Abstract
Dok-R has previously been shown to associate with the epidermal growth factor receptor (EGFR) and become tyrosine phosphorylated in response to EGF stimulation. The recruitment of Dok-R to the EGFR, which is mediated through its phosphotyrosine binding (PTB) domain, results in attenuation of mitogen-activated protein kinase (MAPK) activation. Dok-R's ability to attenuate EGF-driven MAPK activation is independent of its ability to recruit rasGAP, a known attenuator of MAPK activity, suggesting an alternate Dok-R-mediated pathway. Herein, we have determined the structural determinants within Dok-R that are required for its ability to attenuate EGF signaling and to associate with c-Src and with the Src family kinase (SFK)-inhibitory kinase, Csk. We demonstrate that Dok-R associates constitutively with c-Src through an SH3-dependent interaction and that this association is essential to Dok-R's ability to attenuate c-Src activity and diminish MAPK and Akt/PKB activity. We further illustrate that EGF-dependent phosphorylation of Dok-R requires SFK activity and, more specifically, that SFK-dependent phosphorylation of tyrosine 402 on Dok-R facilitates the inducible recruitment of Csk. We propose that recruitment of Csk to Dok-R serves to bring Csk to c-Src and down-regulate its activity, resulting in a concomitant attenuation of MAPK and Akt/PKB activity. Furthermore, we demonstrate that Dok-R can abrogate c-Src's ability to protect the breast cancer cell line SKBR3 from anoikis and that an association with c-Src and Csk is required for this activity. Collectively these results demonstrate that Dok-R acts as an EGFR-recruited scaffolding molecule that processively assembles c-Src and Csk to attenuate signaling from the EGFR.
The precise spatial and temporal control of signals emanating from an activated receptor tyrosine kinase (RTK) depends in part on the diverse repertoire of recruited proximal signaling proteins. These recruited proteins can serve to both augment the signal from the receptor and to attenuate the signal, the balance of which is crucial to normal cell physiology (13). Docking proteins have been shown to play a pivotal role in transducing signals from activated RTKs. In addition to being constitutively bound to signaling molecules, these specialized types of polypeptides also become tyrosine phosphorylated upon recruitment to RTKs. These tyrosine phosphorylation events establish high-affinity phoshotyrosine-based binding sites for the recruitment of additional signaling molecules (17, 34). As such, docking proteins function much like a scaffold protein, locally enriching the quantity and diversity of signaling proteins necessary to elicit a defined response to RTK activation.
One family of docking proteins that appears to have a negative role in RTK or cytokine signaling is the Dok family of proteins. Based on amino acid sequence homology, the Dok family of proteins consists of five members, including Dok, Dok-R, DokL, Dok4, and Dok5 (4, 6, 10, 14, 19, 25, 47). Structural characteristics of this family make them most similar to the insulin receptor substrate family of proteins (6). The Dok family of proteins contains three distinct protein domains or regions, which include an amino-terminal pleckstrin homology (PH) domain, a central phosphotyrosine binding (PTB) domain, and a carboxy-terminal proline-rich region (PRR). Dok4 and Dok5 have been shown to potentiate signals emanating from the c-Ret receptor (14), while Dok, Dok-R, and DokL have all been shown to primarily mitigate signals downstream of a wide array of receptor and nonreceptor tyrosine kinases (6, 20, 25, 31, 48, 49). It has recently been proposed that family members Dok, Dok-R, and DokL are phylogenetically distinct from Dok4 and Dok5 and that they therefore be considered a separate subgroup of the family based upon functional differences and different patterns of expression (14).
Based upon structure-function analysis, it seems apparent that Dok, Dok-R, and DokL mediate negative signaling events by recruiting and locally enriching negative signaling proteins in the proximal region of transduction cascades. For example Dok, Dok-R, and DokL have been shown to inducibly interact with the lipid phosphatase SHIP1 (24, 25, 33), while Dok and DokL also interact with Csk, a potent negative regulator of c-Src family kinase members (38).
Both Dok and Dok-R have been shown to inducibly interact with the GTPase activating protein p120 RasGAP, suggesting a negative regulatory role in Ras/Raf/Mek/mitogen-activated protein kinase (MAPK) signaling pathway (4, 10, 19, 47). Supporting the significance of the Dok/RasGAP association, Songyang and Baltimore et al. demonstrated in v-src-transformed cells that overexpression of Dok leads to a dramatic reduction in Ras GTP loading and a concomitant decrease in cellular transformation (40). Furthermore, genetic ablation of the Dok locus initiates leukemogenesis, an event which correlates with increased and prolonged MAPK activation (11). In addition, Gugasyan and Lock et al. demonstrated that bone marrow cells retrovirally infected with Dok-R are deficient in their ability to reseed lymphoid organs and that this defect is dependent on intact RasGAP binding sites (15). Notwithstanding, a clear consensus in the literature with regards to the functional importance of this association has not been developed. Kashige and Kobayashi et al. demonstrated that Bcr-Abl-dependent recruitment of RasGAP to p62 Dok results in dramatically decreased RasGAP activity, suggesting the existence of alternate mechanisms, other than the Dok/Dok-R-RasGAP association, in attenuating tyrosine kinase-driven activation of MAPK (21). Consistent with this notion, Zhao and Aelst et al. found that Dok-dependent attenuation of MAPK activity following platelet-derived growth factor (PDGF) stimulation was not dependent on its ability to recruit RasGAP or Nck, but rather was a function of some unknown mechanism involving phosphatidylinositol 3-kinase (PI3-kinase) activity (49). Studies by our group on epidermal growth factor receptor (EGFR) signaling (20) have further indicated that RasGAP recruitment by Dok-R is not essential for inhibition of MAPK. And finally, further contributing to the unknown role of RasGAP in modulating the down-regulation of signaling from the Dok family of proteins, there are data demonstrating that DokL, which intrinsically lacks RasGAP binding sites, retains the ability to attenuate MAPK activity in Bcr-Abl-transformed cells (6).
Herein we attempt to elucidate in further detail what mechanisms other than RasGAP may mediate Dok-R-dependent attenuation of MAPK. Optimal recruitment of Dok-R to the activated EGFR and its subsequent phosphorylation require both a functional PH domain and PTB domain, which is required for Dok-R's ability to inhibit downstream activation of MAPK (20). Importantly, these effects were shown not to be a consequence of Dok-R displacing the Shc/Grb2/Sos complex from the receptor (20), suggesting a more specific role for Dok-R in down-regulating MAPK activity than mere competition for binding sites on the EGFR. Moreover, expression of a form of Dok-R no longer able to interact with RasGAP illustrates that recruitment of RasGAP to a phosphorylated Dok-R is not required to mitigate MAPK activation downstream of EGFR (20). Collectively these results support a role for attenuation of MAPK through a rasGAP-independent mechanism that requires recruitment of Dok-R to the EGFR. Here we describe a novel mechanism whereby Dok-R facilitates the formation and recruitment of a multi-protein complex to the activated EGFR, resulting in the attenuation of signals from this receptor. We present data to support a model whereby Dok-R is constitutively associated with c-Src and upon recruitment to the EGFR Dok-R becomes tyrosine phosphorylated on tyrosine residue 402, which serves to recruit the negative regulator kinase, Csk. We show that this processive recruitment of a Dok-R/c-Src/Csk complex to the EGFR serves to provide the scaffolding that ultimately leads to the attenuation of c-Src kinase activity as well as diminished MAPK and Akt/PKB activity.
MATERIALS AND METHODS
Plasmids.
The plasmid encoding hemagglutinin (HA)-Dok-R, HA-Dok-R ΔPRR, and HA-Dok-R ΔC-PRR have previously been described elsewhere (29). HA-Dok-R ΔPRR, HA-Dok-RΔC-PRR were engineered to contain premature TAG stop codons situated at nucleotides 815 and 1010, respectively. HA-Dok-R Y402F, built from the wild-type HA-Dok-R plasmid, was engineered to express a phenylalanine residue in place of tyrosine 402. This construct and all other Dok-R constructs were confirmed for sequence integrity. Streptavidin-tagged Csk SH2 and SH3 were a generous gift of Kari Alitalo. Bacterial glutathione S-transferase (GST) fusion proteins, including Lck, Abl, Vav, Crk, Src, spectrin, p85, and Fyn, were a kind gift of Jane McGlade. The plasmid encoding GST-tagged Erk-2 has previously been described (20). Adenoviruses expressing Flag Dok-R, Flag Dok-R ΔPRR were constructed and purified by Andrea Gambotto, Pittsburgh Viral Vector Core Facility.
Cell lines and cell culture.
