Abstract
Background:
A multistakeholder core outcome set created for asthma trials showed that asthma-specific quality of life (QoL) was a critically meaningful outcome. However, the definition and measurement methods were undetermined. The adverse effects (AEs) of corticosteroids may be a vital clinical trial outcome. Nevertheless, the AE burden from the patient perspective has not yet been elucidated in an asthma population.
Objective:
To characterize patient burden of AEs in oral (OCS) and inhaled corticosteroids (ICS) and how this relates to QoL within an asthma population.
Methods:
We used a convergent parallel mixed-methods design with quantitative surveys of known ICS and OCS AEs that were distributed through the Allergy & Asthma Network database, social channels, and the Asthma UK newsletter. Participants rated the AEs that were (1) most burdensome and (2) most desired to be eliminated. Qualitative interviews and focus groups were performed to better understand patient views on barriers reported in the quantitative data, and to identify patient-important barriers that were not a part of the quantitative survey.
Results:
The 3 most burdensome AEs for OCS were bone mineral density, infectious complications, and weight gain, whereas weight gain was the most desired to be eliminated. The 3 most burdensome AEs for ICS were pneumonia, hoarse voice, and oral thrush, with concordant results for the most desired to be eliminated. In the focus groups, OCS AEs were concordant with quantitative findings. Focus groups identified unmeasured psychosocial effects, such as embarrassment.
Conclusion:
The most burdensome AEs may not be those that would cause patients to stop therapy. Furthermore, qualitative focus groups suggest a psychosocial burden associated with ICS, which needs further investigation.
Introduction
Asthma is 1 of the most common chronic diseases in the world, affecting more than 300 million people worldwide.1 Life expectancy for these individuals is similar to that of the general population, and improved pharmacotherapy is 1 factor that has led to drastic declines in the mortality and hospitalization rates.2
Inhaled and oral steroids are usually used to treat asthma. Inhaled corticosteroids (ICS) are the most effective controllers used in treatment and are a first-line medication.3 Oral corticosteroids (OCS) are also effective in asthma management, particularly in acute exacerbations and in the long term in severe asthma.4 Unfortunately, adverse effects (AEs) associated with corticosteroids may be intolerable for some patients. For ICS, several AEs have been reported by patients, including mostly localized effects such as oral candidiasis and dysphonia.5 Oral corticosteroids also have a range of AEs, with more systemic findings than those of ICS, affecting several body systems.6 Cooper et al surveyed patients and showed that those with strong concerns regarding the use of OCS and ICS had lower adherence to their medication regimen.7 This observation was supported by another study linking fears of AEs and decreased adherence in a minority population with low income (87% African American or Hispanic participants, with 64% of patients earning <US$30,000 per year).8 The importance of corticosteroids in asthma control, the high risk of adverse events, and the impact of AEs on adherence suggest that corticosteroid AEs may be an important clinical trial outcome.
A recent study sought to develop a core set of outcomes recommended for all clinical drug trials in patients with moderate-to-severe asthma using a multistakeholder modified Delphi consensus.9 This process involved a wide range of stakeholders including clinicians, regulators, and researchers, in addition to patients and other key stakeholders. Interestingly, outcomes related to OCS and ICS AEs were felt to be important by patient participants but perceived as redundant with other outcome measures by the clinician participants. The study also found that asthma-specific quality of life (QoL) was a critically meaningful outcome that was nearly unanimously agreed upon. However, the essential components of an asthma-specific QoL instrument were not determined. These results have prompted us to further explore the perception of burden of AEs on patients using steroids, in addition to determining constituents of asthma-specific QoL.
An optimal asthma-specific QoL is a patient-reported outcome that accurately measures the perceived impact that asthma has on a patient’s QoL.10 Currently, there are several independent instruments used to measure asthma-specific QoL. Some of these instruments include components such as symptomology and functional status, whereas some evaluate other factors such as social impact and emotional well-being.11 There is currently no consensus on the vital components of an asthma-specific QoL tool. A structured review comparing 6 of the most used QoL instruments found that each instrument differed in almost all the criteria considered, bringing into question whether the different instruments were measuring the same thing.10 In addition, the National Institutes of Health and other federal agencies convened an expert group to recommend standardized measures of asthma QoL for use in future clinical research. Subcommittees reviewed 11 known instruments and concluded that none of the instruments met their standards.11 The authors reported that the current measures of asthma QoL measured different domains, with most predominantly measuring indicators of asthma control, but failed to provide a reliable score measuring the intended construct or lacked adequate psychometric data. They also mentioned that a patient’s perspective of burden from disease can vary on the basis of the patient’s own priorities that need to be considered. In the Delphi study, participants also communicated that existing QoL measures did not capture symptom impact on QoL. These findings suggest that patients bring a unique perspective in evaluating treatment efficacy, and their experiences can help researchers determine outcome variables that are most impactful.
Despite efforts for a patient-centered approach to asthma, the AE burden of steroids from the patient perspective and its influence on QoL and treatment decision making have not yet been elucidated in an asthma population. The primary aim of this study was to use a convergent mixed-methods approach to quantify the AE burden from short- and intermediate-term OCS and ICS use and conduct qualitative focus groups to better understand the impact of AEs on patient QoL among adults with asthma. Gaining a better understanding of the effect of OCS and ICS from the patient perspective will aid the asthma community, healthcare providers, and other healthcare stakeholders in improving the quality of asthma care.
