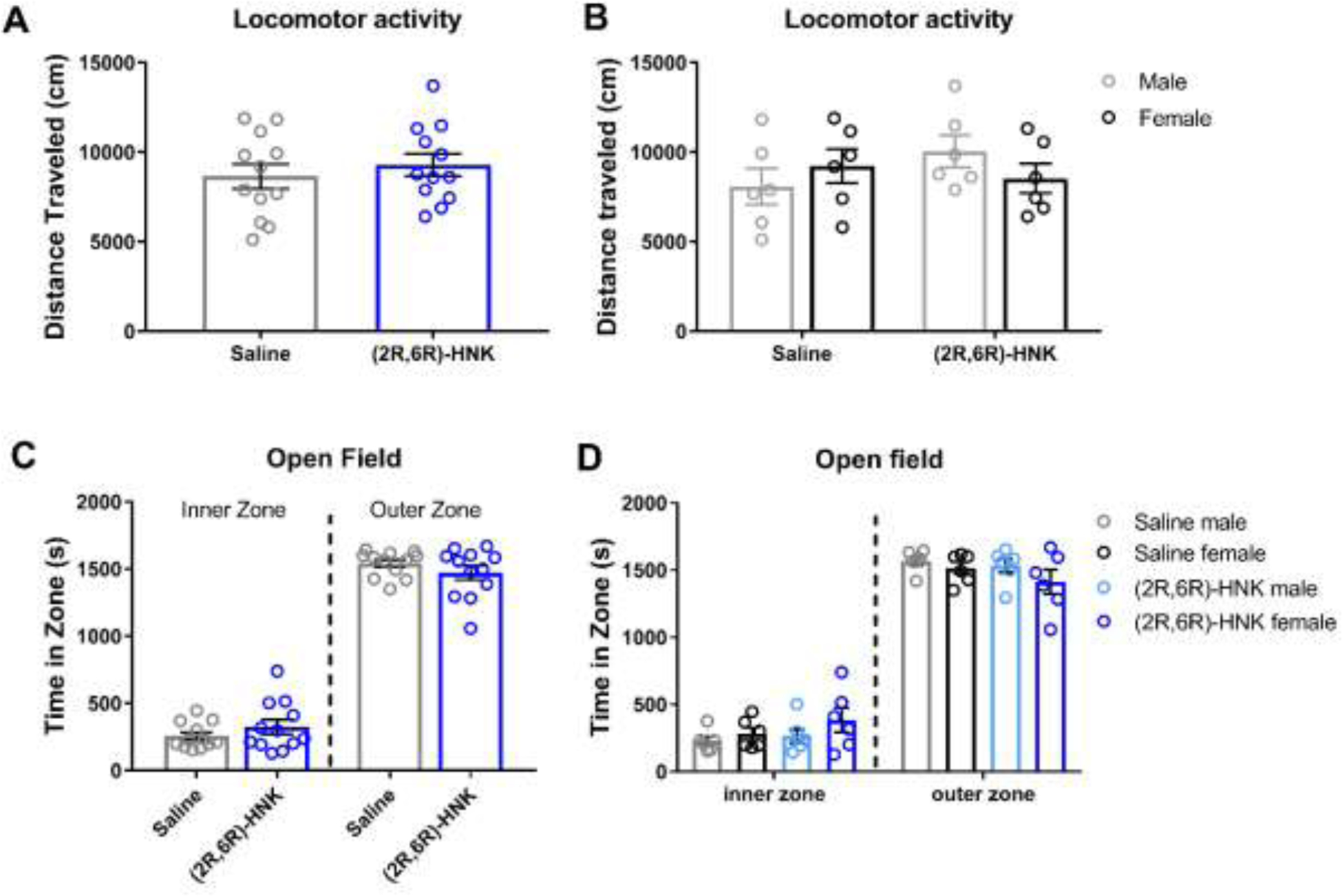
(A) In the open field test, (2R,6R)-HNK (30 mg/kg, s.c., n = 12 per group) did not alter distance traveled when injected 1 h prior to testing, and (B) no sex-dependent effects on locomotor activity were observed. (C) (2R,6R)-HNK did not alter time spent in the inner and outer zones in the open field, and (D) no sex-dependent effects on time spent in the inner/outer zones were observed. Data were analyzed via a Student’s t-test or two-way ANOVA. Data are expressed as means (±SEM).
