Abstract
Ethanol (EtOH) exposure during late pregnancy leads to enduring impairments in learning and memory that may stem from damage to components of the posterior limbic memory system, including the retrosplenial cortex (RSC) and anterior thalamic nuclei (ATN). In rodents, binge-like EtOH exposure during the first week of life (equivalent to the third trimester of human pregnancy) triggers apoptosis in these brain regions. We hypothesized that this effect induces long-lasting alterations in the function of RSC-projecting ATN neurons. To test this hypothesis, vesicular GABA transporter-Venus mice (expressing fluorescently tagged GABAergic interneurons) were subjected to binge-like EtOH vapor exposure on postnatal day (P) 7. This paradigm activated caspase 3 in the anterodorsal (AD), anteroventral (AV), and reticular thalamic nuclei at P7 but did not reduce neuronal density in these areas at P60–70. At P40–60, we injected red retrobeads into the RSC and performed patch-clamp slice electrophysiological recordings from retrogradely labeled neurons in the AD and AV nuclei 3–4 days later. We found significant effects of treatment on instantaneous action potential (AP) frequency and AP overshoot, as well as sex x treatment interactions for AP threshold and overshoot in AD neurons. A sex x treatment interaction was detected for AP number in AV neurons. EtOH exposure also reduced the frequency and amplitude of spontaneous excitatory postsynaptic currents and increased the charge transfer of spontaneous inhibitory postsynaptic currents. These results highlight a novel cellular mechanism that could contribute to the lasting learning and memory deficits associated with developmental EtOH exposure.
Keywords: Fetal, ethanol, development, cortex, retrosplenial, thalamus, apoptosis, glutamate, GABA, electrophysiology, neonatal
1. Introduction
Fetal Alcohol Spectrum Disorder (FASD) poses a significant public health concern, with a relatively high prevalence (approximately 2–7%) and substantial financial costs for society (Greenmyer et al., 2020; May et al., 2020; May et al., 2021). Ethanol (EtOH) consumption during pregnancy can occur at any stage, including the last trimester, where alcohol consumption rates ranging from 5–8% have been detected in specific populations in the United States (Umer et al., 2020). This period is critical for significant brain growth, myelination, and early synaptic development (Cudd, 2005; Dukes et al., 2017; Ethen et al., 2009; Himes et al., 2015; Kostovic and Jovanov-Milosevic, 2006; Murphy et al., 2014). Moreover, third-trimester-equivalent alcohol exposure (TTAE) can also occur in premature babies through exposure to EtOH vapor from hand sanitizers used in incubators or intravenous medications containing EtOH as an excipient (Akinmboni et al., 2018; Hsieh et al., 2018). TTAE, especially when it occurs binge-like, has detrimental effects on child behavior and cognition (Niclasen et al., 2014).
Among the cognitive effects of TTAE are long-lasting learning and memory deficits. Experimental evidence suggests that these deficits could be attributed to damage to the hippocampal-diencephalic-cingulate network (Bird et al., 2020; Bird et al., 2021; Bjorkquist et al., 2010; Dodge et al., 2020; Farber et al., 2010; Lebel et al., 2011; Lewin et al., 2018; Olney et al., 2002; Willoughby et al., 2008; Wozniak et al., 2004). This network consists of interconnected regions, including the retrosplenial cortex (RSC) and anterior thalamic nuclei (ATN) (Bubb et al., 2017). This study focuses on investigating how TTAE affects the ATN neurons projecting to the RSC. Several ATN efferents reach the RSC through the cingulum bundle, originating mainly from the anteroventral (AV) and anterodorsal (AD) nuclei (Bubb et al., 2020; Yamawaki et al., 2019). Axons of AV neurons primarily synapse with apical tuft dendrites of layer 5 pyramidal neurons in layer 1 of the RSC (Odagiri et al., 2011; Yamawaki et al., 2019). In vivo optogenetic experiments have shown that stimulation of ATN→RSC axons transiently increases pyramidal neuron activity in the RSC, followed by brief suppression (Yamawaki et al., 2019). The ATN plays a crucial role in allocentric navigation and path integration, with ATN lesions leading to impaired generation of directional trajectories based on head direction cell activity (Clark and Harvey, 2016). The AD nucleus exhibits a relatively constrained network of interconnections, which may be indicative of its more specialized function as a pivotal component in the head direction navigational system (Nelson, 2021). In contrast, the AV nucleus has a more expansive array of connections, potentially aligning with its broader involvement in cognitive processes, including discrimination learning and attention (Nelson, 2021). Importantly, AD→RSC synaptic connections play a pivotal role in shaping contextual memory formation, while AV→RSC connections serve to modulate memory specificity (Roy et al., 2021). The thalamic reticular nucleus (TRN) provides inhibitory input to both AD and AV thalamic neurons, playing a critical role in modulating their function (Takata, 2020).
Both preclinical and clinical studies have documented alcohol-induced alterations in the structure and function of the thalamus and/or RSC (Bird et al., 2020; Bird et al., 2021; Bjorkquist et al., 2010; Dodge et al., 2020; Fagerlund et al., 2006; Farber et al., 2010; Lebel et al., 2011; Lewin et al., 2018; Meintjes et al., 2010; Meintjes et al., 2014; Olney et al., 2002; Roediger et al., 2021; Willoughby et al., 2008; Woods et al., 2018; Wozniak et al., 2004). Individuals with FASD exhibit decreased thalamic activation during virtual spatial memory tasks (Woods et al., 2018). Furthermore, a single binge-like EtOH exposure at postnatal day 7 (P7) can induce significant neuronal apoptosis in these regions of rodent and primate brains (Bird et al., 2020; Ikonomidou et al., 2000; Olney et al., 2002; Saito et al., 2007; Smiley et al., 2023; Wozniak et al., 2004). EtOH-induced apoptotic neurodegeneration was observed in both the AD and AV nuclei (Farber et al., 2010; Ikonomidou et al., 2000; Smiley et al., 2023; Wozniak et al., 2004). Importantly, long-lasting reductions in AD nucleus volume (Wozniak et al., 2004) and neuronal numbers in both the AD and AV nuclei were observed in adult mice exposed to EtOH at P7 (Smiley et al., 2023). However, it remains uncertain whether the surviving ATN neurons have functional deficits that could contribute to long-lasting contextual and spatial memory impairments (Lewin et al., 2018; Wozniak et al., 2004), as well as other cognitive deficits (Phillips et al., 2019).
To investigate the hypothesis that TTAE disrupts the function of ATN neurons involved in visuospatial learning, mice were exposed to high levels of EtOH in vapor chambers at P7. This treatment activated apoptotic pathways in ATN neurons. When the mice reached late adolescence, red retrobeads were injected into the RSC, and patch-clamp slice electrophysiological recordings were performed on retrogradely-labeled neurons in the AD and AV nuclei. This methodology allowed for the identification of various functional alterations in these neurons.
2. Materials and Methods
Unless otherwise indicated, all chemicals were purchased from Sigma Aldrich (St. Louis, MO).
2.1. Animals
For all experiments, we used transgenic mice expressing Venus yellow fluorescent protein in GABAergic and glycinergic interneurons (under the control of the vesicular GABA transporter (VGAT), referred as Venus-VGAT mice), which were generously provided by Dr. Yanagawa at Gunma University Graduate School of Medicine in Maebashi, Japan (Wang et al., 2009). We opted to employ these mice to facilitate the assessment of the impact of EtOH on caspase-3 activation and neuronal densities within the thalamic reticular nucleus. Furthermore, we intend to utilize these mice in subsequent investigations into the function of these neurons. Mice were housed with water and standard chow available ad libitum on a 12-h reverse light/dark cycle (lights on at 8 p.m.). Breeding and genotyping procedures for Venus-VGAT mice were conducted as described previously (Bird et al., 2018).
2.2. EtOH vapor chamber exposure
P7 Venus-VGAT mouse pups and their dams were randomly assigned for exposure to EtOH or air as described previously (Bird et al., 2020). Exposure to EtOH or air (control) occurred in vapor chambers (Morton et al., 2014) for 4h (approximately from 10 a.m. to 2 p.m). Blood EtOH concentrations following vapor chamber exposures have been described previously (Bird et al., 2020) and have been shown to average roughly 360 mg/dl. Mice were either sacrificed 8 h later for analysis of cleaved caspase-3 (CC3) expression, or were saved for subsequent immunohistochemistry or red retrobead infusion and electrophysiology experiments.
2.3. Quantification of apoptotic cell death at postnatal day 7 and neuronal densities at postnatal days 60–70
P7 Venus-VGAT pups were deeply anesthetized with ketamine (250 mg/kg intraperitoneally) and transcardially perfused 8 h after the cessation of vapor chamber exposure or at P60–70. Mice were perfused transcardially with 32°C phosphate buffered saline (PBS) containing procaine hydrochloride (1 g/L; Sigma-Aldrich, St. Louis, MO) and heparin (1 USP unit/mL; Sagent Pharmaceuticals, Schaumburg, IL) for 2 min, followed by room-temperature 4% paraformaldehyde (PFA; Sigma-Aldrich) in PBS for 2 min, and then with ice-cold 4% PFA in PBS for 5 minutes. Brains were extracted and placed in 4% PFA in the dark at 4 oC on a rotating shaker for 48 h and then cryoprotected in 30% sucrose (w/v in PBS) for 48 h. Brains were sectioned in the coronal plane (50 μm thickness) with a cryostat (Model 505E, Microm GmbH, Walldorf, Germany) and stored at −20 oC in multiplate wells containing a cryoprotectant solution (0.05 M phosphate buffer pH 7.4, 25% glycerol and 25% ethylene glycol).
Four 50 μm serial coronal sections containing the AD, AV, and reticular thalamic nuclei (equivalent to between Bregma −0.47 to Bregma −0.83 in the adult mouse (Paxinos and Franklin, 2013) were processed from 4 air-exposed and 5 EtOH exposed pups for CC3 immunohistochemistry. Sections were incubated for 2 h in blocking/permeabilization solution containing 1% bovine serum albumin, 5% donkey serum (Jackson ImmunoResearch, West Grove, PA) and 0.2% Triton X-100. Sections were next incubated with rabbit anti-CC3 primary antibody (1:400 dilution, catalog # 9661, Cell Signaling, Danvers MA) for 72 h in a humidity chamber protected from light at 4 °C. Sections were then incubated for 2 h in secondary donkey anti-rabbit IgG Alexa Fluor 555 antibody (1:1000 dilution, catalog # A-31572, Thermo Fisher) at room temperature (22–24°C). Nuclei were stained in PBS for 20 min with 600 nM 4’6-diamidino-2-phenylindole hydrochloride (DAPI). Sections were rinsed with PBS, placed onto Superfrost Plus microscope slides (VWR, Radnor, PA) and covered with glass coverslips (VWR) using Fluoromount G mounting media (Southern Biotech, Birmingham, AL).
Fluorescence microscopy was performed as described previously (Bird et al., 2020; Bird et al., 2018) using a Zeiss AxioPlan2 inverted fluorescence microscope (White Plains, NY) equipped with a Nuance spectral imaging system (PerkinElmer, Hopkinton, MA) using a 10X objective lens. Images containing the AD, AV, and reticular thalamic nuclei were collected in each hemisphere of the 4 brain sections resulting in 8 images analyzed per animal with three fluorescence channels: blue for DAPI nuclear staining, green for Venus-VGAT positive interneurons, and red for CC3 positive cells. Images were analyzed by an investigator in a blind fashion using Fiji (Image J software, Bethesda, Maryland). Using the polygon selection tool, the investigator outlined the AD nucleus, AV nucleus and TRN in each image and quantified the number of CC3 positive cells per mm2 in each nucleus. The average density of CC3 and DAPI-positive cells was calculated for each animal’s anterior thalamic nuclei. Images were quantified automatically by a second investigator in a blinded fashion using a brightness threshold and the particles tool in ImageJ. The counts independently obtained by the two investigators where then averaged.
For quantification of neuronal density at P60–70, brains were processed as described above. Sections containing the AD nucleus, AV nucleus and TRN were stained with mouse anti-NeuN monoclonal antibody (MAB377, MilliporeSigma, Burlington, MA) using the procedures previously described (Bird et al., 2021). The secondary antibody was Alexa-Fluor 555 donkey anti-mouse antibody (Thermo-Fisher, Waltham, MA). Sections were also stained with the nuclear dye DAPI. Images were acquired with the Nuance Spectral Imaging System and quantified, as described above.
