Abstract
Vascular diseases, such as atherosclerosis and thrombosis, are major causes of morbidity and mortality worldwide. Traditional in vitro models for studying vascular diseases have limitations, as they do not fully recapitulate the complexity of the in vivo microenvironment. Organ-on-a-chip (OOC) systems have emerged as a promising approach for modeling vascular diseases by incorporating multiple cell types, mechanical and biochemical cues, and fluid flow in a microscale platform. This review provides an overview of recent advancements in engineering OOC systems for modeling vascular diseases, including the use of microfluidic channels, extracellular matrix scaffolds, and patient-specific cells. We also discuss the limitations and future perspectives of OOCs for modeling vascular diseases.
Keywords: Vasculature, Vascular disease, Organ-on-a-chip, Microfluidics, Hemodynamics, Endothelial cell
Graphical Abstract
1. Introduction
The vascular system plays several essential physiological functions within the human body, including the transport of nutrients and oxygen to vital organs, aiding the immune response by delivering cells and antibodies, and providing a robust barrier function to actively regulate the movement of cells, ions, and molecules between blood and the brain.1 Disturbances in the vascular system can lead to cardiovascular disease, which is responsible for over one-thirds of all global mortalities.2 Structurally, vasculature exhibits fractal branching that enables efficient mass transfer. The larger vessels in the vasculature primarily consist of three tissue layers: the intima layer composed of endothelial cells (ECs), the media layer composed of smooth muscle cells (SMCs), and the adventitia composed of fibroblasts and collagen bundles and other extracellular matrix (ECM) components. The EC layer is crucial as it regulates permeability, angiogenesis, immune cell transport, and hemostasis within the vasculature depending on the mechanical (e.g., tissue stiffness) and biochemical triggers present (i.e., hormones) at the specific organ sites in which they reside. For this reason, dysfunction of the EC, driven by a complex interplay of various factors (genetic, mechanical, biochemical) drives inflammatory precursors to begin the cascade to most common vascular diseases such as atherosclerosis, aneurysms, peripheral artery disease (PAD), and thrombosis.3
Disease modeling offers a means to peer into the underlying factors that initiate vascular disease and to gauge the downstream progression of the diseases with respect to clinical prognosis. In addition, disease modeling is important to evaluate the safety and efficacy of novel drugs. In the early stage of research in vascular disease modeling, significant efforts were placed on the use of animal models and 2-dimensional (2D) in vitro models. For example, zebrafish and mouse models have been successfully used for recapitulating abnormal morphology of the vasculature.4,5 Animal models, while considered a gold standard in vascular research, have limitations such as ethical restrictions and limited availability of large animal models with physiological relevance to humans. Two-dimensional in vitro models composed of ECs, SMCs, and human leukemia monocyte cells in separate layered compartments have also been largely implemented to understand cell-to-cell communication of vascular layers, and resident immune cells.6 However, these models respectively lack complex vascular geometries, and cell heterogeneity seen in vivo.7 Common cone-and-plate viscometers have served as an in vitro option for studying the influence of shear forces on endothelial cells and blood rheology.8 However, they are hindered by limited throughput and imaging capabilities.
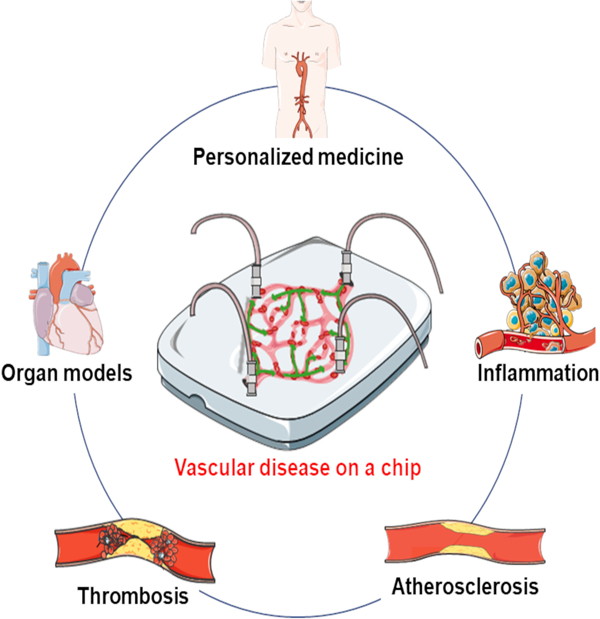
Despite their utility, the field is gradually shifting its focus away from animal and 2D models and towards 3-dimensional (3D) in-vitro microfluidic models and organ-on-a-chip (OOC) models, which allow for the replication of more complex geometries using human-specific cells with the inclusion of fluid flow. Microfluidics and OOC devices offer clear advantages over the traditional tissue culture in terms of creating a native-like microenvironment for disease progression. They allow for media perfusion that resembles the disrupted blood flow pattern, which plays a key role in platelet adhesion and endothelial cell activation during the early phase of atherosclerosis.9 The perfusion can also introduce inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and low-density lipoprotein (LDL) into the stream to further progress the disease phenotype. Microfluidic in vitro models can also take advantage of the natural ECM and synthetic materials, such as collagen, laminin, fibronectin, methylacrylated gelatin (GelMA) and viscoelastic polyester, to recapitulate the chemical ligand and mechanical properties of the early and late phases of atherosclerosis.10 On the other hand, the endothelial and pericyte cell sources can include human primary cells or human pluripotent stem cell-derived cells, enhancing their biological relevance to humans. Microfluidic devices and OOCs can facilitate disease onset, progression and probing the disease mechanisms to develop and test potential therapeutic strategies. An ideal OOC vascular model aims to incorporate co-seeded SMCs and EC layers, an elastic cell seeding interface that can respond to pulsatile flow and mimic vessel geometries that support the generation of physiological and pathophysiological flow.11 This review will focus on the new advances in OOC systems implemented for modeling arteriosclerosis, atherosclerosis, thrombosis, inflammation and other vascular-related diseases, and point out the advantages and limitations of such models (Figure 1).
Figure 1. Schematic illustration of the advanced microfluidic chips for vascular disease modeling and personalized medicine application.
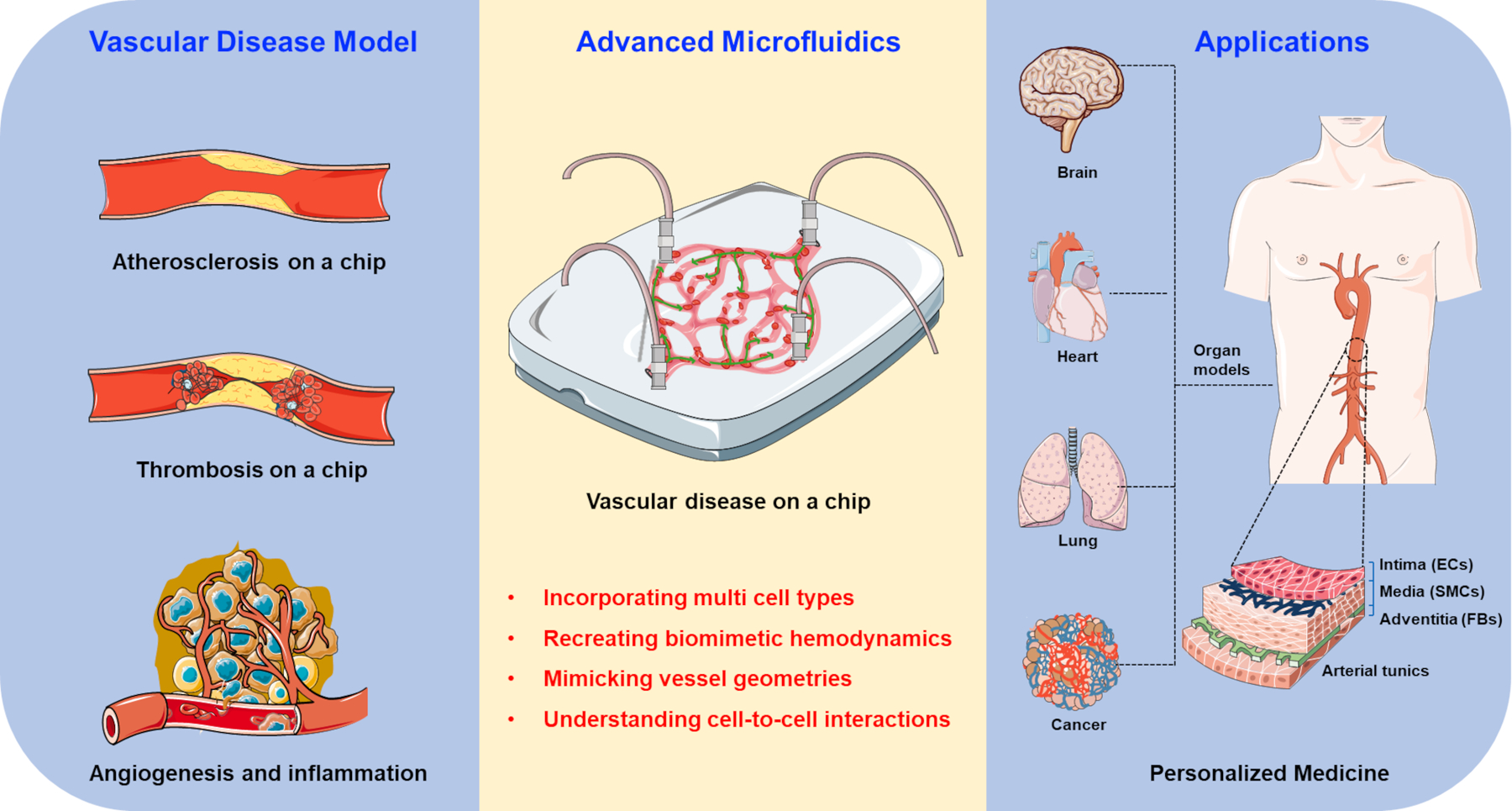
OOC models provide advantages in incorporating various cells, simulating rheological factors, and mimicking pathological microenvironments towards the construction of vascular disease models for investigating pathogenesis of the disease, drug development, and personalized medicine.
2. Organ-on-a-chip technology for vascular disease modeling
2.1. Hallmarks of vasculature-on-a-chip models
OOCs and microfluidic designs have greatly advanced our understanding of vascular diseases. Their potential in clinical applications, including coagulation monitoring, antithrombotic therapy, and point-of-care diagnosis, holds great promise. Microfluidic models require minimal amounts of blood samples, offer tight control of dimensions, lower costs, and are easy to fabricate with repeatability for large-scale experiments. Another advantage of these systems is the ability to create vessel-like structures that mimic the microvasculature in size and complexity, while its transparency allows for visualization and quantification using image analysis systems.12 Effort has been placed on developing perfusable OOC models that offer controlled hemodynamics over microchannels layered with ECs and SMCs, or through self-assembled vascular networks suspended in hydrogel scaffolds.13 This is because the natural vascular microenvironment requires a precisely regulated level of wall shear stress (WSS) to maintain healthy function. Moreover, they allow for the inclusion of different anatomical structures observed in blood vessels, such as atherosclerotic plaques and bifurcations, in the design of the flow path, as well as an independent control of multiple factors, such as endothelial cell type, platelet number, coagulation proteins, shear stress, and leukocyte fractions, facilitating the study of vascular diseases.13,14 Microfluidic devices also hold promise for patient-specific vascular disease modeling (Figure 1). Their ability to incorporate patient-specific vessel anatomy, ECs with genetic mutations, and blood samples, make it possible to create in vitro models that closely mimic the in vivo disease microenvironment. This level of patient-specific modeling is critical for translational research and personalized medicine.15
2.2. Fabrication of vascular disease models on a chip
2.2.1. Material Selection
The choice of materials and fabrication techniques is critical for developing disease-specific organ-on-a-chip models that accurately recapitulate the vascular microenvironment. Various materials and fabrication methods have been explored to mimic the complexity of vascular tissues and provide physiologically relevant platforms for disease modeling.
