Abstract
p21-activated kinase 1 (Pak1) induces cytoskeleton reorganization in part by regulating microtubule dynamics through an elusive mechanism. Using a yeast two-hybrid screen, we identified tubulin cofactor B (TCoB) (a cofactor in the assembly of the α/β-tubulin heterodimers) as an interacting substrate of Pak1. Pak1 directly phosphorylated TCoB in vitro and in vivo on serines 65 and 128 and colocalized with TCoB on newly polymerized microtubules and on centrosomes. TCoB interacted with the GTPase-binding domain of Pak1 and activated Pak1 in vitro and in vivo. In contrast to wild-type TCoB, an S65A, S128A double mutant and knock-down of the endogenous TCoB or Pak1 reduced microtubule polymerization, suggesting that Pak1 phosphorylation is necessary for normal TCoB function. Overexpression of TCoB dramatically increased the number of γ-tubulin-containing microtubule-organizing centers, a phenotype reminiscent of cells overexpressing Pak1. TCoB was overexpressed and phosphorylated in breast tumors. These findings reveal a novel role for TCoB and Pak1 in regulating microtubule dynamics.
Recent studies showing that mammalian p21-activated kinase 1 (Pak1) phosphorylates histone H3.3 (10) and stathmin (5), undergoes phosphorylation during mitosis (22), is enriched in microtubule-organizing centers (MTOCs) (1), and induces mitotic spindle abnormalities in breast cancer cells (26) suggest that in addition to its established involvement in actin cytoskeletal signaling, Pak1 may play an important role in mitosis and microtubule dynamics. Microtubules are involved in a variety of cellular processes, such as chromosomal segregation, morphogenesis, motility, organelle positioning, and vesicle transport (6, 7, 13, 14). Microtubules exhibit dynamic instability due to stochastic transitions at microtubule ends between phases of growth and shrinkage (6), and reversible phosphorylation is shown to play an important role in microtubule dynamics (3).
Microtubule subunits are heterodimers composed of one α-tubulin polypeptide and one β-tubulin polypeptide (7). Correct incorporation of these subunits into the polymer requires a complex folding process and is facilitated by a subfamily of chaperones known as CCT/TriC/c-cpn and a set of tubulin-specific cofactors (A to E) (11, 24). CCT-discharged quasi-native α-tubulin and β-tubulin are captured and stabilized by cofactors B and E or cofactors A and D, respectively (25). Cofactor-tubulin complexes associate to form a supercomplex containing tubulin cofactor C (TCoC), TCoD, TCoE, and α- and β-tubulin; GTP hydrolysis by the bound tubulin triggers the release of native α/β heterodimers (23). TCoC, TCoD, and TCoE all function in the assembly of the tubulin heterodimers, while TCoA and TCoB are specific to β- and α-tubulin, respectively (21).
To identify novel Pak1-interacting proteins with potential functions in the microtubule-mitotic axis, we performed a yeast two-hybrid screen of a human mammary gland cDNA expression library. We have identified TCoB (GenBank accession number XM_009339) as a novel Pak1-interacting substrate. Using various biochemical, molecular, and cell biology approaches, we have provided evidence that Pak1 phosphorylation of TCoB is essential for the polymerization of new microtubules and thus provides novel insights into the molecular mechanism of Pak1 regulation of microtubules.
MATERIALS AND METHODS
Two-hybrid library screening.
Pak1 baits were constructed by amplifying amino acids (aa) 1 to 270 and 271 to 545 by PCR and subcloning the products into a Gal4-binding domain vector (pGBD vector; Clontech). Both baits were used to screen a mammary gland cDNA library fused to the Gal4 activation domain (Clontech), as described previously (27). A total of 2 × 106 clones were screened. Positive clones were isolated and sequenced at the University of Texas M. D. Anderson Cancer Center core sequencing facility. Three positive clones representing tubulin cofactor B were obtained in the screening. Positive clones were also verified by cotransformations followed by selection on agar plates lacking adenosine, histidine, tryptophan, and leucine and also by β-galactosidase assay.
Plasmid construction.
Red fluorescent protein (RFP)-, glutathione S-transferase (GST)- and T7-tagged TCoB constructs were generated by amplifying the selective region of TCoB from the two-hybrid clones obtained from the library screening and by subcloning this region into DsRed2 (Clontech), pGEX 5X (Amersham), and pcDNA3.1 (Invitrogen) vectors, respectively. TCoB-S65A, TCoB-S128Ala, and TCoB-S65AS128A double mutations were generated by site-directed mutagenesis by using a quick-change mutagenesis kit from Stratagene. By amplifying the indicated regions via PCR, we created TCoB fragments and subcloned them into pGEX vectors.
Cell cultures, reagents, transfection, and cell extracts.
MCF-7, SKBR3, and MCF-7/HER2 human breast cancer cells and IMR90 human lung fibroblasts were maintained in Dulbecco's modified Eagle's medium-F12 (1:1) supplemented with 10% fetal calf serum. Antibodies against Pak1 were purchased from Zymed Laboratories Inc. and Cell Signaling; antibodies against T7 were purchased from Novagen; antibody against γ-tubulin and Glu was obtained from Sigma; antibody for Pak2 and Pak3 was purchased from Santa Cruz; antibody against phosphorylated (phospho-T423) Pak1 was described previously (10). Transient transfection studies were performed with a FuGENE 6 kit (Roche Biochemical) in accordance with the manufacturer's instructions. Small interfering RNA (siRNA) against TCoB was designed and synthesized by QiagenXeragon against TCoB target sequence 5′-AATGGGAAACGCTACTTCGAA-3′. siRNA transfections were performed by using Oligofectamine (Invitrogen) according to the manufacturer's protocol. siRNA against Pak1 (catalog number 6361), siRNA against Pak2 (catalog number 6366), and control fluorescein isothiocyanate siRNA (catalog number 6201) were purchased from Cell Signaling. Phosphoserine antibody (catalog number 37430) was purchased from QIAGEN. Anti-peptide antibody (rabbit) against TCoB was generated against the C-terminal 14 amino acids (aa 231 to 244 [NH3-VGDFPEEDYGLDEI-COOH]) and was affinity purified by using GST-TCoB.
Immunoblotting.
For preparation of cell extracts, cells were washed three times with phosphate-buffered saline (PBS) and lysed in buffer (50 mM Tris-HCl [pH 7.5], 120 mM NaCl, 1% Triton X-100, 100 mM NaF, 200 mM NaVO5, 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail [GIBCO]) for 15 min on ice. The lysates were centrifuged with an Eppendorf centrifuge at 4°C for 15 min. Cell lysates containing equal amounts of protein were then resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred onto nitrocellulose, and probed with the appropriate antibodies using an enhanced chemiluminescence method. Cell lysates for immunoprecipitation were prepared by using Nonidet P-40 (NP-40) lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.5% NP-40, 1× protease inhibitor mixture, 1 mM sodium vanadate). Immunoprecipitation was performed for 2 h at 4°C using 1 μg of antibody/mg of protein.
Pak1 kinase assay.
