Abstract
Prenatal exposure to inflammation via maternal infection, allergy, or autoimmunity increases one’s risk for developing neurodevelopmental and psychiatric disorders. Many of these disorders are associated with altered social behavior, yet the mechanisms underlying inflammation-induced social impairment remain unknown. We previously found that a rat model of acute allergic maternal immune activation (MIA) produced deficits like those found in MIA-linked disorders, including impairments in juvenile social play behavior. The neuropeptides oxytocin (OT) and arginine vasopressin (AVP) regulate social behavior, including juvenile social play, across mammalian species. OT and AVP are also implicated in neuropsychiatric disorders characterized by social impairment, making them good candidate regulators of social deficits after MIA.
We profiled how acute prenatal exposure to allergic MIA changed OT and AVP innervation in several brain regions important for social behavior in juvenile male and female rat offspring. We also assessed whether MIA altered additional behavioral phenotypes related to sociality and anxiety. We found that allergic MIA increased OT and AVP fiber immunoreactivity in the medial amygdala and had sex-specific effects in the nucleus accumbens, bed nucleus of the stria terminalis, and lateral hypothalamic area. We also found that MIA reduced ultrasonic vocalizations in neonates and increased the stereotypical nature of self-grooming behavior. Overall, these findings suggest that there may be sex-specific mechanisms underlying MIA-induced behavioral impairment and underscore OT and AVP as ideal candidates for future mechanistic studies.
Keywords: Maternal Immune Activation, Allergy, Social Play, Social Behavior, Oxytocin, Vasopressin, Sex Differences
1. INTRODUCTION
Inflammation caused by maternal infection, allergy, autoimmunity, or environmental pollutants during pregnancy increases the offspring’s risk for attention-deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and schizophrenia (Brown et al., 2004; Canetta et al., 2014; Chen et al., 2014; Han et al., 2021; Instanes et al., 2017; Roberts et al., 2013; Zerbo et al., 2015). The neurobiological mechanisms that mediate the risk conferred by prenatal inflammatory experiences remain poorly understood. Rodent models of maternal immune activation (MIA) allow for the interrogation of how prenatal inflammation alters brain development to increase the likelihood for neurobehavioral impairment. Broadly, rodent studies of MIA produce offspring with behaviors that mimic symptoms associated with neurodevelopmental disorders, such as sensorimotor gating issues (Haddad et al., 2022; Mattei et al., 2014; Smith et al., 2007), anxiety-like behavior (Canetta et al., 2016; Penteado et al., 2014; Smith et al., 2007), locomotor hyperactivity (Breach et al., 2021), repetitive behaviors (Choi et al., 2016; Kirsten & Bernardi, 2017; Kirsten et al., 2012; Malkova et al., 2012), and cognitive deficits (Breach et al., 2021; Canetta et al., 2016; Fernandez de Cossio et al., 2021; Kirsten et al., 2012).
Most disorders associated with MIA involve deficits in social behavior, and most MIA rodent models induce social impairment (Bauminger et al., 2003; Block et al., 2022; Breach et al., 2021; Carbone et al., 2023; Choi et al., 2016; Gzielo et al., 2021; Kirsten et al., 2010; Malkova et al., 2012; Normand et al., 2019; Schwartzer et al., 2013; Smith et al., 2020; Smith et al., 2007; Taylor et al., 2012). We previously found that prenatal exposure to acute allergic MIA impairs juvenile social play in rats (Breach et al., 2021). Social play is an adaptive behavior that can be observed across many mammalian species, especially during the juvenile period (Pellegrini et al., 2007; Thor & Holloway, 1984). Among children, social play is correlated with the development of important skills, contributing to social competence (Newton & Jenvey, 2011; Palagi, 2018). Similarly, rodent studies suggest that juvenile social play facilitates the development of multiple cognitive and socioemotional skills (Pellis et al., 2023). Thus, understanding the mechanisms of MIA-induced social impairment has positive implications for other aspects of development.
The nonapeptides oxytocin (OT) and arginine vasopressin (AVP) are logical candidates for investigation. OT and AVP influence socioemotional behavior across many species (Bredewold & Veenema, 2018; Caldwell, 2017; Guastella et al., 2010; Guastella et al., 2011; Kosfeld et al., 2005; Shahrestani et al., 2013; Thompson et al., 2006). Several studies also implicate OT and AVP in disorders linked to MIA. For example, certain polymorphisms in genes related to OT or AVP signaling are associated with a diagnosis of ASD or schizophrenia (Loparo & Waldman, 2015; Montag et al., 2013), and with social deficits in ASD and ADHD (Baribeau et al., 2017; Kalyoncu et al., 2019). Autistic children have reduced AVP in the cerebrospinal fluid, and AVP negatively correlates with symptom severity (Oztan et al., 2018). Lower levels of peripheral OT also correlate with higher symptom severity in schizophrenia and ADHD (Sasaki et al., 2015; Strauss et al., 2019). Intranasal administration of OT or AVP improves social deficits in ASD (Huang et al., 2021; Parker et al., 2019). Collectively, these findings suggest OT and AVP as likely candidates in the regulation of social deficits in animal models of MIA.
Despite the wealth of evidence suggesting that OT and AVP may regulate social deficits in MIA-associated neuropsychiatric and developmental disorders, only one other study has looked at these nonapeptides following MIA, with analyses limited to AVP (Taylor et al., 2012). In the present study, we investigated whether acute exposure to allergic MIA perturbed OT and AVP fiber density in several brain regions important for social behavior: the lateral septum, nucleus accumbens, bed nucleus of the stria terminalis, the hypothalamus, and medial amygdala. We also expanded upon our previous work by assessing additional behavioral phenotypes associated with sociality and anxiety.
We chose to employ a model of allergy-induced MIA due to the body of evidence demonstrating that maternal allergy or atopy increases risk for neurodevelopmental impairment (Chen et al., 2016; Han et al., 2021; Instanes et al., 2017; Lyall et al., 2014). Despite this evidence, most rodent studies of MIA simulate bacterial or viral infection via injection of lipopolysaccharide (LPS) or polyinosinic-polycytidylic acid (Poly I:C), respectively. While the mechanisms of these two models are slightly different (e.g. LPS activates toll-like receptor (TLR)4 while Poly I:C activates TLR3), both trigger the NFkB inflammatory pathway to produce Th1-like cytokine responses (Meyer, 2014). In contrast, allergy or atopy acts through an alternative inflammatory cascade to trigger a Th2-like cytokine response (Schwartzer et al., 2015; Sharkhuu et al., 2010). Thus, allergy-induced inflammation may have unique effects on fetal development that warrant investigation. Additionally, rates of allergies are also increasing (Gupta et al., 2019; Zhang & Zhang, 2019), making acute allergic MIA an increasingly relevant perturbation to study in the context of neurodevelopment.
Importantly, we included both male and female rats in this study due to the fact that disorders associated with MIA exhibit sex biases, such that males are more frequently diagnosed (Danielson et al., 2018; Zablotsky et al., 2017), have earlier onset (Abel et al., 2010), or exhibit different symptomology compared to females (Breach & Lenz, 2023). Additionally, the effects of OT and AVP on social or anxiety-like behavior can be sex-specific in rodents (Bredewold & Veenema, 2018) and humans (Feng et al., 2015; Thompson et al., 2006). Overall, the current study is the most comprehensive analysis of MIA-induced changes to OT and AVP signaling in the brain to date. Our findings have implications for our broader understanding of immune regulation of neurodevelopment and social behavior.
2. METHODS
2.1. Animals and Prenatal Allergic Inflammation Paradigm
Methodological details provided in accordance with previously published guidelines (Kentner et al., 2019) are available in the Supplementary Methods. All experimental procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Experiments were conducted with Sprague Dawley rats. All rats were group housed (except during gestation) and were maintained on a 12-hour light/dark cycle (lights on from 6:00 AM - 6:00 PM) in individually ventilated cages. Food and water were available ad libitum. See Figure 1 for the experimental timeline.
Figure 1:
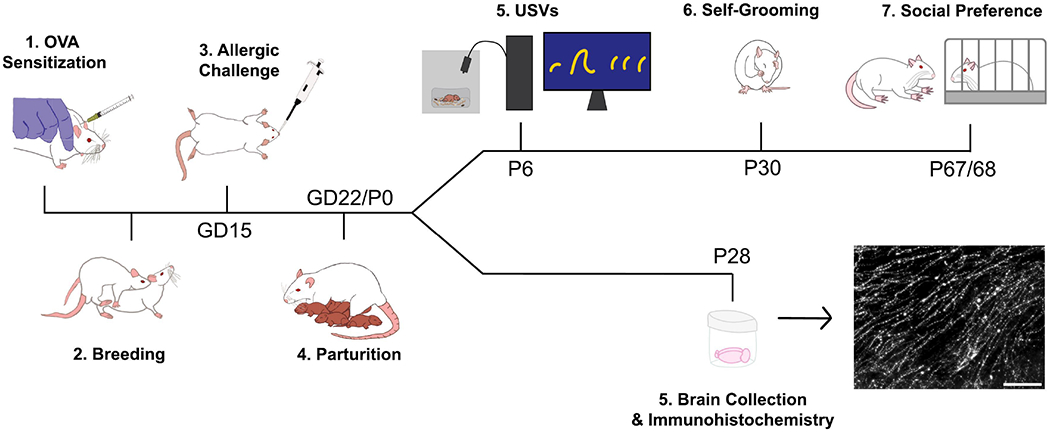
Experimental timeline. Vehicle and MIA offspring subjected to behavioral testing underwent isolation-induced ultrasonic vocalization testing on P6, self-grooming assessment on P30, and social preference testing on P67 or 68. Offspring employed for OT and AVP analyses were sacrificed on P28 and brains were collected and processed for OT and AVP immunohistochemistry.
Adult virgin female rats purchased from Envigo Laboratories (Indianapolis, IN) were randomly assigned to either the allergic inflammation (MIA) or control (Vehicle; Veh) group. Rats that were randomly assigned to the allergic inflammation group (n = 7) were sensitized to ovalbumin (e.g., egg albumin) using the procedure conducted in our previous studies (Breach et al., 2022; Breach et al., 2021; Lenz et al., 2019). Allergy dams received a subcutaneous injection of ovalbumin-alum adjuvant preparation, which was composed of 1mg ovalbumin (OVA grade V, Sigma, A5503-1G) prepared at 4mg/ml in pyrogen-free 0.9% saline and precipitated at a 1:1 ratio with Al(OH)3 (Thermo Scientific, 77161). Two weeks following the first injection, a second injection of the ovalbumin-alum preparation was administered. Female rats assigned to the control group (n = 7) were administered injections of saline vehicle at the same two timepoints to control for handling and injection stress.
