Abstract
Ocular hypertension is caused by dysregulated outflow resistance regulation by the conventional outflow (CO) pathway. The physiology of the CO pathway can be directly studied during ex vivo ocular perfusions. In addition to measuring outflow resistance generation by the CO tissues, perfusion media that is conditioned by CO pathway cells can be collected upon exiting the eye as effluent. Thus, contents of effluent include factors contributed by upstream cells that report on the (dys)functionality of the outflow tissues. Two methods have been used in the past to monitor effluent contents from perfused eyes, each with their limitations. To overcome these limitations, we designed and printed a metabolic chamber to accommodate eyes of different sizes during perfusions. To test this new chamber, human eyes were perfused for 4 hours at constant flow rate of 2.5μl/minute, while pressure was continuously monitored and effluent was collected every hour. Facility was 0.28 ± 0.16μl/min/mmHg for OD eyes and 0.33 ± 0.11μl/min/mmHg for OS eyes (n=3). Effluent samples were protein rich, with protein concentration ranging from 2700 to 10,000μg/ml for all eyes and timepoints (N=3). Effluent samples expressed proteins that were actively secreted by the TM and easily detectible including MYOC and MMP2. Taken together, our model provides a reliable method to collect effluent from ex vivo human eyes, while maintaining whole globe integrity.
7. Introduction:
Ocular hypertension is the primary risk factor for glaucoma (Flammer and Mozaffarieh 2007), a leading cause of blindness worldwide (Tham, Li et al. 2014). Importantly, ocular hypertension is also the only modifiable risk factor for glaucoma, caused by dysregulated removal of aqueous humor (AH) from the eye by the conventional outflow (CO) pathway. To leave the eye, AH passes through the components of the CO pathway in the following order: trabecular meshwork (TM), Schlemm’s canal (SC), collector channels, intrascleral venous plexus and episcleral veins. While some resistance to AH outflow is generated in the distal portion of the CO pathway (McDonnell, Dismuke et al. 2018), the majority of outflow resistance is produced by the innermost region of the TM called the “juxtacanalicular (JCT)” region, where the inner wall of SC and the adjacent TM cells interact (Overby, Stamer et al. 2009, Tamm 2009).
Due to the avascularity of the CO tissues, the physiology of the CO pathway can be studied in isolation ex vivo by perfusing ocular preparations with mock AH (perfusate). In addition to measuring outflow resistance generation by the CO tissues, perfusate that is conditioned by CO pathway cells (effluent) can be collected as it exits the eye. Effluent contains many factors that can inform (dys)functionality of the outflow tissues. For example, TM cells secrete many autocrine and paracrine factors into passing AH/perfusate such as TGFB2, MMP2, MMP9, MYOC, ANGPTL7, NO, and VEGF (Alexander, Samples et al. 1991, Tripathi, Li et al. 1994, Fingert, Stone et al. 2002, De Groef, Van Hove et al. 2013, Hase, Kase et al. 2021, Reina-Torres, De Ieso et al. 2021, Praveen, Patel et al. 2022) that provide information about tissue homeostasis and health. Importantly, previous studies have demonstrated the utility of effluent isolation and analysis to inform ocular physiology/pathology (Table 1). For example, McDonnell et al. (2020) used effluent collected at various timepoints from human anterior segments transduced with an adenovirus engineered to have a shear stress-sensitive promoter (eNOS), driving a secreted reporter protein, secretory alkaline phosphatase (SEAP), to assess IOP-mediated effects on shear stress in the conventional outflow tract. In another study, Perruccio et al. (2008) used effluent collected from human anterior segments to monitor effects of dexamethasone on pigment epithelium derived factor secretion by conventional outflow cells. Comes and Borrás (2007) collected effluent from human anterior segments treated with glucocorticoid receptor (GR)-siRNA in the presence of dexamethasone to examine MYOC secretion by conventional outflow tissues. In a separate study, Perkumas et al. (2006) found myocilin associated with exosomes isolated from effluent collected from human anterior segments in organ culture. In an interesting study by Bahler et al. (2004), effluent was collected from human anterior segments with high initial pressures, and reinfused this effluent into the same eyes after pressure returned to baseline, resulting in a subsequent elevation of IOP. Lastly, Johnson et al. (1990) perfused whole globe human and bovine eyes with microspheres (0.18μm - l.1μm) and collected effluent to determine the percentage of microspheres of specific size passing through the human outflow system as a function of time, to inform filtration characteristics of the ocular outflow system.
Table 1:
Summary of previous studies monitoring contents in effluent from perfused eyes
| Study | Measured in effluent | Method | Advantages | Disadvantages |
|---|---|---|---|---|
| (McDonnell, Perkumas et al. 2020) | Secretory alkaline phosphatase and nitrite | Human anterior segment organ culture perfusion |
|
|
| (Perruccio, Rowlette et al. 2008) | Pigment epithelium derived factor | |||
| (Comes and Borrás 2007) | Myocilin | |||
| (Perkumas, Hoffman et al. 2007) | Myocilin-associated exosomes. |
|
||
| (Bahler, Fautsch et al. 2004) | Collected effluent from human anterior segments with high initial pressure. | |||
| (Spiga and Borrás 2010) | Matrix metalloproteinase-1 | |||
| (Comes, Buie et al. 2011) | Myocilin and fibronectin | |||
| (Johnson, Johnson et al. 1990) | Microspheres (0.18μm - l.1μm) | Perfusion of whole human eyes submerged in silicone oil |
|
|
| (Sit, Gong et al. 1997) | Total protein |
In these studies effluent was either collected from the outside of anterior segments in organ culture clamped into perfusion chambers, (Johnson and Tschumper 1987) or isolated from perfused whole human eyes submerged in silicone oil (Johnson, Johnson et al. 1990). For the first method, anterior segments in organ culture are clamped into perfusion chambers, where the integrity of the whole globe is lost, and collection volumes from chamber are inconsistent because of the clamp ring, and a difficult dissection and custom perfusion chambers are required (Johnson and Tschumper 1987, Bahler, Fautsch et al. 2004, Comes and Borrás 2007, Perkumas, Hoffman et al. 2007, Perruccio, Rowlette et al. 2008, McDonnell, Perkumas et al. 2020). The second method was developed to solve some of these issues by collecting effluent from perfused whole human eyes submerged in silicone oil (Johnson, Johnson et al. 1990). The denser silicone oil caused the effluent to form bubbles as it exited the episcleral veins at the sclera, allowing the collection from the oil surface using gentle suction from a Pasteur pipette. Since the viscosity of the oil is much higher than that of the effluent, the authors noted very little oil entered the Pasteur pipette. However, there are several limitations to this method. Firstly, the eyes are submerged in silicone oil, which is an unnatural environment and may not be conducive of normal outflow physiology. Moreover, there is a risk of silicone oil contamination in the effluent, whereby samples collected from the anterior chamber had to first be mixed with a little silicone oil to duplicate the conditions under which the perfusate was collected. Finally, there is risk of effluent loss when trying to separate the effluent from the oil. In response to limitations imposed by both methods, we designed and tested a new method to optimize collection of effluent from perfused whole eyes.
