Summary:
Using a co-surgeon model has been suggested to improve perioperative outcomes and reduce the risk of complications. Therefore, we evaluated if a co-surgeon model compared with a single microsurgeon model could decrease the surgical time, length of stay, rate of complications, and healthcare-associated costs in adult patients undergoing microvascular breast reconstruction (MBR). A comprehensive search was performed across PubMed MEDLINE, Embase, and Web of Science. Studies evaluating the perioperative outcomes and complications of MBR using a single-surgeon model and co-surgeon model were included. A random-effects model was fitted to the data. Seven retrospective comparative studies were included. Ultimately, 1411 patients (48.23%) underwent MBR using a single-surgeon model, representing 2339 flaps (48.42%). On the other hand, 1514 patients (51.77%) underwent MBR using a co-surgeon model, representing 2492 flaps (51.58%). The surgical time was significantly reduced using a co-surgeon model in all studies compared with a single-surgeon model. The length of stay was reduced using a co-surgeon model compared with a single-surgeon model in all but one study. The log odds ratio (log-OR) of recipient site infection (log-OR = −0.227; P = 0.6509), wound disruption (log-OR = −0.012; P = 0.9735), hematoma (log-OR = 0.061; P = 0.8683), and seroma (log-OR = −0.742; P = 0.1106) did not significantly decrease with the incorporation of a co-surgeon compared with a single-surgeon model. Incorporating a co-surgeon model for MBR has minimal impact on the rates of surgical site complications compared with a single-surgeon model. However, a co-surgeon optimized efficacy and reduced the surgical time and length of stay.
Takeaways
Question: Is there value in having two microsurgeons in breast reconstruction?
Findings: There is great value in having two surgeons in microsurgery.
Meaning: Having two microsurgeons is safer, and outcomes are better in breast reconstruction.
INTRODUCTION
To improve healthcare quality, perioperative outcomes of complex procedures such as operative time and length of stay are often subject to continuous assessment.1 Due to the inherent intricacies of reconstructive microsurgical procedures and their associated extended anesthesia time, surgeons are often required to balance patient safety, efficiency, and optimal clinical results.1 On this matter, previous reports have found that when approaching complex microsurgical cases by specialized multidisciplinary teams, outcomes are usually improved.2,3
To optimize perioperative results, several strategies have been developed. For instance, the use of virtual surgical planning and computed tomography angiography to identify adequate perforators has caused a reduction in surgical time and shorter length of stay.4–6 Furthermore, the enhanced recovery after surgery protocol has been increasingly implemented across the United States, demonstrating reduced use of opioid analgesia and length of stay without negatively altering patient-reported outcomes or the rate of complications.7–9
Other strategies to optimize perioperative outcomes include running simultaneous operating rooms by a single surgeon. Using this approach, the critical portion of a long procedure is performed by the attending surgeon, while the remaining portion can be safely completed by a competent surgical assistant/resident.1 Nonetheless, some cases may require the attending surgeon to be present for almost all the procedure, making the use of simultaneous operating rooms unsafe or unfeasible.1 Another strategy to optimize perioperative outcomes can be the incorporation of a co-surgeon.10–15 With this modality, critical steps of complex microsurgical reconstructive procedures can occur concurrently rather than in sequence.1
Reduced operative time, shorter length of stay, and lesser healthcare-associated costs have been reported with a co-surgeon model for the ablative segment of breast oncologic procedures (mastectomy),10–12 whereas reduced estimated blood loss,14,15 complication rates,14 requirements of narcotics,15 operative time, and improved operating room utilization costs have been reported in the field of orthopedic and neurological surgery with a co-surgeon.13–15 The purpose of this review was to evaluate the role of a co-surgeon model for microvascular breast reconstruction (MBR) in plastic surgery. Our research question was as follows: In adult patients undergoing MBR (population), can a co-surgeon model (intervention) when compared with a single microsurgeon model (comparison) decrease the surgical time, length of stay, the rate of complications, and healthcare-associated costs (outcome) during the perioperative period (time)?
PATIENTS AND METHODS
Literature Search
A comprehensive search was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16,17 We searched PubMed, MEDLINE, Embase (Elsevier, Netherlands), and Web of Science (Clarivate Analytics PLC) from database inception through March 3, 2023. The following terms were used in different combinations: “co-surgeons,” “cosurgeons,” “co-surgeon,” “cosurgeon,” “surgeon,” “microsurgeons,” microsurgery,” “free flap,” “flap,” “microvascular,” “microsurgical,” “reconstruction,” “microsurgery/methods”[MeSH], “surgeons”[MeSH], and “perforator flap”[MeSH]. (See appendix, Supplemental Digital Content 1, which shows search strategy across different databases. http://links.lww.com/PRSGO/D74.) We manually searched the bibliography of relevant articles to complement our electronic search.
Selection Process
We included case-control studies, cohort studies, and randomized clinical trials evaluating the perioperative outcomes and complications of MBR using a single-surgeon model and co-surgeon model (two microsurgeons). Only articles written in English were included. Primary studies from different institutions were selected to decrease the probability of overlapping data. We excluded animal studies, reviews, case reports, conference abstracts, and poster presentations. We excluded articles reporting on MBR with simultaneous vascularized lymph node transfer, lymphovenous anastomosis/bypass, or innervated breast reconstruction (neurotization). Data evaluating clinical outcomes of three or more microsurgeons were not included in our analysis.
A two-stage screening process was performed by two independent reviewers (L.E. and J.M.E.). During the first stage, titles and abstracts were evaluated after duplicated references were removed. A full-text assessment was conducted during the second stage. The senior author (O.J.M.) addressed any conflicts for eligibility during the selection process.
