Abstract
Background:
To systematically evaluate the efficacy and safety of subthreshold micropulse laser (SML) combined with anti-vascular endothelial growth factor (VEGF) drugs for the treatment of diabetic macular edema (DME).
Methods:
The randomized controlled trials on SML combined with anti-VEGF drugs for DME were retrieved from China National Knowledge Infrastructure, Wan Fang Data, VIP Data, Sino Med (China Biomedical Literature Database), PubMed, Web of Science, The Cochrane Library, and Embase by computer from inception to April 19, 2022. The observation group was treated with SML combined with anti-VEGF drugs, while the control group was treated with anti-VEGF agents alone or SML. And the references of the included literature were manually searched. The Meta-analysis was performed using Revman 5.4 and STATA SE 15.
Results:
This study finally included 15 randomized controlled trials involving 891 eyes for Meta-analysis. The results showed that there was no statistically significant difference between the 2 groups in best-corrected visual acuity at 1, 3, 6, 9, and 12 months after treatment. There was no statistical difference between the 2 groups in central macular thickness (CMT) at 1, 3, and 6 months after treatment (P > .05). CMT in the observation group was lower than that in the control group at 9 and 12 months (P < .05). There was no statistical difference between the 2 groups in total macular volume at 3, 6, 9, and 12 months in CMT (P > .05). The number of anti-VEGF drugs injections in the observation was lower than that in the control group (P < .05). The occurrence of complications between the 2 groups was not statistically significant difference (P > .05).
Conclusion:
SML in combination with anti-VEGF drugs in patients with DME are comparable in reducing the number of anti-VEGF drugs injections and CMT, thereby reducing the financial burden on patients. It does not differ in best-corrected visual acuity and total macular volume.
Keywords: anti-VEGF drugs, diabetic macular edema, meta-analysis, subthreshold micropulse laser
1. Introduction
The latest epidemiological results show that the prevalence of diabetes mellitus (DM) was as high as 11.2% in people over 18 years of age in China, exceeding the global level. And the incidence of diabetic complications in China remain the highest among the world.[1] It has been reported that 1 out of fifteen diabetic patients has diabetic macular edema (DME), which will have profound clinical and public health implications.[2] Diabetes mellitus can cause a variety of complications, including diabetic cataracts, neovascular glaucoma, diabetic optic neuropathy, diabetic retinopathy (DR), and so on. Among them, DR is the most common ocular microvascular complication and the leading cause of vision loss in working-age people.[2] DR includes 3 forms: non-proliferative diabetic retinopathy, proliferative diabetic retinopathy, and DME.[2] DME is also the most common cause of vision loss in patients with DM.[3] There are various clinical treatments for DME,[4] among which anti-vascular endothelial growth factor (VEGF) drugs remain the first-line treatment for DME.[5] Previous studies have shown that anti-VEGF drugs can improve clinical symptoms and signs in patients with DME. However, anti-VEGF drugs require frequent injections to maintain the therapeutic effect. That increases the financial burden on patients and the occurrence of complications, such as subconjunctival hemorrhage, infection, and cerebrovascular accidents.[6,7]
Although the conventional laser can reduce macular edema, it destroys the target tissue by thermal damage, resulting in dark vision loss, visual field defects, laser spot enlargement, and secondary choroidal neovascularization in some patients. With continuous technological advances, there has been an evolution from conventional laser to subthreshold micropulse laser (SML). Unlike conventional continuous lasers, SML is a new laser that consists of large repetitive pulse lasers. SML selectively acts on retinal pigment epithelium (RPE) cells to exert modulatory effects and reduce inflammatory responses and macular edema.[8,9] The subthreshold micropulse laser includes 4 types according to wavelength: 810 nm, 532 nm, 577 nm, and 670 nm.[8]
Recently, many scholars have used SML in combination with anti-VEGF drugs in the treatment of DME, but the results remain controversial.[10–13] Most of the results showed that the combination therapy can significantly improve patients visual acuity and reduce the number of drug injections, but some scholars came to the opposite conclusion.[10,14–24] And there is no relevant evidence-based medical literature to confirm effect of the combination therapy. Therefore, in this study, we conducted a meta-analysis by searching randomized controlled trials (RCTs) of SML combined with anti-VEGF drugs in the treatment of DME published in China and English. This study will offer more evidence to support the clinical efficacy of SML combined with anti-VEGF drugs for the treatment of DME.
2. Materials and methods
2.1. Search strategy
This research was registered at PROSPERO (https://www.crd.york.ac.uk/prospero/, registration number CRD42022359632). This systematic review and meta-analysis were conducted according to the PRISMA guidelines (http://prisma-statement.org/?AspxAutoDetectCookieSupport=1).
The source of China National Knowledge Infrastructure, Wan Fang Database, VIP Database, China Biomedical Literature Database, PubMed, Web of Science, The Cochrane Library, and Embase, was conducted by computer. The search period was from the establishment of the database to April 19,2022. The search language was limited to Chinese and English. Search terms include: “diabetic macular edema,” “randomized,” “subthreshold micropulse laser,” “bevacizumab,” “ranibizumab “, “conbercept,” “aflibercept,” “anti-VEGF drug.”
2.2. Inclusion criteria
Study type: only RCTs were included; Study subjects: age > 18 years, diagnosed with diabetes, regardless of gender, race, and nationality; Interventions: the observation group was treated with SML combined with anti-VEGF drugs, and the control group was treated with anti-VEGF drugs or SML alone; The reported outcome indicators included at least one of the following: best-corrected visual acuity (BCVA), central macular thickness (CMT), total macular volume (TMV), the number of anti-VEGF drug injections, and complications.
2.3. Exclusion criteria
Non-RCTs; Studies with nondiabetic macular edema; Interventions that do not meet the requirements, such as traditional laser combined with anti-VEGF drugs; Animal experiments, case reports, conference papers, reviews; Duplicate publications; Literature for which the full text is not available or for which the original data cannot be extracted.