8. Materials and Supplies:
- Perfusate media (referred to as DBG):
- 500mg anhydrous glucose (MP Biomedical, catalog number 152527)
- DPBS, calcium, magnesium (Gibco™, catalog number 14040182)
60ml syringe (BD, catalog number 309653)
20ml syringe (BD, catalog number 302830)
10ml syringe (BD, catalog number 302995)
Pressure Monitoring Tubing with Fixed Male Luer Lock and Female Fitting, Low Pressure, 36” (Medline, catalog number DYNJPMTBG36MF)
Pressure Monitoring Tubing with Fixed Male Luer Lock and Female Fitting, Low Pressure, 24” (Medline, catalog number DYNJPMTBG24MF)
Pressure Monitoring Tubing with Fixed Male Luer Lock and Female Fitting, Low Pressure, 12” (Medline, catalog number DYNJPMTBG12MF)
Disposable Transfer Pipets (VWR®, catalog number 414004-018)
Stopcock 4 Way (Smiths Medical, catalog number MX5341L)
25G x .75 in. BD Vacutainer® Safety-Lok™ Blood Collection Set with 12 in. tubing and luer adapter (BD, catalog number 367285)
BD Luer-Lok™ Syringe (0.5ml) with attached needle (27G) (BD, catalog number 305620)
28 mm Diameter Syringe Filters, 0.2 μm Pore PES Membrane, Sterile, Individually Packaged (Corning®, catalog number 431229)
We used the 202S Isotemp Digital Water Bath (Fisher Scientific, catalog number 15-462-2S). However, any temperature-controlled water bath with an adjustable lid (Fig. 1D) can be used.
2X Stamped Steel Two-Hole Support Stands with Rods (18 inch) (VWR®, catalog number 76293-250)
3X Cast Aluminum Clamp Holder (VWR®, catalog number 76293–416)
3X Clamp, three-prong extension, dual adjustment (VWR®, catalog number 80063-590)
Legato 210 Two-Syringe Pump - Infuse/Withdrawal (WPI, catalog number SPLG210)
Figure 1: Design drawings for eye mount and images of metabolic chamber with human eyes.
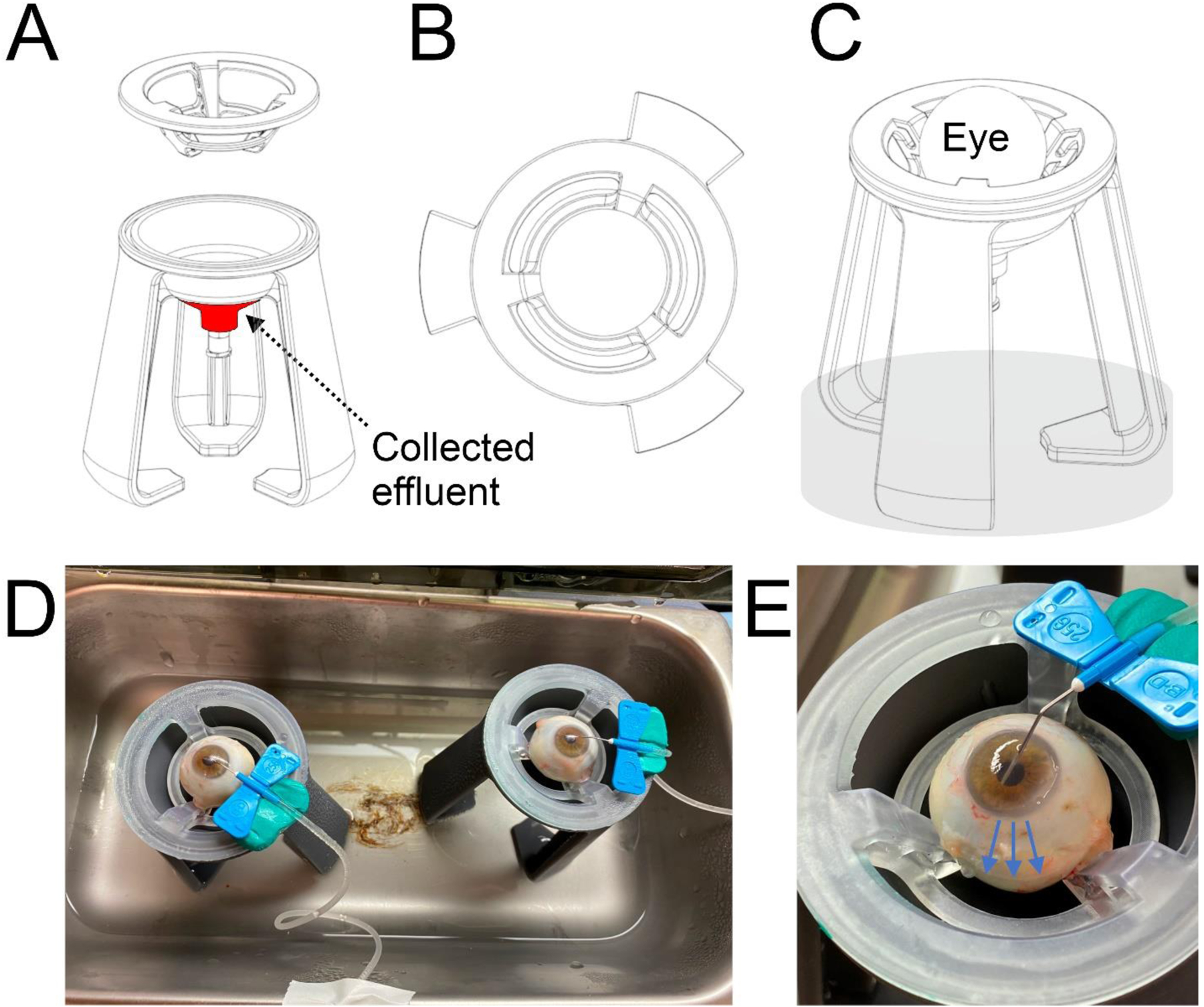
(A) Side view of stand with removable insert to suspend the eye. Effluent collects at the tip of the funnel (red). (B) Top view of insert used to suspend the eye. (C) Side view of fully assembled eye mount with eye mounted, and water level in chamber is represented in transparent gray. (D) Humidified and temperature-controlled water bath (closed during perfusion) with eyes cannulated and set up on eye mounts. (B) Close up view of cannulated human eye set on removable insert. Effluent runs down surface of eye and collects at funnel tip (blue arrows).
9. Detailed Methods:
9.1. Preparation:
-
1
The night before, prepare DBG by adding 1mg/ml of anhydrous glucose to DPBS (containing calcium and magnesium). Prepare 4×50mL tubes of the perfusate and warm to 37°C overnight to degas.
-
2
Enucleated human eyes (3 pairs) were obtained from Miracles in Sight/BioSight. For the purposes of this study, we perfused human eyes with death to perfusion time no greater than 24 hours. This time range was selected to be consistent with previous studies from short term perfusions of whole human eyes (Johnson, Johnson et al. 1990, Allingham, de Kater et al. 1992, Ethier and Chan 2001, Camras, Stamer et al. 2012, Ren, Li et al. 2016).
-
3
Place eyes on ice as soon as possible after death.
-
4
Set up perfusion apparatus as per Fig. 2 and Fig 3A, B, and C (set in zero pressure sensor configuration). NOTE: Fig. 3B is set to perfusion configuration). Syringe pump should be off. Check manifolds to clear any bubbles and leaks. If bubbles need to be cleared, ensure manifold is refilled with NaN3. Fill all tubing and reservoirs with DBG as per Fig. 2 and avoid creating any bubbles. NOTE: Push fluid slowly through tubing to prevent bubble formation. If bubbles form at junctions, detach luer-locked tubing, fill, and reattach with fluid-fluid contact. Sherwood et al. (2016) provides a detailed summary as to the perfusion hardware and software set up.
-
5
The eye mounts are funnels on a stabilizing platform (Fig. 1A), with removable inserts to suspend the eyes (Fig. 1B). In collaboration with a local 3D printing company (Touchstone 3D, Cary, NC, USA), we designed and printed the eye mount (see supplementary files 1 and 2 for 3D blueprint). The eye mounts were printed on an SLA printer and were printed from a Formlabs material called Tough 2000. This material is not biocompatible, but it is possible to print our design using biocompatible materials. For the purposes of this new method, a biocompatible material is not necessary as the eye mount is solely used to support the eye and collect the effluent. The eye mounts are designed to accommodate eyes of different sizes that include human, bovine, rabbit and porcine. Fill water bath so that the legs of the eye mounts are submerged by 3cm of water (Fig. 1C, shaded area)
-
6
Place a probe-fitted thermometer inside the water bath chamber so that the probe is in the air and not submerged in the water. The water bath temperature was set so that the humidified air temperature in the chamber is close to 37°C, to warm and prevent drying of the eyes. Keep checking the temperature throughout the experiment and adjust water bath temperature to keep the air temperature around 37°C.
