Abstract
Background:
Evidence suggests that MAPK pathway activation, as measured by ERK1/2 phosphorylation (p- ERK), predicts overall survival (OS) in recurrent glioblastoma patients receiving anti-PD-1 therapy. We aimed to validate these findings in independent cohorts.
Methods:
In a 24-patient clinical trial on recurrent glioblastoma and high-grade gliomas, we examined the link between p-ERK levels and overall survival (OS). Patients received intravenous nivolumab, followed by maximal safe resection and an intracerebral injection of either ipilimumab alone or combined with nivolumab. Bi-weekly adjuvant nivolumab was then administered up to five times (NCT03233152). Using REMARK criteria, we conducted independent analyses for p-ERK quantification and statistical evaluations. Additional comparative analysis included prior cohorts, totaling 65 patients. Cox proportional hazards models and meta-analysis were employed to assess p-ERK as a predictive biomarker post-immunotherapy.
Results:
Lower median p-ERK+ cell density was observed compared to prior studies, likely due to tissue handling variances. Nonetheless, high p-ERK was associated with prolonged OS, particularly in IDH wild-type glioblastomas (P=0.036). Median OS for high and low p-ERK patients were 55.6 and 30 weeks, respectively. Multivariable analysis reinforced p-ERK’s significance in survival prediction (P=0.011). Meta-analysis across three cohorts (n=65) supported the survival benefit of elevated tumor p-ERK levels (P=0.0424).
Conclusions:
This study strengthens the role of p-ERK as a predictive biomarker for OS in glioblastoma patients on immune checkpoint blockade. Future research should focus on further validation in prospective trials and the standardization of preanalytical variables influencing p-ERK quantification.
Keywords: glioblastoma, p-ERK, immune checkpoint blockade, predictive biomarker
INTRODUCTION
Glioblastoma is a devastating disease with limited treatment options, particularly at time of recurrence. At diagnosis, established treatments include radiation, chemotherapy, and tumor- treating fields1. However, at recurrence, no therapy has been shown to prolong overall survival (OS) in unselected patients. Furthermore, the five-year survival rate for glioblastoma patients is less than 10% underscoring the critical need for innovative and effective therapies1.
One promising area of research in cancer therapy is immune checkpoint inhibition, which involves the use of therapeutic monoclonal antibodies targeting the programmed cell death protein 1 (PD-1), programmed death-ligand 1(PD-L1), or cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). However, immune checkpoint inhibition has not shown efficacy in unselected glioblastoma patients2–4. The highly immunosuppressive tumor microenvironment that characterizes glioblastoma constitutes a significant barrier to effective immune checkpoint blockade5. Yet, several cases of clinical benefit and response to this form of immunotherapy where PD-1/PD-L1 blockade has been evaluated in glioblastoma patients have been reported6–8.
Recent analyses identified molecular features associated with the response to PD-1 blockade. Specifically, activating mutations in BRAF and PTPN11, which drive ERK signaling of the MAPK pathway, were enriched in recurrent glioblastomas from patients that responded to anti-PD-1 therapy8. Considering these results, we evaluated the predictive value of ERK1/2 phosphorylation (p-ERK), as an indicator of MAPK pathway activation, in glioblastomas from patients treated with adjuvant PD-1 blockade7. In two independent cohorts of recurrent glioblastoma patients, we determined that in pretreatment tumor samples (obtained within weeks prior to initiating immunotherapy), p-ERK demonstrated being predictive of OS following adjuvant anti-PD-1 therapy7. Although this study provided supportive evidence on a means of identifying glioblastoma patients that are likely to respond to PD-1 blockade, these studies were not performed in a blinded fashion. Further rigorous validation in additional independent cohorts of glioblastoma patients was deemed necessary before this biomarker could be considered for patient selection in the clinical setting.
Considering the evidence of tolerability and early indications of activity of intratumoral administration of ipilimumab in extracranial metastases of solid tumors9,10, a phase I clinical trial was conducted to explore the potential of the combination of ipilimumab plus nivolumab in patients with recurrent glioblastoma11. This trial investigated a novel approach by performing an intracerebral administration of ipilimumab with or without nivolumab in combination with intravenous nivolumab in patients with resectable high-grade gliomas and glioblastoma at recurrence.
In this study, we aimed to investigate the predictive value of p-ERK in a cohort of high- grade gliomas and glioblastoma patients treated with intracerebral and intravenous immune checkpoint blockade within this trial. We analyzed p-ERK in tumor samples from patients treated with intracerebral administration of either nivolumab only or nivolumab plus ipilimumab followed by postoperative immunotherapy. To add rigor to our analysis, we investigated this biomarker in line with the REMARK criteria12. Utilizing a systematic approach involving multiple teams, one team was dedicated to p-ERK quantification, another consolidated relevant clinical, demographic, and outcome data, while a third specialized statistics team managed pre- determined analyses. Building on this primary analysis, we embarked on a rigorous validation journey across three distinct cohorts of glioblastoma patients. Such an extensive validation was essential to not only fortify our initial findings but also ensure their generalizability and robustness. The combined results from this multifaceted study underscore the pivotal role of p- ERK as a predictor of OS in glioblastoma patients treated with immune checkpoint blockade.
MATERIALS AND METHODS
Study design and analysis by REMARK criteria.
This was an analysis of a clinical trial in which p-ERK was evaluated as a biomarker for response to immune checkpoint blockade (anti-PD-1 and anti-CTLA-4 therapies) in patients with recurrent glioblastoma. All patients included in the analysis received treatment with either ipilimumab (10 mg) or ipilimumab (5 mg) plus nivolumab (10 mg) followed by postsurgical intravenous nivolumab (10 mg) (NCT03233152)11. Within the treated patients, there were two cohorts: cohort 1 (intracerebral injection of nivolumab only) and cohort 2 (intracerebral injection of nivolumab plus ipilimumab). The study was conducted in accordance with institutional ethical regulations and the Declaration of Helsinki principles. All patients provided written informed consent. Clinicopathological data are provided in Supplementary Table I.
We collected the tumor samples from 27 patients enrolled in the phase I trial cohort that was previously published for which we had tumor samples available for analysis. We employed the same methodology described previously for the staining and quantification of p-ERK cell density in tumor samples from these patients7. A neuropathologist evaluated whether the samples contained a sufficient amount of tumor tissue and delineated these regions for further quantification of p-ERK cell density. Out of 27 tumor samples, we analyzed 24 samples from patients whose tumors were of good quality and acceptable for immunohistochemistry (IHC) analysis (Fig. 1a). To strengthen the rigor of the analysis performed in this cohort, we adhered to the REMARK criteria for our workflow12. In this way, we specified the conditions to be analyzed a priori which included the association of p-ERK cell density with OS evaluated by log-rank test and Cox proportional hazards model in the complete patient cohort (recurrent IDH mutant high-grade glioma and recurrent glioblastoma) and only in wild-type IDH glioblastoma patients (Fig. 1b). We specified that the staining for p-ERK and the quantification of p-ERK cell density to be done in a blinded fashion with regards to outcomes and other clinicopathological characteristics.
