Abstract
Upon infection with the cecum-dwelling nematode Trichuris muris, the majority of inbred strains of mice launch a Th2-type immune response and in doing so expel the parasite before patency. In contrast, there are a few mouse strains which develop a nonprotective Th1-type response resulting in a chronic infection and the presence of adult worms. Of the Th2 cytokines known to be associated with the resistant phenotype (interleukin-4 [IL-4], IL-5, IL-9, and IL-13), comparatively little is known about the contribution that IL-9 makes towards the protective immune response. In this study we demonstrate that IL-9 is expressed early during the Th2-type response and that its elevation in vivo results in the enhancement of intestinal mastocytosis and the production of both the immunoglobulin E (IgE) and IgG1 isotypes. In addition, elevated IL-9 levels in vivo facilitated the loss of T. muris from the intestine. That IL-9 is important in promoting worm expulsion was also seen following infection of IL-9-transgenic mice, which constitutively overexpress the cytokine. These animals displayed an extremely rapid, but immune mediated, expulsion of the parasite. Also evident in these animals was a pronounced intestinal mastocytosis, which was previously shown by us to be responsible for the expulsion of the related nematode Trichinella spiralis from these animals. Taken together with observations of IL-9 production following infection with other helminths, the results imply that IL-9 contributes to the general mast cell and IgE response characteristic of these infections and, more specifically, enhances resistance to T. muris.
A clear dichotomy in immune responsiveness to the intestinal nematode Trichuris muris is seen upon infection of different inbred strains of mice. Those that are susceptible (e.g., AKR mice) mount a nonprotective Th1-type response. This results in a chronic infection in which adult worms develop, persist, and are unable to be expelled by their host. In contrast, resistant strains (e.g., BALB/K or C57BL/6 mice) mount a Th2-type response which leads to a short-lived infection in which worm expulsion is initiated and completed before the adult worms develop (7, 11).
This association between Th2-type cytokines and host protection extends to other intestinal nematode infections. Such nematodes include Nippostrongylus brasiliensis, Heligmosomoides polygyrus, Trichinella spiralis, and Strongyloides venezuelensis (reviewed in reference 15). A number of investigators have examined the contribution that an individual Th2 cytokine makes during the protective immune response to these infections. In the main, these studies have focused on interleukin-4 (IL-4), and certainly evidence of a strong role for this cytokine has emerged over recent years. However, in some situations its importance is likely to stem from its role in mediating Th2-cell development and ultimately the production of other, perhaps important, Th2 cytokines (reviewed in reference 1). In addition, there are circumstances in which the presence of IL-4 is not critical for a resistant phenotype (2, 22). It is therefore important to evaluate the contributions of other cytokines during the worm expulsion process.
IL-9 is one such cytokine that is produced by CD4+ T cells during Th2-type responses in vivo, including during infections with intestinal helminths (17, 19, 37). Indeed, mice that are resistant to T. muris produce high levels of IL-9 which negatively correlate with the Trichuris worm burden (10, 11). However, the functional role of this cytokine during helminth infection is not well characterized, even though several biological targets in vitro have been elucidated. These targets include CD4+ T cells (33, 41), bone marrow-derived mast cells (20), B cells (5, 29), and certain erythroid progenitors (4).
Through a variety of approaches, we have assessed the contribution that IL-9 makes during the immune response to T. muris. We demonstrate that IL-9 gene expression correlates with the resistant phenotype and is expressed early following infection. Elevating its levels in vivo results in the enhancement of several Th2-mediated changes that are often considered hallmarks of helminth infection. These include mucosal mastocytosis, moderate levels of mouse mast cell protease-1 (MMCP-1) in serum, and immunoglobulin E (IgE) and IgG1 antibody production. Moreover, elevating IL-9 levels in vivo facilitates worm expulsion, and IL-9-transgenic mice, which constitutively overexpress the cytokine, display extremely rapid expulsion kinetics. As IL-9 has previously been shown to influence expulsion of the related nematode T. spiralis (13), this study extends knowledge of the cytokine’s protective role to T. muris and suggests its importance in other intestinal nematode infections.
MATERIALS AND METHODS
Animals.
AKR (H-2k), BALB/K (H-2k), and C57BL/6 (H-2b) mice were purchased from Harlan Olac Ltd. (Bicester, United Kingdom). IL-9-transgenic mice (H-2q) were generated by microinjecting a transgene construct into the pronuclei of fertilized eggs of FVB mice as previously described (31). These FVB mice were used as wild-type controls. The transgenic mice were generated at the Ludwig Institute for Cancer Research, Brussels, Belgium, and then bred in the facility at the University of Manchester, Manchester, United Kingdom. All experimental groups consisted of four to six male animals which were infected when they were 6 to 8 weeks old.
Parasite.
The techniques used for the maintenance, infection, and recovery of T. muris have previously been described (42). The E/N isolate used was originally obtained from the Wellcome Research Laboratories, London, United Kingdom. Mice were infected with approximately 300 T. muris eggs on day 0 and killed at various time points postinfection (p.i.).
IL-9 complex methodology.
IL-9 was delivered in vivo to AKR mice by intravenous injection of 10 μg of recombinant IL-9 complexed with 50 μg of a neutralizing anti-IL-9 monoclonal antibody (2C12) on days 7, 11, 15, and 18 p.i., using a protocol previously described (14). Animals were killed on day 35 p.i.
IL-9-secreting T-cell line.
IL-9 levels were raised in vivo by intraperitoneal injection of 107 TS1.G6 cells into C57BL/6 mice 7 days prior to infection with T. muris. These cells represent an IL-9 factor-dependent T-cell line transfected with the IL-9 gene and constitutively secrete IL-9 in vitro. Their administration to C57BL/6 mice in vivo has previously been described (40). Animals were killed on day 17 p.i.
