Abstract
Background:
Recent studies have shown that approximately 20% of patients have 4–5 year PFS on BRAF/MEK inhibitors. The long-term safety and efficacy in these patients with more durable responses have not been studied.
Methods:
This retrospective multicenter cohort study assessed response, progression, and adverse events in patients from 8 institutions in 4 countries with > 4-year PFS following BRAF/MEK inhibitors.
Results:
Among 146 patients, 112 (76.7%) remained progression-free at median follow-up of 7.8 years from treatment start; 131 (89.7%) were alive. Among progressors (n=34), 21 (62%) were on treatment at progression. Among those who discontinued treatment for reasons other than progression (toxicity, preference, etc.) (n=68, with median 49 months treatment duration), 13 (19%) progressed (median 15.3 months from treatment cessation to progression). Surgery or radiation for single-organ progression resulted in durable benefit in 11 of 22 patients (50%). Subsequent systemic therapy included immune therapy (24% responded) and BRAF/MEK rechallenge (56% responded). 13 (8.9%) patients had ongoing toxicities at last follow-up, 10 (77%) of which remained on active treatment; all cardiac adverse events had resolved (n=9). 24 (16.4%) patients developed any new primary cancer, and 28 (19%) patients experienced other major health events.
Conclusions:
Over 75% of patients with 4-year PFS from BRAF/MEK inhibitors had continued durable antitumor responses after nearly 8 year median follow-up, with similar results in patients who discontinued therapy for reasons other than progression. Long-term toxicities were uncommon and low-grade. These findings highlight the often-favorable outcomes in patients with extended benefit from BRAF/MEK inhibitors.
Keywords: melanoma, targeted therapy, BRAF/MEK inhibitors, adverse events
Introduction
Greater understanding of the role of oncogenic mutations and immune dysfunction in melanoma has led to the development of molecular targeted therapy and immunotherapy, revolutionizing the treatment of advanced melanoma over the past decade. BRAF/MEK inhibitors selectively target mutations in the signaling protein BRAF, blocking the mutated BRAF protein and its downstream signaling partner MEK. Historically, metastatic melanoma was nearly universally fatal (1); recently the five-year survival was 50% with combination immunotherapy (2) and approximately 30–40% with combination BRAF/MEK targeted therapy (3) or single-agent PD-1 blockade (4).
Conventional dogma suggested that most patients ultimately progress on targeted therapy, however, emerging data have shown that a subset of responses are long-lasting, particularly in patients with low-volume disease and normal lactate dehydrogenase (5, 6). Specifically, the progression-free survival (PFS) rate at 4–5 years for patients treated with BRAF/MEK inhibitors is approximately 15–21% (3, 7). However, it is not clear whether these patients have delayed recurrences beyond this timeframe. Further, BRAF inhibitors paradoxically activate the oncogenic MAPK signaling pathway in non-BRAF mutated cells, thus leading to a theoretical risk of secondary cancers and perhaps other pathologic processes resulting from long-term pathway modulation (8). The rate of secondary malignancies, delayed organ dysfunction (e.g. cardiomyopathy, retinopathy), chronic toxicities (e.g. pyrexia, gastrointestinal symptoms), or other adverse health outcomes has not been well-described. To address this, we identified a cohort of patients with at least 4-year PFS following single-agent BRAF inhibition or combination BRAF/MEK inhibitor therapy to assess the durability of response, pattern of relapses, and spectrum of adverse events.
Methods
Following IRB/ethics committee approval from each institution, retrospective deidentified data were collected from 8 participating institutions. Patients with advanced/unresectable melanoma treated with BRAF inhibitor monotherapy or combined BRAF + MEK inhibitors (vemurafenib +/− cobimetinib, dabrafenib +/− trametinib, and encorafenib +/− binimetinib) and those who experienced at least 4 years of PFS without additional therapy were included. Those who transitioned from one BRAF +/− MEK inhibitor regimen to another in the absence of progression (e.g. for toxicity reasons) were included. Patients who discontinued therapy prior to 4 years in the absence of progression were included if they experienced a total PFS of at least 4 years. Patients who received concomitant or sequential immunotherapy prior to 4 years were excluded (immunotherapy given after 4 years was captured as a subsequent therapy). Patients without evaluable follow-up were also excluded.
Patient demographics, tumor characteristics (melanoma subtype, mutational status, AJCC 8th Edition stage), and treatment details prior to BRAF/MEK inhibitor therapy were collected. We evaluated treatment dose, duration, reason for discontinuation, and best investigator-assessed response to therapy per RECIST 1.1 criteria (9) as reflected in clinical notes and scan reports to determine objective response rate (complete + partial response) and clinical benefit rate (objective responses + stable disease). Staging intervals were per institutional practice (every 2–3 months). In addition to assessing toxicities secondary to BRAF/MEK inhibitor therapy (type, grade (Common Terminology Criteria for Adverse Events, version 5.0), duration, and treatment) (10), we recorded any new cancer diagnoses and other health events or diagnosis of new medical conditions following therapy. Progression details (date, anatomic location, subsequent treatment, and outcomes) following BRAF/MEK inhibition were also analyzed. Descriptive statistics were used to analyze categorical and continuous variables. Kaplan-Meier curves assessed survival (using GraphPad, version 9.5.1). Characteristics of patients who progressed vs. those without progression were compared using T tests and chi-square testing.
