Abstract
SAGA (Spt-Ada-Gcn5-Acetyltransferase), an evolutionarily conserved transcriptional coactivator among eukaryotes, is a large multi-subunit protein complex with two distinct enzymatic activities, namely HAT (Histone acetyltransferase) and DUB (De-ubiquitinase), and is targeted to the promoter by the gene-specific activator proteins for histone covalent modifications and PIC (Pre-initiation complex) formation in enhancing transcription (or gene activation). Targeting of SAGA to the gene promoter is further facilitated by the 19S RP (Regulatory particle) of the 26S proteasome (that is involved in targeted degradation of protein via ubiquitylation) in a proteolysis-independent manner. Moreover, SAGA is also recently found to be regulated by the 26S proteasome in a proteolysis-dependent manner via the ubiquitylation of its Sgf73/ataxin-7 component that is required for SAGA’s integrity and DUB activity (and hence transcription), and is linked to various diseases including neurodegenerative disorders and cancer. Thus, SAGA itself and its targeting to the active gene are regulated by the UPS (Ubiquitin-proteasome system) with implications in diseases.
Keywords: SAGA, UPS, transcription, Sgf73, ataxin-7
1. Introduction:
Transcription of the protein-coding genes is initiated at the promoter via the assembly of RNA polymerase II and GTFs (General transcription factors) such as TFIIA (Transcription factor IIA), TFIIB (Transcription factor IIB), TFIID (Transcription factor IID), TFIIE (Transcription factor IIE), TFIIF (Transcription factor IIF) and TFIIH (Transcription factor IIH) in forming the PIC (Pre-initiation complex) (1–6). Such PIC formation (and hence transcription initiation) is stimulated by the activator protein (or activator) bound at the specific DNA sequence upstream of the core promoter (known as upstream activating sequence or UAS) (Figure 1). Activators facilitate the PIC formation via the co-activators (1–6). One such transcriptional co-activator is SAGA (Spt-Ada-Gcn5-Acetyltransferase). It is an evolutionarily conserved large complex among eukaryotes with two distinct enzymatic activities such as HAT (Histone acetyltransferase) for histone H3 acetylation and DUB (De-ubiquitinase) for de-ubiquitylation of mono-ubiquitylated-histone H2B (3, 7–14). SAGA is recruited to the active gene, and regulates chromatin and PIC formation (via HAT and DUB activities, and Spt3 and Spt8 components) in controlling transcription (3, 11, 12, 15–20) (Figure 1). Thus, SAGA is a key player in gene expression or protein biosynthesis. Despite its crucial role in protein biosynthesis, it is intriguingly found to be regulated by the protein degradation machine, 26S proteasome, in a proteolysis-dependent as well as -independent manners in orchestrating gene expression, as described below.
Figure 1:
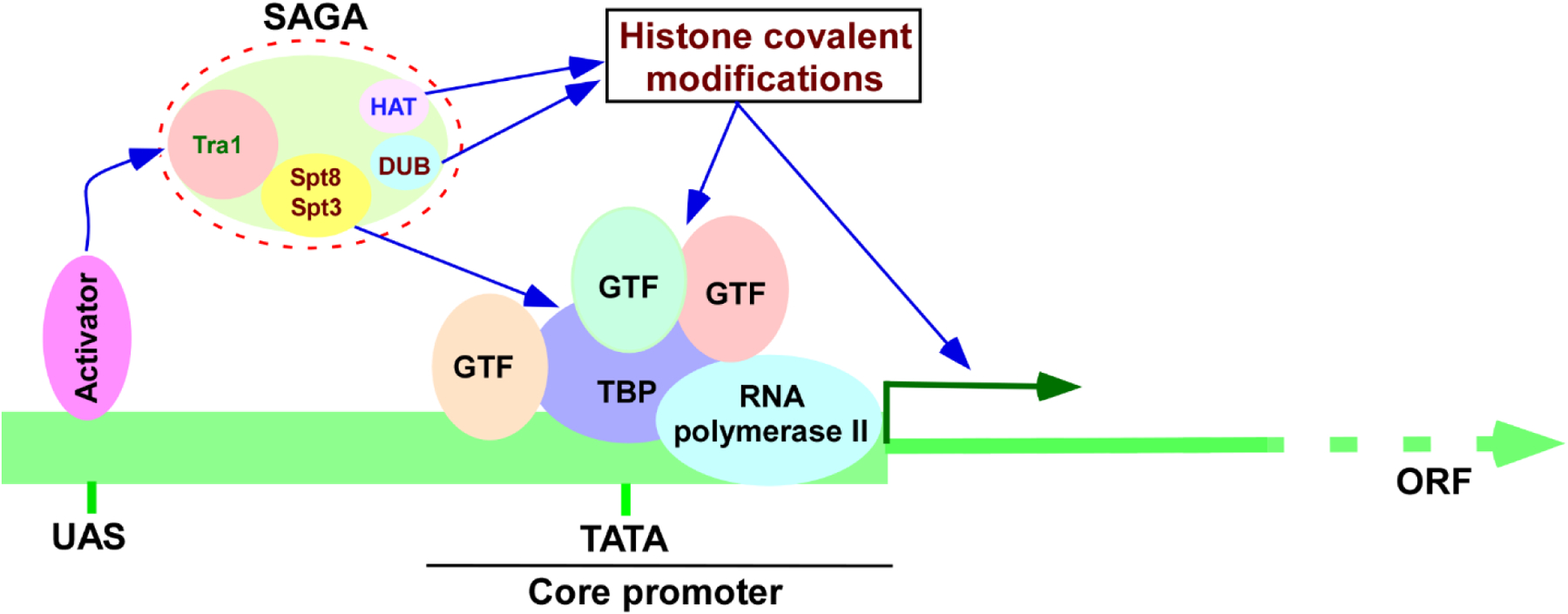
Schematic diagram showing transcription initiation. UAS, upstream activating sequence; TBP, TATA-box binding protein; GTF, general transcription factor; ORF, open reading frame; HAT, histone acetyltransferase; and DUB, de-ubiquitylase.
2. SAGA and its targeting to the active gene by activator and histone covalent modifications:
The evolutionarily conserved SAGA was initially discovered as a HAT (20). In yeast, SAGA consists of many different proteins such as Ada1, Ada2, Ada3, Gcn5, Spt3, Spt7, Spt8, Spt20, Sgf11, Sgf29, Sgf73, Ubp8, Sus1, Tra1, Taf5, Taf6, Taf9, Taf10 and Taf12. These proteins are organized into multiple structural and functional modules such as DUB, HAT, TAF (TBP-associated factor) and SPT (Suppressor of Ty) (Figure 2) (12). The SPT module maintains SAGA’s overall structural integrity (via Spt7, Spt20 and Ada1), interacts with TBP (TATA-box-binding protein) to nucleate the PIC formation (via Spt3 and Spt8) for transcription initiation, and is involved in recruiting SAGA to the active gene via interaction of its Tra1 component with activator (12, 15–19) (Figures 1 and 2). TAF module regulates SAGA and its functions (12, 21, 22). The HAT and DUB modules are involved in catalyzing the acetylation of histone H3 and de-ubiquitylation of mono-ubiquitylated histone H2B, respectively (3, 12, 13, 20, 23–26). Gcn5 and Ubp8 are the respective components of the HAT and DUB modules of SAGA for these enzymatic activities. However, these two individual components do not have catalytic activities, but rather they need to be present within the module or SAGA for their enzymatic activities for histone covalent modifications (3, 12, 13, 23–26). In addition to be targeted by the activator, SAGA-gene association is also regulated by histone covalent modifications. For example, the bromodomain of the Gnc5 component of SAGA interacts with acetylated-histone H3 and stabilizes the association of SAGA with the active gene (3, 27–34). Likewise, SAGA is found to interact with K4 (lysine4)-methylated histone H3 through the tudor domain of its Sgf29 component (35). Thus, while activator targets SAGA, histone covalent modifications stabilize SAGA-gene association.
Figure 2:

Schematic diagram showing different components and modules of SAGA. SPT, suppressor of Ty element; and TAF, TBP-associated factor.