2.4. Red retrobead stereotaxic injection into the RSC
Late adolescent/young adult Venus-VGAT mice (P40-P60) that were exposed to either air or vaporized EtOH at P7 were anesthetized with isoflurane and placed in a stereotaxic alignment system (Kopf Instruments, Tujunga, CA) for injection of red retrobeads (Lumafluor Inc, Durham, NC). Retrobeads (0.25 μl, diluted 1:3 with artificial cerebrospinal fluid (aCSF, recipe provided below in Section 2.5) were injected bilaterally into the RSC using the coordinates: AP - 1.8, ML ± 0.4, DV - 0.75. Injections occurred over 3 min using a syringe pump (Kent Scientific, Torrington, CT). Mice recovered for 3–4 days before brain slices were collected for either image analysis or electrophysiology. To verify RSC retrobead injection site and subsequent expression levels in the AD and AV thalamus, mice were perfused and coronal sections were prepared with a cryostat, as described in Section 2.3. Sections were stained with DAPI and imaged as described in Section 2.3
2.5. Whole-cell patch-clamp electrophysiology
Slices were prepared from retrobead injected mice using a protective cutting/recovery method, as previously described (Bird et al., 2021) (Ting et al., 2018). After deep anesthetization with ketamine (250mg/kg intraperitoneally), mice were transcardially perfused over 2.5 minutes with 25 ml of protective N-methyl-D-glucamine (NMDG)-containing aCSF composed of (in mM): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 3 sodium pyruvate, 5 ascorbic acid, 10 MgSO4, and 0.5 CaCl2 saturated with 95%O2/5%CO2 at 4°C (pH 7.3–7.4 with HCl, 300–310 mOsm). Following perfusion, brains were quickly extracted and incubated in the same NMDG-containing solution for 1 min. Coronal brain slices (300 μM) containing the AD and AV thalamus (Bregma − 0.47 to −0.95) were made using a vibrating slicer (Leica model VT1000 S, Leica Microsystems). Slices were transferred to warm (32–34°C) NMDG-containing aCSF, and over the course of 25 min, the NaCl concentration of the solution was gradually increased to 52 mM by the addition of NMDG aCSF containing 2M NaCl. Slices were then transferred to a slice holding chamber (Model # BSC-PC, Warner Instruments, Hamden CT) for 1 hour containing a holding solution at room temperature composed of (in mM): 95 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea (Alfa Aesar, Ward Hill, MA), 3 sodium pyruvate, 1 MgSO4, 2 CaCl2, and 5 ascorbic acid saturated with 95% O2/5%CO2 at 4°C (pH 7.3–7.4, 300–310 mOsm).
Recordings took place in a chamber containing a slice support (Model # RC-27L, Warner Instruments). The composition of the aCSF was (in mM): 125 NaCl, 1.3 KCl, 1.3 NaH2PO4, 26 NaHCO3, 10 glucose, 1 MgSO4, 2 CaCl2, and 0.4 ascorbic acid. The recording chamber was warmed to 32–34°C using a dual-temperature automatic temperature controller (Model TC-344B, Warner Instruments) and an in-line solution heater (Model SH-27B, Warner Instruments. Solutions were applied at a 2 ml/min rate using a peristaltic pump (Master Flex model 7518–10, Cole-Palmer, Vernon Hills, IL). Slices were stabilized using platinum wire (Catalog # 43288, Alfa Aesar) and imaged with a BX51WI microscope (Olympus, Center Valley, PA) mounted on a manual X-Y translator (Model MT-500, Sutter Instruments, Novato, CA) using infrared light. Slices were visualized with a complementary metal-oxide semiconductor digital camera (Model 01-ROL-BOLT-M-12, Q-Imaging, Surrey, Canada) with a Plan 4x (0.10 N.A.) lens and a LUMPlan F1/IR 40x water immersion (0.8 N.A.) lens (Olympus). Fluorescence was visualized with a mercury arc lamp (Olympus), and red retrobead positive cell bodies in the AD/AV thalamus were identified using an excitation filter ET246/22x, beam splitter T565lpxr, and emission filter ET590lp (Chroma Technology Corp. Bellows Falls, VT). Recording electrodes (2–3 MΩ) were pulled from filament-containing borosilicate capillary glass (Catalog # BF150–86-10) using a DMZ Universal Puller (Zeitz-Instruments Vertriebs GmbH, Martinsried, Germany) and positioned with a micromanipulator (Scientifica, Clarksburg, NJ). Signals were collected with an Axopatch 700B amplifier connected to a Digidata Model 1550B amplifier using Clampex software (Version 11, Molecular Devices, Sunnyvale, CA). Data were acquired at a sampling rate of 10 kHz and was filtered at 2–4 kHz. Recordings in which the access resistance changed by more than 20% were discarded. Analysis of electrophysiology data was performed offline using Clampfit version 11 (Molecular Devices).
Recordings were obtained from retrobead positive AD and AV thalamic nuclei neurons (indicating that they project to the RSC). We focused on these neurons because they showed the highest levels of CC3 after P7 ethanol exposure. The internal solution used to record action potentials and spontaneous excitatory postsynaptic currents (sEPSCs) was composed of (in mM): 140 K-methanesulfonate, 0.5 EGTA, 15 HEPES, 4 KCl, 2 Mg-ATP, 0.3 Na-GTP, and 10 phosphocreatine disodium salt, pH 7.25 (adjusted with KOH) and 305 mOsm. Action potentials were generated in the current clamp mode. The current was injected to maintain cells near −70 mV before a sequence of depolarizing currents (200, 400, 600, 800, 1000 pA) was injected into the cells to evoke action potentials. Spontaneous EPSCs were collected for 5 min in voltage-clamp mode with a holding potential of −70mV in the presence of 25 μM gabazine (SR95531; Hello Bio, Princeton, NJ). A subset of sEPSCs were blocked by applying 3 mM kynurenic acid to block all excitatory glutamatergic activity to confirm that sEPSCs were mediated by glutamate ionotropic receptors. Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded for 5 min at a holding potential of 0 mV with an internal solution composed of (in mM): 140 Cs-methanesulfonate, 0.5 EGTA, 15 HEPES, 2 tetramethylammonium chloride, 2 Mg-ATP, 0.3 Na-GTP, 10 phosphocreatine disodium salt, and 4 QX-314-Cl (Hello Bio, Princeton, NJ) pH 7.25 (adjusted with CsOH) and 305 mOsm. In a ubset of experiments, sIPSCs were blocked by applying 25 μM gabazine to confirm that they were GABAA receptor-mediated.
Action potentials were analyzed using ClampFit software (Version 11, Molecular Devices). For each level of current injected, the following action potential parameters were obtained: threshold, instantaneous firing frequency (1/interspike interval (in seconds) of the first two action potentials), number per 500 ms train, amplitude, overshoot, spike frequency adaptation index (the ratio of the first interspike interval over the final interspike interval for a series of action potentials) and afterhyperpolarization. sEPSC and sIPSC recordings were analyzed using the Mini Analysis Program (Synaptosoft, Decatur, GA); for each sEPSC and sIPSC recording, the average event frequency, amplitude, total charge, decay time, and rise time 10–90%, were calculated.
2.6. Statistical Analyses
Statistical analyses were performed using Prism version 10 (GraphPad Software, San Diego, CA). All data are presented as mean ± SEM. The results of statistical analyses are summarized in the Supplementary Data Table. The unit of determination for all measures was a single animal. If action potential, sEPSC, or sIPSC recordings were collected from multiple neurons from a single animal, those results were averaged together to generate a single data point. Outliers for each measure were removed with a ROUT 1% test. Data were then analyzed with the Shapiro-Wilks normality test. Data that did not pass the test were log-transformed and re-analyzed with this test. Raw or log-transformed data that passed the normality test were analyzed by two- or three-way ANOVA. When normality was not established with log transformation, Aligned Rank Transform (ART) ANOVA was chosen as a nonparametric tool to analyze the interaction and main effects (Leys, 2010; Wobbrock et al., 2011). Three-way ART ANOVAs were conducted with Sex, Current, and EtOH exposure as the between-subject factors using R Statistical Software v4.0.4 (Team, 2021), tidyverse package v1.3.0 (Wickham, 2019), readxl package v1.3.1 (https://CRAN.R-project.org/package=readxl), and ARTool package v0.11.1 (Elkin, 2021). Post hoc testing utilized the ART-C contrast testing procedure within the ARTool package v0.11.1 (Elkin, 2021) with a Sidak correction of p values for multiple tests. Two-way ART ANOVAs were conducted with Sex and EtOH exposure as the between-subject factors using the same process.
3. RESULTS
3.1. EtOH exposure at P7 activated apoptotic pathways in anterior thalamic neurons.
Seven-day-old mouse pups and their dams were exposed to air or EtOH for 4 h in vapor chambers. This paradigm results in peak blood alcohol concentrations in the pups near 360 mg/dl (Bird et al., 2020). Pup brains were collected 8 h after EtOH vapor exposure to measure levels of CC3 expression in the AD, AV, and TRN. Fig 1A–B show that EtOH exposure increased the density of CC3+ cells in the ATN (for reference, a panoramic image illustrating the location of the ATN is shown in Fig 1C). Fig 1D shows that EtOH exposure significantly increased the percentage of DAPI+ cells that were CC3+. The raw data did not follow a normal distribution. However, log-transformed data passed the Shapiro-Wilk normality test and were analyzed by two-way ANOVA (Supplementary Data Tables). This test revealed a significant effect of treatment (p < 0.0001) but not ATN region (p = 0.3) and a significant interaction (p = 0.0003) (Supplementary Data Tables). Posthoc analysis with the Holm-Šídák’s test yielded treatment p values of 0.0001, 0.0001, and 0.032 for the AD, AV, and TRN, respectively.
Figure 1. Ethanol (EtOH) exposure at postnatal day 7 activated apoptotic pathways in anterior thalamic neurons.
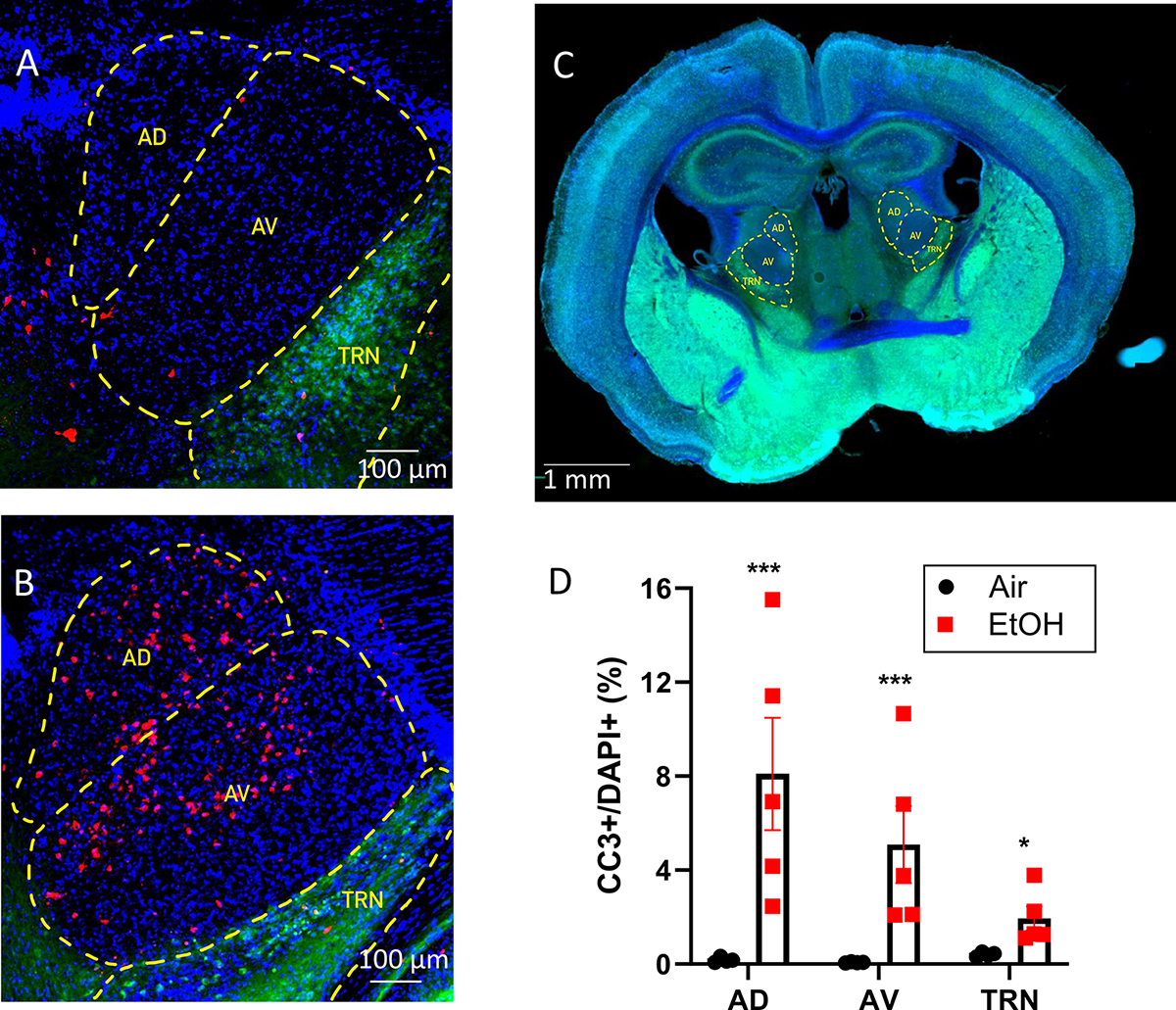
Images from VGAT-Venus mice from the Air (control) (A) and 8 hours post EtOH vapor conditions (B). Red: cleaved caspase 3 (CC3), blue: DAPI, green: Venus. AD, anterodorsal thalamic nucleus; AV, anteroventral thalamic nucleus; TRN, thalamic reticular nucleus. C) Panoramic image illustrating the AD, AV, and TRN location in a coronal brain slice of a P7 VGAT-Venus mouse. Blue: DAPI; green: Venus. D). Summary data of the effect of EtOH treatment on the percentage of DAPI-positive cells that were positive for cleaved caspase 3 (CC3) in the AD nucleus (p<0.0001), AV nucleus (p<0.0001) and TRN (p=0.032) (Šídák’s multiple comparisons test for control vs. EtOH). Control, n=4 pups (2 males and 2 females from 2 litters) and EtOH, n=5 pups (4 males and 1 female from 3 litters).