Cos1 monkey fibroblasts (American Type Culture Collection), SKBR3 breast carcinoma cell lines (gift of Joyce Slingerland), and HT29 (gift of Jorge Filmus) were all maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 1× penicillin, and 1× streptomycin (Life Technologies). All transient cell transfections were performed with 5 μg or 10 μg of plasmid DNA utilizing Lipofectamine (Gibco) according to the manufacturer's instructions. Adenoviral infections were performed in normal 10% DMEM at a final multiplicity of infection of 25.
EGF cell stimulation, PMA, and PP1 treatment.
Cos1 cells were serum starved for a period of at least 18 h prior to EGF stimulation for the indicated times. Purified recombinant EGF (R&D) diluted to 100 ng/ml in serum-free DMEM was applied to the indicated samples and allowed to incubate at 37°C in 5% CO2. Immediately after timed EGF stimulation, cells were rinsed in ice-cold phosphate-buffered saline and lysed on ice. PP1 and phorbol myristate acetate (PMA) were suspended in reagent-grade dimethyl sulfoxide (DMSO) (Sigma). Cells were treated with indicated concentrations of either PP1, PMA, or vehicle.
Antibodies used for immunoprecipitation and Western blotting.
Commercially available antibodies were used as follows: monoclonal anti-Src GD-11, monoclonal anti-phosphotyrosine 4G10, and polyclonal Dok-R (Upstate Biotech); monoclonal anti-phospho-specific p42,44, monoclonal anti-phosphoserine 473 Akt/PKB, and monoclonal anti-Akt (Cell Signaling Technology); monoclonal anti-HA horseradish peroxidase-conjugated 12CA5 and monoclonal anti-HA 12CA5 (Roche); monoclonal anti-actin AC-40 and monoclonal anti-Flag M2 (Sigma); polyclonal anti-phospho Y527 Src (BioSource); polyclonal anti-GST Z-5 and polyclonal anti-EGFR 1005 (Santa Cruz); and monoclonal anti-Csk (Transduction Laboratories). Coimmunoprecipitation and Western blotting procedures have previously been described (19, 20).
In vitro Src kinase assay.
c-Src activity was measured using an in vitro kinase assay as described previously (7). Briefly, equal quantities of cell lysate were immunoprecipitated with 1 μg of monoclonal anti-Src antibody and 30 μl of 50% agarose-bound protein A. Immunoprecipitates were washed three times with 500 μl of lysis buffer and three times with 100 μl of 10 mM HEPES (pH 8.0). Beads were then resuspended in 35 μl of reaction mixture (45 mM HEPES, pH 8.0, 150 mM NaCl, 50 mM MgCl2, 10 μM Na3VO4, 2 μM ATP, and 10 μCi of [γ-32P]ATP) containing 0.04 μg/μl acid-treated enolase (see below) and incubated at 25°C for 15 min. Reactions were stopped by addition of 6 μl of 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and boiling for 5 min. Samples were run through a 7.5% SDS-polyacrylamide gel, and the dried gels were analyzed by autoradiography. The protocol for acid treatment of enolase was adapted from Z. Shen and F. Moran et al. (39). Briefly, 0.6 μl of enolase suspension (Sigma) was mixed with 0.6 μl of 60 mM HEPES (pH 8.0), 2.4 mM dithiothreitol, and 60% glycerol and added to 1.2 μl of 500 mM acetic acid. After incubation at 37°C for 15 min, the reaction was stopped with 2.4 μl of 100 mM Tris-HCl (pH 8.0) and 20 mM MgCl2.
Cell death ELISA.
SKBR3 and HT29 cells were infected with one of the adenovirus vectors ad-LacZ (vector control), ad-Dok-R, or ad-Dok-R ΔPRR. Twenty-four h postinfection, cells were trypsinized and counted in trypan blue. Fifty thousand viable cells were added to each well of a six-well plate either coated with 1% agarose (suspension) or uncoated (monolayer) and were then cultured for an additional 24 h in 10% DMEM at 37°C with 5% CO2. Samples were analyzed according to the manufacturer's specifications (Roche, Cell Death ELISA [enzyme-linked immunosorbent assay] Plus).
In vitro pull-down assay.
Recombinant GST fusion proteins were raised as previously described. Purity of each GST construct and quantification were accomplished via PAGE analysis. Each pull-down experiment utilized 2 μg of purified recombinant GST fusion protein. Cos1 cells were lysed, and pull-downs were executed as previously described (29). Peptide sequences were as follows: Dok-R Y402, biotin-PQATEYDNVILKK; and Dok-R pY402, biotin-PQATEpYDNVILKK.
RESULTS
The carboxyl portion of the proline-rich region of Dok-R is required for EGF-dependent Erk-2 attenuation.
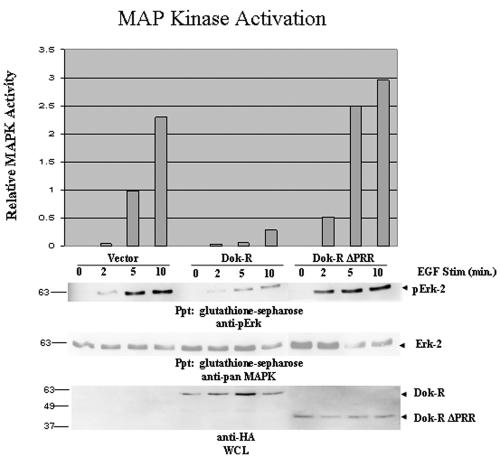
Previously we demonstrated that Dok-R was able to attenuate EGF-induced Erk-2 activity in EGF-stimulated Cos-1 cells in a RasGAP-independent manner (20); thus, we set out to define the alternative pathway(s) that were utilized by Dok-R to attenuate signaling from the EGFR. Towards this goal, a truncation mutant was engineered for these studies, which completely deletes the PRR (Dok-R ΔPRR). The effect of an empty vector, Dok-R, and Dok-R ΔPRR on Erk-2 activation was monitored by cotransfection of Cos1 cells with these constructs and GST-Erk-2 as previously described (20). Serum-starved EGF-stimulated cells transfected with vector cDNA produced the predicted time-dependent increase in Erk-2 activation as assessed by immunoblotting for phospho-specific p42,44 MAPK (Fig. 1). As previously reported, expression of Dok-R in EGF-stimulated cells resulted in a dramatic decrease in the induction of Erk-2 activation as well as a delay in the activation kinetics (Fig. 1), while Dok-R ΔPRR completely lost this Erk-2 attenuating capacity, which demonstrates that the key residues for mediating this attenuation are found within the PRR.
FIG. 1.
The carboxy-terminal region of Dok-R is required for suppression of EGF-dependent MAPK activation. Cos1 cells were transiently transfected with GST-Erk-2 and either vector Dok-R or Dok-R ΔPRR. Serum-starved Cos1 cells were left unstimulated (lane 0) or stimulated (Stim) with 100 ng/ml of EGF for the indicated times (2, 5, or 10 min). Cleared lysates were prepared, and glutathione Sepharose was used to specifically purify the exogenous GST-Erk-2. Immunoblot analysis with phospho-specific MAPK, pan-MAPK (GST-pull-downs) as well as anti-HA (WCL) was performed. The graph represents relative MAPK activation determined via anti-phosho-MAPK immunoblot analysis when normalized to pan-Erk-2 levels using densitometry (Molecular Dynamics and ImageQuant 5.0). Results are representative of four independent experiments and serve to demonstrate that the PRR of Dok-R is necessary to attenuate EGF-dependent MAPK activation.
c-Src kinase activity is necessary for full EGF-dependent activation of MAPK.
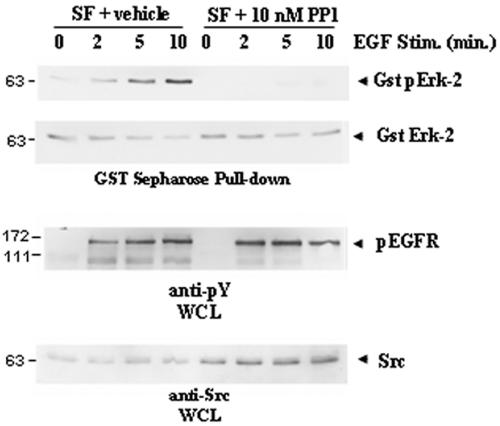
Since our previous studies had demonstrated that Dok-R-mediated attenuation of Erk-2 activity is RasGAP independent, we sought to analyze other cognate MAPK activators downstream of the EGFR. EGF-dependent recruitment and activation of c-Src have been shown to contribute to the potentiation of several distinct signaling pathways which culminate in Erk-2 activation (1). Thus, we were interested in examining whether Dok-R may be playing a role in attenuating signals from the activated EGFR through modulation of c-Src-dependent kinase activity. In order to determine whether Src-family kinases (SFKs) are involved in Erk-2 activation in our cell system, Cos1 cells transfected with the Erk-2-GST plasmid were serum starved and stimulated with EGF in the presence or absence of the SFK inhibitor PP1. Basal Erk-2 phosphorylation was apparent in the untreated cells prior to EGF stimulation, and there was a time-dependent increase in Erk-2 activation up to and including 10 min of stimulation (Fig. 2). Cells that had been pretreated with PP1 demonstrated a potent loss of Erk-2 phosphorylation in both the unstimulated and stimulated samples. Longer exposure of the immunoblots illustrated that although there is a detectable pErk-2 signal in the PP1-treated lanes, the activation is severely impaired (data not shown). Because high levels of PP1 are known to inhibit EGFR activation in certain systems (16), we probed our samples for phosphorylated EGFR and demonstrated that the levels of PP1 used in these experiments did not dramatically affect the activation of the EGFR, suggesting that these effects can be attributed to inhibition of SFKs.
