Abstract
Background
Proton pump inhibitors (PPIs) are the most effective drugs to reduce gastric acid secretion. PPIs are one of the most commonly prescribed classes of medications worldwide. Apart from short‐term application, maintenance therapy with PPIs is recommended and increasingly used in certain diseases, such as Zollinger‐Ellison syndrome and gastro‐oesophageal reflux disease, especially for people with erosive oesophagitis or Barrett's oesophagus. Although PPIs are generally safe, their efficacy and safety of long‐term use remains unclear. The question of whether the long‐term use of PPIs could promote the development of gastric pre‐malignant lesions has been widely investigated, but results are inconsistent. Limited insight on this problem leads to a dilemma in decision making for long‐term PPI prescription.
Objectives
To compare the development or progression of gastric pre‐malignant lesions, such as atrophic gastritis, intestinal metaplasia, enterochromaffin‐like (ECL) cell hyperplasia, and dysplasia, in people taking long‐term (six months or greater) PPI maintenance therapy.
Search methods
We searched the following databases (from inception to 6 August 2013): the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and CINAHL. In addition, we searched the reference lists of included trials and contacted experts in the field.
Selection criteria
We searched for randomised controlled trials (RCTs) in adults (aged 18 years or greater) concerning the effects of long‐term (six months or greater) PPI use on gastric mucosa changes, confirmed by endoscopy or biopsy sampling (or both).
Data collection and analysis
Two review authors independently performed selection of eligible trials, assessment of trial quality, and data extraction. We calculated odds ratios (OR) for analysis of dichotomous data and mean differences for continuous data, with 95% confidence intervals (CI).
Main results
We included seven trials (1789 participants). Four studies had high risk of bias and the risk of bias in the other three trials was unclear. In addition, it was difficult to assess possible reporting bias. We pooled 1070 participants from four RCTs to evaluate corporal atrophy development revealing an insignificantly increased OR of 1.50 (95% CI 0.59 to 3.80; P value = 0.39; low‐quality evidence) for long‐term PPI users relative to non‐PPI users. In five eligible trials, corporal intestinal metaplasia was assessed among 1408 participants, also with uncertain results (OR 1.46; 95% CI 0.43 to 5.03; P value = 0.55; low‐quality evidence). However, by pooling data of 1705 participants from six RCTs, our meta‐analysis showed that participants with PPI maintenance treatment were more likely to experience either diffuse (simple) (OR 5.01; 95% CI 1.54 to 16.26; P value = 0.007; very‐low‐quality evidence) or linear/micronodular (focal) ECL hyperplasia (OR 3.98; 95% CI 1.31 to 12.16; P value = 0.02; low‐quality evidence) than controls. No participant showed any dysplastic or neoplastic change in any included studies.
Authors' conclusions
There is presently no clear evidence that the long‐term use of PPIs can cause or accelerate the progression of corpus gastric atrophy or intestinal metaplasia, although results were imprecise. People with PPI maintenance treatment may have a higher possibility of experiencing either diffuse (simple) or linear/micronodular (focal) ECL cell hyperplasia. However, the clinical importance of this outcome is currently uncertain.
Keywords: Humans; Enterochromaffin‐like Cells; Enterochromaffin‐like Cells/pathology; Gastritis, Atrophic; Gastritis, Atrophic/chemically induced; Hyperplasia; Hyperplasia/chemically induced; Intestines; Intestines/pathology; Maintenance Chemotherapy; Maintenance Chemotherapy/adverse effects; Metaplasia; Metaplasia/chemically induced; Precancerous Conditions; Precancerous Conditions/chemically induced; Precancerous Conditions/pathology; Proton Pump Inhibitors; Proton Pump Inhibitors/adverse effects; Randomized Controlled Trials as Topic; Stomach Neoplasms; Stomach Neoplasms/chemically induced; Stomach Neoplasms/pathology; Time Factors
Plain language summary
Effect of the long‐term use of proton pump inhibitors on the rate of pre‐cancerous lesions in the stomach
Review question
Does long‐term proton pump inhibitor (PPI) use increase the risk of having pre‐cancerous lesions (changes in the stomach lining that are not cancer but could become cancerous over time) in the stomach?
Background
PPIs are the most effective drugs used to reduce gastric acid secretion (called antacids) and they are commonly prescribed worldwide. Although generally safe, their effectiveness and safety for long‐term use remains unclear. It has been suggested that the long‐term use of PPIs could promote the development of pre‐cancerous lesions in the stomach, which might subsequently increase the occurrence of stomach cancer. Therefore, the safety issues of long‐term PPI treatment needs to be addressed.
Study characteristics
We searched databases in August 2013 for randomised controlled trials (clinical trials where people are randomly allocated to one of two or more treatment groups) conducted in adults (aged 18 years or over) who did not have gastric cancer at the start of the trial. Treatment had to be with PPI for six months or more and be compared with no treatment, surgery/endoscopic treatment (where a tube is passed down the food pipe and into the stomach), or any other antacid treatment.
We found seven randomised controlled trials with 1789 participants. Some trials only partially reported gastric pre‐cancerous lesions, and there was a substantial proportion of participants with missing data.
Key results
We concluded that there was no clear evidence to support the notion that the long‐term use of PPIs could promote the development of pre‐cancerous lesions. However, there was a potentially elevated risk of developing a thickening of the stomach lining (hyperplasia) among participants with long‐term PPI use, which is considered as a possible pre‐condition of gastric carcinoid (a relatively benign (non‐cancerous) tumour that develops within the stomach lining).
Quality of the evidence
Currently, available evidence was of low or very low quality, due to their study design and the large proportion of missing data. We therefore suggest future well‐designed clinical trials should be performed for providing better understandings regarding this question.
Summary of findings
Summary of findings for the main comparison. Proton pump inhibitor compared with control for gastric pre‐malignant lesions.
| Proton pump inhibitor compared with control for gastric pre‐malignant lesions | ||||||
| Patient or population: people with pre‐malignant lesions Settings: Intervention: PPI Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PPI | |||||
| Number of participants with worsening scores in corporal atrophy | 21 per 1000 | 31 per 1000 (12 to 75) | OR 1.5 (0.59 to 3.8) | 1070 (4 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
| Number of participants with worsening scores in corporal intestinal metaplasia | 6 per 1000 | 9 per 1000 (3 to 30) | OR 1.46 (0.43 to 5.03) | 1408 (5 studies) | ⊕⊕⊝⊝ low2,3 | ‐ |
| Increase in the number of participants with simple (diffuse) hyperplasia after treatment | 2 per 1000 | 9 per 1000 (3 to 27) | OR 5.01 (1.54 to 16.26) | 1705 (6 studies) | ⊕⊝⊝⊝ very low2,4,5 | ‐ |
| Increase in the number of participants with linear/micronodular (focal) hyperplasia, comparing final biopsies with baseline biopsies | 3 per 1000 | 14 per 1000 (4 to 40) | OR 3.98 (1.31 to 12.16) | 1705 (6 studies) | ⊕⊕⊝⊝ low2,4 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; PPI: proton pump inhibitor. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Two of these four trials had high risk of bias. 2 The relatively few participants or few events (or both) resulted in wide CIs around the estimate of the effect. 3 Three of these five trials had high risk of bias. 4 Risk of bias was high in three of these six trials, and moderate in the other three. 5 High heterogeneity was detected, without clear explanation.
Background
Description of the condition
Gastric cancer, as the fourth most common cancer and second leading cause of cancer‐related deaths worldwide (Ferlay 2010). It is a fatal disease placing a heavy burden on human health. The Lauren's classification divides gastric cancer into two major histologic types: intestinal or diffuse (Lauren 1965). Abundant studies have demonstrated that the intestinal type of gastric cancer is the most common histological type among the older population (aged over 50 years) and that it develops through a well‐recognised cascade of events (inflammation‐metaplasia‐dysplasia‐carcinoma sequence). Generally, this process is known as Correa cascade of multi‐step gastric carcinogenesis (Correa 1992; Kapadia 2003), where a progression occurs from normal mucosa, through chronic non‐atrophic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, and ultimately to carcinoma. The progression is usually triggered by colonisation of Helicobacter pylori (H. pylori), which is the most recognised risk factor for gastric cancer. Pathologically, atrophy gastritis should be diagnosed based on biopsy results that indicate the presence of chronic inflammatory cells and the disappearance of normal glands. Subsequently, with the reduction of normal glands and their replacement by intestinal glands, intestinal metaplasia develops in the atrophic mucosa and is believed to constitute the background in which dysplasia and further carcinoma develop (Dinis‐Ribeiro 2011). Since corpus gastric atrophy, intestinal metaplasia, and epithelial dysplasia of stomach have been tightly associated with an increased risk of gastric cancer (Fukao 1993; Genta 1998; Kapadia 2003), avoiding these pre‐malignant lesions is considered vital for reducing the incidence and mortality of gastric cancer (Dinis‐Ribeiro 2011).
Despite declining incidence rates of gastric cancer, there was an unexpected reverse trend regarding the incidence of non‐cardia gastric cancer among white people aged 25 to 39 years in the US (Anderson 2010). Cross‐sectional population‐based analyses in Sweden between 1990 and 1999 revealed that while the incidence of non‐cardia gastric cancer fell, as expected, among people aged 55 to 64 years, the prevalence of atrophic gastritis surprisingly rose in people aged 35 to 44 years (Held 2004). Given that the prevalence of H. pylori infection was gradually decreasing (Asfeldt 2008; Shiota 2013), other potential causes related to gastric pre‐malignant lesions need to be investigated.
Gastric carcinoid is rare tumour that develops within the gastric mucosa and originates from enterochromaffin‐like (ECL) cells. Although it is relatively benign, the tumour can invade locally into deeper structures of the gastrointestinal tract wall. Furthermore, it has been reported that both solitary and multiple gastric carcinoids can become malignant and metastasise (Hwang 2006). Therefore, concerns about the progression of ECL cell hyperplasia may also have great clinical relevance.
Description of the intervention
Proton pump inhibitors (PPIs) are the most effective drugs to reduce gastric acid secretion. Since their introduction in the late 1980s, they have dramatically improved the medical treatment of acid‐related gastric disorders (Wolfe 2000), including peptic ulcer disease (Dekkers 1999; Holt 1991; Poynard 1995), eradication of H. pylori, treatment and prevention of gastroduodenal ulcers associated with non‐steroidal anti‐inflammatory drugs (NSAIDs) (Agrawal 2000; Rostom 2002), Zollinger‐Ellison syndrome (Norton 1999), and management of gastro‐oesophageal reflux disease (GORD) or Barrett's oesophagus (Cooper 2006; Hetzel 1988; Marks 1994; Vigneri 1995; Wilkinson 1999). PPIs are one of the most commonly prescribed classes of medications worldwide. Apart from short‐term application, maintenance therapy with PPIs is recommended and increasingly used in certain diseases, such as Zollinger‐Ellison syndrome and GORD, particularly for people with erosive oesophagitis or Barrett's oesophagus (Peters 1999; Horwhat 2007). GORD occurs in one‐third of adults (Haag 2003). For those people with a propensity of oesophagitis to relapse, maintenance acid‐suppressive therapy is often necessary.
Gastric hydrogen potassium ATPase (H+/K+‐ATPase) is a vital proton pump that enables the exchange of hydrogen (H+) and potassium (K+) ions across the canalicular membrane in acid‐secreting parietal cells. Once absorbed by the small intestines, PPIs are distributed to the gastric parietal cells, where they are accumulated and protonated to an active form in the acidic environment. The action of PPIs can irreversibly inhibit H+/K+‐ATPase, and thus cause profound suppression of acid secretion (Dajani 2000). Table 2 lists the most commonly used PPIs.
