Abstract
Background:
Phthalates are a group of chemicals with ubiquitous exposure worldwide. Exposures to phthalates during pregnancy may play a role in autism spectrum disorder (ASD) etiology by disrupting hormone levels or directly impacting fetal neurodevelopment. However, there is little research quantifying the aggregate effect of phthalates on child ASD-related behaviors.
Methods:
We used data from two prospective pregnancy and birth cohorts – the Health Outcomes and Measures of the Environment (HOME) and the Early Autism Risk Longitudinal Investigation (EARLI). HOME is a general population cohort while participants in EARLI were at higher familial risk for ASD. Using quantile g-computation and linear regression models, we assessed the joint and individual associations of a mixture of six phthalate metabolites during pregnancy with child ASD-related traits measured by Social Responsiveness Scale (SRS) scores at ages 3–8 years.
Results:
Our analyses included 271 participants from HOME and 166 from EARLI. There were imprecise associations between the phthalate mixture and SRS total raw scores in HOME (difference in SRS scores per decile increase in every phthalate = 1.3; 95% confidence interval [CI]: −0.2, 2.8) and EARLI (difference in SRS scores per decile increase in every phthalate = −0.9; 95% CI: −3.5, 1.7).
Conclusions:
The cohort-specific effect sizes of the pthalates–SRS associations were small and confidence intervals were imprecise. These results suggest that if there are associations between phthalate metabolites during pregnancy and child SRS scores, they may differ across populations with different familial liabilities. Further studies with larger sample sizes are warranted.
INTRODUCTION
Phthalates are a ubiquitous group of chemicals used widely in food packaging,1 personal care products,2 and household furnishings.3 Exposure to phthalates during pregnancy is related to adverse reproductive outcomes,4,5 preterm birth,6 gestational diabetes,7 and neurodevelopmental deficits.8 While some individual phthalates have been associated with autism spectrum disorder (ASD)-related traits,9–11 research exploring the effect of phthalates as a mixture has been limited.
Animal studies have found that urinary phthalate metabolite concentrations during pregnancy caused abnormal social behaviors,12 emotional instability,13 damage in spatial and learning memory,14,15 decreased cognitive flexibility,16 and increased anxiety,15 all of which contribute to ASD diagnoses in humans. For example, one study found that di(2-ethylhexyl) phthalate (DEHP) exposure during pregnancy induced ASD-related behavioral abnormalities in rat offspring.17
Results from human studies of the association between urinary phthalate metabolite concentrations during pregnancy and ASD-related outcomes are inconclusive.18,19 One case–control study showed that metabolites of DEHP, including mono-2-ethylhexyl phthalate (MEHP), were elevated in children with ASD compared to controls.20 Another case–control study found that DEHP and MEHP were higher in ASD cases compared to controls.21 On the other hand, Miodovnik et al. (2011) conducted a cohort study where they showed a positive association between urinary monoethyl phthalate (MEP) concentrations during pregnancy and Social Responsiveness Scale (SRS) scores among offspring.22 However, Braun et al. (2014) showed less clear associations between phthalate metabolites, including MEP, with SRS scores in the Health Outcomes and Measures of the Environment (HOME).23
To date, there is little evidence on the associations between phthalate metabolite mixtures during pregnancy and autism-related traits among children.9 A previous study from our group has used quantile regression to assess the individual associations between nine urinary phthalate metabolites during pregnancy and child autistic traits in HOME and EARLI.24 While we found positive associations between SRS scores and DEHP, mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), and mono-isobutyl phthalate (MiBP) concentrations, further research is needed to better understand the aggregate effect of phthalate exposures given that they have been regulated as a class25 and phthalate exposures tend to co-occur due to shared sources of exposure. Furthermore, these exposures may have nonlinear, interaction effects on health outcomes.
To address these gaps, we assessed the association between exposure to a mixture of phthalates during pregnancy and child developmental traits related to autism in early childhood using two prospective cohort studies. We used quantile g-computation to estimate the combined impact of a chemical mixture on autism-related traits and the proportions of contributions from each element within the mixture,26 and compared these findings to those obtained from individual exposure models.
MATERIALS AND METHODS
Participants
We used data from mother–singleton pairs who participated in two prospective pregnancy and birth cohorts – the Health Outcomes and Measures of the Environment (HOME) Study and the Early Autism Risk Longitudinal Investigation (EARLI) Study. Details about these two cohorts have been published elsewhere.27,28 Briefly, HOME enrolled adult pregnant women (16±3 weeks of pregnancy) who lived in a home built in or before 1978 in the Cincinnati metropolitan area.27 EARLI enrolled pregnant women (< 28 weeks of pregnancy) who previously had a child with ASD across four different study sites in the U.S., and followed the subsequent child through early development.28 By nature of the high recurrence risk of ASD, these younger siblings were at higher risk of developing ASD and other developmental delays themselves.
Outcome
The primary outcome of this analysis was autism-related traits as measured by the SRS total raw scores. SRS forms were administered at 4–8 years in HOME (median: 50.1 months) and at 3 years in EARLI (median: 36.3 months).29 The SRS is a validated caregiver-reported measure of ASD-related behaviors that includes 65 Likert-style items.29,30 The total raw score was a sum of these 65 items, with the maximum of 195 points.29,30 Higher total raw scores indicate higher social impairment and greater degree of traits related to autism. Raw scores can also be converted to T-scores to facilitate clinical evaluation and decision.31 For example, having a SRS T-score 76 indicated that a child had clinically significant social impairment and health professionals have been increasingly using this to decide if the child should be referred to the next phases of ASD diagnosis, such as a comprehensive developmental evaluation.31,32 Hence, we used SRS-T scores in secondary analyses.
Exposures
Phthalates were measured in maternal urine samples in the second (around 16 weeks of pregnancy) and third (around 26 weeks of pregnancy) trimesters in HOME and throughout pregnancy in EARLI.24 We published details on sample handling and quality controls in our previous study.24 Briefly, we quantified the concentrations of the phthalates and phthalate metabolites in urine samples using on-line solid phase extraction coupled with isotope dilution-high performance liquid chromatography (HPLC)-electrospray ionization-tandem mass spectrometry.24,33 All the urine samples were refrigerated at −20°C before they were aliquoted and shipped to the Centers for Disease Control and Prevention National Center for Environmental Health (CDC-NCEH) laboratories for analyses.
