Abstract
Human checkpoint kinase 1 (Chk1) is an essential kinase required to preserve genome stability. Here, we show that Chk1 inhibition by two distinct drugs, UCN-01 and CEP-3891, or by Chk1 small interfering RNA (siRNA) leads to phosphorylation of ATR targets. Chk1-inhibition triggered rapid, pan-nuclear phosphorylation of histone H2AX, p53, Smc1, replication protein A, and Chk1 itself in human S-phase cells. These phosphorylations were inhibited by ATR siRNA and caffeine, but they occurred independently of ATM. Chk1 inhibition also caused an increased initiation of DNA replication, which was accompanied by increased amounts of nonextractable RPA protein, formation of single-stranded DNA, and induction of DNA strand breaks. Moreover, these responses were prevented by siRNA-mediated downregulation of Cdk2 or the replication initiation protein Cdc45, or by addition of the CDK inhibitor roscovitine. We propose that Chk1 is required during normal S phase to avoid aberrantly increased initiation of DNA replication, thereby protecting against DNA breakage. These results may help explain why Chk1 is an essential kinase and should be taken into account when drugs to inhibit this kinase are considered for use in cancer treatment.
To maintain genomic stability cells have evolved mechanisms that ensure the order and fidelity of cell cycle events, such as DNA replication and cell division (11). When DNA is damaged or replication is inhibited, cells respond by activation of evolutionarily conserved signal transduction pathways that delay cell cycle progression and induce repair of the damaged DNA (43). These signal transduction pathways include protein sensors that recognize aberrant DNA structures and activate kinases, thereby inducing phosphorylation cascades that ultimately lead to cell cycle arrest and DNA repair (43).
The ATR kinase plays a central role in the cellular response to several types of DNA damage occurring in S and G2 phases of the cell cycle, including aberrant replication intermediates and DNA double-strand breaks (DSBs) (1). ATR is activated in response to formation of single-stranded DNA (ssDNA), which is induced during DNA damage processing (37, 45). Single-stranded DNA is recognized and coated by the ssDNA binding protein replication protein A (RPA), which subsequently recruits and activates the ATRIP-ATR complex (45).
Among the ATR targets are proteins such as p53, H2AX, and Chk1 (10, 15, 36, 38). The latter kinase is phosphorylated on serine 317 and serine 345, respectively, by ATR, and these sites are required for the ability of Chk1 to amplify the signal by phosphorylating several additional targets (29, 40). ATR-mediated phosphorylation of Chk1 requires the DNA-binding protein claspin, which may serve to recruit Chk1 to the DNA lesions where ATR resides (13). Homozygous disruption of either Chk1 or ATR in mice causes early embryonic lethality (2, 4, 15, 33). It is not clear why Chk1 function is essential, and only a few Chk1 targets have been identified.
Cdc25 phosphatases have been identified as bona fide Chk1 target proteins (9, 24). Cdc25s regulate cell cycle progression by activating the cyclin-dependent kinases (Cdks) (24). Chk1-mediated phosphorylation and inhibition of Cdc25 phosphatases (and thereby Cdks) has been implicated in cell cycle checkpoint control of G1/S, S, and G2/M phases (9, 17, 18, 24, 29, 41). Cdk activity is rate limiting for initiation of DNA replication, at least in part by contributing to the activation of the Mcm2-7 DNA helicase complex that catalyzes the unwinding of the DNA duplex during replication (21). Cdk activity facilities loading of the replication protein Cdc45 to replication origins (46), which is thought to support Mcm2-7-mediated unwinding of DNA (20), as well as loading of DNA polymerases onto DNA (34).
When DNA is damaged in S phase, Chk1 may play a prominent role in restraining initiation of DNA replication from the yet unfired origins (8). In the budding yeast the absence of checkpoint control leads to accumulation of ssDNA and replication fork reversal at stalled replication forks (28). Such abnormal DNA structures may lead to a loss of genome integrity. We previously suggested that during physiological S phase in the absence of exogenously added DNA damage or replication interference, Chk1 may restrain unscheduled DNA synthesis by actively regulating target proteins such as Cdc25A (29, 30, 41). This hypothesis was supported by recent studies of the control of DNA replication initiation in Xenopus egg extracts, where it was shown that the ATR and ATM signaling pathways control origin firing via the downstream targets Chk1, Cdk2, and Cdc25A in the absence of DNA damage (19, 27). Physiological regulation of Chk1 is also under the control of the upstream regulators claspin and the Rad9-Hus1-Rad1 complex, suggesting that DNA replication per se generates lesions that signal to the checkpoint machinery (30). However, it is unknown to which extent such Chk1-mediated control of S-phase events might be required for the process of normal replication. One possibility is that Chk1 would be required to limit excessive activity of Cdks or other replication factors, which could lead to aberrant replication events.
To address this issue, we have studied the effects of Chk1 inhibition in unperturbed cells. We have found that inhibition or depletion of Chk1 causes a rapid and strong phosphorylation of ATR targets in S-phase cells, which was associated with increased initiation of DNA replication, massive induction of ssDNA, and generation of DNA strand breaks. We propose a model where Chk1 is required during normal S-phase progression to avoid aberrantly increased initiation of DNA replication, thereby guarding against generation of potentially harmful DNA lesions.
MATERIALS AND METHODS
Cell lines, drugs, and siRNA treatment.
Human U-2-OS osteosarcoma cells and AT fibroblasts GM 09607B (Coriell Cell Repositories) were grown in Dulbecco modified Eagle medium with 10% fetal bovine serum. AT fibroblasts AT22IJE stably transfected with YZ5 ATM cDNA (44) were grown in the presence of 100 μg of hygromycin/ml. The CEP-3891 Chk1 inhibitor was provided by Cephalon, Inc., and used at a concentration of 500 nM. UCN-01 was a gift from R. J. Schultz (Drug Synthesis and Chemical Branch, National Cancer Institute) and was used at 300 nM. Caffeine was purchased from Sigma. Chk1 and Cdc45 small interfering RNA (siRNA) oligonucleotide sequences were purchased from Dharmacon (Dharmacon SMARTpool reagents M-003255-02 [Chk1] and M-003232-02 [Cdc45]; four siRNAs combined into a single pool). The ATR siRNA sequence was 5′-GAGUUCUCAGAAGUCAACCdTdT-3′. Duplexes directed against green fluorescent protein (GFP) siRNA or a nontargeting sequence 5′-GCGCGCUUUGUAGGAUUCGdTdT-3′ (control siRNA) were used as a control. The OligofectAMINE reagent (Invitrogen) was used for transfection.
