Abstract
Background:
The HIV Prevention Trials Network (HPTN) 083 trial demonstrated that long-acting cabotegravir (CAB-LA) was more effective than tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in preventing HIV in cisgender men and transgender women (TGW) who have sex with men. In this report, we characterize the TGW cohort included in HPTN 083.
Methods:
An enrollment minimum of 10% TGW was set for HPTN 083. Socio-demographic characteristics, use of gender affirming hormone therapy (GAHT), and behavioral assessments were conducted throughout the trial. Laboratory testing and safety evaluations were performed. This is a secondary analysis of the HPTN 083 study (NCT02720094).
Findings:
HPTN 083 enrolled 570 TGW (304 TDF/FTC; 266 CAB-LA). TGW were primarily from Asia (225/570; 39.5%) and Latin America (205/570; 36.0%); 57.9% (330/570) reported GAHT use. Intimate partner violence was common, and 47.4% (270/570) and 30.2% (172/570) reported emotional or physical abuse, respectively; 56.7% (323/570) reported a history of childhood sexual abuse. Approximately one-quarter of TGW disagreed that they were at risk for HIV, and 24.9% (142/570) screened in for depressive symptoms. Incidence rates of syphilis, rectal gonorrhea, and chlamydia was 16.3%, 11.7%, and 20.6%, respectively. Frequency of adverse events was comparable between arms. Nine seroconversions occurred among TGW during the study blinded phase (7 TDF/FTC; 2 CAB-LA); overall incidence was 1.20 per 100 person-years (HR: 0.34; 95% CI: 0.08-1.56). CAB concentrations did not differ by GAHT use.
Interpretation:
HIV prevention strategies for TGW cannot be addressed separately from social and structural vulnerabilities. TGW were well-represented in HPTN 083 and should continue to be prioritized in HIV prevention studies. CAB-LA is a safe and effective PrEP option for TGW.
Funding:
This work was sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), and jointly funded by NIAID and ViiV Healthcare.
Keywords: cabotegravir, transgender women, HIV prevention, PrEP, interactions, gender-affirming hormone therapy
INTRODUCTION
HPTN 083 is an ongoing Phase 2b/3 randomized multicenter double-blind, double-dummy clinical trial that demonstrated the superiority of injectable cabotegravir (CAB-LA) to daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for HIV prevention in cisgender men and transgender women who have sex with men (MSM and TGW; 66% relative risk reduction).1 While previous HIV pre-exposure prophylaxis (PrEP) efficacy trials have not proritized transgender and gender diverse populations, including TGW2,3, HPTN 083 set enrollment targets for the inclusion of TGW.1
TGW are disproportionately affected by HIV, with a global prevalence of 19.9%, which is 66-fold higher than the HIV prevalence among the general population of individuals aged 15 and older.4 Within the context of overall HIV prevalence across geographic regions, HIV infections among TGW are highest in Latin America, followed by Asia, and North America.4 Transgender and gender diverse persons experience health disparities, including a high prevalence of HIV and sexually transmitted infections (STIs), substance use disorders, and mental health conditions that are driven by a complex array of individual, interpersonal, and structural factors.5,6 These factors impede progress at each stage of the HIV care continuum. Detailed knowledge of HIV care in these populations is further compromised by non-standarized or inaccurate data capture of gender identity and lack of representation in public health surveillance initiatives.7,8 These barriers to positive health outcomes could be minimized by the provision of HIV treatment and prevention services in a gender-affirmative environment.9
PrEP represents a significant opportunity for improving health within TGW communities, but marginalization within the public health system, medical and research mistrust, knowing one’s HIV status, and stigma are all barriers to PrEP initiation.10,11 Challenges in the uptake and persistence of oral TDF/FTC have been observed among TGW in PrEP efficacy and demonstration trials.12,13 In the Pre-Exposure Prophylaxis Initiative (iPrEx) trial, none of the TGW participants who acquired HIV had quantifiable concentrations of tenofovir (TFV) in plasma or TFV-diphosphate (TFV-DP) in peripheral blood mononuclear cells.14 In the iPrEx open label extension (OLE), TGW who reported use of feminizing hormones were less likely to have protective intraerythrocytic TFV-DP concentrations collected as dried blood spots (DBS) as compared to cisgender men or TGW not using hormones.14 Recent data from a baseline survey of a multi-site cohort of TGW in the Eastern and Southern United States (US) reported high participant awareness of PrEP, but low uptake.15 In light of the adherence challenges with daily oral PrEP, alternative agents, including long-acting injectables, have been pursued to increase the range of biomedical HIV prevention options available to individuals most at risk. Further, pharmacologic assessments of novel biomedical products in the intended populations, inclusive of transgender and gender diverse populations, is needed.
Gender affirming hormone therapy (GAHT) is a key component of the standard of care for transgender persons, and is administered to achieve changes consistent with a person’s embodiment goals, gender identity, or both.16 Data from a US survey reported that 71% of transgender respondents had ever used GAHT.17 GAHT consisting of estrogen and anti-androgenic agents is commonly used to induce feminization and mitigate gender dysphoria; however, gonadotropin-releasing hormone agonists and other progestogens have also been utilized as GAHT.18 There is heterogeneity in GAHT dosages and formulations, and the use of GAHT can result in changes in fat deposition, renal changes, and alterations in protein activity in pharmacologic pathways.2 While medically provided GAHT is safe, individuals should be monitored to minimize adverse events.18
This report describes selected participant characteristics, safety, prevention efficacy, body weight changes and pharmacokinetics of CAB-LA in TGW during the blinded phase of HPTN 083.
