Abstract
Smad family proteins play a pivotal role in transmitting the transforming growth factor-β (TGF-β) superfamily signals from the cell surface to the nucleus. In response to ligand stimulation, Smad4 forms a complex with respective receptor-specific Smads, and the complex translocates into the nucleus and regulates gene expression. Thus, the nuclear entry of the Smad complex is one of the key steps in signal transduction. However, little is known about regulatory mechanisms for nucleocytoplasmic transport of Smads. Here we report identification of a functional, leucine-rich nuclear export signal (NES) in Smad4, which regulates subcellular distribution of Smad4. We then show evidence suggesting that the NES-dependent cytoplasmic localization of Smad4 is important for ensuring optimal TGF-β responsivenesses in transcriptional activation. Moreover, we show that the NES of Smad4 is specifically inactivated by the stimulus-dependent hetero-oligomerization with receptor-specific Smads during the TGF-β-induced nuclear translocation of Smad4. Taken together, these results suggest an important regulatory role of the NES of Smad4 in TGF-β signaling.
INTRODUCTION
Members of the transforming growth factor-β (TGF-β) superfamily play important regulatory roles in cell proliferation, cell differentiation and various developmental processes (Massagué, 1998; Whitman, 1998), and alterations in the activity of those factors that are involved in signaling pathways underlie several human developmental defects, hyperproliferative disorders and various forms of cancer (Heldin et al., 1997; Massagué, 1998). Signals from TGF-β family members are transduced to the nucleus by members of a family of intracellular proteins called Smads (Heldin et al., 1997; Massagué, 1998; Whitman, 1998; Zhang and Derynck, 1999; Attisano and Wrana, 2000). In response to ligand stimulation, receptor-specific Smads are phosphorylated directly by specific type I receptor kinases (Macias-Silva et al., 1996; Zhang et al., 1996; Kretzschmar et al., 1997; Nakao et al., 1997) and form activated complexes with a common mediator Smad, called Smad4 (Lagna et al., 1996; Zhang et al., 1997), a tumor-suppressor gene product (Hahn et al., 1996). These complexes translocate into the nucleus where they regulate transcription of target genes (Zhang and Derynck, 1999; Attisano and Wrana, 2000). Thus, the nuclear entry of the Smad complex is one of the key steps in signal transduction. Recently, SARA (for Smad anchor for receptor activation) was identified as a Smad-interacting protein that facilitates the access of receptor-specific Smads to activated TGF-β receptor bridging the receptor and Smad2/3 (Tsukazaki et al., 1998). Upon stimulation the resulting phosphorylation causes the release of Smad2/3 from SARA, allowing Smad to the nucleus (Tsukazaki et al., 1998). It has also been reported that Smads associate with microtubule cytoskeleton in the absence of signaling (Dong et al., 2000). However, little is known about the molecular mechanisms mediating the nuclear translocation of Smads in response to TGF-β.
Here we report identification of a functional, leucine-rich nuclear export signal (NES) (Gerace, 1995; Nigg, 1997; Mattaj and Englmeier, 1998) in Smad4, which regulates nuclear transport of Smad4 and thereby controls Smad-dependent transcription. Moreover, we present evidence suggesting that the stimulus-dependent nuclear transport of Smad4 involves specific inactivation of the NES of Smad4, which is induced by its hetero-oligomerization with receptor-specific Smads.