3. Enhancement of SAGA targeting to the active gene by the 19S RP subunit of the 26S proteasome in a proteolysis-independent manner:
3.1. The 19S RP ATPase activity enhances the targeting to SAGA to the activator.
In addition to the above factors, SAGA’s targeting to the active gene is further regulated by the 26S proteasome in a proteolysis-independent manner (3, 4, 36–39). The 26S proteasome is a non-lysosomal targeted protein degradation machine via K48-linked poly-ubiquitylation of the substrate protein, which occurs through the sequential catalytic actions of E1 activating enzyme, E2 ubiquitin conjugase and substrate-specific E3 ubiquitin ligase (Figure 3) (3, 4, 40–43). It consists of 20S CP (Core particle) and 19S RP (Regulatory particle). The 20S CP is responsible for the proteolytic degradation, while the 19S RP contains ATPases and has specificity for ubiquitin protein conjugates (or poly-ubiquitin chain of the substrate protein). The 19S RP ATPase activity is required for unfolding of ubiquitylated-protein bound with the 19S RP for feeding it into the center of the 20S CP for proteolytic degradation by trypsin, chymotrypsin and caspase-like peptidases (40, 41). Further, the 19S RP ATPase activity helps to open the gate/channel of the 20S CP to translocate the unfolded protein to the proteolytic center (40, 41, 44–47). Thus, the 26S proteasome is involved in targeted degradation of poly-ubiquitylated proteins in an ATP-dependent fashion. However, small unfolded proteins can be degraded by the 26S proteasome independently of ubiquitylation and/or 19S RP, as these proteins can pass through the small opening/channel of 20S CP to the proteolytic center (48, 49). While the small unfolded proteins can undergo non-targeted proteasomal degradation, the 26S proteasome is mostly involved in ubiquitylation-mediated targeted protein degradation. Thus, ubiquitylation (via specific interaction of E3 ubiquitin ligase with substrate protein subsequent to the sequential actions of E1 activating enzyme and E2 ubiquitin conjugase) and consequent 26S proteasome-mediated degradation constituting the ubiquitin-proteasome system (UPS) are generally involved in targeted protein degradation. However, not all ubiquitylated-proteins are targeted for the 26S proteasomal degradation. For example, K63-linked poly-ubiquitylated-proteins are not targeted for the 26S proteasomal degradation, but rather function in cellular signaling and protein transport (50–52). Further, K6-linked ubiquitylation participates in mitophagy and DNA damage response (50–52), while K29-linked ubiquitylation is involved in neurodegenerative disorders (50–52). Moreover, mono-ubiquitylated proteins (or K48-linked poly-ubiquitylation with less than 4 ubiquitin moieties) are not targeted for the 26S proteasomal degradation. Thus, UPS is involved in regulating cellular functions/processes in the proteolysis-dependent and -independent manners.
Figure 3:
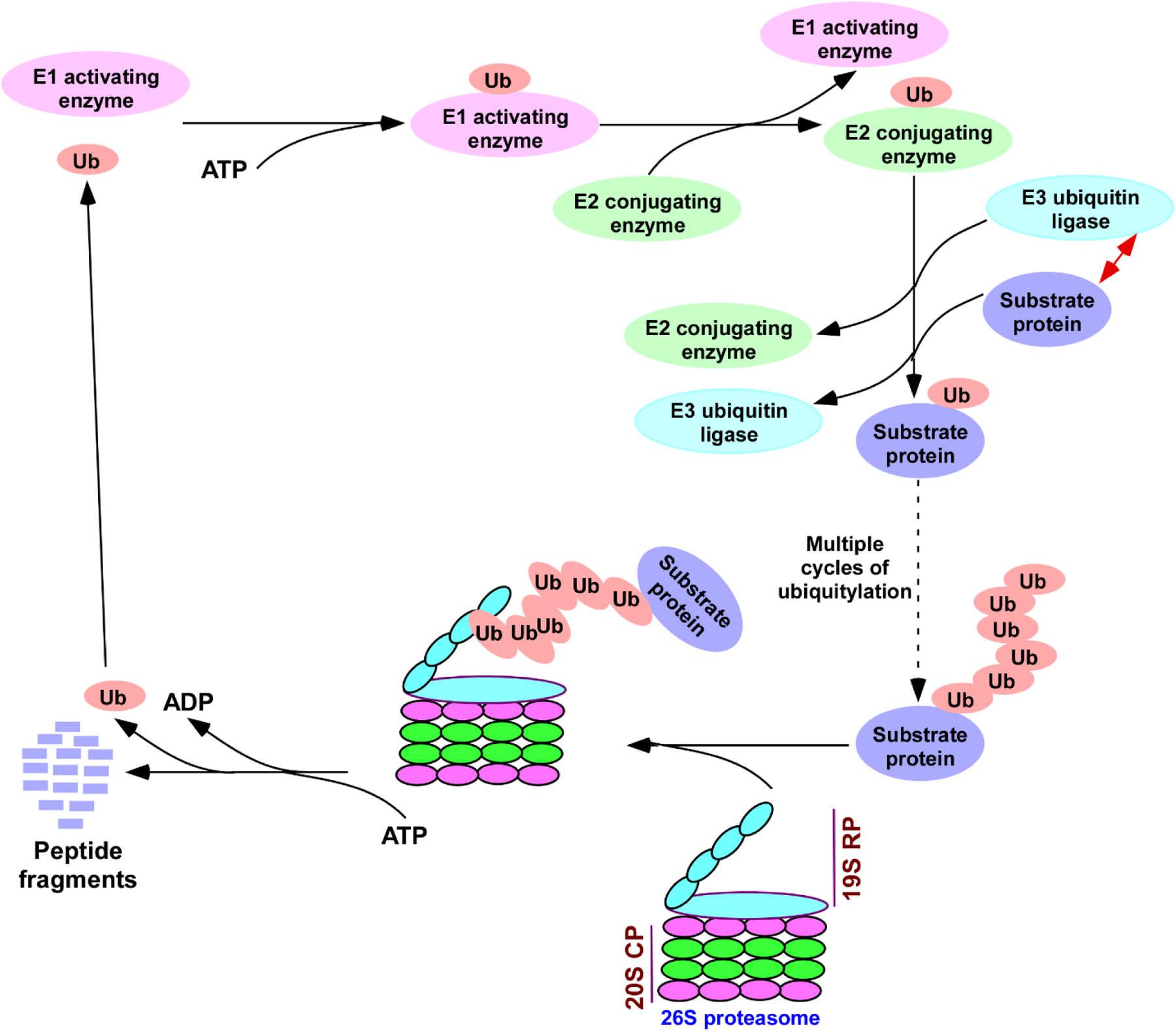
Schematic diagram for ubiquitylation and 26S proteasomal degradation of the substrate protein. The E1 activating enzyme forms a thio-ester bond with 76 amino acids long ubiquitin (Ub) followed by binding with E2 conjugating enzyme, and subsequently, C-terminal forms an isopeptide bond with lysine on the substrate protein with the help of an E3 ubiquitin ligase. This process continues for multiple cycles to generate poly-ubiquitylated protein which is then targeted for degradation by the 26S proteasome complex. 19S RP, 19S regulatory particle; and 20S CP, 20S core particle. ATP, Adenosine triphosphate; and ADP, Adenosine diphosphate.
Although the 26S proteasome is involved in protein degradation, its 19S RP subunit is intriguingly found to enhance the interaction of the co-activator, SAGA, with activator in an ATPase-dependent manner, independently of the 20S CP (3, 4, 36–39). Consistently, SAGA’s recruitment to the activator binding site is enhanced by the 19S RP (36, 37, 39). Such function of the 19S RP is mediated via its interactions with SAGA as well as activator (37, 53). It is possible that the 19S RP might be functioning as a physical bridge between activator and SAGA (as the 19S RP interacts with both activator and SAGA; 37, 53) to enhance SAGA targeting to the active gene. However, it was found that the ATPase activity of the 19S RP, but not its physical integrity, is required for enhancing the SAGA targeting to the activator (36). Thus, the catalytic activity of the 19S RP for ATP hydrolysis is required for SAGA targeting to the active gene.
3.2. The 19S RP is regulated by TOR (Target of rapamycin).
Like SAGA, another transcriptional co-activator NuA4 (Nucleosome acetyltransferase of H4 that interacts with activator and facilitates recruitment of TAFs to the core promoters of the TFIID-regulated genes; 38, 54, 55) is also regulated by the 19S RP in a proteolysis-independent manner (38, 39). We find that the targeting of NuA4 to the activator at the TFIID-dependent ribosomal protein genes (that engage nearly half of cellular RNA polymerase II for transcription; 56) is enhanced by the 19S RP, independently of the 20S CP (38, 39). Thus, 19S RP is required to facilitate the targeting of the co-activators, SAGA and NuA4, to the activators for stimulation of the PIC formation in promoting transcription initiation. Intriguingly, while the 19S RP facilitates the targeting of the co-activator to the activator, it is further regulated by nutrient-responsive TOR (38, 57) that is inactive under amino acids starving conditions, but active in the presence of amino acids/nutrients (58). The recruitment of the co-activator, NuA4, to the ribosomal protein genes for the PIC formation for transcription initiation is reduced following pharmacological inhibition of TORC1 (TOR complex 1) by rapamycin (38), and such decreased targeting of NuA4 is correlated with reduced recruitment of 19S RP (57). Further, the recruitment of the 19S RP to the ribosomal protein genes is decreased following rapamycin inhibition of TORC1 (57). Together, these results support the TORC1 regulation of the19S RP in controlling NuA4 recruitment to the ribosomal protein genres for PIC formation (and hence transcription initiation) (57). Such TOR regulation of the 19S RP (and thus NuA4) is attributed to be mediated via the phosphorylation of the 19S RP by TORC1 kinase (57). Like NuA4, the coactivator SAGA is also regulated by TOR (59). These results decipher the regulation of transcription initiation or gene activation by the TOR signaling pathway in response to nutrients, thus providing insights into the gene-environment/nutrients interactions.