Cortisone treatment.
IL-9-transgenic mice and their wild-type controls were given 1.25 mg of hydrocortisone-21-acetate (Sigma Chemical Co., Poole, United Kingdom) subcutaneously on days −3, −1, 1, and 4 p.i. Animals were killed on days 12 and 34 p.i.
Histology.
The cecum tip was removed at autopsy and fixed in Carnoy’s fluid for 6 h prior to processing by standard histological techniques. Sections were stained in 0.5% toluidine blue (pH 0.3), and the number of mast cells in 20 cecal crypt units (CCU) was determined for each animal.
Enzyme-linked immunosorbent assay.
Levels of MMCP-1 in serum were measured with an MMCP-1 enzyme-linked immunosorbent assay kit purchased from Moredun Animal Health Ltd., Penicuik, United Kingdom, by using a technique described previously (21). Briefly, rabbit anti-MMCP was used as the capture antibody. Tenfold serial dilutions of serum were made from 1/100 to 1/100,000 for IL-9-transgenic mice and from 1/10 to 1/10,000 for all other strains. Horseradish peroxidase-conjugated rabbit anti-mouse MMCP-1 was then added, and quantification was made by reference to purified MMCP-1.
Parasite-specific IgG1 and IgG2a levels were determined as described previously (10). Essentially, T. muris excretory/secretory antigen was used as the target antigen at 5 μg/ml. Double dilutions of sera were made from 1/20 to 1/2,560. Parasite-specific IgG1 and Ig2a were detected by using biotinylated rat anti-mouse IgG1 (Serotec Ltd., Oxford, United Kingdom) and biotinylated rat anti-mouse IgG2a (Pharmingen, Cambridge, United Kingdom).
Total serum IgE levels were determined as previously described (10). A rat anti-mouse IgE (Serotec Ltd.) was used as the capture antibody, and IgE was detected by using a polyclonal horseradish peroxidase-conjugated goat anti-mouse IgE (Nordic Immunological Labs, Maidenhead, United Kingdom). An IgE monoclonal antibody specific for dinitrophenol (Sigma Chemical Co.) was used as a standard.
RT-PCR.
BALB/K and AKR mice were infected on day 0. On days 1, 4, 11, 21, and 35 p.i., mesenteric lymph nodes (MLN) were collected from five animals from each group and pooled, and single-cell suspensions were made as previously described (7). Cells (5 × 106) were centrifuged at 2,000 × g for 10 min, resuspended in RNAzol B (Biogenesis, Bournemouth, United Kingdom), snap frozen, and stored at −80°C until use. MLN cells from an uninfected group were also taken. Total RNA was later extracted from all samples according to the manufacturer’s instructions (Biogenesis), and reverse transcription-PCR (RT-PCR) was performed as previously described (36). The IL-4 and hypoxanthine-guanine phosphoribosyltransferase (HPRT) primers were based on previously published sequences (25, 27). The IL-9 primers were designed from the genomic DNA sequence (30) and were as follows: sense, CATCCTTGCCTCTGTTTTGCT; antisense, CGGAGAGACACAAGCAGCTGG. The amplification program consisted of 1 min at 94°C, 1 min at the annealing temperature, and 2 min at 72°C, for 30 cycles. The annealing temperature for HPRT and IL-4 was 60°C, whereas that for IL-9 was 55°C. Both the cycle number and annealing temperature were previously determined to be optimal for the primer pairs used. The number of cycles performed was determined to be below the reaction saturation point, enabling analysis during the linear relationship between input RNA and PCR product. Both positive and negative controls, including reverse transcriptase and PCR reagent blanks, were run with each amplification. The amplified product was detected by Southern blot analysis with specific internal probe sequences end labelled with [γ-32P]ATP. The sequences for IL-4 and HPRT have previously been described (36). The IL-9 probe, TCCACCGTCAAAATGCAG, was designed from its genomic DNA sequence (30). Blots were exposed to a storage phosphor screen and scanned on a phosphorimager (BAS 2000 TR; Fujix, Fuji, Japan). Values were individually normalized to those for HPRT and expressed relative to those for uninfected controls, which were arbitrarily given a value of 1.
Statistical analysis.
Significant differences between experimental groups were calculated by using the Mann-Whitney U test, with P < 0.05 considered significant. All data are expressed as mean values ± standard errors (SE).
RESULTS
Early IL-9 gene expression following infection of resistant mice.
IL-9 is known to be produced in large quantities by in vitro-stimulated MLN cells from resistant, but not susceptible, mice following T. muris infection (11). In these earlier studies IL-9 production, in conjunction with IL-4, was evident by day 21 p.i. We wanted to look earlier than this time point and to determine the kinetics of IL-9 gene expression in comparison with those of IL-4 gene expression. We therefore infected resistant BALB/K and susceptible AKR mice with the parasite. Following establishment (day 11 infectivity: BALB/K, 95.6 worms ± 9.6; AKR, 87.0 ± 13.3), BALB/K mice commenced expulsion, which was complete by day 21 p.i. (BALB/K, 0.4 ± 0.2; AKR, 115.0 ± 13.9). In contrast, AKR mice failed to expel the parasite, harboring adult worms on day 35 p.i. (AKR, 81.6 ± 19.1).
