Abstract
Mindfulness meditation is a contemplative practice informed by Buddhism that targets the development of present-focused awareness and non-judgment of experience. Interest in mindfulness is burgeoning, and it has been shown to be effective in improving mental and physical health in clinical and non-clinical contexts. In this report, for the first time, we used electroencephalography (EEG) combined with a neurophenomenological approach to examine the neural signature of “cessation” events, which are dramatic experiences of complete discontinuation in awareness similar to the loss of consciousness, which are reported to be experienced by very experienced meditators, and are proposed to be evidence of mastery of mindfulness meditation. We intensively sampled these cessations as experienced by a single advanced meditator (with over 23,000 hours of meditation training) and analyzed 37 cessation events collected in 29 EEG sessions between November 12, 2019, and March 11, 2020. Spectral analyses of the EEG data surrounding cessations showed that these events were marked by a large-scale alpha-power decrease starting around 40 seconds before their onset, and that this alpha-power was lowest immediately following a cessation. Region-of-interest (ROI) based examination of this finding revealed that this alpha-suppression showed a linear decrease in the occipital and parietal regions of the brain during the pre-cessation time period. Additionally, there were modest increases in theta power for the central, parietal, and right temporal ROIs during the pre-cessation timeframe, whereas power in the Delta and Beta frequency bands were not significantly different surrounding cessations. By relating cessations to objective and intrinsic measures of brain activity (i.e., EEG power) that are related to consciousness and high-level psychological functioning, these results provide evidence for the ability of experienced meditators to voluntarily modulate their state of consciousness and lay the foundation for studying these unique states using a neuroscientific approach.
Keywords: cessation, fruition, Nirodha, EEG, mindfulness, meditation, alpha activity, theta activity
Introduction
Scriptures dated over two thousand years ago describe an extraordinary event that can happen during the course of intensive meditation practice (Ñāṇamoli & Bodhi, 1995). In the event known as cessation (or nirodha in Pāli), the practitioner briefly loses consciousness. Upon re-awakening, the meditator is said to experience significant changes to how their mind works, including a sudden sense of profound mental and perceptual clarity (Grabovac, 2015; Ingram, 2018; Sayadaw, 1994). The idea that a meditator can “turn off” consciousness from within may have broad implications for our understanding of how cognition works (Laukkonen et al., 2022, in press). Moreover, the neural correlates of ‘cessation’ may be revealing about the necessary brain activity for the emergence and deconstruction of consciousness. Unlike propofol (general anesthesia) or ketamine-induced unconsciousness, the neural correlates of cessation are not confounded by the presence of a drug. The transition into and out of cessation is also faster and more discontinuous than sleep, and therefore may provide a precise window on changes in level of consciousness.
Meditation research has undergone several phases in the last four to five decades. In the first phase centering around the 1970s, a handful of methodologically weaker studies were conducted on different kinds of meditation, including predominately transcendental meditation (e.g., Wallace, 1970). In the second phase beginning around the 1980s, mindfulness especially in the context of attention, emotion, and stress regulation became a hot topic, garnering widespread popularity in media, and applications in clinical psychology (Kabat-Zinn et al., 2011; Kabat-Zinn and Burney, 1981). Scientific research also surged exponentially, with a recent trend toward careful consideration of methodology and critiques of over-selling the “panacea” of mindfulness (Van Dam et al., 2018). Arguably, we are now in a third phase of empirical research that, in addition to ongoing research on mindfulness, investigates the cognitive-neuroscientific mechanisms underlying different forms of meditation and what they reveal about the mind (Dahl et al., 2015; Lutz et al., 2008; Slagter et al., 2011). Within this third phase, there is a select number of researchers who are focusing on advanced stages or states of meditation involving highly experienced meditators (Berkovich-Ohana, 2017; Dor-Ziderman et al., 2013; Laukkonen & Slagter, 2021; Metzinger, 2020; Paoletti et al., 2022), propelled by a need for a deeper phenomenological understanding of the mental (and neural) activity during meditation (Sparby and Sacchet, 2022).
The present research falls into this subset which aims to, in a complementary way, unveil the mechanisms underlying unusual states of (non)consciousness triggered by meditation, and in doing so, garner novel insights about how the mind works. Below we briefly introduce the novel state under investigation here and then review existing evidence and theory about the state.
From a Theravada Buddhist perspective, cessations occur during prolonged meditation as a culmination of the stages of insight (Berkovich-Ohana, 2017; Grabovac, 2015; Ingram, 2018; Sayadaw, 2016, 1994). Put simply, the stages of insight are signposts along the meditator’s journey that are characterized by specific changes to emotions, cognitions, perceptions, feelings, and consciousness. Cessations are specifically associated with the final stage, sometimes called “fruition” or phala in Pali (Berkovich-Ohana, 2017; Grabovac, 2015; Sayadaw, 2016). An expert meditator can quickly and efficiently pass through these stages, thus creating the conditions for a ‘cessation’ (absence of consciousness and associated inner transformations) to spontaneously occur. Notably, these spontaneous cessation events tend to occur during deep meditation and are relatively short episodes – typically lasting only several seconds (Sayadaw, 1994).
During cessation, consciousness is momentarily absent (as reported by the meditator), and there may also be subsequent awareness of this absence having occurred. However, unlike sleep, there is almost no awareness of time having passed. For the purposes of the present paper, the nuances of the Buddhist context will be put aside in favor of focusing on the cognitive and neuroscientific implications of a meditation-induced unconsciousness. For a recent and detailed review of cessation, its different forms, and its historical and phenomenological context, see (Laukkonen et al., 2023).
So far, to our knowledge, there has been only one neuroimaging study investigating cessation. Berkovich-Ohana (2017) analyzed data from two adept meditators as they experienced three cessations each (i.e., a total of six). The authors reported global long-range gamma (25–45 Hz) phase synchronization during states of cessation as compared to non-cessation states, but no difference in functional connectivity in the other frequency bands. From a general non-meditation perspective, unconsciousness induced through drugs such as ketamine (Blain-Moraes et al., 2014) and propofol (Kallionpää et al., 2020; Lee et al., 2013) is commonly associated with alpha anteriorization, i.e., a spatial shift in alpha power from posterior to frontal regions. An important research endeavor would be to examine whether the cessation-induced loss of awareness has similar neural and experiential characteristics to that of drug-induced unconsciousness.
Current theoretical perspectives on cessation are limited. However, one proposal is that a cessation in consciousness occurs due to the gradual deconstruction of hierarchical predictive processing as meditation deepens, ultimately resulting in the absence of consciousness (Laukkonen et al., 2022, in press; Laukkonen & Slagter, 2021). In particular, it was proposed that advanced stages of meditation may disintegrate a normally unified conscious space, ultimately resulting in a breakdown of consciousness itself (Tononi, 2008, 2004). This idea is supported by the fact that meditation often involves an active deconstruction of the elements of experience (Dahl et al., 2015; Laukkonen et al., 2023).