Production of a U-2-OS clone with tetracycline-inducible Cdk2 short-hairpin RNA (shRNA).
DNA oligonucleotides targeting human Cdk2 (forward strand, 5′-GATCCCCGTTTCAGTATTAGATGCACTTCAAGAGAGTGCATCTAATACTGAAACTTTTT-3′; reverse strand, 5′-AGCTAAAAAGTTTCAGTATTAGATGCACTCTCTTGAAGTGCATCTAATACTGAAACGGG-3′) were ligated into the pSUPERIOR.puro vector (OligoEngine) according to the manufacturer's instructions by using a Rapid Ligation Kit (Roche). The resulting plasmid was then transfected into U-2-OS cells expressing a tetracycline repressor (T-Rex; Invitrogen). After 24 h, puromycin (5 μg/ml) was added to the culture medium, and clones were expanded and tested for the efficiency of Cdk2 knockdown after addition of tetracycline (2 μg/ml; Calbiochem).
Antibodies and immunochemistry.
Immunoblotting was performed as described previously (29). Where indicated, cells were preextracted before cell lysis for 5 min on ice in a buffer containing 0.5% Triton X-100 in 20 mM HEPES (pH 7.4), 50 mM NaCl, 3 mM MgCl2, and 300 mM sucrose, and extracts were sonicated and treated with DNase (0.1 U/ml) to extract chromatin-bound proteins. Phospho-H2AX (γ-H2AX) mouse and rabbit antibodies (Ser139) were purchased from Upstate Biotechnology (catalog nos. 05-636 and 07-164). Phospho-Chk1 antibody (Ser317) and phospho-p53 (Ser15) were purchased from Cell Signaling. Antibodies to Chk1 (DCS-310), Mcm7 (DCS-141), p53 (DO-1), and Cdk7 (MO-1) have been described (29). Goat antibody to ATR (sc-1887) and rabbit antibodies to Cdc45 (sc-20685) and Cdk2 (sc-163) were purchased from Santa Cruz, and rabbit antibody to phospho-SMC1 (Ser 966) was purchased from Ab-cam. Anti-bromodeoxyuridine (BrdU) mouse antibody (RPN20AB) was purchased from Amersham. Fluorescence-conjugated anti-mouse immunoglobulin G (IgG) and anti-rabbit immunoglobulin G IgG were purchased from Molecular Probes (Alexa Fluor 488, 568, and and 594).
Immunofluorescence.
Cells cultured on glass coverslips were treated as indicated in the figure legends, fixed with 4% formaldehyde (12 min. at room temperature), permeabilized in phosphate-buffered saline (PBS)-0.25% Triton X-100 (5 min at room temperature), and stained with the antibodies as specified in the figure legends, followed by DNA staining with ToPro 3 (1 min, diluted 1:20,000: Molecular Probes). Where indicated, cells were preextracted before fixation for 5 min on ice (0.5% Triton X-100 in 20 mM HEPES [pH 7.4], 50 mM NaCl, 3 mM MgCl2, 300 mM sucrose). To measure induction of ssDNA, cells prelabeled with BrdU (3.3 μM, 24 h) were fixed and permeabilized as described above and then stained with anti-BrdU (1 h, diluted 1:300). Confocal images were acquired by a Zeiss 510 laser-scanning microscope.
Flow cytometry.
For two-parameter flow cytometry analysis to assay the cell cycle position of γ-H2AX-positive cells, cells were fixed in 70% ethanol, incubated with PBS-0.25% Triton X-100 for 15 min on ice, and stained with mouse antibody to γ-H2AX (1:500) for 1 h at room temperature, followed by 30 min of incubation with conjugated anti-mouse IgG (Alexa Fluor 488 at 1:500). DNA was then counterstained by 0.1 mg of propidium iodide (PI)/ml containing RNase for 30 min at 37°C and analyzed with a FACSCalibur flow cytometer (BD Biosciences) using CellQuest software.
Measurement of DNA synthesis.
DNA synthesis was assessed as described previously (29).
PFGE and alkaline elution.
The pulsed-field gel electrophoresis (PFGE) assay to measure induction of DNA DSBs was performed as described previously (16). Alkaline elution assay, which measures the induction of both single-strand breaks and DSBs, was performed as described previously (26).
TUNEL assay.
For dual staining with the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay and γ-H2AX, cells cultured on glass coverslips were fixed in 4% paraformaldehyde (12 min at room temperature) and permeabilized by incubation with PBS 0.25% Triton X-100 (5 min). To reduce background staining of the TUNEL assay, cells were then incubated with proteinase K solution (10 μg/ml in PBS) for 5 min at room temperature, and the TUNEL assay was then performed according to the manufacturer's instructions (ApoAlert DNA Fragmentation Assay Kit; BD Biosciences). After the last wash, cells were incubated with PBS-0.25% Triton X-100 for 5 min at room temperature and stained with mouse antibody to γ-H2AX (1:500) for 1 h, followed by a 30-min staining with anti-mouse IgG (Alexa Fluor 568) and finally a 1-min staining with the DNA stain ToPro 3 (Molecular Probes at a dilution of 1:20,000). Confocal images were obtained as described above.
RESULTS
Chk1 inhibition triggers phosphorylation of ATR targets.
