Abstract
PURPOSE
We aimed to investigate the prognostic role of baseline and longitudinal levels of neutrophil-to-lymphocyte ratio (NLR) in patients with metastatic colorectal cancer (mCRC) treated with chemotherapy + bevacizumab (CT + B) or chemotherapy only. Additionally, we investigated whether treatment outcomes were mediated by the longitudinal biomarker.
METHODS
Data from an Italian randomized phase III trial were used. The main end point was progression-free survival (PFS). To address research questions, a series of joint models of longitudinal and survival data were specified, and the direct and indirect treatment effects were quantified.
RESULTS
Data for 239 patients, 113 (47.3%) treated with CT + B and 126 (52.7%) with CT only, were included in the analyses. The effect of NLR seemed to be mediated by the longitudinal trajectory of the biomarker. Only in the patient subgroup treated with CT + B, the baseline NLR retained a direct effect on PFS. Regarding the effect of treatment on PFS, two scenarios were observed. In the subgroup of patients with low baseline, NLR bevacizumab showed a direct protective effect only (hazard ratio [HR], 0.66 [95% CI, 0.45 to 0.98]), whereas in the subgroup with high baseline NLR, there was evidence for an adverse direct effect (HR, 1.63 [95% CI, 1.03 to 2.57]) and a protective indirect—which is mediated by the longitudinal biomarker—effect (HR, 0.71 [95% CI, 0.55 to 0.90]).
CONCLUSION
In our study, inflammatory indexes collected longitudinally showed a significant adverse prognostic role, thus suggesting the collection and use of such data for better clinical decision making. In the specific setting, we considered this is particularly important as the treatment effect seemed to be modified by both the baseline and longitudinal inflammation statuses. However, further research is needed to understand the possible factors underlying these results.
Our results showed the important contribution of NLR measurements other than the baseline and encourage their collection and use in the clinical management.
INTRODUCTION
The prognostic role of inflammatory indexes has been documented in several clinical investigations on metastatic cancers.1 Most studies on metastatic colorectal cancer (mCRC) only considered baseline pretreatment values, a few of them included time-dependent measurements at follow-up,2-5 and none of them applied a joint modeling of longitudinal and survival data.
CONTEXT
Key Objective
The prognostic role of inflammatory indexes, such as neutrophil-to-lymphocyte ratio (NLR), has been documented, but most studies on metastatic colorectal cancer (mCRC) only considered baseline pretreatment values. We applied a joint modeling of longitudinal and survival data to disentangle the contribution of baseline and current NLR measurement on progression-free survival (PFS).
Knowledge Generated
Our study supports that baseline inflammatory indexes have mostly a negative indirect effect on PFS, that is, an effect mediated by longitudinal inflammatory markers. Additionally, we found that bevacizumab showed a protective effect that, in the specific subgroup of patients with high baseline NLR, was partially mediated through a reduction of inflammation. However, further investigation is needed in this specific subgroup because of an observed unfavorable direct effect of bevacizumab.
Relevance
Our results showed the important contribution of NLR measurements other than the baseline and encourage their collection and use in the clinical management of mCRC patients.
In mCRC, bevacizumab combined with fluoropyrimidine-based chemotherapy (CT) is considered a standard first- and second-line treatment. Validated predictors of sensitivity or resistance to bevacizumab are not yet available. Recently, several studies have investigated this issue, focusing on the vascular endothelial growth factor (VEGF) pathway but not the tumor microenvironment and inflammatory response.6
Previously, we investigated the prognostic and the predictive role of baseline inflammatory indexes on survival in patients enrolled in phase III multicenter randomized Italian Trial in Advanced Colorectal cancer (ITACa) trial.7 In this trial, data on inflammatory biomarkers were also longitudinally collected for the duration of treatments.
This study aimed to investigate the relationship between baseline and longitudinal levels of neutrophil-to-lymphocyte ratio (NLR) and progression-free survival (PFS) in patients with mCRC who received chemotherapy + bevacizumab (CT + B) or only chemotherapy in the ITACa trial. Second, we investigated whether the effects of treatment on PFS were mediated by the longitudinal inflammatory biomarker.
Achieving these objectives by separate analyses of longitudinal and time-to-event data may be inefficient or even biased.8 Thus, a valid approach was provided by joint models in which two linked submodels, one for the biomarker repeated measurements and one for the time-to-event (eg, PFS) outcome, were specified.9 In this way, all the information in the data is simultaneously considered, and a valid and efficient inference about the dependence between the two underlying processes is produced.