FIG. 2.
SFK activity is required for full activation of EGF-dependent MAPK activity. GST-Erk-2-transfected Cos1 cells were serum starved (serum free [SF]) for 16 h prior to being treated with SFK inhibitor PP1 (10 μM) or vehicle (DMSO) for 2 h. Cells were either left unstimulated (lane 0) or stimulated (Stim.) with EGF at 100 ng/ml for indicated times (2, 5, or 10 min). GST-Erk-2 was purified from cleared lysates with glutathione Sepharose, and this exogenous Erk-2 was assayed for activation via immunoblotting with phospho-specific MAPK antibody. Inhibition of SFKs (PP1) but not vehicle dramatically decreases EGF-dependent MAPK activation in Cos1 cells. Differences in MAPK activation could not be attributed to total Erk-2 levels (anti-pan-MAPK). Treatment with PP1 at 10 μM does not influence activation of the EGFR (anti-pY EGFR).
Dok-R potently attenuates c-Src kinase activity.
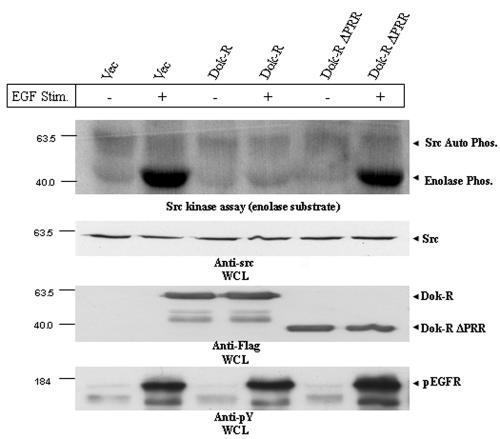
Our results demonstrate that EGF-mediated Erk-2 activation requires the activity of one or more SFKs. We next wanted to examine if Dok-R-mediated attenuation of EGF-dependent Erk2 activation was facilitated through modulation of c-Src kinase activity. To aid in delivering our constructs of interest into cells, we developed recombinant adenoviruses expressing Dok-R and Dok-R ΔPRR. Cos1 cells were infected with either ad-LacZ (vector), ad-Dok-R, or ad-Dok-R ΔPRR and incubated for the indicated times in the presence or absence of EGF. c-Src was immunoprecipitated from equal amounts of protein lysate, and in vitro kinase assays were performed using enolase as an exogenous substrate (7). Several independent in vitro c-Src kinase assays demonstrated that Dok-R potently inhibits EGF-induced c-Src kinase activity, whereas this inhibition is completely abrogated in Dok-R ΔPRR-infected cells, as they responded similarly to vector-infected cells (Fig. 3). Interestingly the levels of c-Src kinase activity in unstimulated Cos1 cells appeared similar in adenovirus vector, ad-Dok-R, and ad-Dok-R ΔPRR samples, indicating that Dok-R does not appear to influence basal c-Src kinase activity, but rather, Dok-R functions to specifically inhibit EGF-induced c-Src kinase activity. Control immunoblots indicate that differences in Src kinase activity could not be accounted for by different levels of total Src protein or activation of the EGFR (see the WCL anti-src IB, anti-4G10 IB results on the figure).
FIG. 3.
Dok-R but not vector or Dok-R ΔPRR inhibits EGF-dependent Src kinase activity. Cos1 cells were infected with adenoviruses engineered to express either vector (Vec) Dok-R or Dok-R ΔPRR. Serum-starved cells were either left unstimulated (−) or stimulated (+) for 5 min with EGF (100 ng/ml). Src immunoprecipitates from infected cells were subjected to an in vitro kinase assay using rabbit enolase as a substrate. Src-mediated enolase phosphorylation (Phos.) in the vector, Dok-R, and Dok-R ΔPRR remained at a basal level in the unstimulated cells. Upon EGF stimulation, cells infected with vector or Dok-R ΔPRR demonstrated a dramatic increase in enolase phosphorylation, while overexpression of Dok-R completely abolished this effect. Immunoblot analysis of Src levels (anti-Src IB) reveals that these effects are not due to differing levels of total Src protein, nor are can they be accounted for by differential EGFR activation (anti-pY EGFR).
Dok-R strongly induces EGF-dependent phosphorylation of c-Src in the negative regulatory tyrosine, 527.
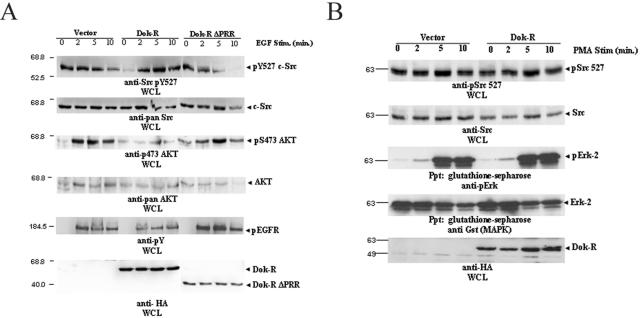
Src kinase activity is tightly and dynamically regulated by the opposing actions of several different phosphatases (8, 9, 12, 35, 50) and almost exclusively, the carboxy-terminal Src kinase, Csk (32). Csk down-regulates c-Src kinase activity by catalyzing the addition of a phosphate group to the negative regulatory tyrosine (Y527) located in the carboxy-terminal region of c-Src. To examine the possibility that Dok-R was mediating its attenuation of c-Src through modulation of the intracellular localization of and/or activity of Csk, we set out to specifically examine the phosphorylation of c-Src tyrosine residue 527. Cos1 cells were transfected with one of the vectors Dok-R and Dok-R ΔPRR and allowed to grow for 24 h under normal growth conditions. Cells were then serum starved and stimulated with EGF as in our previous experiments. Immunoblot analysis with an anti-phosphotyrosine 527 (pY527) Src-specific antibody reveals that Y527 is hyperphosphorylated in the presence of overexpressed Dok-R (Fig. 4A). Importantly, these results are in accordance with our previous in vitro kinase assay results (Fig. 3) and they suggest that the attenuation of c-Src activity is dependent upon recruitment of Dok-R to the activated EGFR. Upon longer exposure of the cells to EGF stimulation, we see a time-dependent loss of c-Src pY527 signal in the vector and Dok-R ΔPRR sample lanes indicative of c-Src activation that is not noted in the Dok-R-transfected lanes. To determine how universal this effect was, we performed the same assay in NIH 3T3 cells and observed a similar, albeit less dramatic, hyperphosphorylation of c-Src tyrosine 527, which most probably reflects the higher expression levels of Dok-R achieved in Cos1 cells (data not shown). Collectively, these results suggest that the PRR of Dok-R is required in both Cos1 and NIH 3T3 cells to promote EGF-dependent hyperphosphorylation of c-Src Y527, and it is this hyperphosphorylation event that results in mitigated c-Src kinase activity.
FIG. 4.
(A) Overexpression of Dok-R facilitates EGF-dependent hyperphosphorylation of autoinhibitory Src Y527. Serum-starved Cos1 cells transfected with either vector Dok-R or Dok-R ΔPRR were either left unstimulated (lane 0) or stimulated (Stim.) with EGF at 100 ng/ml (2, 5, or 10 min) for the indicated times. Cleared lysates were prepared and subjected to SDS-PAGE. Immunoblot analysis of WCL demonstrates that overexpression of Dok-R but not vector or Dok-R ΔPRR results in an EGF-dependent hyperphosphorylation of Src on tyrosine 527. These results cannot be accounted for by overall Src levels (anti-Src immunoblot) or differences in EGFR activation (anti-pY EGFR). Coincident with the Dok-R-dependent hyperphosphorylation of Src Y527 is a dramatic decrease in Akt activation (anti-Akt pS 473), which cannot be accounted for by overall Akt protein levels (anti-pan-Akt). (B) Dok-R does not inhibit Src kinase or MAPK activation in response to PMA stimulation. Cos1 cells cotransfected with one of either vector or Dok-R and GST-Erk2 were left unstimulated (lane 0) or stimulated for indicated times (2, 5, and 10 min) with 200 nM PMA. Cleared lysates were prepared and subjected to SDS-PAGE. Immunoblot analysis demonstrates that overexpression of Dok-R does not result in PMA-dependent inhibition of Src kinase activity (anti-Src pY527) or MAPK activation (Gst Ppt/anti-pErk) when compared to vector-transfected cells.