Methods
This study used a convergent design with a mixed-methods approach, incorporating both quantitative and qualitative methods. The quantitative component involved using surveys to obtain data on the perceived burden of AEs to ICS and OCS, whereas the qualitative component involved interviews and focus groups, which allowed us to better understand patient views on barriers reported in the quantitative data and to identify patient-important barriers that were not a part of the quantitative survey. The described quantitative and qualitative analyses were conducted independently and sequentially, and findings were integrated during interpretation using a convergent design.12 The quantitative survey was collected between February 2020 and April 2020. The qualitative interviews and focus groups were completed between March 2021 and June 2021.
Quantitative Inhaled Corticosteroids and Oral Corticosteroids Survey
Three members (AT, RM, VT) of the Green Park Collaborative’s coreASTHMA project team9 developed the survey used in collaboration with the Allergy & Asthma Network and Asthma UK to determine which ICS or OCS AEs were most burdensome to patients. It was written at an eighth-grade level and distributed online through the Allergy & Asthma Network database and social channels and the Asthma UK newsletter. No incentives were given for completing the surveys.
Respondents were queried regarding their steroid use and whether they used short- or long-term OCS in the last 12 months; the dose was also queried. The full survey is presented in eFigure 1. Respondents were also asked to rate a list of known AEs, comprising 25 from OCS and 9 from ICS (alone or combination inhalers), on a scale of 1 to 5, ranging from least burdensome to most burdensome. The list of AEs was compiled from a targeted literature review, which included only immediate and intermediate (occur in <3 years) AEs (eTable 1). An option to select “Don’t Know/No Opinion” was offered if they had no opinion or had uncertainties that made it impossible to form an opinion. Respondents were then asked to select the 1 AE they would eliminate from each corticosteroid category by switching their treatment, if given the choice. Mean scores for the most burdensome AEs and AEs most desired to be eliminated were calculated.
Demographic information was also collected. The inclusion criteria for analysis were (1) the respondent completed the survey within 24 hours of opening the survey, and (2) the respondent rated at least 1 AE.
Qualitative Interviews and Focus Groups
An expert advisory panel was convened (eTable 2) to help generate a semistructured interview guide (eFig 2). Using this guide, interviews were conducted individually with 11 patients who were members of the Asthma & Allergy Network who recently participated in our core outcome set.9 Results from the 11 patient interviews were subsequently used to generate a guide for the focus groups (eFig 3).
Participants were recruited for the focus groups using convenience sampling, through advertisements from the Allergy and Asthma Network.13 Each participant of the focus groups was given a $100 Amazon gift card for their participation. A total of 3 2-hour virtual in-depth focus groups, each comprising 5 participants with moderate-to-severe asthma, were conducted by our team to determine ways to improve the assessment of QoL in asthma clinical trials to increase patient-centeredness. Focus groups were led by a coinvestigator, and a list of questions was prepared to encourage open discussions on the impact of asthma on QoL. To enhance rigor, a single moderator (RM) was assigned to lead all focus group sessions. In addition, a second coinvestigator (AR) was present during the virtual calls to observe participant interactions, which included identifying any issues related to body language and verbal cues, and provided technical support to the primary moderator. This allowed the primary moderator to focus on facilitating the conversation. Questions involved multiple topics including (but not limited to) the impact of asthma on family, financial and social well-being, mental health, and treatment satisfaction (eFig 3). Focus groups were recorded and transcribed, with responses remaining anonymous.
The focus groups were analyzed qualitatively using an inductive approach.14 Transcripts were reviewed by 2 reviewers (AT and RM) and independently coded. Disagreements in coding were discussed until consensus was reached. These codes were then grouped into themes, which were further grouped into major categories. The study was approved by institutional review board of Advarra (approval number Pro00038849).
Results
Quantitative Inhaled Corticosteroids and Oral Corticosteroids Survey Results
There were 192 respondents to the survey, and of these, 124 met the prespecified inclusion criteria. Most respondents were between 35 and 74 years old (81%), identified as cis-female (85%), and had an asthma diagnosis (95%) (Table 1). All respondents resided in either the United States (78%) or United Kingdom (22%). A total of 82 patients reported short-course OCS use (≤30 days), and 42 reported having a long course of OCS (>30 days) in the past 12 months; 105 reported daily ICS use. Daily doses of OCS, if a patient had been taking them, were queried, but data regarding whether the doses were referring to short- or long-term use was not collected, preventing the assessment of cumulative dosing.
Table 1.
Baseline Characteristics and Medical history of Survey Respondents
| Characteristics | Number (%) |
|---|---|
| Age, y | |
| ≤24 | 6 (5) |
| 25–34 | 11 (9) |
| 35–44 | 12 (10) |
| 45–54 | 21 (17) |
| 55–64 | 43 (35) |
| 65–74 | 24 (19) |
| 75+ | 7 (6) |
| Gender | |
| Cisgender-female | 105 (85) |
| Cisgender-male | 18 (15) |
| Other | 1 (1) |
| Location | |
| United States | 97 (78) |
| United Kingdom | 27 (22) |
| Asthma diagnosis | |
| Yes | 118 (95) |
| No | 6 (5) |
| Severe persistent asthma diagnosis | |
| Yes | 54 (44) |
| No | 65 (52) |
| Not known | 5 (4) |
| Short-course OCS use (≤30 days) in past 12 mo | |
| Yes | 82 (66) |
| No | 42 (34) |
| Long course OCS use (>30 days) in past 12 mo | |
| Yes | 24 (19) |
| No | 100 (81) |
| OCS dose in past 12 mo (if applicable) | |
| Low (<7.5mg prednisone) | 12 (10) |
| Medium (7.5–30mg prednisone) | 21 (17) |
| High (30–100mg prednisone) | 36 (29) |
| It decreased over time | 19 (15) |
| Don’t know | 36 (29) |
| Current daily ICS ± LABA use | |
| Yes | 105 (85) |
| No | 19 (15) |
Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting beta agonist; OCS, oral corticosteroid.