We restricted our work to consider the phthalates where 80% of reported values were above the lower limits of detection (LLODs): MBP, MBzP, mono-3-carboxypropyl phthalate (MCPP), MEP, MiBP, and 4 metabolites of DEHP – MEHP, mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP). We imputed concentrations of the phthalates with the lower limit of detection (LLOD) divided by a square root of two if the concentration was lower than the LLOD34 and corrected for urinary dilution by dividing the phthalate concentration (μg/L) by urinary creatinine concentration (g/L).35 We then reported phthalates as μg analyte per g creatinine.35 Because of their high correlation and common parent diester, DEHP metabolites were summed as ΣDEHP (μg/g creatinine) = [MEHP (μg/g creatinine)/278 (g/mol) + MEOHP (μg/g creatinine)/292 (g/mol) + MEHHP (μg/g creatinine)/294 (g/mol) + MECPP (μg/g creatinine)/308 (g/mol)] × 308 g/mol where 278, 292, 294, and 308 were the molecular weights of MEHP, MEOHP, MEHHP, and MECPP, respectively. We multiplied the sum of the molar concentrations of the 4 metabolites by the molecular weight of MECPP so that the ΣDEHP was on a comparable unit with the other phthalates.24 The LLODs for the phthalates ranged from 0.2 μg/L for MCPP to 1.2 μg/L for MEHP in HOME and from 0.3 μg/L creatinine for MBzP to 1.2 μg/L for MEP in EARLI (eTable 1 http://links.lww.com/EDE/C79). For pregnant women with more than one sample, we calculated average phthalate concentrations across pregnancy.
Covariates
We selected covariates a priori based on previous literature using a directed acyclic graph (DAG), and data availability (eFigure 1 http://links.lww.com/EDE/C79). The selected covariates included maternal age at delivery (years), maternal race/ethnicity (Hispanic or Latino/a/e/x, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income (US$), child sex at birth (male, female), child age at SRS administration (months), and study site (Cincinnati metropolitan area, Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis).24,36 Although child sex at birth and child age at SRS administration were not associated with exposure to phthalates during pregnancy, we included them to improve the precision of the estimates because they were associated with SRS scores.37,38 We used Multiple Imputation by Chained Equations (MICE) to impute any missing values in the phthalates and covariates and selected the first imputed dataset for further analyses.
Statistical analyses
We presented descriptive statistics as N’s and percentages for categorial variables and medians (minimums, maximums) for continuous variables. We visualized the distributions of each of the phthalates and SRS scores using violin plots and histograms. We quantified the bivariate associations between each pair of phthalates using Spearman’s correlation coefficients and visualized using heatmaps.
We fit four quantile g-computation models with SRS total raw score as the outcome and the 6 phthalate metabolites (i.e., ΣDEHP, MBP, MBzP, MCPP, MEP, and MiBP) as the independent variables.39,40 Model 1 was a joint marginal structural model given by where was SRS total raw score, was the phthalates decile, were the covariates, was a link function in a generalized linear model (e.g., the identity function in the case of a linear regression model for the expected value of was the model intercept, was the expected change in the outcome, given a one quantile increase in all exposures simultaneously, is an “index” that represented a joint value of the phthalates, and was a set of model coefficients for the covariates . Model 2 further included product terms between each pair of the phthalates. Model 3 used polynomial splines for the phthalates and the output corresponded to the estimates of the marginal structural model given by where was the change in the outcome for an additional unit of squared joint exposure to the phthalates, over-and-above the linear effect given by , and was the quadratic term of the index that represented a joint value of exposure to the phthalates. Model 4 included indicator terms for each quantile of each phthalate.
In each of the four models above, we adjusted for the covariates noted above. To compare fit of the adjusted models, we calculated the Akaike information criterion (AIC) as and Bayesian information criterion (BIC) as where was the number of estimated parameters, was the maximum value of the likelihood function, and was the number of data points. The model with the lowest AIC and BIC was selected as the best fit to the data.
Next, we calculated the weights of each phthalate within this mixture, defined as the proportion of the overall effects contributed by each phthalate in either the positive or negative direction.39–41 These weights represented the contribution of the individual mixture components to the overall mixture effect.42,43 Hence, the weight could range from −1 to 1, with all negative weights (i.e., negative partial mixture effects) summed to −1 and all positive weights (i.e., positive partial mixture effects) summed to 1.44 Weights are determined empirically by the data and model, and are not researcher specified. As a comparison, we also specified separate linear regression models with SRS total raw score as the outcome and each of the 6 log2-transformed phthalates (i.e., ΣDEHP, MBP, MBzP, MCPP, MEP, and MiBP) as the independent variable. The adjusted model further included all the covariates specified above. As a sensitivity analysis, we re-fitted all the models specified above with SRS total T-score as the outcome and stratified the models by cohort and child sex.
We conducted all data management and statistical analyses using the R Statistical language version 4.1.2 45 on Windows 10 ×64 (build 22000). Code to recreate our results is available at https://github.com/emmayu001/qgcomp-Pthalates-SRS. Data requests should be addressed to the Principal Investigators of the two cohorts (HOME: JMB, KY; EARLI: MDF).
Ethics statement
This analysis was reviewed and approved by the Institutional Review Board (IRB) of Drexel University (IRB number: 2010008124). HOME was reviewed and approved by the Ethics Committee of Cincinnati Children’s Hospital Medical Center IRB (01–8-5, 2008–0022) and EARLI was reviewed and approved by the IRBs of the Drexel University, the Children’s Hospital of Philadelphia, the Kaiser Foundation Research Institute, the Veterans Affairs Northern California Health Care System Human Research Protection Program, and University of California, Davis (120100673).24 All women provided written informed consent for themselves and their kids.
RESULTS
After excluding twin births, the final analytic sample included 437 participants, the majority of whom were from HOME (n = 271, 62%) (Table 1; eFigure 2 http://links.lww.com/EDE/C79). Around 97% (n = 262) of HOME participants provided urinary phthalate data from both the 16-week and 26-week visits while around 11% (n = 17) of EARLI participants provided more than one measurement of urinary phthalate (eTable 2 http://links.lww.com/EDE/C79). For example, 51% (n = 80) EARLI participants provided urinary phthalate measurements in the 2nd trimester while 2% (n = 3) EARLI participants provided urinary phthalate measurements in both the 2nd and the 3rd trimesters (eTable 2 http://links.lww.com/EDE/C79).
Table 1.
Sociodemographic characteristics of the mother-child pairs and distributions of six phthalate metabolites during pregnancy in the analytic sample of HOME and EARLI (N = 437).