Variables of Interest
Two independent reviewers extracted data on the number of patients, number of free flaps, follow-up, age, body mass index, comorbidities (eg, diabetes, hypertension), smoking status (current/nonsmoker), history of abdominal surgery, adjuvant systemic chemotherapy or radiotherapy, timing of reconstruction (immediate/delayed), type of free flaps used, laterality of the reconstruction (bilateral/unilateral), and surgical technique.
Perioperative surgical outcomes analyzed in this study included surgical time or anesthesia time (minutes), flap weight, number of perforators, length of stay (days), and healthcare-related costs. The complications analyzed for this review were as follows: return to the operating room (RTOR), red blood cell transfusion, pulmonary embolism, deep venous thrombosis, pneumothorax, seroma, surgical site infection, wound disruption, hernia, hematoma, fat necrosis, and flap loss.
A co-surgeon was defined as a microsurgeon who assisted partially or entirely during a case of MBR. RTOR was defined as any major complications requiring re-operation or the event of nonelective (urgent) operating room takeback. Wound dehiscence, delayed wound healing, and wound disruption were all incorporated within the spectrum of events defined as wound disruption. Superficial or deep surgical site infections were defined as surgical site infection.
Statistical Analysis
Estimates of the patients’ characteristics were calculated as weighted means (∑ni=1[xi*wi]/∑ni=1wi). For studies with an intervention group (co-surgeon model) and a control group (single-surgeon model), the analysis was carried out using the log odds ratio (log-OR) as the outcome measure with Jamovi.1.2.27.0 (Jamovi, Sydney, Australia). Back-transform from log-OR into OR was performed for all models. A random-effects model was fitted to the data. The amount of heterogeneity (ie, tau²) was estimated using the Sidik-Jonkman estimator.18 In addition to the estimate of tau², the Q-test for heterogeneity and the I² statistic were reported.19 Heterogeneity was regarded as moderate, substantial, and considerable when I2 was between 30%–60%, 50%–90%, and more than 90%, respectively.20 A regression test, using the standard error of the observed outcomes as a predictor, was used to check for funnel plot asymmetry.21
Quality Assessment
The level of evidence was assessed with the Oxford Center for Evidence-Based Medicine.22 The Risk of Bias in Non-randomized Studies - of Interventions (ROBINS-I) instrument was used to evaluate the risk of bias in nonrandomized cohort studies.23,24 Funnel plots were used to qualitatively assess the risk of bias for the models evaluating RTOR and total flap loss.
RESULTS
Study Characteristics
Seven retrospective comparative studies were included after the two-stage screening process was completed (Fig. 1).1,25–30 The number of patients and flaps and the types of flaps used in each study are reported in Table 1. Most studies presented their outcomes using a two-cohort methodology evaluating the results of a single surgeon versus co-surgeon model.25,27–30 Gösseringer et al presented a comparative study evaluating outcomes using one, two, and three microsurgeons.26 The cohort in which three microsurgeons participated was excluded.26 Haddock et al presented a three-cohort analysis: a single surgeon group and two co-surgeon cohorts.1 The two co-surgeon cohorts were as follows: (1) a CSR-I group (in which co-surgery was performed by both surgeons for the entire case) and (2) CSR-II (where co-surgeons appropriately assisted in two concurrent or staggered cases and therefore both surgeons were not present for the entire case).1 For this study by Haddock et al,1 the former group (CSR-I) was included in our analysis, and the latter was excluded, as insufficient data were provided for the segments in which both microsurgeons were present (CSR-II).1
Fig. 1.
PRISMA flowchart for the selection of studies. Outcomes of the systematic review of the literature by record identification, screening, and analysis in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement flow diagram. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Table 1.
Authors and Characteristics of Included Studies
| Authors | Period | Type | LOE | Patients* | Type of Flaps | Flaps* | Follow-up (Mo) |
|---|---|---|---|---|---|---|---|
| Haddock et al1 | 2011–2016 | Retr. | IV | 128 | DIEP | 256 | 1 |
| Bauermeister et al25 | 2010–2016 | Retr. | IV | 50 | MS-TRAM (74%) DIEP (24%) SIEA (2%) |
77 | NR |
| Gösseringer et al26 | 2011–2013 | Retr. | IV | 100 | DIEP (100%) | 100 | NR |
| Razdan et al27 | 2014–2016 | Retr. | IV | 136 | MS-TRAM DIEP SIEA |
272 | NR |
| Weichman et al28 | 2011–2014 | Retr. | IV | 157 | DIEP (60%) MS-TRAM (20.2%) SIEA (1.6%) PAP (12.5%) TUG (0.4%) SGAP (2%) Stacked DIEP (3.2%) |
248 | NR |
| Asaad et al29 | 2010–2017 | Retr. | III | 8680† | NR | 13,537† | 3 |
| Mericli et al30 | 2016–2018 | Retr. | III | 150† | B/L DIEP (59.3%) DIEP/TRAM (18%) B/L TRAM (14%) B/L PAP or TUG (7.3%) SIEA/DIEP (NR) SIEA/TRAM (NR) |
300† | 15 |
Before propensity score matching.
The number of patients/flaps included in the formal analysis could be different depending on the subjects included in the “single surgeon” and “co-surgeon” group.
B/L, bilateral; DIEP, deep inferior epigastric perforator; LEO, level of evidence; MS, muscle-sparing; PAP, profunda artery perforator; Retr., retrospective; SIEA, superficial inferior epigastric artery; SGAP, superior gluteal artery perforator; TRAM, transverse rectus abdominis muscle; TUG, transverse upper gracilis; NR, not reported.
