Abstract
Importance:
Biomarkers may improve prediction of cardiovascular events for patients with stable coronary artery disease (CAD), but their importance in addition to clinical tests of inducible ischemia and CAD severity is unknown.
Objectives:
To evaluate the prognostic value of multiple biomarkers in stable outpatients with obstructive CAD and moderate or severe inducible ischemia.
Design and setting:
The ISCHEMIA and ISCHEMIA CKD trials randomized 5,956 participants with CAD to invasive or conservative management from July 2012 to January 2018; 1,064 participated in the biorepository.
Main outcome measures:
Primary outcome was cardiovascular death, myocardial infarction (MI), or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest. Secondary outcome was cardiovascular death or MI. Improvements in prediction were assessed by cause-specific hazard ratios (HR) and area under the receiver operating characteristics curve (AUC) for an interquartile increase in each biomarker, controlling for other biomarkers, in a base clinical model of risk factors, left ventricular ejection fraction (LVEF) and ischemia severity. Secondary analyses were performed among patients in whom core-lab confirmed severity of CAD was ascertained by computed cardiac tomographic angiography (CCTA).
Exposures:
Baseline levels of interleukin-6 (IL-6), high sensitivity troponin T (hsTnT), growth differentiation factor 15 (GDF-15), N-terminal pro-B-type natriuretic peptide (NT-proBNP), lipoprotein a (Lp(a)), high sensitivity C-reactive protein (hsCRP), Cystatin C, soluble CD 40 ligand (sCD40L), myeloperoxidase (MPO), and matrix metalloproteinase 3 (MMP3).
Results:
Among 757 biorepository participants, median (IQR) follow-up was 3 (2–5) years, age was 67 (61–72) years, and 144 (19%) were female; 508 had severity of CAD by CCTA available. In an adjusted multi-marker model with hsTnT, GDF-15, NT-proBNP and sCD40L, the adjusted HR for the primary outcome per interquartile increase in each biomarker was 1.58 (95% CI 1.22, 2.205), 1.60 (95% CI 1.16, 2.20), 1.61 (95% 1.22, 2.14), and 1.46 (95% 1.12, 1.90), respectively. The adjusted multi-marker model also improved prediction compared with the clinical model, increasing the AUC from 0.710 to 0.792 (P<0.01) and 0.714 to 0.783 (P<0.01) for the primary and secondary outcomes, respectively. Similar findings were observed after adjusting for core-lab confirmed atherosclerosis severity.
Conclusions and relevance:
Among ISCHEMIA biorepository participants, biomarkers of myocyte injury/distension, inflammation, and platelet activity improved cardiovascular event prediction in addition to risk factors, LVEF, and assessments of ischemia and atherosclerosis severity. These biomarkers may improve risk stratification for patients with stable CAD.
Keywords: stable coronary artery disease, biomarkers, risk prediction, coronary atherosclerosis, inducible ischemia, ISCHEMIA trial
Introduction:
Coronary artery disease (CAD) is the leading cause of death and disability worldwide, and affects over 18 million Americans, resulting in approximately 400,000 deaths annually.1 Among patients with stable CAD, it remains challenging to predict who will have a cardiovascular event.2–4 Contemporary risk assessment in stable CAD includes clinical risk scores,5,6 stress testing, and assessment of coronary anatomy.7,8 Even with these tools, an urgent need remains to improve risk stratification for among patients with stable CAD.8 Biomarkers of processes underpinning the pathogenesis of CAD and cardiovascular events may provide important prognostic information.
Few studies have investigated multiple biomarkers simultaneously 8,9 or evaluated the prognostic value of biomarkers added to assessments of ischemia (i.e., stress testing) and atherosclerosis severity.10–12 Prior studies are limited to patients undergoing angiography,13,14 combined patients with stable and unstable syndromes,15,16 or correlated biomarkers with ischemia or atherosclerosis.12,17 Additionally, few if any patients with chronic kidney disease and CAD were included in prior studies. The ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) and ISCHEMIA-Chronic Kidney Disease (CKD) trials (collectively, the ISCHEMIA trials) randomized patients with stable CAD with moderate or severe ischemia to an initial invasive strategy of catheterization and revascularization with guideline-directed medical therapy compared with an initial strategy of guideline-directed medical therapy alone.18,19 The objectives of this substudy were to test the hypotheses that one or more blood biomarkers would be associated with adjudicated cardiovascular events, and that addition of multiple biomarkers to traditional clinical risk factors and testing—including core-lab measured severity of ischemia and atherosclerosis—would improve prediction of cardiovascular events.
Methods:
The design and primary results of ISCHEMIA trials have been reported.18–20 ISCHEMIA enrolled patients with known or suspected CAD based on the finding of moderate or severe ischemia on stress imaging (echocardiography, nuclear perfusion or cardiac magnetic resonance imaging), or severe ischemia on exercise electrocardiography.20 Patients meeting criteria for ischemia severity with an estimated glomerular filtration rate [eGFR] ≥30 ml/kg/1.73m2 were enrolled in the main ISCHEMIA trial, and patients with an eGFR <30 ml/kg/1.73m2 were enrolled in ISCHEMIA-CKD. Blinded coronary computed tomography angiography (CCTA) was performed in most (76%) ISCHEMIA patients with the goal of excluding patients with left main coronary stenosis ≥50% or no obstructive epicardial stenosis.20,21 Participants with kidney impairment (eGFR <60 ml/min/1.73 m2) or known coronary anatomy were not required to undergo a CCTA.18,20 Overall, ISCHEMIA randomized 5,956 patients (5,179 from ISCHEMIA and 777 from ISCHEMIA CKD) to an invasive or a conservative approach, and tested the hypothesis that an initial invasive approach would improve clinical outcomes over an initial conservative approach.20,22 In ISCHEMIA and ISCHEMIA-CKD, the initial invasive approach did not reduce the risk of the primary or secondary endpoints.19,23
ISCHEMIA Trials biorepository and sample selection and study outcomes
Venous blood samples were obtained from consenting participants at baseline within 6 weeks of enrollment and prior to receipt of assigned treatment strategy. Plasma was frozen in aliquots and stored at −70°C or colder until analysis. Details of biomarker analyses are provided in the Supplemental Methods and Supplemental Table 1. All biomarkers were measured at the Uppsala Clinical Research Center Laboratory at Uppsala University (Uppsala, Sweden), accredited to SS-EN ISO 15189.24,25 We used the primary (cardiovascular death, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest) and secondary outcome (cardiovascular death or MI) from the ISCHEMIA trial.20 In sensitivity analyses, we considered the individual endpoints of cardiovascular death, MI, and all-cause death.
Statistical methods
Clinical variables, stress testing, and CCTA findings are presented as median, 25th percentile, and 75th percentile for continuously measured variables and frequencies and percentages for categorical variables. We calculated five-point descriptive summaries of the biomarker distributions and pairwise age- and sex-adjusted Spearman correlation coefficients between the biomarker variables. We compared baseline characteristics, stress testing and CAD severity by CCTA across tertiles of biomarker distributions using the Kruskal-Wallis test and chi-square test for continuous and categorical variables, respectively.
We plotted the cumulative incidence of each study outcome by biomarker tertiles and used the Fine-Gray method to assess differences across groupings. Cox proportional hazards regression modeling was used to estimate cause-specific hazard ratios in separate models for each biomarker. We adjusted for six pre-specified participant baseline characteristics (age, sex, diabetes, dialysis, eGFR among non-dialysis patients, and LVEF),19,23 in addition to ischemia severity. Biomarkers were entered by tertile to allow for nonlinear association and to facilitate clinical interpretation. We evaluated the added prognostic value of individual biomarkers and with multiple biomarkers modeled simultaneously and built Cox proportional hazards regression models with biomarkers measured continuously. Simulations in a Cox setting have shown that having at least 10 events per covariates is a prudent approach to avoiding estimation problems.26 Therefore, to align with recommendations for the number of events per covariate (particularly when risk prediction is an objective),26–28 we used a subset of biomarkers in the multiple marker model, modeled biomarker variables linearly, and used only the six pre- specified baseline covariates and ischemia severity. Previous studies in patients with stable CAD3,9,13,16,29–31 informed biomarker selection, along with data availability, biomarker variability, and correlations with other biomarkers. Hazard ratios for biomarkers reflect an increase from the 25th percentile to the 75th percentile, henceforth referred to as an ‘interquartile increase’. Model accuracy and discrimination was estimated with a time-dependent Brier score and time-dependent area under the curve (AUC), respectively.32,33 The time-dependent Brier score is a summary of predictive accuracy that simultaneously measures both calibration and discrimination. For a given time point, the Brier score is computed as the sum of the squared errors between the observed event status and estimated survival. Performance measures were computed accounting for the competing risk of non-cardiovascular death with a cause-specific approach.34 Each model was compared to a base model with only baseline covariates. Performance measures were computed within-sample and may be interpreted as an upper bound for the true predictive performance. A higher AUC and lower Brier score indicate a better model.
