Abstract
Background:
The efficacy and safety of traditional Chinese medicine (TCM) combined with Silibinin in the treatment of nonalcoholic fatty liver disease (NAFLD) are still inconclusive. This meta-analysis intends to evaluation to explore the clinical efficacy and quality assessment of traditional Chinese medicine in combination with Silymarin in the treatment of NAFLD, aiming to aims to provide evidence-based data analysis for researchers and clinical practitioners involved in TCM research for NAFLD, with the hope of facilitating wider adoption and application
Methods:
In this meta-analysis, we searched PubMed, Embase, Cochrane Library, CNKI, Wanfang, CQVIP and CBM databases from the establishment of the databases to Oct 2023. The study proposed to include studies that reported combination of TCM with Silibinin and Silibinin alone in the treatment of NAFLD, excluding studies for which full text was not available or for which data extraction was not possible; studies using animal studies; reviews and systematic reviews. All data were processed by STATA15.1 statistical software.
Results:
16 randomized controlled trials (RCTs) were included in this meta-analysis. The sample size ranged from 48 to 120, with a total of 1335 patients, including 669 in the Combined treatment group and 384 in the Silibinin group. The findings indicated that the total effective rate of combined treatment group was significantly higher than that of Silibinin alone. Levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglycerides (TG), and gamma glutamyl transpeptidase (GGT) of combined treatment group were all significantly lower than that of western medicine alone. Additionally, after treating NAFLD with a combination of TCM and Silibinin, the TCM syndrome score were significantly lower than those observed with Silibinin alone.
Conclusion:
Traditional Chinese medicine in conjunction with Silibinin capsules has shown significant efficacy in the treatment of NAFLD, improving clinical symptoms, blood lipid levels, and liver function. Furthermore, it is essential to engage in multi-omics research, investigate iron death, and explore the gut microbiota as potential observational indicators for the diagnosis and inclusion criteria. Conducting more high-quality clinical experiments is necessary to further validate these findings.
Keywords: efficacy, meta-analysis and systematic review, nonalcoholic fatty liver disease, Silibinin, traditional Chinese medicine
1. Introduction
nonalcoholic fatty liver disease (NAFLD) is a prevalent clinical manifestation of metabolic syndrome, primarily distinguished by the accumulation of excessive fat in the liver, defined as more than 5% fat content.[1,2] Importantly, NAFLD occurs independently of autoimmune factors, drug-induced effects, viral hepatitis, or a history of excessive alcohol consumption.[3] NAFLD can progress from nonalcoholic simple fatty liver to nonalcoholic steatohepatitis, liver fibrosis, and even advance to liver cirrhosis and hepatocellular carcinoma.[4] Cirrhosis is a late-stage organ failure of the liver that often requires a liver transplant, and liver cancer typically develops decades after cirrhosis or chronic hepatitis B infection. Both of these conditions are common causes of liver disease-related deaths in patients.[5,6] According to statistics, the global prevalence of NAFLD in the general population has reached 25%, with rates among Asian populations ranging from 15% to 40%.[7]
Research has shown that insulin resistance and genetic susceptibility play a role in the development of this disease.[8] Insulin resistance can promote the accumulation of lipids in the liver, causing the initial impact on the liver. The accumulated lipids can further trigger events such as inflammation and oxidative stress, leading to cellular toxicity and damaging liver function, constituting the ‘two-hit’ theory recognized by many scholars in the pathogenesis of nonalcoholic fatty liver disease.[9] At present, Silibinin capsules are commonly used in clinical treatment for nonalcoholic fatty liver disease. They can eliminate reactive oxygen species within liver cells, facilitate detoxification, and subsequently promote liver function recovery. However, using them as a standalone treatment may not achieve the desired therapeutic effect. The pathogenesis of NAFLD is complex, and the disease progression is extensive. While there has been some progress in drug therapy, there is still no specific medication approved for the treatment of NAFLD. Traditional Chinese medicine (TCM) has gained increasing attention in the treatment of NAFLD, as it offers a unique advantage with its multi-component and multi-target effects in liver disease therapy. In recent years, multiple studies have indicated that a combination of traditional Chinese and Western medicine in treating NAFLD is more effective than Western medicine alone in improving abnormal liver function, reducing blood lipids, and enhancing insulin resistance, among other aspects.[10,11] Based on this, our study includes randomized controlled trials of traditional Chinese medicine in combination with Silymarin capsules for the treatment of NAFLD in accordance with the standards. We intend to use meta-analysis and systematic evaluation to explore the clinical efficacy and quality assessment of traditional Chinese medicine in combination with Silymarin in the treatment of NAFLD. This research aims to provide evidence-based data analysis for researchers and clinical practitioners involved in TCM research for NAFLD, with the hope of facilitating wider adoption and application.
2. Methods
2.1. Literature inclusion and exclusion criteria
Inclusion criteria:
Subjects: patients with schizophrenia
Interventions: Combination of TCM and Silibinin in the treatment of NAFLD
Control: Silibinin alone
Outcome indicators: Total effective rate, changes in the levels of triglycerides (TG), total cholesterol (TC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT) and TCM syndrome score.
Study design: Randomized controlled trial (RCT)
Exclusion criteria:
Duplicate publications; studies for which full text was not available or for which data extraction was not possible; studies using animal studies; reviews and systematic reviews.
3. Search strategy
In this meta-analysis, we searched PubMed, Embase, Cochrane Library, CNKI, Wanfang, CQVIP and CBM databases from the establishment of the databases to Aug 2023. The mesh terms used were: “nonalcoholic Fatty Liver Disease” AND “Chinese medicine” “Chinese traditional medicine” “traditional Chinese medicine” “Integrated traditional Chinese and western medicine” “Combination of traditional Chinese and western medicine” AND “Silibinin” AND “Randomized Controlled Trial” “Randomized.” Specific search strategies in English are as follows: ((nonalcoholic Fatty Liver Disease[Title/Abstract]) AND (((((Chinese medicine[Title/Abstract]) OR (Chinese traditional medicine[Title/Abstract])) OR (traditional Chinese medicine[Title/Abstract])) OR (Integrated traditional Chinese[Title/Abstract] AND western medicine[Title/Abstract])) OR (Combination of traditional Chinese[Title/Abstract] AND western medicine[Title/Abstract]))) AND (Randomized[Title/Abstract])
4. Literature screening and data extraction
Two researchers conducted the search for information, the screening of information and the capture of information respectively. Any questions or disagreements were made after consultation with a third party. Information was collected by author, year, study design, number of cases and outcome indicators.
