Abstract
Early in solid tumor development, antigens are presented in tumor-draining lymph nodes (tdLN), a process which is required to set up immune surveillance. Recent evidence indicates that tdLNs fuel systemic tumor-specific T cell responses, which may halt cancer progression and facilitate future responses to immunotherapy. These protective responses, however, are subject to progressive dysfunction exacerbated by LN metastasis. Here, we discuss emerging preclinical and clinical literature that indicates that the tdLN is a critical reservoir for systemic immunity that can potentiate immune surveillance. We discuss the impact of lymph node metastasis and argue that a better understanding of the relationship between lymph node metastasis and systemic immunity is necessary to direct regional disease management in the era of immunotherapy.
Keywords: Metastasis, immune surveillance, lymph node, immunotherapy
Introduction
Solid tumor progression is often associated with regional lymphatic dissemination that can precede emergence of distant, visceral metastases. The sometimes-sequential nature of tumor metastasis, from locoregional to systemic disease, motivates the early resection of the primary tumor and regional (lymph node, LN) disease to minimize future spread. Consequently, identification of tumor cells in the sentinel LN (SLN), the first tumor-draining lymph node (tdLN) identified in patients by lymphoscintigraphy at the time of wide excision, is a significant negative prognostic factor [1]. Consistent with sequential metastasis, preclinical models provide direct evidence that tumor cells in LNs are capable of accessing the hematogenous vasculature and disseminating systemically [2,3]. Recent clinical trials in melanoma and breast cancer, however, indicate that immediate complete LN dissection following positive SLN biopsy does not extend disease-free survival over observation [4,5] and the therapeutic value of even SLN biopsy remains hotly debated [6]. These data present at least two non-mutually exclusive possibilities: 1) that even at early-stages, tumors are already disseminated systemically, or 2) that LN metastasis may have a negative impact on systemic outcomes independent of its role as a depot for future metastasis. In both cases, hematogenous and lymphogenous metastasis may happen in parallel. Indeed, in colorectal [7] and breast cancer [8], the majority of patients’ distant and LN metastases do not share a common clonal origin but rather derive separately from distinct clones in the primary.
That LN metastasis might have a negative impact on systemic outcomes independent of sequential metastasis supports an emerging hypothesis that LN metastasis promotes immune suppression in tdLNs that subsequently impacts systemic immune surveillance and facilitates hematogenous spread [9]. This is consistent with a long history of evidence describing the progressive expansion of suppressive leukocyte populations within tdLNs over time and with the development of LN metastasis [10]. Despite evidence for local immune suppression, however, tdLNs are also potent sources of anti-tumor immunity and therefore potential targets for successful immunotherapy [11–14]. Understanding best how to leverage the protective potential of tdLNs in patients will depend on generating mechanistic insight into the immune potential of tdLNs over time, their contribution to systemic responses, and the consequences of LN metastasis for local and systemic immune function. Here, we review the role of the tdLN as the primary site of tumor antigen presentation, outline recent data supporting a role for tdLNs in systemic response to immunotherapy, and explore the emerging understanding for how LN metastasis impacts both local and systemic immune surveillance.
Antigen presentation and immune surveillance
LNs are secondary lymphoid organs found throughout the body that serve as critical coordinating points for immune surveillance where naïve and memory T and B cells home to LNs to survey for cognate antigen. Antigen reaches the LN through a network of lymphatic vessels that unidirectionally drain fluid, cells, and soluble factors from peripheral tissues to LNs to be filtered before returning to the blood. Lymphatic transport is therefore required to initiate peripheral tissue immune responses as the route for the active migration of antigen presenting cells (APC) and transport of soluble antigen [15]. Though lymphatic vessels may be remodeled during tumor development through a process termed lymphangiogenesis, they remain the critical route for antigen presentation and thereby necessary for the induction of anti-tumor adaptive immune responses and enhancement of local inflammation [16,17] (see Box 1). Tumor-associated lymphangiogenesis, driven in large part by the overexpression of vascular endothelial growth factor C, is sufficient to enhance APC migration and subsequent CD8+ T cell priming, thereby improving response to immunotherapy [18–20].
BOX: A Lymphatic Niche?
Stromal cells that form the lymph node (LN) scaffold are active players in regulating the position, survival, and in some cases, the function, of local leukocytes. The lymphatic endothelium, in particular, lines the subcapsular sinus (SCS), interfollicular zones, and medulla of the LN, creating distinct anatomical niches that govern the influx and efflux of peripheral tissue material [90]. Indeed, the SCS is a point of convergence for lymph-borne antigen, dendritic cells (DCs), effector [91] and stem-like T cells [60,92], and metastasizing tumor cells. More than a simple structural scaffold, lymphatic endothelial cells (LECs) themselves exhibit intrinsic immunologic function, including the scavenging of exogenous antigen for either cross-presentation [18,93,94] or long-term archiving [95]; the regulated release of nitric oxide to control local T cell proliferation [96]; inhibition of DC maturation [97]; and maintenance of peripheral tolerance at least in part through high constitutive expression of PD-L1 [98]. In fact, LECs have the highest expression of PD-L1 in the LN stroma and express at least as much, if not more, than migratory DCs [99]. Indeed, the majority of T cells primed by LECs undergo apoptosis [18,93,94], however, a subset persist to memory and exhibit features of central and stem-like memory [94]. It is, therefore, intriguing to speculate that the lymphatic endothelium, which expands in response to tumor-drainage and inflammation [10], may provide an anatomical and functional niche that contributes to the persistence of stem-like, resident, and or effector T cell populations residing within the LN. The remodeling of this lymphatic niche by tumor cell arrival may therefore disrupt the necessary signals that maintain quiescence and long-term function.