Previous research on cessation is limited for various reasons. First, few expert meditators have reached a level of meditation where cessations occur. Second, cessations are difficult to predict and, therefore, challenging to reliably induce in laboratory conditions (Ingram, 2018). Third, capturing cessations requires a careful neurophenomenological methodology that combines self-report with continuous neural recording with high temporal sensitivity. In the present study, we overcame these challenges in the following way. We recruited one expert meditator who reported being able to enter and report multiple cessation events as they emerged throughout repeated meditation sessions. Using a neurophenomenological approach, systematic ‘first-person’ phenomenological criteria of cessations were used to select events for subsequent EEG-based analysis (Lutz and Thompson, 2003). This approach of extensively sampling the event of interest (i.e., cessation) in a single participant has proved to be a powerful tool in neuroscience to derive insights beyond a single measurement (Poldrack et al., 2015). The participant for this study was ideal for this role, having extensive experience with the cessations and prior experience in neuroimaging environments (EEG, fMRI). Based on our prior studies (van Lutterveld et al., 2017) and other studies that have identified meditative states to be primarily associated with alpha power (Fell et al., 2010; Lomas et al., 2015), we hypothesized that cessation events would be associated with the large-scale modulation of brain activity in the alpha band. Nevertheless, we conduct exploratory analyses to examine power differences in other canonical frequency bands (delta, theta and beta), given evidence of the meditative state modulating EEG power across multiple frequency bands (Fell et al., 2010; Lomas et al., 2015)
Methods
Participant
The present report is a case study of a single male adept meditator (author DMI), aged 51 years old at the time of data collection. The participant is a long-term meditation practitioner and teacher with 26 years of meditation experience at the time of data acquisition. Throughout his meditation practice, the participant has practiced a variety of traditions including Mahasi noting, formed and formless jhanas, Kasina meditation, and Vipassana or Insight meditation (Ingram, 2018). Based on an estimated one hour of daily practice since the start of practice and approximately one year of retreat at 14 hours per day, we estimate the participant to have a total practice amount of 23,110 hours. Important to the current study, the participant reported being able to incline towards cessations as the target of meditation. The Western Institutional Review Board (WIRB) approved the study, and the participant provided informed consent.
EEG Data Acquisition
Data were recorded with a Quick-20 EEG system using a cap with 20 dry electrodes (Cognionics, San Diego, CA, USA; refer to Supplementary Figure S2 for a layout) using a sampling rate of 500 Hz. Twenty-nine EEG runs were acquired (length ranging between 426–3,653 s) while the participant performed meditation of two primary types (Vipassana, specifically “review practice”, where the inclination was to the target of cessation (Bhikkhu, 2010), and fire kasina (Bhikkhu, 2010; Stein and Ingram, 2017), a traditional Theravada practice focusing on internally-generated visual sensations) with eyes closed. Cessations were accompanied by an involuntary movement artifact (eye movements such as fluttering and sometimes squinting of the eye muscles) which also contained a large eye blink1 that can be easily distinguished from the rest of the run conducted with eyes closed, and these eye blinks were used to identify onsets of cessations by examining the raw EEG data. We also ensured that our identification of the cessation was accurate by examining the inter-rater reliability in marking the time of the eye-blink: the participant and one of the other authors independently inspected the EEG runs to assess the onsets of cessations and reported the same location for the eye-blinks. This eye-blink approach was validated in a run with seven cessations in which cessations were marked in the time frame afterward by button-press. Over these seven cessations, the median time between (cessation-related) artifact onset and marker was 3.6 s (range 2.1 – 7.2). The short (<10 s) interval between the cessation-related artifact and the button presses confirm that these events were very short, as described in previous literature (Laukkonen et al., 2023; Sayadaw, 1994). Despite being short in duration, these experiences have unique phenomenology that is distinguished and systematically graded (see Supplementary Text) by the advanced meditator studied here. Figure 1 provides an example of a single cessation event, and these events were visually consistent across runs (see Supplementary Figure S1 for additional examples). Button-presses were excluded from the other runs, which minimized confounding monitoring processes (van Lutterveld et al., 2013).
Figure 1.
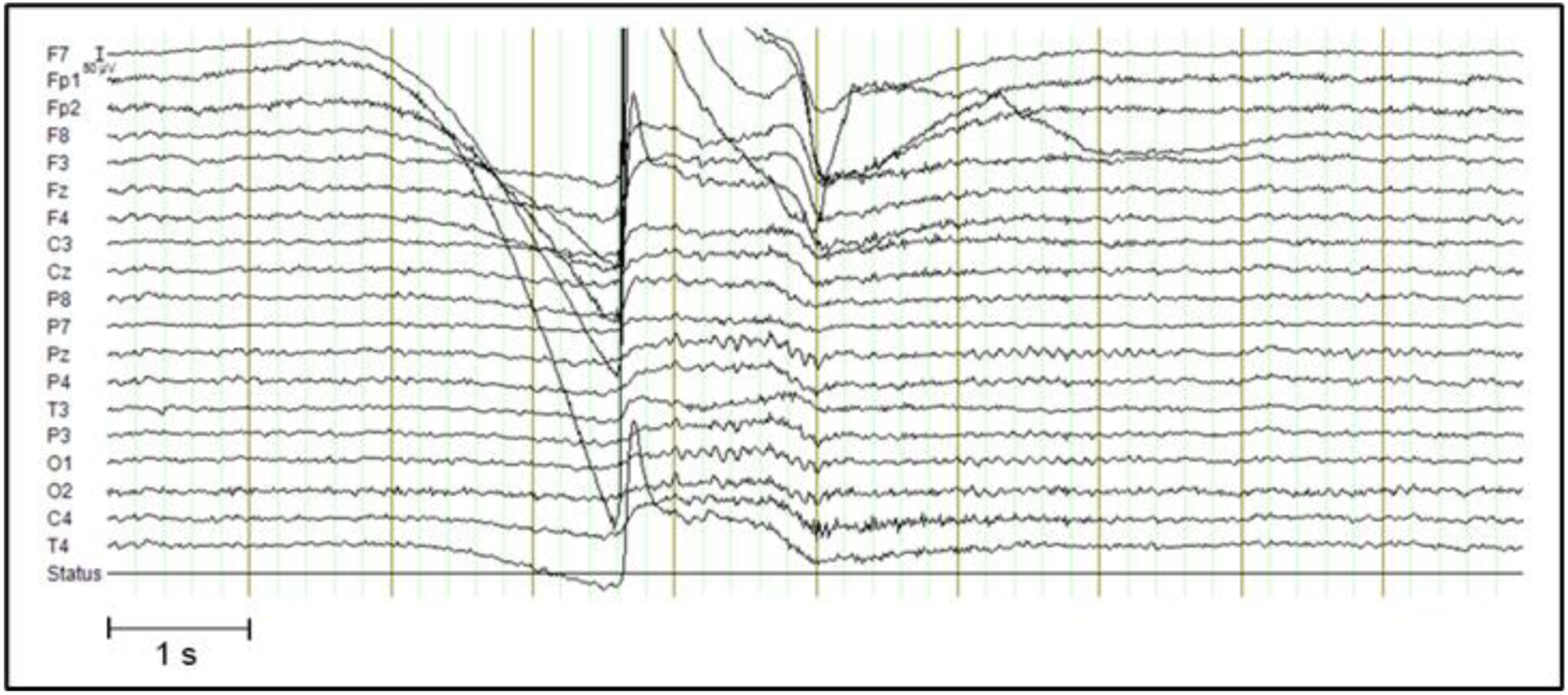
Example of a cessation. Artifactual data around the cessation (± 3 seconds) was not analyzed. See Supplementary Figure S1 for other examples.
To compare the experience of cessation to non-cessation control portions of the meditation, specific segments of the EEG data that were not marked as cessation were selected. This selection involved identifying time points in the EEG time course of the entire meditation run using an automated algorithm and labeling them as “control markers”. These markers were required to meet the following criteria: at least 40 s after the start or 40 s before the end of the run, at least 160 s from the nearest cessations to minimize chances of carry-over effects from cessations, and not overlapping with other control segments. 27 control markers – the maximum possible number of markers based on these selection criteria – were randomly added to the data.