METHODS
Study Design
For this study, data from the first line of the ITACa trial (EudraCT no. 2007-004539-44) and on ClinicalTrials.gov (identifier: NCT01878422) were used.10 In this phase III multicenter trial, 370 patients were originally randomly assigned to receive CT with or without bevacizumab (B), and the main end point was PFS as defined by the time from random assignment to disease progression or death from any cause, whichever occurred first. Tumor responses were radiologically evaluated every 8 weeks, according to the RECIST until disease progression or withdrawal.
Overall, 176 patients received CT (either fluorouracil, leucovorin, and irinotecan or fluorouracil, leucovorin, and oxaliplatin) + B while 194 patients received CT alone. Patients were recruited between November 14, 2007, and March 6, 2012, and the last follow-up update occurred on August 31, 2016. Information on neutrophils and lymphocytes measured before any systemic treatment administration (at baseline), and at each 14 day treatment cycle, was available for 239 of the 370 patients. NLR was obtained as the ratio between the absolute neutrophil and absolute lymphocyte counts. Further details on the study design, eligibility criteria, and endpoint definition have been previously reported.10 Patient characteristics are summarized in Table 1.
TABLE 1.
Pre-Random Assignment Patient Characteristics
| Characteristic | All (n = 239) | Original Cohort (N = 370) | CT (n = 126) | CT + B (n = 113) | P |
|---|---|---|---|---|---|
| Age at enrollment, years, mean ± SD | 64.99 ± 10.72 | 64.5 ± 10.3 | 65.32 ± 10.60 | 64.62 ± 10.89 | .617 |
| Sex, No. (%) | .342 | ||||
| Female | 90 (37.66) | 147 (39.73) | 51 (40.48) | 39 (34.51) | |
| Male | 149 (62.34) | 223 (60.27) | 75 (59.52) | 74 (65.49) | |
| Study arm, No. (%) | |||||
| CT | 126 (52.72) | 194 (52.43) | |||
| CT + B | 113 (47.28) | 176 (47.57) | |||
| CT regimen, No. (%) | .906 | ||||
| FOLFOX4 | 145 (60.67) | 221 (59.73) | 76 (60.32) | 69 (61.06) | |
| FOLFIRI | 94 (39.33) | 149 (40.27) | 50 (39.68) | 44 (38.94) | |
| KRAS status, No. (%) | .748 | ||||
| Wt | 154 (64.44) | 235 (63.51) | 80 (63.49) | 74 (65.49) | |
| Mut | 85 (35.56) | 135 (36.49) | 46 (36.51) | 39 (34.51) | |
| Tumor localization, No. (%) | .124 | ||||
| Rectum | 64 (26.78) | 92 (24.86) | 39 (30.95) | 25 (22.12) | |
| Colon | 175 (73.22) | 278 (75.14) | 87 (69.05) | 88 (77.88) | |
| ECOG PS, No. (%) | .156 | ||||
| 0 | 194 (81.17) | 298 (80.54) | 98 (77.78) | 96 (84.96) | |
| ≥1 | 45 (18.83) | 72 (19.46) | 28 (22.22) | 17 (15.04) | |
| Stage at diagnosis,a No. (%) | .743 | ||||
| I-III | 54 (23.68) | 90 (26.24) | 29 (24.58) | 20 (21.51) | |
| IV | 174 (76.32) | 253 (73.76) | 89 (75.42) | 73 (78.49) |
Abbreviations: B, bevacizumab; CT, chemotherapy; CT + B, chemotherapy + bevacizumab; ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFIRI, fluorouracil, leucovorin, and irinotecan; FOLFOX4, fluorouracil, leucovorin, and oxaliplatin; SD, standard deviation.
The sum does not add to the total because of missing data.
Statistical Analyses
Data were summarized by mean ± standard deviation (SD) or median and first (IQ) and third (IIIQ) quartiles for continuous variables and through natural frequencies and percentages for categorical ones. The association between categorical variables was tested by using the Pearson χ2 test or the Fisher exact test, whereas those between a continuous and a categorical variable were tested using the Student t test or the analogous nonparametric Wilcoxon-Mann Whitney test. To reach the study's main objective, a joint modeling approach was used. Joint models for longitudinal and time-to-event data consist of two joint submodels: one for the biomarker (NLR) trajectory over time (longitudinal submodel) and the other for the survival outcome (PFS; survival submodel). Because of the skewed distribution of NLR, the analyses were performed on log-transformed values, hereafter lNLR.
To model the lNLR trajectory over time, a random intercept and random slope linear mixed-effects model was specified. To better approximate the nonlinear lNLR profile over time, natural cubic splines were used. To model the survival outcome, a Weibull model was considered.
The general form of the longitudinal submodel is as follows:
where for the ith patient, is a Gaussian distributed error term with zero mean and variance and the trajectory function which depends on fixed or time-dependent variables and subject-specific random terms.9,11 Therefore, the trajectory function is the expected value of the longitudinal biomarker.