Using myelin basic protein (MBP) or GST-TCoB fusion protein, we performed in vitro kinase assays in HEPES buffer (50 mM HEPES, 10 mM MgCl2, 2 mM MnCl2, 1 mM dithiothreitol) containing 100 ng of purified GST-Pak1 enzyme, 10 μCi of [γ-32P]ATP, and 25 μM cold ATP. MBP was purchased from Upstate Biotechnology. GST proteins were purified by using glutathione Sepharose (Amersham Biosciences) according to the manufacturer's instructions. The reaction was carried out in a volume of 30 μl for 30 min at 30°C and then stopped by the addition of 10 μl of 4× SDS buffer. We analyzed the reaction products by SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiographed them. In some in vitro kinase assays, MBP (20 μg) was added to the reaction. For stimulation of Pak1 autophosphorylation, purified GST-Cdc42 (6.7 mM) was preloaded with 0.18 mM GTPγ-S for 10 min in a volume of 20 μl in 20 mM Tris buffer (pH 7.5) containing 50 mM NaCl. The activated Cdc42 or purified GST-TCoB (6.7, 13.3, or 20 mM) was then incubated with Pak1 enzyme in HEPES buffer containing 1 μl of γATP in a final reaction volume of 30 μl. Purified human Pak1 enzyme (catalog number GLO110-100) and Cdc42 (catalog number GLO128-001) were purchased from Alexis Biochemicals. His-BAD (catalog number 14-357) was purchased from Upstate biotechnology and used as a positive control.
GST pull-down assay.
In vitro transcription and translation of the Pak1 and TCoB proteins were performed by using the TNT-coupled transcription-translation system (Promega) as described previously (10). In brief, using a T7-TNT kit (Promega), we translated 1 μg of cDNA in the pcDNA 3.1 vector in vitro in the presence of [35S]methionine in a reaction volume of 50 μl. The reaction mixture was diluted to 1 ml with NP-40 lysis buffer (25 mM Tris, 50 mM NaCl, 1% NP-40). An aliquot (250 μl) was used for each GST pull-down assay. Translation and product size were verified by subjecting 2 μl of the reaction mixture to SDS-PAGE and autoradiography. The GST pull-down assays were performed by incubating equal amounts of GST, GST-Pak1, and GST-TCoB fusion proteins immobilized on GST Sepharose beads (Amersham Biosciences) with in vitro-translated 35S-labeled Pak1 or TCoB protein. Bound proteins were isolated by incubating the mixture for 2 h at 4°C, washing five times with NP-40 lysis buffer, eluting the proteins with 2× SDS buffer, and separating the proteins by SDS-PAGE. The bound proteins were then visualized by fluorography.
Immunofluorescence and confocal microscopy studies.
We determined the cellular localization of proteins by indirect immunofluorescence as described previously (10). Briefly, cells grown on glass coverslips were fixed in methanol at −20°C for 4 min. The cells were incubated with primary antibodies for 2 h, washed three times in PBS, and then incubated with secondary antibodies conjugated with Alexa 546 (red), Alexa 633 (blue), or Alexa 488 (green) dye from Molecular Probes (Eugene, Oreg.). The DNA dye Topro-3 (Molecular Probes) was used to costain the DNA (blue). Cells treated only with the secondary antibodies or when available with the peptide against which the primary antibody was raised served as controls.
Confocal scanning analysis was performed by using either a Zeiss LSM510 or an Olympus FV300 laser scanning confocal microscope in accordance with established methods, utilizing sequential laser excitation to minimize the possibility of fluorescent emission bleed-through. Each image is a three-dimensional reconstructed image of stacks of serial Z sections at the same cellular level and magnification. Colocalization of two proteins is shown as yellow for red and green, turquoise for green and blue, pink for red and blue, and white for the overlap of three-color fluorescence. Images were exported to Microsoft Photo Editor for adjustment of contrast prior to assembly into figures using Microsoft Power Point.
Nocodazole treatments for microtubule biogenesis assays were performed as previously described (4, 28). Briefly, cells were treated with 4 μM nocodazole for 30 min, rinsed twice with PBS, and then incubated in medium with 10% serum for 15 min to allow for new microtubule biogenesis. Cells were then washed in PHEM cytoskeletal buffer {100 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 100 mM HEPES, 1 mM EGTA, 2 mM MgSO4, 0.2% Triton X-100 (pH 7.0)} for 2 min to extract unpolymerized tubulin and then processed as described above. A mouse monoclonal antibody against tyrosinated microtubules (selectively present on newly polymerized microtubules) was used for colocalization of TCoB or Pak1 selectively with this microtubule population. In siRNA experiments, attenuated microtubule regrowth was defined as siRNA-positive cells with less than 25% abundance of newly formed, tyrosinated microtubules compared with neighboring, untransfected cells.
Immunohistochemistry.
For immunohistochemical detection of Pak1 and TCoB, sections were deparaffinized with xylene and rehydrated with graded ethanol. Sections were incubated in 0.3% H2O2 and methanol for 30 min to inactivate endogenous peroxidase. The sections were then boiled for 10 min in 0.01 M citrate buffer and cooled for 30 min at room temperature to expose antigenic epitopes. The sections were incubated with 2% normal goat serum in 1% bovine serum albumin and PBS for 30 min and then incubated with anti-Pak1 (Cell Signaling) at a 1:25 dilution and peptide affinity-purified anti-TCoB at a 1:10 dilution overnight and at room temperature. The sections were washed three times with 0.05% Tween in PBS for 10 min and incubated with secondary antibody and then developed with diaminobenzidine-H2O2 and counterstained with Mayer's hematoxylin.
Reproducibility.
The results presented are representative of at least three replicate experiments.
RESULTS AND DISCUSSION
TCoB interacts with Pak1 in vivo.
To identify novel Pak1-interacting proteins with potential functions in the microtubule-mitotic axis, we performed a yeast two-hybrid screen of a human mammary gland cDNA expression library by using either the N-terminal or the C-terminal half of Pak1. One Pak1 N-terminal-interacting protein was identified following sequencing of positive clones as TCoB (GenBank accession number XM_009339). To confirm our yeast two-hybrid screening results, we have performed cotransfection experiments with full-length TCoB and N-terminal or C-terminal Pak1 plasmids. Cotransfection of TCoB and Pak1 constructs gave yeast-transformed colonies the ability to grow in medium lacking adenosine, histidine, leucine, and tryptophan. The cotransformants also turned blue in a β-galactosidase assay, thereby confirming the specific interaction of these proteins in yeast (Fig. 1A). Only the N-terminal region of Pak1 showed interactions suggesting that the kinase domain is not involved in Pak1 binding to TCoB.
FIG. 1.