Regarding OCS, the most burdensome AEs were weight gain or obesity, decreased bone mineral density, and infectious complications, with mean ratings of 3.77, 3.68, and 3.61 (of 5), respectively. The least burdensome AEs included elevated blood pressure, skin effects, and diarrhea, rated as 2.73, 2.62, and 2.39, respectively (Fig 1).
Figure 1.
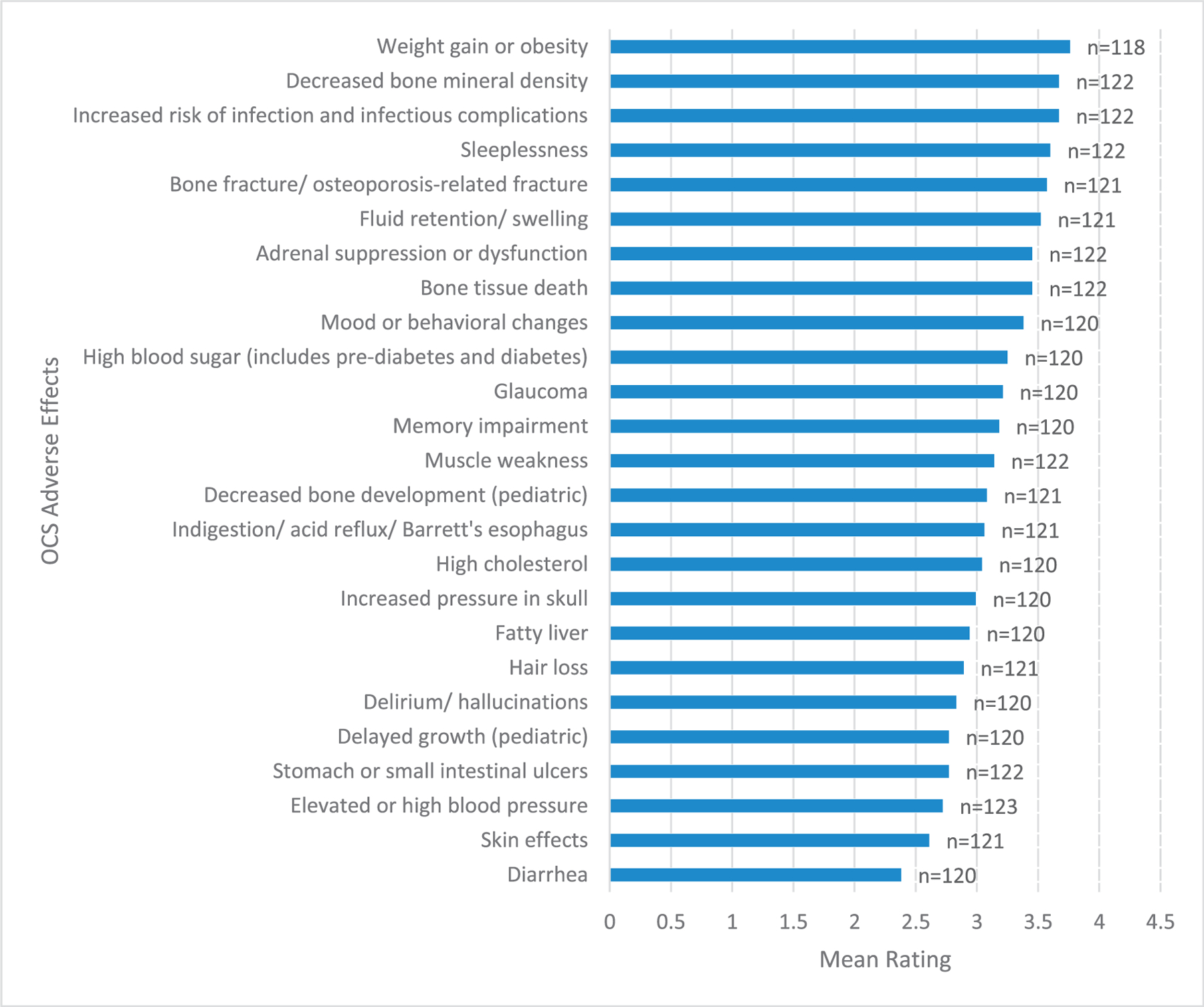
Most burdensome OCS adverse effects organized by mean rating of responses.
OCS, oral corticosteroids.
For ICS, the most burdensome AEs were pneumonia, hoarse voice, and oral thrush, with mean ratings of 3.19, 3.14, and 3.09 (of 5), respectively. The least burdensome AEs were cough with inhalation, tongue enlargement, and nose bleeds, rated as 2.46, 2.42, and 2.39, respectively (Fig 2).
Figure 2.