Results
Patient and disease characteristics
Among 146 patients, 57.5% (n=84) were male with a median age of 55 years (range 18–86) (Table 1). Among patients with known race data (n=128, 87.7%), all patients identified as white. The most common primary site was cutaneous (82.2%, n=120). At treatment initiation, 94.5% (n=138) had stage IV disease; 38.4% (n=56) had M1c and 15.8% (n=23) had M1d disease. 85.6% (n=125) had BRAFV600E mutation, 6.2% (n=9) had BRAFV600K mutation, and 8.2% (n=12) had unspecified BRAFV600 mutation. 47 (32.2%) patients received systemic treatment prior to BRAF/MEK inhibitor therapy. 71.9% (n=105) received BRAF/MEK inhibitor combination therapy, most commonly dabrafenib/trametinib (63%, n=92). The median follow-up duration was 93.8 months; 112 patients (76.7%) remained on therapy for at least 4 years, and 68 patients (46.6%) remained on therapy at the time of last follow-up (with median treatment duration 75.5 months). In the remaining 78 patients, treatment was discontinued for toxicity (17%, n=25, Supplementary Table 1), patient/physician preference (26%, n=38), progression (7%, n=10), or other reasons (e.g. treatment unrelated adverse events, alternative treatment was given) (3%, n=5). At the time of last follow-up (median 93.8 months from treatment start), 131 (89.7%) patients were alive; 97 (66.4%) had no evidence of disease, 34 (23.3%) were alive with active disease, 12 (8.2%) died of melanoma, and 3 (2.1%) died of other causes. The median time from date of last staging to date of last follow-up was 29.4 days.
Table 1:
Patient and Disease Characteristics
| Number of patients (%) | |
|---|---|
| General Demographics | |
| Gender (male) | 84 (57.5) |
| Age (years; median, range) | 55, 18–85 |
| Race | |
| White | 128 (87.7%) |
| Unknown | 18 (12.3%) |
| Primary Site | |
| Cutaneous | 120 (82.2) |
| Acral | 3 (2.1) |
| Unknown primary | 23 (15.8) |
| BRAF Mutation Status | |
| BRAF V600E | 125 (85.6) |
| BRAF V600K | 9 (6.2) |
| BRAF (not specified) | 12 (8.2) |
| Other Mutation Status | 10 (6.8) |
| Stage (AJCC 8th Edition) | |
| III | 8 (5.5) |
| IV | 138 (94.5) |
| M Status | |
| M1a | 29 (19.9) |
| M1b | 30 (20.5) |
| M1c | 56 (38.4) |
| M1d | 23 (15.8) |
| Metastases | |
| Liver | 24 (16.4) |
| Bone | 24 (16.4) |
| Number of Organs Involved with Metastases | |
| 0–1 | 49 (33.6) |
| 2–3 | 78 (53.4) |
| 4+ | 11 (7.5) |
| ECOG | |
| 0 | 107 (73.3) |
| 1 | 35 (24.0) |
| 2 | 2 (1.4) |
| 3 | 1 (0.7) |
| Unknown | 1 (0.7) |
| LDH | |