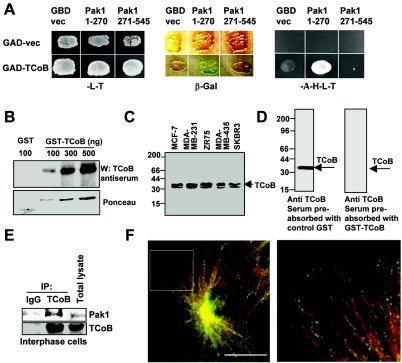
Interaction of Pak1 and TCoB. (A) Yeast cells were cotransfected with control Gal4 activation domain (GAD) vector (vec) or GAD-TCoB along with Gal4 DNA binding domain (GBD) vector, GBD-Pak1 (aa 1 to 270), or GBD-Pak1 (aa 271 to 545). Cotransformants were plated onto selection plates lacking leucine and tryptophan (−L−T) or adenosine, histidine, leucine, and tryptophan (−A−H−L−T). Growth was recorded after 72 h. The blue color in the β-galactosidase (β-Gal) colony filter-lift assays indicates specific interaction of two proteins. (B) Characterization of TCoB antibody. Indicated amounts of GST or GST-TCoB protein were separated on an SDS-PAGE gel and Western blotted (W) with TCoB antibody (1:5,000) (upper panel). Ponceau stain of the membrane representing the amount of GST protein used is shown in the bottom panel. (C) Total cell lysates (100 μg) from indicated breast cancer cells were Western blotted with TCoB antiserum (1:1,000) and developed by enhanced chemiluminescence. (D) TCoB antiserum was preabsorbed with GST or GST-TCoB and then used for Western blotting with MCF-7 cell lysates (100 μg). (E) Interaction of endogenous TCoB with endogenous Pak1. Immunoprecipitation (IP) was performed with control IgG or TCoB antibody using cell lysates from interphase cells. (F) Localization between endogenous TCoB and α-tubulin. Methanol-fixed Vero cells probed with antibodies against TCoB and α-tubulin are shown. Colocalization between TCoB and α-tubulin is shown at a higher resolution. The right panels show the indicated region in the left panel at 300% digital magnification. The left panel was taken with a ×100 objective. Red, tubulin; green, TCoB. Bar, 10 μm.
To further characterize Pak1 interactions with TCoB, we have generated a polyclonal antibody which recognizes endogenous TCoB. The antibody specifically recognizes GST-TCoB (Fig. 1B) as well as endogenous TCoB in a wide variety of cell lines (Fig. 1C), and the specificity was confirmed by preabsorption assays with GST-TCoB protein (Fig. 1D). Using this antibody, we examined whether Pak1 interacts with TCoB in vivo by immunoprecipitation assay. TCoB and not the control immunoglobulin G (IgG) antibody specifically immunoprecipitated Pak1 (Fig. 1E).
We next examined the localization of endogenous TCoB by using the affinity-purified TCoB antibody. A significant amount of endogenous TCoB was observed to colocalize with microtubules (Fig. 1F). However, exclusive localization of TCoB at the plus ends of microtubules was not observed, indicating that the TCoB cytoskeleton-associated protein, glycine-rich (CAP-GLY) motif is not sufficient to confer the plus-end tracking activity observed with other CAP-GLY-containing proteins and that TCoB may have another microtubule-associated function outside of heterodimer assembly.
TCoB is a Pak1 physiological substrate.
Because Pak1 is a serine/threonine kinase, we examined whether TCoB is a substrate of Pak1. MCF-7 cells were metabolically labeled with [32P]orthophosphoric acid, and the status of TCoB phosphorylation was determined after treatment with physiological signals that activate Pak1, including epidermal growth factor (EGF), sphingosine, and serum (2). Results show that signals that activate Pak1 increase the phosphorylation of both endogenous TCoB (Fig. 2A) and epitope-tagged T7-TCoB (Fig. 2B).
FIG. 2.
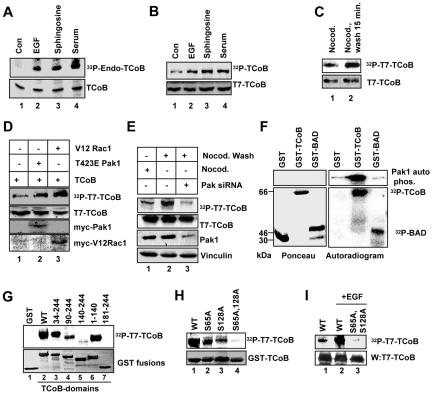
Phosphorylation of TCoB by Pak1 in vitro and in vivo. (A) In vivo phosphorylation of TCoB. MCF-7 cells were serum starved for 24 h and then labeled with [32P]orthophosphoric acid overnight. Cells were then treated with EGF (100 ng/ml), lipids (sphingosine [100 μM]), or 10% serum for 30 min. Cell lysates were immunoprecipitated with TCoB antibody, resolved on an SDS-PAGE gel, and transferred to membranes. After autoradiography, the membranes were blotted with a TCoB antibody (lower panel). Con, control. (B) MCF-7 cells were transfected with T7-tagged TCoB, serum starved, labeled with [32P]orthophosphoric acid, and treated with EGF (100 ng/ml), lipids (sphingosine [100 μM]), and serum (10%) for 30 min. Cell lysates were immunoprecipitated with T7 antibody, and phosphorylation of TCoB was analyzed by autoradiography. After autoradiography, membranes were blotted with a T7 antibody (lower panel). (C) MCF-7 cells were transfected with T7-TCoB, serum starved, labeled with [32P]orthophosphoric acid, and treated with nocodazole (nocod.) for 60 min. Nocodazole was washed out with fresh medium, and 15 min later, the cells were lysed and TCoB was immunoprecipitated with T7 antibody and autoradiographed. (D) MCF-7 cells were cotransfected with T7-TCoB along with cytomegalovirus (CMV) control vector or CMV-T423E Pak1 or CMV-V12 Rac1 plasmid. Twenty-four hours later, cells were labeled with [32P]orthophosphoric acid, and the phosphorylation status of TCoB was determined by immunoprecipitation followed by autoradiography. An aliquot of total lysate was analyzed for expression of transfected plasmids. (E) MCF-7 cells were transfected with T7-TCoB along with or without Pak1 siRNA. Cells were labeled with [32P]orthophosphoric acid and treated with nocodazole, and T7-TCoB was immunoprecipitated and autoradiographed. Knock down of Pak1 was analyzed by Western analysis of total lysate. (F) In vitro kinase assay using GST, GST-TCoB, and GST-BAD as substrates and purified human Pak1 as an enzyme. (G) In vitro Pak1 kinase assay using GST fusion of TCoB fragments containing the indicated serial deletions of TCoB. WT, wild type. (H) In vitro Pak1 kinase assay using wild-type TCoB, TCoB-S65A, TCoB-S128A, and TCoB-S65, S128A mutant as substrates. (I) Effect of EGF on in vivo phosphorylation of wild-type TCoB and the TCoB S65A, S128A mutant.
Because tubulin cofactors play a role in the polymerization of microtubules (21, 25), we examined whether TCoB is phosphorylated during microtubule regrowth by using a well-established in vivo microtubule regrowth assay (4, 28). MCF-7 cells were transfected with T7-TCoB, metabolically labeled with [32P]orthophosphoric acid, and treated with nocodazole to depolymerize existing microtubules. Microtubule regrowth was initiated by washing cells with nocodazole-free medium. Fifteen minutes later, we assessed the status of TCoB phosphorylation by immunoprecipitation. Our results showed increased TCoB phosphorylation during microtubule regrowth (Fig. 2C). Cotransfection of T7-TCoB with constitutively active Pak1 (26) or its upstream activator, active Rac1 (19), also upregulated TCoB phosphorylation (Fig. 2D).