A sensitivity analysis was performed to compare performance between a base model with baseline characteristics (age, sex, diabetes, dialysis, eGFR among patients not on dialysis, LVEF and baseline ischemia severity), a base model + two biomarkers (hsTnT and NT-proBNP) and a base model + four biomarkers (hsTnT, NT-proBNP, GDF15 and sCD40L) among all participants in the ISCHEMIA biomarker biorepository.
To explore the importance of biomarkers when CAD severity is known, we replicated analyses among participants with a core-lab confirmed CCTA (508/757, 67%). Given the smaller sample size for analysis in which severity of CAD is known, covariate adjustment was limited to age, sex, LVEF and ischemia severity. We present only the estimated cause-specific hazard ratios because previous research has demonstrated that association studies may be less sensitive to the number of events per covariate compared to prognostic modeling.27,28
Treatment group was included as a stratum variable in Cox models to handle proportional hazards violation assumption by treatment strategy in ISCHEMIA.19 All biomarkers were natural log-transformed to reduce skewness, and those with values below the detection limit were substituted with one-half the detection limit value.9,35 We conducted analyses in R statistical software,36 using the R package riskRegression.37,38
Results
A total of 1,064 ISCHEMIA Trials (ISCHEMIA and ISCHEMIA-CKD) participants consented for the biorepository. This nested cohort study included 757 participants with at least 9 of 10 biomarkers (Supplemental Figure 1). Sample characteristics are presented in Table 1. Baseline characteristics were similar between biorepository participants in this biomarker substudy (N=757) compared with those excluded (N=307) (Supplemental Table 2). Characteristics of participants in the combined ISCHEMIA Trials have been reported.39
Table 1.
Baseline characteristics of ISCHEMIA Biorepository Biomarker Cohort
| Characteristic | Study Population No. (%)a |
|---|---|
| No. | 757 |
| Demographics | |
| Age at randomization, years | |
| No. | 757 |
| Median (Q1, Q3) | 67 (61, 72) |
| Follow-up time, years | |
| No. | 757 |
| Median (Q1, Q3) | 3 (2, 5) |
| Female sex | 144/757 (19%) |
| Race | |
| White | 634/755 (84%) |
| Black | 84/755 (11%) |
| Asian | 20/755 (3%) |
| Other or multiple ethnic groups | 17/755 (2%) |
| Hispanic or Latino ethnicity | 38/750 (5%) |
| Cigarette smoking | |
| Current smoker | 85/757 (11%) |
| Former smoker | 412/757 (54%) |
| Never smoker | 260/757 (34%) |
| Randomized to invasive | 368/757 (49%) |
| Randomized to conservative | 389/757 (51%) |
| Clinical history | |
| Diabetes | 344/757 (45%) |
| Insulin treated | 141/341 (41%) |
| Non-insulin treated or diet controlled | 200/341 (59%) |
| Hypertension | 644/755 (85%) |
| BMI, kg/m2 | |
| No. | 754 |
| Median (Q1, Q3) | 29 (26, 33) |
| Obese, BMI ≥30 kg/m2 | 343/754 (45%) |
| eGFR, mL/min/1.73 m2 | |
| No. | 757 |
| Median (Q1, Q3) | 74 (58, 90) |
| eGFR <60 ml/min/1.73 m2 | 206/757 (27%) |
| Baseline dialysis | 49/757 (6%) |
| Left ventricular ejection fraction | |
| No. | 757 |
| Median (Q1, Q3) | 60 (54, 65) |
| Left ventricular ejection fraction <45% | 52/756 (7%) |
| Prior myocardial infarction | 175/754 (23%) |
| Prior PCI | 203/757 (27%) |
| Prior CABG | 43/757 (6%) |
| History of heart failure | 34/757 (4%) |
| History of cerebrovascular disease | 85/756 (11%) |
| History of peripheral artery disease | 45/755 (6%) |
| Family history of premature CAD | 209/645 (32%) |
| Ischemia severity by stress testing | |
| Severe | 329/756 (44%) |
| Moderate | 355/756 (47%) |
| Mild/none | 69/756 (9%) |
| Uninterpretable | 3/756 (0%) |
| CCTA findings | |
| CCTA performed | 508/757 (67%) |
| Any obstructive disease ≥70% stenosis by CCTA | 392/508 (77%) |
| Multivessel disease ≥70% stenosis by CCTA | 185/508 (36%) |
| Vessels ≥70% stenosis by CCTA | |
| 0 | 60/508 (12%) |
| 1 | 134/508 (26%) |
| 2 | 84/508 (17%) |
| 3 or more | 65/508 (13%) |
| Non evaluable | 165/508 (32%) |
Continuously measured variables are summarized with the median, 25th percentile (Q1), and 75th percentile (Q3).
The median (interquartile range, IQR) age was 67 years (61, 72), and median follow-up was 3 (2–5) years; 19% of participants were female (Table 1). Hypertension (85%), diabetes (45%), and obesity (45%) were common; 27% of participants had an eGFR <60 ml/min/1.73 m2 and 6% of patients were on dialysis at baseline. Twenty-seven and 23% of participants had a prior percutaneous coronary intervention and MI, respectively, and 4% of the cohort had a history of heart failure. Ninety-one percent of participants in the biomarker substudy had at least moderate ischemia by stress imaging. A CCTA was performed in 508 (67%) participants, 36% of whom had multivessel coronary artery disease ≥70% stenosis. Over a median follow-up of 3 years, there were 146 and 128 primary and secondary endpoints, respectively.
Biomarkers, Participant Characteristics and Clinical Testing in the Biorepository
Supplemental Table 3 summarizes biomarker distributions and percent below detection limits. Most biomarkers were detectable in all patients, with a maximum percent below detection of 7% for hsCRP. Eighteen percent (137/757) of IL-6 assays were missing due to insufficient sample volume; all others had <1% missing due to insufficient volume. The age- and sex-adjusted correlation between most biomarkers was weak to moderate, apart from GDF-15 and Cystatin-C (rho ≈ 0.7) (Supplemental Figure 2).
Supplemental Tables 4 and 5 present relationships between baseline characteristics, stress testing, and CCTA findings for a representative biomarker, hs-TnT. Participant risk factors and comorbidities including hypertension, diabetes requiring insulin, obesity, impaired renal function, baseline dialysis, history of heart failure, cerebrovascular disease and peripheral artery disease were generally more common across increasing biomarker tertiles (lowest to highest). This pattern was consistent for all biomarkers except Lp(a) and sCD40L, for which the distribution of risk factors and comorbidities was unchanged from tertile 1 to tertile 3 (data not shown). The proportion of participants with severe ischemia did not vary across tertiles of hsTnT (Supplemental Table 5) or any other biomarker (data not shown). In contrast, the proportion of patients with multivessel CAD (multivessel CAD ≥70%, multivessel CAD ≥50% stenosis) increased from tertile 1 to tertile 3 of hsTnT (Supplemental Table 5), NTproBNP and Lp(a) (data not shown). For all other biomarkers the proportion of multivessel CAD ≥70% was similar across tertiles (data not shown).
Biomarkers and Cardiovascular Events
Figures 1a and 1b show the unadjusted cumulative incidence of the primary and secondary endpoint by biomarker tertiles (see Figure 2 for tertile cutoff values). The cumulative incidence of the primary and secondary outcome differed across tertiles for all biomarkers except MPO and Lp(a) (Figure 1a and 1b; Fine-Gray p<0.05).
Figure 1a. Cumulative incidence of the primary outcome (Cardiovascular death, MI, hospitalization for unstable angina or heart failure, or resuscitated cardiac arrest) by tertilesa of biomarker distributions.