3.2. Neonatal EtOH exposure did not significantly affect neuronal densities in the AD, AV, or TRN at P60–70.
We investigated if the P7 EtOH-induced increase of CC3+ cells results in a long-term reduction of neuronal numbers in the ATN. We exposed VGAT-Venus pups to air or EtOH at P7 and collected brains at P60–70. Neuronal densities were determined using NeuN immunohistochemistry (Fig 2A). The raw data did not follow a normal distribution. However, log-transformed data passed the Shapiro-Wilk normality test and were analyzed by three-way ANOVA (Supplementary Data Tables). There were significant effects of ATN region (p < 0.0001) and treatment (p = 0.037) on neuronal densities. There was a significant sex x treatment interaction (p = 0.041) but no ATN region x sex (p = 0. 06), ATN region x treatment (p = 0.46), or ATN region x sex x treatment (p = 0.45) interactions. Posthoc analysis with the Holm-Šídák’s test yielded treatment p values ranging from >0.75 to >0.9 for both males and females in the AD nucleus, AV nucleus, and TRN (Supplementary Data Tables).
Figure 2. Neonatal ethanol (EtOH) exposure did not significant affect neuronal densities in anterior thalamic nuclei at postnatal days 60–70.
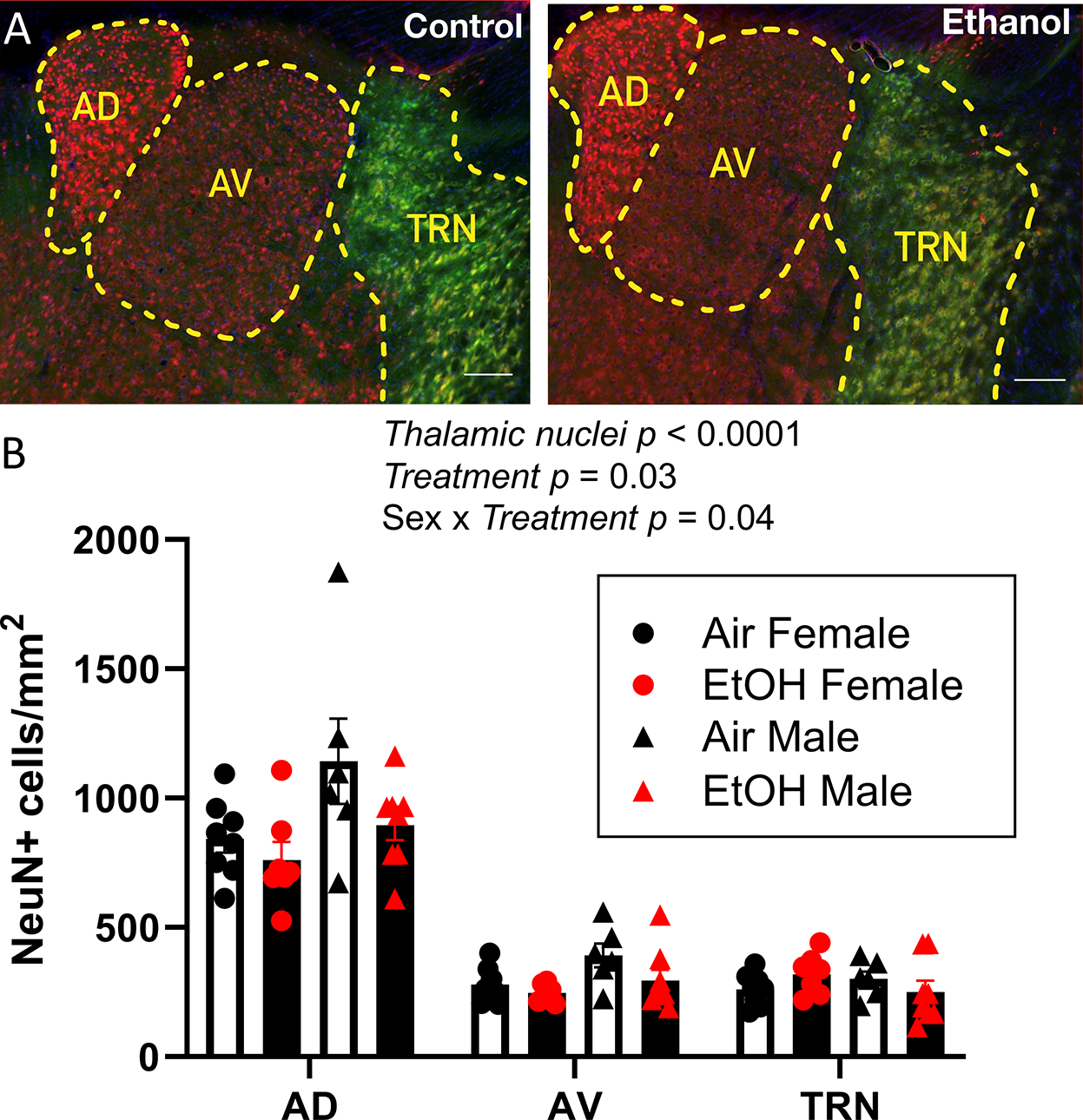
A) Sample images of neurons stained with anti-NeuN antibodies (red) and Venus+ GABAergic interneurons (green) from the Air (control) and EtOH groups (scale bars 100 μm). B) Summary graph illustrating the effect of third trimester-equivalent EtOH exposure on neuronal densities in the anterodorsal (AD), anteroventral (AV), or thalamic reticular (TRN) nuclei. Data were analysed by three-way ANOVA of log-transformed data. Posthoc analysis with the Holm-Šídák’s test yielded treatment p values ranging from >0.75 to >0.9 for both males and females in the AD nucleus, AV nucleus, and TRN. Control female, n=8 pups from 6 litters; EtOH female, n=7 pups from 4 litters; control male, n=6 pups from 3 litters; EtOH male, n=8 pups from 6 litters.
3.3. Neonatal EtOH exposure significantly affected ATN neuron excitability at P40–60.
To investigate how TTAE affects anterior thalamic neurons that project to the RSC, we injected adolescent Venus-VGAT mice at P40–60 with red retrobeads into the RSC (Fig 3). These beads are taken up at the axonal terminals and retrogradely transported back to cell bodies. Injection of red retrobeads into the RSC (Fig 3A–B) led to robust labeling of cells in both the AD and AV thalamus (Fig 3D). We performed whole-cell patch-clamp electrophysiology on red retrobead-expressing cells in the AD and AV thalamus on Venus-VGAT adolescent mice exposed to air (control) or vaporized EtOH at P7 (Fig 3C–D).
Figure 3. Schematic representation of retrobead injection into the retrosplenial cortex to label neurons in the anterodorsal (AD) and anteroventral (AV) thalamic nuclei.
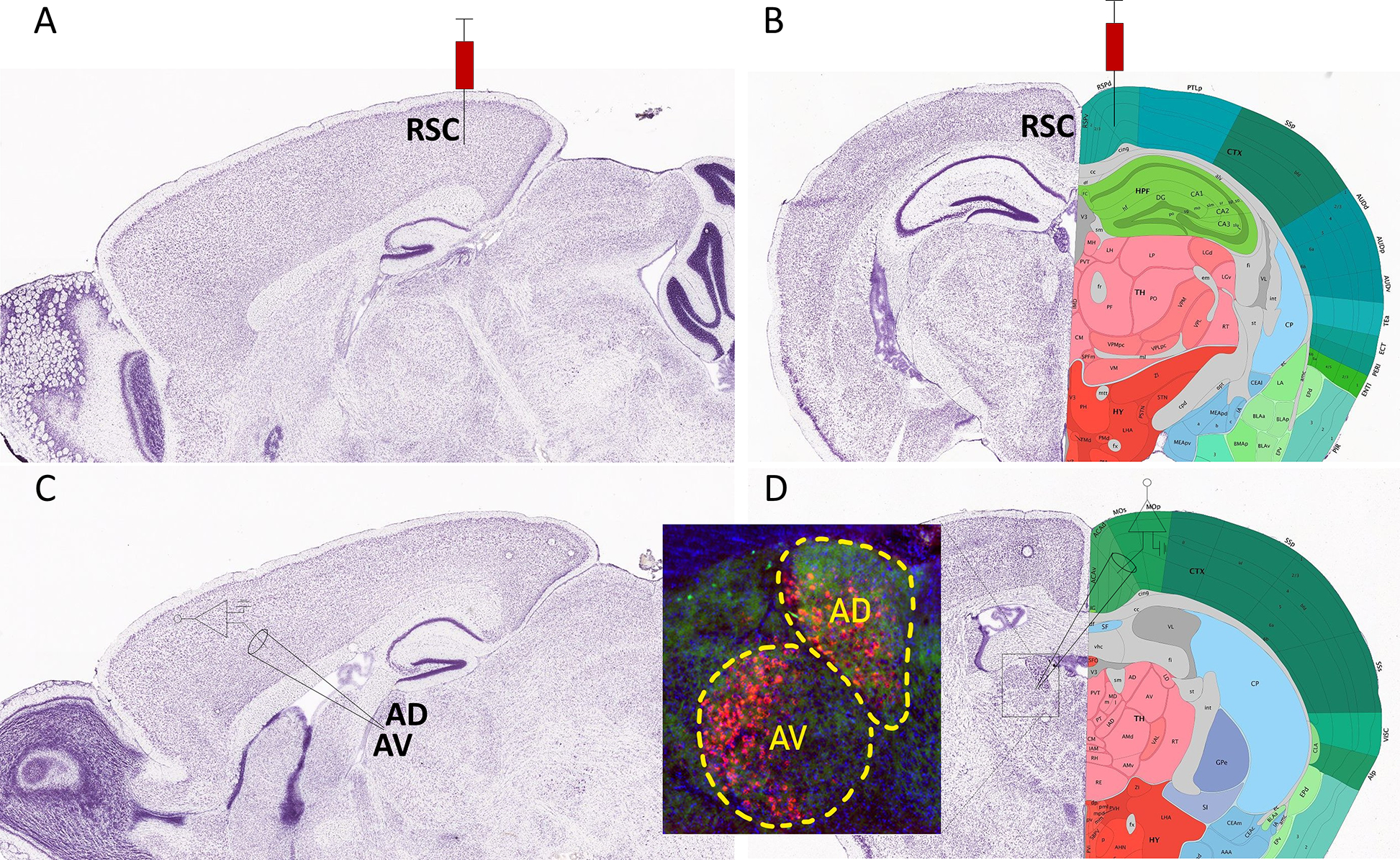
A) Parasagittal image of a Nissl stained section (image #18) from the Allen Reference Atlas – Mouse Brain (postnatal day 56) illustrating the site of red retrobead injection in the retrosplenial cortex (RSC). B) Nissl stained image with anatomical annotations of a coronal section (image #75) from the same atlas also showing the site of injection in the RSC. C) Parasagittal image of a Nissl stained section (image #16) from the same atlas illustrating the site of electrophysiological recordings in the AD and AV thalamic nuclei. D) Nissl-stained image with anatomical annotations of a coronal section (image #62) from the same atlas also showing the site of electrophysiological recordings in the AD and AV thalamic nuclei. The inset shows an image of red retrobeads in the AD and AV nuclei from a mouse that received an injection in the RSC. Allen Mouse Brain Atlas, mouse.brain-map.org.
We first assessed the effect of EtOH exposure on the passive membrane properties of the neurons. Two-way ANOVA of log-transformed data revealed a significant effect of sex (p = 0.016) and treatment (p = 0.035) on the membrane capacitance but no sex x treatment interaction in AD neurons; no significant effects were detected in AV neurons (Table 1 and the Supplementary Table). Two-way ANOVA of log-transformed data did not find any significant effects on the membrane resistance of AD neurons (Table 1 and the Supplementary Table). Two-way ANOVA of ART-transformed data found a significant effect of sex (p = 0.033) on the membrane resistance of AV neurons (Table 1 and the Supplementary Data Tables).
Table 1.
Effect of EtOH exposure on membrane capacitance and membrane resistance of anterodorsal (AD) and anteroventral (AV) thalamic nuclei neurons
| Cm Air, pF (n) | Cm EtOH, pF (n) | Significant p values | Rm Air, MΩ (n) | Rm EtOH, MΩ (n) | Significant p values | |
|---|---|---|---|---|---|---|
| Female AD | 66.81 ± 4.7 (33) | 68.21 ± 2.94 (44) | Sex = 0.016 Treatment = 0.035 |
74.2 ± 5.13 (29) | 72.34 ± 4.86 (45) | N/A |
| Male AD | 53.96 ± 2.67 (34) | 62.39 ± 2.41 (41) | 75.21 ± 4.08 (41) | 72.65 ± 4.64 (43) | ||
| Female AV | 96.41 ± 4.78 (29) | 96.61 ± 4.48 (33) | N/A | 80.7 ± 6.71 (29) | 69.92 ± 4.38 (33) | Sex = 0.033 |
| Male AV | 89.57 ± 3.9 (33) | 91.18 ± 3.82 (30) | 62.38 ± 4.78 (33) | 64.21 ± 3.7 (30) |
For more details of statistical analysis, see Supplementary Table. Numbers in parenthesis represent the number of animals from the following number of litters: AD control female = 18, AD EtOH female = 19; AD control male = 25, AD EtOH male = 21; AV control female = 17, AV EtOH female = 18; AV control male = 20, AV EtOH male = 17.