To examine if this effect was specific to EGFR-mediated signaling events, we cotransfected Cos1 cells with either vector or Dok-R and GST-Erk-2. Serum-starved cells were either left unstimulated or stimulated with PMA for the indicated times. Transfection of Dok-R did not alter the activation of Erk-2 (anti-pErk) or the phosphorylation of c-Src pY527 (anti-Src pY527) in PMA-stimulated cells (Fig. 4B) when compared to vector-transfected controls. These results suggest that c-Src-independent pathways leading to MAPK activation are not perturbed by overexpression of Dok-R protein but rather Dok-R functions to specifically inhibit EGF-induced c-Src activation (Fig. 3 and 4A).
Dok-R is a substrate of c-Src family kinases.
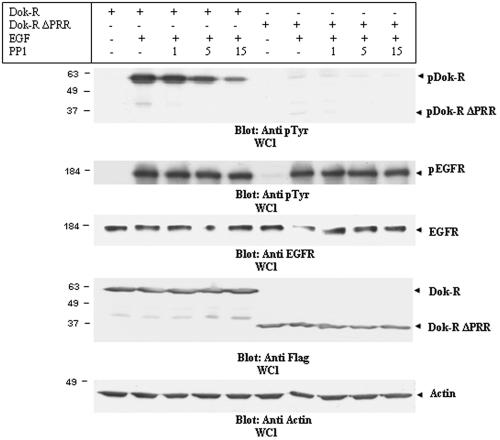
The Dok family of proteins is known to be tyrosine phosphorylated by several distinct protein tyrosine kinases, including Tek/Tie2, Hck, Src, and Abl (19, 27, 29). In previous studies, we have demonstrated that Dok-R becomes tyrosine phosphorylated upon EGF stimulation in Cos1 cells (20). In these studies, the kinase that phosphorylated Dok-R was not determined experimentally; thus, we set out to further define the kinase(s) responsible for EGF-dependent Dok-R phosphorylation. Our initial experiments using the SFK inhibitor PP1 (Fig. 2) suggested that in Cos1 cells SFK activity is absolutely required for full activation of Erk-2; thus, we focused our studies on SFKs. Cos1 cells infected with either ad-Dok-R or ad-Dok-R ΔPRR were treated with PP1 or vehicle (DMSO) prior to stimulation with EGF. Precleared whole-cell lysates (WCL) were analyzed for evidence of stimulation-dependent Dok-R tyrosine phosphorylation via immunoblot analysis with an antiphosphotyrosine antibody (Fig. 5). Inhibition of SFK activity with PP1 resulted in a dramatic reduction of Dok-R phosphorylation. Interestingly the inability of c-Src to physically associate with Dok-R ΔPRR (see Fig. 7A) does not fully abrogate its SFK-dependent phosphorylation, as indicated by the fact that Dok-R ΔPRR to a small degree is tyrosine phosphorylated and this phosphorylation is lost upon treatment with as little as 1 μM PP1. PP1-sensitive phosphorylation of Dok-R ΔPRR suggests that at least one tyrosine residue upstream of the PRR is phosphorylated upon EGF stimulation and that this specific phosphorylation event is mediated by an SFK member. These results may be consistent with those of Songyang et al., as it has recently become apparent that Dok-R, like Dok, becomes phosphorylated on tyrosine residue 142 (unpublished data; Biosource USA), the analogous residue to Dok tyrosine 146 (40). Our results demonstrate that, upon recruitment of Dok-R to the activated EGFR, it becomes tyrosine phosphorylated and the phosphorylation of these tyrosine residues is primarily mediated by an SFK.
FIG. 5.
Dok-R is a substrate of SFKs. Cos1 cells transfected with either Dok-R or Dok-R ΔPRR were serum starved for 16 h prior to a two-hour pretreatment with PP1 (1, 5, and 15 μM) or vehicle (−). Following the pretreatment, cells were either left unstimulated (−) or stimulated (+) for 5 min with 100 ng/ml EGF. Cleared lysates were resolved via SDS-PAGE. Immunoblot analysis of lysates with an anti-pY antibody demonstrates that Dok-R and, to a much lesser degree, Dok-R ΔPRR become tyrosine phosphorylated in response to EGF stimulation. Increasing concentrations of PP1 are able to completely abolish phosphorylation of Dok-R ΔPRR, while phosphorylation of Dok-R is severely impaired in a dose-dependent manner by PP1 treatment. Changes in Dok-R phosphorylation are not a reflection of changes in total Dok-R or Dok-R ΔPRR protein levels (anti-HA, WCL), nor are they due to PP1-dependent changes in EGFR activation (anti-pY EGFR) or EGFR levels (anti-EGFR).
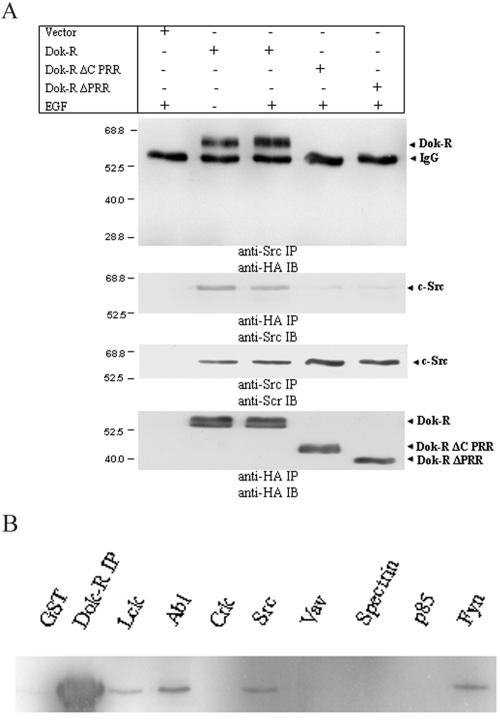
FIG. 7.
Dok-R and Src constitutively coimmunoprecipitate from Cos1 cells, and this association is mediated through Dok-R's PRR. (A) Serum-starved Cos1 cells cotransfected with Src and either vector Dok-R, Dok-R ΔC PRR, or Dok-R ΔPRR were either left unstimulated (−) or stimulated (+) with EGF at 100 ng/ml for 5 min. Cleared lysates were prepared, and immunoprecipitations (IP) were performed for either Src or HA (Dok-R constructs). Reciprocal experiments were performed in which Src immunoprecipitations were immunoblotted (IB) for HA or HA immunoprecipitations were immunoblotted for Src. In both cases, coimmunoprecipitation of Dok-R and Src was noted and was not dependent on EGF stimulation (compare lanes 2 and 3). Membranes were stripped and reprobed (Src IP/Src IB and HA IP/HA IB) and serve to demonstrate that the inability of Dok-R ΔC PRR, and Dok-R ΔPRR to coimmunoprecipitate with Src was not due to a relative lack of these proteins in the immunoprecipitation. (B) The SH3 domain of Src is capable of binding Dok-R in vitro. Cos1 cell lysates transfected with HA Dok-R were mixed with GST or GST-SH3 domain fusions of Lck, Abl, Crk, Src, Vav, spectrin, p85, or Fyn and separated by SDS-PAGE. Anti-HA immunoblots revealed that specifically the SH3 domains of Lck, Abl, Src, and Fyn precipitated Dok-R and not GST or the other GST-SH3 fusion proteins.
Endogenous coimmunoprecipitation of Dok-R/EGFR/c-Src/Csk.
