Abstract
Background:
We previously discovered that Korean red ginseng aqueous extract (RGAE) potentiates the TMEM16A channel, improved mucociliary transport (MCT) parameters in CF nasal epithelia in vitro, and thus could serve as a therapeutic strategy to rescue the MCT defect in cystic fibrosis (CF) airways. The hypothesis of this study is that RGAE can improve epithelial Cl− secretion, MCT, and histopathology in an in-vivo CF rat model.
Methods:
Seventeen 4-month old CFTR−/− rats were randomly assigned to receive daily oral control (saline, n=9) or RGAE (Ginsenosides 0.4mg/kg/daily, n=8) for 4 weeks. Outcomes included nasal Cl− secretion measured with the nasal potential difference (NPD), functional microanatomy of the trachea using micro-optical coherence tomography, histopathology, and immunohistochemical staining for TMEM16a.
Results:
RGAE-treated CF rats had greater mean NPD polarization with UTP (control = −5.48 +/− 2.87 mV, RGAE = −9.49 +/− 2.99 mV, p <0.05), indicating, at least in part, potentiation of UTP-mediated Cl− secretion through TMEM16A. All measured tracheal MCT parameters (airway surface liquid, periciliary liquid, ciliary beat frequency, MCT) were significantly increased in RGAE-treated CF rats with MCT exhibiting a 3-fold increase (control, 0.45+/−0.31 vs. RGAE, 1.45+/−0.66 mm/min, p<0.01). Maxillary mucosa histopathology was markedly improved in RGAE-treated cohort (reduced intracellular mucus, goblet cells with no distention, and shorter epithelial height). TMEM16A expression was similar between groups.
Conclusion:
RGAE improves TMEM16A-mediated transepithelial Cl− secretion, functional microanatomy, and histopathology in CF rats. Therapeutic strategies utilizing TMEM16A potentiators to treat CF airway disease are appropriate and provide a new avenue for mutation-independent therapies.
Keywords: Red ginseng, ginsenoside, mucociliary transport, mucociliary clearance, cystic fibrosis, CFTR, TMEM16A, TMEM16A potentiator, calcium-activated chloride channel, cystic fibrosis rat, nasal potential difference, micro-optical coherence tomography, functional microanatomy
INTRODUCTION
The primary phenotype associated with the loss of CFTR function in cystic fibrosis airway disease is a hyper-concentrated mucus gel that mucociliary transport (MCT) mechanisms struggle to remove. Chronic bacterial infection ensues, progressing to loss of lung function.1 Treatments largely target the symptoms and physiologic manifestations of the underlying disease,2 with the exception of CFTR modulators, which can partially rescue the function of mutated CFTR to improve clinical outcomes in genetically suited patients.3 However, they cannot treat all patients nor restore lung function to normal levels. Effective, mutation-independent therapeutics are urgently needed.
Longstanding investigations into restoring Cl− transport via alternative Cl− channel pathways represent one such therapeutic strategy. The TMEM16A channel (anoctamin-1) was identified as the gene encoding the primary airway calcium-activating Cl− channel in 2008.4 Like CFTR; TMEM16A conducts both Cl− and HCO3− across the airway epithelium and is expressed in both the surface epithelium and submucosal glands. TMEM16A is activated by the endogenous ligand, ATP, via the P2Y2 receptor leading to the release of intracellular Ca2+ as a second messenger. Initial excitement about this alternative Cl− channel pathway to rescue the transport defect in CF was tempered when denufosol, a stable purinergic ligand that activates TMEM16A, failed to benefit CF patients, but rather induced adverse effects, such as cough.5 The failure was attributed to rapid desensitization of the P2Y2 receptor and/or depletion of intracellular Ca2+ stores. Several other studies suggested TMEM16A maintains excessive mucus secretion during inflammatory airway disease and supports airway smooth muscle contraction,6 leading some to advocate for inhibiting, not activating, it as a strategy for CF airway disease.
Korean red ginseng (Panax ginseng Meyer) has been utilized worldwide as alternative medicine, and its main bioactive components are steroidal saponins called ginsenosides.7,8 Early research suggest the anti-inflammatory and neuroprotective character of these phytocompounds are applicable to a variety of conditions including respiratory, cardiovascular, and neurodegenerative disorders.9–11 Our previous in vitro study demonstrated that the red ginseng aqueous extract (RGAE) was a significant potentiator of the TMEM16A channel and improved MCT parameters in wild type and CF murine epithelial cell culture models.12 Evidence for potentiating activity was based upon stimulation of TMEM16A-mediated Cl− secretion in the absence of cytosolic increase in Ca2+ and synergistic improvement in UTP-mediated Cl− secretion as judged by whole cell patch clamp in human embryonic kidney cells expressing TMEM16A. Based on this previous study, we presumed RGAE could serve as a rescue mechanism in CF airways by potentiating the TMEM16A. In theory, a TMEM16A potentiator bypasses excessive stimulation of the purinergic Ca2+ signaling pathway and augments Cl− secretion through the channel during endogenous activation, but without P2Y2 receptor desensitization. Another advantage of this compound is that, unlike the recently discovered TMEM16A potentiator ETX001,13 ginsenosides are orally bioavailable.
We previously developed the CFTR−/− rat, which has dysfunctional MCT, goblet cells with distended mucus, hypertrophy of airway submucosal glands, and increased viscosity of respiratory secretions that first occur between 3 and 6 months, suggesting potential for ameliorating these abnormalities pharmacologically.14–17 The hypothesis of this study is that orally administered RGAE will improve epithelial Cl− secretion via TMEM16A, reverse the MCT defect in the airway, and normalize histopathology in the in-vivo CF rat model.
METHODS
CFTR−/− rat model
All animal use was approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committees (IACUC) prior to initiation of the study. All experiments used Sprague Dawley CFTR−/− rats (Horizon Discovery Waterbeach, United Kingdom). This rat strain was bred and genotyped, as previously described.18 In brief, heterozygote (CFTR+/−) males and females were paired to generate wild type (WT) and knockout pups, and litters remained with lactating dams until 21 days of age. CFTR−/− rats were maintained on a standard rodent diet, with supplemental DietGel 76A (ClearH20, Portland, ME) and 50% Go-LYTLEY (Braintree Laboratories Inc. Braintree, MA) added to the water from weaning to reduce mortality from gastrointestinal obstruction.