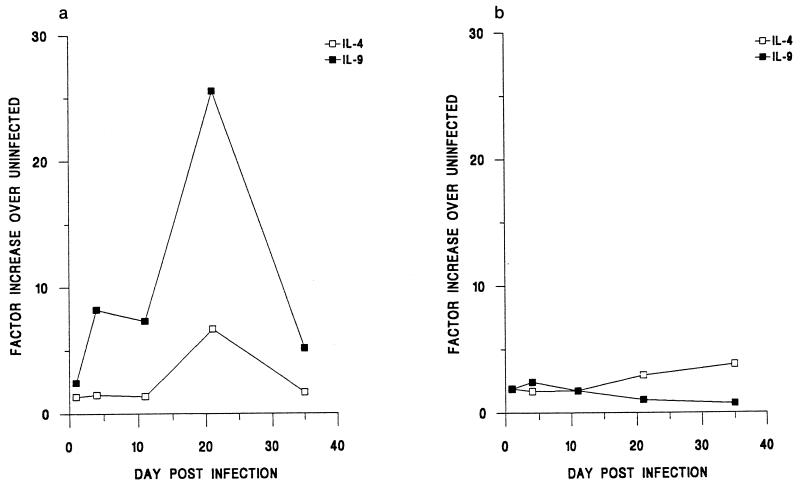
Figure 1 shows the levels of IL-4 and IL-9 gene expression in the MLN in both strains of mice throughout infection. Clearly apparent was the ability of BALB/K mice to express both of these Th2 cytokines in response to infection. In contrast, AKR mice expressed low levels, barely above those for the uninfected controls, throughout the time period. Interestingly, in the BALB/K mice an increase in IL-9 gene expression was observed by day 4 p.i., whereas a rise in IL-4 was not observed until day 21 p.i. Therefore, elevated IL-9 gene expression correlates with the resistant phenotype and is apparent before an observed increase in IL-4 gene expression.
FIG. 1.
Kinetics of IL-9 and IL-4 gene expression in the MLN following infection of BALB/K (a) and AKR (b) mice with T. muris. Values were individually normalized to those for the HPRT gene and are expressed relative to those for uninfected control animals, which were arbitrarily given a value of 1.
Elevated IL-9 levels in vivo enhance several Th2-mediated characteristics of infection.
Considering the lack of IL-9 gene expression in the susceptible AKR mice, we wanted to determine the effects of raising its levels in vivo in these animals. AKR mice were infected on day 0 and given recombinant IL-9 in the form of a complex on the days indicated. Figure 2a shows the numbers of intestinal mast cells on day 35 p.i. Clearly apparent were significantly greater numbers of intestinal mast cells in the IL-9-treated group following infection (P < 0.05). It is important to note that there was no increase in the number of mucosal mast cells in the uninfected animals following the administration of IL-9. This suggests that the cytokine may be acting in synergy with other mast cell growth factors, such as IL-3 and stem cell factor (SCF), as has been shown in vitro (18, 20).
FIG. 2.
Effects of manipulating host IL-9 levels in AKR mice through the administration of an IL-9 complex following infection with T. muris. (a) Mean numbers of intestinal mast cells per 20 CCU ± SE. (b and c) Levels of parasite-specific IgG1 (b) and IgG2a (c) in serum, expressed as the mean optical density (OD) ± SE against dilution of serum. (d) Mean adult worm burden ± SE. *, stunted damaged worms; NS, not significant. All values were determined on day 35 p.i.
We next went on to examine the levels of parasite-specific IgG in serum. Mice susceptible to T. muris have both IgG1 and IgG2a responses, whereas in resistant strains the latter isotype is absent (10). The high levels in serum of both parasite-specific IgG1 and IgG2a found in this experiment were therefore expected (Fig. 2b and c). However, the levels of IgG1 were elevated in the IL-9-treated group, suggesting the involvement of IL-9 in its production. It is known that in vitro IL-9 can enhance the secretion of this antibody from activated B cells (29). The results shown here therefore extend this observation to the in vivo setting.
Figure 2d shows the numbers of worms found in the AKR mice on day 35 p.i. Fewer were found in the IL-9-treated group, and all of these were stunted in their growth, suggesting a detrimental effect of IL-9 on worm survival. However, the reduction in worm burden was found not to be significant (P < 0.06). A larger dose of recombinant IL-9 or a more effective chaperone may have resulted in a more pronounced difference.