Discussion
Growing evidence indicates that (2R,6R)-HNK, a secondary ketamine metabolite, effectively reduces symptoms associated with depression and stress in rodent models (Elmer et al., 2020; Zanos et al., 2019; Chen et al., 2020) but lacks the side effects and abuse potential associated with ketamine (Zanos et al., 2019; Pham et al., 2018; Bonaventura et al., 2022). Because (2R,6R)-HNK has exhibited efficacy across multiple preclinical models of psychiatric disorders, we sought to examine (2R,6R)-HNK’s effects in animal models of OUD. In a mouse model of oxycodone dependence, we revealed that 24 h pretreatment with (2R,6R)-HNK (10 and 30 mg/kg, s.c.) decreased multiple somatic withdrawal-like symptoms, as well as global withdrawal scores. However, 1 h pretreatment with (2R,6R)-HNK only reduced paw tremors and the sum of global withdrawal scores but not GWS Z-scores. In prior behavioral experiments using (2R,6R)-HNK, multiple pretreatment timepoints were rarely examined within the same study. However, Zanos and colleagues did show that both 1 h and 24 h pretreatment with (2R,6R)-HNK reduced immobility time in the forced-swim test to a similar degree (Zanos et al., 2016). In contrast, the same paper revealed that BDNF and AMPAR subunits (GluA1 and GluA2) were significantly increased in the hippocampus 24 h, but not 1 h, after (2R,6R)-HNK administration. These data indicate (2R,6R)-HNK has rapid and sustained effects in the brain which may contribute to time-dependent differences in behavior and molecular adaptations.
In the naloxone-precipitated withdrawal experiments, we did not observe dose-dependent effects of (2R,6R)-HNK (10 vs. 30 mg/kg, s.c.) on global withdrawal scores. A lack of dose-dependent effect within this range has been reported in other behaviors (Zanos et al., 2016; Fukumoto et al., 2019). For example, one study found that 1 or 24 h pretreatment with 5, 25, 75, or 125 mg/kg of (2R,6R)-HNK attenuated forced swim test scores to a similar degree, and no significant differences were reported between these doses in the forced swim test (Zanos et al., 2016). However, dose-dependent effects of (2R,6R)-HNK (5, 10, 25, 75 mg/kg) were reported in learned helplessness behaviors. Thus, in some behaviors, (2R,6R)-HNK has similar efficacy across a wide dose range, and future studies using lower doses (< 5 mg/kg) may be required to observe dose-dependent responses. Additionally, our results indicate that not all behaviors were affected by (2R,6R)-HNK during naloxone-precipitated withdrawal. As the majority of naloxone-precipitated withdrawal papers only report the sum of withdrawal behaviors (GWS) or a single withdrawal-like behavior, it difficult to compare and contrast our findings with others. Also, our data highlight the importance of reporting GWS as Z-scores, as one behavior can greatly influence the global withdrawal score when it is reported as a sum.
In addition to showing effects in withdrawal-like behaviors, we also found that both 1 and 24 h pretreatment with (2R,6R)-HNK attenuated reinstatement of oxycodone CPP. These data align with a number of recent studies showing that low-dose ketamine attenuates morphine- and cocaine-seeking behaviors and incentive-motivational value of reward-related cues in rodents (McKendrick et al., 2020; Maltbie et al., 2019; Fitzpatrick & Morrow, 2017), as well as heroin use in humans (Krupitsky et al., 2002). While the mechanism of action of (2R,6R)-HNK in the reinstatement of oxycodone CPP remains unclear, some putative targets of (2R,6R)-HNK have been linked to opioid-induced relapse-like behaviors. For example, using patch-clamp techniques, Moaddel and colleagues found that (2R,6R)-HNK, but not ketamine, inhibited α7 nicotinic acetylcholine receptors at concentrations less than 1 μM (Moaddel et al., 2013). In preclinical OUD studies, inhibition of α7 nicotinic receptors systemically or within the ventral hippocampus attenuated reinstatement of morphine and heroin CPP (Wright et al., 2019; Palandri et al., 2021). Interestingly, the ventral hippocampus is also a key brain region in ketamine’s antidepressant response (Carreno et al., 2016) and may also be a target brain region of (2R,6R)-HNK during reinstatement. In addition to the ventral hippocampus, (2R,6R)-HNK has been shown to modulate activity in the rodent nucleus accumbens (NAc) and basolateral amygdala (Yao et al., 2018; Bonaventura et al., 2022; Xu et al., 2023), brain regions that are involved in opioid-seeking behaviors (Scofield et al., 2016; Fuchs & See, 2002). Additional studies are needed to understand the brain regions, cell types, and receptors that mediate (2R,6R)-HNK’s effects on opioid withdrawal and seeking behaviors.
