Abstract
β-Cell apoptosis is a key event contributing to the pathogenesis of type 1 diabetes mellitus. In addition to apoptosis being the main mechanism by which β cells are destroyed, β-cell apoptosis has been implicated in the initiation of type 1 diabetes mellitus through antigen cross-presentation mechanisms that lead to β-cell-specific T-cell activation. Caspase-3 is the major effector caspase involved in apoptotic pathways. Despite evidence supporting the importance of β-cell apoptosis in the pathogenesis of type 1 diabetes, the specific role of caspase-3 in this process is unknown. Here, we show that Caspase-3 knockout (Casp3−/−) mice were protected from developing diabetes in a multiple-low-dose streptozotocin autoimmune diabetes model. Lymphocyte infiltration of the pancreatic islets was completely absent in Casp3−/− mice. To determine the role of caspase-3-dependent apoptosis in disease initiation, a defined antigen-T-cell receptor transgenic system, RIP-GP/P14 double-transgenic mice with Casp3 null mutation, was examined. β-cell antigen-specific T-cell activation and proliferation were observed only in the pancreatic draining lymph node of RIP-GP/P14/Casp3+/− mice, but not in mice lacking caspase-3. Together, our findings demonstrate that caspase-3-mediated β-cell apoptosis is a requisite step for T-cell priming, a key initiating event in type 1 diabetes.
Type 1 diabetes mellitus is an autoimmune disease whereby antigen-specific T-cells selectively destroy insulin-producing pancreatic β cells. The activated T cells first invade the islets, leading to insulitis. This is followed by destruction of the islets, mediated by a complex interaction between the activated lymphocytes, cytokines, and macrophages (28). Apoptosis is a fundamental process involved in the destruction of β cells (7, 28). More recently, β-cell apoptosis has also been shown to facilitate cross-presentation of islet antigens, an important initial step for priming or activation of β-cell-specific T cells required for disease onset (4, 39, 45, 56). β-cell-derived antigens are presented by antigen-presenting cells (APCs), such as dendritic cells in the local pancreatic draining lymph nodes (PDLN) (3, 9, 16). Although cross-presentation has classically been thought to induce T-cell tolerance (1, 22), recent evidence suggests that T-cell immunity may also occur, resulting in autoimmune diseases (4, 15, 30).
Caspases are evolutionarily conserved cysteine-aspartyl specific proteases that play a key role in apoptosis. In mammals, there are over 14 caspases, of which some are involved in apoptosis and others in cytokine activation (10, 44, 53). There are two main apoptotic pathways: the extrinsic or cell death receptor pathway involving the tumor necrosis factor superfamily of receptors and the intrinsic or mitochondrial pathway (46, 53). Caspase-8 and caspase-9 are the upstream caspases involved in the extrinsic and intrinsic pathways, respectively. Caspase-3, -6, and -7 are effector caspases downstream of both pathways. Gene targeting strategies have provided valuable tools to study the physiologic function of individual caspases in vivo and have shown their roles not only in apoptosis but also in other fundamental cellular processes. Caspase-3 and -9 have been shown to play a critical role in developmental neuronal apoptosis (14, 21, 54). Caspase-3 is required in Fas-mediated cell death in activated peripheral T cells and hepatocytes but not in immature T cells from the thymus (14, 21, 51, 54). In B lymphocytes, caspase-3 plays a negative regulatory role in cell cycle progression (52); in keratinocytes, caspase-3 is critical in cellular differentiation (35). Caspase-8 is required for cardiac development and T-cell homeostasis (41, 42, 49). Collectively, these studies highlight the tissue- and context-specific functions of individual caspases and their intricate regulation in all facets of cellular function.
Several in vitro studies have suggested that caspase-dependent apoptotic pathways are essential for β-cell apoptosis (12, 25, 26). Cultured human islet cells were shown to undergo apoptosis in response to elevated glucose concentrations by upregulating Fas, a receptor which activates the extrinsic apoptotic pathway (26). Furthermore, β-cell expression of c-Flip, a molecule known to inhibit caspase-8-mediated apoptosis, was shown to inhibit Fas-induced apoptosis and induce β-cell proliferation (25). In vivo studies of nonobese diabetic (NOD) mice and diabetes-prone BB/S rats also demonstrate presence of islet apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) (6, 23, 32). In 2003, Reddy et al. examined pancreatic islets of NOD mice during cyclophosphamide-accelerated diabetes, which showed caspase-3 immunoreactivity predominantly in intraislet macrophages but not islet cells, suggesting that apoptotic β cells are cleared rapidly after diabetes onset (37). Even though islet apoptosis has been shown to be an essential event in both type 1 and type 2 diabetes (6, 27, 32), the specific in vivo role of individual caspases in β-cell apoptosis and disease progression in diabetes models remains to be elucidated.
Here, we use a genetic approach to study the role of caspase-3 in β-cell apoptosis, using Caspase-3 knockout (Casp3−/−) mice in the multiple-low-dose streptozotocin (MLDS) autoimmune diabetes model. Streptozotocin (STZ) is a glucose analog that selectively destroys pancreatic β cells (11, 38). High-dose administration of STZ has been shown to directly cause DNA damage, resulting in massive necrosis of β cells (40). However, MLDS has been shown to cause selective β-cell destruction that in turn induces immune reactions against islets (24). Nude athymic mice do not develop diabetes with STZ treatment, suggesting that the STZ mechanism relies on T-cell functions to mediate its effects on islets (29).
Our results show that Casp3−/− mice are protected from developing diabetes and that their islets are resistant to apoptosis, following MLDS administration. Casp3+/− mice exhibit insulitis following MLDS, contributing to autoimmune diabetes initiation. In contrast, Casp3−/−mice do not develop insulitis. This key finding has led to examining the role of caspase-3 in the antigen cross-presentation pathway. For this purpose, we introduced a Casp3 null mutation into the RIP-GP/P14 double-transgenic model that expresses a defined β-cell antigen and T-cell receptor specific for this defined antigen. These mice express a glycoprotein from lymphocytic choriomeningitis virus (LCMV) under the control of the rat insulin promoter (RIP) and is specifically recognized by CD8 T cells (P14) in the islets (34, 36). Using these mice, we show that caspase-3 is required for inducing activation and proliferation of β-cell-specific T cells in the local PDLN. We show that caspase-3-dependent apoptosis is essential for initiating autoimmune diabetes by facilitating β-cell-antigen presentation for T-cell activation and invasion of the islets.