Characteristics of Patients
Ultimately, 1411 patients (48.23%) underwent MBR using a single-surgeon model, representing 2339 flaps (48.42%). On the other hand, 1514 patients (51.77%) underwent MBR using a co-surgeon model, representing 2492 flaps (51.58%). Three studies only included bilateral reconstructions (Table 2).1,27,30 One study included only unilateral reconstructions.26 Three studies included both unilateral and bilateral reconstructions.25,28,29 The mean or median age of patients ranged from 47.4 to 54 years.1,25–30 The mean or median body mass index of patients ranged from 25.4 to 31.2 kg per m2.1,25–30 One study included only delayed reconstructions.26 The rest of the studies included delayed and immediate reconstructions in their analysis.1,25,27–30
Table 2.
Demographic and Surgical Characteristics of Included Subjects
| Study | Modality | Patients | Flaps | Laterality | Age (y) | BMI (kg/m2) | Timing |
|---|---|---|---|---|---|---|---|
| Haddock et al1 | SS | 35 (27%) | 70 (27%) | Bilateral: 35 (100%) | 50 (range, 32––66) |
29.2 (range, 19.2–37.1) |
Immediate: 50% Delayed: 50% |
| CoS | 69 (54%) | 138 (54%) | Bilateral: 69 (100%) | 51 (range, 30––68) |
31.2 (range, 24–44) |
Immediate: 15.2% Delayed: 84.8% |
|
| Bauermeister et al25 | SS | 27 (54%) | 40 (52%) | Bilateral: 13 (48.1%) Unilateral: 14 (51.9%) |
51 (IQR, 48.5–56) |
30.9 (IQR, 26.6–33.1) |
Delayed: 27 (100%) |
| CoS | 23 (46%) | 37 (48%) | Bilateral: 14 (60.9%) Unilateral: 9 (39.1%) |
54 (IQR, 45–57.5) |
28.3 (IQR, 26.1–31.6) |
Immediate: 3 (13%) Delayed: 20 (87%) |
|
| Gösseringer et al26 | SS | 16 (16%) | 16 (16%) | Unilateral: 16 (100%) | 52.6 ± 9.29 | 25.4 ± 3.1 | Delayed: 16 (100%) |
| CoS | 64 (64%) | 64 (64%) | Unilateral: 64 (100%) | 51.8 ± 8.5 | 26 ± 3.4 | Delayed: 64 (100%) | |
| Razdan et al27 | SS | 80 (59%) | 160 (59%) | Bilateral: 80 (100%) | 48.6 | 30.7 | Immediate: 54 (67.5%) Delayed: 26 (32.5%) |
| CoS | 56 (41%) | 112 (41%) | Bilateral: 56 (100%) | 48.1 | 30.6 | Immediate: 49 (87.5%) Delayed: 7 (12.5%) |
|
| Weichman et al28 | SS | 54 (34.4%) | 78 (31.5%) | Unilateral: 30 (55.6%) Bilateral: 24 (44.4%) |
51.48 ± 9.7 | 28.81 ± 5.8 | Immediate: 64 (82.1%) Delayed: 14 (25.9%) |
| CoS | 103 (65.6%) | 170 (68.5%) | Unilateral: 36 (35%) Bilateral: 67 (65%) |
48.8 ± 8.2 | 25.7 ± 4.9 | Immediate: 126 (74.1%) Delayed: 44 (25.9%) |
|
| Asaad et al29 | SS | 1149* (50%) | 1875* | Bilateral: 726 (63%) Unilateral: 423 (37%) |
50 ± 8 | NR | Immediate: 655 (57%) Delayed: 494 (43%) |
| CoS | 1149* (50%) | 1871* | Bilateral: 722 (63%) Unilateral: 427 (37%) |
50 ± 8 | NR | Immediate: 649 (56%) Delayed: 500 (43%) |
|
| Mericli et al30 | SS | 50* (50%) | 100* (50%) | Bilateral: 50 (100%) | 47.6 | 29.8 | Immediate: 50.9% Delayed: 49.1% |
| CoS | 50* (50%) | 100* (50%) | Bilateral: 50 (100%) | 47.4 | 30.1 | Immediate: 51.3% Delayed: 48.7% |
After propensity score matching.
CoS, co-surgeon model; IQR, interquartile range; NR, not reported; SS, single surgeon.
The percentages of patients with a medical history of diabetes or hypertension were similar between the co-surgeon and single-surgeon groups in most studies.1,25,29,30 Likewise, the proportion of smokers who underwent reconstruction with a co-surgeon and single-surgeon model were similar in most studies.1,25–28,30 The percentage of patients who had systemic chemotherapy or radiotherapy was also comparable between the co-surgeon and single surgeon cohorts in most studies (Table 3).1,25–27,29,30
Table 3.