While the specific mechanisms by which (2R,6R)-HNK reverses the behavioral adaptations seen in OUD are unclear, several direct or indirect, putative targets of (2R,6R)-HNK have also been implicated in OUD models, including AMPA receptors (Russell et al., 2016; Zanos et al., 2016), mTOR (Ucha et al., 2022; Yao et al., 2018), α7 nicotinic receptors (nAChRs) (Wright et al., 2019; Moaddel et al., 2013), BDNF-TrkB (Chen et al., 2012; Koo et al., 2014; Lumsden et al., 2019; Ju et al., 2022), and opioid receptors (Joseph et al., 2021). For example, the efficacy of (2R,6R)-HNK in animal models of depression has been associated with changes in AMPA receptor activity (Yao et al., 2018; Zanos et al., 2016). Treatment with AMPA receptor antagonist NBQX prior to (2R,6R)-HNK administration blocked its antidepressant effects, indicating that the antidepressant properties of (2R,6R)-HNK rely on an AMPAR-dependent mechanism (Zanos et al., 2016). Further, acute (2R,6R)-HNK administration increased GluA1 and GluA2 expression in the mouse hippocampal synaptoneurosomes 24 h after treatment (Zanos et al., 2016), suggesting that a single dose of (2R,6R)-HNK induces long-lasting neuroadaptations in AMPA receptor signaling.
Importantly, GluA1 in the NAc and central amygdala has been shown to play a critical role in morphine CPP as well as morphine withdrawal (Cai et al., 2013; Cai et al., 2020), with lower NAc GluA1 expression linked to potentiated morphine CPP (Hou et al., 2022) and withdrawal (Russel et al., 2016). Thus, the effects of (2R,6R)-HNK on GluA1 during oxycodone withdrawal and seeking behaviors is an area of research that merits further investigation.
(2R,6R)-HNK has attracted attention as a novel treatment for psychiatric disorders, yet discrepancies in (2R,6R)-HNK’s mechanism of action and behavioral effects do exist. For instance, several studies have reported that (2R,6R)-HNK induces alterations in a number of receptors and signaling pathways (Zanos et al., 2016; Collo et al., 2018; Yao et al., 2018; Shaffer et al., 2019; Li et al., 2022; Herzog et al., 2021; Yao et al., 2018; Lumsden et al., 2019; Ju et al., 2022), as well as depression- and anxiety-like behaviors (Pham et al., 2018; Chou et al., 2018; Highland et al., 2019). Contrasting these findings, target deconvolution studies conducted by Bonaventura et al. (2022) report (2R,6R)-HNK as having an inert pharmacologic profile in vitro, including a negligible affinity for NMDA receptors, glutamate receptors, and opioid receptors (Bonaventura et al., 2022). Despite reporting no clear pharmacologic targets, the same study found a significant increase in metabolic activity in the NAc and a reduction in depression-like behaviors in mice treated with (2R,6R)-HNK. Thus, it is possible that (2R,6R)-HNK is exerting its effects in vivo through an unidentified mechanism of action that is not easily detected in cell-based screening assays (Bonaventura et al., 2022). Additionally, differences in mouse strain and sex, the behavioral model used, and conditions prior to (2R,6R)-HNK treatment (e.g., naïve vs. stressed) may also contribute to the discrepancies seen in the literature (Hashimoto & Shirayama, 2018; Highland et al., 2021; Chen et al., 2020; Georgiou et al., 2022).
Some limitations should be considered when interpreting the current study. First, the withdrawal experiments were conducted using naloxone-precipitated withdrawal, and the effects of (2R,6R)-HNK on spontaneous withdrawal remain unknown. Nonetheless, precipitated opioid withdrawal in patients seeking treatment for OUD remains an important clinical issue (Oakley et al., 2021; D’Onofrio et al., 2023), and new evidence suggests that low-dose ketamine administration alleviates buprenorphine-precipitated opioid withdrawal symptoms in patients with OUD (Hailozian et al., 2022), and promotes heroin abstinence in humans (Krupitsky et al., 2002; Lalanne et al., 2016; Krupitsky et al., 2007). Second, the drug-seeking experiments were conducted using CPP procedures in which the mice receive limited oxycodone exposure, and the oxycodone is administered by the experimenter. Testing the effects of (2R,6R)-HNK in operant drug self-administration experiments will be an important future study for understanding the translatable applications of (2R,6R)-HNK in OUD. Third, the long-term behavioral adaptations induced by (2R,6R)-HNK remain unclear. In the current experiments, we reported effects of (2R,6R)-HNK when injected 1 h and 24 h before CPP reinstatement and precipitated withdrawal test, and previous studies have also reported neurobehavioral effects within this time frame following an acute (2R,6R)-HNK injection (Elmer et al., 2020; Zanos et al., 2019; Zanos et al., 2016; Chen et al., 2020). However, the long-lasting (> 24 h) therapeutic effects of acute and/or repeated (2R,6R)-HNK in OUD models will be necessary for understanding its therapeutic utility in humans. Last, the side effects of repeated exposure to (2R,6R)-HNK in OUD models is another unknown. While there appears to be little to no side effects following a single injection of (2R,6R)-HNK, daily administration of (2R,6R)-HNK (30 mg/kg) in mice over a period of 3–4 weeks was shown to impair memory in novel object recognition and passive-avoidance tasks (Riggs et al., 2021). Therefore, it is important for future studies to optimize the long-term dosing regimen of (2R,6R)-HNK in animal models of SUD.