RESULTS AND DISCUSSION
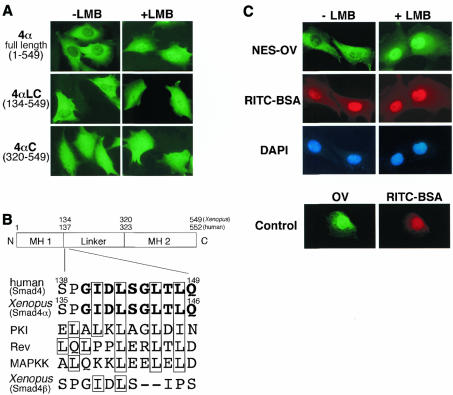
We previously identified two Smad4 proteins in Xenopus, a Xenopus ortholog of Smad4, XSmad4α, and another Smad4-like molecule, XSmad4β (Masuyama et al., 1999). When overexpressed in cultured cells, XSmad4α was present exclusively in the cytoplasm [see Figure 1A, 4α full length (1–549), –LMB], whereas XSmad4β was predominantly nuclear (Masuyama et al., 1999). We treated the cells with leptomycin B (LMB), a specific inhibitor of the NES-dependent intracellular transport (Fornerod et al., 1997; Fukuda et al., 1997; Ossareh-Nazari et al., 1997; Wolff et al., 1997). The LMB treatment induced rapid nuclear accumulation of XSmad4α [Figure 1A, 4α full length (1–549), +LMB]. Human Smad4 behaved identically; the expressed human Smad4 was completely cytoplasmic in the absence of LMB and became nuclear after LMB treatment (see Figure 2A, WT). In contrast, the subcellular localization of XSmad4β did not change after LMB treatment (data not shown). To narrow down the region responsible for LMB sensitivity, we constructed two N-terminal deleted forms of XSmad4α, 4αLC (residues 134–549) and 4αC (residues 320–549). As shown in Figure 1A, in response to LMB treatment the location of 4αLC was changed from the cytoplasm to the nucleus, whereas the location of 4αC was unchanged: predominantly nuclear irrespective of the LMB treatment. Thus, an LMB-sensitive NES sequence appeared to reside in residues 134–320, a linker region (see Figure 1B). The C-terminal portion of the linker region was recently shown to contain sequences required for the activation of Smad-dependent transcriptional responses (de Caestecker et al., 2000). We found a putative leucine-rich NES sequence in the N-terminal portion of the linker region in Smad4 (XSmad4α) (Figure 1B), which was absent from XSmad4β (Figure 1B) and other Smad proteins (not shown). To test whether this sequence functions as an NES, a peptide (140GIDLSGLTLQ149) was cross-linked to ovalbumin (OV) and the resultant conjugate (termed NES-OV) was injected into the nuclei of cells (Figure 1C). Within 30 min after injection, almost all the NES-OV was exported from the nucleus, whereas co-injected RITC–BSA (tetramethyl rhodamine isothiocyanate–bovine serum albumin) remained in the nucleus. The nuclear export of NES-OV was completely inhibited by LMB. It was also confirmed that OV without NES was not exported from the nucleus (Figure 1C).
Fig. 1. A leucine-rich NES in Smad4. (A) C2C12 cells were transfected with Myc-tagged XSmad4α, 4αLC or 4αC. At 12 h after transfection, cells were treated with or without LMB (2 ng/ml) for 1 h, then fixed and stained with anti-Myc antibody. (B) Schematic representation of the primary structure of Smad4 showing a putative NES sequence in the linker region (residues 138–149 in human Smad4 and residues 135–146 in Xenopus Smad4α). The sequence is absent from XSmad4β (see the bottom). The NES sequences of MAPKK, PKI and Rev are aligned. Important hydrophobic residues in the sequences are boxed. (C) A putative NES sequence in human Smad4 can function as NES. Ovalbumin (OV) conjugated with a putative NES peptide (residues 140–149) (NES-OV) was co-injected with RITC-BSA into the nuclei of rat fibroblastic 3Y1 cells in the absence or presence of LMB (2 ng/ml). OV was injected into the nuclei of cells together with RITC-BSA in the absence of LMB as a control. At 30 min after injection, cells were fixed and stained with anti-OV antibody. LMB was added 30 min before injection.
