Abstract
Valvular heart diseases (VHDs) significantly impact morbidity and mortality rates world-wide. Early diagnosis improves patient outcomes. Artificial intelligence (AI) applied to electrocardiogram (ECG) interpretation presents a promising approach for early VHD detection. We conducted a meta-analysis on the efficacy of AI models in this context. We reviewed databases including PubMed, MEDLINE, Embase, Scopus, and Cochrane until August 20, 2023, focusing on AI for ECG-based VHD detection. The outcomes included pooled accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value. The pooled proportions were derived using a random-effects model with 95% confidence intervals (CIs). Study heterogeneity was evaluated with the I-squared statistic. Our analysis included 10 studies, involving ECG data from 713,537 patients. The AI algorithms mainly screened for aortic stenosis (n = 6), mitral regurgitation (n = 4), aortic regurgitation (n = 3), mitral stenosis (n = 1), mitral valve prolapse (n = 2), and tricuspid regurgitation (n = 1). A total of 9 studies used convolution neural network models, whereas 1 study combined the strengths of support vector machine logistic regression and multilayer perceptron for ECG interpretation. The collective AI models demonstrated a pooled accuracy of 81% (95% CI 73 to 89, I2 = 92%), sensitivity was 83% (95% CI 77 to 88, I2 = 86%), specificity was 72% (95% CI 68 to 75, I2 = 52%), PPV was 13% (95% CI 7 to 19, I2 = 90%), and negative predictive value was 99% (95% CI 97 to 99, I2 = 50%). The subgroup analyses for aortic stenosis and mitral regurgitation detection yielded analogous outcomes. In conclusion, AI-driven ECG offers high accuracy in VHD screening. However, its low PPV indicates the need for a combined approach with clinical judgment, especially in primary care settings.
Keywords: aortic stenosis, artificial intelligence, electrocardiograms, mitral regurgitation, valvular heart disease
Valvular heart diseases (VHDs) affect 2.5% of the population and are a significant cause of morbidity and mortality.1 Late detection or misdiagnosis of VHDs can lead to severe complications, including heart failure, arrhythmias, and increased risk of death.2 The most prevalent VHDs are aortic stenosis (AS) and mitral regurgitation (MR), followed by aortic regurgitation, mitral stenosis, mitral valve prolapse (MVP), and tricuspid regurgitation.3–5 As VHDs continue to progress and potentially worsen with age and co-morbid conditions, early detection becomes important for potential interventions.3 Currently, VHDs are suspected based on ambiguous clinical symptoms and inconsistent physical examination results, especially murmurs.6 Diagnostic echocardiography is the gold standard for assessing VHDs; however, it is resource-intensive, making it impractical for large-scale screening.6 Electrocardiograms (ECGs), in contrast, are ubiquitous and are typically the first tests performed for cardiovascular diseases, including in the primary care setting. However, their utility is currently limited to detecting only specific conditions based on the criteria established by human experts.7 However, the recent surge in artificial intelligence (AI) applications, especially machine learning and deep learning, offers a novel avenue for enhancing the use of ECG as a diagnostic tool for VHDs.7 This article provides a comprehensive review and meta-analysis of VHD detection using AI-driven ECG interpretation.
Methods
This systematic review was performed according to Cochrane Collaboration guidance. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.8
We searched PubMed, MEDLINE, Cochrane Library, Embase, Elton B. Stephens Company, Web of Science, and Cumulated Index to Nursing and Allied Health Literature databases from the inception through August 20, 2023. Additional manual searches from the reference list of the included studies were performed. Search terms such as “artificial intelligence,” “deep learning,” “machine learning,” “valvular heart disease,” and “electrocardiogram” were used in various combinations. The identification and assessment of studies for inclusion was systematically performed with the inclusion and exclusion criteria described in the next section.
A total of 2 reviewers (S.S. and R.C.) independently selected and abstracted data from eligible studies. Discrepancies were resolved by discussion and consensus. The results were reviewed by senior investigators (S.V. and M. E.H.). The eligibility criteria were (1) AI use for screening or detection of VHDs; (2) use of ECG for screening or detection of VHDs; and (3) reporting of at least 1 outcome of interest, that is, positive predictive value (PPV), negative predictive value (NPV), sensitivity, specificity, or accuracy. All nonhuman studies and studies with incomplete information on the outcomes of interest or studies evaluating other cardiovascular conditions were excluded.