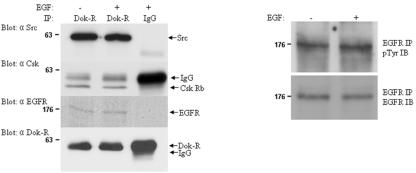
The preceding structure-function studies required transfection and viral experimental approaches; thus, to further validate that these interactions occur in vivo, we tested for the presence of this multiprotein complex in an instance where the EGFR was presumed to be activated in vivo. Embryonic day-12.5 CD1 mouse embryos were harvested on ice and then disaggregated in EDTA/EGTA. In an attempt to further insure maximal activation of the EGFR, half of the cell suspension was stimulated with EGF while the other half was left unstimulated. Cell lysates were prepared, and a single immunoprecipitation was conducted for each of the stimulated and unstimulated samples using equal quantities of either covalently cross-linked anti Dok-R Sepharose or irrelevant control rabbit immunoglobulin G (IgG) Sepharose (non-cross-linked). Each immunoprecipitation was split into four equal portions, and individual Western blots for c-Src, EGFR, Csk, and Dok-R were performed (Fig. 6). The pretreatment of disaggregated embryonic cells with EGF did not increase the relative phosphorylation levels of EGFR, suggesting that the EGFR is already fully activated in early embryos (Fig. 6). Immunoprecipitations utilizing the anti Dok-R antibody specifically associated with large amounts of c-Src and Csk, while EGFR was copurified with Dok-R, albeit at smaller amounts. There was a complete absence of c-Src, Csk, EGFR, and Dok-R in the immunoprecipitations with control rabbit IgG. Attempts to resolve Dok-R from IgG heavy chain have proved futile (see bottom panel, Fig. 6). As such, covalent cross-linking of the anti-Dok-R antibody facilitates relative determination of the quantity of Dok-R immunoprecipitated in this experiment. The total quantity of IgG heavy chain noted in the Csk Western blot is approximately equal to that in the Dok-R Western blot. The increase in the signal pertaining to the Dok-R immunoprecipitation/Western blot when compared to the Dok-R immunoprecipitation/Csk Western blot can be attributed solely to the quantity of Dok-R that was removed from the embryonic lysates. The large amount of IgG heavy chain noted in the rabbit IgG immunoprecipitation/Dok-R Western blot is a reflection of the fact that this IgG was not covalently cross-linked to the beads. The high activation state of the EGFR in lysates produced from early embryos precluded us from detailing the inducibility of these interactions.
FIG. 6.
Dok-R/Src/Csk/EGFR can be coimmunoprecipitated in vivo. Disaggregated embryonic day-12.5 mouse embryos were either left unstimulated or stimulated with EGF for 10 min. Lysates prepared from these samples were subjected to immunoprecipitation (IP) with either antisera to Dok-R (covalently cross-linked to Sepharose beads) or control rabbit IgG (non-cross-linked). Immunoprecipitations containing the Dok-R antisera copurified c-Src, Csk, and EGFR, while these proteins were not detected in the rabbit IgG control lanes. Western blots were performed to assess the relative activation of EGFR in response to EGF stimulation. Based on this analysis, it appears that the EGFR is already maximally phosphorylated in the context of the embryonic tissue used for this experiment (see EGFR IP/pTyr immunoblot [IB] and EGFR IP/EGFR IB).
Constitutive interaction between Dok-R and c-Src.
Towards further defining the type of interactions occurring between Dok-R/Src/Csk/EGFR, we performed detailed structure-function studies utilizing the Cos transfection system described earlier. SFKs have been shown to interact in both inducible and constitutive manners with numerous signaling molecules (43); thus, we sought to determine if Dok-R was able to associate with c-Src. Cos1 cells were cotransfected with plasmids encoding c-Src and either vector Dok-R, a truncation mutant engineered to delete the final 76 amino acids of the PRR (Dok-R ΔC PRR), or Dok-R ΔPRR. Transfected serum-starved cells were either left unstimulated or stimulated for 5 min with EGF. Immunoprecipitations for either c-Src or Dok-R (Fig. 7A) demonstrated that these two proteins were found associated with each other in a non-EGF stimulation-dependent manner. Furthermore, neither of the truncation mutants Dok-R ΔC PRR and Dok-R ΔPRR was able to associate with c-Src, demonstrating the region on Dok-R required for interaction with c-Src resides in the last 76 amino acids of Dok-R. To delineate the region on c-Src responsible for the constitutive interaction with Dok-R, purified GST-SH3 fusion proteins were tested for their ability to bind Dok-R in in vitro pull-down assays. Pull-down experiments with several different GST-SH3 domains demonstrated that Dok-R was unable to associate with purified SH3 domains of Vav, spectrin, p85, and Crk, whereas it is able to associate with the SH3 domain of c-Src, Lck, Fyn, and Abl (Fig. 7B). These results illustrate that c-Src is bound to Dok-R in a constitutive fashion and that this association requires the SH3 domain of c-Src and the last 76 amino acids of Dok-R.
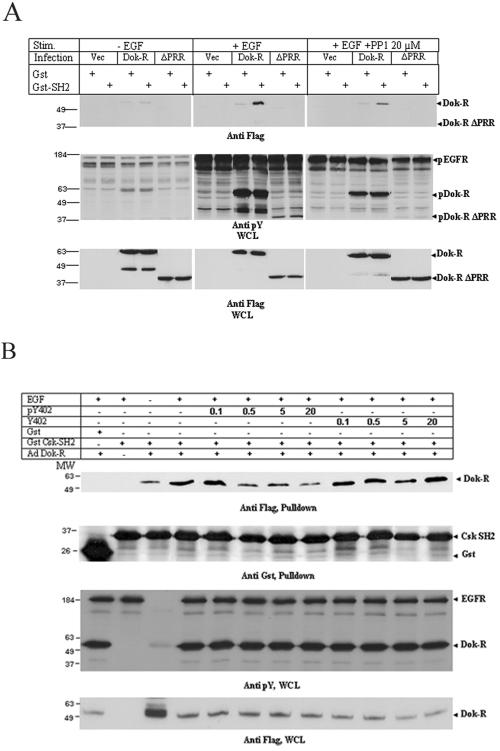
Recruitment of Csk to Dok-R is mediated by phosphorylation of tyrosine 402.
Since previous reports have demonstrated that Dok and DokL both inducibly interact with Csk (25, 38), we decided to explore the possibility that Dok-R serves as a scaffolding protein that recruits Csk into close proximity of c-Src. As noted earlier the relatively high degree of EGFR activation found in mouse embryonic lysates (Fig. 6) precluded us from defining the nature of the Dok-R/Csk interaction. As such, we set out to specifically determine whether the SH2 domain of Csk could associate with Dok-R or Dok-R ΔPRR isolated from EGF-stimulated Cos1 cells. To this end, Escherichia coli purified Csk SH2 was tested for its ability to associate with Dok-R in an in vitro pull-down assay. We demonstrated that the SH2 of Csk is able to interact with Dok-R in an EGF-inducible manner and that this interaction is dependent on the PRR (Fig. 8A). Furthermore, this interaction is severely impaired upon pretreatment of the Cos1 cells with PP1, suggesting the tyrosine on Dok-R that is responsible for this interaction is phosphorylated by an SFK member. Lending credence to this result is the fact that the most carboxy-terminally located tyrosine in Dok-R-Y402 is nested in a consensus sequence (YXXV) that has been previously identified to be both phosphorylated by SFK members and bound by Csk. To confirm this fact, we performed a peptide competition assay to test the hypothesis that Dok-R Y402 is the tyrosine that mediates the interaction with Csk. Phosphorylated and nonphosphorylated peptides spanning the region surrounding Dok-R Y402 were manufactured and tested for their ability to compete for Csk SH2 binding in the presence of Dok-R. Results clearly demonstrate that the phosphorylated peptide, but not the nonphosphorylated peptide, competes for Csk SH2 binding and that this competition is concentration specific (Fig. 8B). Note that we were not able to fully compete off all of the Dok-R-bound Csk SH2 even at high molar equivalents; however, we believe that this is due to a minor nonspecific association that occurs between Dok-R and the GST moiety (see Fig. 8A, lanes 3 and 4).
FIG. 8.
(A) Dok-R, but not vector or Dok-R ΔPRR, inducibly interacts with the SH2 domain of Csk. Cos1 cells were infected with adenoviruses engineered to express either vector Dok-R or Dok-R ΔPRR. The cells were either left unstimulated, stimulated with EGF, or stimulated with EGF plus PP1. Cleared lysates of these cells were subjected to GST Sepharose alone or GST-Csk SH2 Sepharose in in vitro pull-down assays. (B) Src-dependent phosphorylation of Dok-R Y402 mediates Csk SH2 binding. Cos1 cells infected with Dok-R or vector adenoviruses were left either unstimulated or stimulated with EGF. Lysates from these cells were collected, and phosphorylated or nonphosphorylated peptides corresponding to the region spanning Dok-R Y402 were tested for their ability to compete for GST-Csk SH2 binding. Peptide concentration was calculated as molar equivalents when compared to total GST-Csk SH2 (i.e., moles of peptide/mole of Csk SH2).
Dok-R attenuates EGF-dependent activation of Akt.
