Abstract
Background
Postoperative delirium occurs in up to 80% of patients undergoing esophagectomy. We performed an exploratory proteomic analysis to identify protein pathways that may be associated with delirium post-esophagectomy.
Objectives:
Identify proteins associated with delirium and delirium severity in a younger and higher-risk surgical population.
Methods:
We performed a case-control study using blood samples collected from patients enrolled in a negative, randomized, double-blind clinical trial. English speaking adults aged 18 years or older, undergoing esophagectomy, who had blood samples obtained were included. Cases were defined by a positive delirium screen after surgery while controls were patients with negative delirium assessments. Delirium was assessed using Richmond Agitation Sedation Scale and Confusion Assessment Method for the Intensive Care Unit, and delirium severity was assessed by Delirium Rating Scale-Revised-98. Blood samples were collected pre-operatively and on post-operative day 1, and discovery proteomic analysis was performed. Between-group differences in median abundance ratios were reported using Wilcoxon-Mann-Whitney Odds (WMWodds1) test.
Results:
52 (26 cases, 26 controls) patients were included in the study with a mean age of 64 (SD 9.6) years, 1.9% were females and 25% were African American. The median duration of delirium was 1 day (IQR: 1–2), and the median delirium/coma duration was 2.5 days (IQR: 2–4). Two proteins with greater relative abundance ratio in patients with delirium were: Coagulation factor IX (WMWodds: 1.89 95%CI: 1.0–4.2) and mannosyl-oligosaccharide 1,2-alpha-mannosidase (WMWodds: 2.4 95%CI: 1.03–9.9). Protein abundance ratios associated with mean delirium severity at postoperative day 1 were Complement C2 (Spearman rs=−0.31, 95%CI [−0.55, −0.02]) and Mannosyl-oligosaccharide 1,2-alpha-mannosidase (rs=0.61, 95%CI = [0.29, 0.81]).
Conclusions:
We identified changes in proteins associated with coagulation, inflammation, and protein handling; larger, follow-up studies are needed to confirm our hypothesis-generating findings.
Keywords: Proteins, critical care, coma, encephalopathy, delirium severity
Introduction
Delirium, a type of an acute brain failure, is characterized by fluctuation in mental status, inattention, altered level of consciousness, and disorganized thinking. Postoperative delirium is highly prevalent, with rates ranging from 15–80%. [1,2] Prior studies have found postoperative delirium rates as high as 50% among both cardiac and major noncardiac thoracic surgery patients. [3–7] Unfortunately, effective pharmacological interventions to treat or prevent delirium are not available, likely due to our limited understanding of delirium pathophysiology. [8–11] The neuroinflammatory hypothesis of delirium, a leading theory, posits circulating proinflammatory cytokines (e.g., interleukin 6, 8) cross an injured blood brain barrier leading to activation of astrocytes and glial cells in the central nervous system, culminating in neuronal injury and delirium. [12–17] A limitation of this model, however, is that it does not account for the pleiotropic effects of cytokines or the role of anti-inflammatory cytokines acting in vivo.
The study of proteins, which serve important roles in intra-cellular communication and cellular processes, may overcome limitations of existing pathophysiologic models, and delineate new pathways involved in delirium. Proteins could also serve as therapeutic targets or permit development of prediction models for patient-level outcomes. For these reasons, there has been increasing interest in conducting proteomics studies to understand delirium. For example, a recent publication found C-Reactive Protein, zinc alpha-2 glycoprotein, and alpha-1 antichymotrypsin proteins associated with postoperative delirium in patients aged 70 years or older undergoing major non-cardiac surgery. [18] Whether these proteins are also dysregulated in other surgical populations at high risk for delirium, e.g., those undergoing esophagectomy (a type of major noncardiac thoracic surgery), is not known.
Therefore, we conducted a global serum protein analysis in order to identify proteins associated with delirium and delirium severity in a younger and high-risk surgical population (i.e., patients undergoing esophagectomy). We hypothesized that serum proteins associated with inflammation would be dysregulated, lending support for the neuroinflammatory hypothesis of delirium. The objective of this descriptive, exploratory study was to identify serum proteins associated with postoperative delirium in patients undergoing esophagectomy.
Methods
Study Design and Setting
This is a case-control study utilizing blood samples obtained from patients enrolled in Preventing Postoperative Delirium after Major Noncardiac Thoracic Surgery (PEPOD2), a negative, randomized, double-blind placebo-controlled single center clinical trial comparing scheduled low dose haloperidol vs. placebo for prevention of postoperative intensive care unit (ICU) delirium (CT.gov: NCT02213900). [8] Cases were defined as patients who underwent esophagectomy and screened positive for delirium at any time during ICU/hospitalization, and controls were defined as patients who underwent esophagectomy but did not screen positive for delirium (see delirium assessments below). The study was approved by the Institutional Review Board of Indiana University and conducted from October 2013 to June 2015. Patients were screened at the Indiana University Thoracic Surgery pre-operative clinic and provided written informed consent. Screening, recruitment, in-hospital assessments, follow-up, and blood sample collection were performed at Indiana University Health University Hospital, a 257-bed tertiary care center affiliated with the University School of Medicine. Patients were followed in the Surgical Intensive Care Unit and hospital until discharge.
Selection of Participants
Inclusion criteria for the parent trial included: English-speaking patients, age 18 years or older and undergoing esophagectomy. Exclusion criteria (based on the parent trial): history of schizophrenia, Parkinson’s disease, severe dementia, alcohol abuse, neuroleptic malignant syndrome, haloperidol allergy, nursing or pregnant patients, those on levodopa or cholinesterase inhibitors, or those with QT prolongation over 500 milliseconds. Patients enrolled in PEPOD and with blood samples available from pre-operative and post-operative time points were included in this study.
Blood Sample Collection and Proteomics Analysis
Venous blood samples were collected using sterile tubes without anticoagulant by experienced clinical nurses. When existing intravascular devices were present, they were used for the blood draw. Samples (up to 10 ml) were collected at time of pre-operative visit, and on post-operative day 1, between 9:00 am and 11:00 am, stored on ice and transported to the laboratory within 1 hour, with precautions taken to prevent hemolysis. The Indiana Clinical and Translational Sciences Institute laboratory processed samples for storage using the following methods: centrifugation at 1000–1200 × g for 15 minutes at 4 ° C and 1 ml aliquots stored at − 80 ° C to limit freeze and thawing cycles. For samples included in proteomic analyses, cases and controls were frequency matched by gender and randomization to intervention in the parent study. Global proteomic analyses of serum were performed by the Indiana University School of Medicine Proteomics Core (see Figure A.1); laboratory methods, assay types, detection and calibration methods are detailed in the supporting information (text document, see “Supplemental Digital Content”, S1, Proteomics Analysis Workflow).