1. Pharmacokinetic properties of proton pump inhibitors.
| Agent | Bioavailability | Cmax | AUC 0‐24 | Excretion | pKa |
| Omeprazole | 45% | 0.7 | 2.0 | Renal | 4.0 |
| Lansoprazole | 85% | 0.5‐1.0 | 2.5 | Biliary | 4.0 |
| Rabeprazole | 52% | 0.4 | 0.8 | Renal | 5.0 |
| Pantaprazole | 77% | 2.5 | 5.0 | Renal | 3.9 |
| Esomeprazole | 64% | 1.5 | 4.3 | Renal | 4.0 |
AUC: area under the curve (mg.h/L); Cmax: maximum plasma concentration (mg/mL)
Although PPIs are generally safe, their efficacy and safety of long‐term use remains inconclusive (Johnson 2013; Suzuki 2008). Many potential adverse effects have been observed and widely discussed (Ali 2009; Thomson 2010). Briefly, the three main concerns regarding the long‐term safety of the PPIs include the effects of prolonged PPI‐induced hypergastrinaemia (an excess of gastrin in the blood), the effects of chronic hypochlorhydria (reduction in the hydrochloric acid content of gastric juice), and the possible association of PPIs with gastric atrophy. Due to the emerging evidence about adverse effects, the US Food and Drug Administration (FDA) has issued a number of broad‐based product warnings, which includes all of the available PPI drugs either for prescription or non‐prescription purchase.
How the intervention might work
Almost every person develops hypergastrinaemia in response to profound acid‐suppressive therapy (Sanduleanu 1999; Schenk 1998). With prolonged hypergastrinaemia, hyperplasia of ECL cells could occur, especially in people with H. pylori infection or with more markedly increase gastrin levels (Waldum 2014). It has been reported that ECL‐like cell hyperplasia can lead to dysplasia, and ultimately gastric carcinoid formation, both in rats (Freston 1994) and in humans (Jianu 2012). Jianu reported that two people with more than 10 years of PPI use developed a well‐differentiated neuroendocrine tumour (Jianu 2012). However, other long‐term studies concerning people using PPIs have not shown an increased risk of carcinoids (Hassall 2011; Klinkenberg‐Knol 2000).
Another safety concern with long‐term PPI use is the effects of sustained hypochlorhydria. First, prolonged gastric acid hyposecretion could result in clinically significant nutritional deficiencies. For example, deficiencies of vitamin B12, iron, and calcium were commonly observed in people following long‐term PPI use (Abraham 2012). Second, gastrointestinal acid secretion plays a protective role against infectious agents. Therefore, prolonged PPI‐induced hypochlorhydria may also increase the risk of contracting gastrointestinal and respiratory microbial infection (e.g. community‐acquired pneumonia and nosocomial pneumonia) (Kader 1998; Laheij 2004; Neal 1996; Reynaert 1995).
Discussions on the long‐term safety of PPIs has gradually shifted to the possible association between PPI treatment and gastritis. For instance, the propensity of people treated with omeprazole (a PPI) to develop chronic atrophic gastritis has been proposed in several studies (Klinkenberg‐Knol 2000; Kuipers 1996). One possible mechanism of this phenomenon is explained in terms of the interrelation between acid secretion and H. pylori status. In people with suppressed acid secretion caused by maintenance PPI use or any other mechanism, H. pylori are harboured both the antrum and body of the stomach, instead of antrum only. This unusual colonisation mode leads to a corpus predominant gastritis (Malfertheiner 2007), which could accelerate the process of gland loss and subsequently result in the appearance of corpus atrophic gastritis. Treatment of H. pylori‐positive animals with six months of omeprazole resulted in them all developing gastritis, and most then progressed to metaplasia during the later stages of disease (Fox 2011). In Kuipers 1996, a gradual development of atrophic gastritis was observed among H. pylori‐positive people with omeprazole maintenance therapy within the first year of treatment; at a mean follow‐up five‐years, approximately one of every three participants developed atrophic gastritis. Consequently, eradication of H. pylori infection prior to long‐term acid suppression with PPIs was suggested as a way to prevent the development of atrophic gastritis (Moayyedi 2000; Schenk 2000). Since persistent corpus‐predominant gastritis and atrophy were considered as major risk factors for the development of gastric cancer (Sipponen 2007), the potential role of maintenance PPI use on the development of corpus atrophy/intestinal metaplasia became the issue of much debate from the mid‐2000s.
Another factor that might potentially affect the development of gastric pre‐malignant lesions (i.e. atrophic gastritis, intestinal metaplasia, and dysplasia) is non‐Helicobacter bacterial overgrowth under sustained low gastric acid conditions. In people without long‐term PPI use, such colonisation might occur after severe atrophic gastritis has developed. However, in PPI users, colonisation of various microbes probably happens at an earlier stage and is related to the further development of more severe gastritis (Sanduleanu 2001). Furthermore, the potent carcinogens produced by these microbes, including nitrosamines and acetaldehyde, may also promote the information of pre‐cancerous lesions, or even cancer itself.
Why it is important to do this review
Since PPIs are widely used, safety issue about this medication should be seriously considered. The question of whether the long‐term use of PPIs could promote the development of gastric pre‐malignant lesions has been widely investigated, but results are inconsistent. Limited insight on this problem leads to a dilemma in decision making for PPI maintenance treatment. In addition, since the incidence of non‐cardia gastric cancer and the prevalence of atrophic gastritis were increased among the young middle‐age population (aged 25 to 44 years), the possible association between long‐term PPI use and gastric pre‐malignant lesions needs to be elucidated. Thus, there is a need for a systematic review to address the question of long‐term safety of PPIs regarding development of pre‐malignant lesions in the stomach.
Objectives
To compare the development or progression of gastric pre‐malignant lesions, such as atrophic gastritis, intestinal metaplasia, enterochromaffin‐like (ECL) cell hyperplasia, and dysplasia, in people taking long‐term (six months or greater) PPI maintenance therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We included studies reported as full text, as abstract only, and unpublished data.
Types of participants
Adults (aged 18 years or greater) without any gastric malignant lesion at baseline, confirmed by endoscopy or biopsy sampling (or both).
Types of interventions
The experimental intervention was PPI use for six months or greater. We included any study with at least one intervention arm and at least one valid control arm. A valid control group included one of the following subset of participants:
with no treatment or placebo;
undergoing anti‐reflux surgery (ARS) or endoscopic anti‐reflux treatment;
other anti‐acid treatment: histamine H2‐receptor antagonists or antacids.
We considered only oral therapies administered at any dosage.
Types of outcome measures
Primary outcomes
Development or progression of gastric pre‐malignant lesions after long‐term PPI use, mainly including atrophic gastritis, intestinal metaplasia, ECL cell hyperplasia, and dysplasia. Classification and grading of these lesions should be carried out by experienced pathologists, using well‐established criteria (e.g. (updated) Sydney system for gastritis (Dixon 1996; Price 1991), and the system described by Solcia 1988 for ECL cell hyperplasia).
Secondary outcomes
No planned secondary outcomes.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs. The literature search identified potential studies in all languages. We translated the non‐English language papers and assessed them fully for potential inclusion in the review as necessary.
We searched the following electronic databases for identifying potential studies:
Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 8, 2013) (Appendix 1);
MEDLINE (1966 to August 2013) (Appendix 2);
EMBASE (1988 to August 2013);
CINAHL (1982 to August 2013).
We also conducted a search of ClinicalTrials.gov (www.clinicaltrials.gov/).
Searching other resources
We handsearched the abstracts from 1995 to 2012 from the American Digestive Disease Week published in Gastroenterology and the United European Gastroenterology Week published in Gut.
We checked reference lists of all primary studies and review articles for additional references. We planned to contact authors of identified trials and ask them to identify other published and unpublished studies if it was necessary.
We contacted manufacturers and experts in the field.
We searched for errata or retractions from eligible trials on PubMed (www.ncbi.nlm.nih.gov/pubmed) and reported the date this was done within the review.
Data collection and analysis
We performed statistical analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using The Cochrane Collaboration's statistical software, Review Manager 5 (RevMan 2013).
Selection of studies
Two review authors (HS, JZ) independently screened titles and abstracts for inclusion all the potential studies identified by the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication. Two review authors (HS, JZ) independently screened the full text to identify eligible studies and recorded reasons for exclusion of the ineligible studies. We resolved disagreements through discussion or, if required, we consulted a third review author (DL). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram, a Characteristics of excluded studies table, and a Characteristics of included studies table.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. One review author (HS) extracted study characteristics from included studies. We extracted the following:
methods: study design, total duration study and run in, number of study centres and location, study setting, withdrawals, date of study;
participants: number of participants, mean age, age range, gender, diagnosis at baseline, diagnostic criteria, inclusion criteria, exclusion criteria, date and reports of endoscopy or biopsy sampling (or both);
interventions: intervention, comparison, concomitant medications, excluded medications;
outcomes: primary and secondary outcomes specified and collected, time points reported;
notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (HS, JZ) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were reported in an unusable way. We resolved disagreements by consensus or by involving a third review author (DL).
One review author (HS) copied across the data from the data collection form into Review Manager 5 (RevMan 2013). We double‐checked that the data were entered correctly by comparing the study reports with how the data were presented in the systematic review. A second review author (JZ) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (HS, JZ) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by involving a third review author (DL). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. In addition, we considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported deviations from it in the Differences between protocol and review section.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) with 95% confidence intervals (CI). We planned to analyse continuous data, if available, as mean differences (MDs) or standardised mean differences (SMDs) with 95% CIs. We ensured that higher scores for continuous outcomes had the same meaning for the particular outcome, explained the direction to the reader, and reported where the directions were reversed if this was necessary.
We undertook meta‐analyses only where this was meaningful (i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) had to be entered into the same meta‐analysis, we halved the control group to avoid double counting of participants.
Unit of analysis issues
We only considered RCTs. Among currently included trials, we found no studies with non‐standard designs. If we have to involve other types of trial (e.g. cluster randomised trial) in future updates, we will first carefully assess them (regarding recruitment bias, baseline imbalance, loss of clusters, and comparability with individually randomised trials) in order to avoid unit‐of‐analysis errors. We will apply proper statistical methods (e.g. multi‐level model and generalised estimating equations) for analysis according to theHandbook for Systematic Review of Interventions (Higgins 2011). Unit of analysis is the group of participants at randomisation.
Where there are data from a cross‐over trial with eligible intervention performances, we will use the presented data within the first phase only, from the time point of randomisation to the point of cross‐over.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing outcome data where possible (e.g. when a study was identified as abstract only).
We conducted all analysis of outcomes using the intention‐to‐treat (ITT) principle. More specifically, in this case, all participants included in the biopsy protocol (endoscopy assessment was planned) were counted (the participants with missing pathological data were assumed to be with negative results), regardless of how the original data were analysed.
Assessment of heterogeneity
We tested for heterogeneity using the Chi2 test, with significance set at P less than 0.1. We used the I2 statistic to estimate the total variation across studies due to heterogeneity; we considered I2 less than 25% as low‐level, 25% to 50% as moderate‐level, and greater than 50% as high‐level heterogeneity (Higgins 2011). If we found high levels of heterogeneity (I2 greater than 50%) for the primary outcome, we planned to explore possible sources of heterogeneity using the sensitivity and subgroup analyses described in Sensitivity analysis.
Assessment of reporting biases
We planned investigate the possibility of selective outcome reporting by searching for study protocols published before the trials started. If the protocol was available, we could compare the outcomes in the protocol and published report. If there was no protocol available, then we compared outcomes listed in the methods section of an article with those whose results were reported. In addition, we attempted to contact study authors for outcome data if we could not identify them in their published reports. Where this was not possible, and the missed information were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results using a sensitivity analysis.
We were unable to pool more than 10 trials in this review. However, if we can include more than 10 trials in future updates, we will create and examine a funnel plot (intervention effect estimate versus standard error of intervention effect estimate) to explore possible publication biases.
Data synthesis
We created a 'Summary of findings' table using GRADEpro software (GRADEprofiler 2008). We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the pre‐specified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We justified all decisions to downgrade or upgrade the quality of studies using footnotes and made comments to aid reader's understanding of the review where necessary. We considered whether there was any additional outcome information that was not able to be incorporated into meta‐analyses, then noted this in the comments, and stated if it supported or contradicted the information from the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses, where feasible:
people with or without H. pylori infection at baseline;
different duration periods of PPI use (six to 12 months, one to three years, more than three years), and different PPI doses (i.e. less than, equal to, or more than omeprazole 20 mg);
PPI maintenance therapy compared with different control groups (i.e. placebo, surgical intervention, or other drugs);
people receiving PPI for different reasons (e.g. GORD, peptic ulcer disease, or NSAID prophylaxis).