RGAE Feeding and Dosage
Four-month old CFTR−/− rats were chosen for this study. Seventeen rats were randomly assigned to receive daily oral placebo (saline, n = 9) or RGAE (Ginsenosides 0.4mg/kg/daily, n = 8) for 4 weeks. RGAE was provided by the Korea Ginseng & Tobacco Central Research Institute, and dosage of ginsenosides (0.4mg/kg/daily) was based on the daily recommended dosage for humans.19,20 RGAE was produced from the roots of 6-yr-old red ginseng (Panax Ginseng Meyer) through a standardized manufacturing process. The general composition of RGAE is ash, 2.5%; total fat, 0.05%; total crude saponin, 70 mg/g; and total ginsenosides, 20 mg/g. Certifications of consistency and purity are provided through Current Good Manufacturing Practices Certifications, Hazard Analysis and Critical Control Points (HACCP), and International Organization for Standardization for Reliable Food Management Safety Systems certification (ISO 22000).
Outcome measures
1). Nasal potential difference measurement:
Rats were anesthetized with ketamine (200 mg/kg) and xylazine (30 mg/kg) by intraperitoneal injection. The potential difference was measured using potassium chloride (KCl)/Calomel electrodes electrode and electronic data capture (AD Instruments, Sydney, Australia) as previously described for mice and humans.21–26 Chemicals were obtained from Sigma (St. Louis, MO). Nasal cavities were perfused sequentially with the following pharmacologic agents: 1) Ringer’s solution containing 148 mM NaCl, 4 mM KCl, 1.2 mM MgCl2, 2.25 mM CaCl2, 2.4 mM K2HPO4, 0.4 mM KH2PO4 (pH 7.4), and 100 μM amiloride - blocks epithelial Na+ channels, as a means to isolate changes in short-circuit current (ΔISC) secondary to effects on Cl− channel activity); 2) a zero-Cl- -containing solution (NaCl in solution #1 replaced by 148 mM Na gluconate, 2.25 mM Ca gluconate, and 4 mM K gluconate; (pH 7.4) & forskolin (20 μM - activate CFTR by Cl− gradient and elevating intracellular cAMP, which results in protein kinase A (PKA)-dependent phosphorylation of the CFTR regulatory domain), 3) INH-172 (10 μM) and GlyH-101 (10 μM) - CFTR inhibitors that permit determination of CFTR-dependent contributions to ISC, and 4) uridine 5-triphosphate (UTP, 150 μM) – activates CaCC. Each condition was studied for 5 to 10 minutes until a stable signal was achieved. The activity was measured from the stable baseline to the highest point of hyperpolarization. Traces were interpreted in a blinded fashion.
2). Micro-optical coherence tomography (μOCT) image acquisition and analysis:
Measurements of functional microanatomic parameters in ex vivo trachea tissue were performed using μOCT, a high-speed, high-resolution microscopic reflectance imaging modality.27–32 Tracheae were excised and immediately placed on gauze soaked in Minimum Essential Media (Thermo Fisher Scientific, Waltham, MA), such that the apical surface of the trachea remained media free, and incubated under physiologic conditions (37°C, 5% CO2, 100% humidity). Tracheae were allowed to equilibrate for 1 hour before imaging. Images were acquired at five controlled points along the tracheal length, using the cranial end as a reference. ASL and PCL were calculated by direct measurement of the visible depth within the image. To account for refractory properties of the liquid, layer thickness measurements were corrected for the refraction index of the liquid (n = 1.33). ASL and PCL depth were calculated from pixels to μm using a ratio of 0.82. CBF was evaluated by means of a time series of images by determining peak amplitude frequency in the temporal Fourier transform of areas demonstrating oscillatory behavior.25,33–40 Mucociliary transport (MCT) rate was determined using the time elapsed, and distance traveled of native particulates in the mucus over multiple frames. For each trachea, images were acquired at standard distances along the ventral surface, with the optical beam scanned along the longitudinal direction. Statistics were performed for each individual replicate. All images were analyzed using ImageJ version 1.50i (National Institutes of Health, Bethesda, MD) and MATLAB® R2016a (The MathWorks, Natick, MA).
3). Maxillary sinus mucosa histology:
After 4 weeks of treatment, rats were euthanized and heads were harvested. The nasal passages were flushed retrograde with 10% buffered formalin followed by immersion in formalin until processed. Specimens were provided to the UAB Comparative Pathology Laboratory (CPL) for sectioning and evaluation. Briefly, samples were decalcified for 4–5 days in Immunocal (Decal Chemical Corporation, Tallman, NY) solution, and rinsed thoroughly. Sections of the nasal cavity and maxillary sinuses were divided into 4 regions at specific anatomic sites as described previously.41,42 Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). The respiratory epithelium of the maxillary sinus at the mid-maxillary walls were measured for epithelial thickness and the density of goblet cells by 2 blinded judges. Additional slices were stained with Alcian Blue-Periodic Acid Schiff (AB-PAS) to examine intracellular mucus in the goblet cells of the nasal septum. Total mucus was calculated from the area normalized to the height of the epithelium. Sections were imaged on a Keyence All-in-One Fluorescence Microscope BZ-X800 with area calculations using the BZ-H4M/Measurement Application (Keyence, Osaka, Japan).
4). TMEM16A immunohistochemistry of CF rat lungs:
Paraffin-embedded CF rat lung slices were subjected to immunohistochemical staining for TMEM16A (Anti-ANO1 primary antibody, rabbit polyclonal, CAT#: MBS176756 (MyBioSource, Inc., San Diego, Ca.) and visualized by 3,3’-diaminobenzidine (DAB) staining to detect TMEM16A expression. Nuclei were stained with hematoxylin. Data were analyzed with ImageJ Fiji software with semi-quantitative determination of protein expression using immunohistochemistry staining and analysis.43 TMEM16A DAB staining intensities were normalized by the epithelial layer length for each sample to provide the value.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism version 9.0. Inferential statistics (mean+/− SD) were computed using 2-tailed unpaired t test. P values of less than 0.05 were considered significant. Statistics are presented as mean +/− standard deviation.
RESULTS
RGAE-treated CF rats exhibited no adverse effects
No adverse effects (e.g., decreased appetite, cough, breathing difficulty, or death) were noted during this study period. There was no statistical difference in weights before and after treatment between the two groups, although RGAE cohorts had less % of weight loss compared to controls (% of weight loss, control = 4.25 +/− 1.12 % vs. RGAE = 3.21 +/− 2.3, p > 0.05) during the study period.