METHODS
Study Design
The study design and primary outcomes of the blinded phase of HPTN 083 (NCT02720094) have been described previously.1 HIV-uninfected participants were randomized to CAB-LA or TDF/FTC study arms, and included a 5-week oral lead-in (step 1), up to 148 weeks of intramuscular injections (step 2), and a 48-week tail, in which all participants were offered open-label TDF/FTC (step 3). Participants randomized to the CAB-LA arm received oral daily CAB (30 mg tablets daily) in step 1, followed by CAB-LA injections (600 mg) in step 2, with placebo tablets in both phases. Participants randomized to the TDF/FTC arm received TDF/FTC tablets in steps 1 and 2 of the study, as well as placebo pills during the oral lead-in and placebo intramuscular injections during step 2. Participants in both arms received open-label daily TDF/FTC in step 3. The first 2 CAB-LA injections were administered 4 weeks apart starting at week 5, followed by injections every 8 weeks thereafter. An enrollment minimum of ≥10% TGW was pre-specified. TGW were randomly assigned to study arms. Full details of the trial design can be found in the trial protocol. The current analyses were not pre-specified in the protocol, and include data on TGW participants enrolled in HPTN 083 collected up to May 14, 2020, when the study was unblinded by an independent Data Safety Monitoring Board.1
Procedures
Participant socio-demographic data were collected at enrollment and throughout the study by a healthcare professional. Feminizing hormones were defined as estrogens (any route of administration), progestogens, or anti-androgens; GAHT use was captured at each study visit. Computer Assisted Self Interview (CASI) methods were employed for sexual behaviors, alcohol use, and recreational drug use. Structured behavioral assessments, conducted with an interviewer, included demographics (gender identification, age, education level, marital and employment status, income), intimate partner violence (emotional and physical), childhood sexual abuse, HIV risk perceptions, and depression. Assessments were conducted at baseline for demographics, and approximately every second injection cycle for variables that can change over time. Counseling for adherence and risk-reduction, and assessment of adverse events, were performed at every visit; injection-site reactions were assessed at post-injection visits. Weight measures occurred at enrollment, and every other study visit. An Alcohol Use Disorders Identification (AUDIT-C) score ≥4 was used as a cutoff to identify potential hazardous drinking or active alcohol use disorders.19 Positive depression screening was considered with the short form Center for Epidemiologic Studies Depression Scale (CES-D) score ≥10.20 Participants answered questions about HIV risk perceptions at select visits throughout the study. Additional details on the scoring of behavioral assessments are found in the Supplementary Material (appendix, pp 2-4) Additional details on study procedures may be found in the primary study report.1
Routine laboratory testing for safety was performed at all study visits, except the week 5 visit. Testing for HIV and STIs was performed as previously described.1,21 An independent adjudication committee reviewed HIV testing results from study sites and the HPTN Laboratory Center, determined HIV infection status, and identified the date of the first HIV positive visit. CAB and TFV measurements in plasma and intraerythrocytic TFV-DP measurements from DBS were performed using validated liquid chromatographic-tandem mass spectrometric assays.21–23 The in vitro protein-adjusted 90% CAB inhibitory concentration (PA-IC90) is 0.166 mcg/mL.24
Adherence to TDF/FTC or CAB-LA injections
Drug concentrations were evaluated in a cohort of 389 randomly selected participants randomized to the TDF/FTC study arm (340 cisgender men; 49 TGW) to assess adherence to oral PrEP during the blinded phase of the study. TFV-DP concentrations ≥700-1249 fmol/punch and ≥1250 fmol/punch were associated with four TDF/FTC doses/week and daily dosing in the preceding 4-8 weeks, respectively.25 Adherence to CAB-LA was defined as administration of injections within 2 weeks of a scheduled visit.
Evaluation of CAB concentrations in TGW
Select visits were evaluated from a select subset of TGW randomized to the CAB-LA study arm to understand the impact of GAHT on CAB pharmacokinetics. Participants included in this analysis received all CAB-LA injections within ± one week of a scheduled visit, had no missed injections through study week 57, and reported (n=30) or denied (n=23) GAHT use.
Statistical Analysis
We compared socio-demographic, behavioral, clinical and laboratory characteristics according to study arm. We estimated prevalence and incidence rates of syphilis and rectal chlamydia and gonorrhea, and used the exact Poisson method to calculate incidence rates and their 95% confidence interval (95% CI). The risk of HIV infection was compared using a Cox proportional hazards model, accounting for geographic region. We compared body weight changes by arm, according to GAHT use and time of initiation, using linear mixed effects regression analysis, mood’s median test and Wilcoxon p-value. We used SAS (version 9.4) for statistical analyses.
Ethical considerations
The HPTN 083 protocol (appendix, pp 23-497) was approved by institutional review boards, ethics committees, and/or ministries of health, or a combination of these entitites, at each participating site. All participants provided written informed consent.
Role of the funding source
The Division of AIDS of the National Institute of Allergy and Infectious Diseases provided regulatory sponsorship of the trial. The Division of AIDS was responsible for clinical monitoring of the trial. ViiV Healthcare and Gilead Sciences donated trial medications and matching placebos. ViiV Healthcare also provided additional funding. The funder of the study commented on the manuscript but had no role in study design, data collection, data analysis, and data interpretation.
RESULTS
Of 4,566 participants enrolled in the study, 570 (12.5%) were TGW; 304 were randomized to TDF/FTC and 266 to CAB-LA. TGW self-identified as follows: transgender female (400/570; 70.2%); female (90/570; 15.8%); gender variant or non-conforming (43/570; 7.5%); genderqueer (32/570; 5.6%); and other (5/570; 0.9%). TGW were primarily enrolled from Asia (225/570; 39.5%) and Latin America (205/570; 36.0%). Gender identity was not captured at post-enrollment study visits. At enrollment, the median age of transgender participants was 23 years (interquartile range [IQR] 21, 28); 47.0% (268/570) had secondary or lower education, and 84.7% (483/570) were single. The median household monthly income was US$ 419 (IQR 226, 837), and 62.1% (354/570) were employed at enrollment (Table 1). Enrollment characteristics for MSM are included in the Supplementary Material (appendix, p 5).
Table 1.
Baseline demographic characteristics among TGW participants, by study arm.