Surgical History, Medical History, and Adjuvant Oncologic Treatment of Included Subjects
| Author | Modality | Single | Previous Abd. Surgery | Smoker | Hypertension | Diabetes | Adj. ChT | RT |
|---|---|---|---|---|---|---|---|---|
| Haddock et al1 | SS | 35 (27%) | 26 (74.3%) | 13 (37.1%) | 15 (42.9%) | 3 (8.6%) | 9 (25.7%) | 13 (18.6%) |
| CoS | 69 (54%) | 56 (81.2%) | 27 (39.1%) | 20 (29%) | 7 (10.1%) | 18 (26.1%) | 35 (25.4%) | |
| Bauermeister et al25 | SS | 27 (54%) | 16 (59%) | 1 (4%) | 7 (26%) | 4 (15%) | 22 (81%) | 6 (22%) |
| CoS | 23 (46%) | 15 (65%) | 2 (9%) | 6 (26%) | 3 (13%) | 17 (74%) | 3 (13%) | |
| Gösseringer et al26 | SS | 16 (16%) | —— | 0 (0%) | — | — | — | 10 (62.5%) |
| CoS | 64 (64%) | — | 1 (1.6%) | — | — | — | 45 (70.3%) | |
| Razdan et al27 | SS | 80 (59%) | — | 0 (0%) | — | — | — | 29 (18.1%) |
| CoS | 56 (41%) | — | 1 (1.8%) | — | — | — | 17 (15.2%) | |
| Weichman et al28 | SS | 54 (34.4%) | — | 0 (0%) | — | — | — | — |
| CoS | 103 (65.6%) | — | 0 (0%) | — | — | — | — | |
| Asaad et al29 | SS | 1149* (50%) | — | — | 365 (32%) | 94 (8%) | — | 107 (9%) |
| CoS | 1149* (50%) | — | — | 368 (32%) | 100 (9%) | — | 123 (11%) | |
| Mericli et al30 | SS | 50* (50%) | — | 1.7% | 4.6% | 6.6% | — | 38.6% |
| CoS | 50* (50%) | — | 2% | 4.5% | 7.2% | — | 39.4% |
After propensity score matching.
Abd., abdominal; Adj, adjuvant; ChT, chemotherapy; RT, radiotherapy.
Surgical Outcomes
Six studies evaluated the surgical or anesthesia time and the length of stay comparing the co-surgeon versus the single-surgeon model (Table 4).1,25–28,30 The surgical time was significantly reduced using a co-surgeon model in all studies compared with a single-surgeon model (Fig. 2).1,25–28,30 In the study by Mericli et al, the role of a co-surgeon was more common during recipient vessel dissection plus elevation of one of the flaps (22.2%) or during recipient vessel dissection only (22.2%).30 The authors from this study highlighted that the greatest reduction in surgical time was achieved when a co-surgeon performed the recipient vessel dissection and anastomoses and the primary surgeon performed flap elevation, inset, and donor-site closure (P < 0.05).30
Table 4.
Surgical Time and Length of Stay Comparing Single Surgeon versus Co-surgeon Model
| Study | Surgical Time (min) | Length of Stay (d) | ||
|---|---|---|---|---|
| Single Surgeon | Co-surgeon | Single Surgeon | Co-surgeon | |
| Haddock et al1 | 678 (range, 423–1063) |
485 (208–868) |
5 (range, 3–11) |
3.9 (range, 2–9) |
| Bauermeister et al25 | 588 (IQR, 450–666) |
402 (IQR, 300–468) |
4.8 (range, 4–8) |
3.7 (range, 4–8) |
| Gösseringer et al26 | 286 ± 84 (range, 215–570) |
265 ± 57 (range, 150–435) |
6.9 ± 1.1 | 6.7 ± 0.62 |
| Razdan et al27 | 608 (range, 419–1097) |
555 (range, 334–1000) |
4.8 ± 1.5 | 5 ± 2.2 |
| Weichman et al28* | 602.9 ± 117 | 468.5 ± 90.1 | 5.4 ± 2.1 | 4.1 ± 1.6 |
| Weichman et al28† | 385.9 ± 126 | 335.8 ± 129 | 5.5 ± 3.5 | 3.7 ± 1.0 |
| Asaad et al29 | — | — | — | — |
| Mericli et al30 | 681 ± 17.5 | 574 ± 10.3 | 5.5 ± 0.7 | 5.2 ± 0.13 |
Bilateral reconstructions for Weichman et al.
Unilateral reconstructions for Weichman et al.
Fig. 2.
Surgical time of included studies. §, Bilateral reconstructions for Weichman et al.28 µ, Unilateral reconstructions for Weichman et al.28
The length of stay was reduced using a co-surgeon model compared with a single-surgeon model in all but one study (Fig. 3).1,25–28,30 Although an increased length of stay was evident using a co-surgeon model compared with single surgeon-model in the study by Razdan et al, the difference was not significant (5.0 ± 2.2 days versus 4.8 ± 1.5 days, P = 0.54).27
Fig. 3.
Length of stay of included studies. §, Bilateral reconstructions for Weichman et al.28 µ, Unilateral reconstructions for Weichman et al.28
Complications
The rates of different complications for the recipient site/breast are summarized in Table 5.1,25–30 Although there was a tendency toward a lower log-OR of RTOR or major complications requiring re-operation using a co-surgeon model compared with a single-surgeon model, no statistical difference was found [log-OR = −0.437; 95% confidence interval (CI) = −1.107 to 0.234; P = 0.201; Fig. 4]. Heterogeneity was moderate for the analysis of RTOR (I2 = 42.18%; Q = 10.689; P = 0.579). Although there was a tendency toward a lower log-OR of flap loss using a co-surgeon model compared with a single-surgeon model, no statistical difference was found (log-OR = −0.419; 95% CI = −1.504 to 0.665; P = 0.4484; Fig. 5). Heterogeneity for the analysis of flap loss was not clinically significant (I2 = 25.85%; Q = 4.418; P = 0.6203).
Table 5.