The active transport of antigen to tdLNs for presentation to T cells depends on APCs expressing the CC motif chemokine receptor 7 (CCR7), which is necessary for migration via lymphatic vessels, both at steady state and in response to inflammation [21,22]. Presentation of tumor-derived antigens to CD8+ T cells is mediated by conventional dendritic cells (cDCs) that can be migratory or tissue-resident and may be capable of cross-presenting antigen. Mice lacking cross-presenting cDCs (cDC1s) fail to activate CD8+ T cells and respond to immunotherapy [23,24]. In tumors, cDC1s, which are critical for CD8 T cell priming, seem to function simultaneously to support both CD8+ and major histocompatibility complex Class II (MHC-II) -dependent CD4+ T cell priming [25], which further licenses cDC1s for optimal CD8+ T cell priming. Migratory CD103+ and CD11b+ DCs can transfer antigen to resident CD8α+ cDC1s in tdLNs [26], as in non-malignant settings [27], and in so doing also transfer contextual cues from the upstream tumor [28]. Further mechanisms of antigen presentation [29] and the multifaceted roles of dendritic cells (DCs) [30] in cancer have both been recently reviewed.
Despite their potential to mobilize tumor-specific immune responses, migratory DCs are often immune-suppressed in the tumor microenvironment; they acquire immune regulatory programs [31], express inhibitory molecules such as programmed cell death ligand 1 (PD-L1) [12,32,33] and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) [34,35], and decrease in number over time [36], all of which ultimately limits CD8+ T cell expansion and function. Reduced numbers of DCs and reduced expression of activation markers (CD40, CD83, CD86), is already evident in SLNs prior to the formation of LN metastasis in breast cancer patients [37,38], although the extent to which this is seen in other solid tumor types may vary [39]. Ultimately, these changes in the antigen presentation interface results in suboptimal priming of CD8+ T cells [40,41] that is imprinted rapidly during priming [42]. Instead of acquiring a transcriptomic and epigenetic profile consistent with effector function, CD8+ T cells primed in tdLNs express low levels of effector molecules and maintain markers of naïve or memory T cells, such as T cell factor 1 (TCF-1), which antagonizes the effector program [43], especially in poorly immunogenic tumors that express low levels of MHC class I [44]. This distinct mode of priming supports the formation of a stem-like CD8+ T cell (TSC, TCF-1+TIM3−) state [40] (also called progenitor exhausted). TSC can self-renew [45] or give rise to differentiated cytotoxic effector T cells by interacting through the CXCL9/CXCL10-CXCR3 axis with MHCIhigh CCR7neg cDC1s in the tumor stroma [46], and through chronic antigen stimulation become terminally exhausted T cells [43]. Although TSC can be found in the tumor microenvironment, they are often enriched in the tdLN in preclinical models of lung, melanoma and prostate cancer [13,36,40,47], which may help to maintain their progenitor state through protection from chronic antigen-induced dysfunction, and represent a critical mechanism for maintaining immune responses under the pressures of persistent antigen. In tdLN, TSC retention depends in part on transforming growth factor β (TGFβ), where TGFβ signaling both seems to restrain differentiation by suppressing the effector program and promote nodal residence by inducing CD103 expression on T cells [48]. While there is some evidence that the specific state of newly primed CD8+ T cells may differ by anatomical location of the tdLN [49] as a consequence of changes in baseline cytokines and effector populations [50], a comprehensive analysis of how anatomically distinct LN microenvironments shape immune potential remains to be carefully investigated.
Systemic immune surveillance
The potential for active tumor-associated antigen presentation in the tdLN, even if suboptimal, supports a reasonable hypothesis that tdLNs might be important for the reinvigoration of anti-tumor immune surveillance in the setting of immunotherapy (Figure 1). Lymphadenectomy at the time of primary resection in mice suppresses CD8+ T cell mediated systemic immune surveillance at distant sites [51] and reduces the efficacy of ICB [14,47]. Consistent with surgical removal, use of the sphingosine-1-phosphate receptor 1 (S1PR1) agonist FTY720 to limit de novo recruitment of lymphocytes from LNs during ongoing immunotherapy blunts responses to ICB [13,52] and the mobilization of interleukin (IL)-7-dependent, memory responses capable of preventing tumor outgrowth upon rechallenge [53]. TdLNs give rise to an expanded pool of fully differentiated anti-tumor T cells in response to programmed cell death protein-1 (PD-1)/PD-L1 blockade [12,14] that become effector cells responsible for mediating tumor control [54–56]. In particular, TSC in tumors depend on constant seeding from the tdLN [13,36,40] since antigen encounter in tumors drives local differentiation, residence, and exhaustion [40,57,58], while suboptimal antigen encounter supports TSC enrichment [59] and enables their egress and trafficking back to the tdLN [57,60]. Importantly, consistent with these circulation patterns, TSC in human tdLNs are clonally related to tumor-infiltrating, exhausted lymphocytes in lung [61], kidney, prostate, bladder cancer [40], hepatocellular carcinoma [47] and head and neck squamous cell carcinoma (HNSCC) [62]. Consistent with all of these data, clonal tracking of CD8+ T cells in human basal and squamous cell carcinoma demonstrated clonal replacement of CD8+ T cells in the primary tumor following anti-PD-1 therapy [63]. Based on these observations, targeting the tdLN could be advantageous for the efficacy of immunotherapy, and indeed pre-clinical ICB targeting the tdLN [11,12,64] drives effective tumor control and exhibits improved responses over systemic administration. Most recently, clinical trials demonstrate that ICB delivered in the neoadjuvant setting, prior to surgical resection, improves overall survival relative to administration in the adjuvant, post-resection, setting in high risk Stage II-IV resectable melanoma [65,66], with similar results reported for breast and lung carcinoma [67]. These data all together point toward the potential therapeutic value of an intact nodal basin for systemic immune surveillance.
