Abstract
Objective:
Endometrial cancer stage is a strong prognostic factor; however, the current stage classification does not incorporate transtubal spread as determined by intraluminal tumor cells (ILTCs). We examined relationships between ILTCs and survival outcomes according to histological subtype and stage and examined whether identification of ILTCs improves prognostic accuracy of endometrial cancer staging.
Methods:
We conducted a retrospective cohort study of women diagnosed with endometrial cancer at five academic hospitals between 2007 and 2012. Pathologists determined ILTC presence (no vs. yes) and location (free in lumen vs. attached to epithelial surface) based on pathology review of hematoxylin and eosin-stained sections of fallopian tubes. Associations between ILTCs with time to recurrence (TTR) and overall survival (OS) were examined with Cox proportional hazards models adjusted for other prognostic factors. Model discrimination metrics were used to assess the addition of ILTCs to stage for prediction of 5-year TTR and OS.
Results:
In the overall study population (N=1,303), ILTCs were not independently associated with TTR (HR=0.95, 95% CI=0.69-1.32) or OS (HR=0.97, 95% CI=0.72-1.31). Among 805 women with stage I disease, ILTCs were independently associated with worse TTR (HR=2.31, 95% CI=1.06-5.05) and OS (HR=2.16, 95% CI=1.14-4.11). Upstaging early-stage cases with ILTCs present did not increase model discrimination.
Conclusion:
While our data do not suggest that endometrial cancer staging guidelines should be revised to include ILTCs, associations between ILTCs and reduced survival observed among stage I cases suggest this tumor feature holds clinical relevance for subgroups of endometrial cancer patients.
Keywords: Uterus Neoplasm, Fallopian Tube, disease-specific mortality, patterns of spread
INTRODUCTION
Mortality from uterine cancer, the most common gynecologic malignancy in the United States, increased by 1.6% per year between 2011 and 2020, one of few solid tumor types to demonstrate rising mortality rates [1]. Stage – which details the degree to which the primary tumor has spread within and beyond the uterus – is the strongest factor influencing mortality [2]. Approximately 80% of women with distant stage uterine cancer die within five years of diagnosis compared to only 5% of women with localized disease [1]. Cancer staging serves at least three vital functions: 1.) guiding post-operative treatment decisions, 2.) stratifying patients into prognostic categories, and 3.) facilitating the exchange of information between clinicians.
The most commonly used guidelines for endometrial cancer (EC, the predominant form of uterine cancer) stage were developed by the International Federation of Gynecologic Oncology (FIGO) in 1958 [3]. Since their introduction, FIGO stage criteria underwent major revisions in 1988, 2009 and 2023. Before 1988, ECs were clinically staged with dilation and curettage (D&C). However, findings from several Gynecologic Oncology Group (GOG) studies revealed the prognostic significance of several uterine risk factors ascertained from surgical hysterectomy specimens, including grade, depth of myometrial invasion, cervical extension, and lymph node involvement [4-6]. Consequently, surgical staging, which allowed more precise knowledge of the extent and natural history of EC, replaced clinical staging. In 2009, several EC substages were collapsed and peritoneal cytology was removed [7]. The FIGO EC stage criteria were again revised in 2023, in which additional categorizations were introduced to incorporate non-aggressive vs. aggressive histology, along with a strong recommendation for the incorporation of molecular subtypes, potentially providing improved recurrence risk assessment and refining adjuvant treatment selection [8, 9]. These changes to staging criteria reflect the importance of continued monitoring of EC patterns of spread to produce the most accurate staging criteria for improvement in the quality and consistency of patient care.
The current FIGO stage criteria incorporate three anatomical modes of spread: direct extension, lymph node extension, and distant metastasis through lymphovascular invasion. One mode of spread that is potentially relevant for EC and not fully incorporated in the current classification is transtubal spread. EC cells have been identified in cytological fallopian tube washings, supporting the possibility that EC cells can be exfoliated through the fallopian tubes into the peritoneal cavity [10, 11]. Furthermore, the presence of intraluminal tumor cells (ILTCs) is related to other adverse endometrial tumor characteristics [12-14]. While these analyses are consistent with the hypothesis that ILTCs co-occur with other adverse tumor characteristics, several gaps remain. First, we lack data on these associations stratified by EC histologic subtype and stage. These relationships may be particularly important for women diagnosed with the serous subtype, as these tumors commonly spread transperitoneally, suggesting the fallopian tubes as a possible avenue of dissemination [12]. Second, we lack conclusive quantification of the clinical significance of transtubal spread on EC survival and whether incorporation of ILTC information improves the prognostic accuracy of stage criteria. Therefore, we examined these questions in a pooled analysis to improve upon sample size limitations of prior, single-institution studies.
METHODS
Study population
This was a retrospective cohort of women newly diagnosed with EC at one of five academic institutions between January 1, 2007 and December 31, 2012. At three of the sites (Ohio State University, Duke University, and Mayo Clinic, Rochester), existing databases of EC patients were available, while at two sites (University of Calgary and University of Pittsburgh), lists of surgical EC patients were obtained from cancer registries. From each institution, we included all women with diagnoses of prognostically unfavorable cancers, including grade 3 endometrioid, serous, carcinosarcoma, mixed epithelial, or clear cell. We included a subset of women diagnosed with grades 1 or 2 endometrioid tumors given our previous work suggesting that transtubal spread may be a less relevant mechanism for these tumors [15]. Among lists of all diagnosed grades 1 or 2 endometrioid ECs, we randomly selected approximately 20% grades 1 or 2 endometrioid ECs from each site. In the pooled dataset, we excluded five women with missing stage. The Institutional Review Board at the Ohio State University approved this study.