Phenomenology
Immediately after each run, the quality of any cessations that occurred was graded by the participant. Here we employed a neurophenomenological approach, where systematic ‘first-person’ descriptions of experience are related to objective or ‘third-person’ neuroimaging data (Lutz and Thompson, 2003). Specifically, our phenomenologically trained subject systematically evaluated the mental and physiological processes relevant to cessations (setup, entrance, event, exit, aftereffects) as he experienced them, and these evaluations were used to cluster and select events (i.e., high-grade cessations) for subsequent EEG-based analysis. See Supplementary Text S1 for additional information on the description and grading of cessations. Forty-six Grade A, twelve Grade B, and eleven Grade C cessations were acquired.
We selected grade A cessations for further analysis as these most likely represented the purest form of cessations. To minimize carry-over effects from adjacent cessations, an interval of at least 80 s between cessations was an additional criterion for inclusion. Furthermore, cessations were required to occur at least 40 s after the start or 40 s before the end of the run. One dataset was excluded from the analysis as it was not readable by the analysis software due to a technical glitch. Subsequent analyses were thus conducted on 37 cessations that met inclusion criteria.
Data Analysis
Preprocessing
Data were preprocessed in a manner similar to van Lutterveld et al. (2017). Briefly, the BrainVision Analyzer software suite was used for preprocessing (BrainProducts, Munich, Germany). Data were visually inspected for bad channels, which were subsequently removed from analysis (no more than 2 channels for any run, see supplementary). EEG data were filtered using a Butterworth Infinite Impulse Response (IIR) filter between 0.5 and 100 Hz with a slope of 48 dB/octave, and subsequently segmented in segments of 1024 samples (2.048 s) in the 39.936 s pre-cessation to 39.934 s post-cessation timeframe. As the EEG system had active shielding to remove electrical artifacts, and our power analyses were restricted to a maximum of 45Hz, we refrained from using a notch filter to remove 50/60 Hz line noise.
After this, automated artifact identification was performed using the following parameters: (1) maximal allowed voltage step: 50 μV/ms; (2) maximal allowed difference of values in 200 ms intervals: 200 μV; (3) minimal allowed amplitude: −100 μV and maximal allowed amplitude: 100 μV; (4) lowest allowed activity in 100 ms intervals: 0.5 μV; All data was visually inspected by an experienced EEG scientist (RvL) to identify artifacts in the data that were not picked up by the automated artifact rejection algorithm, these were mainly motion/muscle activity. Segments containing artifacts were removed from the analysis. Identical to the cessations, we extracted ~80 s time series around control markers. The control data segments underwent the same preprocessing steps as the cessation data.
Spectral Analysis
The power spectrum was calculated using a Fast Fourier transform with a Hanning window taper between 1 and 45 Hz at 0.5 Hz frequency intervals. In order to isolate the specific contribution of oscillatory contributions to spectral power (isolated from 1/f activity), we performed Irregularly Resampled AutoSpectral Analysis (IRASA; Wen & Liu, 2016) on the preprocessed signal using Fieldtrip software (Oostenveld et al., 2011). This method allows the examination of task-related EEG power by removing the non-oscillatory, potentially baseline, power traces (He, 2014). The resulting spectra from the IRASA algorithm was divided into the following frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–45 Hz). The power spectrum was calculated for each data segment separately.
In order to look at the temporal dynamics of spectral power before and after the cessation onset, data were binned into four consecutive time frames. Timeframe 1 ran from 39.93–21.50 s pre-cessation (segments 1–9), and timeframe 2 from 21.50–3.07 s pre-cessation (segments 10–18), while timeframe 3 ran from 3.07–21.50 s post-cessation (segments 22–30) and timeframe 4 from 21.50–39.93 s post-cessation (segments 31–39). The segment containing the cessation as well as the segments directly preceding and following the cessation were omitted from the analysis due to artifacts (because of movements associated with the cessation).
Time-averaged Power Differences
For each time frame, we averaged the power spectrum across all segments within that time frame for each of the 37 cessation runs to look at time-averaged differences in power between cessation and control data. Similar time-averaged power values were derived for the 27 control runs. Cluster-based permutation testing of t-statistics with Fieldtrip was used to determine channels of significant differences between cessation and control data for each time frame and frequency band. Cluster randomization analysis was used to control for multiple comparisons of power differences with an initial cluster-forming threshold of p = 0.05 repeated for 7,500 permutations. Two-tailed hypothesis testing was used for all analyses. A cluster was considered significant if its Monte Carlo probability for each tail exceeded the threshold of 0.05/2 = 0.025. Following our hypothesis, we focus on the modulation of alpha power surrounding cessations but also conduct exploratory analyses for the other frequency bands. Due to the exploratory nature of these analyses, we did not correct for multiple comparisons for the different frequency bands.
Linear Trends in Power Differences
Since cluster-based permutation tests do not provide definitive spatial information on differences in power spectrum between conditions (Sassenhagen and Draschkow, 2019), and our time-averaged analyses lack temporal resolution, we also looked at linear trends in EEG power pre and post-cessation for a-priori-defined regions. Specifically, we look at the temporal dynamics of power in specific regions-of-interest (ROIs). These ROIs corresponded to the major cortical areas (frontal, frontopolar, central, right temporal, left temporal, parietal, and occipital; see supplementary for ROI definitions).
For each of the 36 time segments (18 pre-cessation, 18 post-cessation), and in each ROI, the power values across all runs were averaged to derive a single power value for each approximately 2 s time segment2. Using the averaged values, modulation of power differences between cessation and control across time was examined in several steps: For each ROI, (1) Friedman’s test was used to look at overall differences in power across the four time frames; (2) If Friedman’s test revealed modulation across time frames for a specific ROI (FDR corrected across the total number of ROIs, i.e., 7), follow-up linear regressions were performed to explore linear changes, with time (i.e., segment number) as a predictor and the averaged power values as the dependent variable. Separate regression analyses (with 10000 parametric bootstraps) were performed for the entire timeframe leading up to cessations and the timeframe after cessations (containing either both time frames 1 and 2 or both time frames 3 and 4). Each set of regression results (for a specific ROI) was corrected for multiple comparisons using FDR correction (Benjamini and Hochberg, 1995), across the number of regressions performed3 for a specific frequency band. In addition to the alpha and theta bands, exploratory analyses were conducted for frequency bands that did not show time-averaged power differences.
Results
Decrease in Alpha Power
For time-averaged analysis of EEG power differences between cessation and control, the channel displaying the absolute maximum t-statistic is reported in the results, along with the cluster p-value. Cluster-based permutation tests across the whole brain revealed a pattern of statistically-marked decreases in alpha band power originating pre-cessation in the occipito-parietal region (timeframe 1, 39.93–21.50 s pre-cessation: max. t(62) = −2.82, cluster p = 0.018, Cohen’s d4 = 0.95) and subsequently intensifying and becoming spatially-widespread until immediately post-cessation (timeframe 3, 3.07–21.50 s post-cessation: max. t(62) = −7.06, cluster p = 1.33e-04, Cohen’s d = 3.79), before returning to pre-cessation levels (timeframe 4, 21.50–39.93 s post-cessation: max. t(62) = −3.10, cluster p = 0.004, Cohen’s d = 0.96) (see Figure 2, right panel). Looking at whole-brain averaged power (Figure 2, right panel) in the different timeframes also confirms that a change in power surrounding cessations drives the effects– i.e., control power does not change across time. Results also revealed a modest but significant increase in theta oscillations in the timeframe immediately pre-cessation (timeframe 2, 21.50–3.07 s pre-cessation: max. t(62) = 3.50, cluster p = 0.006, Cohen’s d = 0.57), most pronounced in the right temporo-parietal area. In the timeframe post-cessation, this changed into a spatially-limited, central decrease in theta power (timeframe 3, 3.07–21.50 s post-cessation: max. t(62) = −3.80, cluster p = 0.009, Cohen’s d = 1.05). Power in the delta and beta frequency bands were not significantly different surrounding cessations5.