| Normal | 90 (61.6) |
| 1–2x upper limit of normal | 24 (16.4) |
| >2x upper limit of normal | 5 (3.4) |
| unknown | 27 (18.5) |
| Treatment | |
| Systemic treatment prior to BRAF/MEK inhibitory therapy | 47 (32.2) |
| Pembrolizumab or nivolumab | 9 (6.2) |
| Ipilimumab | 10 (6.8) |
| Ipilimumab + nivolumab | 12 (8.2) |
| Interferon | 11 (7.5) |
| Chemotherapy | 9 (6.2) |
| Other (IL-2, Ebacadostat, Urelumab, Tremelilumab) | 12 (8.2) |
| BRAF monotherapy | 41 (28.1) |
| BRAF/MEK combination therapy | 105 (71.9) |
| BRAF/MEK Inhibitor Drug | |
| Dabrafenib | 6 (4.1) |
| Dabrafenib/trametinib | 92 (63.0) |
| Encorafenib/binimetinib | 9 (6.2) |
| Vemurafenib | 33 (22.6) |
| Vemurafenib/cobimetinib | 6 (4.1) |
| Duration on Therapy (months; median) | 62.0 |
| Reasons for Stopping Therapy (% of those who stopped therapy) | |
| Ongoing | 68 (46.6) |
| Progression | 10 (6.8) |
| Toxicity* | 25 (17.1) |
| Completion (physician/patient preference) | 38 (26.0) |
| Other** | 5 (3.4) |
| Best Investigator Assessed RECIST 1.1 Response to Therapy | |
| PD | 0 |
| SD | 4 (2.7) |
| PR | 38 (26.0) |
| CR | 104 (71.2) |
| Duration from Treatment Start to Date of Best Response (months; median) | 7.2 |
| Follow-up | |
| Follow-up Duration* (months, median) | 93.8 |
| Status at Last Follow-up | |
| Alive with no evidence of disease | 97 (66.4) |
| Alive with evidence of disease | 34 (23.3) |
| Died from melanoma | 12 (8.2) |
| Died from other cause | 3 (2.1) |
See Supplemental
from treatment start
Progression after BRAF/MEK Inhibitor Therapy
Among the 34 patients (23.3%) who experienced disease progression after initiating BRAF/MEK inhibitor therapy, the median PFS was 67.5 months (Figure 1); 21 (62%) were ECOG 0 and 18 (60% with known values) had normal LDH levels at the time of progression (Table 2). There were no statistically significant associations between progression status and baseline metastatic stage (p=0.3), number of organs involved (p=0.9), ECOG performance status (p=0.3), LDH level (p=0.7), and BRAF monotherapy vs BRAF/MEK combination therapy (p=0.5). Of 104 patients who had complete response as best response, 23 (22%) ultimately progressed, compared with 11 of 42 (26%) patients with partial response or stable disease as best response. Similarly, among patients who stopped therapy before 4 years vs. continued beyond 4 years, there was similar incidence of progression (8 of 34 [24%] vs. 26 of 112 [23%], p=0.97).
Figure 1.
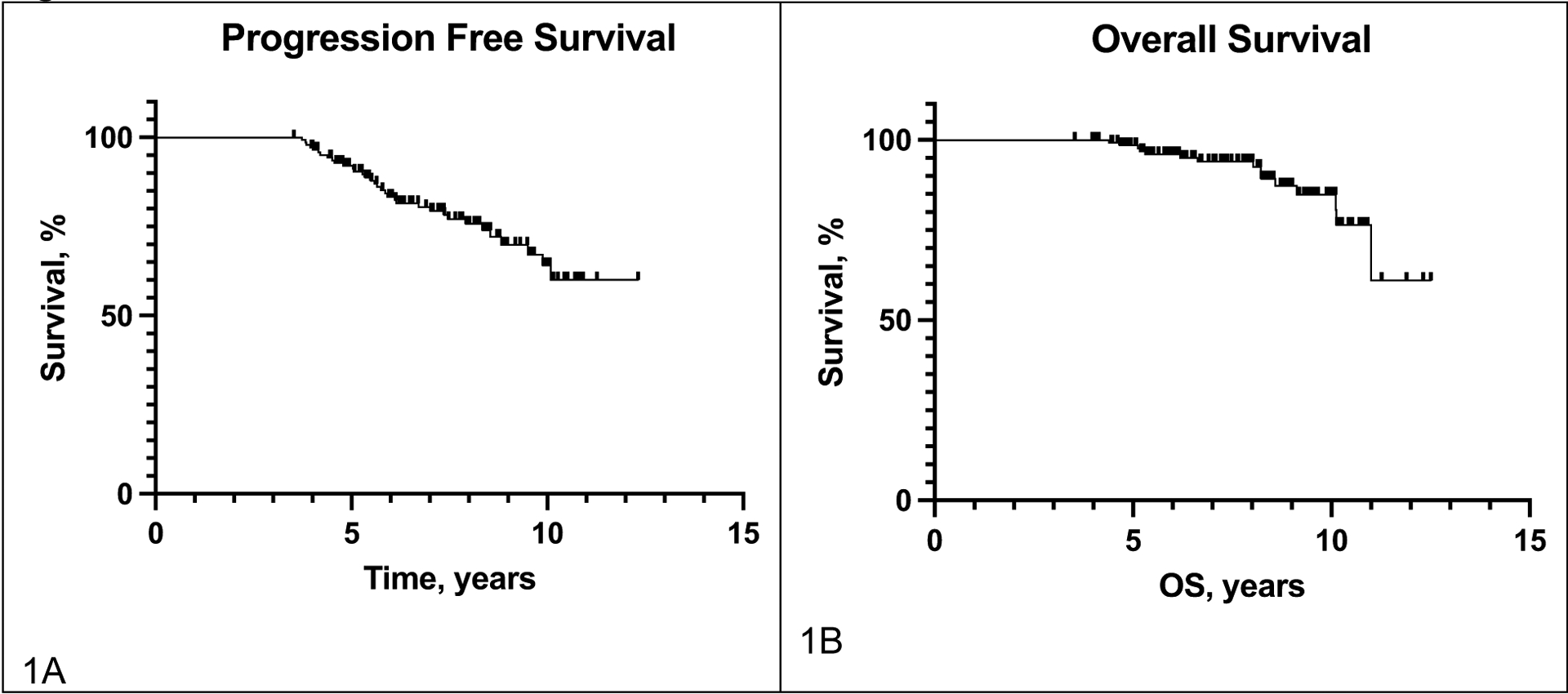
Survival
Table 2.