Data collection
Information on age at diagnosis (continuous and categorized as <55, 55-64, ≥65), self-reported race (White vs. non-White), body mass index (BMI) (< 25, 25-30, ≥30 kg/m2), history of tubal ligation, use of an intrauterine manipulator during surgery, type of EC hysterectomy (laparoscopic, abdominal, other), lymphovascular space invasion (LVSI) (no vs. yes), percent of myometrial invasion (<50%, ≥50%), stage (I, II, III, IV, according to 2009 FIGO) [7], histology (endometrioid, serous, carcinosarcoma, clear cell, mixed carcinoma), grade (1, 2, 3, applicable to endometrioid tumors only), and treatment were collected from electronic medical records and final pathology reports. For women diagnosed before 2009, we re-classified stage based on the FIGO 2009 criteria. Race, history of tubal ligation and intrauterine manipulation at surgery were unavailable for Calgary data.
Dates of EC hysterectomy, recurrence (unavailable for the Calgary data), and death were available and used to define time to recurrence (TTR) and overall survival (OS). For each outcome, follow-up began at the date of hysterectomy and ended at the date of recurrence or EC death (TTR) or death from any cause (OS). Women were censored at date of last follow-up check; for TTR, women who did not experience recurrence and died due to non-EC causes were censored at the date of death.
Determination of ILTC status
Digital images (scanned at 20X) or glass slides of hematoxylin-eosin (H&E) stained sections of fallopian tubes from four of the five sites were reviewed by one gynecologic pathologist (AAS) for presence of ILTCs. The number of sections per woman varied based on availability, but at least one cross-section of the diameter of each fallopian tube was reviewed per case. ILTCs were identified when viable tumor fragments either as tumor cells or tumor cells associated with fibrous or fibro-vascular stroma were in the same plane of focus as the tissue section of fallopian tube, and features of postoperative extraneous tissue were absent [16]. We also included data from the University of Calgary, in which three pathologists (MAD, MR, MAB) utilized the Sectioning and Extensively Examining the Fimbriated End (SEE-FIM) protocol to assess ILTC status in EC patients [16]. Intra- and inter-rater agreement for this site has been published. To compare the agreement between the main study pathologist (AAS) and the University of Calgary team, AAS reviewed a random sample of 36 University of Calgary EC cases (~10% of the sample). McNemar’s test was used to evaluate inter-rater agreement between the Ohio State University (OSU) pathologist and the final ILTC determination in the Calgary dataset. Inter-rater agreement was 97.2% and the kappa was 0.79, indicating almost perfect agreement.
Statistical analysis
Because of the potential for between site heterogeneity, all models included site as a stratification variable. We first assessed associations between ILTCs and clinical/tumor characteristics using univariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Variables significantly (p<0.05) associated with ILTCs in the univariable models were included in a multivariable logistic regression model. Next, we estimated site-specific and pooled hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between ILTCs with TTR and OS using Cox proportional hazards regression. Unadjusted HRs and HRs adjusted for age at diagnosis, total number of reviewed slides (2 vs >2), stage, and histological subtype are presented. We did not include adjuvant treatment as an adjustment factor as it occurs as an intermediate in the pathway between ILTC determination and survival outcomes. However, results from sensitivity analyses including adjuvant treatment as a covariate did not materially change our findings.
We also examined associations between survival and ILTCs (present vs. absent) and ILTC location (attached to the lumen vs. floating in the lumen) stratified by histological subtype or stage with adjustment for age at diagnosis, total number of reviewed slides, stage (histological subtype-stratified models only), and histological subtype (stage-stratified models). As an additional sensitivity analysis, we examined pooled associations between ILTC presence and survival outcomes excluding each site individually to assess whether any specific site unduly influenced the results.
To examine whether ILTC presence improved the prognostic accuracy of FIGO stage, we defined a new stage variable in which patients with stage I or II EC with ILTCs present were restaged as stage IIIa. We compared two Cox proportional hazards models predicting survival (OS or TTR) from clinical features: one included current stage as a predictor and the second included the re-defined stage variable. Risk prediction accuracy of the linear predictors from these models was compared using two discrimination metrics: 1) receiver operating characteristics (ROC) curves evaluating prediction of survival at 5 years, estimated according to Heagerty et al.[17] to account for censoring; and 2) C-statistics assessing how well the risk scores order the survival times within the first 5 years following the method by Uno et al.[18] We performed this analysis overall and by histological subtype, adjusting for age, number of slides, and histology (in the overall model). We also compared discrimination of multivariate models that did and did not include ILTC presence as a variable in the model, rather than using it to update the stage variable; we did this overall and stratified by histology and by stage. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) or R version 4.0.2. All P values were two-sided with the probability of a Type I error set at <5%.
RESULTS
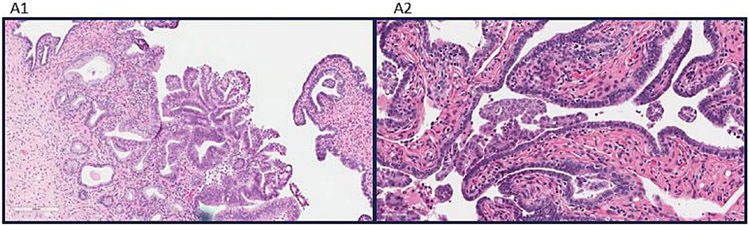
Figure 1 shows examples of ILTCs attached and floating in the lumen. ILTC prevalence ranged from 4.4% (Mayo Clinic) to 15.6% (University of Pittsburgh), with a pooled prevalence of 11.1%. Median age (interquartile range [IQR]) of the pooled sample was 65 (59-72) and 11.5% were non-White women. Table 1 shows distributions of patient and tumor characteristics according to ILTC presence, univariable and multivariable-adjusted ORs. In the multivariable model, higher ILTC odds were associated with age (55-64 vs. ≥65: OR=2.12, 95% CI=1.40-3.22), stage (stage III vs. stage I OR 3.98, 95% CI=2.48-6.41; stage IV vs. stage I OR: 8.64, 95% CI=4.97-15.04), and number of H&E slides reviewed (>2 vs. 2 OR: 2.79, 95% CI=1.81-4.32).