5. Literature quality assessment
Two researchers carried out separate independent evaluations of the quality of the literature, using the Review manager 5.3[12] software risk assessment tool to evaluate the included literature according to random sequence generation, allocation concealment, blinding, whether research results were blinded to review, completeness of outcome data, using the Cochrane Risk Assessment Scale, and gender, selection of reported research outcomes, other biases, etc, and in cases of disagreement, through discussion or consultation with third parties. The Meta-analysis was performed in accordance with the relevant items in the Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) statement.[13]
6. Data synthesis and statistical analysis
Data were analyzed using STATA 15.1.[14] Weighted Mean differences (WMD) with 95%CI were used as continuous variables. I2 was used to evaluate cell heterogeneity. If the test for heterogeneity was P ≥ .1 and I2 ≤ 50%, homogeneity between studies was indicated and the studies were analyzed together using a fixed effects model; if P < .1 and I2 > 50%, significant heterogeneity between this group was indicated; if there was a difference, the source of the difference was identified using sensitivity analysis (We did a sensitivity analysis to exclude each of these trials one by one, and then did a combined analysis of the remaining trials). If the differences were still large, the random-effects model was used or the results of the combined study were discarded in favor of descriptive analysis. Publication bias was analyzed using funnel plots.
7. Results
7.1. Literature search results
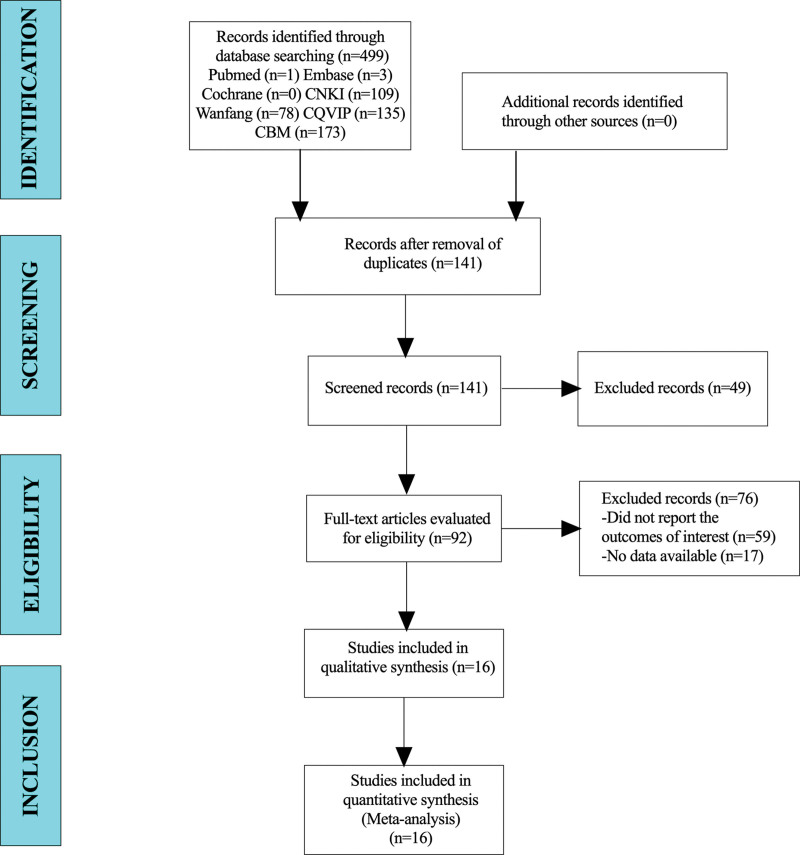
A total of 499 articles were collected for this study. After excluding duplicate trials, 141 patients were included in this study. A total of 92 articles were identified after reading their titles and abstracts. Finally, 16 studies were included in the meta-analysis (Fig. 1).
Figure 1.
Flow diagram for selection of studies.
8. Baseline characteristics and quality assessment of the included studies
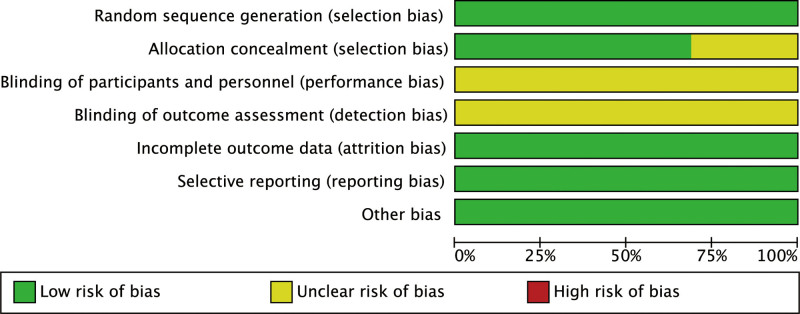
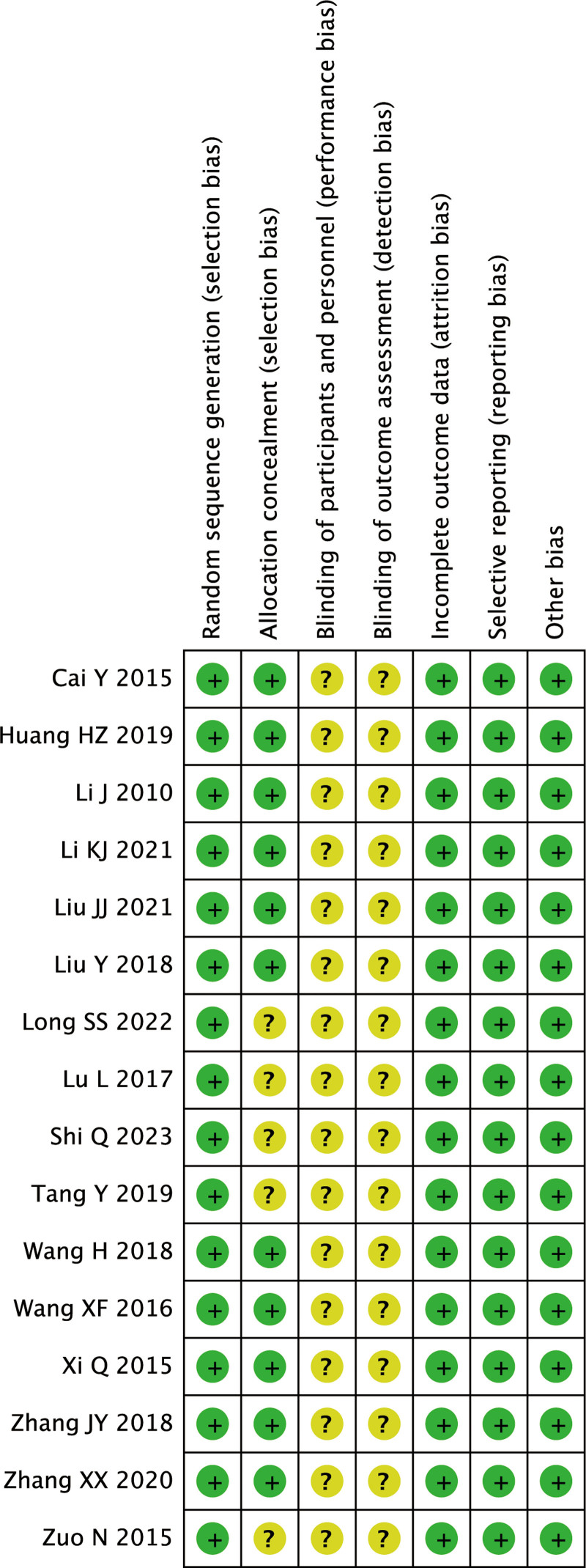
16 RCT studies were included in this meta-analysis. The sample size ranged from 48 to 120, with a total of 1335 patients, including 669 in the Combined treatment group and 384 in the Silibinin group. The age distribution of patients in the combined treatment group was 18 to 65, and the age distribution of patients in the control group was 20 to 66, suggesting that the ages were comparable (Table 1). The quality assessment results of the 16 RCTs are shown in Figures 2–3. The findings showed that all studies included in this review described the formation of random sequences. Eleven articles described “Allocation concealment,” but none described whether blinding was used.
Table 1.
Baseline characteristics and quality assessment of the included studies.