| Participants Enrolled | TDF/FTC (n=304) | CAB-LA (n=266) |
|---|---|---|
| n (%) | n (%) | |
| Self–identification | ||
| Female | 43 (14.1) | 47 (17.7) |
| Transgender male | 0 (0.0) | 0 (0.0) |
| Transgender female | 213 (70.1) | 187 (70.3) |
| Gender queer | 18 (5.9) | 14 (5.3) |
| Gender variant or gender non-confirming | 27 (8.9) | 16 (6.0) |
| Other self-identification | 3 (1.0) | 2 (0.8) |
| Prefer not to answer | 0 (0.0) | 0 (0.0) |
| Age | ||
| 18-29 | 244 (80.3) | 227 (85.3) |
| 30-39 | 42 (13.8) | 29 (10.9) |
| 40+ | 18 (6.0) | 10 (3.7) |
| Median (IQR) | 23 (21, 28) | 23 (21, 27) |
| Geographic Region | ||
| US | 71 (23.4) | 54 (20.3) |
| Latin America | 113 (37.2) | 92 (34.6) |
| Asia | 110 (36.2) | 115 (43.2) |
| Africa | 10 (3.3) | 5 (1.9) |
| Education | ||
| Secondary or lower | 143 (47.1) | 125 (47.0) |
| Technical Training | 22 (7.2) | 24 (9.0) |
| College/University or Higher | 139 (45.7) | 117 (44.0) |
| Marital Status | ||
| Married/Civil Union/Legal Partnership | 7 (2.3) | 3 (1.1) |
| Living with Primary or Main Partner | 14 (4.6) | 16 (6.0) |
| Have primary or Main Partner, not living together | 19 (6.3) | 26 (9.8) |
| Single / Divorced / Widowed | 264 (86.8) | 219 (82.3) |
| Other | 0 (0.0) | 2 (0.8) |
| Employment Status | ||
| Full or part time | 188 (61.8) | 166 (62.4) |
| Not employed | 116 (38.2) | 100 (37.6) |
| Household monthy income (US Dollars) 1 | ||
| Median (IQR) | 482 (279, 955) | 366 (195, 698) |
CAB-LA: Cabotegravir; IQR: interquartile range; TDF/FTC: Tenofovir disoproxil fumarate/emtricitabine; TGW: transgender women.
Income of countries other than the US was converted from local currency to US Dollars using the exchange rate on Jan 1, 2017.
Select enrollment behavioral characteristics for TGW in HPTN 083 are presented in Table 2. At enrollment, 39.5% (220/557) reported recreational drug use. Problematic alcohol use was commonly reported, with 44.5% (248/557) having an AUDIT-C score ≥4. Intimate partner violence was also prevalent, with 47.4% (270/570) and 30.2% (172/570) of TGW reporting emotional or physical abuse, respectively. Additionally, 56.7% (323/570) reported a history of childhood sexual abuse, and 24.9% (142/570) of TGW had an enrollment CES-D score ≥10. Enrollment behavioral characteristics did not differ between study arms. As a comparison, enrollment behavioral data for MSM, categorized by study arm, are included in Supplementary Material (appendix, p 6).
Table 2.
Baseline behavioral characteristics among TGW participants, by study arm.
| Participants Enrolled | TDF/FTC (n=304) | CAB-LA (n=266) |
|---|---|---|
| n (%) | n (%) | |
| Any recreational drug use in the previous 6 months | 116/295 (39.3) | 104/262 (39.7) |
| Positive alcohol screening 1 | 137/295 (46.4) | 111/262 (42.4) |
| Sex partners in the previous month- Median (IQR) | 4 (2, 8) | 3 (2, 7) |
| Sex partners living with HIV | ||
| 1 | 9/295 (3.1) | 4/262 (1.5) |
| 2 + | 6/295 (2.0) | 1/262 (0.4) |
| Receptive anal sex acts – Median (IQR) | 5 (3, 10) | 4 (2, 10) |
| Intimate partner violence | ||
| Emotional Abuse2 | 143/304 (47.0) | 127/266 (47.7) |
| Physical Abuse3 | 92/304 (30.3) | 80/266 (30.1) |
| Childhood sexual abuse before 18 years old | 176/304 (57.9) | 147/266 (55.3) |
| HIV risk perception categorization 4 | ||
| Disagrees that they are at risk | 80/304 (26.3) | 29/266 (29.7) |
| Neutral | 113/304 (37.2) | 95/266 (35.7) |
| Agrees that they are risk | 82/304 (27.0) | 67/266 (25.2) |
| Positive depression screening 5 | 72/304 (23.7) | 70/266 (26.3) |
CAB-LA: Cabotegravir; IQR: interquartile range; TDF/FTC: Tenofovir disoproxil fumarate/emtricitabine; TGW: transgender women.
Alcohol Use Disorders Identification (AUDIT-C) score of 4 or greater;
Emotional abuse: being belittled or called stupid, or having an upset or suspicious partner;
Physical abuse: being punched, beaten, forced to perform sex acts, or abused when partner drinks;
Based on the sentences: 1) I am worried about getting infected with HIV, 2) My sexual experiences put me at risk for HIV, 3) I think that I really could get HIV, 4) I am unlikely to get infected with HIV, 5) It is likely that I will be infected with HIV within the next year. Response options ranged from “strongly disagree (1)” “disagree (2)” “neither agree nor disagree (3)” “agree (4)” “strongly agree (5)” and “don’t know”. Average scores of < 2.5 suggest lower agreement with statements around being at risk for HIV, while scores > 3.5 suggest higher agreement with statements around being at risk for HIV; and scores ranging from 2.51-3.49 were categorized as neutral;
Based on short-form (10-item) Center for Epidemiologic Studies Depression (CES-D) Scale ≥10.
Among TGW participants, at enrollment, the median number of sex partners in the previous month was three (IQR 2, 8), and 3.6% (20/570) reported having at least one sex partner living with HIV. For the assessment of self-perceived HIV risk, approximately 1/4 (109/570; 27.9%) of participants had an average item response that would correspond to generally disagreeing that they are at risk for HIV; this did not differ between study arms (80/304, 26.3% TDF/FTC vs. 29/266, 29.7% CAB-LA; p=0.42) (Table 2).
During the blinded phase of the study, adverse events grade 2 or higher among TGW were comparable between the TDF/FTC (270/304, 88.8%) and CAB-LA (246/266, 92.5%) arms; the frequency of grade 2+ adverse events was consistent with MSM (3706/3992, 92.8%). Serious adverse events were reported in 5.1% (29/570) of TGW; rates were comparable between TDF/FTC (12/304, 4.0%) and CAB-LA (17/266, 6.4%) study arms, and consistent with the observed frequency among MSM (212/3992, 5.3%). (Table 3, appendix, p 7). Two deaths were recorded among TGW during the study period, one in each arm; deaths were not study-product related.
Table 3.
Grades 2+ and 3+ adverse events, serious adverse events and injection site reaction summary by Cohort and Study arm
| Total TGW Participants Enrolled | TGW | MSM | ||
|---|---|---|---|---|
| TDF/FTC (n=304) | CAB-LA (n=266) | TDF/FTC (n=1978) | CAB-LA (n= 2014) | |
| n (%) | n (%) | n (%) | n (%) | |
| Number of Participants with Grade 2+ AEs 1 | 270 (88.8) | 246 (92.5) | 1846 (93.3) | 1860 (92.4) |
| Number of Participants with Grade 3+ AEs 1 | 76 (25.0) | 66 (24.8) | 691 (34.9) | 661 (32.8) |
| Serious adverse events 1 | 12 (4.0) | 17 (6.4) | 109 (5.5) | 103 (5.1) |
| Participants who received at least one injection | 266 (87.5) | 250 (94.0) | 1815 (91.8) | 1867 (92.7) |
| Number of participants who have reported any injection site reaction (ISR) | 77 (28.9) | 217 (86.8) | 575 (31.7) | 1507 (80.7) |
CAB-LA: Cabotegravir; MSM: men who have sex with men; TDF/FTC: Tenofovir disoproxil fumarate/emtricitabine; TGW: transgender women.