The general form of the survival submodel is as follows:
where γ is the vector of regression coefficients, are vectors of time-fixed covariates, α is a tuning parameter, and is the trajectory function.
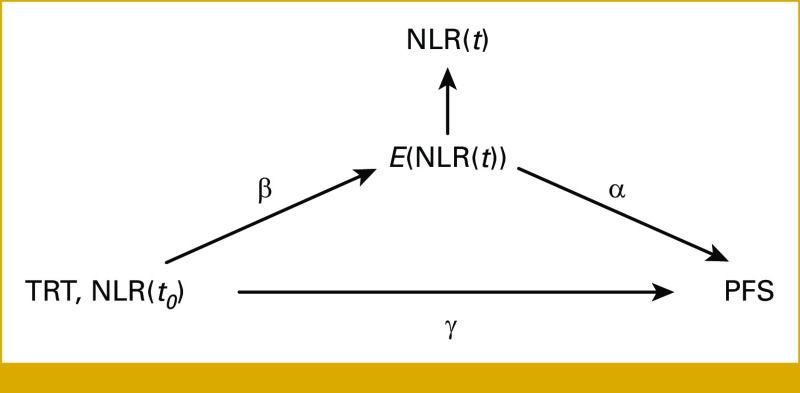
The joint model approach is useful to obtain information on the net direct effect of treatment or pre-random assignment covariates on survival after adjusting for the longitudinal trajectory. Following Ibrahim et al,12 we report in Figure 1 the underlying causal graph of our joint model. The vector of regression coefficients of the longitudinal submodel (β) contains the effects of the treatment and pre-random assignment covariates on the longitudinal outcome while the elements of the vector of regression coefficients γ are the effects of the treatment and the pre-random assignment covariates on survival. Therefore, the effect of the treatment or pre-random assignment covariates is decomposed in a direct effect expressed by the appropriate elements of the vector γ and an indirect effect which is the combination of the β vector and the coefficient α of the trajectory function. Under the assumption of no confounding between treatment or pre-random assignment covariates and the longitudinal outcome and no confounding between the longitudinal outcome and the survival outcome given treatment or pre-random assignment covariates, the indirect effect of treatment and pre-random assignment covariates is simply obtained as β·α, a condition known as assumption of sequential ignorability be satisfied.13 The CIs of the direct and indirect effects were computed using the delta method. For details on the two submodels, see the Data Supplement (Statistical Methods).
FIG 1.
Causal graph of the joint model. TRT denotes treatment, NLR(t0) baseline biomarker value, NLR(t) current biomarker value, E(NLR(t)) trajectory function, and PFS time-to-event outcome. The regression coefficients are denoted by α, β, and γ. NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival.
All tests were two-sided. Overall, a threshold of 0.050 for the P value (P) was considered. The analyses were performed with R version 4.2.0 and JM package version 1.5-2.
Ethical Approval
This study was reviewed and approved by the IRCCS IRST and Area Vasta Romagna Ethics Committee (CEIIAV), approval no. 0063711 of September 19, 2007; it was conducted in accordance with the 1964 Declaration of Helsinki, with Good Clinical Practice (GCP) guidelines and with EQUATOR guidelines.
Consent to Participate
The participants provided their written informed consent to participate in this study.
Consent for Publication
No identifiable human data were included in the manuscript.
RESULTS
Patient Characteristics
The analysis included 239 of 370 patients enrolled in the ITACa trial with available baseline and longitudinal NLR measurements; the Data Supplement (Fig S1) shows the study flowchart. Table 1 shows the distribution of pre-random assignment characteristics for all patients and by treatment group. No substantial differences with the original cohort and between treatment groups were observed. The mean ± SD baseline lNLR was equal to 1.02 ± 0.53; 143 (59.8%) patients had a value lower than 1.10 that is, the logarithm of the cutoff of 3.0 for NLR used in our previous study.7 Among pre-random assignment covariates, a higher percentage of patients with Eastern Cooperative Oncology Group performance status ≥1 was observed among patients with higher baseline NLR value as compared with those with lower levels (30.21% v 11.29%), as reported in the Data Supplement (Table S1).
At the last follow-up update, 224 patients experienced disease progression or died (resulting in a censoring of 6%); the median follow-up time obtained by the reverse Kaplan-Meier method was 1,585 days (Min-max: 182-1,944). The median PFS was 376 (95% CI, 322 to 447) and 282 (95% CI, 246 to 316) days for patients with lNLR <1.10 and receiving CT + B and CT, respectively, and 204 (95% CI, 140 to 284) and 254 (95% CI, 208 to 303) days for patients with lNLR ≥1.10 and receiving CT + B and CT, respectively.