| Author | Yr | Study design | Sample size | Sex (male/female) | Age | Duration of disease (yr) | Measurements | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined treatment group | Control group | Combined treatment group | Control group | Combined treatment group | Control group | Combined treatment group | Control group | Combined treatment group | Control group | |||
| Haizhen[15] | 2019 | RCT | 54 | 54 | 30/24 | 28/26 | 41.4 ± 11.5 | 41.9 ± 11.3 | 2.3 ± 0.7 | 2.5 ± 0.7 | Baogan Jiangzhi Decoction + Silibinin Capsules | Silibinin Capsules |
| Zhang et al[16] | 2020 | RCT | 30 | 30 | 24/6 | 23/7 | 48.5 ± 9.4 | 50.2 ± 7.2 | 3.3 ± 1.2 | 3.1 ± 1.0 | Baogan Jiedu Decoction + Silibinin Capsules | Silibinin Capsules |
| Hui and Wei[17] | 2018 | RCT | 50 | 50 | 27/23 | 24/26 | 45.9 ± 12.0 | 44.6 ± 11.7 | / | / | Danshao Shugan Granules + Silibinin Capsules | Silibinin Capsules |
| Xiaofeng et al[18] | 2016 | RCT | 40 | 40 | 32/8 | 31/9 | 44.9 | 45.2 | 1 | 1 | Gan Zhi Ping Granule + Silibinin Capsules | Silibinin Capsules |
| Ying et al[19] | 2018 | RCT | 25 | 25 | 15/10 | 14/11 | 34.5 ± 6.6 | 33.8 ± 7.0 | 5.2 ± 1.6 | 5.3 ± 2.0 | Huanglian Jiedu Decoction + Silibinin Capsules | Silibinin Capsules |
| Jie et al[20] | 2010 | RCT | 33 | 32 | 20/13 | 18/14 | 36.8 ± 11.7 | 38.3 ± 12.8 | / | / | Jianpi Shugan Granules + Silibinin Capsules | Silibinin Capsules |
| Nan et al[21] | 2015 | RCT | 42 | 42 | 21/21 | 20/22 | 36.7 ± 8.6 | 37.3 ± 11.7 | / | / | Jianpi Shugan Granules + Silibinin Capsules | Silibinin Capsules |
| Zhang et al[22] | 2018 | RCT | 46 | 46 | 9/37 | 8/38 | 52.9 ± 9.4 | 53.0 ± 9.4 | 4.5 ± 1.9 | 4.4 ± 2.0 | Jiangzhi Ligan Decoction + Silibinin Capsules | Silibinin Capsules |
| Shi[23] | 2023 | RCT | 40 | 40 | 28/12 | 30/10 | 40.2 ± 3.1 | 41.3 ± 2.8 | 1.3 ± 0.2 | 1.4 ± 0.1 | Qinggan Huashi Huoxue Decoction + Silibinin Capsules | Silibinin Capsules |
| Qi et al[24] | 2015 | RCT | 45 | 45 | 30/15 | 31/14 | 47.8 ± 9.6 | 43.6 ± 12.0 | 1.4 ± 1.2 | 1.7 ± 1.3 | Shugan Jianpi Decoction + Silibinin Capsules | Silibinin Capsules |
| Lin and Qing[25] | 2017 | RCT | 24 | 24 | 12/12 | 12/12 | 45–65 | 44–66 | / | / | Self-Proposed TCM Prescription + Silibinin Capsules | Silibinin Capsules |
| Panyu et al[26] | 2021 | RCT | 54 | 54 | 25/29 | 26/28 | 53.2 ± 8.3 | 52.8 ± 8.7 | 1.5 ± 0.2 | 1.5 ± 0.2 | Xiaozhi Hugan Decoction + Silibinin Capsules | Silibinin Capsules |
| Yue et al[27] | 2015 | RCT | 35 | 35 | 28/7 | 30/5 | 18–56 | 20–54 | / | / | Xingqi Huatan Method + Silibinin Capsules | Silibinin Capsules |
| Yan and Jie[28] | 2019 | RCT | 56 | 54 | 45/11 | 40/14 | 25–65 | 20–66 | / | / | Quzhi Prescription + Silibinin Capsules | Silibinin Capsules |
| Shuan-Shuang et al[29] | 2022 | RCT | 60 | 60 | 28/32 | 36/24 | 47.4 ± 11.8 | 46.7 ± 12.2 | 2.0 ± 1.2 | 2.2 ± 1.2 | Self-Proposed Xiaopi Huatan Granules + Silibinin Capsules | Silibinin Capsules |
| Kaijie et al[30] | 2021 | RCT | 35 | 35 | 23/12 | 21/14 | 42.4 ± 4.6 | 43.2 ± 5.1 | 4.1 ± 1.7 | 4.2 ± 1.9 | Self-Proposed TCM Prescription + Silibinin Capsules | Silibinin Capsules |
RCT = randomized controlled trial, TCM = traditional Chinese medicine.