Outcome Measures
The main outcome was the relationship between protein abundance and postoperative delirium in patients undergoing esophagectomy. The secondary outcome was to measure the association of protein abundance and delirium severity. We chose to measure protein expression at two time points based on expert opinion and clinician input: prior to surgery (pre-operative clinic visit) when patients did not have delirium, and on post-operative day 1 when patients were admitted to the intensive care unit and at greatest risk for delirium. [19]
Assessment of Delirium
Patients were assessed for delirium twice daily by trained research assistants using the Richmond Agitation Sedation Scale (RASS3) and the Confusion Assessment Method for the ICU (CAM-ICU4). [20,21] Because delirium is a fluctuating disorder, research assistants conducted 2 delirium assessments each day, one in the morning (9:00–11:00 am) and one in the afternoon (3:00–5:00 pm), to maximize delirium identification. Delirium duration was defined as the total number of days a participant was CAM-ICU-positive on the morning or afternoon assessment during the hospitalization. The CAM-ICU score was determined by examining the patient for (a) acute or fluctuating changes in mental status, (b) inattention, (c) altered level of consciousness, and (d) disorganized thinking. Patients were considered delirious if they displayed (a) and (b), plus (c) and/or (d). Delirium severity was measured once daily using Delirium Rating Scale-Revised-98 (DRS-R-985). [22] DRS-R-98 is a validated scale which captures impairments in the following domains: attention, sleep-wake cycle, memory, visuospatial ability, language, thought processes and content, motor function, and mood. The 16-item scale (rated 0–3 each, maximum 39 points) was performed by trained research staff and higher scores indicate greater delirium severity.
Other Data
Data on patients’ characteristics were obtained using the parent trial’s study records. Demographics, comorbidities (assessed using Charlson Comorbidity Index), laboratory values, exposure to medications and anesthetics, intra-operative and post-operative clinical interventions, and clinical outcomes (admission to ICU, length of stay, duration of mechanical ventilation) were assessed using study and medical records. [23] Functional status was assessed by self-report using Katz (activities of daily living) and Lawton scales (instrumental activities of daily living) at pre-operative visit. Potential confounders and modifiers such as severity of illness were assessed by Acute Physiology and Chronic Health Evaluation score (APACHE II6) and American Society of Anesthesiologists (ASA7) Class. [24–26] Cognitive function at baseline was assessed by Repeatable Battery for the Assessment of Neuropsychological Status (RBANS8) at time of the pre-operative visit. [27]
Efforts to Reduce Bias
Bias was reduced through the following approaches: use of blinded outcome assessments, protocolized blood samples collected within a randomized clinical trial, and laboratory analyses performed without knowledge of clinical information. We performed frequency matching using the gender and randomization to intervention as the main matching variables. Frequency matching for males was used to compare similar number of controls within each age group and randomization (there were only 7 female cases and 8 female controls). As described in the next section, we compared protein abundance ratios, consistent with high impact publications. [28,29]
Calculation
We used Fisher’s exact test when comparing categorical baseline and clinical characteristics between patients with and without delirium, and t-test for comparing age. For continuous characteristics, we used the Mann-Whitney U-test for skewed (non-normal) variables and the two sample t-test for normal variables. Normality was assessed using the Wilkes-Shapiro test and graphical methods. Data was not transformed to achieve normal distribution. Abundance ratio was defined as v1/v0, where v1 is the post-operative protein concentration and v0 is the pre-operative protein concentration. We tested for differences in abundance ratios between patients with and without delirium using the Mann-Whitney U-test as it is robust to outliers and present the Wilcoxon-Mann-Whitney-odds and 95% confidence interval. [23] Spearman correlations were used to assess the relationship between protein abundance ratios and delirium severity at postoperative day 1. As this was a preliminary exploratory analysis, we did not adjust our results for multiple comparisons. Due to the lack of published proteomics results in esophagectomy patients at time of research design, our study did not utilize a pre-determined sample size. Two-sided tests with a p-value < 0.05 were used for all significance testing. All statistical analyses were performed using SAS v9.4.
Results
The results have been reported in accordance with the STrengthening Reporting of OBservational studies in Epidemiology – Molecular Epidemiology (STROBE-ME9) guidelines and the recommendations for biomarker identification and qualification in clinical proteomics. Twenty-six patients in the esophagectomy group developed postoperative delirium (defined as cases) and were compared with 26 patients without delirium. There was no loss to follow up during the study period (see Figure A.2). The mean age of participants in the study was 64 years (SD=9.6), 25% of the patients were female which is slightly above the national average of female patients undergoing elective esophagectomy in the United States, and 1.9% were African American. [30,31] Malignancy was the primary surgical indication in both cases and controls (no delirium: 80.8% vs. delirium: 88.5%, p=0.704). [32] Delirium occurred early in the post-operative stay: 1/26 patients (3.9%) experienced delirium on the day of surgery (after the procedure) and 15/26 (57.7%) on postoperative day 1. The median duration of delirium was 1 day (IQR: 1–2), and the median delirium/coma duration was 2.5 days (IQR: 2–4). Consistent with prior studies, patients with delirium had greater mean ICU days (delirium-positive: 5.0 days vs. delirium-negative: 2.9 days, p=0.003), and mean duration of mechanical ventilation (delirium-positive: 1.7 days vs. delirium-negative: 1.2 days, p=0.035). Additional demographic and clinical characteristics are shown in Table 1.
Table 1.