Figure 2: Schematic depicting perfusion setup.
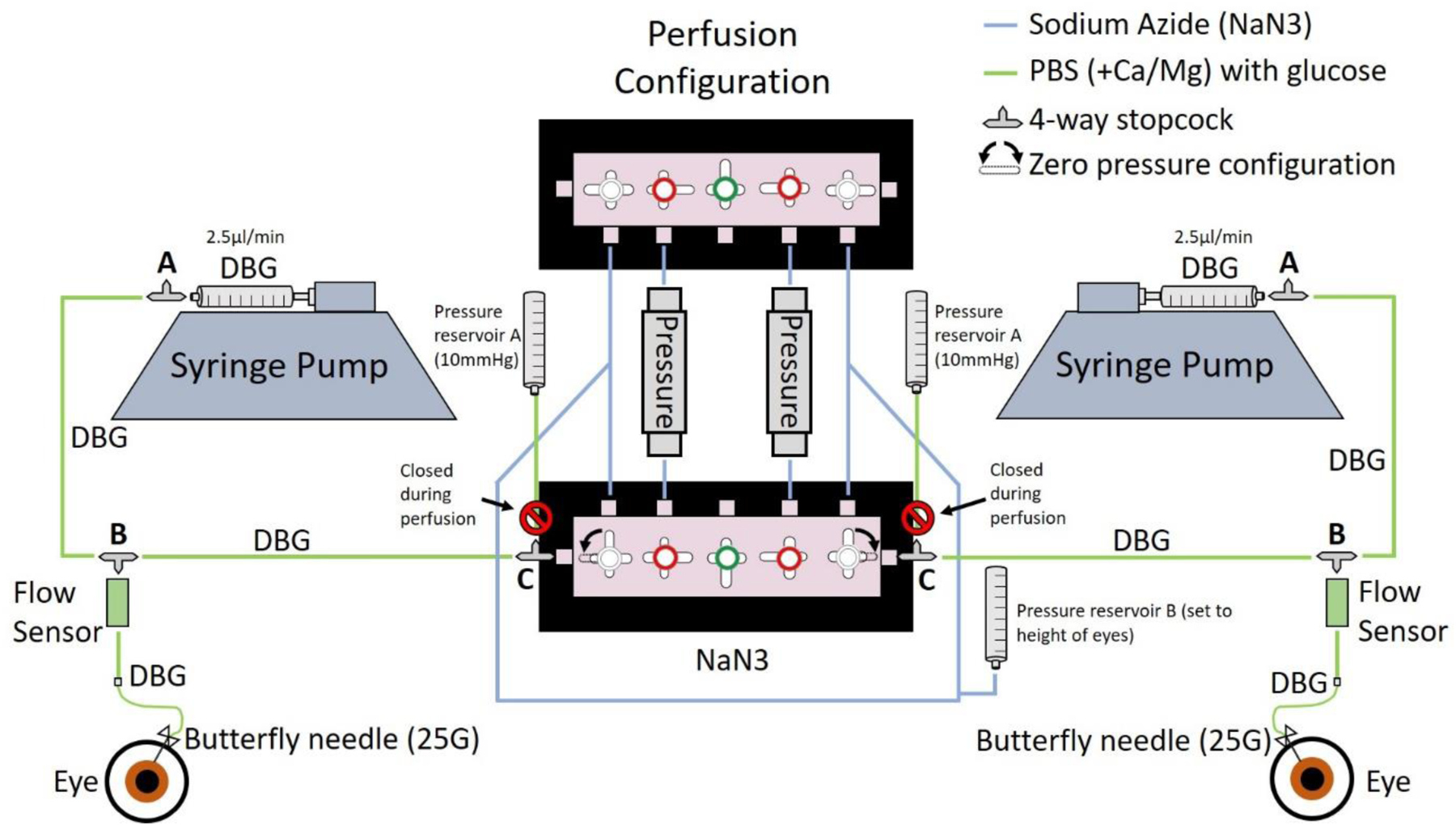
Schematic depicting the perfusion setup. The syringe pump is set to constant flow (2.5μl/min), and each pressure reservoir A (closed to eye during perfusion) is set to 10mmHg (supported by stand and clamps as listed in materials) to re-inflate the eye following aqueous humor evacuation. Pressure reservoir ‘B’ is connected to both the black and the white side of the perfusions system. This reservoir is for zeroing the pressure at the height of the eyes prior to starting the perfusion. Phosphate buffered saline with calcium and magnesium (DBG) is used as perfusate. Sodium azide (NaN3) is a preservative reagent and is used to fill tubing that is not contributing to flow during the perfusion. A 25G butterfly needle is used for cannulation.
Figure 3: Images showing system setup and diagram depicting needle placement.
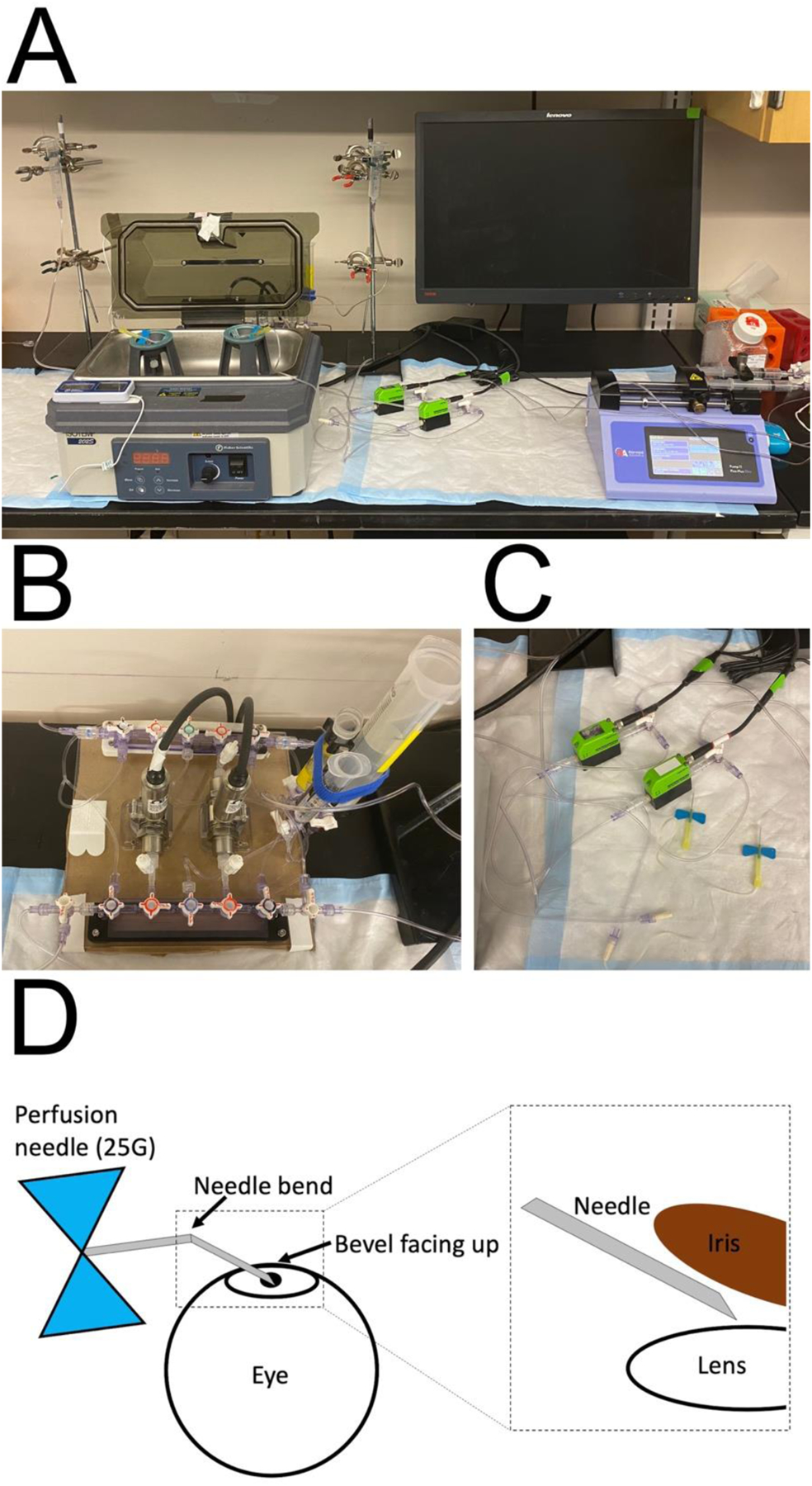
(A) Image showing entire experimental setup. Eye mounts are housed in humidification chamber, tubing is arranged and connected to manifold, flow sensor, reservoirs, and perfusion needles, according to Figure 2. Syringe pump set up is also shown. (B) An image of the manifold setup in perfusion configuration. (C) An image showing the tubing setup connected to the flow sensors. (D) A schematic demonstrating the configuration of the needle prior to cannulation. The needle is bent in the middle at approximately 30°, with bevel facing up. (Inset) Following cannulation, the needle is placed in the posterior chamber, between the iris and the lens, with the bevel facing upward toward the iris.