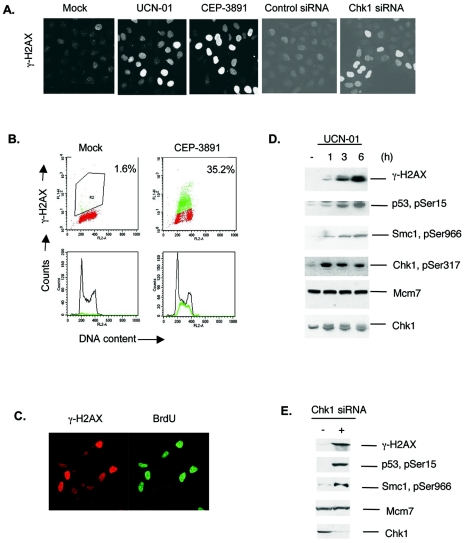
To investigate the role of Chk1 during unperturbed cell cycle progression, we treated cells with two independent inhibitors of Chk1 kinase: UCN-01 and CEP-3891. Both inhibitors triggered rapid and massive phosphorylation of histone H2AX (γ-H2AX) in a subset of exponentially growing U-2-OS cells (Fig. 1A, left panels). A similar effect was obtained by depletion of Chk1 with siRNA (Fig. 1A, right panels). Flow cytometry analysis of cells stained with γ-H2AX and PI showed that the response occurred mainly in S-phase cells (Fig. 1B). Furthermore, when cells were first synchronized in G1 or S phase by release from nocodazole arrest and then treated with UCN-01, only the S phase cells showed an induction of γ-H2AX (data not shown). The γ-H2AX-positive cells were actively replicating their DNA, since they readily incorporated BrdU during a short pulse of BrdU at 3 h after CEP-3891 treatment (Fig. 1C). In addition to γ-H2AX, several other ATR/ATM targets, such as Chk1, p53, SMC1, and RPA, were also rapidly phosphorylated in response to Chk1 inhibition by UCN-01 or CEP-3891 (Fig. 1D and data not shown) and by depletion of Chk1 with siRNA (Fig. 1E). These responses were not limited to U-2-OS cells as similar effects of UCN-01 and CEP-3891 were seen in other cell types, including HCT116 colon cancer cells, AT transformed fibroblasts, Chinese hamster cells, and normal human fibroblasts, although the magnitude of the responses varied among the cell types (data not shown).
FIG. 1.
Chk1 inhibition causes massive phosphorylation of ATR targets in S-phase cells. (A) γ-H2AX measured at 3 h after Chk1 inhibition by UCN-01 (300 nM) or CEP-3891 (500 nM) or after transfection with Chk1 siRNA (100 nM, 48 h) in U-2-OS cells. Immunofluorescence of cells stained with an antibody to γ-H2AX is shown. (B) Flow cytometry analysis of cells stained with γ-H2AX and PI. Numbers indicate the percentages of γ-H2AX-positive cells. (C) BrdU incorporation in γ-H2AX-positive cells. A short pulse of BrdU (5 min) was administered to U-2-OS cells at 3 h after treatment with CEP-3891 (500 nM), and cells were then processed for immunofluorescence analysis with antibody to γ-H2AX, followed by DNase treatment and staining with an antibody to BrdU. (D) Phosphorylation of Chk1, p53, Smc1, and H2AX increase after Chk1 inhibition. Extracts from U-2-OS cells were prepared at 0, 1, 3, and 6 h after treatment with UCN-01 (300 nM) and processed for Western blotting. Mcm7 protein is a loading marker. (E) Phosphorylation of γ-H2AX, p53, and Smc1 increase after depletion of Chk1 by siRNA transfection. Extracts from U-2-OS cells were prepared at 48 h after transfection with control siRNA (−) or Chk1 siRNA (+) and processed for Western blotting.
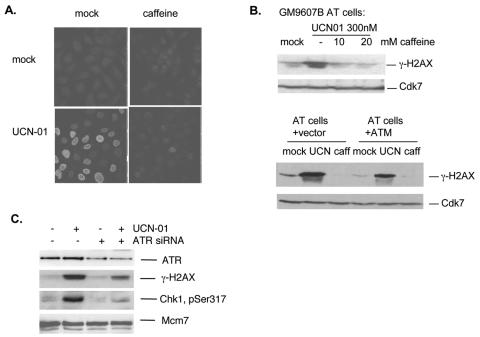
To explore which kinase was likely responsible for these effects, we used caffeine to inhibit the ATR and ATM kinases (25). Caffeine inhibited γ-H2AX induction when added together with UCN-01 in both U-2-OS cells and human AT cells (Fig. 2A and 2B, top panel), indicating that this response was caffeine sensitive but ATM independent. H2AX was phosphorylated to a similar extent in AT cells transfected with empty vector or reconstituted with wild-type ATM (Fig. 2B, bottom panel). We concluded that the responsible kinase was likely ATR, a notion confirmed by siRNA-mediated downregulation of ATR. Thus, as seen in Fig. 2C, transfection with ATR siRNA caused a significant reduction in γ-H2AX and Chk1 phosphorylation after UCN-01 treatment, suggesting that ATR is indeed the kinase responsible for these effects.
FIG. 2.
ATR (but not ATM) is required for H2AX phosphorylation in response to Chk1 inhibition. (A) Caffeine inhibits γ-H2AX in response to Chk1 inhibition. U-2-OS cells were treated for 4 h with caffeine (10 mM), UCN-01 (300 nM), or caffeine plus UCN-01 and then processed for immunofluorescence staining of γ-H2AX. (B) Western blots of extracts of GM9607B AT fibroblasts prepared at 3 h after treatment with UCN-01 (300 nM) in the presence of 0, 10, or 20 nM caffeine are shown in the top panel, and Western blots of AT cells (AT22IJE-T) transfected with control vector or recombinant ATM protein treated with UCN-01 (300 nM) or caffeine (10 mM) in the bottom panel. (C) U-2-OS cells were transfected with 200 nM ATR siRNA (+) or GFP siRNA (−) and then treated with UCN-01 (300 nM) for 3 h at 48 h after transfection. Extracts were then prepared and processed for Western blotting. Mcm7 protein is a loading marker.
Chk1 inhibition triggers increased initiation of DNA replication.