Polydimethylsiloxane (PDMS) is one of the most commonly used materials to fabricate microchannels in OOCs due to its several advantages including optical transparency, biocompatibility, moldability and gas permeability. To improve the biocompatibility of PDMS channels and promote clot formation, various biointerfaces, including collagen16,17, fibronectin18,19, tissue factors20,21, von Willebrand factor (VWF)22, and poly-D-lysine23, have been applied to coat the channels. However, due to the non-specific absorption of hydrophobic small molecules into PDMS, recent research is geared towards application of alternative polymers including thermoplastics and polyesters.24–26 Polyester materials exhibiting lower Young’s moduli such as poly(octamethylene maleate (anhydride) (POMaC),26 poly (octamethylene maleate (anhydride) 1,2,4-butanetricarboxylate) (1,2,4 polymer),27 and poly(itaconate-co-citrate-co-octanediol) (PICO)28 could better mimic the inherent softness of vascular tissue, which is critical for maintaining proper cell functionality. Other polymers and thermoplastics such as polyethylene terephthalate (PET)29, polysulfone30, polycarbonate (PC)31, polystyrene (PS)32 and polyacrylamide33 have also shown promising alternatives for PDMS. Furthermore, the fabrication of microfluidic channels using ECM components such as alginate34, gelatin34,35, Matrigel36, collagen37–40, agarose35, and fibrin41, either individually or in combination, can effectively replicate cell behavior found in native tissues. For instance, an interpenetrating polymer-network (IPN) of agarose/gelatin hydrogel was cast onto a master mold to create channels with the diameter of post-capillary venules that can mimic vascular elasticity with the Young’s modulus of 20 kPa using 1% (w/v) agarose and 1% (w/v) gelatin.35 The IPN hydrogel was also capable of recapitulating permeability and self-healing properties of the endothelial barrier.35 Application of photo-crosslinkable synthetic hydrogels such as GelMA and polyethylene glycol-diacrylate (PEGDA) is another effective technique to preserve endothelial function and vascular perfusability.42–45
2.2.2. Fabrication techniques
To create microfluidic channels and OOC devices using moldable materials like PDMS, silicon master molds are typically first created through photolithography. This process allows for precise replication of channel arrays on photosensitive substrates, such as SU-8, at nano/micro-scale resolutions. The protrusions on the master mold are then transferred to PDMS as channel cavities using a soft lithography process.46–51 Novel methods for fabricating master molds and PDMS-based channels have been extensively reviewed elsewhere.46
Photolithography is a time-consuming and expensive technique, and it can often form channels with rectangular cross-section. Researchers have undertaken various approaches to design microfluidic channels with circular cross-sectional shapes, aiming to better replicate the flow patterns found within natural blood vessels. One method involved the creation of circular channels by introducing air through uncured PDMS, allowing for the formation of circular geometry.52 Another technique utilized extrusion to fabricate channels with circular cross-sections by using sacrificial Pluronic tubes embedded within a GelMA substrate that contained encapsulated fibroblasts.42 PDMS microtubes were also fabricated via extrusion where a heated metal filament was immersed in liquid PDMS, causing the PDMS to coat the wire due to its surface tension.53 Additionally, circular channels were generated through a thermal expansion process, where air trapped within partially cured PDMS was expanded to create the desired circular configuration. 17,54 A more complex approach involved the construction of multilayered structures encompassing a circular channel. In this method, a blunt needle served as a sacrificial structure, allowing the formation of a circular channel surrounded by layers of collagen and fibrin, providing a more intricate microenvironment for biological studies.41 Application of a pre-stretched elastomeric substrate could also form complex PDMS channels with curved cross-sections via buckling of the PDMS.55
Recent advancements have seen a surge in the exploration of 3D printing technology for the fabrication of microfluidic channels. This approach is gaining attention due to its cost-effectiveness, rapidity, flexibility and user-friendly nature, making it a promising alternative to traditional photolithography. One notable 3D printing technique involves the use of coaxial needles in an extrusion-based process. For example, this method enables the creation of vein- and artery-like conduits by employing a hybrid bioink composed of alginate/gelatin or alginate/gelatin in combination with alginate/GelMA for single and double-layered conduits, respectively.34 Furthermore, a multilayered vascular tube was successfully fabricated by extruding nanofibrillar collagen with specific alignments, offering a novel approach to construct intricate microfluidic systems that closely mimic natural vasculature.39 The extrusion approach provides a versatile means of achieving complex vascular structures.56,57 Additionally, stereolithography printing (SLA) has been harnessed to generate circular channels.58 For instance, researchers have utilized SLA printing technology to construct miniaturized models of coronary arteries with the diameter of 400 µm and resolution of 25 µm based on computed tomography angiography (CTA) imaging data.59 In another innovative application of SLA printing, the cardiovascular system’s vasculature has been reconstructed using high-contrast magnetic resonance venography (MRV) images.19 These diverse 3D printing techniques demonstrate the versatility and potential of this technology for creating complex and physiologically relevant microchannels for various biomedical applications.44,60–62
Another innovative approach to model vascular diseases on microfluidic chips involves embedding microchannels within microwell plate platforms. These platforms offer several advantages, including the ease of adding cells and media directly through simple pipetting or tilting actions, eliminating the need for a separate pumping system and streamlining the experimental procedure. One notable example is the integrated vasculature for assessing dynamic events (InVADE) system, which features a porous channel with stiffness mimicking that of natural vasculature, connecting sequential microwells (Figure 2a). To construct this channel, a soft polyester is first cast into two individual PDMS molds and UV cured to create the upper and lower halves of the microchannels. The two halves are then aligned and bonded together using another round of UV treatment to form the complete microchannels through 3D stamping process.63–65 Graddient is another platform which incorporates a collagen microchamber positioned beneath a microwell array. This arrangement allows for precise control over nutrient depletion within the microchamber, offering researchers a valuable tool for studying vascular conditions.31 In a different design, an endothelialized nylon membrane is integrated beneath tissue chambers to establish a vascular barrier.30 These designs incorporate nano and microporosity within the channels or separating membranes, facilitating essential cell communication and nutrient exchange.66
Figure 2. Vascular disease on a chip.
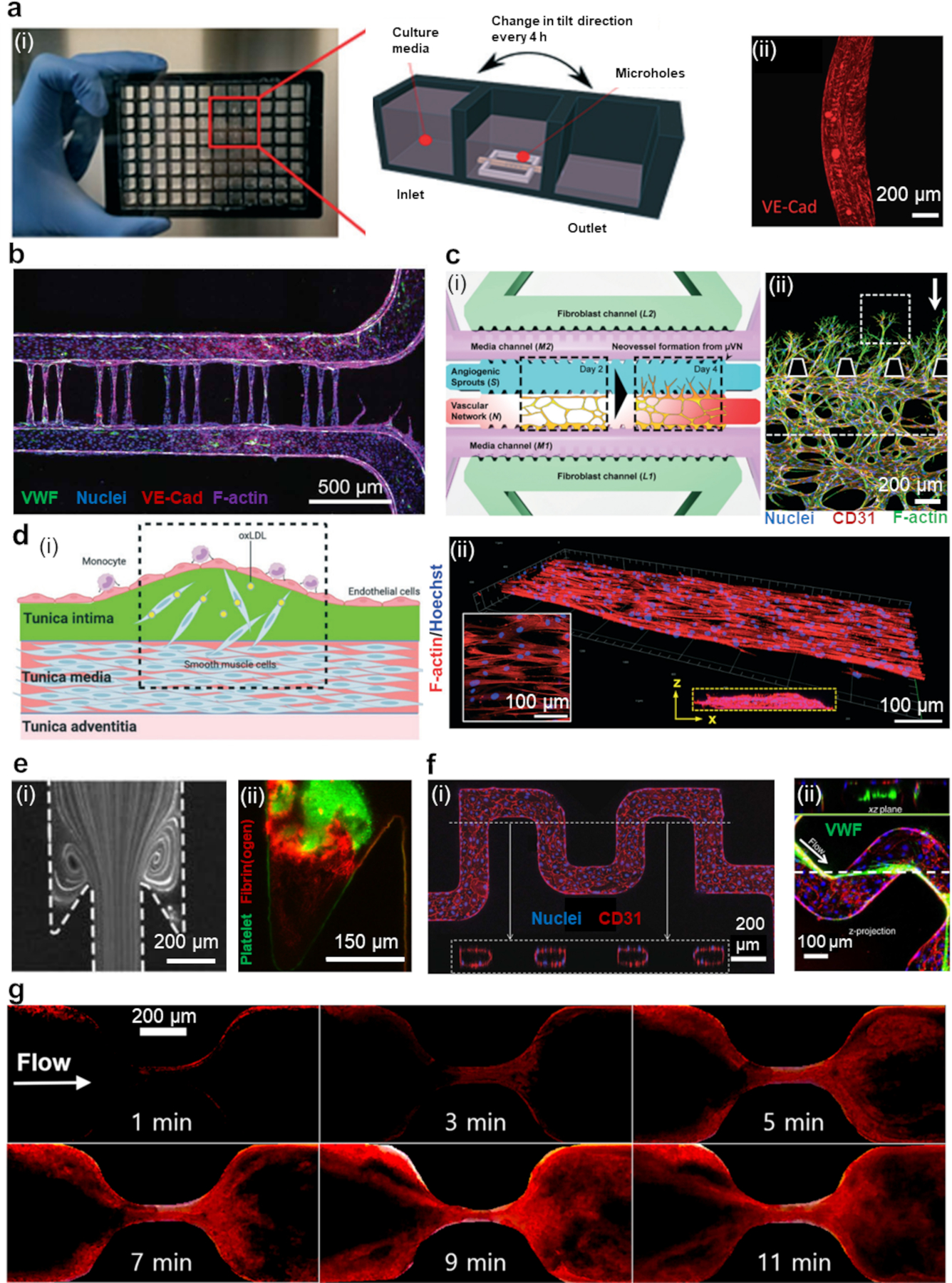
(a) (i) a photo of an InVADE platform. (ii) ECs after 48 hours after HCoVNL63 infection. Copyright 2022, Royal Society of Chemistry.66 (b) The promotion of capillary growth in microvessel devices by using photoablation as a guide. Copyright 2020, American Association for the Advancement of Science.38 (c) (i) A diagram depicting the process of angiogenesis in the assay. (ii) Distinct shapes of angiogenic sprouts that have formed over three days, white arrow indicates the direction of flow. Copyright 2016, Royal Society of Chemistry.69 (d) (i) Visual representation of the dysfunction of ECs/SMCs in the arterial wall during early stages of atherosclerosis, as replicated in a microfluidic system. (ii) Fluorescence image of SMCs co-cultured with ECs. The inset in the white box shows the alignment of actin filament, while the yellow dotted box indicates the z-stack of confocal sections. Copyright 2021, Royal Society of Chemistry.36 (e) (i) Streamlines after perfusion of beads at 150° expansion mimicking venous valve flow. (ii) Thrombus formation. Copyright 2018, American Heart Association.84 (f) (i) Tortuous microvessels that have been endothelialized. (ii) VWF fibers at the inner corners of vessel turns. Copyright 2015, Nature Publication Group.37 (g) Thrombus growth in a stenosed channel. Copyright 2022, Springer.17
Furthermore, laser ablation is employed as a technique to create an array of capillary-sized channels within a polymeric matrix. This approach provides a versatile means of engineering microfluidic systems for the study of vascular diseases, enabling researchers to simulate physiological conditions and interactions between cells and their microenvironment more effectively (Figure 2b).38,40,67
Spontaneous vascularization through culturing ECs in microchambers/microchannels filled with natural hydrogels such as Matrigel, collagen, fibrin/fibrinogen, and decellularized ECM is an alternative way of creating perfusable microvascular networks and modeling angiogenesis.12 In this case, the middle channel with ECs is usually surrounded with two parallel channels providing media for cell growth and vascularization.68–70 Blood vessel organoids that can self-organize and reproduce angiogenesis have also been employed as a model for disease processes in specific organs.71 OOCs were also employed to demonstrate the impact of interstitial flow (IF) on vasculogenesis and pathological angiogenesis.69 In the design, ECs and stromal fibroblasts were co-cultured to form a microvascular network with neovessel sprouting and angiogenic remodeling in response to IF (Figure 2c).69 Angiogenic sprouting was elevated when the flow and the sprouting were in opposite directions. The anisotropic arrangement of cells is also important for mimicking the natural properties of blood vessels.39,72,73 He et al. provided a detailed guide on how to create a tissue-engineered vascular graft anisotropic properties using aligned ECM nanofibers and MSCs.72