At least one week following the sensitization procedure, females were paired with breeder males (one male: two females) between 4:00 - 6:00 PM and left to breed overnight. Breeder males were grouped with one control and one experimental female whenever possible. Between 7:00 - 8:00 AM the following morning, females were separated from breeder males and vaginal lavages were performed to check for the presence of sperm. The date of sperm-positive lavage was designated as gestational day (GD)0 and the pregnant rat was moved to her own cage for the rest of gestation. To verify pregnancy, bodyweight was monitored throughout breeding and gestation (approximately every three days). On projected GD15 (based on the date of sperm-positive lavage), pregnant rats were lightly anesthetized with isoflurane and exposed to either the allergen or vehicle via intranasal infusion of 1% ovalbumin in saline or saline vehicle (50uL/nare). This procedure induces inflammation in both the dam and fetuses (Breach et al., 2021; Lenz et al., 2019), confers significant social and cognitive impairment in offspring (Breach et al., 2021), and alters the developmental trajectory of dendritic spine density across several brain regions (Breach et al., 2022). Following intranasal infusion, dams were returned to their home cage and remained undisturbed for the rest of pregnancy, except for regular husbandry practices.
As GD22 approached, cages were checked daily to determine the day of birth for the offspring, noted as postnatal day (P)0. All dams gave birth on projected GD22. Observations of maternal behavior were conducted on a subset of dams (4 MIA, 4 vehicle) in the home cage during three to four 10-minute-long sessions between P0-P8, and the mean percent of total time spent in various behaviors was calculated. Offspring sex was determined between P0-P3. No more than two rats/sex/litter were assigned to each experimental endpoint. 18 vehicle (8 male, 10 female) and 21 MIA (9 male, 12 female) offspring were employed for immunohistochemical analysis while 20 vehicle (10 male, 10 female) and 20 MIA (10 male, 10 female) offspring were employed for behavioral testing. Supplementary Methods contain more details on litters.
2.2. Behavioral Testing
On P5, two pups/sex/litter were randomly selected for behavioral testing. These pups received unique paw tattoos to facilitate identification throughout the study. Offspring selected for behavioral testing were then assessed on ultrasonic vocalization (USV) behavior on P6, self-grooming behavior on P30, and sociability between P67-68.
2.2.1. Isolation-Induced Ultrasonic Vocalizations
The protocol used for assessing USVs was adapted from Potasiewicz et al (Potasiewicz et al., 2020). During the light cycle on P6 (between 8:30 - 10:00 AM), the first pup to be tested from a litter was removed from its home cage and placed in a plastic tub (15.5 x 11 x 7.5 cm) containing a small amount of home cage nesting material. The pup’s body temperature was taken using an infrared thermometer (Hunan Electric General Guokang Medical, GK128B), and then the pup was placed into a sound-attenuating chamber made of foam (27 x 27 x 27 cm) for 5 minutes. An ultrasound microphone (Pettersson Elektronik) was used to record vocalizations and the recorded signals were filtered and amplified using the Ultravox system (Noldus Information Technology). Following testing, the pup’s body weight was recorded, and temperature was taken again to assess body temperature changes during testing. The pup was then placed in a huddle maintained at 37 ± 1°C by a heating pad while the remaining three pups from the litter were being tested. The pups were returned to their home cages after all littermates were tested. The mean time to complete testing for a litter was 29 minutes (range 27-35 minutes). The number of vocalizations was detected and quantified using DeepSqueak 3.0.4 in MATLAB (Coffey et al., 2019). Audio files were run through DeepSqueak’s “Rat detector YOLO R1” neural network, then a post-hoc denoiser excluded non-USV related noise from the dataset. Call statistics for accepted USVs (30-130 kHz) were calculated using the detection network output.
2.2.2. Novelty-Induced Self-Grooming Behavior
Because juvenile Sprague Dawley rats groom themselves the most from P27-P33 (Thiels et al., 1990), we chose to assess self-grooming behavior on P30. Shortly after the beginning of the dark cycle (6:00 PM), rats were transported from the vivarium to the behavioral testing hallway. Each rat was brought into the testing room and placed into a plexiglass behavioral arena (49.5 x 38 x 32 cm) with a transparent wall to enable visualization of grooming behavior. Rats were video recorded for 10 minutes under red light from two angles (arial view and side view) before being returned to their home cages. The frequency and duration of grooming bouts were scored, and the microstructure of each grooming bout was recorded by an experimenter blind to condition according to a protocol adapted from Kauleff et al. (Kalueff et al., 2007). Grooming bout structure was coded using the following key: (0) no grooming, (1) paw licking/snout stroking, (2) face/head washing, (3) body licking, (4) leg licking, and (5) tail/genital grooming. Since grooming has cephalocaudal syntax, the following grooming transitions were considered ‘correct’, or ‘stereotyped’: 0-1, 1-2, 2-3, 3-4, 4-5, and 5-0. Transitions considered ‘incorrect’ are chaotic and included skipped (e.g. 2-4), reversed (e.g. 3-1), prematurely terminated/interrupted (e.g. 3-0), or incorrectly initiated transitions (e.g. 0-5). A ‘complete’ syntactic chain was counted if a rat grooming bout contained a sequence of 01234 or 012345, as tail/genital grooming is not always considered part of the cephalocaudal syntactic chain (Kalueff et al., 2007). The percentage of correct transitions, interrupted grooming bouts, and syntactic chain completion were calculated for each animal. The frequency of rearing behavior was also scored.
2.2.3. Three-Chamber Social Preference
On P67 or 68, rats underwent sociability testing in a three-chamber apparatus during the light phase of the light cycle (between 8:00 AM - 5:30 PM). Immediately prior to the testing trial, rats were placed into the center of a three-chamber apparatus (91.5 x 61 x 13.5 cm) and allowed to explore the center compartment for five minutes. Following this habituation period, the rat was placed into a holding cage while a novel breeder male previously habituated to the handling procedure was secured underneath a wire cage in one of the side chambers. An empty wire cage was secured to the opposite side chamber. The chamber doors were then removed, and the experimental rat was returned to the center of the chamber and allowed to explore for 10 minutes. The location and identity of the novel breeder male was counterbalanced across groups. The testing phase was recorded from a camera suspended above the apparatus, and an experimenter blind to treatment scored the time spent investigating the stimulus rat versus the empty wire cage. Investigation was defined as the rat directing its nose towards the object within approximately three centimeters of the cage or physically interacting with the cage (e.g. climbing). The social preference index was calculated as the total time spent investigating the stimulus rat divided by the time spent investigating either the rat or the empty wire cage.
2.3. Tissue Collection and Immunohistochemical Staining
On P28, rats were deeply anesthetized via intraperitoneal injections of Euthasol (Virbac) and then transcardially perfused with 0.01M phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Brains were collected, post-fixed overnight in 4% paraformaldehyde, and then stored in 30% sucrose solution. Once saturated with sucrose, brain tissue was sectioned coronally onto slides into 6 series at 30um using a CM1950 Cryostat (Leica Biosystems).
For OT immunostaining, brain sections from one series of tissue were washed in 0.01M PBS and incubated in 50% methanol for 30 minutes. Then, sections were rinsed in PBS and subjected to antigen retrieval in Tris-EDTA (10 mM Tris base, 1 mM EDTA solution, 0.05% Tween 20, pH 9.0) for 10 minutes at 90°C. Sections were then permeabilized for 60 minutes in 0.4% Triton X-100 in PBS (PBST), blocked for 60 minutes in 5% normal donkey serum (NDS) in PBST, and incubated overnight in primary antiserum against oxytocin (Millipore Sigma, rabbit, AB911, 1:500) at 4°C. On day 2, sections were rinsed five times in PBS and incubated in the dark for 120 minutes at room temperature in secondary solution containing donkey anti-rabbit (Invitrogen, A32795, 1:200) secondary antibody in 2.5% NDS in PBST. Sections were subsequently rinsed in PBS, counterstained with DAPI, and coverslipped with Prolong Diamond Antifade Mountant (Invitrogen; P36970).
For AVP immunostaining, brain sections from a separate series of tissue from the same animals were washed in 0.01M PBS and incubated in 50% methanol for 30 minutes. Then, sections were rinsed in PBS and subjected to antigen retrieval in Tris-EDTA for 10 minutes at 90°C. Sections were then blocked for 60 minutes in 5% NDS in PBST, and incubated overnight in primary antiserum against vasopressin (BMA Biomedicals, rabbit, T4563 1:14,000) in 2% NDS in PBS-T at 4°C. On day 2, sections were rinsed five times in PBST and incubated in the dark for 120 minutes at room temperature in secondary solution containing donkey anti-rabbit (Invitrogen, A32795, 1:200) secondary antibody in 2% NDS in PBST. Sections were subsequently rinsed in PBS, counterstained with DAPI, and coverslipped with Prolong Diamond Antifade Mountant (Invitrogen, P36970).
2.4. Oxytocin and Vasopressin Cell Counting Analysis
The majority of OT and AVP is synthesized in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus (Caldwell, 2017). Thus, we counted OT and AVP immunoreactive (ir) cells in these regions. Regions were identified based on gross morphological criteria while referencing the Paxinos and Watson atlas (Paxinos & Watson, 1998). Approximate locations of the regions relative to bregma (as would be found in the adult rat brain) are −0.92 mm through −2.12 mm for both the PVN and SON.
For each rat, one image/ROI/hemisphere was acquired at 10x for up to four (PVN) or five (SON) sections per animal with a Zeiss Axioimager M2 microscope and a CX9000 Digital Camera using Stereoinvestigator Software. Immunoreactive cells were counted manually using FIJI and the average number of cells/um2 of ROI area was calculated.
Previous work in mice has also shown that extrahypothalamic OT neurons in the bed nucleus of the stria terminalis (BNST) influence behavior relating to social anxiety in mice (Duque-Wilckens et al., 2020). Thus, we acquired images at 2.5x across all hemispheres containing the anterior (−0.26 through −0.40 mm) and posterior (−0.80 through −1.30 mm) BNST using the same microscope employed for PVN and SON imaging. The average number of cells per hemisphere for each animal was quantified. AVP cells in the BNST and medial amygdala are also known to modulate social behavior (Rigney et al., 2019); however, similar to a previous study conducted in juvenile rats (Reppucci et al., 2018), we did not see consistent expression of sufficient AVP cells in these regions to enable quantification. Experimenters were blind to condition during all image acquisition and quantification for all analyses.
2.5. Oxytocin and Vasopressin fiber area analysis
We focused our fiber area analyses on several brain regions that receive nonapeptide innervation and are important for regulation of social behavior: the lateral septum (LS), nucleus accumbens core and shell (NAcc and NAcs), the anteromedial and medioventral bed nucleus of the stria terminalis (BNSTam and BNSTmv), lateral hypothalamic area (LHA), and medial amygdala (MeA). As with cell counting analyses, regions were identified based on gross morphological criteria (see Table 1 for region-specific coordinates and imaging information). Z-stack max projection images were acquired by an experimenter blind to condition using the same microscope employed for cell count imaging. Images were adjusted to ensure equivalent contrast and debris was excised manually prior to thresholding and area quantification in FIJI. As conducted in other work (DiBenedictis et al., 2017), the values for all images for one section were summed and the mean fiber area/section were calculated for each animal. For all regions, experimenters were blind to condition during image acquisition and quantification. Importantly, since OT and AVP are released from both axons and dendrites (Caldwell, 2017; van den Pol, 2012), the staining methods used in this study label both types of processes. Thus, the term “fiber” refers to both axons and dendrites. Changes in fiber area may also reflect an increase in the number of axons or dendrites or an increase in the neuropeptide content within them.