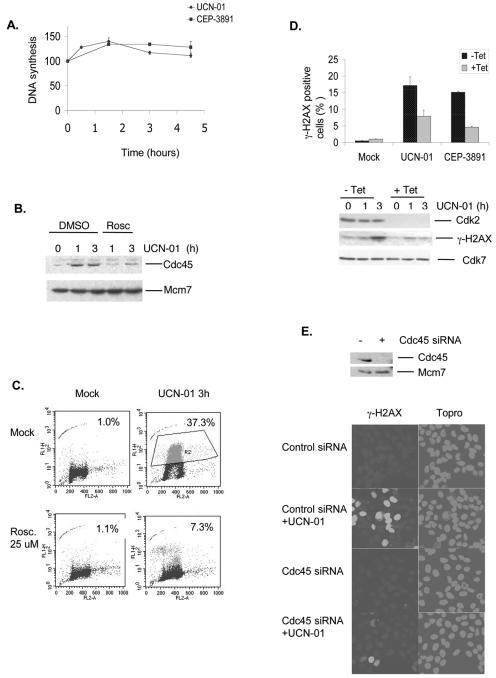
We previously showed that in the absence of DNA damage, Chk1 inhibition by UCN-01 or CEP-3891 caused a rapid and transient increase in Cdk activity, mediated by decreased Chk1 phosphorylation of the Cdc25A phosphatase (29, 41). In line with this, Chk1 inhibition by UCN-01 or CEP-3891 caused a transient increase in the rate of DNA synthesis (Fig. 3A). Similarly, when the rate of DNA synthesis was measured at 24 h after transfection with Chk1 or control siRNA, the Chk1-depleted cells showed a 1.3 (±0.1)-fold-higher rate of DNA synthesis compared to the cells transfected with control siRNA (data not shown). To examine whether the increased rate of DNA synthesis reflected increased initiation, elongation, or both, we measured the amount of nonextractable Cdc45 protein after treatment with UCN-01 (Fig. 3B). We found that UCN-01 caused a marked increase in the amount of nonextractable Cdc45 protein, which was inhibited by addition of the Cdk inhibitor roscovitine (Fig. 3B). Since Cdc45 loading onto chromatin has been implicated in initiation rather than elongation processes, these results strongly suggest that Chk1 inhibition caused increased initiation of DNA replication, which was mediated by the increased Cdk activity. Moreover, Cdk inhibition by roscovitine or shRNA-mediated downregulation of Cdk2 prevented phosphorylation of H2AX in response to UCN-01 or CEP-3891 treatment (Fig. 3CD). Depletion of Cdc45 by siRNA also prevented phosphorylation of H2AX in response to UCN-01 treatment (Fig. 3E). Taken together, these results suggested that the increased Cdk2 activity and increased initiation of DNA replication likely contributed to the activation of ATR seen after Chk1 inhibition. Consistent with the notion that high levels of Cdk2 activity may contribute to such a response, we also found that transient transfections with constitutively active Cdk2 (Cdk2AF) or Cdc25A, both manipulations that increase Cdk2 activity, caused massive γ-H2AX in U-2-OS cells (data not shown). Furthermore, when UCN-01 or CEP-3891 was added to U-2-OS cells pretreated with 2 mM thymidine for 24 h to synchronize cells at the beginning of S phase, a more rapid and stronger γ-H2AX induction was seen, a response that could be also inhibited by addition of roscovitine (data not shown), similarly to the response of exponentially growing cells. Since replication elongation is absent after thymidine synchronization, this result further supported the involvement of increased initiation as a mechanism behind ATR activation in Chk1-deficient cells.
FIG. 3.
Chk1 inhibition causes increased initiation of DNA replication. (A) Chk1 inhibition increases the overall rate of DNA synthesis. U-2-OS cells were treated with UCN-01 (100 nM) or CEP (500 nM) for the indicated times, and DNA synthesis was measured by the determining the uptake of [3H]thymidine. DNA synthesis after treatment is shown relative to nontreated cells (100%). The results are the averages of three independent experiments performed with dishes in duplicate. Error bars indicate the standard error of the mean. (B) Chk1 inhibition causes increased levels of nonextractable Cdc45 protein. U-2-OS cells were treated with UCN-01 (300 nM) for 0, 1, or 3 h in the absence or presence of the Cdk inhibitor roscovitine (25 μM), incubated with extraction buffer, and processed for Western blotting. Mcm7 protein is a loading marker. (C) γ-H2AX in Chk1-deprived cells is inhibited by roscovitine. U-2-OS cells were treated with UCN-01 for 3 h in the absence or presence of roscovitine (25 μM), stained with γ-H2AX and PI, and analyzed by flow cytometry. FL-1, γ-H2AX; FL2-A, PI. (D) Cdk2 depletion prevents induction of γ-H2AX in response to Chk1 inhibition. U-2-OS cells expressing tetracycline (Tet)-dependent Cdk2 shRNA were incubated with Tet− or Tet+ medium as indicated for 48 h and then treated with UCN-01 (300 nM) or Cep-3891 (500 nM). In the top panel is shown flow cytometry analysis of cells stained with γ-H2AX and PI at 3 h after treatment with UCN-01 or CEP-3891. The percentages of γ-H2AX-positive cells were calculated as described for panel C. Error bars indicate the deviations from two independent experiments. In the bottom panel are shown Western blots of extracts prepared at 1 and 3 h after treatment with UCN-01 (300 nM). (E) γ-H2AX in Chk1-deprived cells is inhibited by Cdc45 siRNA. U-2-OS cells transfected with GFP siRNA (−) or Cdc45 siRNA (+) were treated with UCN-01 (300 nM) for 3 h at 48 h after transfection and stained for γ-H2AX and with the DNA stain Topro3. Western blots show downregulation of the Cdc45 protein after transfection with Cdc45 siRNA.
Chk1 inhibition causes increased RPA binding to ssDNA.