RGAE increased UTP-mediated transepithelial Cl− transport in vivo
To characterize the electrophysiology of the upper airway after 4 weeks of treatment of RGAE, transepithelial potential difference was monitored in response to a series of pharmacologic ion channel regulators (Figure 1). As expected, CFTR−/− rats showed no Cl−-dependent secretion when CFTR-mediated Cl− transport was stimulated by Cl−-free Ringer’s lactate with forskolin or blocked by CFTR inhibitors. However, CFTR−/− rats treated with RGAE had a much greater mean NPD polarization with UTP (control = −5.48 +/− 2.87mV, RGAE = −9.49 +/− 2.99mV, p <0.05), indicating, at least in part, potentiation of UTP-mediated Cl− secretion through TMEM16A.
Figure 1.
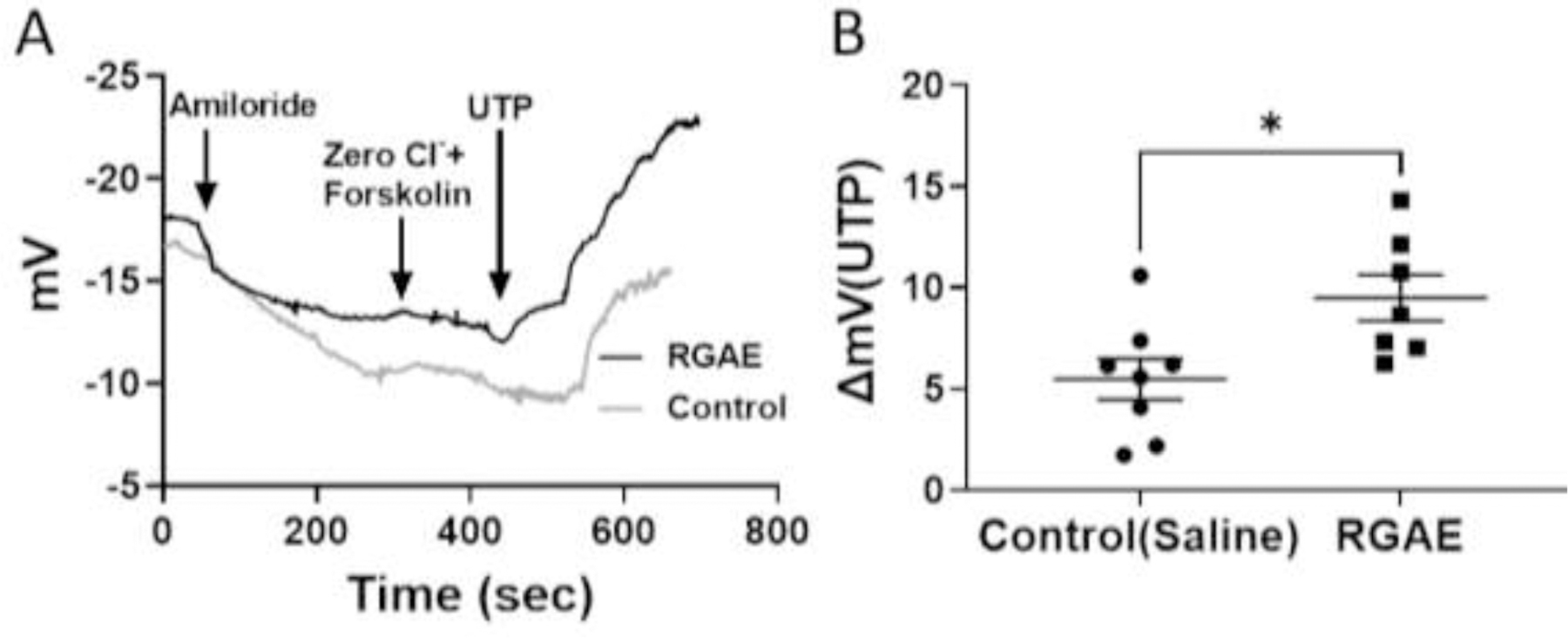
Representative NPD tracings showing PD after serial administration of 1) amiloride (100 μM), 2)Cl− free forskolin (20 μM), 3)INH-172 (10 μM) and GlyH-101 (10 μM), and 4) UTP (150 μM) (A), followed by summary data (B) in RGAE-treated and control CF rats. RGAE treatment caused NPD hyper-polarization with UTP (control = −5.96 +/− 2.3 mV, RGAE = −12.51 +/− 1.04 mV, p <0.05; unpaired t test), indicating potentiation of UTP-mediated Cl− secretion through TMEM16A.
RGAE improved the functional microanatomy of CF rat tracheas
We used μOCT to visualize and quantify the functional microanatomy of the trachea airway surface (Figure 2). Excised tracheas from CFTR KO rats who received RGAE had significantly greater ASL depth (control, 8.70+/−2.06 μm vs RGAE, 25.50+/− 18.04 μm; p < 0.05), PCL depth (control, 5.75+/−0.71 μm vs RGAE = 7.23+/− 1.08 μm; p < 0.01), and CBF (control, 7.22+/− 0.79 Hz vs RGAE = 8.75+/− 0.86 Hz; p<−0.01). The mean MCT14 rate was also markedly improved 3-fold (control, 0.45+/−0.31 vs. RGAE, 1.45+/−0.66 mm/min, p<0.01).
Figure 2.