Figure 1. The tumor-draining lymph node supports systemic anti-tumor immune surveillance.
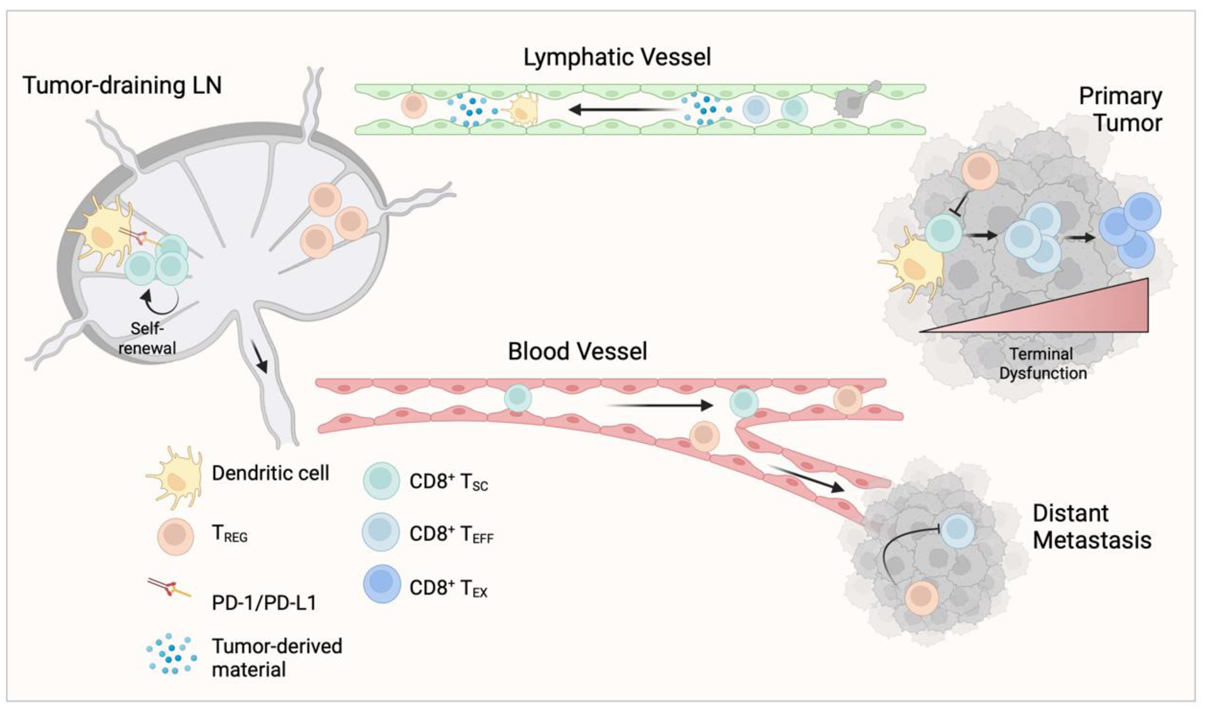
The afferent lymphatic vasculature transports tumor-derived material (e.g. antigens), leukocytes, and tumor cells from the primary tumor to the tumor draining lymph node (tdLN). Dendritic cells in the tdLN express PD-L1 and exhibit a more immature phenotype, resulting in suboptimal priming of CD8+ T cells, characterized by a failure to activate full effector function. Further contributing to these dysfunctional CD8+ T cell states, regulatory T cells (TREG) expand in tdLNs, even before metastasis, and home to sites of tumor where they inhibit effector function. Tumor-primed CD8+ T cells retain a more stem-like phenotype (TSC), self-renew, and circulate from the tdLN through the blood to sites of tumor, particularly in response to immunotherapy, where they are necessary to seed the intratumoral T cell repertoire. Within tumor, a second antigen encounter provided by local antigen presenting cells induce TSC differentiation to an effector (TEFF) and then towards terminal differentiation and exhaustion (TEX). CD8+ T cells that remain in the TSC fate or receive only weak antigen stimulation also egress the tumor to return to the tdLN.
Interestingly, however, early clinical data emerging from neoadjuvant trials indicates that LN metastasis may limit response to neoadjuvant ICB. In resectable non-small cell lung cancer (NSCLC), the presence of LN metastasis was associated with poor response to neoadjuvant ICB and shorter regression-free survival [68]. Consistent with the impact of the tdLN on emerging systemic responses, these clinical data indicate that metastasis may further disrupt the tumor-specific immune response. Indeed, flow cytometric profiling of DCs from SLNs of melanoma patients revealed a decrease in the number of mature LN DCs in metastatic SLN as compared to non-metastatic SLN [39]. Furthermore, the increase in diameter of the metastatic melanoma lesions correlated with progressive reduction of CD83 expression, a marker of mature DCs that stabilizes MHC Class II surface expression [39]. The decline in CD83 on DCs was also observed in metastatic LNs from breast cancer patients [37]. Consistent with the changes in APC maturation, CD8+ T cells in metastatic SLN exhibit signs of reduced activation and function. Compared to non-metastatic SLN, CD8+ T cells from metastatic SLN have reduced expression of the costimulatory protein inducible T cell co-stimulator (ICOS) and human leukocyte antigen (HLA)-DR, markers of T cell activation [37]. Further, scRNAseq performed on SLNs from melanoma [69] and HNSCC [62] patients showed increased exhaustion profiles in CD8+ T cells and a decrease in stem-like CD8+ T cells in metastatic LNs. Consistent with their transcriptional program, CD8+ T cells isolated from melanoma tdLN and activated ex vivo demonstrated reduced effector potential and increased expression of checkpoint molecules [69]. In HNSCC, exhausted T cells colocalized with DCs expressing increased regulatory molecules (CD39, indoleamine 2,3-dioxygenase (IDO), TIM3, PD-L1), TREG expressing higher suppressive markers (TIM3, CD39), and naïve CD4+ T cells expressing elevated TCF-1, suggestive of an immunosuppressive niche unique to the metastatic LN [62]. Furthermore, HNSCC patients with LN metastasis demonstrated reduced expansion of circulating CD8+ T cells in response to ICB [62]. This is all consistent with mouse models, where despite the fact that increased tumor-associated lymphangiogenesis can boost DC transport and T cell priming [19,20], the concomitant seeding of LN metastases appears to generate a less functional CD8+ T cell response over time [18].
