Abstract
Background/Objectives:
Bone health of children with acute recurrent pancreatitis (ARP) and chronic pancreatitis (CP) is not well studied.
Methods:
This retrospective study was performed at three sites and included data from INSPPIRE-2.
Results:
Of the 87 children in the study: 46 had ARP (53%), 41 had CP (47%). Mean age was 13.6 ± 3.9 years at last DXA scan. The prevalence of low height-for-age (Z-score < −2) (13%, 10/78) and low bone mineral density (BMD) adjusted for height (Z-score <−2) (6.4%, 5/78) were higher than a healthy reference sample (2.5%, p<0.0001 and p=0.03, respectively).
Conclusion:
Children with ARP or CP have lower height and BMD than healthy peers. Attention to deficits in growth and bone mineral accrual in children with pancreatic disease is warranted.
Keywords: Bone health, children, pancreatitis
Introduction
Compromised bone strength and increased fracture susceptibility pose significant morbidity and health care costs.1 Inadequate bone mineral accretion in growing children may have lifelong consequences.2 Bone mineral deficits among children with underlying chronic medical conditions can add an increased burden to the child and their families. Many chronic conditions, especially those associated with inflammation or nutritional deficits, such as Crohn’s disease and kidney disorders,3–10 increase a child’s risk for poor bone health. Physical activity helps with bone health and decreased physical activity due to illness may also be a contributing factor. Acute recurrent or chronic inflammatory conditions affecting the pancreas may also pose similar risks to bone health in children, but few studies have examined this topic.11
Acute recurrent pancreatitis (ARP) and chronic pancreatitis (CP) affect children’s growth, nutritional status and overall wellbeing.12 Growth concerns and nutritional risks are more pronounced for children who have exocrine pancreatic insufficiency and endocrine insufficiency (prediabetes or diabetes mellitus) in addition to ARP/CP13. It is recommended that children with ARP/CP are screened every 3–6 months for nutritional deficiencies14. Fat soluble vitamin (A D, E, K) deficiencies have been reported in adults with CP.12, 15–23 Comparable data on vitamin status from children with pancreatitis are sparse. The increased risk of chronic inflammation and nutrient deficiencies place individuals with CP at higher risk for low bone mineral density (BMD) even when they are on pancreatic enzyme replacement therapy (PERT).24 Although pediatric data is sparse, adults with pancreatitis have been shown to have increased risk for low bone density. Adults with advanced CP had BMD in the osteopenia (T-score of −1 to −2.5) or osteoporosis range (T-score <−2.5) and more advanced CP stages were associated with worse BMD markers.25 Moreover, a higher percentage of adult CP patients had BMD values consistent with osteoporosis compared to controls (34% vs. 10%).26, 27 In another study, 55% of adults with CP had either osteopenia or osteoporosis.26, 27 In the PROCEED cohort, the prospective, an observational cohort study of CP in the Consortium for the Study of Chronic Pancreatitis, Diabetes and Pancreatic Cancer (CPDPC), more than half of the tested subjects demonstrated osteoporosis or osteopenia, indicating that bone disease should be carefully considered in adults with CP.28 This increased rate of low bone density in adults with pancreatitis indicates the importance of determining whether the same risks exists in children, in which the presence of low bone density would be expected to lead to greater long term health effects due to duration and lack of development of peak bone mass.
The objectives of this study were to evaluate bone health in children with ARP or CP and to assess whether the loss of endocrine and exocrine pancreatic function was associated with risk for worsened bone health. We hypothesized that children with ARP or CP would have lower lumbar spine BMD compared to a healthy reference group. This multicenter study was initiated at three major medical centers, then expanded to include data from the INternational Study Group for Pediatric Pancreatitis: In search for a cuRE (INSPPIRE) network of ARP or CP in children, and is the first multi-center study to investigate bone health in a large cohort of children with pediatric ARP or CP.29
Methods
This retrospective cross-sectional study was performed at three clinical sites, Cincinnati Children’s Hospital Medical Center, University of Iowa Stead Family Children’s Hospital, and Children’s Hospital of Philadelphia. The study also included data from the INSPPIRE-2 cohort, which at the time of the study, had over 600 pediatric subjects enrolled.30 We included data from those who were < 18 years of age at the time of pancreatitis diagnosis, had a BMD measurement by dual energy x-ray absorptiometry (DXA) within the last 15 years, and results available for analysis. We included DXA scans performed on patients up to age 23 years. All had a diagnosis of either ARP or CP as per published criteria31 at time of data inclusion, and DXA scans were performed after the pancreatitis diagnosis. Exclusion criteria were other diagnoses that would be expected to significantly affect bone density, such as osteogensis imperfecta.