2.2.3. Cell type
Due to the intricate cell composition and diverse microenvironments present at pathological sites, there is an increasing research emphasis on carefully selecting appropriate cell types for the development of diseased vascular models in vitro to mimic this condition and study its mechanisms 74,75. The goal is to faithfully replicate the disease by simulating not only the cellular composition but also the interactions between different cell types.76 In most cases, cells line was employed to fabricate human physiology by organ-on-a-chip technologies, especially for initial device validation. Primary cells such as human umbilical vein endothelial cells are most employed. Stromal cells such as cardiac fibroblasts, for example, can maintain the structural integrity of healthy myocardial tissue and are required to generate a contractile tissue from iPSC derived cardiomyocytes.77 However, in the infarcted area, they need to undergo differentiation into myofibroblasts, eventually becoming the predominant cell type.78 The inclusion of vasculature, with abundant endothelial cells, could support the barrier of tissue compartments while enabling their communication.79 Transient reactivation of the embryonic-restricted genes such as E26 transformation-specific variant 2 (ETV2) in primary cells such as human umbilical vein endothelial cells (HUVECs), was shown to resent the cells to adaptable, vasculogenic cells, which can form perfusable vascular plexus.80 Pluripotent stem cells can be deliberately differentiated into specific cell types, eliminating the necessity for genetic modifications and the repeated passaging of cells that could lead to alterations in cell genotype and phenotype.81. This also enables generation of sophisticated vascular organoids.82 In Table 1, different cell types along with applications, channel designs, materials for channel constructions as well as the associated key advances are summarized.
Table 1.
An overview of various OOCs applications for vascular diseases, including their design features, materials of construction, biofunctional interfaces, and key advances in the field.
| Application | Design | Materials of construction | Biofunctional interfaces | Key advances |
|---|---|---|---|---|
| Atherosclerosis | Straight channel16,33 Cross-connected parallel channels36 Membrane-separated channels18,83 Stenosis-integrated channel52 Multi-layered micro conduit34 |
PDMS16,18,52,83 Polyacrylamide33 GelMA43 Collagen/Matrigel36 Alginate/Gelatin (or GelMA)34 |
HUVECs BAECs SMCs HAECs HUASMCs Platelet- agonists VWF Fibrinogen Collagen Fibronectin Matrigel |
Effects of various shear stresses and channel stiffness were shown.16,18,33 Channels with circular cross-sections were created by air pumping through uncured PDMS.52 Multi-stepped parallel channels enabled capillary-guided hydrogel confinement and ECM patterning for cross-connected channels without physical barriers.36 Coaxial 3D printing of two-layered hydrogels was used for bioprinting of vein- and artery-like conduits.34 |
| Thrombosis | Stenosis-integrated channel17,19,37,59,84–86 Continues channel22,87–91 H-shaped channel92 T-shaped channel93,94 Y-shaped channel20,42,95 Branching channels35,37,96 |
PDMS/ glass19–22,59,84,87,88,92,97 PDMS17,85,89,93 Glass86 GelMA42 Collagen37 PDMS/silica96 Commercial devices90,91 Agarose/gelatin35 |
HUVECs HDMVECs HLMVECs Collagen Collagen/TF VWF Fibroblasts Fibronectin |
Venous valvular stasis was modeled by incorporating undercuts in an expanding channel.84 Platelet adhesion was estimated through correlation mapping.92 Platelet aggregation was achieved via embedding an array of thrombus-sized posts in channels.22 Optical irradiation induced vascular injury.89 Circular channels were SLA printed based on CTA images of CAD59 or MRV images of CVS19. A valve membrane was used to form wound.94 Channels were made by extrusion of sacrificial Pluronic tubes in GelMA containing encapsulated fibroblasts.42 Thermal expansion of air trapped inside partially cured PDMS created circular channels.17,54 EDTA-quenching at the downstream of thrombotic site enabled accurate occlusion timing.20,21 UV laser induced different wettability properties.96 |
| Ischemic | Cross-connected parallel channels78,98,99 Stenosis-integrated channel100 Microwell-plate format31 Channel crosses microchamber32 |
PDMS/glass78,98–100 PC31 PS32 |
Cortical neurons Astrocytes HUVECs iECs iCMs Collagen |
Microgrooves connecting parallel channels enabled neuronal segregation and axonal growth for modeling CNS axonal injury.98 Graddient: a collagen microchamber was positioned beneath a microwell array, allowing control of nutrient depletion within the microchamber.31 Oxygen and nutrient gradients formed in a microchamber via a crossing microchannel separated by diffusion ports.32 |
| Pulmonary arterial hypertension | Membrane-separated channels29 Cross-connected parallel channels23,101 |
PDMS/glass23,101 PDMS/PET membrane29 |
PAH-ECs PAH-SMCs PAH-ADCs Collagen Matrigel Fibronectin Poly-D-lysine |
5 parallel channels separated via micro pillars were utilized to create perivascular, adventitial, medial, intimal and luminal layers of a pulmonary artery.23,101 |
| Vascular dysfunctions, inflammation and malformation | Branching channels35 Cross-connected parallel channels38,40,102 Microwell-plate format66,103 Straight channel39 |
Agarose/gelatin35 Collagon38–40 POMaC66 PDMS/glass102 |
HUVECs HDMVECs HLMVECs iECs iSMCs HBVPCs HUASMCs HPMECs Pericytes Fibroblasts AECs Fibrin Collagen |
IPN hydrogel channels mimicked vascular stiffness, permeability and self-healing of the endothelial barrier.35 Laser ablation created an array of capillary-sized channels within a collagen matrix.38,40 InVADE: A porous channel with vascular-mimicking stiffness links sequential microwells.66 A multilayer vascular tube was fabricated via extrusion of nanofibrillar collagen with certain alignments.39 |
| Various organ-on-a-chip models | Cross-connected parallel channels68,70,104 Connected microchambers105 Straight channel41,106 Membrane-separated channels107 Microwell-plate format30 |
PDMS/glass68,104,105 PDMS70,106,107 Collagen/fibrin41 Polysulfone30 |
HUVECs Im-HUVECs iECs iCMs Omental ADs BMSCs hCB-ECs PSCs AECs hFOB Fibroblasts Fibrin |
Hexagonal chambers were interconnected by two-way ports to model the perivascular and endosteal niches within human bone marrow.105 A multilayered collagen/Fibrin surrounding a circular channel was made by using a blunt needle as a sacrificial structure.41 Highly perusable and irregular vessels were formed in diamond-shaped microchambers connected to two parallel channels from opposite corners.70 An endothelialized nylon membrane was integrated under tissue chambers to form a vascular barrier.30 |
2.3. Characterization of vascular models on a chip
The transparency of microfluidic channels makes optical microscopy the primary method for characterization. It offers direct visualization of vital parameters like cell morphology, clot formation dynamics, and occlusion times. Immunostaining further enhances this technique, allowing researchers to investigate specific characteristics of cells, platelets, and coagulation factors, such as their spatial distribution and interactions, using fluorescent-based microscopy.34,36,84 Advanced approaches incorporate confocal microscopy with z-stack imaging, delivering detailed insights into cell morphologies and surface protein distributions, particularly valuable when conducting 3D and multilayered cell culture within microfluidic setups.36,68,102,103,105 Beyond optical microscopy, additional imaging methods like SEM and histology can be employed for comprehensive analysis.22,41,42,86,91 Furthermore, cells and media retrieved from the device enable further investigations such as RNA sequencing, secretomics and metabolomics 16,20,78
2.3.1. Snapshot imaging
Snapshot imaging in OOC systems corresponds to capturing a single moment in time for in-depth analysis.16,18,37,39,52,66,78,87,88 These observations provide valuable insights into disease events, helping identify rapid changes in vascular cell behavior in response to specific stimuli. Snapshot imaging typically occurs after cell fixation, where cells are chemically preserved at a specific moment.70,106 Researchers can then stain the cells with fluorescent markers or antibodies to visualize specific cellular structures or proteins. Markers of endothelial inflammation and activation such as vascular cell adhesion molecule-1 (VCAM-1), VWF and YAP, could be examined to assess endothelial mechanoresponse to different shear stresses.16 Staining the nucleus, actin cytoskeleton and VE-cadherin of endothelial cells is a common strategy to evaluate cell morphology and tight junction after mechanical or chemical stimulation for a certain amount of time.33 Additionally, snapshot imaging is usually applied in drug screening application to evaluate the efficacy of drugs such as aspirin, vitamin D and metformin at a certain time point after administration.36,52 Furthermore, fixation enables SEM imaging and histology to reveal the morphology of cells, platelets and thrombus inside the microchannels.22,42,86
Snapshot imaging provides critical static insights into vascular processes, but it may not fully capture the dynamic, time-dependent interactions seen in chronic vascular diseases, where understanding disease progression relies on monitoring changes over time.
2.3.2. Long-term observation
Long-term imaging techniques offer a profound means to explore the intricacies of chronic vascular diseases, providing a comprehensive view of disease progression over time. This approach is particularly adept at capturing the dynamic interplay of factors and responses in conditions like atherosclerosis and thrombosis, where disease development unfolds gradually. Long-term observation can be realized through real-time imaging, which continuously records images or videos of microfluidic channels. Time-lapse microscopy, involving periodic image capture over extended periods, serves as another powerful tool for prolonged observation.