Recipient Site Complications
| Single Surgeon | Co-surgeon | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | RTOR | Flap Loss | Fat Necrosis | SSI | Wound | Seroma | Hematoma | RTOR | Flap Loss | Fat Necrosis | SSI | Wound | Seroma | Hematoma |
| Haddock et al1 | 8.6% | 1.4% | 4.3% | 4.3% | 21.4% | 7.1% | 2.9% | 4.3% | 1.4% | 5.1% | 3.6% | 16.7% | 2.2% | 1.5% |
| Bauermeister et al25 | 4% | 4% | — | 4% | 15% | 0% | 0% | 4% | 0% | — | 0% | 26% | 0% | 4% |
| Gösseringer et al26 | 25% | 12.5% | — | — | — | — | — | 14% | 0% | — | — | — | — | — |
| Razdan et al27 | 16.2% | 3.75% | 5% | 5% | — | 1.25% | 1.25% | 3.57% | 0.9% | 0% | 0% | — | 0.9% | 0% |
| Weichman et al28 | — | 0% | 2.6% | — | 2.6% | — | 2.5% | — | 0.6% | 4.7% | — | 4.1% | — | 2.9% |
| Asaad et al29 | 5.83% | 0.44% | — | 1.57% | — | 1.65% | 1.22% | 7.31% | 0.52% | — | 2.44% | — | 0.96% | 1.57% |
| Mericli et al30 | 13.9% | 0% | — | 3.2% | 11.8% | 3.7% | 5.7% | 5% | 0% | — | 0% | 7.6% | 0% | 4% |
SSI, surgical site infection.
Fig. 4.
Effect of co-surgeon on the rate of RTOR or major complications requiring re-operation. Point estimates and 95% CIs are shown (random-effects calculations for the meta-analysis).
Fig. 5.
Effect of co-surgeon on the rate of flap loss. Point estimates and 95% CIs are shown (random-effects calculations for the meta-analysis).
We evaluated the rate of recipient site infection, wound disruption, hematoma, and seroma. [See Supplemental Digital Content 2, which shows the effect of co-surgeon on the rate of recipient site infection, wound disruption, seroma, hematoma, and donor-site wound disruption. Point estimates and 95% CIs are shown (random-effects calculations for the meta-analysis.) http://links.lww.com/PRSGO/D75.] The log-OR of recipient site infection (log-OR = −0.227; 95% CI = −1.211 to 0.757; P = 0.6509), wound disruption (log-OR = −0.012; 95% CI = −0.746 to 0.721; P = 0.9735), hematoma (log-OR = 0.061; 95% CI = −0.656 to 0.777; P = 0.8683), and seroma (log-OR = −0.742; 95% CI = −1.654 to 0.169; P = 0.1106) did not significantly decrease with the incorporation of a co-surgeon for MBR compared with a single-surgeon model. Heterogeneity was not clinically relevant for any of these models.
Donor-site complications or systemic complications were not thoroughly evaluated in all studies. However, the most consistent donor-site complication assessed in the included citations was wound disruption. Although there was a tendency towards a lower log-OR of donor-site wound disruption using a co-surgeon model compared with single-surgeon model, no statistical difference was found (log-OR = −0.593; 95%CI = −1.46 to 0.274; P = 0.1802). Heterogeneity was moderate for this analysis (I2 = 45.52%; Q = 4.354; P = 0.3602). (See table, Supplemental Digital Content 3, which shows systemic complications and donor-site morbidity. http://links.lww.com/PRSGO/D76.)
Cost Analysis
Although Haddock et al1 acknowledged that costs related to operative minutes can vary to a great extent due to the region, type of procedure, and the inclusion/exclusion of fixed overhead costs,31 they determined a cost savings of $4751.66 using a co-surgeon model compared with a single-surgeon model per case.1 Using the estimates from previous reports,32–34 Weichman et al estimated that a co-surgeon model would save as much as $7226.10 in bilateral reconstructions and $5865.90 in unilateral reconstruction.28
Initially, using time-driven activity-based costing principles for assistant, associate, and full professors, the implementation of a co-surgeon model increased the surgeon personnel costs relative to the single-surgeon model, according to Mericli et al ($2507.7 ± $52.27 versus $2148.9 ± $85.3; P < 0.001).30 However, when evaluating the total cost (facility and surgeon personnel cost), using a co-surgeon model saved $1015.5 per case compared with a single-surgeon model ($8491.3 ± $157.7 versus $9506.80 ± $282; P = 0.002).30 The authors highlighted that the greatest reduction in costs was achieved when the primary surgeon performed the flap elevation, inset, and donor-site closure, and a co-surgeon performed recipient vessel dissection and the anastomoses.30
Using a national database, Asaad et al demonstrated that 90-day overall healthcare costs (including surgery, admission, and follow-up) were higher, implementing a co-surgeon model compared with a single-surgeon model (US $76,227 versus $61,340; P < 0.001).29 Using multivariable regression analysis, a co-surgeon model was identified to be a significant predictor for higher healthcare costs relative to a single-surgeon model (+13.5%).29
Quality Assessment
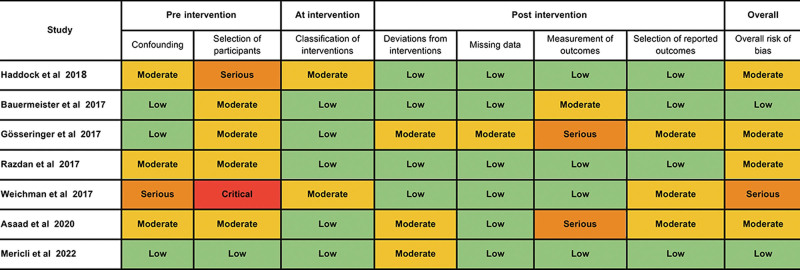
Five studies had a level of evidence of IV,1,25–28 whereas two studies had a level of evidence of III.29,30 Two studies had a propensity score-matched methodology, which decreased the impact of confounders or effect modifiers.29,30 One study used the MarketScan Commercial Claims and Encounters database, which limited the granularity of data for clinical outcomes.29 The funnel plot graphic for RTOR (P = 0.1914) and flap loss (P = 0.4846) suggested no evidence of publication bias, which was further supported by the Egger test meta-regression models (Fig. 6). Most studies had a moderate risk of bias (Fig. 7). These issues were mostly related to confounding factors altering the postoperative outcomes and concerns regarding patient selection for MBR with a co-surgeon model.