Included are only adverse events that were assigned Medical Dictionary for Regulatory Activities (MedDRA), version 23.1 terms by clinical staff. Injection-site reactions and sexually transmitted infections are not included. Inappropriately enrolled participants, including enrolled participants who were later found to have failed to meet a key inclusion/exclusion criteria, and participants who did not receive any oral trial drug are excluded. In cases in which a participant had multiple events with the same MedDRA term, only one event is counted.
Among TGW participants, 516 received at least one injection (266 TDF/FTC; 250 CAB-LA). Injection site reactions were more commonly reported in the CAB-LA arm (217/266; 86.8%) as compared to the TDF/FTC arm (77/304; 28.9%) (Table 3). Permanent discontinuation of injections occurred in 51/516 TGW (9.9% TDF/FTC; 9.6% CAB-LA); one participant (0.4%) randomized to CAB-LA permanently discontinued study product due to injection site reactions.
In a random subset of participants randomized to the TDF/FTC study arm, intraerythrocytic TFV-DP concentrations consistent with 4-6 doses of TDF/FTC per week (≥700-1249 fmol/punch) were observed in 33.7% of evaluated samples from TGW; concentrations consistent with daily adherence (TFV-DP ≥1250 fmol/punch) were observed in 24.3% of evaluated samples (appendix, p 9). Among TGW, TDF/FTC persistence waned over time; based on TFV-DP concentrations ≥700 fmol/punch, adherence decreased from 66.7% at study week 4 to 40% at week 105. Among participants included in the adherence cohort, the proportion of TGW specimens with TFV-DP concentrations ≥700 fmol/punch was significantly lower than for MSM (141/246 (58.0%) versus 1331/1791 (74.3%) respectively, p=<0.0001). Coverage of CAB-LA injections among TGW randomized to the CAB arm was 91.8%, as defined by injections having been received with a delay of less than two weeks; injection coverage among MSM randomized to CAB-LA was 91.6%.
At enrollment, the prevalence of active syphilis, rectal gonorrhea, and chlamydia was 6.9% (39/569), 7.2% (41/570), and 16.8% (96/570), respectively, and prevalence was comparable between study arms. During study follow-up, syphilis, rectal gonorrhea, and chlamydia incidence was 16.3, 11.7%, and 20.6%, respectively. STI rates were similar to those observed among MSM (appendix, p 10).
HIV incidence during the blinded phase of HPTN 083 has been previously reported.1 Nine TGW acquired HIV during the blinded phase of the study (7 TDF/FTC; 2 CAB-LA), with an overall incidence of 1.20 per 100 person-years (py). HIV incidence rates were 1.80 per 100 py (95% CI: 0.73-3.72) and 0.54 per 100 py (95% CI: 0.07-1.95) in TGW participants randomized to TDF/FTC and CAB-LA, respectively, with a hazard ratio (HR) of 0.34 (95% CI: 0.075-1.56); the HR is consistent with the overall HPTN 083 trial (Figure 1A). None of the incident HIV cases in TGW randomized to the TDF/FTC arm had plasma TFV or intraerythrocytic TFV-DP concentrations consistent with prevention-effective PrEP adherence, and five of seven did not have quantifiable drug concentrations at the first HIV positive visit. Of the two TGW participants who acquired HIV in the CAB arm, one acquired HIV during the CAB oral lead-in phase; CAB concentrations were ≥8x PA-IC90 (1.33 mcg/mL) at the first HIV positive visit. The other participant acquired HIV 849 days after their last injection and had unquantifiable CAB concentrations at the first HIV positive visit.
Figure 1.
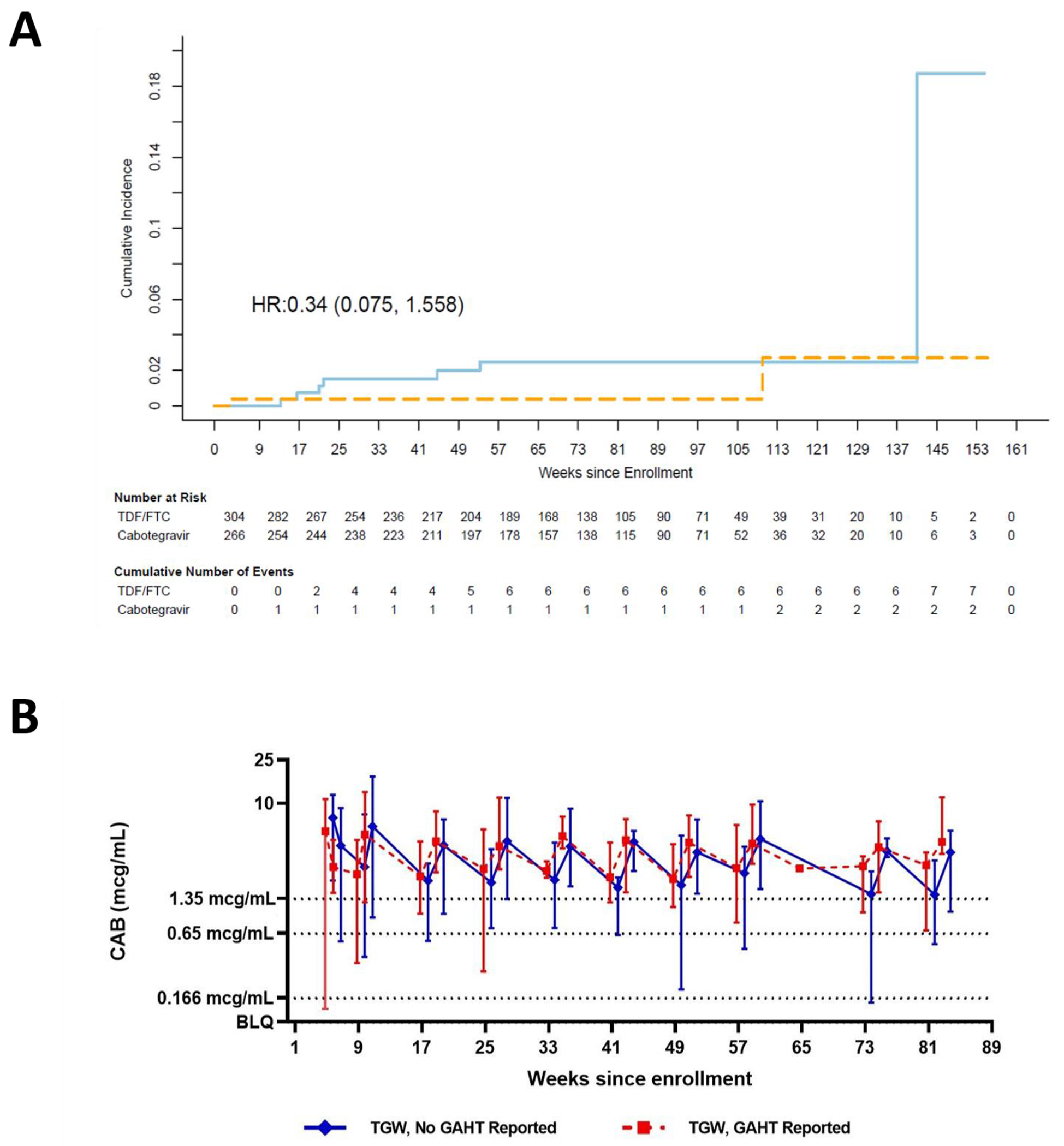
(A) Kaplan-Meier estimates of cumulative incidence of HIV infection among TGW in Intent to Treat Cohort. Solid light-blue line: TDF/FTC arm; Dashed orange line: Cabotegravir arm. TGW: transgender women, HR: Hazard ratio, TDF/FTC: tenofovir disoproxil fumarate/emtricitabine. (B) Median CAB concentrations in TGW in the absence (solid blue line) or presence (dotted red line) of GAHT. Error bars show the lower 5% and upper 95% of CAB concentrations observed at each time point. Dotted horizontal line: 1x PA-IC90 (0.166 μg/mL); dashed horizontal line: 4x PA-IC90 (0.664 μg/mL); solid horizontal line: 8x PA-IC90 (1.33 μg/mL). CAB: cabotegravir; GAHT: gender-affirming hormone therapy; PA-IC90: in vitro protein-adjusted 90% CAB inhibitory concentration.