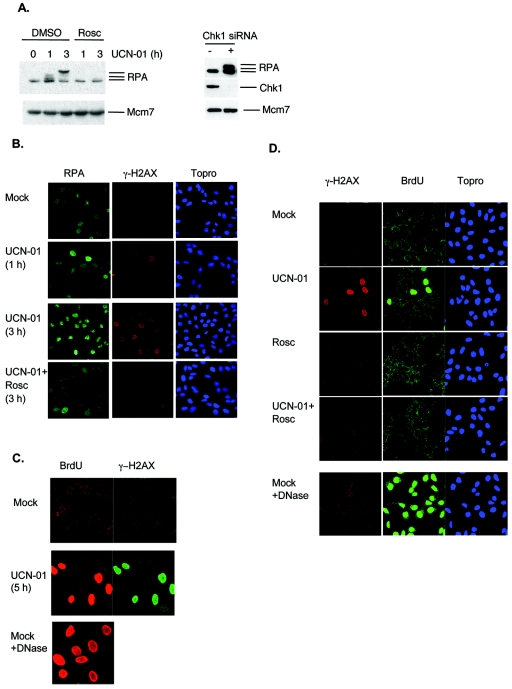
Activation of ATR depends on association of RPA with ssDNA (45). To test whether RPA-coated ssDNA contributed to the responses to Chk1 inhibition, we measured the amount of nonextractable RPA protein and the extent of formation of ssDNA in response to UCN-01 treatment (Fig. 4). We found a significant increase in the amount, as well as hyperphosphorylation, of nonextractable RPA protein at 1 and 3 h after UCN-01 treatment, which were inhibited by addition of roscovitin (Fig. 4A, left panel). Similar effects were observed when Chk1 was depleted by siRNA (Fig. 4A, right panel). Immunofluorescence analysis revealed that the γ-H2AX-positive cells also showed elevated levels of nonextractable RPA protein (Fig. 4B). Furthermore, a simple assay to measure formation of ssDNA based on the ability of the anti-BrdU antibody to detect incorporated BrdU in the absence of DNA denaturation showed massive induction of ssDNA in cells strongly positive for γ-H2AX, which was inhibited by addition of roscovitine (Fig. 4C and D). Similar effects were seen after CEP-3891-mediated inhibition of Chk1 (data not shown). These results support the concept that ATR was activated due to an abnormal increase in RPA coated ssDNA, which likely occurred as a consequence of increased initiation of DNA replication caused by Chk1 inhibition.
FIG. 4.
Chk1 inhibition causes formation of ssDNA. (A) Increased levels of nonextractable RPA protein after Chk1 inhibition. U-2-OS cells were treated with UCN-01 (300 nM) for 0, 1, or 3 h in the absence or presence of roscovitine (25 μM; left panel) or transfected with control (−) or Chk1 (+) siRNA (100 nM, 48 h; right panel), incubated with extraction buffer, and processed for Western blotting. Mcm7 protein is a loading marker. (B) U-2-OS cells were treated with UCN-01 (300 nM) in the absence or presence of roscovitine (25 μM), incubated with extraction buffer, and stained with antibodies to RPA and γ-H2AX and the DNA stain Topro3. (C) Detection of incorporated BrdU in the absence of DNase treatment after Chk1 inhibition. U-2-OS cells prelabeled with BrdU were treated with UCN-01 (100 nM) for 5 h and stained with antibodies to BrdU and γ-H2AX. DNase-treated cells are included to show that the whole-cell population was labeled with BrdU. (D) Roscovitine inhibits the formation of ssDNA. U-S-OS cells prelabeled with BrdU were treated with UCN-01 (300 nM) for 3 h and stained with antibodies to BrdU and γ-H2AX and with the DNA stain Topro3. DNase-treated sample is included to show incorporation of BrdU.
Chk1 inhibition leads to DNA breakage.
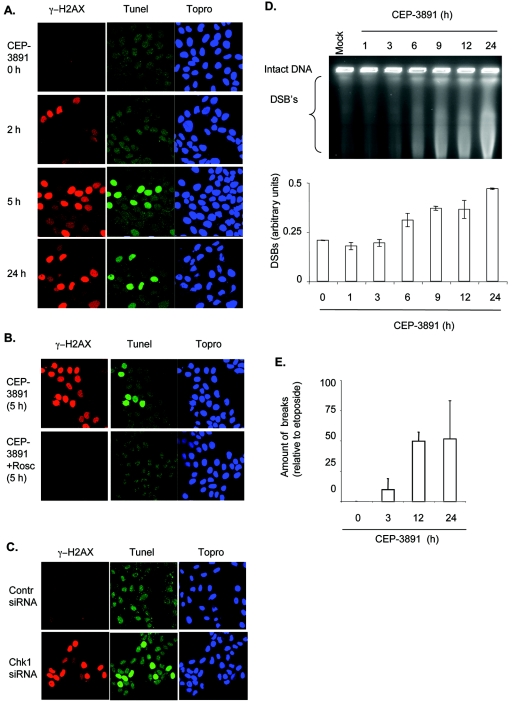
Appearance of γ-H2AX is often used as a marker for DSBs. In order to test whether γ-H2AX was associated with strand breaks after Chk1 inhibition, we first used the TUNEL assay, followed by staining for γ-H2AX with or without CEP-3891 treatment (Fig. 5A). Many of the cells with a strong γ-H2AX signal at 5 h after CEP-3891 treatment were positive for TUNEL staining, suggesting a massive induction of DNA breaks (Fig. 5A). The addition of roscovitine inhibited both the γ-H2AX and TUNEL staining, a finding consistent with the contribution of increased initiation of DNA replication in triggering these responses (Fig. 5B). The positive TUNEL staining was clearly not due to induction of apoptosis since caspase activity was not induced, and there were no signs of apoptotic nuclear morphology (32; data not shown). Similar effects were seen after depletion of Chk1 by siRNA (Fig. 5C). Consistent with the data obtained with CEP-3891, many, but not all, of the cells with a strong γ-H2AX signal after depletion of Chk1 with siRNA were positive for TUNEL staining (Fig. 5C). To verify the presence of DSBs, we also measured the induction of DSBs by the PFGE assay (Fig. 5D). By this assay, DSBs were detectable from around 6 h after addition of CEP-3891 (Fig. 5D). Finally, we used the alkaline elution assay to measure presence of single-strand breaks (and DSBs) (Fig. 5E). Again, the results clearly showed that DNA breaks were present after Chk1 inhibition (Fig. 5E), which was also confirmed by DNA alkaline unwinding (7) analyses (data not shown). Taken together, these results showed that Chk1 inhibition results in massive induction of DNA breaks in S-phase cells, likely as a consequence of aberrant events related to increased initiation of DNA replication.
FIG. 5.