We then examined how P7 exposure affected action potential firing in response to current injection in the AD thalamic nucleus (Fig 4). EtOH exposure reduced the instantaneous frequency of action potentials (ART three-way ANOVA: treatment p < 0.0001; injected current p < 0.0001; sex p = 0.81; there were no significant interactions between stimulation intensity, and treatment, and/or sex) (Fig 4B; Supplementary Data Tables). EtOH exposure reduced action potential amplitude (ART three-way ANOVA: injected current p < 0.0001; injected current x treatment p < 0.01; sex x treatment p < 0.0001; Šídák’s multiple comparisons test: p = 0.005 for females and 0.044 for males) (Fig 4C; Supplementary Data Tables). Fig 4C shows that this effect was particularly evident in females at the 200 pA current injection. The effect of P7 vapor chamber exposure on other action potential parameters in AD neurons are shown in Table 2. ART three-way ANOVA revealed a significant effect of injected current on action potential threshold (p = 0.0001) and a sex x treatment interaction (p = 0.0002); Šídák’s multiple comparisons test yielded a p = 0.001 for females and 0.6 for males (Table 2; Supplementary Data Tables). ART three-way ANOVA revealed a significant effect of injected current on action potential number (p = 0.0001) but no other significant effects (Table 2; Supplementary Data Tables). ART three-way ANOVA revealed a significant effect of injected current (p = 0.0001) and treatment (p = 0.048) on action potential overshoot, as well as an injected current x treatment interaction (p = 0.008) and sex x treatment interaction (p = 0.004) in AD neurons; however, the Šídák’s multiple comparisons test did not reveal any significant effects (Table 2; Supplementary Data Tables). Two-way ANOVA showed a significant effect of sex on adaptation index in AD neurons (p = 0.001) but no significant effect of treatment nor a sex x treatment interaction (Table 2; Supplementary Data Tables). EtOH exposure did not affect action potential afterhyperpolarization (ART three-way ANOVA: treatment p = 0.25; injected current p < 0.0001; sex p = 0.17; there were no significant interactions between stimulation intensity, and treatment, and/or sex) (Table 2; Supplementary Data Tables).
Figure 4. Neonatal ethanol (EtOH) exposure disrupted action potential firing in retrosplenial cortex-projecting anterodorsal (AD) thalamic nucleus neurons at postnatal days 40–60.
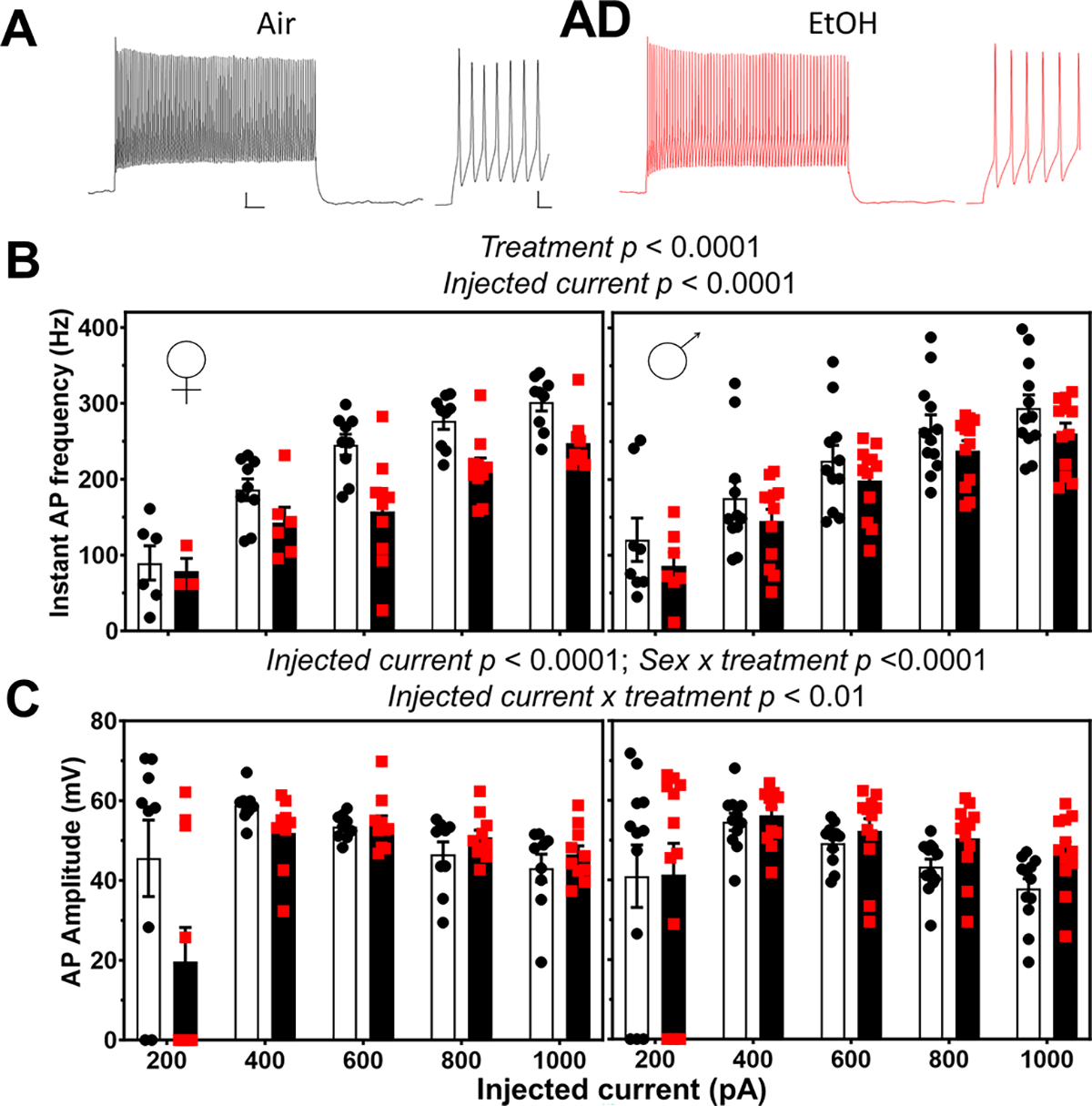
A) Representative traces of action potential firing evoked by current injection (600 pA) in AD nucleus neurons from the Air (control) and EtOH groups. Whole trace scale bar = 50 ms X 10 mV; expanded trace scale bar = 5 ms X 10 mV. B) Summary graphs illustrating that EtOH treatment (red squares) reduced the instantaneous frequency of action potentials in females and males with respect to control (black circles) (data were analyzed by three-way ANOVA of aligned rank transformed data; Supplementary Data Tables). C) Mean effect of EtOH exposure on action potential amplitude in females and males (data were analyzed by three-way ANOVA of aligned rank transformed data; Supplementary Data Tables). Control female, n=9 pups from 6 litters; EtOH female, n=10 pups from 6 litters; control male, n=12 pups from 9 litters; EtOH male, n=12 pups from 7 litters.
Table 2.
Effect of EtOH exposure on action potential parameters of anterodorsal thalamic nuclei neurons
| Injected current (pA) | Females | Males | Significant P Values | |||
|---|---|---|---|---|---|---|
| Control (n) | EtOH (n) | Control (n) | EtOH (n) | |||
| Threshold | 200 | −34.93 ± 3.44 (7) | −29.97 ± 4.68 (4) | −33.73±2.74 (9) | −34.08±2.42 (9) | <0.0001 (Injected current) 0.0002 (Sex x Treatment) |
| 400 | −34.92 ± 0.77 (9) | −29.55 ± 1.75 (9) | −33.38±1.74 (11) | −33.19±1.03 (12) | ||
| 600 | −32.25 ± 0.91 (9) | −29.60 ± 0.63 (9) | −30.45±1.65 (11) | −31.89±0.94 (12) | ||
| 800 | −30.45 ± 1.26 (9) | −29.17 ± 0.73 (10) | −28.44±1.37 (12) | −31.42±1.47 (12) | ||
| 1000 | −31.75 ± 1.16 (9) | −27.72 ± 0.78 (9) | −28.08±1.19 (12) | −30.86±2.55 (12) | ||
| Number/ 500 ms pulse | 200 | 5.278 ± 2.06 (9) | 0.375 ± 0.26 (8) | 8.37 ± 2.35 (12) | 8.25 ± 3.04 (12) | <0.0001 (Injected current) |
| 400 | 35.61 ± 8.32 (9) | 22.56 ± 6.64 (10) | 36.37 ± 7.18 (12) | 35.41 ± 6.08 (12) | ||
| 600 | 70.55 ± 9.24 (9) | 51.81 ± 7.82 (10) | 67.77 ± 8.37 (11) | 61 ± 8.4 (12) | ||
| 800 | 87.44 ± 8.63 (9) | 73.68± 8.36 (10) | 81.66 ± 8.09 (12) | 77.91 ± 11.34 (12) | ||
| 1000 | 79.16 ± 12.33 (9) | 92 ± 5.23 (10) | 80.20 ± 10.23 (12) | 90.54 ± 12.66 (12) | ||
| Overshoot amplitude (mV) | 200 | 18.38 ± 4 (9) | 7.68 ± 3.38 (10) | 15.64 ± 3.21 (12) | 15.80 ± 3.15 (12) | 0.048 (Treatment) <0.0001 (Injected current) 0.004 (Sex x Treatment) 0.008 (Treatment x Current) |
| 400 | 23.86 ± 1.35 (9) | 22.39 ± 1.42 (9) | 19.46 ± 2.27 (12) | 22.97 ± 1.32 (12) | ||
| 600 | 21.22 ± 0.95 (9) | 23.74 ± 1.39 (10) | 18.84 ± 1.10 (11) | 22.29 ± 1.74 (12) | ||
| 800 | 17.91 ± 1.79 (9) | 21.97 ± 1.32 (10) | 16.13 ± 0.86 (12) | 21.47 ± 1.89 (12) | ||
| 1000 | 15.62 ± 2.06 (9) | 18.56 ± 1.80 (10) | 13.23 ± 1.14 (12) | 19.75 ± 2.28 (12) | ||
| Adaptation index | 1000 | 0.67 ± 0.067 (10) | 0.67 ± 0.049 (7) | 0.65 ± 0.079 (9) | 0.72 ± 0.028 (10) | 0.001 (Sex) |
| After-hyperpolarization amplitude (mV) | 200 | −7.11 ± 0.55 (9) | −5.73±1.18 (10) | −7.05±0.81 (12) | −6.41±0.77 (12) | <0.0001 (Injected current) |
| 400 | −8.72 ± 0.90 (9) | −7.86±1.14 (10) | −8.76±0.88 (12) | −8.39±0.93 (12) | ||
| 600 | −9.00 ± 0.85 (9) | −7.91 ±1.17 (10) | −9.43±1.020 (12) | −9.56±1.05 (12) | ||
| 800 | −10.44 ± 0.81 (9) | −8.55±1.28 (10) | −10.38±1.12 (12) | −10.37±1 (12) | ||
| 1000 | −13.36 ± 1.79 (9) | −13.41±3.25 (10) | −14.52±2.05 (12) | −13.08±1.86 (12) | ||
For more details of statistical analysis, see Supplementary Table. Numbers in parenthesis represent the number of animals from the following number of litters: control female = 6 litters; EtOH female = 6 litters; control male = 9 litters; EtOH male = 7 litters.
We studied the effect of P7 exposure on action potential firing in response to current injection in the AV thalamus (Fig 5). EtOH exposure did not have a significant impact on the instantaneous frequency of action potentials, but there was a significant effect of injected current and sex (ART three-way ANOVA: treatment p < 0.62; injected current p < 0.0001; sex p = 0.022; Supplementary Data Tables). There was a significant interaction between sex and treatment (p = 0.043) (Fig 5B; Supplementary Data Tables). However, Šídák’s multiple comparisons test showed no significant differences between the control and EtOH groups in females (p = 0.99) or males (p = 0.27). ART three-way ANOVA revealed effects of injected current (p = 0.0004) and sex (p = 0.001), as well as a significant injected current x sex interaction (0.009) for action potential amplitude; Šídák’s multiple comparisons test did not show significant differences between males and females as a function of injected current (Fig 5C and Supplementary Data Tables). The effect of P7 vapor chamber exposure on other action potential parameters in AV neurons are shown in Table 3. ART three-way ANOVA revealed significant effects of injected current on action potential threshold (p = 0.0001) (Table 3; Supplementary Data Tables). ART three-way ANOVA revealed a significant effect of injected current (p < 0.0001) and sex (p < 0.0001) on action potential number, as well as an injected current x sex interaction (p = 0.033) and sex x treatment interaction (p = 0.036); however, the Šídák’s multiple comparisons test did not reveal any significant effects (Table 3; Supplementary Data Tables). ART three-way ANOVA revealed a significant effect of injected current (p = 0.0006) on action potential overshoot (Table 3; Supplementary Data Tables). Two-way ANOVA showed no significant effects on adaptation index (Table 3; Supplementary Data Tables). P7 vapor chamber exposure had no significant effects effect on action potential afterhyperpolarization (ART three-way ANOVA: treatment p = 0.49; injected current p < 0.0001; sex p = 0.59; there were no significant interactions between stimulation intensity, and treatment, and/or sex) (Table 3; Supplementary Data Tables).
Figure 5. Neonatal ethanol (EtOH) exposure disrupted action potential firing in retrosplenial cortex-projecting anteroventral (AV) thalamic nucleus neurons at postnatal days 40–60.