Fig. 6.
Forrest plot evaluating the risk of bias for the rate of flap loss.
Fig. 7.
Assessment of risk of bias with ROBINS-I, a tool for assessing risk of bias in nonrandomized studies of interventions.
DISCUSSION
Apprehensions regarding the technical complexity, prolonged surgical time, surgical-site morbidity, poor reimbursement patterns, and prolonged recovery with free tissue transfer continue to represent obstacles to the wide adoption of autologous breast reconstruction.35–41 Therefore, implant-based breast reconstruction remains the most common technique.35–41 In this setting, current efforts from surgeon scientists and researchers have been focused on strategies to reinforce patient safety and improve the postoperative course of autologous breast reconstruction.42–44 The incorporation of a co-surgeon to optimize operative time and reduce general anesthesia duration has been promoted as a versatile strategy for increasing effectiveness while reducing the rate of complications related to MBR.1,25,30,45
In this systematic review, although we identified a tendency toward lower log-OR of RTOR, flap loss, and donor site wound disruption using a co-surgeon model compared with a single-surgeon model, we did not find that morbidity was significantly affected by the addition of a co-surgeon. On the other hand, adding a co-surgeon optimized efficacy by reducing the surgical time and length of stay. Several studies have established a robust correlation between operation time and complications46–50; however, the association between this reduction in operative time and a reduction in the rate of complication is still uncertain for the specific case of a co-surgeon model. For instance, it has been hypothesized that by using a co-surgeon and the associated time-savings, morbidity can be reduced, as the primary surgeon can take additional time to inset the flap more carefully.30
In terms of cost-effectiveness, conflicting outcomes have been reported in published studies. For instance, one study found that the co-surgeon model was an independent predictor for higher healthcare costs (+13.5%) versus single-surgeon models.29 Nonetheless, this study only evaluated the 90-day outcomes and did not account for a faster operating room turnover associated with reduced surgical time per case.30 Furthermore, co-surgeons may engage in other clinical activities during the segments and their presence is not required, thus providing the opportunity or potential to increase productivity. On this matter, studies evaluating the cost-effectiveness of a co-surgeon model did not consider variables associated with co-surgeon reimbursement such as the use of modifiers, the different coding strategies, institution-specific and insurer-specific contractual details, and insurance company carve-outs, among others.30 Finally, it is important to highlight that although a shorter anesthesia time may not signify a reduction in the charge billed to insurance companies, it may reduce costs incurred by healthcare institutions.29
Working closely with a peer attending or surgeon is not a novel model in healthcare.51 Aside from the objective outcomes and quantitative parameters that can be evaluated, the intangible advantages of a co-surgeon model need to be discussed. Current practice in surgical and medical specialties favors the implementation of multidisciplinary teams, as it has been shown to improve patient outcomes and team dynamics.51 Shared decision-making, mentorship, supervision, and the combination of experience and skills are facilitated by interactions with other attendings.51,52 These features are of special consideration when approaching unacquainted, challenging, or less common procedures.51 Furthermore, operating with an experienced surgeon offers a sense of peer-to-peer support, boosts confidence, and reduces the cognitive load and burnout among surgeons and the whole surgical team.51 On this matter, further studies are required to determine if the incorporation of a co-surgeon model improves satisfaction and wellness among the members of the surgical team.
The co-surgeon approach, besides allowing for mentorship, is a good opportunity to learn new techniques, tips, and tricks to improve perioperative outcomes in a bi-directional way.12 For instance, it can reduce or prevent fatigue when performing long surgical procedures and reduce the risk of error, an indicator of difficult assessment.27,51,53 Although the co-surgeon model is both operator and procedure dependent, a judicious assessment of the surgical skills and personal reflection can guide the decision to incorporate a second set of hands from another colleague.51,54 In academic medical centers, a co-surgeon model can improve teaching and supervision, making residents more familiar with these types of procedures.
Limitations
The presence of multiple surgeons, residents, or physician assistants is a common occurrence during MBR.55 Unfortunately, these operative characteristics were not ubiquitously analyzed in all included studies.55 In other fields of reconstructive microsurgery such as limb reconstruction, the addition of a second operating attending did not significantly reduce surgery time, hospital length of stay, need for revision surgery, or complication rates.56 This may reflect limitations regarding the external validity and reproducibility of the outcomes of several studies included in our meta-analysis.
The inclusion of a co-surgeon increases over the years for MBR in most practices,29 as it concurrently does the experience of surgeons.29 In this setting, an increased experience of surgeons due to the learning curve can be regarded as an effect modifier affecting the outcomes of surgical time and length of stay. Additionally, in most studies, it is difficult to assess how the concept of a co-surgeon matures over time. Certainly, most surgical teams learn over time how to optimize the assistance of a second microsurgeon, thereby generating an important reduction in the overall cost of care.29
CONCLUSIONS
Our results indicate that with the incorporation of a co-surgeon model for MBR, the rates of complications for donor site and recipient site are comparable relative to a single-surgeon model. On the other hand, adding a co-surgeon optimized efficacy and reduced the surgical time and length of stay. Due to heterogeneity in how outcomes are measured, conflicting results were reported in several studies evaluating the cost-effectiveness of a co-surgeon model compared with a single-surgeon model. The co-surgeon model is unlikely to be necessary for all microsurgical cases but can be exceedingly valuable when planning for long, complex cases or when more than one flap is required (eg, bilateral reconstruction, stacked deep inferior epigastric perforator and profunda artery perforator flaps).