Overall, our findings suggest that (2R,6R)-HNK exhibits time-dependent effects in animal models of oxycodone withdrawal and relapse-like behavior. Continued efforts to identify the molecular and cellular mechanisms that underlie (2R,6R)-HNK’s therapeutic effects in oxycodone dependence, as well as testing the effectiveness of (2R,6R)-HNK in other drug abuse models (e.g., fentanyl, psychostimulants) will be essential for determining (2R,6R)-HNK’s potential as a safe and effective treatment for substance use disorders.
Highlights:
24 h pretreatment with (2R,6R)-HNK attenuated naloxone-precipitated withdrawal in oxycodone-dependent mice.
1 h pretreatment with (2R,6R)-HNK had limited effects on naloxone-precipitated withdrawal in oxycodone-dependent mice.
1 h and 24 pretreatments with (2R,6R)-HNK reduced drug-primed reinstatement of oxycodone CPP.
(2R,6R)-HNK did not alter locomotor activity or thigmotaxis.
Acknowledgments:
This work was supported by the Connecticut Institute for the Brain and Cognitive Sciences (IBACS) and the UConn Summer Undergraduate Research Fund (SURF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: All authors have seen and agree with the contents of the manuscript, and there is no financial interest to report.
Author Disclosures: The authors have no financial conflicts of interest to disclose.
References
- Albott CS, Lim KO, Forbes MK, Erbes C, Tye SJ, Grabowski JG, Thuras P, Batres-y-Carr TM, Wels J, & Shiroma PR (2018). Efficacy, Safety, and Durability of Repeated Ketamine Infusions for Comorbid Posttraumatic Stress Disorder and Treatment-Resistant Depression. The Journal of Clinical Psychiatry, 79(3). 10.4088/JCP.17m11634 [DOI] [PubMed] [Google Scholar]
- Babigian CJ, Wiedner HJ, Wahlestedt C, & Sartor GC (2022). JQ1 attenuates psychostimulant- but not opioid-induced conditioned place preference. Behavioural Brain Research, 418, 113644. 10.1016/j.bbr.2021.113644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura J, Gomez JL, Carlton ML, Lam S, Sanchez-Soto M, Morris PJ, Moaddel R, Kang HJ, Zanos P, Gould TD, Thomas CJ, Sibley DR, Zarate CA, & Michaelides M (2022). Target deconvolution studies of (2R,6R)-hydroxynorketamine: An elusive search. Molecular Psychiatry, 27(10), 4144–4156. 10.1038/s41380-022-01673-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo I, Bluitt M, & McElligott Z (2021). Examining opioid withdrawal scoring and adaptation of global scoring systems to male and female C57BL/6J mice [Preprint]. Neuroscience. 10.1101/2021.10.11.463944 [DOI] [Google Scholar]
- Cai Y-Q, Hou Y-Y, & Pan ZZ (2020). GluA1 in central amygdala increases pain but inhibits opioid withdrawal-induced aversion. Molecular Pain, 16, 174480692091154. 10.1177/1744806920911543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y-Q, Wang W, Hou Y-Y, Zhang Z, Xie J, & Pan ZZ (2013). Central Amygdala GluA1 Facilitates Associative Learning of Opioid Reward. Journal of Neuroscience, 33(4), 1577–1588. 10.1523/JNEUROSCI.1749-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper M, Contreras KM, Walentiny DM, Beardsley PM, & Damaj MI (2021). Validation and characterization of oxycodone physical dependence in C57BL/6J mice. European Journal of Pharmacology, 903, 174111. 10.1016/j.ejphar.2021.174111 [DOI] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, & Lodge DJ (2016). Activation of a ventral hippocampus–medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Molecular Psychiatry, 21(9), 1298–1308. 10.1038/mp.2015.176 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2023). Drug Overdose Deaths: Prescription Opioids. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- Chen BK, Luna VM, LaGamma CT, Xu X, Deng S-X, Suckow RF, Cooper TB, Shah A, Brachman RA, Mendez-David I, David DJ, Gardier AM, Landry DW, & Denny CA (2020). Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology, 45(9), 1545–1556. 10.1038/s41386-020-0714-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Larochelle MR, Weaver DT, Lietz AP, Mueller PP, Mercaldo S, Wakeman SE, Freedberg KA, Raphel TJ, Knudsen AB, Pandharipande PV, & Chhatwal J (2019). Prevention of Prescription Opioid Misuse and Projected Overdose Deaths in the United States. JAMA Network Open, 2(2), e187621. 10.1001/jamanetworkopen.2018.7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-L, Tao P-L, Chu C-H, Chen S-H, Wu H-E, Tseng LF, Hong J-S, & Lu R-B (2012). Low-Dose Memantine Attenuated Morphine Addictive Behavior Through its Anti-Inflammation and Neurotrophic Effects in Rats. Journal of Neuroimmune Pharmacology, 7(2), 444–453. 