We used the primary outcome in subgroup analyses.
We used the same model for the main analyses and subgroups analyses in order to ensure comparability between results. To detect the between‐subgroup variance, we examined differences between subgroups by visual inspection of the CI values: non‐overlapping CIs indicate a statistically significant difference in treatment effect between subgroups. In addition, we planned to apply the approach of Borenstein 2008 (undertaking standard tests for heterogeneity across subgroup results rather than across individual study results) to investigate differences between two or more subgroups formally using Review Manager 5 (RevMan 2013). To ensure that there are adequate studies to justify subgroup analyses, we could only perform subgroup tests for outcomes with 10 or more trials contributing to the data.
Sensitivity analysis
We performed sensitivity analysis defined a priori to assess the robustness of our conclusions. We achieved this by repeating the analyses in order to explore the influence of the following factors on effect size:
including all randomised participants in the analysis;
exclusion of unpublished studies;
exclusion of lower‐quality studies (studies at high or unclear risk of bias related to randomisation, blinding or attrition);
exclusion of studies with moderate/high level of heterogeneity;
exclusion of trials that used other anti‐acid treatments in the control arm;
use of different criteria to assess the occurrence or severity of pre‐malignant lesions: exclusion of studies using unpublished criteria or criteria with no established reliability or validity;
use of a fixed‐effect model.
Results
Description of studies
We have summarised the characteristics of the studies in the Characteristics of included studies tables.
Results of the search
The electronic search in August 2013 identified 492 citations (69 from CENTRAL, 340 from MEDLINE, 183 from EMBASE, 71 from CINAHL), excluding duplicates. Handsearching found four related abstracts from the American Digestive Disease Week published in Gastroenterology (Gardner 1999; Lee 1996; Richter 2000; Richter 2003). Of the 496 reports, we considered 27 to be highly relevant after referring to their abstracts, and, therefore, we obtained the full texts for detailed assessment. Finally, five studies met the inclusion criteria of our protocol (Fiocca 2012; Genta 2003; Lundell 1999; Lundell 2006; Meining 1998). In addition, we identified six more eligible studies from the reference list of these papers (Dent 1994; Gough 1996; Hallerbäck 1994; Johnson 2001; Nishi 2005; Vakil 2001).
Five papers were available only as abstracts (Ficoca 2010; Gardner 1999; Lee 1996; Richter 2000; Richter 2003). Ficoca 2010 was later published as a whole report (Fiocca 2012); and for the Lee 1996 abstract, the reported study was the same as the one stated in Nishi 2005; in addition, the two identical RCTs described in Richter 2000 were then reported separately in Vakil 2001 and Johnson 2001, and their safety data were pooled and presented in one paper (Genta 2003). We were unable to access the full‐texts of the other two studies even after contacting with the authors (Gardner 1999; Richter 2003).
Figure 1 shows the details of the search results.
1.
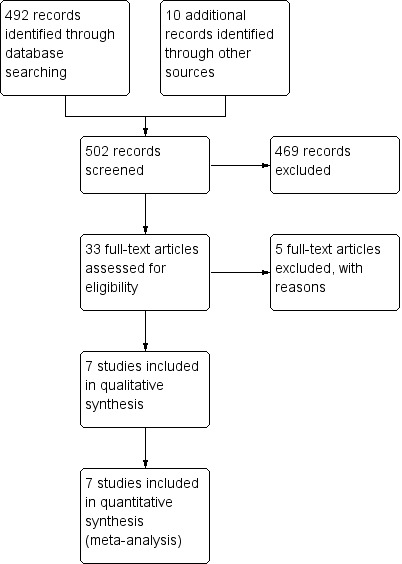
Study flow diagram.
Included studies
After careful evaluation of eligible articles, we included seven trials, corresponding to seven individual RCTs (Dent 1994; Fiocca 2012; Gough 1996; Hallerbäck 1994; Johnson 2001; Lundell 1999; Vakil 2001). The Characteristics of included studies table summarises the details of the included studies.
Since the data concerning the changes of gastric mucosa for two included studies (Johnson 2001; Vakil 2001) were analysed and only reported together in one review (Genta 2003), we extracted relevant data for meta‐analysis from the review. In most situations, it would be misleading to use aggregate (unweighted) data from two studies and use them as if they came from one study. By doing so, we would have violated the standard meta‐analysis methodology; at minimum, we would underestimate heterogeneity. However, the two RCTs were designed and conducted in an identical fashion (identical interventions, identical inclusion and exclusion criteria, identical outcomes, and similar sizes: 318 and 375 participants) by the same team of investigators. The only reason that they were conducted as two RCTs instead of a single large RCT was the regulatory requirements of the FDA. Therefore, we have included these aggregate data as a single RCT, labelled Johnson+Vakil.
Study design
With the exception of the studies comparing PPI with surgery (Fiocca 2012; Lundell 1999), where blinding of participants and doctors was impossible, all other RCTs used double‐blinded and parallel group design. All included trials were multicentre trials, conducted in the US, Europe, and Australia.
Participants
In Fiocca 2012, participants were people with chronic symptomatic GORD. For the other studies, the primary inclusion criterion was endoscopically defined erosive reflux oesophagitis of at least grade 2 severity for recruitment. For healing the oesophagitis, all participants had undergone eight to 12 weeks of healing treatment before randomisation, to verify symptom response and healing of oesophagitis. The participants with successful healing in this run‐in period, with no more than mild symptoms, were then randomised to different groups. The mean age of participants was 50.5 years, with range of 18 to 86 years, and 68% of them were men.
Interventions
Two similar RCTs with 688 participants compared different doses of esomeprazole (10, 20, and 40 mg/day) with placebo (Johnson 2001; Vakil 2001). Three RCTs with 465 participants compared PPI maintenance with ranitidine maintenance (Dent 1994; Gough 1996; Hallerbäck 1994), among which two RCTs compared omeprazole (20 mg/day) with ranitidine (150 mg, twice per day) (Dent 1994; Hallerbäck 1994), and one RCT compared lansoprazole (15/30 mg/day) with high‐dose ranitidine (300 mg, twice per day) (Gough 1996). Instead of drug treatment, two RCTs with 636 participants compared PPI maintenance treatment with surgical options (Fiocca 2012; Lundell 1999). One RCT compared esomeprazole (20 and 40 mg/day) with ARS (Lundell 1999), while one RCT compared laparoscopic anti‐reflux surgery (LARS) with long‐term esomeprazole (20 mg/day) (Fiocca 2012).
We did not identify any studies that assessed the effectiveness of PPIs other than esomeprazole, omeprazole, and lansoprazole.
Outcome measures
The primary outcome of our review, the development or progression of gastric pre‐malignant lesions (mainly including atrophic gastritis, intestinal metaplasia, ECL hyperplasia, and dysplasia) in PPI treatment and control groups were only partly reported in few studies.
Instead of disease diagnosis, most trials pathologically evaluated the changes of the gastric mucosa and scored them using the Sydney system (Price 1991) or updated Sydney system (Dixon 1996); and recorded the changes of gastric endocrine cell growth according to the classification described by Solcia and co‐workers (Solcia 1988; Solcia 1995). However, studies reported the changes in gastritis scores in different ways, which caused difficulties for pooling the data. One RCT reported only the overall prevalences of atrophy/intestinal metaplasia at baseline and each follow‐up time point (Fiocca 2012), while three RCTs listed the number (proportion) of participants with worsening/improving scores of atrophy and intestinal metaplasia separately (Johnson 2001; Lundell 1999; Vakil 2001).
The proportions of participants with each degree of ECL hyperplasia, classified as normal, simple (diffuse), linear, and micronodular (focal), at baseline biopsy and final biopsy were reported in tables for all studies, except one (Gough 1996). The authors stated that "there was no morphological evidence that lansoprazole was associated with the induction of neuroendocrine cell hyperplasia or neoplasia in any of the biopsies from patients examined in the study", without mentioning any specific number (Gough 1996).
For secondary objectives (subgroup analysis), two US studies only included H. pylori‐negative participants (Johnson 2001; Vakil 2001). Two studies reported the changes of gastric mucosa (atrophy and intestinal metaplasia) separately for H. pylori‐positive and H. pylori‐negative subgroups (Fiocca 2012; Lundell 1999).
Excluded studies
We excluded two studies since only abstracts of them were published, and we could not access their full texts even after contacting the authors (Gardner 1999; Richter 2003). Furthermore, we excluded three potential articles. After careful evaluations, one study (Lundell 2006) was an extension of previously included study (Lundell 1999), with more limited proportion of available participants. Thus, we excluded this paper due to replication. One trial with eligible design offered insufficient data for meta‐analysis as during the endoscopy follow‐up, the number of participants remaining in each intervention group was not reported separately (Meining 1998). Since we could not obtain more information by contacting with the authors, we excluded this study. We excluded another trial because the results of our interests were not measured (Nishi 2005). We confirmed this by both referring to its full‐text and contacting its authors.
Risk of bias in included studies
Figure 2 summarises the risk of bias in the included studies. Overall, four studies had a high risk of bias (Fiocca 2012; Gough 1996; Hallerbäck 1994; Lundell 1999). In addition, since none of those trials had accessible protocols with adequate information about the outcomes of our interests, it was difficult to assess the possible reporting bias. The risk of bias in the other three trials was unclear (Dent 1994; Johnson 2001; Vakil 2001).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In two studies, the exact method for 'randomisation' was not stated explicitly (Hallerbäck 1994; Vakil 2001), although in one study, some methods for allocation concealment were indicated (Vakil 2001). The other studies all described how the random sequence was generated. Only three included studies described allocation concealment specifically (Gough 1996; Johnson 2001; Vakil 2001).
Blinding
In two trials comparing PPI with surgery, blinding of participants and researchers was not feasible, so we classified them as having high risk of bias (Fiocca 2012; Lundell 1999). In four trials, we assessed blinding as adequate (Dent 1994; Gough 1996; Johnson 2001; Vakil 2001). One study did not provide enough details for evaluating this risk of bias, thus, we graded this as unclear risk of bias (Hallerbäck 1994).
Incomplete outcome data
Since in most cases our outcomes of interest were not the main outcomes of those trials, the quality of data reporting was poor. Three studies included less than 75% randomised participants for analysis (Fiocca 2012; Gough 1996; Hallerbäck 1994). None of the included studies used ITT analyses.
Selective reporting
For all trials, there was no prior protocol available. Therefore, we have insufficient information for judgement of reporting bias and we assessed all trials as being at unclear risk.
Other potential sources of bias
One trial was funded by a pharmaceutical company (AstraZeneca), which may cause some potential risks of bias; we graded this trial as unclear risk of bias (Fiocca 2012). We rated the remaining six trials as low risk of bias, since there was no obvious potential source of bias.
Effects of interventions
See: Table 1
We extracted summary data from the seven included studies. In all studies, pathological changes of stomach mucosa were measured both at baseline (the beginning of maintenance treatment) and the completion of follow‐up, and reported as number/percentage of people with negative/positive results (dichotomous variables). For the trials with multiple intervention groups (e.g. different doses of PPI drug) but one control group, we summed the number of participants for primary analysis, irrespective of dose level they received, for detecting the global effect of PPI use as a whole. For the trials with different reporting patterns about the same outcome, we undertook meta‐analyses only where this was meaningful (i.e. if measurements were similar enough for pooling to make sense).
Primary outcomes
The effect of proton pump inhibitor use on the risks of gastric preliminary lesion
Corporal atrophy and intestinal metaplasia of gastric mucosa
Six studies performed an evaluation on the changes of corporal atrophy/intestinal metaplasia scores (Dent 1994; Fiocca 2012; Gough 1996; Johnson 2001; Lundell 1999; Vakil 2001). Three studies listed the proportion of participants with worsening/improving scores of corporal atrophy/intestinal metaplasia scores separately (Johnson 2001; Lundell 1999; Vakil 2001). In Gough 1996, since none of tissue sections examined had evidence of aggregated inflammatory changes, the number of people with worsening atrophy/intestinal metaplasia could be inferred. However, Dent 1994 only reported the total number of participants with deteriorated scores of corporal atrophy, concluding that there were no difference between different groups, without stated explicitly the exact number in each group. In addition, Fiocca 2012 described the results as the numbers (proportions) of participants with atrophy or intestinal metaplasia at baseline and each time point of follow‐up, but did not mentioned the changing trends of individuals. In this study, for intestinal metaplasia, the number of participants with increased scores could be calculated because at baseline no person was classified as positive (0%). For corporal atrophy, the exact number of participants with increased atrophy scores could not be known from the literature; and we obtained no reply following our request for more information from the authors.