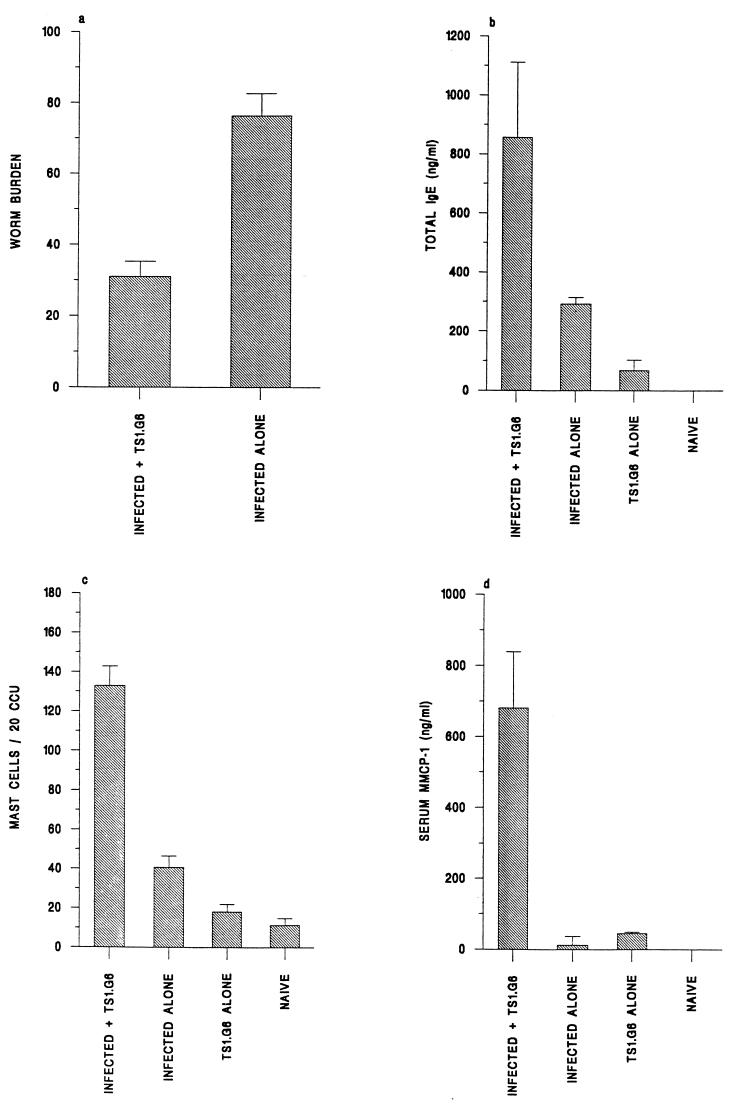
In order to elevate IL-9 levels in vivo more effectively, we utilized the TS1.G6 cell line. These cells are known to secrete high levels of biologically active IL-9 in vitro and are derived from C57BL/6 mice (40). We therefore administered TS1.G6 cells to C57BL/6 mice prior to infection with T. muris. As this strain is resistant to T. muris, the experiment was terminated on day 17, a time point when the infection is still ongoing (9). Any differences in the kinetics of the worm expulsion process would therefore be observed. Figure 3a shows the numbers of worms recovered from normally infected animals and from those receiving the TS1.G6 cell line. A significant reduction in worm burden was observed: those receiving the cell line had approximately 50% fewer worms than the control group (infected mice given TS1.G6, 31.0 ± 4.2; infected mice alone, 76.3 ± 6.3). The results therefore suggest that IL-9 can facilitate the loss of T. muris in these animals.
FIG. 3.
Effects of manipulating host IL-9 levels in C57BL/6 mice through the administration of TS1.G6, an IL-9-secreting T-cell line, following infection with T. muris. (a) Mean worm burden ± SE. (b) Levels of total IgE in serum, expressed as means ± SE. (c) Mean numbers of intestinal mast cells per 20 CCU ± SE. (d) Levels of MMCP-1 in serum, expressed as means ± SE. All values were determined on day 17 p.i.
The levels of parasite-specific IgG1 and IgG2a in serum were below detection at this day 17 time point (data not shown). However, levels of total IgE in serum were measurable and are shown in Fig. 3b. An elevated total IgE response was seen in animals in which IL-9 levels were raised, indicating the involvement of IL-9 in the production of this Th2-controlled antibody isotype. Intestinal mastocytosis was also examined, and the results are shown in Fig. 3c. Once again IL-9 was found to influence this cell population, because an enhanced mast cell response was found in the IL-9-treated group (infected mice given TS1.G6, 132.8 ± 17.3; infected mice alone, 40.7 ± 6.3). In addition, a profound elevation in the levels of MMCP-1, a protease known to be produced by mucosal mast cells in vivo (28), in serum was observed in these animals (Fig. 3d). Collectively, the results suggest the involvement of IL-9 in the expulsion of T. muris and the promotion of several Th2-mediated characteristics of helminth infection, including intestinal mastocytosis and IgE and IgG1 production.
IL-9-transgenic mice rapidly expel T. muris.
We wanted to determine the effects of IL-9 overproduction on infection with T. muris. IL-9-transgenic mice and their wild-type FVB littermates were therefore infected on day 0 and analyzed on days 13, 21, and 34 p.i. Worm burden analysis revealed that both of these strains had virtually completed expulsion by day 13 p.i. (wild type, 11.5 ± 6.0; transgenic mice, 3.8 ± 4.1), and no worms were found at the later time points (data not shown). This was surprising, because previously examined resistant mouse strains typically commence expulsion during the third week of infection (9). The expulsion kinetics are therefore very fast, for both strains, and suggest that the specific arm of the immune response may not have been involved.