Figure 2.
Risk of bias graph.
Figure 3.
Risk of bias summary.
8.1. Results of meta-analysis
8.1.1. Total effective rate.
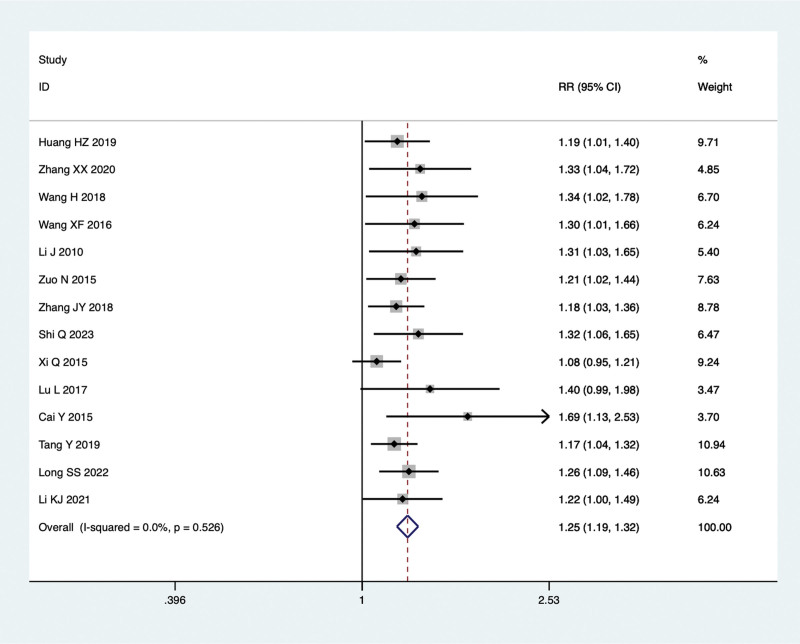
Fourteen studies were conducted to compare the total effective rate of TCM combined with Silibinin and Silibinin alone in the treatment of NAFLD. As no significant heterogeneity was observed (I²=0.0%, P = .526), a fixed-effect model was employed for the meta-analysis. The findings indicated that the total effective rate of combined treatment group was significantly higher than that of Silibinin alone (RR = 1.25, 95% CI: 1.19 to 1.32, P = .000) (Fig. 4).
Figure 4.
Comparing the differences in total effective rate between combined treatment group and Silibinin group in the treatment of NAFLD. NAFLD = non-alcoholic fatty liver disease.
8.1.2. TC levels.
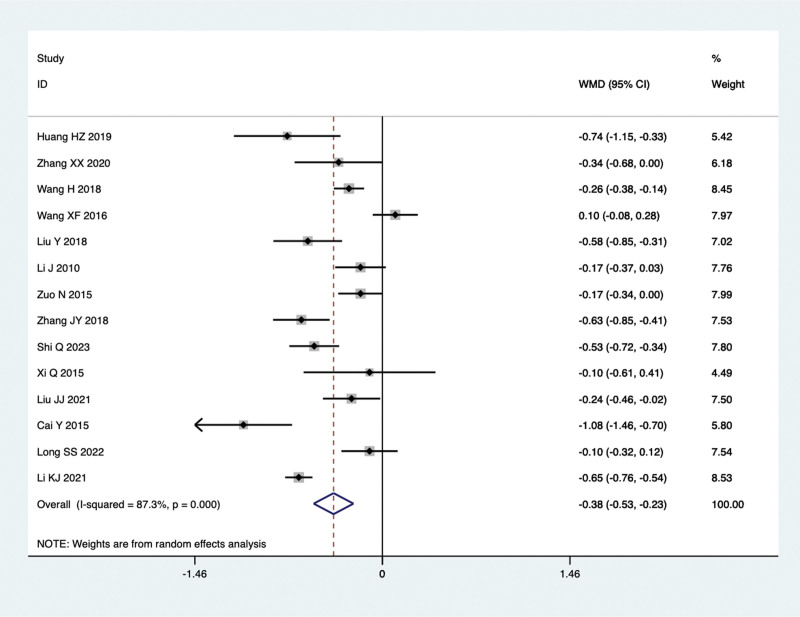
Fourteen studies conducted comparisons of TC levels in the treatment of NAFLD with TCM in combination with Silibinin versus Silibinin alone. Since there was significant heterogeneity (I2 = 87.3%, P = .000), a random-effect model was used for the meta-analysis. The summarized results indicated that after treating NAFLD with a combination of TCM and Silibinin, the TC levels were significantly lower than those observed with Silibinin alone (WMD = −0.38, 95% CI: −0.53 to −0.23, P = .000) (Fig. 5).
Figure 5.
TC levels in the combined treatment group and the Silibinin group after treating NAFLD. NAFLD = non-alcoholic fatty liver disease.