Finally, we pooled 1070 participants from four RCTs to evaluate the corporal atrophy development (Gough 1996; Johnson 2001; Lundell 1999; Vakil 2001). In total, 14 out of 736 participants with PPI maintenance therapy had increased scores of gastric atrophy; and seven out of 334 participants in the control group. There was no statistically significant difference between groups (OR 1.50; 95% CI 0.59 to 3.80; P value = 0.39; Analysis 1.1). Most participants had either a decreased or unchanged atrophy score.
1.1. Analysis.

Comparison 1 Atrophy (proton pump inhibitor (PPI) versus control), Outcome 1 Number of participants with worsening scores in corporal atrophy.
Five trials assessed corporal intestinal metaplasia among 1408 participants (Fiocca 2012; Gough 1996; Johnson 2001; Lundell 1999; Vakil 2001). Only a few participants (seven from PPI group versus three from control group) scored worse on their final biopsies, and there was no evidence that the worsening scores of intestinal metaplasia were associated with long‐term PPI use (OR 1.46; 95% CI 0.43 to 5.03; P value = 0.55; Analysis 2.1).
2.1. Analysis.

Comparison 2 Intestinal metaplasia (proton pump inhibitor (PPI) versus control), Outcome 1 Number of participants with worsening scores in corporal intestinal metaplasia.
Enterochromaffin‐like cell hyperplasia
All seven studies investigated changes in ECL cell morphology. Nevertheless, one trial reported inadequate information about the exact increased number of participants with ECL hyperplasia (Gough 1996). In one trial, linear and micronodular ECL hyperplasia were reported together as a single category (focal hyperplasia) (Hallerbäck 1994), although all the other RCTs described simple, linear, and micronodular ECL hyperplasia separately. In order to pool data from as many as possible trials in our meta‐analysis, we treated linear and micronodular hyperplasia as a single entity accordingly. One trial reported all related data in a proportional histogram (Fiocca 2012); therefore, we performed data extraction for this trial by manually measuring the heights of the histogram bars.
Overall, six trials (1705 participants) evaluated diffuse (simple) ECL cell hyperplasia (Dent 1994; Fiocca 2012; Hallerbäck 1994; Johnson 2001; Lundell 1999; Vakil 2001). One trial had no participants with diffuse ECL hyperplasia at the last visit (Lundell 1999). In one trial, the number of participants with such pathological changes decreased in both omeprazole treatment groups (omeprazole 20 mg group decreased from five (at entry biopsies) to two (at final biopsies), and omeprazole 10 mg group decreased from three (at entry biopsies) to two (at final biopsies); while among control group treated with ranitidine 150 mg twice daily, one more simple hyperplasia participant was identified at the completion of the study (the number increased from one (at entry biopsies) to two (at final biopsies) (Hallerbäck 1994). In the other four RCTs, increase in the number of participants with diffuse ECL hyperplasia was only observed in PPI therapy group. Although in each individual trial there was no significant difference, our meta‐analysis revealed a notably increased risk of developing diffuse ECL hyperplasia among long‐term PPI users compared with non‐PPI users (31/1123 participants with long‐term PPI versus 1/582 participants with no PPI; OR 5.01; 95% CI 1.54 to 16.26; P value = 0.007; Analysis 3.1). Since high heterogeneity (I2 = 56%) was detected when results from Hallerbäck 1994 were included, we performed a sensitivity analysis by excluding this study, which produced similar results.
3.1. Analysis.

Comparison 3 Enterochromaffin‐like cell hyperplasia (proton pump inhibitor (PPI) versus control), Outcome 1 Increase in number of participants with simple (diffuse) hyperplasia after treatment.
Six trials (1705 participants) evaluated linear/micronodular (focal) ECL cell hyperplasia (Dent 1994; Fiocca 2012; Hallerbäck 1994; Johnson 2001; Lundell 1999; Vakil 2001). There was an increased number of participants with focal ECL cell hyperplasia in every PPI treatment group, but only in two out of seven control groups. More specifically, 24 more participants were diagnosed with focal ECL cell hyperplasia at final biopsies in PPI maintenance groups compared with two more participants in control groups. Similar to diffuse ECL cell hyperplasia, according to our analysis, PPI users had a higher risk of developing linear/micronodular (focal) ECL cell hyperplasia relative to non‐PPI users (24/1123 participants with long‐term PPI versus 2/582 participants with no PPI; OR 3.98; 95% CI 1.31 to 12.16; P value = 0.02; Analysis 3.2).
3.2. Analysis.

Comparison 3 Enterochromaffin‐like cell hyperplasia (proton pump inhibitor (PPI) versus control), Outcome 2 Increase in number of participants with linear/micronodular (focal) hyperplasia, comparing final biopsies with baseline biopsies.
Dysplasia/neoplasia
None of the included studies reported participants showing any dysplastic or neoplastic changes.
Subgroup analysis
The effect of proton pump inhibitor use on the risks of gastric preliminary lesion based on different Helicobacter pylori status
Two RCTs described the gastric atrophy/intestinal metaplasia changes in different H. pylori status subgroups separately (Fiocca 2012; Lundell 1999). Two identical RCTs, which used serologically H. pylori‐negative as one of their criteria for enrolment, technically only performed their trials among H. pylori‐uninfected population (Johnson 2001; Vakil 2001). However, Fiocca 2012 only reported the outcome as the overall prevalence of participants with atrophy/intestinal metaplasia at baseline and end of follow‐up, without mentioning the changing trends of individuals and we could not obtain 'the number of participants with worsening scores of corporal atrophy' from this publication. On the basis of the present data, a lower percentage of participants experienced events of interest among H. pylori‐negative people compared with H. pylori‐positive people (atrophy: 8/893 with H. pylori‐positive versus 3/93 with H. pylori‐negative; intestinal metaplasia 2/1184 with H. pylori‐positive versus 8/139 with H. pylori‐negative). Furthermore, in the H. pylori‐positive group, more participants with events occurred in the PPI users compared with the non‐PPI group (atrophy: 3/40 participants with PPI versus 0/53 participants with non‐PPI; intestinal metaplasia 5/69 participants with PPI versus 3/70 with participants non‐PPI). However, we could not conduct a subgroup analysis since we did not have an adequate number of studies.
One trial compared changes of ECL cell hyperplasia between control and PPI group separately regarding different H. pylori statuses (Lundell 1999); and two trials discussed such changes in H. pylori‐uninfected people (Johnson 2001;Vakil 2001). One trial had no H. pylori‐negative participants with ECL cell hyperplasia (Lundell 1999). While two trials assessed 22 more H. pylori‐uninfected PPI users to be with simple hyperplasia at final biopsies, and eight more with linear/micronodular hyperplasia (Johnson 2001;Vakil 2001); the numbers for H. pylori‐uninfected placebo users were both zero. For H. pylori‐infected people (participants of Lundell 1999), micronodular hyperplasia was found in two of 40 PPI users at entry biopsies, then in six of 40 at final biopsies. Corresponding observations in the ARS group were three of 53 at baseline and two of 53 at the end of long‐term follow‐up. We could not conduct a subgroup analysis since we did not have an adequate number of studies.
The effect of proton pump inhibitor use on the risks of gastric preliminary lesion based on different duration/type/dose of proton pump inhibitor use
Five studies investigated the effects/influences of PPI maintenance therapy within one year (12 months or less): two studies had observation periods of six months (Johnson 2001; Vakil 2001), and three studies had observation periods of 12 months (Dent 1994; Gough 1996; Hallerbäck 1994). Another two studies were designed with much longer term of follow‐up (five years with Fiocca 2012 and three years with Lundell 1999). We could not conduct a subgroup analysis since we did not have an adequate number of studies. This was also the case with different types/doses of PPI drugs.
The effect of proton pump inhibitor use on the risks of gastric preliminary lesion compared with different types of control groups
Two identical trials compared esomeprazole maintenance therapy with placebo (Johnson 2001; Vakil 2001). Two studies compared PPI maintenance therapy with surgical interventions (LARS/ARS) (Fiocca 2012; Lundell 1999). Three studies compared PPI maintenance therapy with ranitidine maintenance therapy (Dent 1994; Gough 1996; Hallerbäck 1994). We could not conduct a subgroup analysis since we did not have an adequate number of studies.
The effect of proton pump inhibitor use on the risks of gastric preliminary lesions based on the presence of different underlying diseases
All included studies had recruited participants with healed erosive oesophagitis. Therefore, we could not perform such a subgroup analysis.
Discussion
Summary of main results
Based on the international literature that we reviewed, there was on clear evidence to associate long‐term of PPI use with increased risks of developing corporal atrophic gastritis or intestinal metaplasia among people with healed erosive oesophagitis. However, people with PPI maintenance treatment may have higher possibility of experiencing either diffuse (simple) or linear/micronodular (focal) ECL hyperplasia.
Furthermore, there were no adequate data for subgroup analyses. Therefore, we cannot conclude that there was any difference in gastric safety profiles among participants with different statuses of H. pylori infection, participants receiving different durations/types/doses of PPI drugs, or people who received this medication for different reasons.
Overall completeness and applicability of evidence
Data regarding the effect of long‐term PPI use on gastric pre‐malignant lesions were insufficient, and the identified studies only partially addressed the objectives of the review. First, since none of the included studies reported our outcomes of interests as the primary outcome of their original trials, or due to other practical reasons, the proportion of participants with missing data was substantial. Second, without a uniform approach for group comparison, different methods of outcome reporting made data extraction and analysis difficult. Third, expect for healed erosive oesophagitis, long‐term PPI use for other underlying diseases, such as peptic ulcer disease or NSAID prophylaxis, was not covered in the identified trials. Other applications of long‐term PPI maintenance treatment should be examined in future studies.
In addition, for many trials, the follow‐up period was too short to estimate the effects of long‐term PPI use. Instead of using gastritis scores to grade corporal atrophy and intestinal metaplasia, we need a more clinical diagnosis of pre‐malignant lesions, such as chronic atrophic gastritis or intestinal metaplasia, in future trials.
Quality of the evidence
We included seven RCTs. Most of them provided little or no information on allocation concealment. Many trials had a large proportion of missing data, but performed analysis without application of the ITT principle. The quality of data reporting was poor. It is also notable that relatively few participants or few events (or both) were included in our analyses, leading to a serious imprecision problem for every outcomes. Publication bias was unclear for all of these trials and thereby 'no serious limitation' was assumed. Other GRADE domains, including indirectness and inconsistency were not significant limitations for our evaluation.
Potential biases in the review process
In the Differences between protocol and review section, we stated that since all relevant studies primarily concerned on the effectiveness of PPI maintenance treatment, not the safety issues of PPIs, they did not exclude participants with pre‐malignant lesion at baseline. Therefore, rather than placing the focus on 'the incidence of gastric pre‐malignant lesions', we targeted 'the development of gastric pre‐malignant lesions' by comparing the biopsy results acquired from last visit to those of the baseline. Accordingly, we have subsequently revised the title, objectives, and measures of primary outcomes. All of these post‐protocol changes could have some potential impact on our findings.
In order to prevent bias in the review procedure, the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group guided and developed the search. We placed no restrictions (e.g. on language) on the search. Two review authors independently conducted study selection, assessment of the risks of bias, and data collection without blinding. We resolved any disagreements through discussion with a third review author. We dealt with missing information and data by repeated attempts to contact the authors by email. In addition, for studies that had only been published as abstracts, we tried to obtain further details by emailing the authors. However, since we did not receive any replies from them, we extracted only available data from the literature and pooled it for our meta‐analysis. These incomplete data could produce potential bias, which may influence the precision and reliability of our results.