At enrollment, 249 (43.7%) TGW participants reported accessing GAHT; an additional 81 (14.2%) reported GAHT use after enrollment. The frequency of reported estrogen, anti-androgenic, and progestogen use was 93.9% (310/330), 76.4% (252/330), and 35.5% (117/330), respectively. The most commonly reported GAHT therapies included estradiol valerate (44.5%, 147/330), spironolactone (32.4%, 107/330), estradiol (28.5%, 94/330), and cyproterone acetate (27.9%, 92/330). There were no differences in types of GAHT accessed between study arms (Table 4; appendix, p 11-12). GAHT was not only utilized by TGW in the study; 32 (0.80%) cisgender men reported GAHT use. At enrollment, 7 participants identifying as cisgender male reported GAHT use, and an additional 25 reported accessing GAHT post-enrollment. Estrogens were most commonly used among these participants (Table 4).
Table 4.
Reported gender affirming hormone therapy (GAHT) use by cohort, study arm, and starting period.
| Reported GAHT Use (TGW) | Reported GAHT Use (MSM) | |||||
|---|---|---|---|---|---|---|
| GAHT Reported at Baseline | GAHT Initiated During Follow-up | Total GAHT Use Reported | GAHT Reported at Baseline | GAHT Initiated During Follow-up | Total GAHT Use Reported | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Participants who self-reported any type of GAHT Hormone therapy type 1 | 249/570 (43.7) | 81/570 (14.2) | 330/570 (57.9) | 7/3996 (0.2) | 25/3996 (0.6) | 32/3996 (0.8) |
| Antiandrogens | 202/249 (81.1) | 50/81 (61.7) | 252/330 (76.4) | 5/7 (71.4) | 16/25 (64.0) | 21/32 (65.6) |
| Estrogens | 239/249 (96.0) | 71/81 (87.7) | 310/330 (93.9) | 6/7 (85.7) | 20/25 (80.0) | 26/32 (81.3) |
| Progestogens | 91/249 (36.5) | 26/81 (32.1) | 117/330 (35.5) | 2/7 (28.6) | 9/25 (36.0) | 11/32 (34.4) |
CAB-LA: Cabotegravir; GAHT: gender affirming hormone therapy; MSM: men who have sex with men; TDF/FTC: Tenofovir disoproxil fumarate/emtricitabine; TGW: transgender women.
Numerator refers to the number of participants who reported each hormone therapy regimens; denominators refer to the number of participants who reported GAHT use at baseline or during follow-up.
Body weight changes from baseline were compared by study arm, GAHT use, and time of initiation. A median increase in body weight of 1.17 kg per year (95% CI: 0.65-1.68) was noted in TGW randomized to the CAB-LA arm, as compared with an increase of 0.69 kg (95% CI: 0.18-1.19) in the TDF/FTC arm; these differences were not statistically significant (mean difference 0.48, 95% CI: −0.24, 1.2 p=0.19) (appendix, p 14). Use of GAHT did not result in significant differences in rates of weight gain among TGW (appendix, p 13).
CAB concentrations were compared in the presence (n=30) or absence (n=23) of GAHT in a select subset of transgender participants who had high adherence to CAB-LA injections. While CAB concentrations were nominally higher in TGW accessing GAHT, there were no statistically significant differences in CAB concentrations at evaluated time points (p = 0.783; Figure 1B). Hormone dosing times were not captured and estrogen measurements were not performed; thus, an impact of CAB on GAHT was not assessed.
DISCUSSION
This is the first report to describe TGW-specific participant characteristics, safety, behavioral, efficacy, weight changes, and pharmacologic data from the HPTN 083 clinical trial. The presented work shows that CAB-LA is a safe and effective HIV prevention strategy for TGW, providing high levels of protection among these participants; these findings are consistent with overall study results.1 Further, these data highlight important considerations for the recruitment and inclusion of transgender participants in HIV prevention research.