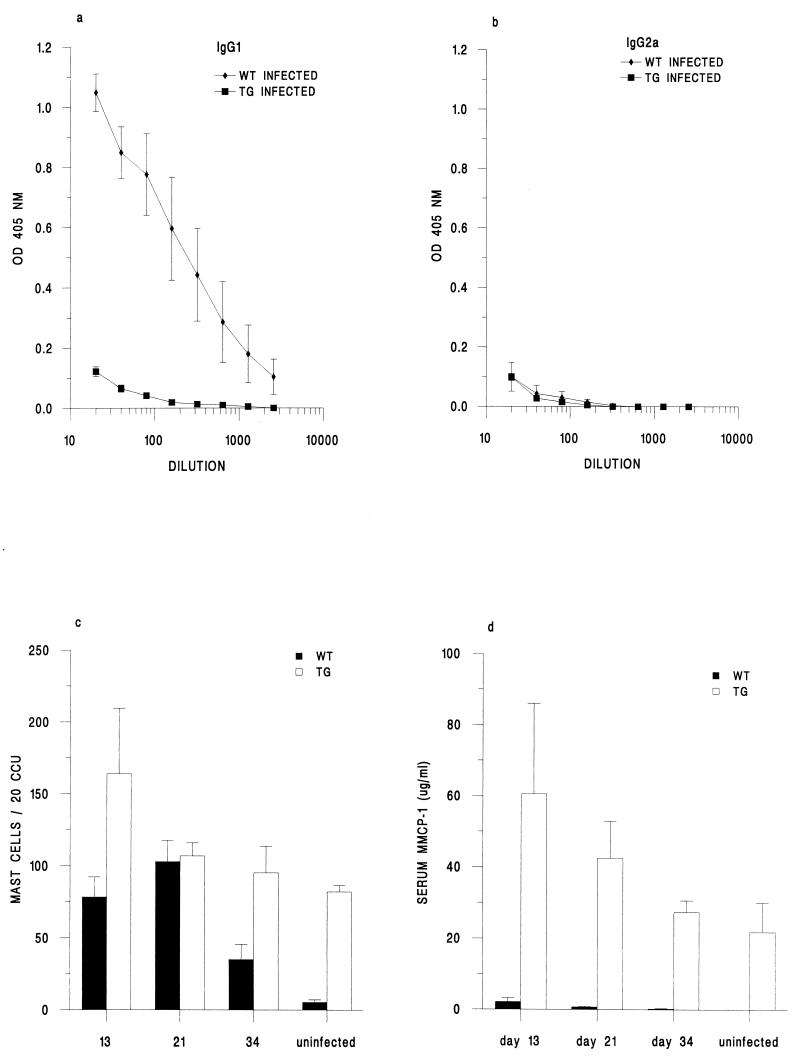
We therefore measured the levels of parasite-specific IgG in serum on day 34 p.i. (Fig. 4a and b). High serum IgG1 levels in the virtual absence of IgG2a were found in the wild-type mice, indicative of a Th2-type response (10). In contrast, both parasite-specific IgG1 and IgG2a were absent in the sera of the IL-9-transgenic mice. Due to the rapid loss of the parasite, this day 34 time point may have been too late for detection. However, we failed to find any parasite-specific IgG antibody, of any isotype, at the earlier time points (data not shown). The two mouse strains therefore appear to respond differently. One possible explanation for this might be that the majority of larvae transverse through the IL-9-transgenic gut so rapidly that insufficient antigen is available for B-cell activation.
FIG. 4.
Infection of IL-9-transgenic (TG) and wild-type (WT) mice with T. muris. (a and b) Levels of parasite-specific IgG1 (a) and IgG2a (b) on day 34 p.i., expressed as mean optical density (OD) ± SE against dilution of serum. (c) Mean numbers of intestinal mast cells per 20 CCU ± SE throughout infection. (d) Levels of MMCP-1 in serum throughout infection, expressed as means ± SE.
Although the intestinal tracts appear to be similar, in terms of their pathology and inflammation, there are differences between the two strains (reference 18 and unpublished observations). Figure 4c shows a pronounced cecal mastocytosis in naive IL-9-transgenic mice, which remained high throughout infection. In contrast, naive wild-type mice had negligible mast cell numbers which increased significantly upon infection but then declined after day 21 p.i. In addition, high levels of MMCP-1 in serum were found in the transgenic mice, both in the naive state and throughout infection, suggesting their functional activity (Fig. 4d). Interestingly, even when cecal mast cell numbers in the transgenic and wild-type animals were comparable (day 21 p.i.), the IL-9-transgenic mice had much greater levels of MMCP-1. No doubt this observation is a result of the high numbers of mast cells present, not just in the cecum but throughout the gastrointestinal tracts of IL-9-transgenic mice (18). Perhaps this pronounced mastocytosis interferes with and reduces the establishment of the parasite. Unfortunately, it is not possible to determine exactly when the worms are lost from their host, as they cannot be counted with any accuracy prior to day 10 p.i. Therefore, in order to address this possibility and to determine whether the expulsion process was immune mediated, we sought to immunosuppress the IL-9-transgenic mice.
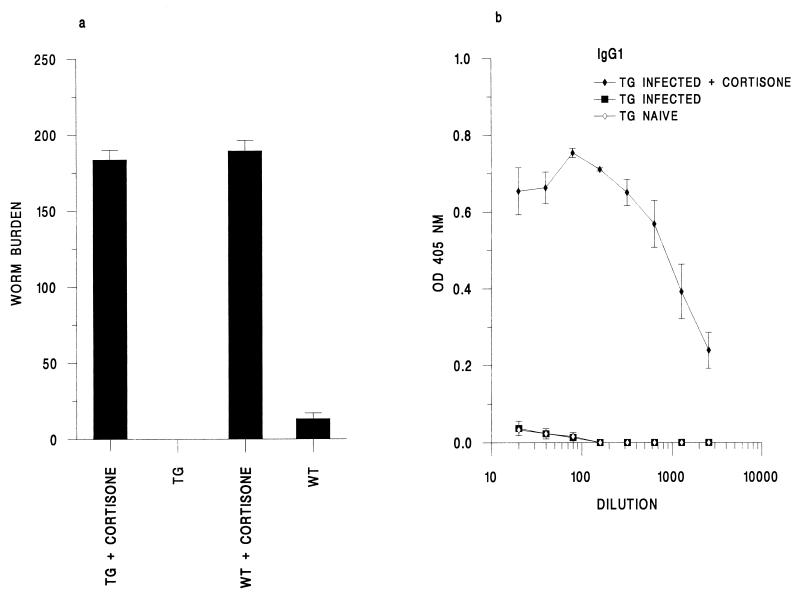
Hydrocortisone acetate is an immunosuppressant and anti-inflammatory drug known to prevent the immune-mediated worm expulsion process (12). We analyzed the effects of this treatment on the outcome of infection. The worm burdens recovered on day 12 p.i. are shown in Fig. 5a. As expected, normally infected wild-type animals had few worms remaining (13.3 ± 3.8) whereas the transgenic mice, in this instance, had completely expelled their worms. However, cortisone treatment resulted in the presence of a full infective dose in both the wild-type and transgenic animals at this time point (wild type, 189.3 ± 6.8; transgenic mice, 176.0 ± 14.3). Therefore, the rapid loss of the parasite is immune mediated and is not a result of the failure of worms to establish themselves in the guts of IL-9 transgenic mice. On day 34 p.i., 30 days following the last cortisone injection, all of the wild-type animals harbored adult worms (215.0 ± 17.8). In contrast, only one of the transgenic mice harbored a full infective dose (worm numbers for three mice, 0, 0, and 180). As it is known that worm expulsion has to occur during the early larval stages, to prevent a Th1-type response and susceptibility, expulsion cannot have been initiated in the wild-type animals before the development of the L3 larvae (12). However, as only one of the transgenic mice harbored adults following cortisone treatment, this is suggestive of their ability to promote worm loss faster than the wild-type strain following cessation of treatment with hydrocortisone.