Progression
| n (%) | |
|---|---|
| Progression | |
| No | 112 (76.7) |
| Yes | 34 (23.3) |
| ECOG at Recurrence | |
| 0 | 21 (61.8) |
| 1 | 8 (23.5) |
| 2 | 3 (8.8) |
| 3 | 2 (5.9) |
| LDH at Recurrence | |
| Normal | 18 (52.9) |
| 1–2x upper limit of normal | 9 (26.5) |
| >2x upper limit of normal | 3 (8.8) |
| Unknown | 4 (11.8) |
| Sites of Progression | |
| 1 | 23 (67.6) |
| 2 | 5 (14.7) |
| 3+ | 6 (17.6) |
| Location of Progression ** | |
| Adrenal | 2 (5.9) |
| Bone | 4 (11.8) |
| Brain | 18 (52.9) |
| Cutaneous/subcutaneous (location of primary) | 6 (17.6) |
| Liver | 4 (11.8) |
| Lung | 7 (20.6) |
| Lymph nodes | 10 (29.4) |
| Other GI (pancreas, peritoneum, small bowel) | 3 (8.8) |
| Treatment of Progression after BRAF/MEK Inhibitor Therapy | |
| Surgery | 10 (29.4) |
| Radiotherapy | 15 (44.1) |
| Systemic Treatment Received for Progression *** | 24 (70.6) |
| Anti-PD-1 monotherapy | 11 (45.8) |
| Response rate | 36.4% |
| Clinical benefit | 54.5% |
| Anti-PD-1/CTLA-4 | 6 (25.0) |
| Response rate | 0.0% |
| Clinical benefit | 33.3% |
| BRAF/MEK inhibitor | 9 (37.5) |
| Response rate | 55.6% |
| Clinical benefit | 77.8% |
| Best Investigator Assessed RECIST 1.1 Response to Therapy | |
| SD | 6 (25.0) |
| PR | 4 (16.7) |
| CR | 3 (12.5) |
| PD | 9 (37.5) |
| NED (no further progression) | 2 (8.3) |
| Subsequent Progression Following Treatment | 19 (55.9) |
Adverse events (not treatment related), other treatment became available in extended access program
10 patients had recurrence at more than one site
One patient received PD-1 monotherapy, anti-PD-1/CTLA-4, and BRAF/MEK inhibitor therapy, clinical benefit includes RECIST stable disease in addition to partial and complete response.
Isolated progression (in one organ) occurred in 23 (68%) patients, while 11 (32%) experienced disseminated progression (5 with 2 sites of progression, 6 with 3+ sites of progression). The most common locations of recurrence were the brain (n=18, 53%), lymph nodes (n=9, 27%), lung (n=7, 21%), and subcutaneous tissue (n=5, 15%). Twenty-one patients (62%) were on treatment at progression while 13 (38%) had previously discontinued treatment due to toxicity or patient preference.
As noted, 68 patients discontinued treatment for reasons other than progression (toxicity, physician/patient preference) at a median treatment duration of 49.0 months and had follow-up of 54.6 months beyond treatment cessation. Of these, 13 (19%) ultimately progressed, with a median duration from treatment cessation to progression was 15.3 months. Among those who discontinued therapy due to toxicity, 16% progressed, while 24% of those who discontinued due to other reasons (completion, physician/patient preference, non-treatment related adverse events, insurance, etc.) progressed. As noted, of the remaining patients treated until last follow-up or progression (n=78), 21 progressed (26%).
Patients who progressed had a median follow-up of 53 months post-progression. During this post-progression period, 10 (29%) patients underwent surgery, 15 (44%) received radiotherapy, and 24 (71%) received systemic treatment. Of 10 patients who underwent surgery to an area of isolated progression, 7 experienced subsequent progression. Twelve of 15 patients who received radiotherapy for progression received it for definitive intent, among which 5 (42%) subsequently progressed. Among those receiving systemic treatment, 11 (46%) received anti-PD-1 with a 36% response rate and 55% with clinical benefit, 6 (25%) received combined anti-PD-1/CTLA-4 (0% response rate, 33% with clinical benefit), and 9 (38%) received BRAF/MEK inhibitor re-treatment (56% response rate, 78% with clinical benefit). Among all patients with progression, 19 (56%) experienced a second episode of progression. 52% of patients with isolated progression (1 organ) experienced subsequent progression whereas 64% of those with systemic progression (2+ organs) experienced subsequent progression. Among 18 patients with CNS progression, 12 (67%) had isolated intra-cranial metastases and six (33%) had at least one other site of metastasis. Three (17%) had surgical resections and 13 (72%) received radiotherapy. 11 received systemic therapy (61%), eight (73%) of which ultimately progressed. Four (22%) patients died of melanoma during evaluable follow up.