The outcomes assessed were accuracy (total correct predictions by AI ÷ total number of ECGs analyzed), sensitiveity (number of true VHDs detected by AI ÷ total number of confirmed VHDs), specificity (number of ÷ ECGs correctly interpreted by AI as negative for VHDs total number of normal ECGs), PPV (number of true VHDs ÷ detected by AI ÷ all VHDs detected by AI), and NPV (number of ECGs correctly interpreted by AI as negative for VHDs all ECGs reported as normal by AI). A subgroup analysis÷was conducted for individual types of VHDs, wherever feasible.
A qualitative bias evaluation was performed using the following key parameters for each study: (1) clear definition of the study population, (2) clear definition of the outcomes and outcome assessment, (3) independent assessment of the outcome parameters, and (4) identification of important confounders and prognostic factors. Publication bias was estimated visually by funnel plots.9,10 If any bias was observed, further bias quantification was measured using the Begg–Mazumdar test,11 Egger test,9 and Duval–Tweedie trim-and-fill method.12 Sensitivity analyses were performed to assess the contribution of each study to the pooled estimate by excluding studies 1 at a time.
We used the random-effects model to pool effect sizes with 95% confidence intervals (CIs) using the MedCalc Statistical Software version 20.023 (MedCalc Software Ltd, 2021; Ostend, Belgium). The heterogeneity of effects was evaluated using the Higgins I2 statistic with mild, moderate, and significant heterogeneity defined as 25%, 50%, and 75%, respectively.13
Results
Our analysis included 10 studies containing ECG data on 713,537 patients1,3–7,14–17 (Figure 1). A total of 6 studies used AI to screen for AS,1,5,14–17 4 studies for MR,1,5,6,15 3 studies for aortic regurgitation,3,5,15 1 study for mitral stenosis,5 2 studies for MVP,4,7 and 1 for tricuspid regurgitation.5 A total of 9 studies used convolution neural network (CNN), and the remaining 1 study used a combination of support vector machine (SVM), logistic regression (LR) and multilayer perceptron (MLP).4 The characteristics of individual studies are listed in Table 1. Supplementary Table 1 lists the time interval between ECGs and echocardiography, verification method to confirm VHDs, and ECG system used in the studies.