8.1.3. TG levels.
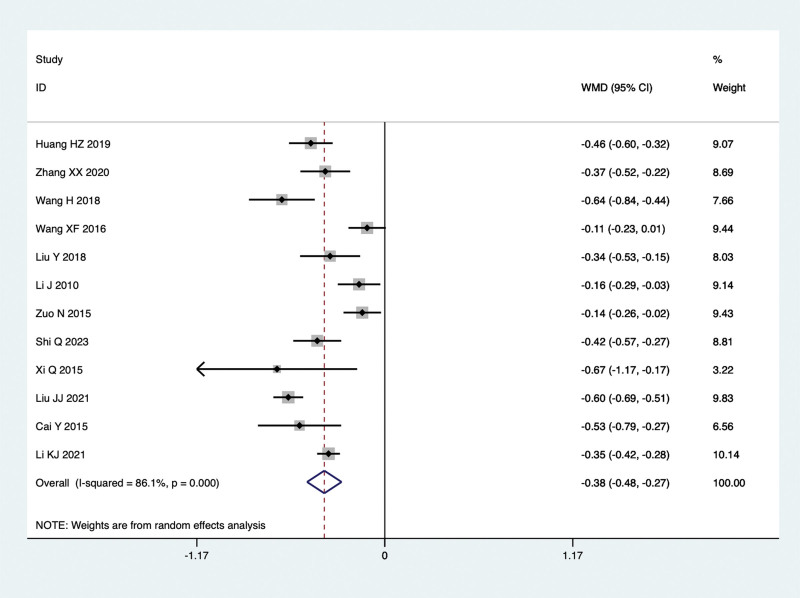
Twelve studies conducted comparisons of TG levels in the treatment of NAFLD with TCM in combination with Silibinin versus Silibinin alone. Since there was significant heterogeneity (I2 = 86.1%, P = .000), a random-effect model was used for the meta-analysis. The summarized results indicated that after treating NAFLD with a combination of TCM and Silibinin, the TG levels were significantly lower than those observed with Silibinin alone (WMD = −0.38, 95% CI: −0.48 to −0.27, P = .000) (Fig. 6).
Figure 6.
TG levels in the combined treatment group and the Silibinin group after treating NAFLD. NAFLD = non-alcoholic fatty liver disease.
8.1.4. ALT levels.
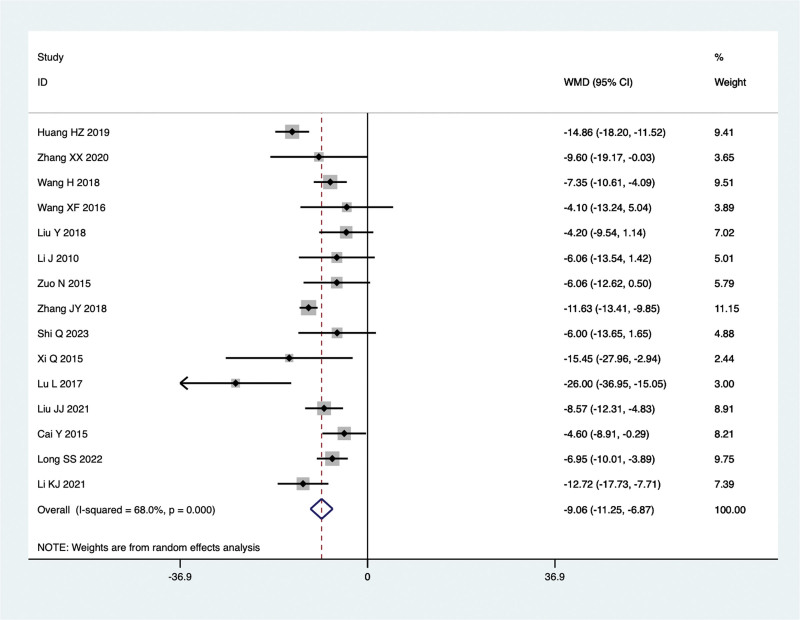
Fifteen studies conducted comparisons of ALT levels in the treatment of NAFLD with TCM in combination with Silibinin versus Silibinin alone. Since there was significant heterogeneity (I2 = 68.0%, P = .000), a random-effect model was used for the meta-analysis. The summarized results indicated that after treating NAFLD with a combination of TCM and Silibinin, the ALT levels were significantly lower than those observed with Silibinin alone (WMD = −9.06, 95% CI: −11.25 to −6.87, P = .000) (Fig. 7).
Figure 7.
ALT levels in the combined treatment group and the Silibinin group after treating NAFLD. ALT = alanine aminotransferase, NAFLD = non-alcoholic fatty liver disease.
8.1.5. AST levels.
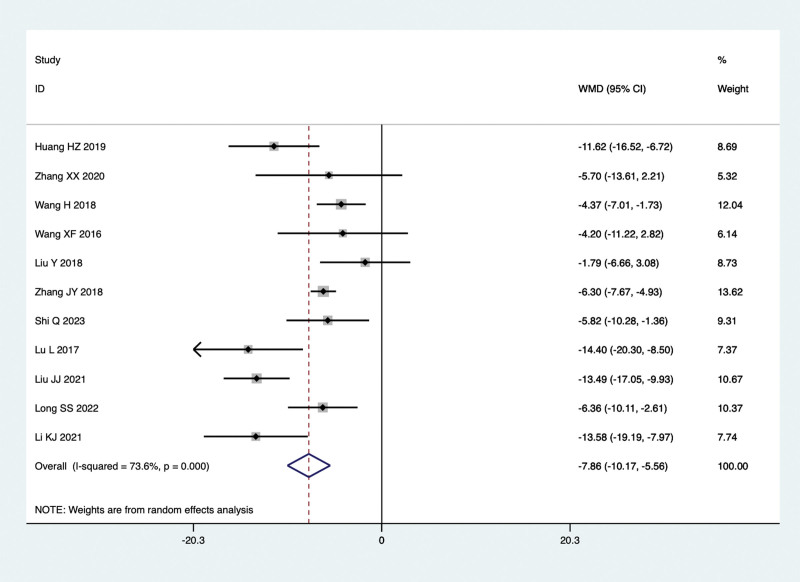
Fifteen studies conducted comparisons of AST levels in the treatment of NAFLD with TCM in combination with Silibinin versus Silibinin alone. Since there was significant heterogeneity (I2 = 68.0%, P = .000), a random-effect model was used for the meta-analysis. The summarized results indicated that after treating NAFLD with a combination of TCM and Silibinin, the AST levels were significantly lower than those observed with Silibinin alone (WMD = −9.06, 95% CI: −11.25 to −6.87, P = .000) (Fig. 8).
Figure 8.
AST levels in the combined treatment group and the Silibinin group after treating NAFLD. AST = aspartate aminotransferase, NAFLD = non-alcoholic fatty liver disease.