Table 1:
List of brain regions analyzed for fiber density analyses. Coordinates are what would be found in the adult brain relative to bregma, based on Paxinos & Watson (1998).
| Region | Approximate Atlas Coordinates | Imaging Magnification | Images per Section | Sections per Animal |
|---|---|---|---|---|
| LS | +1.20 to −0.40 mm | 20x | 6 | 3 |
| NAcc (OT only) | +1.70 to +0.70 mm | 40x | 12 | 2-3 |
| NAcs | +1.70 to +0.70 mm | 20x | 6 | 2-3 |
| BNSTam | −0.26 to −0.40 mm | 20x | 2 | 2-3 |
| BNSTmv | −0.26 to −0.40 mm | 20x | 2 | 2-3 |
| LHA | −0.92 to −2.12 mm | 20x | 4 (OT), 2 (AVP) | 4-5 (OT), 2 (AVP) |
| MeA | −1.80 to −3.30 mm | 20x | 4 | 2-4 |
2.6. Statistical Analyses
Ultrasonic vocalization data, overall grooming measures, and social preference data were each analyzed via two-way ANOVAs using GraphPad Prism software. Maternal observation data was analyzed using a two-way repeated measures ANOVA in GraphPad Prism. Grooming bout microstructure was analyzed via three-way repeated measures ANOVA using GraphPad Prism. For immunohistochemical analysis, initial inspection of the data suggested potential effects of litter and cohort. Thus, statistical testing for these data was conducted using R statistical software to enable more appropriate analyses via the lme4 and lmerTest libraries. For each stain (OT and AVP) in each region, normality assessments were conducted using Shapiro-Wilks testing and qqplot visualization. As appropriate, common box cox transformations were applied to the data based on lambda values to normalize non-normal distributions (See Supplementary Table 2). Initial linear mixed effects models were fit to the data with sex, maternal treatment, and their interaction as fixed effects with litter and cohort included as random intercepts. Simplified random effects models (e.g. just cohort or just litter) or fixed-effects only models (two-way ANOVAs) were used when there was insufficient data supporting the estimation of random effects. P-values for all linear mixed effects analyses were obtained through likelihood ratio testing of the full model with the effect in question against the model without the effect in question.
For all data, group outliers were detected and removed via ROUT (Q = 1%) prior to statistical testing. Statistical significance was set at α = 0.05. Sidak’s post-hoc tests were conducted when significant interactions were found, and adjusted P-values are reported. Effect sizes for ANOVA tests are reported as η2. Effect sizes for linear mixed models are reported as β values, which, due to the categorical nature of our independent variables, represent standardized mean differences (equivalent to Cohen’s d). Graphs were generated in GraphPad Prism and aesthetically modified in Adobe Illustrator. Data are presented in original units as mean ± SEM with individual datapoints depicted over bars. Detailed information on box-cox transformations, specific statistical tests run, and statistical test output are available in the Supplementary Tables.
3. RESULTS
3.1. Maternal Behavior and USVs
MIA did not affect maternal behavior, as no group differences or interactions were found in our maternal observations (Fig. 2; F’s ≤ 0.47, p’s ≥ 0.79). Regardless of treatment group, dams spent most of their time either nursing pups or off the nest (F1.6, 9.5 = 21.37, p = 0.005, η2 = 0.76). However, MIA reduced the number of calls made during the USV testing session (Fig. 3A; F1, 36 = 5.6, p = 0.02, η2 = 0.13), which suggests that MIA impairs offspring communication independent of maternal care. Sex did not affect USV call number (F1, 36 = 0.16, p = 0.69), and there was no interaction (F1, 36 = 0.045, p = 0.83). Neither MIA nor sex affected body weight (F’s1, 36 ≤ 1.18, p’s ≥ 0.28; mean = 13.85 g; SEM = 0.27) or the change in body temperature (F’s1, 36 ≤ 1.14, p’s ≥ 0.29; mean = −0.30 °C; SEM = 0.028;) over the 5-minute USV testing period.
Figure 2:
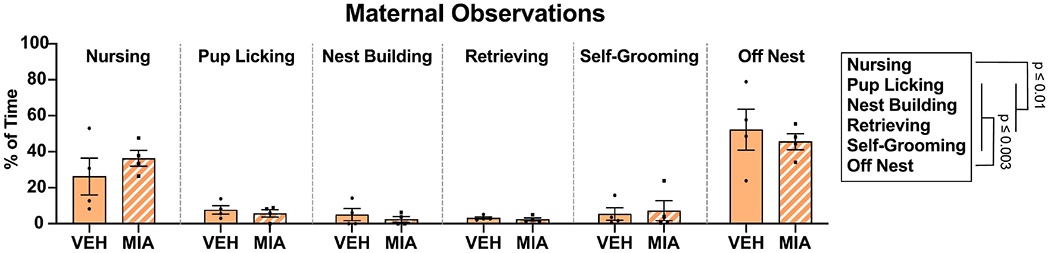
Maternal observation data. MIA did not affect maternal behavior as measured between P0-8. Dams spent most of the time either nursing pups or off the nest.
Figure 3:
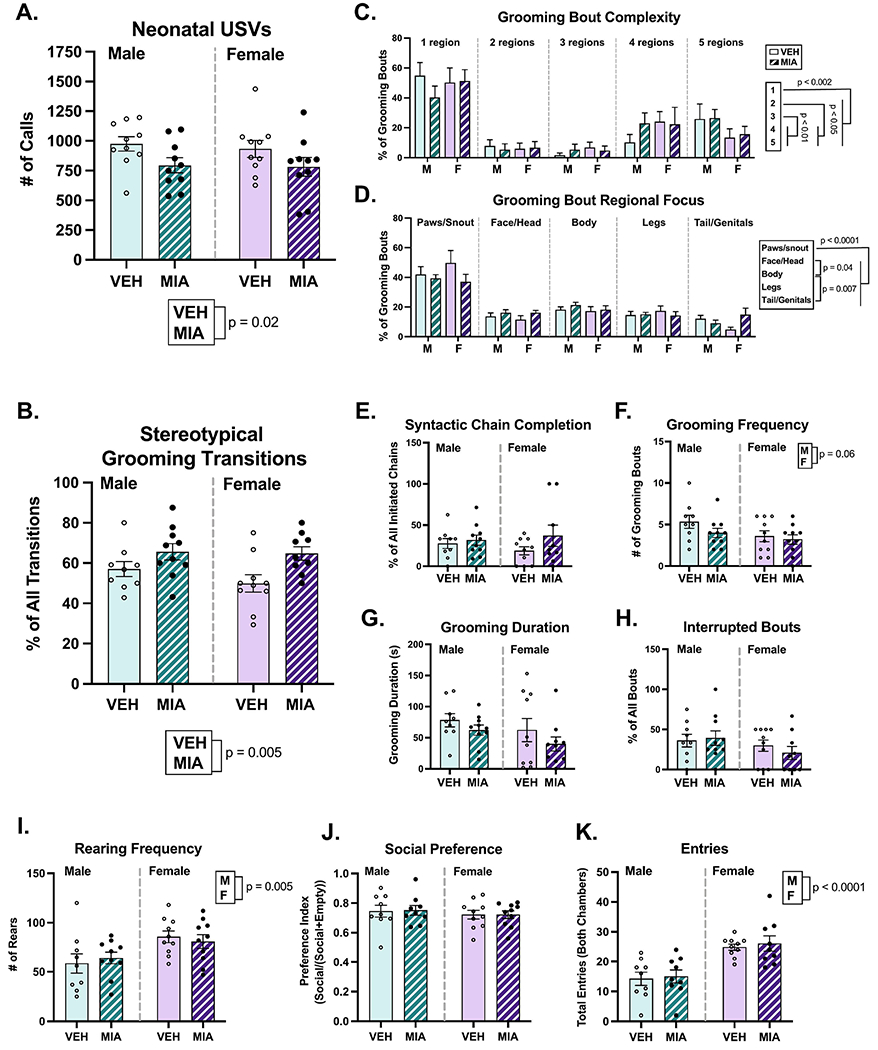
MIA reduces neonatal ultrasonic vocalization behavior and increases stereotypical grooming behavior. A.) MIA offspring exhibited fewer isolation-induced ultrasonic vocalizations. B.) MIA increased the proportion of grooming transitions that fit stereotypical grooming syntax. C-D.) While no effect of sex or MIA was found on juvenile self-grooming bout microstructure, all rats primarily groomed one region in a given grooming bout, with the focus being on the paws and snout. E-H.) No other significant effects on grooming behavior were noted. I.) Females reared more during the grooming assessment compared to males. J-K.) MIA did not alter adult social preference behavior, but females exhibited more exploratory behavior in the chamber. M = male, F = female.
3.2. Self-Grooming
In the grooming assessment (Fig. 3B–I), MIA increased the proportion of stereotypical (i.e. ‘correct’) grooming transitions (Fig. 3B; F1, 34 = 9.20, p = 0.005, η2 = 0.20), suggesting that MIA animals are more rigid in their grooming patterns compared to vehicle animals. No other effects of MIA were found on grooming syntactic chain completion (F1, 34 = 2.06, p = 0.16), grooming frequency (F1, 35 = 1.83, p = 0.19), grooming duration (F1, 35 = 2.21, p = 0.15), grooming bout interruptions (F1, 34 = 0.13, 0.72), or rearing frequency (F1, 35 = 0.005, p = 0.94).
Regarding sex differences, female rats reared significantly more often than male rats (F1, 35 = 9.22, p = 0.005, η2 = 0.21). Conversely, males engaged in grooming bouts more often than females, though this difference was non-significant (F1, 35 = 3.903, p = 0.06, η2 = 0.10). No effects of sex were found on overall time spent grooming (F1, 35 = 2.19, p = 0.15).
Regarding grooming microstructure, a three-way repeated measures ANOVA revealed a main effect of bout complexity (F2.65, 89.99 = 23.65, p < 0.0001, η2 = 0.40), such that all rats were more likely to engage in grooming bouts directed at just one region compared to grooming 2, 3, 4, or 5 regions in a given bout (p’s < 0.002). Although rats were also more likely to groom 4 or 5 regions within one bout compared to 2 or 3 regions (p’s < 0.05). In a separate three-way repeated measures ANOVA, a main effect of region (F1.90, 64.53 = 23.65, p < 0.0001, η2 = 0.52) demonstrated that rats spent the largest proportion of their time grooming their paws and snout versus all other regions (p’s < 0.0001). Neither sex nor treatment affected grooming bout complexity (F’s1, 34 ≤ 1.30, p’s ≥ 0.26) or focus (F’s1, 34 ≤ 0.1514, p’s ≥ 0.70), and there were no additional interactions.
3.3. Social Preference
Neither sex (Fig. 3J; F1, 34 = 0.68, p = 0.42) nor MIA condition (F1, 34 = 0.009, p = 0.93) affected the social preference index measured in the sociability test. Females exhibited a higher number of entries to both the social and nonsocial chambers during the testing period (Fig. 3K; F1, 33 = 28.26, p < 0.0001, η2 = 0.46), but there were no effects of MIA on entries (F1, 33 = 0.24, p = 0.63).