2.4. Literature screening and data extraction
All retrieved literature was imported into Endnote X9, and 2 evaluators independently completed literature screening, data extraction, and literature quality assessment according to inclusion and exclusion criteria. If any disagreement existed, then 2 evaluators would negotiate to solve the problem. If there were still disagreements after negotiation, the third evaluator would solve the problem. The basic information included: year of publication, first author, number of cases, age, duration of diabetes, interventions (including observation and control groups), and outcome indicators.
2.5. Quality evaluation of the literature
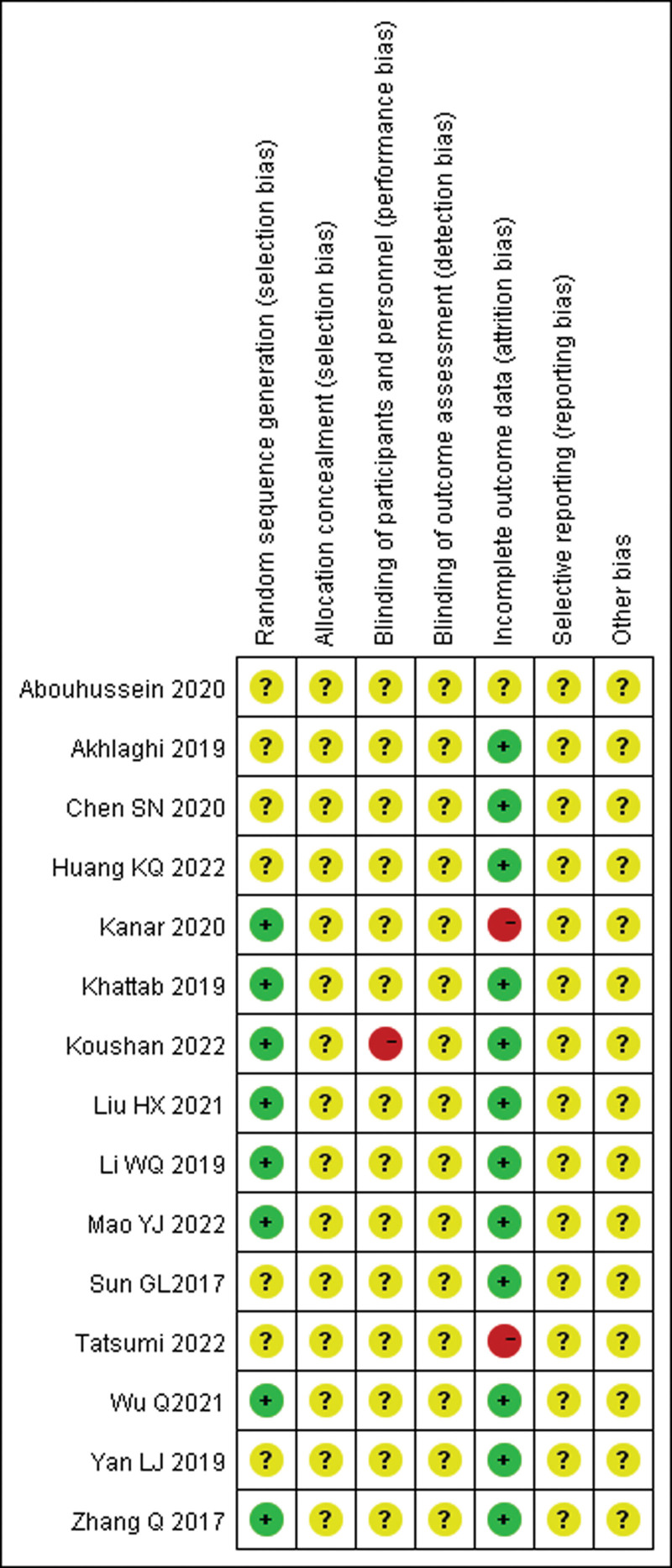
A quality evaluation of all included RCTs was completed independently by 2 researchers with reference to the Cochrane Handbook Risk of Bias Assessment Tool,[25] which including the generation of random sequences, allocation concealment, blinding of investigators and subjects, blinding of study outcome assessment, completeness of outcome data, selective reporting of outcome bias, and other biases. The results of the bias evaluation for each article were divided into 3 grades: “high risk of bias,” “low risk of bias,” and “unclear risk of bias.”
2.6. Statistical methods
Revman 5.4 (https://training.cochrane.org/online-learning/core-software/revman) and Stata SE15 software (https://bbs.pinggu.org/thread-7307635-1-1.html) provided by the Cochrane Collaboration Network were used to complete the Meta-analysis. The weighted mean difference and its 95% confidence interval (CI) were used as the effect analysis statistic for measurement data, while the relative risk ratio or the ratio of ratios and its 95% CI were used for count data. I2 test was used for the heterogeneity test, and I2 ≥ 50% indicated large homogeneity between studies, and Meta-analysis was performed using the random-effects mode. I2 < 50% suggests low homogeneity, and Meta-analysis was performed using the fixed-effect model. Differences were considered statistically significant at P < .05. Sensitivity analysis was performed using 1-by-1 exclusion. Publication bias analysis was performed by Egger test using STATA 15, and P < .05 showed the presence of publication bias.