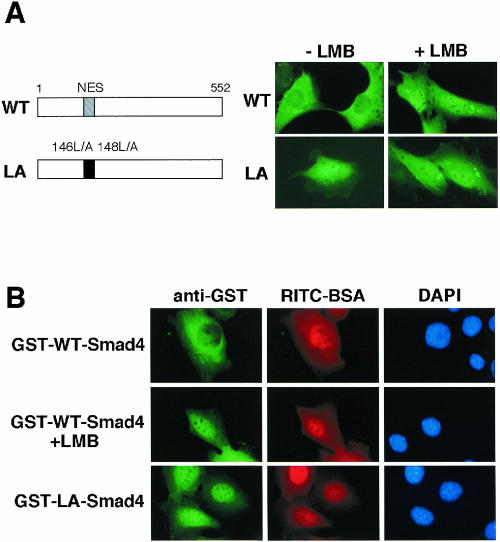
Fig. 2. Regulation of subcellular distribution of Smad4 by its NES. (A) Expression of wild-type (WT) Smad4 and LA-Smad4 (Leu146 and Leu148 are replaced by Ala) in C2C12 cells. C2C12 cells were transiently transfected with plasmids encoding a Myc-tagged form of WT-Smad4 or LA-Smad4. At 12 h after transfection, cells were treated with or without LMB (2 ng/ml) for 1 h, and stained with anti-Myc antibody. (B) Nuclear export of Smad4. A GST fusion WT Smad4 protein (GST–WT-Smad4) or a GST fusion LA-Smad4 protein (GST–LA-Smad4) was co-injected with RITC–BSA into the nucleus of C2C12 cells. The cells were incubated for 30 min in the absence or presence (in the case of GST–WT-Smad4) of LMB (2 ng/ml), and then fixed and stained with anti-GST antibody.
We then constructed a mutant form of human Smad4 (LA-Smad4) in which presumably critical hydrophobic residues in the putative NES sequence (146Leu and 148Leu, see Figure 2A) were replaced by alanines to disrupt NES. When expressed in C2C12 cells, wild-type (WT) Smad4 localized exclusively in the cytoplasm, whereas LA-Smad4 localized primarily in the nucleus even in the absence of LMB (Figure 2A). We then produced glutathione S-transferase (GST) fusion forms of WT Smad4 and LA-Smad4 in bacteria, purified them, and injected them into the nucleus of C2C12 cells. At 30 min after injection, the injected GST–WT Smad4 was completely exported from the nucleus to the cytoplasm, whereas the injected GST–LA-Smad4 remained in the nucleus (Figure 2B, GST–WT-Smad4 and GST–LA-Smad4). In the presence of LMB, the nuclear export of the nuclear injected GST–WT Smad4 was completely suppressed (Figure 2B, GST–WT-Smad4, +LMB). Thus, the putative NES sequence identified in the linker region of Smad4 is a functional NES and determines the cytoplasmic localization of Smad4.
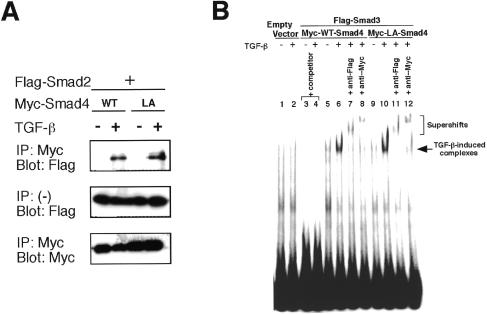
To determine whether LA-Smad4 can interact with receptor-specific Smads, we analyzed the association of Smad2 or Smad3 with LA-Smad4. As shown in Figure 3A, like WT Smad4, LA-Smad4 formed a complex with Smad2 in response to TGF-β in C2C12 cells. We also observed the TGF-β-dependent interaction of Smad3 with LA-Smad4 (data not shown). Similar results were obtained in COS7 cells and SW 480.7 cells (data not shown). We also performed electrophoretic mobility shift assays (EMSAs) using nuclear extracts of SW480.7, a Smad4-deficient cell line, to characterize the DNA-binding activity of LA-Smad4. The TGF-β-inducible DNA-binding complex was detected in cells transfected with LA-Smad4 and Smad3, as well as in cells transfected with WT Smad4 and Smad3 (Figure 3B). These data suggest that LA-Smad4 is functional.