One of the most consistent observations across preclinical models and in patient LN biospecimens is the expansion and accumulation of TREG, which promote an immunosuppressive microenvironment and may further alter CD8+ T cell responses [9,37,39,70–73]. Single cell profiling of human breast cancer metastatic LN [72] and an orthotopic murine model of breast cancer with spontaneous LN metastasis [71] both found expansion of LN TREG upon metastasis. TREG isolated from metastatic LNs were also more proliferative and expressed higher levels of the costimulatory markers ICOS, glucocorticoid-induced TNFR-related protein (GITR), and OX-40 and high levels of checkpoint molecules PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [71,72]. Activated TREG can directly suppress the effector T cell response, and the expression of checkpoint molecules by TREG may indicate that ICB may activate these populations in addition to the effector CD8+ response [74]. Plasmacytoid DCs were initially implicated in the adaptive induction of LN TREG through expression of indoleamine 2,3-dioxygenase [75], but more recently, TREG expansion was also linked to the establishment of LN metastasis [9,72] and even suggested to be a direct result of elevated MHC Class II expression on breast cancer cells that metastasize to LNs [71]. Importantly, this expansion of TREG in LNs may directly impact the development of distant metastasis. Profiling of human metastatic melanoma tdLN by Nanostring whole-slide gene expression and multiplex imaging correlated increased forkhead box P3 (FOXP3) expression, indicative of TREG expansion, with higher rates of distant recurrence within 24 months of primary resection [76]. In an implanted model of melanoma that spontaneously metastasized to the tdLN, the presence of tumor cells in the tdLN induced an expansion of TREG in the tdLN. Adoptive transfer and antibody depletion experiments established that these TREG, once in circulation, home back to sites of tumor and are sufficient to generate an antigen-specific state of immune suppression that promotes primary tumor growth [73] and experimental lung metastases [9]. These data indicate that local changes in tdLNs as a result of metastasis may be sufficient to alter systemic immune surveillance.
Locoregional tumor control
Given the impact of LN metastasis on the tdLN immune repertoire, and consequently systemic immune surveillance, it is reasonable to predict that mechanisms that protect tdLNs from metastasis could have a significant impact on patient outcome and may provide new targets for the protection or reinvigoration of systemic immune responses. While little is known about the active mechanisms that limit the formation of LN metastasis, it is evident that tumor cells must adapt to the unique microenvironment of lymphoid tissue [77]. Two recent manuscripts, in particular, associate preclinical LN metastasis with acquisition of an interferon (IFN) signature [9,71], which may reflect an adaptive response to local cytotoxicity. While other tdLN populations, for example the subcapsular sinus macrophage and others (reviewed [10]), contribute to locoregional control, we focus here on two recently appreciated cytotoxic populations, lymph node resident T cells (TRM) and natural killer (NK) cells, that may represent an important protective barrier to regional dissemination within the LN.
Lymph node resident memory T cells
Resident memory T cells (TRM) are a distinct subset of non-circulating memory T cells, typically defined by their high expression of CD69 and CD103 and relatively low expression of CD62L and S1PR1 when compared to their circulating counterparts. TRM patrol previously infected barrier tissues and provide localized protection against reinfection either through direct cytolytic activity or by orchestrating the recruitment of innate and adaptive immune cells (reviewed in [78]). TRM are capable of protecting barrier tissue from tumor recurrence in preclinical models [79,80]. Tumor infiltrating lymphocytes with a resident-like phenotype are seen in many solid tumors and associated with improved outcome in breast [81] and hepatitis B virus-associated hepatocellular carcinoma [82]. In addition to barrier tissues, however, TRM also form in secondary lymphoid organs following acute viral infections [83], where they may be able to respond rapidly to reencounter with pathogen. The potential for anti-tumor immune surveillance to establish a LN resident population raises the interesting possibility that there might be at least a temporary barrier to LN infiltration and metastasis. In a model of melanoma-induced vitiligo, in addition to forming broadly in skin [80], TRM cells developed in LNs and persisted over time [84]. Consistent with the preclinical data, LN TRM were also identified in melanoma patients and exhibited evidence of clonal expansion, hinting at likely tumor-specificity [84]. Importantly, LN TRM offered local protection upon experimental melanoma rechallenge and a high LN TRM score stratified for longer survival of nearly 48 months in human metastatic LNs [84]. These data provide the first evidence that a TRM population generated in LNs during tumor development could provide a protective, local mechanism to interrupt regional metastasis.