Traditionally, transgender and gender diverse persons have not been prioritized in HIV efficacy trials.18 However, TGW were successfully enrolled and retained in HPTN 083, representing the largest cohort of TGW included in a registrational trial to date. By adopting a pre-specified recruitment target, HPTN 083 provided a unique opportunity to evaluate the efficacy of CAB-LA in TGW, as well as further expand insights into TDF/FTC use in this population. While inclusion of self-identifying TGW was a recruitment goal, we also observed a spectrum of genders within the study. Of participants included in our analysis under the umbrella of TGW, 14% of participants self-identified as queer, gender variant, gender non-conforming, or other gender identities. Across the entire cohort, 57.9% of transgender and gender-diverse participants accessed GAHT prior to or after enrollment. Further, 32 self-identified cisgender MSM reported GAHT use, raising important considerations for how to adequately capture the range of gender identities in HIV research, consider the profile of GAHT users, and be responsive to the evolution of gender identity and preferred pronouns and labels over time.16 In HPTN 083, self-reported gender identity was not longitudinally assessed during study follow up, but should be added to the list of demographic variables assessed throughout study participation.
Various co-occurring contextual variables may be important to consider in implementing PrEP to TGW individuals globally based on these data. Recreational drug and alcohol use were commonly reported and nearly 25% screened in for clinically significant depressive symptoms. TGW experienced a high frequency of intimate partner violence. More than half of TGW reported a history of childhood sexual abuse. Income levels were low and rates of unemployment high. These syndemic problems have been reported previously among transgender and gender diverse persons26,27; these data confirm that this is observed across geographies.
In our cohort, at enrollment, approximately more than one quarter (27.9%) of TGW participants had an average response across items assessing HIV risk perceptions indicating disagreement regarding their risk for HIV acquisition. These data were lower than perceived HIV risk captured in other studies, including those from Latin America and Asia; this may be attributed to methodologic differences in how perceived HIV risk was evaluated.28,29 Risk perception is an important variable in HIV-related health behavior changes, as it is a direct30 or indirect31 component of various social cognitive models of HIV risk. HIV prevention among TGW cannot be addressed in isolation from the socio-behavioral context within which HIV risk occurs, and low risk perception highlights the structural gaps in PrEP acceptance and uptake.
The enrollment prevalence of STIs was high across the entire study cohort, and rates among TGW ranged from 6.9% to 16.8% for syphilis, rectal gonorrhea, and chlamydia. Throughout trial conduct, participants continued to be sexually active and had high rates of STIs. These data underscore not only the ongoing incidence of rectal STIs, but also risk for HIV acquisition and the benefits of PrEP uptake and use. The implications of rectal STIs include low rates of condom use, and re-emphasize the need for a broad menu of choices for HIV prevention and STI management acceptable to populations at-risk.
Within HPTN 083, both TDF/FTC and CAB-LA were highly effective in the prevention of HIV among TGW, and CAB demonstrated superior efficacy. Although the TGW-specific HR was not statistically significant, the magnitude and direction of the effect was consistent with overall trial results. In the TGW participants randomized to the TDF/FTC arm who acquired HIV, none had drug concentrations at the first HIV positive visit consistent with protection-effective oral TDF/FTC adherence; these findings are largely consistent with results from the entire cohort.21 During the blinded phase of the trial, no TGW participants who received on-time CAB-LA injections acquired HIV. However, one TGW participant randomized to CAB acquired HIV during the oral phase of the study. The participant acquired HIV 27 days after enrollment (CAB concentration at first HIV positive visit: 6.30 mcg/mL), and had a CAB concentration ≥8x PA-IC90 at the preceding visit. Although pill counts were used to assess adherence during the trial, direct observation was not performed; thus, it is unclear if the participant used oral CAB consistently. It is also possible that the participant acquired HIV shortly after enrollment, and was not detected until the week 4 visit.
Although data were unavailable to perform a bi-directional drug-hormone interaction evaluation, we compared drug concentrations among TGW in the presence or absence of GAHT use in a small subset (n=53) of transgender participants randomized to the CAB-LA study arm. CAB concentrations were nominally higher in TGW who reported GAHT use; however, differences were not statistically significant. Of note, pharmacologic parameters, including steady state CAB concentrations with repeated dosing, were higher in cisgender women than cisgender men.22,32 These differences may be influenced by BMI or body size. While we were able to compare CAB concentrations in the background of GAHT, we were unable to characterize the impact of CAB on GAHT. This remains an important research gap.
Given the challenges faced by TGW to adhere to oral TDF/FTC PrEP, an injection every 2 months is convenient and discreet, and might address the barriers to daily oral pill-taking, such as competing life priorities, HIV stigma, discrimination, and violence. Of note, 82.6% of TGW participants were younger than 30 years of age, making the preserved advantage of CAB-LA among a young and at-risk population of TGW a remarkable advance in global HIV prevention.12,33
LIMITATIONS
There are limitations to the presented work. While there was a target enrollment goal of ≥10% of TGW in HPTN 083, the enrolled sample size was not large enough for a fully-powered statistical comparison. Further, gender identity was only captured at enrollment, and additional data on participant feelings surrounding gender were not captured throughout the study. Lastly, hormone concentrations were not evaluated, and the study design did not lend itself to a formal drug-hormone interaction analysis.
CONCLUSIONS
TGW-specific results in this study are consistent with the overall HPTN 083 study result, which found that CAB-LA administered intramuscularly every 8 weeks was well-tolerated, safe and highly effective in preventing HIV infection in MSM and TGW at substantial risk for acquiring HIV.13 CAB concentrations were not statistically different between TGW who reported GAHT use as compared to TGW who did reported not using GAHT. However, additional studies, which capture detailed GAHT dosing information and hormone concentrations, are needed to more fully characterize the relationship between CAB and GAHT.
Supplementary Material
Research in context.
Evidence before this study
Despite its successes, tenofovir (TFV)-based oral PrEP has not homogenously decreased HIV incidence, and transgender women (TGW) continue to be disproportionately impacted by HIV. Long-acting injectable cabotegravir (CAB-LA) is an integrase strand transfer inhibitor, and its superiority as compared to TFV-based oral PrEP has been demonstrated in two randomized clinical trials, HPTN 083 (among gay, bisexual, and other cisgender men (MSM) and TGW who have sex with men) and HPTN 084 (among cisgender women). We carried out a bibliographic search at PubMed database on 01 February 2023. The following search strategy was used: (“HIV” OR “human immunodeficiency virus”) AND (“transgender women” OR “transsexual women” OR “trans women”) AND (“cabotegravir”) AND (“pre-exposure prophylaxis” OR “PrEP”) AND (“trial”), with no restriction of publication date or language. This search retrieved only three publications, all related to HPTN 083 (one presenting the main results, another describing a cost-effectiveness analysis, and the last characterizing HIV infections that occurred during the study). No publication has focused on TGW-specific issues with respect to CAB-LA.
