Abstract
Skin expands and regenerates in response to mechanical stretch. This important homeostasis process is critical for skin biology and can be exploited to generate extra skin for reconstructive surgery. Atmospheric oxygen uptake is important in skin homeostasis. However, whether and how cutaneous atmospheric oxygen uptake changes during mechanical stretch remains unclear, and relevant research tools to quantify oxygen flux are limited. Herein, we used the scanning micro-optrode technique (SMOT), a noninvasive self-referencing optical fiber microsensor, to achieve real-time measurement of cutaneous oxygen uptake from the atmosphere. An in vivo mechanical stretch induced-skin expansion model was established, and an in vitro Flexcell Tension system was used to stretch epidermal cells. We found that oxygen influx of skin increased dramatically after stretching for 1 to 3 days and decreased to the non-stretched level after 7 days. The enhanced oxygen influx of stretched skin was associated with increased epidermal basal cell proliferation and impaired epidermal barrier. In conclusion, mechanical stretch increases cutaneous oxygen uptake with temporal-spatial characteristics, correlating with cell proliferation and barrier changes, suggesting a fundamental mechanistic role of oxygen uptake in the skin in response to mechanical stretch. Optical fiber microsensor-based oxygen uptake detection provides a non-invasive approach to understand skin homeostasis.
Keywords: Optical fiber sensor, skin expansion, cutaneous oxygen uptake, mechanical stretch, surgery
Background
It has been observed since the nineteenth century that human skin can directly take up oxygen from the atmosphere. Human epidermis is almost exclusively supplied by atmospheric oxygen rather than the oxygen from dermal circulation1–3. Keratinocyte sensing of atmosphere oxygen contributes significantly to the mammalian systemic adaption to environmental hypoxia4. A recent study revealed that differential atmospheric oxygen level regulated the axis of keratinocytes proliferation-differentiation5. In addition, our previous work showed that atmospheric oxygen influx correlated with regeneration efficiency in a Xenopus wound healing model6. Thus, the atmospheric oxygen uptake has an essential role in maintaining epidermal homeostasis.
As the outmost surface of the human body, skin is constantly exposed to mechanical stress. Mechanical stress is involved in many cutaneous physiological and pathological process7,8. For example, mechanical stretch is a key factor in the pathogenesis of hypertrophic scars9,10 and psoriasis11. Human skin can be stretched and enlarged remarkably during pregnancy or obesity, and also in skin expansion for skin grafts in plastic surgery up to tens of folds of area size. Epidermis responds to stretch at the single-cell level in skin expansion with distinct proliferation and differentiation subpopulations mediated by mechano-sensing, biochemical signaling, and regulated transcriptomics12–15. However, oxygen uptake by the skin has not been studied when the mammalian epidermal homeostasis is disrupted during mechanical stretching.
The optical fiber technique has been clinically used in medical diagnosis and therapeutic monitoring since the 1960s16,17. An optical fiber can be placed non-invasively close to external organs such as the skin or cornea, providing a convenient and safe approach for tissue assessment. Because oxygen has a quenching effect on fluorescence, oxygen concentration can be indicated by detecting the fluorescent substance’s degree of fluorescence18. A new development of this optical sensing technique coupled with self-referencing gives a quantitative measurement of flux of oxygen with high spatial-temporal resolution6,19. Based on this novel approach, here we used a scanning micro-optrode technique (SMOT), a noninvasive self-referencing optical fiber microsensor, to determine spatiotemporal changes in oxygen uptake in a mechanical stretch induced skin expansion model and assessed the epidermal proliferation and barrier function.
Methods
Animal model
Animal procedures were approved by the Institutional Animal Care and Use Committees at University of California, Davis (protocol #20909). Female Wistar rats aged 4–6 weeks were obtained from Envigo (Indianapolis, IN, USA). Based on previous methods with modifications20,21, the skin expansion model was established to apply mechanical stretch to the skin. Briefly, under anesthesia, 10 ml silicone expanders (Guangzhou Wanhe Plastic Materials Co., Ltd., Guangzhou, China) were implanted into the dorsum subcutaneously. Sterile physiological saline was injected into the expander after 7 days post-operation to 120 mmHg of the intracapsular pressure monitored with a modified sphygmomanometer.
Oxygen flux measurement
The oxygen flux was measured by SMOT as previously described in detail19. In brief, hair on the dorsum was removed with hair remover cream (Veet) prior to measurements. During measurements (under anesthesia), the tip of the optrode was brought to measurement position ~ 10 μm from interfollicular epidermis and at least three measurements were taken at each position (outside stretch area, and edge, middle, and center of stretch area; see Fig. 3a). All procedures were conducted at room temperature 21.1 – 22.4 °C. The rat body temperature was 36.0 – 36.4 °C.
Figure 3. Spatial characteristic of atmospheric oxygen flux in rat skin expansion model.

(a) Schematic of skin expansion model and measuring points A-D. Point A is located outside the stretched area. Point D is at the center of the stretched area, point B is at the edge of the stretched area and point C is at the midpoint between B and D. (b) Results of the oxygen flux in rat skin. “A, B, C and D” represent point A-D for different sites as shown above. Data are presented as means ± SD. Each dot represents one experiment. * P < 0.05. ** P < 0.01.*** P < 0.001.