3.4. Cell Counts
In the PVN (Fig. 4A–C), neither sex (χ2(2)’s ≤ 1.91, p’s ≥ 0.39) nor MIA (χ2(2)’s ≤ 2.92, p’s ≥ 0.23) altered the density of AVP or OT immunoreactive cells. Conversely, in the SON (Fig. 4D–F), a main effect of sex (χ2(2) = 6.33, p = 0.04, β = −0.28) revealed that males had higher OT immunoreactive cell density compared to females, though no effect of MIA (χ2(2) = 4.25, p = 0.12) or interaction (χ2(1) = 3.16, p = 0.07) was found. No effects of sex (χ2(2) = 0.51, p = 0.77) or treatment (χ2(2) = 0.29, p = 0.86) on AVP cells in the SON were found. Regarding the BNST (Fig. 3G–J), neither sex nor MIA altered the number of OT immunoreactive cells in the anteromedial (χ2(2)’s ≥ 2.77, p’s ≥ 0.25), medioventral (χ2(2)’s ≤ 2.96, p’s ≥ 0.23), or posterior subregions (χ2(2)’s ≤ 2.22, p’s ≥ 033).
Figure 4:
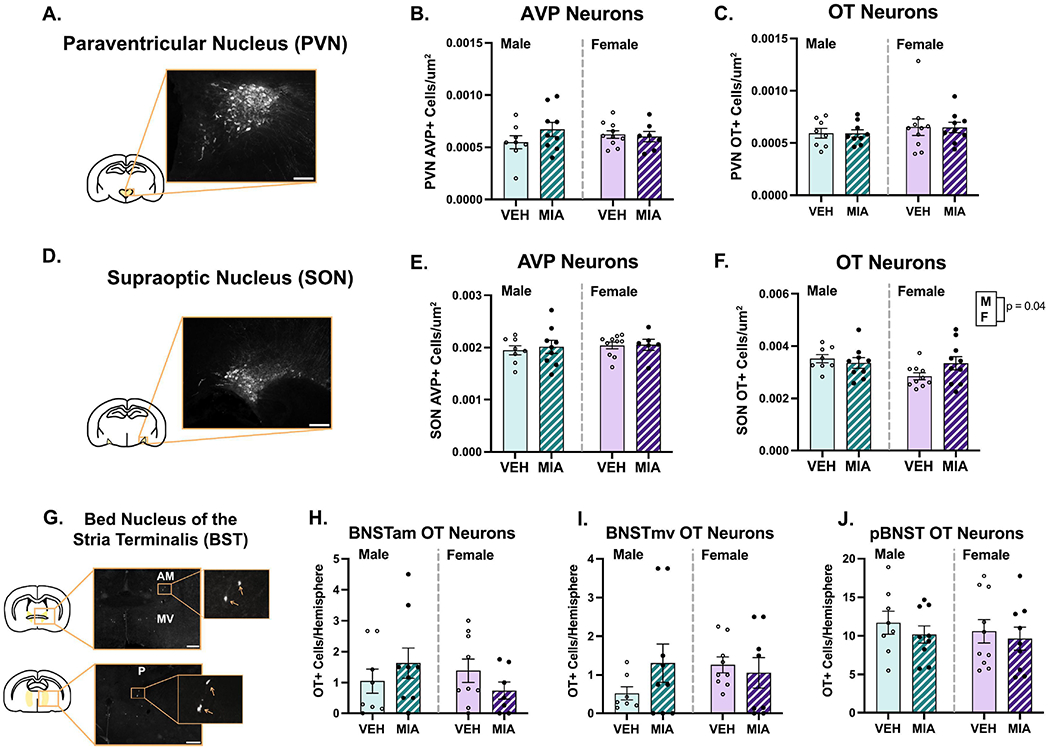
MIA does not significantly alter OT or AVP neuron number. A.) Schematic of a coronal brain section highlighting the approximate location of the PVN with an example image of AVP+ cells. B-C.) No effects of sex or MIA on the PVN were documented. D.) Schematic of a coronal brain section highlighting the approximate location of the SON with an example image of AVP+ cells. E-F.) No effects of MIA were found on AVP or OT neurons in the SON. Males had significantly more OT neurons in the SON than females. G.) Schematic of coronal brain sections highlighting the anterior and posterior BNST with example images of OT+ cells. Boxes correspond to enlargements of images for visualization purposes. H-J.) MIA did not affect OT cell number in any BST subregion. Scale bars = 100μm (A. and D.) or 400μm (G.). The contrast on images was enhanced for visualization purposes.
3.5. Fiber Analysis
Several sex differences were found across the regions analyzed. Males had slightly higher OT fiber density compared to females in the LS (Fig. 5C; χ2(2) = 5.95, p = 0.05, β = −0.33) and NAc shell (Fig. 6E; χ2(2) = 7.66, p = 0.02, β = −0.39). A similar difference was observed in the NAc core, although it was non-significant (Fig. 6D; χ2(2) = 4.75, p = 0.09, β = −0.33). Males also had higher AVP fiber density compared to females in the NAc shell (Fig. 6B; χ2(2) = 16.90, p = 0.00021, β = −0.48; core not analyzed), and medioventral BNST (Fig. 7B; χ2(2) = 8.23, p = 0.02, β = −0.044), although the effect size was small due to an MIA-induced increase in AVP in females (χ2(2) = 8.22, p = 0.004, β = 0.45). Males also had higher AVP fiber density in the lateral hypothalamic area (Fig. 8C; χ2(2) = 5.57, p = 0.06, β = −0.19). No effects of sex were found in the other regions examined (see Supplementary Table 2).
Figure 5:
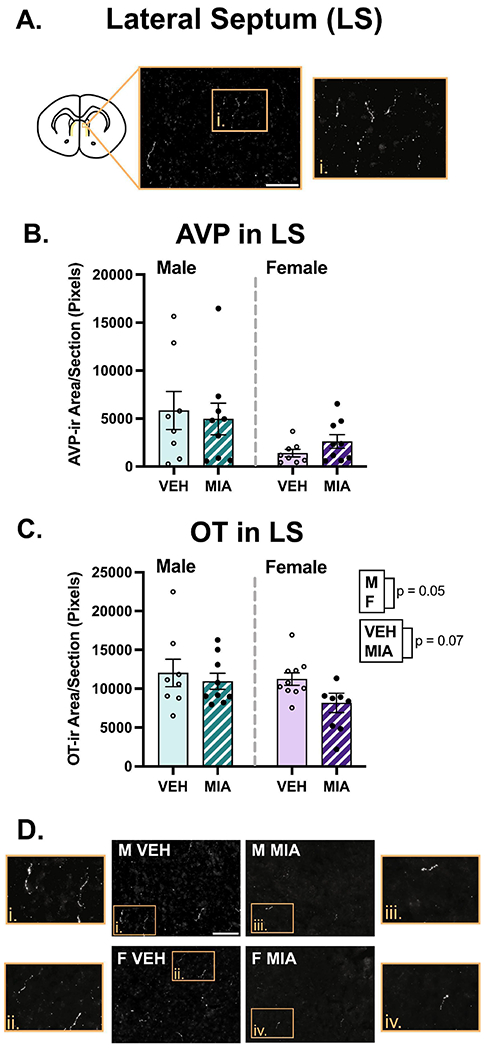
Lateral septum (LS) fiber analysis. A.) Schematic of a coronal section highlighting the LS with an example image of OT staining. B.) MIA did not significantly affect AVP in the LS. C.) MIA produced a non-significant reduction in OT in the LS, and males had more OT compared to females. D.) Representative images of OT staining. Scale bars = 75μm. Boxes correspond to enlargements of images. The contrast on images was enhanced for visualization purposes.
Figure 6:
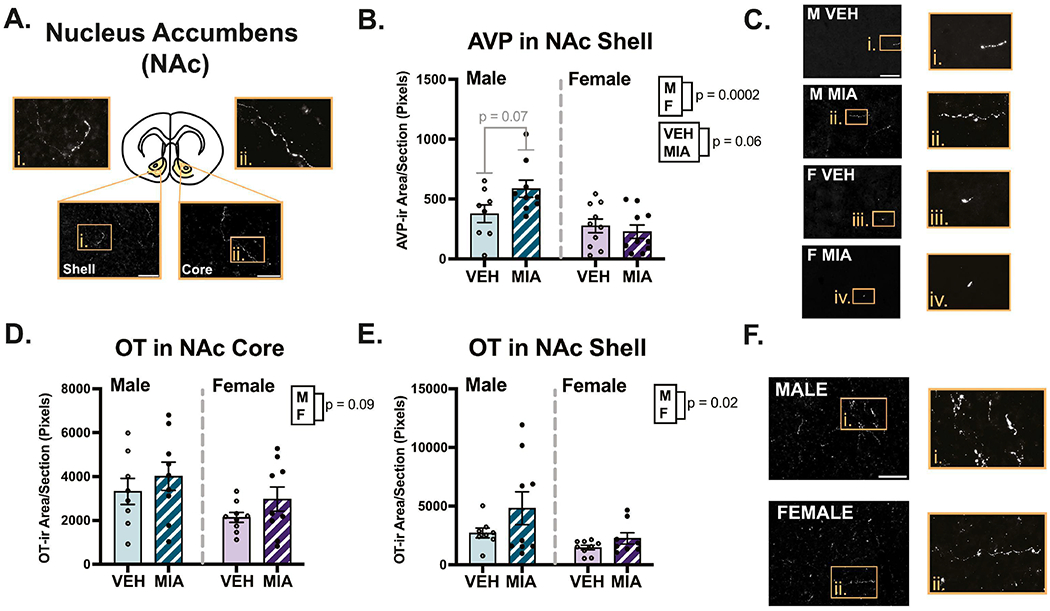
Nucleus accumbens (NAc) fiber analysis. A.) Schematic of a coronal section highlighting the NAc core and shell, with example images of OT staining. B.) MIA produced a non-significant (p = 0.06) increase in AVP innervation in the shell, although a significant interaction reveals this was driven by males. Males also had more AVP compared to females. C.) Representative AVP staining in the shell. D-E.) MIA did not significantly affect OT innervation in the NAc, though males had significantly more OT in the shell compared to females. F.) Representative images of OT staining in the NAc shell. Scale bars = 75μm for shell, 50μm for core. Boxes correspond to enlargements of images and the contrast on images was enhanced for visualization purposes.