3. Results
3.1. Literature screening process and results
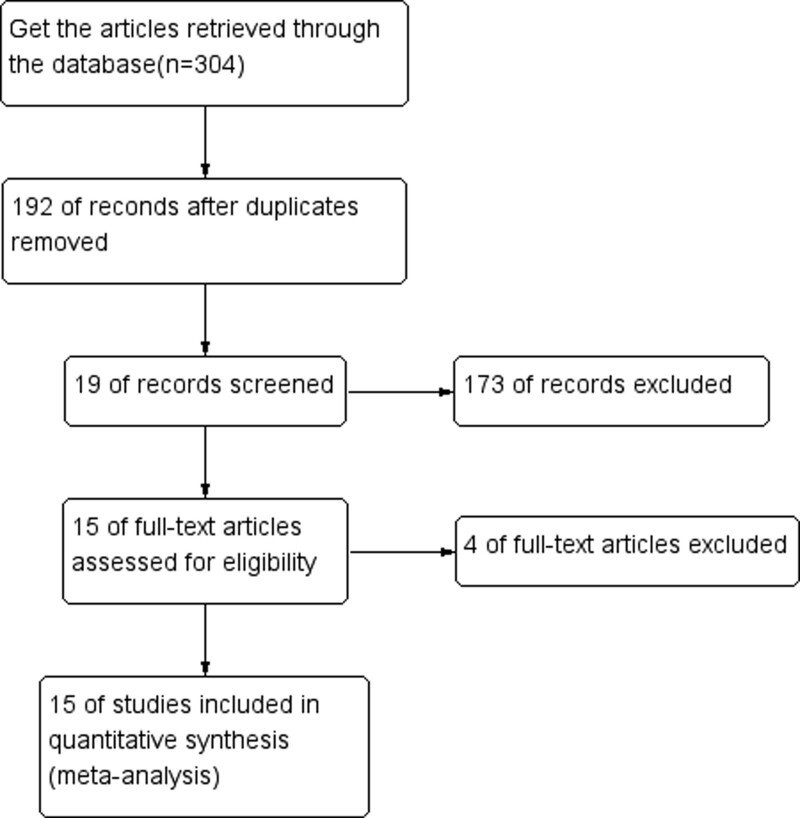
Based on the above search strategy, a total of 304 papers were collected. After eliminating duplicates, 192 papers were obtained. One hundred seventy-three papers were removed after reading the titles and abstracts of the papers. The remaining 19 papers were left after reading carefully for the full text. A total of 15 RCTs were finally included in this Meta-analysis, and all RCTs were single-center studies. (Fig. 1)
Figure 1.
Literature screening process.
3.2. Basic information for inclusion in the literature (Table 1)
Table 1.
Basic characteristics of RCTs.
| Author | Year | Number of cases T/C (eyes) | Age | Duration of diabetes | Interventions (T/C) | The course of treatment (mo) | Outcome |
|---|---|---|---|---|---|---|---|
| Sun GL[15] | 2017 | 15/15 | 58.27 ± 6.85 57.69 ± 6.39 |
NA | SML + ranibizumab/ Ranibizumab |
12–17 13–20 |
①②④⑤ |
| Li WQ[16] | 2019 | 36/32 | 57.2 ± 10.1 60.6 ± 12.3 |
NA | SML + conbercept/ Conbercept |
12 | ① ②③ ④ |
| Huang KQ[13] | 2022 | 26/26 | 62.31 ± 5.48 63.77 ± 5.37 |
62.81 ± 20.01/ 64.04 ± 20.44 (mo) |
SML + ranibizumab/ Ranibizumab |
9 | ① ② ④ |
| Mao YJ[18] | 2022 | 34/34 | 50.35 ± 10.14 51.47 ± 11.23 |
6.74 ± 2.03/ 6.80 ± 2.11 (yr) |
SML + aflibercept/ Aflibercept |
12 | ① ② ④⑤⑧ |
| Zhang Q[14] | 2021 | 35/35 | 56.0 ± 7.7/ 53.3 ± 9.1 |
13.5 ± 4.2/ 12.9 ± 4.1 (yr) |
SML + aflibercept/ Aflibercept |
12 | ① ② ④ |
| Chen SN[25] | 2020 | 30/28 | 56.17 ± 5.44/ 58.68 ± 5.92 |
NA | SML + ranibizumab/ Ranibizumab |
12 | ① ②③ ④⑦ |
| Yan LJ[21] | 2019 | 40/38 | 59.7 ± 4.5/ 56.9 ± 4.4 |
12.7 ± 3.3/ 13.4 ± 3.7 (yr) |
SML + ranibizumab/ Ranibizumab |
NA | ① ② ④ |
| Wu Q[20] | 2021 | 36/36 | 56.8 ± 10.2 56.3 ± 9.5 |
NA | SML + ranibizumab/ Ranibizumab |
9 | ① ②③ ④⑥ |
| Liu HX[26] | 2021 | 44/44 | 69.57 ± 5.31 68.16 ± 3.28 |
6.72 ± 1.31/ 6.72 ± 1.31 (yr) |
SML + ranibizumab/ SML |
1 | ① ②⑤⑧ |
| Akhlaghi[10] | 2019 | 42/42 | 60.86 ± 8.57 60.86 ± 8.57 |
NA | SML + bevacizumab/ Bevacizumab |
4 | ① ② |
| Tatsumi[9] | 2020 | 22/21 | NA | NA | SML + aflibercept/ Aflibercept |
24 | ① ② ④⑤ |
| Abouhussein[17] | 2020 | 20/20 | 60.4 ± 4.2/ 59.5 ± 4.3 |
NA | SML + aflibercept/ Aflibercept |
12 | ① ② ④⑤ |
| Khattab[11] | 2019 | 27/27 | 59.4 ± 4.3/ 55.7 ± 3.4 |
17.8 ± 3.4/ 17.4 ± 4.2 (yr) |
SML + aflibercept/ Aflibercept |
18 | ① ② ⑤ |
| Kanar[19] | 2019 | 28/28 | 63.43 ± 10.14/ 62.64 ± 9.03 |
18.76 ± 2.08/ 18.28 ± 2.24 (yr) |
SML + aflibercept/ Aflibercept |
12 | ① ②③ ④⑥ |
| Koushan[22] | 2022 | 15/15 | 59.8 ± 9.47/ 58.8 ± 9.28 |
NA | SML + aflibercept/ Aflibercept + sham SML |
12 | ① ②③ ④ |
①BCVA; ②CMT; ③TMV; ④The number of anti-VEGF drugs; ⑤Complications.
RCTs = randomized controlled trials, SML = subthreshold micropulse laser.
3.3. Evaluation of the quality of the literature on RCTs included in the literature (Fig. 2)
Figure 2.
The assessment of risk of bias for include RCTs. RCTs = randomized controlled trials.