10.1007/s11481-011-9337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou D, Peng H-Y, Lin T-B, Lai C-Y, Hsieh M-C, Wen Y-C, Lee A-S, Wang H-H, Yang P-S, Chen G-D, & Ho Y-C (2018). (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology, 139, 1–12. 10.1016/j.neuropharm.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Dakwar E, Nunes EV, Hart CL, Foltin RW, Mathew SJ, Carpenter KM, Choi C. J. Jean, Basaraba CN, Pavlicova M, & Levin FR (2019). A Single Ketamine Infusion Combined With Mindfulness-Based Behavioral Modification to Treat Cocaine Dependence: A Randomized Clinical Trial. American Journal of Psychiatry, 176(11), 923–930. 10.1176/appi.ajp.2019.18101123 [DOI] [PubMed] [Google Scholar]
- Dhalla IA, Mamdani MM, Sivilotti MLA, Kopp A, Qureshi O, & Juurlink DN (2009). Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. Canadian Medical Association Journal, 181(12), 891–896. 10.1503/cmaj.090784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, Hawk KF, Perrone J, Walsh SL, Lofwall MR, Fiellin DA, & Herring A (2023). Incidence of Precipitated Withdrawal During a Multisite Emergency Department–Initiated Buprenorphine Clinical Trial in the Era of Fentanyl. JAMA Network Open, 6(3), e236108. 10.1001/jamanetworkopen.2023.6108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Tapocik JD, Mayo CL, Zanos P, & Gould TD (2020). Ketamine metabolite (2R,6R)-hydroxynorketamine reverses behavioral despair produced by adolescent trauma. Pharmacology Biochemistry and Behavior, 196, 172973. 10.1016/j.pbb.2020.172973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enga RM, Jackson A, Damaj MI, & Beardsley PM (2016). Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. European Journal of Pharmacology, 789, 75–80. 10.1016/j.ejphar.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, aan het Rot M, Lapidus KAB, Wan L-B, Iosifescu D, & Charney DS (2014). Efficacy of Intravenous Ketamine for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry, 71(6), 681. 10.1001/jamapsychiatry.2014.62 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CJ, & Morrow JD (2017). Subanesthetic ketamine decreases the incentive-motivational value of reward-related cues. Journal of Psychopharmacology, 31(1), 67–74. 10.1177/0269881116667709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, & See RE (2002). Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology, 160(4), 425–433. 10.1007/s00213-001-0997-7 [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Fogaça MV, Liu RJ, Duman C, Kato T, Li XY, Duman RS. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A. 2019. Jan 2;116(1):297–302. 10.1073/pnas.1814709116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi DH, Jaffe JH, McNary S, Kavanagh GJ, Hayes M, & Currens M (2003). Short-term outcomes after brief ambulatory opioid detoxification with buprenorphine in young heroin users: Outcomes after opioid detoxification. Addiction, 98(4), 453–462. 10.1046/j.1360-0443.2003.00334.x [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Mou T-CM, An X, Gerhard DM, Dryanovski DI, Potter LE, Highland JN, Jenne CE, Stewart BW, Pultorak KJ, Yuan P, Powels CF, Lovett J, Pereira EFR, Clark SM, Tonelli LH, Moaddel R, Zarate CA, … Gould TD (2022). Experimenters’ sex modulates mouse behaviors and neural responses to ketamine via corticotropin releasing factor. Nature Neuroscience, 25(9), 1191–1200. 10.1038/s41593-022-01146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailozian C, Luftig J, Liang A, Outhay M, Ullal M, Anderson ES, Kalmin M, Shoptaw S, Greenwald MK, & Herring AA (2022). Synergistic Effect of Ketamine and Buprenorphine Observed in the Treatment of Buprenorphine Precipitated Opioid Withdrawal in a Patient With Fentanyl Use. Journal of Addiction Medicine, 16(4), 483–487. 10.1097/ADM.0000000000000929 [DOI] [PubMed] [Google Scholar]
- Hartberg J, Garrett-Walcott S, & De Gioannis A (2018). Impact of oral ketamine augmentation on hospital admissions in treatment-resistant depression and PTSD: A retrospective study. Psychopharmacology, 235(2), 393–398. 10.1007/s00213-017-4786-3 [DOI] [PubMed] [Google Scholar]