8.1.6. GGT levels.
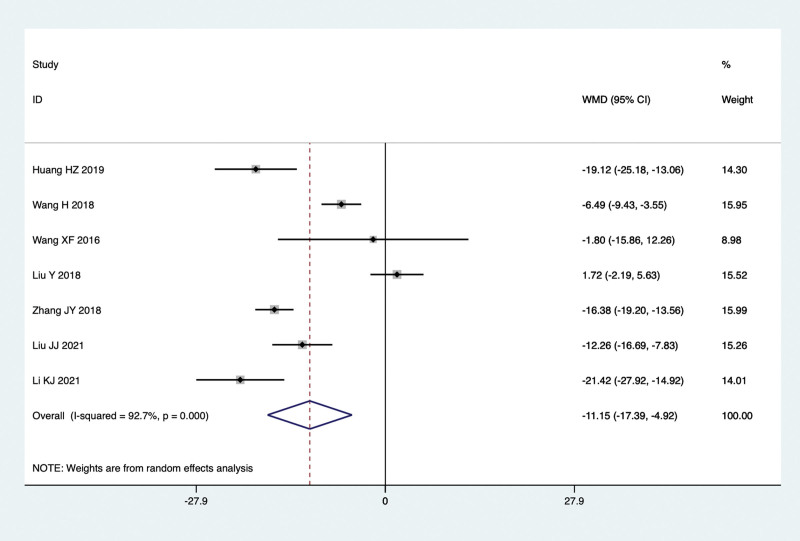
Seven studies conducted comparisons of GGT levels in the treatment of NAFLD with TCM in combination with Silibinin versus Silibinin alone. Since there was significant heterogeneity (I2 = 92.7%, P = .000), a random-effect model was used for the meta-analysis. The summarized results indicated that after treating NAFLD with a combination of TCM and Silibinin, the GGT levels were significantly lower than those observed with Silibinin alone (WMD = −11.15, 95% CI: −17.39 to −4.92, P = .000) (Fig. 9).
Figure 9.
GGT levels in the combined treatment group and the Silibinin group after treating NAFLD. GGT = gamma glutamyl transpeptidase, NAFLD = non-alcoholic fatty liver disease.
8.1.7. TCM syndrome score.
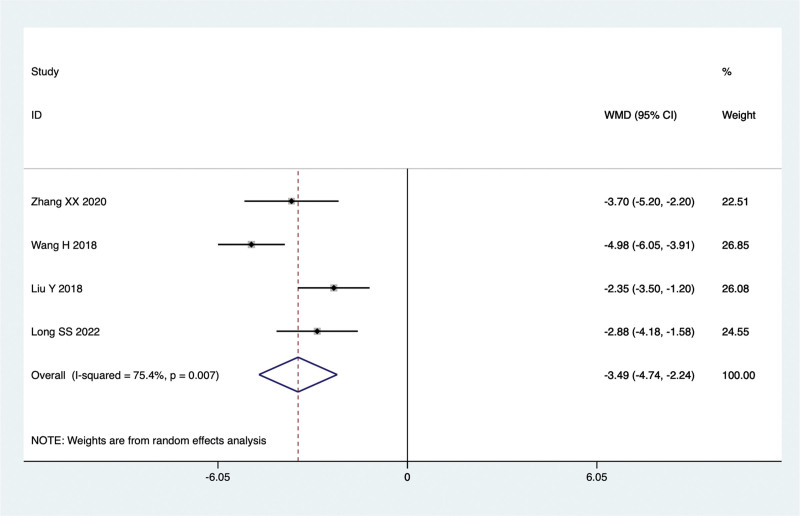
Four studies conducted comparisons of TCM syndrome score in the treatment of NAFLD with TCM in combination with Silibinin versus Silibinin alone. Since there was significant heterogeneity (I2 = 75.4%, P = .007), a random-effect model was used for the meta-analysis. The summarized results indicated that after treating NAFLD with a combination of TCM and Silibinin, the TCM syndrome score were significantly lower than those observed with Silibinin alone (WMD = −3.49, 95% CI: −4.74 to −2.24, P = .000) (Fig. 10).
Figure 10.
TCM syndrome score in the combined treatment group and the Silibinin group after treating NAFLD. NAFLD = non-alcoholic fatty liver disease, TCM = traditional Chinese medicine.
8.2. Sensitivity analysis
We did a sensitivity analysis to exclude each of these trials one by one, and then did a combined analysis of the remaining trials. By doing a Meta-analysis, we found that all the Meta-analyses did not have much effect on the results of the Meta-analysis, indicating that the results of the Meta-analysis were stable and reliable.
8.3. Publication bias
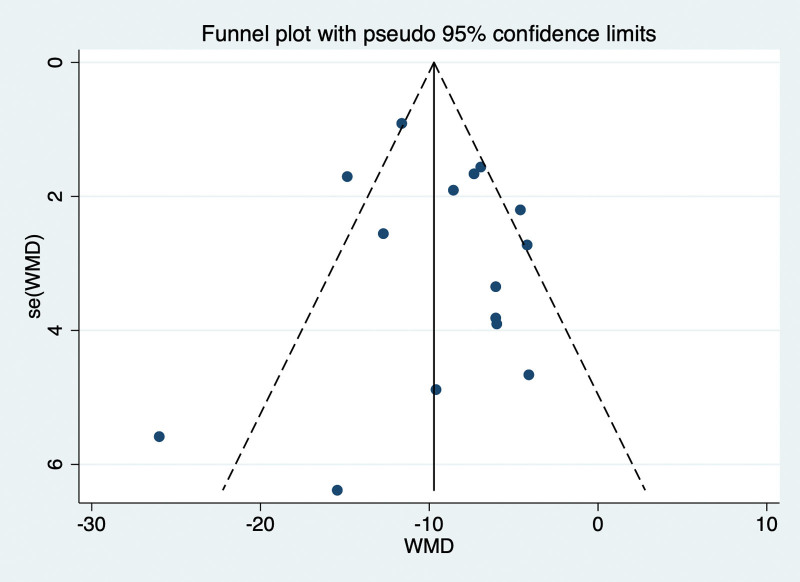
The funnel plot drawn in this study is as follows, and it can be seen that the funnel diagram is basically symmetrical. The P value obtained by Egger test was .524, indicating that no significant publication bias was found in this paper (Fig. 11).
Figure 11.
Funnel plot for evaluating the publication bias of this meta-analysis.