Figure 1.
(A1) Intraluminal tumor cells (ILTCs) attached to the epithelium of the mucosa of the tube (hematoxylin and eosin 400× and inset 100×). (A2) ILTCs floating in the lumen (hematoxylin and eosin 400× and inset 100×).
Table 1.
Pooled odds ratios and 95% confidence intervals for associations between tumor characteristics and presence of intraluminal tumor cells
| Characteristics | Presence of intraluminal tumor cells | |||||
|---|---|---|---|---|---|---|
| Absent (n=1,159) |
Present (n=144) | |||||
| No. (%) | No. (%) |
OR (95% CI)a | pb | OR (95% CI)c | pb | |
| Age | 0.01 | 0.002 | ||||
| <55 | 169 (14.6) | 17 (11.8) | 0.96 (0.55, 1.70) | 1.49 (0.79, 2.78) | ||
| 55-64 | 374 (32.3) | 65 (45.1) | 1.68 (1.16, 2.45) | 2.12 (1.40, 3.22) | ||
| ≥65 | 616 (53.2) | 62 (43.1) | 1.00 | 1.00 | ||
| Race d | 0.90 | |||||
| White | 715 (84.3) | 70 (79.6) | 1.00 | --- | ||
| Non-White | 132 (15.6) | 18 (20.5) | 1.15 (0.63, 2.10) | --- | ||
| BMI (kg/m2)e | 0.27 | |||||
| Normal weight (<25) | 277 (23.9) | 33 (22.9) | 1.00 | --- | ||
| Overweight (25-29.9) | 305 (26.3) | 49 (34.0) | 1.38 (0.86, 2.22) | --- | ||
| Obese (≥30) | 546 (47.1) | 56 (38.9) | 0.92 (0.57, 1.47) | --- | ||
| History of Tubal Ligation d,f | 0.50 | |||||
| No | 701 (82.7) | 76 (86.4) | 1.00 | --- | ||
| Yes | 126 (14.9) | 10 (11.4) | 0.67 (0.34, 1.34) | --- | ||
| Intrauterine manipulator use d,g | 0.11 | |||||
| No | 652 (76.9) | 65 (73.9) | 1.00 | --- | ||
| Yes | 178 (21.0) | 18 (20.5) | 0.59 (0.30, 1.18) | --- | ||
| Hysterectomy type | 0.18 | |||||
| Laparoscopic | 373 (32.2) | 37 (25.7) | 1.00 | --- | ||
| Abdominal | 666 (57.5) | 83 (57.6) | 1.20 (0.75, 1.91) | --- | ||
| Other | 120 (10.4) | 24 (16.7) | 1.79 (0.95, 3.36) | --- | ||
| Number of H&E slides reviewed | <0.0001 | <0.0001 | ||||
| 2 | 845 (72.9) | 65 (45.1) | 1.00 | 1.00 | ||
| >2 | 314 (27.1) | 79 (54.9) | 3.41 (2.25, 5.18) | 2.79 (1.81, 4.32) | ||
| Stage | <0.0001 | <0.0001 | ||||
| I | 764 (65.9) | 41 (28.5) | 1.00 | 1.00 | ||
| II | 60 (5.2) | 7 (4.9) | 1.88 (0.81, 4.41) | 2.05 (0.86, 4.90) | ||
| III | 235 (20.3) | 52 (36.1) | 4.04 (2.60, 6.27) | 3.98 (2.48, 6.41) | ||
| IV | 100 (8.6) | 44 (30.6) | 9.36 (5.75, 15.25) | 8.64 (4.97, 15.04) | ||
| Histology | <0.0001 | 0.02 | ||||
| Low-grade endometrioid | 326 (28.1) | 25 (17.4) | 1.00 | 1.00 | ||
| High-grade endometrioid | 248 (21.4) | 28 (19.4) | 1.81 (1.02, 3.21) | 1.09 (0.59, 2.01) | ||
| Serous | 282 (24.3) | 60 (41.7) | 3.95 (2.36, 6.63) | 1.76 (0.96, 3.22) | ||
| Carcinosarcoma | 127 (11.0) | 10 (6.9) | 1.12 (0.52, 2.42) | 0.48 (0.20, 1.11) | ||
| Clear cell | 72 (6.2) | 5 (3.5) | 1.30 (0.48, 3.58) | 0.57 (0.19, 1.74) | ||
| Mixed epithelial | 102 (8.8) | 15 (10.4) | 1.87 (0.94, 3.74) | 0.99 (0.47, 2.12) | ||
| Undifferentiated | 2 (0.2) | 1 (0.7) | 4.84 (0.42, 55.27) | 0.59 (0.05, 7.52) | ||
| LVSI h | <0.0001 | |||||
| Absent | 773 (66.7) | 60 (41.7) | 1.00 | --- | ||
| Present | 379 (32.7) | 84 (58.3) | 2.37 (1.65, 3.41) | --- | ||
| Myometrial invasion | 0.003 | |||||
| <50% | 725 (62.6) | 67 (46.5) | 1.00 | --- | ||
| ≥50% | 434 (37.5) | 77 (53.5) | 1.72 (1.21, 2.45) | --- | ||
Unadjusted ORs and 95% CIs
Wald Chi-Square p-value
ORs and 95% CIs adjusted for age (<55, 55-64, ≥65), FIGO 2009 stage (I, II, III, IV), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, clear cell, mixed epithelial, undifferentiated), number of HE slides reviewed (2, >2), and stratified by site (Ohio State University, Duke University, University of Calgary, Mayo Clinic, University of Pittsburgh)
Unavailable in the University of Calgary dataset and unknown for 1 individual at OSU
Unknown for 37 individuals at University of Calgary
Unknown for 17 individuals at Mayo Clinic; 2 at Ohio State University , 4 at University of Pittsburgh
Unknown for 23 individuals at OSU
Unknown for 7 individuals at University of Calgary
Median follow-up (IQR) was 5.6 years (2-15). In the pooled univariable models, ILTC presence was significantly associated with reduced TTR (HR=1.88, 95% CI=1.40-2.53) and OS (HR=1.81, 95% CI=1.38-2.38, Table 2). These associations were also noted for individual sites, although statistical significance was not achieved for each individual site and survival outcome. In the multivariable-adjusted pooled analyses, ILTC presence was unrelated to TTR (HR=0.95, 95% CI=0.69-1.32) or OS (HR=0.97, 95% CI=0.72-1.31) in the overall study population.