Figure 1. Analysis workflow and application of the REMARK criteria.
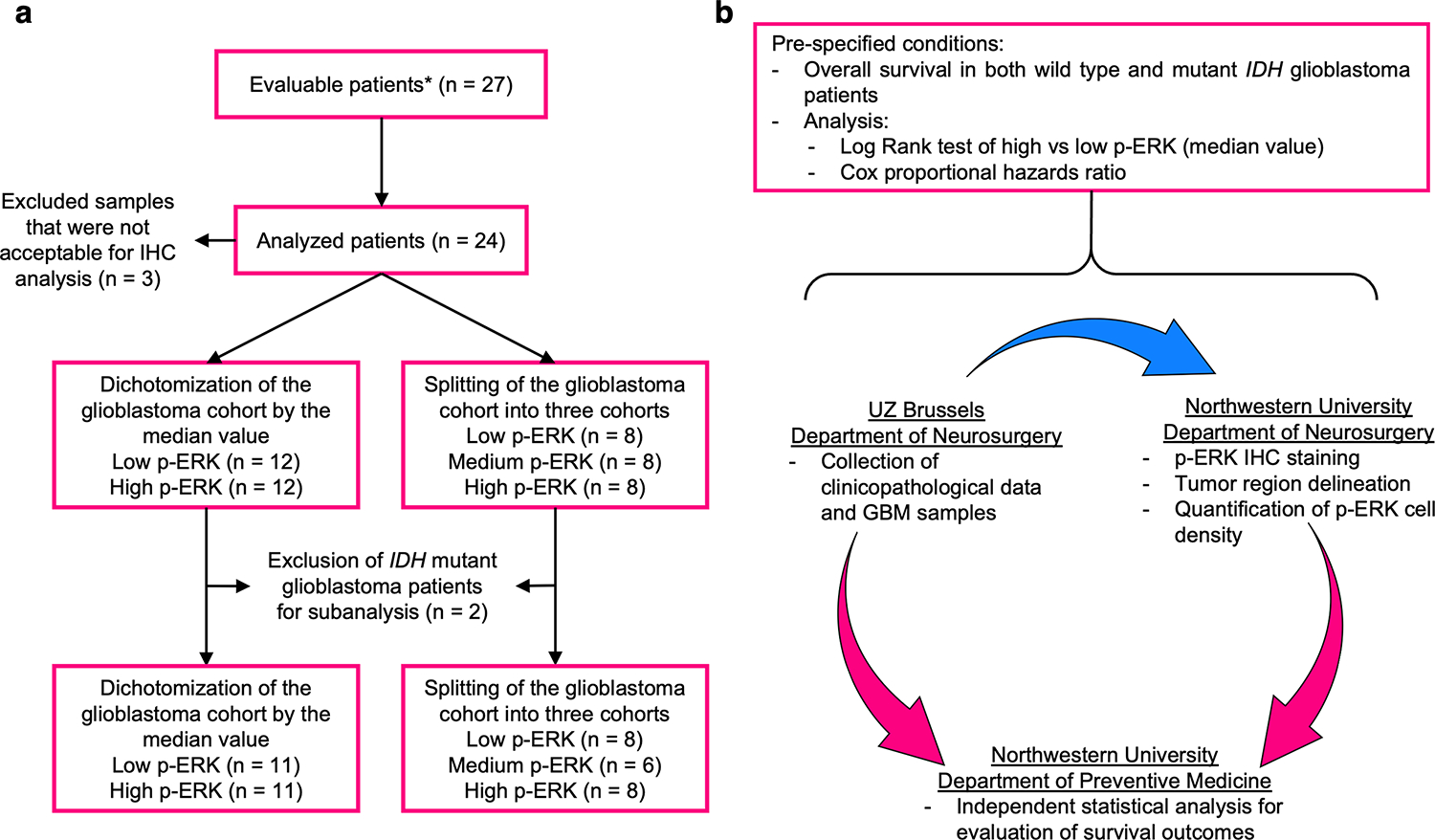
a, Diagram showing the selection of glioblastoma patient samples and formation of groups based on p-ERK cell density. b, Survival analysis workflow using the REMARK criteria for both complete patient cohort and wild-type IDH glioblastoma patients. The blue arrow represents the transfer of tumor samples and the pink arrows represent the transfer of data. *Patients from the phase I clinical trial with tissue available for analysis.
After the initial analysis, to enhance the statistical power and robustness, we integrated data from two other independent cohorts with the current one. This pooling resulted in an amalgamated dataset from three different cohorts.
IHC staining.
Tumor samples from glioblastoma patients were preserved in paraffin blocks. IHC and H&E staining was performed using standard immunoperoxidase staining of FFPE tissue section of 5 μm from glioblastomas obtained during surgery. Paraffin sections were deparaffinized with xylene in the stainer followed by heat-mediated antigen retrieval with sodium citrate buffer. Next, slides were stained against mouse anti-phospho-p44/42 (p-ERK1/2) (Cell Signaling Technologies, 1:500 dilution). Sections were counterstained with hematoxylin, dehydrated, and mounted with coverslips. Staining was performed in the DAKO Autostainer Link48 slide stainer (Agilent Technologies). Slides were scanned with the Hamamatsu K.K. Nanozoomer 2.0 HT and visualized with NDP.view2 Viewing software for tumor delineation.
Quantification of p-ERK cell density in tumor samples.
Following the scanning of stained slides. A neuropathologist outlines the tumoral regions on each sample in a blinded fashion regarding treatments, survival outcomes, and other clinical characteristics. In delineated regions, HistoQuest v.6.0 software (TissueGnostics) was employed for the quantification of p-ERK cell density in the delineated tumor regions (Supplementary Fig. 1). In brief, we employed a segmentation method in which the nucleus was identified and separated from the cytoplasm using a ring mask with an interior radius of −0.45 μm and an exterior radius of 0.91 μm with a cytoplasm cell mask. With this, the cytoplasm was defined for quantification. For nuclei detection, the software parameters were adjusted to detect hematoxylin (red, green, blue (RGB): 30, 45, 84) and DAB chromogen (RGB: 94, 48, 14) in combination with hematoxylin. A mean intensity threshold of 150 was set up to define p-ERK+ cells. For slides where there were multiple tissues for a given tumor, these tissues were quantified, and the resulting values were averaged to generate a single value for one tumor sample. Similar to our previous study7, p-ERK cell density was defined as the number of p-ERK+ cells in a given area (mm2).
For the quantification of p-ERK staining intensity in endothelial cells, these cells were subjected to segmentation by creating ROIs. Next, we employed HistoQuest v.6.0 software (TissueGnostics) to quantify the mean intensity of the sum of brown vessels where the ROI was created.
Survival and statistical analysis.