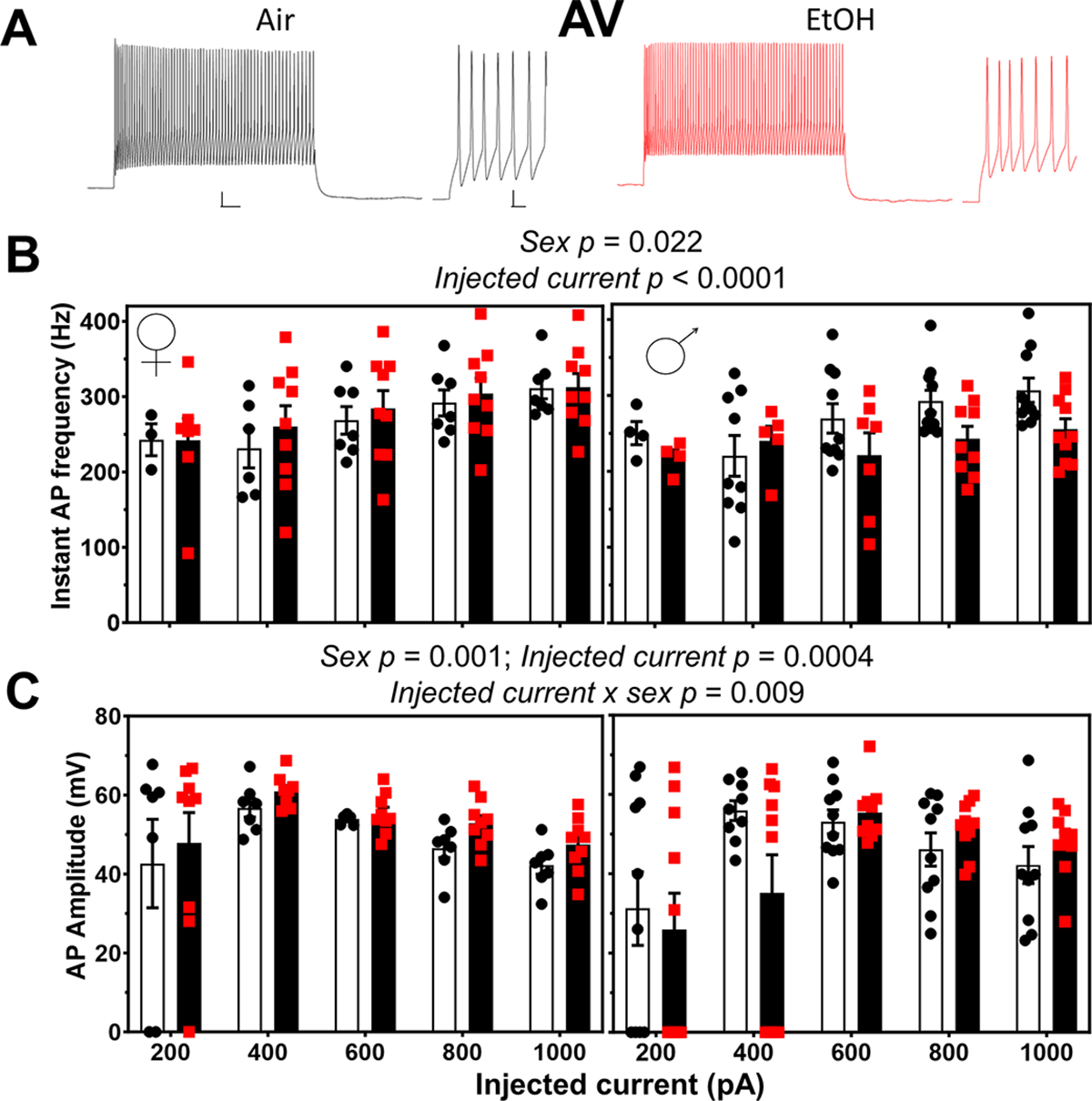
A) Representative traces of action potential firing evoked by current injection (600 pA) in AV nucleus neurons from the Air (control; black circles) and EtOH (red squares) groups. Whole trace scale bar = 50 ms X 10 mV; expanded trace scale bar = 5 ms X 10 mV. B) Mean effect of EtOH treatment on the instantaneous frequency of action potentials in females and males (data were analyzed by three-way ANOVA of aligned rank transformed data; Supplementary Data Tables). C) Mean effect of EtOH exposure on action potential amplitude in females and males (data were analyzed by three-way ANOVA of aligned rank transformed data; Supplementary Data Tables). Control female, n=7 pups from 5 litters; EtOH female, n=9 pups from 6 litters; control male, n=10 pups from 8 litters; EtOH male, n=10 pups from 5 litters.
Table 3.
Effect of EtOH exposure on action potential parameters of anteroventral thalamic nuclei neurons
| Injected current (pA) | Females | Males | Significant P Values | |||
|---|---|---|---|---|---|---|
| Control (n) | EtOH (n) | Control (n) | EtOH (n) | |||
| Threshold | 200 | −39.43 ± 0.94 (5) | −35.78 ± 3.61 (8) | 34.51 ± 4.01 (6) | 35.45± 4.20 (5) | <0.0001 (Injected current) |
| 400 | −37.16 ± 1.48 (7) | −36.77 ± 2.91 (9) | −36.59 ± 1.57 (9) | −36.95± 1.67 (6) | ||
| 600 | −33.61 ± 1.07 (7) | −35.04 ± 2.05 (9) | −34.24 ± 1.99 (10) | −33.12± 1.92 (10) | ||
| 800 | −30.22 ± 0.86 (7) | −32.26 ± 2.08 (9) | −31.45 ± 1.61 (10) | −29.58± 1.58 (10) | ||
| 1000 | −28.23 ± 0.77 (7) | −29.58 ± 1.62 (9) | −31.28 ± 2.53 (10) | −27.94± 1.66 (10) | ||
| Number/ 500 ms pulse | 200 | 1.33 ± 0.43 (7) | 1.83 ± 0.47 (9) | 0.96 ± 0.34 (10) | 0.85 ± 0.29 (10) | <0.0001 (Sex) <0.0001 (injected current) 0.036 (Sex x Treatment) 0.033 (Sex x Current) |
| 400 | 4.11 ± 2 (6) | 9.94 ± 3.13 (9) | 3.11 ± 0.73 (9) | 1.6 ± 0.54 (10) | ||
| 600 | 29.14 ± 9.49 (7) | 36.83 ± 10.63 (9) | 25.18 ± 5.58 (10) | 17.2 ± 6.26 (10) | ||
| 800 | 52.80 ± 12.53 (7) | 55.33 ± 10.35 (9) | 52.78 ± 7.37 (10) | 40.4 ± 8.63 (10) | ||
| 1000 | 72.71 ± 9.21 (7) | 77.22 ± 7.66 (9) | 49.92 ± 9.89 (9) | 54.1 ± 10.35 (10) | ||
| Overshoot amplitude (mV) | 200 | 14.49 ± 4.05 (7) | 16.10 ± 2.74 (9) | 10.51 ± 3.25 (10) | 8.19 ± 3.32 (10) | <0.0001 (Injected current) |
| 400 | 18.17 ± 2.32 (7) | 20.41 ± 1.59 (9) | 19.69 ± 1.13 (9) | 12.94 ± 3.87 (10) | ||
| 600 | 20.07 ± 1.46 (6) | 20.43 ± 0.98 (9) | 19.38 ± 1.56 (10) | 22.38 ± 1.54 (10) | ||
| 800 | 17.45 ± 1.98 (7) | 20.79 ± 1.19 (9) | 17.00 ± 2.84 (10) | 21.95 ± 1.55 (10) | ||
| 1000 | 15.48 ± 1.93 (7) | 19.21 ± 1.40 (9) | 15.66 ± 2.30 (10) | 20.66 ± 1.45 (10) | ||
| Adaptation index | 1000 | 0.42 ± 0.028 (7) | 0.48 ± 0.056 (9) | 0.44 ± 0.05 (8) | 0.47 ± 0.040 (8) | |
| After-hyperpolarization amplitude (mV) | 200 | −5.09 ± 1.079 (7) | −6.43 ± 1.69 (9) | −6.29 ± 0.93 (10) | −5.96 ± 1.15 (10) | <0.0001 (Injected current) |
| 400 | −6.58 ± 1.34 (7) | −8.68 ± 1.64 (9) | −9.08 ± 1.27 (10) | −8.05 ± 1.63 (10) | ||
| 600 | −7.18 ± 1.36 (7) | −9.26 ± 1.50 (9) | −9.87 ± 1.31 (10) | −8.61 ± 1.49 (10) | ||
| 800 | −7.38 ± 1.31 (7) | −9.97 ± 1.83 (9) | −9.99 ± 1.4 (10) | −9.13 ± 1.49 (10) | ||
| 1000 | −10.88 ± 3.25 (7) | −13.657 ± 2.62 (9) | −16.88 ± 3.56 (10) | −11.11 ± 2.01 (10) | ||
For more details of statistical analysis, see Supplementary Table. Numbers in parenthesis represent the number of animals from the following number of litters: control female = 5 litters; EtOH female = 6 litters; control male = 8 litters; EtOH male = 5 litters.
3.4. Neonatal EtOH exposure significantly affected excitatory and inhibitory synaptic transmission in ATN neurons at P40–60.
Next, we examined how developmental EtOH exposure affects spontaneous excitatory neurotransmission in the ATN (Fig 6–7). In the AD thalamic nucleus (Fig 6A–F), two-way ANOVA revealed that developmental EtOH exposure significantly reduced sEPSC frequency (p = 0.041; Fig 6B) and amplitude (p = 0.014; Fig 6C), but had no effect on total charge (p = 0.58 on log-transformed data; Fig 6D), rise time 10–90% (p = 0.15; Fig 6E), or decay time (p = 0.24; Fig 6F) (Supplementary Data Table). There was a significant effect of sex on sEPSC frequency (p = 0.038) but not amplitude (p = 0.08), total charge (p = 0.23), rise time 10–90% (p = 0.27), or decay time (p = 0.94) (Fig 6, Supplementary Data Tables). No significant sex x treatment interactions were detected on any of these parameters (Supplementary Data Tables). In the AV thalamic nucleus (Fig 7A–F), two-way ANOVA revealed that developmental EtOH exposure did not significantly affect sEPSC frequency (p = 0.14; Fig 7B), amplitude (p = 0.73; Fig 7C), total charge (p = 0.65; Fig 7D), rise time 10–90% (p = 0.36; Fig 7E), or decay time (p = 0.75; Fig 7F) (Supplementary Data Tables). There were no significant effects of sex or sex x treatment interactions on these parameters (Supplementary Data Tables).
Figure 6. Neonatal ethanol (EtOH) exposure reduced the frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) in retrosplenial cortex-projecting anterodorsal (AD) thalamic nucleus neurons at postnatal days 40–60.
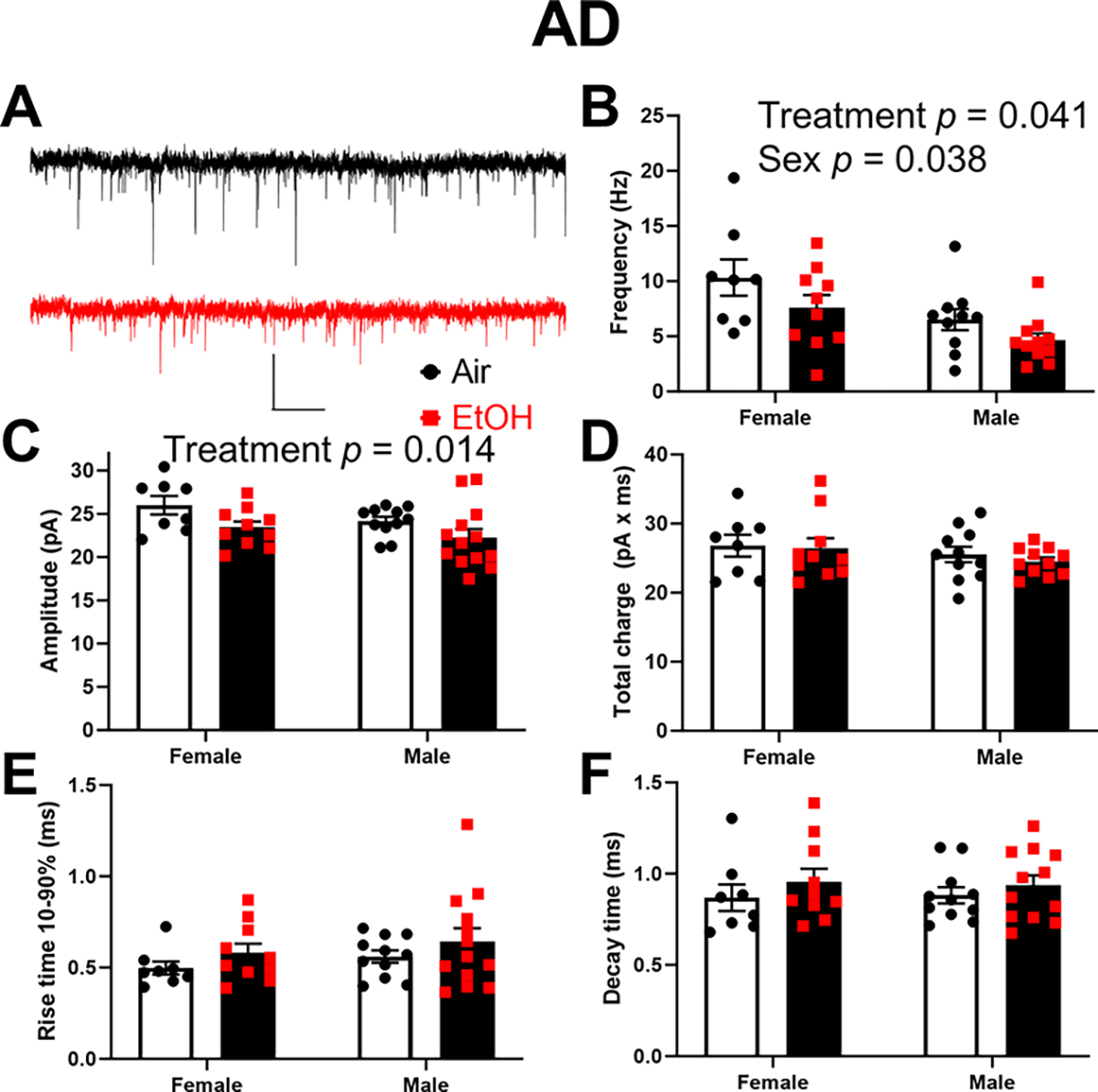
A) Representative sEPSC traces from the Air (control) (black trace) and EtOH groups (red trace). Scale bar is 0.4 s X 40 pA. B) Mean effect of EtOH exposure on sEPSC frequency (two-way ANOVA revealed the indicated significant effects; see Supplementary Data Tables for more details). C) Mean effect of EtOH exposure on sEPSC amplitude (two-way ANOVA revealed the indicated significant effect; see Supplementary Data Tables for more details). D) Mean effect of EtOH exposure on sEPSC total charge. E) Mean effect of EtOH exposure on sEPSC rise time 10–90%. F) Mean effect of EtOH exposure on sEPSC decay time. Control female, n=8 pups from 6 litters; EtOH female, n=10 pups from 7 litters; control male, n=11 pups from 8 litters; EtOH male, n=13 pups from 7 litters.