Clinical Characteristics of Cases and Controls.
| Variables | No Delirium (N =26) | Delirium (N = 26) | P-valuea |
|---|---|---|---|
| Demographics | |||
| Age in years, mean (SD) | 63.4 (9.8) | 64.6 (9.6) | 0.670 |
| African-American, n (%) | 0 (0.0) | 3.9 (1) | 1.000 |
| Caucasian, n (%) | 26 (100.0) | 25 (96.1) | |
| Female, n (%) | 6 (23.1) | 7 (26.9) | 1.000 |
| Education | 0.611 | ||
| High School Graduate, n (%) | 16 (64.0) | 13 (52.0) | |
| Some College, n (%) | 7 (28.0) | 7 (28.0) | |
| Bachelor’s Degree or more, n (%) | 2 (8.0) | 5 (20.0) | |
| Body Mass Index, median (IQR) | 28.9 (24.9–33.1) | 25.0 (21.3–32.0) | 0.120 |
| Comorbidities and Functional Status | |||
| Pre-operative chemotherapy, n (%) | 19 (76.0) | 18 (75.0) | 1.000 |
| Esophagectomy due to malignancy, n (%) | 23 (88.5) | 21 (80.8) | 0.703 |
| Charlson Comorbidity Index, median (IQR) | 2.0 (2.0–3.0) | 3.0 (2.0–5.0) | 0.360 |
| Activities of Daily Living (ADL), median (IQR) | 6.0 (6.0–6.0) | 6.0 (6.0–6.0) | 1.000 |
| Instrumental Activities of Daily Living (IADL), median (IQR) | 8.0 (7.0–8.0) | 8.0 (7.0–8.0) | 0.704 |
| Severity of Illness | |||
| APACHE II, median (IQR) | 18.5 (13.0–26.0) | 24.0 (18.0–27.0) | 0.166 |
| American Society of Anesthesiologists Class | |||
| Class III, n (%) | 26 (100.0) | 26 (100.0) | |
| Baseline Cognitive Function | |||
| Pre-operative RBANS score, median (IQR) | 98.0 (87.0–100.0) | 90.0 (78.0–99.0) | 0.385 |
| Pre-operative RBANS Percentile, median (IQR) | 45.0 (19.0–50.0) | 25.0 (8.0–47.5) | 0.428 |
| Clinical Outcomes | |||
| Admission to ICU in postoperative setting, n (%) | 26 (100) | 26 (100.0) | |
| ICU length of stay in days, median (IQR) | 3.1 (2.2–3.7) | 4.4 (3.0–7.2) | 0.006 |
| Mechanical Ventilation in days, median (IQR) | 1.1 (0.9–1.2) | 1.3 (1.0–2.1) | 0.022 |
| Allocation | 1.000 | ||
| Intervention b , n (%) | 10 (38.5) | 9 (34.6) | |
APACHE, Acute Physiology and Chronic Health Evaluation Score; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
Categorical variables assessed by Fisher’s exact test, continuous variables assessed by Mann-Whitney U-Test, age was compared using t-test.
Allocated to haloperidol intervention arm in the parent trial.
Note: RBANS available on 17/26 Non-Delirious Patients: 20/26 Delirious Patients
As shown in Table 2, there were no significant differences between delirium and no delirium groups in intraoperative or postoperative management, including surgical approach/technique, duration of surgery, intraoperative medications, fluids, and transfusions. [8]
Table 2.
Intraoperative and postoperative management characteristics in patients with postoperative delirium compared to those without delirium.
| Variables | No Delirium (N =26) | Delirium (N = 26) | P-valuea |
|---|---|---|---|
| Type of esophagectomy | 1.000 | ||
| Ivor Lewis, n (%) | 24 (92.3) | 23 (88.5) | |
| Other, n (%) | 2 (7.7) | 3 (11.5) | |
| Duration of surgery in hours, median (IQR) | 5.0 (4.2–5.3) | 4.9 (4.2–5.6) | 0.985 |
| Duration of anesthesia in hours, median (IQR) | 6.3 (5.5–6.8) | 6.7 (5.7–7.3) | 0.284 |
| Intravenous fluids in mL, median (IQR) | 3000.0 (2500.0–3500.0) | 3175.0 (2700.0–3500.0) | 0.369 |
| Estimated blood loss in mL, median (IQR) | 300.0 (150.0–300.0) | 300.0 (100.0–300.0) | 0.855 |
| Intraoperative medications | |||
| Opioids, n (%) | 26 (100.0) | 26 (100.0) | |
| Opioid dose in mg, median (IQR) | 31.7 (31.7–51.7) | 30.4 (25.0–41.7) | 0.077 |
| Benzodiazepines, n (%) | 26 (100.0) | 25 (96.2) | 1.000 |
| Benzodiazepine dose in mg, median (IQR) | 38.8 (12.5–62.5) | 33.8 (12.5–62.5) | 0.596 |
| Ketamine, n (%) | 3 (11.5) | 3 (11.5) | 1.000 |
| Ondansetron, n (%) | 5 (19.2) | 3 (11.5) | 0.703 |
| Dexamethasone, n (%) | 4 (15.4) | 0 (0.0) | 0.110 |
| Postoperative | |||
| Management | |||
| Epidural analgesia, n (%) | 25 (96.2) | 22 (84.6) | 0.350 |
| Opioids, n (%) | 26 (100.0) | 26 (100.0) | |
| Opioid use in days, median (IQR) | 5.0 (3.0–7.0) | 4.0 (3.0–6.0) | 0.761 |
| Benzodiazepines, n (%) | 8 (30.8) | 13 (50.0) | 0.258 |
| Benzodiazepine use in days, median (IQR) | 0.0 (0.0–1.0) | 0.5 (0.0–2.0) | 0.273 |
| Propofol n (%) | 15 (57.7) | 15 (57.7) | 1.000 |
Categorical variables assessed by Fisher’s exact test, continuous variables assessed by Mann-Whitney U-Test
Proteins Associated with Postoperative Delirium
We identified 6 proteins with differences in abundance ratio between patients with postoperative delirium compared to those without postoperative delirium (Table 3). Two proteins had increased relative abundance ratio in patients with delirium compared to those in the no delirium group: coagulation factor IX (WMWodds (Wilcoxon-Mann-Whitney odds): 1.95 95%CI: 1.01–4.55) and mannosyl-oligosaccharide 1,2-alpha-mannosidase (WMWodds: 2.45 95%CI: 1.01–11.13). Four proteins had increased relative abundance ratios in the delirium-negative group compared to delirium positive patients as shown in Table 3. Results of bioinformatics network analysis using the STRING web database showed co-expression of angiotensinogen and ceruloplasmin (see Figure A.3).
Table 3.
