Abstract
Regulatory T (Treg) cells expressing the transcription factor forkhead box P3 (Foxp3) mediate peripheral immune tolerance both to self-antigens and the commensal flora. Their defective function due to inborn errors of immunity or acquired insults is associated with a broad range of autoimmune and immune dysregulatory diseases. While their function in suppressing autoimmunity and enforcing commensalism is established, a broader role for Treg cells in tissue repair and metabolic regulation has emerged, enabled by unique programs of tissue adaptability and specialization. In this review, we focus on the myriad roles played by Treg cells in immune tolerance and host homeostasis and the potential to harness these cells in novel therapeutic approaches to human diseases.
Keywords: regulatory T cells, Foxp3, immunological tolerance, autoimmunity, interleukin-2, regulatory T cell therapy
Introduction
Immunological tolerance denotes a state of non-responsiveness of the immune system towards self-tissues. Tolerance to self was originally thought to be the default state of an immune system that, as Paul Ehrlich argued, could not be imagined to fall into the trap of self-toxicity (horror autotoxicus) 1. Despite the early demonstration of auto-antibody formation against blood elements, it took the classical experiments of Ray Owen and Peter Medawar showing that immune tolerance is an acquired state learned during the development of the immune system for this concept to be firmly established 2.
The question of immune tolerance arises in the context of an adaptive immune response that is inherently prone to autoreactivity. In principle, adaptive immunity offers the host the incisive advantage of anticipating infections by randomly generating lymphocytes bearing unique antigen receptors that recognize hitherto unencountered pathogens. Lymphocyte clones bearing pathogen-specific antigen receptors expand during an infection and are then maintained in a poised state ready for reactivation, thus providing long-term memory of the initial pathogen encounter. However, the same process may also generate autoreactive antigen receptor-bearing lymphocytes. This conundrum presented a major challenge as to how the immune system minimizes responses to self-antigens while maintaining immune responses to unencountered foreign antigens. To achieve a generalized state of self-tolerance, in which the immune system does not routinely attack self tissues, self-reactive lymphocytes in the generative lymphoid organs are largely removed through a negative selection process that collectively induces a state of central tolerance. During this developmental checkpoint, immature T cells bearing T cell receptors (TCR) with too strong of an affinity for self-peptide major histocompatibility complex (self-peptide-MHC) molecules presented on the surface of thymic epithelial cells are eliminated through induction of death by apoptosis (Figure 1) 3-5. Meeting a similar fate, T cells whose TCRs fail to bind self-peptide-MHC molecules with low affinity and consequently do not undergo positive selection also undergo apoptosis through a process dubbed “death by neglect” (Figure 1) 6, 7. Central tolerance, which is leaky by its very nature, is abetted by a process of peripheral tolerance in which regulatory T (Treg) cells actively suppress autoreactivity and restrain overly exuberant immune responses to foreign antigens (Figure 1). The recognition that a host harbors commensal microbial communities, including bacteria, fungi and viruses, that act as part of an “extended self” expands the range of immunological tolerance and in particular the role for Treg cells in enabling commensalism. The scope of Treg cell function has further expanded to encompass a key role in tissue repair and metabolic control, thus linking tolerance mechanisms with organismal homeostasis. In this review we will discuss fundamental concepts in tolerance induction by Treg cells and explore the potential for novel therapies based on manipulation of Treg cells.
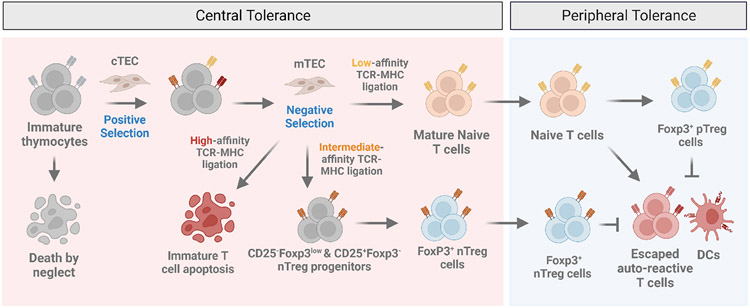
Figure 1. Central and peripheral immunological tolerance.
Although T cells originate from the fetal liver and adult bone marrow, the thymus is the major site of T cell maturation. The selection of developing T cells is dependent on T cell receptor (TCR) recognition of self-peptide-major histocompatibility complex (self-peptide-MHC) molecules and results in the elimination of potentially harmful T cell clones. Positive selection is a process mediated by cortical thymic epithelial cells (cTEC) during which immature thymocytes whose TCRs fail to recognize self-peptide-MHC undergo a default pathway of apoptosis; a phenomenon known as “death by neglect”. Thymocytes whose TCRs recognize peptide-MHC presented by medullary thymic epithelial cells (mTEC) with high avidity undergo a process of negative selection resulting in apoptosis. In an alternative process that is incompletely understood, thymocytes bearing TCRs with intermediate avidities towards self-peptide-MHC are selected onto the Treg cell lineage via at least two distinct progenitor subsets including a CD25+Foxp3− and CD25− Foxp3low regulatory T cell population. The elimination of potentially harmful self-reactive T cells in the thymus through negative selection is the basis for0 central tolerance. In contrast, peripheral tolerance is induced in mature lymphocytes by self-antigens, including components of the extended self and innocuous environmental antigens by a pool of regulatory T cells derived from the thymus (nTreg) or generated in the periphery (pTreg) that act in concert to maintain organismal-wide immunological tolerance.
Natural and Peripheral Foxp3+ Regulatory T Cells in Immune Tolerance
Reflecting a duality in its developmental ontology, the Treg cell compartment is composed of two distinct populations that act synergistically to maintain peripheral immunological tolerance (Figure 2). Tolerance to self-antigens and tissues is enforced by natural regulatory T (nTreg) cells, which are selected in the thymus on the basis of high-avidity TCR interactions and thought to arise from two distinct nTreg progenitor subsets that collectively generate a broad self-biased TCR repertoire 8-10. Selection of nTreg cells in the thymus can also occur through a process of clonal diversion during which thymocytes reactive against peripheral tissue antigens are redirected towards the Treg lineage in a manner that is dependent on the transcriptional autoimmune regulator (Aire)11-13. In contrast to nTreg cells, peripheral regulatory T (pTreg) cells play a critical and non-redundant role in the maintenance of tolerance to environmental antigens and components of the “extended-self”, including antigens derived from the commensal flora and diet as well as neoantigens arising from developing tumors 14 (Figure 2). Unlike nTregs, pTreg cells are generated extrathymically in mucosal interfaces, where the bioavailability of commensal metabolites such as retinoic acid and specialized antigen presenting cells including CD101+ dendritic cells promote their differentiation 15. Treg cells can be also induced (iTreg) from conventional naïve CD4+ T cells in-vitro upon TCR-stimulation in the presence of TGF-β and interleukin-2 15, 16 (Figure 2). However, the functional relevance of in-vitro derived iTreg cells has been contentious, as these cells exhibit incomplete CpG demethylation at the Foxp3 locus resulting in unstable expression of Foxp3 and a susceptibility towards destabilization 17, 18. Although no definitive markers exist to segregate nTreg and pTreg cell populations phenotypically, nTreg cells are characterized by heightened expression of the Ikaros transcription factor family member Helios and additionally in mice the cell surface glycoprotein neuropilin-1 (Nrp-1) 19, 20. It is now appreciated that the TCR repertoires of nTreg and pTreg cells are largely distinct and share minimal overlap 21 22. The essentiality of nTreg and pTreg subsets in optimal protection from deleterious autoimmunity is evidenced by the absolute requirement of both populations in the prevention of lethality induced by Foxp3-deficiency 22. In summary, a division of labor between nTreg and pTreg cells ensures comprehensive coverage over a wide range of antigens expressed by the body’s own cells and tissues as well as innocuous antigens originating from the environment, diet and commensal microorganisms that constitute the extended self.
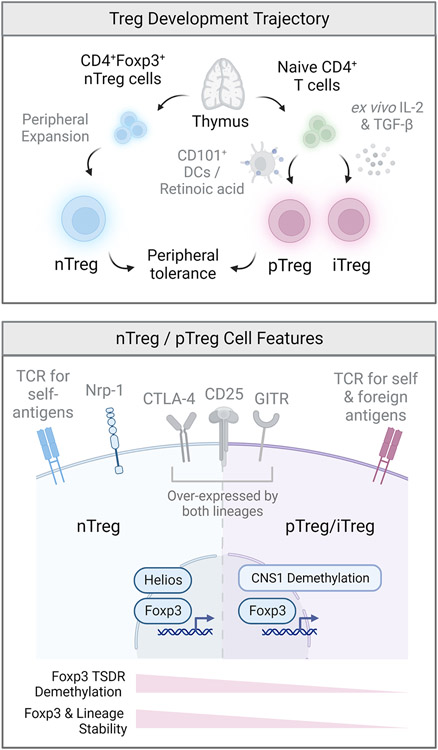
Figure 2. Developmental trajectory and subsets of regulatory T cells.
The regulatory T cell pool is composed of two major populations that act in concert to maintain peripheral tolerance. Natural regulatory T (nTreg) cells develop in the thymus and play a critical role in the maintenance of tolerance to self-antigens. Peripheral regulatory T (pTreg) cells are generated from conventional naïve CD4+ T cells at mucosal interfaces and barrier sites and are critically important for the maintenance of tolerance towards dietary, environmental and commensal antigens. Regulatory T cells can also be induced (iTreg) in-vitro from conventional naïve CD4+ T cells following TCR ligation in the presence of transforming growth factor beta (TGF-β). Although all three populations express high levels of the transcription factor Foxp3 and share the expression of several canonical Treg cell surface molecules including CTLA-4, CD25 and GITR, the TCR repertoires of nTreg and pTreg/iTreg and are distinct and non-overlapping. Although not uniquely expressed by nTreg cells, the Ikaros family transcription factor Helios and cell surface glycoprotein neuropilin-1 (Nrp-1) are highly expressed in nTreg as opposed to pTreg/iTreg populations. pTreg/iTreg cells exhibit a greater susceptibility towards destabilization and loss of Foxp3 expression, largely due to the absence of an nTreg-like specific demethylation signature at conserved non-coding sequences within the locus of Foxp3 and other Treg signature genes.