Figure 1.
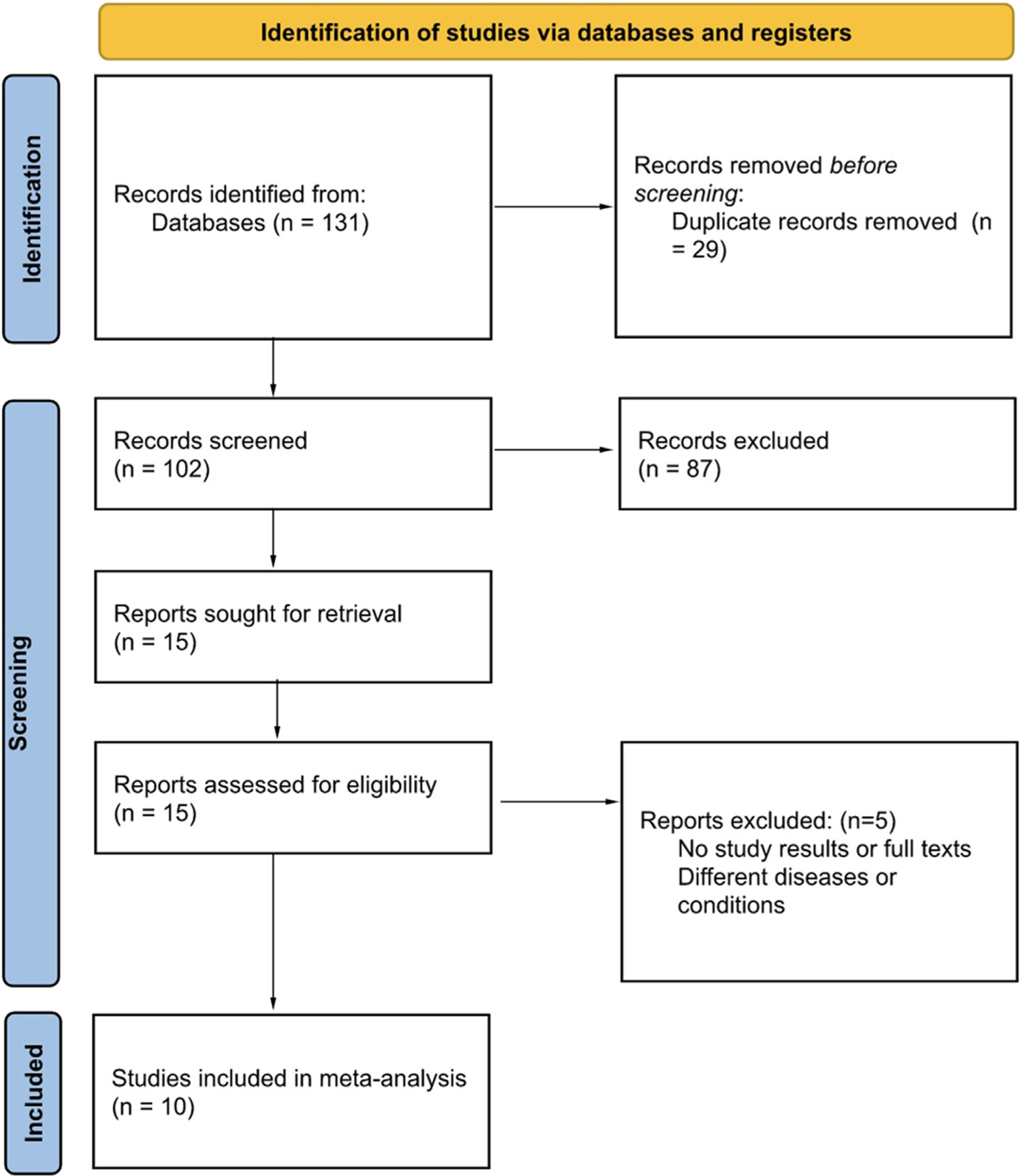
PRISMA 2020 flow diagram depicting the search strategy.
Table 1.
Study characteristics
| Study | Year | Aim | Model | Training strategy | Testing strategy |
|---|---|---|---|---|---|
| Cohen-Shelly | 2021 | ECG screening for moderatesevere AS | CNN | 129,788 random subjects | 102,926 random subjects |
| Elias | 2022 | ECG identification of moderate or severe AS, AR, and MR | CNN (ValveNet) | 43,165 patients | 21,048 patients |
| Hata | 2020 | ECG classification of AS | CNN | 128 ECG data | 44 ECG data |
| Kwon | 2020 | ECG detection of significant AS | MLP and CNN | 39,371 ECGs from 25,733 patients | 10,865 ECGs from 10,865 patients (external validation) |
| Kwon JM | 2020 | ECG detection of MR | CNN | 56,670 ECGs from 24,202 patients | 10,865 ECGs of 10,865 patients (external validation) |
| Lin | 2021 | ECG prediction of MVP | SVM, LR, MLP | 1654 subjects | 552 subjects |
| Sawano | 2022 | ECG diagnosis of significant AR | 2D-CNN, FC-DNN | 19,136 ECGs from 10,460 patients | 6,036 ECGs from 3,269 patients |
| Tison | 2019 | ECG detection of MVP | CNN-HMM–heuristic filter | 170 manually segmented ECGs | 36,186 sinus rhythm ECGs |
| Ulloa-Cerna | 2022 | ECG prediction of moderate or severe valvular disease (AS, AR, MR, MS, TR) | CNN with classification pipeline (min–max scaling, mean imputation, XGBoost classifier, and calibration) | 2,232,130 ECGs from 484,765 adults | 276058 patients |
| Vaid | 2023 | ECG identification of AS and MR | MLP and CNN | 617,338 ECG-Echo pairs from 123,096 patients (MR) 617,338 ECG-Echo pairs for 128,628 patients (AS) |
617,338 ECG-Echo pairs from 123,096 patients (MR) 617,338 ECG-Echo pairs for 128,628 patients (AS) |
2D-CNN = two-dimensional convolutional neural network; AR = aortic regurgitation; AS = aortic stenosis; CNN = convolutional neural network; ECG = electrocardiogram; FC-DNN = fully connected deep neural network; HMM = hidden Markov model; LR = logistic regression; MLP = multilayer perceptron; MR = mitral regurgitation; MS = mitral stenosis; MVP = mitral valve prolapse; SVM = support vector machine; TR = tricuspid regurgitation.