Fluorescent beads and dyes can be used in long term observation techniques to study the flow dynamics within microchannels and constrictions52,83,84, as well as measure channel permeability and perfusability35,38. Moreover, different components of blood such as blood cells, platelets and fibrinogen, are usually fluorescently stained before perfusion into the microchannels to allow for monitoring thrombus formation and platelet coverage overtime.17,84,85,87,91,96 The use of genetically modified cells capable of expressing fluorescence is another approach for long-term imaging. For instance, red fluorescent protein (RFP)-expressing breast cancer cells can be tracked at 48-hour intervals in microfluidic devices to study migration patterns.105 Green fluorescence protein (GFP)-expressing HUVECs are also another commonly-used cells in the fabrication of vasculature on a chip.77,108 Additionally, long-term models are instrumental in investigating gradual changes in endothelial integrity under shear stress,33 and tracking cells such as leukocytes and neutrophils in channels.20,22,83
2.3.3. Integration of sensors
Incorporating sensors into microfluidic devices offers valuable data generation capabilities for assessing vascular disease status. For instance, integrating a disposable pressure sensor into a PDMS microfluidic device enables the measurement of channel occlusion by tracking pressure changes over time.85 In an alternative approach, a set of specialized electronic components, such as a digital pressure regulator, solenoid valves and pressure sensors were custom-designed to independently regulate four bioreactors.51 A pressure sensor was also integrated into microfluidic chip using a capillary to track pressure changes. It monitored alterations in pressure over time by observing the movement of the liquid-gas boundary inside the capillary.109 Furthermore, the occlusion time can be determined by utilizing the pressure sensor integrated into the pump. In a particular investigation, a roller pump was manually tuned to sustain a consistent pressure head of 30 mmHg. Once the flow rate reached a minimum threshold, the pump was deactivated, establishing the occlusion time.86 Advanced detection tools, like surface acoustic wave lysis devices and multiplexed sensors, can also be incorporated into microfluidic chips to quantitatively and rapidly measure miRNAs, particularly in the context of ischemia-reperfusion injury. These tools have been used to detect miRNA markers in effluents from different stages of myocardial infarction, facilitating a comprehensive assessment of disease progression.99
3. Examples of vascular disease modeling on a chip
3.1. Atherosclerosis on a chip
Arteriosclerosis, a chronic vascular disorder presented by vessel narrowing and arterial occlusion, is the principal cause of cardiovascular morbidity and mortality worldwide. It is a complex disease that involves initial chronic vascular inflammation initiated by lipid retention, endothelial dysfunction due to the disturbances of blood, the excessive infiltration of immune cells, and phenotypic switching of SMCs that eventually results in plaque rupture and thrombosis.91,110 Disturbed hemodynamic conditions and immune inflammation are considered two pivotal points for modeling atherosclerosis-on-a-chip in vitro.14
Elevated shear stress and disturbed flow of blood preferentially occur at the arterial bifurcations and branching points, resulting in endothelial dysfunction as well as atherosclerotic lesions.16 Considering the spatial and temporal variations of hemodynamic forces at the site of the lesion, microfluidics has gained considerable attention in simulating these rheological factors due to the significant advantage of geometrical complexity and hierarchical microstructure of microchannels and the elaborate control of flow within the chips. For instance, using microchannels with 50% and 80% stenosed regions, monocyte cells were perfused over inflamed ECs at different WSS of 1-10dyn/cm2 to study the effect of microscale perturbation of flow on monocyte recruitment.83 Shear-induced platelet aggregation at 2D and 3D stenosis regions (50%−85%) was observed under varying WSS of 100-1000dyn/cm2. When the inlet WSS was lower than 10dyn/cm2, the blood flow remained laminar, and platelet binding was minimal.52
Additional cyclic tensile and compressive forces can be applied to the chips, thus simulating cyclical mechanical strain and circumferential stretch of the ECs and SMCs for modeling pulsatile blood flow and muscular contractions in vascular tissues.43 The employment of synergistic cyclic stretch and fluid shear stress could enhance EC alignment and spreading, as well as crosstalk between ECs and SMCs in vitro.18,111 For instance, Zheng et al. utilized a normal flow shear stress (FSS) of 5.07 Pa and a normal cardiac cycle frequency (CS) of 1.17 Hz, which corresponds to the frequency at which the human heart undergoes one complete cycle of contraction and relaxation. These parameters were chosen to represent physiological and pathophysiological conditions of human artery hemodynamics.18 Pathophysiological conditions were simulated with abnormal FSS at 1.16Pa while maintaining a normal CS, and high CS at 2Hz with normal FSS represented tachycardia-induced hemodynamics. Lastly, combining abnormal FSS with high CS simulated severe atherosclerotic conditions in human arteries.18
The adjustable stiffness of the chip substrate by means of various polymers could contribute to recreating pathological vascular stiffness, which is strongly correlated with the microenvironment of early atherosclerotic lesions. For instance, polyacrylamide/acrylamide ratios were manipulated in a study done by Shin et al. to achieve physiologically relevant elasticity (Young’s modulus) ranging from 2.4 to 19.2kPa and a pathologically high elasticity of 153.6kPa. The applied shear stress of approximately 9dyn/cm2 represented an intermediate value, falling between the normal and atheroprone shear ranges.33
Hyperlipidemia, hyperglycemia, and inflammatory response are linked to the increased oxidative stress and endothelial activation that is clinically associated with atherosclerosis.43 The over-retention of oxide low-density lipoprotein cholesterol (ox-LDL) in the subendothelial ECM will initiate endothelial inflammation, following the phenotypic switching of SMCs from a quiescent state to a proliferate state and leukocyte recruitment, eventually leading to migration of SMCs to intima layer to initiate the growth of unstable plaques. In some cases, hyperlipidemic factors and inflammatory cytokines, such as ox-LDL, lipopolysaccharides (LPS), TNF-α and IL-1β were directly incorporated into the microfluidic chips as determination of atherosclerosis-prone biochemical conditions.36 These small molecules enable precise control of the diseased microenvironment at various stages of pathological development within the chip by means of the dynamic flow of cytokine-contained culture medium.
The coating of the chip substrate with hydrogels is an effective tool for recapitulating the interface of the arterial wall comprised of the tunica intima, formed by EC-layer, and tunica media with SMCs (Figure 2d).34,36 Based on such engineered 3D arterial walls, the dynamic migration of SMCs could be monitored in response to inflammatory factors, which is essential for investigating the pathogenesis of atherosclerosis. Overall, microfluidics exhibited remarkable advantages in modeling diseased vascular models in terms of scales, matrix composition, cellular fidelity, and architectural variability.112
3.2. Thrombosis on a chip
Similar to atherosclerosis, thrombosis can lead to severe complications due to the occlusion of blood flow. While atherosclerosis is caused by fatty deposits on artery walls, thrombosis involves coagulation and clot formation that blocks blood flow. By manipulating the flow rates and channel dimensions, microfluidic systems are able to mimic thrombosis at various shear rates, ranging from ~100 s−1 to ~10000 s−1 or more, which could be induced via arterial pathology and stenotic disease.113 They can also replicate complex flow patterns such as pulsatile motion and shear gradients, aiding study of conditions like thrombosis near venous valves.114 Through altering channel diameter, microfluidic devices can generate stenosis regions with increased shear rates, enabling assessment of clot formation kinetics and location in a controlled environment. For instance, maximum WSS of 3-100dyn/cm2 with an stenosis of ~55% have been shown a good approximation to recapitulate the physiological conditions of thrombosis in a stenosed arterioles.85 When the channel geometry includes sudden expansions and undercuts, it can create pockets that exhibit primary and secondary vortices, resembling the flow patterns found in venous valves. In these pockets, a fibrin gel initially forms within the secondary vortex.84 A 3:1 stenosis ratio could be selected to mimic the ratio of vessel diameter/the distance between valve leaflets in the human greater saphenous vein and superficial femoral vein (Figure 2e).84
By optimization of channel design, stenosis geometry, and flow rate, it is possible to induce platelet adhesion at desired locations and retrieve the intact platelet aggregations.86,92 A maximum shear rate of 10000s−1 was shown to be required for rapid platelet accumulation in an 80% stenosis region.86 Moreover, arrays of micro posts in channels can induce localized shear gradients and be used to study neutrophil-platelet thrombi interactions in a 3D flow field.22 Under a shear rate of 1000s−1, platelets could adhere to the VWF-coated posts (80µm diameter) in 3min, resulting in thrombi formation with 70-90µm widths.22
Comprehensive assessment of thrombosis can be achieved by endothelialized microchannels, allowing for induction of cell injury through inflammation, oxidative stress, and physical stimulation to initiate coagulation cascade and thrombosis.13 For instance, cell stimulation can be conducted by optical irradiation of the channels. As reported, perfusion of blood containing microplastics after the stimulation overexpresses fibrinogen and promotes thrombosis.89 Alternatively, through a T-junction, ferric chloride can be introduced to a perpendicular endothelialized channel to induce oxidative damage and create FeCl3 thrombosis model with 50mM FeCl3 concentration at the wall of the lumen.93 Endothelialized microchannels with normal and stenotic geometries can be employed to compare thrombosis formation under physiologically relevant shear rates using human whole blood perfusion.59 However, channel endothelialization is challenging as the thrombotic relevant shear rates dissociate the cells. Fixation of endothelium can enhance their robustness while supporting thrombus formation at an arterial shear rate.87 In another strategy, lumens lined with ECs can be embedded in fibroblasts containing hydrogel through 3D printing to trigger fibrosis and thrombosis.42 Similarly, endothelialized networks with various geometries and diameters could be fabricated in collagen type I matrix. At a WSS of 5dyn/cm2, the cells could secrete 1-6mm VWFs without sufficient metalloprotease ADAMTS13, leading to platelet binding and channel occlusion (Figure 2f).37
Thrombogenic agents such as collagen, tissue factors, fibrin, fibrinogen, and VWF are usually incorporated in microchannels to initiate clot formation.17,88 Choi et al., for instance, used a collagen coated PDMS channel with circular cross-section and showed thrombus growth process in an 83.73% stenosis region (Figure 2g).17 The channels can be coated with collagen and tissue factor patch to simulate plaque rupture and platelet activation, allowing for determination of “time to occlude” and investigation of anti-platelet drug effects.20,21 As another example, collagen or VWF coated microchannels have been used to study vaccine-induced immune thrombotic thrombocytopenia, where blood containing anti-PF4 antibody could induce clot formation on the chip.90,91 Modifying the micro topography and wettability of microchannels has also been shown to alter their thrombogenic properties.96
Thrombosis models have also been established by application of patient-specific blood/cell samples. For instance, whole blood from hematological thrombocytopenic patients was perfused into collagen and tissue factor-coated microchannels to study hemostasis via total thrombus formation analysis (T-TAS).15 Additionally, laminin-coated microfluidics equipped with a gas exchanger, high-throughput microscopic imaging, and data analysis methods were employed to associate the Sickle red blood cells adhesion profile to inherited haemoglobin (Hb) disorders in different patients.35 Interestingly, Zhao et al. used MRV images of cerebral venous sinus thrombosis (CVST) patients to 3D print patient-specific cerebral venous sinus (CVS) on a chip, where they could recapitulate Virchow’s triad and assess thrombosis.19
3.3. Organ specific models