Natural Killer cells
Natural Killer (NK) cells are an essential component of tumor immune surveillance, and therapies to enhance and restore NK cell mediated cytotoxicity continue to be developed [85]. NK cells are located in the LN medulla and paracortex, are recruited from blood, and form long-lasting interactions with DCs and are potent secretors of the cytokine IFNγ [86], which may also promote the cytotoxicity of T cells in the paracortex. NK cells identify target cells that lack of MHC Class I, triggering NK cell-mediated lysis. NK cell surveillance is implicated in preventing hematogenous metastasis to visceral organs [87] and reactivation of cancer cells from dormancy [88]. NK cells may also, however, directly limit LN metastasis [9,70,89]. In spontaneous models of LN metastasis, NK cells are recruited to inflamed tdLNs in a L-selectin-dependent manner, where they reduce both the incidence and overall burden of LN metastasis in preclinical melanoma [89]. Interestingly, the progressive adaptation of melanoma cell lines for LN tropism revealed upregulation of MHC Class I as an essential modification to permit tumor cell seeding of the LN [9], which may indicate that evasion of NK-mediated surveillance is a critical first step to metastasis. Interestingly, the expansion of TREG in the tdLN, described above, may also serve to suppress NK cell surveillance and thereby further support a microenvironment conducive to metastatic outgrowth. In a spontaneous mouse model of breast cancer, LN TREG limited the accumulation and activation of NK cells in the tdLN [70]. Importantly, while depletion of TREG reduced LN metastasis, concomitant depletion of NK cells completely reversed this phenotype, providing additional evidence that the evasion of NK cell function is key to an increased incidence of LN metastasis [70].
These emerging data indicate that tdLNs likely harbor mechanisms that protect against tumor invasion and the progressive suppression of their function may underscore regional metastasis in some patients. Together with the evidence for disrupted systemic immune surveillance, a model emerges whereby the chronic education of the tdLN basin features a dynamic interplay between multiple mechanisms that shift the balance between immune surveillance and metastasis (Figure 2). What tips these scales remains an important question for future mechanistic and clinical study.
Figure 2. Metastasizing tumor cells evade local mechanisms of protection and induce immune dysfunction.
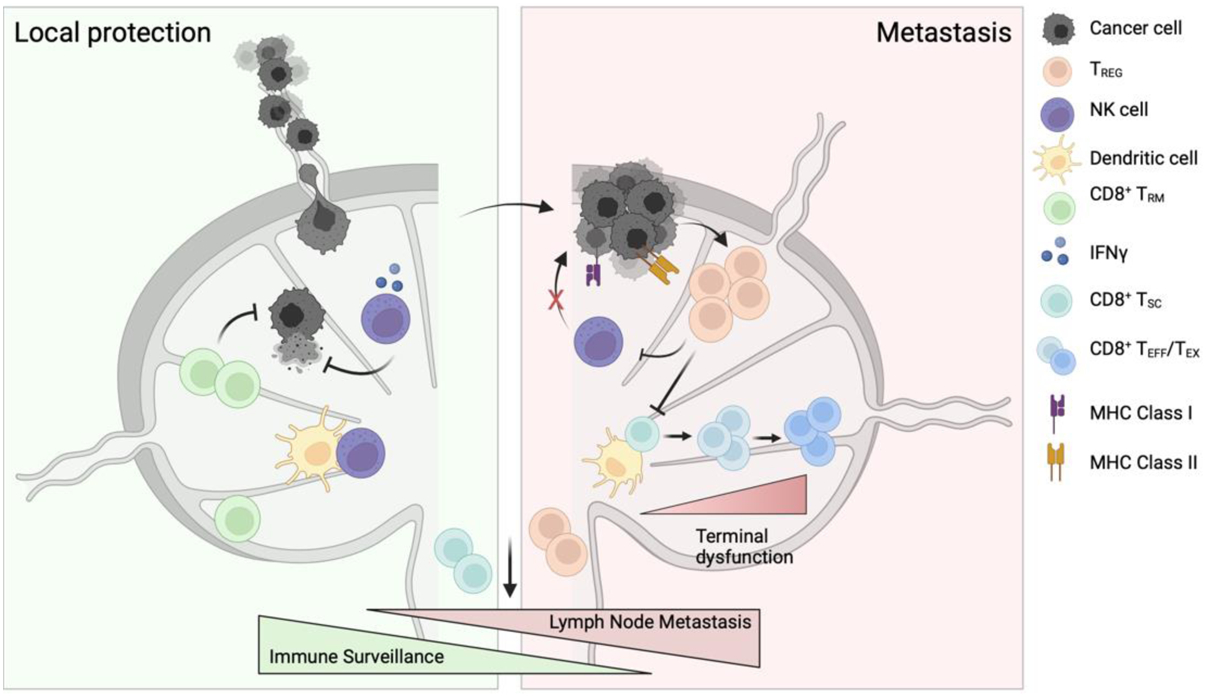
Local protection. Lymph nodes harbor defense mechanisms that limit tumor cell seeding. Cytotoxic resident memory CD8+ T cells (TRM) and natural killer (NK) cells provide rapid cytotoxicity in response to tumor arrival, limiting tumor cell seeding and protecting the antigen-specific, stem-like CD8+ T cells (TSC) that circulate and mediate primary tumor control. Metastasis. Eventual seeding of the lymph node by cancer cells may depend on upregulation of MHC Class I, allowing them to evade killing by NK cells. The seeding of tumor cells then further conditions the lymph node by expanding regulatory T cells (TREG), in part by MHC Class II expression, and promoting a decrease in dendritic cell maturation and local antigen presentation that drives the TSC population towards dysfunction (TEFF/TEX). TREG further suppress NK cell cytotoxicity, limit the priming of effector CD8+ T cell responses, and recirculate to exert their suppressive function at distant tumor locations.