We detected high heterogeneity (I2 = 56%) when comparing the changes of diffuse ECL hyperplasia between the PPI group and control group. This was caused by the inclusion of results from one trial (Hallerbäck 1994). We found no clear explanation for this. However, since only one event happened in the control group, it could have occurred by chance. We performed a sensitivity analysis by excluding this study, which produced similar results. Moreover, the use of aggregate data from two included studies (Johnson 2001; Vakil 2001) as a single RCT (labelled Johnson+Vakil) could be a source of potential bias (e.g. underestimating heterogeneity), albeit the similarity of these two trials has been clearly stated.
Finally, the time lag between the search date and publication date should also be noted when interpreting the findings of our review.
Agreements and disagreements with other studies or reviews
The propensity of long‐term PPI treatment to worsen oxyntic mucosa gastritis (atrophic gastritis/intestinal metaplasia) has been proposed by many researchers, particularly for people with H. pylori infection (Klinkenberg‐Knol 2000; Kuipers 1996; McColl 2000; Pounder 2000). However, clinical trials with insignificant results about this association exist (Brunner 2012; Geboes 2001; Rindi 2005; Solcia 1988; Uemura 2000). In Kuipers 2004, there was no progression of atrophy even in H. pylori‐infected people. Consequently, consistent with our results, there was little evidence in support of the initial concern that PPI maintenance therapy accelerates the development of progression of corpus gastric atrophy or intestinal metaplasia. Other reviews focusing on this topic arrived at the same conclusion (Eslami 2013; Laine 2000).
It is still controversial whether the PPI‐related hypergastrinaemia increases ECL cell numbers, as well as linear or micronodular hyperplasia. A positive association was indicated in many investigations, and might be more apparent amongH. pylori‐positive people (Eissele 1997; Kuipers 1996; Laine 2000; Lamberts 2001; Lundell 2006). In our analysis, both the development of diffuse (simple) and linear/micronodular (focal) ECL hyperplasia were related to long‐term PPI use, albeit with low‐quality evidence. This did not agree with the results from a very similar review published in 2013 (Eslami 2013). The possible reason for this inconsistency may be that fewer participants were pooled for their meta‐analysis ‐ although they found six individual studies with the data on ECL hyperplasia, only two of the studies were included in their final analysis.
In addition, it seems to be universally acknowledged that, up to 2013, there is no evidence that the incidence of gastric dysplasia and neoplasia increases among people with long‐term PPI maintenance therapy.
Authors' conclusions
Implications for practice.
In conclusion, as of 2013, there is no clear evidence that the long‐term use of proton pump inhibitors (PPIs) can cause or accelerate the progression of corpus gastric atrophy or intestinal metaplasia, although the results were imprecise.
People with PPI maintenance treatment may have higher possibility of experiencing either diffuse (simple) or linear/micronodular (focal) enterochromaffin‐like (ECL) hyperplasia. However, the clinical importance of this outcome is uncertain.
Implications for research.
Further studies to clarify this question are required before any definitive conclusion can be drawn. Well‐designed and well‐conducted randomised controlled trials with long‐term follow‐up periods should be conducted in order to reduce the risk of bias when evaluating the effects of long‐term PPI use on the gastric mucosa. In addition, there is a need to minimise the proportion of randomised participants with missing pathological data; and the comparisons of all pathological outcomes should be diagnosed and reported in a uniform manner. Since all the included trials enrolled participants with erosive oesophagitis, long‐term PPI use for other underlying diseases, such as peptic ulcer disease or non‐steroidal anti‐inflammatory drug prophylaxis, should be examined in future studies.
Acknowledgements
We thank Karin Dearness, Managing Editor, Cochrane Upper Gastrointestinal and Pancreatic Diseases Group for providing administrative and logistical support, and Racquel Simpson, Trials Search Co‐ordinator, Cochrane Upper Gastrointestinal and Pancreatic Diseases Group for developing and executing the search strategies.
Appendices
Appendix 1. CENTRAL search strategy
*Precancerous Conditions/
Gastritis, Atrophic/
(gastric adj2 premalignant).tw.
Metaplasia/
(intestin* adj1 metaplasia).tw.
dysplasia.tw.
or/1‐6
Proton Pump Inhibitors/
((proton adj2 pump adj2 inhibitor$) or PPI or PPIs).tw.
Omeprazole/
(omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).tw.
Esomeprazole Sodium/
(Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
(lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
(rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
(Dexlansoprazole or Kapidex or Dexilant).tw.
(pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
or/8‐17
7 and 18
Appendix 2. MEDLINE search strategy
*Precancerous Conditions/
Gastritis, Atrophic/
(gastric adj2 premalignant).tw.
Metaplasia/
(intestin* adj1 metaplasia).tw.
dysplasia.tw.
or/1‐6
Proton Pump Inhibitors/
((proton adj2 pump adj2 inhibitor$) or PPI or PPIs).tw.
Omeprazole/
(omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).tw.
Esomeprazole Sodium/
(Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
(lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
(rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
(Dexlansoprazole or Kapidex or Dexilant).tw.
(pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
or/8‐17
7 and 18
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/20‐27
exp animals/ not humans.sh.
28 not 29
19 and 30
Appendix 3. EMBASE search strategy
precancer/
atrophic gastritis/
(gastric adj2 premalignant).tw.
intestine metaplasia/
(intestin* adj1 metaplasia).tw.
gastrointestinal dysplasia/
or/1‐6
proton pump inhibitor/
((proton adj2 pump adj2 inhibitor$) or PPI or PPIs).tw.
omeprazole/
(omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).tw.
exp esomeprazole/
(Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
exp lansoprazole/
(lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
rabeprazole/
(rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
(Dexlansoprazole or Kapidex or Dexilant).tw.
pantoprazole/
(pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
or/8‐20
7 and 21
random:.tw. or placebo:.mp. or double‐blind:.tw.
22 and 23
Data and analyses
Comparison 1. Atrophy (proton pump inhibitor (PPI) versus control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with worsening scores in corporal atrophy | 3 | 1070 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.59, 3.80] |
Comparison 2. Intestinal metaplasia (proton pump inhibitor (PPI) versus control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with worsening scores in corporal intestinal metaplasia | 4 | 1408 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.43, 5.03] |
Comparison 3. Enterochromaffin‐like cell hyperplasia (proton pump inhibitor (PPI) versus control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Increase in number of participants with simple (diffuse) hyperplasia after treatment | 5 | 1705 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.01 [1.54, 16.26] |
| 2 Increase in number of participants with linear/micronodular (focal) hyperplasia, comparing final biopsies with baseline biopsies | 5 | 1705 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.98 [1.31, 12.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dent 1994.
| Methods | RCT, multicentre | |
| Participants | 159 outpatients aged 18‐86 years Number randomised: DO 53; WO 55; DR 51 Number evaluated: DO 39; WO 40; DR 40 Age (mean ± SD): DO 59 ± 13; WO 62 ± 13; DR 62 ± 12 years Diagnosis: people who had endoscopically defined erosive reflux oesophagitis of at least grade 2 severity Inclusion: people who were healed by omeprazole in an 8‐week healing phase (verified by endoscopy and were asymptomatic or had only mild symptoms) Exclusion: pregnancy or lactation, inadequate contraception, treatment with prokinetic agents or anti‐secretory therapy; concurrent peptic ulcer or complications of ulcer disease; GI malignancy; oesophagitis resulting from systemic disease, infection, intubation, or other mechanical trauma, burns, irradiation, or physical deformity; history of oesophagogastric surgery other than simple closure of a perforation or failed ARS without myotomy, vagotomy, or a drainage procedure; ongoing upper GI haemorrhage (bleeding associated with peptic mucosal damage had to be controlled adequately before entry); concurrent or past disease, e.g. severe cardiac, hepatic, or renal disease; clinically significant unexpected abnormalities in the assessments before entry or laboratory screen variables; any condition known to be associated with poor patient compliance; treatment with any other investigational drug during the 4 weeks before or after the initial endoscopy |
|
| Interventions | DO: daily omeprazole (20 mg every morning) WO: weekend omeprazole weekend (20 mg in the morning on Fridays, Saturdays, and Sundays) DR: daily ranitidine (150 mg twice daily) Treatment duration: up to 12 months |
|
| Outcomes | Presence and grading of corpus atrophic gastritis before and after 12 months' eminence therapy Number of participants with increased simple/linear/micronodular hyperplasia after 12 months' maintenance therapy Number of participants with dysplasia after 12 months' maintenance therapy |
|
| Notes | The authors only reported the total number of participants with deteriorated scores of corporal atrophy, without stating explicitly the exact number in each group. Therefore, for the analysis 'number of participants with worsening scores of corporal atrophy', we did not include data from this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 119 randomised participants were included in analysis (74.8%); no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol available |
| Other bias | Low risk | No obvious potential source of bias |
Fiocca 2012.
| Methods | RCT, multicentre | |
| Participants | 554 participants aged 18‐70 years Number randomised: LARS 288; ESO 266 Number evaluated: LARS 158; ESO 180 Men: LARS 72%; ESO 82% Diagnosis: people with chronic symptomatic GORD. Diagnosis of GORD established based on typical clinical history and presence of oesophageal mucosal breaks at endoscopy or pathological pH‐metry (or both) Inclusion: all participants were eligible for either LARS or medical treatment, and after 3‐month run‐in period, which allowed medical treatment with ESO 40 mg to verify symptom response and healing of oesophagitis, oesophagitis had to be no more than Los Angeles grade B at the time of randomisation, and GORD symptoms no more than mild Exclusion: people with a history of oesophageal, gastric, or duodenal surgery; current or historical evidence of Zollinger‐Ellison syndrome; primary oesophageal disorders (achalasia, scleroderma, and primary oesophageal spasm); inflammatory bowel disease; dysplastic changes in a columnar‐lined oesophagus or any condition associated with abnormal absorption from the GI tract; any other significant concomitant disease; potential for poor compliance (at the discretion of the investigator) |
|
| Interventions | LARS: laparoscopic surgery had to be performed within 3 months of randomisation and consisted of a crural repair and the creation of a short floppy total fundoplication ESO: esomeprazole (20 mg once daily) Treatment duration: up to 5 years |
|
| Outcomes | Overall prevalence of atrophy at each time point (antrum/corpus), based on the status of H. pylori infection Overall prevalence of intestinal metaplasia at each time point (antrum/corpus), based on the status of H. pylori infection Overall prevalence of ECL cell hyperplasia (diffuse/liner/micronodular) at each time point |
|
| Notes | Only the overall prevalences of atrophy/intestinal metaplasia at baseline and each follow‐up time point. For the analysis 'the number of participants with worsening scores of corporal atrophy', we did not include data from this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in blocks of 4 to either LARS or maintenance medical treatment with ESO 20 mg once daily. The randomised design was selected to avoid bias in the selection of participants for medical or surgical treatment |
| Allocation concealment (selection bias) | Unclear risk | Unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 338 randomised participants included in analysis (61.0%); no ITT |
| Selective reporting (reporting bias) | Unclear risk | Study protocol available, but no information about pathological measurement |
| Other bias | Unclear risk | Study funded in total by AstraZeneca |
Gough 1996.