Histology and Immunohistochemistry
Skin tissue were obtained, fixed and embedded in paraffin. Cross sections were stained with hematoxylin and eosin. For immunohistochemistry staining, sections were antigen repaired and blocked and incubated with primary antibody. Then the sections were washed and incubated with corresponding HRP-conjugated secondary antibody. Slides were scanned using a slide scanner (Pannoramic SCAN II; 3DHistech Kft. Budapest, Hungary), and images were randomly captured by a slide viewer (CaseViewer; 3DHistech Kft. Budapest, Hungary). The quantitative analysis of positive expression was conducted using ImageJ (National Institutes of Health, Bethesda, MD, USA). The following primary and secondary antibodies were used: anti-Ki67 (1:200; Abcam, Cambridge, UK), anti-Loricrin (1:500; Abcam, Cambridge, UK) and Goat anti-Rabbit Secondary Antibody (1:500; Jackson Immuno Research Laboratories, West Grove, PA, USA).
Cell culture and mechanical stretching
Normal human primary epidermal keratinocytes (HEKs) were purchased from ScienCell Research Laboratories. The HEKs were cultured in keratinocyte medium containing 1% keratinocyte growth supplement and 1% penicillin-streptomycin (all from ScienCell Research Laboratories, Carlsbad, CA, USA). HaCat cells were cultured in DMEM medium (HyClone) containing 10% FBS (Gibco, Australia) and 1% penicillin-streptomycin. The cultures were maintained in incubator at 37°C in a humidified atmosphere with 5% CO2.
To apply mechanical stretch to HaCat cells and HEKs, cells were seeded on six-well flexible silicone rubber BioFlex plates (Flexcell International Corporation, Hillsborough, NC, USA) at a density of 5×105 cells/well in 2ml of medium. Cells were cultured for 24 h to reach 60%−80% confluence before mechanical stretch was applied. Cyclic mechanical stretch was applied in a sinusoidal pattern with 10% amplitude at 0.5 Hz for 24h using an FX-5000T™ Flexcell Tension Plus device (Flexcell International Corporation, Hillsborough, NC, USA) as previously reported22. HaCat cells and HEKs cultured in the same plates but not stretched served as controls.
Western blot
Cells were lysed and protein fractions were run on SDS-PAGE gel and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked and then incubated with primary antibodies against Loricrin (1:1000; Affinity Biosciences, USA) and GAPDH (1:10000; Bioworld, Minnesota, USA). The blots were then incubated with HRP-conjugated secondary antibodies and visualized using an enhanced chemiluminescence detection system (Millipore, Bedford, MA, USA). Quantitative analysis was performed for immunoreactive bands using ImageJ software.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan) was used to assess cell proliferation. After stretching, cells were reseeded in 96-well plates at a density of 1 × 104 cells/well. After culturing for 24 h and 48 h in complete medium, each well received 90 μl medium mixed with 10 μl CCK-8 reagent and was further incubated at 37°C for 2 h. Subsequently, the optical density (OD) at 450 nm (630 nm as reference) was measured using an Infinite M200 PRO microplate reader (TECAN, Switzerland).
Statistical analyses
Data are presented as mean ± SD. A two-tailed Student’s t-test or analysis of variance (ANOVA) was used to assess the statistical significance. The differences were considered statistically significant when P < 0.05.
Results
Rat skin takes up atmospheric oxygen
We first determined cutaneous uptake of atmospheric oxygen by unstretched skin in anesthetized rats. Oxygen concentration at the near and far positions close to the skin surface were detected during the excursion of the micro-optrode (Figure 1a). The tip of the micro-optrode is coated with oxygen sensitive fluorophore. The quenching effect of oxygen allows the measurement of the oxygen level (Figure 1b). To eliminate the possible effects of hair remover on oxygen flux, we detected the oxygen flux on footpad (no hair) pre- and post-hair remover treatment (Supplementary Figure 1a). The results showed that hair remover did not significantly affect the oxygen flux (P >0.05) (Supplementary Figure 1b). Freshly isolated skin ex vivo had similar oxygen influx (−48.7 ± 11.03 pmol cm−2 s−1) as that of the in vivo skin (−47.32 ± 8.79 pmol cm−2 s−1), which supports the notion that atmospheric oxygen can be taken up directly by the skin. In contrast, after the skin was fixed in 4% paraformaldehyde (PFA), oxygen influx returned to a value near zero (−6.32 ± 3.98 pmol cm−2 s−1) (P < 0.001) (Figure 1c, d). This indicates that dead skin lost the ability to take up atmospheric oxygen.
Figure 1. Atmospheric oxygen flux at rat skin.