No significant publication bias was observed in the primary and safety outcomes analyses using funnel plots. However, it should be noted that AI research, particularly, in its early phases of application in cardiovascular medicine, tends to highlight positive results. The sensitivity analysis revealed that a single study did not drive the results. We deemed all the studies at a high risk of bias because of the pooling of different VHDs with different underlying etiologies and variability of co-morbidities in the study groups.
The pooled accuracy of AI for the diagnosis of VHDs using ECG was 81% (95% CI 73 to 89, I2 = 92%), sensitivity was 83% (95% CI 77 to 88, I2 = 86%), specificity was 72% (95% CI 68 to 75, I2 = 52%), PPV was 13% (95% CI 7 to 19, I2 = 90%), and NPV was 99% (95% CI 97 to 99, I2 = 50%) (Figure 2).
Figure 2.

Pooled accuracy, sensitivity, positive predictive value, and negative predictive value of AI-driven ECG diagnosis of valvular heart diseases from meta-analysis of the included studies.
The subgroup analysis for detection of AS showed a pooled accuracy of 86% (95% CI 73 to 96, I2 = 93%), sensitivity of 88% (95% CI 81 to 93, I2 = 68%), specificity of 71% (95% CI 65 to 76, I2 = 32%), PPV of 12% (95% CI 9 to 16, I2 = 15%), and NPV of 99% (95% CI 98 to 100, I2 = 0%) (Table 2). Similarly, the subgroup analysis for screening of MR revealed a pooled accuracy of 86% (95% CI 62 to 99, I2 = 95%), sensitivity of 88% (95% CI 83 to 92, I2 = 28%), specificity of 69% (95% CI 61 to 76, I2 = 55%), PPV of 23% (95% CI 5 to 48, I2 = 95%), and NPV of 97% (95% CI 90 to 100, I2 = 85%) (Table 2).
Table 2.
Pooled outcomes for types of valvular heart diseases
| Pooled rates | Accuracy | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| AS | 86.36 (73.05 to 95.62) I2 = 93.22% 5 studies |
87.56 (81.27 to 92.70) I2 = 68.60% 4 studies |
70.88 (65.36 to 76.10) I2 = 32.26% 4 studies |
12.21 (8.96 to 15.88) I2 = 15.39% 4 studies |
98.84 (97.57 to 99.65) I2 = 0.00% 4 studies |
| MR | 86.00 (62.03 to 98.94) I2 = 95.97% 3 studies |
87.50 (82.78 to 91.56) I2 = 28.48% 3 studies |
68.73 (60.65 to 76.27) I2 = 55.71% 3 studies |
22.96 (5.44 to 47.85) I2 = 95.46% 3 studies |
96.89 (89.79 to 99.92) I2 = 85.53% 3 studies |
Values in pooled proportions (95% confidence intervals).
AS = aortic stenosis; MR = mitral regurgitation; NPV = negative predictive value; PPV = positive predictive value.
Discussion
Our study fills a critical gap by providing the most comprehensive meta-analysis to date evaluating the value of ECG-based AI for screening for VHDs. The pooled accuracy, sensitivity, specificity, and NPV of AI were exceptionally high, confirming the significant potential of this technology. However, the PPV was notably low. Several factors could contribute to this low PPV. A potential reason could be the inherent variability in ECG recordings. In addition, factors such as patient positioning, electrode placement, and minor arrhythmias can affect ECG readings, potentially influencing the AI’s interpretation.18
The observed low PPV, juxtaposed with a high NPV, has significant clinical implications. Although AI-driven ECG interpretation exhibits a strong potential as a first-line screening tool to exclude VHDs, its current capacity to positively diagnose the disease is limited.1 Compared with traditional diagnostic methods, AI-driven ECG interpretation provides faster results; however, reliability remains a concern. In primary care or first-contact medical scenarios where ruling out VHDs is paramount, AI-driven ECG interpretation could be instrumental. Nevertheless, for diagnostic purposes or in specialty clinics, it may be prudent not to rely solely on AI for definitive diagnosis. Collaborative diagnosis, where AI interpretation is vetted with clinical judgment, may mitigate this limitation.