Median protein abundance ratio by delirium status.
| Accession Number | Protein Name (Gene) | Patients with protein identified (n) | No Delirium (Median IQR) | Delirium Median (IQR) | WMWodds (95% CI) | P-valuea |
|---|---|---|---|---|---|---|
| Increased Protein Abundance Ratiob in Delirium Cases | ||||||
| P00740 | Coagulation factor IX (F9) | N=52 | 1.13 (0.99–1.18) | 1.18 (1.08–1.28) | 1.95 (1.01–4.55) | 0.047 |
| P33908 | Mannosyl-oligosaccharide 1,2-alpha-mannosidase (MAN1A1) | N=31c | 1.36 (1.34–1.42) | 1.50 (1.35–1.60) | 2.45 (1.01–11.13) | 0.049 |
| Increased Protein Abundance Ratiob in Patients without Delirium | ||||||
| P01019 | Angiotensinogen (AGT) | N=52 | 1.19 (1.14–1.23) | 1.13 (1.05–1.23) | 0.50 (0.21–0.96) | 0.038 |
| P00450 | Ceruloplasmin (CP) | N=52 | 1.02 (0.98–1.08) | 0.99 (0.93–1.02) | 0.50 (0.21–0.96) | 0.038 |
| P06681 | Complement C2 (C2) | N=52 | 1.01 (0.96–1.08) | 0.97 (0.91–1.00) | 0.44 (0.17–0.86) | 0.016 |
| Q06033 | Inter-alpha-trypsin inhibitor heavy chain H3 (ITIH3) | N=52 | 1.27 (1.21–1.36) | 1.20 (1.13–1.27) | 0.49 (0.21–0.95) | 0.036 |
P-value assessed by Mann-Whitney U-Test. WMWOdds: Wilcoxon-Mann-Whitney-odds.
Relative abundance; >1: more abundant at post-operative time, <1: less abundant at post-operative time.
Protein identified in a total of 31 patients (14 patients with delirium and 17 patients with no delirium).
Relationship of Protein Abundance Ratio and Delirium Severity in Patients with Postoperative Delirium
In patients who developed postoperative delirium, protein abundance ratios (post-operative value/pre-operative value) of Complement C2 (Spearman correlation: rs=−0.32, 95%CI [−0.56, −0.01]), and Mannosyl-oligosaccharide 1,2-alpha-mannosidase (MAN1A110) (rs=0.71, 95%CI = [0.42, 0.86]) were moderately associated with mean DRS-R-98 delirium severity score on post-operative day 1.
Discussion
Postoperative delirium in patients undergoing esophagectomy is a prevalent complication and likely occurs due to the interaction of multiple pathophysiologic pathways, limiting the development of effective treatments. [33] To identify potential biological mechanisms associated with postoperative delirium after esophagectomy, we conducted a hypothesis-generating proteomics analysis using blood samples from pre-operative and post-operative time points. We hypothesized proteins related to inflammatory pathways would be dysregulated, supporting leading neuroinflammatory hypotheses for postoperative delirium. In addition, we hypothesized our analysis would identify novel candidate pathways that may help in designing future studies on risk stratification, prediction of delirium severity, or conversely, protection from postoperative delirium.
Our analysis found proteins involved in coagulation and protein glycosylation functions had higher median abundance ratios in postoperative delirium cases compared to delirium negative patients. Our results suggest coagulation factor 9 and mannosyl-oligosaccharide 1,2-alpha-mannosidase (MAN1A1) may have a role in postoperative delirium. These findings are supported by prior studies where mutations in mannosidase alpha class 1B member 1 (MAN1B111) and MAN1A1 have been associated with intellectual disability in pediatric populations. [33] Coagulation factor 12 has also been previously associated with cognitive impairment in murine models. [34]
Recent plasma proteomics analyses also suggest involvement of the coagulation cascade in mild cognitive impairment (MCI12), postoperative delirium, and other neurodegenerative diseases. [35–37] One study reported a significant increase in coagulation factor XI in patients diagnosed with probable Alzheimer’s disease compared to other groups. Furthermore, a decrease in cognitive function was associated with low coagulation factor IX. [38] Additionally, Girard et al. have previously described relationships between low Protein C levels and increased risk of delirium in critically ill patients, suggesting that deranged coagulation is involved in delirium pathogenesis. [36] Together, these findings support the biological plausibility of coagulation cascades being associated with postoperative delirium.
Our study may also shed light on proteins that have protective effects against postoperative delirium. We found the median relative abundance ratios of angiotensinogen, ceruloplasmin, complement C2, and inter-alpha-trypsin inhibitor (ITIH313) were higher in patients who did not develop delirium compared to those who did. As we discuss in the next section, our findings appear consistent with other neurocognitive studies.
In murine models for vascular dementia, and in Alzheimer’s disease cohorts, angiotensinogen genes were downregulated or dysregulated. [39–41] Ceruloplasmin, involved in ferroxidase activity and copper transport, was decreased in studies involving Alzheimer’s patients, with iron retention noted in brain tissues. [42,43] Increased activity of complement C2, part of the classical complement pathway, has also been associated with cognitive impairment. Higher complement levels were noted in the brain tissue of Alzheimer’s patients, and those with vascular angiopathies. [44–46] By contrast, our findings of greater complement abundance ratios in patients without delirium suggest patients with postoperative delirium may have had increased consumptive processes, requiring further study. Follow-up proteomics studies are needed to assess whether proteins dysregulated in dementia are also associated with delirium.
In addition to the above-mentioned proteins, we also found higher abundance ratios of ITIH3, an endopeptidase inhibitor, in patients who did not develop postoperative delirium. ITIH3 is in the same family as SERPINA3 (a protease inhibitor) which has been associated with postoperative delirium. [47] The exact pathway through which ITIH3 may be involved in postoperative delirium is not clear, though polymorphisms in this gene have been targeted for schizophrenia treatment when negative symptoms predominate. [48]
Taken together, our research findings appear to be consistent with other recent proteomics studies in the field of postoperative delirium. Complement, zinc alpha-2 glycoprotein (AZGP114) and alpha-1 chymotrypsin (SERPINA315alpha-1 chymotrypsin) were dysregulated in a recent serum proteomic study in elderly non-cardiac surgery patients. [18] As AZGP1 is involved in lipid metabolism, and SERPINA3 inhibits enzymes which convert angiotensin-1 to active angiotensin-2, we believe our results may add candidate proteins for a new delirium model that includes not only inflammatory pathways but also lipid homeostasis and vascular autoregulation. Our findings, if confirmed by larger studies, also suggest dysregulated protein pathways may overlap across different surgical populations.