Following the REMARK criteria for biomarker validation, we investigated p-ERK as a predictive of OS in 24 evaluable tumor samples of recurrent glioblastoma patients from a phase I clinical trial. Prespecified conditions to analyze were established a priori, which included the association of p-ERK cell density with OS evaluated by log-rank test and Cox proportional hazards model in the complete patient cohort (wild-type and IDH mutant glioblastoma patients) and only in the wild-type IDH glioblastoma patients. OS was defined as the time from informed consent. Age was also determined at the time when informed consent was obtained. OS was censored for patients that were alive at the time of the cutoff date. We ensured that the staining and quantification of p-ERK were performed in a blinded fashion concerning outcomes and other clinicopathological characteristics. The p-ERK quantification results, survival outcomes, and clinicopathological characteristics were sent to an independent statistician for survival analysis. The dichotomization of the cohort into high and low p-ERK glioblastoma patients was determined using the median of all values in the cohort derived from software-based quantification of p-ERK cell density. Patients were also divided into three groups (high, intermediate, and low) based on p-ERK cell density. We employed a two-sided log-rank test for survival analysis between high and low p-ERK groups. For exploratory purposes, patients were divided into 3 groups (n=8 per group) based on p-ERK cell density.
To support our primary findings regarding p-ERK’s predictive capacity for survival post- immunotherapy in glioblastoma patients, we pooled data from two previously documented cohorts7,13, and the present cohort11, which expanded the sample size to 65 glioblastoma patients. Focusing on these cohorts, we scaled p-ERK values by the median within each cohort and applied a Cox proportional hazards model. The initial approach utilized a Cox univariable model for p-ERK followed by a multivariable Cox model to adjust for potential confounders. The model considered levels of the biomarker, age, steroid use, and differences in cohorts. The pooling of data from multiple cohorts enhances the statistical power of this analysis. For a comprehensive validation, we obtained the hazard ratios for p-ERK from the multivariable Cox model and undertook a meta-analysis using a random-effects model integrating data from the three distinct cohorts, inclusive of the current study cohort. R v.4.0.2 and GraphPad Prism v. 9.5.1 were used for statistical analyses.
Data availability.
The data generated in this study are available upon request from the corresponding author.
RESULTS
Patient baseline characteristics.
We sought to evaluate p-ERK as a predictive biomarker in a cohort of recurrent high-grade glioma and glioblastoma patients treated with immune checkpoint blockade where tumor samples and clinical outcomes were collected prospectively11. This cohort included 2 patients with IDH mutant high-grade recurrent gliomas and 25 patients recurrent with wild-type IDH glioblastoma. As part of the phase I clinical trial, patients received a fixed dose of nivolumab (10 mg) through intravenous administration and underwent a maximal safe resection within 24 hours. Following this, patients received an injection of either ipilimumab (10 mg) in cohort 1 or a combination of ipilimumab (5 mg) and nivolumab (10 mg) in cohort 2, directly into the brain tissue surrounding the brain cavity. Nivolumab was administered intravenously again every two weeks post-surgery.
To identify potentially clinically significant p-ERK cut-points, we dichotomized p-ERK at the median value of 540.5 cells/mm2, categorizing glioblastoma samples into high p-ERK (>540.5 cells/mm2) and low p-ERK (<= 540.5 cells/mm2). For a more nuanced analysis, we further divided samples into low (<= 55), medium (55–895), and high (>895) p-ERK groups. The baseline and clinical characteristics were similar between groups (Table I).
Table 1.
Demographics and clinical data of glioblastoma patients.
| Patient characteristics | |||
|---|---|---|---|
|
| |||
| High p-ERK (n=12) | Low p-ERK (n=12) | Total number of patients (n=24) | |
|
| |||
| Cohort (%) | |||
| Cohort 1 (nivo) | 2 (16.67) | 1 (8.33) | 3 (12.5) |
| Cohort 2 (nivo + ipi) | 10 (83.33) | 11 (91.67) | 21 (87.5) |
| Age | |||
| Mean ± s.d. | 59.2 (10.5) | 51.8 (10.1) | 55.5 (10.8) |
| Median (Range) | 62.2 (41.5–72.4) | 53.4 (37.7–70.3) | 55.1 (37.7–72.4) |
| ECOG PS (%) | |||
| 0 | 3 (25) | 7 (58.33) | 10 (41.67) |
| 1 | 7 (58.33) | 5 (41.67) | 12 (50) |
| 2 | 2 (16.67) | 0 | 2 (8.33) |
| IDH status, n (%) | |||
| Wild type | 11 (91.67) | 11 (91.67) | 22 (91.67) |
| Mutant | 1 (8.33) | 1 (8.33) | 2 (8.33) |
| Use of corticosteroids (%) | |||
| No | 10 (83.33) | 6 (50) | 16 (66.67) |
| Yes | 2 (16.67) | 6 (50) | 8 (33.33) |
s.d: standard deviation
High p-ERK: > 540.5 cells/mm2 Low p-ERK: <= 540.5 cells/mm2
Out of 27 evaluable subjects, 25 had died at the time of analysis from tumoral progression. The median OS for all evaluable patients was 38.3 weeks (95% two-sided CI 30– 75.7) with 25th and 75th quantiles corresponding to 20.1 and 87 weeks, respectively (Supplementary Fig. 2). In the analysis cohort that included 24 evaluable subjects, we determined that there were 22 deaths. The median OS for the analysis cohort (n=24) was 36.1 weeks (95% two-sided CI 30–87) with 25th and 75th quantiles corresponding to 19.3 and 97.3 weeks, respectively (Fig. 2).
Figure 2. Survival outcomes of high-grade glioma patients.