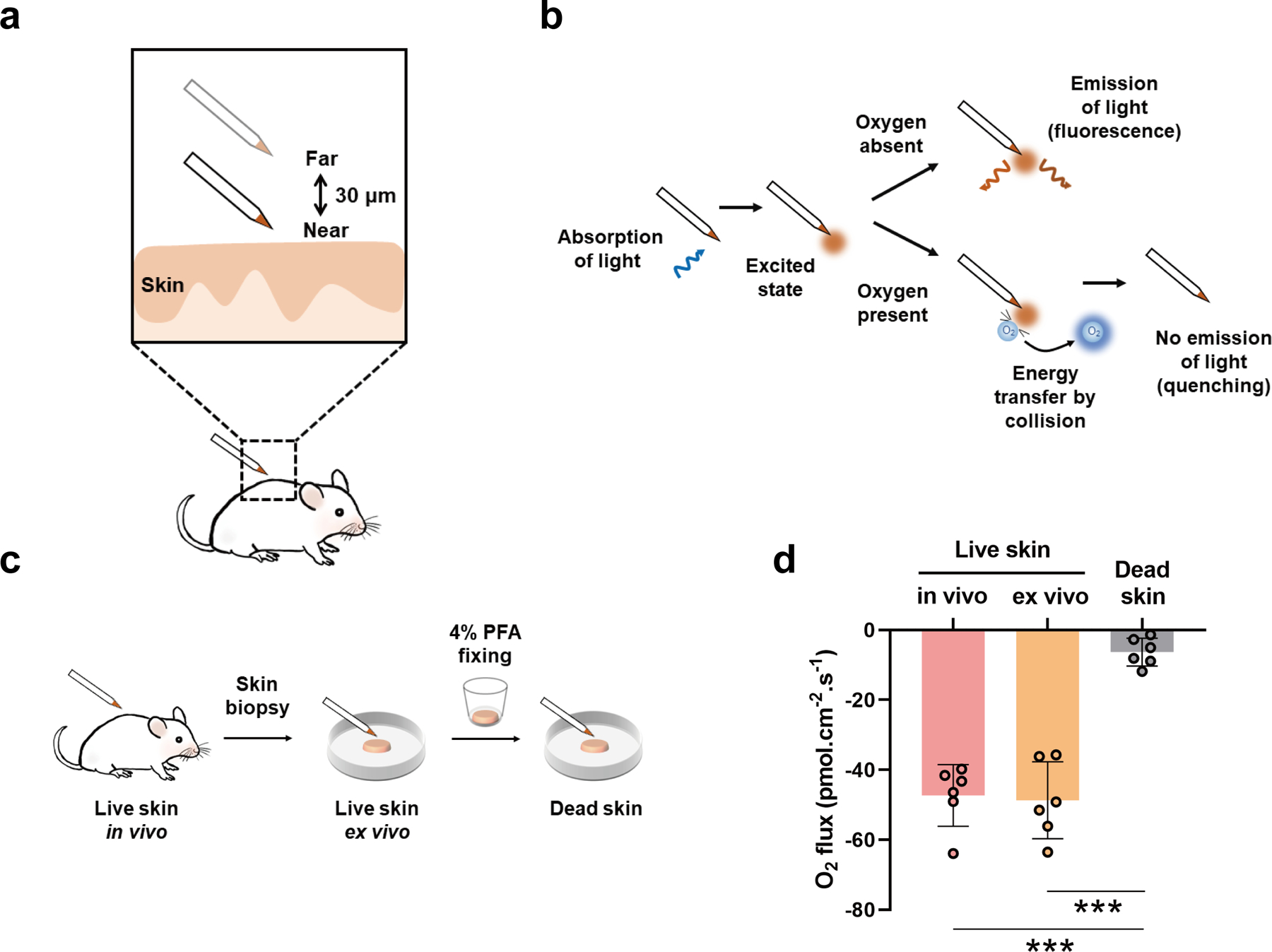
(a, b) Schematic of the fluorescence quenching-based oxygen measurement using the scanning micro-optrode technique (SMOT). (c) Schematic of measurements in the live/dead rat skin. (d) Results of the oxygen flux in the live/dead rat skin. Data are presented as means ± SD. Each dot represents one rat. *** P < 0.001.
Mechanical stretch increases cutaneous atmospheric oxygen influx in a time-dependent manner
To detect the cutaneous atmospheric oxygen influx in response to mechanical stretch, we measured the oxygen flux at the center area of stretched skin at 6 hours, 1, 3 and 7 days after expander inflation (Figure 2a). The oxygen flux of non-stretched skin was also measured as control. The results showed that there was no significant increase in oxygen influx at 6 hours post-inflation. However, oxygen influx was significantly increased after 1 day (P < 0.05) and 3 days (P < 0.001) stretching respectively, compared to non-stretched rat skin. Interestingly, the oxygen influx decreased to baseline after 7 days (P > 0.05) stretching, which showed no significance compared to the non-stretched skin (Figure 2b). Taken together, these data show that mechanical stretch increases the epidermal oxygen uptake in a time-dependent manner, and we chose 3 days post-stretching for subsequent experiments.
Figure 2. Atmospheric oxygen flux in stretched rat skin at different time points.