Fig. 3. An NES-disrupted Smad4 (LA-Smad4) is functional. (A) C2C12 cells were co-transfected with the indicated vectors and treated with TGF-β (10 ng/ml). Cell lysates were immunoprecipitated with anti-Myc antibody and the precipitates analyzed by anti-Flag immunoblotting. (B) An EMSA was performed using a 32P-labeled probe and nuclear extracts from SW480.7 cells, which were co-transfected with the indicated vectors and treated with or without TGF-β for 30 min. Bands corresponding to specific TGF-β-induced complexes are indicated. One hundred molar excesses of non-radiolabeled oligonucleotides were added as competitor. Supershift assays were performed by the use of nuclear extracts with the indicated antibodies. The supershifted complexes are indicated.
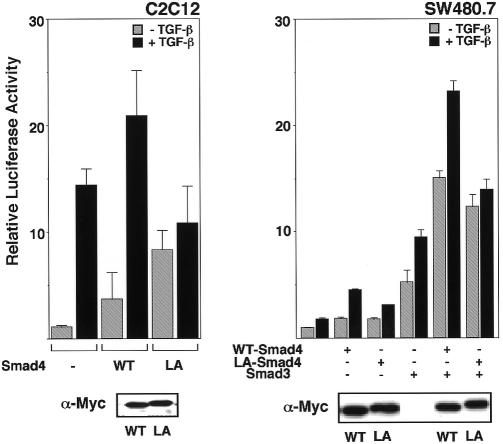
Next we examined the role of the NES of Smad4 in TGF-β signaling. We investigated the effect of expression of LA-Smad4 on a TGF-β-induced transcriptional activation. We used a TGF-β-responsive promoter that drives expression of a luciferase reporter gene, p3TP-Lux (Figure 4). When WT Smad4 was expressed in C2C12 cells, both the basal and the TGF-β-induced levels of transcriptional activation were increased, and the extent of TGF-β-induced enhancement was in the range of 5- to 10-fold (Figure 4, left, WT). In contrast, expression of LA-Smad4 increased the basal level of activity but decreased the TGF-β-induced level, so that the extent of TGF-β-induced activation decreased in the range of 1.1- to 1.5-fold (Figure 4, left, LA). In a Smad4-deficient cell line SW480.7, the extent of TGF-β-induced enhancement of transcriptional activation in LA-Smad4-introduced cells was also significantly lower than that in WT Smad4-introduced cells (Figure 4, right). However, the TGF-β-induced enhancement of transcriptional activation in SW480.7 was lower than that in C2C12, so we newly co-expressed Smad3, with Smad4. In this experiment, expression of WT Smad4 induced an efficient, TGF-β-responsive transcriptional activation, whereas the TGF-β responsiveness was almost completely lost in LA-Smad4-introduced cells (Figure 4, right). These results taken together suggest that cytoplasmic localization of Smad4 under unstimulated conditions, which is directed by its NES, is essential for ensuring proper and optimal TGF-β responsiveness.
Fig. 4. The role of the NES of Smad4 in TGF-β-induced transcriptional activation. C2C12 cells or SW480.7 cells were transiently transfected with the 3TP-lux reporter plasmid and a Myc-tagged form of the vectors indicated. Cells were incubated with or without TGF-β (10 ng/ml) and luciferase activity was then measured. The data are averages of three separate experiments. Expression levels of WT Smad4 and LA-Smad4 were examined by immunoblotting with anti-Myc antibody.