Data on height, vitamin D status, exocrine and endocrine sufficiency, mobility status and fracture history were abstracted from the electronic medical record at the 3 clinical sites and entered into a REDCap database. INSPPIRE-2 data included information from participating centers on lumbar spine BMD, as well as information on exocrine, endocrine and vitamin D status, height and weight. Height-for-age Z-scores (HAZ) were calculated using the CDC growth reference. Z-scores for individuals age > 20 years were calculated as if they were age 20 years. Vitamin D insufficiency was defined as serum 25-hydroxy vitamin D level below 30 ng/ml. Exocrine insufficiency was diagnosed by fecal elastase of <100 mcg/g stool or an endoscopic pancreatic function test. Endocrine insufficiency, diabetes and prediabetes, was as defined by the American Diabetes Association.32
Overall, there were six sites that used Hologic fan beam densitometers (Hologic, Inc., Marborough, MA, USA) and five that used a GE-Lunar Prodigy (Madison, WI, USA) densitometer. We used published equations to convert GE-Lunar BMD results to be equivalent to Hologic BMD results.33 Data from lumbar spine scans was used because whole body scans were not widely available. Scan analysis software used to quantify BMD on both Hologic and GE Lunar systems accommodated pediatric as well as adult scans. We calculated age-, sex- and race specific BMD Z-scores (BMDZ) using reference ranges by Zemel et al34, which are suitable for Hologic derived BMD results. BMDZ was adjusted for height-for-age Z-scores (HAZ) as recommended to account for spurious effects due to short stature.
Statistical Analysis
Data were analyzed using SAS®, version 9.4 (SAS Institute, Cary, NC). Depending on distributions, continuous data were summarized as mean ± standard deviation (SD) or median with interquartile range (IQR: 25th - 75th percentiles). Categorical data were summarized as frequency counts and percentages. Chi-square or Fisher’s exact tests were used to compare differences in proportions. Binomial proportions tests were used to compare study proportions to 2.5%, the expected value associated with −2 standard deviation (i.e., a Z-score of −2). One-sample t-tests were used to compare our study mean Z-scores to 0, which is the expected value of a healthy population. Generalized linear models were used to assess BMDZ trends over time from the development of first acute pancreatitis (AP) or CP. Due to the majority of participants not having multiple DXA scans, the last DXA available after AP diagnosis was used for each subject in generalized linear models. When lumbar spine BMDZ was the outcome, the covariates tested in each model were time from AP or CP diagnosis, HAZ (in models when BMDZ was not adjusted for HAZ), endocrine insufficiency, and exocrine insufficiency. HAZ was also examined over time from either AP or CP diagnoses. For individuals over the age of 20 years at time of DXA (n=4), the reference values of 20 years of age were used to calculate Z-scores. P <0.05 was considered statistically significant.
Results
Ninety pediatric patients had DXA data available after their pancreatitis diagnosis. Two patients were excluded due to BMDZ scores that were extremely low compared to the rest of the cohort (−6, and −7) and the presence of multiple complex medical diagnoses upon clinical review. One subject was excluded because they only had a whole body DXA scan and no lumbar spine scan. Of the 87 patients that met study criteria, 38 were from the 3 clinical centers and 49 from the INSPIRE-2 cohort; 46 (53%) had ARP and 41 (47%) had CP, and 52% were male (Table 1). Exocrine insufficiency was present in 30% of the cohort, and endocrine insufficiency (prediabetes or diabetes) was present in 7% out of the cohort included with available bone health measurements.
Table 1.
ARP/CP Bone Health patient characteristics
| n | n (%) or mean ± SD or median (25th-75th percentile) | Min-max | |
|---|---|---|---|
| Female | 42 (48) | ||
| Other | 16 (18) | ||
| Ethnicity (Non-Hispanic) (%) | 86 | 74 (86) | |
| Age 1st AP attack (years) | 85 | 8.8 ± 4.3 | 0.9–17.2 |
| CP | 41 (47) | ||
| Age at 2nd AP attack (years) | 79 | 9.8 ± 4.3 | 1.5–17.9 |
| Age at CP diagnosis (years) | 41 | 9.4 ± 4.4 | 2.9–24.3 |
| Time 1st AP attack to 2nd AP attack (months) | 79 | 5.6 (2.7–15.0) | 0.0–91.2 |
| Time 1st AP attack to CP (months) | 39 | 7.1 (0.5–39.0) | 0.0–179.0 |
| Number of AP attacks | 82 | 4 (2–6) | 1–12 |
| No | 55 (67) | ||
| Endocrine insufficiency (%) | 85 | 6 (7) |
ARP (Acute Recurrent Pancreatitis), CP (Chronic Pancreatitis), AP (Acute Pancreatitis)
The mean age at the last DXA scan was 13.6 ± 3.9 years, Table 2. The mean HAZ was −0.38, which was statistically lower than the healthy reference population, p=0.04. The proportion of patients with low HAZ (<−2) (13%, 10/78) was higher than expected for a healthy population (2.5%, p<0.0001). The proportion of patients with low BMDZ (<−2) (15%, 13/87) and low HAZ-adjusted spine BMDZ (<−2) (6.4%, 5/78) were higher than expected for a healthy population (2.5%, p<0.0001 and p=0.03 respectively). Vitamin D insufficiency was present in 37/53 (70%) patients (Table 2). Of those with vitamin D insufficiency, 16% (6/37) had spine BMDZ <−2 and 6% (2/32) had HAZ-adjusted BMDZ <−2.