| HOME (N=271) | EARLI (N=166) | Pooled (N=437) | |
|---|---|---|---|
| MATERNAL CHARACTERISTICS | |||
| Maternal age at delivery (years) | 29.0 (19.0, 45.0) | 34.0 (22.0, 44.0) | 32.0 (19.0, 45.0) |
| Maternal race/ethnicity, n(%) | |||
| Hispanic or Latino | 7 (3%) | 33 (20%) | 40 (9%) |
| Non-Hispanic black | 84 (31%) | 15 (9%) | 99 (23%) |
| Non-Hispanic white | 168 (62%) | 92 (55%) | 260 (59%) |
| Other/multiple races | 12 (4%) | 26 (16%) | 38 (9%) |
| Parity a | |||
| 0–2 | 251 (93%) | 136 (82%) | 387 (89%) |
| >2 | 20 (7%) | 30 (18%) | 50 (11%) |
| Pre-pregnancy BMI (kg/m2) | 24.7 (15.8, 51.3) | 26.6 (17.8, 58.4) | 25.5 (15.8, 58.4) |
| Household annual income, $; n(%) | |||
| 0–10,000 | 46 (17%) | 6 (4%) | 52 (12%) |
| 10,001–20,000 | 19 (7%) | 4 (2%) | 23 (5%) |
| 20,001–30,000 | 20 (7%) | 7 (4%) | 27 (6%) |
| 30,001–50,000 | 42 (16%) | 24 (14%) | 66 (15%) |
| 50,001–75,000 | 42 (16%) | 29 (18%) | 71 (16%) |
| 75,001–100,000 | 58 (21%) | 31 (19%) | 89 (20%) |
| 100,001–200,000 | 44 (16%) | 53 (32%) | 97 (22%) |
| >200,000 | 0 (0%) | 12 (7%) | 12 (3%) |
| MBP (ug/g creatinine), median (min, max) | 26.3 (4.5, 557.0) | 14.5 (2.2, 217.6) | 21.6 (2.2, 557.0) |
| MBzP (ug/g creatinine), median (min, max) | 11.1 (0.6, 185.1) | 6.7 (0.3, 238.6) | 9.2 (0.3, 238.6) |
| MCPP (ug/g creatinine), median (min, max) | 2.4 (0.6, 55.2) | 2.4 (0.4, 2811.1) | 2.4 (0.4, 2811.1) |
| DEHP (ug/g creatinine), median (min, max) b | 88.0 (16.3, 3809.8) | 41.3 (7.4, 1135.7) | 63.6 (7.4, 3809.8) |
| MEP (ug/g creatinine), median (min, max) | 152.3 (11.1, 9158.9) | 34.5 (2.5, 2279.4) | 94.0 (2.5, 9158.9) |
| MiBP (ug/g creatinine), median (min, max) | 5.3 (0.8, 48.3) | 10.3 (1.8, 317.7) | 6.9 (0.8, 317.7) |
| CHILD CHARACTERISTICS | |||
| Child sex at birth, n(%) | |||
| Female | 149 (55%) | 76 (46%) | 225 (52%) |
| Male | 122 (45%) | 90 (54%) | 212 (48%) |
| Age at SRS administration (months), median (min, max) | 50.1 (45.2, 125.2) | 36.3 (35.0, 50.2) | 48.6 (35.0, 125.2) |
| SRS total raw scores (points), median (min, max) c | 30.0 (1.0, 126.0) | 29.0 (6.0, 174.0) | 30.0 (1.0, 174.0) |
| SRS total T-scores (unitless), median (min, max) | 50.0 (35.0, 90.0) | 45.0 (36.0, 101.0) | 48.0 (35.0, 101.0) |
| STUDY SITE, n(%) | |||
| HOME | 271 (100%) | 0 (0%) | 271 (62%) |
| Drexel | 0 (0%) | 41 (25%) | 41 (9%) |
| Johns Hopkins University | 0 (0%) | 39 (23%) | 39 (9%) |
| Kaiser Permanente | 0 (0%) | 58 (35%) | 58 (13%) |
| UC Davis | 0 (0%) | 28 (17%) | 28 (6%) |
All the pregnant women in EARLI had prior pregnancies by study design.
DEHP (ug/g creatinine) was calculated as ΣDEHP (ug/g creatinine) = [MEHP (ug/g creatinine)/278 (g/mol) + MEOHP (ug/g creatinine)/292 (g/mol) + MEHHP (ug/g creatinine)/294 (g/mol) + MECPP (ug/g creatinine)/308 (g/mol)] × 308 g/mol where 278, 292, 294, and 308 are the molecular weights of mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), respectively.
SRS had 65 items, each of which was scored as 0 (“not true”), 1 (“sometimes true”), 2 (“often true”), or 3 (“almost always true”). The total raw score was a sum of all these 65 items.
All values are sample sizes (column percentages) for categorial variables and medians (minimums, maximums) for continuous variables unless otherwise specified. Maternal urinary phthalates concentrations were imputed with the lower limit of detection (LLOD) divided by square root of two if lower than the LLOD and corrected for urinary dilution using creatinine.
BMI, body mass index; DEHP, di(2-ethylhexyl) phthalate; EARLI, the Early Autism Risk Longitudinal Investigation Study; HOME, the Health Outcomes and Measures of the Environment Study; MBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate; SRS, Social Responsiveness Scale.
The medians (minimums, maximums) of maternal age at delivery were 29 (19, 45) years and 34 (22, 44) years in HOME and EARLI, respectively (Table 1). In the combined cohorts, the majority of participants were non-Hispanic white (n = 260, 59.5%), had 0–2 parity (n = 387, 88.6%), and had a household annual income of less than $100,000 (n = 239, 54.7%) (Table 1). There were 225 (51.5%) female births and 212 (48.5%) male births (Table 1).
In comparison to pregnant women in HOME, pregnant women in EARLI had lower medians for four out of the six phthalate metabolites (Table 1). In both cohorts, the four ΣDEHP metabolites were strongly correlated (Spearman correlation coefficients > 0.7)46 (eFigure 3 http://links.lww.com/EDE/C79). In HOME, MBzP was not correlated with MEP and ΣDEHP; MEP was negligibly correlated with MCPP and ΣDEHP; and ΣDEHP was not correlated with MBzP and MEP (Spearman correlation coefficients between −0.1 to 0.1)46 (eFigure 3 http://links.lww.com/EDE/C79). In EARLI, MCPP was negligibly correlated with MBzP and MEP was negligibly correlated with MBzP (Spearman correlation coefficients between −0.1 to 0.1)46 (eFigure 3 http://links.lww.com/EDE/C79). The other pairs of phthalates had weak to moderate correlations with each other46 (eFigure 3 http://links.lww.com/EDE/C79).
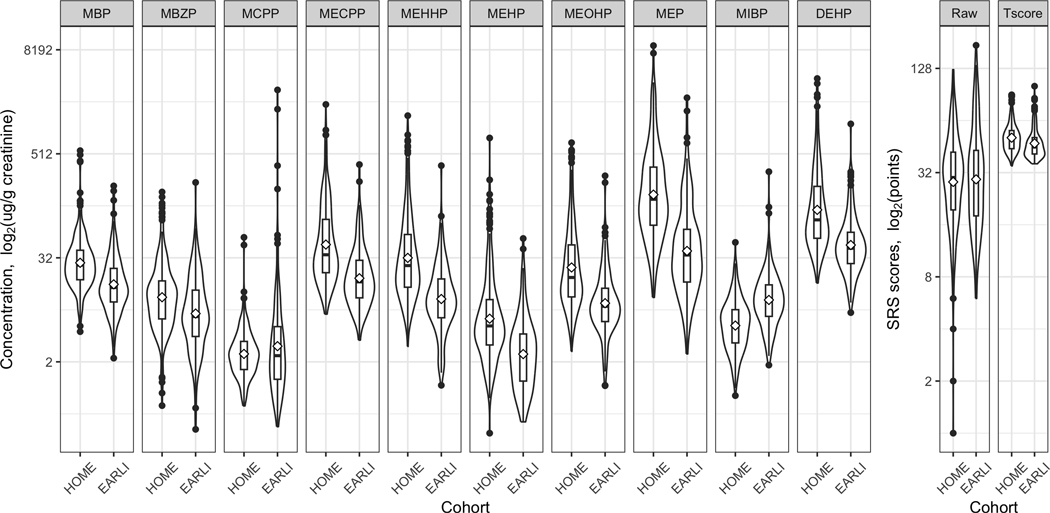
The medians (minimums, maximums) of SRS total raw scores in HOME and EARLI were 30.0 points (1.0, 126.0) and 29.0 (6.0, 174.0) points, respectively (Table 1, Figure 1). The median of SRS total T-scores in HOME (50.0) was higher than that in EARLI (45.0) (Table 1, Figure 1).