Treg Specialization in Non-Lymphoid Tissues
Unique populations of Treg cells with specialized functions have been identified in non-lymphoid tissues. As a result, a newly found appreciation for the phenotypic heterogeneity and functional diversity of Treg cells has emerged, highlighting the importance of Treg cells not only as sentinels of peripheral tolerance but also as orchestrators of tissue homeostasis. Within the central nervous system (CNS), Treg cells were shown to exert regenerative functions by virtue of promoting the remyelination and differentiation of oligodendrocyte progenitor cells 23. Treg cells also participate in the resolution of acute lung injury 24,promote wound healing following myocardial infraction and control the development of atherosclerosis 25,26, 27. A unique population of Foxp3+ Treg cells residing in the visceral adipose tissue (VAT) plays an important role in the regulation of metabolism, including control of insulin sensitivity and VAT inflammation 28. VAT Treg cells exhibit a unique transcriptome, which is largely driven by the peroxisome proliferator-activated receptor-γ (PPARγ), the master transcriptional regulator of adipocyte differentiation. Importantly, PPARy is necessary and sufficient for the accumulation, phenotypic adaptation and function of VAT Tregs, consistent with the notion that lineage defining-transcription factors license the specialization of Treg cells in distinct tissue microenvironments. In addition, VAT Treg cells exhibit a unique TCR repertoire when compared to their counterparts in lymphoid tissues and are dependent on IL-33 signaling for their accumulation and proliferation 29, 30.
Another population of Treg cells with dedicated tissue reparative function is found in injured skeletal muscle 31. These Treg cells rapidly expand following acute muscle injury and play an important role in muscle repair and regeneration 15,32. Similar to VAT Tregs, muscle Treg cells exhibit a tissue-adapted transcriptome and clonally expanded TCR repertoire with a preferential reliance on IL-33 signaling 31, 33. Moreover, Treg cells were shown to play an important role in the prevention of infectious lung tissue injury through production of the epidermal growth factor amphiregulin (AREG), which also acts on progenitor muscle cells to enhance muscle regeneration 31, 34. Interestingly, AREG produced by CNS Treg cells is critical for protection against neurotoxic astrogliosis by suppressing pro-inflammatory IL-6-STAT3 signaling in astrocytes 35. These results highlight the importance of AREG production by Treg cells as a general mechanism directing the regeneration and repair of various tissues following injury. In contrast, a number of factors secreted by tissue-Treg cells play a privileged and non-redundant role in the regeneration of particular tissues and/or cell types, including the growth regulatory protein CCN3, which accelerates the differentiation and remyelination of oligodendrocytes in the CNS 23. With the exception of placental and colonic lamina propria Treg cell populations, the majority of tissue-resident Treg cells are thought to be thymically derived and express high levels of nTreg-associated markers Helios and Nrp-1 36. Consistent with thymic derivation but not peripheral conversion, VAT and muscle Treg cells are clonally expanded and exhibit a unique TCR repertoire that is not shared with conventional T cells in the same tissue 36. Collectively, these studies highlight an important functional dualism that extends beyond the classical role of Treg cells in the maintenance of immunological tolerance.
Treg cells at the environmental interfaces
The environmental interfaces in the airways, gut and skin pose special challenges for the immune system because they are heavily colonized by the commensal microbiota. Treg cells play a critical role in shaping and enforcing this commensalism, safeguarding barrier integrity and orchestrating immune responses to inciting agents. The diversity of the tissues involved, their respective metabolic attributes and the different loads of resident microbiota ranging from very high in the gut to very low in the lower airways, are reflected in the heterogeneity of tissue-specific barrier-resident Treg cell populations.
Intestinal Treg cells
Intestinal Treg cells are an important subset of immune cells that play a crucial role in maintaining immune tolerance in the gut. These specialized cells are key regulators of the immune response, helping to prevent immune-mediated damage to the intestines and promoting overall intestinal health. Experimental depletion or functional dysregulation of Treg cells exacerbates intestinal inflammation, which can be alleviated following Treg cell reconstitution 23. Intestinal Treg cells are enriched in the lamina propria, where they represent more than 25% of CD4+ T cells 37, 38. This enrichment is lost in germ-free or antibiotic-treated mice, highlighting the essentiality of the microbiome in intestinal Treg cell differentiation and persistence 39, 40. Indeed, the intestinal Treg pool is largely composed of pTreg cells, as illustrated by the high abundance of lamina propria Foxp3+ cells lacking expression of the nTreg cell-associated markers Helios and neuropilin-1 19, 20. Interestingly, specific deletion of the Foxp3 intronic enhancer CNS1, which leads to a defect in pTreg generation, results in TH2-type pathologies in the gut in the absence of systemic inflammation, highlighting a critical role for pTreg cells in the maintenance of gut homeostasis 41. Intestinal Treg cells exhibit a certain degree of plasticity, as they can adapt to the local microenvironment through the expression of effector T (Teff) cell transcription factors, including upregulation of BCL6, GATA3, RORγt and T-bet. A specialized subset of follicular regulatory T (Tfr) cells characterized by the expression of PD-1, CXCR5, and BCL6 play an important role in mucosal IgA production, expansion of follicular helper T (TFH ) cell populations, and control of the germinal center reaction 42. Roughly 40% of intestinal Treg cells express the Teff cell transcription factor RORγt and are induced by microbial products in a STAT3-dependent manner 43-45. Recent studies have shown the differentiation of this population involves antigen presentation by specific MHCII+ antigen-presenting cells, including type 3 innate lymphoid cells (ILC3) and RORγt -expressing Thetis cells 46-49. RORγt + Treg cells are critically involved in the maintenance of gut homeostasis and tolerogenic immune responses to allergens. For instance, susceptibility of mice to food allergy is associated with a preferential decrease in RORγt Tregs; the replenishment of RORγt+ Tregs by treatment with Clostridial and Bacteroidetes species can prevent the development of food allergy 40.
GATA3+ Treg cells represent another Treg cell subset in the intestine and account for 15-20% of the intestinal Treg cell compartment 50. The majority of these cells are Helios+ and their induction is microbiota-independent, indicative of their nTreg origin 45. This population expresses interleukin 1 receptor-like 1 (ST2) and can respond to alarmins during intestinal inflammation, including its ligand IL-33 produced by intestinal epithelial cells 51. IL-33 in combination with antigen recognition and IL-2 promotes GATA3 upregulation and enhances Foxp3 and ST2 expression to support intestinal Treg cell proliferation 51. Moreover, GATA3 and Foxp3 form a complex following TCR stimulation which promotes the stability and accumulation of Tregs in the intestine 50, 52. As a result, GATA3-deficient Treg cells fail to accumulate in the inflamed intestine and exhibit defects in Foxp3 expression 50, 53. In summary, the unique microbial and metabolic attributes of the intestinal mucosal interface is reflected in a diversity of specialized Treg cell populations that serve to enable tissue homeostasis and repair, license microbial commensalism and prevent adverse immune responses to foods and the commensal bacteria.
Lung Treg cells
Treg cells play a pivotal role in the maintenance of tolerance to allergens at environmental interfaces in the airways and prevention of deleterious inflammatory reactions against innocuous airborne antigens. Although both nTreg and pTreg cells are present in the lung, a critical role for pTreg cells in the maintenance of tolerance to airborne antigens is supported by the observation that mice unable to generate pTreg cells develop severe TH2-type pathologies in the lung, including asthma-like airway allergic inflammation 41. Consistent with this finding, adoptive transfer of allergen-specific Treg cells markedly attenuates allergen-induced allergic airway inflammation 54. Lung antigen-specific pTreg cells can be developed through the consorted action of several myeloid cell populations in the lung, including plasmacytoid dendritic cells, conventional dendritic cells and lung-resident tissue macrophages. Airborne antigens including pollen, dust mites and fungal spores are major targets for tolerogenic Treg cells in the human lung 55. These results emphasize that during steady state, lung Treg cells are coordinately mobilized to maintain tolerance to potentially harmful allergens as well as innocuous airborne antigens.
In addition to supporting steady state homeostasis in the lung, Treg cells play a central role in the pathogenesis of asthma. CD4+CD25+ mucosal Treg cells were shown to be critical for reversal of airway hyperresponsiveness following experimental asthma exacerbation 56. ST2+ lung Treg cells play a major role in actively restraining γδ T cell activation and function through the production of IL-35 during allergic airway inflammation induced by house dust mite exposure 57. Accordingly, allergic pulmonary inflammation is exacerbated in mice lacking functional ST2+ Treg cells. Interestingly, administration of IL-33 impairs established immunological tolerance to inhaled antigens by promoting the development of dysfunctional TH2 cell-like mucosal ST2+ Treg cells 58. Reprogramming of lung Treg cells towards a pathogenic TH2 cell-like phenotype is also a feature of allergic airway disease induced in OVA-tolerized mice subjected to recurrent respiratory infections with respiratory syncytial virus 59. These studies suggest that inflammatory triggers may comprise established tolerance and increase susceptibility to allergic disease in later life.