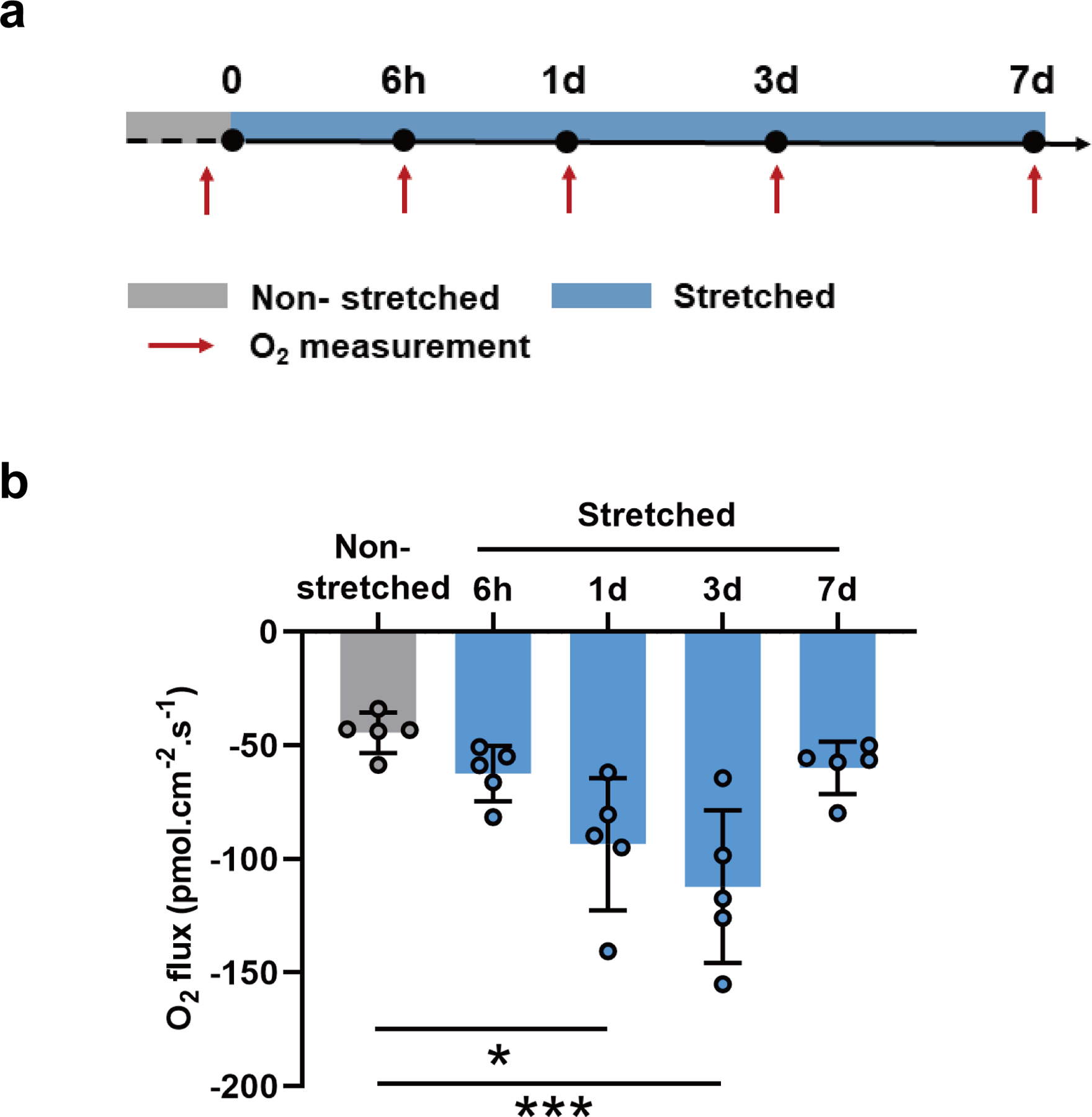
(a) Schematic timecourse of measurements in rat skin expansion. (b) Results of the oxygen flux in rat skin. “Control” represents non-stretched rat skin. “6h, 1d, 3d, 7d” represent stretched rat skin for different periods in hours and days. Data are presented as means ± SD. Each dot represents one rat. * P < 0.05. *** P < 0.001.
Spatial characteristic of oxygen influx at stretched skin
To define the spatial characteristic of the atmospheric oxygen uptake in rat skin expansion, we measured the oxygen flux at different sites in the rat skin expansion model after 3 days stretching. According to the shape contour of the expanded area, points A-D were selected to map the spatial characteristic of oxygen influx in skin expansion (Figure 3a). Point A is located outside the stretched area. Point B, C and D located in the stretched area. Point D is at the center of the stretched area, point B is at the edge of the stretched area and point C is at the midpoint between B and D. The overall results showed a significant increase of oxygen influx at point D compared to point A. For some rats (rat 1, 3 and 5), point B and C also showed an increase of oxygen influx compared to point A. However, there is no significant difference in point B, C and D on the whole (Figure 3b). Taken together, these findings indicate that rat skin, especially in the center site of the expanded area, takes up more atmospheric oxygen after stretching.