Added value of this study
This is the first report to describe TGW-specific participant characteristics, safety, behavioral, efficacy, weight changes and pharmacologic data from the phase 2b/3 HPTN 083 clinical trial. With 570 (12.5%) TGW enrolled, HPTN 083 had the largest cohort of TGW included in a registrational trial to date. TGW were successfully enrolled and retained in HPTN 083. The adoption of a pre-specified recruitment minimum for TGW allowed a unique opportunity to evaluate CAB-LA efficacy and expanded insights into TFV-based oral PrEP use in this population. In our analysis, we also considered gender fluidity, a topic of intense interest. Among participants included under the umbrella of TGW, 14% self-identified as queer, gender variant, gender non-conforming, or other gender identities. The gender-fluidity discussion also emerged with the identification of 32 participants who self-identified as MSM yet reported use of gender-affirming hormone therapy (GAHT). Our results showed that CAB-LA was a safe and effective HIV prevention strategy for TGW and provided high levels of protection among these participants. However, TGW had important structural vulnerabilities, such as low income and unemployment, and approximately one-quarter of participants disagreed with statements about them being at risk for HIV. Finally, HPTN 083 was the first to report CAB concentrations in TGW within the context of self-reported GAHT status.
Implications of all the available evidence
Adherence to TFV-based PrEP represents an ongoing challenge for TGW, and CAB-LA administration every 2 months is a convenient and discreet strategy that may overcome the barriers to daily oral pill-taking. Characterizing the impact of CAB-LA on GAHT remains an important research gap. Further research will need to consider gender fluidity as it is becoming more commonly recognized as part of gender identity, and as the profile of GAHT users continues to evolve. Self-reported gender identity should be assessed longitudinally during future studies. The frequency of mental health distress, as shown by the 24.9% screening in for depressive symptoms, highlights the need to address TGW mental health across geographies as an urgent and pressing issue that requires special attention. HIV prevention among TGW cannot be addressed separately from social and structural aspects. There is a critical need for a broad menu of choices for HIV prevention that are sexually congruent and acceptable to populations at-risk.
Acknowledgements
The authors thank HPTN 083 study team and participants.
Declaration of interests
MAM has received grant funding from ViiV/GSK and Gilead Sciences for industry-sponsored research. RJL reports receiving study products and additional support for study conduct to sites from ViiV Healthcare and was given study products from Gilead Sciences during the conduct of the study. RJL received advisory scientific board fees, and travel support from Merck Inc outside the submitted work. JFR is a paid employee and stockholder of Gilead Sciences. AR is a paid employee and stockholder of ViiV Healthcare. CP reports grants from the National Institutes of Health (NIH), advisory board fees from Fenway Institute, and UCLA, outside the submitted work, and participated as DSMBs at UNC and MGH. JF reports grants from ViiV Health Care UK and the National Institute of Allergy and Infectious Diseases (NIAID), during the conduct of the study. SF was a paid employee and stockholder of GSK during the conduct of the study. MSC reports grants from the National Institutes of Health (NIH), scientific and medical advisory board fees, respectively, from Aerium and Atea, honoraria for lectures from NY course, MJH Life Sciences, Clinical Care Solutions, Virology Education, Amgen, Medscape, UpToDate and for Educational Panel from Astra Zeneca. MSC reports travel support from GSK and was co-Chair in HPTN and CoVPN. All other authors declare no competing interests.
Contributor Information
Mark A Marzinke, Johns Hopkins University, Baltimore, USA.
Brett Hanscom, Fred Hutchinson Cancer Center, Seattle, USA.
Zhe Wang, Fred Hutchinson Cancer Center, Seattle, USA.
Steven A Safren, University of Miami, Coral Gables, FL, USA.
Christina Psaros, Massachusetts General Hospital, Boston, USA.
Deborah Donnell, Fred Hutchinson Cancer Center, Seattle, USA.
Paul A Richardson, Johns Hopkins University, Baltimore, USA.
Philip Sullivan, Johns Hopkins University, Baltimore, USA.
Susan H Eshleman, Johns Hopkins University, Baltimore, USA.
Andrea Jennings, FHI 360, Durham, North Carolina, USA.
Kailazarid Gomez Feliciano, FHI 360, Durham, North Carolina, USA.
Emilia Jalil, Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz (INI-Fiocruz), Av Brasil 4365, Manguinhos, 21040-360, Rio de Janeiro, Brazil.
Carolina Coutinho, Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz (INI-Fiocruz), Av Brasil 4365, Manguinhos, 21040-360, Rio de Janeiro, Brazil.
Nadir Cardozo, Fundación Huésped CRS, Buenos Aires, Argentina.
Bernardo Maia, Hospital Emílio Ribas, Sao Paolo, Brazil.
Taimur Khan, Fenway Health CRS, Boston, USA.
Yashna Singh, Desmond Tutu HIV Centre, Institute of Infectious Disease and Molecular Medicine and Department of Medicine, University of Cape Town, Cape Town, South Africa.
Keren Middelkoop, Desmond Tutu HIV Centre, Institute of Infectious Disease and Molecular Medicine and Department of Medicine, University of Cape Town, Cape Town, South Africa.
Julie Franks, Harlem Prevention Center CRS, New York, USA.
Javier Valencia, Barranco CRS, Lima, Peru.
Naiymah Sanchez, FHI 360, Durham, North Carolina, USA.
Jonathan Lucas, FHI 360, Durham, North Carolina, USA.
James F Rooney, Gilead Sciences, Foster City, CA, USA.
Alex R Rinehart, ViiV Healthcare, Research Triangle, North Carolina, USA.
Susan Ford, ViiV Healthcare, Research Triangle, North Carolina, USA.
Adeola Adeyeye, National Institutes of Health, Rockville, MD, USA.
Myron S Cohen, University of North Carolina at Chapel Hill, Chapel Hill, USA.
Marybeth McCauley, FHI 360, Durham, North Carolina, USA.
Raphael J Landovitz, Center for Clinical AIDS Research and Education, David Geffen School of Medicine, University of California, Los Angeles, USA.