Figure 1. Profiles of maternal urinary phthalates during pregnancy and offspring SRS scores in HOME and EARLI (N = 437).
All the phthalates were imputed with the lower limit of detection (LLOD) divided by a square root of two if lower than the LLOD and corrected for urinary dilution using urinary creatinine concentrations.
DEHP, di(2-ethylhexyl) phthalate; EARLI, the Early Autism Risk Longitudinal Investigation Study; HOME, the Health Outcomes and Measures of the Environment Study; MBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MECPP, mono-2-ethyl-5-carboxypentyl phthalate; MEHHP, mono-2-ethyl-5-hydroxyhexyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEOHP, mono-2-ethyl-5-oxohexyl phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate; SRS, Social Responsiveness Scale.
Overall association between phthalates mixture and SRS scores
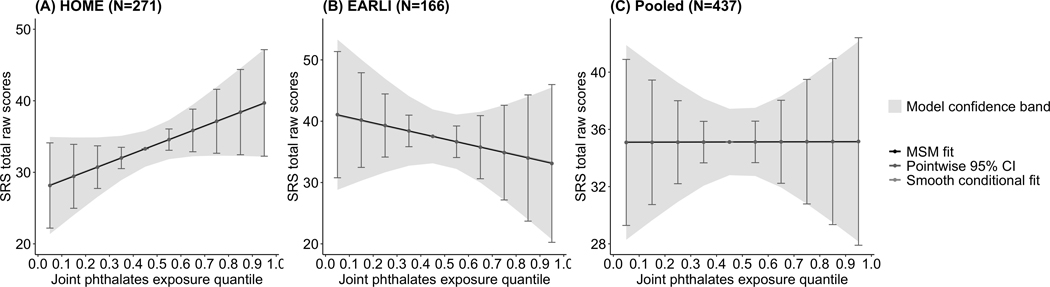
The simplest quantile g-computation model, i.e., model 1, had the lowest AIC (3969.3) and BIC (4054.9) compared to the other three models; because it fit the data best, we applied this model to all sensitivity analyses (eTable 3 http://links.lww.com/EDE/C79). After adjustment for covariates, concentrations of phthalate metabolites during pregnancy were positively associated with offspring SRS total raw scores in HOME (difference per decile increase in every phthalate = 1.3; 95% CI: −0.20, 2.8) but negatively associated with offspring SRS total raw scores in EARLI (difference per decile increase in every phthalate = −0.90; 95% CI: −3.5, 1.7) (Table 2, Figure 2); both estimates are notably imprecise.
Table 2.
Associations between prenatal exposure to a mixture of 6 phthalates and offspring Social Responsiveness Scale (SRS) total raw scores in HOME and EARLI: quantile g-computation models.
| Unadjusted modela | Adjusted model | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HOME (N=271) | EARLI (N=166) | Pooled (N=437) | HOMEb (N=271) | EARLIc (N=166) | Pooledd (N=437) | ||
| ψ1 (95% CI) | 1.3 (−0.35, 2.9) | −1.6 (−3.9, 0.65) | −0.36 (−1.8, 1.1) | 1.3 (−0.21, 2.8) | −0.88 (−3.5, 1.7) | 0.01 (−1.4, 1.5) | |
| Percentile | |||||||
| Minimum – 10th | −5.1 (−11, 1.4) | 6.6 (−2.6, 15) | 1.4 (−4.4, 7.3) | −5.1 (−11, 0.83) | 3.5 (−6.8, 13) | −0.03 (−5.8, 5.8) | |
| 10th – 20th | −3.8 (−8.7, 1.0) | 4.9 (−2.0, 11) | 1.1 (−3.3, 5.5) | −3.8 (−8.3, 0.62) | 2.6 (−5.1, 10) | −0.02 (−4.4, 4.3) | |
| 20th – 30th | −2.5 (−5.8, 0.69) | 3.3 (−1.3, 7.9) | 0.73 (−2.2, 3.7) | −2.6 (−5.5, 0.41) | 1.8 (−3.4, 6.9) | −0.01 (−2.9, 2.9) | |
| 30th – 40th | −1.3 (−2.9, 0.35) | 1.6 (−0.65, 3.9) | 0.36 (−1.1, 1.8) | −1.3 (−2.8, 0.21) | 0.88 (−1.7, 3.5) | −0.01 (−1.5, 1.4) | |
| 40th – 50th | Reference | Reference | Reference | Reference | Reference | Reference | |
| 50th – 60th | 1.3 (−0.35, 2.9) | −1.6 (−3.9, 0.65) | −0.36 (−1.8, 1.1) | 1.3 (−0.21, 2.8) | −0.88 (−3.5, 1.7) | 0.01 (−1.4, 1.5) | |
| 60th – 70th | 2.5 (−0.69, 5.8) | −3.3 (−7.9, 1.3) | −0.73 (−3.7, 2.2) | 2.6 (−0.41, 5.5) | −1.8 (−6.9, 3.4) | 0.01 (−2.9, 2.9) | |
| 70th – 80th | 3.8 (−1.0, 8.7) | −4.9 (−11, 2.0) | −1.1 (−5.5, 3.3) | 3.8 (−0.62, 8.3) | −2.6 (−10, 5.1) | 0.02 (−4.3, 4.4) | |
| 80th – 90th | 5.1 (−1.4, 11) | −6.6 (−16, 2.6) | −1.4 (−7.3, 4.4) | 5.1 (−0.83, 11) | −3.5 (−14, 6.8) | 0.03 (−5.8, 5.8) | |
| 90th – Maximum | 6.4 (−1.7, 14) | −8.2 (−20, 3.2) | −1.8 (−9.2, 5.5) | 6.4 (−1.0, 13) | −4.4 (−17, 8.4) | 0.04 (−7.2, 7.3) | |
Included di(2-ethylhexyl) phthalate (DEHP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), monoethyl phthalate (MEP), and mono-isobutyl phthalate (MiBP) as the independent variables. DEHP (ug/g creatinine) was calculated as ΣDEHP (ug/g creatinine) = [MEHP (ug/g creatinine)/278 (g/mol) + MEOHP (ug/g creatinine)/292 (g/mol) + MEHHP (ug/g creatinine)/294 (g/mol) + MECPP (ug/g creatinine)/308 (g/mol)] × 308 g/mol where 278, 292, 294, and 308 are the molecular weights of mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), respectively.