The median number (IQ-IIIQ) of NLR measurements per patient was 12 (7-14).7-14 The Data Supplement (Fig S2) shows the individual lNLR profiles of a dozen randomly selected patients. There is a large variability of the observed trajectories among patients; this justified the use of a mixed random-effects model as described in the Methods section. The Data Supplement (Fig S3) shows the distribution, over time, of all 2,756 lNLR measurements, including the individual predicted trajectories obtained by fitting the mixed-effects model of equation S1 (Data Supplement).
Longitudinal and Survival Joint Model
The results of fitting our joint model—longitudinal submodel for lNLR and survival submodel for PFS—are shown in Table 2 (we did not report the regression coefficients corresponding to the B-splines because they are not directly interpretable).
TABLE 2.
Parameter Estimate of the Joint Model
| Variable | Coefficient | 95% CI | P |
|---|---|---|---|
| Longitudinal submodela | |||
| Intercept | 0.39 | 0.29 to 0.48 | |
| Treatment (CT + B v CT) | 0.01 | –0.11 to 0.14 | .862 |
| Baseline lNLR (≥1.10 v <1.10) | 0.52 | 0.38 to 0.65 | <.001 |
| Treatment × baseline lNLR | –0.26 | –0.47 to –0.06 | .012 |
| Variable | Coefficient | 95% CI | P | HR | 95% CI |
|---|---|---|---|---|---|
| Survival submodel | |||||
| Treatment (CT + B v CT) in baseline lNLR <1.10 group | –0.41 | –0.79 to –0.03 | .037 | 0.66 | 0.45 to 0.98 |
| Baseline lNLR (≥1.10 v <1.10) in CT + B group | 0.56 | 0.10 to 1.02 | .017 | 1.75 | 1.10 to 2.77 |
| Treatment × baseline lNLR | 0.90 | 0.30 to 1.50 | .003 | ||
| Assoc | 1.39 | 0.95 to 1.82 | <.001 | 4.00 | 2.60 to 6.17 |
Abbreviations: B, bevacizumab; CT, chemotherapy; CT + B, chemotherapy + bevacizumab; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio.
The regression coefficients for the natural cubic splines were omitted as not directly interpretable.
Longitudinal Submodel
On average, the longitudinal NLR values were lower than the baseline measurements (Data Supplement, Eq S1). The random intercept and random slope standard deviations and the correlation coefficient as estimated by the longitudinal submodel were equal to 0.374, 0.002, and –0.392, respectively. This implies that the variability of NLR measurements among patients decreases over time. The regression coefficients reported in Table 2 show that the higher baseline NLR CT patients group has an average intercept of NLR of 0.91 (0.39 + 0.52), the higher baseline NLR CT + B patient group has an average intercept of 0.65 (0.39 + 0.52 – 0.26), the lower baseline NLR CT patient group has an average intercept of 0.39, and lower baseline NLR CT + B patient group has an average intercept of 0.40 (0.39 + 0.01).
In Table 2, baseline lNLR predicts the longitudinal lNLR. Particularly, the higher the baseline NLR, the higher the expected longitudinal trajectory (β = 0.52 [95% CI, 0.32 to 0.65]). Moreover, the regression coefficients for bevacizumab show a mitigating effect of it on inflammation indexes in the group with high baseline lNLR (β is equal to –0.26 + 0.01 in the group with high baseline lNLR compared with 0.01 in the low lNLR group).
We tested if the effect of baseline NLR vanishes over time introducing an appropriate interaction term between baseline NLR and the measurement times in the longitudinal submodel, and we found no evidence against the null hypothesis (P = .207, results not shown).
Figure 2 shows the observed values and the predicted individual lNLR trajectories by the longitudinal submodel. The number of lNLR measurement points below zero is greater in the bottom panels corresponding to the patient groups with low baseline NLR values, consistently with an association between the baseline and longitudinal NLR values.
FIG 2.