Table 2.
Site-specific and pooled hazard ratios and 95% confidence intervals for ILTC presence, time to recurrence and overall survival
| Site | N | ILTC prevalence |
Time to recurrence (TTR) | Overall survival (OS) | ||||
|---|---|---|---|---|---|---|---|---|
| EC death or recurrence, n (%)a |
HR (95% CI)b | HR (95% CI)c | deaths, n (%)a |
HR (95% CI)b | HR (95% CI)c | |||
| Ohio State University | 295 | 11.9% | 86 (29.2) | 1.97 (1.15, 3.40) | 0.72 (0.38, 1.36) | 84 (28.5) | 1.99 (1.14, 3.49) | 0.87 (0.46, 1.66) |
| University of Calgaryd | 367 | 15.3% | 30 (8.2) | 1.50 (0.65, 3.51) | 0.87 (0.34, 2.28) | 60 (16.4) | 1.58 (0.88, 2.84) | 0.93 (0.48, 1.83) |
| Duke University | 188 | 11.2% | 92 (48.9) | 1.63 (0.92, 2.88) | 1.24 (0.64, 2.39) | 99 (52.7) | 1.31 (0.73, 2.35) | 0.78 (0.38, 1.58) |
| Mayo Clinic | 344 | 4.4% | 88 (25.6) | 2.00 (0.87, 4.60) | 0.72 (0.29, 1.74) | 102 (29.7) | 2.69 (1.30, 5.57) | 1.17 (0.53, 2.58) |
| University of Pittsburgh | 109 | 15.6% | 42 (38.5) | 2.46 (1.26, 4.82) | 2.26 (0.90, 5.65) | 61 (56.0) | 2.22 (1.21, 4.08) | 1.25 (0.59, 2.62) |
| Poolede | 1,303 | 11.1% | 338 (25.9) | 1.88 (1.40, 2.53) | 0.95 (0.69, 1.32) | 406 (31.2) | 1.81 (1.38, 2.38) | 0.97 (0.72, 1.31) |
row percentage
Unadjusted HRs and 95% CIs
HRs and 95% CIs adjusted for age at diagnosis (<55, 55-64, ≥65), number of HE slides reviewed (2, >2), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, clear cell, mixed epithelial, undifferentiated), and FIGO 2009 stage (I, II, III, IV)
Recurrences were unavailable in the University of Calgary dataset - only EC deaths are counted in the TTR outcome for this site
Stratified by site (Ohio State University, Duke University, University of Calgary, Mayo Clinic, University of Pittsburgh)
Table 3 shows associations between ILTC presence, TTR, and OS stratified by histological subtype or stage. Among women diagnosed with serous tumors, ILTC presence was significantly associated with reduced TTR (HR=2.40, 95% CI=1.57-3.66) and OS (HR=1.85, 95% CI=1.25-2.73); however, these associations were attenuated in the multivariable-adjusted models. Among women diagnosed with stage I tumors, presence of ILTCs was associated with worse TTR (HR=2.31, 95% CI=1.06-5.05) and OS (HR=2.16, 95% CI=1.14-4.11) in multivariable-adjusted models. Supplemental Table 1 characterizes associations between ILTC location with TTR and OS. In multivariable-adjusted models, among women diagnosed with carcinosarcomas, ILTCs floating in the lumen were significantly associated with reduced TTR (HR=5.80, 95% CI=1.14-29.61) and OS (HR=39.59, 95% CI=5.12-306.17). Similarly, among women diagnosed with stage I tumors, ILTCs floating in the lumen were significantly associated with reduced TTR (HR= 2.64, 95%CI=1.16- 6.01) and OS (HR= 3.54, 95% CI=1.82-6.86).
Table 3.