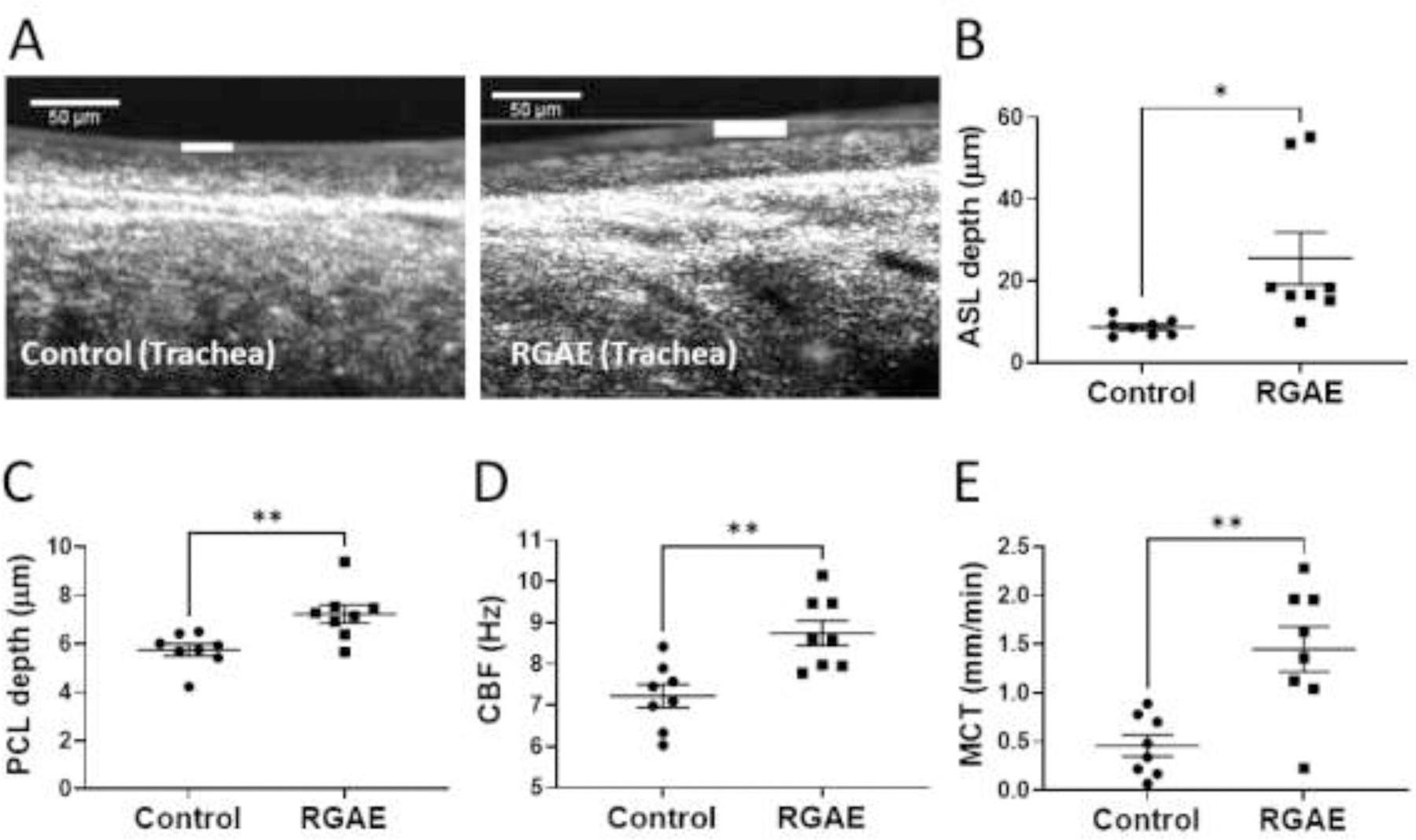
Representative μOCT images (A) and summary data for ASL (B), PCL (C), CBF (D), and MCT (E) recorded in tracheas excised from control and RGAE-treated CF rats (*P<0.05, **P<0.01; unpaired t test). RGAE significantly improved all markers of functional anatomy (ASL – airway surface liquid, PCL – periciliary liquid, CBF – ciliary beat frequency, MCT – mucociliary transport, RGAE – red ginseng aqueous extract). White line = ASL depth. Representative μOCT videos are provided as supplementary data.
Goblet cell abundance and epithelial height in the maxillary sinus mucosa were decreased in RGAE-treated CF rats
The two rat cohorts had normally developed epithelium consisting of a pseudostratified columnar epithelium comprised of both goblet and ciliated epithelial cells. As described previously, CF rats exhibit abnormal goblet cell histology with an excessive number distended with thick mucus when compared to wild type rats and thickened submucosa with mucus-filled submucosal glands by 3–6 months of age.17,18 CF rats treated with RGAE had decreased goblet cell number (goblet cells/mm) and lacked the mucus-distended appearance that was present in the saline controls (control, 42.67+/− 11.68 vs. 29.48+/−11.64; p<0.05). The epithelial layer was also thinner in the CF rats treated with RGAE presumably due to the “loss” of distended goblet cells (control, 72.33+/−11.83 μm vs. RGAE, 56.50+/−8.64 μm, p<0.01) (Figure 3).
Figure 3.
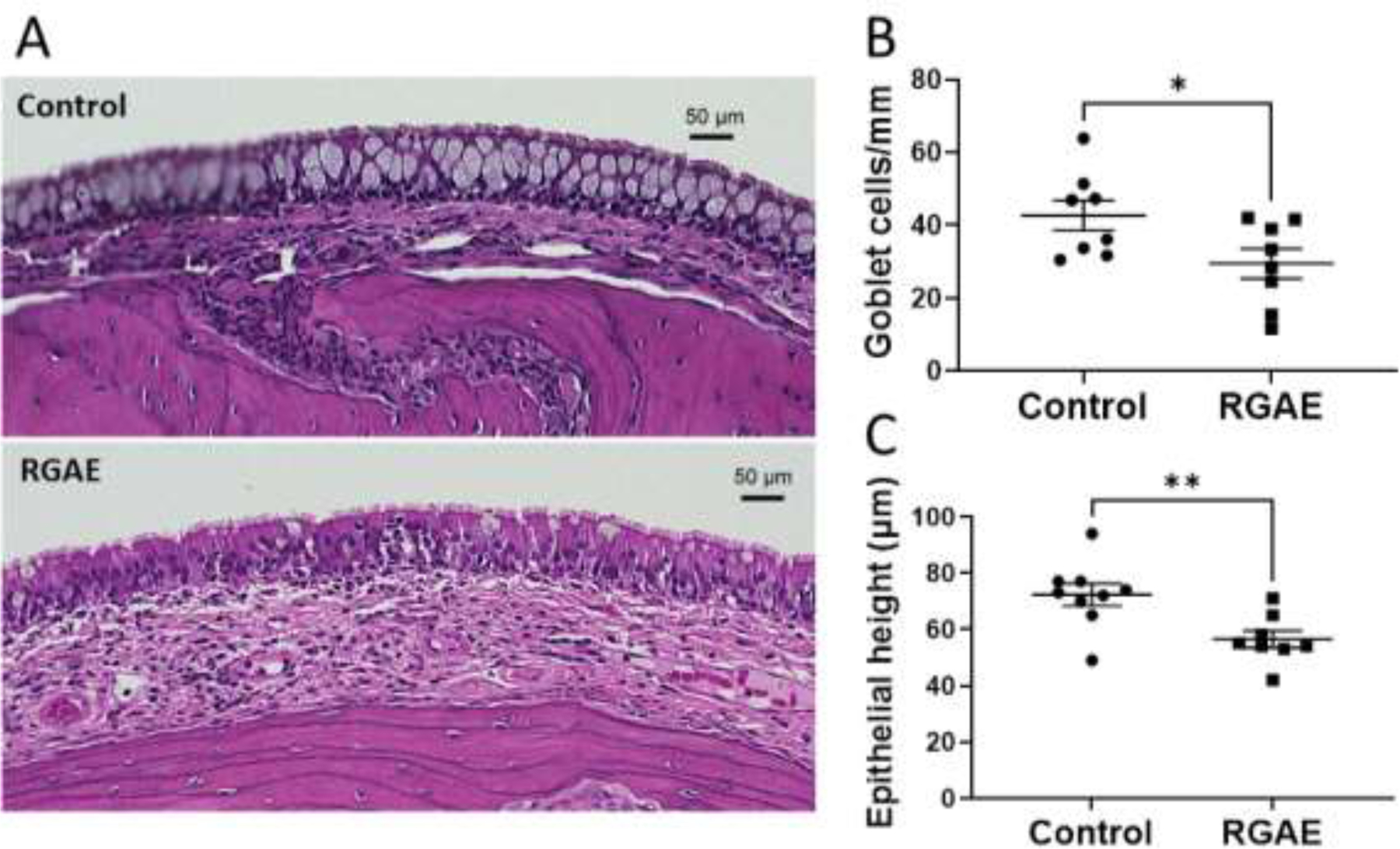
Representative hematoxylin/eosin-stained maxillary sinus mucosa (A) of 10× H&E stained mucosa from control and ginseng-treated CF rats. Summary data regarding number of goblet cells (B) and epithelial height (C). RGAE-treated CF rats demonstrate marked improvement in histopathology (*P<0.05, **P<0.01, unpaired t test).