3.4. Results of efficacy analysis
3.4.1. BCVA.
LogMAR visual acuity and ETDRS visual acuity were included and analyzed separately.
3.4.1.1. LogMAR visual acuity
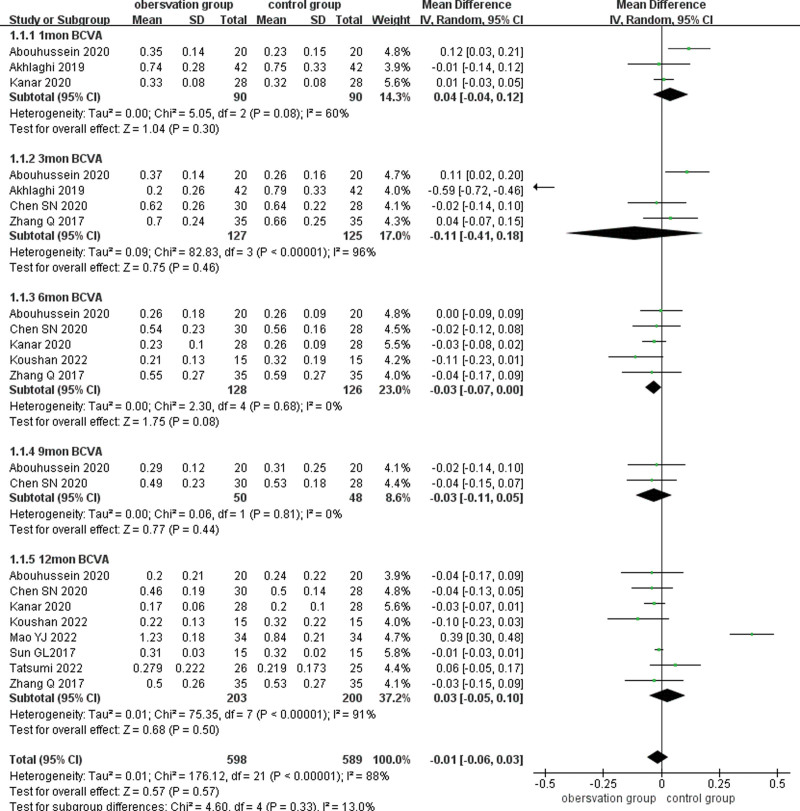
Nine studies reported BCVA (LogMAR) after treatment, and subgroup analyses of BCVA were performed according to different follow-up times (1, 3, 6, 9, and 12 months). The results existed large heterogeneity (P < .001, I2 = 88%), using a random-effects model combined with effect size analysis. There was no statistical difference in BCVA at 1 month between the 2 groups (MD = 0.04, 95% CI: −0.04 to 0.12, P = .30). Four studies reported BCVA at 3 months after treatment and there was no statistically significant difference in BCVA between the 2 groups (MD = −0.11,95% CI: −0.41 to 0.18, P = .46). Five papers reported BCVA at 6 months after treatment and there was no statistically significant difference in BCVA between the 2 groups (MD = −0.03, 95% CI: −0.07 to 0.00, P = .08). Two papers reported BCVA at 9 months after treatment and there was no statistically significant difference in BCVA between the 2 groups (MD = −0.03, 95% CI: −0.07 to 0.00, P = .08). Two papers reported BCVA at 9 months after treatment and there was no statistically significant difference in BCVA between the 2 groups (MD = −0.03, 95% CI: −0.11 to 0.05, P = .44). Eight studies reported BCVA at 12 months after treatment and there was no statistically significant difference in BCVA between the 2 groups (MD = 0.03, 95% CI: −0.05 to 0.10, P = .50).(Fig. 3)
Figure 3.
Forest plots comparing BCVA subgroups at different times after treatment in the observation and control groups. BCVA = best-corrected visual acuity.
3.4.1.2. ETDRS visual acuity
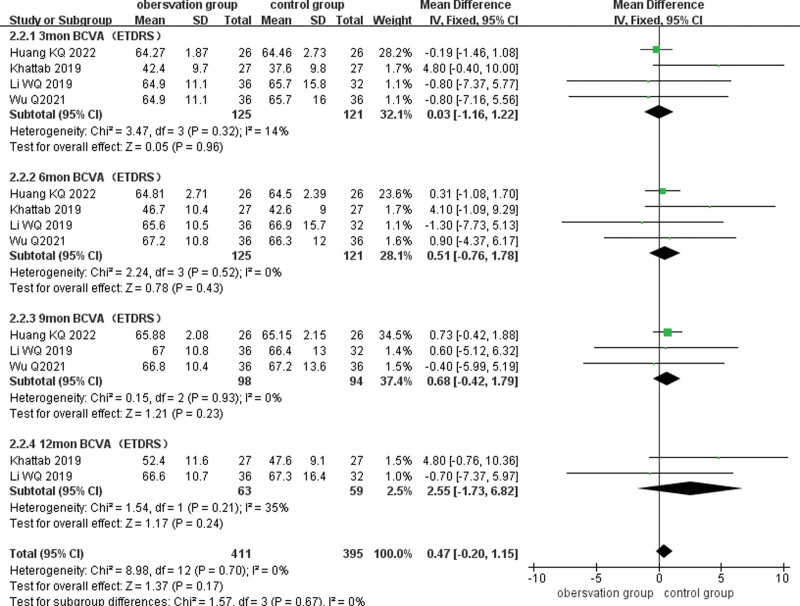
Four of the included analyses reported BCVA (ETDRS) after treatment, and subgroup analyses of BCVA were performed according to different follow-up times (3, 6, 9, and 12 months), and the combined results showed low heterogeneity (P = .70, I2 = 0%), using a fixed-effects model combined with effect size analysis. Four studies reported BCVA at 3 months after treatment and there was no statistically significant difference in BCVA between the 2 groups (MD = 0.03, 95% CI: −1.16 to 1.22, P = .96). Four studies reported BCVA at 6 months after treatment and there was no statistical difference in BCVA between the 2 groups (MD = 0.51, 95% CI: −0.42 to 1.79, P = .23). Three studies reported BCVA at 9 months after treatment and there was no statistical difference between the 2 groups (MD = 0.68, 95% CI: −0.42 to 1.79, P = .23). Two papers reported BCVA at 12 months after treatment and there was no statistically significant difference between the 2 groups (MD = 2.55, 95% CI: −1.73 to 6.82, P = .24). (Fig. 4)
Figure 4.