Figure 7:
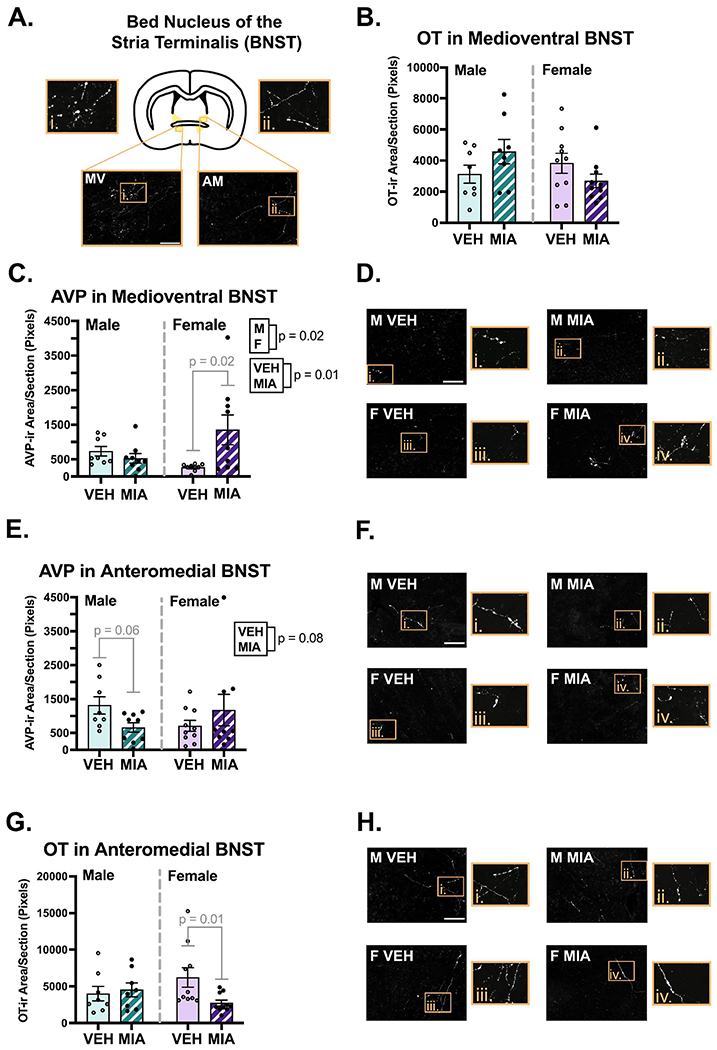
Bed nucleus of the stria terminalis (BNST) fiber analysis. A.) Schematic of a coronal section of the BNST with an example image of OT staining in the anteromedial (AM) and medioventral (MV) subregions. B.) A significant interaction suggests MIA increases OT in medioventral BNST of males and decreases it in females. C-D.) MIA selectively increases AVP in the medioventral BNST in males, with representative images. E-F.) MIA reduced AVP in the anteromedial BNST in males, with representative images. G-H.) MIA reduced OT in the anteromedial BNST of females, with representative images. Scale bars = 75μm. Boxes correspond to enlargements of images, and the contrast on images was enhanced for visualization purposes.
Figure 8:
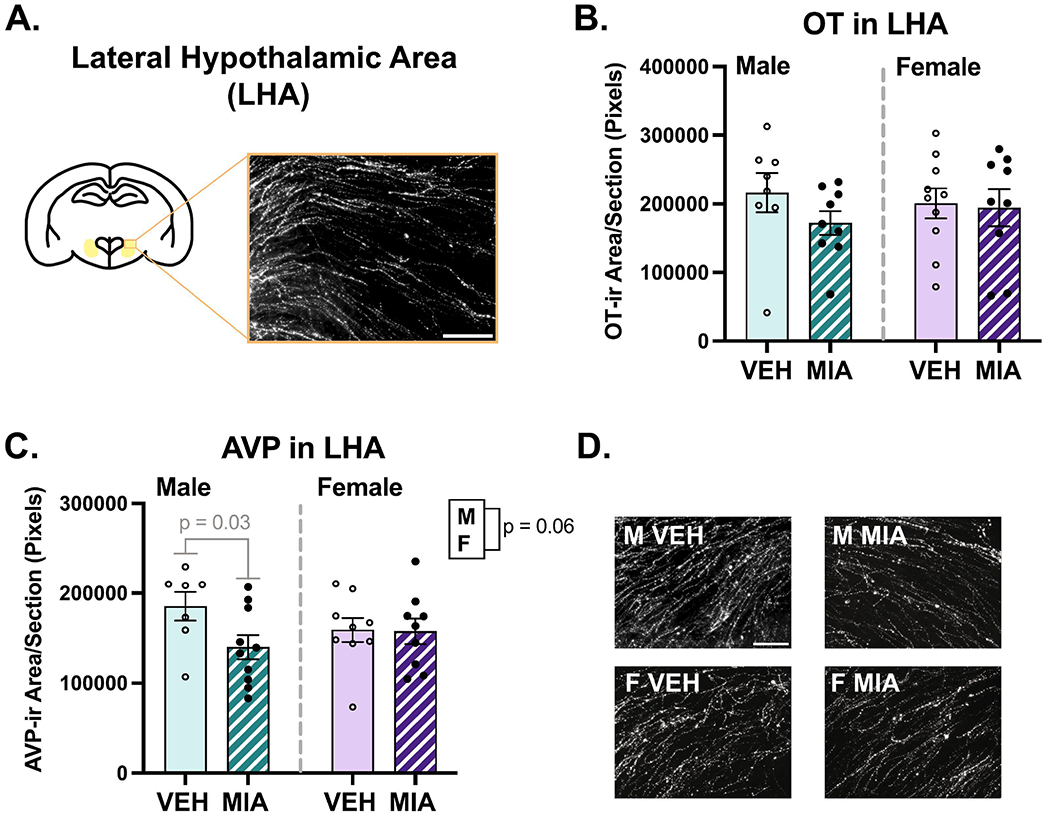
Lateral hypothalamic area (LHA) fiber analysis. A.) Schematic of a coronal section highlighting the lateral hypothalamic area. B.) MIA did not affect OT in the LHA. C-D.) MIA selectively reduced AVP in the LHA of males and males had more AVP than females (though p = 0.06), with representative images. Scale bars = 75μm.
MIA had variable effects on nonapeptide fiber density across regions examined. In the MeA, MIA significantly increased the density of OT (Fig. 9D; F1, 32 = 4.78, p = 0.04, η2 = 0.12) and AVP (Fig. 9B; χ2(2) = 5.84, p = 0.05, β = 0.41). MIA also produced a non-significant decrease in OT fiber density in the LS (Fig. 5C; χ2(2) = 5.39, p = 0.07, β = −0.29). Interestingly, MIA had sex-specific effects on OT and AVP fiber density in several regions, with the most dynamic effects found in the BNST. In both the anteromedial and medioventral subregions of the BNST (Fig. 7), significant interactions (F’s1, 32 ≥ 4.39, p’s ≤ 0.04, η’s ≥ 0.12) revealed that MIA reduced OT fiber density in females and not males; post hoc tests for sex-specific comparisons only reached significance in the anteromedial subregion (p = 0.01). Conversely, for AVP, there was non-significant decrease because of MIA in the anteromedial BNST (Fig. 7E; χ2(2) = 4.97, p = 0.08, β = −0.16), that was driven by a significant interaction (χ2(1) = 4.20, p = 0.04, β = 0.32), whereby MIA decreased AVP in males and not females (p = 0.06). In the medioventral subregion, MIA significantly increased AVP fiber density (Fig. 7C; χ2(2) = 9.10, p = 0.01, β = 0.16), and an interaction (χ2(1) = 8.22, p = 0.004, β = 0.45) indicated this was due to a significant increase in females (p = 0.02).
Figure 9:
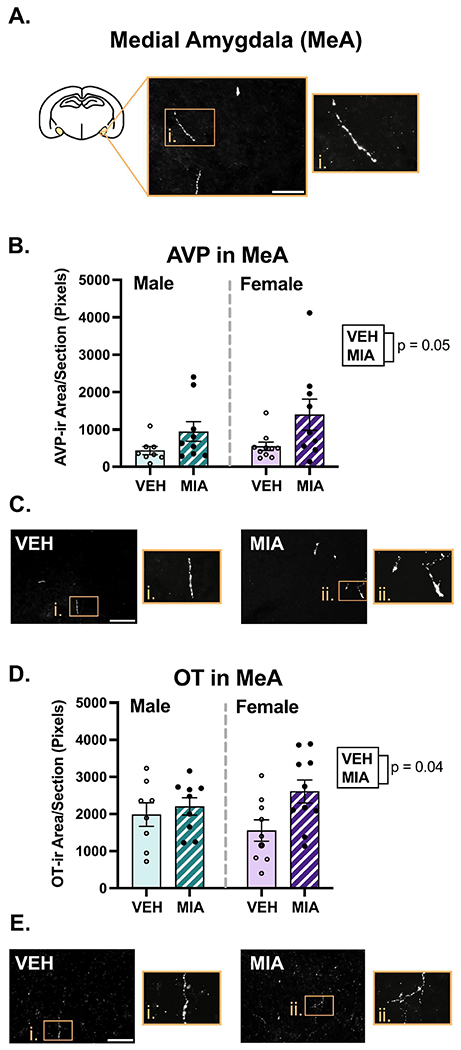
Medial amygdala fiber analysis. A.) Schematic of a coronal section with the medial amygdala highlighted. B-C.) MIA increased AVP in the MeA with representative images. D-E.) MIA increased OT in the MeA, with representative images. Scale bars = 75μm. Boxes correspond to enlargements of images. The contrast on images was enhanced for visualization purposes.
Additionally, sex-specific effects were documented in in the NAc and hypothalamus. In the NAc shell, MIA produced a non-significant increase AVP fiber density (Fig. 6B; χ2(2) = 5.36, p = 0.06, β = 0.017), and a significant interaction (χ2(1) = 4.68, p = 0.03, β = −0.27) revealed this was driven by MIA males (p = 0.07). In the lateral hypothalamic area, a significant interaction (Fig. 8C; χ2(1) = 3.73, p = 0.05, β = 0.22) revealed that MIA decreased AVP fiber density in males (p = 0.03). No other interactions were found (see Supplementary Table 2).
4. DISCUSSION
We previously showed that acute allergic MIA in rats produces multiple behaviors similar to those found in neurodevelopmental and psychiatric disorders, including behavioral hyperactivity, cognitive inflexibility, and reduced social play behavior (Breach et al., 2021). In this study, we assessed whether allergic MIA altered additional behavioral phenotypes relating to sociality and anxiety, and profiled how potential neuronal mediators of social impairment, OT and AVP, were affected in juvenile animals following MIA.
4.1. Acute allergic MIA impairs communication and increases stereotypical self-grooming behavior.
MIA reduced ultrasonic vocalizations in neonatal offspring, showing that offspring communication is impaired as early as postnatal day six in this model. This goes along with previous work from other models of MIA showing reduced (Carbone et al., 2023; Kirsten et al., 2012; Malkova et al., 2012) or increased USVs in neonates exposed to MIA (Choi et al., 2016; Schwartzer et al., 2013). A few studies suggest that males are particularly susceptible to MIA-induced changes to vocalization (Bilbo et al., 2018; Block et al., 2022), yet the present study found effects in both males and females. These disparate findings could be attributable to differences in the MIA inflammatory cascade or species under investigation (mice versus rats). Nonetheless, USVs function as a communication tool (Takahashi et al., 2010), and calls emitted from neonates elicit prompt care from mothers (Branchi et al., 2001). Thus, reduced vocalizations found in our MIA offspring could reflect impaired communicative ability, altered motivational states, or reduced emotional distress.
We also found that MIA increased stereotypical grooming transitions, without affecting total time or frequency of grooming behavior. These data correspond with findings from other rodent models of neurodevelopmental disorders, which also found repetitive self-grooming patterns and increased stereotypical transitions (Liu et al., 2021; Pearson et al., 2011; Reynolds et al., 2013). Self-grooming behavior is a complex, sequentially patterned, and ethologically relevant measure of repetitive behavior in rodents (Kalueff et al., 2016), and it may also be indicative of anxiety-like behavior (Estanislau et al., 2019). Here, MIA-induced increases in stereotypical grooming transitions may reflect inflexible repetitive behavior or rigid stress-coping behavior.