MATERIALS AND METHODS
Mice.
Generation of Casp3−/− mice and RIP-GP/P14 double-transgenic mice has been previously described (34, 36, 54). All the mice were backcrossed to C57BL/6 mice for at least 10 generations. Approximately half of Casp3−/− mice survive to adulthood in the C57BL/6 background and appear generally healthy. The RIP-GP/P14 double-transgenic mice were crossed to Casp3−/− mice to generate RIP-GP/P14/Casp3+/− mice. These mice were intercrossed to generate RIP-GP/P14/Casp3+/− and RIP-GP/P14/Casp3−/− mice. All genotyping was done using PCR protocols as previously described (34, 54). All mice were maintained in the pathogen-free animal colony at the Ontario Cancer Institute (Toronto, Canada) in accordance with protocols approved by the research institute.
Induction of diabetes and glucose monitoring.
The mice were injected intraperitoneally with STZ (40 mg of STZ/kg of body weight) for 5 consecutive days (40). Tail vein glucose was monitored using One Touch Ultra (Life Scan; Johnson & Johnson).
Histology.
Paraffin-embedded pancreatic tissue was stained with hematoxylin and eosin (H&E) and examined by light microscopy. To prepare specimen for TUNEL staining, paraffin sections were dewaxed and equilibrated in phosphate-buffered saline (PBS; pH 7.4), followed by incubation in 20 μg of proteinase K (Roche Biochemicals)/μl in 10 mM Tris-HCl (pH 7.5) for 15 min at 37°C. Following washing, the reaction mixture containing terminal deoxynucleotidyltransferase, labeled nucleotides, and DNA polymerase was then applied to sections in a humidified chamber for 90 min at 37°C, according to the manufacturer's instructions (Roche Biochemicals). Some sections were then washed in PBS and prepared for visualization with the fluorescent microscope. Anti-fluorescein antibody peroxidase conjugate was added to other sections for 30 min at 37°C. Sections were washed and incubated with 3,3-diaminobenzidine (Sigma) substrate and visualized by light microscopy.
Islet isolation.
To isolate the islets, 2 ml of 2-mg/ml collagenase (type IV; Sigma) was injected into the common bile duct for pancreatic digestion. The digested pancreas was removed and incubated at 37° for 20 to 28 min. The digest was washed twice by resuspension in Hank's balanced salt solution (HBSS) and centrifuged at 400 × g for 1 min at 4°C. The pellet was resuspended in HBSS and filtered through gauze. Islets were then handpicked in HBSS. Islets were maintained in suspension culture in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and incubated at 37°C and 5% CO2.
In vitro STZ treatment.
STZ was dissolved in 0.1 mM sodium citrate (pH 4.5) at 4°C and was added to each well of 96-well tissue culture plates containing 30 to 40 islets to obtain the required final STZ concentrations (0, 0.25, and 0.5 mM). Islet were incubated overnight (18 h) at 37°C in humidified air and 5% CO2.
Islet morphology and viability analysis of STZ-treated islets.
Islet morphology in culture was assessed with a Zeiss inverted microscope. To assess viability, STZ-treated islets were trypsinized to generate single-cell suspensions. For the annexin V-7-amino-actinomycin D (7-AAD) binding assay, cells were resuspended with annexin V binding buffer and incubated with 1 μl of annexin V/100 μl of binding buffer and 0.5 μm of 7-AAD for 15 min at room temperature in the dark. Cells were washed again with annexin V binding buffer, and the percentage of 7-AAD+ and annexin V+ cells was determined by flow cytometry with a FACSCalibur (Becton Dickinson).
Immunohistochemistry.
Pancreatic tissue was fixed overnight in a solution of freshly prepared 4% paraformaldehyde in 0.1 M PBS, pH 7.4, at 4°C. Samples were dehydrated and prepared as paraffin blocks and stained with H&E as mentioned above. For detection of insulin, glucagon, and CD3ɛ on sections, monoclonal anti-insulin, anti-glucagon, and anti-CD3ɛ antibodies (1:100; DAKO), respectively, were used. The appropriate primary antibody was added in blocking buffer and incubated overnight at 4°C. Sections were washed and incubated in biotinylated secondary antibody at 1:2,000 for 2 h at room temperature, followed by washing and incubation with avidin biotin complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, Calif.) at 1:100 for 1 h at room temperature. Sections were washed and incubated with 3,3-diaminobenzidine (Sigma).
Islet insulin and glucagon content.
Insulin and glucagon composition per islet were measured with Image-Pro Plus software (Media Cybernetics, Silver Spring, Md.). Slides immunostained for insulin and glucagon were scanned with a Super Coolscan 8000 ED (Nikon, Tokyo, Japan). Representative pancreas sections for each mouse genotype were chosen for analysis. The Image-Pro Plus system was used to quantify the insulin- and glucagon-stained area and the total islet area to determine the percentage of islets stained for insulin and glucagon, respectively.
T-cell activation analyses.
Lymph nodes were isolated from RIP-GP/P14/Casp3+/− and RIP-GP/P14/Casp3−/− mice 4 days post-MLDS administration. Isolated PDLNs and pooled nondraining lymph nodes (NDLNs) (inguinal and axillary) were prepared into single-cell suspensions. Lymphocytes were stained with antibodies recognizing CD8α and CD69 (BD PharMingen). Detection of biotin-conjugated antibodies was performed using streptavidin-conjugated red 670 (GIBCO BRL). Live events were collected based on forward and side scatter profiles on a FACScan flow cytometer (Becton Dickinson) and analyzed using CELLQuest software (Becton Dickinson). The percentage of CD69-positive cells was determined by gating on the CD8+ cells.
Bone marrow chimeras.
Casp3+/+, Casp3+/−, and Casp3−/− donor mice were intravenously injected with CD4+ and CD8+ T-cell-depleting antibodies 3 days and 1 day prior to bone marrow transfer. Recipient mice received 9 Gy of γ irradiation just prior to bone marrow transfer. Bone marrow from donor mice was harvested from femurs and washed in HBSS. A total of 107 bone marrow cells in 200 μl of HBSS were transferred intravenously into sex-matched recipients. Eight weeks later, peripheral blood was stained for Thy 1.1 (donor) and Thy 1.2 (recipient) to determine bone marrow reconstitution.
Peptide gp33 immunization.
Mice were immunized with an intravenous administration of 10 μg of the LCMV glycoprotein peptide p33 (KAVYNFATM) in HBSS (36).