Intracellular mucus was decreased in RGAE-treated CF rat nasal septum epithelium
Similarly, when additional sections of the nasal cavities were stained with AB- PAS and examined for intracellular mucus, the RGAE-treated rats demonstrated a marked decrease in total intracellular mucus normalized to epithelial height in μm2 per μm (control, 7.45+/−3.15 vs. RGAE, 4.27+/−2.75, p<0.05) (Figure 4).
Figure 4.
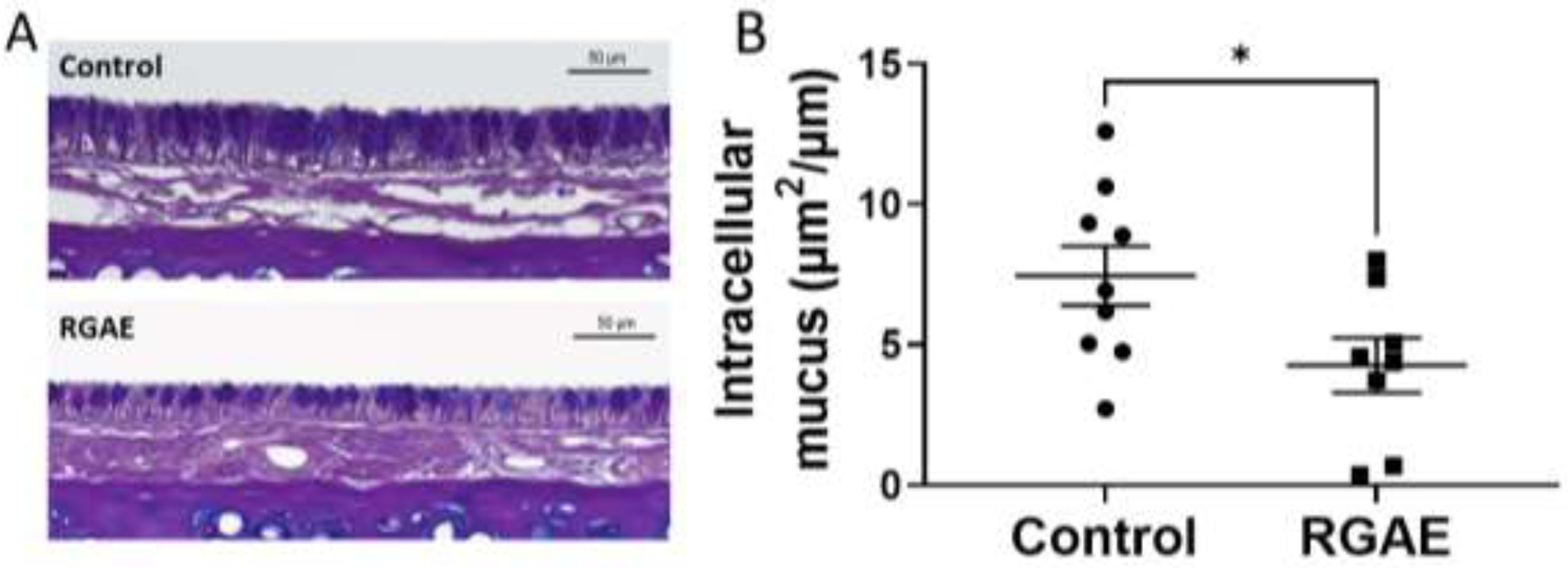
Representative Alcian Blue – Periodic Acid Schiff staining of nasal septum epithelium (A) from control and ginseng-treated CF rats. Summary data demonstrates significant reduction in intracellular mucus in RGAE-treated CF rats (*P<0.05, unpaired t test).
TMEM16A expression was similar between RGAE-treated and control CF rats.
In order to assess whether RGAE might influence expression of TMEM16A protein as a potential reason for the observed effects, immunohistochemical staining for TMEM16A was performed on the CF rat lung slices of the rat cohorts. TMEM16A protein expression was not increased in RGAE-treated rats (0.0048+/−0.0033 vs. control, 0.0049+/−0.0030, p= 0.94) (Figure 5)
Figure 5.