OOCs enable investigating the reciprocal effect of diseased organs on vascular dysfunctions. Due to space limitations, we only mention a few representative examples here and invite readers to consult recent reviews on the topic.7,11,71,115,116 Vascularized tumors on chip, particularly, have recently gained much attention, demonstrating the efficacy of OOCs for such complex interactions.68,105,117 For instance, Heinrich et al. utilized a multi-layered vascularized OOC model of pancreatic cancer to investigate fibrosis-induced compression of vasculature.41 They found that activation of pancreatic stellate cells and ECM production led to vessel compression, and that cancer-associated fibroblast modulatory therapeutics could inhibit this effect. Vascularized human bone marrow niches were also designed via two 865 μm wide hexagonal chambers connected by 80 μm long two-way ports.105 Researchers have also employed a vascularized OOC model of the human omentum and ovarian tumor microenvironment to investigate the effect of stromal cells on tumor cell growth and vascular permeability, revealing the critical role of tumor cells in ascites formation.68 A lung alveolus-on-a-chip with alveolar epithelium and endothelium was also developed by Jain et al.107 Perfusing blood in the chip allowed for analysis of thrombosis and platelet-endothelial interactions during inflammation-induced acute lung injury and testing of potential therapeutics.107 Soon et al. replicated brain arteriovenous malformations via mosaic culture of wild-type and KRAS-mutated endothelial cells in a microfluidic platform, resulting in large, leaky microvessels.70 MEK inhibitor could improve tight junctions and barrier functionality.70 Cherubini et al. introduced hydrogel-laden microfluidics for generating fetal-like vessels on a chip, allowing for the study of pericyte-endothelial interactions and stromal cells effect on vascular remodeling.104 Another work modelled personalized intracranial aneurysms on a chip using patient-specific cells and flow parameters, where the effect of shear stress on elongation and numbers of pluripotent stem cell-derived ECs (hiPSC-ECs) was compared with those of HUVECs.106 In cardiac tissues, vascular cells determine the magnitude of diastolic dysfunction upon inflammatory agent stimulation.118
3.4. Inflammation and other vascular dysfunctions
OOCs can also provide insight into vascular dysfunction in infections and chronic inflammation. Qiu et al. developed a vasculature-on-a-chip mimicking vessel stiffness and endothelial barrier function.35 The chip allowed real-time observation of microvascular obstruction and endothelial permeability, revealing how sickle-cell disease or malaria-infected erythrocytes cause reversible endothelial dysfunction.35 Another microvascular model was used to investigate microcirculatory obstruction and hematologic disorders in malaria by mimicking arteriole-capillary-venule transition and observing red blood cell (RBC) movement.38 The design had ~11,600 channels (5µm diameter) per mm2 which could be tuned to match the channel density in the human heart. The model showed a swift buildup of plasmodium falciparum-infected RBCs in capillary. Using the InVADE design, researchers modeled the interaction of SARS-CoV-2 virus with endothelial cells and peripheral blood mononuclear cells (Figure 2g).66 The virus disrupted endothelial barrier function and elevated inflammatory cytokines. Angiopoietin-1 derived peptide mitigated the inflammation and boosted barrier function against the infection.66 A new 64-chip microfluidic platform has also been developed to create vascularized-lower respiratory tract, allowing for study of SARS-CoV-2 infectivity and thrombotic events.103 In an innovative design, the use of nanofibrillar collagen with longitudinal and circumferential alignments to create vascular channels with 1mm diameter, enabled investigation of anti-inflammatory function.39 Aligned patterning may have therapeutic benefit in preventing atherosclerotic lesion formation.39
Furthermore, other vascular conditions such as ischemia-reperfusion 31,32,76,78,98–100, Hutchinson-Gilford progeria syndrome 119, vascular malformation 102, and pulmonary arterial hypertension injury 23,29,101 have been modeled on a chip. For example, in a microfluidic design, microgrooves connecting parallel channels enabled neuronal segregation and axonal growth for modeling central nervous system (CNS) injury‑ischemia. While neuronal cell bodies and dendrites were confined within the soma chamber, axons extended into the axon terminal chamber via 450 μm-long microgrooves.98 Amar et al. also showed a microfluidic model for ischemia-reperfusion injury (IRI) where re-perfusion of the channel was possible after thrombolytic treatment.100 Considering the distinctive attributes of ischemic stroke, marked by a swift decline in oxygen and nutrient levels, replicating both hypoxia and nutrient deprivation via microfluidic devices becomes crucial.31 Traditional in vitro models relying on Petri dishes struggle to accurately reproduce the oxygen levels observed during a stroke. McBain et al. introduced Graddient design where a microchamber loaded with collagen and astrocytes was situated beneath a microwell array, enabling precise regulation of nutrient depletion within the microchamber for continuous real-time monitoring.31 In a polystyrene-made microfluidic device developed by Denecke et al., one could simultaneously generate oxygen and nutrient gradients within a microchamber. The design replicated ischemia induced necrosis and penumbra, and illustrated that under conditions of hypoxia and nutrient deficiency, signaling abilities of astrocytes within the penumbral region significantly changed.32 The elevated shear stress and disturbed blood flow, occurring predominantly at arterial bifurcations and branching points, were stimulated by adjusting the geometric complexity and hierarchical microstructure of microchannels, applying additional cyclic tensile and compressive forces, as well as precise control over the flow of liquids within the chips 76,102,120,121. The benefits of adjustable parameters within these devices enable the simulation of blood-related disorders, such as vascular malformation and pulmonary arterial hypertension (PAH).122 For instance, to study PAH, parallel channels separated via micro pillars were utilized to create perivascular, adventitial, medial, intimal and luminal layers of a pulmonary artery.23,101 Different shear stresses in the range of 0.1 to 15 dyn/cm2 were applied in this study.23 In another work, human pulmonary artery endothelial cells (HPAECs) and human pulmonary artery smooth muscle cells (HPASMCs) were cultured on two channels separated via a PET membrane with 400nm pore size. Under the human lung arterioles shear stress of 6 dyn/cm2, PAH was investigated.29 Overall, these models have enabled a better understanding of the cellular and molecular events underlying this phenomenon and the development of potential therapeutic interventions.
4. Limitations of organ-on-a-chip models and Future Outlook
In this review we demonstrated that organ-on-a-chip systems have a great potential for modeling vascular diseases. Despite this promise, significant challenges remain in replicating the intricate biology of these diseases accurately. Here we will provide a discussion of these obstacles and propose potential directions for improvement.
Vascular disease modeling in vitro will often require multiple cell types and the appropriate ECM that compose the natural vasculature, which is often challenging to mimic. For example, most of the models are composed of microchannels seeded with endothelial cells, lacking the existence of other cell types, such as smooth-muscle cells and immune cells, which are crucial for mimicking the environment required for recapitulating disease conditions. Atherosclerosis, for example, will benefit from the incorporation of monocytes and macrophages for better mimicking plaque formation. ECM components that were used for cell seeding are often derived from the tumor (Matrigel or other Matrigel-like products) with high batch-to-batch variability or blood clotting material (fibrinogen) and cannot recapitulate the healthy microenvironment of the vasculature.123 Thus, ECM products with defined composition tailored to the target diseases would be an ideal replacement.
Often, the scale of the vessels in the system will be different from the scale needed to recapitulate the disease conditions exactly. It is difficult to reproduce branching form the µm scale of capillaries to the mm scale of coronary arteries. Developing platforms with the right dimension and the appropriate shear stress is still not achieved at all length scales (e.g <10µm and >1mm). Often, these platforms require external pumping to achieve a specific fluid flow, which can be difficult to scale up and will affect throughput in drug testing.
Another critical limitation that is poorly studied in current OOC systems is the lack of systemic circulation. Systemic infection and inflammation are the leading cause of hemostatic abnormalities. This aspect is challenging to mimic as most of the OOCs are composed of very few organ- or tissue-specific compartments, which commonly do not include immune system organs. The addition of PBMCs66 or CD14+ immune cells only partially recapitulates the cascade of in vivo immune responses as they commonly lack immunogenic cells, i.e. neutrophils and eosinophils, as well as the immune organs, i.e. spleen, thymus, or lymph nodes, that correspond to the adaptive immunity. The use of organ-specific endothelial cells is currently performed only rarely, and will be required for high fidelity modeling.
The longevity of the Organ-on-a-Chip culture is typically limited to 14–21 days, where the vasculature lining and added PBMCs can regress or die off due to the unfit in vitro microenvironment, or lack of the replacement. This will hinder the opportunity of modeling chronic diseases. Lastly, like other organ-on-a-chip devices, the common use of PDMS will affect the reliability of therapeutic drug screening, therefore, replacement with low-absorbable materials, such as polystyrene or polycarbonate will be required.
Highlights:
Organ-on-a-chip (OOC) systems have emerged as a powerful tool for studying vascular diseases in a more physiologically relevant environment.
OOCs can model various aspects of vascular diseases, including atherosclerosis, thrombosis, and inflammations.
Organ specific models offer new opportunities for drug discovery and personalized medicine.
Sources of Funding:
Our work is funded by the Canadian Institutes of Health Research (CIHR) Foundation Grant FDN- 167274, Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN 326982-10), Stem Cell Network Impact Award 920530, National Institutes of Health Grant 2R01 HL076485. M.R. was supported by Killam Fellowship and Canada Research Chair.
Abbreviations:
- 1,2,4 polymer
Poly(octamethylene maleate (anhydride) 1,2,4-butanetricarboxylate)
- 2D
2-dimensional
- 3D
3-dimensional
- ADCs
Adventitial cells
- ADs
Adipocytes
- AECs
Primary human alveolar epithelial cells
- BAECs
Primary bovine aortic endothelial cells
- BMSCs
Bone marrow stromal cells
- CAD
Coronary artery disease
- CNS
Central nervous system
- CTA
Computed tomography angiography
- CVS
Cerebral venous sinus
- CVST
Cerebral venous sinus thrombosis
- ECs
Endothelial cells
- ECM
Extracellular matrix
- EDTA
Ethylenediaminetetraacetic acid
- ETV2
E26 transformation-specific variant 2
- FSS
Flow shear stress
- GelMA
Methylacrylated gelatin
- GFP
Green fluorescence protein
- HAECs
Primary human aortic endothelial cells
- Hb
Haemoglobin
- HBVPCs
Human brain vascular pericytes
- hCB-ECs
Human cord blood derived endothelial cells
- HDMVECs
Human dermal microvascular endothelial cells
- hFOB
Human fetal osteoblast
- hiPSC-EC
Human induced pluripotent stem cell-derived endothelial cells
- HLMVECs
Human lung microvascular endothelial cells
- HPAECs
Human pulmonary artery endothelial cells
- HPASMCs
Human pulmonary artery smooth muscle cells
- HPMECs
Human pulmonary microvascular endothelial cells
- HUASMC
Human umbilical artery SMCs
- HUASMCs
Human umbilical arterial smooth muscle cells
- HUVECs
Human umbilical vein endothelial cells
- iCMs
Induced cardiomyocytes
- iECs
Induced endothelial cells
- IF
Interstitial flow
- IL-1β
Interleukin-1β
- Im-HUVECs
Immortalized human umbilical vein endothelial cells
- InVADE
Integrated vasculature for assessing dynamic events
- IPN
Interpenetrating polymer-network
- IRI
Ischemia-reperfusion injury
- iSMCs
Induced smooth muscle cells
- LDL
Low-density lipoprotein
- LPS
Lipopolysaccharides
- MRV
Magnetic resonance venography
- OOC
Organ-on-a-chip
- ox-LDL
Oxide low-density lipoprotein cholesterol
- PAD
Peripheral artery disease
- PAH
Pulmonary arterial hypertension
- PAH-ECs
PAH-afflicted pulmonary arterial endothelial
- PC
Polycarbonate
- PDMS
Polydimethylsiloxane
- PEGDA
Polyethylene glycol diacrylate
- PET
Polyethylene terephthalate
- PICO
Poly(itaconate-co-citrate-co-octanediol)
- POMaC
Poly (octamethylene maleate (anhydride)
- PS
Polystyrene
- PSCs
Pancreatic stellate cells
- RBC
Red blood cell
- SLA
Stereolithography
- SMCs
Smooth muscle cells
- TF
Tissue factor
- TNF-α
Tumor necrosis factor-α
- T-TAS
Total thrombus formation analysis
- VCAM-1
Vascular cell adhesion molecule-1
- VWF
Von Willebrand factor
- WSS
Wall shear stress
- YAP
Yes-associated protein
Footnotes
Disclosures: M.R. and Y.Z. are inventors on a patent licensed to Valo Health and they are receiving licensing revenue from this invention.