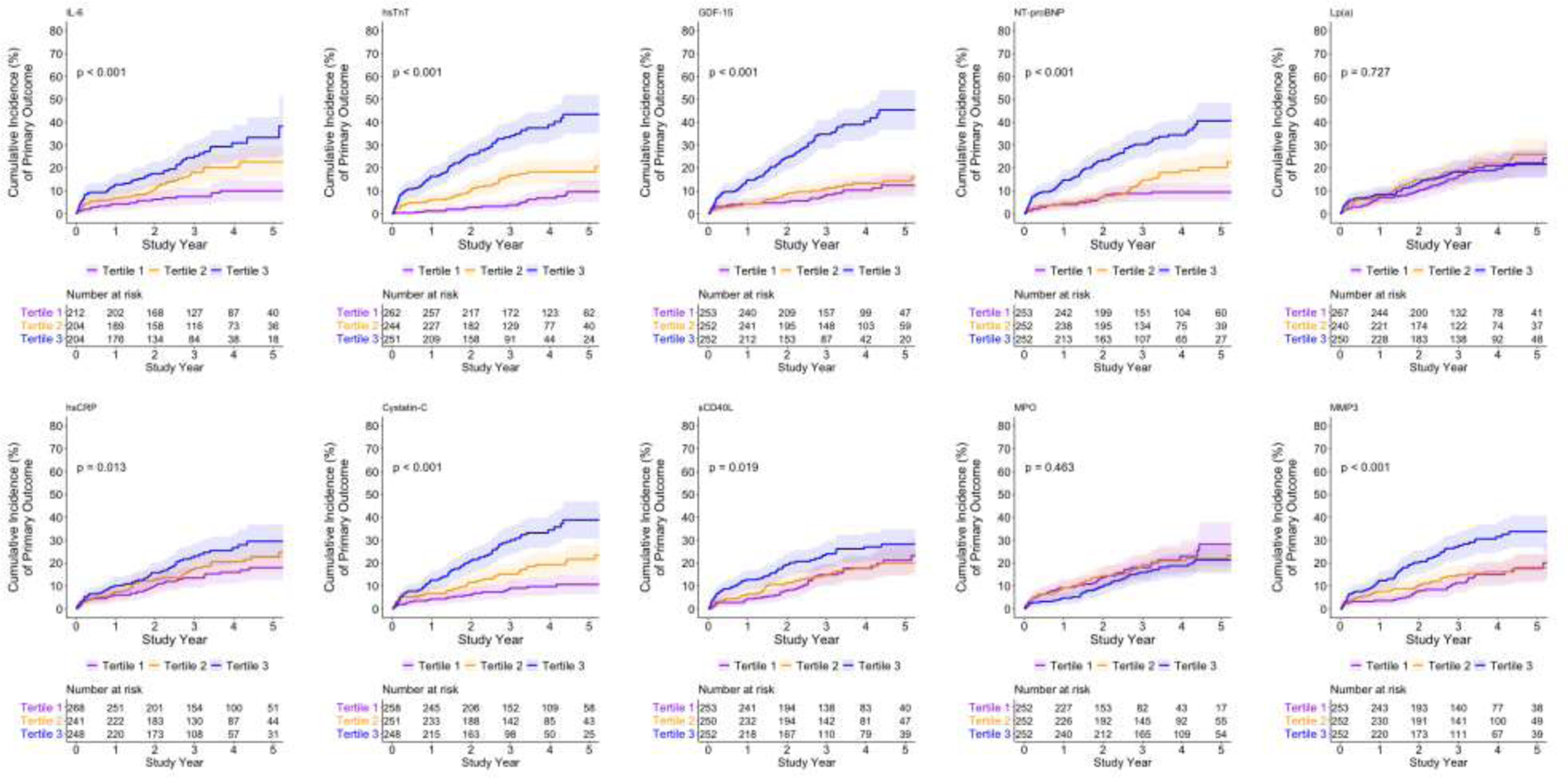
a See Figure 2 for biomarker values of tertile cutoffs.
Figure 1b. Cumulative incidence of the secondary outcome (Cardiovascular death or MI) by tertilesa of biomarker distributions.
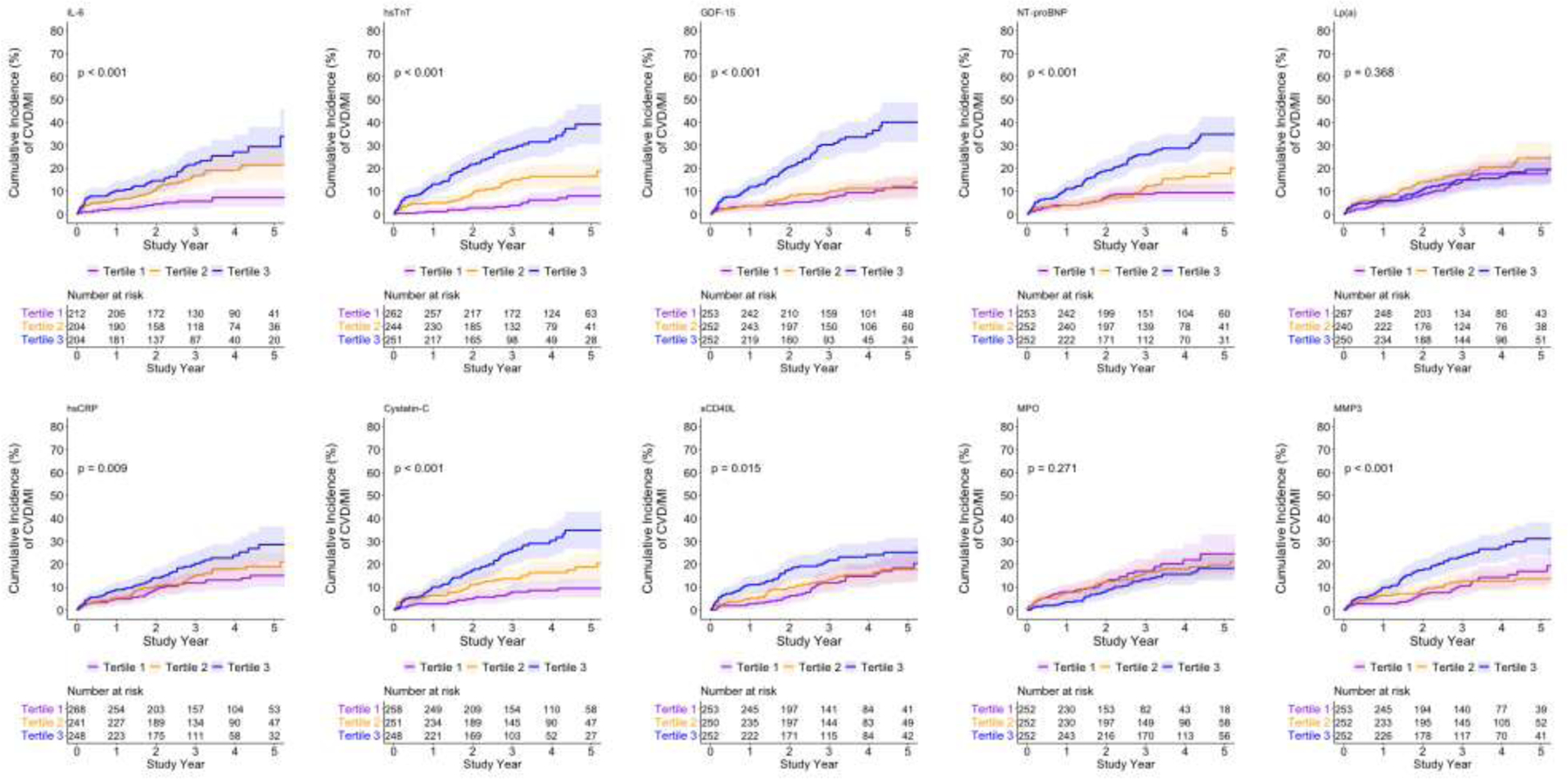
a See Figure 2 for biomarker values of tertile cutoffs.
Figure 2a. Forest plot of the adjusted association of the primary outcome and biomarker tertiles in the ISCHEMIA biorepository biomarker substudy.