Recent studies support the hypothesis that dysregulated Treg cells play a critical role in the pathogenesis of allergic asthma. Expression of Notch4 on tissue resident lung pTreg cells is involved in the potentiation of type 2 innate lymphoid cell (ILC2) activation via a β-catenin-dependent Treg cell-intrinsic pathway that induces the expression of growth and differentiation factor 15 (GDF15) 60. GDF15 reinforces allergic airway inflammation by directly acting on ILC2s to promote their expansion and activation.
It is important to note that a failure by Treg cells to initiate lung-tissue reparative programs can in turn lead to unabated inflammation, as demonstrated by aggravated inflammatory immune responses in the lung by Treg cells incapable of producing the tissue repair factor amphiregulin 34, 61 62. Indeed, lung pTreg cell Notch4 expression was increased as a function of severity in subjects with COVID-19 infections 61. Mechanistically, Notch4 suppressed the induction of AREG by IL-18, a tissue reparative factor necessary for the prevention of severe lung inflammation 61. Collectively, these studies demonstrate the importance of Treg cells in the prevention of airway inflammation.
Skin Treg cells
Treg cells are highly abundant in the skin and represent a sizable portion of the CD4+ T cell compartment in humans and mice 63, 64. Homing of Treg cells to the skin is accomplished through several cutaneous chemokine receptors including CCR4 and CCR6 65-67. During neonatal life, epithelial cells within skin hair follicles drive the influx of CCR6-expressing Treg cells via the chemokine CCL20, which supports their accumulation in areas with high hair density 64, 68. Within the skin, Treg cells exhibit an effector memory-like phenotype and unique TCR repertoire collectively indicative of tissue residence and specialization 69-71. Expression of nTreg cell-associated markers Helios and Nrp-1 in skin Treg cells suggests that these cells are largely thymically derived 72 71. Functional specialization of skin Treg cells is acquired by their heightened expression of the canonical TH2 transcription factor GATA3, which governs their ability to control type 2 immune responses (Figure 3) 50, 53, 73. Lineage-specific deletion of GATA3 in Treg cells results in preferential exacerbation of TH2 cytokine responses in the skin and potentiates fibroblast activation to drive skin fibrosis 74, indicating that GATA3 safeguards the identity and functional specialization of skin Treg cells during inflammatory immune responses.
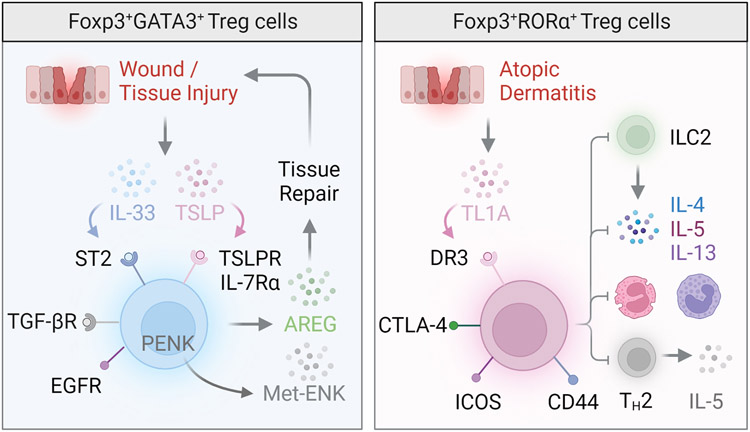
Figure 3. Regulatory T cells in the skin.
The skin Treg cell compartment is largely composed of nTreg cells that express the transcription factor GATA-3 (left), although a RORα+ population involved in the control of cutaneous inflammation has also been reported (right). These RORα+ skin Treg cells express the tumor necrosis factor receptor superfamily member 25, also known as death receptor 3 (DR3), and sequester TNF ligand–related molecule 1 (TL1A) to curtail type 2 innate lymphoid cell activity, eosinophil and basophil accumulation, and type 2 inflammation during atopic dermatitis. GATA-3+ skin Treg cells expressing the transcription factor IRF4 play a key role in skin wound repair and healing. Expression of alarmin receptors including the IL-33 receptor (ST2), IL-18 receptor and thymic stromal lymphopoietin receptor (TSLPR) enable skin Treg cells to sense and respond to cues associated with cutaneous damage and stress. Skin Treg cells also express the epidermal growth factor receptor (EGFR), which is thought to contribute to the accumulation of Treg cells in wounded skin and promote wound closure, likely though the production of the tissue reparative factor amphiregulin (AREG). Following UV light exposure, production of the opioid precursor proenkephalin (PENK) by UVB-exposed skin Treg cells contributes to generation of the neuropeptide methionine enkephalin (Met-ENK), which in turn promotes keratinocyte outgrowth and wound healing.
Another transcription factor enriched in skin Treg cells is the retinoic acid-related orphan receptor α (RORα), whose deletion results in uncontrolled cutaneous inflammation driven by ILC2s (Figure 3) 75. Similar to GATA3, RORα opposes the destabilization and skewing of skin Treg cells towards pathogenic IL-4 producing Teff-like cells. In an experimental model of psoriasis, Treg cells were shown to be critical for control of cutaneous inflammation by limiting the ability of pathogenic GM-CSF-producing CD4+ T cells to invade and expand within lesional skin and draining lymph nodes 76. In contrast to their classical “immunosuppressive” role, skin Treg cells can delay barrier repair to facilitate innate inflammation and protection against Staphylococcus aureus infection 77. Interestingly, adhesion of skin Treg cells through the c-type-lectin receptor Laylin impairs their suppressive capacity, suggesting that motility itself acts a cue in the regulation of immune suppression 78. Skin Treg cells also produce tissue-reparative factors such as AREG which can promote keratinocyte growth and wound healing 79. Collectively, these studies demonstrate that although skin Treg cells are functionally poised to restrain TH2-associated skin pathology, they nevertheless exhibit spatiotemporal and contextual plasticity, which enables them to direct both pro-inflammatory and anti-inflammatory responses in the maintenance of skin homeostasis.
The duality of Treg cells as cellular agents directing the response to injury in the skin is now well appreciated 80, 81. GATA3+ skin Treg cells express receptors for alarmins including IL-33, IL-18 and TSLP that can be released from non-hematopoietic cells following damage (Figure 3). Treg cells have been shown to facilitate cutaneous wound healing and suppress pro-inflammatory macrophage accumulation and IFN-γ secretion through epidermal growth factor receptor (EGFR) signaling 80. EGFR-deficient-Treg cells in the skin are decreased numerically and this decrease is associated with delayed wound closure and healing (Figure 3) [40]. Epidermal regeneration after injury requires Treg cell mediated attenuation of IL-17A-associated inflammation and suppression of CXCL5 expression from interfollicular epidermal cells 81. Taken together, these studies highlight the modular nature of skin Treg cells in broadly enabling skin homeostasis.
The Molecular Versatility of Treg Cells
Much like Teff cells, which adopt unique transcriptional programs resulting in the generation of highly specialized anti-pathogen immune responses (i.e TH1, TH2 etc.), Treg cells exhibit remarkable molecular versality and can effectively co-opt lineage-defining transcription factors (i.e Tbet, GATA3) required for the specification of particular immune response types 82. Molecular co-option represents an important mechanism by which Treg cells assume immune response-specific identities to prevent deleterious immunopathology. For instance, the transcription factors Tbet and STAT3 endow Treg cells with the preferential capacity to suppress TH1 and TH17 pro-inflammatory responses respectively, as evidenced by the precipitation of dysregulated TH1 and TH17 immune-responses in mice with Treg-cell-specific knockout of Tbet and STAT3 83, 84. In Treg cells, immune response type-specific functional adaptation is facilitated by the integration of discrete environmental cues and expression of TH-type specific molecules by Treg cells. For example, during pathogenic TH17 responses, expression of the CCR6 chemokine and interleukin-6 receptor and interleukin-1 receptor enable Treg cells to traffic to inflammatory sites where they can compete for cytokines known to promote TH17 differentiation including IL-6 and IL-1 83. During TH1 immune responses Treg cells upregulate Tbet, the master TH1 transcriptional regulator, and begin to express the TH1 cell-associated chemokine receptor CXCR3, which enables them to co-colocalize with CXCR3+ Teff cells 85.
Several studies support the notion of a division of anti-inflammatory labor by Treg cells. Absence of the interferon regulatory factor-4 (IRF4) in Treg cells results in unabated TH2 inflammation and aberrant antibody production 86. Moreover, Tregs can co-opt the transcriptional repressor BCL6 to gain entry into germinal centers via expression of CXCR5, where they can support antigen-specific plasma cell differentiation and curtail the outgrowth of non-antigen-specific B cells 87. Collectively, these studies underscore an important dualism in the functional plasticity of Treg cells, highlighting the essentiality of molecular co-option and Teff cell feature assimilation by Treg cells in the resolution of diverse inflammatory processes. It is now appreciated that in addition to being a cornerstone for the maintenance of peripheral tolerance, Treg cells play disproportionately important roles in enforcing tissue homeostasis broadly, where they adapt unique functional, phenotypic and tissue-reparative properties 88. This additional layer of Treg cell tissue “specialization” and the consequences of its disruption in peripheral tolerance breakdown will be reviewed in the following section.