9.2. Load calibration files and check pressure sensors:
-
7
Open perfusion software by double clicking the desktop icons which will open the W and B projects.
-
8
In each project, double click iPerfuse.
-
9
iPerfuse > Press the right facing arrow below ‘Edit’ in the top left corner to run the code.
-
10
iPerfuse > Setup > Basepath > User, enter your name in the text field. Hit the save button to the right to store this as default.
-
11
Perfuse > Setup > Basepath > Experiment, enter the name of your experiment. All data acquisition for this experiment will be saved in the same folder, separated by day of perfusion.
-
12
iPerfuse > Setup > Basepath > Press AutoName
-
13
iPerfuse > Setup > Pressure Sensor > Load
-
14
Calib_Liquid_Black/White.txt from folder origin (choose relevant one for software)
-
15
iPerfuse > Setup > Pressure Sensor > Save
-
16
iPerfuse > Setup > Pressure Sensor > Run. Pressure should be ~0.1
-
17
iPerfuse > Setup > Flow Sensor > Q Offset. Q-offset should be close to 0
-
18
Press “Run” to stop acquisition.
9.3. Zeroing pressure sensors to height of eyes:
-
19
Set manifolds to perfusion configuration and close off to pressure reservoir A at stopcock C (Fig. 2).
-
20
Fill empty containers with PBS and set in the eye mounts (to prevent wetting the mounts before use). NOTE: the packaging for the filters acts as an effective container here.
-
21
Use plyers to bend the perfusion needle at about halfway the length of the needle, at about a 30-degree angle to allow the needle to sit comfortably on the eye. The bevel should be facing up when cannulating (Fig. 3D).
-
22
Rest needles attached to the system in the PBS bath. You might need to use putty for extra support. Make sure PBS water level is about the same height as limbus of eye.
-
23
iPerfuse > Acquisition > Case > Eye
-
24
iPerfuse > Acquisition > Live
-
25
Check the pressure is zero. If it is not, adjust water level in the zero pressure reservoir until pressure is zero for black and white sides (Fig. 2).
-
26
Remove any PBS in eye mounts and dry.
-
27
Press “Live” to stop acquisition.
9.4. Cannulation:
NOTE: Refer to Fig. 2 and Supp. Video 1.
-
28
Set the eyes on the mounts with pupils facing up (Fig. 1C).
-
29
Place some putty on the edge of the support to use as a support for the needle. Try to keep putty dry.
-
30
iPerfuse > Acquisition > Live
-
31
Close flow from the syringe pump at the 4-way stopcock attached to flow sensor (stopcock B) but keep flow open to manifold and needle.
-
32
Open flow from each pressure reservoir A with stopcock C. Stopcock C should be open in all directions at this stage, so reservoir A is open to pressure and flow sensors.
-
33
Adjust water level (and if necessary, adjust height of the reservoirs with the clamps) of each pressure reservoir A until system pressure is as close to 10mmHg as possible for both white and black side.
-
34
Close flow from pressure reservoir A at stopcock C, and open flow to syringe pump at stopcock B. Stopcock B should be open in all directions at this stage. NOTE: Refer to Fig. 1 and 2
-
35
Remove any PBS in eye mounts and dry.
-
36
Use a 27G needle attached to a 0.5mL syringe to withdraw 200μL of aqueous humor (AH) from the anterior chamber of each eye. Be very careful not to insert the needle into the lens, and only draw the AH. Insert the needle through the center of the cornea. Gently withdraw AH from the anterior chamber, remove the needle, and place AH in a labelled tube. Store AH at −80C until/if needed for analysis.
-
37Then recannulate the eyes with the bent 25G needle (Fig. 1E) attached to the perfusion setup (Fig. 2) as follows:
- Place some putty on the edge of the support to use as a support for the needle. Try to keep putty dry.
- Turn on syringe pump at 10 μL/min to ensure there is DBG flowing through the needle, and then cannulate into the posterior chamber (to prevent anterior chamber deepening) by inserting the needle through the same insertion point in the cornea as used for extracting AH. Use tweezers to stabilize the eye. Set the needle tip between the iris and lens. Be sure the bevel of the needle is facing upwards.
- Tape needle tubing to bath to prevent agitation of the needles when opening and closing the chamber lid.
- Turn off syringe pump
- Close flow from the syringe pump at the 4-way stopcock attached to flow sensor (stopcock B).
- Open flow from each pressure reservoir A (10mmHg) at each stopcock C, which should be open in all directions at this stage. These reservoirs should now be open to pressure and flow sensors and should be set at 10mmHg. This step serves to reinflate the eyes to even pressures following the AH removal.
- Check that the pressures of both eyes are similar are about 10mmHg and wait until flow rate has stabilized (approximately 10 minutes). If there are large fluctuations in flow rates, check for any bubbles in the needles, tubing, or manifolds. If the flow rates are too low (<1 μL/min), check if the needle is being pushed against the iris, thereby blocking flow. If the flow rates are too high (>8 μL/min), check for any leaks in the tubing, needle, or manifolds.
- Close flow from pressure reservoir A at stopcock C, and open flow to syringe pump at stopcock B. Stopcock B should be open in all directions at this point.
9.5. Perfusion:
-
38
Set syringe pump to infuse at 2.5 μL/min and start infusion.
-
39
iPerfuse > Acquisition > Live (to stop Live)
-
40
iPerfuse > Acquisition > Record
-
41
To allow pressure to stabilize within the eye, perfuse eyes initially for 1 hour (see Supp. Fig. 1 for pressure dynamics over time), and then remove any effluent that has collected at the bottom of the funnel (Fig. 1A) and discard. This interval also allows for the effluent to pass through the episcleral veins to reach the surface of the eye, as it would likely be contaminated with blood (Sit, Gong et al. 1997).
-
42
The effluent exits via the episcleral veins and runs down the side of the eye (Fig. 1E) and pools at the tip of the funnel (Fig. 1A). During the perfusion, gently open the chamber lid and use a transfer pipette to remove effluent at 2H, 3H, and 4H mark by gently lowering the transfer pipette between the eye and the wall of the eye mount until the tip of the transfer pipette contacts the effluent. Put effluent in labelled tubes and store at −80C until needed. For reference, see Supp. Video 2.