References
- 1.Rafii S, Butler JM, Ding B-S. Angiocrine functions of organ-specific endothelial cells. Nature 2016;529:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Lin L, Qiao L. Recent developments in organ-on-a-chip technology for cardiovascular disease research. Analytical and Bioanalytical Chemistry 2023:1–15. [DOI] [PubMed]
- 3.Thondapu V, Bourantas CV, Foin N, Jang I-K, Serruys PW, Barlis P. Biomechanical stress in coronary atherosclerosis: emerging insights from computational modelling. European heart journal 2017;38:81–92. [DOI] [PubMed] [Google Scholar]
- 4.Bowley G, Kugler E, Wilkinson R, Lawrie A, van Eeden F, Chico TJA, Evans PC, Noël ES, Serbanovic-Canic J. Zebrafish as a tractable model of human cardiovascular disease. British Journal of Pharmacology 2022;179:900–917. doi: 10.1111/bph.15473 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Sano S, Ogawa H, Horitani K, Evans MA, Yura Y, Miura-Yura E, Doviak H, Walsh K. Murine models of clonal haematopoiesis to assess mechanisms of cardiovascular disease. Cardiovascular Research 2021;118:1413–1432. doi: 10.1093/cvr/cvab215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noonan J, Grassia G, MacRitchie N, Garside P, Guzik TJ, Bradshaw AC, Maffia P. A novel triple-cell two-dimensional model to study immune-vascular interplay in atherosclerosis. Frontiers in Immunology 2019;10:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Wang EY, Lai FB, Cheung K, Radisic M. Organs-on-a-chip: a union of tissue engineering and microfabrication. Trends in Biotechnology 2023. [DOI] [PMC free article] [PubMed]
- 8.Ye C, Ali S, Sun Q, Guo M, Liu Y, Gao Y, Huo B. Novel cone-and-plate flow chamber with controlled distribution of wall fluid shear stress. Computers in Biology and Medicine 2019;106:140–148. doi: 10.1016/j.compbiomed.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Huang N, Yang Z. Revealing the Role of Zinc Ions in Atherosclerosis Therapy via an Engineered Three‐Dimensional Pathological Model. Advanced Science 2023:2300475. [DOI] [PMC free article] [PubMed]
- 10.Ryu J-K, Bouchoux C, Liu HW, Kim E, Minamino M, de Groot R, Katan AJ, Bonato A, Marenduzzo D, Michieletto D. Bridging-induced phase separation induced by cohesin SMC protein complexes. Science advances 2021;7:eabe5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini V, Mallone A, Nasrollahi F, Ostrovidov S, Nasiri R, Mahmoodi M, Haghniaz R, Baidya A, Salek MM, Darabi MA, et al. Healthy and diseased in vitro models of vascular systems. Lab on a Chip 2021;21:641–659. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor C, Brady E, Zheng Y, Moore E, Stevens KR. Engineering the multiscale complexity of vascular networks. Nat Rev Mater 2022;7:702–716. doi: 10.1038/s41578-022-00447-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai Y, Hardy ET, Lam WA. Hemostasis-on-a-chip / incorporating the endothelium in microfluidic models of bleeding. In: Platelets Taylor & Francis; 2023:2185453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers DR, Lam WA. Vascularized Microfluidics and Their Untapped Potential for Discovery in Diseases of the Microvasculature. Annual Review of Biomedical Engineering 2021;23:407–432. doi: 10.1146/annurev-bioeng-091520-025358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samanbar S, Piñeyroa J, Moreno-Castaño AB, Pino M, Torramade-Moix S, Martinez-Sanchez J, Moskowitz KA, Alexander A, Escolar G, Diaz-Ricart M. Hemostatic Ability of Thrombosomes® in Blood from Thrombocytopenic Patients Using the Total Thrombus Formation Analysis System (T-TAS) and Confocal Microscopy in Microfluidic Chambers. In: Blood 2022:11242–11243. [Google Scholar]
- 16.Walther BK, Rajeeva Pandian NK, Gold KA, Kiliç ES, Sama V, Gu J, Gaharwar AK, Guiseppi-Elie A, Cooke JP, Jain A. Mechanotransduction-on-chip: vessel-chip model of endothelial YAP mechanobiology reveals matrix stiffness impedes shear response. Lab Chip 2021;21:1738–1751. doi: 10.1039/d0lc01283a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JS, Ham DH, Kim JH, Marcial HBF, Jeong PH, Choi JH, Park WT. Quantitative image analysis of thrombus formation in microfluidic in-vitro models. Micro and Nano Systems Letters 2022;10. doi: 10.1186/s40486-022-00166-3 [DOI] [Google Scholar]
- 18.Zheng W, Huang R, Jiang B, Zhao Y, Zhang W, Jiang X. An Early-Stage Atherosclerosis Research Model Based on Microfluidics. Small 2016;12:2022–2034. doi: 10.1002/smll.201503241 [DOI] [PubMed] [Google Scholar]
- 19.Zhao YC, Zhang Y, Wang Z, Jiang F, Kyanian K, Aye SSS, Hong T, Vatankhah P, Nasser A, Sun A, et al. Novel Movable Typing for Personalized Vein-Chips in Large Scale: Recapitulate Patient-Specific Virchow’s Triad and its Contribution to Cerebral Venous Sinus Thrombosis. In: Advanced Functional Materials 2023:2214179. [Google Scholar]
- 20.Berry J, Peaudecerf FJ, Masters NA, Neeves KB, Goldstein RE, Harper MT. An “occlusive thrombosis-on-a-chip” microfluidic device for investigating the effect of anti-thrombotic drugs. In: Lab on a Chip Royal Society of Chemistry; 2021:4104–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry J, Harper MT. Protease-activated receptor antagonists prevent thrombosis when dual antiplatelet therapy is insufficient in an occlusive thrombosis microfluidic model. In: Research and Practice in Thrombosis and Haemostasis Elsevier Masson SAS; 2022:e12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju LA, Kossmann S, Zhao YC, Moldovan L, Zhang Y, De Zoysa Ramasundara S, Zhou F, Lu H, Alwis I, Schoenwaelder SM, et al. Microfluidic post method for 3-dimensional modeling of platelet-leukocyte interactions. In: Analyst 2022:1222–1235. [DOI] [PubMed]
- 23.Al-Hilal TA, Keshavarz A, Kadry H, Lahooti B, Al-Obaida A, Ding Z, Li W, Kamm R, McMurtry IF, Lahm T. Pulmonary-arterial-hypertension (PAH)-on-a-chip: fabrication, validation and application. Lab on a Chip 2020;20:3334–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shakeri A, Jarad NA, Khan S, Didar TF. Bio-functionalization of microfluidic platforms made of thermoplastic materials: A review. Analytica Chimica Acta 2022;1209:339283. doi: 10.1016/j.aca.2021.339283 [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019;176:913–927. e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Masse S, Kim J, Reis L, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nature Materials 2016;15:669–678. doi: 10.1038/nmat4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai BFL, Lu RXZ, Hu Y, Davenport Huyer L, Dou W, Wang EY, Radulovich N, Tsao MS, Sun Y, Radisic M. Recapitulating Pancreatic Tumor Microenvironment through Synergistic Use of Patient Organoids and Organ‐on‐a‐Chip Vasculature. Advanced Functional Materials 2020;30:2000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davenport Huyer L, Bannerman AD, Wang Y, Savoji H, Knee-Walden EJ, Brissenden A, Yee B, Shoaib M, Bobicki E, Amsden BG, Radisic M. One-Pot Synthesis of Unsaturated Polyester Bioelastomer with Controllable Material Curing for Microscale Designs. Adv Healthc Mater 2019;8:e1900245. doi: 10.1002/adhm.201900245 [DOI] [PubMed] [Google Scholar]
- 29.Wojciak-Stothard B, Ainscough A, Smith T, Rhodes C, Fellows A, Howard L, Wharton J, Wilkins M, Edel J. A microfluidic chip for pulmonary arterial hypertension 2021.