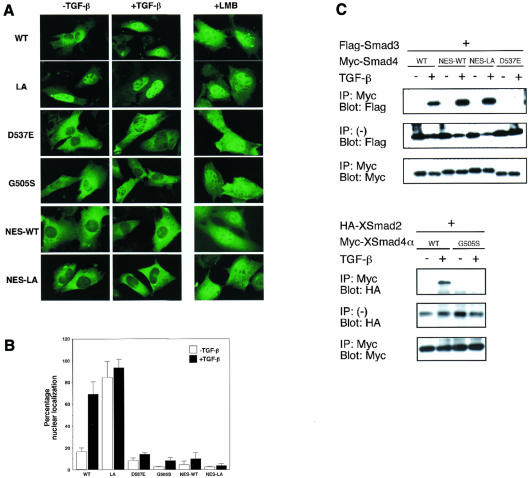
Our results show that either LMB treatment or disruption of NES of Smad4 induces nuclear accumulation of Smad4 (see Figures 1A, C and 2). Moreover, we have found that a fusion protein between green fluorescent protein and WT Smad4 also translocates to the nucleus from the cytoplasm by LMB treatment in spite of its larger molecular weight (∼85 kDa; data not shown). Therefore, Smad4 has the ability to enter the nucleus constitutively even in the absence of ligand stimulation, and appears to shuttle constantly between the nucleus and the cytoplasm. As Smad4 apparently localizes to the cytoplasm under unstimulated conditions, the nuclear export activity due to the NES may be stronger than the ability to enter the nucleus. We then tested whether or not the stimulus-induced hetero-oligomerization is required for the stimulus-dependent nuclear entry of Smad4, since it is well known that nuclear entry of Smad4 is accompanied by hetero-oligomerization (Liu et al., 1997; Hoodless et al., 1999). To this end, we constructed two mutants of Smad4, which were previously shown to be unable to hetero-oligomerize (Shi et al., 1997). One is a mutant identified as the Drosophila/C. elegans developmental mutant, in which Gly505 is replaced by serine (G505S) (Sekelsky et al., 1995; Savage et al., 1996). The other is D537E, which corresponds to a mutant form of Smad2 (D450E) found in colon cancer (Eppert et al., 1996). It was confirmed that both mutants of Smad4 are unable to hetero-oligomerize with Smad2 or Smad3 in response to TGF-β stimulation (Figure 5C and data not shown). We then examined subcellular distribution of various forms of Smad4 before and after TGF-β stimulation. When co-expressed with Smad3 or Smad2 in C2C12 cells, WT Smad4 translocated from the cytoplasm to the nucleus in response to TGF-β stimulation (Figure 5A and B, WT). In contrast, both mutants, D537E and G505S, stayed in the cytoplasm even after TGF-β stimulation (Figure 5A and B, D537E, G505S). Essentially the same results were obtained in SW480.7 cells (data not shown). These results suggest that the stimulus-dependent nuclear entry of Smad4 requires its hetero-oligomerization with receptor-specific Smads, which inactivates the NES of Smad4 probably by steric masking. In agreement with this idea, the overexpressed WT Smad4 was unable to translocate to the nucleus upon TGF-β stimulation in the absence of co-transfected Smad3 or Smad2 (data not shown), as previously reported by Liu et al. (1997).
Fig. 5. Nuclear translocation of Smad4 in response to TGF-β stimulation. (A) Smad4 enters the nucleus by making a complex with receptor-specific Smads upon TGF-β stimulation. C2C12 cells were transfected with Myc-tagged forms of WT Smad4, two mutants of Smad4 [XSmad4α(G505S) and Smad4(D537E)], an extra NES-fused WT Smad4 (NES-WT) or an extra NES-fused LA-Smad4 (NES-LA), together with Smad3 or XSmad2 [in the case of XSmad4α(G505S)] and stimulated with TGF-β (10 ng/ml) for 1 h or treated with LMB for 1 h, and then the cells were fixed and stained with anti-Myc antibody. (B) Quantification of the data shown in (A). The percentages of cells in which nuclear staining was stronger than, or equal to, cytoplasmic staining were indicated. 150–200 cells were examined in each case. (C) TGF-β-induced hetero-oligomerization. C2C12 cells were co-transfected with the vectors indicated and treated with TGF-β as in (A). Cell lysates were immunoprecipitated with anti-Myc antibody and the precipitates analyzed by anti-Flag (upper) or anti-HA (lower) immunoblotting.