Adoptive transfer of CFSE-labeled P14 TCR transgenic cells.
Single-cell suspensions of spleen cells from P14 TCR transgenic mice were prepared, and CD8+ T cells were purified using anti-CD8α-coated beads (Miltenyi Biotech) and a magnetic activated cell sorter (Miltenyi Biotech). The purified CD8+ T cells were then labeled with 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE), washed in serum-free RPMI 1640 (GIBCO BRL), and resuspended in serum-free medium at 106 cells per 200 μl containing 5 μM CFSE (Molecular Probes). The purified CD8+ T cells were incubated for 10 min at 37°C in an atmosphere containing 5% CO2 and then washed in RPMI 1640 containing 10% fetal calf serum (Sigma-Aldrich). A total of 5 × 106 CD8-purified, CFSE-labeled cells were then transferred intravenously into nonirradiated RIP-GP/Casp3+/− and RIP-GP/Casp3−/− mice 48 h post-MLDS. Recipients were sacrificed, and PDLNs and NDLNs were harvested and stained with an antibody recognizing Vα2 (BD PharMingen) for fluorescence-activated cell sorter analysis. The lymphocytes from PDLNs and NDLNs were gated on Vα2+ lymphocytes, and CFSE profiles were determined.
RESULTS
Casp3−/− mice were protected from MLDS-induced diabetes.
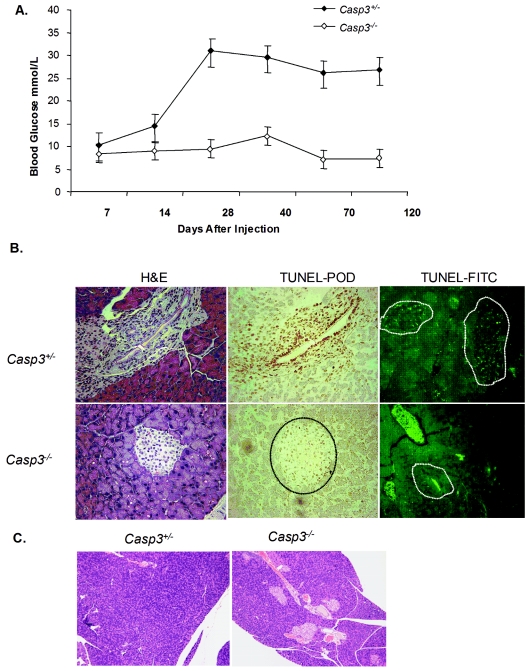
To examine the role of caspase-3 in the MLDS-diabetes model, Casp3+/− and Casp3−/− mice were injected with STZ as described in Materials and Methods, and tail vein glucose levels were monitored weekly for up to 120 days. By day 14 post-STZ treatment, average blood glucose levels of Casp3+/− mice reached 15 mM and continued to increase, whereas Casp3−/− mice remained normoglycemic throughout the study period (Fig. 1A). H&E staining and matched TUNEL labeling of pancreas specimens at 14 days revealed TUNEL-positive cells in the islets of Casp3+/− mice but not Casp3−/− mice (Fig. 1B). At 120 days postinjection, there were no detectable islets present in the Casp3+/− pancreas samples, whereas the islets were normal in number and appearance in the Casp3−/− mice (Fig. 1C).
FIG. 1.
Casp3−/− mice are protected from MLDS-induced diabetes. (A) Fourteen Casp3+/− mice and 12 Casp3−/− mice were injected with MLDS, and tail vein glucose was monitored weekly. (B) Matched H&E-stained (left) and TUNEL-peroxidase-stained (right) pancreatic sections (magnification, ×32) 14 days post-MLDS (three random sections were examined per mouse, with four mice per group). The dashed lines depicts the islet areas that correspond to H&E staining. (C) H&E-stained islets 120 days post-MLDS (magnification, ×8).
Hormone content in the islets of Casp3+/− and Casp3−/− islets post-MLDS diabetes.
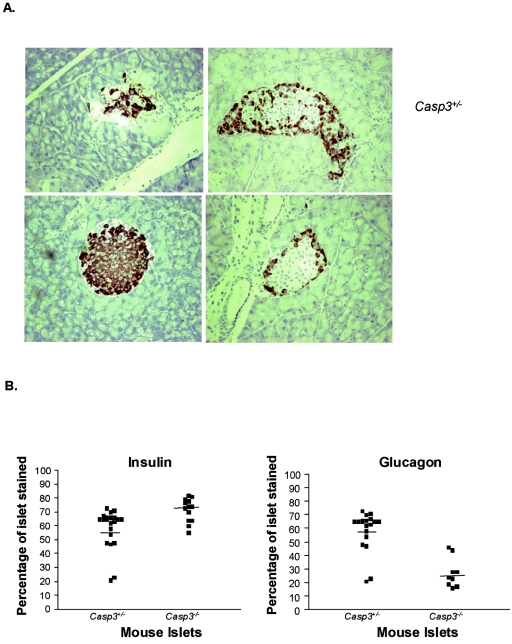
To further assess the effect of STZ, immunohistochemical analyses for insulin and glucagon were performed on pancreatic sections. As expected, the majority of islets from Casp3−/− pancreases isolated 14 days post-MLDS stained positive for insulin (Fig. 2A). Furthermore, the glucagon-positive α cells were confined to the rim of the islets, suggesting that the architecture of Casp3−/− islets was normal. In contrast, islet cells from Casp3+/− mice were architecturally distorted, containing significantly fewer insulin-positive cells and proportionally more glucagon-positive cells (Fig. 2A). Quantitative image analysis was used to assess the proportion of insulin and glucagons positive cells per islet in Casp3+/− and Casp3−/− pancreatic sections. The percentage of Casp3+/− and Casp3−/− islets stained for insulin were 58.48% ± 3.10% and 71.62% ± 2.35% (P = 0.002), respectively, and 58.63% ± 3.36% and 27.22% ± 3.65% (P = 0.001), respectively, for glucagon (Fig. 2B). Together, these findings further support that Casp3−/− islets are protected from destruction.
FIG. 2.
Hormone staining of pancreases post-MLDS. (A) At 14 days post-MLDS, pancreases were immunohistochemically stained for insulin (left) and glucagon (right) (magnification, ×16). (B) The percentage of insulin- and glucagon-positive cells per islet was significantly different between Casp3+/− and Casp3−/− mice (insulin levels, P = 0.002; glucagon levels, P = 0.001). Each black square represents an islet of Casp3+/− or Casp3−/− mice, as indicated. The results are representative examples of experimental groups containing four mice per genotype.