3.3. SAGA in complementation of the 19S RP-regulated co-activator, NuA4.
While the co-activator, NuA4, is predominantly involved in regulating TAF/TFIID-dependent transcription initiation at the ribosomal protein genes (38, 54, 55), these genes become SAGA-dependent in the absence of the Eaf1 component of NuA4 (that maintains NuA4’s overall structural integrity; 60, 61) for the PIC formation (55). Intriguingly, SAGA-dependent transcription initiation occurs independently of TFIID at the TFIID-dependent ribosomal protein genes in the absence of NuA4 occurs independently of TFIID (55). Thus, activator, Rap1, at the ribosomal protein genes can target both NuA4 and SAGA (55), but leads to the TFIID-dependent transcription initiation via NuA4 (55). However, in the absence of NuA4, the TFIID-independent transcription initiation occurs via SAGA (55). Therefore, although Rap1 can potentially target both SAGA and NuA4 (55), it prefers NuA4 for TFIID-dependent transcription initiation under normal conditions (55), but requires SAGA for transcription initiation in the absence of NuA4 (55). Likewise, inorganic phosphate (Pi)-regulated gene, PHO84, is controlled by NuA4 and SAGA under different growth conditions (39). Transcription initiation of PHO84 occurs predominantly via SAGA in the presence of Pi in the growth medium (39). In contrast, NuA4, but not SAGA, is found to participate in transcription initiation of PHO84 in a TFIID-dependent manner, when the growth medium did not have Pi (or had a very low level of Pi) (39). Thus, activator at PHO84 targets both SAGA and NuA4 under two different growth conditions (39). Such differential requirement of SAGA and NuA4 for PHO84 transcription initiation is attributed to their distinct requirements for chromatin regulation under two different growth conditions (39). Under both conditions, the targeting of SAGA and NuA4 to the activators for PHO84 transcription initiation is dependent on the 19S RP (39), consistent with the role of the 19S RP in enhancing the targeting of co-activator to the activator.
In addition to their roles in sense transcription initiation, NuA4 and SAGA might also participate in the anti-sense transcription initiation. To address this, the roles of SAGA and NuA4 were analyzed for anti-sense transcription initiation from the 3’-end of the coding sequence of GAL10, whose sense transcription initiation is dependent on SAGA, but not NuA4 (62–64). Intriguingly, GAL10 anti-sense transcription is not found to be dependent on SAGA, but requires NuA4 HAT activity (62, 63). However, the requirement of such NuA4 HAT activity in GAL10 anti-sense transcription is not dependent on the 19S RP and TOR (63) that are required for NuA4 recruitment for sense transcription initiation (38, 39, 57). This could be due to different recruitment mechanism of NuA4 during anti-sense transcription in comparison to the sense-transcription (64).
3.4. The 19S RP ATPases function co-operatively in regulating transcription initiation.
AS described above, sense transcription initiation is dependent on the 19S RP for enhanced targeting of the co-activator to the activator in stimulating the PIC formation, independently of the 20S CP. The 19S RP consists of two subcomplexes, known as “base” and “lid”. The 19S base contains six different ATPases (Rpt1, Rpt2, Rpt3, Rpt4, Rpt5, Rpt6) and three non-ATPases (Rpn1, Rpn2 and Rpn10), while the 19S lid has eight non-ATPases, namely Rpn3, Rpn5, Rpn6, Rpn7, Rpn8, Rpn9, Rpn11 and Rpn12 (4, 40, 41, 47). The 19S lid interfaces with the base via Rpn10 that interacts with poly-ubiquitin chain of the substrate protein, and subsequently, substrate protein gets unfolded for translocation to the central chamber of the 20S CP in an ATPase-dependent manner for proteolytic degradation (40, 41, 44). Thus, the ATPase activity of the 19S base is required for targeted degradation of the poly-ubiquitylated proteins. The 19S RP ATPase activity is also required for targeting SAGA as well as NuA4 to the activator in facilitating the PIC formation for transcription initiation, as described above (3, 4, 36–39). The mutation/inactivation of the individual ATPase component of the 19S base impairs the PIC formation and hence transcription (36). Intriguingly, mutation/inactivation of one ATPase component of the 19S base is not compensated by its other five active ATPases for the PIC formation (36). Thus, the six different ATPases within the 19S base appear to cross-talk (or function co-operatively) to regulate the PIC formation, and hence transcription (36). These six different ATPases of the 19S base are organized into to the ring-shaped structure with a central pore. Malik et al (36) indicated that these ATPases of the 19S base function co-operatively to regulate the PIC and hence transcription. It is likely that the six different ATPases of the 19S base organized into the ring-shaped structure with a central pore generate a cyclic force/energy following ATP hydrolysis with a chaperonin activity. In fact, Braun et al (65) have demonstrated the molecular chaperonin activity of the 19S base. The co-operativity of the six different ATPases within the 19S base might be contributing to the molecular chaperonin activity by generating cyclic force/energy subsequent to the ATP hydrolysis, analogous to the previous studies (66–69) implicating circular motion or co-operativity among ATPases in the ring-shaped homohexameric chaperone. Such molecular chaperonin activity might be favoring to structure the activation domain of activator for enhanced interaction with co-activator, as activation domains are generally unstructured, and become structured upon binding to the coactivator/target (e.g., 70–87). Consistently, 19S RP has been shown to enhance the targeting of coactivators, SAGA and NuA4, to the activators (3, 4, 36–39). Further, the 19S RP might also help to properly assemble co-activator and other factors for transcription complex assembly at the promoter for transcription initiation. In agreement, the PIC formation (and hence transcription) is impaired at both SAGA and NuA4-regulated genes when the 19S RP ATPase was inactivated (36, 38, 39). Collectively, these studies demonstrate that 19S RP is involved in gene activation via enhanced targeting of the co-activator to the activator for proper transcription complex assembly at the promoter in an ATPase-dependent manner, but independently of the proteolytic function of the 26S proteasome.
4. Regulation of SAGA at the level of its Sgf73/ataxin-7 component by the 26S proteasome in a proteolysis-dependent manner:
In addition to be controlled by the 26S proteasome in a proteolysis-independent manner, SAGA is also recently found to be regulated via targeted ubiquitylation and proteasomal degradation of its Sgf73/ataxin-7 component that anchors the DUB module with SAGA for SAGA’s overall structural and functional integrities in regulation of chromatin, PIC formation and transcription (12, 88–93). The DUB module of SAGA contains Sgf73, Ubp8, Sgf11 and Sus1 (3, 12, 23–26). In mammalian cells, the related proteins (e.g., USP27X and USP51) of Ubp8’s homologue (i.e., USP22) form other DUBs with ataxin-7 to regulate histone H2B ubiquitylation outside SAGA (94, 95). Although Ubp8 has the potential DUB activity, it needs to be presented within the DUB module or SAGA for its histone H2B de-ubiquitylation activity (3, 12, 23–26). In addition to anchoring the DUB module with SAGA, Sgf73 regulates the enzymatic activity of the SAGA’s DUB module in controlling histone H2B de-ubiquitylation (3, 12, 23–26). Therefore, altered level or abundance of Sgf73/ataxin-7 is likely to affect SAGA’s integrity and DUB activity, and hence chromatin and transcription with associated cellular pathologies. In fact, depletion of human Sgf73/ataxin-7 (also known as ATXN7) or its increased level is linked to various diseases such as impaired retinal development, early childhood blindness, neurodegenerative disorders, defect in neuronal tube development, oncogenesis and attention-deficit/hyperactivity disorder (e.g., 96–115). Thus, it is crucial to know how Sgf73/ataxin-7’s abundance is regulated, which can help to decipher disease pathogenesis with targeted therapeutic potentials. However, the regulation of Sgf73/ataxin-7’s abundance was largely unknown until our recent studies (116) demonstrating its ubiquitylation and proteasomal degradation, as described below.