Figure 2.
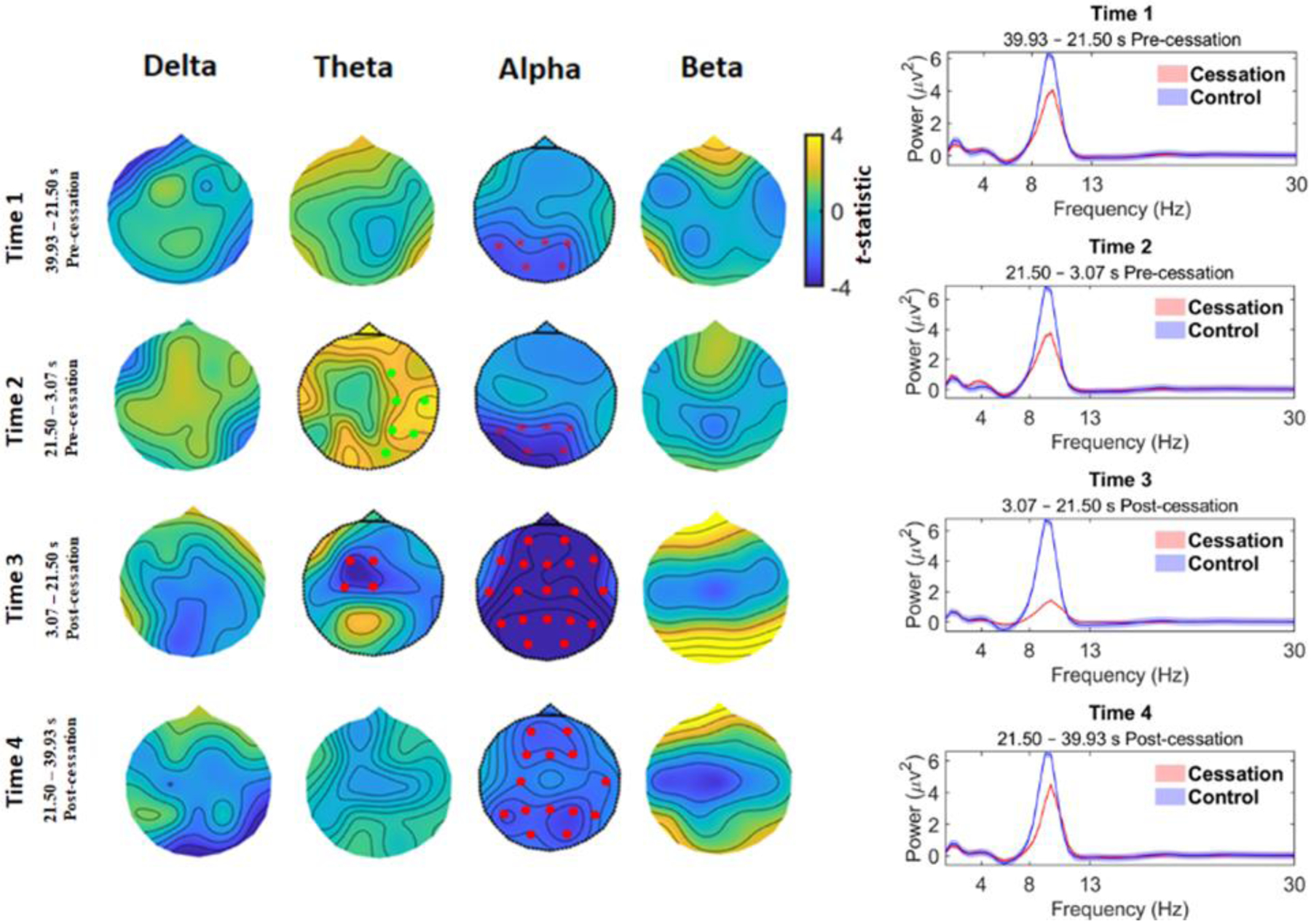
Time-averaged EEG results. Left: The comparison of cessation versus control for changes in spectral activity reveals significant decreases for the alpha band originating 40-sec pre-cessation in the occipito-parietal cortex and gradually spreading to the whole brain at timeframe 3 immediately post-cessation. At timeframe 4 post-cessation, alpha power starts to return closer to pre-cessation levels. There is also a spatially limited (right temporo-parietal) and modest increase in theta power immediately pre-cessation (timeframe 2), that evolves into a theta decrease immediately post-cessation. Red dots correspond to clusters (cessation < control), p < 0.01 and asterisks for clusters p < 0.05. Green dots correspond to clusters where (cessation > control), p < 0.01. Right: Grand-average spectral power for cessation (red line) and control (blue line) in the four timeframes.
Linear Trends Results
Friedman’s test revealed overall differences in power in the alpha band across time for all ROIs (see Supplementary Table S1 for statistics). Examination of linear trends by ROIs confirmed that alpha power decreased in the pre-cessation timeframe, and this decrease was most pronounced in the occipital and parietal regions of the brain (Figure 3, Left). For these regions, time negatively predicted alpha power differences in the entire pre-cessation time frame (time frames 1 and 2 combined; occipital: β = −0.16, 95% CI = [−0.26, −0.06], p = 0.009; parietal: β = −0.07, 95% CI = [−0.13,−0.02], p = 0.022), and positively predicted alpha power differences in the entire post-cessation time frame (time frames 3 and 4 combined; occipital: β = 0.36, 95% CI = [0.25,0.47], p = 5.99e-04; parietal: β = 0.20, 95% CI = [0.14,0.27], p = 4.99e-04). This trend of pre-cessation decreases in power, and post-cessation return to normal was also significant at the whole-brain level. For all other ROIs, time predicted alpha differences (i.e., return towards pre-cessation levels) only in the post-cessation time frame.
Figure 3.