-
43
After the perfusion is complete, click iPerfuse > Acquisition > Record – to stop recording. Pressure was continuously monitored to calculate outflow facility (Fig. 4B)
Figure 4: Effluent during whole eye perfusions contains abundant levels of protein.
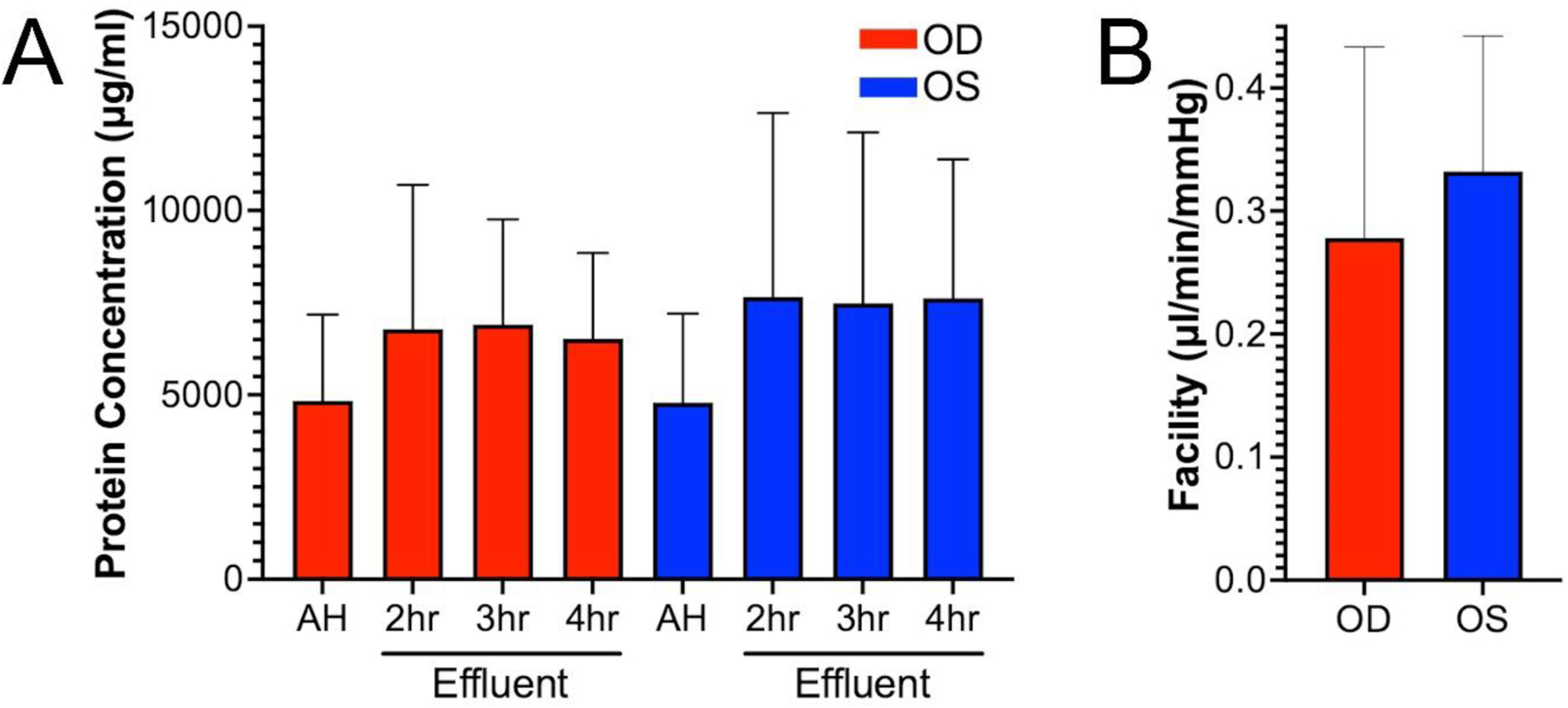
(A) Protein concentration in the effluent samples collected from 3 pairs of human donor eyes was determined. For AH, protein concentration ranged from 2500 to 7400μg/ml for all eyes. For effluent, protein concentration ranged from 2700 to 10,000μg/ml for all eyes and timepoints. N=3. (B) Facility was calculated by averaging the pressure readings for each eye over a 3-hour period (between 1 and 4hrs). Then we used the constant flow rate (2.5μl/min) to calculate average facility over that time-period. Facilities were 0.28±0.16 and 0.33±0.11μl/minute/mmHg for OD and OS, respectively (mean±SD, n=3).
9.6. End of Day
-
44
Remove needles from globes, dispose of in sharps
-
45
If fixation is required, make a small incision at the equator of each eye and submerge eyes in 4% PFA overnight at 4°C.
NOTE: Change to PBS the day after and store at 4°C until ready to process tissue.
-
46
Empty all fixative into waste bottles and throw away tubing and plastics.
-
47
Drain eye baths and clean with 70% ethanol.
-
48
Rinse eye mounts with DI water and clean with 70% ethanol.
9.7. Effluent quality control:
A number of different types of proteins and molecules are secreted by the TM and deposited into the effluent, such as MMP-1, −2, −3, −9 (Samples, Alexander et al. 1993, Pang, Hellberg et al. 2003), TIMP-1 (Pang, Hellberg et al. 2003), MYOC (Tawara, Okada et al. 2000), connective tissue growth factor (Tomarev, Wistow et al. 2003) interleukin-6 (Liton, Luna et al. 2005), transforming growth factor-beta 1 (Tripathi, Li et al. 1993), prostaglandin E2 (Uchida, Shimizu et al. 2021, Uchida, Shimizu et al. 2021), and adenosine (Wu, Li et al. 2012). Furthermore, Yun et al. (1989) used immunofluorescence to show that the TM also secretes major constituents of the ECM including collagens (types I, III, IV, V and VI), laminin, fibronectin, and basement membrane-associated proteoglycans. So, to confirm fluid collected from the human eyes was effluent and not derived from condensation or another unintended source, we checked three parameters. Importantly, we used protein lysate from cultured TM cells treated with dexamethasone (not from perfused human eyes used in this experiment) only as a positive control (for MYOC) and negative control (for MMP) for the purposes of this experiment.
9.7.1. Protein concentration:
To monitor protein content in AH effluent from the 3 pairs of human donor eyes, we performed a BCA assay (Pierce™ BCA Protein Assay Kit, catalog number 23227) (Fig. 4A). For AH, protein concentration ranged from 2500 to 7400μg/ml for all eyes. For effluent, protein concentration ranged from 2700 to 10,000μg/ml for all eyes and timepoints. N=3. For OD eyes, AH protein concentration (4841 ± 2340μg/ml) was not significantly different to effluent at 2H (6783 ± 3915μg/ml, p=0.59), 3H (6913 ± 2850μg/ml, p=0.47) or 4H (6523 ± 2329μg/ml, p=0.43). For OS eyes, AH protein concentration (4796 ± 2411μg/ml) was also not significantly different to effluent at 2H (7659 ± 4985μg/ml, p=0.52), 3H (7487 ± 4630μg/ml, p=0.52) or 4H (7621 ± 3765μg/ml, p=0.45). These data suggest that the effluent samples collected were protein rich.