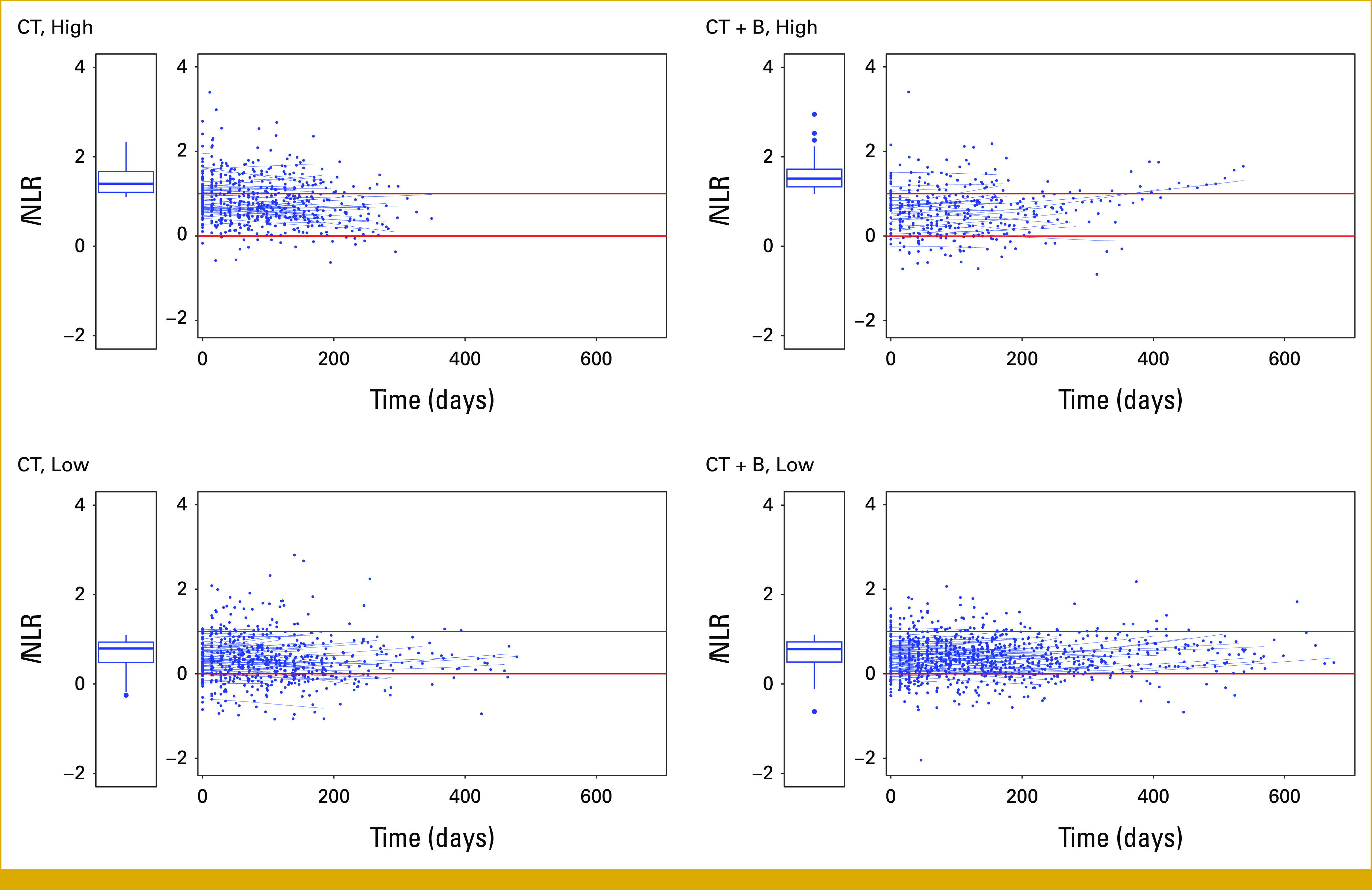
Observed values and predicted individual trajectories for lNLR over time from the mixed random-effects model excluding the baseline lNLR values by treatment arm and baseline lNLR value (low: lNLR <1.10; high: ≥1.10). Here, time equals to zero corresponds to the first postbaseline time point available for each patient. Boxplots refer to lNLR values at baseline in each group of patients. CT, chemotherapy; CT + B, chemotherapy + bevacizumab; NLR, neutrophil-to-lymphocyte ratio.
Among patients with high baseline NLR values (top panels), the CT + B group shows a favorable pattern with a smaller number of NLR measurements points above zero compared with the CT group.
Survival Submodel
Considering the results of the survival submodel reported in Table 2, we found a strong effect of the current longitudinal NLR measurements (ie, the trajectory function) on PFS (as measured by the estimate of the assoc parameter, corresponding to a hazard ratio for a unit increase of lNLR of 4.0 [95% CI, 2.6 to 6.2]; Data Supplement, Eq S2). In the survival submodel, there is a strong interaction term between the treatment arm and baseline lNLR: Bevacizumab shows a protective effect in the subgroup with low baseline lNLR (hazard ratio [HR], 0.66 [95% CI, 0.45 to 0.98]), and baseline lNLR has an unfavorable prognostic value for patients allocated to the CT + B treatment arm (HR, 1.75 [95% CI, 1.10 to 2.77]).