Treg Cell Destabilization and Peripheral Tolerance Breakdown
The contentious topic of Foxp3 instability was borne out of the use of Foxp3-cre transgenes that were inherently leaky in nature and would readily label non-Treg cells with a transiently active Foxp3 locus 89-91. Notwithstanding these pitfalls, it is now appreciated that Treg cells can be phenotypically and functionally destabilized, particularly during persistent inflammatory immune responses. While the capacity of Treg cells to co-opt different transcriptional programs endows them with the ability to control immune response specific outcomes, it also serves as a vulnerability towards their pathological reprogramming and functional degeneration. Pathological reprogramming of Treg cells is largely a feature of the p/iTreg cell compartment and several human polymorphisms associated with inflammatory disorders directly impact the stability and pathogenic potential of allergen-specific pTreg cells. For example, mice carrying a tyrosine (Y) to phenylalanine (F) mutation at position 709 of the murine interleukin-4 receptor alpha chain (Il4raF709), which models human polymorphisms linked to atopy and asthma, exhibit enhanced sensitivity to food allergens and intensified allergen-induced airway hyperreactivity 92, 93. The formation of allergen-specific pTreg cells in ovalbumin-allergic Il4raF709 mice was shown to be impaired as a result of pathogenic TH2 cell-like pTreg cell reprogramming (Figure 4) 94. Mechanistically, the acquisition of a TH2 cell-like phenotype by pTreg cells resulted from elevated IL-4R-STAT6 signaling, which was also evident in pTreg cells from children with food allergy (Figure 4). Importantly, engraftment of Il4raF709 CD4+ T cells into Treg-deficient mice was sufficient to confer susceptibility to food allergy, while Treg cell-specific deletion of Shp1, which mimics the effects of the Il4raF709 mutation, induced the reprogramming of Treg cells into TH2-like cells and imparted disease susceptibility. These results demonstrate a requisite role for enhanced IL-4R signaling in oral tolerance breakdown and suggest that blockade of the IL-4-IL-4R pathway may enhance mucosal allergen-specific pTreg function in the re-establishment of oral tolerance. It is important to note that nTreg cells can also loose Foxp3 and contribute to immunopathology during inflammatory immune responses. Loss of Foxp3 in antigen-specific nTreg cells was shown to occur during the pathogenesis of experimental autoimmune encephalitis (EAE) 95. These autoreactive “exTreg” cells produced high levels of IFN-γ and were sufficient to induce EAE following adoptive transfer.
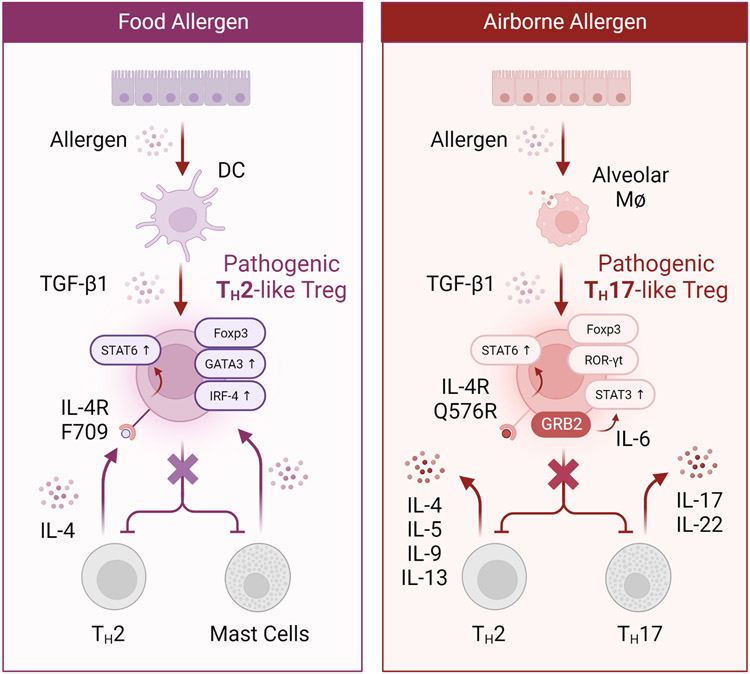
Figure 4. Pathological reprogramming of regulatory T cells in the intestine and lung.
Left: Food allergic Il4raF709 mice exhibit deficits in allergen-specific Treg cells. OVA-specific Il4raF709 Treg cells fail to suppress food allergy as a result of undergoing pathogenic TH2 cell-like reprogramming due to excessive IL-4R-STAT6 signaling. These pathogenic TH2 cell-like Tregs are characterized by heightened expression of canonical TH2 program components such as IRF4 and GATA-3. Importantly, allergen-specific Treg cells of human subjects with food allergy harbor similar TH2 cell-like Tregs, suggesting that blockade of the IL-4R pathway may offer a viable therapeutic strategy for the induction of long-lasting tolerance. Right: Destabilization of IL-4raQ576R pTreg cells in asthma towards a pathogenic TH17 cell-like state leads to the exacerbation of pulmonary inflammation. IL-4raQ576-dependent recruitment of the growth-factor-receptor-bound protein 2 (GRB2) results in mitogen-activated protein kinase (MAPK) activation and heightened autocrine IL-6-STAT3 signaling which collectively derails protective pTreg cell responses in the lung during allergic airway inflammation. Blocking the IL-4R or IL-6R pathways may prevent the destabilization of allergen-specific pTreg cells into TH2 and TH17 like cells, respectively, and afford novel Treg intervention strategies for the re-establishment of tolerance.
Using mice expressing a coding variant in the interleukin-4 receptor alpha chain (IL-4RR576) that is associated with increased asthma severity, signaling via IL-4RR576 was shown to amplify the expression of Notch4 on lung pTreg cells by recruiting the growth factor receptor-bound protein 2 (GRB2), which in turn mediates super-induction of Notch4 via IL-6 production, ultimately leading to exacerbated lung inflammation 96. The IL-4RR576 polymorphism was also shown to redirect TGF β-dependent lung pTreg cell differentiation towards the TH17 cell lineage, which occurs via GRB2-MAPK-dependent autocrine IL-6 signaling, highlighting an additional mechanism by which IL-4RR576 reprograms lung pTreg cells towards a pathogenic TH17-like state (Figure 4) 97. Interestingly, treatment with anti-IL-6 mAb in a severe asthmatic patient homozygous for the IL-4raR576 mutation resulted in immunological and clinical improvement, highlighting the therapeutic potential of intercepting pro-inflammatory signals involved in the destabilization of pTreg cells 98. In autoimmune arthritis, synovial fibroblast-derived IL-6 was shown to mediate the conversion of Foxp3+ pTreg cells into TH17 cells with potent arthritogenic and autoreactive properties 99. Taken together, these studies highlight a critical role for IL-6 dependent T H17 reprogramming of pTreg cells in asthma pathogenesis and point to intervention strategies aimed at re-stabilizing pTreg cell responses relevant to asthma or other inflammatory disorders.
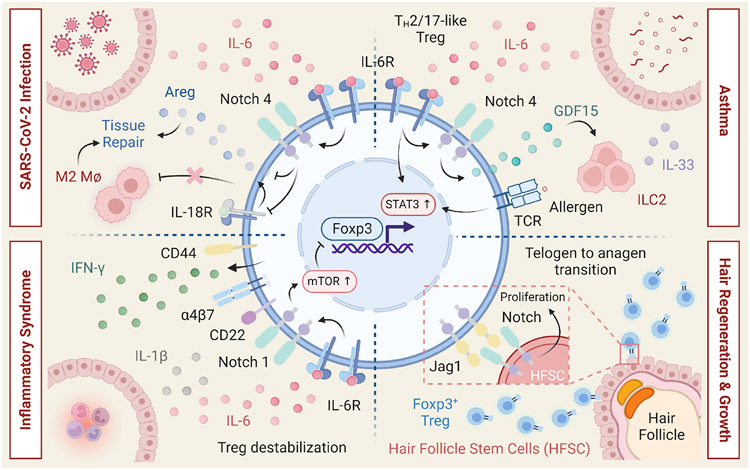
Additional examples of Treg cell destabilization involve the Notch signaling axis (Figure 5) 60, 61, 100-102. Enforced Notch signaling disrupts peripheral tolerance by promoting TH1 reprogramming of both nTreg and pTreg cells in a cell-intrinsic and canonical pathway-dependent manner 100. In contrast, lineage specific disruption of Notch signaling enhances Treg cell fitness and function in the settings of inflammation. Consistent with these results, genetic disruption or pharmacological Notch-1 blockade induces tolerance in murine cardiac and lung transplantation models 101. Moreover, activation of Notch signaling was shown to negatively impact the immunosuppressive functions of Treg cells during experimental autoimmune uveitis, chronic hepatitis and allergic rhinitis 103 104, 105. Notch4 expression licenses allergic airway inflammation in asthma by disrupting the capacity of pTreg cells to control ILC2 activation in a Treg cell-intrinsic manner that involves Wnt signaling and GDF15 (Figure 5) 60. Interestingly, Notch4 also participates in an alternative mechanism that involves the restraint of AREG production by pTreg cells during severe COVID-19 infection, which results in the exacerbation of lung inflammation (Figure 5) 61. In addition, Treg cells in multisystem inflammatory syndrome in children (MIS-C) are destabilized through a Notch1-dependent mechanism that promotes the expression of CD22 and leads to aberrant mTORC1 activity (Figure 5) 106. Collectively these studies illustrate a critical role for Notch signaling in negatively regulating Treg cells and suggest that therapeutic targeting of individual Notch receptors may offer opportunities to restore tolerance in a tissue and disease-specific manner.
Figure 5. Multifaced roles of Notch signaling in regulatory T cells.
Notch signaling plays pleiotropic roles within the Treg cell compartment and is critically involved in the control of Treg cell function in the periphery. Upper Right: In experimental models of asthma, Notch4 expression on lung pTreg cells licenses allergic airway inflammation through a process that involves the destabilization of pTreg cells towards TH2 and TH17 cell fates. During allergic airway inflammation, Notch4 expression on lung pTreg cells also potentiates ILC2 activation and expansion, via a growth and differentiation factor 15 (GDF15)dependent mechanism that abrogates pTreg cell-mediated suppression of ILC2s. Upper Left: During severe SARS-CoV-2 infection, pTreg cell Notch4 represses production of the tissue reparative factor amphiregulin (AREG) by inhibiting IL-18 production, which promotes severe lung inflammation. Lower Left: In multisystem inflammatory syndrome in children (MIS-C), subjects harboring mutations in the Notch-related genes, upregulation of Notch1 on Treg cells leads to super-induction of CD22 and augmented TCR signaling, collectively promoting Treg-cell destabilization in an mTOR-dependent manner. Lower Right: Expression of the Notch ligand Jagged-1 by skin Treg cells promotes the proliferation and function of hair follicle stem cells to support hair follicle regeneration and hair growth.