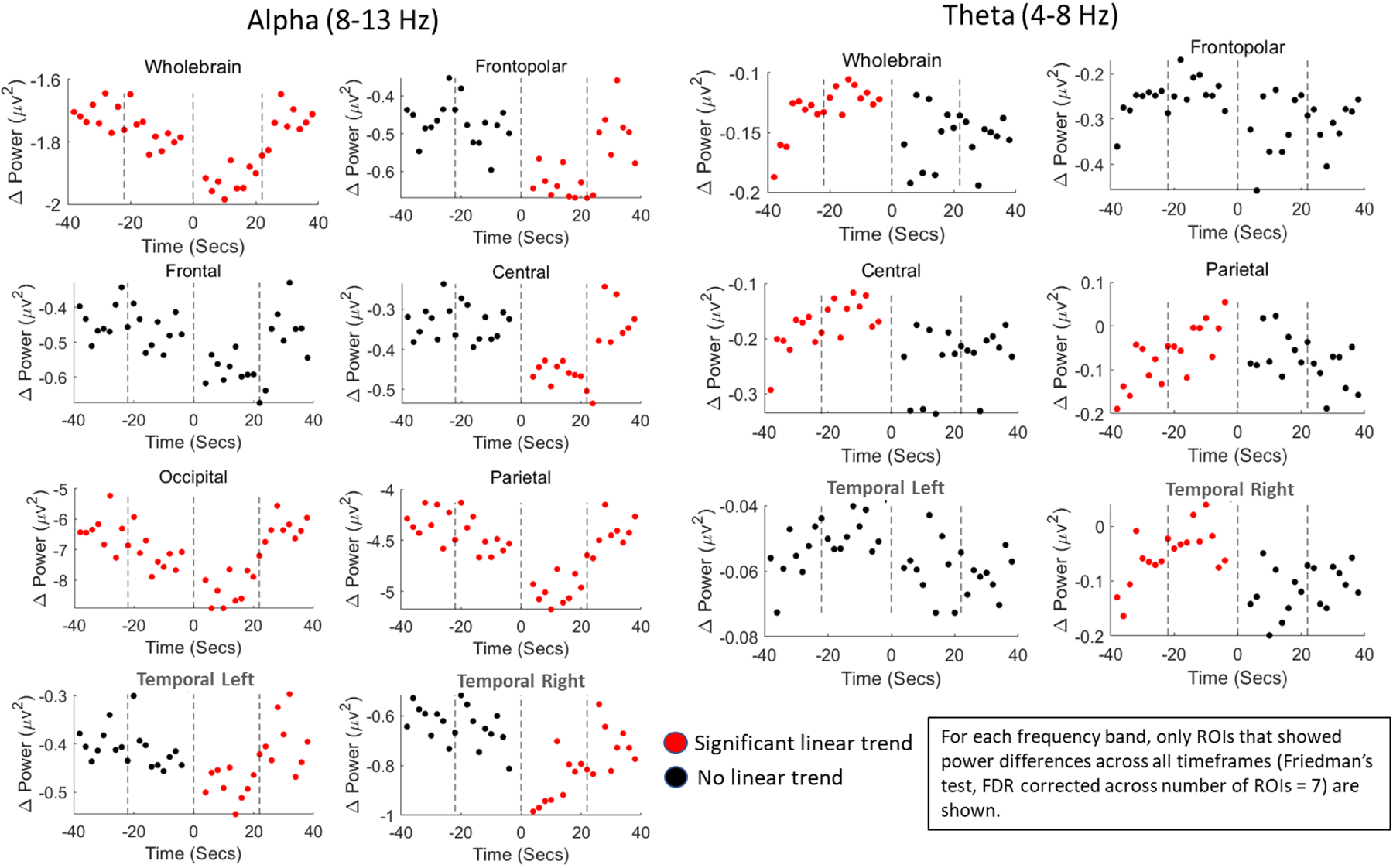
Linear trends in power differences (cessation - control) conducted at the region-of-interest (ROI) level. Left: In the occipital and parietal regions, alpha power linearly decreased for the entire pre-cessation timeframe, with the lowest values being immediately post-cessation. Subsequently, in timeframes 3–4 (3.07–39.93 s post-cessation), power differences linearly returned to pre-cessation levels. For all other ROIs, this linear trend was only significant for the post-cessation timeframe (red dots indicate a significant linear trend for that timeframe). Right: There were also modest increases in theta power for the central, parietal, and right temporal ROIs in the pre-cessation timeframe (timeframes 1–2, 39.93–3.07 s pre-cessation), such that power differences between control and cessation became close to zero. There were no significant post-cessation trends in theta power.
Linear regression analysis also revealed that theta band power differences between cessation and control data in the central, parietal, and right temporal regions were reduced in the entire pre-cessation timeframe, as pre-cessation theta power linearly increased during that timeframe (time frames 1 and 2 combined; central: β = 0.01, 95% CI = [0.004, 0.02], p = 0.007; parietal: β = 0.02, 95% CI = [0.01,0.03], p = 0.001; right temporal: β = 0.01, 95% CI = [0.003, 0.02], p = 0.015). There were no linear trends for any ROI in the post-cessation timeframes for the theta band (Figure 3; Right; Supplementary Table S2).
Discussion
Our study is the first to examine the electrophysiological signature of a unique state of altered consciousness, namely cessations, as extensively sampled from an adept meditator during mindfulness meditation. We found that cessations were marked by a large-scale alpha-power decrease that was already visible around 40 seconds prior to their onset, and that this alpha-power was lowest immediately following a cessation. Region-of-interest (ROI) based examination of this finding revealed that the alpha-suppression was most pronounced over the occipital and parietal regions of the brain, and linearly decreased with time for the entire pre-cessation duration.
Following the cessation, alpha-power also showed a linear trend of return to pre-cessation (i.e., 40 s prior) levels. Additionally, there were modest increases in theta power for the central, parietal, and right temporal ROIs during the pre-cessation timeframe. By relating cessations to objective and intrinsic measures of brain activity (i.e., EEG power) related to consciousness, these results validate the ability of experienced meditators to voluntarily modulate their state of consciousness and lay the foundation for studying these unique states using a neuroscientific approach.
The gradual decrease in alpha power prior to cessation is consistent with our theoretical assumption that cessations are the consequence of attenuated hierarchical processing in the brain. Increased alpha power has been established as an index of top-down processing and high-level psychological functioning (Klimesch, 2012). Specifically, alpha oscillations may aid in predicting and thereby facilitating the processing of lower-level sensory inputs (Mayer et al., 2015), effectively increasing the brain’s efficiency in interacting with the environment through inhibitory mechanisms. Alpha-suppression, therefore, may be related to the disintegration of this hierarchical cognition and related inhibition. Our finding that this alpha-power decrease follows a linear trend of decline in the occipito-parietal areas suggests that it is indeed an attenuation of the posterior alpha rhythm commonly associated with inhibition of external attention (in favor of internally focused attention; Cooper et al., 2003). In contrast to the anteriorization of alpha in drug-induced unconsciousness (Kallionpää et al., 2020), the suppression in post-cessation global alpha power may be an indication of renewed wakefulness (Bazanova and Vernon, 2014), which some meditators (including the participant in the current study) describe as a kind of inner “reset”, and differentiates this experience from general anesthesia.
In this regard, the hierarchical cognitive processing/representations made by humans, here associated with suppressed alpha oscillations, is a process best described as “abstraction” (Badcock et al., 2019; Friston, 2008; Gilead et al., 2020; Huntenburg et al., 2018; Raut et al., 2020; Taylor et al., 2015). Laukkonen & Slagter (2021) proposed that meditation gradually reduces this tendency to abstract by bringing the mind closer to the present. Thus, as meditation deepens, high-level abstractions that increase the temporal depth of processing – such as counterfactual and future-oriented judgments – decrease. It is possible that reductions in abstraction may also release the processes underlying wakefulness, resulting in a state without consciousness, as in cessations (Laukkonen et al., 2022, in press).
Interestingly, a number of previous studies have reported an increase in alpha power during mindfulness meditation (Lee et al., 2018; Lomas et al., 2015), which enhances present-moment awareness, whereas we found cessation events are experienced following a gradual decrease in whole-brain alpha oscillations. A decrease in resting-state alpha synchronization is also found in disorders of consciousness (e.g., coma), where alpha power decreases (and delta increases) with increasing levels of unconsciousness (vegetative state < minimally conscious state) (Wutzl et al., 2021). Of note, although theta power changes in these states are less studied and report mixed results, there is a trend of higher power ratio index (i.e., the ratio of power in delta and theta frequency bands to that in alpha and beta frequency bands) with increasing unconsciousness levels (Coleman et al., 2005). Therefore, cessations may be more closely related to a state of unconsciousness than other states of meditation. More generally, our findings highlight the need for examining distinct stages/states in meditation, as it can help to relate the state-specific phenomenological experience to its unique underlying neural representation.