Unadjusted and adjusted pooled hazard ratios and 95% confidence intervals for associations between ILTC presence, time to recurrence, and overall survival stratified by histological subtype and stage
| Time to recurrence (TTR) | Overall survival (OS) | ||||||
|---|---|---|---|---|---|---|---|
| ILTC presence | EC death or recurrence, n (%)a |
HR (95% CI)b | HR (95% CI)c | ILTC presence | deaths, n (%)a |
HR (95% CI)b | HR (95% CI)c |
| Low-grade endometrioid, N=351 | |||||||
| Absent (n=326) | 18 (5.5) | 1.00 | 1.00 | Absent (n=326) | 25 (7.7) | 1.00 | 1.00 |
| Present (n=25) | 2 (8.0) | 2.03 (0.45, 9.12) | 0.36 (0.06, 2.14) | Present (n=25) | 3 (12.0) | 1.35 (0.38, 4.84) | 0.53 (0.13, 2.26) |
| High-grade endometrioid, N=276 | |||||||
| Absent (n=248) | 73 (29.4) | 1.00 | 1.00 | Absent (n=248) | 71 (28.6) | 1.00 | 1.00 |
| Present (n=28) | 7 (25.0) | 0.84 (0.38, 1.83) | 0.64 (0.28, 1.46) | Present (n=28) | 9 (32.1) | 1.13 (0.56, 2.29) | 0.85 (0.41, 1.77) |
| Serous, N=342 | |||||||
| Absent (n=282) | 93 (33.0) | 1.00 | 1.00 | Absent (n=282) | 126 (44.7) | 1.00 | 1.00 |
| Present (n=60) | 32 (53.3) | 2.40 (1.57, 3.66) | 1.19 (0.74, 1.90) | Present (n=60) | 36 (60.0) | 1.85 (1.25, 2.73) | 1.02 (0.66, 1.59) |
| Carcinosarcoma, N=137 | |||||||
| Absent (n=127) | 62 (48.8) | 1.00 | 1.00 | Absent (n=127) | 68 (53.5) | 1.00 | 1.00 |
| Present (n=10) | 5 (50.0) | 1.78 (0.68, 4.70) | 1.26 (0.38, 4.22) | Present (n=10) | 6 (60.0) | 1.33 (0.54, 3.29) | 1.08 (0.38, 3.04) |
| Clear cell, N=77 | |||||||
| Absent (n=72) | 12 (16.7) | 1.00 | 1.00 | Absent (n=72) | 19 (26.4) | 1.00 | 1.00 |
| Present (n=5) | 3 (60.0) | 2.23 (0.49, 10.20) | 0.70 (0.08, 6.14) | Present (n=5) | 3 (60.0) | 1.56 (0.37, 6.68) | 0.47 (0.08, 2.66) |
| Mixed epithelial, N=117 | |||||||
| Absent (n=102) | 25 (24.5) | 1.00 | 1.00 | Absent (n=102) | 32 (31.4) | 1.00 | 1.00 |
| Present (n=15) | 5 (33.3) | 2.14 (0.77, 5.97) | 1.55 (0.47, 5.09) | Present (n=15) | 7 (46.7) | 2.19 (0.93, 5.18) | 1.89 (0.68, 5.26) |
| Stage I, N=805 | |||||||
| Absent (n=764) | 82 (10.7) | 1.00 | 1.00 | Absent (n=764) | 122 (16.0) | 1.00 | 1.00 |
| Present (n=41) | 8 (19.5) | 1.77 (0.85, 3.71) | 2.31 (1.06, 5.05) | Present (n=41) | 12 (29.3) | 1.95 (1.07, 3.58) | 2.16 (1.14, 4.11) |
| Stage II, N=67 | |||||||
| Absent (n=60) | 22 (36.7) | 1.00 | 1.00 | Absent (n=60) | 22 (36.7) | 1.00 | 1.00 |
| Present (n=7) | 0 (0.0) | NEd | NE | Present (n=7) | 0 (0.0) | NE | NE |
| Stage III, N=287 | |||||||
| Absent (n=235) | 112 (47.7) | 1.00 | 1.00 | Absent (n=235) | 124 (52.8) | 1.00 | 1.00 |
| Present (n=52) | 21 (40.4) | 0.79 (0.49, 1.26) | 0.89 (0.54, 1.48) | Present (n=52) | 26 (50.0) | 0.85 (0.55, 1.31) | 0.97 (0.61, 1.54) |
| Stage IV, N=144 | |||||||
| Absent (n=100) | 67 (67.0) | 1.00 | 1.00 | Absent (n=100) | 73 (73.0) | 1.00 | 1.00 |
| Present (n=44) | 26 (59.1) | 0.84 (0.51, 1.38) | 0.73 (0.43, 1.24) | Present (n=44) | 27 (61.4) | 0.73 (0.44, 1.20) | 0.57 (0.33, 0.99) |
row percentage
Unadjusted HRs and 95% CIs
HRs and 95% CIs adjusted for age (<55, 55-64, ≥65), FIGO 2009 stage (I, II, III, IV), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, clear cell, mixed epithelial, undifferentiated), number of HE slides reviewed (2, >2) and stratified by site (Ohio State University, Duke University, University of Calgary, Mayo Clinic, University of Pittsburgh)
Not evaluated (NE)
Performance statistics comparing the original FIGO 2009 with FIGO staging updated to include ILTC presence are shown in Table 4. This change restaged 48 individuals: 28 Stage IA, 13 Stage IB, and 7 Stage 2. We observed no significant changes in either the area under the ROC curve or the C-statistic for prediction of either TTR or OS when using this updated staging variable. In overall and histology-stratified models including ILTC as a variable in addition to stage, age, and number of slides, there were no significant changes in model discrimination (data not shown). In stage-stratified models, including an indicator of ILTC presence also did not improve model discrimination. In the overall analysis, the areas under the ROC curves for prediction of survival and recurrence at 5 years were 0.82, suggesting that stage, histology, and age are reasonably good at predicting death within 5 years. However, c-statistics approached 0.5, suggesting that these predictors do not successfully order the survival times within the first five years.
Table 4.