9. Discussion
According to the clinical symptoms and characteristics of patients, NAFLD can be classified within the realm of TCM as conditions related to “fat-dampness,” “hypochondriac pain,” “accumulation,” and “liver stagnation.” Its pathological location is the liver, with pathological factors such as phlegm-dampness, damp-heat, qi stagnation, and blood stasis. These various pathological factors can intersect, leading to a complex presentation that may include both deficiency and excess conditions. It can be differentiated into pattern types such as liver depression with spleen deficiency, phlegm-dampness obstruction, damp-heat retention, or phlegm-blood stasis intermingling, among others. For clinical treatment of NAFLD with concurrent liver damage, the main therapeutic approach involves behavioral interventions, liver protection, and managing enzymes, while also focusing on blood sugar and lipid control.[31] Silibinin capsules have antioxidative and anti-inflammatory properties. They work by inhibiting the generation of fatty acids, reducing the accumulation of lipids and peroxidation in the liver. Moreover, they improve mitochondrial function and enhance lipid exchange between the interior and exterior of cells, thereby alleviating cellular lipid stress. These actions have been shown to be clinically effective and safe.[32]
This study summarizes clinical randomized controlled trials of TCM in combination with Silibinin for the treatment of NAFLD. By employing various software for analysis, assessing bias risks, and evaluating evidence quality, it aims to provide evidence-based medicine data for the combined treatment of NAFLD using both traditional Chinese and Western medicine. The results indicate that, regardless of treatment duration, administration methods, medication forms, or geographical distribution, the total effective rate of TCM in combination with Silibinin is significantly superior to the control group, with statistically significant differences. This suggests a clinical advantage for TCM in conjunction with Silibinin. TCM offers the advantage of targeting multiple mechanisms, making it an important source for the development of new approaches and drugs for NAFLD. However, due to the lack of repetition in the TCM methods used in the included literature, it is unclear which specific TCM approach is superior.
In terms of observed indicators, it was found that liver function markers (ALT, AST, GGT), lipid metabolism markers (TG, TC), and TCM syndrome scores showed more significant improvements in the combined treatment of traditional Chinese and Western medicine. In fact, during the data collection process for this study, it was noted that some researchers used fasting insulin, insulin index, leptin, adiponectin, and other parameters as observed indicators. However, due to limited available data, these parameters were eventually excluded from this study. NAFLD is closely associated with metabolic syndrome, type 2 diabetes, cardiovascular diseases, and colorectal tumors. Therefore, we need more evidence to support the inclusion of additional observational indicators. Mass spectrometry-based multi-omics studies are gaining popularity, and it is promising to obtain biomarkers for NAFLD from genomics, transcriptomics, proteomics, and metabolomics. The “gut-liver axis” has been shown to be of great significance in NAFLD, and iron death, closely related to lipid peroxidation, has also been implicated in the pathophysiology of NAFLD.[33] Therefore, utilizing multi-omics, iron death, or studying the gut microbiota associated with NAFLD as observational indicators can help elucidate the mechanisms of traditional Chinese medicine in the context of NAFLD.
This study has certain limitations: Traditional Chinese medicine prescription and medication lacked standardization. Included literature primarily used classic formulas, clinical experience, or research on commercially available patent medicines as the basis for interventions. The diversity in intervention measures, along with variations in herbal flavors and dosages in traditional Chinese medicine compound components, made it challenging to compare results and interpret them consistently, which reduced the comparability between trials. Lack of follow-up records. None of the 16 RCTs included in this study had any follow-up data, preventing a deeper understanding of the prognosis of NAFLD when treated with traditional Chinese medicine in conjunction with Silymarin capsules.
10. Conclusion
Traditional Chinese medicine in conjunction with Silibinin capsules has shown significant efficacy in the treatment of NAFLD, improving clinical symptoms, blood lipid levels, and liver function. Furthermore, it is essential to engage in multi-omics research, investigate iron death, and explore the gut microbiota as potential observational indicators for the diagnosis and inclusion criteria. Conducting more high-quality clinical experiments is necessary to further validate these findings.
Author contributions
Conceptualization: Xiang Zhang, Qiujun Zhou.
Data curation: Xiaoliang Jin, Qiujun Zhou.
Formal analysis: Xiang Zhang, Qiujun Zhou.
Funding acquisition: Zhenghao Jiang.
Methodology: Xiaoliang Jin.
Software: Xiaoliang Jin.
Writing – original draft: Xiang Zhang, Xiaoliang Jin, Qiujun Zhou.
Writing – review & editing: Zhenghao Jiang, Xiang Zhang, Xiaoliang Jin, Qiujun Zhou.
Abbreviations:
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- GGT
- gamma glutamyl transpeptidase
- NAFLD
- non-alcoholic fatty liver disease
- RCTs
- randomized controlled trials
- RR
- relative risk
- TC
- total cholesterol
- TCM
- Traditional Chinese medicine
- TG
- triglycerides
- WMD
- weighted mean differences
XZ and ZJ contributed equally to this work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
This study was funded by Zhejiang Traditional Chinese Medicine Scientific Research Fund Project (2022ZB125).
The authors have no conflicts of interest to disclose.
How to cite this article: Zhang X, Jiang Z, Jin X, Zhou Q. Efficacy of traditional Chinese medicine combined with Silibinin on nonalcoholic fatty liver disease: A meta-analysis and systematic review. Medicine 2024;103:5(e37052).
Contributor Information
Xiang Zhang, Email: xiang1979.cool@163.com.
Zhenghao Jiang, Email: 247576522@qq.com.
Xiaoliang Jin, Email: jin_xiaoliang@foxmail.com.