Concluding Remarks
The clinical landscape for treatment of solid tumors is rapidly evolving to incorporate a growing toolbox of immunotherapies and an increasing appreciation for the importance of proper sequencing of targeted and conventional therapies, including surgery. The recent successes of neoadjuvant therapy along with the failure to demonstrate survival benefit with complete LN dissection calls for at least a rethink of regional disease management. The data presented here argue that the tdLN basin harbors tremendous immune potential, but also points out the progressive dysfunction that emerges as regional disease advances. There remains a clear need to expand on our understanding of the immunobiology of the tdLN (see Outstanding Questions) using spontaneous models of LN metastasis paired with clinically relevant surgical regimens and immunotherapy. Clinically, expanded efforts to understand whether and to what extent LN metastasis limits response to immunotherapy in the neoadjuvant setting will be necessary to guide patient selection for trial and to surgical regional disease management. If LN metastasis indeed limits ongoing therapy, a mechanistic understanding of the direct impact of metastasis on the heterogenous LN populations that mediate both local and systemic tumor control may lead to novel therapies that preserve systemic immune surveillance. Given that patients may present with LN involvement at diagnosis, therapies that disrupt metastasis-induced immune suppression or control macro-metastatic outgrowth, may have a more significant clinical impact than those aimed at preventing initial seeding. Ultimately, the application of emerging data to shift the clinical paradigm, will require close partnership between basic scientists and the surgical and medical oncologists to understand what is both feasible and effective for patients.
Outstanding Questions.
Does lymph node metastasis limit response to neoadjuvant immunotherapy? If it does, what are the clinical features of lymph node burden that determine outcome?
In the clinic, does targeting immune checkpoint blockade to tumor draining lymph nodes improve response? What is the impact both on lymph node and distant tumor burden?
What are the therapeutically relevant ways in which to target immune suppression in the tumor draining lymph node and would this boost systemic immune surveillance?
To what extent can lymph node immune suppression be reversed and when? Is early primary tumor removal sufficient to restore immune surveillance?
What are the necessary adaptations that allow tumor cell seeding of lymph nodes and what are the mechanisms of local immune evasion?
Where are lymph node resident memory T cells, what is their natural evolution, and could they underlie the heterogeneity in risk for regional and distant recurrence?
If sentinel lymph node biopsy is performed, can non-sentinel lymph nodes within the regional basin be reinvigorated for therapeutic response?
Are baseline responses in tumor draining lymph nodes tissue-specific, either quantitively or qualitatively (e.g. gut vs skin vs brain)?
What are the mechanistic consequences of lymph node metastasis on systemic immune surveillance beyond regulatory T cells? How does this evolve with time?
When do the primary tumor-draining lymph nodes no longer matter?
Highlights.
Lymphatic transport facilitates antigen presentation in tumor-draining lymph nodes and priming against tumor-associated antigen imprints a distinct functional program in CD8+ T cells.
The tumor-draining lymph node sustains systemic anti-tumor immune surveillance and promotes the efficacy of immune checkpoint therapy.
Lymph node metastasis exacerbates immune suppression including reduced DC maturation, CD8+ T cell and NK cell dysfunction, and expansion of regulatory T cells.
Tumors evade protective mechanisms that safeguard lymph nodes from metastatic growth.
In the era of immunotherapy regional disease management protocols may need to consider the potential impact, both good and bad, of the draining lymph node on response and recurrence.
Acknowledgements
AWL is supported by the National Institutes of Health (R01CA238163, U54 CA263001, P50 CA225450), the Department of Defense (ME200052), the Cancer Research Institute (Lloyd J. Old STAR Award), the Mark Foundation for Cancer Research (19–047-ELA), the American Cancer Society (RSG-18–169-01-LIB), and the American Association for Cancer Research (AACR-BMS Midcareer Female Investigator Grant). KSV is supported by the NYU Clinical and Translational Science Institute (TL1 TR001447). DJ is supported by the METAvivor Organization (Early Career Investigator Grant), the American Association for Cancer Research and Breast Cancer Research Foundation (Career Development Award), and the Karin Grunebaum Cancer Research Foundation. Figures were generated with BioRender.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
AWL reports consulting services for AGS Therapeutics.
References
- 1.Morton DL et al. (2014) Final Trial Report of Sentinel-Node Biopsy versus Nodal Observation in Melanoma. N Engl J Med 370, 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padera TP et al. (2002) Lymphatic Metastasis in the Absence of Functional Intratumor Lymphatics. Science 296, 1883–1886 [DOI] [PubMed] [Google Scholar]
- 3.Brown M et al. (2018) Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359, 1408–1411 [DOI] [PubMed] [Google Scholar]
- 4.Leiter U et al. (2019) Final Analysis of DeCOG-SLT Trial: No Survival Benefit for Complete Lymph Node Dissection in Patients With Melanoma With Positive Sentinel Node. J Clin Oncol 37, 3000–3008 [DOI] [PubMed] [Google Scholar]
- 5.Faries MB et al. (2017) Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. New England Journal of Medicine 376, 2211–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X et al. (2023) Debating Sentinel Lymph Node Biopsy for Melanoma in the Modern Adjuvant Era. JCO DOI: 10.1200/JCO.23.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naxerova K et al. (2017) Origins of lymphatic and distant metastases in human colorectal cancer. Science 357, 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venet D et al. (2020) Phylogenetic reconstruction of breast cancer reveals two routes of metastatic dissemination associated with distinct clinical outcome. EBioMedicine 56, 102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reticker-Flynn NE et al. (2022) Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 185, 1924–1942.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Bois H et al. (2021) Tumor-draining lymph nodes: At the crossroads of metastasis and immunity. Sci Immunol 6, eabg3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas SN et al. (2014) Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824 [DOI] [PubMed] [Google Scholar]
- 12.Dammeijer F et al. (2020) The PD-1/PD-L1-Checkpoint Restrains T cell Immunity in Tumor-Draining Lymph Nodes. Cancer Cell 38, 685–700.e8 [DOI] [PubMed] [Google Scholar]