Forest plots comparing BCVA subgroups at different times after treatment in the observation and control groups. BCVA = best-corrected visual acuity.
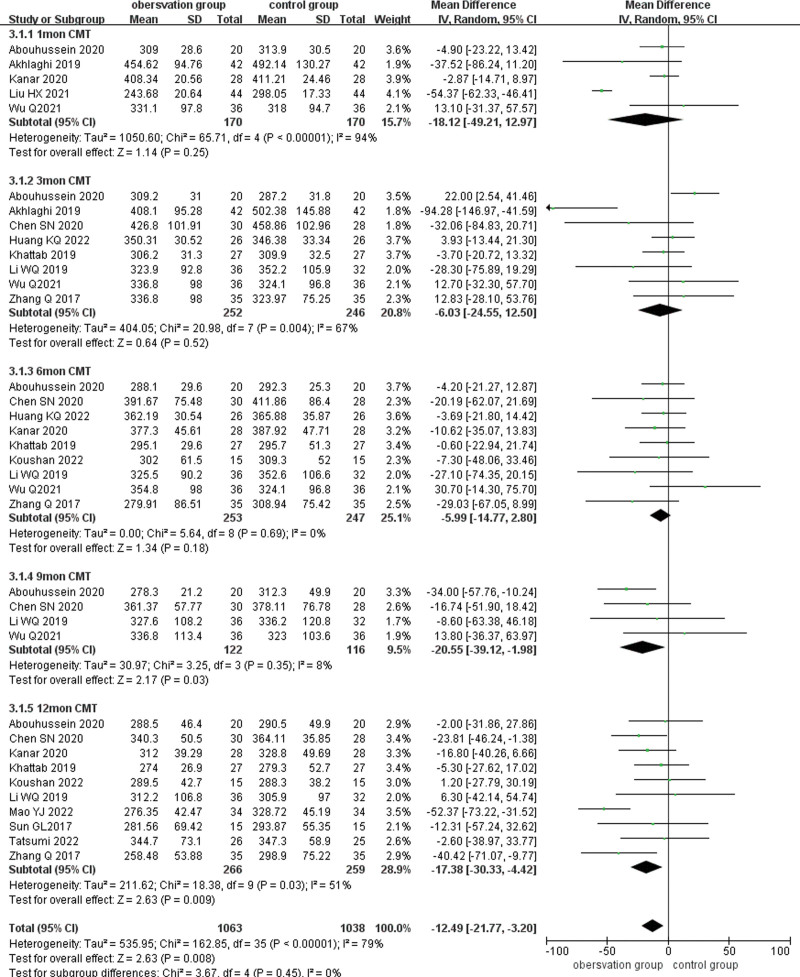
3.4.2. CMT.
Fourteen papers reported CMT after treatment, and CMT was analyzed in subgroups according to different follow-up times (1, 3, 6, 9, and 12 months), and the results showed large heterogeneity (P < .001, I2 = 78%), using a random-effects model combined with effect size analysis. Five papers reported CMT at 1 month after treatment and there was no statistically significant difference in CMT between the 2 groups (MD = −18.12, 95% CI: −49.21 to 12.97, P = .25). Eight papers reported CMT at 3 months after treatment and there was no statistical difference in CMT between the 2 groups (MD = −6.03,95% CI: −24.55 to 12.50, P = .52). Nine papers reported CMT at 6 months after treatment and there was no statistically significant difference in CMT between the 2 groups (MD = −20.55, 95% CI: −39.12 to −1.98, P = .18). Four studies reported CMT at 9 months after treatment and there was a statistically significant difference between the 2 groups (MD = −6.03, 95% CI: −24.55 to 12.50, P = .03). Ten papers reported CMT at 12 months after treatment and result showed that CMT at 12 months after treatment was lower than that of the control group (MD = −17.50, 95% CI: −30.50 to −4.51, P = .008). (Fig. 5)
Figure 5.
Forest plot comparing CMT subgroups at different times after treatment in the observation and control groups. CMT = central macular thickness.
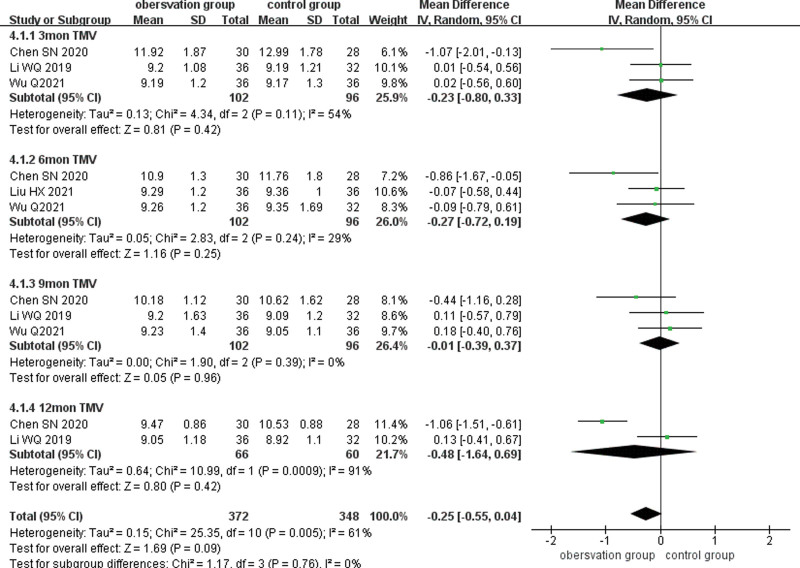
3.4.3. TMV.
Four studies reported TMV after treatment, and subgroup analyses of TMV after treatment were performed according to different follow-up times (3, 6, 9, and 12 months), and the results existed large heterogeneity between studies (P = .005, I2 = 61%), using a random-effects model. Three studies reported TMV at 3 months after treatment and there was no statistically significant difference between the 2 groups (MD = −0.23,95% CI: −0.80 to 0.33, P = .42). Three studies reported TMV at 6 months after treatment and there was no statistical difference between the 2 groups (MD = −0.27,95% CI: −0.72 to 0.19, P = .25). Three studies reported TMV at 9 months after treatment and there was no statistical difference between the 2 groups (MD = −0.01, 95% CI: −0.39 to 0.37, P = .96). Two studies reported TMV at 12 months after treatment and there was no statistical difference between the 2 groups (MD = −0.48, 95% CI: −1.64 to 0.69, P = .42). (Fig. 6)
Figure 6.