Increased theta power is another key feature of meditation and may signify a state of relaxed alertness (Fell et al., 2010; Lomas et al., 2015). Generally, theta oscillations may reflect the inhibition of task-irrelevant brain areas (Snipes et al., 2022), both during cognitive demands (Cavanagh and Frank, 2014; Ratcliffe et al., 2022) and temporally local sleep (Siclari and Tononi, 2017). The increase in theta oscillations prior to cessations observed in our study may, therefore, indicate inhibition of certain regions in favor of facilitation in others. This is consistent with the notion that advanced stages of mindfulness meditation engages more effortless control of cognition (Tang, 2017; Tang et al., 2015). Theta increases in our study may be a marker of this selective control to attain cessation (i.e., controlled internal attention).
Interestingly, our findings of pre-cessation alpha suppression, combined with increased theta is similar to some findings in psychedelic experiences (Timmermann et al., 2019), which is pertinent given parallels that have been drawn between these two domains of inquiry (Millière et al., 2018; Senthilingam, 2016; Timmermann et al., 2023). Specifically, the collapse of alpha coupled with the emergence of theta might be neural signatures of ‘visionary’ states (Carhart-Harris, 2007; Maquet et al., 1996), where the mind is profoundly engaged in endogenous processing such as dreaming. However, psychedelic states have also been associated with a reduction in theta power (Carhart-Harris et al., 2016; Valle et al., 2016), and may therefore be neurally different from cessations. The cessations reported in the current study are transient states of (non)consciousness and are different from the relatively longer psychedelic experiences. Nevertheless, the slow and linear modulation of pre and post-cessation alpha (spanning at least 40 s in both directions) suggest a complex temporal dynamic and possibly distinct phenomenological experiences at each timeframe. Given the revamped interest and emerging evidence for the efficacy of using psychedelics as a treatment for treating psychiatric conditions, especially depression (Davis et al., 2021), future research may benefit from examining how the neurophenomenology of advanced meditative states (such as cessations) relates to that of psychedelic experiences and how it may similarly affect psychopathology.
The only other EEG study to investigate the neuroscience of cessations (Berkovich-Ohana, 2017) reported a significant increase in overall long-range gamma (25–45 Hz) synchronization with a sample of only six fruition events. In the present study, we focus on whole-brain and regional power signatures to characterize cessations and find limited evidence for gamma-modulation related to cessations (Supplementary Figure S2), and generally refrain from interpreting gamma power changes as they concur with muscle artifacts (Whitham et al., 2007). Although inter-regional synchronization and intra-regional power represent separate neural processes, several other differences between the studies (e.g., a greater number of cessation events in our data, the difference in signaling method used by the meditators to indicate a cessation, and the use of different meditators in their study) may explicate the distinct results.
Limitations
The current study used a single case design in which we extensively sampled cessation events from a single adept meditator (cessation N = 37). This approach of extensively sampling events of interest (i.e., cessation) in a single participant has proved to be a powerful tool in neuroscience to derive insights beyond a single or several repeats of a measurement (Levenson et al., 2012; Poldrack et al., 2015). Despite this case-study design being a major strength of the current study, such a protocol simultaneously reduces the generalizability of the current results, that is, it may be that the alpha suppression effect observed may be idiosyncratic to the participant. Nevertheless, the EEG power values in our study (especially for ROIs that showed significant time-related trends surrounding cessions) showed moderate to good inter-run reliability (Supplementary Table S3), providing additional confidence in the neural homogeneity of these cessation events. Our post hoc identification of cessation events was also possible due to the participant’s distinctive involuntary eye-blink response to cessations, and future research may identify additional objective markers of cessations to examine if they and other phenomenological qualities of the experience can explain heterogeneity of neural measures (e.g., variability in alpha power). Similarly, we rely on the phenomenology reports of a single participant from a specific tradition of meditation practice, to define the quality of cessations, and as with all subjective reports, these reports may suffer from response biases. Future studies with multiple participants of varying tradition and proficiency in levels of meditation practice will allow for the examination of the broader validity of the current findings. We should also note that the alpha suppression we observed immediately following a cessation partially resembles the ‘Berger effect’—that is, the suppression of alpha amplitude in response to eyes opening from an eyes-closed state. However, whereas the Berger effect occurs only after the opening of eyes, we observe a linear trend of alpha-suppression almost 40 secs prior to the onset of cessations (marked by an involuntary eye-blink and related eye movements), which is indicative of a gradual cognitive process unrelated to the Berger effect.
Additionally, the control datasets used in our study differ from the cessations in that they do not contain an eye-blink and may be said to ignore any associated anticipatory processes related to these blinks. However, the eye blink associated with a cessation event is involuntary, whereas a control condition with voluntary eye blinks may erroneously introduce findings related to this difference in volition (Pfurtscheller and Da Silva, 1999). The comparison of these two conditions may, therefore, be rendered invalid. In contrast, by comparing the cessations with blink-free control datasets, we are able to examine the unconfounded modulation of alpha power surrounding cessations.
Conclusion
In today’s research climate, studies on altered states of consciousness are growing in popularity (Carhart-Harris and Friston, 2019; Millière et al., 2018b). Within studies on meditation, unique states of consciousness such as non-dual awareness and pure consciousness (Josipovic, 2019, 2010; Travis and Pearson, 2000) and other alterations in self-experiences (Nave et al., 2022) are becoming high-interest topics of research. Some even consider that these unique states hold the keys to understanding consciousness itself (Metzinger, 2020). Our findings provide initial insight into the mechanisms that underly the unusual capacity to induce momentary void of consciousness during cessations through the large-scale modulation of brain activity. We provide the groundwork for future studies that aim to examine distinct altered states of consciousness that emerge from advanced meditation and therefore may be informative regarding the nature of consciousness in general.
Supplementary Material
Acknowledgments
The authors jointly affirm that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained. The Western Institutional Review Board (WIRB) approved the study, and the participant provided informed consent. Dr. Sacchet and the Meditation Research Program are supported by the National Institute of Mental Health (Project Number R01MH125850), Dimension Giving Fund, Ad Astra Chandaria Foundation, Brain and Behavior Research Foundation (Grant Number 28972), BIAL Foundation (Grant Number 099/2020), Emergence Benefactors, The Ride for Mental Health, Gatto Foundation, and individual donors.
Footnotes
Conflict of interest statement: Authors AC, RvL, REL, and HAS have no conflict of interest. DMI has authored the book Mastering the Core Teachings of the Buddha that this paper references. He volunteers to run as Board Chair and Acting CEO of an international research charity, Emergence Benefactors, whose fundraising and public relations campaigns might benefit from the publication of this study. MDS directs the Meditation Research Program at Massachusetts General Hospital, which has received research funding from co-author and case study subject DMI.
Data will be made available on request.
We are unaware of the specific reason(s) why these involuntary eye-blinks/movements coincide with cessations, and this is a research question open to inquiry. Anecdotal evidence from other advanced meditators suggests that this physiological reaction to cessation may be idiosyncratic and that other meditators may experience a combination of eye movements and body twitches.
In order to examine the validity of averaging these cessation power values across runs, we also examined the inter-run reliability of these power values, using intra-class correlation (ICC; Ten Hove et al., 2022) across runs. Specifically, ICC estimates and their 95% confident intervals were calculated using custom R code (R Core Team, 2013) from Ten Hove et al. (2022) based on a mean-rating (k = 37), inter-run consistency, 2-way random-effects model. Results of this ICC analysis for each ROIs is presented in Supplementary Table S3. ICC values indicated moderate (0.5 < ICC < 0.75) to good (0.75 < ICC < 0.90) realiability, especially for ROIs that showed significant time-related trends surrounding cessions (Supplementary Table S3).