Sgf73/ataxin-7 was previously found to interact with the 19S RP subunit of the 26S proteasome (117). This suggests that Sgf73/ataxin-7 might be regulated by the UPS, and alteration of such UPS regulation could be associated with cellular pathologies. To test this possibility, we recently inhibited pharmacologically the proteolytic function of the 26S proteasome by a peptide aldehyde, MG132 (Carbobenzoxy-Leu-Leu-leucinal), and then analyzed the effect of such proteasomal inhibition on the stability of Sgf73/ataxin-7 in yeast (116). Our results revealed that Sgf73/ataxin-7 abundance is increased at the level of protein, but not mRNA, following pharmacological inhibition of the proteasome (116). Likewise, the genetic inhibition of the proteolytic activity of the 26S proteasome also increased the stability of Sgf73/ataxin-7 (116). These results support the proteasomal regulation of Sgf73/ataxin-7 (116). Since proteasomal degradation generally occurs via ubiquitylation, Sgf73/ataxin-7 is likely to be ubiquitylated for 26S proteasomal degradation. Indeed, we find that Sgf73/ataxin-7 undergoes ubiquitylation (116). Like in yeast, human Sgf73/ataxin-7 is also found to undergo ubiquitylation and proteasomal degradation (116). Thus, our results demonstrate a conserved UPS regulation of the Sgf73/ataxin-7 component of SAGA in controlling its cellular abundance in yeast and humans (116), hence implicating the enzymes/factors associated with the ubiquitylation and proteasomal degradation of Sgf73/ataxin-7 in cellular pathologies/diseases. Further, sumoylation has been implicated to regulate Sgf73/ataxin-7 ubiquitylation and proteasomal degradation in human cells (118).
5. UPS regulation of Sgf73/ataxin-7 controls SAGA assembly:
Since protein ubiquitylation is a dynamic process, Sgf73 is thus likely to undergo both ubiquitylation and de-ubiquitylation by E3 ubiquitin ligase and ubiquitin protease (that reverses ubiquitylation through interaction with ubiquitylated-protein), respectively. Mutations/malfunctions/misregulations of these enzymes would affect the level of ubiquitylated-Sgf73, and hence its proteasomal degradation (and consequently cellular abundance). For example, mutation or loss of function of E3 ubiquitin ligase of Sgf73 would impair Sgf73 ubiquitylation and subsequent proteasomal degradation, leading to its increased cellular abundance. Likewise, mutation or loss of function of ubiquitin protease of Sgf73 would increase the level of ubiquitylated-Sgf73, contributing to its increased proteasomal degradation (and hence decreased cellular level). To analyze the effect of UPS regulation of Sgf73/ataxin-7 on SAGA’s integrity, we monitored the recruitment of the SAGA components to the GAL1 UAS (where activator Gal4 and its co-activator, SAGA, are recruited; 15–17) in the presence of increased as well as decreased abundances of Sgf73/ataxin-7 (116, 119). We find that the association of SAGA with the GAL1 UAS is impaired in the absence of Sgf73/ataxin-7, supporting the requirement of Sgf73/ataxin-7 for SAGA’s structural integrity (119), consistent with biochemical studies (92). Intriguingly, the DUB association with SAGA is found to be impaired in the presence of high abundance of Sgf73/ataxin-7 (116). Thus, both increased and decreased levels of Sgf73/ataxin-7 affect SAGA’s overall structural integrity. Therefore, UPS-mediated fine-tuning of Sgf73/ataxin-7 is required for SAGA assembly.
While impaired structural integrity of SAGA is expected in the absence of Sgf73/ataxin-7 (as Sgf73/ataxin-7 anchors SAGA with DUB), decreased DUB association with SAGA in the presence of high abundance of Sgf73/ataxin-7 is somewhat surprising. How does increased abundance of Sgf73 impair the assembly of DUB with SAGA? Previous studies demonstrated the existence of DUB-depleted, but Sgf73/ataxin-7-associated SAGA in the absence of Ubp8 (92), suggesting that Sgf73/ataxin-7-associated SAGA interacts with Sgf11, Sus1 and Ubp8 to assemble DUB-containing SAGA. However, free and excess Sgf73 can compete with Sgf73-associated, but DUB-depleted, SAGA for interactions with Sgf11, Sus1 and Ubp8 to form DUB outside SAGA, thereby impairing the formation of fully assembled DUB-containing SAGA, as schematically shown in Figure 4. In agreement, Kohler et al (92) have demonstrated the existence of the DUB module outside SAGA, which might not exhibit targeted DUB activity at the active gene. It is likely that free/excess Sgf73 undergoes ubiquitylation and proteasomal degradation. Such targeted degradation of free/excess Sgf73 would allow Sgf73-associated, but DUB-depleted, SAGA to form fully assembled SAGA (Figure 4). Consistently, we observed DUB-depleted SAGA when cellular abundance of Sgf73/ataxin-7 was high (that could be resulted by the absence of Sgf73/ataxin-7 ubiquitylation and proteasomal degradation) (116). Thus, ubiquitylation and proteasomal degradation of free/excess Sgf73/ataxin-7 is likely to favor the formation of fully assembled DUB-containing SAGA, which needs to be further elucidated. Further, too much degradation of Sgf73/ataxin-7 in the absence of its de-ubiquitylation would impair DUB assembly and its association with SAGA as well as DUB activity (92, 119). Therefore, fine-tuning of Sgf73/ataxin-7 by UPS contributes to proper assembly of SAGA.
Figure 4:

Schematic diagram showing UPS (Ubiquitin-proteasome system) regulation of SAGA. Free and excess Sgf73/ataxin-7 impairs DUB-containing SAGA assembly by competing with Sgf73-associated, DUB-depleted, SAGA to favor the formation of DUB outside SAGA. The ubiquitylation and proteasomal degradation of free/excess Sgf73/ataxin-7 allows Sgf73-associated and DUB-depleted form of SAGA to form fully assembled DUB-containing SAGA.
6. Fine-tuning of Sgf73/ataxin-7 by UPS orchestrates transcription:
As described above, decreased DUB association with SAGA was observed in response to increased abundance of Sgf73 (that can be resulted by the absence of its ubiquitylation and proteasomal degradation) (116). Such decreased DUB association with SAGA would increase histone H2B ubiquitylation level due to decreased de-ubiquitylation. Increased level of histone H2B ubiquitylation impairs transcription elongation, as Wyce et al (120) demonstrated that high level histone H2B ubiquitylation acts a barrier for transcription elongation. Further, decreased level of histone H2B ubiquitylation also impairs transcription elongation (121–126). These observations point to the fine-tuning of histone H2B ubiquitylation by DUB and E3 ubiquitin ligase for optimal transcription. Therefore, increased level of Sgf73/ataxin-7 in the absence of its ubiquitylation and proteasomal degradation is likely to impair transcription elongation (via impaired DUB assembly with SAGA, and hence increased histone H2B ubiquitylation). Indeed, increased abundance of Sgf73/ataxin-7 was found to (i) impair cellular growth in 6-AU (6 Aza-uracil), (ii) slow down RNA polymerase II processivity/movement at the ORF (Open reading frame), (iii) enhance the level of RNA polymerase II at the 5’-end in comparison to the 3’-end of the ORF, and (iv) decrease mRNA/transcript level (116). These results support the defect in transcription elongation (116). Such transcription elongation defect in response to increased abundance of Sgf73/ataxin-7 resulting in the accumulation of RNA polymerase II at the 5’-end of the ORF might interfere with the transitioning of the initiating RNA polymerase II to the elongation form. This would lead to increased residence time of the PIC at the promoter, and hence increased crosslinking of the PIC components with promoter (and consequently increased chromatin immunoprecipitation signals). Indeed, chromatin immunoprecipitation analysis revealed increased level of TBP as well as RNA polymerase II at the promoter in the presence of high abundance of Sgf73/ataxin-7 (116). Although increased chromatin immunoprecipitation signals for the PIC components could be resulted from the defect in transcription elongation, it is also possible that the DUB-depleted SAGA in the presence of increase abundance of Sgf73/ataxin-7 (as described above) might be more efficient in recruiting TBP to nucleate the PIC formation in comparison to the DUB-containing SAGA, as the DUB module being located close to the TBP binding surface (90) could be inhibitory to the TBP recruitment. Nonetheless, our results revealed that increased abundance of Sgf73 (that can be resulted by the absence of its ubiquitylation and proteasomal degradation) impairs transcription (116). Further, decreased level of Sgf73/ataxin-7 (that can be resulted from the mutation or the loss of function of ubiquitin protease) impairs transcription (116, 119). Thus, increased and decreased levels of Sgf73/ataxin-7 in response to altered UPS impairs transcription (116, 119). Likewise, transcription in humans is also altered in response to increased or decreased level of Sgf73/ataxin-7 (103, 127). Therefore, UPS fine-tunes Sgf73/ataxin-73 in yeast and humans for optimal transcription.
In addition to regulating SAGA’s integrity and functions, Sgf73/ataxin-7 is also required for the assembly of TREX-2 (Transcription export 2) complex in exporting mRNA from nucleus to the cytoplasm for translation (92). Thus, increased or decreased level of Sgf73/ataxin-7 in response to altered UPS regulation would affect the TREX-2 assembly, and hence mRNA export, which remains to be investigated. Further, UPS regulation of Sgf73/ataxin-7 might affect longevity and the SAGA variant, SLIK (SAGA like) with impact on retrograde response pathway (11, 128), as Sgf73/ataxin-7 regulates ageing (129, 130), and is an integral component of SLIK involved in retrograde response (11, 128). Moreover, Sgf73/ataxin-7-mediated SAGA’s DUB activity is also involved in DNA repair via modulation of histone H2B ubiquitylation (131–133). Thus, Sgf73/ataxin-7 ubiquitylation and proteasomal degradation might also be involved in maintaining genomic integrity.