Table 2.
ARP/CP Bone Health Last DXA available after first AP
| n | Mean ± SD or n (%) | |
|---|---|---|
| Age at DXA (years) | 87 | 13.6 ± 3.9 |
| Height-for-age Z-score | 78 | −0.38 ± 1.63 |
| Body mass index Z-score | 68 | 0.42 ± 1.07 |
| Artifact on DXA (%) | 36 | 5 (14) |
| Low vitamin D (<30 ng/mL) (%) | 53 | 37 (70) |
| Spine BMDZ | 87 | −0.37 ± 1.46 |
| HAZ adjusted BMDZ | 78 | −0.13 ± 1.18 |
| Low Spine BMDZ (<−2) (%) | 87 | 13 (15) |
| Low HAZ adjusted BMDZ (<−2) (%) | 78 | 5 (6.4) |
| Low HAZ (<−2) (%) | 78 | 10 (13) |
AP (acute pancreatitis)
ARP (acute recurrent pancreatitis)
CP (chronic pancreatitis)
DXA (dual energy x-ray absorptiometry)
HAZ (Height-for-age Z-score)
BMDZ (Bone Mineral Density Z-score)
In multivariable regression models evaluating lumbar spine BMDZ, there was no significant association between BMDZ and time since diagnosis of AP or CP, (Figure 1, A and B). Likewise, there was no significant association between HAZ-adjusted BMDZ and time since diagnosis of either AP or CP (Figure 1 C and D). HAZ was positively associated with BMDZ in AP and CP patients (p<0.001 and p=0.004, respectively). Neither endocrine and exocrine insufficiency (from AP (p=0.06) or CP (p=0.08)). Exocrine insufficiency alone (from AP (p=0.53) or CP (p=0.20)) were not significantly associated with spine BMDZ, nor was exocrine insufficiency alone associated with HAZ-adjusted lumbar spine BMDZ. Pancreatitis patients who had endocrine and exocrine insufficiency had significantly (p=0.04) lower/higher HAZ-adjusted BMDZ when adjusted for time since AP diagnosis. This association did not meet statisitical significance when evaluating at time from CP diagnosis (p=0.08). All 6 patients who had endocrine insufficiency also had exocrine insufficiency (none in the sample had only endocrine insufficiency).
Figure 1. Spine BMDZ and HAZ-adjusted BMDZ from time of first AP and CP diagnosis.