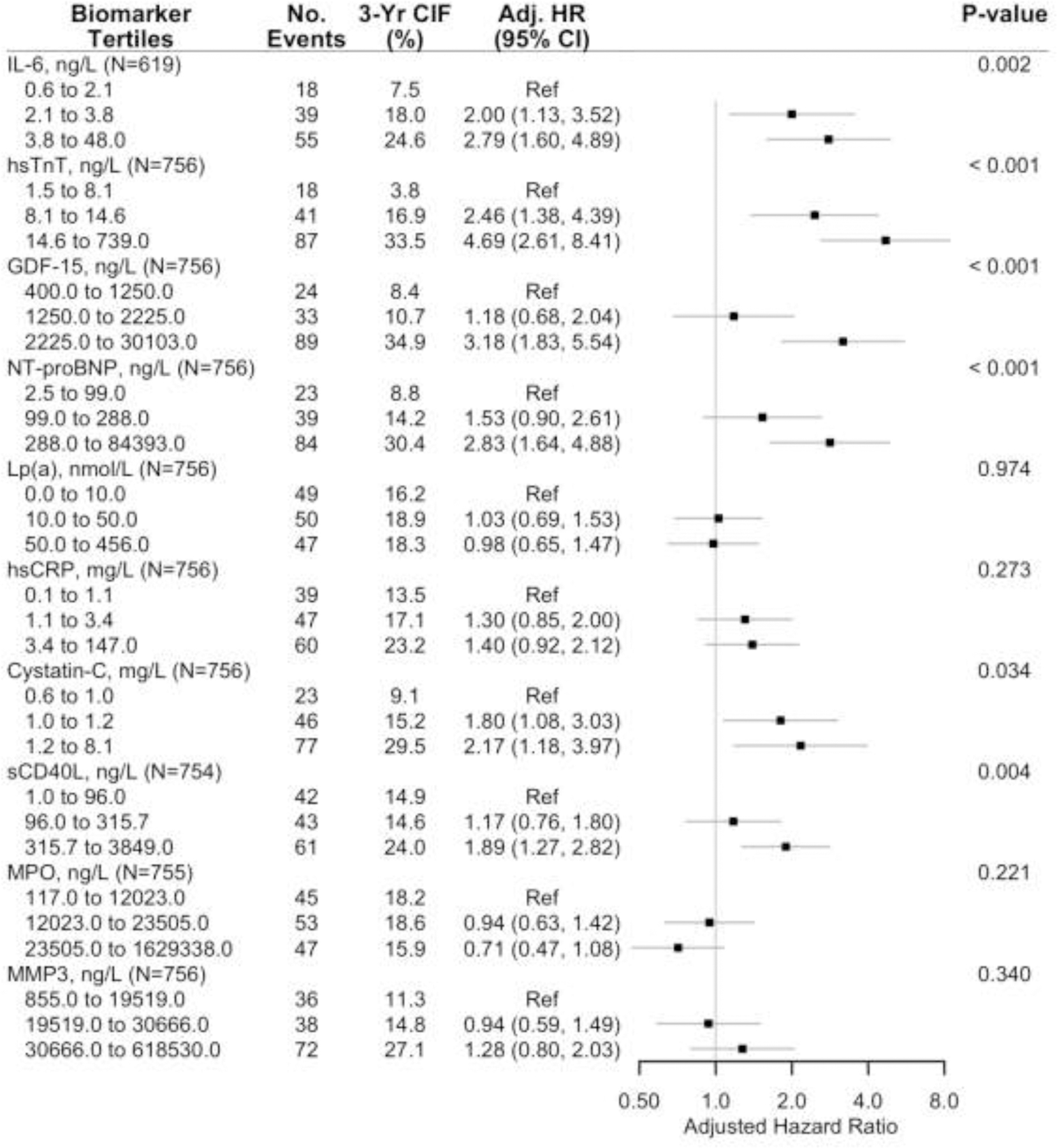
a Adjusted cause-specific hazard ratios for the association of the primary outcome by tertiles of each biomarker (controlling for sex, age, diabetes, dialysis, eGFR among non-dialysis patients, left ventricular ejection fraction and ischemia severity).
b Primary outcome was the composite of cardiovascular death, myocardial infarction, hospitalization for unstable angina or heart failure, or resuscitated cardiac arrest.
Abbreviations: No., number, 3-yr CIF, 3-year Cumulative Incidence Function, Adj. HR, adjusted hazard ratio.
The 3-year unadjusted cumulative incidence of the primary and secondary outcome appeared greater across increasing tertiles of most biomarkers except Lp(a) and MPO (Figures 2a and 2b). After adjusting for age, sex, diabetes, dialysis, eGFR among those not on dialysis, LVEF and ischemia severity there was a significant increasing hazard for the primary (Figure 2a) and secondary (Figure 2b) outcome across biomarker tertiles. IL-6, hsTnT, GDF-15, NT-proBNP, Cystatin-C, and sCD40-L were each individually associated with the primary and secondary outcomes.
Figure 2b. Forest plot of the adjusted association of secondary outcome and biomarker tertiles, in the ISCHEMIA biorepository biomarker substudy.
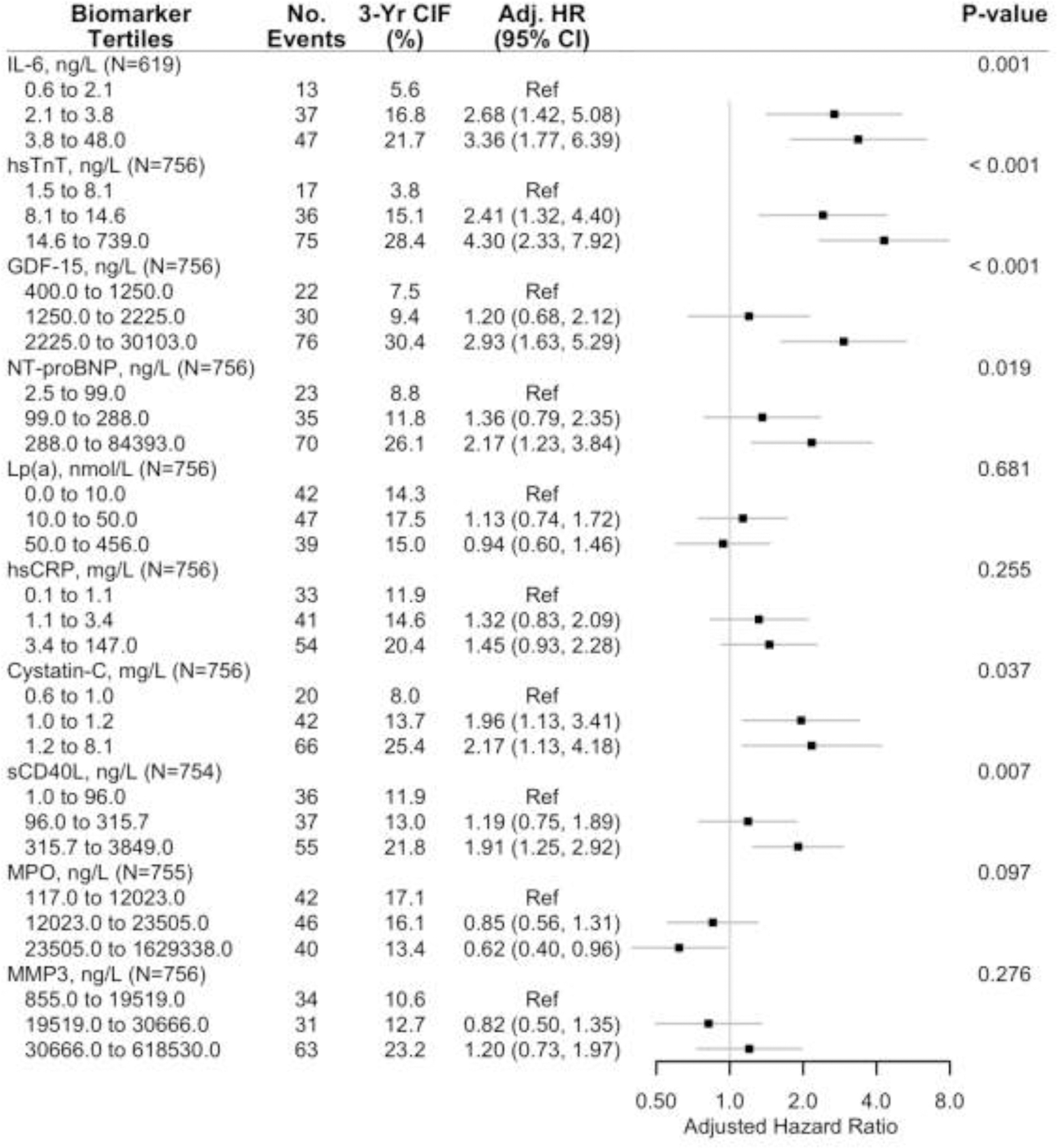
a Adjusted cause-specific hazard ratios for the association of the primary outcome by tertiles of each biomarker (controlling for sex, age, diabetes, dialysis, eGFR among non-dialysis patients, left ventricular ejection fraction and ischemia severity).
b Secondary outcome was the composite of cardiovascular or myocardial infarction.