FIG. 5.
Effects of cortisone treatment on IL-9-transgenic (TG) and wild-type (WT) animals. (a) Mean worm burden ± SE on day 12 p.i. (b) Levels of parasite-specific IgG1 in serum in IL-9 transgenic mice on day 34 p.i., expressed as mean optical density (OD) ± SE against dilution of serum.
The antibody responses on day 34 p.i. reflected the chronicity of infection. Normally infected wild-type mice, which expelled their infection, mounted a parasite-specific IgG1 response in the absence of IgG2a (data not shown). Following cortisone treatment, they made high levels of both antibody isotypes, which is suggestive of an ongoing Th1-type response (data not shown). The IL-9-transgenic mice again failed to make any parasite-specific antibody but following cortisone treatment made high levels of IgG1 (Fig. 5b). Therefore, they can mount a specific antibody response, presumably because of their greater antigenic load.
DISCUSSION
IL-9 production is seen during Th2-type responses in vivo, including during intestinal nematode infections (15, 19, 38). One key finding from these studies is the upregulation of IL-9 gene expression early following infection. For example, a rise in the local levels of IL-9 is seen as early as 6 h following a primary infection by H. polygyrus (38), and in the case of N. brasiliensis, IL-9 gene expression is coincident with the arrival of the worms in the gut (15). Here, we analyzed for the first time the expression of this cytokine following infection with T. muris. We found a significant increase in neither the IL-9 nor the IL-4 message following infection of the susceptible AKR (Th1-type) mouse strain. However, in the resistant BALB/K strain, IL-9 gene expression was upregulated early during the Th2-type response, in accordance with the above- described model systems. We also measured the cytokines secreted by their restimulated MLN cells and found these levels to correlate with the level of message obtained (data not shown).
In the BALB/K mice the expression of IL-9 preceded that of IL-4, although further studies are needed to confirm this observation and to examine expression prior to 24 h p.i. This is an interesting observation, because CD4+ T cells are a known cellular source of IL-9, and IL-4 is known to promote IL-9 gene expression in T cells (33, 34). However, a recent study has also shown the induction of IL-9 mRNA before IL-4 message as well as the ability of IL-4 knockout mice to express the IL-9 gene (24). As their early IL-9 peak disappeared following anti-CD4 treatment, it appears that IL-4-independent regulation of IL-9 gene expression in vivo can occur. Furthermore, a non-T-cell source of early IL-9 has been described, which may help to explain its rapid production in our system (38). Investigations are needed to address this issue and to determine the role of IL-9 in subsequent Th2 cell development.
The elevation of host IL-9 levels had a significant effect on intestinal mastocytosis. It is well known that IL-9 can influence the growth of bone marrow-derived mast cells and their functional activity in vitro (6, 20). However, evidence of its role as a mast cell growth factor in vivo is only now beginning to emerge. The control of mast cell proliferation and function is complex but is believed to be under the influence of a number of cytokines, including IL-3, IL-4, and IL-10 (32, 35, 39). Whether IL-9 acts independently of these cytokines in vivo is not yet clear. In the studies detailed here, the administration of IL-9 in the absence of antigen was not sufficient to promote mastocytosis, suggesting the involvement of other factors. However, the presence of very high levels of IL-9 did appear to circumvent the need for additional cytokines: naive IL-9 transgenic mice had greatly elevated numbers of intestinal mast cells, yet no other systemic or local cytokine production was found (unpublished observations). SCF is a stromal-cell-derived factor involved in the development of mast cells in the bone marrow and their subsequent migration into the periphery (16). Interestingly, when SCF function is blocked in the transgenic animals, their intestinal mast cell numbers profoundly decrease (13). As both transgenic and wild-type mice constitutively express SCF, these findings together suggest that both IL-9 and SCF are required for the high-level mastocytosis observed (18).
The elevation of serum MMCP-1 levels following the administration of IL-9 and the high circulating levels in the IL-9-transgenic mice suggest the functional activity of the intestinal mast cells. It is known that mucosal- but not connective-tissue-type mast cells produce this protease following their activation (23). Indeed, IL-9-transgenic mice have numbers of connective-tissue-type mast cells comparable to those in their wild-type counterparts (18). Taken together, the results suggest the specific involvement of IL-9 in the development of the mucosal mast cell lineage.