Mechanical stretch impairs epidermal barrier and increases epidermal keratinocytes proliferation in vitro and in vivo
We further investigated the reasons why oxygen influx increased in the stretched rat skin. It is likely that stretch induced epidermal barrier breaking leading to the increased atmospheric oxygen sink. We detected the epidermal barrier function in skin expansion by staining loricrin, a major component of cornified cell envelope, in the rat skin. The results showed that loricrin decreased significantly in 3-days-stretched rat skin compared to the non-stretched rat skin (Figure 4a, d). Meanwhile, the in vitro cell stretching assay also showed decreased loricrin expression level in HEKs and HaCat cells (Figure 4g) after mechanical stretching for 24h. These data indicate that impaired epidermal barrier after mechanical stretching might give rise to the increased oxygen influx in skin expansion. In addition, we detected the state of epidermal cell proliferation after stretching, because cell proliferation increased demand for oxygen. Ki67 positive cells were counted in the 3-days-stretched rat skin. The results showed that epidermal basal cell proliferation increased significantly in 3-days-stretched rat skin compared to the non-stretched rat skin (Figure 4a, c). Next, H&E staining showed increased thickness of epidermis in the 3-days-stretched rat skin compared to the non-stretched rat skin (Figure 4a, b), which may result from the increase of hyperproliferative epidermal cells in response to stretch. Moreover, the in vitro cell stretching assay also showed increased proliferative capacity in HEKs (Figure 4e) and HaCat cells (Figure 4f) after mechanical stretching for 24h. This enhanced capacity to proliferate lasted at least 48h even when detached from mechanical stretch stimulation. Together, these data illustrate that mechanical stretch-induced cell proliferation as well as epidermal barrier impairment might be correlated to the increased oxygen influx in skin expansion.
Figure 4. Assessment of epidermal barrier function and cell proliferation in vitro and in vivo.

(a) H&E, Ki67 and Loricrin staining in non-stretched rat skin and 3 days stretched rat skin. Scale bars: 50 μm. (b) Quantification of epidermal thickness. (c) Quantification of Ki67 positive cells per HPF. (d) Quantification of loricrin. (e, f) CCK-8 assay of human primary epidermal keratinocytes (HEK) and HaCat cells after stretching for 24h. (g) Western blot of loricrin in HEK and HaCat cells after stretching for 24h. Data are presented as means ± SD. Each dot represents one experiment. * P < 0.05. * *P < 0.01. *** P < 0.001.
Discussion
Skin homeostasis maintenance is a complex process. In response to extrinsic mechanical stimuli, intrinsic physiological reactions are orchestrated to restore the cutaneous homeostasis. In this context, the cutaneous oxygen uptake alterations in response to external mechanical stretch appear very interesting and remain to be elucidated. In our study, a real-time non-invasive SMOT was utilized to directly quantify the cutaneous atmospheric oxygen flux. We demonstrated that rat skin in vivo and ex vivo could directly take up atmosphere oxygen. Moreover, the cutaneous oxygen uptake increased significantly in response to mechanical stretch during skin expansion and was associated with enhanced epidermal cell proliferation and impaired epidermal barrier.
Cutaneous respiration has been known for a long time23,24. According to a review from Fitzgerald et al., the absorption of atmospheric oxygen through skin surface is approximately 30 to 100 ml m−2 h−1 24. M Stücker et al. (2002) developed non-invasive measurements of transcutaneous oxygen flux with an oxygen fluxoptode consisting of multiple layers of membrane. They reported the oxygen influx from the atmosphere at 0.53 ± 0.27 ml m−2 min−1 at normal human skin surface1. Based on the same measuring principle of oxygen-induced fluorescence quenching, we used the SMOT to detect the oxygen influx in vivo in an improved non-invasive self-referencing (near and far positions) mode without direct contact with the skin. The value we detect at rat skin under resting conditions varies in the range of −47.32 ± 8.79 pmol cm−2 s−1. Interestingly, we found that the oxygen influx in rat palm (foot) skin was less than the dorsal skin. One possible explanation is that the palm is more cornified than the dorsal skin which leads to the thickness barrier and thus lower oxygen diffusion.
Effective tissue regeneration requires an adequate supply of oxygen, which regulates cell proliferation, differentiation, and angiogenesis5,25,26. Continuous delivery of dissolved oxygen has been reported to significantly improve chronic wound healing27. The use of hyperbaric oxygen therapy for rapid skin expansion can effectively increase capillary blood flow to the expanded skin and skin growth28. Skin expansion usually involves stages including expander inflation, stretching, skin growth and relaxation. During this sequential process, mechanical stretch triggers a series of morphological and physiological changes in the epidermis and dermis29, while the atmospheric oxygen influx conditions are not well known. Here, our data reveal a spatio-temporal pattern of atmospheric oxygen influx during skin expansion. We observed that the oxygen influx increased at 1 to 3 days post-expansion and then returned to the control level at 7 days post-expansion. One possibility for this is relaxation of skin over time, because of the viscoelastic properties and skin growth which diminishes the pressure inside the expander. It is reported that after 7 days, there was no measurable pressure inside the expander30. Thus, the decreased atmospheric oxygen influx after 7 days stretching might result from the decreased strain that the expander imposed on the skin. Next, we observed the spatial characteristics of cutaneous atmospheric oxygen influx during stretch-mediated skin expansion. After stretching for 3 days, the overall spatial distribution trend showed enhanced cutaneous atmospheric oxygen uptake in the stretched area, especially the center portion of the area. Meanwhile, we noticed that the differences of atmospheric oxygen influx existed between different sites of stretched area in one rat. Adrián Buganza Tepole et al. showed that spatio-temporal evolution of the grown skin is co-related to the shape of the expander31.Besides, C Marquardt et al. reported that the intracutaneously measured tissue oxygen partial pressure exhibited a linear correlation with the force32. Therefore, we presumed the geometric shape of expander and thus spatial strain distribution difference may be a factor in the differential oxygen influx in the stretched area. However, an accurate stress profile of the expander has not been quantified. Some studies have used finite element analysis to model tissue expansion, providing a method to map the stress distribution of the skin during expansion33–35. Additionally, we observed strong inter-individual fluctuations of atmospheric oxygen influx. In our experiment, animal age, gender and skin humidity have been controlled. On the one hand, this may be explained by the fact that anesthesia condition, the breath triggered body movement and skin temperature inconsistencies could be the interference factors upon in vivo detection. On the other hand, the amount of skin deformation of each rat may vary due to the differential skin texture and elasticity in response to stretch, although we have controlled the intracapsular pressure of expander when expanding.