A number of molecules with specialized functions play prominent roles in the suppressive capacity of Treg cells. Within the gastrointestinal tract, TGF-β1 was shown to safeguard the functions of colonic Treg by supporting their accumulation and retention through maintenance of integrin αEβ7 (CD103) expression 107. While Treg cell-specific monoallelic inactivation of Tgfb1 results in impaired RORγt+ Treg cell differentiation, biallelic deficiency results in fatal autoimmunity characterized by dysregulated autoimmune humoral responses 108, 109. These results demonstrate that Treg-derived TGF-β1 plays a privileged and non-redundant role in the regulation of oral tolerance and autoimmunity. Treg-derived interleukin-10 (IL-10) represents another immunomodulatory cytokine that plays a non-redundant role in restraining autoimmunity at mucosal interfaces, including the colon and lungs, but is dispensable for the control of systemic autoimmunity 89, 110. Similarly, RORγt expression in Treg cells is important for the maintenance of colonic homeostasis and TH2-associated pathology is exacerbated in the absence of RORγt + Treg cells 44, 45. The importance of RORγt + Treg cells is additionally illustrated by the demonstration that protection against food allergy by commensal bacteria is dependent on the induction of RORγt Tin nascent Treg cells 40. Taken together, these results demonstrate critical roles for TGF-β1, IL-10 and RORγt as factors crucial for the establishment and maintenance of immunological tolerance at particular environmental interfaces and/or inflammatory settings.
In summary, a comprehensive understanding of the inflammatory triggers and molecular mechanisms governing the susceptibility of Treg cells towards functional destabilization may inform conceptually novel approaches aimed at re-invigorating Treg stability in the settings of persistent inflammation.
Resetting Tolerance: Treg Cell Therapies
Since their initial description almost three decades ago, Treg cells have taken center stage as master orchestrators of peripheral immunological tolerance. A growing understanding of the fundamental mechanisms involved in the induction, differentiation, maintenance and suppressive functions of Treg cells has stimulated therapeutic approaches to selectively manipulate these cells to deliver precision immunotherapy for immune dysregulatory diseases. Amongst several approaches, the use of autologous Treg cell products as adoptive cell therapies have already been shown to be safe in a number of clinical trials 111-113. Currently, Foxp3 gene editing approaches and designer IL-2 muteins engineered to preferentially elicit the activity of Treg cells have been propelled to the forefront of Treg-based cell therapy 114, 115. Below we summarize recent developments in therapeutic approaches aimed at the normalization or amplification of Treg cell function, including novel gene editing approaches used to generate and modify Tregs as cellular therapies for inflammatory and autoimmune disorders.
Foxp3 and Treg Gene Editing
In seminal studies demonstrating an essential role for Foxp3 in the development and function of Treg cells, ectopic expression of Foxp3 was shown to confer a regulatory phenotype in conventional murine CD4+ T cells 116-118. These early observations suggested that conventional CD4+ T cells may be therapeutically repurposed for induction of tolerance in autoimmune disease and transplantation settings through enforced expression of Foxp3 (Figure 6A).
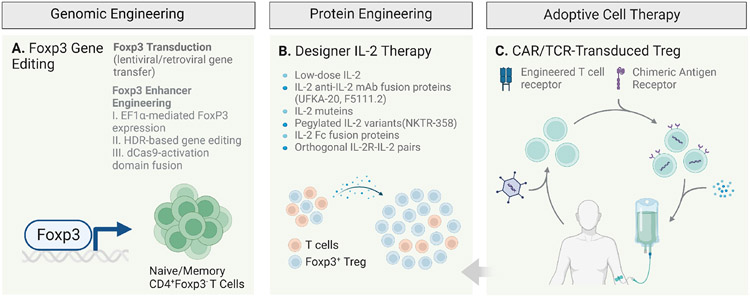
Figure 6. Regulatory T cell therapies.
A number of therapeutic approaches are currently attempting to harness the potential of Treg cells as therapeutics for autoimmune and immune dysregulatory disorders. These strategies include: A. genomic engineering strategies to artificially induce long-lasting Foxp3 expression and reprogram conventional CD4+ T cells into Foxp3-expressing Treg-like cells capable of inducing tolerance. B. Modified IL-2 reagents including IL-2 muteins, pegylated-IL-2 variants, IL-2:anti-IL-2 complexes and fusion proteins as well as orthogonal IL-2-IL-2R pairs. C. Adoptive cell therapies (ACTs) utilizing autologous ex-vivo expended polyclonal or antigen-specific Treg cells with engineered TCRs including chimeric antigen receptor (CAR) Treg cells.
Subsequent studies in human systems demonstrated that retroviral gene transfer of FOXP3 into naive CD25−CD45RO−CD4+ T cells results in the acquisition of several Treg cell features, including in-vitro suppressive activity 119, 120. Lentiviral-mediated expression of FOXP3 promotes the induction of a homogenous and highly suppressive population of Treg-like cells but only when FOXP3 is under the control of an activation-independent promoter 121. Using a bi-directional lentiviral vector in which expression of FOXP3 is under control of the human elongation factor 1 alpha (EF1α), transduction of naïve or memory CD4+ T cells was shown to result in high and stable expression of FOXP3 (Figure 6A). In a similar approach utilizing homology directed repair (HDR) based gene editing, insertion of strong promoter into the endogenous FOXP3 locus resulted in the acquisition of a stable regulatory phenotype 122 (Figure 6A). Nevertheless FOXP3-transduced Treg cells exhibited several differences as compared to nTreg cells, including a methylated Treg specific demethylation region (TSDR) and reduced expression of the Ikaros family transcription factor Helios previously shown to be important for Treg cell differentiation and function 123, 124. A number of functional genetic perturbation approaches, including loss-of-function CRISPR/Cas9 screens, have also been recently employed in the identification of novel regulatory networks and transcriptional regulators of Foxp3 activity and Treg cell identity 125-128.
The utilization of induced pluripotent stem cells (iPSCs) for the generation of Tregs has captured considerable attention as a promising approach to produce readily accessible Tregs. iPSCs offer an appealing and cost-effective solution to address the challenges associated with engineered adoptive Treg cell therapy, encompassing issues related to production, efficacy, immunogenicity, and application. Moreover, IPSCs utilization allows for precise antigenic targeting through genetic modifications 129, 130. Numerous studies have successfully generated iPSC-derived Tregs that closely resemble natural Tregs and have demonstrated their efficacy in suppressing autoimmune arthritis, Type 1 Diabetes (T1D), and Graft versus host disease 131-133
Using a catalytically inactive Cas9 variant (dCas9), the feasibility of targeting regulatory regions of FOXP3 was shown by fusing dCas9 with a transcriptional activation domain in order to induce prolonged upregulation of endogenous FOXP3 134 (Figure 6A). Notably, epigenetic imprinting of Treg cells that precedes FOXP3 expression may represent an important barrier in the generation of functionally stable Treg cells following induction of FOXP3 135-137. As previously discussed, selective gene knockout of immune response specific components (IL-6R, STAT3, STAT6, GATA3 etc.) involved in the functional plasticity of Treg cells may render these cells resistant to phenotypic destabilization and functional dysregulation. Conversely, enforced expression of chemokine receptors (CXCR3, CXCR5, CCR6 etc.) may enhance the trafficking of Treg cells to particular inflammatory sites and reduce the potential of broad off-target immunosuppression. A complete understanding of the elements involved in the specification and maintenance of the Treg lineage beyond Foxp3 will be instrumental for the rational design of gene editing approaches aimed at generating bone-fide Treg cells.
Designer Treg IL-2 Therapy
IL-2 is pleiotropic cytokine that plays a critical role in the induction, homeostatic proliferation, phenotypic stability and suppressor function of Treg cells 138-140. As a result of a diminished threshold for IL-2R signaling in maintaining Treg cell activation and proliferation, a number of therapeutic approaches leveraging IL-2 have been developed to specifically target Treg cells in-vivo (141; 142) (Figure 6B). Previous studies have shown the utility of in-vivo administration of immune complexes of IL-2 and specific anti-IL-2 mAbs in stimulating the activation and proliferation of Treg cells 143, and in the treatment of preclinical models of autoimmunity and graft rejection in mice 144. Treg cell-biased IL-2:anti-IL-2 immune complexes can be achieved by using anti-IL-2 mAbs that sterically block the IL-2:IL-2Rβ interaction while imparting exquisite selectivity towards CD25high cells 145, 146.
Attempts to harness the therapeutic potential of IL-2 in the form of designer muteins engineered to preferentially stimulate the activity of Treg cells have recently gained attention 147 (Figure 6B). The activity of different IL-2 muteins, including IL-2 Fc fusion proteins and pegylated IL-2 variants, are currently being assessed across several autoimmune indications as precision Treg immunotherapies (Figure 6B). Site-specific pegylation of IL-2 can also support IL-2Rα bias and lead to preferential and sustained activation of Treg cells 148, elicit tolerogenic activity in non-human primates and ameliorate disease progression in an experimental model of systemic lupus erythematosus 149. Several IL-2 Fc fusion proteins with increased dependency towards CD25 and attenuated IL-2Rβ affinity have been shown to exhibit preferential Treg cell activity in-vivo 150-152. The status of clinical trials assessing the activity of IL-2 Treg-biased muteins in autoimmune indications have been recently reported 153.