To demonstrate the functional significance of Pak1 in the phosphorylation of TCoB during microtubule biogenesis, we selectively knocked down Pak1 with a Pak1-specific siRNA. Down-regulation of Pak1 expression substantially reduced the phosphorylation of TCoB in the nocodazole regrowth assay (Fig. 2E, compare lane 2 to lane 3). To examine whether TCoB is a direct substrate of Pak1, we performed an in vitro kinase assay using purified GST-TCoB and purified Pak1 enzyme (Fig. 2F). The protein BAD, an established Pak1 substrate (18), was used as a positive control. The results showed that the Pak1 enzyme could phosphorylate TCoB and BAD in vitro in a similar fashion. The autophosphorylation of Pak1 enzyme was substantially higher when GST-TCoB was used as a substrate than when GST alone or His-BAD was used (Fig. 2F, middle panel, compare lanes 1 and 3 with lane 2).
We next mapped the Pak1 phosphorylation sites in TCoB by using GST fusions of various TCoB domains. Deletion of TCoB N-terminal aa 1 to 33 (Fig. 2G, lane 3) or the CAP-GLY domain (aa 140 to 244) (Fig. 2G, lane 6) had no effect on the ability of Pak1 to phosphorylate TCoB. However, deletion of the N-terminal aa 1 to 89, which contain the N-terminal functional region, partially reduced the ability of Pak1 to phosphorylate TCoB (50% reduction) (Fig. 2G, lane 4). Deletion of aa 1 to 139, which contain the N-terminal functional region and part of the coiled-coil region, severely reduced the ability of Pak1 to phosphorylate TCoB (Fig. 2G, lane 5). The reduced ability of Pak1 to phosphorylate a TCoB mutant lacking aa 1 to 89 and the complete loss of Pak1 phosphorylation in a TCoB mutant lacking aa 1 to 139 suggested that Pak1 phosphorylated TCoB at at least two or more sites.
Examination of TCoB aa 1 to 140 revealed two potential Pak1 consensus phosphorylation sites, serine 65 and serine 128. Pak1 phosphorylates a number of substrates on serine/threonine residues, preferably in the context of preceding basic residues such as (K/R)(R/X)X(S/T) (2, 9). Single-point mutation of either serine to alanine partially reduced the ability of Pak1 to phosphorylate TCoB (Fig. 2H, lanes 2 and 3, respectively). Double mutation of both serines to alanine in the full-length TCoB severely reduced the phosphorylation in vitro (Fig. 2H, lane 4) and nearly abolished TCoB phosphorylation in vivo upon stimulation with EGF (Fig. 2I).
TCoB and Pak1 interaction domains.
To identify the domains of the Pak1-TCoB interaction, we examined the ability of in vitro-translated Pak1 to bind with the full-length TCoB as well as various TCoB domains expressed as GST fusion proteins. An earlier study identified three functional domains in TCoB: the N-terminal functional domain (aa 1 to 118); the central coiled-coil domain (aa 119 to 163), which is required for maintenance of cellular α-tubulin levels; and the C-terminal CAP-GLY domain (aa 164 to 244), which is required for binding of α-tubulin (16). Our results showed that wild-type TCoB interacted with Pak1 (Fig. 3A, lane 2). Deletion of parts of the N-terminal functional domain had no effect on the binding of TCoB to Pak1 (Fig. 3A, lanes 3 and 4). However, partial deletion of the central coiled-coil region abolished TCoB binding with Pak1 (Fig. 3A, lane 5). The C-terminal CAP-GLY domain alone did not show any interaction with Pak1 (Fig. 3A, lane 6). These results suggested that the Pak1-binding site in TCoB was localized in the central coiled-coil domain.
FIG. 3.
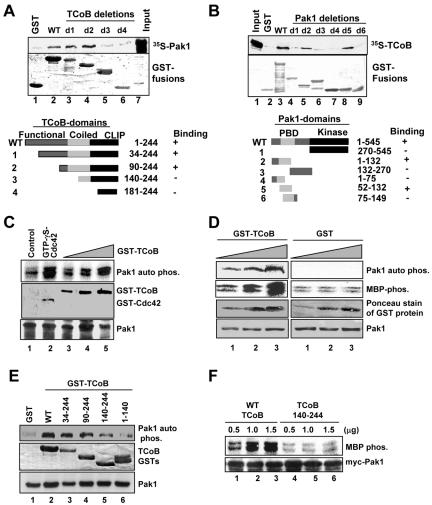
Identification of the TCoB domains that interact with Pak1. (A) Pak1 cDNA encoding aa 1 to 545 was translated in vitro. 35S-labeled Pak1 was incubated with GST-TCoB or GST fusions of various lengths of TCoB in the GST pull-down assays, and the interaction was then analyzed by SDS-PAGE followed by autoradiography. WT, wild type; CLIP, MHC class II-invariant peptide. (B) Identification of the Pak1 domain that interacts with TCoB. Wild-type GST-Pak1 (aa 1 to 545) and GST-Pak1 fusion proteins containing the kinase domain (aa 270 to 545), N-terminal domain (aa 1 to 132), PIX binding domain (aa 132 to 270), NCK binding domain (aa 1 to 75), CRIB domain (aa 52 to 132), and autoinhibitory domain (aa 75 to 149) were incubated with in vitro-translated TCoB (amino acids 1 to 244). Binding was analyzed with GST pull-down assay followed by SDS-PAGE and autoradiography. PBD, protein binding domain. (C) TCoB stimulates Pak1 activity in vitro. Pak1 enzyme was incubated with either GTP-γS-loaded Cdc42 (6.7 mM) or purified TCoB (6.7, 13.3, or 20 mM) and incubated in kinase buffer containing [γ-32P]ATP, and Pak1 autophosphorylation (auto phos.) was measured by autoradiography. (D) Pak1 enzyme was incubated with 16.7, 33.3, or 50 mM GST protein or GST-TCoB for 30 min, and the ability of Pak1 to phosphorylate MBP was analyzed by in vitro kinase reaction. Ponceau stains for GST loading and Pak1 immunoblot are shown below. (E) Ability of various TCoB domains to stimulate Pak1 activity as determined by in vitro kinase assay. (F) MCF-7 cells were cotransfected with Myc-tagged Pak1, with or without increasing amounts of wild-type TCoB or TCoB (aa 140 to 244). Cells were serum starved for 24 h, and Pak1 activity was measured by immunoprecipitation with Myc-tagged antibody followed by in vitro kinase assay using MBP as a substrate.