| Methods | RCT, multicentre | |
| Participants | 266 participants aged 18‐86 years Number randomised: L15 96; L30 89; R600 81 Number evaluated: L15 38; L30 24; R600 22 Age (mean): L15 57.8; L30 57.0; R600 56.1 years Diagnosis: participants had originally been endoscopically diagnosed with grade 2 or 3 oesophagitis Inclusion: all participants had undergone 8 weeks' healing treatment with lansoprazole 30 mg once daily. Participants were considered to be healed, and thus eligible to enter the maintenance phase, if they had no oesophagitis and were asymptomatic at the end of 8 weeks of treatment Exclusion: women who were pregnant or lactating, people with Zollinger‐Ellison syndrome, varices, gastric or duodenal ulcers, known gastric malignancy, scleroderma, or active biliary or pancreatic disease |
|
| Interventions | L15: lansoprazole (15 mg once daily; Lederle, Gosport, UK) L30: lansoprazole (30 mg once daily) R600: ranitidine (300 mg twice daily; Glaxo, Greenford, UK) Treatment duration: up to 12 months |
|
| Outcomes | Number of participants with atrophic gastritis/intestinal metaplasia before and after 12 months' maintenance therapy | |
| Notes | Histopathological assessment of gastric biopsies was performed in participants from 10 of the 21 centres, selected for practical and logistic reasons The authors only stated that "there was no morphological evidence that lansoprazole was associated with the induction of neuroendocrine cell hyperplasia or neoplasia in any of the biopsies from patients examined in the study", without mention any specific number. Therefore, for analyses concerning ECL hyperplasia, we did not include data from this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (block size of 6) was incorporated into the study supplies with participant kits identical in appearance except for the randomisation number |
| Allocation concealment (selection bias) | Low risk | Randomisation (block size of 6) was incorporated into the study supplies with participant kits identical in appearance except for the randomisation number |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 84 randomised participants were included in analysis (31.6%); no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol available |
| Other bias | Low risk | No obvious potential source of bias found |
Hallerbäck 1994.
| Methods | RCT, multicentre | |
| Participants | 392 participants aged 18‐80 years Number randomised: E20 131; E10 133; R 128 Number evaluated: E20 103; E10 87; R 72 Diagnosis: people with erosive or ulcerative oesophagitis, grade ≥ 2 Inclusion: all participants had undergone 8‐12 weeks of healing treatment with omeprazole 20 mg once daily. Participants were considered to be healed, and thus eligible to enter the maintenance phase, if they had gross endoscopic appearance not more than grade 1 and no or mild symptoms after 8 or 12 weeks of treatment Exclusion: oesophageal structures needing dilation, concomitant active gastric or duodenal ulcers, and oesophageal abnormalities caused by general or systemic diseases; history of oesophagogastric surgery other than simple closure of ulcers; malignancy or other diseases with a potential to interfere with the evaluation of the study; pregnancy or lactation; treatment with other investigational drugs within 1 month before inclusion; drug or alcohol abuse |
|
| Interventions | E20: omeprazole 20 mg once daily E10: omeprazole 10 mg once daily R: ranitidine 150 mg twice daily Treatment duration: up to 12 months |
|
| Outcomes | Prevalence of ECL cell hyperplasia (diffuse/liner/micronodular) at baseline and after 12‐month maintenance treatment | |
| Notes | No results on corporal gastritis changes reported. Therefore, for analysis concerning corpus atrophy/intestinal metaplasia, we did not include data from this study. For ECL hyperplasia, we reported the linear and micronodular data together as a single category (focal hyperplasia) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 262 randomised participants were included in analysis (66.8%); no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol available |
| Other bias | Low risk | No obvious potential source of bias was found |
Johnson 2001.
| Methods | RCT, multicentre | |
| Participants | 318 participants Number randomised: E40 82; E20 82; E10 77; placebo 77 Number evaluated: E40 81; E20 81; E10 76; placebo 77 Diagnosis: people with erosive oesophagitis Inclusion: men and non‐pregnant, non‐lactating women were eligible for enrolment if they fulfilled the following requirements: 1. aged 18‐75 years inclusive; 2. had confirmed healing of erosive oesophagitis and no record of any serious adverse event related to study medication in prior healing study (4‐8 weeks' duration); and 3. were negative for H. pylori by histology. Women were postmenopausal, surgically sterilised, or using medically acceptable contraception throughout the study Exclusion: people were excluded from the healing (and thus maintenance) studies if they had endoscopic evidence of either Barrett's oesophagus (> 3 cm) or significant dysplastic changes in the oesophagus. In addition, people with significant GI disease; any other notable medical conditions or diseases (such as malignancy, pancreatitis, cardiac, pulmonary, liver, or renal diseases); or known hypersensitivity to ESO or Gelucilt were excluded. Use of or need for the following medications also resulted in exclusion: a proton pump inhibitor (other than the one used in protocol for the healing study) within 28 days before the baseline visit; a histamine H2‐receptor antagonist daily during the 2 weeks before the baseline oesophagogastroduodenoscopy (occasional use less than daily was permitted during this period); and continuous concurrent therapy with quinidine, diazepam, phenytoin, mephenytoin, warfarin, anticholinergics, prostaglandin analogues, anti‐neoplastic agents, salicylates (unless < 165 mg daily for cardiovascular prophylaxis), steroids (oral or intravenous), pro‐motility drugs, sucralfate, or non‐steroidal anti‐inflammatory drugs, or a combination within 1 week of randomisation |
|
| Interventions | E40: esomeprazole 40 mg E20: esomeprazole 20 mg E10: esomeprazole 10 mg Placebo Treatment duration: up to 6 months |
|
| Outcomes | Numbers and percentages of participants showing an worsening/improvement of atrophic gastritis scores after 6 months' maintenance therapy Numbers and percentages of participants showing an worsening/improvement of intestinal metaplasia scores after 6 months' maintenance therapy Prevalence of ECL cell hyperplasia (diffuse/liner/micronodular) at baseline and after 6 months' maintenance therapy |
|
| Notes | Outcomes about gastric mucosal changes were reported in another article together with another very similar US 6‐month double‐blind trial (Vakil 2001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blinded blocks of 4 allocation numbers were used in a 1 : 1 : 1 : 1 ratio for randomisation |
| Allocation concealment (selection bias) | Low risk | To maintain blinding, all study drugs were identical in appearance, and investigators used individually sealed and blinded randomisation envelopes to allocate treatment for each participant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 315 randomised participants were included in analysis (99.0%); no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol available |
| Other bias | Low risk | No obvious potential source of bias was found |
Johnson+Vakil.
| Methods | Not an individual trial, but a meta‐analysis using data from 3 different trials, 2 of which (Johnson 2001 and Vakil 2001) met our inclusion criteria | |
| Participants | 693 participants combined between Johnson and Vakil studies, 688 of which contributed data to the analyses | |
| Interventions | E40: esomeprazole 40 mg E20: esomeprazole 20 mg E10: esomeprazole 10 mg Placebo Treatment duration: up to 6 months |
|
| Outcomes | Numbers and percentages of participants showing an worsening/improvement of atrophic gastritis scores after 6 months' maintenance therapy Numbers and percentages of participants showing an worsening/improvement of intestinal metaplasia scores after 6 months' maintenance therapy Prevalence of ECL cell hyperplasia (diffuse/liner/micronodular) at baseline and after 6 months' maintenance therapy |
|
| Notes | This meta‐analysis is the sole source of data from the Johnson 2001 and Vakil 2001 trials | |
Lundell 1999.
| Methods | RCT, multicentre | |
| Participants | 310 participants Number randomised: ARS 155; LOT 155 Number evaluated: ARS 155; LOT 143 Diagnosis: people with chronic GORD symptoms and documented oesophagitis at endoscopy who were also considered suitable for ARS Inclusion: eligible participants were initially given either omeprazole 20 or 40 mg daily to control symptoms and to heal the oesophagitis, usually for 4‐8 weeks, but according to the protocol, a treatment period of up to 4 months was allowed |
|
| Interventions | ARS: anti‐reflux surgery within 3 months of randomisation (median time 61 days) LOT: long‐term omeprazole 20 or 40 mg daily |
|
| Outcomes | Numbers and percentages of participants showing an worsening/improvement of atrophic gastritis scores after 3 years' maintenance therapy, based on the status of H. pylori infection Numbers and percentages of participants showing an worsening/improvement of intestinal metaplasia scores after 3 years' maintenance therapy, based on the status of H. pylori infection Prevalence of ECL cell hyperplasia (diffuse/liner/micronodular) at baseline and after 3 years' maintenance therapy, based on the status of H. pylori infection |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program, which randomised participants in blocks specific for each participating centre |
| Allocation concealment (selection bias) | Unclear risk | Unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 298 randomised participants were included in analysis (96.1%); no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol available |
| Other bias | Low risk | No obvious potential source of bias was found |
Vakil 2001.
| Methods | RCT, multicentre | |
| Participants | 375 participants Number randomised: E40 92; E20 98; E10 91; placebo 94 Number evaluated: E40 92; E20 98; E10 91; placebo 92 Diagnosis: people with erosive oesophagitis Inclusion: men and non‐pregnant, non‐lactating women, aged 18‐75 years inclusive, who had confirmed healing of erosive oesophagitis, no record of any serious adverse event related to study medication in the healing study, and who were negative for H. pylori by histology eligible for entry into maintenance study. Women were required to be postmenopausal, surgically sterilised, or using a medically acceptable contraception throughout the study Exclusion: people were excluded from the healing (and thus maintenance) studies if they had endoscopic evidence of either Barrett's oesophagus (> 3 cm) or significant dysplastic changes in the oesophagus. In addition, people with significant GI disease, any other notable medical conditions or diseases (such as malignancy, pancreatitis, cardiac, pulmonary, liver, or renal diseases), or known hypersensitivity to ESO or Gelucilt were excluded. Use of or need for the following medications also resulted in exclusion: a proton pump inhibitor (other than the one used as protocol for the healing study) within 28 days before the baseline visit; a histamine H2‐receptor antagonist daily during the 2 weeks before the baseline oesophagogastroduodenoscopy (occasional use less than daily was permitted during this period); and continuous concurrent therapy with quinidine, diazepam, phenytoin, mephenytoin, warfarin, anticholinergics, prostaglandin analogues, anti‐neoplastic agents, salicylates (unless < 165 mg daily for cardiovascular prophylaxis), steroids (oral or intravenous), pro‐motility drugs, sucralfate, non‐steroidal anti‐inflammatory drugs, or a combination within 1 week of randomisation |
|
| Interventions | E40: esomeprazole 40 mg E20: esomeprazole 20 mg E10: esomeprazole 10 mg Placebo All drugs/placebo were taken orally once daily in the morning Treatment duration: up to 6 months |
|
| Outcomes | Numbers and percentages of participants showing an worsening/improvement of atrophic gastritis scores after 6 months' maintenance therapy Numbers and percentages of participants showing an worsening/improvement of intestinal metaplasia scores after 6 months' maintenance therapy Prevalence of ECL cell hyperplasia (diffuse/liner/micronodular) at baseline and after 6 months' maintenance therapy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unspecified |
| Allocation concealment (selection bias) | Low risk | Blinded randomisation envelopes that were collected and checked by the monitor at the end of the study to ensure the integrity of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 373 randomised participants were included in analysis (99.5%); no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol available |
| Other bias | Low risk | No obvious potential source of bias was found |
ARS: anti‐reflux surgery; ECL: enterochromaffin‐like; ESO: esomeprazole; GI: gastrointestinal; GORD: gastro‐oesophageal reflux disease; H. pylori: Helicobacter pylori; ITT: intention to treat; LARS: laparoscopic anti‐reflux surgery; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gardner 1999 | Only an abstract available, and no information was acquired by contacting the authors |
| Lundell 2006 | An extension of previously included study Lundell 1999, with more limited proportion of available participants |
| Meining 1998 | The design of this study was eligible. However, there was inadequate data for meta‐analysis. During the endoscopy follow‐up, the numbers of participants remained in each intervention group were not reported separately |
| Nishi 2005 | Referring to the full‐text, the our outcomes of interests were not measured in this study; and the authors offered no related information |
| Richter 2003 | Only an abstract was available, and no information was acquired by contacting the authors |
Differences between protocol and review
According to the original inclusion criteria in our protocol, we intended to include studies focused on adults (aged 18 years or greater) without any malignant or pre‐malignant lesion at baseline, confirmed by endoscopy or biopsy sampling (or both). However, since all relevant studies primarily concerned the effectiveness of PPI maintenance treatment, not the safety issues of this drug, they did not exclude the participants with pre‐malignant lesions at baseline. Therefore, we changed our inclusion criteria to 'adults (aged 18 years or greater) without any malignant lesion at baseline, confirmed by endoscopy or biopsy sampling (or both).