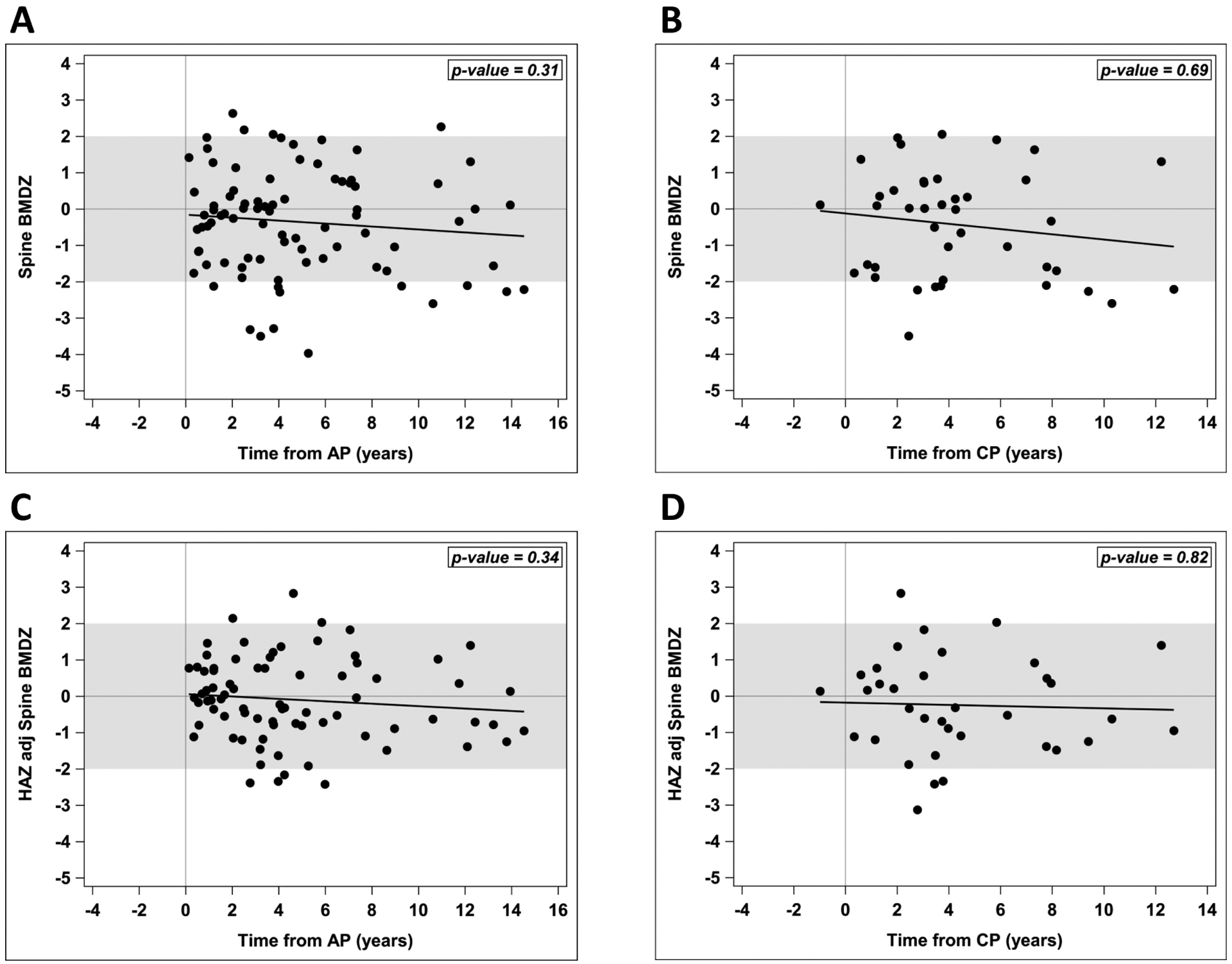
(A, B) Spine bone mineral density Z-scores (BMDZ) did not change significantly over the years from first acute pancreatitis (AP) or chronic pancreatitis (CP) diagnosis. (C, D) Height-for-age Z-scores (HAZ) adjusted BMDZ did not change significantly over the years from first AP or CP diagnosis. Analysis done using generalized linear regression models.
We evaluated all available height measures, regardless if there was a DXA scan at that timepoint. HAZ did not significantly change over time from first AP attack (p=0.50) or from CP diagnosis (p=0.67). HAZ was not significantly different in ARP versus CP groups over time (p=0.76), Figure 2. Using the last height available for each patient, the mean HAZ of −0.4 ± 1.5 for these ARP and CP patients was significantly lower than the expected value of 0 (p=0.02) (Figure 3).
Figure 2. HAZ over time in ARP and CP groups.