- Hashimoto K (2019). Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psychiatry and Clinical Neurosciences, 73(10), 613–627. 10.1111/pcn.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, & Shirayama Y (2018). What Are the Causes for Discrepancies of Antidepressant Actions of (2R,6R)-Hydroxynorketamine? Biological Psychiatry, 84(1), e7–e8. 10.1016/j.biopsych.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Herzog DP, Perumal N, Manicam C, Treccani G, Nadig J, Rossmanith M, Engelmann J, Jene T, Hasch A, van der Kooij MA, Lieb K, Gassen NC, Grus FH, & Müller MB (2021). Longitudinal CSF proteome profiling in mice to uncover the acute and sustained mechanisms of action of rapid acting antidepressant (2R,6R)-hydroxynorketamine (HNK). Neurobiology of Stress, 15, 100404. 10.1016/j.ynstr.2021.100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland JN, Farmer CA, Zanos P, Lovett J, Zarate CA, Moaddel R, & Gould TD (2022). Sex-dependent metabolism of ketamine and ( 2R,6R )-hydroxynorketamine in mice and humans. Journal of Psychopharmacology, 36(2), 170–182. 10.1177/02698811211064922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland JN, Morris PJ, Zanos P, Lovett J, Ghosh S, Wang AQ, Zarate CA, Thomas CJ, Moaddel R, & Gould TD (2019). Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of ( 2R,6R )-hydroxynorketamine. Journal of Psychopharmacology, 33(1), 12–24. 10.1177/0269881118812095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan Ezquerra-Romano I, Lawn W, Krupitsky E, & Morgan CJA (2018). Ketamine for the treatment of addiction: Evidence and potential mechanisms. Neuropharmacology, 142, 72–82. 10.1016/j.neuropharm.2018.01.017 [DOI] [PubMed] [Google Scholar]
- Iyer V, Woodward TJ, Pacheco R, & Hohmann AG (2022). A limited access oral oxycodone paradigm produces physical dependence and mesocorticolimbic region-dependent increases in DeltaFosB expression without preference. Neuropharmacology, 205, 108925. 10.1016/j.neuropharm.2021.108925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph TT, Bu W, Lin W, Zoubak L, Yeliseev A, Liu R, Eckenhoff RG, Brannigan G. Ketamine Metabolite (2R,6R)-Hydroxynorketamine Interacts with μ and κ Opioid Receptors. ACS Chem Neurosci. 2021. May 5;12(9):1487–1497. doi: 10.1021/acschemneuro.0c00741. Epub 2021 Apr 27. 10.1021/acschemneuro.0c00741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovaisa T, Laurinenas G, Vosylius S, Sipylaite J, Badaras R, & Ivaskevicius J (2006). Effects of ketamine on precipitated opiate withdrawal. Medicina (Kaunas, Lithuania), 42(8), 625–634. [PubMed] [Google Scholar]
- Ju L, Yang J, Zhu T, Liu P, & Yang J (2022). BDNF-TrkB signaling-mediated upregulation of Narp is involved in the antidepressant-like effects of (2R,6R)-hydroxynorketamine in a chronic restraint stress mouse model. BMC Psychiatry, 22(1), 182. 10.1186/s12888-022-03838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Lobo MK, Chaudhury D, Labonté B, Friedman A, Heller E, Peña CJ, Han M-H, & Nestler EJ (2014). Loss of BDNF Signaling in D1R-Expressing NAc Neurons Enhances Morphine Reward by Reducing GABA Inhibition. Neuropsychopharmacology, 39(11), 2646–2653. 10.1038/npp.2014.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno DM, Ribeiro E, Peña CJ, Walker D, Bagot RC, Cahill ME, Anderson SAR, Labonté B, Hodes GE, … Nestler EJ (2015). Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nature Neuroscience, 18(3), 415–422. 10.1038/nn.3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Burakov A, Romanova T, Dunaevsky I, Strassman R, & Grinenko A (2002). Ketamine psychotherapy for heroin addiction: Immediate effects and two-year follow-up. Journal of Substance Abuse Treatment, 23(4), 273–283. 10.1016/S0740-5472(02)00275-1 [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Dunaevsky IV, Romanova TN, Slavina TY, & Grinenko AY (2007). Single Versus Repeated Sessions of Ketamine-Assisted Psychotherapy for People with Heroin Dependence. Journal of Psychoactive Drugs, 39(1), 13–19. 10.1080/02791072.2007.10399860 [DOI] [PubMed] [Google Scholar]
- Lalanne L, Nicot C, Lang J-P, Bertschy G, & Salvat E (2016). Experience of the use of Ketamine to manage opioid withdrawal in an addicted woman: A case report. BMC Psychiatry, 16(1), 395. 10.1186/s12888-016-1112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du Y, Wang C, Lu G, Sun H, Kong Y, Wang W, Lian B, Li C, Wang L, Zhang X, & Sun L (2022). (2R,6R)-hydroxynorketamine acts through GluA1-induced synaptic plasticity to alleviate PTSD-like effects in rat models. Neurobiology of Stress, 21, 100503. 10.1016/j.ynstr.2022.100503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lin D, Wu B, & Zhou W (2016). Ketamine abuse potential and use disorder. Brain Research Bulletin, 126, 68–73. 10.1016/j.brainresbull.2016.05.016 [DOI] [PubMed] [Google Scholar]
- Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, Lovett J, Kim S, Wang F-H, Schmidt S, Jenne CE, Yuan P, Morris PJ, Thomas CJ, Zarate CA, Moaddel R, Traynelis SF, Pereira EFR, Thompson SM, … Gould TD (2019). Antidepressant-relevant concentrations of the ketamine metabolite (2 R ,6 R )-hydroxynorketamine do not block NMDA receptor function. Proceedings of the National Academy of Sciences, 116(11), 5160–5169. 10.1073/pnas.1816071116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltbie EA, Gopinath KS, & Howell LL (2019). Effects of ketamine treatment on cocaine-induced reinstatement and disruption of functional connectivity in unanesthetized rhesus monkeys. Psychopharmacology, 236(7), 2105–2118. 10.1007/s00213-019-05204-4 [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, Brietzke E, Dodd S, Gorwood P, Ho R, Iosifescu DV, Lopez Jaramillo C, Kasper S, Kratiuk K, Lee JG, Lee Y, Lui LMW, Mansur RB, Papakostas GI, … Stahl S (2021). Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. American Journal of Psychiatry, 178(5), 383–399. 10.1176/appi.ajp.2020.20081251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick G, Garrett H, Jones HE, McDevitt DS, Sharma S, Silberman Y, & Graziane NM (2020). Ketamine blocks morphine-induced conditioned place preference and anxiety-like behaviors in mice [Preprint]. Neuroscience. 10.1101/2020.01.22.915728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, & Wainer IW (2013). Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. European Journal of Pharmacology, 698(1–3), 228–234. 10.1016/j.ejphar.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Komiya S, Uzawa N, Inoue K, Itoh T, Aoki S, Shibasaki M, & Suzuki T (2013). Involvement of supraspinal and peripheral naloxonazine-insensitive opioid receptor sites in the expression of μ-opioid receptor agonist-induced physical dependence. European Journal of Pharmacology, 715(1–3), 238–245. 10.1016/j.ejphar.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Oakley B, Wilson H, Hayes V, & Lintzeris N (2021). Managing opioid withdrawal precipitated by buprenorphine with buprenorphine. Drug and Alcohol Review, 40(4), 567–571. 10.1111/dar.13228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palandri J, Smith SL, Heal DJ, Wonnacott S, & Bailey CP (2021). Contrasting effects of the α7 nicotinic receptor antagonist methyllycaconitine in different rat models of heroin reinstatement. Journal of Psychopharmacology, 35(10), 1204–1215. 10.1177/0269881121991570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng S-X, Fabresse N, Alvarez J-C, Landry DW, Brachman RA, Denny CA, & Gardier AM (2018). Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine–Induced Sustained Antidepressant-like Effects. Biological Psychiatry, 84(1), e3–e6. 10.1016/j.biopsych.2017.10.020 [DOI] [PubMed] [Google Scholar]
- Riggs LM, An X, Pereira EFR, & Gould TD (2021). (R,S)-ketamine and (2R,6R)-hydroxynorketamine differentially affect memory as a function of dosing frequency. Translational Psychiatry, 11(1), 583. 10.1038/s41398-021-01685-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Puttick DJ, Sawyer AM, Potter DN, Mague S, Carlezon WA, & Chartoff EH (2016). Nucleus Accumbens AMPA Receptors Are Necessary for Morphine-Withdrawal-Induced Negative-Affective States in Rats. Journal of Neuroscience, 36(21), 5748–5762. 10.1523/JNEUROSCI.2875-12.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Powell SK, Brothers SP, & Wahlestedt C (2015). Epigenetic Readers of Lysine Acetylation Regulate Cocaine-Induced Plasticity. The Journal of Neuroscience, 35(45), 15062–15072. 10.1523/JNEUROSCI.0826-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Powell SK, Wiedner HJ, Wahlestedt C, & Brothers SP (2016). Nociceptin receptor activation does not alter acquisition, expression, extinction and reinstatement of conditioned cocaine preference in mice. Brain Research, 1632, 34–41. 10.1016/j.brainres.2015.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassano-Higgins S, Baron D, Juarez G, Esmaili N, & Gold M (2016). A REVIEW OF KETAMINE ABUSE AND DIVERSION: Review: Ketamine. Depression and Anxiety, 33(8), 718–727. 10.1002/da.22536 [DOI] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith ACW, Roberts-Wolfe D, & Kalivas PW (2016). The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacological Reviews, 68(3), 816–871. 10.1124/pr.116.012484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer CL, Dutra JK, Tseng WC, Weber ML, Bogart LJ, Hales K, Pang J, Volfson D, am Ende CW, Green ME, & Buhl DL (2019). Pharmacological evaluation of clinically relevant concentrations of (2R,6R)-hydroxynorketamine. Neuropharmacology, 153, 73–81. 10.1016/j.neuropharm.2019.04.019 [DOI] [PubMed] [Google Scholar]