To understand the possible reasons involved in mechanical stretch-induced atmospheric oxygen influx peaking at 3 days post-expansion, we next investigated the epidermal barrier and cell proliferation conditions from the standpoint of oxygen flux following passive diffusion in a supply-demand way. Atmospheric air contains about 20.9% oxygen, higher than epidermis (0.2–0.8%) and dermis (>7%)3, which would theoretically result in the atmospheric oxygen flowing down a concentration gradient when the epidermal barrier is compromised. The stratum corneum is a significant epidermal barrier to gas diffusion from the atmosphere. When the epidermal barrier is disrupted, the skin permeability as well as oxygen diffusion is increased36–38. Stratum corneum components, including loricrin, filaggrin and involucrin, are functionally important to accomplish the barrier function39,40. Qiao et al. reported that down-regulation of loricrin, filaggrin and involucrin level in HaCat cells after mechanical stretch may contribute to the psoriasis progression11. Also, our previous work showed cyclic mechanical stretch inhibited loricrin expression in keratinocytes22. Moreover, human epidermal stem cells seeded on stiff substrates showed less expression of loricrin and filaggrin than that on soft substrates41. These indicate that skin barrier function is affected by mechanical stimulation. In accordance with these works, here we found that the epidermal barrier was impaired after stretching characterized by down-regulation of loricrin in vitro and in vivo. This might give an explanation for the robust atmospheric oxygen influx after stretching.
As determined by our experiments, mechanical stretch promoted epidermal keratinocyte proliferation in vitro and in vivo, in general agreement with previous reports13,22,42. Cell hyperproliferation consumed a large amount of oxygen. This might result in an aggravated hypoxic microenvironment in the epidermis. Our previous work verified higher levels of HIF-1α expression in stretched skin compared to non-stretched skin in a rat skin expansion model43, and the canonical HIF-1a pathway functioned as a predominant pathway activated by tissue expansion44. These data indicate the epidermal hypoxic microenvironment in skin expansion. Thus, we presumed that the atmospheric oxygen of relatively higher concentration sank down to the epidermis because the diffusion gradient is a key factor in oxygen delivery. Our previous work on a Xenopus tail model showed peak oxygen influx at 24h post amputation, which also indicated the increased oxygen demand due to cell proliferation6.
There are some limitations to our study. First, because of the fine tip of the micro-optrode, we choose interfollicular epidermis as the detecting points, while the follicular sites are not detected in our current experiment. Secondly, our data now enables us to know mechanical stretch increased cutaneous atmospheric oxygen uptake, we further hope to map the dynamic changes of strain distribution and specify the strain-oxygen influx relationship during the expanding process. Moreover, the specific mechanism of oxygen influx in the stretch-mediated skin expansion, especially the epidermis homeostasis maintenance, remains to be investigated. It is of great interest to further compare the oxygen uptake volume of epidermis alone and dermis alone with the advanced micro-optrode technique. Finally, translational studies on the oxygen uptake in pathological skin and the related effect of therapeutic agents in the view of oxygen influx will continue.
Conclusion
In conclusion, this study used a new optical sensing technology to measure oxygen uptake at skin, and provides the first evidence (to our knowledge) that skin responds to mechanical stretch by increasing atmospheric oxygen uptake with a spatiotemporal pattern, which correlates with down-regulation of barrier (e.g. loricrin) and increased keratinocyte proliferation. Such homeostasis responses are important for the physiology of highly stretchability that is characteristic of the skin more than other tissues. The self-referencing optical fiber microsensor provides a practical tool for monitoring dynamics of cutaneous oxygen uptake with superior spatiotemporal resolution and may be used in cutaneous disorder assessment and therapy.
Supplementary Material
Acknowledgments
This work was supported in part by NIH 1R01EY019101. Work in Zhao Laboratory is also supported by NEI Core Grant (P-30 EY012576, PI. Jack Werner / Marie Burns). S. S. was sponsored by China Scholarship Council, Shanghai Pujiang Program (2021PJD034) and National Natural Science Foundation of China (82102343). The researchers are grateful to Louis and Karen Burns family donation, Un-restricted Grant from Research to Prevent Blindness, Inc. S. S. would like to thank Brad Shibata for technical supporting in histology.