Absence of insulitis in islets of Casp3−/−mice.
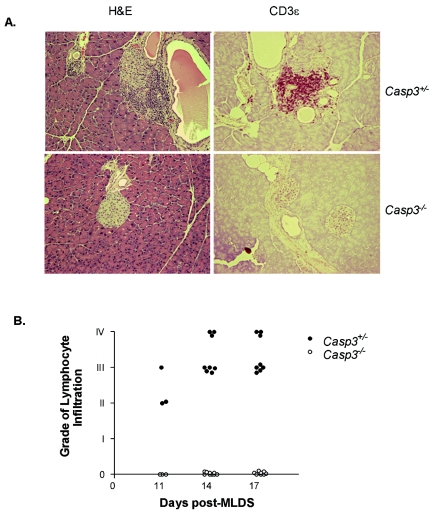
Pancreases of Casp3+/− mice isolated 14 days post-MLDS administration showed the presence of T-cell infiltration by H&E staining and CD3ɛ staining, in keeping with MLDS leading to autoimmune destruction of the islets (Fig. 3A). Intriguingly, no lymphocyte infiltration was detected in islets of Casp3−/− mice at any of the time points examined (11, 14, 17, and 120 days post-MLDS). In Casp3+/− mice, the mean percentage of islets that displayed infiltration per pancreas was 45%. The severity of lymphocyte infiltration in the islets of Casp3+/− mice was scored by the following grade: grade 0, normal; grade I, periductal lymphocyte infiltrate; grade II, peri-insulitis; grade III, insulitis (lymphocytic infiltrate invading islets); and grade IV, severe insulitis (massive lymphocytic infiltrate with islet destruction) (8). Most of the islets showing infiltration were high grade, between grades III and IV (Fig. 3B).
FIG. 3.
Lymphocyte infiltration in the islets post-MLDS. (A) H&E (left) and anti-CD3ɛ (right); staining magnification, ×20. Absence of lymphocyte infiltration in the islets of Casp3−/− mice (bottom), and presence of lymphocyte infiltration in the islets of Casp3+/− mice (top). (B) Severity of lymphocyte infiltration, indicated by grade, at days 11, 14, and 17 post-MLDS of Casp3+/− islets (closed circles) and Casp3−/− islets (open circles). Grade 0, normal; grade I, periductal lymphocyte infiltrate; grade II, peri-insulitis; grade III, insulitis (lymphocytic infiltrate invading islets); and grade IV, severe insulitis (massive lymphocytic infiltrate with islet destruction). Data are representative of experimental groups consisting of four mice per group.
In vitro analysis of STZ-induced islet apoptosis.
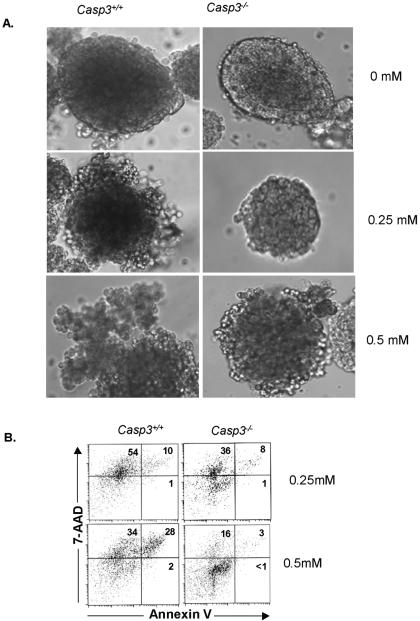
To directly assess the protective effect of caspase-3's absence in STZ-induced islet apoptosis, islets were isolated and tested in vitro. Pancreatic islet cells were isolated from Casp3+/+ and Casp3−/− mice and cultured overnight with or without STZ. Islet cultures incubated with no STZ showed no change in islet morphology. Casp3+/+ islets cultured in 0.25 mM STZ showed distorted architecture. At 0.5 mM STZ, Casp3+/+ islets were completely disintegrated into single cells. In contrast, Casp3−/− islet morphology remained intact at both concentrations of STZ and showed no evidence of single-cell disintegration (Fig. 4A). To examine islet viability, the annexin V-7-AAD binding assay was done. Annexin V has high affinity for phosphatidylserine, which is externalized in the earlier stages of apoptosis and is one of the key features of apoptosis; 7-AAD is a DNA intercalator. Analysis of Casp3+/+ and Casp3−/− islets cultured in 0.25 mM STZ showed no significant difference in the percentage of annexin V+ cells (similar to profiles of islets in culture with no STZ) (data not shown). However, there was a significantly greater percentage of annexin V+ cells in Casp3+/+ islets (28%) than in Casp3−/− islets (3%) at 0.5 mM STZ (Fig. 4B). These data show that the absence of caspase-3 in the islets gives direct protection from STZ-induced apoptosis.
FIG. 4.
Casp3−/− islets are protected from STZ treatment in vitro. (A) Photomicrographs of Casp3+/+ islets and Casp3−/− islets, following overnight culture with STZ. Casp3−/− islets show reduced disruption of islet integrity following STZ treatment compared to Casp3+/+ islets (magnification, ×8). (B) Annexin V-7-AAD profiles of Casp3+/+ and Casp3−/− islets at 0.25 and 0.5 mM STZ.
Protection from lymphocyte infiltration was due to the absence of caspase-3 in the islets and not in the lymphocytes.
Since the Casp3−/− mice used here have caspase-3 deleted in all tissues, it was not clear whether the protection from insulitis in Casp3−/− mice following MLDS was due to caspase-3 being absent in the β cells or in the lymphocytes. To address this issue, we performed bone marrow chimeric experiments. Casp3+/+, Casp3+/−, and Casp3−/− lymphocytes were used as donors and reciprocally as recipients. Thy 1.1 and Thy 1.2 lymphocyte surface markers were used to distinguish between the donor and recipient cells. Casp3+/+ donor mice were positive for the lymphocyte marker Thy 1.1, whereas recipient mice were Thy 1.2 positive. In all irradiated mice examined for immune reconstitution, the recipient mice were reconstituted with donor cells (data not shown).