Interestingly, a wide dynamic range of IL-2R signaling generates productive Treg cell biological responses in-vitro, and distinct minimal thresholds of IL-2R signaling are needed to achieve specific Treg functional outcomes in-vivo 154. The capacity of Treg-biased IL-2 muteins to be utilized in combinatorial approaches is supported by the demonstration that Fc-IL-2 mutein mediated enrichment of Treg cells can promote the in-vivo expansion of antigen-specific Treg cells 155. By using engineered orthogonal IL-2R-IL-2 pairs that specifically bind to one another but not their natural counterparts, it was shown that ortho-IL-2 can effectively and preferentially expand ortho-IL-2Rβ-expressing Treg cells leading to improved hematopoietic stem cell engraftment 156, 157. The use of messenger RNAs to encode IL-2 muteins with extended half-life and Treg bias has also been recently explored 158. Lastly, the application of scRNA-seq analysis has begun to delineate Treg cell subset alterations and distinct gene expression profiles following expansion with IL-2 muteins and holds promise for the identifying tissue-specific patterns of modulation in Treg cells 159. The constraints of number, function, and stability represent significant limitations in the context of Treg therapies 160, 161. To circumvent these limitations, combination therapies involving IL-2 and CAR-Tregs have emerged as a potential solution. In fact, the creation of a membrane-bound IL-2 molecule that facilitates autocrine IL-2 signaling in CAR-Tregs has been demonstrated to enhance the expansion, functionality, and durability of CAR-Tregs in a humanized preclinical mouse model 162. Collectively, these studies have begun to provide a molecular blueprint for the therapeutic engineering of Treg potentiating molecules and hold tremendous promise for a wide range of autoimmune and inflammatory disorders.
Tregs as cellular therapies
The therapeutic utility of Treg cells as adoptive cell therapies (ACT) was first highlighted by the demonstration that transfer of CD4+CD25+ but not conventional CD4+CD25− cells rescues the lymphoproliferative disease of Foxp3 and CTLA-4-deficient mice 116-118. In 2009, the first use of autologous Treg cells as an ACT in human subjects with GvHD was reported 111. Although limited to two patients, this case study and a subsequent phase 1 trial in children with recent-onset type 1 diabetes (T1D) from the same group collectively demonstrated the feasibility, safety and potential therapeutic benefit of autologous Treg cell products following their massive expansion ex-vivo under good manufacturing practice conditions 112 163. In 2015, the safety of expanded autologous polyclonal Treg cells in T1D was reported 113. Expansion of Treg cells ex-vivo resulted in the correction of dysfunction associated with Treg cells in T1D subjects, including impaired IL-2-induced STAT5 phosphorylation, and these cells outperformed Treg cells from healthy individuals in in-vitro suppression assays. Despite exhibiting superior activity following ex-vivo expansion, the majority of the infused Treg cells were absent from circulation 3 months following infusion, with a precipitous drop already apparent 2 weeks following infusion. As high concentrations of IL-2 are required for optimal expansion of Treg cells ex-vivo, it was thought that the bioavailability of IL-2 may critically govern longevity of the Treg cell product. Supporting this notion, ex-vivo expanded polyclonal Treg cells become highly dependent on IL-2 and serum IL-2 levels rapidly decline following infusion of Treg cells 161. To understand whether persistence of Treg cells following infusion depends on the bioavailability of IL-2, Dong et al. examined the effects of low dose IL-2 in combination with Treg ACT in patients with T1D 160. Although combination therapy led to an increase in the number of exogenous Treg cells in peripheral blood, cytotoxic cells including subsets of activated NK and granzyme/perforin-producing CD8+ T cells were also expanded in the combinatorial arm. These observations provide a strong impetus for the clinical assessment of Treg-biased IL-2 muteins/variants in combination with autologous polyclonal Treg cell therapy and underscore the importance of the pleiotropic effects of IL-2, even with the redirection of specificity of IL-2 towards Treg cells.
The safety and activity of autologous Treg ACT has been investigated in a number of autoimmune indications and transplant settings including liver and kidney transplantation 164; 165; 166; 167 168, multiple sclerosis 169, GvHD 170, and Crohn’s disease 171. Several clinical trials are currently assessing the safety of autologous polyclonal or antigen-specific Treg cells as ACTs (NCT02711826, NCT03239470, NCT04661254, NCT04817774, and NCT04817774) (Figure 6C). For example, the safety and tolerability of chimeric antigen receptor (CAR) Treg cells targeting HLA-A2, an antigen commonly mismatched in transplantation, is currently underway in the settings of kidney transplantation and end stage renal disease (NCT04817774). As antigen-specific Treg cells have been shown to exhibit superior activity in preclinical models of autoimmunity when compared to polyclonal Treg cells, it will be interesting to understand whether redirection of Treg cells towards autoantigens via TCR and CAR engineering approaches leads to sustained antigen-specific tolerance induction in-vivo in humans 172. In summary, combinatorial modalities leveraging TCR/CAR-Treg engineering approaches coupled with designer cytokine therapy promise to deliver selective and individually disease-tailored Treg cell immunotherapy (Figure 6B-C).
Concluding Remarks and Future Directions
In the past two decades the identification of Foxp3 Treg cells has ushered a revolution in our understanding of fundamental processes governing immunological tolerance; beyond this classical role of Treg cells, a new role for these cells has recently emerged in the maintenance tissue homeostasis and repair. The harnessing of these mechanisms has the potential to deliver innovative therapeutic approaches in autoimmune and inflammatory diseases as well as chronic degenerative diseases.
Acknowledgement
We apologize to colleagues whose work we were not able to discuss and cite due to space limitations. We thank Dr. Dillon Patterson and Dr. Joseph Pereira for careful reading of this manuscript. This work was supported by the Office of Faculty Development at Boston Children's Hospital to M.B and by the National Institutes of Health grants R01 AI115699, AI065617 and AI126915 to T.A.C, 1F31CA281090-01 to P.G., P01 AI108545 to A.H.S., RO1DK127278 to M.C.H and RO1CA276866 to M.H.C and A.H.S.
Abbreviations used:
- Foxp3
forkhead box P3
- Treg
regulatory T
- self-peptide-MHC
self-peptide major histocompatibility complex
- nTreg
natural regulatory T
- pTreg
peripheral regulatory T
- iTreg
induced regulatory T
- CNS
central nervous system
- AREG
amphiregulin
- Aire
autoimmune regulator
- VAT
visceral adipose tissue
- PPARγ
peroxisome proliferator-activated receptor-γ
- Nrp-1
neuropilin-1
- Teff
effector T
- Tfr
follicular regulatory T
- TFH
follicar helper T
- ST2
interleukin 1 receptor-like 1
- ILC2
type 2 innate lymphoid cell
- ILC3
type 3 innate lymphoid cells
- GDF15
growth and differentiation factor 15
- GRB2
growth factor-receptor bound protein 2
- RORα
retinoic acid-related orphan receptor α
- RORγt
RAR-related orphan receptor gamma
- EGFR
epidermal growth factor receptor
- EAE
experimental autoimmune encephalitis
- IRF4
interferon regulatory factor-4
- MIS-C
multisystem inflammatory syndrome in children
- ACT
adoptive cell therapy
- T1D
type 1 diabetes
- CAR
chimeric antigen receptor
- cTEC
cortical thymic epithelial cells
- mTEC
medullary thymic epithelial cells
- DR3
death receptor 3
- TL1A
TNF ligand-related molecule 1
- TSLRPR
thymic stromal lymphopoietin receptor
- PENK
proenkephalin
- Met-ENK
methionine enkephalin
- MAPK
mitogen-activated protein kinase
- TSDR
Treg specific demethylation region
- HDR
homology directed repair
- EF1α
elongation factor 1 alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
S.H. has consulted for Merck KGaA. P.G. has consulted for RA Capital and Astro Therapeutics. A.H.S. currently has funding from Quark and AbbVie, unrelated to the submitted work. A.H.S. serves on advisory boards for SQZ Biotechnologies, Selecta, Elpiscience, Monopteros, Bicara, Fibrogen, IOME, Corner Therapeutics and Alixia. She also is on scientific advisory boards for the Massachusetts General Cancer Center, Program in Cellular and Molecular Medicine at Boston Children’s Hospital, the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center, Bloomberg-Kimmel Institute for Cancer Immunotherapy, Glaxo Smith Kline, Janssen and Amgen. She is an academic editor for the Journal of Experimental Medicine. A.H.S. has patents/pending royalties on the PD-1 pathway from Roche and Novartis. M.C.H. has patents pending on the PHD3 pathway, is on the scientific advisory board for Alixia, Minovia, and MitoQ, and had research funding from Roche.