The production of both IgE and IgG1 was enhanced following elevation of host IL-9 levels. IL-4 is thought to be the principal cytokine involved in their production, both in vitro and in vivo (37), although recent reports have suggested an IL-4-independent regulation of IgE production under certain circumstances (26). It is known that IL-9 can act in synergy with IL-4 to increase the secretion of both of these antibody isotypes in vitro (29). Whether IL-9 acts independently of IL-4 to promote their production in our system is unclear. The absence of a parasite-specific IgG1 response in the transgenic animals was surprising and might appear to contradict a role for IL-9 in promoting its production. Indeed, we have previously observed very high levels of this antibody following infection of IL-9-transgenic mice with T. spiralis (13). However, cortisone treatment, which allowed parasite survival, did result in the production of parasite-specific IgG1. Therefore, it appears that during a normal infection of IL-9-transgenic mice, there is insufficient antigenic stimulation for measurable antibody production, owing to the rapid worm expulsion.
An important finding from this study was the ability of IL-9 to promote the loss of T. muris from its intestinal niche. In essence, we raised IL-9 levels by three methods, with the IL-9 complex method and the transgenic system being the least and most effective systems, respectively. However, it must be stressed that the results of the latter experiment need to be treated with some caution, as the levels of IL-9 present are extraordinarily high. Therefore, the results may not reflect the role of IL-9 under physiological conditions. However, it is quite clear from the studies using IL-9 complexes and IL-9-producing T cells in vivo that raising IL-9 levels more moderately also results in changes similar to those seen in IL-9-transgenic mice. Taken together, the results of the present study present compelling evidence for an important role for IL-9 in protective immunity to helminths. The mechanisms by which IL-9 promotes resistance remain to be determined. Other studies have convincingly shown that mast cells, eosinophils, and antibody-dependent mechanisms are not essential for worm expulsion (3, 8). Certainly, in the transgenic animals the worms were expelled quickly and effectively in the absence of any detectable serum antibody response. Experiments to establish the physiology of the IL-9-transgenic gut, in terms of gut motility and fluid absorption, are under way, as this may exert an influence. As IL-9 is expressed early during the specific Th2-type response, our working hypothesis is that IL-9 helps mediate resistance through its ability to promote T-cell growth and hence potentiate Th2-cell development. Future experiments will address this issue.
ACKNOWLEDGMENTS
We thank Neil Humphreys for his excellent technical support and Catherine Betts for her help and advice with the RT-PCR analysis.
The financial support of the BBSRC is gratefully acknowledged.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft A J, McKenzie A N J, Grencis R K. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 3.Betts, C. J., and K. J. Else. Effector mechanisms of Trichuris muris elimination: the potential roles of mast cells, eosinophils and antibody-mediated cytotoxicity. Submitted for publication.
- 4.Donahue R E, Yang Y-C, Clark S C. Human P40 T-cell growth factor (interleukin-9) supports erythroid colony formation. Blood. 1990;75:2271–2275. [PubMed] [Google Scholar]
- 5.Dugas B, Renauld J-C, Pène J, Petit-Frère C, Braquet P, Bousquet J, Van Snick J, Mencia-Huerta J M. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23:1687–1692. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 6.Eklund K K, Ghildyal N, Austen K F, Stevens R L. Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14-derived transcripts that encode late-expressed mouse mast cell proteases. J Immunol. 1993;151:4266–4273. [PubMed] [Google Scholar]
- 7.Else K J, Grencis R K. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology. 1991;72:508–513. [PMC free article] [PubMed] [Google Scholar]
- 8.Else K J, Grencis R K. Antibody-independent effector mechanisms in resistance to the intestinal nematode parasite Trichuris muris. Infect Imm. 1996;64:2950–2954. doi: 10.1128/iai.64.8.2950-2954.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Else K J, Wakelin D. The effects of H-2 and non-H-2 genes on the expulsion of the nematode Trichuris muris from inbred and congenic mice. Parasitology. 1988;96:543–550. doi: 10.1017/s0031182000080173. [DOI] [PubMed] [Google Scholar]
- 10.Else K J, Entwistle G M, Grencis R K. Correlations between worm burden and markers of Th1 and Th2 cell subset induction in an inbred strain of mouse infected with Trichuris muris. Parasite Immunol. 1993;15:595–600. doi: 10.1111/pim.1993.15.10.595. [DOI] [PubMed] [Google Scholar]
- 11.Else K J, Hültner L, Grencis R K. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of Th-cell subsets in resistant and susceptible mice. Immunology. 1992;75:232–237. [PMC free article] [PubMed] [Google Scholar]
- 12.Else K J, Hültner L, Grencis R K. Modulation of cytokine production and response phenotypes in murine trichuriasis. Parasite Immunol. 1992;14:441–449. doi: 10.1111/j.1365-3024.1992.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner H, Humphreys N, Renauld J-C, Van Snick J, Grencis R K. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman F D, Madden K B, Morris S C, Holmes J M, Boiani N, Katona I M, Maliszewski C R. Anti-cytokine antibodies as carrier proteins: prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 15.Finkelman F D, Shea-Donahue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F. Cytokine regulation of host defence against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 16.Galli S J, Zsebo K M, Geissler E N. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 17.Gessner A, Blum H, Röllinghoff M. Differential regulation of IL-9 expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 18.Godfraind, C., J. Louahed, H. Faulkner, A. Vink, G. Warnier, R. K. Grencis, and J.-C. Renauld. 1998. Intraepithelial infiltration by mast cells with both connective tissue type characteristics in the gut, trachea and kidneys of interleukin-9 transgenic mice. J. Immunol., in press. [PubMed]