Forest plot comparing TMV subgroups at different times after treatment in the observation and control groups. TMV = total macular volume.
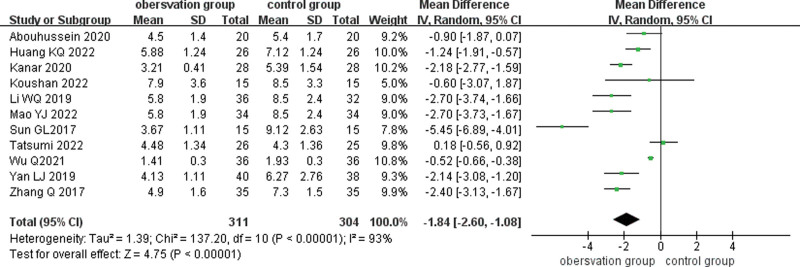
3.4.4. Number of anti-VEGF drug injections.
Eleven studies compared the complications of SML combined with anti-VEGF drugs for DME, and there was large heterogeneity (I2 = 93%, P < .001) and were analyzed using a random-effects model. Meta-analysis showed that the number of vitreous cavity injections of anti-VEGF drugs was lower in the observation group than in the control group (MD = −1.85, 95% CI: −2.61 to −1.08, P < .001). (Fig. 7)
Figure 7.
Forest plot comparing the number of anti-VEGF drugs injected into the vitreous cavity in the observation and control groups. VEGF = vascular endothelial growth factor.
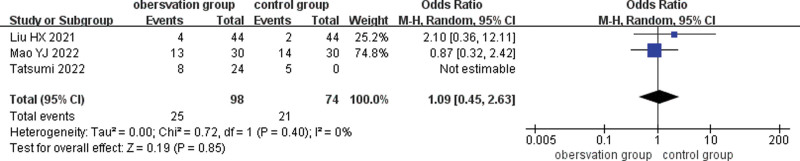
3.4.5. Complications.
Three studies compared the complications of SML combined with anti-VEGF drugs for DME, and results existed low heterogeneity (I2 = 0%, P = .57), indicating no heterogeneity between studies, and were analyzed using a fixed-effects model. There was no statistically significant difference in occurrence of adverse events between 2 groups (ratio of ratios = 1.28. 95% CI: 0.61–2.66, P = .51). (Fig. 8)
Figure 8.
Forest plot comparing complications during treatment in the observation and control groups.
3.5. Sensitivity analysis
Sensitivity analysis was performed for each of the 5 outcome indicators of BCVA, CMT, TMV, the number of anti-VEGF drugs, and complications. The result showed that changing the model had no significant effect on the combined results. By removing every literature, there was no statistically significantly reduced ieterogeneity, suggesting stable and reliable results.
3.6. Analysis of publication bias
Publication bias was detected for the combined results when the included literature over 3 papers. Egger test was performed by STATA 15. The results showed that there was a publication bias in BCVA (ETDRS) and CMT 9 months after treatment (P = .039, P = .013, respectively). The remaining outcome indicators had no publication bias. (Table 2)
Table 2.
Analysis of publication bias.
| Research content | Inclusion of literature (article) | The P value of Egger test |
|---|---|---|
| BCVA (LogMAR) | ||
| 1 mo | 3 | P = .19 |
| 3 mo | ||
| 6 mo | 5 | P = .315 |
| 12 mo | 8 | P = .903 |
| BCVA (ETDRS) | ||
| 3 mo | 4 | P = .488 |
| 6 mo | 4 | P = .327 |
| 9 mo | 3 | P = .039 |
| CMT | ||
| 1 mo | 5 | P = .388 |
| 3 mo | 8 | P = .083 |
| 6 mo | 9 | P = .825 |
| 9 mo | 4 | P = .013 |
| 12 mo | 10 | P = .382 |
| TMV | ||
| 3 mo | 3 | P = .138 |
| 6 mo | 3 | P = .150 |
| 9 mo | 3 | P = .071 |
| Number of injections | 11 | P = .545 |
| Adverse reactions | 3 | P = .207 |
BCVA = best-corrected visual acuity, CMT = central macular thickness, TMV = total macular volume.