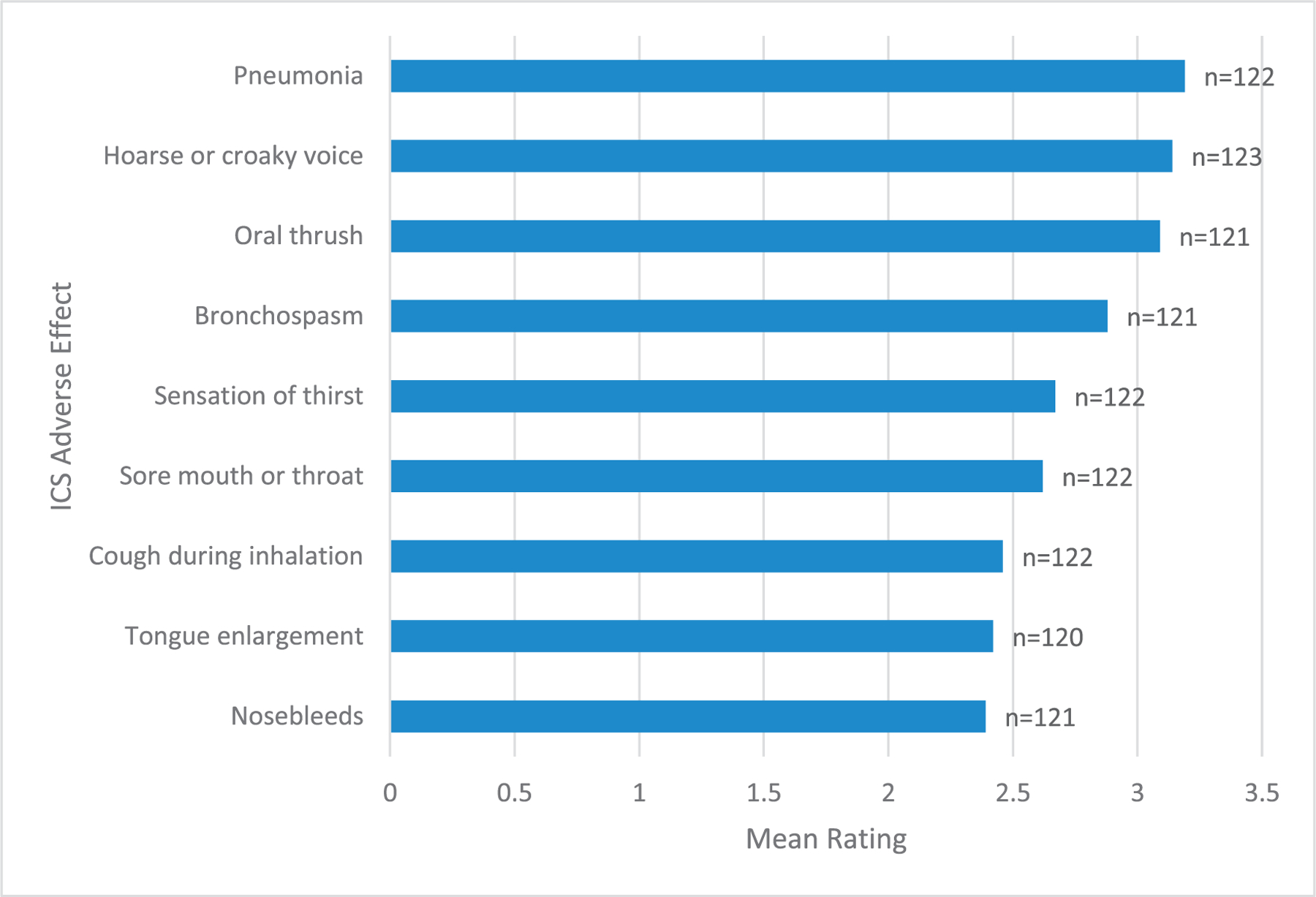
Most burdensome ICS adverse effects (with or without LABA) organized by mean rating of responses.
ICS, inhaled corticosteroids; LABA, long-acting beta agonist.
Participants were also given the option to choose the AE they most desired to be eliminated from each treatment option. For OCS, the top 3 effects most desired to be eliminated were weight gain or obesity, decreased bone density, and sleeplessness (Fig 3). For ICS, the most desired to be eliminated AEs were pneumonia, hoarse voice, and oral thrush (Fig 4).
Figure 3.