For our primary outcomes, we added ECL cell hyperplasia as the focus of primary outcomes. This was because this histological change, as an origin of gastric carcinoid tumour that has the chance of progressing to malignancy or metastasising, may have potentially clinical relevance long term. In addition, since people with pre‐existing pre‐malignant lesions were not excluded at baseline, the measure of 'the incidence of gastric pre‐malignant lesions observed at the end of follow‐up' no longer serves our objectives. Therefore, we used 'the development or progression of gastric pre‐malignant lesions' as the primary outcome in this review. Specifically, for corporal atrophy and intestinal metaplasia, we measured the primary outcome as 'the number of participants with worsening scores of corporal atrophy/intestinal metaplasia'; and for ECL hyperplasia, we evaluated the number of participants with diffuse (simple) and linear/micronodular (focal) hyperplasia and compared them between baseline and final visit ‐ 'increase in the number of participants with simple/focal ECL hyperplasia after treatment' in order to measure our primary outcome.
Since we considered that the control group that received 'other anti‐acid treatment' was not completely 'controlled', we added a subgroup analysis concerning on different control methods (placebo, surgical intervention, or other drug) to our original protocol.
Due to the changes stated above, we revised the statements in the title and review objectives accordingly, from 'the incidence of gastric pre‐malignant lesions' to 'the development of gastric pre‐malignant lesions'.
Contributions of authors
Conceiving the protocol: HS.
Designing the protocol: HS.
Co‐ordinating the protocol: HS.
Designing search strategies: RS.
Writing the protocol: HS, JZ.
Providing general advice on the protocol: DL.
Performing previous work that was the foundation of the current study: HS, DL, JZ.
Sources of support
Internal sources
Karolinska Institutet, Sweden.
Shandong Provincial Hospital, China.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Dent 1994 {published data only}
- Dent J, Yeomans ND, Mackinnon M, Reed W, Narielvala FM, Hetzel DJ, et al. Omeprazole v ranitidine for prevention of relapse in reflux oesophagitis. A controlled double blind trial of their efficacy and safety. Gut 1994;35(5):590‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiocca 2012 {published data only}
- Fiocca R, Mastracci L, Attwood SE, Ell C, Galmiche JP, Hatlebakk J, et al. Gastric exocrine and endocrine cell morphology under prolonged acid inhibition therapy: results of a 5‐year follow‐up in the LOTUS trial. Alimentary Pharmacology and Therapeutics 2012;36(10):959‐71. [DOI] [PubMed] [Google Scholar]
Gough 1996 {published data only}
- Gough AL, Long RG, Cooper BT, Fosters CS, Garrett AD, Langworthy CH. Lansoprazole versus ranitidine in the maintenance treatment of reflux oesophagitis. Alimentary Pharmacology and Therapeutics 1996;10(4):529‐39. [DOI] [PubMed] [Google Scholar]
Hallerbäck 1994 {published data only}
- Hallerbäck B, Unge P, Carling L, Edwin B, Glise H, Havu N, et al. Omeprazole or ranitidine in long‐term treatment of reflux esophagitis. The Scandinavian Clinics for United Research Group. Gastroenterology 1994;107(5):1305‐11. [DOI] [PubMed] [Google Scholar]
Johnson+Vakil {published data only}
- Genta RM, Rindi G, Fiocca R, Magner DJ, D'Amico D, Levine DS. Effects of 6‐12 months of esomeprazole treatment on the gastric mucosa. American Journal of Gastroenterology 2003;98(6):1257‐65. [DOI] [PubMed] [Google Scholar]
Johnson 2001 {published data only}
- Genta RM, Rindi G, Fiocca R, Magner DJ, D'Amico D, Levine DS. Effects of 6‐12 months of esomeprazole treatment on the gastric mucosa. American Journal of Gastroenterology 2003;98(6):1257‐65. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Benjamin SB, Vakil NB, Goldstein JL, Lamet M, Whipple J, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double‐blind, placebo‐controlled study of efficacy and safety. American Journal of Gastroenterology 2001;96(1):27‐34. [DOI] [PubMed] [Google Scholar]
Lundell 1999 {published data only}
- Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Thor K, Andersson A, et al. Lack of effect of acid suppression therapy on gastric atrophy. Gastroenterology 1999;117(2):319‐26. [DOI] [PubMed] [Google Scholar]
Vakil 2001 {published data only}
- Genta RM, Rindi G, Fiocca R, Magner DJ, D'Amico D, Levine DS. Effects of 6‐12 months of esomeprazole treatment on the gastric mucosa. American Journal of Gastroenterology 2003;98(6):1257‐65. [DOI] [PubMed] [Google Scholar]
- Vakil NB, Shaker R, Johnson DA, Kovacs T, Baerg RD, Hwang C, et al. The new proton pump inhibitor esomeprazole is effective as a maintenance therapy in GERD patients with healed erosive oesophagitis: a 6‐month, randomized, double‐blind, placebo‐controlled study of efficacy and safety. Alimentary Pharmacology and Therapeutics 2001;5(7):927‐35. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gardner 1999 {published data only}
- Gardner JD, Rindi G, Dayal Y, Fiocca R, Perdomo C, Jaskir J, et al. Evolution of Helicobacter pylori infection, gastritis and enterochromaffine‐like cell hyperplasia in 443 patients with gastroesophageal reflux disease treated for one year with rabeprazole. Gastroenterology 1999;16(4 part 2):G0731. [Google Scholar]
Lundell 2006 {published data only}
- Lundell L, Havu N, Miettinen P, Myrvold HE, Wallin L, Julkunen R, et al. Changes of gastric mucosal architecture during long‐term omeprazole therapy: results of a randomized clinical trial. Alimentary Pharmacology and Therapeutics 2006;23(5):639‐47. [DOI] [PubMed] [Google Scholar]
Meining 1998 {published data only}
- Meining A, Kiel G, Stolte M. Changes in Helicobacter pylori‐induced gastritis in the antrum and corpus during and after 12 months of treatment with ranitidine and lansoprazole in patients with duodenal ulcer disease. Alimentary Pharmacology and Therapeutics 1998;12(8):735‐40. [DOI] [PubMed] [Google Scholar]
Nishi 2005 {published data only}
- Nishi T, Makuuchi H, Weinstein WM. Changes in gastric ECL cells and parietal cells after long‐term administration of high‐dose omeprazole to patients with Barrett's esophagus. Tokai Journal of Experimental and Clinical Medicine 2005;30(2):117‐21. [PubMed] [Google Scholar]
Richter 2003 {published data only}
- Richter J, Fraga P, Mack M, Dermovsesian A. Comparison of the clinical efficacy and safety of pantoprazole vs ranitidin over three years to prevent relapse in patients with healed erosive esophagitis. Gastroenterology 2003;124:A232. [Google Scholar]
Additional references
Abraham 2012
- Abraham NS. Proton pump inhibitors: potential adverse effects. Current Opinion in Gastroenterology 2012;28(6):615‐20. [DOI] [PubMed] [Google Scholar]
Agrawal 2000
- Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs. ranitidine in healing nonsteroidal anti‐inflammatory drug‐associated gastric ulcers: results of a double‐blind, randomized, multicenter study. Archives of Internal Medicine 2000;160(10):1455‐61. [DOI] [PubMed] [Google Scholar]
Ali 2009
- Ali T, Roberts DN, Tierney WM. Long‐term safety concerns with proton pump inhibitors. American Journal of Medicine 2009;112(10):896‐903. [DOI] [PubMed] [Google Scholar]
Anderson 2010
- Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age‐specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303(17):1723‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asfeldt 2008
- Asfeldt AM, Straume B, Steigen SE, Løchen ML, Florholmen J, Bernersen B, et al. Changes in the prevalence of dyspepsia and Helicobacter pylori infection after 17 years: the Sørreisa gastrointestinal disorder study. European Journal of Epidemiology 2008;23(9):625‐33. [DOI] [PubMed] [Google Scholar]
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Chichester: John Wiley & Sons, 2008. [Google Scholar]
Brunner 2012
- Brunner G, Athmann C, Schneider A. Long‐term, open‐label trial: safety and efficacy of continuous maintenance treatment with pantoprazole for up to 15 years in severe acid‐peptic disease. Alimentary Pharmacology & Therapeutics 2012;36(1):37‐47. [DOI] [PubMed] [Google Scholar]
Cooper 2006
- Cooper BT, Chapman W, Neumann CS, Gearty JC. Continuous treatment of Barrett's oesophagus patients with proton pump inhibitors up to 13 years: observations on regression and cancer incidence. Alimentary Pharmacology & Therapeutics 2006;23(6):727‐33. [DOI] [PubMed] [Google Scholar]
Correa 1992
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process ‐ First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Research 1992;52(24):6735‐40. [PubMed] [Google Scholar]
Dajani 2000
- Dajani EZ. Gastroesophageal reflux disease: pathophysiology and pharmacology overview. Journal of the Association for Academic Minority Physicians 2000;11(1):7‐11. [PubMed] [Google Scholar]
Dekkers 1999
- Dekkers CP, Beker JA, Thjodleifsson B, Gabryelewicz A, Bell NE, Humphries TJ. Comparison of rabeprazole 20 mg versus omeprazole 20 mg in the treatment of active duodenal ulcer: a European multicentre study. Alimentary Pharmacology and Therapeutics 1999;13(2):179‐86. [DOI] [PubMed] [Google Scholar]
Dinis‐Ribeiro 2011
- Dinis‐Ribeiro M, Areia M, Vries AC, Marcos‐Pinto R, Monteiro‐Soares M, O'Connor A, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2011;44(1):74‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dixon 1996
- Dixon MF, Genta RM, Yardley JH. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. American Journal of Surgical Pathology 1996;20:1161‐81. [DOI] [PubMed] [Google Scholar]
Eissele 1997
- Eissele R, Brunner G, Simon B, Solcia E, Arnold R. Gastric mucosa during treatment with lansoprazole: Helicobacter pylori is a risk factor for argyrophil cell hyperplasia. Gastroenterology 1997;112(3):707‐17. [DOI] [PubMed] [Google Scholar]
Eslami 2013
- Eslami L, Nasseri‐Moghaddam S. Meta‐analyses: does long‐term PPI use increase the risk of gastric premalignant lesions?. Archives of Iranian Medicine 2013;16(8):449‐58. [PubMed] [Google Scholar]
Ferlay 2010
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. [DOI] [PubMed] [Google Scholar]
Ficoca 2010
- Fiocca R, Mastracci L, Attwood S, Ell C, Galmiche J, Hatlebakk J, et al. Exocrine and endocrine gastric mucosal changes under medical or surgical antireflux therapy. Results of a 5‐yr follow up in the lotus trial. Gastroenterology 2010;138(5):S649‐50. [Google Scholar]
Fox 2011
- Fox JG, Kuipers EJ. Long‐term proton pump inhibitor administration, H pylori and gastric cancer: lessons from the gerbil. Gut 2011;60(5):567‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freston 1994
- Freston JW. Omeprazole, hypergastrinemia, and gastric carcinoid tumors. Annals of Internal Medicine 1994;121(3):232‐3. [DOI] [PubMed] [Google Scholar]
Fukao 1993
- Fukao A, Hisamichi S, Ohsato N, Fujino N, Endo N, Iha M. Correlation between the prevalence of gastritis and gastric cancer in Japan. Cancer Causes Control 1993;4(1):17‐20. [DOI] [PubMed] [Google Scholar]
Geboes 2001
- Geboes K, Dekker W, Mulder CJ, Nusteling K, Dutch Study Group. Long‐term lansoprazole treatment for gastro‐oesophageal reflux disease: clinical efficacy and influence on gastric mucosa. Alimentary Pharmacology and Therapeutics 2001;15(11):1819‐26. [DOI] [PubMed] [Google Scholar]
Genta 1998
- Genta RM. Review article: gastric atrophy and atrophic gastritis ‐ nebulous concepts in search of a definition. Alimentary Pharmacology and Therapeutics 1998;12(Suppl 1):17‐23. [DOI] [PubMed] [Google Scholar]
Genta 2003