References
- [1].van Dijk AM, Schattenberg JM, Holleboom AG, et al. Referral care paths for non-alcoholic fatty liver disease-Gearing up for an ever more prevalent and severe liver disease. United European Gastroenterol J. 2021;9:903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].de Groot J, Santos S, Geurtsen ML, et al. Risk factors and cardio-metabolic outcomes associated with metabolic-associated fatty liver disease in childhood. EClinicalMedicine. 2023;65:102248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chacon C, Arteaga I, Martinez-Escude A, et al. Clinical epidemiology of non-alcoholic fatty liver disease in children and adolescents The LiverKids: study protocol. PLoS One. 2023;18:e0286586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhu S, Wu Z, Wang W, et al. A revisit of drugs and potential therapeutic targets against non-alcoholic fatty liver disease: learning from clinical trials. J Endocrinol Invest. 2023. doi:10.1007/s40618-023-02216-y. [DOI] [PubMed] [Google Scholar]
- [5].Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med. 2017;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–82. [DOI] [PubMed] [Google Scholar]
- [8].Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–48. [DOI] [PubMed] [Google Scholar]
- [9].Bagherniya M, Nobili V, Blesso CN, et al. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: a clinical review. Pharmacol Res. 2018;130:213–40. [DOI] [PubMed] [Google Scholar]
- [10].Gong P, Long H, Guo Y, et al. Chinese herbal medicines: the modulator of nonalcoholic fatty liver disease targeting oxidative stress. J Ethnopharmacol. 1169;318:27. [DOI] [PubMed] [Google Scholar]
- [11].Cao Y, Fang X, Sun M, et al. Preventive and therapeutic effects of natural products and herbal extracts on nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Phytother Res. 2023;37:3867–97. [DOI] [PubMed] [Google Scholar]
- [12].Yan C, Xiong Y, Chen L, et al. A comparative study of the efficacy of ultrasonics and extracorporeal shock wave in the treatment of tennis elbow: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2019;14:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sarkis-Onofre R, Catala-Lopez F, Aromataris E, et al. How to properly use the PRISMA Statement. Syst Rev. 2021;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhu ZG, Wang JR, Pan XY. Efficacy of scraping therapy on blood pressure and sleep quality in stage I and II essential hypertension: a systematic review and meta-analysis. J Integr Med. 2023;S2095-4964:00097–3. [DOI] [PubMed] [Google Scholar]
- [15].Haizhen H. Clinical study on baogan jiangzhi tang combined with silibinin for nonalcoholic steatohepatitis. New Chin Med. 2019;51:158–61. [Google Scholar]
- [16].Zhang XX, Yandong L, Chuan Y, et al. Decoction retention enema combined with silybin in the treatment of non-alcoholic steatohepatitis. Acta Chin Med Pharmacol. 2020;48:47–51. [Google Scholar]
- [17].Hui W, Wei Z. Clinical observation of 100 cases on “Danshao Shugan Granules”combined with “Silibinin capsules”in the treatment of humid heat and spleen deficiency type of nonalcoholic steatohepatitis. J Integrative Chin Western Hepatol. 2018;28:14–6. [Google Scholar]
- [18].Xiaofeng W, Wenjun M, Xiaodan F, et al. Ganzhiping granules for the treatment of non-alcoholic fatty liver disease. Jilin Tradit Chin Med. 2016;36:454–6. [Google Scholar]
- [19].Ying L, Xianzhong H, Meiling X, et al. Observation of the therapeutic effect of huanglian jiedu decoction combined with silibin capsule on non-alcoholic steatohepatitis with dampness-heat accumulation. Chin J Basic Med Tradit Chin Med. 2018;24:1258–61. [Google Scholar]
- [20].Jie L, Xinxing W, Shugan J. Pill combined with Silybin Capsule in the treatment of non-alcoholic steatohepatitis: Clinical Observation. Chin J Tradit Chin Med Inform. 2010;17:68–9. [Google Scholar]
- [21].Nan Z, Huabin T, Shugan J. Pill combined with silybin capsule for the treatment of non-alcoholic steatohepatitis. Northern Pharm. 2015;12:44–5. [Google Scholar]
- [22].Zhang J, Zhang Y, Liu QQ. Effects of jiangligan decoction combined with silybin on liver function and blood lipid level in patients with non-alcoholic fatty liver disease. Modern Distance Educ Chin Med. 2023;21:107–10. [Google Scholar]
- [23].Shi Q. Clinical observation of qinggan huoxue decoction and silybin capsule in the treatment of non-alcoholic fatty liver. Modern Distance Educ Chin Tradit Med. 2023;21:80–3. [Google Scholar]
- [24].Qi X, Yazhu L, Chunrong S, et al. Shugan Jianpi huoxue fang combined with Silibin capsule for Steatosis. Shaanxi J Tradit Chin Med. 2015;36:520–2. [Google Scholar]
- [25].Lin L, Qing W. Observation of the curative effect of silibinin combined with chinese medicine on 24 cases of non-alcoholic fatty liver disease. Inner Mongolia Tradit Chin Med. 2017;36:27. [Google Scholar]
- [26].Panyu L, Yuqing Y, Miaoqing Y, et al. Effects of Xiaozhihugan Decoction combined with silybin on glucose and lipid metabolism and liver function in patients with nonalcoholic fatty liver disease. World J Integrated Chin Western Med. 2021;16:1177–80. [Google Scholar]
- [27].Yue C, Chz Z, Xueshu W. Clinical observation of treating non-alcoholic fatty liver with qi-eliminating phlegm. Chin Med Rev. 2015;21:83–5. [Google Scholar]
- [28].Yan T, Jie L. Observation on the curative effect of integrated Chinese and western medicine in the treatment of non-alcoholic steatohepatitis. J Practical Chin Med. 2019;35:454–5. [Google Scholar]
- [29].Shuan-Shuang L, Wei J, Yan-nan D, et al. Self-prepared xiaoxihuatan granule combined with silybin capsule in the treatment of non-alcoholic steatohepatitis (phlegm-dampness internal obstruction syndrome). Chinese Medicine Review 2022;28:66–70. [Google Scholar]
- [30].Kaijie L, Shujing Z, Jian H, et al. Efficacy of self-formulated Chinese medicine combined with silybin in the treatment of non-alcoholic fatty liver disease. J Hebei North Univ (Natural Science Edition). 2018;34:35–7. [Google Scholar]
- [31].Junxiang L, Jing C, Yunliang W. Consensus opinion on the diagnosis and treatment of integrative chinese and western medicine in non-alcoholic fatty liver disease (2017). Chin J Integrative Chin Western Med Digest. 2017;25:805–11. [Google Scholar]
- [32].Zhi-Peng Z, Yan-Qiu M, Zhi W. Research Progress on biological activity and mechanism of silybin and its derivatives. Chin Herbal Med. 2021;52:3717–24. [Google Scholar]
- [33].Jayachandran M, Qu S. Non-alcoholic fatty liver disease and gut microbial dysbiosis- underlying mechanisms and gut microbiota mediated treatment strategies. Rev Endocr Metab Disord. 2023;24:1189–204. [DOI] [PubMed] [Google Scholar]