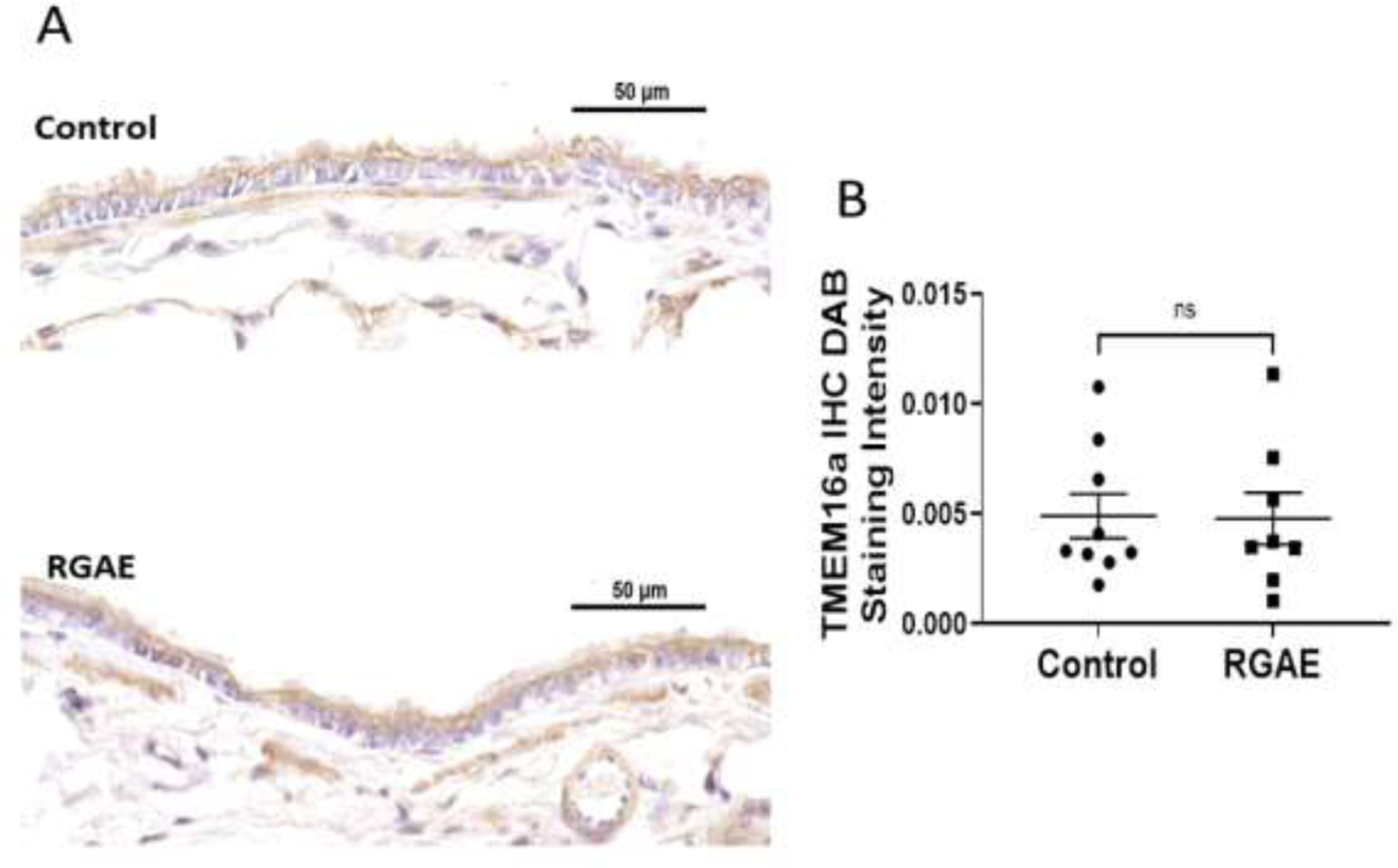
Representative TMEM16A immunohistochemical staining between control and RGAE-treated CF rats (A). Summary data demonstrates no increase in TMEM16a staining intensity in RGAE treated CF rats (B).
DISCUSSION
Ginsenosides are the active ingredients within Korean red ginseng with Rb1 recently identified to activate TMEM16A in guinea pig intestine.44,45 In our previous in vitro study, RGAE significantly increased Cl− transport in CFTR−/− murine nasal sinus epithelium and HEK cells expressing the TMEM16A in a Ca2+-independent fashion indicating TMEM16A potentiation as the likely mechanism of action. Because RGAE significantly increased MCT parameters in CFTR−/− mice, this suggested a translational potential to improve MCT parameters in a CF animal model in vivo.12 Indeed, the findings of the current study support this strategy. RGAE amplified UTP-mediated Cl− secretion, increased all measures of functional microanatomy, and improved the appearance of maxillary sinus mucosa histology in CFTR−/− rats.12 Similarly, total intracellular mucus was significantly decreased in CF rat nasal cavities treated with RGAE. Expression of TMEM16A was similar between RGAE and control rat lungs and thus RGAE did not confer its effect through overexpression of TMEM16A.
Thick mucus contributes to the pathophysiology of inflammatory airway diseases, including CRS, asthma, chronic obstructive pulmonary disease, and CF.46–50 During inflammation, TMEM16A is known to be upregulated particularly in mucus producing cells, with only little expression in ciliated cells.51 Combined with the clinical failure of earlier studies targeting TMEM16A through purinergic activation, interest has recently turned towards inhibiting TMEM16A in the airway to decrease mucus exocytosis.5,52 In our study, RGAE-treated CF rats had significantly lower epithelial height, fewer identifiable goblet cells in the sinus mucosa, and overall decreased intracellular mucus in nasal epithelium. Thus, our results contradict reports that TMEM16A inhibition is required to reduce the goblet cell population.53,54 We hypothesize that the decreased population of goblet cells, decreased intracellular mucus, and improved goblet cell and submucosal gland architecture is secondary to stimulation of mucus exocytosis during the 4 weeks of treatment, a finding that has been found to be defective in CF.55 Combined with improved mucus clearance by augmented MCT, this would be expected to reduce goblet cell hyperplasia over time. While some postulate that mucus release by resolving mucus exocytosis has the potential to exacerbate cough and increase mucus expression into the airway, RGAE-treated CF rats experienced no adverse pulmonary events. Furthermore, we observed no ramifications of excess mucus release upon initial treatment. All MCT parameters increased in CF rat tracheas, including a 3-fold increase in MCT in rats ingesting RGAE. This finding suggests that either 1) exocytosis of mucus from goblet cells with entrapped mucus has no impact on clearance on the time scales we examined them; or 2) potentiation of TMEM16A Cl− transport can overcome any deleterious impact of increased mucus load during an acute treatment period.
In the airways, the primary phenotype associated with the loss of CFTR function is a hyper-concentrated mucus gel that MCT mechanisms struggle to remove. Chronic bacterial infection ensues, progressing to loss of lung function.6 Current treatments for CF largely target the symptoms and physiologic manifestations of the underlying disease,7 with the exception of CFTR modulators, which can partially rescue the function of mutated CFTR to improve clinical outcomes in genetically suited patients.8 However, they cannot treat all patients nor restore lung function to normal levels. Our discovery of an oral, bioavailable TMEM16A potentiator that rescues the anion secretory defect and CF-mediated MCT dysfunction can be complementary to current therapies and could target patients with no options for CFTR modulator therapy.