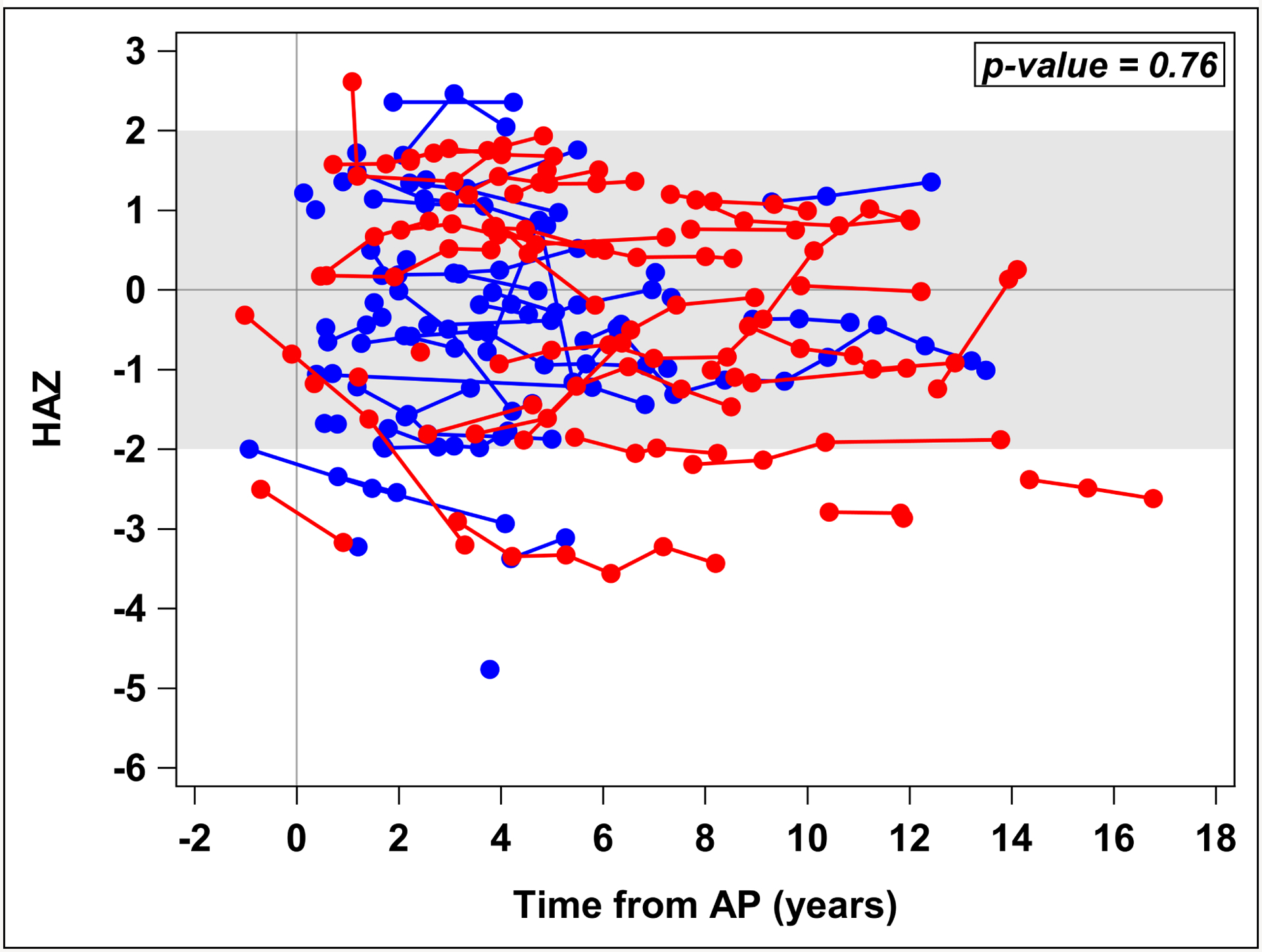
Scatter plot figure showing height-for-age Z-scores (HAZ) did not significantly differ between acute recurrent pancreatits (ARP) and chronic pancreatitis (CP) patients over time (p=0.76) [red=CP, blue=ARP]. Analysis done using generalized linear regression models with repeated measures.
Figure 3. HAZ of the ARP/CP cohort compared to the general population.
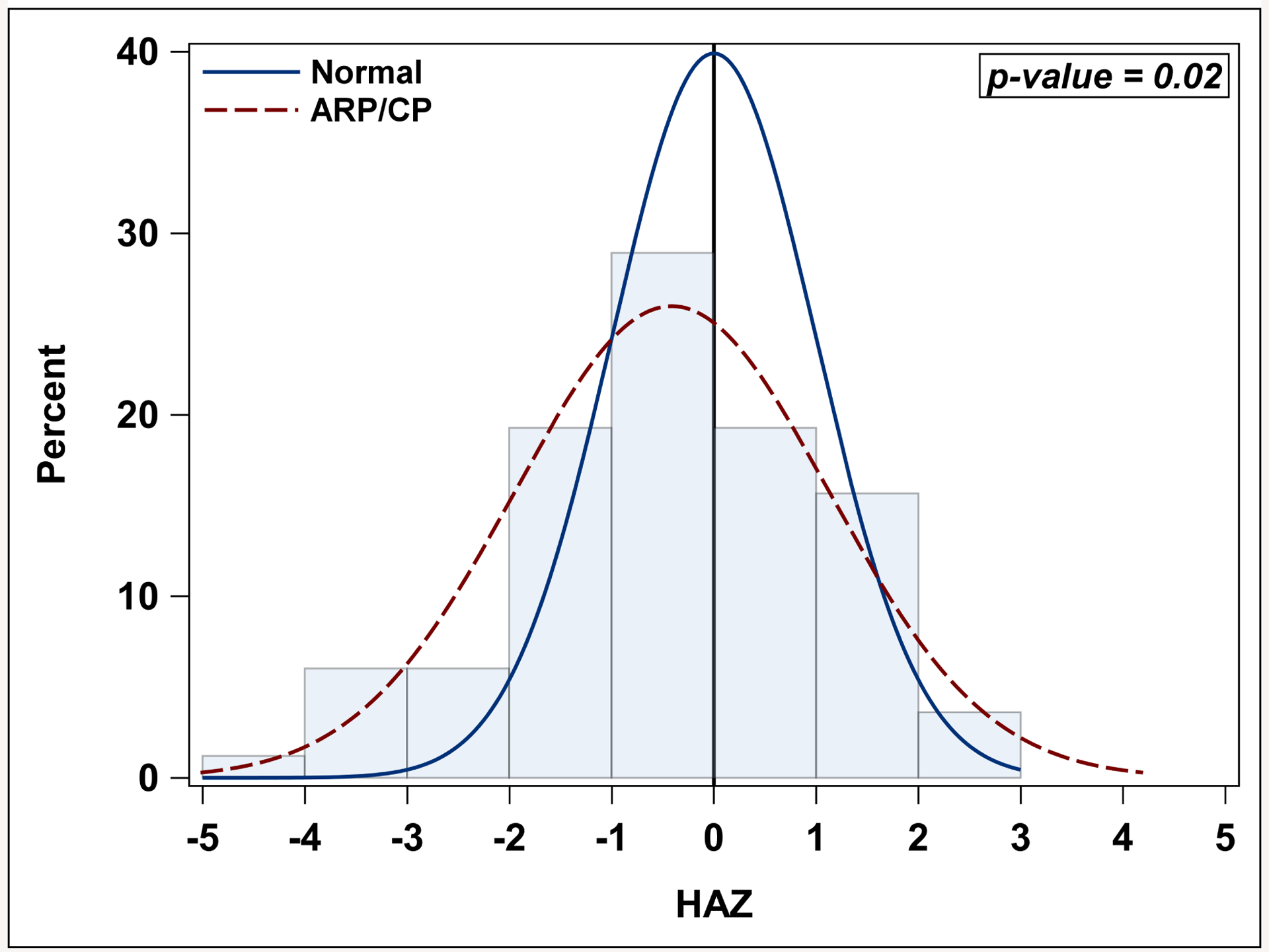
Histogram figure demonstrating HAZ for acute recurrent pancreatits (ARP)/ chronic pancreatitis (CP) patients is significantly lower than the general population Z-score of 0 (p=0.02). Analysis was done using one-sample t-test.
Fracture history and mobility status were only available for the 38 patients from the three main sites. Of these, 32 (84%) were mobile and 6 (16%) had impaired mobility (5 partially mobile and 1 fully wheelchair bound). In the impaired mobility group 83% (5/6) had spine BMDZ scores <−2 compared to 6% (2/32) in the mobile group (p=0.0002). The prevalence of HAZ-adjusted BMDZ of < −2 was 17% (1/6) in the impaired mobility group and 3% (1/32) in the mobile group (p=0.29). Fracture history was known for 32 patients, and 19% (6/32) had one or more fractures. All 6 who sustained fractures had at least one fracture after ARP diagnosis. The impaired mobility group had a significantly higher proportion with fractures (67%, 4/6) than the mobile group (8%, 2/26) (p=0.006).
Discussion
In this pediatric multicenter study with largest sample size ever reported, the prevelance of low BMDZ (Z-score < −2) was greater in children with ARP or CP than expected in a healthy population. Although children with ARP or CP had lower HAZ than general population, they had a lower HAZ- adjusted BMDZ than a healthy population as well. The presence of exocrine or endocrine dysfunction was not associated with higher rates of low BMD in our cohort.
Few studies have examined bone health in pediatric patients with pancreatitis including cases of ARP, which adds to the novelty of our study. CP had been attributed to low BMD, at a major center in India when examined, 18.6% (14/75) of children were affected. Children with low BMD had a lower BMI than the subjects with normal bone mineral density, suggesting that undernutrition in presence of pancreatic disease can affect bone health.11 In the PROCEED cohort of adults with CP, nearly half of subjects tested had osteopenia or osteoporosis.28
Comparing our data with adult data for ARP and CP patients suggest that compromised bone health may be a prolonged process that may start in the pediatric age group and advance over time into the adulthood as chronic inflammatory changes progress over the patients’ lifetime. This would amplify the prevalence of poor bone health into adulthood for those with pancreatitis beginning in childhood.