Chk1 inhibition causes DNA breaks. (A) TUNEL staining is increased after Chk1 inhibition. U-2-OS cells were treated with CEP-3891 (500 nM) for 0, 2, 5, or 24 h, and processed for the TUNEL assay, followed by staining with the γ-H2AX antibody and the DNA-stain Topro3. (B) The formation of DNA breaks is inhibited by roscovitine. U-2-OS cells were treated with CEP-3891 (500 nM) for 5 h in the absence or presence of roscovitine (25 μM) and stained as described for panel A. (C) TUNEL staining after depletion of Chk1 with siRNA. U-2-OS cells were transfected with control or Chk1 siRNA (100 nM, 48 h) and stained as described for panel A. (D) Chk1 inhibition causes DSBs as measured by PFGE. U-2-OS cells were treated with CEP-3891 (500 nM) for the indicated times and processed for the PFGE assay. The results of a representative experiment are shown. (E) Chk1 inhibition causes DNA breaks as measured by the alkaline elution assay. U-2-OS cells were treated with CEP-3891 (500 nM) for the indicated times, and samples were analyzed by the alkaline elution assay. The amount of breaks in response to CEP-3891 is shown relative to the breaks after treatment with etoposide (3 μM, 1 h, 100% breaks). The results shown are the average from two independent experiments.
DISCUSSION
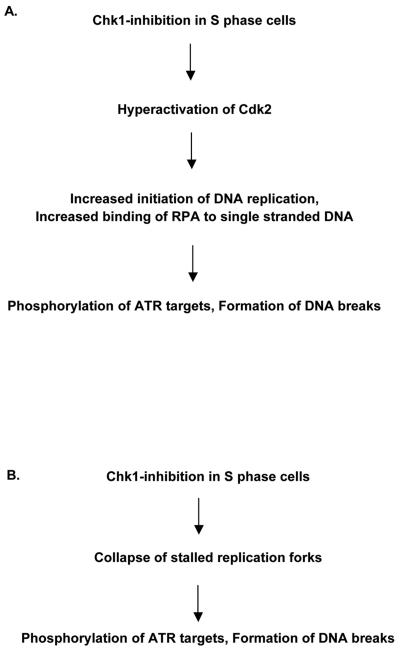
The results presented here show that Chk1 plays an important role during normal S-phase progression by minimizing the occurrence of aberrant replication-associated events. These results are consistent with a recent study where conditional Chk1 heterozygosity caused accumulation of DNA damage during DNA replication in mice (14). Our study shows that as a consequence of Chk1 inhibition in human S-phase cells, ATR is rapidly activated (within 1 h). This is likely due to stabilization of Cdc25A and the accompanying hyperactivation of Cdk2, resulting in increased loading onto chromatin of the key replication factor Cdc45, increased activation of replication origins, and subsequent increased association of RPA to ssDNA (Fig. 6A). As a further consequence of this process, the genome is destabilized, as evidenced by massive accumulation of DNA strand breaks. Based on our results we propose that Chk1-mediated control of the initiation of DNA synthesis is required for normal S-phase progression of human cells. These data are consistent with and mechanistically further extend the recent findings in Xenopus cell-free system demonstrating the critical involvement of ATR in preventing unscheduled replication (19, 27).
FIG. 6.
Proposed models for how Chk1 inhibition causes activation of ATR and massive DNA lesions in S-phase cells. (A) In response to Chk1 inhibition, Cdc25A is stabilized and the Cdk activity is rapidly increased, which leads to increased initiation of DNA replication (as measured here by increased loading of Cdc45 protein to chromatin and an increased rate of overall DNA synthesis). This is accompanied by an increased binding of RPA to ssDNA and subsequent phosphorylations of ATR targets. During this process, DNA is destabilized, leading to formation of DNA strand breaks. (B) In response to Chk1 inhibition, replication forks may collapse due to a lack of Chk1-mediated maintenance of stalled replication forks during normal S-phase progression. This may result in the formation of ssDNA and DNA breaks and in phosphorylations of ATR targets.
An alternative, but not mutually exclusive, possibility is that Chk1 inhibition may cause DNA breaks and activation of ATR due to lack of Chk1-mediated maintenance of stalled replication forks during normal S phase (Fig. 6B). Chk1, as well as ATR, are required to prevent collapse of stalled replication forks after treatment with replication inhibitors such as aphidicolin (3, 8). ATR also likely prevents replication fork collapse during normal S-phase progression, as ATR is required to avoid chromosome breaks in mitosis in the absence of exogenous treatment (3). It is possible that Chk1 is also needed to prevent fork collapse during normal S-phase progression and that this may contribute to the responses reported here. Although the pattern of BrdU incorporation appeared normal in cells with massive γ-H2AX after Chk1 inhibition, clearly demonstrating that a massive collapse of replication forks did not occur (Fig. 1C), we cannot exclude that the integrity of a small portion of replication forks was perturbed. However, taken into account our observations that Cdk2 and Cdc45 are required for the responses (Fig. 3), and that transient transfections with Cdk2AF or Cdc25A caused massive γ-H2AX (data not shown), the model outlined in Fig. 6A seems more plausible.
In response to Chk1 inhibition by drugs, measurements of both cyclin A-, E-, and B-associated Cdk activities showed an ca. two- to threefold transient and rapid increase, which occurs due to lack of Chk1-mediated phosphorylation of the Cdc25s (29, 32). The results of our present study clearly indicate that increased Cdk activity in response to Chk1 inhibition generates increased initiation of DNA replication, which ultimately leads to induction of DNA strand breaks. In this context, it is interesting that constitutive overexpression of cyclin E causes chromosomal instability by an unknown mechanism likely associated with defective DNA replication (23, 31). One possibility is that high levels of cyclin E may cause DNA damage and chromosomal instability due to increased initiation of DNA replication in a similar way as described here, although a recent study (6) proposed that cyclin E-mediated impairment of prereplication complex assembly in early G1 phase may be involved. On the other hand, more studies are required to explore whether increased Cdk activity is sufficient to trigger the whole cascade of responses that we have observed after Chk1 inhibition in S-phase cells. At the present stage we cannot exclude that Chk1 may target additional factors involved in control of replication initiation. For example, it was suggested that ATR-Chk1, together with Cdk2, may regulate phosphorylation of Mcm4 in a complex manner after treatment with hydroxyurea (12), and Chk1-mediated control of Mcm4 or other origin-binding proteins could likely be involved.