Performance criteria of multivariate models with FIGO 2009 staging and updated staging with ILTC reclassification (overall and stratified by histology), and multivariate models with and without ILTC status (stratified by stage)
| Time to recurrence (TTR) | Overall survival (OS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Performance Metric |
FIGO 2009 | FIGO 2009 plus ILTCs |
Difference | p | Performance Metric |
FIGO 2009 | FIGO 2009 plus ILTCs |
Difference | p |
| Overall (N=1,303) | |||||||||
| AUC for 5-year survival | 0.823 | 0.819 | −0.004 (−0.011, 0.003) | 0.23 | AUC for 5-year survival | 0.816 | 0.816 | 0.000 (−0.007, 0.008) | 0.90 |
| C-statistic | 0.492 | 0.495 | 0.003 (−0.007, 0.013) | 0.55 | C-statistic | 0.496 | 0.488 | −0.007 (−0.023, 0.008) | 0.36 |
| Low-grade endometrioid (N=351) | |||||||||
| AUC for 5-year survival | 0.909 | 0.901 | −0.008 (−0.065, 0.028) | 0.67 | AUC for 5-year survival | 0.75 | 0.747 | −0.003 (−0.076, 0.079) | 0.89 |
| C-statistic | 0.543 | 0.572 | 0.029 (−0.107, 0.164) | 0.68 | C-statistic | 0.538 | 0.541 | 0.003 (−0.123, 0.130) | 0.96 |
| High-grade endometrioid (N=276) | |||||||||
| AUC for 5-year survival | 0.758 | 0.767 | 0.009 (−0.033, 0.020) | 0.63 | AUC for 5-year survival | 0.754 | 0.763 | 0.009 (−0.035, 0.036) | 0.61 |
| C-statistic | 0.513 | 0.509 | −0.004 (−0.019, 0.012) | 0.63 | C-statistic | 0.495 | 0.496 | 0.001 (−0.029, 0.032) | 0.93 |
| Serous (N=342) | |||||||||
| AUC for 5-year survival | 0.784 | 0.783 | −0.001 (−0.033, 0.028) | 0.95 | AUC for 5-year survival | 0.746 | 0.745 | −0.000 (−0.020, 0.027) | 0.98 |
| C-statistic | 0.513 | 0.504 | −0.010 (−0.034, 0.015) | 0.43 | C-statistic | 0.534 | 0.54 | 0.006 (−0.018, 0.030) | 0.64 |
| Carcinosarcoma (N=137) | |||||||||
| AUC for 5-year survival | 0.8 | 0.809 | 0.009 (−0.003, 0.056) | 0.33 | AUC for 5-year survival | 0.812 | 0.823 | 0.011 (−0.007, 0.040) | 0.25 |
| C-statistic | 0.525 | 0.525 | 0.000 (0.000, 0.000) | >0.99 | C-statistic | 0.519 | 0.486 | −0.033 (−0.173, 0.107) | 0.64 |
| Mixed epithelial (N=117) | |||||||||
| AUC for 5-year survival | 0.782 | 0.775 | −0.007 (−0.049, 0.031) | 0.62 | AUC for 5-year survival | 0.717 | 0.716 | −0.001 (−0.032, 0.057) | 0.93 |
| C-statistic | 0.494 | 0.522 | 0.029 (−0.211, 0.268) | 0.81 | C-statistic | 0.532 | 0.538 | 0.006 (−0.066, 0.078) | 0.86 |
| Clear cell (N=77) a | |||||||||
| AUC for 5-year survival | - | - | - | - | AUC for 5-year survival | - | - | - | - |
| C-statistic | - | - | - | - | C-statistic | - | - | - | - |
| Performance Metric |
Multivariate Model without ILTCs |
Multivariate Model with ILTCs |
Difference | p | Performance Metric |
Multivariate Model without ILTCs |
Multivariate Model with ILTCs |
Difference | p |
| Stage I (N=805) | |||||||||
| AUC for 5-year survival | 0.751 | 0.745 | −0.007 (−0.016, 0.015) | 0.5 | AUC for 5-year survival | 0.751 | 0.756 | 0.005 (−0.006, 0.026) | 0.53 |
| C-statistic | 0.496 | 0.501 | 0.004 (−0.009, 0.017) | 0.52 | C-statistic | 0.489 | 0.496 | 0.007 (−0.007, 0.021) | 0.32 |
| Stage II (N=67) | |||||||||
| AUC for 5-year survival | 0.615 | 0.462 | −0.153 (−0.259, 0.126) | 0.37 | AUC for 5-year survival | 0.57 | 0.659 | 0.089 (−0.073, 0.162) | 0.27 |
| C-statistic | 0.556 | 0.562 | 0.006 (−0.224, 0.236) | 0.96 | C-statistic | 0.618 | 0.62 | 0.002 (−0.140, 0.144) | 0.98 |
| Stage III (N=287) | |||||||||
| AUC for 5-year survival | 0.582 | 0.584 | 0.003 (−0.013, 0.034) | 0.74 | AUC for 5-year survival | 0.661 | 0.658 | −0.003 (−0.023, 0.043) | 0.76 |
| C-statistic | 0.536 | 0.522 | −0.014 (−0.061, 0.034) | 0.57 | C-statistic | 0.497 | 0.497 | 0.000 (−0.010, 0.010) | >0.99 |
| Stage IV (N=144) | |||||||||
| AUC for 5-year survival | 0.671 | 0.598 | −0.073 (−0.070, 0.075) | 0.54 | AUC for 5-year survival | 0.715 | 0.751 | 0.036 (−0.043, 0.113) | 0.34 |
| C-statistic | 0.548 | 0.549 | 0.002 (−0.021, 0.024) | 0.88 | C-statistic | 0.527 | 0.529 | 0.002 (−0.047, 0.050) | 0.95 |
No individuals' stage changed when including ILTC status among cases with clear cell EC; thus, no comparison could be made.