References
- 1.Silverstein AM. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat Immunol 2001; 2:279–81. [DOI] [PubMed] [Google Scholar]
- 2.Brent L. The discovery of immunologic tolerance. Hum Immunol 1997; 52:75–81. [DOI] [PubMed] [Google Scholar]
- 3.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell 1987; 49:273–80. [DOI] [PubMed] [Google Scholar]
- 4.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 1988; 333:742–6. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002; 298:1395–401. [DOI] [PubMed] [Google Scholar]
- 6.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, et al. T-cell-receptor affinity and thymocyte positive selection. Nature 1996; 381:616–20. [DOI] [PubMed] [Google Scholar]
- 7.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol 2014; 14:377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001; 2:301–6. [DOI] [PubMed] [Google Scholar]
- 9.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity 2008; 28:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR, et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat Immunol 2019; 20:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA. Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity 2016; 44:1102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013; 339:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 2015; 348:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol 2013; 4:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 2012; 30:733–58. [DOI] [PubMed] [Google Scholar]
- 16.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol 2007; 178:4022–6. [DOI] [PubMed] [Google Scholar]
- 17.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol 2009; 9:83–9. [DOI] [PubMed] [Google Scholar]
- 19.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 2012; 209:1713–22, S1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010; 184:3433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009; 30:626–35. [DOI] [PubMed] [Google Scholar]
- 22.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 2011; 35:109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dombrowski Y, O'Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci 2017; 20:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 2009; 119:2898–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014; 115:55–67. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 2012; 125:1652–63. [DOI] [PubMed] [Google Scholar]
- 27.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 2006; 12:178–80. [DOI] [PubMed] [Google Scholar]
- 28.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012; 486:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, et al. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell 2018; 174:285–99 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013; 155:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med 2014; 6:258ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, et al. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016; 44:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015; 162:1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019; 565:246–50. [DOI] [PubMed] [Google Scholar]
- 36.Munoz-Rojas AR, Mathis D. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol 2021; 21:597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 2016; 16:295–309. [DOI] [PubMed] [Google Scholar]
- 38.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol 2016; 138:639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500:232–6. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORgammat pathway to suppress food allergy. Nat Med 2019; 25:1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 2012; 482:395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 2011; 17:983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, et al. Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol 2016; 9:444–57. [DOI] [PubMed] [Google Scholar]
- 44.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 2015; 349:989–93. [DOI] [PubMed] [Google Scholar]
- 45.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015; 349:993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 2022; 610:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, et al. A RORgammat(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature 2022; 610:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akagbosu B, Tayyebi Z, Shibu G, Paucar Iza YA, Deep D, Parisotto YF, et al. Novel antigen-presenting cell imparts T(reg)-dependent tolerance to gut microbiota. Nature 2022; 610:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephen-Victor E, Chatila TA. An embarrassment of riches: RORgammat(+) antigen-presenting cells in peripheral tolerance. Immunity 2022; 55:1978–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest 2011; 121:4503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014; 513:564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 2012; 13:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 2011; 35:337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 2005; 202:1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bacher P, Heinrich F, Stervbo U, Nienen M, Vahldieck M, Iwert C, et al. Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. Cell 2016; 167:1067–78 e16. [DOI] [PubMed] [Google Scholar]
- 56.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med 2006; 203:2649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faustino LD, Griffith JW, Rahimi RA, Nepal K, Hamilos DL, Cho JL, et al. Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat Immunol 2020; 21:1371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen CC, Kobayashi T, Iijima K, Hsu FC, Kita H. IL-33 dysregulates regulatory T cells and impairs established immunologic tolerance in the lungs. J Allergy Clin Immunol 2017; 140:1351–63 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med 2012; 18:1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harb H, Stephen-Victor E, Crestani E, Benamar M, Massoud A, Cui Y, et al. A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Nat Immunol 2020; 21:1359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harb H, Benamar M, Lai PS, Contini P, Griffith JW, Crestani E, et al. Notch4 signaling limits regulatory T-cell-mediated tissue repair and promotes severe lung inflammation in viral infections. Immunity 2021; 54:1186–99 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mock JR, Garibaldi BT, Aggarwal NR, Jenkins J, Limjunyawong N, Singer BD, et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol 2014; 7:1440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015; 43:1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017; 169:1119–29 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol 2006; 177:4488–94. [DOI] [PubMed] [Google Scholar]
- 66.Iellem A, Colantonio L, D'Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+CD25+ suppressor T cells. Eur J Immunol 2003; 33:1488–96. [DOI] [PubMed] [Google Scholar]
- 67.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med 2007; 204:1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong HA, et al. Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin. Cell Host Microbe 2017; 21:467–77 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, et al. Memory regulatory T cells reside in human skin. J Clin Invest 2014; 124:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol 2016; 16:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delacher M, Imbusch CD, Weichenhan D, Breiling A, Hotz-Wagenblatt A, Trager U, et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat Immunol 2017; 18:1160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamazaki S, Nishioka A, Kasuya S, Ohkura N, Hemmi H, Kaisho T, et al. Homeostasis of thymus-derived Foxp3+ regulatory T cells is controlled by ultraviolet B exposure in the skin. J Immunol 2014; 193:5488–97. [DOI] [PubMed] [Google Scholar]
- 73.Harrison OJ, Linehan JL, Shih HY, Bouladoux N, Han SJ, Smelkinson M, et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019; 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalekar LA, Cohen JN, Prevel N, Sandoval PM, Mathur AN, Moreau JM, et al. Regulatory T cells in skin are uniquely poised to suppress profibrotic immune responses. Sci Immunol 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malhotra N, Leyva-Castillo JM, Jadhav U, Barreiro O, Kam C, O'Neill NK, et al. RORalpha-expressing T regulatory cells restrain allergic skin inflammation. Sci Immunol 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartwig T, Zwicky P, Schreiner B, Yawalkar N, Cheng P, Navarini A, et al. Regulatory T Cells Restrain Pathogenic T Helper Cells during Skin Inflammation. Cell Rep 2018; 25:3564–72 e4. [DOI] [PubMed] [Google Scholar]
- 77.Moreau JM, Dhariwala MO, Gouirand V, Boda DP, Boothby IC, Lowe MM, et al. Regulatory T cells promote innate inflammation after skin barrier breach via TGF-beta activation. Sci Immunol 2021; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehta P, Gouirand V, Boda DP, Zhang J, Gearty SV, Zirak B, et al. Layilin Anchors Regulatory T Cells in Skin. J Immunol 2021; 207:1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shime H, Odanaka M, Tsuiji M, Matoba T, Imai M, Yasumizu Y, et al. Proenkephalin(+) regulatory T cells expanded by ultraviolet B exposure maintain skin homeostasis with a healing function. Proc Natl Acad Sci U S A 2020; 117:20696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nosbaum A, Prevel N, Truong HA, Mehta P, Ettinger M, Scharschmidt TC, et al. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J Immunol 2016; 196:2010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathur AN, Zirak B, Boothby IC, Tan M, Cohen JN, Mauro TM, et al. Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity 2019; 50:655–67 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Georgiev P, Charbonnier LM, Chatila TA. Regulatory T Cells: the Many Faces of Foxp3. J Clin Immunol 2019; 39:623–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 2009; 326:986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 2017; 546:421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity 2012; 37:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 2009; 458:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011; 17:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol 2016; 34:609–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science 2010; 329:1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 2012; 36:262–75. [DOI] [PubMed] [Google Scholar]
- 91.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009; 10:1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol 2011; 127:795–805 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tachdjian R, Al Khatib S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol 2010; 125:1128–36 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 2015; 42:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 2013; 39:949–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benamar M, Harb H, Chen Q, Wang M, Chan TMF, Fong J, et al. A common IL-4 receptor variant promotes asthma severity via a T(reg) cell GRB2-IL-6-Notch4 circuit. Allergy 2022; 77:3377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med 2016; 22:1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Esty B, Harb H, Bartnikas LM, Charbonnier LM, Massoud AH, Leon-Astudillo C, et al. Treatment of severe persistent asthma with IL-6 receptor blockade. J Allergy Clin Immunol Pract 2019; 7:1639–42 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014; 20:62–8. [DOI] [PubMed] [Google Scholar]
- 100.Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat Immunol 2015; 16:1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Magee CN, Murakami N, Borges TJ, Shimizu T, Safa K, Ohori S, et al. Notch-1 Inhibition Promotes Immune Regulation in Transplantation Via Regulatory T Cell-Dependent Mechanisms. Circulation 2019; 140:846–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xia M, Harb H, Saffari A, Sioutas C, Chatila TA. A Jagged 1-Notch 4 molecular switch mediates airway inflammation induced by ultrafine particles. J Allergy Clin Immunol 2018; 142:1243–56 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rong H, Shen H, Xu Y, Yang H. Notch signalling suppresses regulatory T-cell function in murine experimental autoimmune uveitis. Immunology 2016; 149:447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin L, Zhou YC, Wu HJ, Zhuo Y, Wang YP, Si CY, et al. Notch Signaling Modulates the Balance of Regulatory T Cells and T Helper 17 Cells in Patients with Chronic Hepatitis C. DNA Cell Biol 2017; 36:311–20. [DOI] [PubMed] [Google Scholar]