When cells treated with CEP-3891 or transfected with Chk1 siRNA were assayed simultaneously for γ-H2AX and DSB induction by the TUNEL assay, we consistently observed that only a subset of the γ-H2AX-positive cells were TUNEL positive (Fig. 5). These results indicate that the induction of massive DNA breaks is a late event likely occurring after the onset of massive phosphorylation of γ-H2AX. Consistent with previous studies (37, 45), the signal that first activates ATR in response to Chk1 inhibition may therefore be ssDNA rather than DSBs. However, we cannot exclude that a small number of DSBs not detectable by the TUNEL assay may be involved.
Interestingly, we observed significantly less phosphorylation of H2AX in response to Chk1 inhibition when Cdk2 was downregulated by conditionally expressed shRNA (Fig. 3D). In agreement with several recent studies reporting that Cdk2 is dispensable for cell cycle progression (22, 35), we have not observed any effects on cell cycle progression by depleting Cdk2 in U-2-OS cells in the absence of UCN-01 or CEP-3891 treatment (data not shown). Taken together, our results demonstrate for the first time that although Cdk2 may be dispensable for normal cell cycle progression, Cdk2 is not dispensable in the situation when cells are exposed to external stress such as Chk1-inhibitors.
It was previously suggested that Chk1 is essential for early embryonic viability due to its control of entry into mitosis (33). Defective Chk1 function was predicted to cause premature activation of cyclin B-Cdk1 and mitotic catastrophe (33). Our results demonstrating that Chk1 is required during normal S-phase progression to avoid replication-associated defects, together with the recent report where conditional Chk1 heterozygosity caused DNA damage during DNA replication in mice (14), add a new possible explanation of this phenomenon. We propose that lack of Chk1-mediated control of the initiation of DNA replication might contribute to lethality of Chk1−/− cells. It is plausible to assume that induction of lethal DNA breaks occurs during S phase of early embryonic cells from Chk1−/− mice through a similar mechanism to the one described here in human cells. On the other hand, Chk1 was previously shown to be dispensable for the viability of somatic cells derived from the chicken cell line DT40 under normal growth conditions in culture (39). However, such Chk1-deficient cells did proliferate significantly more slowly in the absence of any externally induced DNA damage or replication stress, which was attributed to an increase in the incidence of spontaneous cell death (39), and which could possibly be due to similar replication defects as we have described in the present study. Alternatively, these highly genetically unstable chicken cells may behave differently than the human cell lines used in our present study.
Due to its critical role in regulating the DNA damage-induced checkpoints, Chk1 has been proposed as a potential target for cancer treatment (reviewed in reference 42). The principle idea of this strategy is to combine standard chemo- or radiotherapy with drugs that inhibit Chk1 kinase in order to inhibit the S and G2 checkpoints. Cancer cells could likely be particularly sensitive to such treatment since they commonly lack normal G1 checkpoint control and may rely more on the S and G2 checkpoints compared to normal cells (5). The results presented here, showing that Chk1 inhibition per se leads to massive induction of DNA breaks in human S-phase cells, demonstrate that much remains to be understood in terms of the role of Chk1 kinase in the absence or presence of genotoxic agents. In particular, it will be important to determine to which extent these S-phase responses may vary between different cancer and normal cell types. Deeper insight into this issue will be important before pharmacological manipulation of Chk1 can be utilized in the clinic to enhance the efficacy of anticancer therapies.
Acknowledgments
This study was supported by Danish Medical Research Council, Alfred Benzon's Fond, Danish Cancer Society, European Union, Novo Nordic Foundation, and John and Birthe Meyer Foundation.
We thank Cephalon, Inc., for providing the CEP-3891 Chk1 inhibitor; Y. Shiloh for AT cells reconstituted with ATM; and Sanne Jul Christiansen for technical assistance.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Brown, E. J., and D. Baltimore. 2000. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14:397-402. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, E. J., and D. Baltimore. 2003. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17:615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Klein, A., M. Muijtjens, R. van Os, Y. Verhoeven, B. Smit, A. M. Carr, A. R. Lehmann, and J. H. Hoeijmakers. 2000. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10:479-482. [DOI] [PubMed] [Google Scholar]
- 5.Dixon, H., and C. J. Norbury. 2002. Therapeutic exploitation of checkpoint defects in cancer cells lacking p53 function. Cell Cycle 1:362-368. [DOI] [PubMed] [Google Scholar]
- 6.Ekholm-Reed, S., J. Mendez, D. Tedesco, A. Zetterberg, B. Stillman, and S. I. Reed. 2004. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J. Cell Biol. 165:789-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erixon, K., and G. Ahnstrom. 1979. Single-strand breaks in DNA during repair of UV-induced damage in normal human and xeroderma pigmentosum cells as determined by alkaline DNA unwinding and hydroxylapatite chromatography: effects of hydroxyurea, 5-fluorodeoxyuridine, and 1-β-d-arabinofuranosylcytosine on the kinetics of repair. Mutat. Res. 59:257-271. [DOI] [PubMed] [Google Scholar]
- 8.Feijoo, C., C. Hall-Jackson, R. Wu, D. Jenkins, J. Leitch, D. M. Gilbert, and C. Smythe. 2001. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furnari, B., N. Rhind, and P. Russell. 1997. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science 277:1495-1497. [DOI] [PubMed] [Google Scholar]
- 10.Guo, Z., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwell, L. H., and T. A. Weinert. 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246:629-634. [DOI] [PubMed] [Google Scholar]