In a sensitivity analysis, we examined whether the removal of any specific site altered the overall association between ILTCs, TTR, and OS (Supplemental Table 2). The HRs resulting from removal of each individual site were within the confidence interval of the estimate with all data; therefore, we suspect that no single institution had an unduly large influence.
DISCUSSION
In this multi-institutional retrospective cohort study, we did not observe improved prognostic accuracy of EC FIGO staging when stage was reclassified based on the presence or absence of ILTCs. In line with our prior findings, however, we did observe that ILTCs were significantly associated with survival among women diagnosed with serous or stage I EC tumors. Among the latter subgroup, a significant two-fold higher risk of death was observed, independent of other clinical characteristics. The positive association between ILTCs and worse survival suggests this tumor feature is potentially clinically relevant for stage I patients, which represents the most common stage of EC diagnosis.
Cancer staging guidelines are an important clinical tool, guiding adjuvant treatment selection, informing post-diagnosis surveillance strategies, and communicating prognosis to patients. Refinement of stage criteria is an ongoing process, informed by new information about clinical behavior and management strategies. For example, the most recent EC stage revisions include new categories that parse patterns of spread based on histology type [8]. In addition, these guidelines call for the inclusion of EC molecular subtypes based on research demonstrating heterogeneous clinical outcomes between these subgroups [19, 20]. As noted in the updated guidelines, the role of ILTCs on EC outcomes is controversial, with a need for well-powered studies to clarify the clinical importance of this tumor feature [8].
The current analysis was predicated on two connected areas of research: 1.) studies showing an inverse association between tubal ligation surgery, EC stage, and survival outcomes [15, 21] and 2.) literature showing that ILTCs, a histological marker of fallopian tube spread, were associated with other aggressive tumor features [12-14] and reduced EC-specific survival in univariate models [14]. In this larger, pooled study with adjustment for prognostic tumor characteristics, we observed two-fold higher risks of EC death/recurrence and deaths from any cause among women with stage I tumors. When examining the location of the ILTCs, cells floating in the lumen were associated with increased risks of EC death/recurrence and deaths from any cause, while ILTCs attached to the lumen were not significantly related to outcomes. Although we expected to find significant associations when modeling ILTCs attached to the lumen as the feature – as this may signal an ability of these cells to attach to the lumen and spread – our null results for this category may be a consequence of small sample size. Similar to the research that guided this investigation, we observed that ILTCs were associated with aggressive tumor features, including non-endometrioid histology, myometrial invasion, lymphovascular space invasion, and advanced stage. This may explain the attenuation of univariable associations with ILTC presence when other clinical characteristics are included in the model.
Despite significant associations between ILTC presence with TTR and OS in subgroups, we did not detect an incremental gain in risk prediction accuracy of an updated staging variable incorporating ILTC status. In these analyses we reclassified women with stage I or II EC with identified ILTCs as stage IIIA, as this designation corresponds to cancer spread to the outer layer of the uterus and/or to the fallopian tubes, ovaries, and ligaments of the uterus. Including ILTC status in models stratified by histology and stage similarly showed no improvement in risk prediction. Even factors important in risk estimation and stratification can produce insignificant changes in the area under the curve (AUC), so this does not imply that ILTC status cannot improve understanding of patients’ prognosis [22]. Moreover, only 48 women (41 stage I) had their stage reclassified in this analysis, which may have resulted in insufficient power to detect improvement, particularly in subgroups of interest.
This study included the largest sample size of EC patients with ILTC assessment, offering an unprecedented investigation of the relationship between ILTCs and EC outcomes; nonetheless, subset analyses were limited by the relatively infrequent identification of ECs with cells in tubal lumina, and this frequency varied widely by study site. Despite pathology review, the prevalence of ILTCs in the pooled study is potentially underestimated given the limited number of slides per case that were available at most sites. This is a potential concern given our observation of higher odds of ILTC detection with a greater number of reviewed slides. One site that contributed to this study, (University of Calgary) utilized the SEE-FIM protocol, which includes longitudinal sectioning of the fimbria and extensive cross sectioning of the remaining tube at 2-mm intervals, to assess ILTCs [23]. SEE-FIM contrasts with classical methods in which fallopian tubes are sampled transversely, with three ring shape sections taken from each tube without specific sampling of the fimbrial ends. Direct comparisons of these methods demonstrate the SEE-FIM protocol is superior in detecting microscopic lesions in fallopian tube epithelium [24]. ILTC prevalence was higher in the University of Calgary series compared with all other institutions, except University of Pittsburgh, possibly reflecting a more sensitive ILTC detection method. Misclassification of ILTC presence is likely non-differential with respect to survival outcomes and may contribute to our null findings.
Relatedly, we noted qualitative differences in fallopian tube H&E slides between the various sites, likely stemming from different clinical processing protocols. For example, at one site, all operating room material is frozen to allow for diagnoses to be issued immediately during or after surgery. Subsequently, tissue is processed as formalin fixed paraffin embedded tissue, which made determination of fimbriae and other structures difficult. These observations prompted a sensitivity analysis that individually excluded each site. These analyses did not reveal a disproportionate effect from any specific site. Other potential limitations of our study include the historical nature of the cohort (diagnoses between 2007 and 2012). Given the time investment needed for pathology review, we sought to expand an existing series of ILTCs and EC prognosis [14] by including additional EC patients diagnosed during a comparable timeframe. EC treatment recommendations have not changed substantially; therefore, we believe the historical nature of this cohort still provides clinical relevance to more contemporary EC patient populations.