Toxicities
Among 120 (82.2%) patients with on-treatment toxicities, 77 (64.2%) were grade 1, 53 (44.2%) were grade 2, and 29 (24.2%) were grade 3+ (Table 3). Dose adjustments due to toxicity were required in 73 (60.8%) patients, with intermittent dosing (n=26, 21.7%) and dose reduction (n=35, 29.2%) as the most common adjustments. Of the 35 patients who required dose reductions due to toxicities, 25 (71.4%) required permanent dose reduction, 8 (22.9%) required transient dose reduction, and the status was unknown for 2 patients (5.7%). Among those requiring long-term dose reduction, the average stable, long-term dose was 62.3% of the initial, full dose, with a range from 33.3% to 83.3%. Seventeen patients switched regimens secondary to on-treatment toxicities (14.2%). The most prevalent toxicity types were fever/chills (n=50, 41.7%), gastrointestinal symptoms (nausea, vomiting, and diarrhea; n=45, 37.5%), and cutaneous manifestations (rash, pruritis, photosensitivity; n=44, 36.7%). Cardiac toxicities were reported in 9 (7.5%) patients with a median onset of 21.6 months (range 1.4–77.4 months) from treatment start (Supplementary Table 2); 7 (78%) were on dabrafenib/trametinib and 2 (22%) on vemurafenib. Six cardiac events were grade 1–2 (67%), 2 (22%) were grade 3 (reduced ejection fraction in both), and 1 (11%) was unknown (ventricular hypertrophy). The most common types were decrease in ejection fraction (n=5, 56%) and prolonged QTc interval (n=2, 22%), with single reports of atrial fibrillation, sinus tachycardia, and ventricular hypertrophy. Three patients (33%) discontinued treatment due to cardiac toxicity, and all cardiac toxicities resolved by last follow-up. Ocular toxicities were reported in 22 (18.3%) patients with a median onset of 4.0 months from treatment start (range 11 days-50.5 months) (Supplementary Table 3). Six patients (27%) were on BRAF inhibitor monotherapy while 16 (73%) were on BRAF/MEK inhibitor combination therapy. Ten (46%) were grade 1, 7 (32%) were grade 2, and 4 (18%) were grade 3 (retinal detachment, uveitis, and iritis). Uveitis was reported in 7 patients (32%), followed by conjunctivitis (n=3, 14%), macular edema (n=3, 14%), retinal detachment (n=2, 9%), retinopathy (n=2, 9%), and blepharitis, blurred vision, and keratitis (n=1 each). All ocular toxicities resolved by last follow-up except for one patient who remained on Vemurafenib with persistent grade 2 chronic anterior uveitis.
Table 3.
Toxicities
| Short-term Toxicities Secondary to BRAF/MEK Inhibitor Therapy | n (%) |
|---|---|
| Short-term Toxicities | 120 (82.2) |
| Grade | n (% of those with short-term toxicities) |
| 1 | 77 (64.2) |
| 2 | 53 (44.2) |
| 3+ | 29 (24.2) |
| Dose Adjustments Secondary to Toxicities | 73 (60.8) |
| Ceased | 3 (2.5) |
| Dose reduction | 23 (19.2) |
| Intermittent dosing | 26 (21.7) |
| Treatment break | 9 (7.5) |
| Treatment break and dose reduction | 12 (10.0) |
| Switched Regimens Secondary to Toxicities | 17 (14.2) |
| Short-term Toxicity Type * | |
| Fever/chills | 50 (41.7) |
| Fatigue | 21 (17.5) |
| Arthritis/arthralgias | 18 (15.0) |
| GI (nausea, vomiting, diarrhea) | 45 (37.5) |
| Cutaneous (rash, pruritis, photosensitivity) | 44 (36.7) |
| Cardiac | 9 (7.5) |
| Ocular | 22 (18.3) |
| Ongoing Toxicities at Last Follow-up | 13 (10.8) |
| Grade | n (% of those with ongoing toxicities) |
| 1 | 8 (61.5) |
| 2 | 5 (38.5) |
| 3 | 1 (7.7) |
| Unknown | 2 (15.4) |
| Ongoing Toxicity Type ** | |
| Anterior uveitis | 1 (7.7) |
| Arthralgias/myalgias | 7 (53.8) |
| Cutaneous | 4 (30.8) |
| Dysgeusia/xerostomia | 1 (7.7) |
| Fatigue | 4 (30.8) |
| Fever | 1 (7.7) |
| Pneumonitis | 1 (7.7) |
| GI upset | 2 (15.4) |
| Neuropathy | 2 (15.4) |
| Neutropenia | 1 (7.7) |
| Intermittent weight gain | 1 (7.7) |
>5% prevalence
8 patients had >/= 2 distinct ongoing toxicities
Erythema nodosum (n=1), erythema (n=1), toe lesion (n=1), rash (n=1)
At the time of last follow-up (median 93.8 months), 13 patients (8.9%) were experiencing ongoing toxicities, 10 (77%) of which were on ongoing BRAF/MEK inhibitors. Among patients with these chronic events, 8 (62%) were grade 1, 5 (39%) were grade 2, and 1 (8%) was grade 3. The most common persistent toxicities overall were arthralgias/myalgias (n=7, 54%), cutaneous (n=4, 31%), and fatigue (n=4, 31%). Conversely, 58 patients on active therapy (85%) had no ongoing toxicities noted. Among the 3 patients with toxicities persisting after therapy discontinuation, one had persistent erythema nodosum (unknown grade), one had dyspepsia (grade 2), and another had arthralgias (grade 1) and lower extremity neuropathy (grade 3).