- 105.Jiao WE, Wei JF, Kong YG, Xu Y, Tao ZZ, Chen SM. Notch Signaling Promotes Development of Allergic Rhinitis by Suppressing Foxp3 Expression and Treg Cell Differentiation. Int Arch Allergy Immunol 2019; 178:33–44. [DOI] [PubMed] [Google Scholar]
- 106.Benamar M, Chen Q, Chou J, Jule AM, Boudra R, Contini P, et al. The Notch1/CD22 signaling axis disrupts Treg function in SARS-CoV-2-associated multisystem inflammatory syndrome in children. J Clin Invest 2023; 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, et al. Transforming Growth Factor-beta Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 2017; 46:660–74. [DOI] [PubMed] [Google Scholar]
- 108.Turner JA, Stephen-Victor E, Wang S, Rivas MN, Abdel-Gadir A, Harb H, et al. Regulatory T Cell-Derived TGF-beta1 Controls Multiple Checkpoints Governing Allergy and Autoimmunity. Immunity 2020; 53:1202–14 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stephen-Victor E, Cui Y, Wang Z, Benamar M, Charbonnier LM, Chatila TA. Essential functions of regulatory T cell TGF-beta1 revealed by differential gene-targeting approaches. Immunity 2021; 54:397–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011; 34:566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol 2009; 133:22–6. [DOI] [PubMed] [Google Scholar]
- 112.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, et al. Administration of CD4+CD25highCD127− regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care 2012; 35:1817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015; 7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dolgin E. T(reg) engineers take aim at autoimmunity. Nat Biotechnol 2021; 39:1317–9. [DOI] [PubMed] [Google Scholar]
- 115.Boardman DA, Levings MK. Emerging strategies for treating autoimmune disorders with genetically modified Treg cells. J Allergy Clin Immunol 2022; 149:1–11. [DOI] [PubMed] [Google Scholar]
- 116.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 117.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. [DOI] [PubMed] [Google Scholar]
- 118.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4:337–42. [DOI] [PubMed] [Google Scholar]
- 119.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest 2005; 115:3276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aarts-Riemens T, Emmelot ME, Verdonck LF, Mutis T. Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4(+)CD25(−) cells. Eur J Immunol 2008; 38:1381–90. [DOI] [PubMed] [Google Scholar]
- 121.Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther 2008; 16:194–202. [DOI] [PubMed] [Google Scholar]
- 122.Honaker Y, Hubbard N, Xiang Y, Fisher L, Hagin D, Sommer K, et al. Gene editing to induce FOXP3 expression in human CD4(+) T cells leads to a stable regulatory phenotype and function. Sci Transl Med 2020; 12. [DOI] [PubMed] [Google Scholar]
- 123.Sebastian M, Lopez-Ocasio M, Metidji A, Rieder SA, Shevach EM, Thornton AM. Helios Controls a Limited Subset of Regulatory T Cell Functions. J Immunol 2016; 196:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015; 350:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cortez JT, Montauti E, Shifrut E, Gatchalian J, Zhang Y, Shaked O, et al. CRISPR screen in regulatory T cells reveals modulators of Foxp3. Nature 2020; 582:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schumann K, Raju SS, Lauber M, Kolb S, Shifrut E, Cortez JT, et al. Functional CRISPR dissection of gene networks controlling human regulatory T cell identity. Nat Immunol 2020; 21:1456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loo CS, Gatchalian J, Liang Y, Leblanc M, Xie M, Ho J, et al. A Genome-wide CRISPR Screen Reveals a Role for the Non-canonical Nucleosome-Remodeling BAF Complex in Foxp3 Expression and Regulatory T Cell Function. Immunity 2020; 53:143–57 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lam AJ, Lin DTS, Gillies JK, Uday P, Pesenacker AM, Kobor MS, et al. Optimized CRISPR-mediated gene knockin reveals FOXP3-independent maintenance of human Treg identity. Cell Rep 2021; 36:109494. [DOI] [PubMed] [Google Scholar]
- 129.Han X, Wang M, Duan S, Franco PJ, Kenty JH, Hedrick P, et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc Natl Acad Sci U S A 2019; 116:10441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 2019; 37:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haque M, Lei F, Xiong X, Das JK, Ren X, Fang D, et al. Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes. JCI Insight 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haque M, Fino K, Sandhu P, Song J. Development of stem cell-derived antigen-specific regulatory T cells against autoimmunity. J Vis Exp 2016;117:e54720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yano H, Sato T, Shinohara T, Koga K, Iriguchi S, Matsuda A, et al. iPSC-Derived HLA-A2 CAR Tregs Showed Suppression of GvHD in a Xenograft Model. Blood 2022; 140:2365–6. [Google Scholar]
- 134.Forstneric V, Oven I, Ogorevc J, Lainscek D, Praznik A, Lebar T, et al. CRISPRa-mediated FOXP3 gene upregulation in mammalian cells. Cell Biosci 2019; 9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol 2007; 8:359–68. [DOI] [PubMed] [Google Scholar]
- 136.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012; 150:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morikawa H, Ohkura N, Vandenbon A, Itoh M, Nagao-Sato S, Kawaji H, et al. Differential roles of epigenetic changes and Foxp3 expression in regulatory T cell-specific transcriptional regulation. Proc Natl Acad Sci U S A 2014; 111:5289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005; 6:1142–51. [DOI] [PubMed] [Google Scholar]
- 139.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, et al. An essential role for the IL-2 receptor in T(reg) cell function. Nat Immunol 2016; 17:1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol 2010; 185:6426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abbas AK, Trotta E, D RS, Marson A, Bluestone JA. Revisiting IL-2: Biology and therapeutic prospects. Sci Immunol 2018; 3. [DOI] [PubMed] [Google Scholar]
- 142.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 2009; 30:204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006; 311:1924–7. [DOI] [PubMed] [Google Scholar]
- 144.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 2009; 206:751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spangler JB, Tomala J, Luca VC, Jude KM, Dong S, Ring AM, et al. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity 2015; 42:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spangler JB, Trotta E, Tomala J, Peck A, Young TA, Savvides CS, et al. Engineering a Single-Agent Cytokine/Antibody Fusion That Selectively Expands Regulatory T Cells for Autoimmune Disease Therapy. J Immunol 2018; 201:2094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dolgin E. IL-2 upgrades show promise at ASCO. Nat Biotechnol 2022; 40:986–8. [DOI] [PubMed] [Google Scholar]
- 148.Zhang B, Sun J, Wang Y, Ji D, Yuan Y, Li S, et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells. Nat Biomed Eng 2021; 5:1288–305. [DOI] [PubMed] [Google Scholar]
- 149.Dixit N, Fanton C, Langowski JL, Kirksey Y, Kirk P, Chang T, et al. NKTR-358: A novel regulatory T-cell stimulator that selectively stimulates expansion and suppressive function of regulatory T cells for the treatment of autoimmune and inflammatory diseases. J Transl Autoimmun 2021; 4:100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khoryati L, Pham MN, Sherve M, Kumari S, Cook K, Pearson J, et al. An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci Immunol 2020; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peterson LB, Bell CJM, Howlett SK, Pekalski ML, Brady K, Hinton H, et al. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J Autoimmun 2018; 95:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Glassman CR, Su L, Majri-Morrison SS, Winkelmann H, Mo F, Li P, et al. Calibration of cell-intrinsic interleukin-2 response thresholds guides design of a regulatory T cell biased agonist. Elife 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Raeber ME, Sahin D, Karakus U, Boyman O. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. EBioMedicine 2023; 90:104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ghelani A, Bates D, Conner K, Wu MZ, Lu J, Hu YL, et al. Defining the Threshold IL-2 Signal Required for Induction of Selective Treg Cell Responses Using Engineered IL-2 Muteins. Front Immunol 2020; 11:1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pham MN, Khoryati L, Jamison BL, Hayes E, Sullivan JM, Campbell DJ, et al. In Vivo Expansion of Antigen-Specific Regulatory T Cells through Staggered Fc.IL-2 Mutein Dosing and Antigen-Specific Immunotherapy. Immunohorizons 2021; 5:782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hirai T, Ramos TL, Lin PY, Simonetta F, Su LL, Picton LK, et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J Clin Invest 2021; 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hirai T, Lin PY, Ramos TL, Simonetta F, Su LL, Picton LK, et al. IL-2 receptor engineering enhances regulatory T cell function suppressed by calcineurin inhibitor. Am J Transplant 2022; 22:3061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de Picciotto S, DeVita N, Hsiao CJ, Honan C, Tse SW, Nguyen M, et al. Selective activation and expansion of regulatory T cells using lipid encapsulated mRNA encoding a long-acting IL-2 mutein. Nat Commun 2022; 13:3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu DR, Wu H, Driver I, Ingersoll S, Sohn S, Wang S, et al. Dynamic changes in the regulatory T-cell heterogeneity and function by murine IL-2 mutein. Life Sci Alliance 2020; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dong S, Hiam-Galvez KJ, Mowery CT, Herold KC, Gitelman SE, Esensten JH, et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 2021; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marek-Trzonkowska N, Mysliwiec M, Iwaszkiewicz-Grzes D, Gliwinski M, Derkowska I, Zalinska M, et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med 2016; 14:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kremer J, Henschel P, Simon D, Riet T, Falk C, Hardtke-Wolenski M, et al. Membrane-bound IL-2 improves the expansion, survival, and phenotype of CAR Tregs and confers resistance to calcineurin inhibitors. Front Immunol 2022; 13:1005582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol 2014; 153:23–30. [DOI] [PubMed] [Google Scholar]
- 164.Sanchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY, Romano M, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant 2020; 20:1125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chandran S, Tang Q, Sarwal M, Laszik ZG, Putnam AL, Lee K, et al. Polyclonal Regulatory T Cell Therapy for Control of Inflammation in Kidney Transplants. Am J Transplant 2017; 17:2945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020; 395:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roemhild A, Otto NM, Moll G, Abou-El-Enein M, Kaiser D, Bold G, et al. Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial. BMJ 2020; 371:m3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harden PN, Game DS, Sawitzki B, Van der Net JB, Hester J, Bushell A, et al. Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Am J Transplant 2021; 21:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chwojnicki K, Iwaszkiewicz-Grzes D, Jankowska A, Zielinski M, Lowiec P, Gliwinski M, et al. Administration of CD4(+)CD25(high)CD127(−)FoxP3(+) Regulatory T Cells for Relapsing-Remitting Multiple Sclerosis: A Phase 1 Study. BioDrugs 2021; 35:47–60. [DOI] [PubMed] [Google Scholar]
- 170.Theil A, Tuve S, Oelschlagel U, Maiwald A, Dohler D, Ossmann D, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy 2015; 17:473–86. [DOI] [PubMed] [Google Scholar]
- 171.Desreumaux P, Foussat A, Allez M, Beaugerie L, Hebuterne X, Bouhnik Y, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology 2012; 143:1207–17 e2. [DOI] [PubMed] [Google Scholar]
- 172.Raffin C, Vo LT, Bluestone JA. T(reg) cell-based therapies: challenges and perspectives. Nat Rev Immunol 2020; 20:158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]