Casp3+/+ and Casp3+/− mice reconstituted with Casp3+/+, Casp3+/−, or Casp3−/− mouse bone marrow developed diabetes with high blood glucose levels 2 weeks post-MLDS and displayed lymphocyte infiltration in their islets in a time frame and severity similar to those in the original experiments performed with Casp3+/− mice (Fig. 3A; Table 1). In contrast, Casp3−/− mice reconstituted with either Casp3+/+ or Casp3−/− bone marrow were protected from diabetes development. These mice did not show evidence of any insulitis, much like Casp3−/− mice without reconstitution as described above.
TABLE 1.
Percentage of islets displaying lymphocyte infiltration in bone marrow chimeras 14 days post-MLDSa
| Donor bone marrow | Recipient mice | Mean percentage of islets infiltrated |
|---|---|---|
| Casp3+/+ | Casp3+/− | 41 |
| Casp3+/+ | Casp3−/− | 0 |
| Casp3−/− | Casp3+/+ | 38 |
| Casp3+/− | Casp3+/+ | 36 |
Results represent mean percentages (mean number of islets, 13 per section; three sections per mouse, with three to six mice per experimental group).
Together, these experiments suggest that the protection from diabetes development and lymphocyte infiltration resulted from the absence of caspase-3 in islets and not in lymphocytes.
Caspase-3 is required for cross-priming of β-cell-specific T cells in PDLNs in the MLDS diabetes model.
The lack of islet infiltration in caspase-3 null islets led us to hypothesize that caspase-3-dependent islet apoptosis may be important for priming β-cell-specific T cells. To this end, the Casp3 null mutation was introduced into the RIP-GP/P14 transgenic system to generate RIP-GP/P14/Casp3+/− and RIP-GP/P14/Casp3−/− mice, which enabled us to examine the effect of caspase-3 on islet apoptosis and subsequent cross-presentation of a nominal β-cell-specific antigen to antigen-specific T cells.
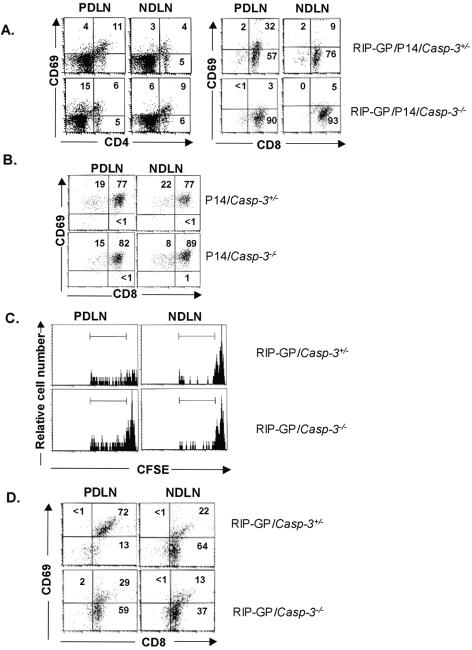
MLDS in the RIP-GP/P14/Casp3+/− and RIP-GP/P14/Casp3−/− mice led to a rise in blood glucose levels similar to that in Casp3+/− mice and Casp3−/− mice, respectively. Analysis of PDLNs isolated from MLDS-treated RIP-GP/P14/Casp3+/− mice showed that 32% of CD8+ T cells were CD69hi, compared to 9% in NDLNs (Fig. 5A). In contrast, for CD8+ T cells isolated from RIP-GP/P14/Casp3−/− PDLNs, only 3% were CD69hi, similar to that of NDLNs (5%) (Fig. 5A). There was no difference in the percentage of CD69hi CD4+ T cells isolated from either RIP-GP/P14/Casp3+/− or RIP-GP/P14/Casp3−/− mice in both the PDLNs and NDLNs, since CD4+ T cells do not recognize the predominant transgenic antigen present in this mouse model.
FIG. 5.
In vivo T-cell activation and proliferation. (A) T-cell activation after MLDS. CD69 profiles of lymphocytes from PDLNs (left) and NDLNs (right) are shown for RIP-GP/P14/Casp3+/− mice (top) and RIP-GP/P14/Casp3−/− mice (bottom). Numbers in quadrants represent the percentages of Vα2+ gated cells. Data are representative of three independent experiments, using five RIP-GP/P14/Casp+/− mice and four RIP-GP/P14/Casp3−/− mice. (B) T-cell activation after immunization with peptide. CD69 profiles of lymphocytes from PDLNs (left) and NDLNs (right) are shown for P14/Casp3+/− mice (top) and P14/Casp3−/− mice (bottom). Numbers in quadrants represent the percentages of Vα2+ gated cells. (C) In vivo proliferation after MLDS. Four days after adoptive transfer of CFSE-labeled T cells into MLDS-treated mice, fluorescence was measured by gating on Vα2+ lymphocytes. Less fluorescence is seen in lymphocytes isolated from RIP-GP/Casp3+/− PDLNs (top left) than in those isolated from RIP-GP/Casp3−/− PDLNs (bottom left). There was no difference in CFSE fluorescence between lymphocytes isolated from NDLNs of RIP-GP/Casp3+/− mice (top right) and RIP-GP/Casp3−/− mice (bottom right). (D) T-cell activation of adoptively transferred T cells after MLDS. CD69 profiles of T cells from PDLNs (left) and NDLNs (right) were determined for RIP-GP/Casp3+/− and RIP-GP/Casp3−/− mice adoptively transferred with CFSE-labeled P14 T cells. The results are representative of experimental groups containing five RIP-GP/Casp3+/− mice and four RIP-GP/Casp3−/− mice.
To show that caspase-3-deficient mice do not display any intrinsic defect in priming of T cells, mice were immunized with peptide gp33, the major epitope on LCMV glycoprotein that is recognized by P14 T cells in the context of H-2Db (36). Analysis of PDLNs isolated from P14/Casp3+/− mice and P14/Casp3−/− mice 24 h after immunization showed no significant difference in the percentage of activated CD8+ T cells as assessed by the presence of the activation marker CD69 (Fig. 5B).
These results show that caspase-3 is required for cross-presentation of β-cell antigen leading to antigen-specific T-cell activation in the local draining lymph nodes. Importantly, the lack of T-cell activation in the caspase-3-deficient mice is not due to an intrinsic defect of the T cells but rather to the absence of caspase-3-dependent islet apoptosis and cross-presentation in the PDLNs.
In vivo proliferation of antigen-specific T cells.