Beatriz Grinsztejn, Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz (INI-Fiocruz), Av Brasil 4365, Manguinhos, 21040-360, Rio de Janeiro, Brazil.
Data Sharing
Data collected for this study may be made available upon request. The data archive will be held at the Fred Hutchinson Cancer Center (Seattle, WA, USA). Requests can be sent to HPTN-Data-Access@scharp.org.
References
- 1.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. New England Journal of Medicine 2021; 385: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirrincione LR, Grieve VLB, Holloway J, Marzinke MA. Inclusion of Transgender and Gender Diverse People in Phase III Trials: Examples from HIV Pharmacologic Prevention Studies. Clinical Pharmacology & Therapeutics; n/a. DOI: 10.1002/cpt.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sevelius JM, Deutsch MB, Grant R. The future of PrEP among transgender women: the critical role of gender affirmation in research and clinical practices. J Int AIDS Soc 2016; 19: 21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stutterheim SE, Dijk M van, Wang H, Jonas KJ. The worldwide burden of HIV in transgender individuals: An updated systematic review and meta-analysis. PLOS ONE 2021; 16: e0260063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reisner SL, Poteat T, Keatley J, et al. Global health burden and needs of transgender populations: a review. Lancet 2016; 388: 412–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AI Scheim, Baker KE, Restar AJ, Sell RL. Health and Health Care Among Transgender Adults in the United States. Annu Rev Public Health 2022; 43: 503–23. [DOI] [PubMed] [Google Scholar]
- 7.Reisner SL, Deutsch MB, Bhasin S, et al. Advancing methods for US transgender health research. Curr Opin Endocrinol Diabetes Obes 2016; 23: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker KE, Streed CG, Durso LE. Ensuring That LGBTQI+ People Count - Collecting Data on Sexual Orientation, Gender Identity, and Intersex Status. N Engl J Med 2021; 384: 1184–6. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Serving the needs of key populations: case examples of innovation and good practice on HIV prevention, diagnosis, treatment and care. World Health Organization, 2017. https://apps.who.int/iris/handle/10665/255610 (accessed Oct 6, 2022). [Google Scholar]
- 10.Rael CT, Martinez M, Giguere R, et al. Barriers and Facilitators to Oral PrEP Use Among Transgender Women in New York City. AIDS Behav 2018; 22: 3627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson EC, Jalil EM, Castro C, Fernandez NM, Kamel L, Grinsztejn B. Barriers and Facilitators to PrEP for Transwomen in Brazil. Glob Public Health 2019; 14: 300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalil EM, Torres TS, Luz PM, et al. Low PrEP adherence despite high retention among transgender women in Brazil: the PrEParadas study. J Int AIDS Soc 2022; 25. DOI: 10.1002/jia2.25896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veloso VG. Evidence for PrEP implementation in Latin America: ImPrEP Project results. 2022. https://conference.aids2022.org/media-2269-evidence-for-prep-implementation-in-latin-america-imprep-project-results (accessed Sept 6, 2022).
- 14.Deutsch MB, Glidden DV, Sevelius J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2: e512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguayo-Romero RA, Cannon CM, Wirtz AL, et al. HIV awareness and prevention strategies among transgender women in the Eastern and Southern United States: findings from the LITE Study. J Int AIDS Soc 2022; 25 Suppl 5: e25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman E, Radix AE, Bouman WP, et al. Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. International Journal of Transgender Health 2022; 23: S1–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James SE & Herman J USTS-Executive-Summary-Dec17.pdf. 2017. https://transequality.org/sites/default/files/docs/usts/USTS-Executive-Summary-Dec17.pdf (accessed Jan 26, 2023).
- 18.Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017; 102: 3869–903. [DOI] [PubMed] [Google Scholar]
- 19.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158: 1789–95. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, O’Brien N, Forrest JI, et al. Validating a Shortened Depression Scale (10 Item CES-D) among HIV-Positive People in British Columbia, Canada. PLoS One 2012; 7: e40793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzinke MA, Grinsztejn B, Fogel JM, et al. Characterization of Human Immunodeficiency Virus (HIV) Infection in Cisgender Men and Transgender Women Who Have Sex With Men Receiving Injectable Cabotegravir for HIV Prevention: HPTN 083. The Journal of Infectious Diseases 2021; 224: 1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landovitz RJ, Li S, Eron JJ, et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 2020; 7: e472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 2018; 62: e01710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials 2013; 14: 192–203. [DOI] [PubMed] [Google Scholar]
- 25.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4: 151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witcomb GL, Claes L, Bouman WP, Nixon E, Motmans J, Arcelus J. Experiences and Psychological Wellbeing Outcomes Associated with Bullying in Treatment-Seeking Transgender and Gender-Diverse Youth. LGBT Health 2019; 6: 216–26. [DOI] [PubMed] [Google Scholar]
- 27.Bouman WP, Claes L, Brewin N, et al. Transgender and anxiety: A comparative study between transgender people and the general population. International Journal of Transgenderism 2017; 18: 16–26. [Google Scholar]
- 28.Malone J, Reisner SL, Cooney EE, et al. Perceived HIV Acquisition Risk and Low Uptake of PrEP Among a Cohort of Transgender Women With PrEP Indication in the Eastern and Southern United States. J Acquir Immune Defic Syndr 2021; 88: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillen-Diaz-Barriga C, Diaz-Sosa D, Torres TS, et al. HIV Risk Perception and Pre-Exposure Prophylaxis (PrEP) Awareness Among Transgender Women from Mexico. AIDS Behav 2022; published online Sept 19. DOI: 10.1007/s10461-022-03836-w. [DOI] [PubMed] [Google Scholar]
- 30.Janz NK, Champion VL, Stretcher VJ. The health belief model. In: Glanz Rimer, Lewis, eds. Health Behavior and Health Education: Theory, Research, and Practice, 3a edição. San Francisco, CA: John Wiley & Sons, 2002: 45–66. [Google Scholar]
- 31.Bandura A Social cognitive theory: an agentic perspective. Annu Rev Psychol 2001; 52: 1–26. [DOI] [PubMed] [Google Scholar]
- 32.Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med 2018; 15: e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinsztejn B, Hoagland B, Moreira RI, et al. Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. The Lancet HIV 2018; 5: e136–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this study may be made available upon request. The data archive will be held at the Fred Hutchinson Cancer Center (Seattle, WA, USA). Requests can be sent to HPTN-Data-Access@scharp.org.