Importantly, a TMEM16A potentiator will only potentiate channels that are endogenously activated. In our study, UTP was administered during the NPD to observe how much activation can be achieved (hyperpolarization, change in transepithelial potential) with maximal stimulation relative to CF rats that did not receive drug. This is similar to measuring hyperpolarization in response to isoproterenol (CFTR activation) in CF patients in CFTR modulator trials.2 Just as CF patients who are prescribed CFTR modulators do not need isoproterenol for the drug to have its observed effect, a TMEM16A potentiator would not require an exogenous activator of TMEM16A through P2Y2 signaling, like defenusol. Thus, the limitations of a treatment strategy with UTP and other P2Y2 analogues, including desensitization and chronic intracellular calcium release, are eliminated with use of a TMEM16A potentiator.
There are several limitations to the current study. While the CF rat has a mucus transport defect and abnormal histopathology, it does not develop spontaneous airway disease. The resolution of abnormalities of RGAE-treated CF rats may not be applicable in another in vivo model with concurrent or spontaneous airway infection and inflammation. Birket et al.14 instilled with CF rat airways with a mucoid clinical isolate of Pseudomonas aeruginosa (PAM57-15) at 6 months and the animals subsequently developed bacterial persistence through 28 days post-infection. Since the model had airway mucus occlusion and lingering inflammation, it will be crucial to repeat these experiments post-infection to simulate overproduced and viscous mucus observed in human CF airways. Other properties of the airway surface, particularly the impact on mucin structure and function will also require evaluation. Moreover, it will be important to assay the pH of the mucus or to analyze HCO3− activity in the ASL. Although orally bioavailable, the absorption of ginsenosides are impacted by intestinal secretions, digestive enzymes, and gut bacteria.56 To improve the bioavailability of these compounds, topical application of ginsenosides through inhalation or irrigation could improve the impact in the target area of the sinuses and lung. While the presumed mechanism of action is TMEM16A potentiation, further characterization of isolated ginsenosides in RGAE using single channel patch clamp analysis is warranted. Finally, other components of RGAE that are related to anti-inflammatory and anti-infective activity could be responsible for the intended effect and thus analysis of single ginsenosides will help determine specificity.
CONCLUSION
RGAE is the first known orally bioavailable compound capable of potentiating TMEM16A. Our in vivo study showed that RGAE significantly improves TMEM16A-mediated transepithelial Cl− secretion, functional microanatomy of the airway, and maxillary sinus mucosa histopathology. A therapeutic strategy utilizing TMEM16A potentiators to treat CF airway disease is promising and provides a new avenue for mutation-independent therapies that target the Cl− transport defect in CF.
Supplementary Material
Highlights.
Mutation-independent therapeutic strategies are needed for all CF patients.
Korean red ginseng aqueous extract (RGAE) has TMEM16a potentiating properties.
CF rats that ingested RGAE exhibited enhanced UTP-mediated Cl− secretion.
RGAE also improved Cl− secretion, mucociliary transport (MCT), and histopathology.
RGAE could serve as a therapeutic strategy to rescue the MCT defect in CF.
ACKNOWLEDGEMENTS
This work was supported by NIH/National Institutes of Allergy and Infectious disease (K08AI146220) and Cystic Fibrosis Foundation K08 Boost Award (CHO20A0-KB) to DYC; National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-05) to SMR; and National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-05), NIH/NCCIM (1R21AT012234-01), and Cystic Fibrosis Foundation Research Grant 002481G221 to BAW. We would like to thank the CFTR Rat Models Core in the UAB CF Research Center for providing animals for this study.
Footnotes
Conflict of Interest Statement: Bradford A. Woodworth, M.D. is a consultant for Cook Medical, Smith and Nephew, and Medtronic. All other authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Current opinion in pulmonary medicine Nov 2014;20(6):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marigowda G, Liu F, Waltz D. Effect of bronchodilators in healthy individuals receiving lumacaftor/ivacaftor combination therapy. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society Mar 2017;16(2):246–249. [DOI] [PubMed] [Google Scholar]
- 3.Zaher A, ElSaygh J, Elsori D, ElSaygh H, Sanni A. A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy. Cureus Jul 2021;13(7):e16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caputo A, Caci E, Ferrera L, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science Oct 24 2008;322(5901):590–594. [DOI] [PubMed] [Google Scholar]
- 5.Moss RB. Pitfalls of drug development: lessons learned from trials of denufosol in cystic fibrosis. The Journal of pediatrics Apr 2013;162(4):676–680. [DOI] [PubMed] [Google Scholar]
- 6.Kunzelmann K, Ousingsawat J, Cabrita I, et al. TMEM16A in Cystic Fibrosis: Activating or Inhibiting? Frontiers in pharmacology 2019;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Kim J-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. Journal of Ginseng Research 2014/07/01/ 2014;38(3):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H-M, Li S-L, Zhang H, et al. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. Journal of Pharmaceutical and Biomedical Analysis 2012/03/25/ 2012;62:258–273. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed T, Raza SH, Maryam A, et al. Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res Bull Jul 2016;125:30–43. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Liu Y, Chen K. Roles and mechanisms of ginsenoside in cardiovascular diseases: progress and perspectives. Sci China Life Sci Mar 2016;59(3):292–298. [DOI] [PubMed] [Google Scholar]
- 11.Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3 (Review). Int J Mol Med Mar 2017;39(3):507–518. [DOI] [PubMed] [Google Scholar]
- 12.Cho D-Y, Skinner D, Zhang S, et al. Korean red ginseng aqueous extract improves markers of mucociliary clearance by stimulating chloride secretion. Journal of Ginseng Research 2019/09/13/ 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danahay H, Fox R, Lilley S, et al. Potentiating TMEM16A does not stimulate airway mucus secretion or bronchial and pulmonary arterial smooth muscle contraction. FASEB bioAdvances Aug 2020;2(8):464–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birket SE, Davis JM, Fernandez CM, et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI insight Jan 11 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma J, Abbott J, Klaskala L, Zhao G, Birket SE, Rowe SM. A Novel G542X CFTR Rat Model of Cystic Fibrosis Is Sensitive to Nonsense Mediated Decay. Frontiers in physiology 2020;11:611294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birket SE, Davis JM, Fernandez-Petty CM, et al. Ivacaftor Reverses Airway Mucus Abnormalities in a Rat Model Harboring a Humanized G551D-CFTR. American journal of respiratory and critical care medicine Nov 1 2020;202(9):1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tipirneni KE, Cho DY, Skinner DF, et al. Characterization of primary rat nasal epithelial cultures in CFTR knockout rats as a model for CF sinus disease. The Laryngoscope Nov 