List of abbreviations
- SMOT
scanning micro-optrode technique
- HEKs
human primary epidermal keratinocytes
- CCK-8
Cell Counting Kit-8
- OD
optical density
- ANOVA
analysis of variance
- PFA
paraformaldehyde
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Stucker M, Struk A, Altmeyer P, Herde M, Baumgartl H, Lubbers DW. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol-London. Feb 1 2002;538(3):985–994. doi: 10.1013/jphysiol.2001.013067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stücker M, Altmeyer P, Struk A, et al. The Transepidermal Oxygen Flux from the Environment is in Balance with the Capillary Oxygen Supply. Journal of Investigative Dermatology. 2000/March/01/ 2000;114(3):533–540. doi: 10.1046/j.1523-1747.2000.00911.x [DOI] [PubMed] [Google Scholar]
- 3.Rezvani HR, Ali N, Nissen LJ, et al. HIF-1alpha in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. The Journal of investigative dermatology. Sep 2011;131(9):1793–805. doi: 10.1038/jid.2011.141 [DOI] [PubMed] [Google Scholar]
- 4.Boutin AT, Weidemann A, Fu Z, et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. Apr 18 2008;133(2):223–34. doi: 10.1016/j.cell.2008.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh R, Szeverenyi I, Lee B, et al. Oxygen-Mediated Control of the Keratinocyte Proliferation-Differentiation Axis. The Journal of investigative dermatology. Jan 2020;140(1):235–238 e3. doi: 10.1016/j.jid.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 6.Ferreira F, Raghunathan V, Luxardi G, Zhu K, Zhao M. Early redox activities modulate Xenopus tail regeneration. Nature communications. Oct 16 2018;9(1):4296. doi: 10.1038/s41467-018-06614-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CK, Lin HH, Harn HI, Hughes MW, Tang MJ, Yang CC. Mechanical forces in skin disorders. J Dermatol Sci. Mar 8 2018;doi: 10.1016/j.jdermsci.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Biggs LC, Kim CS, Miroshnikova YA, Wickstrom SA. Mechanical forces in the skin: roles in tissue architecture, stability, and function. The Journal of investigative dermatology. Jul 18 2019;doi: 10.1016/j.jid.2019.06.137 [DOI] [PubMed] [Google Scholar]
- 9.Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. Oct 2007;21(12):3250–61. doi: 10.1096/fj.07-8218com [DOI] [PubMed] [Google Scholar]
- 10.Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nature medicine. Jan 2012;18(1):148–52. doi: 10.1038/nm.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao P, Guo W, Ke Y, et al. Mechanical Stretch Exacerbates Psoriasis by Stimulating Keratinocyte Proliferation and Cytokine Production. The Journal of investigative dermatology. Jul 2019;139(7):1470–1479. doi: 10.1016/j.jid.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 12.Reichelt J Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol. Dec 2007;86(11–12):807–16. doi: 10.1016/j.ejcb.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 13.Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. The Journal of investigative dermatology. Mar 2004;122(3):783–90. doi: 10.1111/j.0022-202X.2004.22328.x [DOI] [PubMed] [Google Scholar]
- 14.Aragona M, Sifrim A, Malfait M, et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature. Aug 2020;584(7820):268–273. doi: 10.1038/s41586-020-2555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Y, Lyu C, Taylor A, et al. Mechanical tension mobilizes Lgr6(+) epidermal stem cells to drive skin growth. Sci Adv. Apr 29 2022;8(17):eabl8698. doi: 10.1126/sciadv.abl8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin LCL, Whelan WM, Vitkin IA. Optical Fiber Sensors for Biomedical Applications. In: Welch AJ, van Gemert MJC, eds. Optical-Thermal Response of Laser-Irradiated Tissue. Springer; Netherlands; 2011:661–712. [Google Scholar]
- 17.Tosi D, Poeggel S, Iordachita I, Schena E. Fiber Optic Sensors for Biomedical Applications. Opto-Mechanical Fiber Optic Sensors. 2018:301–333. [Google Scholar]
- 18.Zhao Y, Zhang H, Jin Q, Jia D, Liu T. Ratiometric Optical Fiber Dissolved Oxygen Sensor Based on Fluorescence Quenching Principle. Sensors (Basel). Jun 25 2022;22(13)doi: 10.3390/s22134811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira F, Luxardi G, Reid B, Ma L, Raghunathan V, Zhao M. Real-time physiological measurements of oxygen using a non-invasive self-referencing optical fiber microsensor. Nat Protoc. Feb 2020;15(2):207–235. doi: 10.1038/s41596-019-0231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M, Li Q, Sheng L, Li H, Weng R, Zan T. Bone marrow-derived mesenchymal stem cells transplantation accelerates tissue expansion by promoting skin regeneration during expansion. Annals of surgery. Jan 2011;253(1):202–9. doi: 10.1097/SLA.0b013e3181f9ba1ah [DOI] [PubMed] [Google Scholar]
- 21.Sheng L, Yang M, Du Z, Yang Y, Li Q. Transplantation of stromal vascular fraction as an alternative for accelerating tissue expansion. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. Apr 2013;66(4):551–7. doi: 10.1016/j.bjps.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Wang J, Zhang N, Zhang YF, Li QF. Identification of biomechanical force as a novel inducer of epithelial-mesenchymal transition features in mechanical stretched skin. American journal of translational research. 2015;7(11):2187–2198. [PMC free article] [PubMed] [Google Scholar]