As described above, mammalian cells have other DUBs formed by USP22 (Ubp8’s homologue)-related proteins (e.g., USP27X and USP51) and other proteins including Sgf73/ataxin-7 (94, 95). These DUBs are involved in de-ubiquitylation of ubiquitylated-histone H2B outside SAGA. Since these DUBs are formed by Sgf73/ataxin-7, the UPS regulation of Sgf73/ataxin-7 is likely to have impact on the formation of these DUBs outside SAGA, and hence histone H2B de-ubiquitylation. According to our proposed model in Figure 4, ubiquitylation and proteasomal degradation of free/excess Sgf73/ataxin-7 would impair the formation of Sgf73/ataxin-7-containing DUBs outside SAGA, but rather favor SAGA assembly for targeted histone H2B de-ubiquitylation and associated functions, which remains to be further elucidated. Nonetheless, above described recent studies reveal a novel and evolutionarily conserved UPS regulatory pathway of gene expression via Sgf73/ataxin-7.
Concluding remarks:
As described above, the 26S proteasome is involved in targeting co-activator, SAGA, to the activator at the active gene and regulating its integrity and functions in a proteolysis-independent as well as -dependent manners, respectively. The 19S RP subunit of the 26S proteasome enhances the targeting of SAGA to the activator for the PIC formation in promoting transcription initiation. However, the molecular basis of such role of the 19S RP in gene activation remains unclear. Further, the 19S RP is regulated by TOR, but the mechanism-of-action of TOR in regulating the 19S RP with downstream functions needs to be further elucidated. In addition to regulating SAGA targeting via proteolysis-independent manner, the 26S proteasome controls SAGA itself at the level of ubiquitylation and proteasomal degradation of its key component, Sgf73/ataxin-7, that anchors the DUB module with SAGA in controlling targeted histone H2B ubiquitylation involved in regulating chromatin and transcription for normal cellular functions/health. Thus, increased and decreased levels of Sgf73/ataxin-7 (or misregulation of Sgf73/ataxin-7) are associated with altered DUB activity and various diseases including neurodegenerative disorder and cancer. Recent results provide a novel upstream UPS regulation of Sgf73/ataxin-7, which when misregulated would alter the abundance of Sgf73/ataxin-7, contributing to cellular pathologies/diseases. However, it is yet unknown how Sgf73/ataxin-7 is regulated by UPS. Further, we do not yet know the enzymes/factors involved in the UPS regulation of Sgf73/ataxin-7. The malfunctions/mutations/misregulations of the factors/enzymes involved in UPS regulation of Sgf73/ataxin-7 would alter the cellular abundance of Sgf73/ataxin-7, which could be associated with diseases. Thus, the enzymes/factors associated with ubiquitylation and proteasomal degradation of Sgf73/ataxin-7 could emerge as disease biomarkers with potentials for targeted therapeutic interventions. Further, it is not clearly understood how increased level of Sgf73/ataxin-7 in the absence of its ubiquitylation and proteasomal degradation (i) impairs transcription elongation and DUB assembly with SAGA, and (ii) enhances the association of the PIC components with promoter. Moreover, SAGA’s DUB is associated with DNA repair. Further, ubiquitylation and proteasomal degradation of Sgf73/ataxin-7 is also involved in ageing and mRNA export. Therefore, Sgf73/ataxin-7 and associated factors/enzymes might also be involved in orchestrating these processes. Thus, there are many important questions to follow up towards gaining more insights into this novel UPS regulation of SAGA and its functions with potential implications in disease pathogenesis and targeted therapies.
Acknowledgements:
The work in the Bhaumik laboratory was supported by the grants from National Institutes of Health (2R15GM088798–03), American Heart Association (15GRNT25700298), Simmons Cancer Institute (Team Science Grant) and SIU School of Medicine (Research Seed and Near-miss grants). We apologize to the authors whose work could not be cited due to limited space.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest:
Authors declare no conflict of interest.
References:
- 1.Roeder RG (2019) 50+ years of eukaryotic transcription: an expanding universe of factors and mechanisms. Nat Struct Mol Biol., 26: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roeder RG (2005) Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett., 579: 909–15. [DOI] [PubMed] [Google Scholar]
- 3.Bhaumik SR (2011) Distinct regulatory mechanisms of eukaryotic transcriptional activation mediated by SAGA and TFIID. Biochimica et Biophysica Acta – Gene Regulatory Mechanisms, 1809: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaumik SR, and Malik S (2008) Diverse regulatory mechanisms of eukaryotic transcriptional activation by the proteasome complex. Crit Rev Biochem Mol Biol., 43: 419–33. [DOI] [PubMed] [Google Scholar]
- 5.Durairaj G, Malik S, and Bhaumik SR (2017) Regulatory mechanisms of eukaryotic gene expression. Gene regulation, Epigenetics and Hormone Signaling (Editor: Mandal S). Wiley-VCH, Germany. Volume 1: 1–28. [Google Scholar]
- 6.Karmakar S, Ponnusamy MP, Bhaumik SR, and Batra SK (2018) RNA polymerase II and associated transcription factors. Encyclopedia in Life Science (eLS). John Wiley & Sons, Ltd., Chichester [Google Scholar]
- 7.Rodriguez-Navarro S (2009) Insights into SAGA function during gene expression. EMBO Rep, 10: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spedale G, Timmers HT, Pijnappel WW (2012) ATAC-king the complexity of SAGA during evolution. Genes Dev, 26: 527–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KK, Sardiu ME, Swanson SK, Gilmore JM, Torok M, Grant PA, Florens L, Workman JL, Washburn MP (2011) Combinatorial depletion analysis to assemble the network architecture of the SAGA and ADA chromatin remodeling complexes. Mol Syst Biol., 7: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker SP, Grant PA (2007) The SAGA continues: expanding the cellular role of a transcriptional co-activator complex. Oncogene, 26: 5329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant PA, Winston F, Berger SL (2021) The biochemical and genetic discovery of the SAGA complex. Biochim Biophys Acta Gene Regul Mech., 1864: 194669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soffers JHM, Workman JL (2020) The SAGA chromatin-modifying complex: the sum of its parts is greater than the whole. Genes Dev., 34: 1287002D1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinola-Lopez JM, Tan S (2021) The Ada2/Ada3/Gcn5/Sgf29 histone acetyltransferase module. Biochim Biophys Acta Gene Regul Mech., 1864: 194629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YC, Dent SYR (2021) Conservation and diversity of the eukaryotic SAGA coactivator complex across kingdoms. Epigenetics Chromatin, 14: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhaumik SR, and Green MR (2001) SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes and Development, 15: 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larschan E and Winston F 2001. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes & Dev, 15: 1946–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhaumik SR, Raha T, Aiello DP, and Green MR (2004) In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer. Genes and Development, 18: 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhaumik SR, and Green MR (2002) Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Molecular and Cellular Biology, 22: 7365–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley AM, Rougeulle C, and Winston F 1999. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes & Dev. 13: 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant PA, Duggan L, Côté J, Roberts SM, Brownell JE, Candau R, Ohba R, Owen-Hughes T, Allis CD, Winston F, Berger SL, Workman JL (1997) Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev, 11: 1640–1650. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan K, Jackson BM, Rhee E, Hinnebusch AG (1998) yTAFII61 has a general role in RNA polymerase II transcription and is required by Gcn4p to recruit the SAGA coactivator complex. Mol. Cell, 2: 683–692 [DOI] [PubMed] [Google Scholar]
- 22.Grant PA, Schieltz D, Pray-Grant MG, Steger DJ, Reese JC, Yates JR, Workman JL (1998) A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell, 94: 45–53. [DOI] [PubMed] [Google Scholar]