4. Discussion
DME is the leading cause of vision loss in patients with DM.[2,3] The pathogenesis of diabetic macular edema is complex and not fully understood, mainly due to a series of inflammatory responses secondary to ischemia and hypoxia, in which multiple inflammatory factors are involved. VEGF is one of them.[28]VEGF has been shown to be one of the most important inflammatory factors in the pathogenesis of DME, and the expression of VEGF in the vitreous of DM patients is 10-fold higher than that of non-DM patients. The upregulation of VEGF leads to the breakdown of the blood-retinal barrier, disrupts vascular permeability, promotes neovascularization, and ultimately leads to the formation of DME. The current treatment modalities for DME are diverse and include vitreous cavity injections of anti-VEGF drugs, glucocorticoids (Triamcinolone acetonide, dexamethasone implant, and fluorescence implant), photocoagulation (conventional retinal laser, subthreshold micropulse laser), surgical treatment, herbal medicine, and combination therapy, among which anti-VEGF drugs are currently the first choice.[29,30]
Common anti-VEGF drugs include the following: nucleic acid aptamers (Pegaptanib), VEGF antibodies (Bevacizumab), VEGF antibody fragments (Ranibizumab), and fusion proteins (Aflibercept, Conbercept).[31] Anti-VEGF drugs alleviate DME by reducing the inflammatory response and inhibiting neovascularization.[32] Although anti-VEGF is effective in treating DME, it requires repeated multiple injections, which not only increases the financial burden but also the potential risks such as infection.[6] In recent years, many scholars have used SML combined with anti-VEGF drugs for the treatment of DME. Subthreshold micropulse laser is a conventional laser split into multiple short, repetitive pulsed lasers, with a single pulsed laser time including ON and OFF period. During the ON period, the laser energy is converted into heat energy in the RPE cells, but the RPE cells start to cool during OFF period to avoid thermal damage to the RPE cells and finally prevent the laser energy from spreading to the surrounding area. The mechanism of action of subthreshold micropulse laser is not fully well understood, and it is speculated that it may be related to the promotion of RPE cell proliferation, tight junctions between RPE cells, restoration of RPE cell function, promotion of subretinal and intraretinal fluid uptake. What’s more, SML could upregulate heat shock protein 70 and pigment epithelium-derived factor, downregulation of VEGF.[33]
Recently, scholars have used SML combined with anti-VEGF drugs for the treatment of DME, but the results are controversial. Several studies have found that SML combined with anti-VEGF drugs can reduce the number of vitreous injections of anti-VEGF drugs.[14–22] However, some scholars believe that combination therapy cannot reduce the number of anti-VEGF drug injections, but increases the financial burden of patients.[10,23] Therefore, in this paper, we conducted this meta-analysis, aiming to compare the clinical efficacy of SML combined with anti-VEGF drugs and provide more reliable evidence for the application of SML combined with anti-VEGF drugs in the treatment of DME.
15 RCTs were included for Meta-analysis. There was not statistically significant difference between 2 groups in BCVA after treatment at 1, 3, 6, 9, and 12 months. The CMT in the observation group was lower than that in the control group at 9 and 12 months after treatment (P < .05). There was no statistically significant difference between 2 groups in TMV at 3, 6, 9, and 12 months. The number of anti-VEGF injections was lower in the observation group than that in the control group, and the difference was statistically significant (P < .05). The occurrence of complications in the 2 groups was no statistical difference between the 2 groups.
This Meta-analysis has some limitations: First, only the literature published in the journal were included, which may lead to some bias; The number of included studies is small and follow-up periods are inconsistent. The duration of diabetes mellitus patients is inconsistent. The above differences may have led to variability in the study results; The time points for observation of outcome indicators were inconsistent. The wavelength and protocol of SML in different studies were inconsistent. The above difference may lead to some bias; The types of anti-VEGF drugs are inconsistent, which may lead to differences in the results. Hence, because of the above limitations of this study, more high-quality, multicenter, large-sample randomized controlled trials are needed in the future to provide more clinical evidence for the clinical use of SML in combination with anti-VEGF drugs in the treatment of DME.
5. Conclusion
In summary, this study shows that SML combined with anti-VEGF drugs does not improve BCVA and TMV well in patients with DME, but it improves macular edema and reduces the number of injections of anti-VEGF drugs, thereby reducing the financial burden on patients. And the combination never increases the risk of ocular or systemic complications.
Author contributions
Conceptualization: Dahua Xu, Mei Chen.
Data curation: Dahua Xu.
Formal analysis: Dahua Xu, Ting Zhu, Lin Huang, Xiaolin Wang.
Investigation: Dahua Xu, Ting Zhu, Xiaolin Wang, Mei Chen.
Methodology: Dahua Xu, Ting Zhu, Lin Huang, Xiaolin Wang, Mei Chen.
Resources: Dahua Xu.
Software: Dahua Xu, Ting Zhu, Lin Huang, Xiaolin Wang.
Writing – original draft: Dahua Xu, Ting Zhu, Mei Chen.
Writing – review & editing: Dahua Xu, Ting Zhu, Mei Chen.
Abbreviations:
- BCVA
- best-corrected visual acuity
- CI
- confidence interval
- CMT
- central macular thickness
- DM
- diabetes mellitus
- DME
- diabetic macular edema
- DR
- diabetic retinopathy
- RCTs
- randomized controlled trials
- RPE
- retinal pigment epithelium
- SML
- subthreshold micropulse laser
- TMV
- total macular volume
- VEGF
- vascular endothelial growth factor
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
DX and TZ contributed equally to this work.
This study is a meta-analysis, so no ethics committee approval is required for this study.
This study has been supported by the Chongqing Science and Health Technology Innovation and Application Development Project of Traditional Chinese Medicine (NO.2020ZY024098).
The authors have no conflicts of interest to disclose.
How to cite this article: Xu D, Zhu T, Huang L, Wang X, Chen M. Clinical efficacy of subthreshold micropulse laser combined with anti-VEGF drugs in the treatment of diabetic macular edema: A meta-analysis. Medicine 2024;103:5(e34583).
Contributor Information
Dahua Xu, Email: xudahua2020@163.com.
Ting Zhu, Email: 793545985@qq.com.
Lin Huang, Email: HUANGLIN3020@163.com.
Xiaolin Wang, Email: wxl4431@163.com.