The Data Supplement (Fig S4) shows the observed trajectory plot by time to event: Consistently with an effect of current longitudinal NLR measurements on survival, an increase in lNLR in the proximity of the event is observed. The increase started up to 8 months before the event and became steeper in the past 2 months (right panel). No pattern of deviations from randomness of the censoring times was observed (left panel).
Direct and Indirect Effects
Baseline NLR Direct and Indirect Effects
Table 3 reports the direct, indirect, and total effects of baseline NLR estimated by the joint model by treatment arm. Higher baseline NLR shows an adverse indirect effect on survival; this is the effect of baseline NLR mediated by the longitudinal NLR trajectory. The HRs are 2.05 (95% CI, 1.53 to 2.73) and 1.42 (95% CI, 1.12 to 1.81) for CT and CT + B arms, respectively (Table 3—indirect effect). In the CT + B arm, baseline lNLR maintains a residual direct effect on survival (HR, 1.75 [95% CI, 1.10 to 2.77]), showing a prognostic value not mediated by the longitudinal NLR trajectory. Notice that there is no evidence of a direct effect of baseline lNLR in the CT arm (HR, 0.71 [95% CI, 0.46 to 1.11]).
TABLE 3.
Separate Effects of Baseline lNLR (≥1.10 v <1.10) as Estimated by the Joint Model by Treatment Arm
| Effects | CT, HR (95% CI) | CT + B, HR (95% CI) |
|---|---|---|
| Direct | 0.71 (0.46 to 1.11) | 1.75 (1.10 to 2.77) |
| Indirect | 2.05 (1.53 to 2.73) | 1.42 (1.12 to 1.81) |
| Total | 1.46 (0.94 to 2.28) | 2.49 (1.54 to 4.03) |
Abbreviations: CT, chemotherapy; CT + B, chemotherapy + bevacizumab; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio.
Bevacizumab Direct and Indirect Effects
Table 4 reports the direct, indirect, and total effects of bevacizumab estimated by the joint model by baseline NLR. We found a protective direct effect (not mediated by the longitudinal NLR trajectory) of bevacizumab on survival in the group with low baseline NLR (HR, 0.66 [95% CI, 0.45 to 0.98]). There is no evidence of an indirect effect in this subgroup of patients (HR, 1.02 [95% CI, 0.85 to 1.21]).
TABLE 4.
Separate Treatment Effects (CT + B v CT) as Estimated by the Joint Model by Baseline lNLR
| Effects | lNLR <1.10, HR (95% CI) | lNLR ≥1.10, HR (95% CI) |
|---|---|---|
| Direct | 0.66 (0.45 to 0.98) | 1.63 (1.03 to 2.57) |
| Indirect | 1.02 (0.85 to 1.21) | 0.71 (0.55 to 0.90) |
| Total | 0.67 (0.45 to 1.02) | 1.15 (0.70 to 1.89) |
Abbreviations: CT, chemotherapy; CT + B, chemotherapy + bevacizumab; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio.
In the subgroup of patients with high baseline NLR, bevacizumab shows a protective indirect effect on survival (mediated by a reduction of the inflammatory index in the longitudinal NLR trajectory; HR, 0.71 [95% CI, 0.55 to 0.90]). However, there was evidence of a negative direct effect on survival of bevacizumab (HR, 1.63 [95% CI, 1.03 to 2.57]), which counterbalanced the protective direct effect. Therefore, the total effect of bevacizumab on survival, in this group of patients, was almost null (HR, 1.15 [95% CI, 0.70 to 1.89]).
DISCUSSION
Previously, we investigated the prognostic and the predictive roles of baseline inflammatory indexes on PFS and overall survival in patients enrolled into the phase III multicenter randomized ITACa trial in patients treated with CT alone or CT + B.7
The prognostic role of inflammatory indexes has been documented in several clinical investigations on metastatic cancers, but it was not clear if baseline measurements maintained a prognostic role when follow-up measurements were available. In this study, we found evidence that the clinical role of baseline measurements in mCRC is mediated by the longitudinal patient trajectory of the inflammatory index, that is, longitudinal measurements are important prognostic factors, and in clinical practice, the baseline measurements are quite uninformative whenever follow-up measurements become available. The effect of one unit increase of lNLR current follow-up measurement on PFS was estimated as HR, 4.00 (95% CI, 2.60 to 6.17).
Particularly, the baseline NLR showed a significant indirect effect on PFS ranging from a HR of 1.42-2.05, depending on the treatment received (Table 3). In addition, NLR baseline measurements may still have a direct effect on the survival outcome but only in the subgroup of patients receiving the combination CT + B (HR, 1.75 [95% CI, 1.10 to 2.77]).