Finally, to see whether the intrinsic NES of Smad4 is specifically inactivated during stimulation, we constructed an extra NES-fused Smad4 (NES-WT). An NES of MAP kinase kinase (Fukuda et al., 1996) (see Figure 1B, MAPKK) was fused to the N-terminus of Smad4. This extra NES-fused Smad4 was shown to hetero-oligomerize with Smad3 normally in response to TGF-β (Figure 5C), but it did not translocate into the nucleus upon TGF-β stimulation even in the presence of co-transfected Smad3 (Figure 5A and B, NES-WT). This suggests that while the intrinsic NES is inactivated by hetero-oligomerization, the extrinsic NES remains active, and that the active extrinsic NES is able to block the TGF-β-induced nuclear translocation of the Smad4–Smad3 complex and therefore inactivation of the intrinsic NES is a critical event for nuclear entry of WT Smad4. It is likely that the extrinsic NES is not masked by hetero-oligomerization. We then fused the extra NES to LA-Smad4, in which the intrinsic NES was disrupted. This extra NES-fused LA-Smad4 (NES-LA-Smad4) was present in the cytoplasm under unstimulated conditions (Figure 5A and B, NES-LA), whereas LA-Smad4 was nuclear (Figure 5A and B, LA). NES-LA-Smad4, however, did not translocate into the nucleus in response to TGF-β stimulation, either (Figure 5A and B, NES-LA). This result is consistent with our idea that if NES, irrespective of the intrinsic or extrinsic NES, is not inactivated, Smad4 is unable to enter the nucleus in response to TGF-β stimulation. Taken together, our results suggest that the specific inactivation of the intrinsic NES of Smad4, which is induced by the stimulus-dependent hetero-oligomerization, is essential for nuclear entry of Smad4.
Previous studies demonstrated that Smad4 is an essential component of signal transduction pathways of the TGF-β superfamily, as a common partner for each pathway-specific Smad (Derynck and Feng, 1997; Heldin et al., 1997; Massagué, 1998; Whitman, 1998; Attisano and Wrana, 2000). Our results identify a leucine-rich NES in Smad4, which suppresses nuclear localization of Smad4 in unstimulated cells and thus ensures the stimulus-dependent responsiveness. Our data further define the importance of the hetero-oligomerization-dependent inactivation of the NES of Smad4 in its nuclear translocation. Most recently, Smad3 is reported to contain a nuclear localization signal (NLS) (Xiao et al., 2000). The nuclear entry of Smad4 after stimulation might thus be stimulated by the NLS of Smad3 or other receptor-specific Smads. It is also likely, however, that Smad4 itself has some NLS because Smad4 has the ability to enter the nucleus, as demonstrated in the present results of LMB treatment. The molecular details of the inactivation mechanism of the NES of Smad4 and the nuclear import mechanism of Smads should be elucidated in future studies.
METHODS
Plasmid construction. The entire coding regions, the deletion series or the mutant forms of XSmad4α and human Smad4 were amplified by PCR. Mutagenesis of Leu146 and Leu148 to Ala in hSmad4 was performed by the QuickChange Site-Directed Mutagenesis Kit (Stratagene) using mutagenic primers 5′-GATCTCTCAGGAGCAACAGCGCAGAGTAAT-3′ and its complementary strand to yield LA-Smad4. To obtain G505S of XSmad4α and D537E of hSmad4 the mutagenic primers 5′-TTCGTTAAAAGTTGGGGCCCTGATTACCCG-3′ and 5′-CTCCAGCTCCTAGAAGAAGTACTTCATACC-3′ and their complementary strands were used, respectively. The nucleotide sequences amplified and the presence of mutations were confirmed by DNA sequencing. All constructs of XSmad4α and hSmad4 were inserted into a Myc tag fused version of pCS2 plasmid. XSmad2 was a kind gift from Dr Melton.
Microinjection. Cultures of 3Y1 cells, microinjection and conjugation of synthetic peptides to OV were performed as described (Fukuda et al., 1996). At 30 min after injection, cells were fixed and stained with anti-OV antibody (Cappel) or anti-GST antibody as previously described (Fukuda et al., 1996). BSA was labeled with tetramethyl rhodamine isothiocyanate to yield RITC–BSA. RITC–BSA was injected to indicate the initial injection site.
Recombinant proteins. GST–WT-Smad4 and GST–LA-Smad4 were prepared by using the expression vector pGEX-6P-2 (Pharmacia Biotech Inc.), expressed in Escherichia coli, and purified on glutathione–Sepharose 4B (Pharmacia Biotech Inc.) as described (Fukuda et al., 1996).