- 23.Köhler A, Zimmerman E, Schneider M, Hurt E, Zheng N (2010) Structural basis for assembly and activation of the heterotetrameric SAGA histone H2B deubiquitinase module. Cell, 141: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samara NL, Datta AB, Berndsen CE, Zhang X, Yao T, Cohen RE, Wolberger C (2010) Structural insights into the assembly and function of the SAGA deubiquitinating module. Science, 328: 1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samara NL, Ringel AE, Wolberger C (2012) A role for intersubunit interactions in maintaining SAGA deubiquitinating module structure and activity. Structure, 20: 1414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheon Y, Kim H, Park K, Kim M, Lee D (2020) Dynamic modules of the coactivator SAGA in eukaryotic transcription. Exp Mol Med, 52: 991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel JA, Grant PA. P. A (2007) Multi-tasking on chromatin with the SAGA coactivator complexes. Mutat Res, 618: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winston F, Allis CD (1999) The bromodomain: a chromatintargeting module? Nat. Struct. Biol, 6: 601–604. [DOI] [PubMed] [Google Scholar]
- 29.Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA (2000) The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J., 19: 6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syntichaki P, Topalidou I, Thireos G (2000) The Gcn5 bromodomain co-ordinates nucleosome remodelling. Nature, 404: 414–417. [DOI] [PubMed] [Google Scholar]
- 31.Hassan AH, Neely KE, Workman JL (2001) Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell, 104: 817–827. [DOI] [PubMed] [Google Scholar]
- 32.Hassan AH, Prochasson P, Neely KE, Galasinski SC, Chandy M, Carrozza MJ, Workman JL (2002) Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell, 111: 369–379. [DOI] [PubMed] [Google Scholar]
- 33.Agalioti T, Chen G, Thanos D. (2002) Deciphering the transcriptional histone acetylation code for a human gene. Cell, 111: 381–392. [DOI] [PubMed] [Google Scholar]
- 34.Yoon S, Qiu H, Swanson MJ, Hinnebusch AG (2003) Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA. Mol. Cell Biol, 23: 8829–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bian C, Xu C, Ruan J, Lee KK, Burke TL, Tempel W, Barsyte D, Li J, Wu M, Zhou BO, Fleharty BE, Paulson A, Allali-Hassani A, Zhou JQ, Mer G, Grant PA, Workman JL, Zang J, Min J. (2011) Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation. EMBO J., 30: 2829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik S, Shukla A, Sen P, Bhaumik SR (2009) The 19S proteasome subcomplex establishes a specific protein interaction network at the promoter for stimulated transcriptional initiation in vivo. J Biol Chem., 284: 35714–35724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee D, Ezhkova E, Li B, Pattenden SG, Tansey WP, Workman JL (2005) The proteasome regulatory particle alters the SAGA coactivator to enhance its interactions with transcriptional activators. Cell, 123: 423–436. [DOI] [PubMed] [Google Scholar]
- 38.Uprety B, Lahudkar S, Malik S, Bhaumik SR (2012) The 19S proteasome subcomplex promotes the targeting of NuA4 HAT to the promoters of ribosomal protein genes to facilitate the recruitment of TFIID for transcriptional initiation in vivo. Nucleic Acids Res., 40: 1969–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferdoush J, Sen R, Kaja A, Barman P, Bhaumik SR (2018) Two Distinct Regulatory Mechanisms of Transcriptional Initiation in Response to Nutrient Signaling. Genetics, 208: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voges D, Zwick IP, Baumeister W (1999) The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem, 68: 1015–1068. [DOI] [PubMed] [Google Scholar]
- 41.Coux O (2002) The 26S proteasome. Prog Mol Subcell Biol., 29: 85–107. [DOI] [PubMed] [Google Scholar]
- 42.Frankland-Searby S, Bhaumik SR (2012) The 26S proteasome complex: an attractive target for cancer therapy. Biochim Biophys Acta., 1825: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durairaj G, Garg P, Bhaumik SR (2009) Nuclear export of mRNA and its regulation by ubiquitylation. RNA Biol., 6: 531–5. [DOI] [PubMed] [Google Scholar]
- 44.Larsen CN, Finley D (1997) Protein translocation channels in the proteasome and other proteases. Cell, 91: 431–434. [DOI] [PubMed] [Google Scholar]
- 45.Groll M, Bajorek M, Kohler A, Moroder L, Rubin DM, Huber R, Glickman MH, Finley D (2000) A gated channel into the proteasome core particle. Nat Struct Biol., 7: 1062–1067. [DOI] [PubMed] [Google Scholar]
- 46.Smith DM, Chang SC, Park S, Finley D, Cheng Y, Goldberg AL (2007) Docking of the proteasomal ATPases’ carboxyl termini in the 20S proteasome’s alpha ring opens the gate for substrate entry. Mol Cell, 27: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glickman MH, Rubin DM, Fried VA, Finley D (1998) The Regulatory Particle of the Saccharomyces cerevisiae Proteasome. Mol Cell Biol., 18: 3149–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarcsa E, Szymanska G, Lecker S, O’Connor CM, Goldberg AL (2000) Ca2+-free calmodulin and calmodulin damaged by in vitro aging are selectively degraded by 26S proteasomes without ubiquitylation. J Biol Chem., 275: 20295–20301 [DOI] [PubMed] [Google Scholar]
- 49.Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O’Malley BW (2007) Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell, 26: 831–842. [DOI] [PubMed] [Google Scholar]
- 50.Tracz M, Bialek W (2021) Beyond K48 and K63: Non-canonical protein ubiquitination. Cell Mol. Biol. Lett, 26, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haakonsen DL, Rape M (2019) Branching Out: Improved Signaling by Heterotypic Ubiquitin Chains. Trends Cell Biol, 29: 704–716. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Xiang Y, Fan M, Fang S, Hua Q (2023) The Ubiquitin-Proteasome System in Tumor Metabolism. Cancers (Basel), 15: 2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang C, Gonzalez F, Rothermel B, Sun L, Johnston SA, Kodadek T (2001) The Gal4 activation domain binds Sug2 protein, a proteasome component, in vivo and in vitro. J Biol Chem., 276: 30956–63. [DOI] [PubMed] [Google Scholar]
- 54.Reid JL, Iyer VR, Brown PO, Struhl K (2006) Coordinate regulation of yeast ribosomal protein genes is associated with targeted recruitment of Esa1 histone acetylase. Mol. Cell, 6: 1297–1307. [DOI] [PubMed] [Google Scholar]
- 55.Uprety B, Sen R, Bhaumik SR (2015) Eaf1p is required for recruitment of NuA4 in targeting TFIID to the promoters of the ribosomal protein genes for transcriptional initiation in vivo. Mol. Cell. Biol, 35: 2947–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warner JR (1999) The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci 24: 437–440. [DOI] [PubMed] [Google Scholar]
- 57.Uprety B, Kaja A, Bhaumik SR (2018) TOR Facilitates the Targeting of the 19S Proteasome Subcomplex To Enhance Transcription Complex Assembly at the Promoters of the Ribosomal Protein Genes. Mol Cell Biol., 38: e00469–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laribee RN. (2018) Transcriptional and Epigenetic Regulation by the Mechanistic Target of Rapamycin Complex 1 Pathway. J Mol Biol., 430: 4874–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laboucarié T, Detilleux D, Rodriguez-Mias RA, Faux C, Romeo Y, Franz-Wachtel M, Krug K, Maček B, Villén J, Petersen J, Helmlinger D. (2017) TORC1 and TORC2 converge to regulate the SAGA co-activator in response to nutrient availability. EMBO Rep., 18): 2197–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell L, Lambert JP, Gerdes M, Al-Madhoun AS, Skerjanc IS, Figeys D, Baetz K. (2008) Functional dissection of the NuA4 histone acetyltransferase reveals its role as a genetic hub and that Eaf1 is essential for complex integrity. Mol Cell Biol., 28: 2244–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auger A, Galarneau L, Altaf M, Nourani A, Doyon Y, Utley RT, Cronier D, Allard S, Côté J (2008) Eaf1 is the platform for NuA4 molecular assembly that evolutionarily links chromatin acetylation to ATP-dependent exchange of histone H2A variants. Mol Cell Biol., 28: 2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malik S, Durairaj G, Bhaumik SR (2013) Mechanisms of antisense transcription initiation from the 3′-end of the GAL10 coding sequence in vivo. Mol. Cell. Biol, 33: 3549–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uprety B, Kaja A, Ferdoush J, Sen R, Bhaumik SR (2016) Regulation of antisense transcription by NuA4 histone acetyltransferase and other chromatin regulatory factors. Mol. Cell. Biol, 36: 992–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barman P, Reddy D, Bhaumik SR (2019) Mechanisms of Antisense Transcription Initiation with Implications in Gene Expression, Genomic Integrity and Disease Pathogenesis. Noncoding RNA, 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braun BC, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, Finley D, Schmidt M (1999) The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat. Cell Biol, 1: 221–226. [DOI] [PubMed] [Google Scholar]