If no predictive value of time was observed for the entire pre-cessation or post-cessation time period, for sake of completeness we also performed regression analyses using only the time frame immediately leading up to the cessation or after the cessation (i.e., only time frame 2 or time frame 3). Results for these analyses are reported in Supplementary Tables S1 and S2. The results were FDR corrected for multiple comparisons across total number of regressions performed – i.e., a maximum of four and a minimum of two regressions.
Refer to Supplementary Text for an explanation of how Cohen’s d was calculated.
We refrained from reporting and interpreting power in the gamma frequency band (i.e., > 30Hz) in the main text since a significant portion of 30–45 Hz scalp power is muscle-related activity (Whitham et al., 2007), but report it in Supplementary Figure S3.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badcock PB, Friston KJ, Ramstead MJD, Ploeger A, Hohwy J, 2019. The hierarchically mechanistic mind: an evolutionary systems theory of the human brain, cognition, and behavior. Cogn. Affect. \& Behav. Neurosci 19, 1319–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazanova OM, Vernon D, 2014. Interpreting EEG alpha activity. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. [Google Scholar]
- Berkovich-Ohana A, 2017. A case study of a meditation-induced altered state: increased overall gamma synchronization. Phenomenol. Cogn. Sci 16, 91–106. 10.1007/s11097-015-9435-x [DOI] [Google Scholar]
- Bhikkhu Ñ, 2010. Visuddhimagga: The Path of Purification.
- Blain-Moraes S, Lee U, Ku S, Noh G, Mashour GA, 2014. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front. Syst. Neurosci 8, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R, 2007. Waves of the unconscious: the neurophysiology of dreamlike phenomena and its implications for the psychodynamic model of the mind. Neuropsychoanalysis 9, 183–211. [Google Scholar]
- Carhart-Harris RL, Friston KJ, 2019. REBUS and the anarchic brain: Toward a unified model of the brain action of psychedelics. Pharmacol. Rev 71, 316–344. 10.1124/pr.118.017160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams LT, Williams TM, Bolstridge M, Sessa B, McGonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, Evans J, Singh KD, Wise RG, Curran HV, Feilding A, Nutt DJ, 2016. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. U. S. A 113, 4853–4858. 10.1073/pnas.1518377113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MR, Menon DK, Fryer TD, Pickard JD, 2005. Neurometabolic coupling in the vegetative and minimally conscious states: preliminary findings. J. Neurol. Neurosurg. \& Psychiatry 76, 432–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NR, Croft RJ, Dominey SJJ, Burgess AP, Gruzelier JH, 2003. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int. J. Psychophysiol 47, 65–74. [DOI] [PubMed] [Google Scholar]
- Dahl CJ, Lutz A, Davidson RJ, 2015. Reconstructing and deconstructing the self: Cognitive mechanisms in meditation practice. Trends Cogn. Sci 19, 515–523. 10.1016/j.tics.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, Griffiths RR, 2021. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA psychiatry 78, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor-Ziderman Y, Berkovich-Ohana A, Glicksohn J, Goldstein A, 2013. Mindfulness-induced selflessness: a MEG neurophenomenological study. Front. Hum. Neurosci 7, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Axmacher N, Haupt S, 2010. From alpha to gamma: Electrophysiological correlates of meditation-related states of consciousness. Med. Hypotheses 75, 218–224. 10.1016/j.mehy.2010.02.025 [DOI] [PubMed] [Google Scholar]
- Friston K, 2008. Hierarchical models in the brain. PLoS Comput. Biol 4, e1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead M, Trope Y, Liberman N, 2020. Above and beyond the concrete: The diverse representational substrates of the predictive brain. Behav. Brain Sci 43. [DOI] [PubMed] [Google Scholar]
- Grabovac A, 2015. The Stages of Insight: Clinical Relevance for Mindfulness-Based Interventions. Mindfulness (N. Y). 6, 589–600. 10.1007/s12671-014-0294-2 [DOI] [Google Scholar]
- He BJ, 2014. Scale-free brain activity: past, present, and future. Trends Cogn. Sci 18, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntenburg JM, Bazin P-L, Margulies DS, 2018. Large-scale gradients in human cortical organization. Trends Cogn. Sci 22, 21–31. [DOI] [PubMed] [Google Scholar]
- Ingram DM, 2018. Mastering the Core Teachings of the Buddha: An Unusually Hardcore Dharma Book (Revised and Expanded). Red Wheel/Weiser. [Google Scholar]
- Josipovic Z, 2019. Nondual awareness: consciousness-as-such as non-representational reflexivity. Prog. Brain Res 244, 273–298. [DOI] [PubMed] [Google Scholar]
- Josipovic Z, 2010. Duality and nonduality in meditation research. Conscious. Cogn 19, 1119–1121. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Burney R, 1981. The clinical use of awareness meditation in the self-regulation of chronic pain. Pain 11, S273. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Siegel D, Hanh TN, Kornfield J, 2011. The mindfulness revolution: Leading psychologists, scientists, artists, and meditation teachers on the power of mindfulness in daily life. Shambhala Publications. [Google Scholar]
- Kallionpää RE, Valli K, Scheinin A, Långsjö J, Maksimow A, Vahlberg T, Revonsuo A, Scheinin H, Mashour GA, Li D, 2020. Alpha band frontal connectivity is a state-specific electroencephalographic correlate of unresponsiveness during exposure to dexmedetomidine and propofol. Br. J. Anaesth 125, 518–528. [DOI] [PubMed] [Google Scholar]
- Klimesch W, 2012. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkonen RE, Sacchet MD, Barendregt H, Devaney KJ, Chowdhury A, Slagter HA, 2023. Cessations of consciousness in meditation: Advancing a scientific understanding of nirodha sam{\=a}patti. [DOI] [PubMed]
- Laukkonen RE, Sacchet MD, Barendregt H, Devaney KJ, Chowdhury A, Slagter HA, 2022. Cessations of consciousness in meditation: Advancing a scientific understanding of Nirodha Samāpatti. [DOI] [PubMed]
- Laukkonen RE, Slagter HA, 2021a. From many to (n)one: Meditation and the plasticity of the predictive mind. Neurosci. Biobehav. Rev 128, 199–217. 10.1016/j.neubiorev.2021.06.021 [DOI] [PubMed] [Google Scholar]
- Laukkonen RE, Slagter HA, 2021b. From many to (n) one: Meditation and the plasticity of the predictive mind. Neurosci. \& Biobehav. Rev. 128, 199–217. 10.1016/j.neubiorev.2021.06.021 [DOI] [PubMed] [Google Scholar]
- Laukkonen RE, Slagter HAA, 2021c. From many to (n)one: Meditation and the plasticity of the predictive mind. Neurosci. Biobehav. Rev 128, 199–217. 10.1016/j.neubiorev.2021.06.021 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Kulubya E, Goldin P, Goodarzi A, Girgis F, 2018. Review of the neural oscillations underlying meditation. Front. Neurosci 12, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA, 2013. Disruption of frontal--parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology 118, 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Ekman P, Ricard M, 2012. Meditation and the startle response: A case study. Emotion 12, 650–658. 10.1037/a0027472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas T, Ivtzan I, Fu CHYY, 2015. A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci. Biobehav. Rev 57, 401–410. 10.1016/j.neubiorev.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ, 2008. Attention regulation and monitoring in meditation. Trends Cogn. Sci 12, 163–169. 10.1016/j.tics.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Thompson E, 2003. Neurophenomenology integrating subjective experience and brain dynamics in the neuroscience of consciousness. J. Conscious. Stud 10, 31–52. [Google Scholar]
- Maquet P, Péters J-M, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G, 1996. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature 383, 163–166. [DOI] [PubMed] [Google Scholar]
- Mayer A, Schwiedrzik CM, Wibral M, Singer W, Melloni L, 2015. Expecting to see a letter: alpha oscillations as carriers of top-down sensory predictions. Cereb. Cortex 26, 3146–3160. [DOI] [PubMed] [Google Scholar]
- Metzinger T, 2020. Minimal phenomenal experience: Meditation, tonic alertness, and the phenomenology of “pure” consciousness. Philos. Mind Sci 1, 1–44. [Google Scholar]
- Millière R, Carhart-Harris RL, Roseman L, Trautwein F-M, Berkovich-Ohana A, 2018a. Psychedelics, meditation, and self-consciousness. Front. Psychol 9, 1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millière R, Carhart-Harris RL, Roseman L, Trautwein F-MM, Berkovich-Ohana A, 2018b. Psychedelics, meditation, and self-consciousness. Front. Psychol 9, 1475. 10.3389/fpsyg.2018.01475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ñ\=aṇamoli B, Bodhi B, 1995. The middle length discourses of the Buddha: A translation of the Majjhima Nikaya.