9.7.2. MYOC expression:
MYOC is secreted into the AH via several ocular tissues including ciliary body (Adam, Belmouden et al. 1997, Ortego, Escribano et al. 1997) and iris (Ortego, Escribano et al. 1997). MYOC is further secreted by the TM into effluent (Tawara, Okada et al. 2000, Fingert, Stone et al. 2002, Chowdhury, Madden et al. 2010). So, we checked to see if MYOC was present in our AH and effluent samples (Fig. 5A and C). To test this, we performed gel electrophoresis as previously described (De Ieso, Kuhn et al. 2022). AH and effluent samples were diluted to a protein concentration of 1mg/ml in 1X laemmli sample buffer with 100mM of dithiothreitol. Protein lysates were boiled for 5 min and 20μg of protein was loaded per well in 12% TGX Stain-Free polyacrylamide gels. Primary antibodies were diluted in 5% BSA in TBS-T, and blots were incubated in primary antibody solution at 4°C overnight, on a rocker. Rabbit polyclonal anti-MYOC primary antibody (homemade; 1/2500) was used to detect MYOC. We normalized to total protein using TGX Stain-Free™ FastCast™ Acrylamide Starter Kit, 12% (catalog number 1610184; Biorad). Dexamethasone-treated TM cell lysate (human TM136) was used as a positive control for MYOC. MYOC was prominent in the positive control, in the AH, and effluent from all samples and time points tested. The concentration of MYOC in the effluent samples was less than AH samples. We conclude this is due to the anterior chamber exchange of AH with protein-free DBG, which limited the contribution of MYOC in the effluent to conventional outflow cells/tissues.
Figure 5: MYOC and MMP2 proteins are actively secreted by conventional outflow cells into effluent during whole eye perfusions.
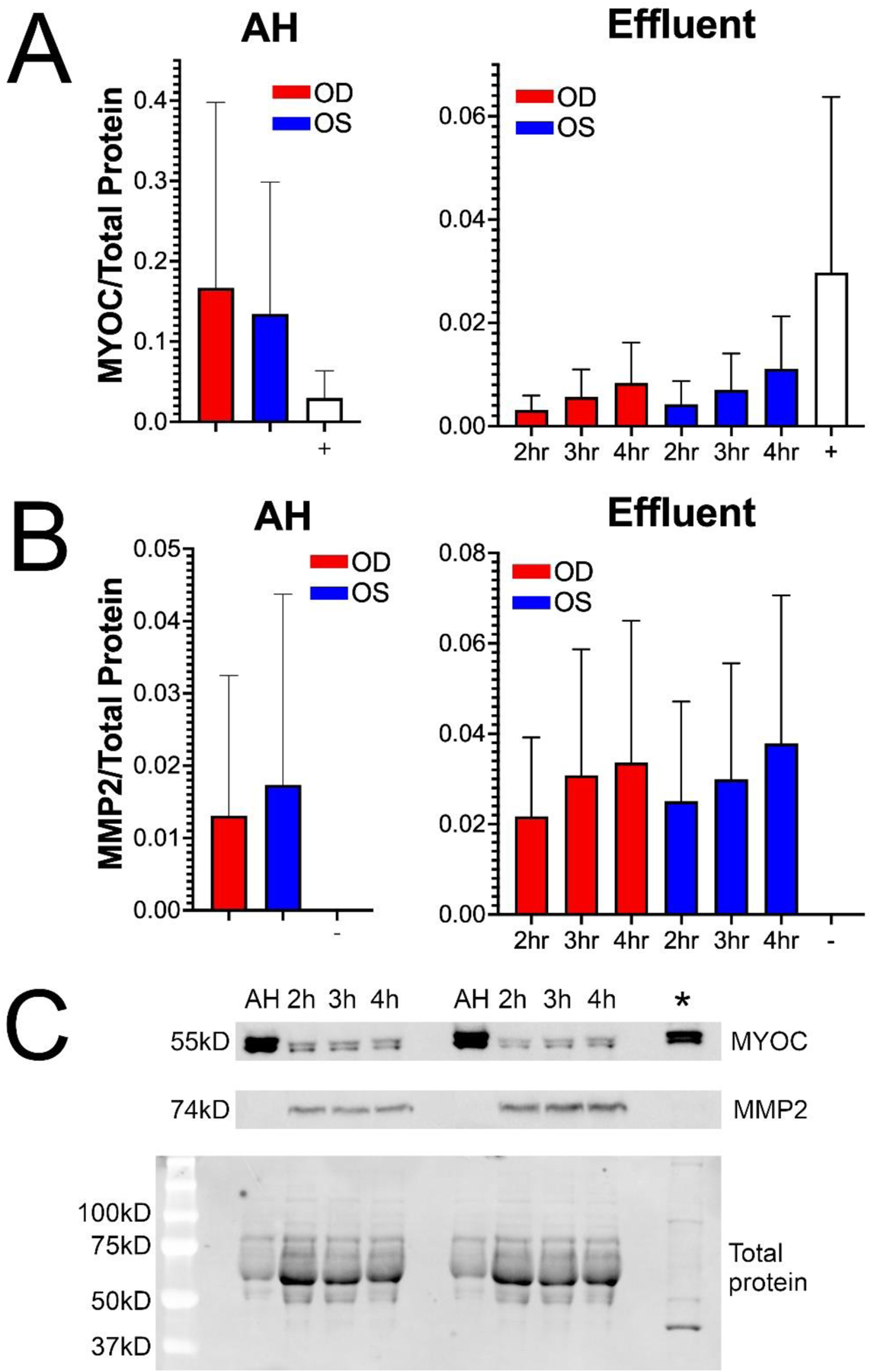
(A) MYOC protein was present in all samples and timepoints as determined by western blot (n=3). As a positive control for MYOC (+), lysates were prepared from dexamethasone-treated cultured human TM cells. (B) MMP2 protein was abundant in effluent at all time points, and trace amounts of MMP2 was present in AH. There was no detectable MMP2 in the dexamethasone-treated cultured human TM cell lysate (n=3). Dexamethasone-treated cultured human TM cell lysate was used as a negative control for MMP2 (−). NOTE: Dexamethasone-treated cultured human TM cell lysate was not from perfused eyes in this experiment. (C) Representative blots from A and B. Total protein analysis was used for normalization.
9.7.3. MMP2 expression:
The TM secretes MMP2 into the AH as it flows through the conventional outflow pathway and exits as effluent (Samples, Alexander et al. 1993), so MMP2 should also be expressed in the effluent samples. Therefore, we performed gel electrophoresis (as described above) to monitor MMP2 levels in the AH and effluent (Fig. 5B and C). Dexamethasone-treated TM cell lysate (human TM136) was used as a negative control for MMP2 as MMP2 is a secreted protein and its expression in cell lysate is downregulated by dexamethasone (Mohd Nasir, Agarwal et al. 2020). We normalized to total protein (as described above). Rabbit monoclonal anti-MMP2 primary antibody (catalog number 87809s; 1/1000; Cell Signaling Technology) was used to detect MMP2. As expected, MMP2 expression was absent in the dexamethasone-treated TM cell lysate. Presence of MMP2 was not detected in AH from 1 pair of human eyes and was detected at low levels in the other 2 pairs of eyes. MMP2 was abundant in all effluent samples from different donor pairs and timepoints, likely deposited by the TM and other conventional outflow pathway cells.
Thus, we showed that the effluent samples were protein rich, and expressed proteins that are known to be actively secreted by the TM including MYOC and MMP2 in perfused eyes.
We measured facility of all eyes to confirm outflow tissues were viable for the length of the experiment (Fig. 4B). Facility was 0.28 ± 0.16μl/min/mmHg for OD eyes and 0.33 ± 0.11μl/min/mmHg for OS eyes (n=3). These facility values are in the normal range for older human eyes, and were similar to previously published ranges for human eyes (Erickson-Lamy, Schroeder et al. 1990, Erickson-Lamy, Rohen et al. 1991, Acott, Kelley et al. 2014, Cha, Xu et al. 2016, Kazemi, McLaren et al. 2018, McDonnell, Dismuke et al. 2018). Thus, these data show that the eyes remained physiologically viable for the length of the experiment. We used eyes with death to perfusion time no greater than 24 hours, and outflow facility within a normal range (0.2– 0.5 μl/min/mmHg) as an endpoint indicator for viable tissue. An additional quality control step may include tissue morphology by light and/or electron microscopy.
9.8. Statistics:
We used paired t-tests to determine statistical significance. Data is presented as mean ± SD. Significance defined as p<0.05. N=3 for all datasets.