- 66.Smith DM, Kafri G, Cheng Y, Ng D, Walz T, Goldberg AL (2005) ATP binding to PAN or the 26S ATPases causes association with the 20S proteasomes, gate opening, and translocation of unfolded proteins. Mol. Cell, 20: 687–698. [DOI] [PubMed] [Google Scholar]
- 67.Rubin DM, Glickman MH, Larsen CN, Dhruvakumar S, Finley D (1998) Active site mutants in the six regulatory particle ATPases reveal multiple roles for ATP in the proteasome. EMBO J., 17: 4909–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Shaw A, Bates PA, Newman RH, Gowen B, Orlova E, Gorman MA, Kondo H, Dokurno P, Lally J, Leonard G, Meyer H, van Heel M, Freemont PS (2000) Structure of the AAA ATPase p97. Mol Cell, 6: 1473–1484. [DOI] [PubMed] [Google Scholar]
- 69.Davies JM, Brunger AT, Weis WI (2008) Improved structures of full-length p97, an AAA ATPase: implications for mechanisms of nucleotide-dependent conformational change. Structure, 16: 715–726. [DOI] [PubMed] [Google Scholar]
- 70.Sigler PB (1988) Transcriptional activation. Acid blobs and negative noodles. Nature, 333: 210–212 [DOI] [PubMed] [Google Scholar]
- 71.Cho HS, Liu CW, Damberger FF, Pelton JG, Nelson HCM, and Wemmer DE (1996) Yeast heat shock transcription factor N-terminal activation domains are unstructured as probed by heteronuclear NMR spectroscopy. Protein Sci., 5: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn S (1993) Structure(?) and function of acidic transcription activators. Cell, 72: 481–483. [DOI] [PubMed] [Google Scholar]
- 73.Kussie PH, Gorina S, Marechal V, Elenbass B, Moreau J, Levine AJ, and Pavletich NP (1996) Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science, 274: 948–953. [DOI] [PubMed] [Google Scholar]
- 74.Blommers MJJ, Fendrich G, Garcia-Echeverria C, and Chene P (1997) On the interaction between p53 and MDM2: Transfer NOE study of a p53-derived peptide ligated to MDM2. J Am Chem Soc, 119: 3425–3426. [Google Scholar]
- 75.Uesugi M, and Verdine GL (1999) The alpha-helical FXXPhiPhi motif in p53: TAF interaction and discrimination by MDM2. Proc. Natl. Acad. Sci. U. S. A, 96: 14801–14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Regier JL, Shen F, and Triezenberg SJ (1993) Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. U. S. A, 90: 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmitz ML, dos Santos Silva MA, Altmann H, Czisch M Holak TA, and Baeuerle PA (1994) Structural and functional analysis of the NF-kappa B p65 C terminus. An acidic and modular transactivation domain with the potential to adopt an alpha-helical conformation. J. Biol. Chem, 269: 25613–25620. [PubMed] [Google Scholar]
- 78.O’Hare P, and Williams G (1992) Structural studies of the acidic transactivation domain of the Vmw65 protein of herpes simplex virus using 1H NMR. Biochemistry, 31: 4150–4156. [DOI] [PubMed] [Google Scholar]
- 79.Donaldson L, and Capone JP (1992) Purification and characterization of the carboxyl-terminal transactivation domain of Vmw65 from herpes simplex virus type 1. J. Biol. Chem, 267: 1411–1414. [PubMed] [Google Scholar]
- 80.Dahlman-Wright K, Baumann H, McEwan IJ, Almlof T, Wright APH, Gustafsson J, and Hard T (1995) Structural characterization of a minimal functional transactivation domain from the human glucocorticoid receptor. Proc. Natl. Acad. Sci. U. S. A, 92: 1699–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leuther KK, Salmeron JM, and Johnston SA (1993) Genetic evidence that an activation domain of GAL4 does not require acidity and may form a beta sheet. Cell, 72: 575–585. [DOI] [PubMed] [Google Scholar]
- 82.Van Hoy M, Leuther KK, Kodadek T, and Johnston SA (1993) The acidic activation domains of the GCN4 and GAL4 proteins are not alpha helical but form beta sheets. Cell, 72: 587–594. [DOI] [PubMed] [Google Scholar]
- 83.Uesugi M, Nyanguile O, Lu H, Levine AJ, and Verdine GL (1997) Induced alpha helix in the VP16 activation domain upon binding to a human TAF. Science, 277: 1310–1313. [DOI] [PubMed] [Google Scholar]
- 84.Hua Q, Jia W, Bullock BP, Habener JF, and Weiss MA (1998) Transcriptional activator-coactivator recognition: nascent folding of a kinase-inducible transactivation domain predicts its structure on coactivator binding. Biochemistry, 37: 5858–5866. [DOI] [PubMed] [Google Scholar]
- 85.Hi R, Osada S, Yumoti N, and Osumi T (1999) Characterization of the amino-terminal activation domain of peroxisome proliferator-activated receptor alpha. Importance of alpha-helical structure in the transactivating function. J. Biol. Chem, 274: 35152–35158. [DOI] [PubMed] [Google Scholar]
- 86.Tell G, Perrone L, Fabbro D, Pellizzari L, Pucillo C, De Felince M, Acquaviva R, Formisano S, and Damante G (1999) Structural and functional properties of the N transcriptional activation domain of thyroid transcription factor-1: similarities with the acidic activation domains. Biochem. J, 329: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee H, Mok KH, Muhandiram R, Park KH, Suk JE, Kim DH, Chang J, Sung YC, Choi KY, and Han KH (2000) Local structural elements in the mostly unstructured transcriptional activation domain of human p53. J Biol Chem., 275: 29426–32. [DOI] [PubMed] [Google Scholar]
- 88.Durand A, Bonnet J, Fournier M, Chavant V, Schultz P (2014) Mapping the deubiquitination module within the SAGA complex. Structure, 22: 1553–9. [DOI] [PubMed] [Google Scholar]
- 89.Morgan MT, Haj-Yahya M, Ringel AE, Bandi P, Brik A, Wolberger C (2016) Structural basis for histone H2B deubiquitination by the SAGA DUB module. Science, 351: 725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H, Dienemann C, Stützer A, Urlaub H, Cheung ACM, Cramer P (2020) Structure of the transcription coactivator SAGA. Nature, 577: 717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee KK, Swanson SK, Florens L, Washburn MP, Workman JL (2009) Yeast Sgf73/Ataxin-7 serves to anchor the deubiquitination module into both SAGA and Slik(SALSA) HAT complexes. Epigenetics Chromatin, 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Köhler A, Schneider M, Cabal GG, Nehrbass U, Hurt E (2008) Yeast Ataxin-7 links histone deubiquitination with gene gating and mRNA export. Nat Cell Biol., 10: 707–15. [DOI] [PubMed] [Google Scholar]
- 93.Köhler A, Pascual-García P, Llopis A, Zapater M, Posas F, Hurt E, Rodríguez-Navarro S (2006) The mRNA export factor Sus1 is involved in Spt/Ada/Gcn5 acetyltransferase-mediated H2B deubiquitinylation through its interaction with Ubp8 and Sgf11. Mol Biol Cell, 17: 4228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Atanassov BS, Mohan RD, Lan X, Kuang X, Lu Y, Lin K, McIvor E, Li W, Zhang Y, Florens L, et al. ATXN7L3 and ENY2 Coordinate Activity of Multiple H2B Deubiquitinases Important for Cellular Proliferation and Tumor Growth. Mol. Cell 2016;62:558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.El-Saafin F, Devys D, Johnsen SA, Vincent SD, Tora L (2022) SAGA-Dependent Histone H2Bub1 Deubiquitination Is Essential for Cellular Ubiquitin Balance during Embryonic Development. Int J Mol Sci., 23: 7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karam A, Trottier Y. (2018) Molecular Mechanisms and Therapeutic Strategies in Spinocerebellar Ataxia Type 7. Adv Exp Med Biol., 1049: 197–218. [DOI] [PubMed] [Google Scholar]