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 5 February 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Haddock NT, Kayfan S, Pezeshk RA, et al. Co-surgeons in breast reconstructive microsurgery: what do they bring to the table? Microsurgery. 2018;38:14–20. [DOI] [PubMed] [Google Scholar]
- 2.Kotsougiani-Fischer D, Fischer S, Warszawski J, et al. Multidisciplinary team meetings for patients with complex extremity defects: a retrospective analysis of treatment recommendations and prognostic factors for non-implementation. BMC Surg. 2021;21:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciudad P, Bolletta A, Kaciulyte J, et al. The breast cancer–related lymphedema multidisciplinary approach (B-LYMA): algorithm for conservative and multimodal surgical treatment. Microsurgery. 2022;43:427–436. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68:131–138. [DOI] [PubMed] [Google Scholar]
- 5.Chang EI, Jenkins MP, Patel SA, et al. Long-term operative outcomes of preoperative computed tomography-guided virtual surgical planning for osteocutaneous free flap mandible reconstruction. Plast Reconstr Surg. 2016;137:619–623. [DOI] [PubMed] [Google Scholar]
- 6.Matsui C, Escandón J, Mohammad A, et al. Dental silicone-based surgical guides to harvest the chimeric scapular flap: preventing iatrogenic vascular injury. Plast Reconstr Surg Glob Open. 2022;10:e4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batdorf NJ, Lemaine V, Lovely JK, et al. Enhanced recovery after surgery in microvascular breast reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:395–402. [DOI] [PubMed] [Google Scholar]
- 8.Offodile AC, II, Gu C, Boukovalas S, et al. Enhanced recovery after surgery (ERAS) pathways in breast reconstruction: systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2019;173:65–77. [DOI] [PubMed] [Google Scholar]
- 9.Tan YY, Liaw F, Warner R, et al. Enhanced recovery pathways for flap-based reconstruction: systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45:2096–2115. [DOI] [PubMed] [Google Scholar]
- 10.Hudson L, Reese E, Hecksher A, et al. Single surgeon versus co-surgeon bilateral mastectomy: comparing outcomes and costs based on health economic modeling from the perspective of the hospital system. J Surg Oncol. 2022;126:239–246. [DOI] [PubMed] [Google Scholar]
- 11.Mallory MA, Tarabanis C, Schneider E, et al. Bilateral mastectomies: can a co-surgeon technique offer improvements over the single-surgeon method? Breast Cancer Res Treat. 2018;170:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallory MA, Valero MG, Hu J, et al. Bilateral mastectomy operations and the role for the cosurgeon technique: a nationwide analysis of surgical practice patterns. Breast J. 2020;26:220–226. [DOI] [PubMed] [Google Scholar]
- 13.Nahm NJ, Ludwig M, Thompson R, et al. Single-event multilevel surgery in cerebral palsy: value added by a co-surgeon. Medicine (Baltim). 2021;100:e26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrader MW, Wood W, Falk M, et al. The effect of two attending surgeons on the outcomes of posterior spine fusion in children with cerebral palsy. Spine Deform. 2018;6:730–735. [DOI] [PubMed] [Google Scholar]
- 15.Chan CYW, Kwan MK. Perioperative outcome in posterior spinal fusion for adolescent idiopathic scoliosis: a prospective study comparing single versus two attending surgeons strategy. Spine (Phila Pa 1976). 2016;41:E694–E699. [DOI] [PubMed] [Google Scholar]
- 16.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2016;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inthout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. [Google Scholar]
- 20.Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 21.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durieux N, Pasleau F, Howick J; OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-based Medicine. Published 2011. Available at https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed January 24, 2024.