MIA did not alter sociability on the three-chamber social preference task in adulthood. This supports findings from our previous study, whereby MIA did not alter paired reciprocal social interaction in adults (Breach et al., 2021). Collectively, these data suggest that social impairment in our model of acute allergic may be restricted to or most pronounced during development. However, there are a few alternative explanations that merit discussion. First, we used breeder males as stimulus animals as opposed to age- and sex-matched peers. It is possible that the breeder males were a more salient social stimulus, and this could have washed out more nuanced differences in social approach. Another explanation could be that it may take multiple habituations to a behavioral testing procedure for social impairments to manifest in this model. For example, we previously gathered juvenile social play data over the course of repeated testing sessions and found impaired play in MIA offspring (Breach et al., 2021). The social interaction and sociability tasks, conversely, took place over the course of a single testing session. Nonetheless, taken in the context of our previous work, these data generally add strength to the face validity of acute allergic MIA model of social impairment, as least during development.
4.2. Acute allergic MIA increases AVP and OT innervation in the medial amygdala.
The medial amygdala (MeA) functions as a critical node of social behavior brain circuitry, as it receives and relays olfactory and other sensory cues to influence and regulate social behavior output (Raam & Hong, 2021). The MeA also regulates the frequency of juvenile play in both sexes (Argue et al., 2017; VanRyzin et al., 2019). Here, we found that acute allergic MIA increased AVP and OT immunoreactivity in the MeA in male and female offspring.
A similar study employing an LPS model of MIA found reduced AVP immunoreactive cells in the juvenile MeA of males (Taylor et al., 2012). While we were unable to quantify AVP cell number in the MeA due to insufficient expression of immunoreactive cells in this region, our findings of increased AVP fibers add support to the idea that MIA perturbs AVP signaling in the MeA. Despite previous work establishing that the MeA receives AVP and OT innervation and expresses their corresponding receptors (DiBenedictis et al., 2017; Smith et al., 2017), it is surprisingly unknown how AVP or OT signaling in the MeA regulates social behavior in juveniles. Furthermore, even in the adult literature, more is known about AVP in the central amygdala, where it facilitates anxiety (Harper et al., 2019; Hernandez et al., 2016; Hernández-Pérez et al., 2018), relative to the MeA. Studies that have examined AVP in the MeA have found that it facilitates social avoidance in response to sick odor in males (females not examined) (Arakawa et al., 2010), and that AVP V1b receptor antagonism suppresses immobility on the forced swim task (Salomé et al., 2006). Thus, elevated AVP in the MeA may enhance social avoidance or contribute to passive coping in this model, but more work testing whether such findings apply to juveniles is merited.
In contrast to AVP, OT action in the adult MeA is generally known to promote pro-social behavior. In studies conducted in adult male OT-knockout mice, OT administration into the MeA restores social recognition memory (Ferguson et al., 2001). Likewise, blockade of the OT receptor (OTR) in the male MeA reduces social approach to conspecific bedding (Arakawa et al., 2010), and impairs social recognition memory for female adults (Lukas et al., 2013). OTR binding density in the MeA also correlates positively with social investigation in males but not females (Dumais et al., 2013), yet a follow up study surprisingly found that modulation of MeA OTR activation does not affect social investigation in either sex (Dumais, Alonso, Bredewold, et al., 2016). Here, it is possible that elevated OT innervation in the MeA may compensate for potential reductions in OTR or it may represent an oversaturation of OT signaling. While receptor-based analyses were beyond the scope of the current study, the question of how MIA-related OT and AVP fiber remodeling relates to changes in regional sensitivity to these nonapeptides remains an important question for potential future investigation.
Previous work has found that AVP and OT in the lateral septum (LS) regulate juvenile social play behavior in rats (Bredewold et al., 2014). We did not find significant alterations in either AVP or OT following allergic MIA. However, MIA may decrease OT fiber immunoreactivity in this region (p = 0.07). OTR antagonism in the LS reduces juvenile social play in a novel cage in females but not males (Bredewold et al., 2014), suggesting that OT facilitates social play in novel environments, at least in females. Other work in adult rodents has found OT in the LS is important for maintenance of social recognition memory in males (females not examined) (Lukas et al., 2013), thus, while the finding requires replication, the non-significant reductions in OT in the LS may contribute to impaired social behavior in our model, but stronger evidence is required.
4.3. MIA altered OT and AVP in several regions in a sex-specific manner.
We found novel sex-specific effects of MIA on OT and AVP fibers in multiple brain regions. In males, allergic MIA reduced AVP immunoreactivity in the anteromedial BNST and LHA and increased AVP in the NAc shell. One study found that lower levels of AVP mRNA in the whole BNST were actually associated with increased juvenile social play in male rats (Paul et al., 2014); however, this study assessed AVP mRNA via in-situ hybridization, which would not detect translated AVP peptides in neuronal processes. Since we were unable to count cells in this region for reasons stated in section 2.4, it is difficult to interpret how these findings would apply in our study. Nonetheless, another study found that AVP release within the anteromedial BNST correlates negatively with aggression amongst adult male rats (Veenema et al., 2010), and ablating or reducing AVP expression in the adult BNST reduces same-sex social investigation and communication behavior in male mice (Rigney et al., 2019; Rigney et al., 2022). Thus, lower levels of AVP in the anteromedial BNST of male MIA offspring may lead to increased aggression and/or impaired social behavior.
Regarding the NAc and LHA, no studies have specifically assessed the effects of AVP on juvenile social play in these regions. However, micro infusion of an AVP V1a receptor antagonist into the NAc during cocaine conditioning blunts the subsequent expression of the conditioned response, and exogenous AVP administration potentiates the expression of the conditioned response in the absence of cocaine (Rodríguez-Borrero et al., 2010). AVP in the LHA stimulates arousal through activation of local orexin neurons (Islam et al., 2022), and activation of LHA orexin neurons relieves anxiety-like behavior induced by chronic stress (Wang et al., 2021). Thus, increased AVP in the NAc may contribute to perseveration of learned associations, while decreased AVP in the LHA could lead to increased anxiety in our MIA males.
In females, MIA reduced OT in the anteromedial BNST and increased AVP in the medioventral BNST. To our knowledge, the only relevant studies that have assessed OT in the anterior subregions of the female BNST were conducted in adult California mice. In this species, OT in the anteromedial BNST reduces female social behavior (Duque-Wilckens et al., 2020; Luo et al., 2022). However, administration of an OTR antagonist into the posterior BNST inhibited social recognition in adult female rats (Dumais, Alonso, Immormino, et al., 2016), suggesting that OT signaling is conducive to social cognition in rats, at least in the posterior subregion. Conversely, AVP in the medioventral BNST correlates negatively with social approach in adult female California mice, yet modulation of AVP synthesis or signaling in this region reduces or has no effect on social investigation (Duque-Wilckens et al., 2016; Rigney et al., 2022).
Ultimately, site-specific manipulation studies are still required to determine exactly how OT and AVP in these regions may contribute to juvenile social behavior in rats. Studies conducted in adult animals of various species consistently find that these neuropeptides are involved in social behavior regulation. Thus, the sex-specific changes discussed in this section represent potential neural substrates for sex-specific mechanisms of neurodevelopmental impairment following exposure to prenatal allergic inflammation.
4.4. Overall sex differences.
In addition to profiling the effects of MIA on OT and AVP in several brain regions important for social behavior, the current study builds upon previous work showing baseline sex differences in OT and AVP innervation (Smith et al., 2019). Under baseline conditions, both OT and AVP are known to regulate social behavior in both juvenile and adult rats, often in a sex-specific manner (Bredewold & Veenema, 2018; Caldwell, 2017; Smith et al., 2019). Here, we found that males had higher OT in the LS and NAc shell, and higher AVP in the NAc shell compared to females. We also generally saw higher AVP in the LS and BNST of males, which has been documented previously (Smith et al., 2019). However, the main effects of sex in these cases were not statistically significant, likely due to variability and interaction effects with MIA. Moreover, sex differences in OT and AVP are nuanced and can differ across studies (Smith et al., 2019), potentially due to differences in tissue processing, analysis method, species, or breed employed. However, our findings are consistent with the general theme seen across studies: when there are sex differences, males tend to have higher nonapeptide content compared to females (Smith et al., 2019), and this may contribute to sex differences seen in social, sexual, or anxiety-like behavior.
5. CONCLUSIONS
Previously, we found that acute prenatal exposure to allergic MIA impaired juvenile social play behavior in offspring. In the current study, we further found further evidence of neurodevelopmental impairment, including reduced neonatal communication behavior and increased stereotypy of self-grooming behavior in juveniles. Additionally, we uncovered several neural substrates that may underlie MIA-induced juvenile social impairment. In males, MIA reduced AVP fiber immunoreactivity in the lateral hypothalamic area and anteromedial BNST whilst increasing it in the nucleus accumbens. Conversely, in females, MIA decreased OT but increased AVP in the BNST. MIA also increased both OT and AVP fiber immunoreactivity in the medial amygdala in both male and female offspring. These findings represent a substantial basis for future investigations of potential sex-specific mechanisms underlying reduced juvenile social play behavior following MIA.
Supplementary Material
Highlights:
Allergic MIA impairs communication behavior and increases self-grooming stereotypy.
MIA increased OT and AVP innervation in the juvenile medial amygdala.
MIA reduced AVP in the lateral hypothalamus and BNST in juvenile males
MIA also increased AVP in the NAc of juvenile males.
In juvenile females, MIA decreased OT but increased AVP in the BNST.
7. ACKNOWLEDGEMENTS
The authors would like to thank Dr. Cole Vonder Haar for advice regarding statistical analyses, Nikhila Elevarthi for assisting with pilot imaging in the hypothalamus, and Brooke Schatz for assisting with sectioning tissue and behavioral testing. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1343012. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Funding:
This work was supported by the National Institutes of Health (NIH) grant R21MH105826 to KML. MRB is supported by the National Science Foundation Graduate Research Fellowship Program (Grant DGE-1343012). HEA was supported by the Ohio State University Undergraduate Research Apprenticeship Program. SC was supported by the Ohio State University Explorations in Neuroscience Internship Program (NIH grant 5R25NS120282-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no competing financial interests.
Data Sharing:
The data presented here are available from the corresponding author upon reasonable request.