We next mapped the TCoB binding site in Pak1. The full-length Pak1-GST fusion protein (Fig. 3B, lane 3) but not GST alone (Fig. 3B, lane 2) efficiently interacted with the 35S-labeled full-length TCoB protein. Furthermore, the N-terminal Pak1 region (aa 1 to 132) (Fig. 3B, lane 5) and the Cdc42/Rac-interactive binding (CRIB) domain of Pak1 (aa 52 to 132) (Fig. 3B, lane 8) efficiently interacted with TCoB. However, binding was not observed with a Pak1 fragment containing aa 75 to 149 (Fig. 3B, compare lanes 8 and 9). Our results suggest that 23 amino acids (between residues 52 and 75) play an important role in the binding of Pak1 to TCoB. Comparison of these 23 amino acids among three Pak isoforms revealed differences in eight amino acids, which are localized to the N-terminal region of the CRIB domain (Pak1, 52-DRFYRSILPGD-61; Pak2, 52-HKIISIFSGT-61; and Pak3, 52-ARLRSIFPGG-61). In vitro binding assays showed no detectable interaction of Pak2 and Pak3 with TCoB (data not shown). These results suggest that even though the GTPase binding domain is conserved among type 1 Paks, the adjacent amino acids are not conserved among type 1 Paks, and therefore, the unique eight amino acids present in Pak1 may account for Pak1 specificity to TCoB.
Because the TCoB-interacting surface of Pak1 overlaps with the CRIB domain and because GTPase binding to the CRIB domain has been shown to activate Pak1 by relieving the autoinhibition of Pak1 (2), we examined whether the interaction between Pak1 and TCoB would relieve Pak1 autoinhibition and thereby increase its activity. We first compared the TCoB-mediated activation of Pak1 with a known activator of Pak1 Cdc42. Results showed that incubation of both Cdc42 and TCoB with the Pak1 enzyme activated Pak1 autophosphorylation (Fig. 3C). However, TCoB activation of Pak1 was less than that of Cdc42 in this in vitro assay, as substantially more TCoB protein was required to achieve a level of activation similar to that of Cdc42 (Fig. 3C). To examine whether the TCoB-mediated increase in autophosphorylation translates into increased Pak1 activity to exogenous substrates, we incubated GST or GST-TCoB with Pak1 enzyme and used MBP as a substrate to measure Pak1 activity. Interestingly, TCoB increased Pak1 activity against the established substrate MBP in a dose-dependent manner (Fig. 3D). Furthermore, as we expected from the data illustrated in Fig. 3C, Pak1 autophosphorylation was markedly increased (Fig. 3D, left panel). Pak1 binding to TCoB was essential, as deletion of the Pak1-binding domain in TCoB (aa 1 to 139) abolished the ability of TCoB to activate Pak1 (Fig. 3E). By exploring whether TCoB stimulates Pak1 kinase activity in vivo, we demonstrated that transient coexpression of T7-TCoB, but not a mutant TCoB lacking the Pak1-binding domain (aa 1 to 139), enhanced Pak1 kinase activity (Fig. 3F).
TCoB phosphorylation mutant blocks polymerization of new microtubules.
We next reasoned that if the TCoB-Pak1 interaction is playing a role in microtubule assembly, there should be increased association during periods of rapid microtubule assembly, such as during cell recovery from treatment with nocodazole, an established microtubule-disrupting agent. Indeed, TCoB and Pak1 were coimmunoprecipitated during this recovery phase (Fig. 4A). We then examined the association of TCoB with newly polymerized microtubules by using confocal microscopy and an established microtubule regrowth assay (4, 28) (Fig. 4B). Since newly polymerized microtubules contain tyrosinated α-tubulin, we used an antibody specific for tyrosinated tubulin (blue) as a marker of these newly polymerized microtubules. Colocalization of TCoB (red) with tyrosinated tubulin (blue) was demonstrated by the appearance of a pink color. We found that nocodazole treatment completely depolymerized microtubules (data not shown). However, 15 min after the cells were washed and free of nocodazole, we observed extensive TCoB protein localization along the growing microtubules during recovery from nocodazole (Fig. 4B, arrows). Our results revealed that endogenous TCoB localizes to newly polymerized microtubules and colocalizes with the tyrosinated α-tubulin microtubules.
FIG. 4.
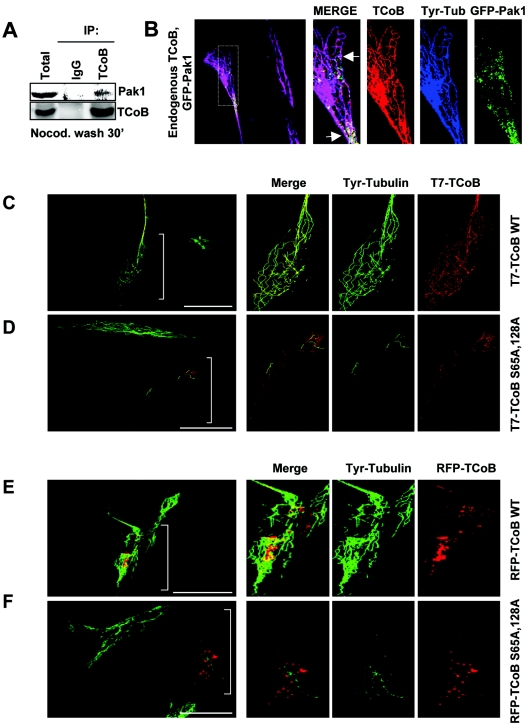
Colocalization of endogenous TCoB with tyrosinated tubulin in newly polymerized microtubules. (A) MCF-7 cells were treated with nocodazole as described above and allowed to recover for 15 min, and cells were then lysed and immunoprecipitated (IP) with either IgG or anti-TCoB. (B) IMR90 cells were treated with nocodazole as described above, fixed in methanol, and stained for TCoB (red) and tyrosinated tubulin (Tyr-Tub) (blue). The insert area is enlarged to show specific localization patterns and colocalization between TCoB and tyrosinated microtubules. (C to F) Effect of wild-type (WT) and mutant TCoB expression on microtubule dynamics. IMR90 cells were transfected with either RFP-tagged (C) or T7-tagged (E) wild-type TCoB or RFP-tagged (D) or T7-tagged (F) mutant TCoB-S65A, S128A that cannot be phosphorylated by Pak1. Cells were treated with nocodazole for 30 min and washed for 15 min, and polymerization of new microtubules was then visualized by confocal microscopy analysis using tyrosinated tubulin antibody (green) and RFP-TCoB (red) or T7-tagged antibody (red). Bar, 50 μm.
We next investigated the role of Pak1 phosphorylation sites within TCoB in microtubule regrowth by using epitope-tagged wild-type TCoB or a TCoB mutant that cannot be phosphorylated by Pak1 (TCoB S65A, S128A). Human IMR90 cells, which have a flat, extended morphology when grown in culture that facilitates examination of cytoskeletal changes, were transiently transfected with RFP-tagged wild-type or mutant TCoB, and after 48 h, the microtubules were disrupted by treating the cells with nocodazole. The cells were then washed to allow for the reformation of microtubules and were then fixed in methanol 15 min later. RFP-labeled TCoB (red) and tyrosinated α-tubulin (green) were localized by confocal microscopy. The majority of either transfected wild-type or mutant RFP-TCoB was localized along the tyrosinated tubules, while a portion seemed to converge in larger aggregates. This localization pattern was also observed for endogenous TCoB, with some cell-to-cell variation (data not shown). Microtubule regrowth was observed in wild-type RFP-TCoB-expressing cells (Fig. 4C), while 80% of the cells expressing the RFP-TCoB S65A, S128A double mutant (which lacks both Pak1 phosphorylation sites) showed attenuated microtubule regrowth compared to neighboring untransfected cells (Fig. 4D). To rule out any possible experimental artifacts resulting from the RFP tag, which can oligomerize when expressed using certain vectors that were not employed in these experiments, these experiments were repeated using wild-type and mutant T7-tagged TCoB (Fig. 4E and F). Similar to the RFP-tagged mutant TCoB, cells transfected with wild-type T7-TCoB appeared similar to neighboring untransfected cells (Fig. 4E), while cells transfected with the T7-tagged TCoB phosphorylation mutant showed attenuated synthesis of new microtubules (Fig. 4F). These results suggested that phosphorylation of TCoB at both sites was important for the regrowth of new microtubules.