Several biologically important kinases are found in signaling pathways that are downstream of other kinases. These kinase-kinase cascades serve to amplify and diversify signals; one such kinase is the serine/threonine kinase, Akt/PKB. In a recent report, Kong and Posner et al. demonstrated that full EGF-dependent activation of Akt requires Src family kinase activity in rat hepatocytes (23). Thus, we set out to determine whether Dok-R's ability to attenuate c-Src and ultimately Erk-2 activation could also result in the attenuation of other kinases such as Akt. Cos1 cells expressing Dok-R or Dok-R ΔPRR were either left unstimulated or stimulated with EGF for the indicated times, and lysates were probed with an activation-specific antibody directed to phosphorylation of Akt serine residue 473 (18). Cells transfected with either empty vector or Dok-R ΔPRR produced very similar levels of activated Akt, such that phospho-Akt levels increased rapidly upon stimulation, persisted for at least 5 min, and then began to decline considerably by 10 min poststimulation (Fig. 4A). In dramatic contrast to this, cells expressing Dok-R demonstrated severely impaired levels of Akt activation. Control immunoblots of lysates from these samples suggests that the stark differences in Akt activation between these experimental groups of samples cannot be accounted for by different levels of EGFR activation (anti-pY blot), total Akt levels (pan-Akt), or overall protein levels (anti-B-actin). The central role that c-Src plays in normal cellular physiology suggests that Dok-R's ability to attenuate c-Src activity may extend to numerous pathways that are downstream of c-Src, including Erk-2 and Akt, the latter playing a key role in mediating cell survival.
Potentiation of anoikis by Dok-R.
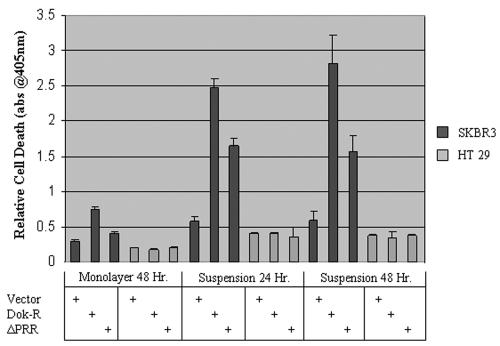
Expression of c-Src or v-Src has been shown to play a pivotal role in counteracting cell detachment-induced apoptosis (anoikis) (5, 46). The breast cancer cell line SKBR3 is known to express high levels of EGFR and c-Src and display increased resistance to anoikis (2, 45). Thus, we utilized this cell line to examine if expression of Dok-R within these cells could sensitize them to anoikis. The colorectal carcinoma cell line, HT29, also known to express very high levels of c-Src (46), was included to examine the relative importance of EGFR signaling. Both cell lines were cultured in monolayer and then infected with one of either ad-LacZ (vector), ad-Dok-R, or ad-Dok-R ΔPRR. After 24 h, the cells were trypsinized and counted, and equal numbers of cells were seeded into six-well dishes that were either coated with agarose (suspension) or not (monolayer). Immunoblot analysis of protein lysates from infected cells demonstrates that both Dok-R cDNAs were expressed to similar levels (data not shown). HT29 cells were unaffected by either of the Dok-R expressing viruses; paradoxically, SKBR3 cells expressing Dok-R ΔPRR were somewhat sensitized to anoikis when compared to parental (ad-LacZ) SKBR23 cells, while cells expressing Dok-R were highly sensitized (Fig. 9).
FIG. 9.
Overexpression of Dok-R sensitizes SKBR3, but not HT29 cells, to cell detachment-induced cell death. SKBR3 and HT29 cells were infected with adenoviruses engineered to express one either vector Dok-R or Dok-R ΔPRR. Twenty-four h postinfection, cells were placed in monolayer or suspension culture for indicated times. Triplicate samples were analyzed for cell death with the Cell Death ELISA Plus kit (Roche). Results are representative of three separate experiments performed in triplicate. abs@405nm, A405.
DISCUSSION
Since their cloning, it has been known that Dok and DokL bind Csk, although the functional significance of this interaction has remained elusive. Our data now clearly demonstrate that Dok-R also binds Csk, further lending support to the notion that Dok, DokL, and Dok-R belong to a subfamily of the larger Dok family. These data provided the impetus for us to test the hypothesis that Dok-R attenuates MAPK activation by modulating EGF-dependent c-Src kinase activity specifically and beg the question that in general this subfamily of proteins may function universally to modulate c-Src kinase activity. As such, one would imagine, based upon shared sequence and structural characteristics, that this subfamily shares similar and/or overlapping functions. These three family members are generally known as context-specific attenuators of receptor and nonreceptor tyrosine kinase signaling. More specifically, they have been shown in many different settings to attenuate downstream activation of MAPK and thus counteract mitogenic cell signaling. The mechanism by which this takes place is still not clear, and herein we present an alternate novel mechanism using the EGFR and Dok-R as prototype members to gain valuable insight into the role of Csk and c-Src in these events. We show processive assembly of an EGF-inducible quaternary complex consisting of the EGFR, Dok-R, c-Src, and Csk. Previous work in our laboratory has shown that Dok-R is recruited to an activated EGFR via association of its PTB domain with phosphorylated tyrosine residues 1086 and 1148 of the EGFR (20). Here we demonstrate that Dok-R is able to shuttle constitutively associated c-Src to the EGFR, whereupon there is a transient activation of c-Src that results in tyrosine phosphorylation of the PRR of Dok-R. Phosphorylation of these sites is SFK dependent and serves to recruit downstream signaling proteins to Dok-R. One such protein is the negative regulator of c-Src, Csk. We show that one of the sites that c-Src specifically phosphorylates on Dok-R, Y402 is nested within an ideal consensus sequence (YXXV) for SH2-dependent binding of Csk. The inducible recruitment of Csk to this complex positions c-Src and Csk in such a way that Csk is able to hyperphosphorylate the negative regulatory tyrosine of c-Src, Y527 (Fig. 10, schematic). Furthermore, the noted hyperphosphorylation of c-Src Y527 correlates with a dramatic loss of EGF-dependent activation of c-Src kinase, MAPK, and Akt that occurs as soon as 2 min poststimulation and persists for at least ten minutes.
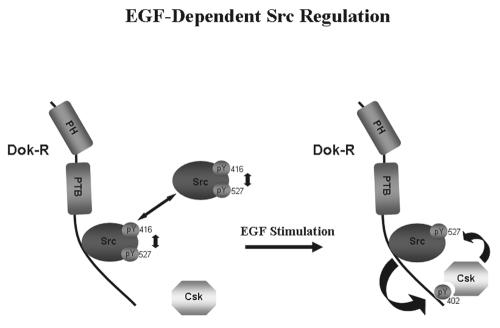
FIG. 10.
Dok-R mediates attenuation of EGF-dependent MAPK and Akt activation through processive recruitment of c-Src and Csk. c-Src is tightly and dynamically maintained between active (pY416) and inactive (pY527) conformations. In the absence of EGF stimulation, a portion of c-Src constitutively interacts with the PRR of Dok-R. Upon EGF stimulation, Dok-R is recruited to the EGFR, where a transient activation of c-Src occurs. c-Src-dependent phosphorylation of Dok-R tyrosine 402 facilitates the recruitment of Csk and subsequent inhibition of Src kinase activity.
EGF-driven cell proliferation is a highly conserved cellular event that requires exquisite regulation. Tyrosine residues 845 and 1101 of the EGFR have been shown to be phosphorylated in vivo in response to recruitment and activation of c-Src (36, 44), while in vitro tyrosine residues 891 and 920 are c-Src-specific substrates (41). Although mutation of tyrosine 845 to phenylalanine does not affect the overall kinase activity of the EGFR, c-Src-dependent phosphorylation of this residue has been shown to be a critical event for EGF-driven mitogenesis (28, 44). Two primary mitogenic pathways that are activated by the EGFR are the Ras and the phospholipase C-γ (PLC-γ) pathway. Full activation of either of these pathways requires EGFR-c-Src complex formation (3, 28, 37). The ability of Dok and Dok-R to inducibly associate with the inhibitor of Ras signaling, RasGAP, suggests that the Dok proteins may impinge on the MAPK pathway by promoting the turnover of GTP-bound Ras to GDP-bound Ras, thus inactivating the Ras-dependent arm of MAPK activation. Some evidence has been presented to support this idea (4, 38, 40, 47, 48). However, we demonstrate here and in previous work (20) that Dok-R is capable of strongly inhibiting EGF-dependent c-Src kinase activity and that the noted attenuation of MAPK associated with enforced Dok-R expression is more likely a consequence of c-Src inhibition than a function of its ability to recruit RasGAP and shut down Ras signaling. Importantly, these two phenomena are difficult to differentiate and ultimately may lead to similar outcomes. For instance, if EGF-dependent c-Src activity is diminished, there will be a concomitant decrease in phosphorylation of EGFR substrates Shc and PLC-γ and thus the Ras signaling pathway. Alternatively, if Dok-R was, through recruitment of RasGAP, attenuating Ras activation, one would observe the same effects, starting from Ras GTP loading down to MAPK activation. Our previous work (20), however, demonstrates that site-directed ablation of the RasGAP binding sites on Dok-R does not mitigate its ability to attenuate MAPK activation, nor does Dok-R displace Shc from being recruited to the EGFR, further lending support to the idea that Dok-R inhibits MAPK activation by modulating c-Src kinase activity.