- 23.Leibsohn E, Appel B, Ullrick WG, Tye MJ. Respiration of Human Skin. Journal of Investigative Dermatology. 1958;30(1):1–8. doi: 10.1038/jid.1958.1 [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald LR. Cutaneous respiration in man. Physiol Rev. Jul 1957;37(3):325–36. doi: 10.1152/physrev.1957.37.3.325 [DOI] [PubMed] [Google Scholar]
- 25.Guan Y, Gao N, Niu H, Dang Y, Guan J. Oxygen-release microspheres capable of releasing oxygen in response to environmental oxygen level to improve stem cell survival and tissue regeneration in ischemic hindlimbs. J Control Release. Mar 10 2021;331:376–389. doi: 10.1016/j.jconrel.2021.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. British Journal of Dermatology. 2010;163(2):257–268. doi: 10.1111/j.1365-2133.2010.09804.x [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. May 2020;6(20):eaba4311. doi: 10.1126/sciadv.aba4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju Z, Wei J, Guan H, Zhang J, Liu Y, Feng X. Effects of hyperbaric oxygen therapy on rapid tissue expansion in rabbits. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2012;65(9):1252–1258. doi: 10.1016/j.bjps.2012.03.028 [DOI] [PubMed] [Google Scholar]
- 29.Razzak MA, Hossain MS, Radzi ZB, Yahya NA, Czernuszka J, Rahman MT. Cellular and Molecular Responses to Mechanical Expansion of Tissue. Front Physiol. 2016;7:540. doi: 10.3389/fphys.2016.00540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pamplona D, Carvalho C. Characterization of human skin through skin expansion. Journal of Mechanics of Materials and Structures. 2012;7(7):641–655. doi: 10.2140/jomms.2012.7.641 [DOI] [Google Scholar]
- 31.Tepole AB, Gosain AK, Kuhl E. Stretching skin: The physiological limit and beyond. International journal of non-linear mechanics. Oct 2012;47(8):938–949. doi: 10.1016/j.ijnonlinmec.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquardt C, Bolke E, Gerber PA, et al. Correlation of cutaneous tension distribution and tissue oxygenation with acute external tissue expansion. Eur J Med Res. Nov 3 2009;14(11):480–6. doi: 10.1186/2047-783x-14-11-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tepole AB, Ploch CJ, Wong J, Gosain AK, Kuhl E. Growing skin: A computational model for skin expansion in reconstructive surgery. Journal of the mechanics and physics of solids. Oct 1 2011;59(10):2177–2190. doi: 10.1016/j.jmps.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han T, Lee T, Ledwon J, et al. Bayesian calibration of a computational model of tissue expansion based on a porcine animal model. Acta biomaterialia. 2022;137:136–146. doi: 10.1016/j.actbio.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janes LE, Ledwon JK, Vaca EE, et al. Modeling Tissue Expansion with Isogeometric Analysis: Skin Growth and Tissue Level Changes in the Porcine Model. Plastic and reconstructive surgery. Oct 2020;146(4):792–798. doi: 10.1097/PRS.0000000000007153 [DOI] [PubMed] [Google Scholar]
- 36.Stucker M, Moll C, Altmeyer P. Cutaneous oxygen supply. With special consideration of skin uptake of oxygen from the atmosphere. Hautarzt. Mar 2004;55(3):273–9. Sauerstoffversorgung der Haut Unter besonderer Berucksichtigung der kutanen Sauerstoffaufnahme aus der Atmosphare. doi: 10.1007/s00105-003-0662-7 [DOI] [PubMed] [Google Scholar]
- 37.Jaszczak P Skin oxygen tension, skin oxygen consumption, and skin blood flow measured by a tc-pO2 electrode. Acta Physiol Scand Suppl. 1991;603(0302–2994 (Print)):53–7. [PubMed] [Google Scholar]
- 38.Heise HM, Lampen P, Stucker M. Reflectance spectroscopy can quantify cutaneous haemoglobin oxygenation by oxygen uptake from the atmosphere after epidermal barrier disruption. Skin Research and Technology. Nov 2003;9(4):295–298. doi:DOI 10.1034/j.1600-0846.2003.00036.x [DOI] [PubMed] [Google Scholar]
- 39.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature reviews Molecular cell biology. Apr 2005;6(4):328–40. doi: 10.1038/nrm1619 [DOI] [PubMed] [Google Scholar]
- 40.Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. BioEssays : news and reviews in molecular, cellular and developmental biology. Sep 2002;24(9):789–800. doi: 10.1002/bies.10144 [DOI] [PubMed] [Google Scholar]
- 41.Totaro A, Castellan M, Battilana G, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nature communications. May 17 2017;8:15206. doi: 10.1038/ncomms15206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietramaggiori G, Liu P, Scherer SS, et al. Tensile forces stimulate vascular remodeling and epidermal cell proliferation in living skin. Annals of surgery. Nov 2007;246(5):896–902. doi: 10.1097/SLA.0b013e3180caa47f [DOI] [PubMed] [Google Scholar]
- 43.Liang X, Huang X, Zhou Y, Jin R, Li Q. Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation. Stem Cells Transl Med. Jul 2016;5(7):960–9. doi: 10.5966/sctm.2015-0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang YM, Huang XL, Chen G, Sheng LL, Li QF. Activated hypoxia-inducible factor-1alpha pathway modulates early events in stretch-induced skin neovascularization via stromal cell-derived factor-1 and vascular endothelial growth factor. The British journal of dermatology. Nov 2014;171(5):996–1005. doi: 10.1111/bjd.12920 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