New Cancers and Health Events
A new primary cancer was diagnosed in 24 (16.4%) patients during or at some point after stopping BRAF/MEK inhibitor therapy, with a median duration of 50.7 months from treatment start to new cancer diagnosis (Table 4). Among these patients, 19 (79%) had localized cancer, 3 (13%) had regional disease, 1 (4%) had metastatic disease, and 1 (4%) had unknown stage. The most common type of new cancer was non-melanoma skin cancer (n=17, 71%), followed by new primary melanoma (n=2, 8%), and head and neck squamous cell carcinoma (n=2, 8%). Other major health events (not obviously related to BRAF/MEK inhibition) occurred in 28 patients (19.2%) during the follow-up period, including development of type 2 diabetes, ST-elevation myocardial infarction, atrial fibrillation, Crohn’s disease, epilepsy, and pulmonary embolism, although no individual event occurred in more than three patients (Supplementary Table 4).
Table 4.
New Cancers following BRAF/MEK Inhibitor Therapy
| New Cancer Diagnosis Following BRAF/MEK Inhibitor Therapy (n=24) | n (% of those with new cancer diagnoses) |
|---|---|
| Duration from Treatment Start to New Cancer Diagnosis (median; months) | 51.1 |
| Stage | |
| Localized | 19 (79.2) |
| Regional | 3 (12.5) |
| Metastatic | 1 (4.2) |
| Unknown | 1 (4.2) |
| Type | |
| Anal SCC | 1 (4.2) |
| Breast | 1 (4.2) |
| Non-melanoma skin cancer | 17 (70.8) |
| Melanoma (new primary) | 2 (8.3) |
| Lymphoma | 1 (4.2) |
| Esophageal adenocarcinoma | 1 (4.2) |
| Head and neck cancer* | 2 (8.3) |
| Lung, adenocarcinoma | 1 (4.2) |
| Prostate | 1 (4.2) |
Tonsillar (n=1), tongue (n=1)
Discussion
Despite the promising results of molecular targeted therapy within a subset of patients who have prolonged responses (6), the long-term efficacy of BRAF/MEK inhibitors are not well characterized. We found that 77% of those with at least 4-year PFS continued to experience durable antitumor responses with a median follow-up of 7.8 years from treatment start. Further, most patients who discontinued therapy for reasons other than progression continued to benefit. Although many patients had toxicities requiring dose-adjustment, ongoing symptoms were uncommon at last follow-up. Finally, although a sizable minority had additional health conditions or new skin cancer diagnoses during their follow-up period (potentially due to age or shared environmental risk factors), there was no dominant safety signal observed.
Most patients in our study had low volume disease (76% with <3 organs involved), normal LDH (61%) at baseline, and normal ECOG performance status (73%), consistent with prior studies showing a favorable long-term outcome to BRAF/MEK inhibition in similar patients (3, 11). While lower disease burden may independently facilitate active anti-tumor immune surveillance, the durable antitumor responses may be attributable to the immunomodulatory effects on melanoma cells and the tumor microenvironment induced by BRAF/MEK inhibitors (changes in antigen display and expression of MHC, decreased regulatory T cells, and increased activity of CD4-CD8+ T cells) (12). These findings reinforce the notion that baseline patient characteristics may be useful for guiding initial treatment choices. However, many long-term survivors also had at least some unfavorable characteristics (>50% with stage IV M1c/d), showing that excellent outcomes can occur in these populations, and suggesting that long-term responders may experience continued benefit regardless of their baseline characteristics. The lack of observed statistically significant associations between progression status and these features likely reflects the unique characteristics of the selected cohort of long-term survivors with favorable outcomes and should not be interpreted as a general trend.
Notably, most patients with prolonged responses (4+ years) continued to experience still more extended benefit, although 23% of long-term survivors ultimately experienced delayed progression. Nearly two-thirds who progressed were on treatment at the time of progression (62%), though the remainder had ceased treatment prior to progression due to toxicity or physician/patient preference, raising the question whether patients may ultimately safely discontinue therapy. A recent study suggested that 38% of patients who discontinued BRAF/MEK inhibitors prior to progression (after a median 29.6 months) ultimately progressed with a median time to progression of 4.7 months (13). We found a lower rate of progression after discontinuation (19%) and a longer latency time to progression after stopping (15 months). These improved outcomes could reflect a selection effect of patients who already had experienced a prolonged PFS benefit and extended treatment (median treatment duration of 49 months in our study), compared with prior studies. These findings suggest that timing of treatment discontinuation may be an important factor in determining risk of disease progression, where later discontinuation (beyond 4–5 years) may be a reasonable approach. Although most patients did continue to benefit, the nearly 1 in 5 risk of subsequent progression highlights the need for individualized treatment decisions based on patient-specific factors, and close monitoring of patients who have ceased treatment.