10. Potential Pitfalls and Troubleshooting:
Taken together, our model provides a reliable method to collect effluent from ex vivo human eyes without the use of silicone oil, while maintaining whole globe integrity.
10.1. The advantages of this model include:
10.1.1. Simplicity of experimental setup and effluent collection:
The experimental setup for this novel method is quick and straightforward. For the anterior segment organ culture model, the human whole globe donor eyes must first be carefully dissected, and the anterior segment must then be clamped onto the mount underneath a clamping ring that must create a watertight seal, while maintaining even pressure applied to the sclera. These extra steps are time consuming and introduce a higher risk for error. Our model does not require any prior dissection, and the whole eye can be set in the eye mount and remain stable without the use of clamps or gauze. There is also minimal risk for leakage, which can potentially occur if the clamp ring is not set properly in the anterior segment organ culture model. Furthermore, the silicone oil whole globe model presents higher risk for effluent contamination with oil, and higher risk for effluent volume loss in the collection process. It is also technically challenging to collect effluent mixed in silicone oil. For our model, risk of effluent contamination is low, and the effluent collection method is easy and maximizes the amount of effluent that can be collected as the effluent samples are isolated and restricted to the small space within the funnel tip.
10.1.2. Minimal mechanical stress on outflow tissues:
In the anterior segment organ culture model, dissection of the tissue involves separation of the anterior segment and removal of lens, iris, and any leftover pigment. While there are advantages to this method, there is an increased risk for tissue damage or stress during the dissection and clamping process. This is particularly relevant since dissection and clamping of eye pairs must be very similar for interpretable results. Without the need to perform any prior dissection (except for the removal of connective tissue for non-human eyes) or mechanical clamping of the tissue to the mount, our new model for effluent collection presents a method with reduced risks for tissue damage or stress when preparing the eyes for the perfusion.
10.1.3. Maintenance of whole globe integrity and physiological environment:
The anterior segment organ culture model does not allow for maintenance of whole globe integrity, with loss of structures such as the lens, iris, and posterior segment. Maintaining whole globe integrity is important to reduce stress on the conventional outflow tissues and to maintain an experimental environment similar to in vivo conditions. Moreover, while the silicone oil whole globe model allows for maintenance of whole globe integrity, it does not facilitate an experimental environment that mimics physiological conditions as the globes are submerged in non-physiological silicone oil. Our model allows for maintenance of whole globe integrity in addition to maintenance of a physiologically similar environment to in vivo conditions. The humidified and temperature-controlled chamber keeps the eyes moist and warm as they would be in vivo, without having to submerge the eyes in oil.
10.1.4. Adaptability:
Our novel method can be easily adapted to accommodate constant pressure perfusions instead of constant flow, for example, if a researcher wished to investigate the change in expression of a particular protein in the effluent following high and low IOP conditions. Furthermore, by performing dual cannulation, anterior chamber exchanges can be performed to perfuse drug, fluorescent microbeads, or fixative. In addition to human, the eye mounts are also compatible with bovine, rabbit, and porcine eyes. Our method has been used in a recent publication, where Kelly et al. (2023) performed dual cannulation for drug exchange, perfusing the anterior chamber of one pig eye with L-NAME and the contralateral eye with control perfusate. The perfusion was performed at constant pressure for 3 hours following a one-hour acclimation. In this study, Kelly et al. analyzed the effluent and found significantly lower nitrite levels in the L-NAME treated eye over time, demonstrating the utility and adaptability of our model and emphasizing that the cells are alive and responsive to drugs such as L-NAME. Thus, the adaptability of our new model ensures broad utility for the system, and for information that can be obtained from the effluent.
10.2. The limitations of this model include:
10.2.1. Shorter experimental duration:
An advantage of the anterior segment organ culture model is that the highly vascular tissues are removed. The majority of the remaining tissue is avascular, such as the TM and cornea, so the duration of a given experiment can be longer as the avascular tissues receive sufficient sustenance from the perfusate to stay viable. The disadvantage of any whole globe perfusion protocol is that the experimental duration lasts over hours rather than days, as the vascular tissues such as the ciliary body and iris break down over time. Since changes in protein expression, secretion, or activation in response to a treatment can take longer than 4 hours, this method is limited to changes that can occur within a 4-hour timeframe.
10.2.2. Loss of protein:
We showed that effluent collected with our model contained high amounts of total protein and we were also able to detect significant amounts of secreted proteins from the outflow pathway. However, as effluent is collected in a funnel after it runs down the side of the eye, there is a risk of protein loss due non-specific sticking to the ocular surface.
Supplementary Material
Acknowledgements:
This work was supported by National Institutes of Health Grants R01EY028608, R01EY022359, P30EY005722 and Research to Prevent Blindness, Inc. The authors thank Dr. Joseph Sherwood and Dr. Fiona McDonnell for helpful discussion regarding the experimental procedures and data analyses; and Broadwing Bio for discussions about utility of custom chamber to accurately collect effluent from perfused human eyes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest:
None
References:
- Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M and Bradley JM (2014). “Intraocular pressure homeostasis: maintaining balance in a high-pressure environment.” Journal of Ocular Pharmacology and Therapeutics 30(2–3): 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam MF, Belmouden A, Binisti P, Brézin AP, Valtot F, Béchetoille A, Dascotte J-C, Copin B, Gomez L and Chaventré A (1997). “Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma.” Human molecular genetics 6(12): 2091–2097. [DOI] [PubMed] [Google Scholar]
- Alexander JP, Samples JR, Van Buskirk EM and Acott TS (1991). “Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork.” Investigative ophthalmology & visual science 32(1): 172–180. [PubMed] [Google Scholar]
- Allingham RR, de Kater AW, Ethier CR, Anderson PJ, Hertzmark E and Epstein DL (1992). “The relationship between pore density and outflow facility in human eyes.” Investigative ophthalmology & visual science 33(5): 1661–1669. [PubMed] [Google Scholar]
- Bahler CK, Fautsch MP, Hann CR and Johnson DH (2004). “Factors influencing intraocular pressure in cultured human anterior segments.” Investigative ophthalmology & visual science 45(9): 3137–3143. [DOI] [PubMed] [Google Scholar]
- Camras LJ, Stamer WD, Epstein D, Gonzalez P and Yuan F (2012). “Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes.” Investigative ophthalmology & visual science 53(9): 5242–5250. [DOI] [PubMed] [Google Scholar]
- Cha ED, Xu J, Gong L and Gong H (2016). “Variations in active outflow along the trabecular outflow pathway.” Exp Eye Res 146: 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury UR, Madden BJ, Charlesworth MC and Fautsch MP (2010). “Proteome analysis of human aqueous humor.” Investigative ophthalmology & visual science 51(10): 4921–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comes N and Borrás T (2007). “Functional delivery of synthetic naked siRNA to the human trabecular meshwork in perfused organ cultures.” Mol Vis 13: 1363–1374. [PubMed] [Google Scholar]
- Comes N, Buie LK and Borrás T (2011). “Evidence for a role of angiopoietin‐like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma.” Genes to Cells 16(2): 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groef L, Van Hove I, Dekeyster E, Stalmans I and Moons L (2013). “MMPs in the trabecular meshwork: promising targets for future glaucoma therapies?” Investigative ophthalmology & visual science 54(12): 7756–7763. [DOI] [PubMed] [Google Scholar]