Figure 7. Neonatal ethanol (EtOH) exposure did not affect spontaneous excitatory postsynaptic currents (sEPSCs) in retrosplenial cortex-projecting anteroventral (AV) thalamic nucleus neurons at postnatal days 40–60.
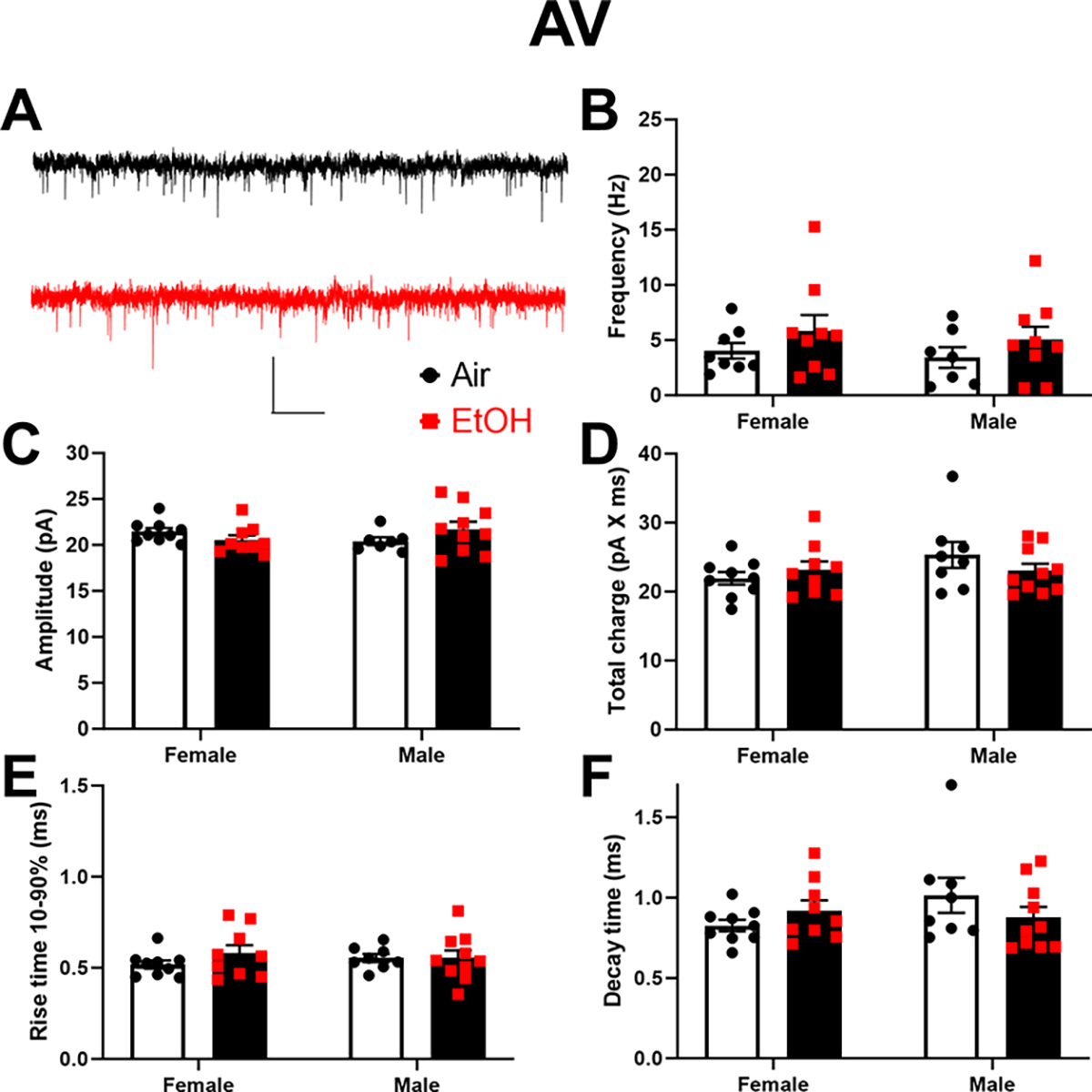
A) Representative sEPSC traces from the Air (control) (black trace) and EtOH groups (red trace). Scale bar is 0.4 s X 40 pA. B) Mean effect of EtOH exposure on sEPSC frequency. C) Mean effect of EtOH exposure on sEPSC amplitude. D) Mean effect of EtOH exposure on sEPSC total charge. E) Mean effect of EtOH exposure on sEPSC rise time 10–90%. F) Mean effect of EtOH exposure on sEPSC decay time. Control female, n=9 pups from 6 litters; EtOH female, n=9 pups from 6 litters; control male, n=8 pups from 7 litters; EtOH male, n=10 pups from 6 litters.
Finally, we characterized the effect of developmental EtOH exposure on inhibitory neurotransmission in the ATN (Fig 8–9). In the AD thalamic nucleus (Fig 8A–F), P7 EtOH exposure significantly increased total charge (p = 0.029; Fig 8D) but not sIPSC frequency (p = 0.35; Fig 8B), amplitude (p = 0.13; Fig 8C), rise time 10–90% (p = 0.16; Fig 8E), or decay time (p = 0.062; Fig 8F) (Supplementary Data Tables). There were no significant effects of sex or sex x treatment interactions on these parameters (Supplementary Data Tables). In the AV thalamic nucleus (Fig 9A–F), P7 EtOH exposure did not significantly affect sEPSC frequency (p = 0.66; Fig 9B), amplitude (p = 0.18; Fig 9C), total charge (p = 0.4; Fig 9D), rise time 10–90% (p = 0.23; Fig 9E), or decay time (p = 0.78; Fig 9F) (Supplementary Data Tables). There were no significant effects of sex or sex x treatment interactions on these parameters (Supplementary Data Tables).
Figure 8. Neonatal ethanol (EtOH) exposure increased the total charge of spontaneous inhibitory postsynaptic currents (sIPSCs) in retrosplenial cortex-projecting anterodorsal (AD) thalamic nucleus neurons at postnatal days 40–60.
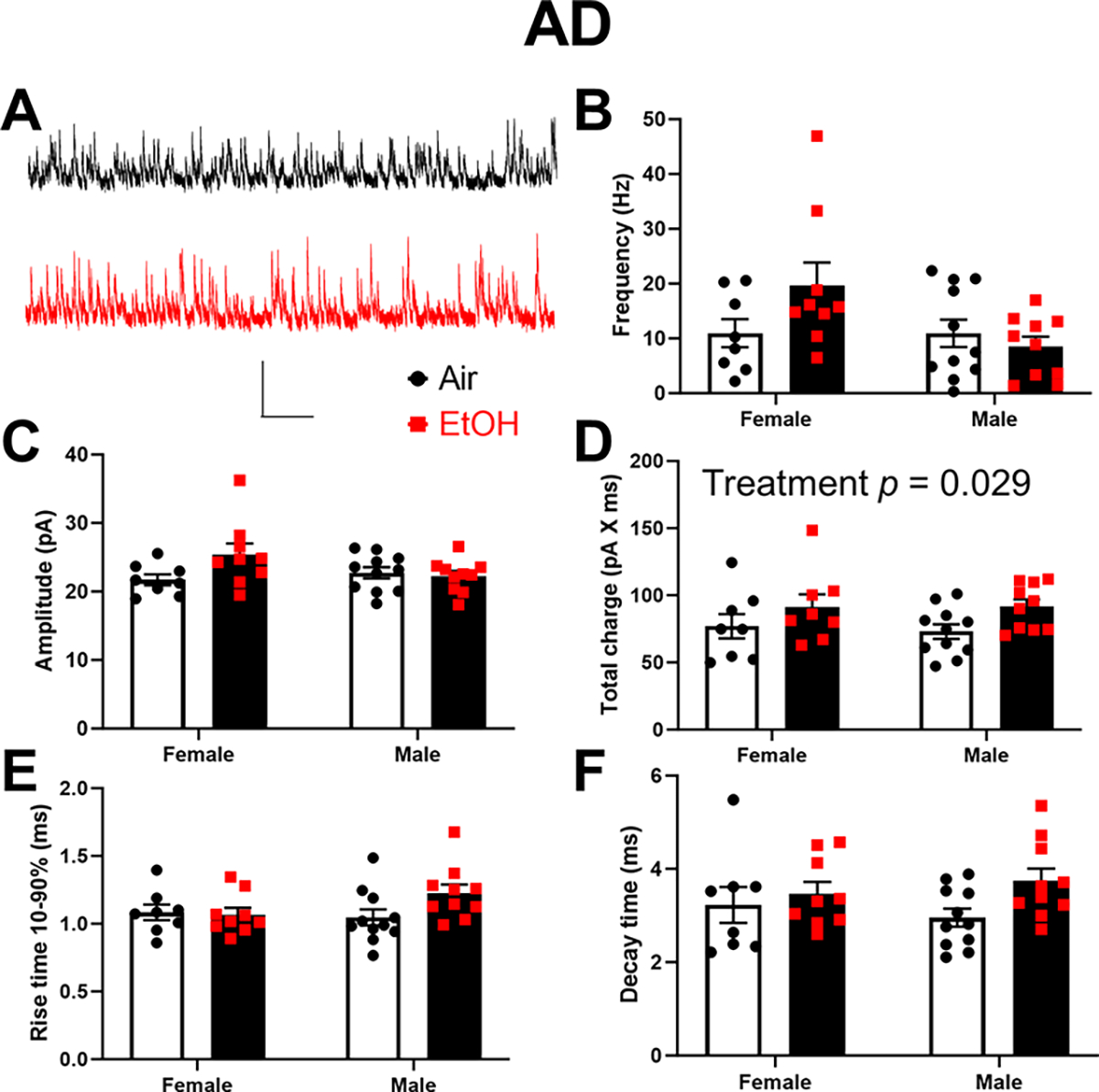
A) Representative sIPSC traces from the Air (control) (black trace) and EtOH groups (red trace). Scale bar is 0.4 s X 40 pA. B) Mean effect of EtOH exposure on sIPSC frequency. C) Mean effect of EtOH exposure on sIPSC amplitude. D) Mean effect of EtOH exposure on sIPSC total charge (two-way ANOVA revealed the indicated significant effect; see Supplementary Data Tables for more details). E) Mean effect of EtOH exposure on sIPSC rise time 10–90%. F) Mean effect of EtOH exposure on sIPSC decay time. Control female, n=8 pups from 6 litters; EtOH female, n=9 pups from 6 litters; control male, n=11 pups from 8 litters; EtOH male, n=10 pups from 7 litters.
Figure 9. Neonatal ethanol (EtOH) exposure increased the total charge of spontaneous inhibitory postsynaptic currents (sIPSCs) in retrosplenial cortex-projecting anterodorsal (AV) thalamic nucleus neurons at postnatal days 40–60.