Included DEHP, MBP, MBzP, MCPP, MEP, MiBP, maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), and child age at Social Responsiveness Scale (SRS) administration (months) as the independent variables.
Included DEHP, MBP, MBzP, MCPP, MEP, MiBP, maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), child age at Social Responsiveness Scale (SRS) administration (months), and three dummy variables for study site (Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables.
Included DEHP, MBP, MBzP, MCPP, MEP, MiBP, maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), child age at Social Responsiveness Scale (SRS) administration (months), and 4 dummy variables for study site (HOME, Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables.
All values are mean differences (95% confidence intervals [CIs]) for the pointwise comparisons between different percentiles as compared to the 40th percentile – 50th percentile unless otherwise specified. ψ1 (95% CI) represents the expected change in the SRS total scores, given a one quantile increase in all 6 phthalates simultaneously. All the phthalates were imputed with the lower limit of detection (LLOD) divided by a square root of two if lower than the LLOD and corrected for urinary dilution using urinary creatinine concentrations.
EARLI, the Early Autism Risk Longitudinal Investigation Study; HOME, the Health Outcomes and Measures of the Environment Study.
Figure 2. Pointwise effects of prenatal exposure to a mixture of six phthalates and offspring Social Responsiveness Scale (SRS) total raw scores in HOME and EARLI: quantile g-computation models.
All the models included di(2-ethylhexyl) phthalate (DEHP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), monoethyl phthalate (MEP), and mono-isobutyl phthalate (MiBP), maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), and child age at SRS administration (months) as the independent variables. The model using only EARLI data further included three dummy variables for study site (Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables while the model using pooled data further included 4 dummy variables for study site (HOME, Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables.
EARLI, the Early Autism Risk Longitudinal Investigation Study; HOME, the Health Outcomes and Measures of the Environment Study; MSM, marginal structural model.
Urinary phthalate metabolite concentrations during pregnancy were imprecisely positively associated with offspring SRS total raw scores among both male children (difference per decile increase in every phthalate = 1.9; 95% CI: −0.4, 4.2) and female children (difference per decile increase in every phthalate = 0.6; 95% CI: −1.4, 2.7) in HOME (eTable 4 http://links.lww.com/EDE/C79). On the other hand, urinary phthalate metabolite concentrations during pregnancy were imprecisely negatively associated with offspring SRS total raw scores among both male children (difference per decile increase in every phthalate = −0.7; 95% CI: −5.4, 4.1) and female children (difference per decile increase in every phthalate = −1.5; 95% CI: −4.1, 1.1) in EARLI (eTable 4 http://links.lww.com/EDE/C79).
Findings were consistent when using SRS T-scores, with associations of less than one absolute value of T-score points for all the subgroups (eTable 5 http://links.lww.com/EDE/C79, eTable 6 http://links.lww.com/EDE/C79, eFigure 4 http://links.lww.com/EDE/C79). Urinary phthalate metabolite concentrations during pregnancy were positively associated with offspring SRS total T-scores in HOME (difference per decile increase in every phthalate = 0.6; 95% CI: −0.1, 1.4) while negatively associated with offspring SRS total T-scores in EARLI (difference per 10 percentiles increase in the phthalates = −0.3; 95% CI: −1.3, 0.6) (eTable 5 http://links.lww.com/EDE/C79).
Finally, results from the quantile g-computation models with two-way interaction terms between each pair of the phthalates are shown in eTable 7 http://links.lww.com/EDE/C79 and quantile g-computation models with polynomial splines in eTable 8 http://links.lww.com/EDE/C79; these results did not suggest a nonlinear dose response.
Contribution of individual phthalates within the mixture to SRS scores
In HOME, ΣDEHP, MiBP, MCPP, and MEP were associated with higher SRS scores, with 42%, 25%, 20%, 13% positive weights on SRS total raw scores, respectively (Figure 3). In EARLI, MBzP (52%), MBP (47%), and MCPP (2%) had positive weights on SRS total raw scores (Figure 3). In contrast, MBP and MBzP had negative weights on SRS total raw scores, contributing to 70% and 30% of the negative weights in HOME (Figure 3). Finally, in EARLI, ΣDEHP, MiBP and MEP had negative weights on SRS total raw scores, contributing to 37%, 37%, and 25% of the negative weights, respectively (Figure 3). Results with SRS total T-scores as the outcome were consistent with results using raw scores (eFigure 5 http://links.lww.com/EDE/C79).
Figure 3. Weights of each phthalate from quantile g-computation model in HOME and EARLI (N = 437).
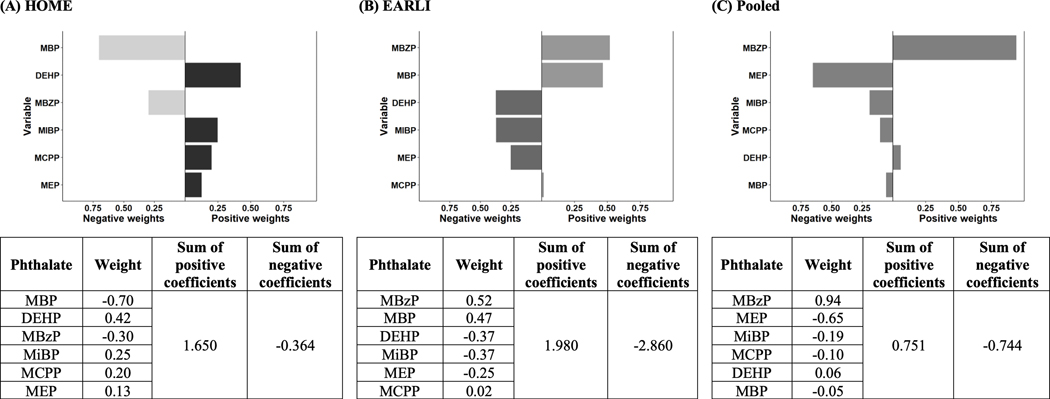
All the models had Social Responsiveness Scale (SRS) total raw scores as the outcome and di(2-ethylhexyl) phthalate (DEHP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), monoethyl phthalate (MEP), and mono-isobutyl phthalate (MiBP), maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), and child age at SRS administration (months) as the independent variables. The model using only EARLI data further included 3 dummy variables for study site (Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables while the model using pooled data further included 4 dummy variables for study site (HOME, Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables.
DEHP, di(2-ethylhexyl) phthalate; EARLI, the Early Autism Risk Longitudinal Investigation Study; HOME, the Health Outcomes and Measures of the Environment Study; MBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
Associations between individual phthalates and SRS scores
Overall, results from the linear regression models indicated overwhelmingly imprecise and null associations between individual phthalates and SRS scores, controlling for maternal age at delivery, maternal race/ethnicity, maternal parity, pre-pregnancy BMI, household annual income, child sex at birth, child age at SRS administration, and study site (Table 3). Notably, in HOME, a doubling of ΣDEHP concentrations during pregnancy was, on average, associated with an SRS raw total score increase of 1.9-points (95% CI: 0.4, 3.4) (Table 3).