Abbreviations: No., number, 3-yr CIF, 3-year Cumulative Incidence Function, Adj. HR, adjusted hazard ratio.
We next evaluated the potential contribution of biomarkers to improve risk prediction. For the primary and secondary outcome, in separate models by individual biomarker, Supplemental Table 6 presents cause-specific hazard ratios for an increase from the 25th to the 75th percentile (“interquartile increase”) in a given biomarker distribution. For example, the IQR increase for NT-proBNP refers to an increase from 75 ng/L to 415 ng/L on the raw (un-transformed) scale. Each interquartile increase in hsTnT, NT-proBNP, or GDF-15 was associated with an approximately 2-fold greater hazard for the primary and secondary outcome; the hazards for the primary and secondary outcome per IQR increase in IL-6, Cystatin-C, sCD40L or MMP3 were more modest (Supplemental Table 6). The base model with clinical risk factors, LVEF and ischemia severity had an area under the receiver operating characteristics curve (AUC) of approximately 0.71 for the primary and secondary outcome (Supplemental Table 7). Compared to the base model, when considered individually hsTnT and GDF-15 significantly improved model discrimination for both composite outcomes (Supplemental Table 7). Predictive accuracy of both the primary and secondary outcome (as assessed by Brier score) was improved significantly by inclusion of hsTnT.
Table 2 presents adjusted cause-specific hazard ratios of biomarkers selected for multi-marker modeling (hsTnT, NT-proBNP, GDF-15 and sCD40L) of the primary and secondary outcomes. Controlling for other biomarkers, participant characteristics and ischemia severity, an interquartile increase in each biomarker was individually associated with an approximately 50% (44%-61%) greater hazard of the primary and secondary outcome (Table 2). When included simultaneously, the addition of hsTnT, NT-proBNP, GDF-15 and sCD40L to the base model substantially improved model discrimination and predictive accuracy. The AUC increased from 0.711 to 0.791 (P=0.001) for the 5-component primary outcome and from 0.712 to 0.783 (P=0.002) for the secondary outcome of cardiovascular death or MI (Table 2, Figure 3a/b). Predictive accuracy as measured by the Brier score also significantly improved for both outcomes.
Table 2.
Multivariable adjusted multi-marker association and prediction models in the ISCHEMIA Biorepository Biomarker Cohort (N=754).
| Primary outcomea | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker (IQR increase) | Adjusted HRc (95% CI) | Base AUC | New AUC | ∆ AUC (95% CI) | P value | Base Brier Score | New Brier Score | ∆ Brier Score (95% CI) | P value |
| hsTnT, ng/L (7.0 – 18.4) | 1.58 (1.22, 2.05) | 0.711 | 0.791 | 0.080 (0.035, 0.126) | 0.001 | 0.129 | 0.117 | −0.012 (−0.020, −0.005) | 0.001 |
| GDF-15, ng/L (1086.0 – 2753.0) | 1.60 (1.16, 2.20) | ||||||||
| NT-proBNP, ng/L (75.0 – 415.0) | 1.61 (1.22, 2.14) | ||||||||
| sCD40L, ng/L (68.5 – 418.5) | 1.46 (1.12, 1.90) | ||||||||
| Secondary outcome b | |||||||||
| Biomarker (IQR increase) | Adjusted HRc (95% CI) | Base AUC | New AUC | ∆ AUC (95% CI) | P value | Base Brier Score | New Brier Score | ∆ Brier Score (95% CI) | P value |
| hsTnT, ng/L (7.0 – 18.4) | 1.54 (1.17, 2.04) | 0.712 | 0.783 | 0.071 (0.026, 0.116) | 0.002 | 0.116 | 0.107 | −0.009 (−0.015, −0.002) | 0.008 |
| GDF-15, ng/L (1086.0 – 2753.0) | 1.57 (1.12, 2.20) | ||||||||
| NT-proBNP, ng/L (75.0 – 415.0) | 1.44 (1.08, 1.94) | ||||||||
| sCD40L, ng/L (68.5 – 418.5) | 1.51 (1.14, 2.00) | ||||||||
Hazard ratios are expressed per increase in biomarker concentration from the 25th to the 75th percentile (termed IQR increase) of the distribution.
Abbreviations: HR, hazard ratio; CI, confidence interval. IQR, interquartile range. eGFR, estimated glomerular filtration rate. LVEF, left ventricular ejection fraction. Biomarker abbreviations as noted in the abbreviations list.
Cardiovascular death, myocardial infarction, resuscitated cardiac arrest, hospitalization for unstable angina or heart failure.
Cardiovascular death or myocardial infarction
Adjusted for age, sex, diabetes, dialysis, eGFR among patients not on dialysis, LVEF, and baseline ischemia severity.
Figure 3. Receiver operating curves at 3 years for the base model and selected biomarkers for the primary (3a) and secondary (3b) outcome in the ISCHEMIA biorepository biomarker substudy, N=757.
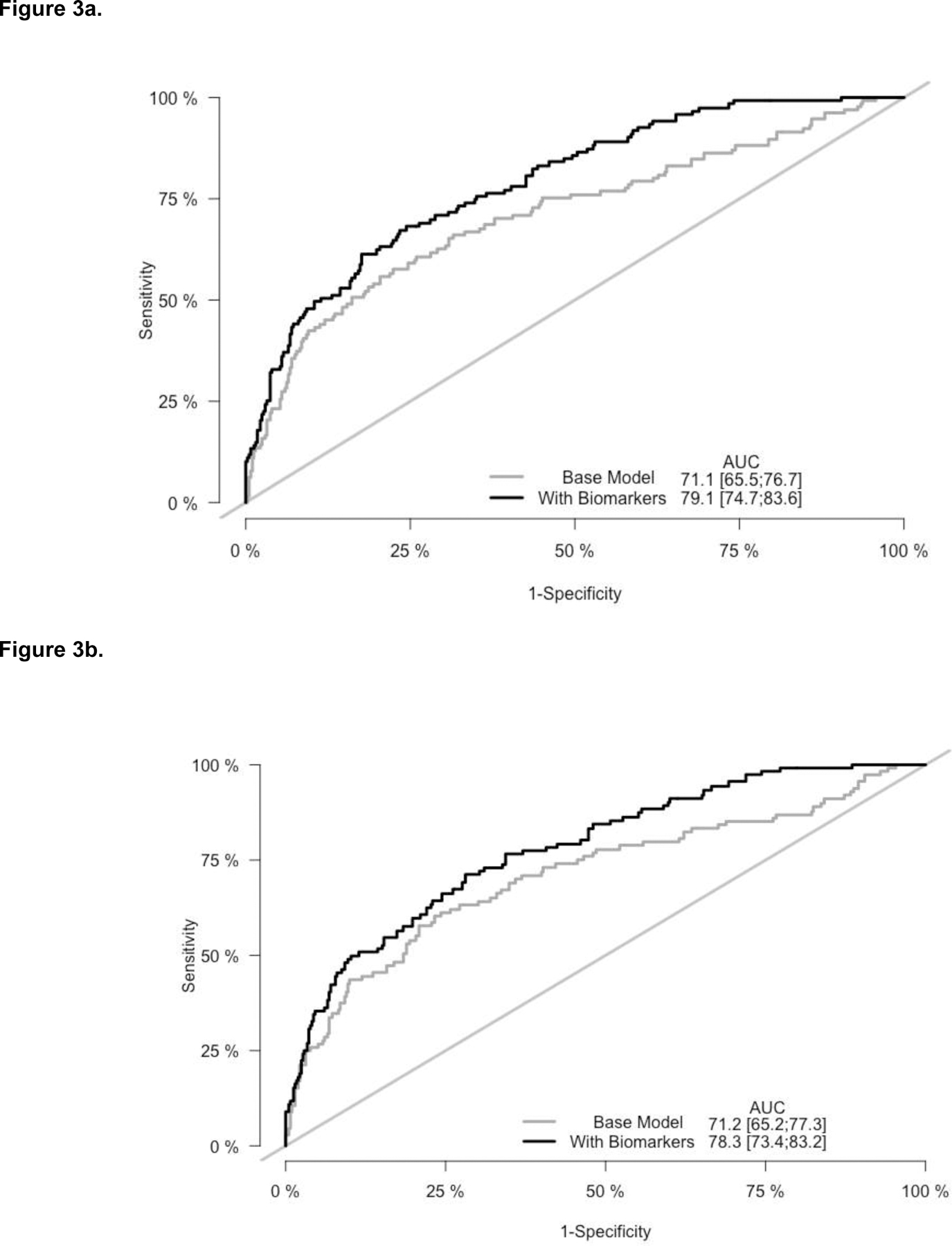
Base model includes adjustment for age, sex, diabetes, dialysis, eGFR among non-dialysis patients, ischemia severity and left ventricular ejection fraction. With biomarkers denotes the base model and 4 biomarkers: hsTnT, GDF-15, NT-proBNP, and sCD40L.