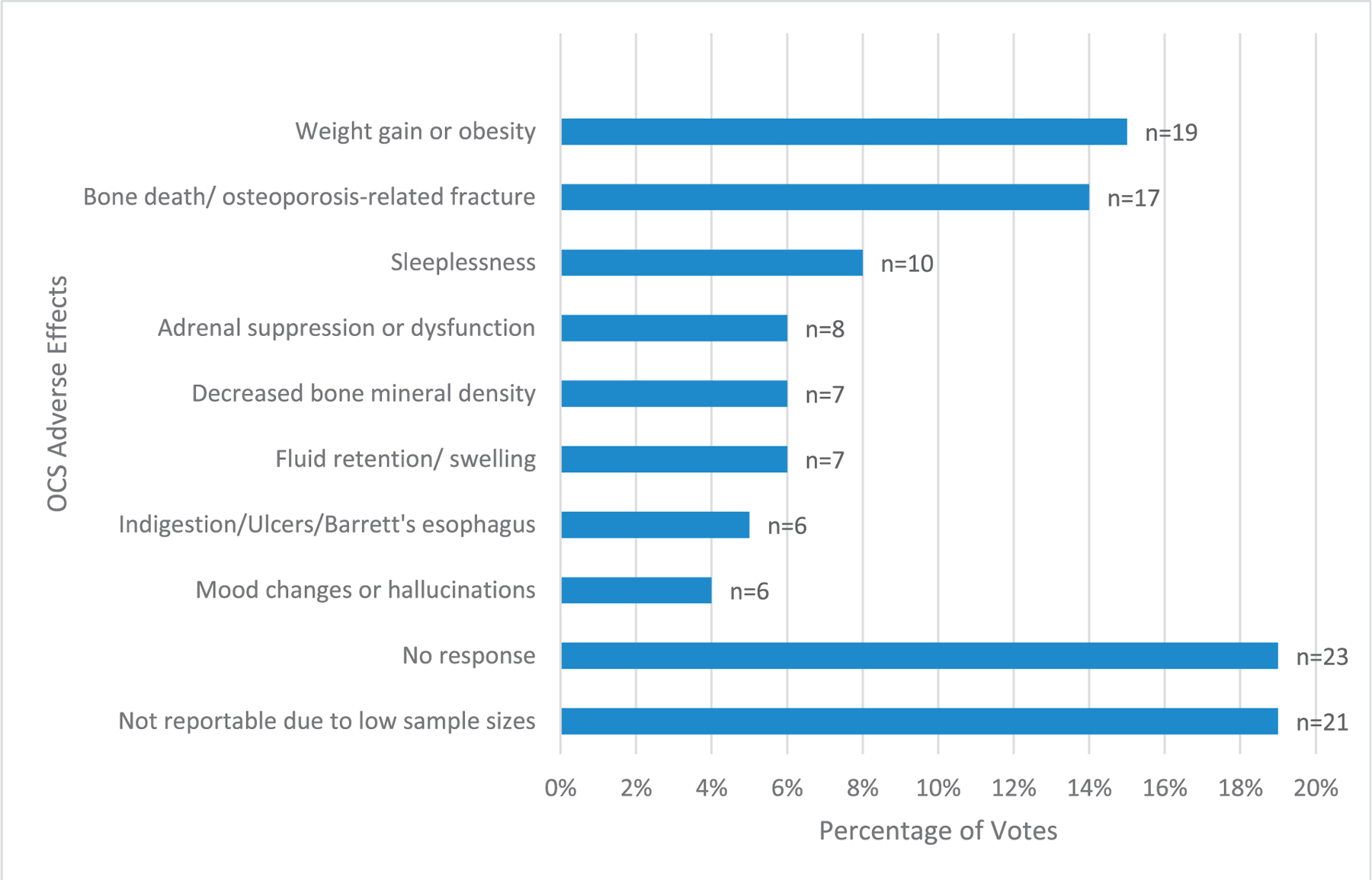
OCS adverse effects most desired to be eliminated, organized by percentage of votes.
Figure 4.
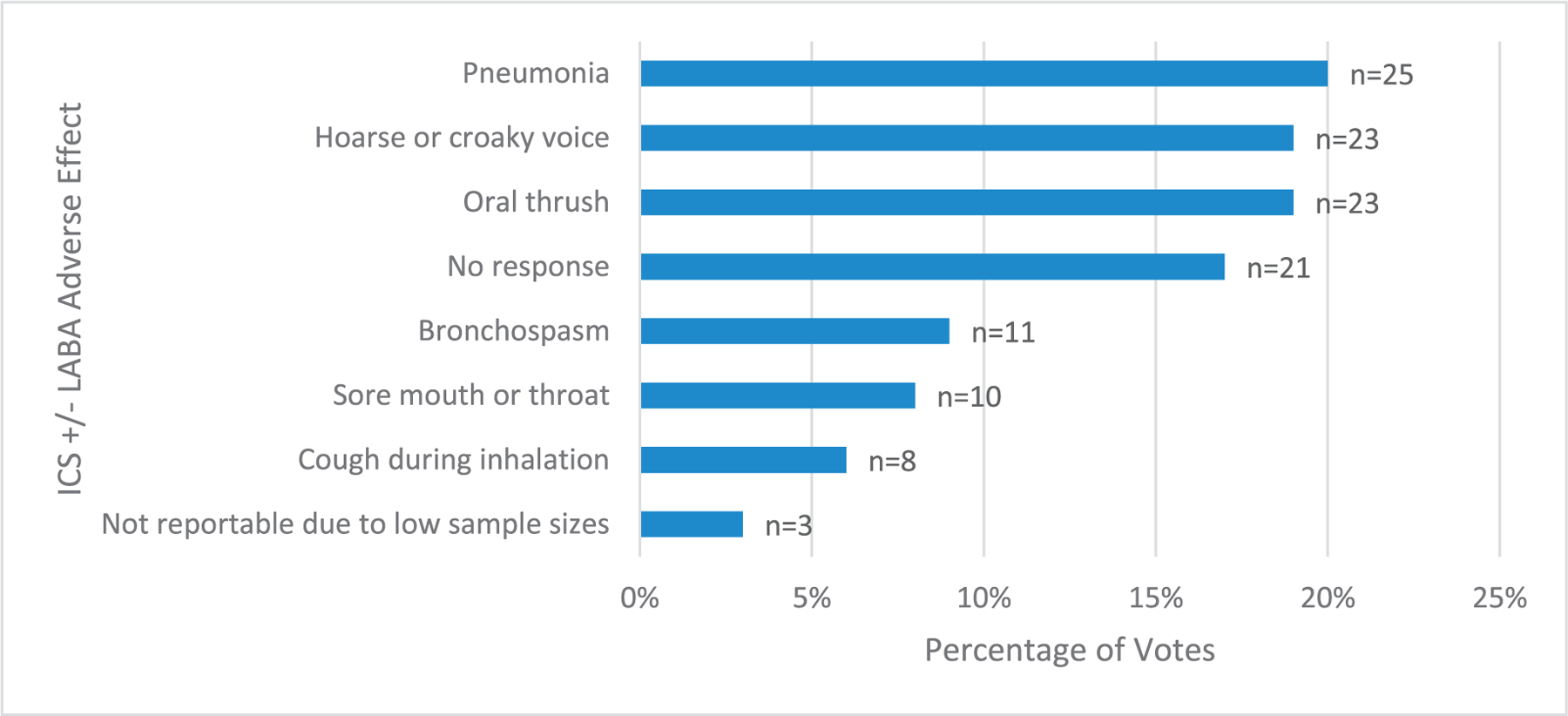
ICS adverse effects (with or without LABA) most desired to be eliminated by switching medical therapy.