Regarding the treatment effect, bevacizumab showed a direct effect on PFS—that is, through a pathway that does not involve inflammatory biomarkers. This corresponded to a HR of 0.66 (95% CI, 0.45 to 0.98) in patients with low baseline NLR and to a HR of 1.63 (95% CI, 1.03 to 2.57) in patients with high NLR at baseline (Table 4).
However, in the subgroup of patients with high baseline NLR, there was also evidence of a significant indirect effect of bevacizumab (through longitudinal NLR levels); this time in the opposite direction as compared with the corresponding direct effect (HR, 0.71 [95% CI, 0.55 to 0.90]; Table 4). Thus, in patients with a high level of NLR at baseline, bevacizumab appeared to be able to reduce inflammation and, as a consequence, indirectly improve survival.
These two opposite effects were then responsible for an almost null total effect of bevacizumab on PFS in this subgroup of patients (HR, 1.15 [95% CI, 0.70 to 1.89]). Through additional analyses aimed at possibly improving our understanding about the possible reason behind these apparently discordant effects, we observed that the subgroup of patients with high baseline NLR and treated with CT + B showed slightly higher baseline NLR values (mean ± SD, 1.60 ± 0.40) as compared with the high baseline NLR in the CT-only group (mean ± SD, 1.47 ± 0.30). We also observed that such subgroup of patients showed a slightly higher tumor burden as measured by the sum of the longest diameters of the RECIST-defined target lesions (mean ± SD, 146 ± 88.2 mm) as compared with the high baseline NLR in the CT-only group (mean ± SD, 122 ± 75.8). This subgroup has a long right tail of frail patients, and we cannot exclude that a residual confounding could have affected our results. Validated predictors of sensitivity or resistance to bevacizumab are still unavailable, notwithstanding several studies have investigated this issue in recent years, primarily focusing on the VEGF pathway and not on the tumor microenvironment and inflammatory response.6 We leave it to future research to investigate possible explanations for these results.
We discuss below the potential limitations and weaknesses of our study.
Our study was a randomized controlled trial with a relatively small sample size (N = 370). In addition, follow-up measurements were available for a subset (239 of 370, 65%) of patients, which, however, showed characteristic comparable with those of the enrolled cohort.
The effect of baseline NLR in our study was mainly an indirect effect, mediated by the longitudinal NLR patient trajectory. However, we made some assumptions about the mechanism of this indirect effect. One is that this indirect effect is time invariant. A different assumption could be that the indirect effect vanishes the further away the follow-up time. We tested the hypothesis of no time-dependent indirect effect of baseline NLR, introducing an appropriate interaction term between baseline NLR and the measurement times in the longitudinal submodel finding no evidence against the null (P = .207). However, this test may have a low power, and we leave further research to deepen this question.
Disentangling direct and indirect effects depends on the validity of the assumption of conditional ignorability, that is, the absence of confounding of the relationship among the exposure, the biomarker, and the survival outcome. More complex models are required to relax this assumption.14 We are confident in the assumption of conditional ignorability on the basis of the observed covariates in a controlled trial like ours.
The mechanism by which the exposure (baseline NLR) may affect survival through the longitudinal biomarker (ie, the indirect effect) can be complex: One possibility is that the exposure may influence the level of biomarker and the ultimate effect on survival is driven by the actual level of the biomarker—that is, a pure indirect effect; another possibility is that the exposure and the biomarker might interact, and the effects might change depending on the level of exposure or biomarker. We checked this assumption, and we found no evidence of exposure—mediator interaction (P = .671).
We are confident that our findings be not related to modeling choices, in sensitivity analyses we checked several parametrizations including or excluding treatment as a predictor of the longitudinal NLR, or time-dependent effects.
In the literature, most studies addressing similar objectives considered only baseline pretreatment values, and few ones included a couple of time-dependent measurements.2-5 To our knowledge, none of them applied a joint modeling of longitudinal and survival data.
Using our long series of repeated measurements, we were able to find evidence of an effect of the current measurement and no evidence of effect of the rate of change (P = .388). In other words, we did not find any prognostic value of the extent of the decrease of NLR at the start of treatment.
Finally, we considered how to identify those patients who could benefit from bevacizumab treatment using biomarkers collected during treatment and addressing mediating pathways. To this purpose, joint modeling of longitudinal and survival data appeared to be most promising and able to disentangle direct and indirect effects—that is, assessing different mechanisms of effect and evaluating treatment responses among the various patient groups.
In conclusion, our study supports that inflammatory indexes are important prognostic indicators in colorectal metastatic cancer. Baseline inflammatory indexes mostly have an (indirect) effect on survival mediated by longitudinal inflammatory markers. Therefore, we provide evidence supporting the use of current longitudinal measurements in clinical practice. Bevacizumab showed a protective direct effect on survival in patients with low baseline NLR and a protective indirect effect in patients with high baseline NLR that is, an effect that was partially mediated through a reduction of inflammation, as measured by longitudinal inflammatory indexes. However, this indirect effect is insufficient to contrast the worse prognosis of the subgroup of patients with high baseline levels of inflammatory indexes treated with bevacizumab. Additional research is needed to best tailor treatment strategies for patients with mCRC.