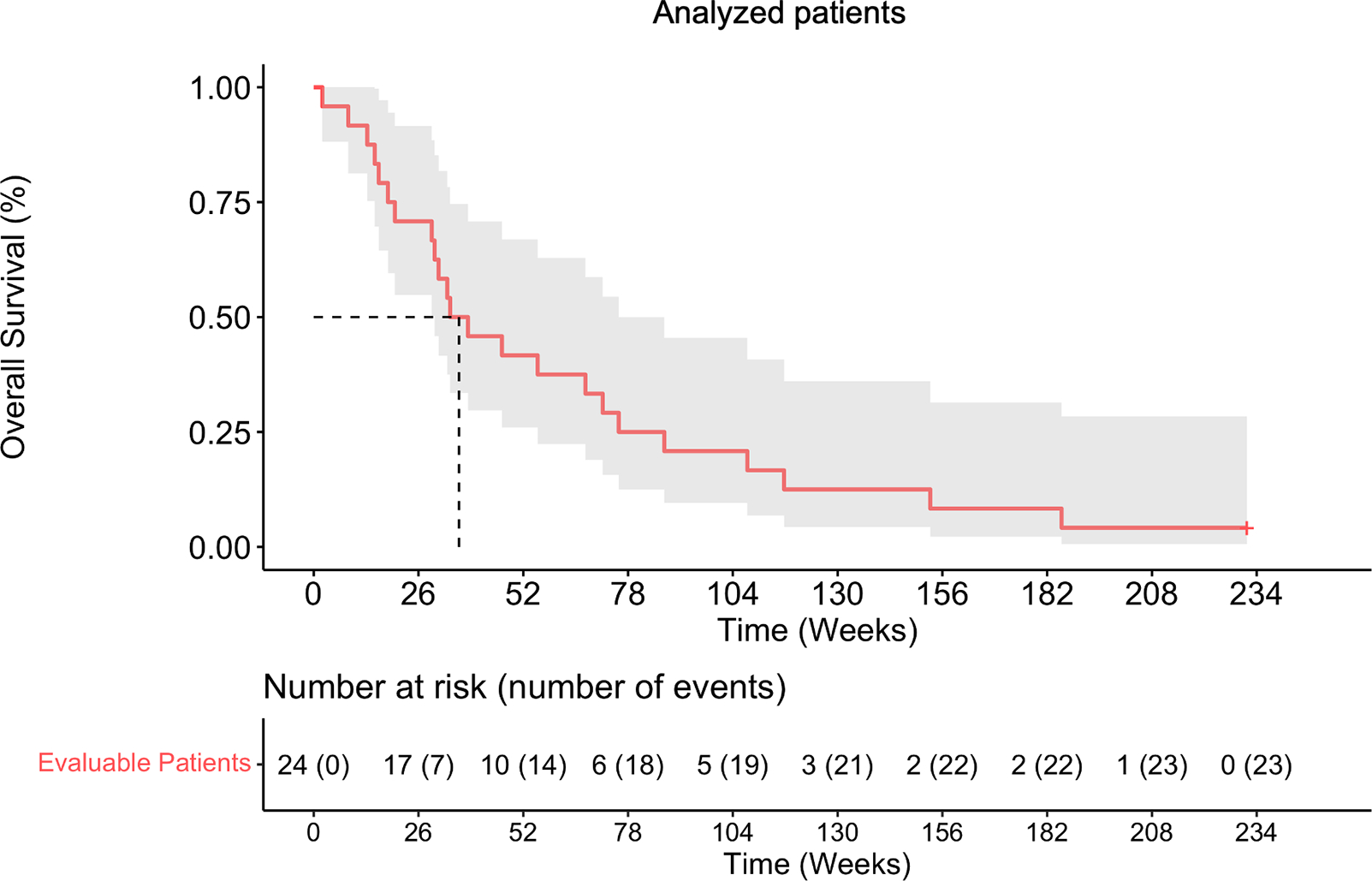
Kaplan-Meier curve showing the OS of analyzed glioblastoma patients.
Comparison of p-ERK values across glioblastoma cohorts.
Considering that preanalytical variables can influence the integrity of phosphorylated proteins in glioblastoma7, we performed a comparison of the distribution of p-ERK cell density in tumor regions obtained from the current clinical trial cohort, and previous cohorts of glioblastoma patients from different institutions7. We determined that the cohort of patients from both Northwestern University and Columbia University (NU/CU) had a median value of 3207 cells/mm2 (25–75%: 1786–4148 cells/mm2), the cohort from UCLA had a median value of 3616 cells/mm2 (25–75%: 1410–5053 cells/mm2), and the current cohort had a median value of 540.5 cells/mm2 (25–75%: 39.16–1287 cells/mm2) (Fig. 3a). Whereas the NU/CU cohort and the UCLA cohort did not show differences in the cell density of p-ERK+ cells in tumors (P = 0.8131, one-way ANOVA), tumors from the UZ Brussels patient cohort had lower values of p-ERK+ cells compared to the NU/CU cohort (P < 0.0001, one-way ANOVA) and the UCLA cohort (P < 0.0001, one-way ANOVA).
Figure 3. Comparison of p-ERK values across independent glioblastoma cohorts.
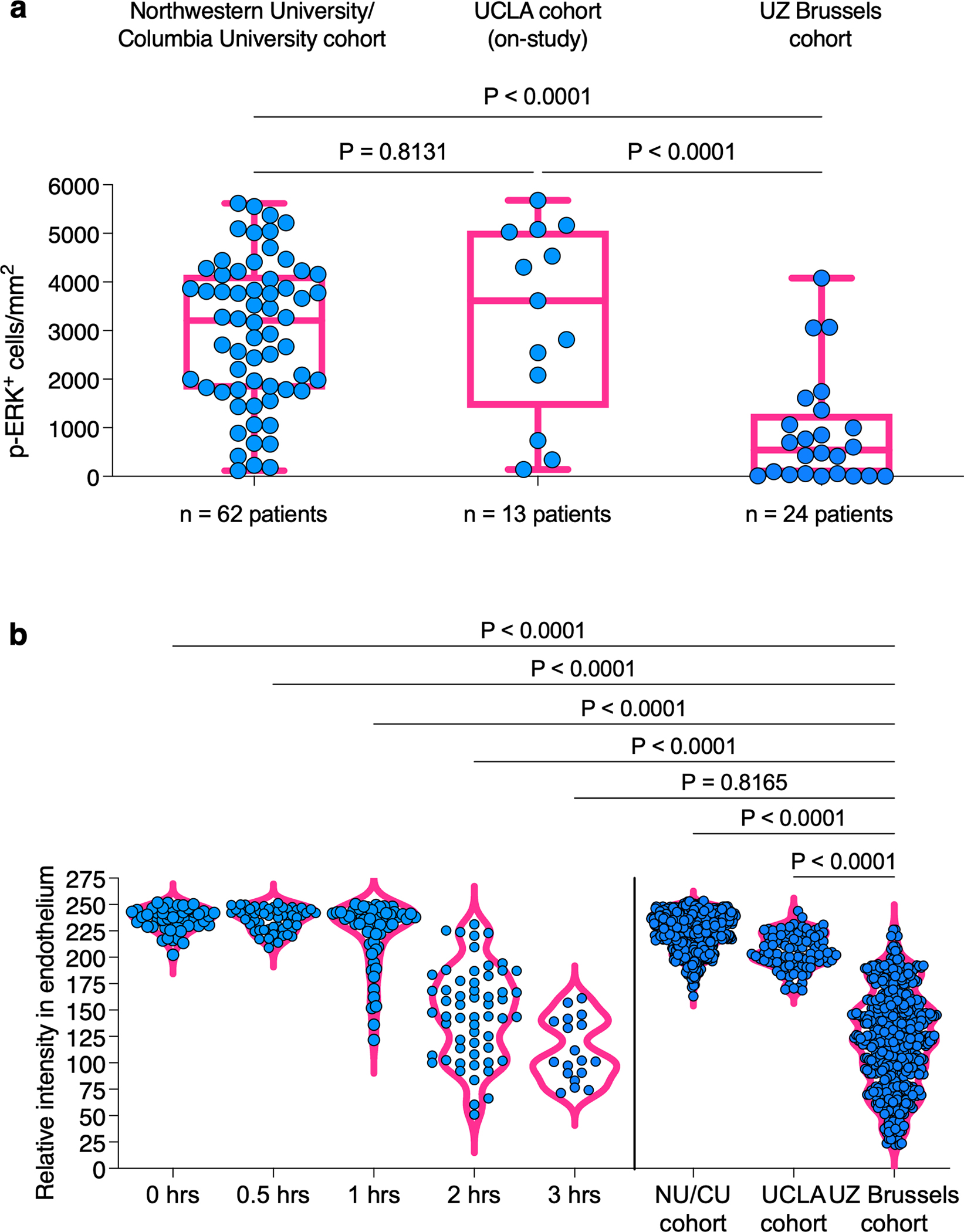
a, Distribution of p-ERK cell density values for three different glioblastoma patient cohorts (NU/CU cohort from Arrieta et al7, UCLA cohort from Cloughesy et al13, and UZ Brussels). b, Intensity of p-ERK staining in endothelial cells from 3 glioblastomas at different periods of ischemic time and glioblastomas across the three cohorts. Each dots represent p-ERK intensity on individual endothelial cells within the same samples. P values by two-sided Kruskal Wallis test with post-hoc Dunn’s multiple comparison test.