Table 3.
Associations between prenatal exposure to individual phthalates and offspring Social Responsiveness Scale (SRS) raw scores in HOME and EARLI: linear regression models (N = 437).
| Phthalate, log2(ug/g creatinine) | Unadjusted modela | Adjusted model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HOME (N=271) | EARLI (N=166) | Pooled (N=437) | HOMEb (N=271) | EARLIc (N=166) | Pooledd (N=437) | |
| MBP | 0.24 (−2.1, 2.6) | −1.3 (−5.3, 2.6) | −0.85 (−2.8, 1.1) | −0.17 (−2.3, 2.0) | 0.52 (−4.5, 3.4) | −0.23 (−2.3, 1.8) |
| MBzP | 2.1 (0.24, 3.9) | 1.5 (−1.5, 4.5) | 1.5 (−0.08, 3.1) | 0.15 (−1.6, 1.9) | 0.89 (−2.1, 3.8) | 0.47 (−1.1, 2.1) |
| MCPP | −0.37 (−3.1, 2.4) | −1.9 (−4.1, 0.28) | −1.4 (−3.0, 0.19) | 1.9 (−0.64, 4.5) | −0.75 (−3.0, 1.5) | −0.12 (−1.7, 1.4) |
| DEHPc | 0.21 (−1.4, 1.8) | −3.6 (−7.4, 0.14) | −1.0 (−2.5, 0.40) | 1.9 (0.43, 3.4) | −2.8 (−6.5, 0.87) | 0.53 (−1.0, 2.1) |
| MEP | 0.68 (−0.72, 2.1) | −2.0 (−4.5, 0.53) | −0.62 (−1.7, 0.48) | 0.36 (−0.91, 1.6) | −1.6 (−4.2, 1.0) | −0.59 (−1.8, 0.64) |
| MiBP | 2.2 (−0.25, 4.7) | −2.2 (−6.2, 1.8) | 0.88 (−1.1, 2.9) | 1.8 (−0.41, 4.1) | −1.6 (−5.4, 2.3) | 0.18 (−1.9, 2.2) |
Included di(2-ethylhexyl) phthalate (DEHP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), monoethyl phthalate (MEP), and mono-isobutyl phthalate (MiBP) as the independent variables. DEHP (ug/g creatinine) was calculated as ΣDEHP (ug/g creatinine) = [MEHP (ug/g creatinine)/278 (g/mol) + MEOHP (ug/g creatinine)/292 (g/mol) + MEHHP (ug/g creatinine)/294 (g/mol) + MECPP (ug/g creatinine)/308 (g/mol)] × 308 g/mol where 278, 292, 294, and 308 are the molecular weights of mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), respectively.
Included DEHP, MBP, MBzP, MCPP, MEP, MiBP, maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), and child age at Social Responsiveness Scale (SRS) administration (months) as the independent variables.
Included DEHP, MBP, MBzP, MCPP, MEP, MiBP, maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), child age at Social Responsiveness Scale (SRS) administration (months), and three dummy variables for study site (Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables.
Included DEHP, MBP, MBzP, MCPP, MEP, MiBP, maternal age at delivery (years), three dummy variables for maternal race/ethnicity (Hispanic or Latino, Non-Hispanic black, Non-Hispanic white, Other/multiple races), maternal parity (0–2, >2), pre-pregnancy BMI (kg/m2), household annual income ($), child sex at birth (male, female), child age at Social Responsiveness Scale (SRS) administration (months), and 4 dummy variables for study site (HOME, Drexel, Johns Hopkins University, Kaiser Permanente, UC Davis) as the independent variables.
All values are regression coefficients (95% confidence intervals) unless otherwise specified. All the phthalates were imputed with the lower limit of detection (LLOD) divided by a square root of two if lower than the LLOD and corrected for urinary dilution using urinary creatinine concentrations.
DEHP, di(2-ethylhexyl) phthalate; MBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
DISCUSSION
We investigated the association between urinary phthalate metabolite concentrations during pregnancy and developmental traits related to autism among children enrolled in two pregnancy and birth cohorts. In HOME, we observed imprecise positive associations of the phthalate mixture and some phthalate metabolites with autism-related traits. In EARLI, we observed imprecise negative associations between the phthalate mixture and SRS scores. Notably, these associations between urinary phthalate metabolite concentrations during pregnancy and developmental traits related to autism were essentially null across quintiles of SRS scores in both cohorts.
Evidence on the associations between urinary phthalate metabolite concentrations during pregnancy and autism-related outcomes is mixed. Two previous studies from our group found that the independent and individual associations between urinary phthalate metabolite concentrations during pregnancy and child SRS scores were predominantly null in HOME using traditional regression methods.23,24 This work used a mixture approach to assess the aggregate association between a mixture of six phthalate metabolites and child SRS scores using g-computation approach. The two lines of work yielded consistent results. Another study found that urinary phthalate metabolite concentrations during pregnancy were not associated with ASD diagnosis in the Markers of Autism Risk in Babies – Learning Early Signs (MARBLES) Study, an enriched-risk cohort similar to EARLI.47 On the other hand, several studies found that urinary phthalate metabolite concentrations during pregnancy were associated with social impairment, which represents a key feature of ASD diagnosis.22,36,48
We detected mixture associations in the opposite directions in the two cohorts. One possible reason is that these two cohorts had different study designs. HOME recruited pregnant women from the general population while EARLI had an enriched-risk design, meaning that all the pregnant women enrolled already had a child with ASD prior to this pregnancy. Risk of recurrence of ASD in siblings to ASD-affected children was estimated to be as high as 18.7%.49 The increased familial liability of ASD and developmental traits related to autism in EARLI might outweigh potential environmental risk. Specifically, there are known differences between the general population and the siblings of ASD-affected children, which was suggested to be attributable to different mechanisms of intergenerational genetic transmission of ASD.50,51
We also cannot rule out potential differences across cohorts due to measurement of autism-related traits. Specifically, HOME administered the first edition form of the SRS for children aged 4–18 years whereas the preschool form of the SRS was used in EARLI, due to the modestly differing ages across these two studies. Prior work supports the comparability of these two forms, and only minor wording changes exist between them to account for age-appropriate examples in items. Thus, these differences are unlikely to fully explain differences in results across the two cohorts. However, it has been noted that scores on preschool forms consistent with ASD may be slightly lower on average than those from school-age forms, which might explain the similar average T-scores in the two cohorts.52(p2)
This analysis has several strengths. First, we used a quantile g-computation method to estimate the association between in utero exposures to a phthalate mixture and offspring developmental traits related to autism. Quantile g-computation can estimate the aggregate effect of correlated exposures instead of estimating the effects of exposures individually.39 Second, we adjusted for a detailed set of confounders and were able to use two cohorts to determine if associations were comparable in different populations. Third, we strived to minimize measurement error of the exposures by calculating the average of up to two samples per woman at 16 weeks and 26 weeks of gestation in HOME. This may reduce within-person variation in concentrations of these compounds that could be related to the timing of urine sample collection. This approach was consistent with our previous study to ensure comparability.24
There are also several limitations to this analysis. First, while many pregnant women in HOME had two measures of urinary phthalates from two visits, most pregnant women in EARLI provided one measure of urinary phthalates. Phthalates are quickly eliminated from human bodies, and hence this may result in misclassification error.24 Some phthalates, e.g., ΣDEHP, need multiple biospecimen per participant because they have high within-participant temporal variability.53 Second, we cannot rule out the possibility of residual confounding and we did not assess effect measure modification by folic acid, which has been observed to modify the association between phthalates and SRS scores in another study.36 However, we controlled for an extensive list of confounders or variables that are associated with SRS scores, in order to minimize potential confounding. And sensitivity analysis with prenatal vitamin use initiated in the first month of pregnancy as an additional covariate in the quantile g-computation models revealed null associations as well (eTable 9 http://links.lww.com/EDE/C79). Third, while using a continuous outcome provided more statistical power compared to a dichotomous outcome, our sample sizes are still modest.