There are several important limitations to our study. Firstly, this was an exploratory study nested within a clinical trial, and the sample size was not informed by a priori selection of candidate proteins. Given the small sample size and hypothesis-generating nature of the study, our findings are not corrected for multiple comparisons or false discovery rates. We acknowledge the possibility that proteins associated with postoperative delirium may have been confounded by the inflammatory pathology of esophageal malignancy. [28] Hence, studies with larger cohorts are needed to better delineate the relationships between proteins identified in our study with postoperative delirium while adjusting for potential confounders. Secondly, our study lacks a validation or confirmatory cohort, including use of protein-specific validation by mass spectrometry or enzyme-linked immunoassays, or comparison with protein levels in the cerebrospinal fluid. [49] Replicating our findings in larger cohorts comprising a broader range of surgical patients, including those without malignancy, may expand our understanding of postoperative delirium. Thirdly, we compared protein expression at two time points: preoperative and postoperative day one. As our study population had short delirium duration (median delirium duration in days: 1, IQR: 1–2), and not all patients experienced delirium on postoperative day 1 (n=16, or 57% experienced delirium on POD 1), additional blood sample collection later in the hospital course may have identified additional proteins. Finally, the time between pre-operative assessments and surgery between study participants was variable between study participants, but we are not aware of any significant interim health events which may have contributed to delirium or cognitive change affecting our results. Similarly, while the patients were enrolled in a randomized trial and there were no significant differences in patients assigned to intervention or control groups, we cannot exclude a potential small effect of haloperidol on the protein expression results in our study. The strengths of our study include the use of matched controls and use of quantitative proteomics with TMT isobaric labeling to achieve high sensitivity and precision results.
Our study describes altered protein expression in esophagectomy patients with postoperative delirium. Further studies are needed to better define abnormal protein expression in postoperative delirium.
Supplementary Material
S1. Supplemental Digital Content; Proteomics Analysis Workflow. In-depth explanation of the digestion and cleaning process of samples along with Protein detection and calibration methods.
Highlights:
Changes in protein pathways are associated with delirium after esophagectomy.
Proteins involved in coagulation, inflammation, and protein handling were identified.
Our results may help develop future targeted therapies for post-operative delirium.
Acknowledgements:
We would like to thank our patients, their families, the healthcare team, and Team Vitality without whom we could not advance the scientific understanding of delirium.
Funding
This work was supported by National Institute of Aging (NIA) [grant numbers 1K76AG074925–01A1, R01 AG067631–04, R01 AGO55391–05, R01 AG067631–05, K23 AG076662–02] and Indiana Health Values Fund Grant [grant number VFR 398]
Figure A.1.
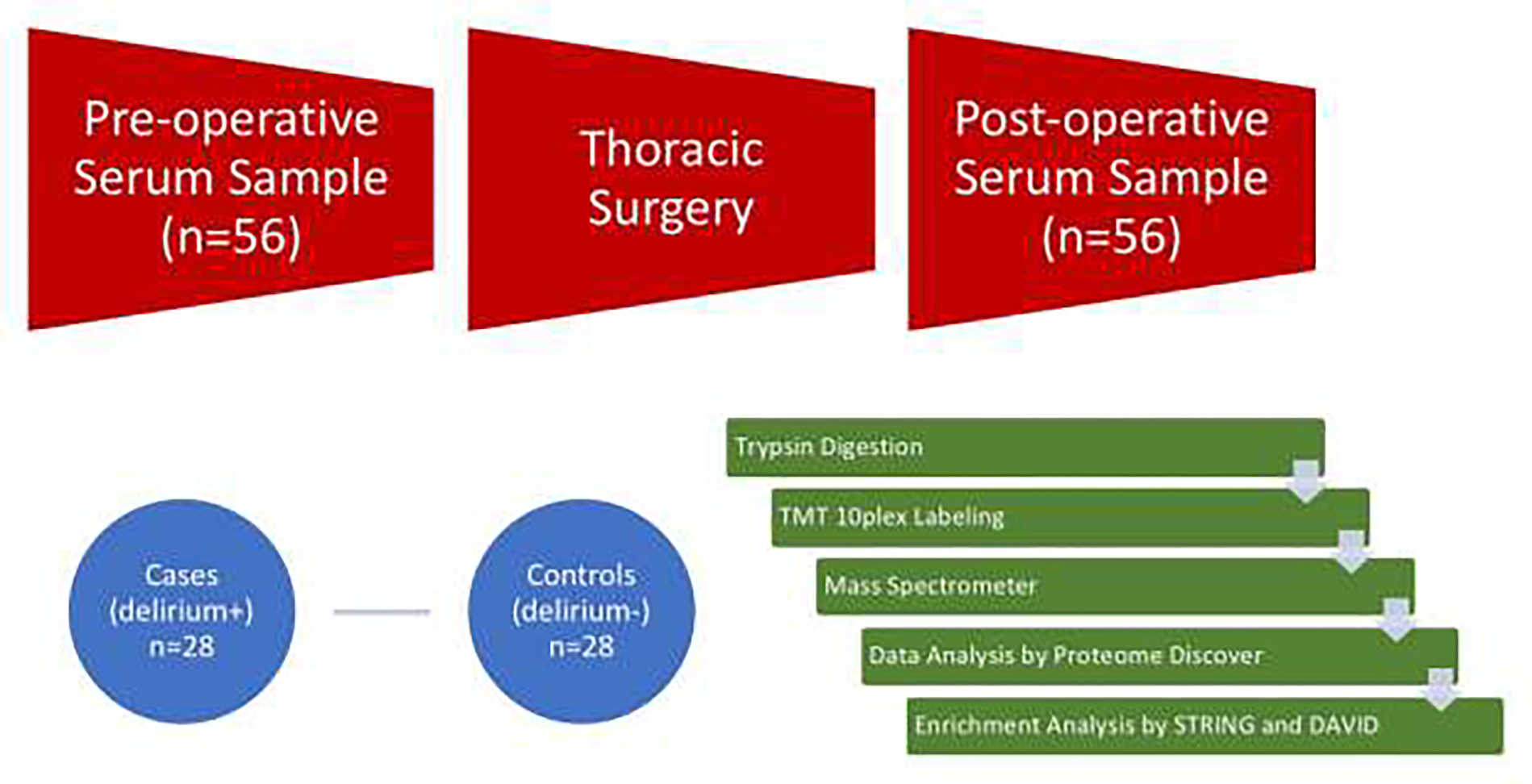
Experimental Workflow.