Primary outcome: Cardiovascular death, MI, hospitalization for heart failure or unstable angina, or resuscitated cardiac arrest.
Secondary outcome: CV death or MI
Sensitivity analysis demonstrated that compared to a clinical model with hsTnT and NT-proBNP, a clinical model with four biomarkers (hsTnT, NT-proBNP, GDF-15 and sCD40L) significantly improved discrimination of the primary and secondary outcomes by AUC but did not improve predictive accuracy (Supplemental Table 8). In an exploratory analysis with all 10 biomarkers entered simultaneously (adjusting for clinical covariates and ischemia severity), hsTnT, GDF-15, and sCD40L were each associated with an increased hazard of the primary and secondary outcome, while NT-proBNP was associated only with an increased hazard for the primary outcome (Supplemental Table 9).
Analyses of the adjusted cause-specific hazard ratios between biomarkers and individual endpoints of MI, CV death, and all-cause death were largely consistent with the primary and secondary outcomes. HsTnT, NT-proBNP and GDF-15 were each associated with MI, CV death, and all-cause death. While not associated with the endpoint of MI, MMP3 and Cystatin C were associated with CV death and all-cause death. sCD40L was significantly associated only with MI (Supplemental Figures 3a–3c).
Biomarkers, severity of CAD, and cardiovascular events
To characterize biomarker performance in addition to core-lab confirmed severity of CAD, we next performed analysis of biomarkers among ISCHEMIA biorepository substudy participants with available CCTA data. In comparison with the biomarker cohort (N=757), participants with a CCTA (N=508) had a lower burden of diabetes and better renal function, Supplemental Table 2.
We next explored associations with biomarkers after adjustment for CAD severity in the subset with core-lab confirmed atherosclerosis burden by CCTA. For the primary and secondary outcome, Supplemental Table 10 presents cause-specific hazard ratios for an interquartile increase in each biomarker. After adjusting for age, sex, LVEF, ischemia severity and core-lab confirmed multivessel CAD ≥70% stenosis, each IQR increase in IL-6, hsTnT, GDF-15, NT-proBNP, or sCD40L was individually associated with the primary and secondary outcome; hsCRP and Cystatin-C were associated with only the secondary outcome (Supplemental Table 10). For the primary and secondary outcomes, Supplemental Table 11 presents adjusted cause-specific hazard ratios of each biomarker selected for multi-marker modeling (namely, hsTnT, NT-proBNP, GDF-15 and sCD40L), adjusting for other biomarkers, clinical characteristics, ischemia severity and multivessel CAD ≥70%. An interquartile increase in each of the selected biomarkers for the multi-marker model was associated with an approximately 50% (42%-87%) greater hazard for the primary and secondary outcomes.
Discussion
In this analysis from the ISCHEMIA Trials biorepository, we found that biomarkers of myocardial injury/distension (hsTnT, NT-proBNP), inflammation (GDF-15), and platelet activity (sCD40L) were associated with and improved prediction of cardiovascular events after adjustment for clinical risk factors, LVEF, severity of ischemia and atherosclerosis. This suggests a clinical utility for biomarker measurement beyond current risk paradigms for stable CAD.
Landmark prospective cohort studies provided important data on the use of biomarkers to enhance cardiovascular risk prediction.30,31,40–44 Data from the current analyses extend knowledge to CAD patients with core-lab confirmed ischemia in whom severity of CAD is known. Biorepositories embedded in randomized clinical trials present a unique opportunity to evaluate biomarkers alongside clinical testing and management. Of the 10,003 outpatients with stable chest pain randomized in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) study,45 4,031 were included in a blood biorepository.46,47 PROMISE analyses demonstrate that high-sensitivity troponin46,47 and IL-648 were associated with CAD characteristics and cardiovascular events. However, PROMISE analyses have not evaluated if biomarkers associate with events when added to inducible ischemia or atherosclerosis severity.45–48 The Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial compared the effect of an Lp-PLA2 inhibitor (darapladib) with placebo on cardiovascular events in 15,828 patients with stable CAD, of whom 13,164 patients were included in a biorepository.24,25 STABILITY demonstrated that NTproBNP, hsTnT,25 and IL-624 provide incremental predictive value when added to clinical testing.25 However, severity of CAD and inducible ischemia were not core-lab confirmed prior to randomization and were not included in multivariable modeling.24,25 Incorporation of atherosclerosis severity in modeling is important because without it, one cannot tell if the biomarker predicts atherosclerosis severity—a well-known prognostic indicator—or is independently associated with higher risk.
It is in the context of these landmark studies that we demonstrate that a multi-marker model improves prediction of cardiovascular events in the setting of moderate-severe stress imaging and core lab-confirmed severity of CAD by CCTA. Biomarkers identified represent complementary pathophysiological processes in stable CAD. Cardiac troponins are an integral part of myocardial contractile apparatus and are released into circulation following acute and chronic injury.49 Naturietic peptides including NT-proBNP reflect myocardial dysfunction, wall stress and ventricular dysfunction.50 GDF-15 is an stress responsive cytokine expressed and secreted in response to inflammation and oxidative stress,51 and sCD40-L is an immunomodulator ligand with platelet activity.52 We show that compared to a clinical model with hsTnT and NT-proBNP alone, the addition of GDF-15 and sCD40L significantly improves model discrimination but does not appreciably change predictive accuracy, as measured by the Brier score which takes into account both model discrimination and calibration. More broadly, this observation suggests that non-myocardial biomarkers, such as markers of inflammation or platelet activity, may have prognostic relevance for the care of patients with stable CAD.
Few, if any, patients with stable CAD and comorbid renal dysfunction have been included in prior biomarker studies.46 In contrast, more than one quarter (28%) of patients in the current analysis had an eGFR <60 ml/min/1.73 m2. Our findings provide preliminary data on the use of biomarkers on the subset of patients with stable CAD and comorbid renal disease.
Our study has limitations. Analyses were conducted post-hoc on existing biorepository data and were unadjusted for multiple comparisons. Second, analyses were performed in a single cohort and caution is warranted for over-interpretation of predictive analyses. External validation is needed. Biomarkers were available in a subset of ISCHEMIA Trials participants in whom sample collection was allowed by country specific regulations. Analyses are limited to samples collected at baseline precluding analyses of change in biomarkers over time. Sites were encouraged to process and store the samples rapidly; however, delay in processing may have occurred and affected the values of some of the biomarkers reported. Analyses were based on hsTnT and recent data indicates high-sensitivity cardiac troponin I (hsTnI) may be more specific for cardiovascular outcomes than hsTnT.53,54 Participants in ISCHEMIA were required to have moderate or severe ischemia prior to randomization.19,20 Therefore, our results may not be applicable to patients without ischemia or with non-obstructive CAD. Finally, the subset of patients with inducible ischemia in whom CAD severity was available is a subset of the overall biorepository and does not include patients with CKD.