To further characterize the proliferative response of β-cell-specific T cells in the PDLNs subsequent to MLDS, in vivo, we adoptively transferred CFSE-labeled P14 transgenic T cells into RIP-GP/Casp3+/− and RIP-GP/Casp3−/− mice 2 days after the last injection of the MLDS regimen. CFSE labeling allows tracking of subsequent cellular divisions in vivo. Four days after the adoptive transfer, mice were sacrificed, and lymphocytes from PDLNs and NDLNs were isolated. Since all adoptively transferred lymphocytes that have caspase-3 present are P14 cells, these adoptive transfer experiments further supported the importance of caspase-3 in the islets, as opposed to the potential effect of caspase-3 in the lymphocytes. We observed that PDLNs of RIP-GP/Casp3+/− mice contained primarily CFSE-low cells, suggesting that the majority of adoptively transferred P14 T cells had undergone cell division (62%). In contrast, PDLNs of RIP-GP/Casp3−/− mice still maintained a CFSE-high peak, reflecting fewer cells that have undergone proliferation (45%) (Fig. 5B). Control NDLNs of both RIP-GP/Casp3+/− and RIP-GP/Casp3−/− mice contained CFSE-high cells, reflecting a lack of cell division, consistent with the absence of cross-presented antigens in these lymph nodes. In addition to the CFSE analysis, the same adoptively transferred CD8+P14 T cells were analyzed for their state of activation. In keeping with the higher levels of proliferation found in the PDLN of RIP-GP/Casp3+/− mice, 72% of the CD8+P14 T cells were CD69hi, in contrast to 29% of the adoptively transferred CD8+P14 T cells which were CD69hi in PDLNs of RIP-GP/Casp3−/− mice (Fig. 5C). These results show that caspase-3-dependent β-cell apoptosis is required for activation of antigen-specific T cells and initiation of autoimmune diabetes.
DISCUSSION
Much evidence shows that β-cell apoptosis is a fundamental process involved in the pathogenesis of type 1 diabetes (6, 27, 32). Despite this notion, a genetic approach to examine the mechanisms of β-cell apoptosis using specific caspase knockout mice has not been done to date. A knockout approach serves as a useful tool to study the mechanisms of apoptosis in vivo. Indeed, many of the caspase knockout mice have shed new insight into the biological functions of many caspases (14, 21, 41, 42, 49, 54). In this study, we provide evidence, using a genetic approach, that caspase-3-dependent β-cell apoptosis is essential for facilitating cross-presentation of β-cell antigen to activate β-cell-specific T cells. We show that mice lacking caspase-3 do not develop autoimmune diabetes, characterized by normoglycemia, intact β cells displaying high insulin content, and absence of β-cell specific T-cell activation in the PDLNs.
Although cross-presentation of autoantigens has generally been thought to induce T-cell tolerance (1, 19, 22), immunity has also been shown to occur in some experimental models (30, 31, 55). Activation of antigen-specific T cells is thought to be promoted through interactions with activated, mature APCs. We show that the caspase-3-dependent β-cell apoptosis provides a source of antigen for cross-presentation and activation of β-cell-specific T cells. Consistent with our results, recent studies demonstrate that apoptotic tumor cells can cross-prime host tumor-specific CD8+ T cells (30, 31, 55). In this way, the significant degree of cell death that occurs in proliferating tumors provides appropriate signals to APCs to promote immunity. In addition, using MLDS in NOD mice expressing transgenic TCRs, Zhang and colleagues showed the STZ-induced β-cell damage can enhance priming of β-cell-specific T cells (56). These studies further support that exaggerated β-cell damage can induce activation of β-cell-specific T cells. In addition, NOD mice exhibit a defect in the clearance of apoptotic β cells (33). Therefore, the apoptotic cells may be a critical determinant contributing to the initiation of autoimmunity by having the capacity to instruct APCs to modulate immune responses so that the outcome is T-cell activation (2, 43).
When would such priming occur in type 1 diabetes? Theoretically, any destruction causing antigen to be shed and presented by nearby APCs may provide an opportunity for β-cell-specific T cells to become activated in predisposed individuals. For example, viruses, toxins, and abnormal physiological tissue turnover have been shown to be possible initiators for diabetes development. Previous studies suggest that virus-mediated destruction of islets can trigger autoimmune diabetes (18). For example, coxsackie virus infection has been associated with autoimmune diabetes in both humans and animal models (20). In keeping with our results, it has been shown that virus-mediated bystander tissue damage can induce immune activation against β cells, much like STZ-induced autoimmune destruction of the islets (5, 17, 18, 20).
Another opportunity where β-cell-specific T cells may encounter excess antigen is during the neonatal period. β cells were once thought to be terminally differentiated adynamic cells. However, mathematical models and enhanced technology have shown that β cells are dynamic throughout the life of the organism (13, 47). In particular, during the neonatal period there is enhanced remodeling associated with β-cell apoptosis. In autoimmune-prone mice such as NOD mice, β-cell death is exaggerated compared to mice that are not prone to spontaneous diabetes (47). It has been speculated that this period may be an opportunity where β-cell antigen is cross-presented to initiate autoimmune diabetes. Consistent with this hypothesis, studies of spontaneously occurring diabetes models such as BDC2.5 transgenic mice and 8.3 NOD mice have shown β-cell-specific T-cell activation in the PDLNs occurring in a synchronous manner following the neonatal remodeling period (16, 50, 56). This event precedes insulitis and diabetes development in these animals. Recently, using the BDC2.5 transgenic model, Turley et al. (2003) showed that the enhanced wave of neonatal β-cell death precipitates the activation of β-cell-specific T cells in the PDLNs (48).
Together, our genetic models show the importance of caspase-3-dependent β-cell apoptosis in initiating T-cell priming, a prerequisite event in the pathogenesis of type 1 diabetes. Understanding the initiating events in diabetes induction may lead to novel therapeutic interventions for the prevention of diabetes and may have implications in enhancing islet survival in islet transplantation. Therapeutically targeting caspase-3 activity in β cells may be relevant for preventing the initiation of autoimmune diabetes.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research, Banting and Best Diabetes Centre and Bickell's Foundation. N.L. is supported by the Canadian Institutes of Health Research/Canadian Diabetes Association and Banting and Best Diabetes Centre studentships. This work was also funded by the NCIC with funds from the Canadian Cancer Society to P.S.O. M.W. is a recipient of the CHIR New Investigator Award and a BBDC Denis Scholar.