References
- [1].Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American diabetes association: national cross sectional study. BMJ. 2020;369:m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–91. [DOI] [PubMed] [Google Scholar]
- [3].Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:1334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tomita Y, Lee D, Tsubota K, et al. Updates on the current treatments for diabetic retinopathy and possibility of future oral therapy. J Clin Med. 2021;10:4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kodjikian L, Bellocq D, Bandello F, et al. First-line treatment algorithm and guidelines in center-involving diabetic macular edema. Eur J Ophthalmol. 2019;29:573–84. [DOI] [PubMed] [Google Scholar]
- [6].Porta M, Striglia E. Intravitreal anti-VEGF agents and cardiovascular risk. Intern Emerg Med. 2020;15:199–210. [DOI] [PubMed] [Google Scholar]
- [7].Zehden JA, Mortensen XM, Reddy A, et al. Systemic and ocular adverse events with intravitreal anti-VEGF therapy used in the treatment of diabetic retinopathy: a review. Curr Diab Rep. 2022;22:525–36. [DOI] [PubMed] [Google Scholar]
- [8].Sabal B, Teper S, Wylęgała E. Subthreshold micropulse laser for diabetic macular edema: a review. J Clin Med. 2022;12:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hirabayashi K, Kakihara S, Tanaka M, et al. Investigation of the therapeutic mechanism of subthreshold micropulse laser irradiation in retina. Graefes Arch Clin Exp Ophthalmol. 2020;258:1039–47. [DOI] [PubMed] [Google Scholar]
- [10].Tatsumi T, Takatsuna Y, Oshitari T, et al. Randomized clinical trial comparing intravitreal aflibercept combined with subthreshold laser to intravitreal aflibercept monotherapy for diabetic macular edema. Sci Rep. 2022;12:10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Akhlaghi M, Dehghani A, Pourmohammadi R, et al. Effects of subthreshold diode micropulse laser photocoagulation on treating patients with refractory diabetic macular edema. J Curr Ophthalmol. 2019;31:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khattab AM, Hagras SM, AbdElhamid A, et al. Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2019;257:1373–80. [DOI] [PubMed] [Google Scholar]
- [13].Abouhussein MA, Gomaa AR. Aflibercept plus micropulse laser versus aflibercept monotherapy for diabetic macular edema: 1-year results of a randomized clinical trial. Int Ophthalmol. 2020;40:1147–54. [DOI] [PubMed] [Google Scholar]
- [14].Huang KQ, Liu LH, Li M, et al. Clinical effect of ranibizumab combined with 577nm micropulse laser in the treatment of severe diabetic macular edema. Int Eye Sci. 2022;22:1377–380. [Google Scholar]
- [15].Zhang Q, Duan YP, JIN ZQ. Effect of aflibercept combined with sub-threshold micropulse laser in the treatment of 30 cases with diabetic macular edema. Her Med. 2021;40:631–34. [Google Scholar]
- [16].Sun GL, Jiang J, Wang CH, et al. High-density micropulse photocoagulation combined with intravitreal injection of ranibizumab for diabetic macular edema. Rec Adv Ophthalmol. 2017;37:279–81. [Google Scholar]
- [17].Li WQ, Song YP, Ding Q. The effect of conbercept combined with 577nm subthreshold micropulse laser photocoagulation on diabetic macular edema. Chin J Ocul Fundus Dis. 2019;35:129–34. [Google Scholar]
- [18].Abouhussein MA, Gomaa AR. Aflibercept plus micropulse laser versus aflibercept monotherapy for diabetic macular edema: 1-year results of a randomized clinical trial. Int Ophthalmol. 2020;40:1147–54. [DOI] [PubMed] [Google Scholar]
- [19].Mao YJ, Yang YY, Yin Q, et al. Clinical effect of aflibercept vitreous injection combined with 577nm micropulse in the treatment of patients with diabetic macular edema. Med Innov China. 2022;19:108–12. [Google Scholar]
- [20].Kanar HS, Arsan A, Altun A, et al. Can subthreshold micropulse yellow laser treatment change the anti-vascular endothelial growth factor algorithm in diabetic macular edema? A randomized clinical trial. Indian J Ophthalmol. 2020;68:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wu Q, Wang Y, Jiao J. Effect of ranibizumab injection combined and 577nm micropulse laser photocoagulation on CMT and TMV of patients with diabetic macular edema. Chin J Laser Med Surg. 2021;30:155–60. [Google Scholar]
- [22].Yan LJ, Ji A. Observation on effect of micropulse laser combined with intravitreal injection of leizhumab in treatment of diabetic macular edema. Drugs Clin Pract. 2019;4:124–26. [Google Scholar]
- [23].Koushan K, Eshtiaghi A, Fung P, et al. Treatment of diabetic macular edema with aflibercept and micropulse laser (DAM study). Clin Ophthalmol. 2022;16:1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Altinel MG, Acikalin B, Alis MG, et al. Comparison of the efficacy and safety of anti-VEGF monotherapy versus anti-VEGF therapy combined with subthreshold micropulse laser therapy for diabetic macular edema. Lasers Med Sci. 2021;36:1545–53. [DOI] [PubMed] [Google Scholar]
- [25].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen SN, Yang PF, Chen S, et al. Comparison of single subthreshold micropulse yellow laser and combined with ranibizumab intravitreal injection for diabetic macular edema. Int Eye Sci. 2020;20:607–12. [Google Scholar]
- [27].Lliu HX. Effects of ranibizumab combined with subthreshold micropulse laser photocoagulation on patients with diabetic retinopathy. Med J Chin People’s Health. 2021;33:1–3. [Google Scholar]
- [28].Noma H, Yasuda K, Shimura M. Involvement of cytokines in the pathogenesis of diabetic macular edema. Int J Mol Sci. 2021;22:3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chauhan MZ, Rather PA, Samarah SM, et al. Current and novel therapeutic approaches for treatment of diabetic macular edema. Cells. 2022;11:1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern. Ophthalmology. 2020;127:P66–P145. [DOI] [PubMed] [Google Scholar]
- [31].Furino C, Boscia F, Reibaldi M, et al. Intravitreal therapy for diabetic macular edema: an update. J Ophthalmol. 2021;2021:6654168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Imazeki M, Noma H, Yasuda K, et al. Anti-VEGF therapy reduces inflammation in diabetic macular edema. Ophthalmic Res. 2021;64:43–9. [DOI] [PubMed] [Google Scholar]
- [33].Frizziero L, Calciati A, Midena G, et al. Subthreshold micropulse laser modulates retinal neuroinflammatory biomarkers in diabetic macular edema. J Clin Med. 2021;10:3134. [DOI] [PMC free article] [PubMed] [Google Scholar]