- Singh MB, Babigian CJ, & Sartor GC (2022). Domain-selective BET inhibition attenuates transcriptional and behavioral responses to cocaine. Neuropharmacology, 210, 109040. 10.1016/j.neuropharm.2022.109040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2011). New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Current Psychiatry Reports, 13(5), 398–405. 10.1007/s11920-011-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J, Harvey M, Voss L, & Denny B (2014). Ketamine – More mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care, 4(2–3), 76–81. 10.1016/j.tacc.2014.03.002 [DOI] [Google Scholar]
- Towers EB, Tunstall BJ, McCracken ML, Vendruscolo LF, & Koob GF (2019). Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology, 151, 189–194. 10.1016/j.neuropharm.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Smith ML, Sullivan B, Heller CY, Garcia C, & Bates M (2011). The Neurobehavioral Pharmacology of Ketamine: Implications for Drug Abuse, Addiction, and Psychiatric Disorders. ILAR Journal, 52(3), 366–378. 10.1093/ilar.52.3.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucha M, Roura-Martínez D, Santos-Toscano R, Capellán R, Ambrosio E, & Higuera-Matas A (2022). Effects of heroin self-administration and forced withdrawal on the expression of genes related to the mTOR network in the basolateral complex of the amygdala of male Lewis rats. Psychopharmacology, 239(8), 2559–2571. 10.1007/s00213-022-06144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin O, Jenne C, Fox ME, Arakawa K, Keller A, & Cramer N (2021). Divergent profiles of fentanyl withdrawal and associated pain in mice and rats. Pharmacology Biochemistry and Behavior, 200, 173077. 10.1016/j.pbb.2020.173077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2015). Opioid Abuse in the U.S. and HHS Actions to Address Opioid-Drug Related Overdoses and Deaths. https://aspe.hhs.gov/reports/opioid-abuse-us-hhs-actions-address-opioid-drug-related-overdoses-deaths-0 [DOI] [PubMed]
- Witkin JM, Kranzler J, Kaniecki K, Popik P, Smith JL, Hashimoto K, & Sporn J (2020). R-(−)-ketamine modifies behavioral effects of morphine predicting efficacy as a novel therapy for opioid use disorder1. Pharmacology Biochemistry and Behavior, 194, 172927. 10.1016/j.pbb.2020.172927 [DOI] [PubMed] [Google Scholar]
- Wright VL, Georgiou P, Bailey A, Heal DJ, Bailey CP, & Wonnacott S (2019). Inhibition of alpha7 nicotinic receptors in the ventral hippocampus selectively attenuates reinstatement of morphine-conditioned place preference and associated changes in AMPA receptor binding. Addiction Biology, 24(4), 590–603. 10.1111/adb.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yu Z, Chen S, Li Z, Long X, Chen M, Lee C-S, Peng H-Y, Lin T-B, Hsieh M-C, Lai C-Y, & Chou D (2023). (2R,6R)-hydroxynorketamine targeting the basolateral amygdala regulates fear memory. Neuropharmacology, 225, 109402. 10.1016/j.neuropharm.2022.109402 [DOI] [PubMed] [Google Scholar]
- Yao N, Skiteva O, Zhang X, Svenningsson P, & Chergui K (2018). Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Molecular Psychiatry, 23(10), 2066–2077. 10.1038/mp.2017.239 [DOI] [PubMed] [Google Scholar]
- Yost JG, Wulf HA, Browne CA, & Lucki I (2022). Antinociceptive and Analgesic Effects of (2 R ,6 R )-Hydroxynorketamine. Journal of Pharmacology and Experimental Therapeutics, 382(3), 256–265. 10.1124/jpet.122.001278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang X-P, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, & Gould TD (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature, 533(7604), 481–486. 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA, & Gould TD (2018). Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacological Reviews, 70(3), 621–660. 10.1124/pr.117.015198 [DOI] [PMC free article] [PubMed] [Google Scholar]