Role of Pak1-TCoB interaction in polymerization of new microtubules.
To assess the significance of the endogenous TCoB and Pak1 interactions in the maintenance of cellular α-tubulin levels and polymerization of new microtubules, we have used a knock-down approach using siRNA specific to Pak1 and TCoB. TCoB-specific siRNA targeted to two different regions of the TCoB mRNA reduced the expression of endogenous TCoB mRNA (Fig. 5A) and protein (Fig. 5B) by greater than 80%, with no or minimal alterations in the α-tubulin protein levels in the cells (Fig. 5B, left panel). Similar results were obtained using siRNA targeting to a different site of TCoB (Fig. 5B, right panel). Also, knock down of Pak1 using two distinct Pak1-specific siRNAs efficiently reduced Pak1 expression but did not affect cellular α-tubulin levels (Fig. 5C). These results suggest that Pak1 and TCoB interactions are not essential for the maintenance of α-tubulin levels in human cells or that the minimal levels of TCoB present in the cells following siRNA may be enough to maintain the cellular TCoB levels. Previously published experiments with yeast also showed that TCoB knock down did not severely affect the cellular α-tubulin levels. Although tubulin-folding cofactor mutants in yeast caused an altered microtubule cytoskeleton, the defects are not clearly attributable to decreases in the steady-state levels of α- or β-tubulin (16). Also, given that in fission yeast, TCoE was shown to bypass the requirement of TCoB (15), it is possible that TCoE may perform a similar role in mammalian cells and thus may account for the maintenance of α-tubulin levels in TCoB siRNA-treated cells.
FIG. 5.
Role of Pak1 and TCoB interaction in tubulin biogenesis and polymerization of new microtubules. (A) Functionality of TCoB siRNAs was analyzed by Northern blotting. (B, C) MCF-7 cells were transfected with control (Con)-, TCoB-, or Pak1-specific siRNAs, and 48 h later, expression of TCoB and Pak1 was measured by Western analysis of total cellular lysates. The effect of TCoB- and Pak1-specific siRNA on the expression of α-tubulin was examined by Western analysis (lower panel). NS, nonspecific. (D) The ability of Pak1-specific siRNA to block constitutive and EGF-induced in vivo phosphorylation of TCoB was examined in untransfected and T7-TCoB-transfected, 32P-labeled MCF-7 cells. WB, Western blot. (E) The ability of TCoB-specific and Pak1-specific siRNA to block endogenous protein expression was examined in IMR90 cells. (F) Effect of TCoB-, Pak1-, and Pak2-specific siRNAs on polymerization of new microtubules. IMR90 cells were cotransfected with fluorescently labeled nonspecific siRNA (blue) together with specific siRNAs (1:10 ratio), and cells were then treated with nocodazole as described above. Microtubule polymerization was visualized by confocal microscopy using a tyrosine tubulin antibody (green) and an antibody specific to endogenous TCoB (red). Pak2 siRNA and nonspecific siRNA were used as negative controls. Bar, 50 μm.
In order to determine if Pak1 siRNA would affect constitutive or signaling-dependent TCoB phosphorylation, vector-transfected or T7-TCoB-transfected MCF-7 cells were cotransfected with Pak1-specific siRNA, serum starved, and then stimulated with EGF to induce Pak1 activity and TCoB phosphorylation. Results shown in Fig. 5D indicate that blocked Pak1 protein expression effectively reduced constitutive and EGF-induced phosphorylation of both endogenous and transfected TCoB. These results emphasize the importance of Pak1 in regulating TCoB phosphorylation and potentially its functions in regulating new microtubule formation.
We next examined the effect of the TCoB and Pak1 knock down on the polymerization of new microtubules. In order to verify expression of TCoB and Pak1 in IMR90 cells and the effectiveness of specific siRNAs in these cells, we transfected either TCoB-specific or Pak1-specific siRNA and examined target protein expression by Western blot (Fig. 5E). Results show that endogenous TCoB and Pak1 expression in IMR90 cells was effectively reduced by transfecting specific siRNA.
Using IMR90 cells as a model system for microtubule changes, cells were transfected separately with Pak1 and TCoB siRNA to validate our biochemical results. Pak2 siRNA and nonspecific control siRNA were used as controls. Pak2 expression in IMR90 cells was validated by Western blot (data not shown). All transfected cells appeared to be viable, with no apparent morphological apoptotic changes either before or after nocodazole treatment (data not shown). Knock down of either TCoB or Pak1 severely reduced the formation of new microtubules (Fig. 5F) in the nocodazole-based microtubule biogenesis assay described above. More than 70% of cells (n = 100) transfected with siRNA against TCoB showed reduced regrowth of microtubules compared with untransfected neighboring cells 15 min after nocodazole removal (Fig. 5F, upper left panel). Similarly, Pak1 knock down also reduced microtubule regrowth in the transfected cells in the nocodazole treatment assay (Fig. 5F, upper right panel). Knock down with control, nonspecific siRNA (Fig. 5F, lower left panel) or knock down of Pak2 by Pak2-specific siRNA (Fig. 5F, lower right panel) did not show any apparent impact on the ability of endogenous TCoB to promote microtubule formation or on TCoB colocalization with newly formed microtubules. Thus, our findings indicated that both Pak1 and TCoB were critical molecules for the generation of new microtubules and that Pak1 phosphorylation of TCoB might be essential for normal function of TCoB.
Role of TCoB in mitotic spindle dynamics.