Determination of p-ERK epitope integrity across cohorts through staining intensity on endothelial cells.
Given that a high percentage of endothelial cells express p-ERK7 which is predominantly expressed during neovasculogenesis14, we evaluated the intensity of p-ERK staining in endothelial cells in glioblastomas from previous and current cohorts and compared them against endothelial cells from tumor samples fixed at different ischemic time points (0, 0.5, 1, 2, and 3 hr)7. We noticed that the p-ERK intensity in endothelial cells from glioblastomas from the UZ Brussels cohort most closely resembled the endothelium staining intensity of the samples that were fixed after 3 h. of ischemic time (P < 0.8165, one-way ANOVA; Fig. 3b). These results suggest some degradation of the p-ERK phosphoepitope across samples in the UZ Brussels cohort and highlight the need to develop and establish standardized protocols and methods with better accuracy to evaluate p-ERK and its association with OS following immune checkpoint blockade in glioblastoma patients.
Association of p-ERK with survival in IDH wild-type glioblastoma patients treated with immune checkpoint blockade.
Whereas we found differences in p-ERK values and intensities in glioblastomas from patients enrolled in the phase I clinical trial, we adhered to our initial pre-specified conditions for OS analysis and used the p-ERK median value to partition groups (high and low p-ERK groups). Thus, through independent statistical analysis of survival outcomes, we evaluated whether p- ERK was associated with increased OS in the complete cohort (IDH wild-type glioblastoma and IDH mutant high-grade glioma patients, n=24) and in IDH wild-type glioblastoma only patients (n=22). By analyzing the complete analysis cohort (n=24), we found that patients with high-p- ERK tumors (> 540.5 cells/mm2) treated with PD-1 blockade had longer median OS (47.6 weeks) compared to those with low-p-ERK tumors (<= 540.5 cells/mm2) although not statistically significant (P = 0.29, log-rank test; Fig. 4a). However, in the subset of IDH wild- type glioblastoma patients, we found that patients with high-p-ERK tumors (> 540.5 cells/mm2) treated with PD-1 blockade exhibited prolonged survival (median OS: 55.6 weeks (95% CI 31, NA)) compared to those with low-p-ERK tumors (median OS: 30 weeks (95% CI 18.34, NA)) (P = 0.036, log-rank test; Fig. 4b). In addition, we also applied the median value (3,207 cells/mm2) obtained from the p-ERK quantification of a previous analysis we performed to dichotomize the current glioblastoma patient cohort7. This dichotomization identified one glioblastoma patient with a tumor classified as high p-ERK that lived 185.57 weeks after therapy (Supplementary Fig. 3).
Figure 4. Association of p-ERK with survival in glioblastoma patients treated with immune checkpoint blockade.
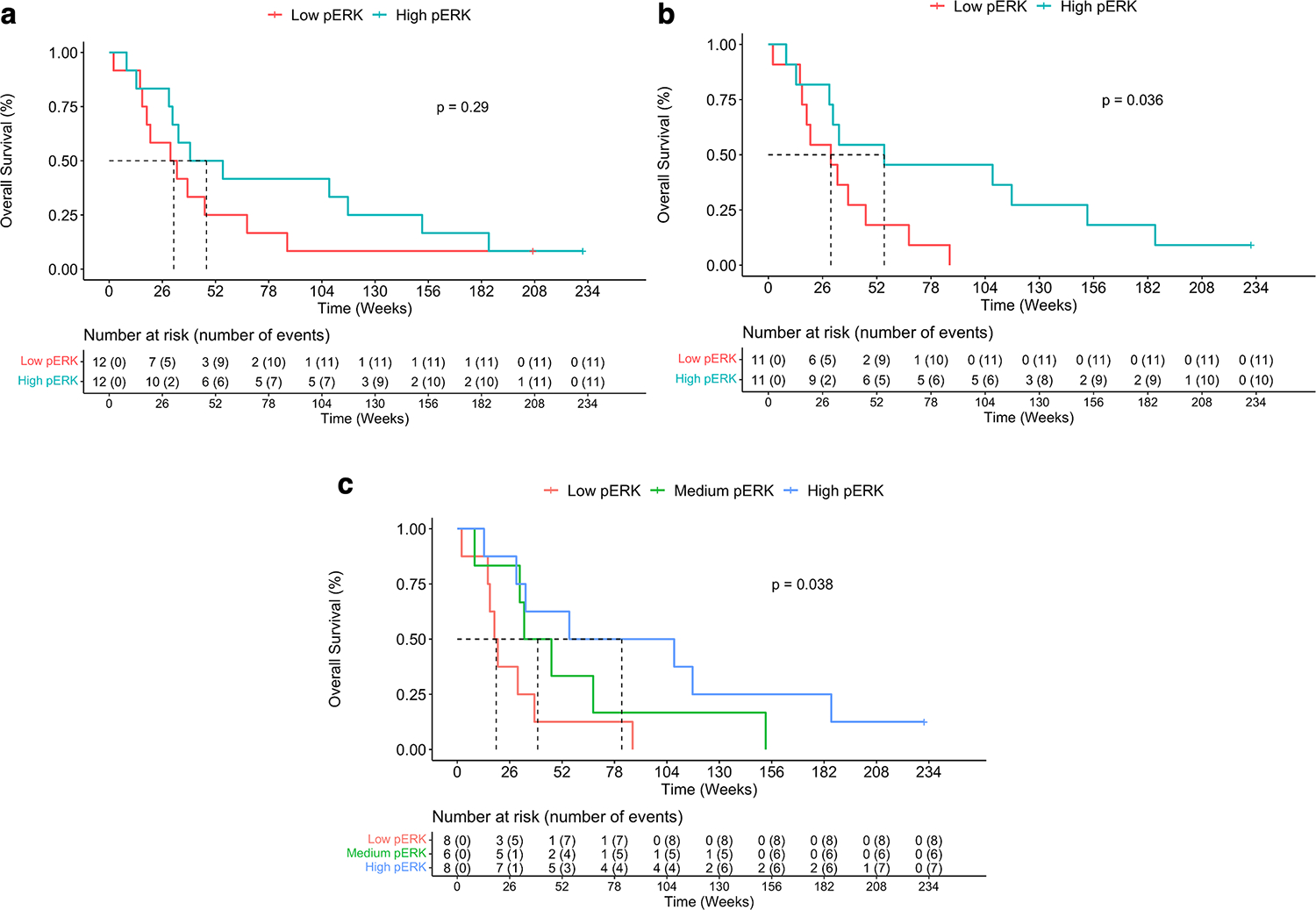
a, OS comparison between high and low p-ERK groups in the complete glioblastoma patient cohort. b, OS comparison between high and low p-ERK groups in wild-type IDH glioblastoma patients. c, Overall survival comparison among high, medium, and low p-ERK groups in the exploratory analysis.