- 19.Grencis R K, Hültner L, Else K J. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991;74:329–332. [PMC free article] [PubMed] [Google Scholar]
- 20.Hültner L, Moeller J, Schmitt E, Jager G, Reisbach G, Ring J, Dörmer P. Thiol-sensitive mast cell lines derived from mouse bone marrow respond to a mast cell growth-enhancing activity different from both IL-3 and IL-4. J Immunol. 1989;142:3440–3446. [PubMed] [Google Scholar]
- 21.Huntley J F, Gooden C, Newlands G F J, MacKeller A, Lammas D A, Wakelin D, Tuohy M, Woodbury R G, Miller H R P. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 1990;12:85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence R A, Gray C A, Osbourne J, Maizels R. Nippostrongylus brasiliensis. Cytokine responses and nematode expulsion in normal and IL-4 deficient mice. Exp Parasitol. 1996;84:65–73. doi: 10.1006/expr.1996.0090. [DOI] [PubMed] [Google Scholar]
- 23.Miller H R P, Huntley J F, Newlands G F J, Mackellar A, Lammas D A, Wakelin D. Granule proteinases define mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology. 1988;65:559–563. [PMC free article] [PubMed] [Google Scholar]
- 24.Monteyne P, Renauld J-C, Van Broeck J, Dunne D W, Brombacher F, Coutelier J-P. IL-4-independent regulation of in vivo IL-9 expression. J Immunol. 1997;159:2616–2623. [PubMed] [Google Scholar]
- 25.Montgomery R A, Dallman M J. Analysis of cytokine gene expression during fetal thymic ontogeny using the polymerase chain reaction. J Immunol. 1991;147:554–560. [PubMed] [Google Scholar]
- 26.Morawetz R A, Gabriele L, Rizzo L V, Noben-Trauth N, Kuhn R, Rajewsky K, Muller W W, Doherty T M, Finkelman F, Coffman R L, Morse H C. Interleukin (IL)-4-independent immunoglobulin switch to immunoglobulin (Ig)E in the mouse. J Exp Med. 1996;184:1651–1661. doi: 10.1084/jem.184.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy E, Hierly S, Sher A, O’Garra A. Detection of in vivo expression of interleukin-10 using a semiquantitative polymerase chain reaction method in Schistosoma mansoni infected mice. J Immunol Methods. 1993;162:211–223. doi: 10.1016/0022-1759(93)90386-l. [DOI] [PubMed] [Google Scholar]
- 28.Newlands G F J, Gibson S, Knox D P, Grencis R, Wakelin D, Miller H R P. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology. 1987;62:629–634. [PMC free article] [PubMed] [Google Scholar]
- 29.Petit-Frère C, Dugas B, Braquet P, Mencia-Huerta J M. Interleukin-9 potentiates the interleukin-4 induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–151. [PMC free article] [PubMed] [Google Scholar]
- 30.Renauld J-C, Goethals A, Houssiau F, Merz H, Van Roost E, Van Snick J. Human P40/IL-9: expression in activated CD4+ T cells, genomic organization, and comparison with the mouse gene. J Immunol. 1990;144:4235–4241. [PubMed] [Google Scholar]
- 31.Renauld J-C, Lugt N, Vink A, Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, Van Snick J. Thymic lymphomas in interleukin-9 transgenic mice. Oncogene. 1994;9:1327–1332. [PubMed] [Google Scholar]
- 32.Rennick D M, Lee F D, Yokota T, Arai K, Cantor H, Nabel G L. A cloned MCGF cDNA encodes a multilineage haematopoietic growth factor: multiple activities of interleukin-3. J Immunol. 1985;134:910–914. [PubMed] [Google Scholar]
- 33.Schmitt E, Van Brandwijk R, Van Snick J, Siebold B, Rude E. TCGF III/P40 is produced by naive murine CD4+ T cells but is not a general growth factor. Eur J Immunol. 1989;19:2167–2170. doi: 10.1002/eji.1830191130. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt E, German T, Goedert S, Hoehn P, Huels C, Koelsch S, Kühn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-β and IL-4 and is inhibited by IFN-γ. J Immunol. 1994;153:3989–3998. [PubMed] [Google Scholar]
- 35.Smith C, Rennick D. Characterization of a murine lymphokine distinct from interleukin-2 and interleukin-3 (IL-3) possessing a T cell growth factor activity and a mast cell activity that synergizes with IL-3. Proc Natl Acad Sci USA. 1986;83:1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smythies L E, Betts C, Coulson P S, Dowling M, Wilson A. Kinetics and mechanism of effector focus formation in the lungs of mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasite Immunol. 1996;18:359–369. doi: 10.1046/j.1365-3024.1996.d01-115.x. [DOI] [PubMed] [Google Scholar]
- 37.Snapper C, Mond J J. Towards a comprehensive view of class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 38.Svetic A, Madden K B, Zhou X, Lu P, Katona I M, Finkelman F D, Urban J F, Gause W C. A primary intestinal helminthic infection rapidly induces a gut associated elevation of Th2-associated cytokines and IL-3. J Immunol. 1993;150:3434–3441. [PubMed] [Google Scholar]
- 39.Thompson-Snipes L, Dhar V, Bond N W, Mosmann T R, Moore K W, Rennick D M. Interleukin-10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uyttenhove C, Druez C, Renauld J-C, Hérin M, Noël H, Van Snick J. Autonomous growth and tumorigenicity induced by P40/interleukin-9 cDNA transfection of a mouse P40-dependent T cell line. J Exp Med. 1991;173:519–522. doi: 10.1084/jem.173.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uyttenhove C, Simpson R J, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci USA. 1988;85:6934–6938. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wakelin D. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology. 1967;57:515–517. doi: 10.1017/s0031182000072395. [DOI] [PubMed] [Google Scholar]