Negative feedback loops in signal transduction serve to limit the intensity and duration of a particular signal and are therefore necessary to maintain cellular homeostasis (13). For instance, the activation of cell signaling events in T cells is tightly regulated and is dependent upon antigen receptor ligation and subsequent activation/inactivation of Lck or Fyn (30). Specifically in the case of T-cell receptor activation, Lck and Fyn phosphorylate key tyrosine residues that reside within receptor-associated chains—immunoreceptor tyrosine-based activation motifs as well as within PAG/Cbp (26). Phosphorylation of PAG/Cbp on tyrosine 317 facilitates the recruitment of Csk. Although little is currently known about Csk activation, it has been shown that binding of Csk to PAG/Cbp and its juxtaposition to Lck and Fyn are sufficient for Csk to phosphorylate the carboxy-terminal inhibitory tyrosine of these SFK members (42). Furthermore, it is the intensity of the original signal that dictates the degree to which the signal will be allowed to propagate, implying a high degree of regulation at the level of both activation and inactivation. Dok-R, like PAG/Cbp, is an adapter/scaffolding protein. Unlike PAG/Cbp, which is constitutively associated with gycosphingolipid-enriched microdomains, Dok-R is inducibly recruited to phosphoinsitol phosphates by virtue of its PH domain. The significance of this difference is not currently known, but it would suggest an even greater level of regulation that is facilitated through EGF-dependent changes in phosphorylation-dephosphorylation status of proximal inositol lipids. Like T-cell receptor activation, signaling events initiated by the EGFR involve activation of the SFK members. Here we show that activation of the EGFR results in SFK-mediated tyrosine phosphorylation of Dok-R, recruitment of Csk, and a subsequent attenuation of c-Src kinase activity, thereby specifically inhibiting c-Src-dependent aspects of EGFR signaling.
Aside from mitogenesis, c-Src is involved in many other cellular functions, including regulation of the cytoskeleton, vascular permeability, and apoptosis. In the case of apoptosis, Windham and Gallick et al. and Coll and Filmus et al. examined the role of c-Src in counteracting cell detachment-induced cell death-anoikis (5, 46). Coll and Filmus et al. found that v-Src-induced activation of Mek/MAPK leads to increased expression of the antiapoptotic protein Bcl-xL, while Windham and Gallick et al. demonstrate that increased levels of c-Src expression and/or activity in colon epithelial cells result in a survival advantage conferred by Akt/PKB activation. Stover et al. have investigated the collaborative role EGFR and c-Src play in recruiting the p85 subunit of the antiapoptotic protein PI3 kinase to the EGFR. Their work demonstrates that c-Src-dependent phosphorylation of EGFR tyrosine residue 920 facilitates recruitment of PI3 kinase in cancer cell lines DLD-1 and MCF 7 (41). Although c-Src-dependent recruitment of p85 to an activated EGFR is interesting, it does not, in and of itself, address whether this interaction is sufficient to activate PI3 kinase and its downstream substrate, Akt/PKB. Kessenbrock et al. have since demonstrated that PP1 treatment of T47D cells blocks EGF-induced activation of the antiapoptotic protein kinase AKT/PKB, suggesting that this event requires c-Src kinase activity (22). Herein we describe a Dok-R-dependent decrease in EGF-driven c-Src kinase activity that correlates with a decrease in both MAPK and, consistent with the above studies, a concomitant decrease in Akt activation. Our current studies demonstrate that Dok-R is able to sensitize SKBR3 cells, one of the two cell lines tested, while the other line, HT29, was completely unaffected. We believe that one possible explanation for this is the EGFR status of each of the cell lines. While both cell lines express high levels of c-Src, SKBR3 cells have much higher levels of EGFR (2; and data not shown). As detailed in our previous studies (20), Dok-R must be recruited to the EGFR to efficiently attenuate MAPK activity. Here, we show that this attenuation is dependent on Dok-R's ability to modulate c-Src kinase activity, suggesting that cell lines possessing intrinsically low levels of EGFR, such as HT29, would not be sensitive to Dok-R-mediated effects. Quite unexpectedly we find that Dok-R ΔPRR was able to partially sensitize SKBR3 cells to cell detachment-induced cell death. Although the origin of this result is not currently known, we are examining the possibility that there are functional Dok-R domains outside of the PRR that participate in apoptosis. One possibility is phosphorylation of Dok-R tyrosine 142. Songyang et al. demonstrated that the equivalent tyrosine in Dok, tyrosine 146, becomes phosphorylated in a v-Src-specific manner and that this phosphorylation event facilitates clustering of additional Dok proteins via homotypic PTB-based interactions (40). If this proves to be the case for Dok-R, the Dok-R ΔPRR may be recruiting wild-type endogenous Dok-R to activated EGF receptors in the SKBR3 cells.
Perhaps the most intriguing concept to arise from this work is the notion that Dok, Dok-R, and DokL are global inhibitors of SFK members. This is not a novel idea though: Lemay and Veillette et al. coined this idea upon cloning of Dok3 (DokL), suggesting that Dok and DokL may inhibit SFKs by virtue of their ability to recruit Csk (25). Until now there has been no evidence to substantiate this hypothesis. Dok-R, unlike Dok, associates with c-Src via a constitutive SH3-mediated interaction not an inducible SH2-based interaction. GST pull-down assays conducted in our laboratory (Fig. 3) serve to demonstrate that the SH3 domains of SFK members c-Src, Lck, and Fyn as well as Abl are able to directly interact with Dok-R. Although we did not examine the binding of additional SFK members, due to the highly conserved nature of their SH3 domains, it seems possible that Dok-R may bind several as yet undetermined SFK members. Taken together with the fact that Dok-R associates with Csk in an inducible fashion and that Dok-R binds many receptor and nonreceptor tyrosine kinases, it is intriguing to consider the possibility that the phenomenon that we see with the EGFR may be a highly conserved manner of attenuating RTK-induced SFK signaling.
REFERENCES
- 1.Belsches, A. P., M. D. Haskell, and S. J. Parsons. 1997. Role of c-Src tyrosine kinase in EGF-induced mitogenesis. Front. Biosci. 2:d501-d518. [DOI] [PubMed] [Google Scholar]
- 2.Belsches-Jablonski, A. P., J. S. Biscardi, D. R. Peavy, D. A. Tice, D. A. Romney, and S. J. Parsons. 2001. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene 20:1465-1475. [DOI] [PubMed] [Google Scholar]
- 3.Biscardi, J. S., R. C. Ishizawar, C. M. Silva, and S. J. Parsons. 2000. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpino, N., D. Wisniewski, A. Strife, D. Marshak, R. Kobayashi, B. Stillman, and B. Clarkson. 1997. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell 88:197-204. [DOI] [PubMed] [Google Scholar]
- 5.Coll, M. L., K. Rosen, V. Ladeda, and J. Filmus. 2002. Increased Bcl-xL expression mediates v-Src-induced resistance to anoikis in intestinal epithelial cells. Oncogene 21:2908-2913. [DOI] [PubMed] [Google Scholar]
- 6.Cong, F., B. Yuan, and S. P. Goff. 1999. Characterization of a novel member of the DOK family that binds and modulates Abl signaling. Mol. Cell. Biol. 19:8314-8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, J. A., and T. Hunter. 1983. Identification and characterization of cellular targets for tyrosine protein kinases. J. Biol. Chem. 258:1108-1115. [PubMed] [Google Scholar]
- 8.Cooper, J. A., and C. S. King. 1986. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol. Cell. Biol. 6:4467-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Hertog, J., C. E. Pals, M. P. Peppelenbosch, L. G. Tertoolen, S. W. de Laat, and W. Kruijer. 1993. Receptor protein tyrosine phosphatase alpha activates pp60c-src and is involved in neuronal differentiation. EMBO J. 12:3789-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Cristofano, A., N. Carpino, N. Dunant, G. Friedland, R. Kobayashi, A. Strife, D. Wisniewski, B. Clarkson, P. P. Pandolfi, and M. D. Resh. 1998. Molecular cloning and characterization of p56dok-2 defines a new family of RasGAP-binding proteins. J. Biol. Chem. 273:4827-4830. [DOI] [PubMed] [Google Scholar]