For exploratory purposes, IDH wild-type glioblastoma patients were divided into 3 groups (high, intermediate, and low) based on p-ERK cell density. We observed an incremental improvement in OS with high p-ERK (> 895, n=8) glioblastoma patients exhibiting a median OS of 81.6 weeks (95% CI 33.86-NA), intermediate p-ERK (55–895, n=6) median OS of 39.9 weeks (95% CI 33.14-NA), and low p-ERK (<= 55, n=8) group with a median OS of 19.3 weeks (95% CI 16.14-NA) (P=0.038, log-rank test; Fig. 4c).
These preliminary results show the potential of p-ERK as a predictive biomarker for glioblastoma patients treated with immune checkpoint inhibitors demonstrated in an additional independent cohort of patients.
Validation of p-ERK as a predictive biomarker across three independent cohorts
To strengthen the major finding that p-ERK predicts response to immune checkpoint blockade in recurrent glioma patients, we explored p-ERK as a predictive biomarker by pooling data from two previously published cohorts7,13 as well as the current cohort11, yielding a comprehensive sample of 65 IDH wild-type glioblastoma patients. Then, we conducted a multivariate Cox proportional hazards model incorporating the p-ERK levels and factors that influence survival such as age, IDH mutation, and steroid usage. Considering the differences in p-ERK values between cohorts, we introduced “cohort” (UZ Brussels, NU/CU, and UCLA) as a stratification factor to separate baseline hazard functions for each cohort while estimating a common hazard ratio for the biomarker. The results of this analysis indeed reveal that p-ERK remains a significant predictor of survival, even after adjusting for the aforementioned covariates (P=0.011; Fig. 5a). The adjusted hazard ratio for p-ERK is 0.711, reinforcing its potential utility as a biomarker.
Figure 5. Multivariable analysis of p-ERK and covariates influencing survival trends in recurrent glioma patients receiving immune checkpoint blockade.
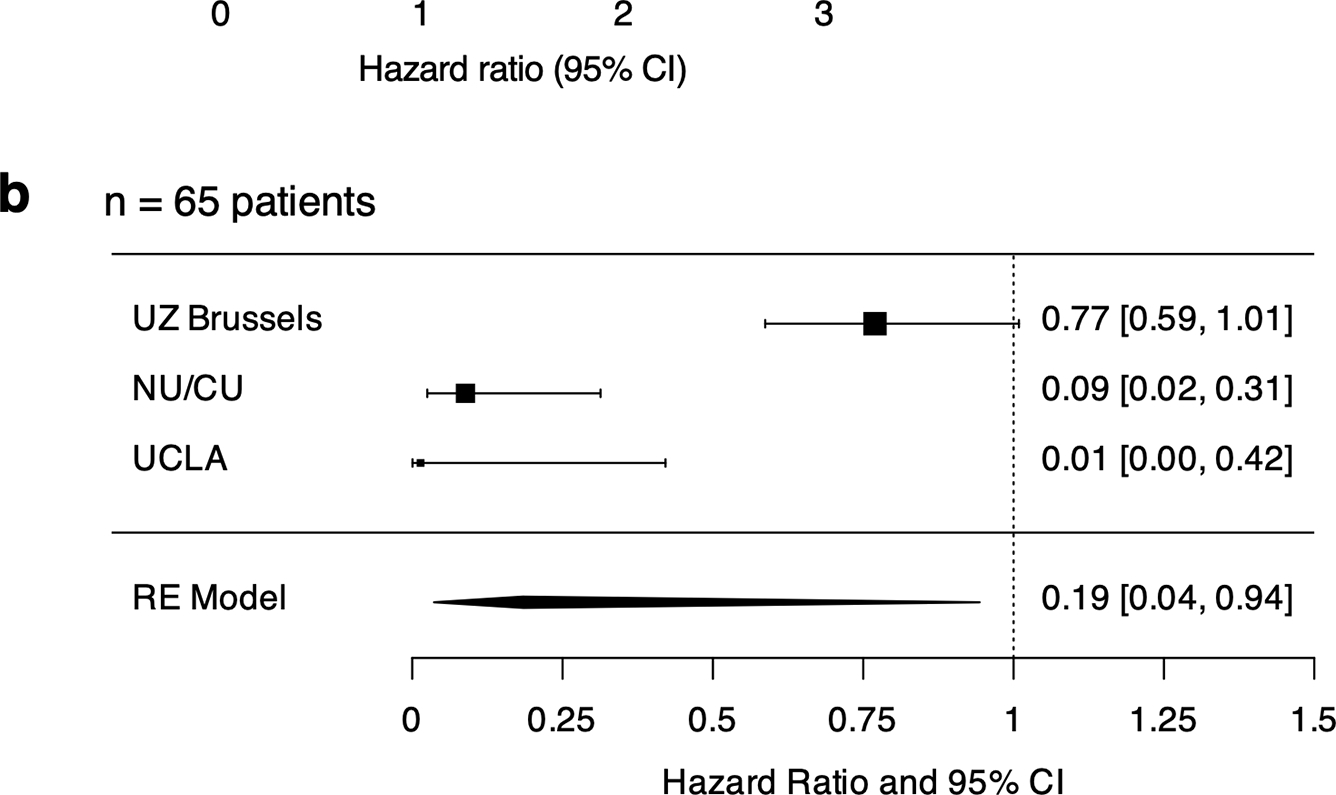
a, Forest plots showing the association of steroids, IDH mutation, age, and p-ERK after adjusting the multivariable Cox model with cohorts considered as a covariate (N= 65 recurrent glioma patients). b, Forest plot showing the results from the meta-analysis employing a random-effects model in three different cohorts (UZ Brussels, NU/CU, and UCLA; N= 65 recurrent glioma patients). c, Forest plots showing the association of steroids, age, and p-ERK after adjusting the multivariable Cox model with cohorts considered as a covariate (N= 56 recurrent glioma patients). Results are presented as HR (95% CI) in a, b, and c. P value by two-sided Wald test in a and c.
To further substantiate our findings, a complementary analytical strategy was employed. After consolidating data across the three cohorts and deriving the multivariable Cox model outputs, we obtained the cohort-specific adjusted hazard ratios. These were used to perform a meta-analysis employing a random-effects model to determine whether overall p-ERK is associated with a decreased risk of death across cohorts. After adjusting for differences among the three cohorts within a random-effects model, we observed the average HR effect across the studies for p-ERK is 0.19 (95% CI: 0.04–0.94; P=0.0424; Fig. 5b). This value suggests that as p- ERK increases, the risk of death decreases.