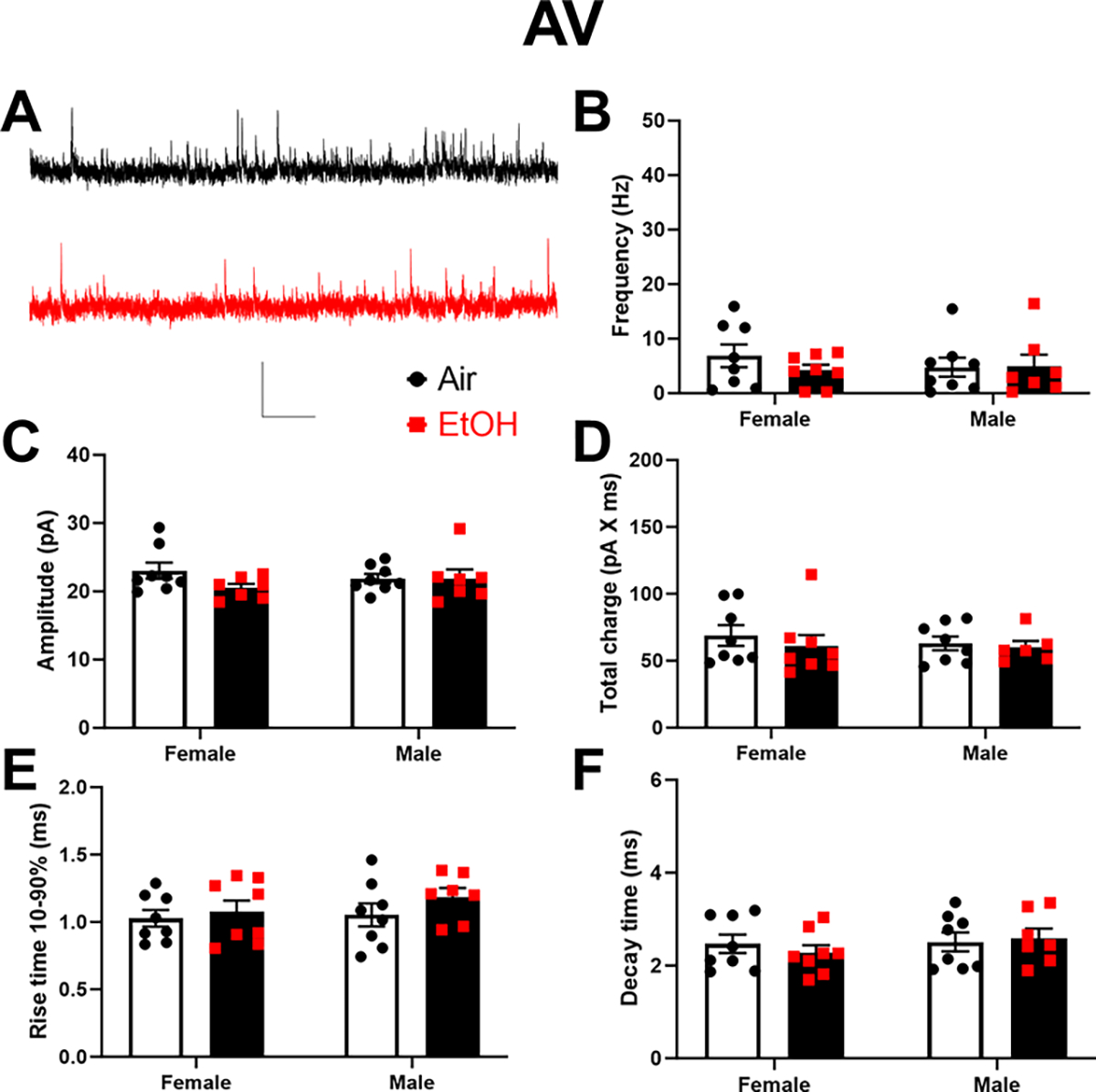
A) Representative sIPSC traces from the Air (control) (black trace) and EtOH groups (red trace). Scale bar is 0.4 s X 40 pA. B) Mean effect of EtOH exposure on sIPSC frequency. C) Mean effect of EtOH exposure on sIPSC amplitude. D) Mean effect of EtOH exposure on sIPSC total charge. E) Mean effect of EtOH exposure on sIPSC rise time 10–90%. F) Mean effect of EtOH exposure on sIPSC decay time. Control female, n=8 pups from 6 litters; EtOH female, n=8 pups from 6 litters; control male, n=8 pups from 5 litters; EtOH male, n=7 pups from 6 litters.
4. Discussion
Our study significantly advances our understanding of the short- and long-term effects of developmental EtOH exposure on excitability and synaptic transmission in anterior thalamic neurons. Specifically, we demonstrate that binge-like EtOH vapor exposure at P7 leads to apoptotic neurodegeneration in the AD, AV, and TRN. Furthermore, this exposure induces lasting functional changes in adolescent mice’s RSC-projecting anterior thalamic neurons. These findings unveil a novel mechanism that could contribute to the cognitive impairments associated with fetal EtOH exposure.
It is important to note that our vapor exposure paradigm closely mimics subcutaneous EtOH injection in postnatal rodents at P7, which has been shown to induce apoptotic neurodegeneration (Ikonomidou et al., 2000; Olney et al., 2002). In line with these previous studies, we observed a similar increase in apoptosis in the ATN following EtOH vapor chamber exposure. Ikonomidou et al (Ikonomidou et al., 2000) found that 18% and 5% of cells underwent EtOH-induced apoptotic neurodegeneration in the laterodorsal and mediodorsal thalamus of P7 rats, respectively. A similar effect was observed in the ATN of both macaques and mice (Farber et al., 2010; Wozniak et al., 2004). In the latter, P7 EtOH exposure caused a ~50% reduction in AD volume that persisted until adolescence and adulthood (i.e., it was detected at P14, P30, and P90) (Wozniak et al., 2004). Smiley et al (Smiley et al., 2023) found that approximately 8% of AD neurons exhibited apoptotic characteristics (pyknotic nuclei) 8 h after subcutaneous administration of EtOH to P7 mice. These investigators also reported that about 50% of AD neurons are eliminated between P7 and P70 and that P7 EtOH exposure caused a reduction in neuronal numbers at P7 that persisted until adulthood (P70) (Smiley et al., 2023). These investigators also observed a long-lasting reduction in neuronal numbers in the AV nucleus (Smiley et al., 2023). In general agreement with these reports, we observed that 8%, 5%, and 2% of cells were CC3+ 8 h after the end of EtOH vapor chamber exposure in the AD, AV, and reticular thalamic nuclei of Venus-VGAT mice, respectively. However, unlike the subcutaneous injection studies, we did not observe a significant reduction in neuronal density in young adult mice’s AD nucleus, AV nucleus, and TRN. This difference may be attributed to the more gradual increase in blood EtOH concentrations in vapor-exposed pups compared to subcutaneously-injected ones (Bird et al., 2020; Ikonomidou et al., 2000). Nevertheless, taken together with the findings of the above-discussed studies, our results further support the notion that the ATN is an essential target of EtOH-induced damage during the mouse-equivalent to the last trimester of human pregnancy.
Previous research has demonstrated that developmental EtOH exposure can lead to enduring reductions in the excitability of specific neuronal populations. For example, exposure of rats to EtOH throughout gestation (via liquid diet) was shown to produce long-lasting effects on both spontaneous and whisker stimulation-induced activity of barrel cortex neurons (Rema and Ebner, 1999). Prenatal EtOH exposure (via intragastric intubation from gestation day 8 to 20) reduced the frequency of evoked action potentials in putative dopaminergic neurons of the ventral tegmental area (Wang et al., 2006). Vapor chamber EtOH exposure during P2-P6 caused a reduction in the frequency and duration of dendritic spikes in layer 5 pyramidal neurons of the somatosensory cortex of adolescent rats (P30-P60) (Granato et al., 2012). The number of somatic action potentials evoked by dendritic current injection was reduced in the EtOH group, as well as the frequency of somatic action potentials evoked by somatic current injection (Granato et al., 2012). Exposure of mice to EtOH between gestational days 10–18 and P4–14 (via oral gavage) reduced the frequency of action potentials evoked by somatic current injection in layer 6 pyramidal neurons of the medial prefrontal cortex (Louth et al., 2016). In general agreement with these studies, we found that EtOH vapor exposure at P7 induced long-lasting alterations in the properties of action potentials evoked by somatic current injection in the RSC-projecting AD and AV neurons that survived ethanol-induced apoptotic neurodegeneration. This effect is not a consequence of alterations in the membrane resistance or conductances that mediate the afterhyperpolarization. Furthermore, it is noteworthy that AD neurons exhibited greater susceptibility to adverse effects of EtOH exposure on their electrophysiological properties compared to AV neurons, despite both AD and AV nuclei showing similar levels of caspase-3 activation at P7. An intriguing hypothesis is that caspase-3, aside from its apoptotic role, plays a distinct role in axonal, dendritic, and synaptic pruning, and this non-apoptotic function may have been differentially engaged in AD and AV neurons (Mukherjee and Williams, 2017). Moreover, RNA sequencing studies have unveiled significant transcriptomic differences between the AD and AV nuclei, which could contribute to determine their differential sensitivity to TTAE (Phillips et al., 2019). Future experiments will be required to unravel the mechanisms underpinning of the varying EtOH sensitivity of AD and AV thalamic nuclei neurons. It is also crucial to delve into the impact of TTAE on the regulation of ATN neuron excitability by synaptic inputs, as well as the communication between the ATN and the RSC.
In addition to alterations in excitability, we also observed long-lasting changes in spontaneous excitatory and inhibitory synaptic transmission in RSC-projecting AD neurons but not AV neurons. These alterations include a decrease in the frequency and amplitude of sEPSCs and an increase in the total charge of sIPSCs. The reduction in sEPSC frequency might be linked to decreased spontaneous firing of glutamatergic axons or reduced glutamate release probability. In contrast, the decrease in sEPSC amplitude suggests changes at the postsynaptic level, such as decreased AMPA receptor function or density. Previous studies have reported sEPSC frequency and amplitude reductions in neurons from rodents that underwent prenatal and perinatal exposure to EtOH (Vaglenova et al., 2008; Wijayawardhane et al., 2007). However, other studies have found increases or no changes (Baculis et al., 2015; Delatour et al., 2020; Louth et al., 2016; Montgomery et al., 2018)
Regarding the increase in sIPSC total charge in RSC-projecting AD neurons, this observation aligns with an enhancement in the function of postsynaptic GABAA receptors. Typically, such an increase would be associated with changes in sIPSC amplitude, rise time, and decay time (Sallard et al., 2021). It is possible that the cumulative impact of subtle changes in these parameters led to a significant overall increase in charge transfer. It is essential to emphasize that GABAA receptors mainly mediate the inhibition of neuronal activity by transferring negative charges into the cell (Sallard et al., 2021). As a result, it is essential to investigate whether the heightened sIPSC charge transfer we observed contributes to the reduction of AD action potential firing (our recordings of action potential firing were conducted without blockers of neurotransmitter receptors). Recent studies on the effects of developmental EtOH exposure have reported alterations in IPSC charge transfer. For example, binge-like prenatal EtOH exposure during embryonic days 13.5 and 16.5 increased both sIPSC charge transfer and frequency in somatosensory cortex layer 5–6 pyramidal neurons of P28-P32 mice (Delatour et al., 2020). Furthermore, our research group detected an increase in the charge transfer of optically-evoked IPSCs at parvalbumin interneuron-to-pyramidal neuron synapses in the RSC of adolescent mice exposed to EtOH vapor at P7 (Bird et al., 2020). Taken together, our findings and those from the above-discussed electrophysiological studies suggest that prenatal and/or perinatal EtOH exposure significantly alters GABAA receptor-mediated synaptic transmission.
In conclusion, our study offers critical insights into the lasting effects of third-trimester-equivalent EtOH exposure on ATN neurons’ excitability and synaptic transmission. ATN neurons target neocortical layer 1, where apical tuft dendrites of layers 2/3 and 5 are located, providing feedback information that is critical for several cognitive processes (Larkum, 2013; Odagiri et al., 2011; Yamawaki et al., 2019). Given that ATN lesions in rodents are associated with learning and memory deficits, our findings raise intriguing questions about the potential link between the electrophysiological changes induced by TTAE and the observed learning and memory impairments in mice exposed to EtOH at P7 (Aggleton and Nelson, 2015; Harvey et al., 2019; Lewin et al., 2018; Wozniak et al., 2004). We hope that our preclinical study will inspire further research on the impact of prenatal EtOH exposure on the function of the ATN and other components of the limbic memory system in humans with FASD. A better understanding of the mechanisms underlying these alterations could pave the way for improved interventions and treatments for individuals affected by this prevalent disorder.
Supplementary Material
Highlights.
Mice were ethanol exposed during the third-trimester-equivalent of human pregnancy
Ethanol caused caspase 3 activation in the anterior and reticular thalamic nuclei
A long-lasting neuronal density reduction was not observed in these areas
We recorded from retrosplenial cortex-projecting anterior thalamic nuclei neurons
These revealed altered excitability and neurotransmission during adolescence
Acknowledgements.
Author Roles: Clark W. Bird (conceptualization; data curation; formal analysis, investigation, visualization, writing - original draft and review & editing), Stefanie S. Mayfield (data curation; formal analysis, investigation, visualization, writing - original draft and review & editing), Katalina M. Lopez (formal analysis, validation, writing - review & editing), Brooke R. Dunn (formal analysis, validation, writing - review & editing), Angela Feng (formal analysis, validation, writing - review & editing), Bryce T. Roberts (formal analysis, validation, writing - review & editing), Roberto N. Almeida (formal analysis, validation, writing - review & editing), Glenna J. Chavez (investigation, writing - review & editing), and C. Fernando Valenzuela (conceptualization; data curation; formal analysis; funding acquisition; project administration; supervision; visualization; writing - original draft and review & editing). During the preparation of this work the authors used ChatGPT and Grammarly to improve the readability of the manuscript. After using these services, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Disclosure/Funding
The National Institutes of Health supported this work under grant R01 AA015614 (CFV). This research was also partially supported by UNM Comprehensive Cancer Center Support Grant NCI P30CA118100 and made use of the Fluorescence Microscopy and Cell Imaging shared resource.