- De Ieso ML, Kuhn M, Bernatchez P, Elliott MH and Stamer WD (2022). “A Role of Caveolae in Trabecular Meshwork Mechanosensing and Contractile Tone.” Frontiers in Cell and Developmental Biology: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Lamy K, Rohen JW and Grant WM (1991). “Outflow facility studies in the perfused human ocular anterior segment.” Experimental eye research 52(6): 723–731. [DOI] [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder A, Bassett-Chu S and Epstein D (1990). “Absence of time-dependent facility increase (washout) in the perfused enucleated human eye.” Investigative ophthalmology & visual science 31(11): 2384–2388. [PubMed] [Google Scholar]
- Ethier CR and Chan DW-H (2001). “Cationic ferritin changes outflow facility in human eyes whereas anionic ferritin does not.” Investigative ophthalmology & visual science 42(8): 1795–1802. [PubMed] [Google Scholar]
- Fingert JH, Stone EM, Sheffield VC and Alward WL (2002). “Myocilin glaucoma.” Survey of ophthalmology 47(6): 547–561. [DOI] [PubMed] [Google Scholar]
- Flammer J and Mozaffarieh M (2007). “What is the present pathogenetic concept of glaucomatous optic neuropathy?” Survey of ophthalmology 52(6): S162–S173. [DOI] [PubMed] [Google Scholar]
- Hase K, Kase S, Kanda A, Shinmei Y, Noda K and Ishida S (2021). “Expression of vascular endothelial growth factor-C in the trabecular meshwork of patients with neovascular glaucoma and primary open-angle glaucoma.” Journal of clinical medicine 10(13): 2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DH and Tschumper RC (1987). “Human trabecular meshwork organ culture. A new method.” Investigative ophthalmology & visual science 28(6): 945–953. [PubMed] [Google Scholar]
- Johnson M, Johnson DH, Kamm RD, DeKater AW and Epstein DL (1990). “The filtration characteristics of the aqueous outflow system.” Experimental eye research 50(4): 407–418. [DOI] [PubMed] [Google Scholar]
- Kazemi A, McLaren JW, Kopczynski CC, Heah TG, Novack GD and Sit AJ (2018). “The effects of netarsudil ophthalmic solution on aqueous humor dynamics in a randomized study in humans.” Journal of Ocular Pharmacology and Therapeutics 34(5): 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RA, McDonnell FS, De Ieso ML, Overby DR and Stamer WD (2023). “Pressure Clamping During Ocular Perfusions Drives Nitric Oxide-Mediated Washout.” Investigative Ophthalmology & Visual Science 64(7): 36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Luna C, Bodman M, Hong A, Epstein DL and Gonzalez P (2005). “Induction of IL-6 expression by mechanical stress in the trabecular meshwork.” Biochemical and biophysical research communications 337(4): 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell F, Dismuke WM, Overby DR and Stamer WD (2018). “Pharmacological regulation of outflow resistance distal to Schlemm’s canal.” American Journal of Physiology-Cell Physiology 315(1): C44–C51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell F, Perkumas KM, Ashpole NE, Kalnitsky J, Sherwood JM, Overby DR and Stamer WD (2020). “Shear stress in schlemm’s canal as a sensor of intraocular pressure.” Scientific Reports 10(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Nasir NA, Agarwal R, Krasilnikova A, Sheikh Abdul Kadir SH and Iezhitsa I (2020). “Effect of dexamethasone on the expression of MMPs, adenosine A1 receptors and NFKB by human trabecular meshwork cells.” Journal of basic and clinical physiology and pharmacology 31(6): 20190373. [DOI] [PubMed] [Google Scholar]
- Ortego J, Escribano J and Coca-Prados M (1997). “Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin.” FEBS letters 413(2): 349–353. [DOI] [PubMed] [Google Scholar]
- Overby DR, Stamer WD and Johnson M (2009). “The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium.” Exp Eye Res 88(4): 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang I-H, Hellberg PE, Fleenor DL, Jacobson N and Clark AF (2003). “Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells.” Investigative ophthalmology & visual science 44(8): 3485–3493. [DOI] [PubMed] [Google Scholar]
- Perkumas K, Hoffman E, McKay B, Allingham RR and Stamer W (2007). “Myocilin-associated exosomes in human ocular samples.” Experimental eye research 84(1): 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruccio EM, Rowlette LLS, Comes N, Locatelli-Hoops S, Notari L, Becerra SP and Borrás T (2008). “Dexamethasone increases pigment epithelium-derived factor in perfused human eyes.” Current eye research 33(5–6): 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveen K, Patel GC, Gurski L, Ayer AH, Persaud T, Still MD, Miloscio L, Van Zyl T, Di Gioia SA and Brumpton B (2022). “ANGPTL7, a therapeutic target for increased intraocular pressure and glaucoma.” Communications biology 5(1): 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Torres E, De Ieso ML, Pasquale LR, Madekurozwa M, van Batenburg-Sherwood J, Overby DR and Stamer WD (2021). “The vital role for nitric oxide in intraocular pressure homeostasis.” Progress in retinal and eye research 83: 100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Li G, Le TD, Kopczynski C, Stamer WD and Gong H (2016). “Netarsudil Increases Outflow Facility in Human Eyes Through Multiple Mechanisms.” Invest Ophthalmol Vis Sci 57(14): 6197–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples JR, Alexander JP and Acott TS (1993). “Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin-1 and dexamethasone.” Investigative ophthalmology & visual science 34(12): 3386–3395. [PubMed] [Google Scholar]
- Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B and Overby DR (2016). “Measurement of outflow facility using iPerfusion.” PLoS One 11(3): e0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit AJ, Gong H, Ritter N, Freddo TF, Kamm R and Johnson M (1997). “The role of soluble proteins in generating aqueous outflow resistance in the bovine and human eye.” Experimental eye research 64(5): 813–821. [DOI] [PubMed] [Google Scholar]
- Spiga M-G and Borrás T (2010). “Development of a gene therapy virus with a glucocorticoid-inducible MMP1 for the treatment of steroid glaucoma.” Investigative ophthalmology & visual science 51(6): 3029–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER (2009). “The trabecular meshwork outflow pathways: structural and functional aspects.” Exp Eye Res 88(4): 648–655. [DOI] [PubMed] [Google Scholar]
- Tawara A, Okada Y, Kubota T, Suzuki Y, Taniguchi F, Shirato S, Nguyen TD and Ohnishi Y (2000). “Immunohistochemical localization of MYOC/TIGR protein in the trabecular tissue of normal and glaucomatous eyes.” Current Eye Research 21(6): 934–943. [DOI] [PubMed] [Google Scholar]
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T and Cheng C-Y (2014). “Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis.” Ophthalmology 121(11): 2081–2090. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Wistow G, Raymond V, Dubois S and Malyukova I (2003). “Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis.” Investigative Ophthalmology & Visual Science 44(6): 2588–2596. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Borisuth N and Tripathi BJ (1993). “Trabecular cells of the eye express messenger RNA for transforming growth factor-beta 1 and secrete this cytokine.” Investigative ophthalmology & visual science 34(8): 2562–2569. [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WA and Tripathi BJ (1994). “Aqueous humor in glaucomatous eyes contains an increased level of TGF-β2.” Experimental eye research 59(6): 723–728. [DOI] [PubMed] [Google Scholar]
- Uchida T, Shimizu S, Yamagishi R, Tokuoka SM, Kita Y, Honjo M and Aihara M (2021). “Mechanical stretch induces Ca2+ influx and extracellular release of PGE2 through Piezo1 activation in trabecular meshwork cells.” Scientific Reports 11(1): 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Shimizu S, Yamagishi R, Tokuoka SM, Kita Y, Sakata R, Honjo M and Aihara M (2021). “TRPV4 is activated by mechanical stimulation to induce prostaglandins release in trabecular meshwork, lowering intraocular pressure.” Plos one 16(10): e0258911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li G, Luna C, Spasojevic I, Epstein DL and Gonzalez P (2012). “Endogenous production of extracellular adenosine by trabecular meshwork cells: potential role in outflow regulation.” Investigative Ophthalmology & Visual Science 53(11): 7142–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun A, Murphy C, Polansky J, Newsome D and Alvarado J (1989). “Proteins secreted by human trabecular cells. Glucocorticoid and other effects.” Investigative ophthalmology & visual science 30(9): 2012–2022. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