Figure A.2.
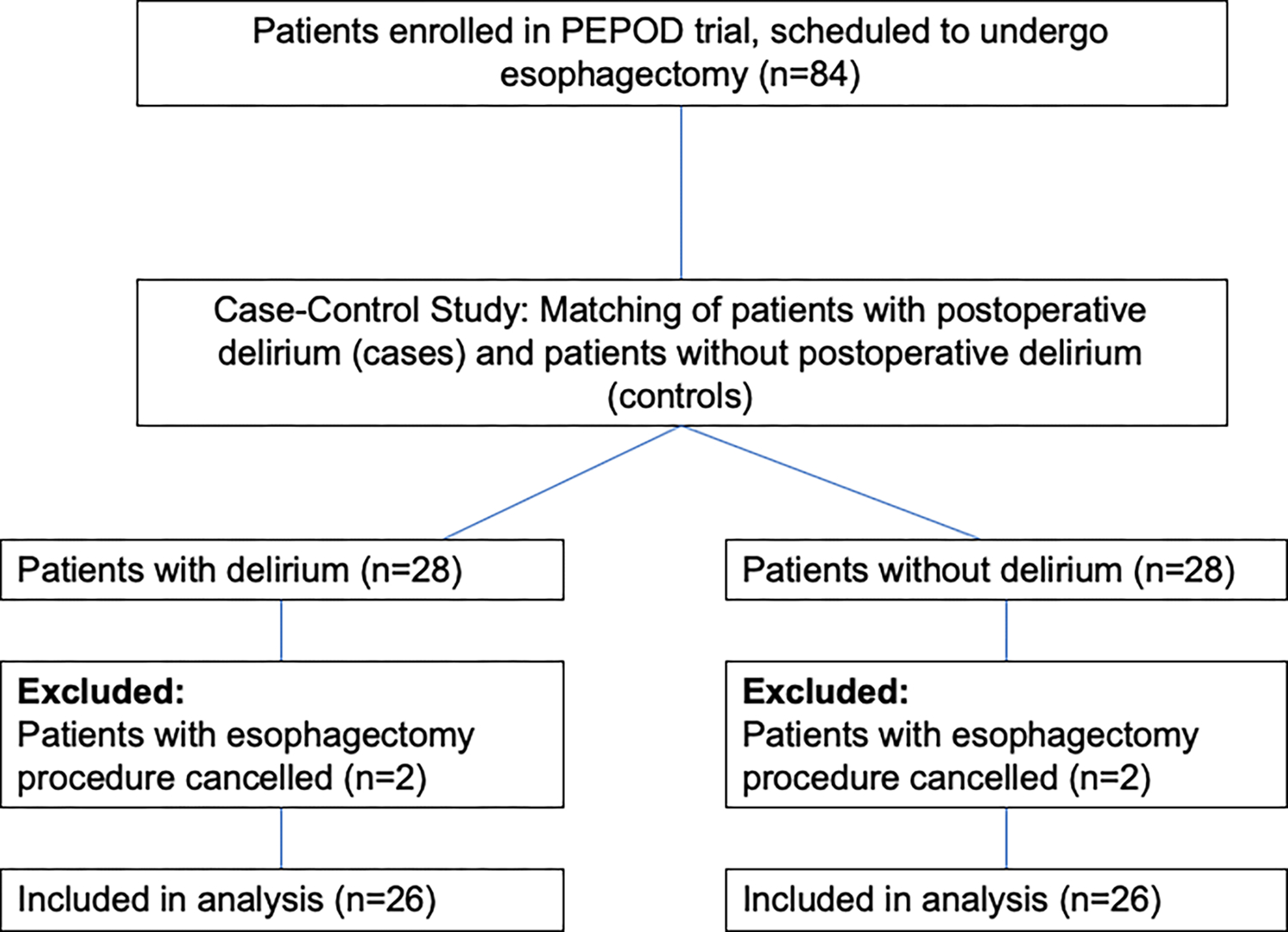
Patients screened for eligibility in the study.
Figure A.3.
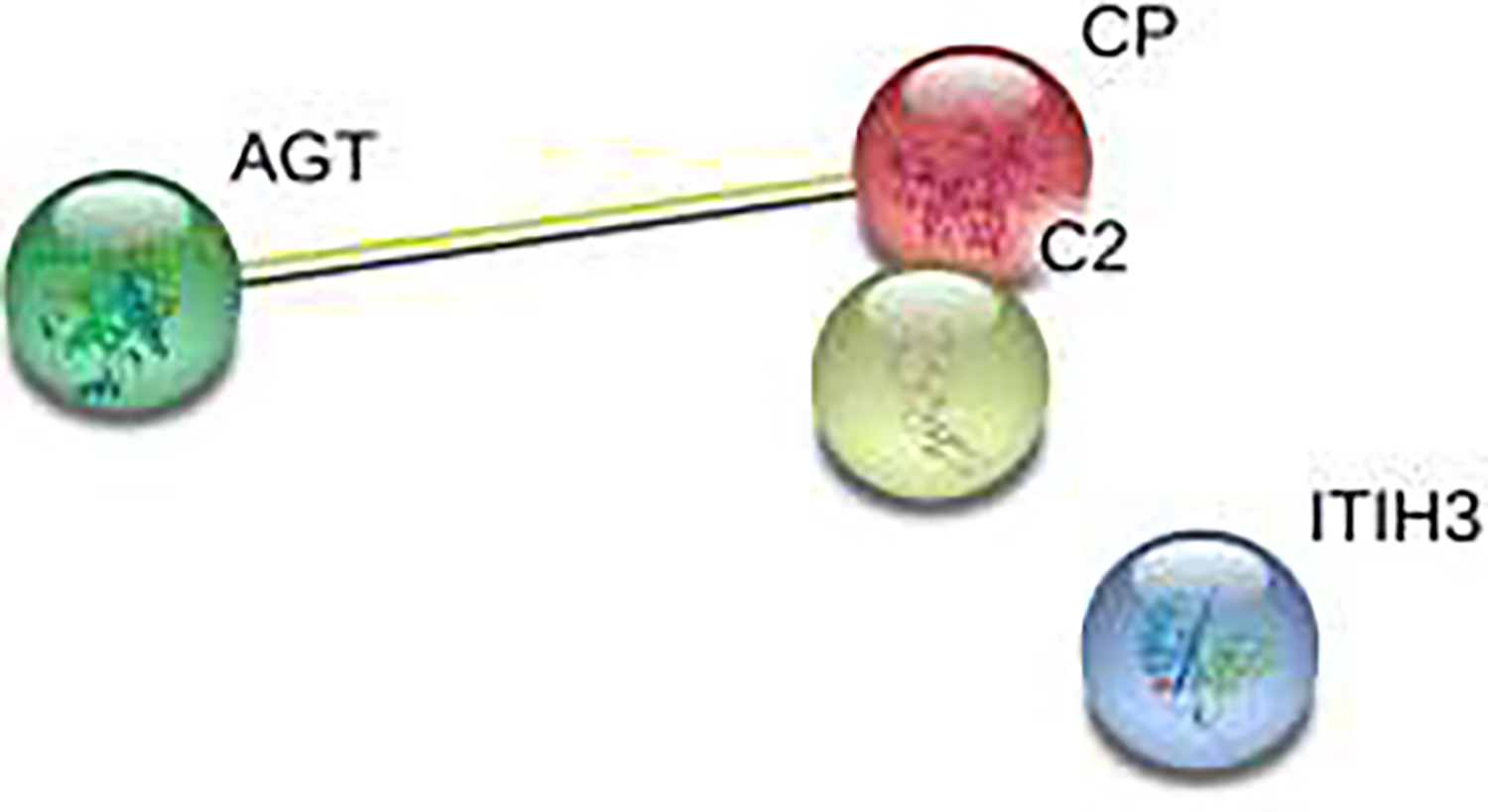
Co-expression of Angiotensinogen and Ceruloplasmin.