ICS, inhaled corticosteroids; LABA, long-acting beta agonist.
Qualitative Asthma Treatment Results
Qualitative data were collected from organized focus groups, where participants with asthma were asked about topics they associated mostly with QoL. The major topics discussed included mental health and emotional wellness, treatment satisfaction and access to care, physical health and functioning, impact on daily life/lifestyle, and financial well-being. Patients’ quotes and major themes are listed in eTable 3.
Although there were no direct questions involving steroid usage, experiences with OCS and ICS kept arising. It was noted that patients who used OCS were burdened predominantly by biological/physical AEs of the medication. For example, “…I know about the prednisone. Made me gain so much weight that I don’t even like taking it.” Another patient-reported poor sleep from prednisone: “Prednisone as we all know is horrible. It’s worse for some people than others, but there have been times when I’ve gone at least 10 days with...not one minute of sleep.” The AEs reported by patients using ICS were more focused on the social stigma of using the medication. For example, a participant reported embarrassment with having to use their ICS in front of others: “If I have to use my inhaler sometimes out, I’ll go into the restroom or I’ll try to pull it out quickly and put it back.” The major themes discussed during the focus groups that involved the burden of medication use are described in Table 2.
Table 2.
Major Steroid Burden Themes Reported by Participants in the Qualitative Group
| Major topics | Themes | Supporting quotes |
|---|---|---|
| Biological/Physical Burden of Steroids: In treating disease, patients experience additional physical harm from the treatments themselves. | Physical effects of steroids: Patients reported known steroid adverse effects such as weight gain, sleep disturbances. Balancing adverse effects with disease management: Patients worked to balance adverse effects of steroids with necessity for controlling disease. |
—“…about the prednisone. It made me gain so much weight that I don’t even like taking it.” —“prednisone as we all know is horrible. It’s worse for some people than others, but there have been times when I’ve gone at least 10 days with zero... With zero, not one minute of sleep. …” —“So I am stuck on prednisone, but you know what? It’s handling me right now and then that’s what I have to do.” |
| Psychosocial Burden of Steroids/Inhalers: Analogous to physical and emotional health, patients experienced emotional harm from treatment in addition to physical. | Frustration: Patients were frustrated with the inability to control disease on an acceptable drug regimen. Embarrassment: Patients were also worried about external perception of illness from medication use. |
—“When I do go to the doctor, I just usually end up, burst out crying, because I don’t wanna do another round of prednisone.” —“If I have to use my inhaler sometimes out, I’ll go into the restroom or I’ll try to pull it out quickly and put it back. There’s still this level of embarrassment of having this condition and having to pull out this medication.” |
Discussion
This study used a convergent mixed-methods approach, combining both quantitative and qualitative methods to provide a comprehensive understanding of patient experiences with ICS and OCS use. The quantitative results highlighted the most burdensome AEs associated with ICS and OCS use, whereas the qualitative data further enriched the understanding of patient perspectives and uncovered additional concerns, such as the psychosocial burden not captured by the quantitative data.
Differing views of the AEs from ICS emerged from our qualitative and quantitative data. In the quantitative surveys, the most burdensome AEs reported by patients were pneumonia, hoarse voice, and oral thrush and were also the 3 most desired AEs that patients would eliminate by switching therapies. However, in our qualitative data, psychosocial burden of ICS was an emergent theme whereas somatic ICS AEs were not. This difference may be partially explained by the importance healthcare providers place on somatic ICS AEs, as indicated by the wide range of interventions to prevent their occurrence, compared with a paucity of interventions targeting psychosocial AEs of ICS. Local effects of ICS are common, with estimated incidence of hoarse voice reported to have an incidence of 5% to 58%,15 and oral thrush of 5% with a range from less than 1% to almost 70% across studies.16 Conversely, studies have been mixed regarding whether there is a risk of pneumonia in patients with asthma on medication.17,18 Although prevalent, the AEs of ICS may be reduced by rinsing with water after inhaler use,19,20 using a spacer device,20 or using different steroid formulations/regimens.16 Psychosocial effects of inhalers are known but not widely studied. Past studies have reported that adolescents with asthma expressed concerns of social stigma from taking inhalers,21,22 and patients in India viewed inhaler use as an impediment to marriage proposals.23 In addition, these effects may be generalizable to other inhaler medications.24 However, there are few studies investigating ways to mitigate this social stigma or the effect of stigma on medication adherence. This is particularly important given the association of depression and anxiety with asthma severity25 and exacerbations,26 and data showing behaviors such as educating individuals about the disease and positive thinking may be protective.21 The consistency of our qualitative data with past results and the lack of inclusion of psychosocial AEs of ICS in the quantitative survey suggest that psychosocial AEs of ICS are important to patients and underrecognized by healthcare practitioners.