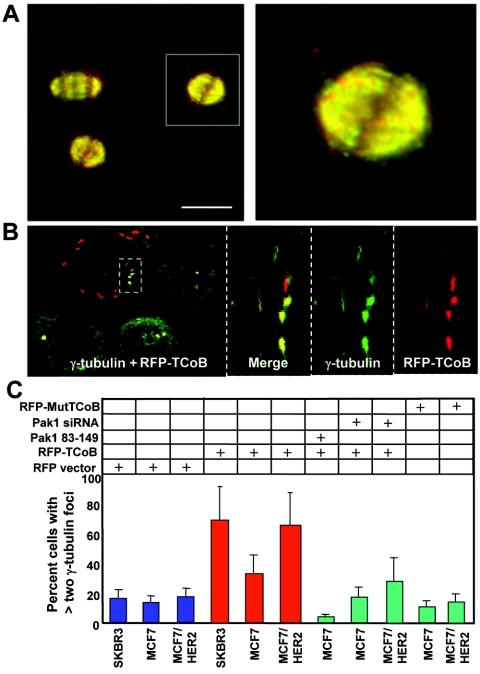
During colocalization studies with endogenous TCoB antibody, we observed colocalization between TCoB and tubulin during interphase and also during mitosis, where TCoB association with staining of the mitotic spindle was prominent (Fig. 6A). Because TCoB is a substrate of Pak1 and since TCoB overexpression increases Pak1 activity (Fig. 3C to F), we examined whether TCoB overexpression could mimic the Pak1 phenotypes (i.e., abnormalities in mitotic spindles) that we had previously shown to be the direct result of Pak1 overexpression (26). Human IMR90 cells were transfected with RFP-TCoB (red) and stained for γ-tubulin (green) as a marker of the centrosomes (Fig. 6B). Thirty percent of the RFP-TCoB-transfected cells exhibited more than two γ-tubulin-containing MTOCs (data not shown). We validated these findings in several mammary epithelial model cell lines (MCF-7, SKBR3, and MCF-7 overexpressing HER2). Overexpression of RFP-TCoB resulted in the appearance of 70% of the transfected SKBR3 cells with more than two MTOCs (Fig. 6C) and in 30% of transfected MCF-7 cells with more than two MTOCs (Fig. 6C). Because SKBR3 cells are known to naturally overexpress HER2, and HER2 signaling is known to activate Pak1 (9), we hypothesized that hyperactivation of Pak1 in SKBR3 cells may contribute to the TCoB-induced increase in the number of MTOCs. Indeed, transfection of RFP-TCoB into MCF-7 clones overexpressing HER2 increased the number of MTOCs to levels similar to those observed in SKBR3 cells (Fig. 6C). Transfection of the RFP-TCoB S65A, S128A mutant that cannot be phosphorylated by Pak1 did not induce multiple MTOCs (Fig. 6C). Furthermore, cotransfection of the Pak1 autoinhibitory fragment (Pak1 amino acids 83 to 149) or Pak1 siRNA along with RFP-TCoB abolished the ability of TCoB to induce multiple MTOCs in MCF-7 cells (Fig. 6C), suggesting that the presence of more than two γ-tubulin dots may be due in part to a failure in cytokinesis in addition to other mitotic events such as centrosome duplication.
FIG. 6.
(A) Colocalization of TCoB with tubulin was observed during mitosis. Methanol-fixed Vero cells probed with antibodies against TCoB and α-tubulin are shown. The right panel shows the indicated region of the left panel at 300% digital magnification. The left panel was acquired with a ×63 objective. Bar, 10 μM. (B) SKBR3 cells were transfected with RFP-TCoB (red), and MTOCs were analyzed by confocal microscopy using antibody against γ-tubulin staining (green). (C) Breast cancer MCF-7, SKBR3, and MCF-7 cells stably overexpressing HER2 were transfected with different TCoB and Pak1 plasmids or with TCoB- or Pak1-specific siRNA as indicated, and MTOCs were analyzed by γ-tubulin staining. Only quantitation of MTOCs in RFP-TCoB-expressing breast cancer cells was shown.
TCoB expression is deregulated in breast tumors.
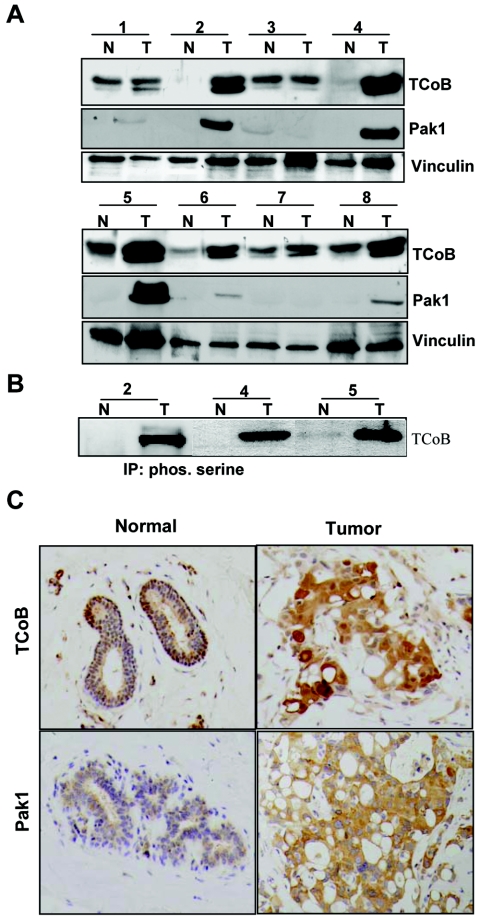
In cancer cells, it has been suggested that any alteration of centrosome homeostasis may lead to centrosome amplification and abnormal multipolar mitoses and consequently chromosome missegregation, causing chromosomal instability and phenotypic diversity. Such changes may help tumor progression, metastasis, and chemoresistance (8, 17). Since both Pak1 (10) and TCoB localized to centrosomes (Fig. 6), and because Pak1 expression is known to be deregulated in breast cancer specimens (26), we examined whether TCoB is overexpressed in breast tumor specimens. Examination of TCoB protein levels in eight paired samples of human breast tumors and adjacent normal tissue showed increased expression of TCoB in five out of eight tumors compared to that in normal tissues (Fig. 7A). TCoB-overexpressing tumors also showed increased expression of Pak1. By immunoprecipitation of human tissue lysates using a phosphoserine-specific antibody followed by Western blot analysis for endogenous TCoB, we found that TCoB is indeed phosphorylated on serine residues in human breast tumors (Fig. 7B). Immunohistochemical examination of representative tumors expressing both TCoB and Pak1 revealed intense cytoplasmic and nuclear TCoB staining and strong cytoplasmic Pak1 staining (Fig. 7C).
FIG. 7.
(A) Breast tumor lysates were analyzed for TCoB expression by Western analysis, and the blots were subsequently reprobed with Pak1 and vinculin antibodies. (B) Total lysates from paired normal (N) and tumor (T) tissue were immunoprecipitated (IP) by using phosphoserine (phos. serine) antibody and Western blotted with endogenous TCoB antibody. (C) Representative paraffin-embedded tissue sections from paired normal and tumor tissue (sample 5 from panel A) were analyzed by immunostaining with antibodies against TCoB and Pak1.
Multiple spindles are widely observed in breast cancer specimens. Members of the EGF receptor family, notably EGFR and HER2, are frequently overexpressed in breast tumors, and overexpression of EGFR/HER2 receptors could lead to centrosome amplification (12, 20). Pak1 is activated by a number of growth factors including EGF and may be deregulated in breast tumors (9). We report here that the coordinate deregulation of TCoB and Pak1 and phosphorylation of TCoB was observed in human breast cancer specimens. Furthermore, both a Pak1 dominant mutant and Pak1 siRNA reduced the ability of TCoB overexpression to promote multiple spindles in cell culture assays. Thus, deregulation of TCoB and Pak1 may contribute to the multiple-spindle phenotype seen in human breast tumors.