Footnotes
The authors report there are no competing interests to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Nelson AJ, 2015. Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits? Neurosci Biobehav Rev 54, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinmboni TO, Davis NL, Falck AJ, Bearer CF, Mooney SM, 2018. Excipient exposure in very low birth weight preterm neonates. J Perinatol 38, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baculis BC, Diaz MR, Valenzuela CF, 2015. Third trimester-equivalent ethanol exposure increases anxiety-like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacology, biochemistry, and behavior 137, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CW, Barber MJ, Post HR, Jacquez B, Chavez GJ, Faturos NG, Valenzuela CF, 2020. Neonatal ethanol exposure triggers apoptosis in the murine retrosplenial cortex: Role of inhibition of NMDA receptor-driven action potential firing. Neuropharmacology 162, 107837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CW, Chavez GJ, Barber MJ, Valenzuela CF, 2021. Enhancement of parvalbumin interneuron-mediated neurotransmission in the retrosplenial cortex of adolescent mice following third trimester-equivalent ethanol exposure. Sci Rep 11, 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CW, Taylor DH, Pinkowski NJ, Chavez GJ, Valenzuela CF, 2018. Long-term Reductions in the Population of GABAergic Interneurons in the Mouse Hippocampus following Developmental Ethanol Exposure. Neuroscience 383, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkquist OA, Fryer SL, Reiss AL, Mattson SN, Riley EP, 2010. Cingulate gyrus morphology in children and adolescents with fetal alcohol spectrum disorders. Psychiatry Res 181, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb EJ, Kinnavane L, Aggleton JP, 2017. Hippocampal - diencephalic - cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci Adv 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb EJ, Nelson AJD, Cozens TC, Aggleton JP, 2020. Organisation of cingulum bundle fibres connecting the anterior thalamic nuclei with the rodent anterior cingulate and retrosplenial cortices. Brain Neurosci Adv 4, 2398212820957160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Harvey RE, 2016. Do the anterior and lateral thalamic nuclei make distinct contributions to spatial representation and memory? Neurobiol Learn Mem 133, 69–78. [DOI] [PubMed] [Google Scholar]
- Cudd TA, 2005. Animal model systems for the study of alcohol teratology. Experimental biology and medicine 230, 389–393. [DOI] [PubMed] [Google Scholar]
- Delatour LC, Yeh PWL, Yeh HH, 2020. Prenatal Exposure to Ethanol Alters Synaptic Activity in Layer V/VI Pyramidal Neurons of the Somatosensory Cortex. Cereb Cortex 30, 1735–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Thomas KGF, Meintjes EM, Molteno CD, Jacobson JL, Jacobson SW, 2020. Reduced Hippocampal Volumes Partially Mediate Effects of Prenatal Alcohol Exposure on Spatial Navigation on a Virtual Water Maze Task in Children. Alcohol Clin Exp Res 44, 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes K, Tripp T, Willinger M, Odendaal H, Elliott AJ, Kinney HC, Robinson F, Petersen JM, Raffo C, Hereld D, Groenewald C, Angal J, Hankins G, Burd L, Fifer WP, Myers MM, Hoffman HJ, Sullivan L, Network P, 2017. Drinking and smoking patterns during pregnancy: Development of group-based trajectories in the Safe Passage Study. Alcohol 62, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin LAK, M.; Higgins JJ; Wobbrock JO, 2021. An Aligned Rank Transform Procedure for Multifactor Contrast Tests. UIST ‘21: The 34th Annual ACM Symposium on User Interface Software and Technology. Association for Computing Machinery, Virtual Event, pp. 754–768. [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA, 2009. Alcohol consumption by women before and during pregnancy. Maternal and child health journal 13, 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlund A, Heikkinen S, Autti-Ramo I, Korkman M, Timonen M, Kuusi T, Riley EP, Lundbom N, 2006. Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 30, 2097–2104. [DOI] [PubMed] [Google Scholar]
- Farber NB, Creeley CE, Olney JW, 2010. Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol Dis 40, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato A, Palmer LM, De Giorgio A, Tavian D, Larkum ME, 2012. Early exposure to alcohol leads to permanent impairment of dendritic excitability in neocortical pyramidal neurons. J Neurosci 32, 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenmyer JR, Popova S, Klug MG, Burd L, 2020. Fetal alcohol spectrum disorder: a systematic review of the cost of and savings from prevention in the United States and Canada. Addiction 115, 409–417. [DOI] [PubMed] [Google Scholar]
- Harvey RE, Berkowitz LE, Hamilton DA, Clark BJ, 2019. The effects of developmental alcohol exposure on the neurobiology of spatial processing. Neurosci Biobehav Rev 107, 775–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes SK, Dukes KA, Tripp T, Petersen JM, Raffo C, Burd L, Odendaal H, Elliott AJ, Hereld D, Signore C, Willinger M, Huestis MA, Prenatal Alcohol in S, Stillbirth N, 2015. Clinical sensitivity and specificity of meconium fatty acid ethyl ester, ethyl glucuronide, and ethyl sulfate for detecting maternal drinking during pregnancy. Clin Chem 61, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, Sapkota A, Wood R, Bearer C, Kapoor S, 2018. Neonatal ethanol exposure from ethanol-based hand sanitisers in isolettes. Arch Dis Child Fetal Neonatal Ed 103, F55–F58. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW, 2000. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287, 1056–1060. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N, 2006. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med 11, 415–422. [DOI] [PubMed] [Google Scholar]
- Larkum ME, 2013. The yin and yang of cortical layer 1. Nat Neurosci 16, 114–115. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER, 2011. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev 21, 102–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M, Ilina M, Betz J, Masiello K, Hui M, Wilson DA, Saito M, 2018. Developmental Ethanol-Induced Sleep Fragmentation, Behavioral Hyperactivity, Cognitive Impairment and Parvalbumin Cell Loss are Prevented by Lithium Co-treatment. Neuroscience 369, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys CS, S., 2010. A nonparametric method to analyze interactions: The adjusted rank transform test. Journal of Experimental Social Psychology 46, 684–688. [Google Scholar]
- Louth EL, Bignell W, Taylor CL, Bailey CD, 2016. Developmental Ethanol Exposure Leads to Long-Term Deficits in Attention and Its Underlying Prefrontal Circuitry. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, Baete A, Russo J, Elliott AJ, Kalberg WO, Buckley D, Brooks M, Ortega MA, Hedrick DM, Tabachnick BG, Abdul-Rahman O, Adam MP, Jewett T, Robinson LK, Manning MA, Hoyme HE, 2020. Fetal Alcohol Spectrum Disorders in a Midwestern City: Child Characteristics, Maternal Risk Traits, and Prevalence. Alcohol Clin Exp Res 44, 919–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, Hooper SR, Hedrick DM, Jackson-Newsom J, Mullis CE, Dobyns E, Kalberg WO, Buckley D, Robinson LK, Abdul-Rahman O, Adam MP, Manning MA, Jewett T, Hoyme HE, 2021. Estimating the community prevalence, child traits, and maternal risk factors of fetal alcohol spectrum disorders (FASD) from a random sample of school children. Drug Alcohol Depend 227, 108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Hoyme HE, Robinson LK, Khaole N, Gore JC, Jacobson SW, 2010. An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res 34, 1450–1464. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJ, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW, 2014. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuroimage Clin 5, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery KS, Bancroft EA, Fincher AS, Migut EA, Provasek V, Murchison D, DuBois DW, 2018. Effects of ethanol and varenicline on female Sprague-Dawley rats in a third trimester model of fetal alcohol syndrome. Alcohol 71, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RA, Diaz MR, Topper LA, Valenzuela CF, 2014. Construction of vapor chambers used to expose mice to alcohol during the equivalent of all three trimesters of human development. Journal of visualized experiments : JoVE 89, 51839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Williams DW, 2017. More alive than dead: non-apoptotic roles for caspases in neuronal development, plasticity and disease. Cell Death Differ 24, 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, Dunney C, Mullally A, Adnan N, Fahey T, Barry J, 2014. A prospective cohort study of alcohol exposure in early and late pregnancy within an urban population in Ireland. Int J Environ Res Public Health 11, 2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, 2021. The anterior thalamic nuclei and cognition: A role beyond space? Neurosci Biobehav Rev 126, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclasen J, Andersen AM, Strandberg-Larsen K, Teasdale TW, 2014. Is alcohol binge drinking in early and late pregnancy associated with behavioural and emotional development at age 7 years? Eur Child Adolesc Psychiatry 23, 1175–1180. [DOI] [PubMed] [Google Scholar]
- Odagiri S, Meguro R, Asano Y, Tani T, Ichinohe N, 2011. Single axon branching analysis in rat thalamocortical projection from the anteroventral thalamus to the granular retrosplenial cortex. Front Neuroanat 5, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C, 2002. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res 133, 115–126. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, 2013. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. Elsevier, San Diego, CA. [Google Scholar]
- Phillips JW, Schulmann A, Hara E, Winnubst J, Liu C, Valakh V, Wang L, Shields BC, Korff W, Chandrashekar J, Lemire AL, Mensh B, Dudman JT, Nelson SB, Hantman AW, 2019. A repeated molecular architecture across thalamic pathways. Nat Neurosci 22, 1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rema V, Ebner FF, 1999. Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. J Neurosci 19, 10993–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger DJ, Krueger AM, de Water E, Mueller BA, Boys CA, Hendrickson TJ, Schumacher MJ, Mattson SN, Jones KL, Lim KO, Wozniak JR, 2021. Hippocampal subfield abnormalities and memory functioning in children with fetal alcohol Spectrum disorders. Neurotoxicol Teratol 83, 106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Zhang Y, Aida T, Choi S, Chen Q, Hou Y, Lea NE, Skaggs KM, Quay JC, Liew M, Maisano H, Le V, Jones C, Xu J, Kong D, Sullivan HA, Saunders A, McCarroll SA, Wickersham IR, Feng G, 2021. Anterior thalamic dysfunction underlies cognitive deficits in a subset of neuropsychiatric disease models. Neuron 109, 2590–2603 e2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Mao RF, Wang R, Vadasz C, Saito M, 2007. Effects of gangliosides on ethanol-induced neurodegeneration in the developing mouse brain. Alcohol Clin Exp Res 31, 665–674. [DOI] [PubMed] [Google Scholar]
- Sallard E, Letourneur D, Legendre P, 2021. Electrophysiology of ionotropic GABA receptors. Cell Mol Life Sci 78, 5341–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Bleiwas C, Marino BM, Vaddi P, Canals-Baker S, Wilson DA, Saito M, 2023. Estimates of total neuron number show that neonatal ethanol causes immediate and lasting neuron loss in cortical and subcortical areas. Front Neurosci 17, 1186529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, 2020. Thalamic reticular nucleus in the thalamocortical loop. Neurosci Res 156, 32–40. [DOI] [PubMed] [Google Scholar]
- Team RC, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Viena, Austria. [Google Scholar]
- Ting JT, Lee BR, Chong P, Soler-Llavina G, Cobbs C, Koch C, Zeng H, Lein E, 2018. Preparation of Acute Brain Slices Using an Optimized N-Methyl-D-glucamine Protective Recovery Method. Journal of visualized experiments : JoVE 132, 53825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umer A, Lilly C, Hamilton C, Baldwin A, Breyel J, Tolliver A, Mullins C, John C, Maxwell S, 2020. Prevalence of alcohol use in late pregnancy. Pediatr Res 88, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Pandiella N, Wijayawardhane N, Vaithianathan T, Birru S, Breese C, Suppiramaniam V, Randal C, 2008. Aniracetam reversed learning and memory deficits following prenatal ethanol exposure by modulating functions of synaptic AMPA receptors. Neuropsychopharmacology 33, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Wang J, Haj-Dahmane S, Shen RY, 2006. Effects of prenatal ethanol exposure on the excitability of ventral tegmental area dopamine neurons in vitro. J Pharmacol Exp Ther 319, 857–863. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kakizaki T, Sakagami H, Saito K, Ebihara S, Kato M, Hirabayashi M, Saito Y, Furuya N, Yanagawa Y, 2009. Fluorescent labeling of both GABAergic and glycinergic neurons in vesicular GABA transporter (VGAT)-venus transgenic mouse. Neuroscience 164, 1031–1043. [DOI] [PubMed] [Google Scholar]
- Wickham H A. M; Bryan J; Chang; D’Agostino McGowan; François R; Grolemund; Hayes A; Henry L; Hester J; Kuhn M; Lin Pedersen T; Miller E; Milton Bache S; Müller K; Ooms J; Robinson D; Paige Seidel; Spinu V; Takahashi K; Vaughan D, Wilke C; Woo K; Yutani H, 2019. Welcome to the tidyverse. Journal of Open Source Software 4, 1686. [Google Scholar]
- Wijayawardhane N, Shonesy BC, Vaglenova J, Vaithianathan T, Carpenter M, Breese CR, Dityatev A, Suppiramaniam V, 2007. Postnatal aniracetam treatment improves prenatal ethanol induced attenuation of AMPA receptor-mediated synaptic transmission. Neurobiol Dis 26, 696–706. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J, 2008. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc 14, 1022–1033. [DOI] [PubMed] [Google Scholar]
- Wobbrock JO, Findlater L, Gergle D, Higgins JJ, 2011. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. 29th Annual Chi Conference on Human Factors in Computing Systems, 143–146. [Google Scholar]
- Woods KJ, Thomas KGF, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM, 2018. Prenatal alcohol exposure affects brain function during place learning in a virtual environment differently in boys and girls. Brain Behav 8, e01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ, 2004. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis 17, 403–414. [DOI] [PubMed] [Google Scholar]
- Yamawaki N, Li X, Lambot L, Ren LY, Radulovic J, Shepherd GMG, 2019. Long-range inhibitory intersection of a retrosplenial thalamocortical circuit by apical tuft-targeting CA1 neurons. Nat Neurosci 22, 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