8. REFERENCES
- Abel KM, Drake R, & Goldstein JM (2010). Sex differences in schizophrenia. Int Rev Psychiatry, 22(5), 417–428. 10.3109/09540261.2010.515205 [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, & Deak T (2010). Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor. Neuroscience, 171(4), 1141–1151. 10.1016/j.neuroscience.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Argue KJ, Vanryzin JW, Falvo DJ, Whitaker AR, Yu SJ, & McCarthy MM (2017). Activation of Both CB1 and CB2 Endocannabinoid Receptors Is Critical for Masculinization of the Developing Medial Amygdala and Juvenile Social Play Behavior. eNeuro, 4(1), ENEURO.0344-0316. 10.1523/eneuro.0344-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, Dupuis A, Paton TA, Scherer SW, Schachar R, Arnold PD, Szatmari P, Nicolson R, Georgiades S, Crosbie J, Brian J, Laboni A, Lerch JP, & Anagnostou E (2017). Oxytocin Receptor Polymorphisms are Differentially Associated with Social Abilities across Neurodevelopmental Disorders. Sci Rep, 7(11618). 10.1038/s41598-017-10821-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauminger N, Shulman C, & Agam G (2003). Peer Interaction and Loneliness in High-Functioning Children with Autism. Journal of Autism and Developmental Disorders, 33, 489–507. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, & Tran PK (2018). Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol, 299(Pt A), 241–251. 10.1016/j.expneurol.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block CL, Eroglu O, Mague SD, Smith CJ, Ceasrine AM, Sriworarat C, Blount C, Beben KA, Malacon KE, Ndubuizu N, Talbot A, Gallagher NM, Chan Jo Y, Nyangacha T, Carlson DE, Dzirasa K, Eroglu C, & Bilbo SD (2022). Prenatal environmental stressors impair postnatal microglia function and adult behavior in males. Cell Reports, 40(5), 111161. 10.1016/j.celrep.2022.111161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Santucci D, & Alleva E (2001). Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res, 125, 49–56. [DOI] [PubMed] [Google Scholar]
- Breach MR, Dye CN, Galan A, & Lenz KM (2022). Prenatal allergic inflammation in rats programs the developmental trajectory of dendritic spine patterning in brain regions associated with cognitive and social behavior. Brain Behav Immun, 102, 279–291. 10.1016/j.bbi.2022.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breach MR, Dye CN, Joshi A, Platko S, Gilfarb RA, Krug AR, Franceschelli DV, Galan A, Dodson CM, & Lenz KM (2021). Maternal allergic inflammation in rats impacts the offspring perinatal neuroimmune milieu and the development of social play, locomotor behavior, and cognitive flexibility. Brain Behav Immun, 95, 269–286. 10.1016/j.bbi.2021.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breach MR, & Lenz KM (2023). Sex Differences in Neurodevelopmental Disorders: A Key Role for the Immune System. Curr Top Behav Neurosci, 62, 165–206. 10.1007/7854_2022_308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, & Veenema AH (2014). Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci, 8, 216. 10.3389/fnbeh.2014.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, & Veenema AH (2018). Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Curr Opin Neurobiol, 49, 132–140. 10.1016/j.conb.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, & Susser ES (2004). Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Archives of General Psychiatry, 61(8), 774. 10.1001/archpsyc.61.8.774 [DOI] [PubMed] [Google Scholar]
- Caldwell HK (2017). Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. The Neuroscientist, 23(5), 517–528. [DOI] [PubMed] [Google Scholar]
- Canetta S, Bolkan S, Padilla-Coreano N, Song LJ, Sahn R, Harrison NL, Gordon JA, Brown A, & Kellendonk C (2016). Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry, 21(7), 956–968. 10.1038/mp.2015.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel H-M, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, McKeague IW, & Brown AS (2014). Elevated Maternal C-Reactive Protein and Increased Risk of Schizophrenia in a National Birth Cohort. American Journal of Psychiatry, 171(9), 960–968. 10.1176/appi.ajp.2014.13121579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Buzzelli V, Manduca A, Leone S, Rava A, & Trezza V (2023). Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats. Int J Mol Sci, 24(4). 10.3390/ijms24043920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, Chen TJ, Pan TL, & Bai YM (2014). Is atopy in early childhood a risk factor for ADHD and ASD? a longitudinal study. J Psychosom Res, 77(4), 316–321. 10.1016/jjpsychores.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Chen SW, Zhong XS, Jiang LN, Zheng XY, Xiong YQ, Ma SJ, Qiu M, Huo ST, Ge J, & Chen Q (2016). Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behav Brain Res, 296, 61–69. 10.1016/j.bbr.2015.08.035 [DOI] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, & Huh JR (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science, 351(6276), 933–939. 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey KR, Marx RE, & Neumaier JF (2019). Deep Squeak: a deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology, 44(5), 859–868. 10.1038/s41386-018-0303-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, & Blumberg SJ (2018). Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J Clin Child Adolesc Psychol, 47(2), 199–212. 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, & Veenema AH (2017). Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol, 525(11), 2549–2570. 10.1002/cne.24216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Bredewold R, & Veenema AH (2016). Role of the oxytocin system in amygdala subregions in the regulation of social interest in male and female rats. Neuroscience, 330, 138–149. 10.1016/j.neuroscience.2016.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, & Veenema AH (2016). Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology, 64, 79–88. 10.1016/j.psyneuen.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, & Veenema AH (2013). Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex-specific ways. Horm Behav, 64(4), 693–701. 10.1016/j.yhbeh.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL, & Trainor BC (2016). Inhibition of vasopressin V1a receptors in the medioventral bed nucleus of the stria terminalis has sex- and context-specific anxiogenic effects. Neuropharmacology, 110, 59–68. 10.1016/j.neuropharm.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, Hao R, Ramos-Maciel S, Rios RA, Jackson K, Flores-Ramirez FJ, Garcia-Carachure I, Pesavento PA, Iniguez SD, Grinevich V, & Trainor BC (2020). Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl Acad Sci U S A, 117(42), 26406–26413. 10.1073/pnas.2011890117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estanislau C, Veloso AWN, Filgueiras GB, Maio TP, Dal-Col MLC, Cunha DC, Klein R, Carmona LF, & Fernandez-Teruel A (2019). Rat self-grooming and its relationships with anxiety, dearousal and perseveration: Evidence for a self-grooming trait. Physiol Behav, 209, 112585. 10.1016/j.physbeh.2019.112585 [DOI] [PubMed] [Google Scholar]
- Feng C, Hackett PD, Demarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, & Rilling JK (2015). Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging and Behavior, 9(4), 754–764. 10.1007/s11682-014-9333-9 [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, & Young LJ (2001). Oxytocin in the Medial Amygdala is Essential for Social Recognition in the Mouse. The Journal of Neuroscience, 21(20), 8278–8285. 10.1523/jneurosci.21-20-08278.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez de Cossio L, Lacabanne C, Bordeleau M, Castino G, Kyriakakis P, & Tremblay ME (2021). Lipopolysaccharide-induced maternal immune activation modulates microglial CX3CR1 protein expression and morphological phenotype in the hippocampus and dentate gyrus, resulting in cognitive inflexibility during late adolescence. Brain Behav Immun, 97, 440–454. 10.1016/j.bbi.2021.07.025 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, & Hickie IB (2010). Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry, 67(12), 1220–1222. 10.1016/j.biopsych.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Unkelbach C, Alvares GA, & Hickie IB (2011). Arginine Vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology, 36(2), 294–297. 10.1016/j.psyneuen.2010.07.023 [DOI] [PubMed] [Google Scholar]
- Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, Schleimer RP, & Nadeau KC (2019). Prevalence and Severity of Food Allergies Among US Adults. JAMA Network Open, 2(1), e185630. 10.1001/jamanetworkopen.2018.5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gzielo K, Potasiewicz A, Litwa E, Piotrowska D, Popik P, & Nikiforuk A (2021). The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats. Brain Sci, 11(3). 10.3390/brainsci11030344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad FL, Patel SV, Doornaert EE, De Oliveira C, Allman BL, Baines KJ, Renaud SJ, & Schmid S (2022). Interleukin 15 modulates the effects of poly I:C maternal immune activation on offspring behaviour. Brain Behav Immun Health, 23, 100473. 10.1016/j.bbih.2022.100473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han VX, Patel S, Jones HF, Nielsen TC, Mohammad SS, Hofer MJ, Gold W, Brilot F, Lain SJ, Nassar N, & Dale RC (2021). Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry, 11(1), 71. 10.1038/S41398-021-01198-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Butler RK, Cook CA, Criswell HE, Stuber GD, & Breese GR (2019). Amygdala Arginine Vasopressin Modulates Chronic Ethanol Withdrawal Anxiety-Like Behavior in the Social Interaction Task. Alcoholism: Clinical and Experimental Research, 43(10), 2134–2143. 10.1111/acer.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez VS, Hernandez OR, Perez de la Mora M, Gomora MJ, Fuxe K, Eiden LE, & Zhang L (2016). Hypothalamic Vasopressinergic Projections Innervate Central Amygdala GABAergic Neurons: Implications for Anxiety and Stress Coping. Front Neural Circuits, 10, 92. 10.3389/fncir.2016.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Párez OR, Crespo-Ramírez M, Cuza-Ferrer Y, Anias-Calderón J, Zhang L, Roldan-Roldan G, Aguilar-Roblero R, Borroto-Escuela DO, Fuxe K, & Perez De La Mora M (2018). Differential activation of arginine-vasopressin receptor subtypes in the amygdaloid modulation of anxiety in the rat by arginine-vasopressin. Psychopharmacology, 235(4), 1015–1027. 10.1007/s00213-017-4817-0 [DOI] [PubMed] [Google Scholar]
- Huang Y, Huang X, Ebstein RP, & Yu R (2021). Intranasal oxytocin in the treatment of autism spectrum disorders: A multilevel meta-analysis. Neurosci Biobehav Rev, 122, 18–27. [DOI] [PubMed] [Google Scholar]
- Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, & Klungsoyr K (2017). Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol Psychiatry, 81(5), 452–459. 10.1016/j.biopsych.2015.11.024 [DOI] [PubMed] [Google Scholar]
- Islam MT, Rumpf F, Tsuno Y, Kodani S, Sakurai T, Matsui A, Maejima T, & Mieda M (2022). Vasopressin neurons in the paraventricular hypothalamus promote wakefulness via lateral hypothalamic orexin neurons. Curr Biol, 32(18), 3871–3885 e3874. 10.1016/j.cub.2022.07.020 [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, & Tuohimaa P (2007). Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc, 2(10), 2538–2544. 10.1038/nprot.2007.367 [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, & Fentress JC (2016). Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci, 17(1), 45–59. 10.1038/nrn.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyoncu T, Özbaran B, Köse S, & Onay H (2019). Variation in the Oxytocin Receptor Gene Is Associated With Social Cognition and ADHD. Journal of Attention Disorders, 23(7), 702–711. 10.1177/1087054717706757 [DOI] [PubMed] [Google Scholar]
- Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, Meyer U, Pearce BD, Pletnikov MV, Yolken RH, & Bauman MD (2019). Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology, 44(2), 245–258. 10.1038/s41386-018-0185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten TB, & Bernardi MM (2017). Prenatal lipopolysaccharide induces hypothalamic dopaminergic hypoactivity and autistic-like behaviors: Repetitive self-grooming and stereotypies. Behav Brain Res, 331, 25–29. 10.1016/j.bbr.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Chaves-Kirsten GP, Chaible LM, Silva AC, Martins DO, Britto LR, Dagli ML, Torrao AS, Palermo-Neto J, & Bernardi MM (2012). Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. J Neurosci Res, 90(10), 1903–1912. 10.1002/jnr.23089 [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Taricano M, Maiorka PC, Palermo-Neto J, & Bernardi MM (2010). Prenatal Lipopolysaccharide Reduces Social Behavior in Male Offspring. Neuroimmunomodulation, 17, 240–251. 10.1159/000290040 [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, & Fehr E (2005). Oxytocin increases trust in humans. Nature, 435(7042), 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Lenz KM, Pickett LA, Wright CL, Galan A, & McCarthy MM (2019). Prenatal Allergen Exposure Perturbs Sexual Differentiation and Programs Lifelong Changes in Adult Social and Sexual Behavior. Sci Rep, 9(1), 4837. 10.1038/s41598-019-41258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Huang X, Xu J, Mao H, Li Y, Ren K, Ma G, Xue Q, Tao H, Wu S, & Wang W (2021). Dissection of the relationship between anxiety and stereotyped self-grooming using the Shank3B mutant autistic model, acute stress model and chronic pain model. Neurobiol Stress, 15, 100417. 10.1016/j.ynstr.2021.100417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loparo D, & Waldman ID (2015). The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Molecular Psychiatry, 20(5), 640–646. 10.1038/mp.2014.77 [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, & Neumann ID (2013). Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology, 38(6), 916–926. 10.1016/j.psyneuen.2012.09.018 [DOI] [PubMed] [Google Scholar]
- Luo PX, Zakharenkov HC, Torres LY, Rios RA, Gegenhuber B, Black AM, Xu CK, Minie VA, Tran AM, Tollkuhn J, & Trainor BC (2022). Oxytocin receptor behavioral effects and cell types in the bed nucleus of the stria terminalis. Horm Behav, 143, 105203. 10.1016/j.yhbeh.2022.105203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Ashwood P, Van de Water J, & Hertz-Picciotto I (2014). Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord, 44(7), 1546–1555. 10.1007/s10803-013-2017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, & Patterson PH (2012). Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun, 26(4), 607–616. 10.1016/j.bbi.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossio LF, Goetz T, Matyash M, Kettenmann H, Winter C, & Wolf SA (2014). Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun, 38, 175–184. 10.1016/j.bbi.2014.01.019 [DOI] [PubMed] [Google Scholar]
- Meyer U (2014). Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry, 75(4), 307–315. 10.1016/j.biopsych.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Montag C, Brockmann E-M, Bayerl M, Rujescu D, Müller DJ, & Gallinat J (2013). Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: A case–control study. The World Journal of Biological Psychiatry, 14(7), 500–508. 10.3109/15622975.2012.677547 [DOI] [PubMed] [Google Scholar]
- Newton E, & Jenvey V (2011). Play and theory of mind: associations with social competence in young children. Early Child Development and Care, 181(6), 761–773. 10.1080/03004430.2010.486898 [DOI] [Google Scholar]
- Normand S, Soucisse MM, Melançon MPV, Schneider BH, Lee MD, & Maisonneuve M-F (2019). Observed Free-Play Patterns of Children with ADHD and Their Real-Life Friends. Journal of Abnormal Child Psychology, 47(2), 259–271. 10.1007/s10802-018-0437-3 [DOI] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Partap S, Sherr EH, Hardan AY, Farmer C, Thurm A, Swedo SE, & Parker KJ (2018). Cerebrospinal fluid vasopressin and symptom severity in children with autism. Annals of Neurology, 84(4), 611–615. 10.1002/ana.25314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagi E (2018). Not just for fun! Social play as a springboard for adult social competence in human and non-human primates. Behavioral Ecology and Sociobiology, 72(6). 10.1007/s00265-018-2506-6 [DOI] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Fung LK, Garner JP, & Hardan AY (2019). A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, & de Vries GJ (2014). Sexually dimorphic role for vasopressin in the development of social play. Front Behav Neurosci, 8, 58. 10.3389/fnbeh.2014.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (1998). The RAt Brain in Stereotaxic Coordinates (4 ed.). Academic Press. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RLH, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, & Blanchard RJ (2011). Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes, Brain and Behavior, 10(2), 228–235. 10.1111/j.1601-183x.2010.00659.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini AD, Dupuis A, & Smith PK (2007). Play in evolution and development. Developmental Review, 27(2), 261–276. [Google Scholar]
- Pellis SM, Pellis VC, Ham JR, & Stark RA (2023). Play fighting and the development of the social brain: The rat’s tale. Neurosci Biobehav Rev, 145, 105037. 10.1016/j.neubiorev.2023.105037 [DOI] [PubMed] [Google Scholar]
- Penteado SH, Teodorov E, Kirsten TB, Eluf BP, Reis-Silva TM, Acenjo MK, de Melo RC, Suffredini IB, & Bernardi MM (2014). Prenatal lipopolysaccharide disrupts maternal behavior, reduces nest odor preference in pups, and induces anxiety: studies of F1 and F2 generations. Eur J Pharmacol, 738, 342–351. 10.1016/j.ejphar.2014.05.058 [DOI] [PubMed] [Google Scholar]
- Potasiewicz A, Gzielo K, Popik P, & Nikiforuk A (2020). Effects of prenatal exposure to valproic acid or poly(I:C) on ultrasonic vocalizations in rat pups: The role of social cues. Physiol Behav, 225, 113113. 10.1016/j.physbeh.2020.113113 [DOI] [PubMed] [Google Scholar]
- Raam T, & Hong W (2021). Organization of neural circuits underlying social behavior: A consideration of the medial amygdala. Curr Opin Neurobiol, 68, 124–136. 10.1016/j.conb.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Gergely CK, & Veenema AH (2018). Activation patterns of vasopressinergic and oxytocinergic brain regions following social play exposure in juvenile male and female rats. J Neuroendocrinol. 10.1111/jne.12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S, Urruela M, & Devine DP (2013). Effects of environmental enrichment on repetitive behaviors in the BTBR T+tf/J mouse model of autism. Autism Res, 6(5), 337–343. 10.1002/aur.1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigney N, Whylings J, Mieda M, de Vries G, & Petrulis A (2019). Sexually Dimorphic Vasopressin Cells Modulate Social Investigation and Communication in Sex-Specific Ways. eNeuro, 6(1). 10.1523/ENEURO.0415-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigney N, Zbib A, Vries GJ, & Petrulis A (2022). Knockdown of sexually differentiated vasopressin expression in the bed nucleus of the stria terminalis reduces social and sexual behaviour in male, but not female, mice. Journal of Neuroendocrinology. 10.1111/jne.13083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, & Weisskopf MG (2013). Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect, 121(8), 978–984. 10.1289/ehp.1206187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Borrero E, Rivera-Escalera F, Candelas F, Montalvo J, Muñoz-Miranda WJ, Walker JR, & Maldonado-Vlaar CS (2010). Arginine vasopressin gene expression changes within the nucleus accumbens during environment elicited cocaine-conditioned response in rats. Neuropharmacology, 58(1), 88–101. 10.1016/j.neuropharm.2009.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé N, Stemmelin J, Cohen C, & Griebel G (2006). Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology, 187(2), 237–244. 10.1007/s00213-006-0424-1 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Hashimoto K, Oda Y, Ishima T, Kurata T, Takahashi J, Kamata Y, Kimura H, Niitsu T, Komatsu H, Ishikawa M, Hasegawa T, Shiina A, Hashimoto T, Kanahara N, Shiraishi T, & Iyo M (2015). Decreased levels of serum oxytocin in pediatric patients with Attention Deficit/Hyperactivity Disorder. Psychiatry Res, 228(3), 746–751. 10.1016/j.psychres.2015.05.029 [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Chang C, Onore CE, & Ashwood P (2015). Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Transl Psychiatry, 5, e543. 10.1038/tp.2015.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, & Ashwood P (2013). Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Translational Psychiatry, 3(3), e240–e240. 10.1038/tp.2013.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, & Guastella AJ (2013). The Impact of a Single Administration of Intranasal Oxytocin on the Recognition of Basic Emotions in Humans: A Meta-Analysis. Neuropsychopharmacology, 38(10), 1929–1936. 10.1038/npp.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkhuu T, Doerfler DL, Krantz QT, Luebke RW, Linak WP, & Gilmour MI (2010). Effects of prenatal diesel exhaust inhalation on pulmonary inflammation and development of specific immune responses. Toxicol Lett, 196(1), 12–20. 10.1016/j.toxlet.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Smith CJ, Kingsbury MA, Dziabis JE, Hanamsagar R, Malacon KE, Tran JN, Norris HA, Gulino M, Bordt EA, & Bilbo SD (2020). Neonatal immune challenge induces female-specific changes in social behavior and somatostatin cell number. Brain Behav Immun, 90, 332–345. 10.1016/j.bbi.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJW, DiBenedictis BT, & Veenema AH (2019). Comparing vasopressin and oxytocin fiber and receptor density patterns in the social behavior neural network: Implications for cross-system signaling. Front Neuroendocrinol, 53, 100737. 10.1016/j.yfrne.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJW, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, & Veenema AH (2017). Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct, 222(2), 981–1006. 10.1007/s00429-016-1260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, & Patterson PH (2007). Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci, 27(40), 10695–10702. 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Chapman HC, Keller WR, Koenig JI, Gold JM, Carpenter WT, & Buchanan RW (2019). Endogenous oxytocin levels are associated with impaired social cognition and neurocognition in schizophrenia. Journal of Psychiatric Research, 112, 38–43. 10.1016/j.jpsychires.2019.02.017 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kashino M, & Hironaka N (2010). Structure of Rat Ultrasonic Vocalizations and Its Relevance to Behavior. PLoS One, 5(11), e14115. 10.1371/journal.pone.0014115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, & de Vries GJ (2012). Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ, 3(1), 15. 10.1186/2042-6410-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels EA, Alberts JR, & Cramer CP (1990). Weaning in Rats: 11. Pup Behavior Patterns. Developmental Psychobiology, 23(6), 495–510. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, & Benson J (2006). Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Sciences, 103(20), 7889–7894. 10.1073/pnas.0600406103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, & Holloway WR (1984). Developmental analyses of social play behavior in juvenile rats. Bulletin of the Psychonomic Society, 22(6), 587–590. 10.3758/bf03333916 [DOI] [Google Scholar]
- van den Pol AN (2012). Neuropeptide Transmission in Brain Circuits. Neuron, 76(1), 98–115. 10.1016/j.neuron.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, Hill MN, & McCarthy MM (2019). Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron, 102(2), 435–449 e436. 10.1016/j.neuron.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, & Neumann ID (2010). Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav, 58(2), 273–281. 10.1016/j.yhbeh.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Wang D, Li A, Dong K, Li H, Guo Y, Zhang X, Cai M, Li H, Zhao G, & Yang Q (2021). Lateral hypothalamus orexinergic inputs to lateral habenula modulate maladaptation after social defeat stress. Neurobiol Stress, 14, 100298. 10.1016/j.ynstr.2021.100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, & Blumberg SJ (2017). Estimated Prevalence of Children with Diagnosed Developmental Disabilities in the United States, 2014-2016. [PubMed]
- Zerbo O, Qian Y, Yoshida C, Grether JK, Van De Water J, & Croen LA (2015). Maternal Infection During Pregnancy and Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 45(12), 4015–4025. 10.1007/s10803-013-2016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, & Zhang L (2019). Increasing Prevalence of Allergic Rhinitis in China. Allergy, Asthma & Immunology Research, 11(2), 156. 10.4168/aair.2019.11.2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented here are available from the corresponding author upon reasonable request.