- Genta RM, Rindi G, Fiocca R, Magner DJ, D'Amico D, Levine DS. Effects of 6‐12 months of esomeprazole treatment on the gastric mucosa. American Journal of Gastroenterology 2003;98(6):1257‐65. [DOI] [PubMed] [Google Scholar]
GRADEprofiler 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEprofiler (GRADEpro). Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Haag 2003
- Haag S, Holtmann G. Reflux disease and Barrett's esophagus. Endoscopy 2003;35(2):112‐7. [DOI] [PubMed] [Google Scholar]
Hassall 2011
- Hassall E, Owen D, Kerr W, Sturby T, Richardson P, El‐Serag H. Gastric histology in children treated with proton pump inhibitors long term, with emphasis on enterochromaffin cell‐like hyperplasia. Alimentary Pharmacology and Therapeutics 2011;33(7):829‐36. [DOI] [PubMed] [Google Scholar]
Held 2004
- Held M. Epidemiological Studies of Helicobacter pylori and its Relation to Cancer and Precancerous Lesions in the Upper Gastrointestinal Tract. Stockholm: Karolinska Institutet, 2004. [Google Scholar]
Hetzel 1988
- Hetzel DJ, Dent J, Reed WD, Narielvala FM, Mackinnon M, McCarthy JH, et al. Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology 1988;95(4):903‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holt 1991
- Holt S, Howden CW. Omeprazole: overview and opinion. Digestive Diseases and Sciences 1991;36(4):385‐93. [DOI] [PubMed] [Google Scholar]
Horwhat 2007
- Horwhat JD, Baroni D, Maydonovitch C, Osgard E, Ormseth E, Rueda‐Pedraza E, et al. Normalization of intestinal metaplasia in the esophagus and esophagogastric junction: incidence and clinical data. American Journal of Gastroenterology 2007;102(3):497‐506. [DOI] [PubMed] [Google Scholar]
Hwang 2006
- Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006;130(7):2217‐28. [DOI] [PubMed] [Google Scholar]
Jianu 2012
- Jianu CS, Fossmark R, Viset T, Qvigstad G, Sørdal O, Mårvik R, et al. Gastric carcinoids after long‐term use of a proton pump inhibitor. Alimentary Pharmacology & Therapeutics 2012;31(7):644‐9. [DOI] [PubMed] [Google Scholar]
Johnson 2013
- Johnson DA, Oldfield EC 4th. Reported side effects and complications of long‐term proton pump inhibitor use: dissecting the evidence. Clinical Gastroenterology and Hepatology 2013;11(5):458‐64. [DOI] [PubMed] [Google Scholar]
Kader 1998
- Kader SA, Mansour AM, Mohran Z, el‐Taoil A, Abdalla KF. A study on the relation between proton pump inhibitor and gastric giardiasis. Journal of the Egyptian Society of Parasitology 1998;28(1):149‐57. [PubMed] [Google Scholar]
Kapadia 2003
- Kapadia CR. Gastric atrophy, metaplasia, and dysplasia: a clinical perspective. Journal of Clinical Gastroenterology 2003;36(5 Suppl):S29‐36, S61‐2. [DOI] [PubMed] [Google Scholar]
Klinkenberg‐Knol 2000
- Klinkenberg‐Knol EC, Nelis F, Dent J, Snel P, Mitchell B, Prichard P, et al. Long‐term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology 2000;118(4):661‐9. [DOI] [PubMed] [Google Scholar]
Kuipers 1996
- Kuipers EJ, Lundell L, Klinkenberg‐Knol EC, Havu N, Festen HP, Liedman B, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. New England Journal of Medicine 1996;334(16):1018‐22. [DOI] [PubMed] [Google Scholar]
Kuipers 2004
- Kuipers EJ, Nelis GF, Klinkenberg‐Knol EC, Snel P, Goldfain D, Kolkman JJ, et al. Cure of Helicobacter pylori infection in patients with reflux oesophagitis treated with long term omeprazole reverses gastritis without exacerbation of reflux disease: results of a randomised controlled trial. Gut 2004;53(1):12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laheij 2004
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA 2004;292(16):1955‐60. [DOI] [PubMed] [Google Scholar]
Laine 2000
- Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long‐term acid suppression with proton pump inhibitors. Alimentary Pharmacology and Therapeutics 2000;14(6):651‐68. [DOI] [PubMed] [Google Scholar]
Lamberts 2001
- Lamberts R, Brunner G, Solcia E. Effects of very long (up to 10 years) proton pump blockade on human gastric mucosa. Digestion 2001;64(4):205‐13. [DOI] [PubMed] [Google Scholar]
Lauren 1965
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. Acta Pathologica et Microbiologica Scandinavica 1965;64:31‐49. [DOI] [PubMed] [Google Scholar]
Lee 1996
- Lee SW, Lieberman DA, Ippoliti AD, Hsieh CY, Weinstein WM. Changes in the parietal cell mass after long‐term therapy with high dose omeprazole in Barrett's esophagus (BE). Gastroenterology 1996;110:A173. [Google Scholar]
Malfertheiner 2007
- Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El‐Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007;56(6):772‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 1994
- Marks RD, Richter JE, Rizzo J. Omeprazole vs. H2‐receptor antagonists in treating patients with peptic stricture and esophagitis. Gastroenterology 1994;106(4):907‐15. [DOI] [PubMed] [Google Scholar]
McColl 2000
- McColl KEL, Murray LS, Gillen D. Omeprazole and accelerated onset of atrophic gastritis. Gastroenterology 2000;118:239. [DOI] [PubMed] [Google Scholar]
Moayyedi 2000
- Moayyedi P, Wason C, Peacock R, Walan A, Bardhan K, Axon AT, et al. Changing patterns of Helicobacter pylori gastritis in long‐standing acid suppression. Helicobacter 2000;5(4):206‐14. [DOI] [PubMed] [Google Scholar]
Neal 1996
- Neal KR, Scott HM, Slack RC, Logan RF. Omeprazole as a risk factor for campylobacter gastroenteritis: case‐control study. BMJ 1996;312(7028):414‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norton 1999
- Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, et al. Surgery to cure the Zollinger‐Ellison syndrome. New England Journal of Medicine 1999;341(9):635‐44. [DOI] [PubMed] [Google Scholar]
Peters 1999
- Peters FT, Ganesh S, Kuipers EJ, Sluiter WJ, Klinkenberg‐Knol EC, Lamers CB, et al. Endoscopic regression of Barrett's oesophagus during omeprazole therapy. Gut 1999;45(4):489‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pounder 2000
- Pounder RE, Williams MP. Omeprazole and accelerated onset of atrophic gastritis. Gastroenterology 2000;118:238‐9. [DOI] [PubMed] [Google Scholar]
Poynard 1995
- Poynard T, Lemaire M, Agostini H. Meta‐analysis of randomized clinical trials comparing lansoprazole with ranitidine or famotidine in the treatment of acute duodenal ulcer. European Journal of Gastroenterology and Hepatology 1995;7(7):661‐5. [PubMed] [Google Scholar]
Price 1991
- Price AB. The Sydney System: histological division. Journal of Gastroenterology and Hepatology 1991;6(3):209‐22. [DOI] [PubMed] [Google Scholar]
RevMan 2013 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Reynaert 1995
- Reynaert H, Fernandes E, Bourgain C, Smekens L, Devis G. Proton‐pump inhibition and gastric giardiasis: a causal or casual association?. Journal of Gastroenterology 1995;30(6):775‐8. [DOI] [PubMed] [Google Scholar]
Richter 2000
- Richter J, Kahrilas P, Hwang C, Marino V, Hamelin B. Six month safety and tolerability of esomeprazole as maintenance therapy in GERD patients with healed erosive esophagitis (EE). Gastroenterology 2000;118(4 part 2):A1299. [Google Scholar]
Rindi 2005
- Rindi G, Fiocca R, Morocutti A, Jacobs A, Miller N, Thjodleifsson B, European Rabeprazole Study Group. Effects of 5 years of treatment with rabeprazole or omeprazole on the gastric mucosa. European Journal of Gastroenterology and Hepatology 2005;17(5):559‐66. [DOI] [PubMed] [Google Scholar]
Rostom 2002
- Rostom A, Dube C, Wells GA, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID‐induced gastroduodenal ulcers. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD002296] [DOI] [PubMed] [Google Scholar]
Sanduleanu 1999
- Sanduleanu S, Stridsberg M, Jonkers D, Hameeteman W, Biemond I, Lundqvist G, et al. Serum gastrin and chromogranin A during medium‐ and long‐term acid suppressive therapy: a case‐control study. Alimentary Pharmacology and Therapeutics 1999;13(2):145‐53. [DOI] [PubMed] [Google Scholar]
Sanduleanu 2001
- Sanduleanu S, Jonkers D, De Bruïne A, Hameeteman W, Stockbrügger RW. Double gastric infection with Helicobacter pylori and non‐Helicobacter pylori bacteria during acid‐suppressive therapy: increase of pro‐inflammatory cytokines and development of atrophic gastritis. Alimentary Pharmacology and Therapeutics 2001;15(8):1163‐75. [DOI] [PubMed] [Google Scholar]
Schenk 1998
- Schenk BE, Kuipers EJ, Klinkenberg‐Knol EC, Bloemena E, Nelis GF, Festen HP, et al. Hypergastrinaemia during long‐term omeprazole therapy: influences of vagal nerve function, gastric emptying and Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics 1998;12(7):605‐12. [DOI] [PubMed] [Google Scholar]
Schenk 2000
- Schenk BE, Kuipers EJ, Nelis GF, Bloemena E, Thijs JC, Snel P, et al. Effect of Helicobacter pylori eradication on chronic gastritis during omeprazole therapy. Gut 2000;46(5):615‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shiota 2013
- Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Review of Gastroenterology and Hepatology 2013;7(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sipponen 2007
- Sipponen P, Graham DY. Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scandinavian Journal of Gastroenterology 2007;42(1):2‐10. [DOI] [PubMed] [Google Scholar]
Solcia 1988
- Solcia E, Bordi C, Creutzfeldt W, Dayal Y, Dayan AD, Falkmer S, et al. Histopathological classification of nonantral gastric endocrine growths in man. Digestion 1988;41(4):185‐200. [DOI] [PubMed] [Google Scholar]
Solcia 1995
- Solcia E, Fiocca R, Villani L, Luinetti O, Capella C. Hyperplastic, dysplastic, and neoplastic enterochromaffin‐like‐cell proliferations of the gastric mucosa. Classification and histogenesis. American Journal of Surgical Pathology 1995;19(Suppl 1):S1‐7. [PubMed] [Google Scholar]
Suzuki 2008
- Suzuki M, Suzuki H, Hibi T. Proton pump inhibitors and gastritis. Journal of Clinical Biochemistry and Nutrition 2008;42(2):71‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 2010
- Thomson AB, Sauve MD, Kassam N, Kamitakahara H. Safety of the long‐term use of proton pump inhibitors. World Journal of Gastroenterology 2010;16(19):2323‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uemura 2000
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Mashiba H, et al. Changes in Helicobacter pylori‐induced gastritis in the antrum and corpus during long‐term acid‐suppressive treatment in Japan. Alimentary Pharmacology and Therapeutics 2000;14(10):1345‐52. [DOI] [PubMed] [Google Scholar]
Vigneri 1995
- Vigneri S, Termini R, Leandro G, Badalamenti S, Pantalena M, Savarino V, et al. A comparison of five maintenance therapies for reflux esophagitis. New England Journal of Medicine 1995;333(17):1106‐10. [DOI] [PubMed] [Google Scholar]
Waldum 2014
- Waldum HL, Hauso O, Fossmark R. The regulation of gastric acid secretion ‐ clinical perspectives. Acta Physiologica 2014;210:239‐56. [DOI] [PubMed] [Google Scholar]
Wilkinson 1999
- Wilkinson SP, Biddlestone L, Gore S, Shepherd NA. Regression of columnar lined Barrett's oesophagus with omeprazole 40 mg daily: results of 5 years continuous therapy. Alimentary Pharmacology and Therapeutics 1999;13(9):1205‐9. [DOI] [PubMed] [Google Scholar]
Wolfe 2000
- Wolfe MM, Sachs G. Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress‐related erosive syndrome. Gastroenterology 2000;118(2 Suppl 1):S9‐31. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Eslami 2008
- Eslami L, Kalantarian S, Nasseri‐Moghaddam S, Majdzadeh R. Long term proton pump inhibitor (PPI) use and incidence of gastric (pre) malignant lesions. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007098.pub2] [DOI] [Google Scholar]