Conclusions:
In this analysis from the ISCHEMIA Trials biorepository, biomarkers of myocyte injury/distension, inflammation, and platelet activity improved prediction of cardiovascular events. At a median follow-up of 3.5 years, high-sensitivity troponin T and NT-proBNP improved prediction of cardiovascular events in a high-risk population of patients with stable CAD when added to models including clinical risk factors, core lab-confirmed severity of CAD and inducible ischemia. Identified biomarkers will require prospective testing and external validation.
Supplementary Material
Key Points.
Question:
Do biomarkers improve risk stratification for cardiovascular events among patients with stable coronary artery disease (CAD) when severity of ischemia and atherosclerosis are known?
Findings:
In this sub-study from the ISCHEMIA biorepository, a multi-marker model of baseline high sensitivity cardiac troponin T (hsTnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), growth differentiation factor 15 (GDF-15) and soluble CD 40 ligand (sCD40L) was independently associated with and improved prediction of cardiovascular events after adjustment for clinical risk factors, ejection fraction, severity of ischemia and atherosclerosis.
Meaning:
Biomarkers of myocyte injury/distension, inflammation, and platelet activity may improve risk stratification for patients with stable CAD in addition to assessments of ischemia and atherosclerosis severity.
Sources of Funding:
NIH grants U01HL105907, U01HL105462, U01HL105561, U01HL105565, R56HL1555528, R01HL165208
Other Support:
This project was supported in part by Clinical Translational Science Award Nos. 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences and by grants from Arbor Pharmaceuticals LLC and Astra Zeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc.; medications provided by Amgen Inc; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck Sharp & Dohme Corp. and Sunivion Pharmaceuticals
Abbreviations list:
- ASCVD
atherosclerotic cardiovascular disease
- AUC
Area under the receiver operating characteristics curve
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- GDF-15
growth/differentiation factor-15
- HR
hazard ratio
- hsCRP
High sensitivity C-reactive protein
- hs-cTnT
high-sensitivity cardiac troponin T
- IL-6
interleukin 6
- Lp(a)
Lipoprotein (a)
- LVEF
left ventricular ejection fraction
- MI
Myocardial infarction
- MACE
major adverse cardiovascular events
- MMP3
matrix metalloproteinase 3
- MPO
myeloperoxidase
- NT-proBNP
N-terminal (NT)-pro hormone B-type natriuretic peptide (BNP)
- sCD40L
soluble CD-40 ligand
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Clinical Trial Registration: ClinicalTrials.gov identifier: NCT01471522; https://clinicaltrials.gov/ct2/show/NCT01471522
Disclosures
Dr. Jonathan D. Newman reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Rebecca Anthopolos reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Harmony R. Reynolds reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study, she receives support from Abbott Vascular (donation of optical coherence tomography catheters for an unrelated research study), Biotelemetry Inc (donation of telemetry monitors for an unrelated research study), and Siemens.
Dr. Sripal Bangalore reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Abbott Vascular; personal fees from Biotronik, Pfizer, Amgen, and Reata outside the submitted work.
Dr. Mavromatis reports grants from National Heart, Lung, and Blood Institute; grants from NHLBI (CV Inflammation Reduction Trial and GMCSF in PAD-3 Trial), grants from CSL Behring, St Jude’s Medical, Medtronic, DalCor Pharmaceuticals, AstraZeneca, Novartis, Regeneron, and Member of American College of Cardiology and Society of Cardiovascular Angiography and Interventions.
Dr. L. Kristin Newby reports grants from the NIH/NHLBI, AstraZeneca Healthcare Foundation, Boehringer Ingelheim, CSL, and Medtronic.
Dr. Judith S. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; Abbott Laboratories (formerly St. Jude Medical, Inc); Royal Philips NV (formerly Volcano Corporation); Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc. Espero BioPharma; and Amgen, Inc; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
Dr. David J. Maron reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Jeffrey S. Berger reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Drs. Held, Wallentin, Kullo, McManus, Ruggles and Rosenberg have no disclosures to report. Macintosh Cornwell has no disclosures to report.
References:
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019;139(10):e56–e66. doi: 10.1161/cir.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Eagle KA, Ginsburg GS, Musunuru K, et al. Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation 2010;121(12):1447–1454. doi: 10.1161/CIRCULATIONAHA.109.904029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty AL, Ku IA, Bibbins‐Domingo K, et al. Traditional Risk Factors Versus Biomarkers for Prediction of Secondary Events in Patients With Stable Coronary Heart Disease: From the Heart and Soul Study. Journal of the American Heart Association 2015;4(7). doi: 10.1161/jaha.114.001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesnier J, Ducrocq G, Danchin N, et al. International Observational Analysis of Evolution and Outcomes of Chronic Stable Angina: The Multinational CLARIFY Study. Circulation 2021;144(7):512–523. doi: 10.1161/CIRCULATIONAHA.121.054567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohula EA, Bonaca MP, Braunwald E, et al. Atherothrombotic Risk Stratification and the Efficacy and Safety of Vorapaxar in Patients With Stable Ischemic Heart Disease and Previous Myocardial Infarction. Circulation 2016;134(4):304–313. doi: 10.1161/CIRCULATIONAHA.115.019861 [DOI] [PubMed] [Google Scholar]
- 6.Dorresteijn JAN, Visseren FLJ, Wassink AMJ, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart 2013;99(12):866–872. doi: 10.1136/heartjnl-2013-303640 [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74(12):1608–1617. doi: 10.1016/j.jacc.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 8.McCarthy CP, McEvoy JW, Januzzi JL. Biomarkers in stable coronary artery disease. American Heart Journal 2018;196:82–96. doi: 10.1016/j.ahj.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 9.McCarthy CP, Kimmenade RRJ van, Gaggin HK, et al. Usefulness of Multiple Biomarkers for Predicting Incident Major Adverse Cardiac Events in Patients Undergoing Diagnostic Coronary Angiography (From the Catheter Sampled Blood Archive in Cardiovascular Diseases [CASABLANCA] Study). The American Journal of Cardiology 2017;120(1):25–32. doi: 10.1016/j.amjcard.2017.03.265 [DOI] [PubMed] [Google Scholar]
- 10.Everett BM, Brooks MM, Vlachos HEA, et al. Troponin and Cardiac Events in Stable Ischemic Heart Disease and Diabetes. N Engl J Med 2015;373(7):610–620. doi: 10.1056/NEJMoa1415921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammadah M, Al Mheid I, Wilmot K, et al. Association Between High-Sensitivity Cardiac Troponin Levels and Myocardial Ischemia During Mental Stress and Conventional Stress. JACC Cardiovasc Imaging 2018;11(4):603–611. doi: 10.1016/j.jcmg.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caselli C, Prontera C, Liga R, et al. Effect of Coronary Atherosclerosis and Myocardial Ischemia on Plasma Levels of High-Sensitivity Troponin T and NT-proBNP in Patients With Stable Angina. Arteriosclerosis, thrombosis, and vascular biology 2016;36(4):757–764. doi: 10.1161/atvbaha.115.306818 [DOI] [PubMed] [Google Scholar]
- 13.Goliasch G, Kleber ME, Richter B, et al. Routinely available biomarkers improve prediction of long-term mortality in stable coronary artery disease: the Vienna and Ludwigshafen Coronary Artery Disease (VILCAD) risk score. Eur Heart J 2012;33(18):2282–2289. doi: 10.1093/eurheartj/ehs164 [DOI] [PubMed] [Google Scholar]
- 14.Kleber ME, Goliasch G, Grammer TB, et al. Evolving biomarkers improve prediction of long-term mortality in patients with stable coronary artery disease: the BIO-VILCAD score. Journal of Internal Medicine 2014;276(2):184–194. doi: 10.1111/joim.12189 [DOI] [PubMed] [Google Scholar]
- 15.Eapen DJ, Manocha P, Patel RS, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. Journal of the American College of Cardiology 2013;62(4):329–337. doi: 10.1016/j.jacc.2013.03.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blankenberg S, McQueen MJ, Smieja M, et al. Comparative Impact of Multiple Biomarkers and N-Terminal Pro-Brain Natriuretic Peptide in the Context of Conventional Risk Factors for the Prediction of Recurrent Cardiovascular Events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2006;114(3):201–208. doi: 10.1161/CIRCULATIONAHA.105.590927 [DOI] [PubMed] [Google Scholar]