- 97.Mohan RD, Abmayr SM, Workman JL (2014) Pulling complexes out of complex diseases: Spinocerebellar Ataxia 7. Rare Dis., 2: e28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohan RD, Dialynas G, Weake VM, Liu J, Martin-Brown S, Florens L, Washburn MP, Workman JL, Abmayr SM (2014) Loss of Drosophila Ataxin-7, a SAGA subunit, reduces H2B ubiquitination and leads to neural and retinal degeneration. Genes Dev., 28: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yanicostas C, Barbieri E, Hibi M, Brice A, Stevanin G, Soussi-Yanicostas N. (2012) Requirement for zebrafish ataxin-7 in differentiation of photoreceptors and cerebellar neurons. PLoS One, 7: e50705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garden GA, La Spada AR (2008) Molecular Pathogenesis and Cellular Pathology of Spinocerebellar Ataxia Type 7 Neurodegeneration. Cerebellum., 7: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Helmlinger D, Hardy S, Abou-Sleymane G, Eberlin A, Bowman AB, Gansmüller A, Picaud S, Zoghbi HY, Trottier Y, Tora L, Devys D (2006) Glutamine-Expanded Ataxin-7 Alters TFTC/STAGA Recruitment and Chromatin Structure Leading to Photoreceptor Dysfunction. PLoS Biol., 4: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCullough SD, Xu X, Dent SYR, Bekiranov S, Roeder RG, Grant PA (2012) Reelin is a target of polyglutamine expanded ataxin-7 in human spinocerebellar ataxia type 7 (SCA7) astrocytes. Proc Natl Acad Sci U S A., 109: 21319–21324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palhan VB, Chen S, Peng G-H, Tjernberg A, Gamper AM, Fan Y, Chait BT, La Spada AR, Roeder RG (2005) Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc Natl Acad Sci U S A., 102: 8472–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weake VM, Lee KK, Guelman S, Lin C-H, Seidel C, Abmayr SM, Workman JL (2008) SAGA-mediated H2B deubiquitination controls the development of neuronal connectivity in the Drosophila visual system. EMBO J., 27: 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Melo-Cardenas J, Xu Y, Wei J, Tan C, Kong S, Gao B, Montauti E, Kirsammer G, Licht JD, Yu J, Ji P, Crispino JD, Fang D. (2018) USP22 deficiency leads to myeloid leukemia upon oncogenic Kras activation through a PU.1-dependent mechanism. Blood, 132: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL, McMahon SB. (2008) The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell, 29: 102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schrecengost RS, Dean JL, Goodwin JF, Schiewer MJ, Urban MW, Stanek TJ, Sussman RT, Hicks JL, Birbe RC, Draganova-Tacheva RA, Visakorpi T, DeMarzo AM, McMahon SB, Knudsen KE. (2014) USP22 regulates oncogenic signaling pathways to drive lethal cancer progression. Cancer Res, 74: 272–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lindenberg KS, Yvert G, Müller K, Landwehrmeyer GB. (2000) Expression analysis of ataxin-7 mRNA and protein in human brain: evidence for a widespread distribution and focal protein accumulation. Brain Pathol., 10: 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yvert G, Lindenberg KS, Devys D, Helmlinger D, Landwehrmeyer GB, Mandel JL (2001) SCA7 mouse models show selective stabilization of mutant ataxin-7 and similar cellular responses in different neuronal cell types. Hum. Mol. Genet, 10: 1679–1692. [DOI] [PubMed] [Google Scholar]
- 110.Rüb U, Brunt ER, Seidel K, Gierga K, Mooy CM, Kettner M, Van Broeckhoven C, Bechmann I, La Spada AR, Schöls L, den Dunnen W, de Vos RA, Deller T. (2008) Spinocerebellar ataxia type 7 (SCA7): widespread brain damage in an adult-onset patient with progressive visual impairments in comparison with an adult-onset patient without visual impairments. Neuropathol Appl Neurobiol., 34: 155–68. [DOI] [PubMed] [Google Scholar]
- 111.Michalik A, Martin JJ, Van Broeckhoven C. (2004) Spinocerebellar ataxia type 7 associated with pigmentary retinal dystrophy. Eur J Hum Genet., 12: 2–15. [DOI] [PubMed] [Google Scholar]
- 112.Holmberg M, Duyckaerts C, Dürr A, Cancel G, Gourfinkel-An I, Damier P, Faucheux B, Trottier Y, Hirsch EC, Agid Y, Brice A. (1998) Spinocerebellar ataxia type 7 (SCA7): a neurodegenerative disorder with neuronal intranuclear inclusions. Hum Mol Genet., 7: 913–8. [DOI] [PubMed] [Google Scholar]
- 113.Yoo SY, Pennesi ME, Weeber EJ, Xu B, Atkinson R, Chen S, Armstrong DL, Wu SM, Sweatt JD, Zoghbi HY. (2003) SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron, 37: 383–401. [DOI] [PubMed] [Google Scholar]
- 114.Dela Peña IJI, Botanas CJ, de la Peña JB, Custodio RJ, Dela Peña I, Ryoo ZY, Kim BN, Ryu JH, Kim HJ, Cheong JH. (2019) The Atxn7-overexpressing mice showed hyperactivity and impulsivity which were ameliorated by atomoxetine treatment: A possible animal model of the hyperactive-impulsive phenotype of ADHD. Prog Neuropsychopharmacol Biol Psychiatry, 88: 311–319. [DOI] [PubMed] [Google Scholar]
- 115.Guha S, Barman P, Manawa A, Bhaumik SR (2022). Nuclear Export of mRNAs with Disease Pathogenesis and Therapeutic Implications. In: Jurga S, Barciszewski J (eds) Messenger RNA Therapeutics. RNA Technologies, vol 13. Springer, Cham. [Google Scholar]
- 116.Barman P, Kaja A, Chakraborty P, Guha S, Roy A, Ferdoush J, Bhaumik SR (2023) A novel UPS regulation of Sgf73/ataxin-7 that maintains the integrity of the co-activator SAGA in orchestrating transcription. Genetics. 2023 Apr 19:iyad071 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim S, Kwak J, Kim M, Lee D (2013) Separation of a functional deubiquitylating module from the SAGA complex by the proteasome regulatory particle. Nat Commun, 4: 2641. [DOI] [PubMed] [Google Scholar]
- 118.Marinello M, Werner A, Giannone M, Tahiri K, Alves S, Tesson C, den Dunnen W, Seeler JS, Brice A, Sittler A (2019) SUMOylation by SUMO2 is implicated in the degradation of misfolded ataxin-7 via RNF4 in SCA7 models. Dis Model Mech., 12: dmm036145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shukla A, Bajwa P, Bhaumik SR (2006) SAGA-associated Sgf73p facilitates formation of the preinitiation complex assembly at the promoters either in a HAT-dependent or independent manner in vivo. Nucleic Acids Res., 34: 6225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wyce A, Xiao T, Whelan KA, Kosman C, Walter W, Eick D, Hughes TR, Krogan NJ, Strahl BD, Berger SL (2007) H2B ubiquitylation acts as a barrier to Ctk1 nucleosomal recruitment prior to removal by Ubp8 within a SAGA-related complex. Mol Cell, 27: 275–88. [DOI] [PubMed] [Google Scholar]
- 121.Shukla A, Bhaumik SR (2007) H2B-K123 ubiquitination stimulates RNAPII elongation independent of H3-K4 methylation. Biochem Biophys Res Commun., 359: 214–20. [DOI] [PubMed] [Google Scholar]
- 122.Sen R, Lahudkar S, Durairaj G, and Bhaumik SR (2013) Functional analysis of Bre1p, an E3 ligase for histone H2B ubiquitylation, in regulation of RNA polymerase II association with active genes and transcription in vivo. Journal of Biological Chemistry, 288: 9619–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiao T, Kao CF, Krogan NJ, Sun ZW, Greenblatt JF, Osley MA, Strahl BD (2005) Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol. Cell Biol, 25: 637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fleming AB, Kao CF, Hillyer C, Pikaart M, Osley MA (2008) H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol. Cell, 31: 57–66. [DOI] [PubMed] [Google Scholar]
- 125.Tanny JC, Erdjument-Bromage H, Tempst P, Allis CD (2007) Ubiquitylation of histone H2B controls RNA polymerase II transcription elongation independently of histone H3 methylation. Genes Dev., 21: 835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sen R, Bhaumik SR (2013) Transcriptional stimulatory and repressive functions of histone H2B ubiquitin ligase. Transcription, 4: 221–6. [DOI] [PubMed] [Google Scholar]
- 127.Ström AL, Forsgren L, Holmberg M (2005) A role for both wild-type and expanded ataxin-7 in transcriptional regulation. Neurobiol Dis., 20: 646–55. [DOI] [PubMed] [Google Scholar]
- 128.Pray-Grant MG, Schieltz D, McMahon SJ, Wood JM, Kennedy EL, Cook RG, Workman JL, Yates JR 3rd, Grant PA. (2002) The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol Cell Biol., 22: 8774–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCormick MA, Mason AG, Guyenet SJ, Dang W, Garza RM, Ting MK, Moller RM, Berger SL, Kaeberlein M, Pillus L, La Spada AR, Kennedy BK (2014) The SAGA histone deubiquitinase module controls yeast replicative lifespan via Sir2 interaction. Cell Rep., 8: 477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mason AG, Garza RM, McCormick MA, Patel B, Kennedy BK, Pillus L, La Spada AR (2017) The replicative lifespan-extending deletion of SGF73 results in altered ribosomal gene expression in yeast. Aging Cell, 16: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng X, Côté V, Côté J (2021) NuA4 and SAGA acetyltransferase complexes cooperate for repair of DNA breaks by homologous recombination. PLoS Genet, 17: e1009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Evangelista FM, Maglott-Roth A, Stierle M, Brino L, Soutoglou E, Tora L (2018) Transcription and mRNA export machineries SAGA and TREX-2 maintain monoubiquitinated H2B balance required for DNA repair. J Cell Biol., 217: 3382–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramachandran S, Haddad D, Li C, Le MX, Ling AK, So CC, Nepal RM, Gommerman JL, Yu K, Ketela T, Moffat J, Martin A (2016) The SAGA Deubiquitination Module Promotes DNA Repair and Class Switch Recombination through ATM and DNAPK-Mediated γH2AX Formation. Cell Rep., 15: 1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]