Immobility is known to affect bone density,35 and we also found this in the subset of ARP and CP patients that had that data available. This is important since the incidence of pancreatitis is higher in patients with cerebral palsy and immobility, who are already at elevated risk for low bone density.36 Mutliple factors may lead to poor growth and affect the bone health in pancreatitis. One factor could be recurrent or chronic inflammation in ARP and CP that may affect bone formation, bone mineral accrual, and overall nutrition. As in most inflammatory conditions, we expect there would be an increased metabolic demand to maintain nutritional balance in pancreatitis. In addition, exocrine or endocrine dysfunction may exacerbate an already negative nutritional balance. It is interesting that bone health in our sample was not statistically associated with duration of pancreatitis, and this may be related to the short duration of follow up during this study period.
While our study highlights an important problem, it is not without limitations. Although the overall sample size of our study was larger than others, it still had limited power to examine the effects of nutrition, effect of endocrine or exocrine status or vitamin status on bone health. Additionally, we had limited data related to risk factors for bone health. For instance, potential confounders susch as anthropometric measures, laboratory values, nutritional parameters, pancreatic enzyme dosing, were not obtained at the same time as the DXA scans. The absence of a control population is a limitation that we attempted to overcome by extrapolating from published normal control data. Also, the mobility status of the patient or the presence of artifacts in the scan field was not captured in the INSPPIRE cohort. The small number of subjects in the INSPPIRE cohort with DXA scans is another limitation. Lastly the limited follow up period may be a factor for not detecting bone effect from pancreatic disease. Nonetheless the broad representation of geographic distribution of the centers makes our findings generalizable.
In conclusion, we found that linear growth is decreased in pediatrics patients with ARP or CP and that a higher proportion of children with pancreatitis have low BMD adjusted for height and age than would be expected. Our findings illustrate the need for a future prospective multi-center effort to obtain DXA scans systematically on patients with pancreatitis, while taking into account factors that may affect their growth, nutrition, and pancreatitis related disease measures. Validation of the study is needed so that bone health surveillance in children with pancreatic disease can be incorporated into clinical practice.
Acknowledgements
The authors acknowledge Mr. Tyler Thompson and all coordinators who assisted with the study. MAH is supported by NIDDK, grant number K23DK118190. The study was supported by NIDDK U01 DK108334, U01DK108328.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Potential Conflicts: SJS consultant for Mirum, Renxxion, Abvie, and UpToDate, AU is a consultant for Cystic Fibrosis Foundation and Abbvie Pharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzti M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12:22–8. [DOI] [PubMed] [Google Scholar]
- 3.Buison AM, Kawchak DA, Schall JI, Ohene-Frempong K, Stallings VA, Leonard MB, et al. Bone area and bone mineral content deficits in children with sickle cell disease. Pediatrics. 2005;116(4):943–9. [DOI] [PubMed] [Google Scholar]
- 4.Burnham JM, Shults J, Petit MA, Semeao EJ, Beck TJ, Zemel BS, et al. Alterations in proximal femur geometry in children treated with glucocorticoids for Crohn disease or nephrotic syndrome: impact of the underlying disease. J Bone Miner Res. 2007;22(4):551–9. [DOI] [PubMed] [Google Scholar]
- 5.Burnham JM, Shults J, Semeao E, Foster B, Zemel BS, Stallings VA, et al. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19(12):1961–8. [DOI] [PubMed] [Google Scholar]
- 6.Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136(1):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly A, Schall JI, Stallings VA, Zemel BS. Deficits in bone mineral content in children and adolescents with cystic fibrosis are related to height deficits. J Clin Densitom. 2008;11(4):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostoufi-Moab S, Brodsky JL, Isaacoff EJ, Tsampalieros A, Ginsberg JP, Zemel BS, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97(10):3584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel BS, Shults J, Leonard MB. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res. 2012;27(4):760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terpstra AM, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Foster BJ, et al. Bone density and cortical structure after pediatric renal transplantation. J Am Soc Nephrol. 2012;23:715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Saini N, Mathias A, Arya A, Jain S, Yachha SK. Prevalence and predictive factors of undernutrition and low bone mineral density in children with chronic pancreatitis. Pancreatology. 2021;21(1):74–80. [DOI] [PubMed] [Google Scholar]