Regarding OCS AEs, somatic AEs were highly prevalent in our quantitative data, in addition to being an emergent theme in our qualitative data. In our quantitative study on OCS, the most burdensome AEs (weight gain, decreased bone density, and infectious complications) reported by patients aligned with the AEs patients preferred most to eliminate, except for infectious complications, which were replaced by sleeplessness. Consistent with the quantitative group, our qualitative study group reported weight gain and sleeplessness as burdensome AEs. These results are consistent with past reports showing association of OCS with weight gain,27 decreases in bone density,28 and sleeplessness,27 even at low doses4 or intermittent dosing.29 Past qualitative work has likewise highlighted the great importance patients with pulmonary diseases such as chronic obstructive pulmonary disease30 and sarcoidosis31 place on avoiding OCS AEs. Although there are interventions to reduce OCS AEs, such as calcium and vitamin D supplementation,32 screening for osteoporosis,31 and minimizing dose, these measures are often insufficient to mitigate all somatic effects.4 These results indicate the continued importance of developing therapies to minimize the reliance on OCS in patients with asthma, and the impact of OCS physical effects on asthma QoL.
It is important to note the burden of AEs is only a piece of the puzzle regarding medication adherence. Although AEs from OCS and ICS were prevalent in our quantitative results, our qualitative results highlighted many other barriers to adherence, including cost of medication, access to expert providers, unpredictability of disease, and comorbid mental health burdens (eTable 3). Because of these and other factors, adherence to asthma medication regimens remains poor, with reported rates ranging from 30% to 70%.33 By determining the AEs that would deter patients with asthma from steroid adherence, future studies can look for ways to further minimize the most burdensome effects. Further studies may also explore the populations most at risk. Given that ICS and OCS remain the cornerstone of asthma management, better mitigation of these AEs, both somatic and psychosocial, will be important to enhance medication compliance and improve asthma treatment. Understanding the role of ICS and OCS in AEs will also be important for developing more patient-centered asthma-specific QoL instruments for research and clinical practice.
There are limitations of our study that merit discussion. The qualitative and quantitative data did not include an in-depth look at patient demographics, which makes patient individuality difficult to consider. For example, sleeplessness was voted to be a more desirable AE to eliminate than were infectious complications. However, occupational factors, such as working fixed hours vs flexible hours or being a retiree, may affect one’s ability to manage sleeplessness and how one rates that AE. Ethnicity and race were also not considered in this study, in both the participants in the study and the individuals who composed the expert panel and interview guides. As a result, this study may have a bias toward Western perspectives, ignoring culturally relevant aspects of the disease and AE perceptions. Asthma severity is another important variable, whereby patients with less severe asthma symptoms or less experience with steroids would more likely be open to switching therapies. Patients who have experienced certain AEs may also have different responses from those who have not. Unfortunately, if a patient previously experienced an AE, this was not documented. The cumulative steroid dose each patient received was also not documented. In addition, the study did not document patients who used strategies to mitigate OCS or ICS, such as mouth rinsing. Individuals who used such strategies may have perceived a different level of burden for specific AEs. Another limitation of this study involves its limited sample size (124 participants) and the use of voluntary surveys to collect data. These limitations put us at risk for voluntary response bias and not being fully representative of the total population. Individuals with limited technology literacy/access were also unlikely to be included. Another limitation that exists is the rating system used for level of burden, given this is a subjective measure and the difference between the nonextreme values may be difficult for participants to discriminate. The study also relied on patients self-reporting their asthma diagnosis and medication use, which may introduce further bias. Moreover, not all AEs were included in the survey, such as cataracts, which were previously shown to have a prevalence of 9% in patients with severe asthma on long-term OCS.34 This is an unfortunate limitation because we are unable to determine how patients view the burden of this AE compared with others. Lastly, the survey period traversed the announcement of the SARS-CoV-2 pandemic, which could have confounded participants’ responses. We anticipate participants might have answered on the basis of their historical experiences, but the effect that the pandemic announcement might have had on survey responses is unclear. In addition, although our study emphasized the psychosocial burden of ICS, it is likely that this finding is not unique to ICS but could also apply to other inhalers.
Using quantitative questionnaires and qualitative interviews and focus groups, this study allowed us to explore aspects of asthma-specific QoL important to patients, especially regarding the perception of burden of AEs from steroids. We found that the most burdensome AEs faced by patients were not fully the same AEs that would cause them to change medications, although there is overlap. Furthermore, patient interviews and focus groups identified social stigma and psychosocial burden associated with ICS and OCS use that may limit adherence. Further understanding of patient perception can assist in better characterization of asthma-specific QoL as a clinical outcome, importantly with incorporation of the medication AE burden. Closer collaboration and direct communication between healthcare providers and patients will allow greater understanding of the effects of asthma interventions on patients’ QoL and most accurately capturing the burden of asthma.
Supplementary Material
Acknowledgments
The authors acknowledge Rachael Moloney and Ayesha Robinson for their efforts in leading and moderating the focus group discussions.