Transfection and transcriptional reporter assay. Cultures of C2C12 cells, transfection and cell staining were performed as described (Masuyama et al., 1999). SW480.7 cells were transfected with FuGENE™ 6 (Boehringer Mannheim). At 20 h after transfection, the cells were treated with 10 ng/ml human TGF-β1 for 20 h. Luciferase activity was measured using the Luciferase Assay System (Promega) and was normalized for transfection efficiency by co-expressed β-galactosidase activities.
Immunoprecipitation and immunoblotting. At 18–24 h after transfection, cells were treated with or without 10 ng/ml human TGF-β1 for 60 min and lysed. Immunoprecipitation was performed by incubation with the 9E10 anti-Myc antibody (Santa Cruz Biotechnology) and protein G–Sepharose (Pharmacia Biotech). The immunoprecipitates and the aliquots of total lysates were subjected to SDS–PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. Immunoblotting was performed by the use of antibodies against Myc, HA (12CA5) or Flag (Kodak), followed by incubation with HRP-anti-mouse IgG antibody.
Electrophoretic mobility shift assay (EMSA). SW480.7 cells were transfected with FuGENE™ 6 (Boehringer Mannheim) and nuclear extracts were prepared from control and TGF-β-treated cells as described (Dennler et al., 1998). EMSA was performed as described (Dennler et al., 1998). For antibody supershift assays, extracts were incubated for 15 min in binding buffer, then 20 min at 37°C with the probe, and 60 min with antibodies on ice. DNA–protein complexes were resolved on a 5% polyacrylamide gel containing 2.5% glycerol in 0.5× TBE buffer and visualized by autoradiography.
Acknowledgments
ACKNOWLEDGEMENTS
We thank H. Hanafusa, M. Adachi, K. Terasawa and Y. Miyata for technical comments and helpful discussion. We are grateful to Dr Douglas A. Melton for providing the Xenopus Smad2 plasmid. This work was supported by grants from the Ministry of Education, Science and Culture of Japan (to E.N.).
REFERENCES
- Attisano L. and Wrana, J.L. (2000) Smads as transcriptional co-modulators. Curr. Opin. Cell Biol., 12, 235–243. [DOI] [PubMed] [Google Scholar]
- de Caestecker M.P., Yahata, T., Wang, D., Parks, W.T., Huang, S., Hill, C.S., Shioda, T., Roberts, A.B. and Lechleider, R.J. (2000) The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain. J. Biol. Chem., 275, 2115–2122. [DOI] [PubMed] [Google Scholar]
- Dennler S., Itoh, S., Vivien, D., ten Dijke, P., Huet, S. and Gauthier, J.-M. (1998) Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J., 17, 3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R. and Feng, X.H. (1997) TGF-β receptor signaling. Biochim. Biophys. Acta, 1333, 105–150. [DOI] [PubMed] [Google Scholar]
- Dong C., Li, Z., Alvarez, R., Jr, Feng, X.-H. and Goldschmidt-Clermont, P.J. (2000) Microtubule binding to Smads may regulate TGFβ activity. Mol. Cell, 5, 27–34. [DOI] [PubMed] [Google Scholar]
- Eppert K. et al. (1996) MADR2 maps to 18q21 and encodes a TGFβ-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell, 86, 543–552. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno, M., Yoshida, M. and Mattaj, I.W. (1997) CRM1 is an export receptor for leucine-rich nuclear export signals. Cell, 90, 1051–1060. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Gotoh, I., Gotoh, Y. and Nishida, E. (1996) Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J. Biol. Chem., 271, 20024–20028. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Asano, S., Nakamura, T., Adachi, M., Yoshida, M., Yanagida, M. and Nishida, E. (1997) CRM1 is responsible for intracellular transport mediated by nuclear export signal. Nature, 390, 308–311. [DOI] [PubMed] [Google Scholar]
- Gerace L. (1995) Nuclear export signals and the fast track to the cytoplasm. Cell, 82, 341–344. [DOI] [PubMed] [Google Scholar]
- Hahn S.A. et al. (1996) DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science, 271, 350–353. [DOI] [PubMed] [Google Scholar]