We thank Kelvin So and Michelle Sleiman for their technical assistance. We also thank Laurent Sabbagh, Jacinth Abraham, Mark Cattral, Kinh Tung Nguyen, and Paul Doherty for critical review of the manuscript.
REFERENCES
- 1.Adler, A. J., D. W. Marsh, G. S. Yochum, J. L. Guzzo, A. Nigam, W. G. Nelson, and D. M. Pardoll. 1998. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 187:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert, M. L. 2004. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 4:223-231. [DOI] [PubMed] [Google Scholar]
- 3.Albert, M. L., M. Jegathesan, and R. B. Darnell. 2001. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2:1010-1017. [DOI] [PubMed] [Google Scholar]
- 4.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs.Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 5.Andreoletti, L., D. Hober, C. Hober-Vandenberghe, S. Belaich, M. C. Vantyghem, J. Lefebvre, and P. Wattre. 1997. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J. Med. Virol. 52:121-127. [DOI] [PubMed] [Google Scholar]
- 6.Augstein, P., A. G. Elefanty, J. Allison, and L. C. Harrison. 1998. Apoptosis and beta-cell destruction in pancreatic islets of NOD mice with spontaneous and cyclophosphamide-accelerated diabetes. Diabetologia 41:1381-1388. [DOI] [PubMed] [Google Scholar]
- 7.Bach, J. F., L. Chatenoud, A. Herbelin, J. M. Gombert, and C. Carnaud. 1997. Autoimmune diabetes: how many steps for one disease? Res. Immunol. 148:332-338. [DOI] [PubMed] [Google Scholar]
- 8.Baik, S. H., I. B. Park, K. M. Choi, Y. H. Kim, N. H. Kim, S. J. Kim, G. G. Song, and D. S. Choi. 1999. BCG vaccine prevents insulitis in low dose streptozotocin-induced diabetic mice. Diabetes Res. Clin. Pract. 46:91-97. [DOI] [PubMed] [Google Scholar]
- 9.Carbone, F. R., C. Kurts, S. R. Bennett, J. F. Miller, and W. R. Heath. 1998. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol. Today 19:368-373. [DOI] [PubMed] [Google Scholar]
- 10.Creagh, E. M., H. Conroy, and S. J. Martin. 2003. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 193:10-21. [DOI] [PubMed] [Google Scholar]
- 11.Elsner, M., B. Guldbakke, M. Tiedge, R. Munday, and S. Lenzen. 2000. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia 43:1528-1533. [DOI] [PubMed] [Google Scholar]
- 12.Federici, M., M. Hribal, L. Perego, M. Ranalli, Z. Caradonna, C. Perego, L. Usellini, R. Nano, P. Bonini, F. Bertuzzi, L. N. Marlier, A. M. Davalli, O. Carandente, A. E. Pontiroli, G. Melino, P. Marchetti, R. Lauro, G. Sesti, and F. Folli. 2001. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes 50:1290-1301. [DOI] [PubMed] [Google Scholar]
- 13.Finegood, D. T., L. Scaglia, and S. Bonner-Weir. 1995. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44:249-256. [DOI] [PubMed] [Google Scholar]
- 14.Hakem, R., A. Hakem, G. S. Duncan, J. T. Henderson, M. Woo, M. S. Soengas, A. Elia, J. L. de la Pompa, D. Kagi, W. Khoo, J. Potter, R. Yoshida, S. A. Kaufman, S. W. Lowe, J. M. Penninger, and T. W. Mak. 1998. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339-352. [DOI] [PubMed] [Google Scholar]
- 15.Heath, W. R., and F. R. Carbone. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47-64. [DOI] [PubMed] [Google Scholar]
- 16.Hoglund, P., J. Mintern, C. Waltzinger, W. Heath, C. Benoist, and D. Mathis. 1999. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 189:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz, M. S., L. M. Bradley, J. Harbertson, T. Krahl, J. Lee, and N. Sarvetnick. 1998. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4:781-785. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz, M. S., A. Ilic, C. Fine, E. Rodriguez, and N. Sarvetnick. 2002. Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J. Clin. Investig. 109:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugues, S., E. Mougneau, W. Ferlin, D. Jeske, P. Hofman, D. Homann, L. Beaudoin, C. Schrike, M. Von Herrath, A. Lehuen, and N. Glaichenhaus. 2002. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity 16:169-181. [DOI] [PubMed] [Google Scholar]
- 20.Jun, H. S., and J. W. Yoon. 2001. The role of viruses in type I diabetes: two distinct cellular and molecular pathogenic mechanisms of virus-induced diabetes in animals. Diabetologia 44:271-285. [DOI] [PubMed] [Google Scholar]
- 21.Kuida, K., T. S. Zheng, S. Na, C. Kuan, D. Yang, H. Karasuyama, P. Rakic, and R. A. Flavell. 1996. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368-372. [DOI] [PubMed] [Google Scholar]
- 22.Kurts, C., H. Kosaka, F. R. Carbone, J. F. Miller, and W. R. Heath. 1997. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J. Exp. Med. 186:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lally, F. J., H. Ratcliff, and A. J. Bone. 2001. Apoptosis and disease progression in the spontaneously diabetic BB/S rat. Diabetologia 44:320-324. [DOI] [PubMed] [Google Scholar]
- 24.Like, A. A., and A. A. Rossini. 1976. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 193:415-417. [DOI] [PubMed] [Google Scholar]
- 25.Maedler, K., A. Fontana, F. Ris, P. Sergeev, C. Toso, J. Oberholzer, R. Lehmann, F. Bachmann, A. Tasinato, G. A. Spinas, P. A. Halban, and M. Y. Donath. 2002. FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc. Natl. Acad. Sci. USA 99:8236-8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maedler, K., G. A. Spinas, R. Lehmann, P. Sergeev, M. Weber, A. Fontana, N. Kaiser, and M. Y. Donath. 2001. Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 50:1683-1690. [DOI] [PubMed] [Google Scholar]
- 27.Mandrup-Poulsen, T. 2001. β-Cell apoptosis: stimuli and signaling. Diabetes. 50:S58-S63. [DOI] [PubMed] [Google Scholar]