- 17.Lee G, Twerenbold R, Tanglay Y, et al. Clinical benefit of high-sensitivity cardiac troponin I in the detection of exercise-induced myocardial ischemia. American Heart Journal 2016;173:8–17. doi: 10.1016/j.ahj.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Hochman JS, Reynolds HR, Bangalore S, et al. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical Trial. JAMA Cardiology 2019;4(3):273–286. doi: 10.1001/jamacardio.2019.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron DJ, Hochman JS, Reynolds HR, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. New England Journal of Medicine Published online March 30, 2020. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed]
- 20.Maron DJ, Hochman JS, O’Brien SM, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) Trial: Rationale and Design. American Heart Journal 2018;201(N Engl J Med 356 2007):124–135. doi: 10.1016/j.ahj.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancini GBJ, Leipsic J, Budoff MJ, et al. Coronary CT Angiography Followed by Invasive Angiography in Patients With Moderate or Severe Ischemia-Insights From the ISCHEMIA Trial. JACC Cardiovasc Imaging Published online January 7, 2021:S1936–878X(20)31019–6. doi: 10.1016/j.jcmg.2020.11.012 [DOI] [PMC free article] [PubMed]
- 22.Bangalore S, Maron DJ, Fleg JL, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches-Chronic Kidney Disease (ISCHEMIA-CKD): Rationale and Design. Am Heart J 2018;205:42–52. doi: 10.1016/j.ahj.2018.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangalore S, Maron DJ, O’Brien SM, et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N Engl J Med 2020;382(17):1608–1618. doi: 10.1056/NEJMoa1915925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Held C, White HD, Stewart RAH, et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc 2017;6(10):e005077. doi: 10.1161/JAHA.116.005077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindholm D, Lindback J, Armstrong PW, et al. Biomarker-Based Risk Model to Predict Cardiovascular Mortality in Patients With Stable Coronary Disease. Journal American College Cardiology 2017;70(7):813–826. doi: 10.1016/j.jacc.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 26.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48(12):1503–1510. doi: 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15(4):361–387. doi: [DOI] [PubMed] [Google Scholar]
- 28.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165(6):710–718. doi: 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 29.Schnabel RB, Schulz A, Messow CM, et al. Multiple marker approach to risk stratification in patients with stable coronary artery disease. Eur Heart J 2010;31(24):3024–3031. doi: 10.1093/eurheartj/ehq322 [DOI] [PubMed] [Google Scholar]
- 30.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation 2003;108(24):2987–2992. doi: 10.1161/01.CIR.0000103681.04726.9C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bibbins-Domingo K, Gupta R, Na B, Wu AHB, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 2007;297(2):169–176. doi: 10.1001/jama.297.2.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med 2013;32(30):5381–5397. doi: 10.1002/sim.5958 [DOI] [PubMed] [Google Scholar]
- 33.Gerds TA, Andersen PK, Kattan MW. Calibration plots for risk prediction models in the presence of competing risks. Statistics in Medicine 2014;33(18):3191–3203. doi: 10.1002/sim.6152 [DOI] [PubMed] [Google Scholar]
- 34.Wolbers M, Koller MT, Witteman JCM, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 2009;20(4):555–561. doi: 10.1097/EDE.0b013e3181a39056 [DOI] [PubMed] [Google Scholar]
- 35.Echouffo-Tcheugui JB, Daya N, Matsushita K, et al. Growth Differentiation Factor (GDF)-15 and Cardiometabolic Outcomes among Older Adults: The Atherosclerosis Risk in Communities Study. Clin Chem 2021;67(4):653–661. doi: 10.1093/clinchem/hvaa332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Team RC. R: A Language and Environment for Statistical Computing; 2018. https://www.R-project.org/
- 37.Ozenne B, Sørensen A Lyngholm, Scheike T, Torp-Pedersen C, Gerds T Alexander. riskRegression: Predicting the Risk of an Event using Cox Regression Models. The R Journal 2017;9(2):440. doi: 10.32614/RJ-2017-062 [DOI] [Google Scholar]
- 38.Gerds TA, Kattan MW. Medical Risk Prediction: With Ties to Machine Learning 1st ed. Chapman and Hall/CRC; 2021. doi: 10.1201/9781138384484 [DOI] [Google Scholar]
- 39.Newman JD, Anthopolos R, Mancini GBJ, et al. Outcomes of Participants with Diabetes in the ISCHEMIA Trials | Circulation. Circulation Published online October 26, 2021. doi: 10.1161/CIRCULATIONAHA.121.054439 [DOI] [PMC free article] [PubMed]
- 40.Hussain A, Sun W, Deswal A, et al. Association of NT-ProBNP, Blood Pressure, and Cardiovascular Events: The ARIC Study. J Am Coll Cardiol 2021;77(5):559–571. doi: 10.1016/j.jacc.2020.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia X, Sun W, Hoogeveen RC, et al. High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation 2019;139(23):2642–2653. doi: 10.1161/CIRCULATIONAHA.118.038772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang TJ, Wollert KC, Larson MG, et al. Prognostic Utility of Novel Biomarkers of Cardiovascular Stress: The Framingham Heart Study. Circulation 2012;126(13):10.1161/CIRCULATIONAHA.112.129437. doi: 10.1161/CIRCULATIONAHA.112.129437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seliger SL, Hong SN, Christenson RH, et al. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation 2017;135(16):1494–1505. doi: 10.1161/CIRCULATIONAHA.116.025505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schopfer DW, Ku IA, Regan M, Whooley MA. Growth differentiation factor 15 and cardiovascular events in patients with stable ischemic heart disease (The Heart and Soul Study). American Heart Journal 2014;167(2):186–192.e1. doi: 10.1016/j.ahj.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 45.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N Engl J Med 2015;372(14):1291–1300. doi: 10.1056/NEJMoa1415516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Januzzi JL, Suchindran S, Hoffmann U, et al. Single-Molecule hsTnI and Short-Term Risk in Stable Patients With Chest Pain. J Am Coll Cardiol 2019;73(3):251–260. doi: 10.1016/j.jacc.2018.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Januzzi JL, Suchindran S, Coles A, et al. High-Sensitivity Troponin I and Coronary Computed Tomography in Symptomatic Outpatients With Suspected CAD: Insights From the PROMISE Trial. JACC Cardiovasc Imaging 2019;12(6):1047–1055. doi: 10.1016/j.jcmg.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferencik M, Mayrhofer T, Lu M, et al. Relationship Of Myocardial Necrosis, Inflammation And Coronary Atherosclerosis To Cardiovascular Outcomes In Patients With Stable Chest Pain: Results From The Promise Trial. Journal of Cardiovascular Computed Tomography 2020;14(3):S83–S84. doi: 10.1016/j.jcct.2020.06.169 [DOI] [Google Scholar]
- 49.Parmacek MS, Solaro RJ. Biology of the troponin complex in cardiac myocytes. Progress in Cardiovascular Diseases 2004;47(3):159–176. doi: 10.1016/j.pcad.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 50.Kragelund C, Grønning B, Køber L, Hildebrandt P, Steffensen R. N-Terminal Pro–B-Type Natriuretic Peptide and Long-Term Mortality in Stable Coronary Heart Disease. New England Journal of Medicine 2005;352(7):666–675. doi: 10.1056/NEJMoa042330 [DOI] [PubMed] [Google Scholar]
- 51.Wollert KC, Kempf T, Wallentin L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin Chem 2017;63(1):140–151. doi: 10.1373/clinchem.2016.255174 [DOI] [PubMed] [Google Scholar]
- 52.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 Ligand System. Journal of the American College of Cardiology 2009;54(8):669–677. doi: 10.1016/j.jacc.2009.03.076 [DOI] [PubMed] [Google Scholar]
- 53.Bay B, Goßling A, Blaum CM, et al. Association of High‐Sensitivity Troponin T and I Blood Concentrations With All‐Cause Mortality and Cardiovascular Outcome in Stable Patients— Results From the INTERCATH Cohort. Journal of the American Heart Association 2022;11(17):e024516. doi: 10.1161/JAHA.121.024516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welsh P, Preiss D, Hayward C, et al. Cardiac Troponin T and Troponin I in the General Population. Circulation 2019;139(24):2754–2764. doi: 10.1161/CIRCULATIONAHA.118.038529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