2017;127(11):E384–E391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuggle KL, Birket SE, Cui X, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PloS one 2014;9(3):e91253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YS, Woo JY, Han CK, Chang IM. Safety Analysis of Panax Ginseng in Randomized Clinical Trials: A Systematic Review. Medicines (Basel) Jun 8 2015;2(2):106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So SH, Lee JW, Kim YS, Hyun SH, Han CK. Red ginseng monograph. J Ginseng Res Oct 2018;42(4):549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho DY, Zhang S, Skinner DF, et al. Ivacaftor restores delayed mucociliary transport caused by Pseudomonas aeruginosa-induced acquired cystic fibrosis transmembrane conductance regulator dysfunction in rabbit nasal epithelia. International forum of allergy & rhinology May 2022;12(5):690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim DJ, McCormick J, Skinner D, et al. Controlled delivery of ciprofloxacin and ivacaftor via sinus stent in a preclinical model of Pseudomonas sinusitis. International forum of allergy & rhinology Apr 2020;10(4):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho DY, Skinner D, Mackey C, et al. Herbal dry extract BNO 1011 improves clinical and mucociliary parameters in a rabbit model of chronic rhinosinusitis. International forum of allergy & rhinology Jun 2019;9(6):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazrak A, Jurkuvenaite A, Ness EC, et al. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. American journal of respiratory cell and molecular biology May 2014;50(5):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander NS, Hatch N, Zhang S, et al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. The Laryngoscope Jun 2011;121(6):1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. The Laryngoscope May 2010;120(5):1051–1056. [DOI] [PubMed] [Google Scholar]
- 27.Leung HM, Birket SE, Hyun C, et al. Intranasal micro-optical coherence tomography imaging for cystic fibrosis studies. Science translational medicine Aug 7 2019;11(504). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tipirneni KE, Grayson JW, Zhang S, et al. Assessment of acquired mucociliary clearance defects using micro-optical coherence tomography. International forum of allergy & rhinology Sep 2017;7(9):920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho DY, Mackey C, Van Der Pol WJ, et al. Sinus Microanatomy and Microbiota in a Rabbit Model of Rhinosinusitis. Frontiers in cellular and infection microbiology 2017;7:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Chu KK, Houser GH, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PloS one 2013;8(1):e54473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. The Laryngoscope Oct 2015;125 Suppl 7(0 7):S1–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PloS one 2014;9(8):e104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Blount AC, McNicholas CM, et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PloS one 2013;8(11):e81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander NS, Blount A, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. The Laryngoscope Jun 2012;122(6):1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. The Laryngoscope Sep 2011;121(9):1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodworth BA, Zhang S, Tamashiro E, Bhargave G, Palmer JN, Cohen NA. Zinc increases ciliary beat frequency in a calcium-dependent manner. American journal of rhinology & allergy Jan-Feb 2010;24(1):6–10. [DOI] [PubMed] [Google Scholar]
- 37.Cho DY, Zhang S, Lazrak A, et al. LPS decreases CFTR open probability and mucociliary transport through generation of reactive oxygen species. Redox biology Jul 2021;43:101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. American journal of rhinology & allergy Sep-Oct 2011;25(5):307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. The Laryngoscope Jul 2010;120(7):1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. The Laryngoscope Nov 2009;119(11):2269–2274. [DOI] [PubMed] [Google Scholar]
- 41.Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol Jul-Aug 1981;1(4):309–312. [DOI] [PubMed] [Google Scholar]
- 42.Grayson J, Tipirneni KE, Skinner DF, et al. Sinus hypoplasia in the cystic fibrosis rat resolves in the absence of chronic infection. International forum of allergy & rhinology Sep 2017;7(9):904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowe AR, Yue W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-protocol Dec 20 2019;9(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SM, Bae BS, Park HW, et al. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng Res Oct 2015;39(4):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo S, Chen Y, Pang C, et al. Ginsenoside Rb1, a novel activator of the TMEM16A chloride channel, augments the contraction of guinea pig ileum. Pflugers Archiv : European journal of physiology Jun 2017;469(5–6):681–692. [DOI] [PubMed] [Google Scholar]
- 46.Kimple AJ, Senior BA, Naureckas ET, et al. Cystic Fibrosis Foundation otolaryngology care multidisciplinary consensus recommendations. International forum of allergy & rhinology Jan 28 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spielman DB, Beswick DM, Kimple AJ, et al. The management of cystic fibrosis chronic rhinosinusitis: An evidenced-based review with recommendations. International forum of allergy & rhinology Dec 21 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tipirneni KE, Zhang S, Cho DY, et al. Submucosal gland mucus strand velocity is decreased in chronic rhinosinusitis. International forum of allergy & rhinology Apr 2018;8(4):509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banks C, Freeman L, Cho DY, Woodworth BA. Acquired cystic fibrosis transmembrane conductance regulator dysfunction. World journal of otorhinolaryngology - head and neck surgery Sep 2018;4(3):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho DY, Woodworth BA. Acquired Cystic Fibrosis Transmembrane Conductance Regulator Deficiency. Advances in oto-rhino-laryngology 2016;79:78–85. [DOI] [PubMed] [Google Scholar]
- 51.Caputo A, Caci E, Ferrera L, et al. TMEM16A, A Membrane Protein Associated with Calcium-Dependent Chloride Channel Activity. Science 2008;322(5901):590–594. [DOI] [PubMed] [Google Scholar]
- 52.Benedetto R, Cabrita I, Schreiber R, Kunzelmann K. TMEM16A is indispensable for basal mucus secretion in airways and intestine. FASEB journal : official publication of the Federation of American Societies for Experimental Biology Mar 2019;33(3):4502–4512. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Wang X, Wang H, et al. TMEM16A-Mediated Mucin Secretion in IL-13-Induced Nasal Epithelial Cells From Chronic Rhinosinusitis Patients. Allergy, asthma & immunology research Jul 2015;7(4):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin Y, Jiang Y, Sheikh AS, Shen S, Liu J, Jiang D. Interleukin-13 stimulates MUC5AC expression via a STAT6-TMEM16A-ERK1/2 pathway in human airway epithelial cells. International immunopharmacology Nov 2016;40:106–114. [DOI] [PubMed] [Google Scholar]
- 55.Yang N, Garcia MA, Quinton PM. Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis. The Journal of physiology Sep 15 2013;591(18):4581–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryu JS, Lee HJ, Bae SH, et al. The bioavailability of red ginseng extract fermented by Phellinus linteus. J Ginseng Res Mar 2013;37(1):108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