- 12.Duggan SN, Smyth ND, O’Sullivan M, Feehan S, Ridgway PF, Conlon KC. The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr Clin Pract. 2014;29(3):348–54. [DOI] [PubMed] [Google Scholar]
- 13.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H, Clinical Practice Guidelines on G, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–9. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen HH, Irtun O, Olesen SS, Drewes AM, Holst M. Nutrition in chronic pancreatitis. World J Gastroenterol. 2013;19(42):7267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M, Wakasugi H, Ibayashi H. Serum vitamin E, lipid peroxide and glutathione peroxidase in patients with chronic pancreatitis. Clin Chim Acta. 1981;110(1):121–5. [DOI] [PubMed] [Google Scholar]
- 16.Funakoshi A, Kimura T, Shinozaki H, Ibayashi H. Comparisons between absorption of vitamin E in patients with chronic pancreatitis and healthy controls: the bioavailability of vitamin E. Tohoku J Exp Med. 1986;148(4):393–401. [DOI] [PubMed] [Google Scholar]
- 17.Kalvaria I, Labadarios D, Shephard GS, Visser L, Marks IN. Biochemical vitamin E deficiency in chronic pancreatitis. Int J Pancreatol. 1986;1(2):119–28. [DOI] [PubMed] [Google Scholar]
- 18.Marotta F, Labadarios D, Frazer L, Girdwood A, Marks IN. Fat-soluble vitamin concentration in chronic alcohol-induced pancreatitis. Relationship with steatorrhea. Dig Dis Sci. 1994;39(5):993–8. [DOI] [PubMed] [Google Scholar]
- 19.Segal I, Gut A, Schofield D, Shiel N, Braganza JM. Micronutrient antioxidant status in black South Africans with chronic pancreatitis: opportunity for prophylaxis. Clin Chim Acta. 1995;239(1):71–9. [DOI] [PubMed] [Google Scholar]
- 20.Van Gossum A, Closset P, Noel E, Cremer M, Neve J. Deficiency in antioxidant factors in patients with alcohol-related chronic pancreatitis. Dig Dis Sci. 1996;41(6):1225–31. [DOI] [PubMed] [Google Scholar]
- 21.Morris-Stiff GJ, Bowrey DJ, Oleesky D, Davies M, Clark GW, Puntis MC. The antioxidant profiles of patients with recurrent acute and chronic pancreatitis. Am J Gastroenterol. 1999;94(8):2135–40. [DOI] [PubMed] [Google Scholar]
- 22.Quilliot D, Walters E, Bonte JP, Fruchart JC, Duriez P, Ziegler O. Diabetes mellitus worsens antioxidant status in patients with chronic pancreatitis. Am J Clin Nutr. 2005;81(5):1117–25. [DOI] [PubMed] [Google Scholar]
- 23.Klapdor S, Richter E, Klapdor R. Vitamin D status and per-oral vitamin D supplementation in patients suffering from chronic pancreatitis and pancreatic cancer disease. Anticancer Res. 2012;32(5):1991–8. [PubMed] [Google Scholar]
- 24.Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27(1):21–7. [DOI] [PubMed] [Google Scholar]
- 25.Mann ST, Stracke H, Lange U, Klor HU, Teichmann J. Alterations of bone mineral density and bone metabolism in patients with various grades of chronic pancreatitis. Metabolism. 2003;52(5):579–85. [DOI] [PubMed] [Google Scholar]
- 26.Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas. 2012;41(7):1119–24. [DOI] [PubMed] [Google Scholar]
- 27.Sikkens EC, Cahen DL, Koch AD, Braat H, Poley JW, Kuipers EJ, et al. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology. 2013;13(3):238–42. [DOI] [PubMed] [Google Scholar]
- 28.Hart PA, Yadav D, Li L, Appana S, Fisher W, Fogel E, et al. High Prevalence of Osteopathy in Chronic Pancreatitis: A Cross-sectional Analysis From the PROCEED Study. Clin Gastroenterol Hepatol. 2022;20(9):2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morinville VD, Lowe ME, Ahuja M, Barth B, Bellin MD, Davis H, et al. Design and implementation of INSPPIRE. J Pediatr Gastroenterol Nutr. 2014;59(3):360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uc A, Perito ER, Pohl JF, Shah U, Abu-El-Haija M, Barth B, et al. INternational Study Group of Pediatric Pancreatitis: In Search for a CuRE Cohort Study: Design and Rationale for INSPPIRE 2 From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47(10):1222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012;55(3):261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes A. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39 Suppl 1: S13–22. [Google Scholar]
- 33.Fan B, Lu Y, Genant H, Fuerst T, Shepherd J. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int. 2010;21(7):1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minaire P Immobilization osteoporosis: a review. Clin Rheumatol. 1989;8 Suppl 2: 95–103. [DOI] [PubMed] [Google Scholar]
- 36.Hawa K, Corker L, Hornung L, Noritz G, Gariepy C, Shaikhkhalil A, et al. Pancreatitis in the Complex Care Population: Presentation, Incidence, and Severity. J Pediatr Gastroenterol Nutr. 2022;75(6):749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