- Nave K, Deane G, Miller M, Clark A, 2022. Expecting some action: Predictive Processing and the construction of conscious experience. Rev. Philos. Psychol 1–19. [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M, 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Leshem R, Pellegrino M, Ben-Soussan TD, 2022. Tackling the Electro-Topography of the Selves Through the Sphere Model of Consciousness. Front. Psychol 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Da Silva FHL, 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol 110, 1842–1857. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Laumann TO, Koyejo O, Gregory B, Hover A, Chen MY, Gorgolewski KJ, Luci J, Joo SJ, Boyd RL, Hunicke-Smith S, Simpson ZB, Caven T, Sochat V, Shine JM, Gordon E, Snyder AZ, Adeyemo B, Petersen SE, Glahn DC, Mckay DR, Curran JE, Göring HHH, Carless MA, Blangero J, Dougherty R, Leemans A, Handwerker DA, Frick L, Marcotte EM, Mumford JA, 2015. Long-term neural and physiological phenotyping of a single human. Nat. Commun 6. 10.1038/ncomms9885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe O, Shapiro K, Staresina BP, 2022. Fronto-medial theta coordinates posterior maintenance of working memory content. Curr. Biol 32, 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut RV, Snyder AZ, Raichle ME, 2020. Hierarchical dynamics as a macroscopic organizing principle of the human brain. Proc. Natl. Acad. Sci 117, 20890–20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenhagen J, Draschkow D, 2019. Cluster-based permutation tests of MEG/EEG data do not establish significance of effect latency or location. Psychophysiology 56, e13335. [DOI] [PubMed] [Google Scholar]
- Sayadaw M, 2016. Manual of insight. Simon and Schuster. [Google Scholar]
- Sayadaw M, 1994. The progress of insight: a treatise on satipatthana meditation. Buddhist Publication Society. [Google Scholar]
- Senthilingam M, 2016. Can meditation and psychedelics have the same benefits for your mind? [WWW Document]. CNN. URL https://www.cnn.com/2016/06/03/health/psychedelics-anxiety-depression-meditation/index.html [Google Scholar]
- Siclari F, Tononi G, 2017. Local aspects of sleep and wakefulness. Curr. Opin. Neurobiol 44, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Davidson RJ, Lutz A, 2011. Mental training as a tool in the neuroscientific study of brain and cognitive plasticity. Front. Hum. Neurosci 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes S, Krugliakova E, Meier E, Huber R, 2022. The theta paradox: 4–8 Hz EEG oscillations reflect both sleep pressure and cognitive control. J. Neurosci 42, 8569–8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparby T, Sacchet MD, 2022. Defining Meditation: Foundations for an Activity-Based Phenomenological Classification System. Front. Psychol 12, 6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S, Ingram DM, 2017. The Fire Kasina, 1st ed. Heptarchia. [Google Scholar]
- Tang Y-Y, 2017. The neuroscience of mindfulness meditation: How the body and mind work together to change our behaviour. Springer. [Google Scholar]
- Tang Y-Y, Hölzel BK, Posner MI, 2015. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci 16, 213–225. 10.1038/nrn3916 [DOI] [PubMed] [Google Scholar]
- Taylor P, Hobbs JN, Burroni J, Siegelmann HT, 2015. The global landscape of cognition: hierarchical aggregation as an organizational principle of human cortical networks and functions. Sci. Rep 5, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann C, Bauer PR, Gosseries O, Vanhaudenhuyse A, Vollenweider F, Laureys S, Singer T, Antonova E, Lutz A, 2023. A neurophenomenological approach to non-ordinary states of consciousness: hypnosis, meditation, and psychedelics. Trends Cogn. Sci 27, 139–159. 10.1016/j.tics.2022.11.006 [DOI] [PubMed] [Google Scholar]
- Timmermann C, Roseman L, Schartner M, Milliere R, Williams LTJ, Erritzoe D, Muthukumaraswamy S, Ashton M, Bendrioua A, Kaur O, others, 2019. Neural correlates of the DMT experience assessed with multivariate EEG. Sci. Rep 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, 2008. Consciousness as integrated information: a provisional manifesto. Biol. Bull 215, 216–242. [DOI] [PubMed] [Google Scholar]
- Tononi G, 2004. An information integration theory of consciousness. BMC Neurosci. 5, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis F, Pearson C, 2000. Pure consciousness: distinct phenomenological and physiological correlates of “consciousness itself.” Int. J. Neurosci 100, 77–89. [PubMed] [Google Scholar]
- Valle M, Maqueda AE, Rabella M, Rodr\’\iguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mañanas MÀ, Barker S, Friedlander P, others, 2016. Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur. Neuropsychopharmacol 26, 1161–1175. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A, Meissner T, Lazar SW, Kerr CE, Gorchov J, others, 2018. Mind the hype: A critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspect. Psychol. Sci 13, 36–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lutterveld R, Diederen KMJ, Koops S, Begemann MJH, Sommer IEC, 2013. The influence of stimulus detection on activation patterns during auditory hallucinations. Schizophr. Res 145, 27–32. [DOI] [PubMed] [Google Scholar]
- van Lutterveld R, van Dellen E, Pal P, Yang H, Stam CJ, Brewer J, 2017. Meditation is associated with increased brain network integration. Neuroimage 158, 18–25. 10.1016/j.neuroimage.2017.06.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RK, 1970. Physiological effects of transcendental meditation. Science (80-. ). 167, 1751–1754. [DOI] [PubMed] [Google Scholar]
- Wen H, Liu Z, 2016. Separating fractal and oscillatory components in the power spectrum of neurophysiological signal. Brain Topogr. 29, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, DeLosAngeles D, Lillie P, 2007. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin. Neurophysiol 118, 1877–1888. [DOI] [PubMed] [Google Scholar]
- Wutzl B, Golaszewski SM, Leibnitz K, Langthaler PB, Kunz AB, Leis S, Schwenker K, Thomschewski A, Bergmann J, Trinka E, 2021. Narrative review: quantitative EEG in disorders of consciousness. Brain Sci. 11, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