- 13.Connolly KA et al. (2021) A reservoir of stem-like CD8 + T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol 6, eabg7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fransen MF et al. (2018) Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 3, e124507, 124507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loo CP et al. (2017) Lymphatic Vessels Balance Viral Dissemination and Immune Activation following Cutaneous Viral Infection. Cell Reports 20, 3176–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund AW et al. (2016) Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest 126, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alitalo AK et al. (2013) VEGF-C and VEGF-D Blockade Inhibits Inflammatory Skin Carcinogenesis. Cancer Research 73, 4212–4221 [DOI] [PubMed] [Google Scholar]
- 18.Lund AW et al. (2012) VEGF-C Promotes Immune Tolerance in B16 Melanomas and Cross-Presentation of Tumor Antigen by Lymph Node Lymphatics. Cell Reports 1, 191–199 [DOI] [PubMed] [Google Scholar]
- 19.Song E et al. (2020) VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577, 689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fankhauser M et al. (2017) Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med 9, eaal4712. [DOI] [PubMed] [Google Scholar]
- 21.Roberts EW et al. (2016) Critical Role for CD103+/CD141+ Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 30, 324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohl L et al. (2004) CCR7 Governs Skin Dendritic Cell Migration under Inflammatory and Steady-State Conditions. Immunity 21, 279–288 [DOI] [PubMed] [Google Scholar]
- 23.Hildner K et al. (2008) Batf3 Deficiency Reveals a Critical Role for CD8α + Dendritic Cells in Cytotoxic T Cell Immunity. Science 322, 1097–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salmon H et al. (2016) Expansion and Activation of CD103+ Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 44, 924–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris ST et al. (2020) cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature 584, 624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruhland MK et al. (2020) Visualizing Synaptic Transfer of Tumor Antigens among Dendritic Cells. Cancer Cell 37, 786–799.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan RS et al. (2006) Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity 25, 153–162 [DOI] [PubMed] [Google Scholar]
- 28.Pirillo C et al. (2023) Cotransfer of antigen and contextual information harmonizes peripheral and lymph node conventional dendritic cell activation. Sci. Immunol 8, eadg8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jhunjhunwala S et al. (2021) Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer 21, 298–312 [DOI] [PubMed] [Google Scholar]
- 30.Zagorulya M and Spranger S (2023) Once upon a prime: DCs shape cancer immunity. Trends in Cancer 9, 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maier B et al. (2020) A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng Q et al. (2020) PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun 11, 4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh SA et al. (2020) PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Cancer 1, 681–691 [DOI] [PubMed] [Google Scholar]
- 34.de Mingo Pulido Á et al. (2018) TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 33, 60–74.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon KO et al. (2021) TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 595, 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenkel JM et al. (2021) Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. Immunity 54, 2338–2353.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Pul KM et al. (2019) Selectively hampered activation of lymph node-resident dendritic cells precedes profound T cell suppression and metastatic spread in the breast cancer sentinel lymph node. J Immunother Cancer 7, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuura K et al. (2006) Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 106, 1227–1236 [DOI] [PubMed] [Google Scholar]
- 39.van den Hout MFCM et al. (2017) Melanoma Sequentially Suppresses Different DC Subsets in the Sentinel Lymph Node, Affecting Disease Spread and Recurrence. Cancer Immunol Res 5, 969–977 [DOI] [PubMed] [Google Scholar]
- 40.Prokhnevska N et al. (2023) CD8+ T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56, 107–124.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schietinger A et al. (2016) Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 45, 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudloff MW et al. (2023) Hallmarks of CD8+ T cell dysfunction are established within hours of tumor antigen encounter before cell division. Nat Immunol DOI: 10.1038/s41590-023-01578-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z et al. (2019) TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision. Immunity 51, 840–855.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escobar G et al. (2023) Tumor immunogenicity dictates reliance on TCF1 in CD8+ T cells for response to immunotherapy. Cancer Cell DOI: 10.1016/j.ccell.2023.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu T et al. (2016) The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 1, eaai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meiser P et al. (2023) A distinct stimulatory cDC1 subpopulation amplifies CD8+ T cell responses in tumors for protective anti-cancer immunity. Cancer Cell 41, 1498–1515.e10 [DOI] [PubMed] [Google Scholar]
- 47.Huang Q et al. (2022) The primordial differentiation of tumor-specific memory CD8+ T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell 185, 4049–4066.e25 [DOI] [PubMed] [Google Scholar]
- 48.Li G et al. (2022) TGF-β-dependent lymphoid tissue residency of stem-like T cells limits response to tumor vaccine. Nat Commun 13, 6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horton BL et al. (2021) Lack of CD8 + T cell effector differentiation during priming mediates checkpoint blockade resistance in non–small cell lung cancer. Sci. Immunol 6, eabi8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zagorulya M et al. (2023) Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. Immunity 56, 386–405.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fear VS et al. (2021) Tumour draining lymph node-generated CD8 T cells play a role in controlling lung metastases after a primary tumour is removed but not when adjuvant immunotherapy is used. Cancer Immunol Immunother 70, 3249–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spitzer MH et al. (2017) Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 168, 487–502.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Micevic G et al. (2023) IL-7R licenses a population of epigenetically poised memory CD8+ T cells with superior antitumor efficacy that are critical for melanoma memory. Proceedings of the National Academy of Sciences 120, e2304319120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller BC et al. (2019) Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurtulus S et al. (2019) Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1−CD8+ Tumor-Infiltrating T Cells. Immunity 50, 181–194.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siddiqui I et al. (2019) Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 50, 195–211.e10 [DOI] [PubMed] [Google Scholar]