While it remains inconclusive if there are specific times of increased susceptibility to xenobiotic exposures during pregnancy, there is an increasing appreciation that the effects of prenatal exposures to xenobiotics on offspring health outcomes may differ by pregnancy stage.54 For example, Watkins et al. (2016) found that the associations between prenatal phthalates concentrations and birth sizes were different by trimester.55 Additionally, Messerlian et al. (2017) indicated that the preconception period may be another critical window of susceptibility of phthalate exposures for offspring neurodevelopment.56 However, due to the limited sample sizes in each trimester and the lack of data in the preconception period, we could not pursue trimester-specific analyses in this study. Further studies using cohorts with larger sample sizes in each trimester are thus warranted.
CONCLUSIONS
We estimated heterogeneous associations between urinary phthalate metabolite concentrations during pregnancy and offspring SRS scores across two prospective pregnancy and birth cohorts. While effect sizes were small and confidence intervals included the null, observed differences could point to a potential role of background familial liability in the associations between phthalates and child developmental traits related to autism. Further studies using other cohorts are warranted.
Supplementary Material
Acknowledgments
We sincerely thank all the participants and staff from HOME and EARLI. We are aware of the recent concern of using the language “risk” in the field of autism research and are moving away from this term. However, EARLI was named years ago before the discussion started, so we kept the original cohort’s name.
Funding statement:
EARLI was funded by the National Institutes of Health (R01ES016443 and R24ES030893) and Autism Speaks (003953). The HOME Study was funded by the National Institutes of Health and the U.S. Environmental Protection Agency (P01ES011261, R01ES014575, R01ES020349, R01 ES024381, R01 ES032836).
Footnotes
Data and code availability:
Once the paper is published, we will publish the code at https://github.com/emmayu001/qgcomp-Phthalates-SRS. Inquiries about data request should be addressed to the Principal Investigators of the two cohorts (HOME: JMB, KY; EARLI: MDF).
Conflict of Interest: Joseph Braun was financially compensated for serving as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water.
References
- 1.Rudel RA, Gray JM, Engel CL, et al. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ Health Perspect. 2011;119(7):914–920. doi: 10.1289/ehp.1003170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harley KG, Kogut K, Madrigal DS, et al. Reducing Phthalate, Paraben, and Phenol Exposure from Personal Care Products in Adolescent Girls: Findings from the HERMOSA Intervention Study. Environ Health Perspect. 2016;124(10):1600–1607. doi: 10.1289/ehp.1510514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitro SD, Dodson RE, Singla V, et al. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ Sci Technol. 2016;50(19):10661–10672. doi: 10.1021/acs.est.6b02023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radke EG, Braun JM, Meeker JD, Cooper GS. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ Int. 2018;121:764–793. doi: 10.1016/j.envint.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yost EE, Euling SY, Weaver JA, et al. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ Int. 2019;125:579–594. doi: 10.1016/j.envint.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radke EG, Glenn BS, Braun JM, Cooper GS. Phthalate exposure and female reproductive and developmental outcomes: a systematic review of the human epidemiological evidence. Environ Int. 2019;130:104580. doi: 10.1016/j.envint.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radke EG, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int. 2019;132:104768. doi: 10.1016/j.envint.2019.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsikantami I, Sifakis S, Tzatzarakis MN, et al. A global assessment of phthalates burden and related links to health effects. Environ Int. 2016;97:212–236. doi: 10.1016/j.envint.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 9.Day DB, Collett BR, Barrett ES, et al. Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environ Int. 2021;147:106330. doi: 10.1016/j.envint.2020.106330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobrosly RW, Evans S, Miodovnik A, et al. Prenatal Phthalate Exposures and Neurobehavioral Development Scores in Boys and Girls at 6–10 Years of Age. Environ Health Perspect. 2014;122(5):521–528. doi: 10.1289/ehp.1307063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel SM, Miodovnik A, Canfield RL, et al. Prenatal Phthalate Exposure Is Associated with Childhood Behavior and Executive Functioning. Environ Health Perspect. 2010;118(4):565–571. doi: 10.1289/ehp.0901470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KI, Chiang CW, Lin HC, et al. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch Toxicol. 2016;90(5):1211–1224. doi: 10.1007/s00204-015-1539-0 [DOI] [PubMed] [Google Scholar]
- 13.Hoshi H, Ohtsuka T. Adult Rats Exposed to Low-Doses of Di-n-Butyl Phthalate During Gestation Exhibit Decreased Grooming Behavior. Bull Environ Contam Toxicol. 2009;83(1):62–66. doi: 10.1007/s00128-009-9729-1 [DOI] [PubMed] [Google Scholar]
- 14.Lin H, Yuan K, Li L, et al. In Utero Exposure to Diethylhexyl Phthalate Affects Rat Brain Development: A Behavioral and Genomic Approach. Int J Environ Res Public Health. 2015;12(11):13696–13710. doi: 10.3390/ijerph121113696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barakat R, Lin PC, Park CJ, et al. Prenatal Exposure to DEHP Induces Neuronal Degeneration and Neurobehavioral Abnormalities in Adult Male Mice. Toxicol Sci. 2018;164(2):439–452. doi: 10.1093/toxsci/kfy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kougias DG, Sellinger EP, Willing J, Juraska JM. Perinatal Exposure to an Environmentally Relevant Mixture of Phthalates Results in a Lower Number of Neurons and Synapses in the Medial Prefrontal Cortex and Decreased Cognitive Flexibility in Adult Male and Female Rats. J Neurosci. 2018;38(31):6864–6872. doi: 10.1523/JNEUROSCI.0607-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Zhao Y, Lu Y, et al. Autism spectrum disorder-like behavior induced in rat offspring by perinatal exposure to di-(2-ethylhexyl) phthalate. Environ Sci Pollut Res. 2022;29(34):52083–52097. [DOI] [PubMed] [Google Scholar]
- 18.Jeddi MZ, Janani L, Memari AH, Akhondzadeh S, yunesian M. The role of phthalate esters in autism development: A systematic review. Environ Res. 2016;151:493–504. doi: 10.1016/j.envres.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 19.Radke EG, Braun JM, Nachman RM, Cooper GS. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ Int. 2020;137:105408. doi: 10.1016/j.envint.2019.105408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Testa C, Nuti F, Hayek J, et al. Di-(2-Ethylhexyl) Phthalate and Autism Spectrum Disorders. ASN Neuro. 2012;4(4):AN20120015. doi: 10.1042/AN20120015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kardas F, Bayram AK, Demirci E, et al. Increased serum phthalates (MEHP, DEHP) and bisphenol A concentrations in children with autism spectrum disorder: the role of endocrine disruptors in autism etiopathogenesis. J Child Neurol. 2016;31(5):629–635. [DOI] [PubMed] [Google Scholar]