Notably, post-progression outcomes remained excellent in some patients. Surgical resection and radiotherapy were effective in a subset of patients with single-site progression, though patients remained at high risk for future progression. Immune checkpoint inhibitors also produced responses in some cases, as did BRAF/MEK inhibitor retreatment (3). Our findings reinforce evidence that rechallenge with BRAF/MEK inhibitor therapy have substantial efficacy, now including in this population with prolonged PFS (13, 14). However, most patients eventually experienced subsequent progression, suggesting that even delayed progressors may have a guarded prognosis. Thus, better therapies remain a priority even in this population.
Safety
Adverse events for BRAF/MEK inhibitor therapy were generally manageable and consistent with previous reports (6). Over half of patients with long-term PFS had required dose adjustment secondary to early-onset toxicities and the most common transient toxicities were fever/chills, gastrointestinal, and cutaneous. Cardiac toxicities, most commonly reduced ejection fraction and prolonged QTc interval, were reported in 6% of patients, but all resolved by last follow-up. Similarly, ocular adverse events occurred in 15% but resolved prior to last follow-up with one exception. These findings suggest cardiac and ocular toxicities may be delayed, are overall mild, and generally reversible.
While acute toxicities have been well elucidated, long-term adverse events have not been well defined (15). 11% of patients with early-onset toxicities and 9% of the original cohort experienced ongoing toxicities at the time of last follow-up, although most (77%) were still on therapy. Only one patient had grade 3 toxicity (neuropathy) although a significant portion remained grade 2+ (46%). There was a wide spectrum of long-term toxicities, the most commonly arthralgias/myalgias, cutaneous adverse effects, and fatigue. No new safety signals were observed after long-term follow-up. These finding suggest that while chronic adverse events are not common, they produce morbidity in a subset of patients. Dissimilar to anti-PD-1, however, these usually resolve with therapy discontinuation (16, 17).
New Cancers and Other Health Events
Twenty-four (16.4%) patients received a new cancer diagnosis at some point after stopping BRAF/MEK inhibitors. Most were localized, with only 1 patient each having regional or metastatic disease and were most often non-melanoma skin cancer (n=17, 70.8%), new primary melanoma (n=2) and head and neck cancer (n=2). Secondary cutaneous malignancies, are generally associated with paradoxical MAPK activation caused by selective BRAF inhibition, though the relevance is less clear when concurrent MEK inhibition is given (8). Prolonged MAPK modulation could theoretically affect numerous pathophysiologic mechanisms outside of cancer; other health effects occurred in 18.5% of patients with long-term survival from BRAF/MEK inhibition. While some patients developed type 2 diabetes mellitus, myocardial infarctions, and cirrhosis, no obvious and dominant signal emerged. However, larger studies would be needed to identify more modest effects of prolonged MAPK modulation on other disease processes.
Conclusions
Limitations of this study include the retrospective nature and limited sample size. While all progression events were attributed to the initial melanoma, not all were confirmed through biopsies, and thus, it is possible some arose from new primaries. A prospective design with more prolonged follow-up on a larger population, including an assessment of ethnicity/race, with systematic monitoring could further characterize the spectrum of long-term outcomes, toxicities, cancers, and health events.
Our study reinforces that long-term survival occurs in a subset of patients, and most patients (>75%) who were progression-free at four years remained so at a median follow-up of nearly eight years. Further, many patients who electively discontinued therapy continued to benefit, thus providing some qualified support for this practice. However, approximately one quarter of patients did progress, and BRAF/MEK rechallenge, immunotherapy, and surgery/radiation for isolated recurrences showed efficacy in this context. It remains unclear who can safely discontinue therapy, therefore, and further research is needed. Furthermore, long-term treatment with BRAF/MEK inhibition was generally well-tolerated, with no new safety signals identified. Overall, the study underscores the need for careful long-term monitoring of patients undergoing BRAF/MEK inhibition, and proactive measures to address any long-term adverse effects that may arise.
Supplementary Material
Highlights.
Durable responses to BRAF/MEK led to favorable long-term outcomes.
>75% with 4-year PFS had continued responses after ~8 year median follow-up.
Similar results in those who stopped therapy for reasons other than progression.
Many had subsequent extended benefit from surgery, radiation, or systemic therapy.
No new safety signals were identified.
Funding information:
RSG receives funding from the SCRIPS Foundation, Burroughs Wellcome Fund. GVL is supported by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant and the University of Sydney Medical Foundation. AMM is supported by an NHMRC Investigator Grant, Nicholas and Helen Moore and Melanoma Institute Australia. LW is supported by a Future Health Research and Innovation Fund/Raine Medical Research Foundation fellowship. DBJ receives funding from the NCI R01CA227481. Susan and Luke Simons Directorship for Melanoma, the James C. Bradford Melanoma Fund, the Van Stephenson Melanoma Fund.