Another combined screening method for detecting VHDs could enhance the PPV, as demonstrated by Ulloa-Cerna et al.5 Furthermore, the significant correlation observed by Vaid et al1 between AI-detected AS and patients who underwent transcatheter procedures underscores the benefits of incorporating AI into clinical practice. This is supported by the high pooled accuracy observed in our study.
The subgroup analyses for detecting AS and MR yielded similar results. The significance of these findings is heightened given the increasing importance of early VHD diagnosis, especially because previous studies have highlighted improved outcomes with timely intervention in asymptomatic patients.19 Especially in resource-limited settings where advanced diagnostic tools such as echocardiography may not be readily available, the potential for AI-driven ECG interpretation is paramount. Such settings could benefit immensely from the cost-effectiveness and rapidity of AI-driven ECGs.
Given the increasing popularity and accessibility of wearable health devices, such as smartwatches and fitness bands, the potential for AI-driven ECG interpretation for patient self-monitoring becomes even more significant. It can empower patients to monitor their heart health and seek timely medical interventions. However, the accuracy of readings from these devices, potential data overload for health care professionals, and patient anxiety from false alarms are challenges that need addressing.
Notably, 1 of the pioneering studies by Tison et al7 used a fusion of CNN and hidden Markov models for MVP detection, revealing an area under the receiver operating characteristic of 0.77 (95% CI 0.76 to 0.78). This accuracy was slightly lower for MVP than for other cardiac pathologies, likely attributed to the subtle impact of MVP on ECG.7 This was further corroborated by a subsequent study by Lin and Zeng,4 which used different machine learning models such as SVM, LR, and MLP.
The heterogeneity in our analysis stemmed largely from the diverse AI models used across studies. For instance, although CNN models were predominant, the synergistic application of SVM, LR, and MLP in the included studies warrants attention. SVM is particularly adept at high-dimensional spaces, making it apt for intricate ECG data. LR offers probabilistic outcomes, presenting a nuanced risk assessment, whereas MLP identifies nonlinear relations in ECG signals. Their combined utility has the potential to provide a more comprehensive interpretation of ECG data, capturing informational facets that CNNs may overlook. Yet, as we venture further into the realm of AI-driven health care, it is crucial to ensure that patient consent is obtained transparently, especially when it comes to data sharing and AI analysis. Ethical guidelines need to be established to ensure that the patients’ rights and privacy are not compromised.
In envisioning future AI-driven ECG interpretation, we anticipate a shift toward studies that combine advanced AI methods with comprehensive clinical validation. This would ideally include real-time validation of AI across diverse patient groups, confirming the method’s broad applicability. Consistently improving the AI in response to real-world data can enhance their precision and versatility. Moreover, collaborations between AI experts and clinicians are necessary to ensure that AI remains relevant to clinical practice.
Our study has several limitations. First, the lack of external validation in some studies curtails the generalizability of our results. These limitations imply that although AI-driven ECG offers a promising avenue for VHD screening, its application in diverse clinical settings requires cautious optimism. Second, the analysis was limited by the inherent bias of the retrospective design of the included studies. In addition, the use of identical data sets for training and testing in some studies could have inadvertently inflated the reported accuracies. Moreover, the limited studies on VHDs other than AS and MR rendered us incapable of conducting a comprehensive subgroup analysis for all VHD types.
In conclusion, although AI-driven ECG offers high accuracy in VHD screening, its low PPV limits definitive diagnosis. Collaboration between AI and clinical judgment can optimize its clinical utility, especially in primary care scenarios. Moving forward, further research should delve into optimizing AI algorithms, considering diverse age groups, ethnicities, and geographical locations. Exploring their utility in diverse patient populations and collaborating with interdisciplinary teams, including cardiologists, data scientists, and ethicists, will be crucial. Assessing their potential in tandem with other diagnostic tools is also recommended.
Supplementary Material
Funding:
T32HL129964 grant supported Dr. Chaudhary.
Footnotes
Declaration of competing interest
The authors have no competing interest to declare.