- 57.Steele MM et al. (2023) T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat Immunol 24, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavil NV et al. (2023) Chronic antigen in solid tumors drives a distinct program of T cell residence. Sci. Immunol 8, eadd5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burger ML et al. (2021) Antigen dominance hierarchies shape TCF1+ progenitor CD8 T cell phenotypes in tumors. Cell 184, 4996–5014.e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z et al. (2022) In vivo labeling reveals continuous trafficking of TCF-1+ T cells between tumor and lymphoid tissue. J Exp Med 219, e20210749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pai JA et al. (2023) Lineage tracing reveals clonal progenitors and long-term persistence of tumor-specific T cells during immune checkpoint blockade. Cancer Cell 41, 776–790.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahim MK et al. (2023) Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 186, 1127–1143.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yost KE et al. (2019) Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 25, 1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francis DM et al. (2020) Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci. Transl. Med 12, eaay3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel SP et al. (2023) Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. New England Journal of Medicine 388, 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rozeman EA et al. (2021) Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat Med 27, 256–263 [DOI] [PubMed] [Google Scholar]
- 67.Provencio M et al. (2023) Perioperative Nivolumab and Chemotherapy in Stage III Non–Small-Cell Lung Cancer. New England Journal of Medicine 0, null [DOI] [PubMed] [Google Scholar]
- 68.Yang H et al. (2022) Multi-scale characterization of tumor-draining lymph nodes in resectable lung cancer treated with neoadjuvant immune checkpoint inhibitors. eBioMedicine 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yaddanapudi K et al. (2022) Single-Cell Immune Mapping of Melanoma Sentinel Lymph Nodes Reveals an Actionable Immunotolerant Microenvironment. Clinical Cancer Research 28, 2069–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kos K et al. (2022) Tumor-educated Tregs drive organ-specific metastasis in breast cancer by impairing NK cells in the lymph node niche. Cell Rep 38, 110447. [DOI] [PubMed] [Google Scholar]
- 71.Lei P-J et al. (2023) Cancer cell plasticity and MHC-II-mediated immune tolerance promote breast cancer metastasis to lymph nodes. J Exp Med 220, e20221847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Núñez NG et al. (2020) Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat Commun 11, 3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shabaneh TB et al. (2018) Oncogenic BRAFV600E Governs Regulatory T-cell Recruitment during Melanoma Tumorigenesis. Cancer Research 78, 5038–5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Gulijk M et al. (2023) PD-L1 checkpoint blockade promotes regulatory T cell activity that underlies therapy resistance. Science Immunology 8, eabn6173. [DOI] [PubMed] [Google Scholar]
- 75.Sharma MD et al. (2007) Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Invest 117, 2570–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Krimpen A et al. (2022) Immune suppression in the tumor-draining lymph node corresponds with distant disease recurrence in patients with melanoma. Cancer Cell 40, 798–799 [DOI] [PubMed] [Google Scholar]
- 77.Lee C et al. (2019) Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 363, 644–649 [DOI] [PubMed] [Google Scholar]
- 78.Masopust D and Soerens AG (2019) Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 37, 521–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park SL et al. (2019) Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature 565, 366–371 [DOI] [PubMed] [Google Scholar]
- 80.Malik BT et al. (2017) Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol 2, eaam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savas P et al. (2018) Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 24, 986–993 [DOI] [PubMed] [Google Scholar]
- 82.Cheng Y et al. (2021) Non-terminally exhausted tumor-resident memory HBV-specific T cell responses correlate with relapse-free survival in hepatocellular carcinoma. Immunity 54, 1825–1840.e7 [DOI] [PubMed] [Google Scholar]
- 83.Schenkel JM et al. (2014) Cutting Edge: Resident Memory CD8 T Cells Occupy Frontline Niches in Secondary Lymphoid Organs. The Journal of Immunology 192, 2961–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molodtsov AK et al. (2021) Resident memory CD8+ T cells in regional lymph nodes mediate immunity to metastatic melanoma. Immunity 54, 2117–2132.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiossone L et al. (2018) Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18, 671–688 [DOI] [PubMed] [Google Scholar]
- 86.Bajénoff M et al. (2006) Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. The Journal of Experimental Medicine 203, 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lo HC et al. (2020) Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nat Cancer 1, 709–722 [DOI] [PubMed] [Google Scholar]
- 88.Correia AL et al. (2021) Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature 594, 566–571 [DOI] [PubMed] [Google Scholar]
- 89.Chen S et al. (2005) Suppression of tumor formation in lymph nodes by L-selectin–mediated natural killer cell recruitment. The Journal of Experimental Medicine 202, 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jalkanen S and Salmi M (2020) Lymphatic endothelial cells of the lymph node. Nat Rev Immunol 20, 566–578 [DOI] [PubMed] [Google Scholar]
- 91.Heim TA et al. (2023) Lymphatic vessel transit seeds precursors to cytotoxic resident memory T cells in skin draining lymph nodes, Immunology; [DOI] [PubMed] [Google Scholar]
- 92.Steele MM et al. (2023) T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat Immunol 24, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirosue S et al. (2014) Steady-State Antigen Scavenging, Cross-Presentation, and CD8+ T Cell Priming: A New Role for Lymphatic Endothelial Cells. The Journal of Immunology 192, 5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vokali E et al. (2020) Lymphatic endothelial cells prime naïve CD8+ T cells into memory cells under steady-state conditions. Nat Commun 11, 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tamburini BA et al. (2014) Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun 5, 3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lukacs-Kornek V et al. (2011) Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol 12, 1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Podgrabinska S et al. (2009) Inflamed Lymphatic Endothelium Suppresses Dendritic Cell Maturation and Function via Mac-1/ICAM-1-Dependent Mechanism. The Journal of Immunology 183, 1767–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tewalt EF et al. (2012) Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120, 4772–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lane RS et al. (2018) IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. Journal of Experimental Medicine 215, 3057–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]