- 28.Mathis, D., L. Vence, and C. Benoist. 2001. β-Cell death during progression to diabetes. Nature 414:792-798. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura, M., S. Nagafuchi, K. Yamaguchi, and R. Takaki. 1984. The role of thymic immunity and insulitis in the development of streptozocin-induced diabetes in mice. Diabetes 33:894-900. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen, L. T., A. R. Elford, K. Murakami, K. M. Garza, S. P. Schoenberger, B. Odermatt, D. E. Speiser, and P. S. Ohashi. 2002. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J. Exp. Med. 195:423-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak, A. K., R. A. Lake, A. L. Marzo, B. Scott, W. R. Heath, E. J. Collins, J. A. Frelinger, and B. W. Robinson. 2003. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 170:4905-4913. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien, B. A., B. V. Harmon, D. P. Cameron, and D. J. Allan. 1997. Apoptosis is the mode of beta-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes 46:750-757. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien, B. A., Y. Huang, X. Geng, J. P. Dutz, and D. T. Finegood. 2002. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes 51:2481-2488. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi, P. S., S. Oehen, K. Buerki, H. Pircher, C. T. Ohashi, B. Odermatt, B. Malissen, R. M. Zinkernagel, and H. Hengartner. 1991. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65:305-317. [DOI] [PubMed] [Google Scholar]
- 35.Okuyama, R., B. C. Nguyen, C. Talora, E. Ogawa, A. Tommasi di Vignano, M. Lioumi, G. Chiorino, H. Tagami, M. Woo, and G. P. Dotto. 2004. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev. Cell 6:551-562. [DOI] [PubMed] [Google Scholar]
- 36.Pircher, H., K. Burki, R. Lang, H. Hengartner, and R. M. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342:559-561. [DOI] [PubMed] [Google Scholar]
- 37.Reddy, S., J. Bradley, S. Ginn, P. Pathipati, and J. M. Ross. 2003. Immunohistochemical study of caspase-3-expressing cells within the pancreas of non-obese diabetic mice during cyclophosphamide-accelerated diabetes. Histochem. Cell Biol. 119:451-461. [DOI] [PubMed] [Google Scholar]
- 38.Rerup, C. C. 1970. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol. Rev. 22:485-518. [PubMed] [Google Scholar]
- 39.Rovere, P., C. Vallinoto, A. Bondanza, M. C. Crosti, M. Rescigno, P. Ricciardi-Castagnoli, C. Rugarli, and A. A. Manfredi. 1998. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J. Immunol. 161:4467-4471. [PubMed] [Google Scholar]
- 40.Saini, K. S., C. Thompson, C. M. Winterford, N. I. Walker, and D. P. Cameron. 1996. Streptozotocin at low doses induces apoptosis and at high doses causes necrosis in a murine pancreatic beta cell line, INS-1. Biochem. Mol. Biol. Int. 39:1229-1236. [DOI] [PubMed] [Google Scholar]
- 41.Sakamaki, K., T. Inoue, M. Asano, K. Sudo, H. Kazama, J. Sakagami, S. Sakata, M. Ozaki, S. Nakamura, S. Toyokuni, N. Osumi, Y. Iwakura, and S. Yonehara. 2002. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 9:1196-1206. [DOI] [PubMed] [Google Scholar]
- 42.Salmena, L., B. Lemmers, A. Hakem, E. Matysiak-Zablocki, K. Murakami, P. Y. Au, D. M. Berry, L. Tamblyn, A. Shehabeldin, E. Migon, A. Wakeham, D. Bouchard, W. C. Yeh, J. C. McGlade, P. S. Ohashi, and R. Hakem. 2003. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17:883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savill, J., I. Dransfield, C. Gregory, and C. Haslett. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965-975. [DOI] [PubMed] [Google Scholar]
- 44.Shi, Y. 2004. Caspase activation: revisiting the induced proximity model. Cell. 117:855-858. [DOI] [PubMed] [Google Scholar]
- 45.Spiotto, M. T., P. Yu, D. A. Rowley, M. I. Nishimura, S. C. Meredith, T. F. Gajewski, Y. X. Fu, and H. Schreiber. 2002. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity 17:737-747. [DOI] [PubMed] [Google Scholar]
- 46.Thornberry, N. A., and Y. Lazebnik 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 47.Trudeau, J. D., J. P. Dutz, E. Arany, D. J. Hill, W. E. Fieldus, and D. T. Finegood. 2000. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes 49:1-7. [DOI] [PubMed] [Google Scholar]
- 48.Turley, S., L. Poirot, M. Hattori, C. Benoist, and D. Mathis. 2003. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 198:1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varfolomeev, E. E., M. Schuchmann, V. Luria, N. Chiannilkulchai, J. S. Beckmann, I. L. Mett, D. Rebrikov, V. M. Brodianski, O. C. Kemper, O. Kollet, T. Lapidot, D. Soffer, T. Sobe, K. B. Avraham, T. Goncharov, H. Holtmann, P. Lonai, and D. Wallach. 1998. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267-276. [DOI] [PubMed] [Google Scholar]
- 50.Verdaguer, J., D. Schmidt, A. Amrani, B. Anderson, N. Averill, and P. Santamaria. 1997. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 186:1663-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo, M., A. Hakem, A. J. Elia, R. Hakem, G. S. Duncan, B. J. Patterson, and T. W. Mak. 1999. In vivo evidence that caspase-3 is required for Fas-mediated apoptosis of hepatocytes. J. Immunol. 163:4909-4916. [PubMed] [Google Scholar]
- 52.Woo, M., R. Hakem, C. Furlonger, A. Hakem, G. S. Duncan, T. Sasaki, D. Bouchard, L. Lu, G. E. Wu, C. J. Paige, and T. W. Mak. 2003. Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat. Immunol. 4:1016-1022. [DOI] [PubMed] [Google Scholar]
- 53.Woo, M., R. Hakem, and T. W. Mak. 2000. Executionary pathway for apoptosis: lessons from mutant mice. Cell Res. 10:267-278. [DOI] [PubMed] [Google Scholar]
- 54.Woo, M., R. Hakem, M. S. Soengas, G. S. Duncan, A. Shahinian, D. Kagi, A. Hakem, M. McCurrach, W. Khoo, S. A. Kaufman, G. Senaldi, T. Howard, S. W. Lowe, and T. W. Mak. 1998. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12:806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, P., M. T. Spiotto, Y. Lee, H. Schreiber, and Y. X. Fu. 2003. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J. Exp. Med. 197:985-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, Y., B. O'Brien, J. Trudeau, R. Tan, P. Santamaria, and J. P. Dutz. 2002. In situ beta cell death promotes priming of diabetogenic CD8 T lymphocytes. J. Immunol. 168:1466-1472. [DOI] [PubMed] [Google Scholar]