Funding
The authors have no funding sources to report.
Footnotes
Disclosures
The authors have no conflicts of interest to report.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2023.08.595.
References
- 1.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr 2019;7:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, Lange P. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J 2019;54(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ. Inhaled corticosteroids. Pharmaceuticals (Basel) 2010;3(3):514–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med 2020;201(3):276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miravitlles M, Auladell-Rispau A, Monteagudo M, et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur Respir Rev 2021;30(160). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 7.Cooper V, Metcalf L, Versnel J, Upton J, Walker S, Horne R. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med 2015;25:15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponieman D, Wisnivesky JP, Leventhal H, Musumeci-Szabó TJ, Halm EA. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Ann Allergy Asthma Immunol 2009;103(1):38–42. [DOI] [PubMed] [Google Scholar]
- 9.Tejwani V, Chang HY, Tran AP, et al. A multistakeholder Delphi consensus core outcome set for clinical trials in moderate-to-severe asthma (coreASTHMA). Ann Allergy Asthma Immunol 2021;127(1):116–122.e7. [DOI] [PubMed] [Google Scholar]
- 10.Apfelbacher CJ, Hankins M, Stenner P, Frew AJ, Smith HE. Measuring asthma-specific quality of life: structured review. Allergy 2011;66(4):439–457. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SR, Rand CS, Cabana MD, et al. Asthma outcomes: quality of life. J Allergy Clin Immunol 2012;129(3 suppl):S88–S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creswell JW, Klassen AC, Clark VP, Smith KC. For the Office of Behavioral and Social Sciences Research, Best Practices for Mixed Methods Research in the Health Sciences National Institutes of Health; 2011:39. http://obssr.od.nih.gov/mixed_methods_research. Accessed September 18, 2023. [Google Scholar]
- 13.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Admin Policy Ment Health 2015;42(5):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval 2006;27(2):237–246. [Google Scholar]
- 15.Galván CA, Guarderas JC. Practical considerations for dysphonia caused by inhaled corticosteroids. Mayo Clin Proc 2012;87(9):901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buhl R Local oropharyngeal side effects of inhaled corticosteroids in patients with asthma. Allergy 2006;61(5):518–526. [DOI] [PubMed] [Google Scholar]
- 17.McKeever T, Harrison TW, Hubbard R, Shaw D. Inhaled corticosteroids and the risk of pneumonia in people with asthma: a case-control study. Chest 2013;144 (6):1788–1794. [DOI] [PubMed] [Google Scholar]
- 18.O’Byrne PM, Pedersen S, Carlsson LG, et al. Risks of pneumonia in patients with asthma taking inhaled corticosteroids. Am J Respir Crit Care Med 2011;183 (5):589–595. [DOI] [PubMed] [Google Scholar]
- 19.Kajiwara A, Kita A, Saruwatari J, et al. Absence of gargling affects topical adverse symptoms caused by inhaled corticosteroids in females. J Asthma 2014;51 (2):221–224. [DOI] [PubMed] [Google Scholar]
- 20.Levy ML, Dekhuijzen PNR, Barnes PJ, et al. Inhaler technique: facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). NPJ Prim Care Respir Med 2016;26(1):16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Simoni A, Horne R, Fleming L, Bush A, Griffiths C. What do adolescents with asthma really think about adherence to inhalers? Insights from a qualitative analysis of a UK online forum. BMJ Open 2017;7(6): e015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naimi DR, Freedman TG, Ginsburg KR, Bogen D, Rand CS, Apter AJ. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol 2009;123(6):1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh V, Sinha HV, Gupta R. Barriers in the management of asthma and attitudes towards complementary medicine. Respir Med 2002;96(10):835–840. [DOI] [PubMed] [Google Scholar]
- 24.Alzayer R, Almansour HA, Basheti I, Chaar B, al Aloola N, Saini B. Asthma patients in Saudi Arabia − preferences, health beliefs and experiences that shape asthma management. Ethn Health 2022;27(4):877–893. [DOI] [PubMed] [Google Scholar]
- 25.Amelink M, Hashimoto S, Spinhoven P, et al. Anxiety, depression and personality traits in severe, prednisone-dependent asthma. Respir Med 2014;108(3):438–444. [DOI] [PubMed] [Google Scholar]
- 26.Anastasia P, Eleni T, Eleftheria M, et al. Depression levels influence the rate of asthma exacerbations in females. J Pers Med 2021;11(6):586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006;55 (3):420–426. [DOI] [PubMed] [Google Scholar]
- 28.van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2006;79(3):129–137. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol 2018;141(1):110–116.e7. [DOI] [PubMed] [Google Scholar]
- 30.Laue J, Melbye H, Risør MB. Self-treatment of acute exacerbations of chronic obstructive pulmonary disease requires more than symptom recognition - a qualitative study of COPD patients’ perspectives on self-treatment. BMC Fam Pract 2017;18(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harper LJ, Love G, Singh R, Smith A, Culver DA, Thornton JD. Barriers to care among patients with sarcoidosis: a qualitative study. Ann Am Thorac Soc 2021;18 (11):1832–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol 2017;76(1):1–9. [DOI] [PubMed] [Google Scholar]
- 33.Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MCJM, Verhamme KMC. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J 2015;45(2):396–407. [DOI] [PubMed] [Google Scholar]
- 34.Barry LE, Sweeney J, O’Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res 2017;18(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