Continued examination of cancer staging criteria are warranted as stage is one of the strongest prognostic factors for most solid tumors. Although ILTCs represent a biologically plausible route of EC spread, our study does not support revising EC stage criteria to include an assessment of ILTCs based on H&E slides. Future studies should compare ILTC assessment via classical methods to a more thorough assessment using SEE-FIM protocols to evaluate ILTC misclassification when using H&E slides. If misclassification is substantial, further studies evaluating whether more thorough assessment of ILTCs can be used to improve staging, at least for particular subgroups of women with EC, should be conducted. At the very least, our study suggests there may be prognostic value of applying SEE-FIM protocols in selected subgroups of EC patients, particularly in light of the abandonment of peritoneal wash cytology.
Supplementary Material
Research Highlights.
In endometrial cancer, the role of transtubal spread, as determined by intraluminal tumor cell presence, is controversial.
Presence of intraluminal tumor cells was not related to recurrence or overall survival in the overall study population.
Among women with stage 1 disease, intraluminal tumor cells were related to worse recurrence and overall survival.
Upstaging women with early-stage endometrial cancer with intraluminal tumor cells did not affect model discrimination.
Funding
This work was supported by the National Cancer Institute (R03CA230673) to ASF, JAS, and AAS. The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure
Drs. Felix, Sinnott, and Suarez reported grants from the National Cancer Institute during the conduct of the study. Dr. Sherman receives research support from Exact Sciences. Dr. Cohn reports his positions of UpToDate author and Gynecologic Oncology Editor in Chief. Ms. Meade, Ms. Hall and Drs. Duggan, Havrilesky, Olawaiye, Mariani, Rodriquez, Brett, Dinoi, Goldfeld, and Elishaev have no conflicts of interest to report.
Data availability statement
The data underlying this article cannot be shared due to the privacy of individuals that participated in the study. Additional summary level data without individual data can be provided.
REFERENCES
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: a cancer journal for clinicians. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2006;95 Suppl 1:S105–43. [DOI] [PubMed] [Google Scholar]
- 3.Odicino F, Pecorelli S, Zigliani L, Creasman WT. History of the FIGO cancer staging system. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2008;101(2):205–10. [DOI] [PubMed] [Google Scholar]
- 4.Boronow RC, Morrow CP, Creasman WT, Disaia PJ, Silverberg SG, Miller A, et al. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63(6):825–32. [PubMed] [Google Scholar]
- 5.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40(1):55–65. [DOI] [PubMed] [Google Scholar]
- 6.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035–41. [DOI] [PubMed] [Google Scholar]
- 7.Creasman W. Revised FIGO staging for carcinoma of the endometrium. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105(2):109. [DOI] [PubMed] [Google Scholar]
- 8.Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. FIGO staging of endometrial cancer: 2023. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2023;162(2):383–94. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahle T. Transtubal spread of tumor cells in carcinoma of the body of the uterus. Surgery, gynecology & obstetrics. 1956;103(3):332–6. [PubMed] [Google Scholar]
- 11.Arikan G, Reich O, Weiss U, Hahn T, Reinisch S, Tamussino K, et al. Are endometrial carcinoma cells disseminated at hysteroscopy functionally viable? Gynecol Oncol. 2001;83(2):221–6. [DOI] [PubMed] [Google Scholar]
- 12.Snyder MJ, Bentley R, Robboy SJ. Transtubal spread of serous adenocarcinoma of the endometrium: an underrecognized mechanism of metastasis. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2006;25(2):155–60. [DOI] [PubMed] [Google Scholar]
- 13.Stewart CJ, Doherty DA, Havlat M, Koay MH, Leung YC, Naran A, et al. Transtubal spread of endometrial carcinoma: correlation of intra-luminal tumour cells with tumour grade, peritoneal fluid cytology, and extra-uterine metastasis. Pathology. 2013;45(4):382–7. [DOI] [PubMed] [Google Scholar]
- 14.Felix AS, Sinnott JA, Vetter MH, Rhoades J, Cohn DE, Backes FJ, et al. Detection of endometrial cancer cells in the fallopian tube lumen is associated with adverse prognostic factors and reduced survival. Gynecol Oncol. 2018;150(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felix AS, Brinton LA, McMeekin DS, Creasman WT, Mutch D, Cohn DE, et al. Relationships of Tubal Ligation to Endometrial Carcinoma Stage and Mortality in the NRG Oncology/ Gynecologic Oncology Group 210 Trial. J Natl Cancer Inst. 2015;107(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriquez M, Felix AS, Brett MA, Samimi G, Duggan MA. Associations Between Intraluminal Tumor Cell Involvement in Serially Examined Fallopian Tubes and Endometrial Carcinoma Characteristics and Outcomes. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2022;41(5):520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–44. [DOI] [PubMed] [Google Scholar]
- 18.Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Statistics in medicine. 2011;30(10):1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802–13. [DOI] [PubMed] [Google Scholar]
- 20.León-Castillo A, de Boer SM, Powell ME, Mileshkin LR, Mackay HJ, Leary A, et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J Clin Oncol. 2020;38(29):3388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Li M, Zhao L, Wang Z, Wang Y, Shen D, et al. Prior Tubal Ligation Might Influence Metastatic Spread of Nonendometrioid Endometrial Carcinoma. Int J Gynecol Cancer. 2016;26(6):1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928–35. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Medeiros F, Kindelberger D, Callahan MJ, Muto MG, Crum CP. Advances in the recognition of tubal intraepithelial carcinoma: applications to cancer screening and the pathogenesis of ovarian cancer. Advances in anatomic pathology. 2006;13(1):1–7. [DOI] [PubMed] [Google Scholar]
- 24.Koc N, Ayas S, Arinkan SA. Comparison of the Classical Method and SEE-FIM Protocol in Detecting Microscopic Lesions in Fallopian Tubes with Gynecological Lesions. Journal of pathology and translational medicine. 2018;52(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared due to the privacy of individuals that participated in the study. Additional summary level data without individual data can be provided.