- 23.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med. 2018;23:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauermeister AJ, Zuriarrain A, Newman M, et al. Impact of continuous two-team approach in autologous breast reconstruction. J Reconstr Microsurg. 2017;33:298–304. [DOI] [PubMed] [Google Scholar]
- 26.Gösseringer N, Mani M, Cali-Cassi L, et al. Benefits of two or more senior microsurgeons operating simultaneously in microsurgical breast reconstruction: Experience in a Swedish medical center. Microsurgery. 2017;37:416–420. [DOI] [PubMed] [Google Scholar]
- 27.Razdan SN, Panchal HJ, Hespe GE, et al. The impact of the cosurgeon model on bilateral autologous breast reconstruction. J Reconstr Microsurg. 2017;33:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weichman KE, Lam G, Wilson SC, et al. The impact of two operating surgeons on microsurgical breast reconstruction. Plast Reconstr Surg. 2017;139:277–284. [DOI] [PubMed] [Google Scholar]
- 29.Asaad M, Xu Y, Chu CK, et al. The impact of co-surgeons on complication rates and healthcare cost in patients undergoing microsurgical breast reconstruction: analysis of 8680 patients. Breast Cancer Res Treat. 2020;184:345–356. [DOI] [PubMed] [Google Scholar]
- 30.Mericli AF, Chu CK, Sisk GC, et al. Microvascular breast reconstruction in the era of value-based care: use of a cosurgeon is associated with reduced costs, improved outcomes, and added value. Plast Reconstr Surg. 2022;149:338–348. [DOI] [PubMed] [Google Scholar]
- 31.Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22:233–236. [DOI] [PubMed] [Google Scholar]
- 32.Zhong T, McCarthy C, Min S, et al. Patient satisfaction and health-related quality of life after autologous tissue breast reconstruction: a prospective analysis of early postoperative outcomes. Cancer. 2012;118:1701–1709. [DOI] [PubMed] [Google Scholar]
- 33.Bodin F, Zink S, Lutz J-C, et al. Which breast reconstruction procedure provides the best long-term satisfaction? Ann Chir Plast Esthet. 2010;55:547–552. [DOI] [PubMed] [Google Scholar]
- 34.Christensen BO, Overgaard J, Kettner LO, et al. Long-term evaluation of postmastectomy breast reconstruction. Acta Oncol. 2011;50:1053–1061. [DOI] [PubMed] [Google Scholar]
- 35.Escandón JM, Sweitzer K, Christiano JG, et al. Subpectoral versus prepectoral two-stage breast reconstruction: a propensity score-matched analysis of 30-day morbidity and long-term outcomes. J Plast Reconstr Aesthetic Surg. 2022;76:76–87. [DOI] [PubMed] [Google Scholar]
- 36.Hu ES, Pusic AL, Waljee JF, et al. Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship Period. Plast Reconstr Surg. 2009;124:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Escandón JM, Escandón L, Ahmed A, et al. Breast reconstruction using the latissimus dorsi flap and immediate fat transfer (LIFT): a systematic review and meta-analysis. J Plast Reconstr Aesthetic Surg. 2022;75:4106–4116. [DOI] [PubMed] [Google Scholar]
- 38.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in US Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. [DOI] [PubMed] [Google Scholar]
- 39.Escandón JM, Nazerali R, Ciudad P, et al. Minimally invasive harvest of the latissimus dorsi flap for breast reconstruction: a systematic review. Int J Med Robot. 2022;18:e2446. [DOI] [PubMed] [Google Scholar]
- 40.Yueh JH, Slavin SA, Adesiyun T, et al. Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125:1585–1595. [DOI] [PubMed] [Google Scholar]
- 41.Manrique OJ, Escandón JM. Breast reconstruction in the era of evidence-based medicine. Ann Transl Med. 2023;11:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mericli AF, McHugh T, Kruse B, et al. Time-driven activity-based costing to model cost utility of enhanced recovery after surgery pathways in microvascular breast reconstruction. J Am Coll Surg. 2020;230:784–794.e3. [DOI] [PubMed] [Google Scholar]
- 43.Kaoutzanis C, Ganesh Kumar N, O’Neill D, et al. Enhanced recovery pathway in microvascular autologous tissue-based breast reconstruction: should it become the standard of care? Plast Reconstr Surg. 2018;141:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Neill AC, Mughal M, Saggaf MM, et al. A structured pathway for accelerated postoperative recovery reduces hospital stay and cost of care following microvascular breast reconstruction without increased complications. J Plast Reconstr Aesthet Surg. 2020;73:19–26. [DOI] [PubMed] [Google Scholar]
- 45.Canizares O, Mayo J, Soto E, et al. Optimizing efficiency in deep inferior epigastric perforator flap breast reconstruction. Ann Plast Surg. 2015;75:186–192. [DOI] [PubMed] [Google Scholar]
- 46.Procter LD, Davenport DL, Bernard AC, et al. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. 2010;210:60–5.e1. [DOI] [PubMed] [Google Scholar]
- 47.Bekelis K, Coy S, Simmons N. Operative duration and risk of surgical site infection in neurosurgery. World Neurosurg. 2016;94:551–555.e6. [DOI] [PubMed] [Google Scholar]
- 48.Kim JYS, Khavanin N, Rambachan A, et al. Surgical duration and risk of venous thromboembolism. JAMA Surg. 2015;150:110–117. [DOI] [PubMed] [Google Scholar]
- 49.Daley BJ, Cecil W, Clarke PC, et al. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee surgical quality collaborative. J Am Coll Surg. 2015;220:550–558. [DOI] [PubMed] [Google Scholar]
- 50.Wong AK, Nguyen TJ, Peric M, et al. Analysis of risk factors associated with microvascular free flap failure using a multi-institutional database. Microsurgery. 2009;35:6–12. [DOI] [PubMed] [Google Scholar]
- 51.Ellis R, Hardie JA, Summerton DJ, et al. Dual surgeon operating to improve patient safety. Br J Oral Maxillofac Surg. 2021;59:752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutton PA, Rooney PS. Multi-consultant operating. Bulletin Royal Colle Surg Engl. 2018;100:329–332. [Google Scholar]
- 53.Nguyen PD, Herrera FA, Roostaeian J, et al. Career satisfaction and burnout in the reconstructive microsurgeon in the United States. Microsurgery. 2015;35:1–5. [DOI] [PubMed] [Google Scholar]
- 54.Hardie JA, Brennan PA. Are you surgically current? Lessons from aviation for returning to non-urgent surgery following COVID-19. Br J Oral Maxillofac Surg. 2020;58:843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jubbal KT, Echo A, Spiegel AJ, et al. The impact of resident involvement in breast reconstruction surgery outcomes by modality: AN analysis of 4,500 cases. Microsurgery. 2017;37:800–807. [DOI] [PubMed] [Google Scholar]
- 56.Ehrl D, Heidekrueger PI, Ninkovic M, et al. Impact of two attendings on the outcomes of microvascular limb reconstruction. J Reconstr Microsurg. 2018;34:59–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.