AGT: Angiotensinogen, CP: Ceruloplasmin: C2: Complement C2; ITIH3: Inter-alpha-trypsin inhibitor heavy chain H3 (ITIH3).
Footnotes
Wilcoxon-Mann-Whitney Odd
Preventing Postoperative Delirium after Major Noncardiac Thoracic Surgery
Richmond Agitation Sedation Scale
Confusion Assessment Method for the ICU
Delirium Rating Scale-Revised-98
Acute Physiology and Chronic Health Evaluation score
American Society of Anesthesiologists
Repeatable Battery for the Assessment of Neuropsychological Status
STrengthening Reporting of OBservational studies in Epidemiology – Molecular Epidemiology
Mannosyl-oligosaccharide 1,2-alpha-mannosidase
Mannosidase alpha class 1B member 1
Mild cognitive impairment
inter-alpha-trypsin inhibitor
Complement, zinc alpha-2 glycoprotein
alpha-1 chymotrypsin
Declaration of Competing Interest.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interv Aging. 2008;3(2):351–5. doi: 10.2147/cia.s2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KH, Ha YC, Lee YK, Kang H, Koo KH. Frequency, risk factors, and prognosis of prolonged delirium in elderly patients after hip fracture surgery. Clin Orthop Relat Res. Sep 2011;469(9):2612–20. doi: 10.1007/s11999-011-1806-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Mo L, Hu H, Ou Y, Luo J. Risk factors of postoperative delirium after cardiac surgery: a meta-analysis. Journal of Cardiothoracic Surgery. 2021;16(1)doi: 10.1186/s13019-021-01496-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi M, Takeuchi H, Fujisawa D, et al. Incidence and risk factors of postoperative delirium in patients with esophageal cancer. Ann Surg Oncol. Nov 2012;19(12):3963–70. doi: 10.1245/s10434-012-2432-1 [DOI] [PubMed] [Google Scholar]
- 5.Brown CH. Delirium in the cardiac surgical ICU. Curr Opin Anaesthesiol. Apr 2014;27(2):117–22. doi: 10.1097/aco.0000000000000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Risk factors of delirium after cardiac surgery: a systematic review. Eur J Cardiovasc Nurs. Dec 2011;10(4):197–204. doi: 10.1016/j.ejcnurse.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Whitlock EL, Torres BA, Lin N, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. Apr 2014;118(4):809–17. doi: 10.1213/ane.0000000000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan BA, Perkins AJ, Campbell NL, et al. Preventing Postoperative Delirium After Major Noncardiac Thoracic Surgery-A Randomized Clinical Trial. J Am Geriatr Soc. Dec 2018;66(12):2289–2297. doi: 10.1111/jgs.15640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan BA, Perkins AJ, Campbell NL, et al. Pharmacological Management of Delirium in the Intensive Care Unit: A Randomized Pragmatic Clinical Trial. J Am Geriatr Soc. May 2019;67(5):1057–1065. doi: 10.1111/jgs.15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Boogaard M, Slooter AJC, Bruggemann RJM, et al. Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium: The REDUCE Randomized Clinical Trial. JAMA. Feb 20 2018;319(7):680–690. doi: 10.1001/jama.2018.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard TD, Exline MC, Carson SS, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med. Dec 27 2018;379(26):2506–2516. doi: 10.1056/NEJMoa1808217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maldonado JR. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. Nov 2018;33(11):1428–1457. doi: 10.1002/gps.4823 [DOI] [PubMed] [Google Scholar]
- 13.Bjornsson GL, Thorsteinsson L, Gudmundsson KO, Jonsson H Jr., Gudmundsson S, Gudbjornsson B. Inflammatory cytokines in relation to adrenal response following total hip replacement. Scand J Immunol. Jan 2007;65(1):99–105. doi: 10.1111/j.1365-3083.2006.01872.x [DOI] [PubMed] [Google Scholar]
- 14.Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. Jan 1995;161(1):17–22. [PubMed] [Google Scholar]
- 15.Hofer S, Bopp C, Hoerner C, et al. Injury of the blood brain barrier and up-regulation of icam-1 in polymicrobial sepsis. J Surg Res. May 15 2008;146(2):276–81. doi: 10.1016/j.jss.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 16.Nishioku T, Dohgu S, Takata F, et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol. May 2009;29(3):309–16. doi: 10.1007/s10571-008-9322-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. Jul 2008;63(7):764–72. doi: 10.1093/gerona/63.7.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasunilashorn SM, Ngo LH, Chan NY, et al. Development of a Dynamic Multi-Protein Signature of Postoperative Delirium. J Gerontol A Biol Sci Med Sci. Jan 16 2019;74(2):261–268. doi: 10.1093/gerona/gly036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Ju JW, Oh SY, Kim J, Jung CW, Ryu HG. Impact of timing and duration of postoperative delirium: a retrospective observational study. Surgery. Mar 15 2018;doi: 10.1016/j.surg.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 20.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. Nov 15 2002;166(10):1338–44. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA Dec 5 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. Spring; 2001;13(2):229–42. doi: 10.1176/jnp.13.2.229 [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 24.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. Sep 21 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 25.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. Autumn; 1969;9(3):179–86. [PubMed] [Google Scholar]
- 26.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. Aug 1981;9(8):591–7. doi: 10.1097/00003246-198108000-00008 [DOI] [PubMed] [Google Scholar]