In conclusion, this report identifies for the first time TCoB as a Pak1-interacting substrate and provides new insight into how Pak1 and TCoB influence microtubule biogenesis. Our results suggest that Pak1 phosphorylates TCoB and that such phosphorylation has a role in the growth of new microtubules. Furthermore, these newly identified roles for TCoB and Pak1 in microtubule dynamics and normal cell function may be coordinately deregulated in human cancers.
Acknowledgments
This work was supported by the U.S. National Institutes of Health grants CA90970, CA80066, and P50CA97007 (to R.K.). H.V.G. was supported in part by the American Heart Association.
We thank Liana Adam for the γ-tubulin confocal microscopy and Sally A. Lewis, New York University Medical Center, for providing cofactor B pet23d vector.
REFERENCES
- 1.Banerjee, M., D. Worth, D. Prowse, and M. Nikolic. 2002. Pak1 phosphorylation on T212 affects microtubules in cells undergoing mitosis. Curr. Biol. 12:1233-1239. [DOI] [PubMed] [Google Scholar]
- 2.Bokoch, G. M. 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72:743-781. [DOI] [PubMed] [Google Scholar]
- 3.Cassimeris, L. 1999. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol. 11:134-141. [DOI] [PubMed] [Google Scholar]
- 4.Cau, J., S. Faure, M. Comps, C. Delsert, and N. Morin. 2001. A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule stabilization. J. Cell Biol. 155:1029-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daub, H. F., K. F. Gevaert, J. F. Vandekerckhove, A. F. Sobel, and A. Hall. 2001. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine. J. Biol. Chem. 276:1677-1680. [DOI] [PubMed] [Google Scholar]
- 6.Desai, A., and T. J. Mitchison. 1997. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13:83-117. [DOI] [PubMed] [Google Scholar]
- 7.Jordan, M. A., and L. Wilson. 1998. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 10:123-130. [DOI] [PubMed] [Google Scholar]
- 8.Kramer, A. F., K. F. Neben, and A. D. Ho. 2002. Centrosome replication, genomic instability and cancer. Leukemia 16:767-775. [DOI] [PubMed] [Google Scholar]
- 9.Kumar, R., and R. K. Vadlamudi. 2002. Emerging functions of p21-activated kinases in human cancer cells. J. Cell. Physiol. 193:133-144. [DOI] [PubMed] [Google Scholar]
- 10.Li, F., L. Adam, R. K. Vadlamudi, H. Zhou, H. F. Zhou, S. Sen, J. Chernoff, M. Mandal, and R. Kumar. 2002. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 3:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Fanarraga, M., J. Avila, A. Guasch, M. Coll, and J. C. Zabala. 2001. Postchaperonin tubulin folding cofactors and their role in microtubule dynamics. J. Struct. Biol. 135:219-229. [DOI] [PubMed] [Google Scholar]
- 12.Montagna, C. F., E. R. Andrechek, H. F. Padilla-Nash, W. J. Muller, and T. Ried. 2002. Centrosome abnormalities, recurring deletions of chromosome 4, and genomic amplification of HER2/neu define mouse mammary gland adenocarcinomas induced by mutant HER2/neu. Oncogene 21:890-898. [DOI] [PubMed] [Google Scholar]
- 13.Ottilie, S., P. J. Miller, D. F. Johnson, C. L. Creasy, M. A. Sells, S. Bagrodia, S. L. Forsburg, and J. Chernoff. 1995. Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO J. 14:5908-5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qyang, Y., P. Yang, H. Du, H. Lai, H. Kim, and S. Marcus. 2002. The p21-activated kinase, Shk1, is required for proper regulation of microtubule dynamics in the fission yeast, Schizosaccharomyces pombe. Mol. Microbiol. 44:325-334. [DOI] [PubMed] [Google Scholar]
- 15.Radcliffe, P. A., D. Hirata, L. Vardy, and T. Toda. 1999. Functional dissection and hierarchy of tubulin-folding cofactor homologues in fission yeast. Mol. Biol. Cell 10:2987-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radcliffe, P. A., and T. Toda. 2000. Characterization of fission yeast alp11 mutants defines three functional domains within tubulin-folding cofactor B. Mol. Gen. Genet. 263:752-760. [DOI] [PubMed] [Google Scholar]
- 17.Salisbury, J. L. 1995. Centrin, centrosomes, and mitotic spindle poles. Curr. Opin. Cell Biol. 7:39-45. [DOI] [PubMed] [Google Scholar]
- 18.Schürmann, A., A. F. Mooney, L. C. Sanders, M. A. Sells, H. G. Wang, J. C. Reed, and G. M. Bokoch. 2000. p21-activated kinase 1 phosphorylates the death agonist Bad and protects cells from apoptosis. Mol. Cell. Biol. 20:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sells, M. A., A. Pfaff, and J. Chernoff. 2000. Temporal and spatial distribution of activated Pak1 in fibroblasts. J. Cell Biol. 151:1449-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherline, P. F., and R. Mascardo. 1982. Epidermal growth factor-induced centrosomal separation: mechanism and relationship to mitogenesis. J. Cell Biol. 95:316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szymanski, D. 2002. Tubulin folding cofactors: half a dozen for a dimer. Curr. Biol. 19:R767-R769. [DOI] [PubMed] [Google Scholar]
- 22.Thiel, D. A., M. Reeder, A. F. Pfaff, T. R. Coleman, M. A. Sells, and J. Chernoff. 2002. Cell cycle-regulated phosphorylation of p21-activated kinase 1. Curr. Biol. 12:1227-1232. [DOI] [PubMed] [Google Scholar]
- 23.Tian, G., A. Bhamidipati, N. J. Cowan, and S. A. Lewis. 1999. Tubulin folding cofactors as GTPase-activating proteins. GTP hydrolysis and the assembly of the alpha/beta-tubulin heterodimer. J. Biol. Chem. 274:24054-24058. [DOI] [PubMed] [Google Scholar]
- 24.Tian, G., Y. Huang, H. Rommelaere, J. Vandekerckhove, C. Ampe, and N. J. Cowan. 1996. Pathway leading to correctly folded beta-tubulin. Cell 86:287-296. [DOI] [PubMed] [Google Scholar]
- 25.Tian, G., S. A. Lewis, B. Feierbach, T. Stearns, H. Rommelaere, C. Ampe, and N. J. Cowan. 1997. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J. Cell Biol. 138:821-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vadlamudi, R. K., L. Adam, R. A. Wang, M. Mandal, D. Nguyen, A. Sahin, J. Chernoff, M. C. Hung, and R. Kumar. 2000. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J. Biol. Chem. 275:36238-36244. [DOI] [PubMed] [Google Scholar]
- 27.Vadlamudi, R. K., F. Li, L. Adam, D. Nguyen, Y. Ohta, T. P. Stossel, and R. Kumar. 2002. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat. Cell Biol. 4:681-690. [DOI] [PubMed] [Google Scholar]
- 28.Waterman-Storer, C. M., R. A. Worthylake, B. P. Liu, K. F. Burridge, and E. D. Salmon. 1999. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1:45-50. [DOI] [PubMed] [Google Scholar]