Declaration of interests
MSC has served on advisory boards or as a consultant for Amgen, BMS, Eisai, Ideaya, MSD, Nektar, Novartis, Oncosec, Pierre-Fabre, Qbiotics, Regeneron, Roche, Merck and Sanofi, and received honoraria from BMS, MSD, and Novartis. AMM has served on advisory boards for BMS, MSD, Novartis, Roche, Pierre-Fabre and QBiotics. EL served as consultant and/or has received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre-Fabre, Sanofi, Sunpharma, Takeda and travel support from Bristol-Myers Squibb, Pierre- Fabre, Sunpharma and Novartis, outside the submitted work. RJS has served on advisory boards or as a consultant for BMS, Marengo, Merck, Novartis, Pfizer, and Replimune. GVL is consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA, Merck Sharpe & Dohme, Novartis, OncoSec, PHMR Ltd, Pierre Fabre, Provectus, Qbiotics, Regeneron. DS has served on advisory boards for BMS, Immunocore, MSD, Neracare, Novartis, Pfizer, Philogen, Pierre Fabre, and Sanofi/Regeneron. LW has served on advisory boards for BMS, MSD and Novartis. DBJ has served on advisory boards or as a consultant for BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, Targovax, and Teiko, has received research funding from BMS and Incyte, and has patents pending for use of MHC-II as a biomarker for immune checkpoint inhibitor response, and abatacept as treatment for immune-related adverse events. All remaining authors have declared no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate: Institutional Review Board approval was obtained from each participating institution. The study was performed in accordance with the Declaration of Helsinki.
CRediT authorship contribution statement
RSG conducted data collection, formal analysis, visualization, project administration, and manuscript writing (original draft, review & editing). DBJ conducted manuscript conceptualization, methodology, data interpretation, manuscript writing (review & editing), and project supervision. All other authors contributed to data collection and manuscript writing (review & editing).
Data availability:
The datasets analyzed are not publicly available due to privacy and confidentiality concerns with patient sensitive information. Access to the datasets can only be granted through a formal request to the corresponding author and approval from the respective institutional review boards.
References:
- 1.Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–46. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med. 2019;381(7):626–36. [DOI] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med. 2019;381(7):626–36. [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Eroglu Z, Infante J, Patel S, Daud A, Johnson DB, et al. Long-Term Outcomes in Patients With BRAF V600–Mutant Metastatic Melanoma Who Received Dabrafenib Combined With Trametinib. Journal of Clinical Oncology. 2018;36(7):667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dummer R, Flaherty KT, Robert C, Arance A, Groot JWBd, Garbe C, et al. COLUMBUS 5-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib Plus Binimetinib Versus Vemurafenib or Encorafenib in Patients With BRAF V600–Mutant Melanoma. Journal of Clinical Oncology. 2022;40(36):4178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibney GT, Messina JL, Fedorenko IV, Sondak VK, Smalley KS. Paradoxical oncogenesis--the long-term effects of BRAF inhibition in melanoma. Nat Rev Clin Oncol. 2013;10(7):390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 10.Common terminology criteria for adverse events (CTCAE), version 5.0: US Department of Health and Human Resources; 2017. [Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf.
- 11.Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol. 2016;34(8):871–8. [DOI] [PubMed] [Google Scholar]
- 12.Ascierto PA, Dummer R. Immunological effects of BRAF+MEK inhibition. Oncoimmunology. 2018;7(9):e1468955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Ahmed T, Maurichi A, Di Guardo L, Stagno AM, Warburton L, et al. BRAF inhibitor cessation prior to disease progression in metastatic melanoma: Long-term outcomes. European Journal of Cancer. 2023;179:87–97. [DOI] [PubMed] [Google Scholar]
- 14.Valpione S, Carlino MS, Mangana J, Mooradian MJ, McArthur G, Schadendorf D, et al. Rechallenge with BRAF-directed treatment in metastatic melanoma: A multi-institutional retrospective study. Eur J Cancer. 2018;91:116–24. [DOI] [PubMed] [Google Scholar]
- 15.Garutti M, Bergnach M, Polesel J, Palmero L, Pizzichetta MA, Puglisi F. BRAF and MEK Inhibitors and Their Toxicities: A Meta-Analysis. Cancers. 2023;15(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrinely JR, Young AC, Quach H, Williams GR, Ye F, Fan R, et al. Survivorship in immune therapy: Assessing toxicities, body composition and health-related quality of life among long-term survivors treated with antibodies to programmed death-1 receptor and its ligand. European Journal of Cancer. 2020;135:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrinely JR Jr., Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021;7(5):744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed are not publicly available due to privacy and confidentiality concerns with patient sensitive information. Access to the datasets can only be granted through a formal request to the corresponding author and approval from the respective institutional review boards.