CRediT authorship contribution statement
Sahib Singh: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Rahul Chaudhary: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Kevin P. Bliden: Methodology, Supervision, Writing – review & editing. Udaya S. Tantry: Methodology, Supervision, Writing – review & editing. Paul A. Gurbel: Methodology, Supervision, Writing – review & editing. Shyam Visweswaran: Conceptualization, Methodology, Supervision, Writing – review & editing. Matthew E. Harinstein: Conceptualization, Methodology, Supervision, Writing –review & editing.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2023.12.015.
References
- 1.Vaid A, Argulian E, Lerakis S, Beaulieu-Jones BK, Krittanawong C, Klang E, Lampert J, Reddy VY, Narula J, Nadkarni GN, Glicksberg BS. Multi-center retrospective cohort study applying deep learning to electrocardiograms to identify left heart valvular dysfunction. Commun Med (Lond) 2023;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Writing Committee M, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:450–500. [DOI] [PubMed] [Google Scholar]
- 3.Sawano S, Kodera S, Katsushika S, Nakamoto M, Ninomiya K, Shinohara H, Higashikuni Y, Nakanishi K, Nakao T, Seki T, Takeda N, Fujiu K, Daimon M, Akazawa H, Morita H, Komuro I. Deep learning model to detect significant aortic regurgitation using electrocardiography. J Cardiol 2022;79:334–341. [DOI] [PubMed] [Google Scholar]
- 4.Lin GM, Zeng HC. Electrocardiographic machine learning to predict mitral valve prolapse in young adults. IEEE Access 2021;9:103132–103140. [Google Scholar]
- 5.Ulloa-Cerna AE, Jing L, Pfeifer JM, Raghunath S, Ruhl JA, Rocha DB, Leader JB, Zimmerman N, Lee G, Steinhubl SR, Good CW, Haggerty CM, Fornwalt BK, Chen R. rECHOmmend: an ECG-based machine learning approach for identifying patients at increased risk of undiagnosed structural heart disease detectable by echocardiography. Circulation 2022;146:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon JM, Kim KH, Akkus Z, Jeon KH, Park J, Oh BH. Artificial intelligence for detecting mitral regurgitation using electrocardiography. J Electrocardiol 2020;59:151–157. [DOI] [PubMed] [Google Scholar]
- 7.Tison GH, Zhang J, Delling FN, Deo RC. Automated and interpretable patient ECG profiles for disease detection, tracking, and discovery. Circ Cardiovasc Qual Outcomes 2019;12:e005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hr objartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, R€ucker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 11.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 12.Duval S, Tweedie RR. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen-Shelly M, Attia ZI, Friedman PA, Ito S, Essayagh BA, Ko WY, Murphree DH, Michelena HI, Enriquez-Sarano M, Carter RE, Johnson PW, Noseworthy PA, Lopez-Jimenez F, Oh JK. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur Heart J 2021;42:2885–2896. [DOI] [PubMed] [Google Scholar]
- 15.Elias P, Poterucha TJ, Rajaram V, Moller LM, Rodriguez V, Bhave S, Hahn RT, Tison G, Abreau SA, Barrios J, Torres JN, Hughes JW, Perez MV, Finer J, Kodali S, Khalique O, Hamid N, Schwartz A, Homma S, Kumaraiah D, Cohen DJ, Maurer MS, Einstein AJ, Nazif T, Leon MB, Perotte AJ. Deep learning electrocardiographic analysis for detection of left-sided valvular heart disease. J Am Coll Cardiol 2022;80:613–626. [DOI] [PubMed] [Google Scholar]
- 16.Hata E, Seo C, Nakayama M, Iwasaki K, Ohkawauchi T, Ohya J. Classification of aortic stenosis using ECG by deep learning and its analysis using grad-CAM. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:1548–1551. [DOI] [PubMed] [Google Scholar]
- 17.Kwon JM, Lee SY, Jeon KH, Lee Y, Kim KH, Park J, Oh BH, Lee MM. Deep learning-based algorithm for detecting aortic stenosis using electrocardiography. J Am Heart Assoc 2020;9:e014717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garc ıa-Niebla J, Llontop-Garc ıa P, Valle-Racero JI, Serra-Autonell G, Batchvarov VN, de Luna AB. Technical mistakes during the acquisition of the electrocardiogram. Ann Noninvasive Electrocardiol 2009;14:389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgartner H, Iung B, Otto CM. Timing of intervention in asymptomatic patients with valvular heart disease. Eur Heart J 2020; 41:4349–4356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