Leveraging our preliminary insights and the link of p-ERK with OS in IDH wild-type glioblastoma patients from the phase I clinical trial (Fig. 4b), we analyzed the data from IDH wild-type glioblastoma patients from the three cohorts, yielding a comprehensive sample of 56 patients. To account for the intrinsic variations in p-ERK values across three cohorts and other pivotal factors that influence OS, we performed a multivariable Cox model. We incorporated biomarker levels, cohort differences, age, and steroid consumption. This model pointed towards a statistically significant reduction in the risk of death in glioblastoma patients treated with immune checkpoint blockade. Specifically, for every unit increase in p-ERK (scaled by the median), there was a decrease in death risk by 32.5% (adjusted HR = 0.675, 95% CI: 0.5162– 0.8827, p = 0.004085; Fig. 5c). Notably, both age (adjusted HR = 1.048, 95% CI: 1.019–1.077, p = 0.000886) and steroid use (adjusted HR = 1.846, 95% CI: 1.033–3.297, p = 0.038273) were significant survival predictors associated with an increase death risk. The concordance statistic of our model, standing at 0.707, showcases the reliable ability of these covariates in predicting survival outcomes.
Through rigorous analyses spanning multiple independent cohorts and employing different statistical survival methodologies, our results firmly establish the prognostic significance of p-ERK levels in recurrent glioblastoma patients. Specifically, elevated p-ERK expression is associated with a reduced risk of mortality, particularly in those undergoing immune checkpoint blockade therapy. This convergence of findings from both multivariable Cox models and meta-analysis accentuates the paramount importance of p-ERK as a pivotal marker, underscoring its potential in guiding therapeutic decisions and stratifying patient risk.
DISCUSSION
The quest to identify glioblastoma patients who are likely to respond to immunotherapy is of paramount importance, particularly in light of the negative outcomes of clinical trials evaluating immune checkpoint blockade in unselected patient populations2–4. Several clinical trials assessing the use of nivolumab, an anti-PD-1 therapy, in glioblastoma patients have failed to demonstrate any survival advantage. The CheckMate 143 clinical trial showed that the median OS of nivolumab-treated patients was similar to that of bevacizumab-treated control patients2. Likewise, the CheckMate 498 clinical study failed to meet its primary endpoint of OS improvement in newly diagnosed glioblastoma patients with unmethylated MGMT3. Nivolumab in combination with TMZ and radiotherapy was also found not to be superior to TMZ, radiotherapy, and placebo in newly diagnosed glioblastoma patients with methylated MGMT promoter4.
Despite these discouraging outcomes, several studies and case reports have shown that certain subsets of glioblastoma patients exhibit prolonged OS and durable radiographic responses following immune checkpoint blockade5,6,8,13,15–17. Clinical evidence has suggested that tumor phenotype and the composition of the tumor microenvironment might influence responses to immunotherapy in glioblastoma5,7,18. This highlights the critical need to identify patients who are likely to respond to immunotherapy and which variables may impact treatment efficacy.
In this clinical study, we sought to evaluate the potential of p-ERK as a predictive biomarker for OS in recurrent glioblastoma patients treated with immune checkpoint blockade. Our analysis, based on a cohort of 24 patients with recurrent glioblastoma enrolled in a phase I clinical trial, provides additional evidence that p-ERK could serve as a valuable biomarker for the prognosis of glioblastoma patients receiving immune checkpoint inhibitors. This finding has important implications for the clinical management of glioblastoma, as it may help identify patients who are more likely to benefit from immunotherapy and optimize treatment strategies.
Our results add to the growing body of literature supporting that MAPK pathway activation in gliomas is associated with favorable outcomes following immunotherapy7,8. We recently showed that activation of the MAPK/ERK pathway, indicated by phosphorylation of ERK1/2, was associated with OS following adjuvant anti-PD-1 immunotherapy in two independent recurrent glioblastoma patient cohorts7. This observation suggests that the activation of this pathway may be a critical determinant of response to PD-1 inhibitors in recurrent glioblastoma patients, including those who do not harbor BRAF/PTPN11 mutations.
Whereas the MAPK pathway signaling is associated with response to immunotherapy in gliomas, no causal relationship has been established to date. Interesting observations suggest that gliomas with activation of the MAPK pathway have a distinct tumor immune microenvironment. We observed that glioblastoma tumors with elevated p-ERK were associated with a distinct microglial phenotype including elevated expression of MHC-class II molecules7. Moreover, we also showed that when gliomagenesis occurs in the absence of CD8 T-cells in murine models, the resultant gliomas exhibit elevated p-ERK and increased abundance of Iba1+ myeloid cells19. Additional recent studies have provided further insight into the relationship between MAPK activation and a distinct microenvironment in gliomas20,21.
The use of the REMARK criteria was important to ensure the rigor of our analysis. By specifying the conditions to be analyzed a priori and employing a blinded approach to p-ERK quantification, we minimized potential sources of bias in our study. Moreover, our use of an independent statistician for survival analysis further bolstered the reliability of our results.
An important aspect of this study was the comparison of p-ERK values in independent glioblastoma cohorts from different institutions. We found significant differences in p-ERK+ cell density and staining intensity, highlighting the need to develop and establish standardized protocols and methods for evaluating p-ERK and its association with OS following immune checkpoint blockade in glioblastoma patients.
Our study has several strengths, including the prospective collection of tumor samples and clinical data, as well as the adherence to the REMARK criteria for survival analysis. Nevertheless, our study also has limitations. The relatively small sample size and the heterogeneity of p-ERK values across cohorts necessitate further investigation in a larger, more diverse patient population. An important limitation of p-ERK to identify glioma patients who might be susceptible to immunotherapy is the relatively unstable integrity of the phosphoepitope, which is subject to degradation with increasing ischemia time. p-ERK determination in tumor- related endothelial cells can help identify samples with sub-optimal quality. Yet our analysis suggests that even in the context of some degradation of p-ERK, this biomarker might segregate patients with favorable outcomes following immunotherapy. We have shown that p-ERK integrity remains reasonable when tumor samples are fixed within 30 minutes to 1 hour after excision. Ideally, timely processing of specimens would overcome this limitation of p-ERK as a biomarker. Ultimately, controlling p-ERK phospho-epitope integrity will be important for implementing this biomarker for patient care in the future.
To supplement our initial findings from a limited sample size and to further bolster the argument for p-ERK as a biomarker, we consolidated data from three independent cohorts and undertook comprehensive statistical analyses. Utilizing a meta-analysis approach and incorporating the Cox proportional hazards model, our findings revealed that elevated p-ERK levels corresponded with a marked reduction in mortality risk.
While clinical trials have offered mixed outcomes, our study delineates that a subset of patients, identifiable through molecular markers like p-ERK, might achieve significant therapeutic advantages. Our investigation underscored the potential of p-ERK as a promising biomarker for discerning glioblastoma patients who may benefit from immune checkpoint blockade. however, variations across different cohorts and the inherent challenges with p-ERK stability emphasize the necessity for methodological standardization, rigorous validation, and continual reassessment. As we move forward, integrating such biomarkers into clinical practice requires a synergistic approach, combining rigorous scientific research with practical clinical considerations. The overarching goal remains clear: enhancing therapeutic strategies for glioblastoma patients to improve survival rates and quality of life.
Supplementary Material
Translational relevance.