- 22.Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. NeuroToxicology. 2011;32(2):261–267. doi: 10.1016/j.neuro.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun JM, Kalkbrenner AE, Just AC, et al. Gestational Exposure to Endocrine-Disrupting Chemicals and Reciprocal Social, Repetitive, and Stereotypic Behaviors in 4- and 5-Year-Old Children: The HOME Study. Environ Health Perspect. 2014;122(5):513–520. doi: 10.1289/ehp.1307261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patti MA, Newschaffer C, Eliot M, et al. Gestational Exposure to Phthalates and Social Responsiveness Scores in Children Using Quantile Regression: The EARLI and HOME Studies. Int J Environ Res Public Health. 2021;18(3):1254. doi: 10.3390/ijerph18031254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prohibition of Children’s Toys and Child Care Articles Containing Specified Phthalates. U.S. Consumer Product Safety Commission. Accessed October 26, 2022. https://www.cpsc.gov/Regulations-Laws--Standards/Rulemaking/Final-and-Proposed-Rules/Prohibition-of-Childrens-Toys-and-Child-Care-Articles-Containing-Specified-Phthalates [PubMed]
- 26.Hamra GB, Buckley JP. Environmental exposure mixtures: questions and methods to address them. Curr Epidemiol Rep. 2018;5(2):160–165. doi: 10.1007/s40471-018-0145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun JM, Kalloo G, Chen A, et al. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017;46(1):24. doi: 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newschaffer CJ, Croen LA, Fallin MD, et al. Infant siblings and the investigation of autism risk factors. 2012;4(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Western Psychological Services. Los Angel CA. Published online 2005. [Google Scholar]
- 30.Bölte S, Poustka F, Constantino JN. Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Res. 2008;1(6):354–363. doi: 10.1002/aur.49 [DOI] [PubMed] [Google Scholar]
- 31.Bruni TP. Test Review: Social Responsiveness Scale–Second Edition (SRS-2). J Psychoeduc Assess. 2014;32(4):365–369. doi: 10.1177/0734282913517525 [DOI] [Google Scholar]
- 32.Aldridge FJ, Gibbs VM, Schmidhofer K, Williams M. Investigating the Clinical Usefulness of the Social Responsiveness Scale (SRS) in a Tertiary Level, Autism Spectrum Disorder Specific Assessment Clinic. J Autism Dev Disord. 2012;42(2):294–300. doi: 10.1007/s10803-011-1242-9 [DOI] [PubMed] [Google Scholar]
- 33.Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):106–112. doi: 10.1016/j.jchromb.2007.10.023 [DOI] [PubMed] [Google Scholar]
- 34.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Environ Hyg. 1990;5(1):46–51. doi: 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- 35.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oulhote Y, Lanphear B, Braun JM, et al. Gestational Exposures to Phthalates and Folic Acid, and Autistic Traits in Canadian Children. Environ Health Perspect. 2020;128(2):027004. doi: 10.1289/EHP5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaat AJ, Shui AM, Ghods SS, et al. Sex differences in scores on standardized measures of autism symptoms: a multisite integrative data analysis. J Child Psychol Psychiatry. 2021;62(1):97–106. doi: 10.1111/jcpp.13242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanken LME, Muetzel RL, Jaddoe VWV, et al. White matter microstructure in children with autistic traits. Psychiatry Res Neuroimaging. 2017;263:127–134. doi: 10.1016/j.pscychresns.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 39.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Env Health Perspect. 2020;128(4):47004. doi: 10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. Response to “Comment on ‘A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures.’” Env Health Perspect. 2021;129(3):38002. doi: 10.1289/EHP8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keil AP. Introduction. Accessed July 8, 2022. https://cran.r-project.org/web/packages/qgcomp/vignettes/qgcomp-vignette.html
- 42.Bulka CM, Avula V, Fry RC. Associations of exposure to perfluoroalkyl substances individually and in mixtures with persistent infections: Recent findings from NHANES 1999–2016. Environ Pollut. 2021;275:116619. doi: 10.1016/j.envpol.2021.116619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letellier N, Zamora S, Yang JA, Sears DD, Jankowska MM, Benmarhnia T. How do environmental characteristics jointly contribute to cardiometabolic health? A quantile g-computation mixture analysis. Prev Med Rep. 2022;30:102005. doi: 10.1016/j.pmedr.2022.102005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parada H, Benmarhnia T, Engel LS, et al. A congener-specific and mixture analysis of plasma polychlorinated biphenyl (PCB) levels and incident breast cancer. Epidemiol Camb Mass. 2021;32(4):499–507. doi: 10.1097/EDE.0000000000001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Published online 2021. https://www.R-project.org/
- 46.Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. 2018;126(5):1763–1768. doi: 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 47.Shin HM, Schmidt RJ, Tancredi D, et al. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health. 2018;17(1):85. doi: 10.1186/s12940-018-0428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alampi JD, Lanphear BP, Braun JM, et al. Association Between Gestational Exposure to Toxicants and Autistic Behaviors Using Bayesian Quantile Regression. Am J Epidemiol. 2021;190(9):1803–1813. doi: 10.1093/aje/kwab065 [DOI] [PubMed] [Google Scholar]
- 49.Ozonoff S, Young GS, Carter A, et al. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167(11):1349–1356. doi: 10.1176/appi.ajp.2010.09101470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. 2009;150B(3):328–334. doi: 10.1002/ajmg.b.30810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Constantino JN, Gruber CP. Social Responsiveness Scale: SRS-2. Western psychological services Torrance, CA; 2012. [Google Scholar]
- 53.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiol Camb Mass. 2016;27(3):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yland JJ, Zhang Y, Williams PL, et al. Phthalate and DINCH urinary concentrations across pregnancy and risk of preterm birth. Environ Pollut. 2022;292:118476. doi: 10.1016/j.envpol.2021.118476 [DOI] [PubMed] [Google Scholar]
- 55.Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: A preliminary analysis. Reprod Toxicol. 2016;65:59–66. doi: 10.1016/j.reprotox.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Messerlian C, Bellinger D, Mínguez-Alarcón L, et al. Paternal and maternal preconception urinary phthalate metabolite concentrations and child behavior. Environ Res. 2017;158:720–728. doi: 10.1016/j.envres.2017.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.