ACKNOWLEDGMENT
The following collaborators are acknowledged: Angela Ragazzini, Monia Dall’Agata, Silvia Ruscelli, Britt Rudnas, Piancastelli Alessandra, Zumaglini Federica, Vertogen Bernadette (IRCCS IRST, Meldola); Luigi Cavanna, Elena Orlandi, Camilla Di Nunzio (“Guglielmo da Saliceto” Hospital, Piacenza); Elisa Pettorelli (University Hospital of Modena); Barbara Venturini (“Infermi” Hospital, Rimini); Jody Corbelli (“Degli Infermi” Hospital, Faenza); Paola Nasuti, Claudia Mucciarini (“Ramazzini” Hospital, Carpi); Gianpiero Romano (“Vito Fazzi” Hospital, Lecce); Andrea Ardizzoni, Elena Rapacchi (University Hospital of Parma); Alba A. Brandes, Rosalba Poggi, Enrico Franceschi, Stefania Bartolini (“Bellaria-Maggiore” Hospital, Bologna); Roberto Faggiuolo, Maria Giovanna Boe (Azienda ASL CN2, Alba-Bra); Antonio Frassoldati, Elena Raisi (AOU “S.Anna”, Ferrara); Mauro Giusto, Petros Giovanis (Azienda ULSS 1, Belluno).
SUPPORT
Supported in part by the research grant no. FARM6FJJAY from the Italian Medicines Agency (AIFA). AIFA played no role in the design, collection of data, analysis, or interpretation of the study, the writing of the manuscript, or the decision to submit the manuscript for publication. The work described in this manuscript is original research and has not been previously published.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Emanuela Scarpi, Elisabetta Petracci, Annibale Biggeri, Oriana Nanni
Administrative support: Oriana Nanni, Giovanni Luca Frassineti, Emanuela Scarpi
Provision of study materials or patients: Alessandro Passardi, Martina Valgiusti, Manlio Monti
Collection and assembly of data: Alessandro Passardi, Emanuela Scarpi
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1. Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–146. doi: 10.1016/j.critrevonc.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 2. Formica V, Luccchetti J, Cunningham D, et al. Systemic inflammation, as measured by the neutrophil/lymphocyte ratio, may have differential prognostic impact before and during treatment with fluorouracil, irinotecan and bevacizumab in metastatic colorectal cancer patients. Med Oncol. 2014;31:166. doi: 10.1007/s12032-014-0166-6. [DOI] [PubMed] [Google Scholar]
- 3. Lin GN, Liu PP, Liu DY, et al. Prognostic significance of the pre-chemotherapy lymphocyte-to-monocyte ratio in patients with previously untreated metastatic colorectal cancer receiving FOLFOX chemotherapy. Chin J Cancer. 2016;35:5. doi: 10.1186/s40880-015-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chua W, Charles KA, Baracos VE, et al. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaneko M, Nozawa H, Sasaki K, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in advanced colorectal cancer patients receiving oxaliplatin-based chemotherapy. Oncology. 2012;82:261–268. doi: 10.1159/000337228. [DOI] [PubMed] [Google Scholar]
- 6. Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 7. Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7:33210–33219. doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000;1:465–480. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 9.Rizopoulos D. Joint Models for Longitudinal and Time-To Event Data: With Applications in R. ed 1. New York, NY: Chapman and Hall/CRC; 2012. [Google Scholar]
- 10. Passardi A, Nanni O, Tassinari D, et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: Final results for first-line treatment from the ITACa randomized clinical trial. Ann Oncol. 2015;26:1201–1207. doi: 10.1093/annonc/mdv130. [DOI] [PubMed] [Google Scholar]
- 11.Skrondal A, Rabe-Hesketh S. Generalized Latent Variable Modeling. Multilevel, Longitudinal, and Structural Equation Models. ed 1. New York, NY: Chapman and Hall/CRC; 2004. [Google Scholar]
- 12. Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28:2796–2801. doi: 10.1200/JCO.2009.25.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robins J. A new approach to causal inference in mortality studies with a sustained exposure period-application to control of the healthy worker survivor effect. Math Model. 1986;7:1393–1512. [Google Scholar]
- 14. Zheng C, Liu L. Quantifying direct and indirect effect for longitudinal mediator and survival outcome using joint modeling approach. Biometrics. 2022;78:1233–1243. doi: 10.1111/biom.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.