- Heldin C.-H., Miyazono, K. and ten Dijke, P. (1997) TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature, 390, 465–471. [DOI] [PubMed] [Google Scholar]
- Hoodless P.A., Tsukazaki, T., Nishimatsu, S.-i., Attisano, L., Wrana, J.L. and Thomsen, G.H. (1999) Dominant-negative Smad2 mutants inhibit Activin/Vg1 signaling and disrupt axis formation in Xenopus. Dev. Biol., 207, 364–379. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Liu, F., Hata, A., Doody, J. and Massagué, J. (1997) The TGF-β mediator Smad1 is directly phosphorylated and functionally activated by the BMP receptor kinase. Genes Dev., 11, 984–995. [DOI] [PubMed] [Google Scholar]
- Lagna G., Hata, A., Hemmati-Brivanlou, A. and Massagué, J. (1996) Partnership between DPC4 and SMAD proteins in TGFβ signaling pathways. Nature, 383, 832–836. [DOI] [PubMed] [Google Scholar]
- Liu F., Pouponnot, C. and Massagué, J. (1997) Dual role of the Smad4/DPC4 tumor suppressor in TGFβ-inducible transcriptional complexes. Genes Dev., 11, 3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M., Abdollah, S., Hoodless, P.A., Pirone, R., Attisano, L. and Wrana, J.L. (1996) MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell, 87, 1215–1224. [DOI] [PubMed] [Google Scholar]
- Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem., 67, 753–791. [DOI] [PubMed] [Google Scholar]
- Masuyama N., Hanafusa, H., Kusakabe, M., Shibuya, H. and Nishida, E. (1999) Identification of two Smad4 proteins in Xenopus. Their common and distinct properties. J. Biol. Chem., 274, 12163–12170. [DOI] [PubMed] [Google Scholar]
- Mattaj I.W. and Englmeier, L. (1998) Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem., 67, 265–306. [DOI] [PubMed] [Google Scholar]
- Nakao A. et al. (1997) TGF-β receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J., 16, 5353–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A. (1997) Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature, 386, 779–787. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B., Bachelerie, F. and Dargemont, C. (1997) Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science, 278, 141–144. [DOI] [PubMed] [Google Scholar]
- Savage C., Das, P., Finelli, A.L., Townsend, S.R., Sun, C.-Y., Baird, S.E. and Padgett, R.W. (1996) Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor β pathway components. Proc. Natl Acad. Sci. USA, 93, 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J.J., Newfeld, S.J., Raftery, L.A., Chartoff, E.H. and Gelbart, W.M. (1995) Genetic characterization and cloning of Mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics, 139, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Hata, A., Lo, R.S., Massagué, J. and Pavletich, N.P. (1997) A structural basis for mutational inactivation of the tumour suppressor Smad4. Nature, 388, 87–93. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T., Chiang, T.A., Davison, A.F., Attisano, L. and Wrana, J.L. (1998) SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell, 95, 779–791. [DOI] [PubMed] [Google Scholar]
- Whitman M. (1998) Smads and early developmental signaling by the TGFβ superfamily. Genes Dev., 12, 2445–2462. [DOI] [PubMed] [Google Scholar]
- Wolff B., Sanglier, J.J. and Wang, Y. (1997) Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol., 4, 139–147. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Liu, X. and Lodish, H.F. (2000) Importinβ mediates nuclear translocation of Smad3. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- Zhang Y. and Derynck, R. (1999) Regulation of Smad signalling by protein associations and signalling crosstalk. Trends Cell Biol., 9, 274–279. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Feng, X., We, R. and Derynck, R. (1996) Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature, 383, 168–172. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Musci, T. and Derynck, R. (1997) The tumor suppressor Smad4/DPC4 as a central mediator of Smad function. Curr. Biol., 7, 270–276. [DOI] [PubMed] [Google Scholar]