- 27.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. Jun 1998;20(3):310–9. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Wang Q, Chang J, et al. Serum proteomics unveil characteristic protein diagnostic biomarkers and signaling pathways in patients with esophageal squamous cell carcinoma. Clin Proteomics. May 24 2022;19(1):18. doi: 10.1186/s12014-022-09357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W, Xie L, He YH, et al. Large-scale and high-resolution mass spectrometry-based proteomics profiling defines molecular subtypes of esophageal cancer for therapeutic targeting. Nat Commun. Aug 16 2021;12(1):4961. doi: 10.1038/s41467-021-25202-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. Jul 2012;256(1):95–103. doi: 10.1097/SLA.0b013e3182590603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita M, Otsu H, Kawano H, et al. Gender differences in prognosis after esophagectomy for esophageal cancer. Surg Today. Mar 2014;44(3):505–12. doi: 10.1007/s00595-013-0573-x [DOI] [PubMed] [Google Scholar]
- 32.Fuchita M, Khan SH, Perkins AJ, et al. Perioperative Risk Factors for Postoperative Delirium in Patients Undergoing Esophagectomy. Ann Thorac Surg. Jul 2019;108(1):190–195. doi: 10.1016/j.athoracsur.2019.01.040 [DOI] [PubMed] [Google Scholar]
- 33.Rymen D, Peanne R, Millon MB, et al. MAN1B1 deficiency: an unexpected CDG-II. PLoS Genet. 2013;9(12):e1003989. doi: 10.1371/journal.pgen.1003989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen ZL, Revenko AS, Singh P, MacLeod AR, Norris EH, Strickland S. Depletion of coagulation factor XII ameliorates brain pathology and cognitive impairment in Alzheimer disease mice. Blood. May 4 2017;129(18):2547–2556. doi: 10.1182/blood-2016-11-753202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girard TD, Ware LB, Bernard GR, et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med. Dec 2012;38(12):1965–73. doi: 10.1007/s00134-012-2678-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mietani K, Hasegawa-Moriyama M, Yagi K, et al. Elevation of serum plasminogen activator inhibitor-1 predicts postoperative delirium independent of neural damage: a sequential analysis. Sci Rep. Oct 12 2022;12(1):17091. doi: 10.1038/s41598-022-21682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begic E, Hadzidedic S, Obradovic S, Begic Z, Causevic M. Increased Levels of Coagulation Factor XI in Plasma Are Related to Alzheimer’s Disease Diagnosis. J Alzheimers Dis. 2020;77(1):375–386. doi: 10.3233/jad-200358 [DOI] [PubMed] [Google Scholar]
- 39.Mateos L, Ismail MA, Gil-Bea FJ, et al. Upregulation of brain renin angiotensin system by 27-hydroxycholesterol in Alzheimer’s disease. J Alzheimers Dis. 2011;24(4):669–79. doi: 10.3233/JAD-2011-101512 [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Swaminathan S, Inlow M, et al. Influence of genetic variation on plasma protein levels in older adults using a multi-analyte panel. PLoS One. 2013;8(7):e70269. doi: 10.1371/journal.pone.0070269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur P, Muthuraman A, Kaur M. The implications of angiotensin-converting enzymes and their modulators in neurodegenerative disorders: current and future perspectives. ACS Chem Neurosci. Apr 15 2015;6(4):508–21. doi: 10.1021/cn500363g [DOI] [PubMed] [Google Scholar]
- 42.Zhao YS, Zhang LH, Yu PP, et al. Ceruloplasmin, a Potential Therapeutic Agent for Alzheimer’s Disease. Antioxid Redox Signal. May 10 2018;28(14):1323–1337. doi: 10.1089/ars.2016.6883 [DOI] [PubMed] [Google Scholar]
- 43.Siotto M, Simonelli I, Pasqualetti P, et al. Association Between Serum Ceruloplasmin Specific Activity and Risk of Alzheimer’s Disease. J Alzheimers Dis. 2016;50(4):1181–9. doi: 10.3233/JAD-150611 [DOI] [PubMed] [Google Scholar]
- 44.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Pathol. Mar 1999;154(3):927–36. doi: 10.1016/S0002-9440(10)65340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker DG, Dalsing-Hernandez JE, Lue LF. Human postmortem brain-derived cerebrovascular smooth muscle cells express all genes of the classical complement pathway: a potential mechanism for vascular damage in cerebral amyloid angiopathy and Alzheimer’s disease. Microvasc Res. Apr 2008;75(3):411–9. doi: 10.1016/j.mvr.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terai K, Walker DG, McGeer EG, McGeer PL. Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res. Sep 26 1997;769(2):385–90. doi: 10.1016/s0006-8993(97)00849-4 [DOI] [PubMed] [Google Scholar]
- 47.Poljak A, Hill M, Hall RJ, et al. Quantitative proteomics of delirium cerebrospinal fluid. Transl Psychiatry. Nov 4 2014;4:e477. doi: 10.1038/tp.2014.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandl EJ, Lett TA, Chowdhury NI, et al. The role of the ITIH3 rs2535629 variant in antipsychotic response. Schizophr Res. Oct 2016;176(2–3):131–135. doi: 10.1016/j.schres.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 49.Wiredu K, Aduse-Poku E, Shaefi S, Gerber SA. Proteomics for the Discovery of Clinical Delirium Biomarkers: A Systematic Review of Major Studies. Anesth Analg. Mar 1 2023;136(3):422–432. doi: 10.1213/ane.0000000000006246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Supplemental Digital Content; Proteomics Analysis Workflow. In-depth explanation of the digestion and cleaning process of samples along with Protein detection and calibration methods.