This study highlights the translational potential of p-ERK as a key predictive biomarker for the efficacy of immune checkpoint inhibitors in patients with recurrent glioblastoma. The immunosuppressive tumor microenvironment of glioblastoma has posed challenges to the effectiveness of immune checkpoint inhibitors. However, the presented data, validated across three cohorts, strongly advocate and offer a pathway towards a more nuanced, personalized therapeutic strategy, aiming to pinpoint those patients most poised for a positive response to immune checkpoint inhibition. Before p-ERK can be considered for patient selection in clinical settings, it is important to establish standardized protocols for evaluating p-ERK to improve accuracy and reliability. Alternatively, the use of cost-effective and readily available tests to measure MAPK pathway activation could be integrated into personalized treatment strategies for glioblastoma patients undergoing immune checkpoint blockade therapy. Amid a backdrop of limited treatment options, these findings offer a renewed hope for devising more effective treatment modalities in glioblastoma management.
ACKNOWLEDGEMENTS
This work was supported by the NIH grant 1R01NS110703–01A1 (AMS), NIH/NCI 1U19CA264338–01 (A.M.S. and R.S.), NIH/NCI 1R01CA245969–01A1 (A.M.S. and R.S,), P50CA221747 SPORE for Translational Approaches to Brain Cancer as well as generous philanthropic support from the Moceri Family Foundation and the Panattoni Family Foundation. We thank K. McCortney, J. Walshon, M. Santa Flowers, and A. Steffens from the Nervous System Tumor Bank supported by the P50CA221747 SPORE for Translational Approaches to Brain Cancer. We thank B. Frederick, B. Shmaltsuyeva, and H. Fan at the Northwestern University Pathology Core Facility funded by the Cancer Center Support Grant (no. NCI CA060553). Most importantly, we thank the patients and their families for their contribution to this research.
Footnotes
CONFLICT OF INTEREST
Please refer to the COI declared by individual authors on the ICMJE forms submitted.
DATA AVAILABILITY
Data will be made available upon reasonable request.
REFERENCES
- 1.Stupp R, Taillibert S, Kanner A, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 12 2017;318(23):2306–2316. doi: 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reardon DA, Brandes AA, Omuro A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. May 2020;doi: 10.1001/jamaoncol.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omuro A, Brandes AA, Carpentier AF, et al. Radiotherapy Combined With Nivolumab or Temozolomide for Newly Diagnosed Glioblastoma With Unmethylated MGMT Promoter: An International Randomized Phase 3 Trial. Neuro Oncol. Apr 14 2022;doi: 10.1093/neuonc/noac099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim M, Weller M, Idbaih A, et al. Phase 3 Trial of Chemoradiotherapy With Temozolomide Plus Nivolumab or Placebo for Newly Diagnosed Glioblastoma With Methylated MGMT Promoter. Neuro Oncol. May 2 2022;doi: 10.1093/neuonc/noac116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrieta VA, Dmello C, McGrail DJ, et al. Immune checkpoint blockade in glioblastoma: from tumor heterogeneity to personalized treatment. J Clin Invest. Jan 17 2023;133(2)doi: 10.1172/JCI163447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukas RV, Rodon J, Becker K, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol. Nov 2018;140(2):317–328. doi: 10.1007/s11060-018-2955-9 [DOI] [PubMed] [Google Scholar]
- 7.Arrieta VA, Chen AX, Kane JR, et al. ERK1/2 phosphorylation predicts survival following anti-PD-1 immunotherapy in recurrent glioblastoma. Nat Cancer. 2021;doi: 10.1038/s43018-021-00260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti- PD-1 immunotherapy in glioblastoma. Nat Med. Mar 2019;25(3):462–469. doi: 10.1038/s41591-019-0349-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray A, Williams MA, Meek SM, et al. A phase I study of intratumoral ipilimumab and interleukin-2 in patients with advanced melanoma. Oncotarget. Sep 27 2016;7(39):64390–64399. doi: 10.18632/oncotarget.10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarze JK, Awada G, Cras L, et al. Intratumoral Combinatorial Administration of CD1c (BDCA-1) + Myeloid Dendritic Cells Plus Ipilimumab and Avelumab in Combination with Intravenous Low-Dose Nivolumab in Patients with Advanced Solid Tumors: A Phase IB Clinical Trial. Vaccines (Basel). Nov 10 2020;8(4)doi: 10.3390/vaccines8040670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duerinck J, Schwarze JK, Awada G, et al. Intracerebral administration of CTLA-4 and PD-1 immune checkpoint blocking monoclonal antibodies in patients with recurrent glioblastoma: a phase I clinical trial. J Immunother Cancer. Jun 2021;9(6)doi: 10.1136/jitc-2020-002296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol. Aug 2005;2(8):416–22. [PubMed] [Google Scholar]
- 13.Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. Mar 2019;25(3):477–486. doi: 10.1038/s41591-018-0337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagasawa-Masuda A, Terai K. ERK activation in endothelial cells is a novel marker during neovasculogenesis. Genes Cells. Nov 2016;21(11):1164–1175. doi: 10.1111/gtc.12438 [DOI] [PubMed] [Google Scholar]
- 15.Arrieta VA, Iwamoto F, Lukas RV, Sachdev S, Rabadan R, Sonabend AM. Can patient selection and neoadjuvant administration resuscitate PD-1 inhibitors for glioblastoma? J Neurosurg. Dec 2019:1–6. doi: 10.3171/2019.9.JNS192523 [DOI] [PubMed] [Google Scholar]
- 16.Roth P, Valavanis A, Weller M. Long-term control and partial remission after initial pseudoprogression of glioblastoma by anti-PD-1 treatment with nivolumab. Neuro Oncol. 03 2017;19(3):454–456. doi: 10.1093/neuonc/now265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of Hypermutated Glioblastoma: A Patient with Germline POLE Deficiency Treated with Checkpoint Blockade Immunotherapy. Cancer Discov. 11 2016;6(11):1230–1236. doi: 10.1158/2159-8290.CD-16-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White K, Connor K, Meylan M, et al. Identification, validation and biological characterisation of novel glioblastoma tumour microenvironment subtypes: implications for precision immunotherapy. Ann Oncol. Mar 2023;34(3):300–314. doi: 10.1016/j.annonc.2022.11.008 [DOI] [PubMed] [Google Scholar]
- 19.Robert KJ, Junfei Z, Takashi T, et al. CD8+ T-cell-mediated immunoediting influences genomic evolution and immune evasion in murine gliomas. Clin Cancer Res. 2020;26:4390–4401. doi: 10.1101/705152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Mohamed E, Tong S, et al. CXCL14 Promotes a Robust Brain Tumor-Associated Immune Response in Glioma. Clin Cancer Res. Jul 01 2022;28(13):2898–2910. doi: 10.1158/1078-0432.CCR-21-2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J, Sun J, Li M, Zhang Z, Yang D, Cao H. MAPK Activated Protein Kinase 3 Is a Prognostic-Related Biomarker and Associated With Immune Infiltrates in Glioma. Front Oncol. 2021;11:793025. doi: 10.3389/fonc.2021.793025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.
Data will be made available upon reasonable request.