- 30.Ronaldson-Bouchard K, Teles D, Yeager K, Tavakol DN, Zhao Y, Chramiec A, Tagore S, Summers M, Stylianos S, Tamargo M, et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat Biomed Eng 2022;6:351–371. doi: 10.1038/s41551-022-00882-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed-McBain C, Turaga RV, Zima S, Campo SA, Riendeau J, Guzman EC, Juang T, Juang DS, Hampton D, Skala M. Microfluidic device with reconfigurable spatial temporal gradients reveals plastic astrocyte response to stroke and reperfusion. Lab on a Chip 2023. [DOI] [PubMed]
- 32.Denecke KM, McBain CA, Hermes BG, Teertam SK, Farooqui M, Virumbrales-Muñoz M, Panackal J, Beebe DJ, Famakin B, Ayuso JM. Microfluidic model to evaluate astrocyte activation in penumbral region following ischemic stroke. Cells 2022;11:2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin Y, Lim S, Kim J, Jeon JS, Yoo H, Gweon B. Emulating endothelial dysfunction by implementing an early atherosclerotic microenvironment within a microfluidic chip. Lab Chip 2019;19:3664–3677. doi: 10.1039/c9lc00352e [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Maharjan S, Kuang X, Wang Z, Mille LS, Tao M, Yu P, Cao X, Lian L, Lv L, et al. Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci Adv 2022;8:eabq6900. doi: 10.1126/sciadv.abq6900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Y, Ahn B, Sakurai Y, Hansen CE, Tran R, Mimche PN, Mannino RG, Ciciliano JC, Lamb TJ, Joiner CH. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nature biomedical engineering 2018;2:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su C, Menon NV, Xu X, Teo YR, Cao H, Dalan R, Tay CY, Hou HW. A novel human arterial wall-on-a-chip to study endothelial inflammation and vascular smooth muscle cell migration in early atherosclerosis. Lab Chip 2021;21:2359–2371. doi: 10.1039/d1lc00131k [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Chen J, López JA. Flow-driven assembly of VWF fibres and webs in in vitro microvessels. In: Nature Communications 2015:7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arakawa C, Gunnarsson C, Howard C, Bernabeu M, Phong K, Yang E, DeForest CA, Smith JD, Zheng Y. Biophysical and biomolecular interactions of malaria-infected erythrocytes in engineered human capillaries. Science Advances 2020;6:eaay7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayama KH, Joshi PA, Lai ES, Gujar P, Joubert L-M, Chen B, Huang NF. Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. Regenerative medicine 2015;10:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA. In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the national academy of sciences 2012;109:9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinrich MA, Uboldi I, Kuninty PR, Ankone MJ, van Baarlen J, Zhang YS, Jain K, Prakash J. Microarchitectural mimicking of stroma-induced vasculature compression in pancreatic tumors using a 3D engineered model. Bioactive Materials 2023;22:18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YS, Davoudi F, Walch P, Manbachi A, Luo X, Dell’Erba V, Miri AK, Albadawi H, Arneri A, Li X, et al. Bioprinted thrombosis-on-a-chip. In: Lab on a Chip Royal Society of Chemistry; 2016:4097–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Huang N, Yang Z. Revealing the Role of Zinc Ions in Atherosclerosis Therapy via an Engineered Three-Dimensional Pathological Model. Advanced Science n/a:2300475. doi: 10.1002/advs.202300475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhusal A, Dogan E, Nguyen H-A, Labutina O, Nieto D, Khademhosseini A, Miri AK. Multi-material digital light processing bioprinting of hydrogel-based microfluidic chips. Biofabrication 2021;14:014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abudupataer M, Chen N, Yan S, Alam F, Shi Y, Wang L, Lai H, Li J, Zhu K, Wang C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomedical microdevices 2020;22:1–10. [DOI] [PubMed] [Google Scholar]
- 46.Shakeri A, Khan S, Didar TF. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab on a Chip 2021;21:3053–3075. [DOI] [PubMed] [Google Scholar]
- 47.Shakeri A, Jarad NA, Leung A, Soleymani L, Didar TF. Biofunctionalization of Glass- and Paper-Based Microfluidic Devices: A Review. Advanced Materials Interfaces 2019;6:1900940. doi: 10.1002/admi.201900940 [DOI] [Google Scholar]
- 48.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab on a Chip 2013;13:1489–1500. [DOI] [PubMed] [Google Scholar]
- 49.Chin L, Yu J, Fu Y, Yu T, Liu A, Luo K. Production of reactive oxygen species in endothelial cells under different pulsatile shear stresses and glucose concentrations. Lab on a Chip 2011;11:1856–1863. [DOI] [PubMed] [Google Scholar]
- 50.Ren L, Liu W, Wang Y, Wang J-C, Tu Q, Xu J, Liu R, Shen S-F, Wang J. Investigation of hypoxia-induced myocardial injury dynamics in a tissue interface mimicking microfluidic device. Analytical chemistry 2013;85:235–244. [DOI] [PubMed] [Google Scholar]
- 51.Parsa H, Wang BZ, Vunjak-Novakovic G. A microfluidic platform for the high-throughput study of pathological cardiac hypertrophy. Lab on a Chip 2017;17:3264–3271. [DOI] [PubMed] [Google Scholar]
- 52.Menon NV, Su C, Pang KT, Phua ZJ, Tay HM, Dalan R, Wang X, Li KHH, Hou HW. Recapitulating atherogenic flow disturbances and vascular inflammation in a perfusable 3D stenosis model. Biofabrication 2020;12:045009. doi: 10.1088/1758-5090/aba501 [DOI] [PubMed] [Google Scholar]
- 53.Xi W, Kong F, Yeo JC, Yu L, Sonam S, Dao M, Gong X, Lim CT. Soft tubular microfluidics for 2D and 3D applications. Proceedings of the National Academy of Sciences 2017;114:10590–10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen TQ, Park W-T. Rapid, low-cost fabrication of circular microchannels by air expansion into partially cured polymer. Sensors and Actuators B: Chemical 2016;235:302–308. [Google Scholar]
- 55.Luan H, Zhang Q, Liu T-L, Wang X, Zhao S, Wang H, Yao S, Xue Y, Kwak JW, Bai W. Complex 3D microfluidic architectures formed by mechanically guided compressive buckling. Science advances 2021;7:eabj3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savoji H, Davenport Huyer L, Mohammadi MH, Lun Lai BF, Rafatian N, Bannerman D, Shoaib M, Bobicki ER, Ramachandran A, Radisic M. 3D printing of vascular tubes using bioelastomer prepolymers by freeform reversible embedding. ACS Biomaterials Science & Engineering 2020;6:1333–1343. [DOI] [PubMed] [Google Scholar]
- 57.Liu C, Campbell SB, Li J, Bannerman D, Pascual-Gil S, Kieda J, Wu Q, Herman PR, Radisic M. High Throughput Omnidirectional Printing of Tubular Microstructures from Elastomeric Polymers. Advanced Healthcare Materials 2022;11:2201346. doi: 10.1002/adhm.202201346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R, Larsen NB. Stereolithographic hydrogel printing of 3D culture chips with biofunctionalized complex 3D perfusion networks. Lab on a Chip 2017;17:4273–4282. [DOI] [PubMed] [Google Scholar]
- 59.Costa PF, Albers HJ, Linssen JEA, Middelkamp HHT, Van Der Hout L, Passier R, Van Den Berg A, Malda J, Van Der Meer AD. Mimicking arterial thrombosis in a 3D-printed microfluidic: In vitro vascular model based on computed tomography angiography data. In: Lab on a Chip Royal Society of Chemistry; 2017:2785–2792. [DOI] [PubMed] [Google Scholar]
- 60.Nie J, Gao Q, Xie C, Lv S, Qiu J, Liu Y, Guo M, Guo R, Fu J, He Y. Construction of multi-scale vascular chips and modelling of the interaction between tumours and blood vessels. Materials Horizons 2020;7:82–92. [Google Scholar]
- 61.Mori N, Morimoto Y, Takeuchi S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017;116:48–56. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Shridhar SV, Gerwitz M, Soman P. An in vitro vascular chip using 3D printing-enabled hydrogel casting. Biofabrication 2016;8:035015. [DOI] [PubMed] [Google Scholar]
- 63.Lai BFL, Huyer LD, Lu RXZ, Drecun S, Radisic M, Zhang B. InVADE: integrated vasculature for assessing dynamic events. Advanced functional materials 2017;27:1703524. [Google Scholar]
- 64.Lai BFL, Lu RXZ, Davenport Huyer L, Kakinoki S, Yazbeck J, Wang EY, Wu Q, Zhang B, Radisic M. A well plate–based multiplexed platform for incorporation of organoids into an organ-on-a-chip system with a perfusable vasculature. Nature protocols 2021;16:2158–2189. [DOI] [PubMed] [Google Scholar]
- 65.Zhang B, Lai BFL, Xie R, Davenport Huyer L, Montgomery M, Radisic M. Microfabrication of AngioChip, a biodegradable polymer scaffold with microfluidic vasculature. Nature protocols 2018;13:1793–1813. [DOI] [PubMed] [Google Scholar]
- 66.Lu RXZ, Lai BFL, Rafatian N, Gustafson D, Campbell SB, Banerjee A, Kozak R, Mossman K, Mubareka S, Howe KL, et al. Vasculature-on-a-chip platform with innate immunity enables identification of angiopoietin-1 derived peptide as a therapeutic for SARS-CoV-2 induced inflammation. Lab on a Chip 2022;22:1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heintz KA, Bregenzer ME, Mantle JL, Lee KH, West JL, Slater JH. Fabrication of 3D biomimetic microfluidic networks in hydrogels. Advanced healthcare materials 2016;5:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibrahim LI, Hajal C, Offeddu GS, Gillrie MR, Kamm RD. Omentum-on-a-chip: A multicellular, vascularized microfluidic model of the human peritoneum for the study of ovarian cancer metastases. Biomaterials 2022;288:121728. doi: 10.1016/j.biomaterials.2022.121728 [DOI] [PubMed] [Google Scholar]
- 69.Kim S, Chung M, Ahn J, Lee S, Jeon NL. Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab on a Chip 2016;16:4189–4199. [DOI] [PubMed] [Google Scholar]
- 70.Soon K, Li M, Wu R, Zhou A, Khosraviani N, Turner WD, Wythe JD, Fish JE, Nunes SS. A human model of arteriovenous malformation (AVM)-on-a-chip reproduces key disease hallmarks and enables drug testing in perfused human vessel networks. Biomaterials 2022;288:121729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salewskij K, Penninger JM. Blood Vessel Organoids for Development and Disease. Circulation Research 2023;132:498–510. doi: doi: 10.1161/CIRCRESAHA.122.321768 [DOI] [PubMed] [Google Scholar]
- 72.He W, Sharma D, Jia W, Zhao F. Fabrication of a completely biological and anisotropic human mesenchymal stem cell-based vascular graft. In: Zhao F, Leong KW, eds. Vascular Tissue Engineering: Methods and Protocols New York, NY: Springer US; 2022:101–114. [DOI] [PubMed] [Google Scholar]