- 12.Ishimi, Y., Y. Komamura-Kohno, H. J. Kwon, K. Yamada, and M. Nakanishi. 2003. Identification of MCM4 as a target of the DNA replication block checkpoint system. J. Biol. Chem. 278:24644-24650. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai, A., and W. G. Dunphy. 2000. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell 6:839-849. [DOI] [PubMed] [Google Scholar]
- 14.Lam, M. H., Q. Liu, S. J. Elledge, and J. M. Rosen. 2004. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell 6:45-59. [DOI] [PubMed] [Google Scholar]
- 15.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 16.Lundin, C., K. Erixon, C. Arnaudeau, N. Schultz, D. Jenssen, M. Meuth, and T. Helleday. 2002. Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Mol. Cell. Biol. 22:5869-5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mailand, N., J. Falck, C. Lukas, R. G. Syljuasen, M. Welcker, J. Bartek, and J. Lukas. 2000. Rapid destruction of human Cdc25A in response to DNA damage. Science 288:1425-1429. [DOI] [PubMed] [Google Scholar]
- 18.Mailand, N., A. V. Podtelejnikov, A. Groth, M. Mann, J. Bartek, and J. Lukas. 2002. Regulation of G2/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 21:5911-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marheineke, K., and O. Hyrien. 2004. Control of replication origin density and firing time in Xenopus egg extracts: role of a caffeine-sensitive, ATR-dependent checkpoint. J. Biol. Chem. 279:28071-28081. [DOI] [PubMed] [Google Scholar]
- 20.Masuda, T., S. Mimura, and H. Takisawa. 2003. CDK- and Cdc45-dependent priming of the MCM complex on chromatin during S-phase in Xenopus egg extracts: possible activation of MCM helicase by association with Cdc45. Genes Cells 8:145-161. [DOI] [PubMed] [Google Scholar]
- 21.Mendez, J., and B. Stillman. 2003. Perpetuating the double helix: molecular machines at eukaryotic DNA replication origins. Bioessays 25:1158-1167. [DOI] [PubMed] [Google Scholar]
- 22.Ortega, S., I. Prieto, J. Odajima, A. Martin, P. Dubus, R. Sotillo, J. L. Barbero, M. Malumbres, and M. Barbacid. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35:25-31. [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan, H., P. V. Jallepalli, C. Rago, V. E. Velculescu, K. W. Kinzler, B. Vogelstein, and C. Lengauer. 2004. Inactivation of hCDC4 can cause chromosomal instability. Nature 428:77-81. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez, Y., C. Wong, R. S. Thoma, R. Richman, Z. Wu, H. Piwnica-Worms, and S. J. Elledge. 1997. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497-1501. [DOI] [PubMed] [Google Scholar]
- 25.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 26.Sehested, M., I. Wessel, L. H. Jensen, B. Holm, R. S. Oliveri, S. Kenwrick, A. M. Creighton, J. L. Nitiss, and P. B. Jensen. 1998. Chinese hamster ovary cells resistant to the topoisomerase II catalytic inhibitor ICRF-159: a Tyr49Phe mutation confers high-level resistance to bisdioxopiperazines. Cancer Res. 58:1460-1468. [PubMed] [Google Scholar]
- 27.Shechter, D., V. Costanzo, and J. Gautier. 2004. ATR and ATM regulate the timing of DNA replication origin firing. Nat. Cell Biol. 6:648-655. [DOI] [PubMed] [Google Scholar]
- 28.Sogo, J. M., M. Lopes, and M. Foiani. 2002. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297:599-602. [DOI] [PubMed] [Google Scholar]
- 29.Sorensen, C. S., R. G. Syljuasen, J. Falck, T. Schroeder, L. Ronnstrand, K. K. Khanna, B. B. Zhou, J. Bartek, and J. Lukas. 2003. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3:247-258. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen, C. S., R. G. Syljuasen, J. Lukas, and J. Bartek. 2004. ATR, Claspin and the Rad9-Rad1-Hus1 complex regulate Chk1 and Cdc25A in the absence of DNA damage. Cell Cycle 3:941-945. [PubMed] [Google Scholar]
- 31.Spruck, C. H., K. A. Won, and S. I. Reed. 1999. Deregulated cyclin E induces chromosome instability. Nature 401:297-300. [DOI] [PubMed] [Google Scholar]
- 32.Syljuasen, R. G., C. S. Sorensen, J. Nylandsted, C. Lukas, J. Lukas, and J. Bartek. 2004.. Chk1 inhibition by CEP-3891 accelerates mitotic nuclear fragmentation in response to ionizing radiation (IR). Cancer Res. 64:9035-9040. [DOI] [PubMed] [Google Scholar]
- 33.Takai, H., K. Tominaga, N. Motoyama, Y. A. Minamishima, H. Nagahama, T. Tsukiyama, K. Ikeda, K. Nakayama, and M. Nakanishi. 2000. Aberrant cell cycle checkpoint function and early embryonic death in Chk1−/− mice. Genes Dev. 14:1439-1447. [PMC free article] [PubMed] [Google Scholar]
- 34.Tercero, J. A., K. Labib, and J. F. Diffley. 2000. DNA synthesis at individual replication forks requires the essential initiation factor Cdc45p. EMBO J. 19:2082-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tetsu, O., and F. McCormick. 2003. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 3:233-245. [DOI] [PubMed] [Google Scholar]
- 36.Tibbetts, R. S., K. M. Brumbaugh, J. M. Williams, J. N. Sarkaria, W. A. Cliby, S. Y. Shieh, Y. Taya, C. Prives, and R. T. Abraham. 1999. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter, J., and J. Newport. 2000. Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase alpha. Mol. Cell 5:617-627. [DOI] [PubMed] [Google Scholar]
- 38.Ward, I. M., and J. Chen. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759-47762. [DOI] [PubMed] [Google Scholar]
- 39.Zachos, G., M. D. Rainey, and D. A. Gillespie. 2003. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 22:713-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao, H., and H. Piwnica-Worms. 2001. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21:4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, H., J. L. Watkins, and H. Piwnica-Worms. 2002. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway inhibits ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. USA 99:14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou, B. B., and J. Bartek. 2004. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat. Rev. Cancer 4:216-225. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408:433-439. [DOI] [PubMed] [Google Scholar]
- 44.Ziv, Y., A. Bar-Shira, I. Pecker, P. Russell, T. J. Jorgensen, I. Tsarfati, and Y. Shiloh. 1997. Recombinant ATM protein complements the cellular A-T phenotype. Oncogene 15:159-167. [DOI] [PubMed] [Google Scholar]
- 45.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]
- 46.Zou, L., and B. Stillman. 1998. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science 280:593-596. [DOI] [PubMed] [Google Scholar]