- 11.Di Cristofano, A., M. Niki, M. Zhao, F. G. Karnell, B. Clarkson, W. S. Pear, L. Van Aelst, and P. P. Pandolfi. 2001. p62(dok), a negative regulator of Ras and mitogen-activated protein kinase (MAPK) activity, opposes leukemogenesis by p210(bcr-abl). J. Exp. Med. 194:275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang, K. S., H. Sabe, H. Saito, and H. Hanafusa. 1994. Comparative study of three protein-tyrosine phosphatases. Chicken protein-tyrosine phosphatase lambda dephosphorylates c-Src tyrosine 527. J. Biol. Chem. 269:20194-20200. [PubMed] [Google Scholar]
- 13.Fiorini, M., M. Alimandi, L. Fiorentino, G. Sala, and O. Segatto. 2001. Negative regulation of receptor tyrosine kinase signals. FEBS Lett. 490:132-141. [DOI] [PubMed] [Google Scholar]
- 14.Grimm, J., M. Sachs, S. Britsch, S. Di Cesare, T. Schwarz-Romond, K. Alitalo, and W. Birchmeier. 2001. Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation. J. Cell Biol. 154:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gugasyan, R., C. Quilici, T. T. I. Stacey, D. Grail, A. M. Verhagen, A. Roberts, T. Kitamura, A. R. Dunn, and P. Lock. 2002. Dok-related protein negatively regulates T cell development via its RasGTPase-activating protein and Nck docking sites. J. Cell Biol. 158:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, B. A. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 17.Hlavacek, W. S., J. R. Faeder, M. L. Blinov, A. S. Perelson, and B. Goldstein. 2003. The complexity of complexes in signal transduction. Biotechnol. Bioeng. 84:783-794. [DOI] [PubMed] [Google Scholar]
- 18.James, S. R., C. P. Downes, R. Gigg, S. J. Grove, A. B. Holmes, and D. R. Alessi. 1996. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem. J. 315:709-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, N., and D. J. Dumont. 1998. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene 17:1097-1108. [DOI] [PubMed] [Google Scholar]
- 20.Jones, N., and D. J. Dumont. 1999. Recruitment of Dok-R to the EGF receptor through its PTB domain is required for attenuation of erk MAP kinase activation. Curr. Biol. 9:1057-1060. [DOI] [PubMed] [Google Scholar]
- 21.Kashige, N., N. Carpino, and R. Kobayashi. 2000. Tyrosine phosphorylation of p62dok by p210bcr-abl inhibits RasGAP activity. Proc. Natl. Acad. Sci. USA 97:2093-2098. (Erratum, 97: 6236.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassenbrock, C. K., S. Hunter, P. Garl, G. L. Johnson, and S. M. Anderson. 2002. Inhibition of Src family kinases blocks epidermal growth factor (EGF)-induced activation of Akt, phosphorylation of c-Cbl, and ubiquitination of the EGF receptor. J. Biol. Chem. 277:24967-24975. [DOI] [PubMed] [Google Scholar]
- 23.Kong, M., C. Mounier, V. Dumas, and B. I. Posner. 2003. Epidermal growth factor-induced DNA synthesis. Key role for Src phosphorylation of the docking protein Gab2. J. Biol. Chem. 278:5837-5844. [DOI] [PubMed] [Google Scholar]
- 24.Latour, S., G. Gish, C. D. Helgason, R. K. Humphries, T. Pawson, and A. Veillette. 2001. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat. Immunol. 2:681-690. [DOI] [PubMed] [Google Scholar]
- 25.Lemay, S., D. Davidson, S. Latour, and A. Veillette. 2000. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell. Biol. 20:2743-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindquist, J. A., L. Simeoni, and B. Schraven. 2003. Transmembrane adapters: attractants for cytoplasmic effectors. Immunol. Rev. 191:165-182. [DOI] [PubMed] [Google Scholar]
- 27.Lock, P., F. Casagranda, and A. R. Dunn. 1999. Independent SH2-binding sites mediate interaction of dok-related protein with RasGTPase-activating protein and Nck. J. Biol. Chem. 274:22775-22784. [DOI] [PubMed] [Google Scholar]
- 28.Maa, M. C., T. H. Leu, D. J. McCarley, R. C. Schatzman, and S. J. Parsons. 1995. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc. Natl. Acad. Sci. USA 92:6981-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Master, Z., J. Tran, A. Bishnoi, S. H. Chen, J. M. Ebos, P. Van Slyke, R. S. Kerbel, and D. J. Dumont. 2003. Dok-R binds c-Abl and regulates Abl kinase activity and mediates cytoskeletal reorganization. J. Biol. Chem. 278:30170-30179. [DOI] [PubMed] [Google Scholar]
- 30.Mustelin, T., and K. Tasken. 2003. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem. J. 371:15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelms, K., A. L. Snow, J. Hu-Li, and W. E. Paul. 1998. FRIP, a hematopoietic cell-specific rasGAP-interacting protein phosphorylated in response to cytokine stimulation. Immunity 9:13-24. [DOI] [PubMed] [Google Scholar]
- 32.Okada, M., S. Nada, Y. Yamanashi, T. Yamamoto, and H. Nakagawa. 1991. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J. Biol. Chem. 266:24249-24252. [PubMed] [Google Scholar]
- 33.Ott, V. L., I. Tamir, M. Niki, P. P. Pandolfi, and J. C. Cambier. 2002. Downstream of kinase, p62(dok), is a mediator of Fc gamma IIB inhibition of Fc epsilon RI signaling. J. Immunol. 168:4430-4439. [DOI] [PubMed] [Google Scholar]
- 34.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 35.Peng, Z. Y., and C. A. Cartwright. 1995. Regulation of the Src tyrosine kinase and Syp tyrosine phosphatase by their cellular association. Oncogene 11:1955-1962. [PubMed] [Google Scholar]
- 36.Poppleton, H. M., G. J. Wiepz, P. J. Bertics, and T. B. Patel. 1999. Modulation of the protein tyrosine kinase activity and autophosphorylation of the epidermal growth factor receptor by its juxtamembrane region. Arch. Biochem. Biophys. 363:227-236. [DOI] [PubMed] [Google Scholar]
- 37.Sato, K., A. Sato, M. Aoto, and Y. Fukami. 1995. c-Src phosphorylates epidermal growth factor receptor on tyrosine 845. Biochem. Biophys. Res. Commun. 215:1078-1087. [DOI] [PubMed] [Google Scholar]
- 38.Shah, K., and K. M. Shokat. 2002. A chemical genetic screen for direct v-Src substrates reveals ordered assembly of a retrograde signaling pathway. Chem. Biol. 9:35-47. [DOI] [PubMed] [Google Scholar]
- 39.Shen, Z., A. Batzer, J. A. Koehler, P. Polakis, J. Schlessinger, N. B. Lydon, and M. F. Moran. 1999. Evidence for SH3 domain directed binding and phosphorylation of Sam68 by Src. Oncogene 18:4647-4653. [DOI] [PubMed] [Google Scholar]
- 40.Songyang, Z., Y. Yamanashi, D. Liu, and D. Baltimore. 2001. Domain-dependent function of the rasGAP-binding protein p62Dok in cell signaling. J. Biol. Chem. 276:2459-2465. [DOI] [PubMed] [Google Scholar]
- 41.Stover, D. R., M. Becker, J. Liebetanz, and N. B. Lydon. 1995. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J. Biol. Chem. 270:15591-15597. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi, S., Y. Takayama, A. Ogawa, K. Tamura, and M. Okada. 2000. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J. Biol. Chem. 275:29183-29186. [DOI] [PubMed] [Google Scholar]
- 43.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 44.Tice, D. A., J. S. Biscardi, A. L. Nickles, and S. J. Parsons. 1999. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA 96:1415-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, S. C., K. Makino, W. Xia, J. S. Kim, S. A. Im, H. Peng, S. C. Mok, S. E. Singletary, and M. C. Hung. 2001. DOC-2/hDab-2 inhibits ILK activity and induces anoikis in breast cancer cells through an Akt-independent pathway. Oncogene 20:6960-6964. [DOI] [PubMed] [Google Scholar]
- 46.Windham, T. C., N. U. Parikh, D. R. Siwak, J. M. Summy, D. J. McConkey, A. J. Kraker, and G. E. Gallick. 2002. Src activation regulates anoikis in human colon tumor cell lines. Oncogene 21:7797-7807. [DOI] [PubMed] [Google Scholar]
- 47.Yamanashi, Y., and D. Baltimore. 1997. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell 88:205-211. [DOI] [PubMed] [Google Scholar]
- 48.Yamanashi, Y., T. Tamura, T. Kanamori, H. Yamane, H. Nariuchi, T. Yamamoto, and D. Baltimore. 2000. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes. Dev. 14:11-16. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao, M., A. A. Schmitz, Y. Qin, A. Di Cristofano, P. P. Pandolfi, and L. Van Aelst. 2001. Phosphoinositide 3-kinase-dependent membrane recruitment of p62(dok) is essential for its negative effect on mitogen-activated protein (MAP) kinase activation. J. Exp. Med. 194:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng, X. M., R. J. Resnick, and D. Shalloway. 2000. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 19:964-978. [DOI] [PMC free article] [PubMed] [Google Scholar]