- 73.Qian Z, Sharma D, Jia W, Radke D, Kamp T, Zhao F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics 2019;9:2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benedicto I, Dorado B, Andrés V. Molecular and cellular mechanisms driving cardiovascular disease in Hutchinson-Gilford progeria syndrome: lessons learned from animal models. Cells 2021;10:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zamorano M, Castillo RL, Beltran JF, Herrera L, Farias JA, Antileo C, Aguilar-Gallardo C, Pessoa A, Calle Y, Farias JG. Tackling ischemic reperfusion injury with the aid of stem cells and tissue engineering. Frontiers in physiology 2021;12:705256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stapleton LM, Farry JM, Zhu Y, Lucian HJ, Wang H, Paulsen MJ, Totherow KP, Roth GA, Brower KK, Fordyce PM. Microfluidic encapsulation of photosynthetic cyanobacteria in hydrogel microparticles augments oxygen delivery to rescue ischemic myocardium. Journal of Bioscience and Bioengineering 2023;135:493–499. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Y, Rafatian N, Wang EY, Feric NT, Lai BF, Knee-Walden EJ, Backx PH, Radisic M. Engineering microenvironment for human cardiac tissue assembly in heart-on-a-chip platform. Matrix Biology 2020;85:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veldhuizen J, Chavan R, Moghadas B, Park JG, Kodibagkar VD, Migrino RQ, Nikkhah M. Cardiac ischemia on-a-chip to investigate cellular and molecular response of myocardial tissue under hypoxia. Biomaterials 2022;281:121336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rogers MT, Gard AL, Gaibler R, Mulhern TJ, Strelnikov R, Azizgolshani H, Cain BP, Isenberg BC, Haroutunian NJ, Raustad NE. A high-throughput microfluidic bilayer co-culture platform to study endothelial-pericyte interactions. Scientific Reports 2021;11:12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palikuqi B, Nguyen DT, Li G, Schreiner R, Pellegata AF, Liu Y, Redmond D, Geng F, Lin Y, Gómez-Salinero JM, et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 2020;585:426–432. doi: 10.1038/s41586-020-2712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei W-J, Wang Y-C, Guan X, Chen W-G, Liu J. A neurovascular unit-on-a-chip: culture and differentiation of human neural stem cells in a three-dimensional microfluidic environment. Neural regeneration research 2022;17:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wörsdörfer P, Dalda N, Kern A, Krüger S, Wagner N, Kwok CK, Henke E, Ergün S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Scientific Reports 2019;9:15663. doi: 10.1038/s41598-019-52204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venugopal Menon N, Tay HM, Pang KT, Dalan R, Wong SC, Wang X, Li KHH, Hou HW. A tunable microfluidic 3D stenosis model to study leukocyte-endothelial interactions in atherosclerosis. APL Bioengineering 2018;2. doi: 10.1063/1.4993762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lehmann M, Schoeman RM, Krohl PJ, Wallbank AM, Samaniuk JR, Jandrot-Perrus M, Neeves KB. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI-dependent manner in an in vitro venous thrombosis model. In: Arteriosclerosis, Thrombosis, and Vascular Biology 2018:1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jain A, Graveline A, Waterhouse A, Vernet A, Flaumenhaft R, Ingber DE. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. In: Nature communications Nature Publishing Group; 2016:10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim DA, Ku DN. Structure of shear-induced platelet aggregated clot formed in an in vitro arterial thrombosis model. In: Blood Advances 2022:2872–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jain A, van der Meer AD, Papa AL, Barrile R, Lai A, Schlechter BL, Otieno MA, Louden CS, Hamilton GA, Michelson AD, et al. Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium. In: Biomedical Microdevices Biomedical Microdevices; 2016:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Trigani KT, Shankar KN, Crossen J, Liu Y, Sinno T, Diamond SL. Anti-GPVI Fab reveals distinct roles for GPVI signaling in the first platelet layer and subsequent layers during microfluidic clotting on collagen with or without tissue factor. In: Thrombosis Research Elsevier Ltd; 2022:112–129. [DOI] [PubMed] [Google Scholar]
- 89.Chen L, Zheng Y, Liu Y, Tian P, Yu L, Bai L, Zhou F, Yang Y, Cheng Y, Wang F, et al. Microfluidic-based in vitro thrombosis model for studying microplastics toxicity. Lab on a Chip 2022;22:1344–1353. doi: 10.1039/d1lc00989c [DOI] [PubMed] [Google Scholar]
- 90.Singh A, Toma F, Uzun G, Wagner TR, Pelzl L, Zlamal J, Freytag V, Weich K, Nowak-Harnau S, Rothbauer U, et al. The interaction between anti-PF4 antibodies and anticoagulants in vaccine-induced thrombotic thrombocytopenia. In: Blood 2022:3430–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leung HHL, Perdomo J, Ahmadi Z, Zheng SS, Rashid FN, Enjeti A, Ting SB, Chong JJH, Chong BH. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nature Communications 2022;13:5206–5206. doi: 10.1038/s41467-022-32946-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeom E, Park JH, Kang YJ, Lee SJ. Microfluidics for simultaneous quantification of platelet adhesion and blood viscosity. In: Scientific Reports Nature Publishing Group; 2016:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciciliano JC, Sakurai Y, Myers DR, Fay ME, Hechler B, Meeks S, Li R, Dixon JB, Lyon LA, Gachet C, Lam WA. Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. In: Blood 2015:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hardy ET, Sakurai Y, Lam WA. Miniaturized Vascularized Bleeding Model of Hemostasis. In: Rasponi M, ed. Organ-on-a-Chip: Methods and Protocols New York, NY: Springer US; 2022:159–175. [DOI] [PubMed] [Google Scholar]
- 95.Qiu Y, Sakurai Y, Lam WA. Monitoring the “Lifetime” of a Thrombus over Long Timescales By Leveraging a Novel Microvasculature-on-Chip Thrombosis Resolution Assay. Blood 2019;134:441. doi: 10.1182/blood-2019-128718 [DOI] [Google Scholar]
- 96.Xu Y, Deng P, Yu G, Ke X, Lin Y, Shu X, Xie Y, Zhang S, Nie R, Wu Z. Thrombogenicity of microfluidic chip surface manipulation: Facile, one-step, none-protein technique for extreme wettability contrast micropatterning. In: Sensors and Actuators, B: Chemical 2021:130085. [Google Scholar]
- 97.Li M, Ku DN, Forest CR. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab on a Chip 2012;12:1355–1362. [DOI] [PubMed] [Google Scholar]
- 98.Huang N, Sheng Z-H. Microfluidic devices as model platforms of CNS injury-ischemia to study axonal regeneration by regulating mitochondrial transport and bioenergetic metabolism. Cell Regeneration 2022;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ellis BW, Ronan G, Ren X, Bahcecioglu G, Senapati S, Anderson D, Handberg E, March KL, Chang H-C, Zorlutuna P. Human Heart Anoxia and Reperfusion Tissue (HEART) Model for the Rapid Study of Exosome Bound miRNA Expression As Biomarkers for Myocardial Infarction. Small 2022;18:2201330. doi: 10.1002/smll.202201330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nemcovsky Amar D, Epshtein M, Korin N. Endothelial cell activation in an embolic ischemia-reperfusion injury microfluidic model. Micromachines 2019;10:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sarkar T, Nguyen T, Moinuddin SM, Stenmark KR, Nozik ES, Saha D, Ahsan F. A protocol for fabrication and on-chip cell culture to recreate PAH-afflicted pulmonary artery on a microfluidic device. Micromachines 2022;13:1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aw WY, Cho C, Wang H, Cooper AH, Doherty EL, Rocco D, Huang SA, Kubik S, Whitworth CP, Armstrong R. Microphysiological model of PIK3CA-driven vascular malformations reveals a role of dysregulated Rac1 and mTORC1/2 in lesion formation. Science Advances 2023;9:eade8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jung O, Tung Y-T, Sim E, Chen Y-C, Lee E, Ferrer M, Song MJ. Development of human-derived, three-dimensional respiratory epithelial tissue constructs with perfusable microvasculature on a high-throughput microfluidics screening platform. Biofabrication 2022;14:025012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cherubini M, Haase K. A Bioengineered Model for Studying Vascular-Pericyte Interactions of the Placenta. In: Margadant C, ed. Cell Migration in Three Dimensions New York, NY: Springer US; 2023:409–423. [DOI] [PubMed] [Google Scholar]
- 105.Glaser DE, Curtis MB, Sariano PA, Rollins ZA, Shergill BS, Anand A, Deely AM, Shirure VS, Anderson L, Lowen JM, et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials 2022;280:121245. doi: 10.1016/j.biomaterials.2021.121245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vivas A, Mikhal J, Ong GM, Eigenbrodt A, Van der Meer AD, Aquarius R, Geurts BJ, Boogaarts HD. Aneurysm-on-a-Chip: Setting Flow Parameters for Microfluidic Endothelial Cultures Based on Computational Fluid Dynamics Modeling of Intracranial Aneurysms. Brain Sciences 2022;12:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jain A, Barrile R, van der Meer AD, Mammoto A, Mammoto T, De Ceunynck K, Aisiku O, Otieno MA, Louden CS, Hamilton GA. Primary human lung alveolus‐on‐a‐chip model of intravascular thrombosis for assessment of therapeutics. Clinical pharmacology & therapeutics 2018;103:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Isosaari L, Vuorenpää H, Yrjänäinen A, Kapucu FE, Kelloniemi M, Pakarinen T-K, Miettinen S, Narkilahti S. Simultaneous induction of vasculature and neuronal network formation on a chip reveals a dynamic interrelationship between cell types. Cell Communication and Signaling 2023;21:132. doi: 10.1186/s12964-023-01159-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y, Chan HN, Michael SA, Shen Y, Chen Y, Tian Q, Huang L, Wu H. A microfluidic circulatory system integrated with capillary-assisted pressure sensors. Lab on a Chip 2017;17:653–662. [DOI] [PubMed] [Google Scholar]
- 110.Simion V, Zhou H, Haemmig S, Pierce JB, Mendes S, Tesmenitsky Y, Pérez-Cremades D, Lee JF, Chen AF, Ronda N, et al. A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nat Commun 2020;11:6135. doi: 10.1038/s41467-020-19664-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng W, Jiang B, Wang D, Zhang W, Wang Z, Jiang X. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab on a Chip 2012;12:3441–3450. doi: 10.1039/C2LC40173H [DOI] [PubMed] [Google Scholar]
- 112.Zhao YC, Zhang Y, Wang Z, Jiang F, Kyanian K, Aye SSS, Hong T, Vatankhah P, Nasser A, Sun A, et al. Novel Movable Typing for Personalized Vein-Chips in Large Scale: Recapitulate Patient-Specific Virchow’s Triad and its Contribution to Cerebral Venous Sinus Thrombosis. Advanced Functional Materials 2023:2214179–2214179. doi: 10.1002/adfm.202214179 [DOI]
- 113.Pandian NK, Mannino RG, Lam WA, Jain A. Thrombosis-on-a-chip: Prospective impact of microphysiological models of vascular thrombosis. Current Opinion in Biomedical Engineering 2018;5:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herbig BA, Yu X, Diamond SL. Using microfluidic devices to study thrombosis in pathological blood flows. In: Biomicrofluidics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shirure VS, Hughes CCW, George SC. Engineering Vascularized Organoid-on-a-Chip Models. Annual Review of Biomedical Engineering 2021;23:141–167. doi: 10.1146/annurev-bioeng-090120-094330 [DOI] [PubMed] [Google Scholar]
- 116.Kim JJ, Hou L, Huang NF. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater 2016;41:17–26. doi: 10.1016/j.actbio.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Del Piccolo N, Shirure VS, Bi Y, Goedegebuure SP, Gholami S, Hughes CCW, Fields RC, George SC. Tumor-on-chip modeling of organ-specific cancer and metastasis. Advanced Drug Delivery Reviews 2021;175:113798. doi: 10.1016/j.addr.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 118.Voges HK, Foster SR, Reynolds L, Parker BL, Devilee L, Quaife-Ryan GA, Fortuna PRJ, Mathieson E, Fitzsimmons R, Lor M, et al. Vascular cells improve functionality of human cardiac organoids. Cell Rep 2023:112322. doi: 10.1016/j.celrep.2023.112322 [DOI] [PubMed]
- 119.Bersini S, Schulte R, Huang L, Tsai H, Hetzer MW. Direct reprogramming of human smooth muscle and vascular endothelial cells reveals defects associated with aging and Hutchinson-Gilford progeria syndrome. Elife 2020;9:e54383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y, Zhan Q, Zhang J, Wang W, Khoo BL, Liu Z, Wei S, Niu J, Xu J, Yu C-C. Accurate prediction of drug-induced heterogeneous response of red cell in vivo using a gravity-driven flow cytometry based on a microfluidic chip. Analytica Chimica Acta 2022;1221:340151. [DOI] [PubMed] [Google Scholar]
- 121.Huang J, Xu Z, Jiao J, Li Z, Li S, Liu Y, Li Z, Qu G, Wu J, Zhao Y. Microfluidic intestinal organoid-on-a-chip uncovers therapeutic targets by recapitulating oxygen dynamics of intestinal IR injury. Bioactive Materials 2023;30:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Llucià-Valldeperas A, Smal R, Bekedam FT, Cé M, Pan X, Manz XD, Wijnker PJ, Vonk-Noordegraaf A, Bogaard HJ, Goumans M-J. Development of a 3-dimensional model to study right heart dysfunction in pulmonary arterial hypertension: First observations. Cells 2021;10:3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hughes CS, Postovit LM, Lajoie GA. Matrigel: A complex protein mixture required for optimal growth of cell culture. PROTEOMICS 2010;10:1886–1890. doi: 10.1002/pmic.200900758 [DOI] [PubMed] [Google Scholar]


