Abstract
Women with a history of recurrent Escherichia coli urinary tract infections (UTIs) are significantly more likely to be nonsecretors of blood group antigens than are women without such a history, and vaginal epithelial cells (VEC) from women who are nonsecretors show enhanced adherence of uropathogenic E. coli isolates compared with cells from secretors. We previously extracted glycosphingolipids (GSLs) from native VEC and determined that nonsecretors (but not secretors) selectively express two extended globoseries GSLs, sialosyl galactosyl globoside (SGG) and disialosyl galactosyl globoside (DSGG), which specifically bound uropathogenic E. coli R45 expressing a P adhesin. In this study, we demonstrated, by purifying the compounds from this source, that SGG and DSGG are expressed in human kidney tissue. We also demonstrated that SGG and DSGG isolated from human kidneys bind uropathogenic E. coli isolates expressing each of the three classes of pap-encoded adhesins, including cloned isolates expressing PapG from J96, PrsG from J96, and PapG from IA2, and the wild-type isolates IA2 and R45. We metabolically 35S labeled these five E. coli isolates and measured their relative binding affinities to serial dilutions of SGG and DSGG as well as to globotriaosylceramide (Gb3) and globotetraosylceramide (Gb4), two other globoseries GSLs present in urogenital tissues. Each of the five E. coli isolates bound to SGG with the highest apparent avidity compared with their binding to DSGG, Gb3, and Gb4, and each isolate had a unique pattern of GSL binding affinity. These studies further suggest that SGG likely plays an important role in the pathogenesis of UTI and that its presence may account for the increased binding of E. coli to uroepithelial cells from nonsecretors and for the increased susceptibility of nonsecretors to recurrent UTI.
Several epidemiological studies have shown that women who are nonsecretors of blood group antigens have a three- to fourfold-increased risk of developing recurrent urinary tract infection (UTI) (5, 17, 32). In addition, uroepithelial cells from nonsecretors have a two- to threefold-greater capacity for adherence of uropathogenic Escherichia coli than do cells from secretors (22). Colonization of the vaginal and periurethral epithelium precedes the development of E. coli UTI, and E. coli isolates expressing pap-encoded adhesins are overrepresented among isolates causing these infections (6). Uropathogenic E. coli isolates expressing pap-encoded adhesins bind to globoseries glycosphingolipids (GSLs) (6, 19), which are amphipathic molecules embedded in the outer leaflets of eukaryotic cell membranes. There are several families of GSLs which are differentiated by their molecular structures, and these molecules serve as bacterial and viral adhesion sites on mammalian cells and as markers of eukaryotic cell differentiation and oncogenesis (4).
In previous investigations, we collected vaginal epithelial cells from secretors and nonsecretors and extracted the GSLs from pooled cells from women in each group (36). We demonstrated that cells from nonsecretors express two unique globoseries GSLs, sialosyl galactosyl globoside (SGG) and disialosyl galactosyl globoside (DSGG) (36). We utilized high-performance thin-layer chromatography (HPTLC), bacterial overlay assays, HPTLC immunostaining, radioimmunoassays, and immunohistochemical staining with a monoclonal antibody (MAb) directed against SGG to show that SGG and DSGG were expressed in vaginal epithelial cells from nonsecretors but not in cells from secretors and that these moieties bound a clinical isolate of E. coli (R45) that expresses P fimbriae carrying a pap-encoded adhesin (36). These studies demonstrated for the first time that the secretor gene influences the biosynthesis of globoseries GSLs in the vaginal epithelium and suggested that genetically determined differences in receptor moieties in this tissue might explain the increased susceptibility of nonsecretors to UTI (32, 36).
In this study, we isolated and purified SGG and DSGG from human kidneys and assessed the in vitro binding of representative Pap-expressing E. coli isolates to SGG and DSGG in order to further elucidate possible mechanisms through which the selective expression of one or both of these molecules in the vaginal or urogenital epithelium of nonsecretors might influence their risk of UTI.
(This work was presented in part at the 32nd annual meeting of the Infectious Diseases Society of America [36a].)
MATERIALS AND METHODS
Purification of SGG and DSGG from human kidney tissue.
Normal human kidney tissue was chosen as an appropriate source from which to purify SGG and DSGG for several reasons. First, it is an available and clinically relevant urinary tract tissue, whereas the vaginal epithelium cannot be harvested in sufficient quantity for the purification of SGG and DSGG. In addition, we chose a human tissue as the source for these compounds, since variations in the structure of the ceramide portions of GSLs may be species specific, and thus structural differences found in animal tissues can have implications for the binding specificities of microorganisms (14). In preliminary studies, using the methods described below, we extracted and purified GSLs from small autopsy samples of normal human kidney tissue and determined that SGG and DSGG were expressed in these tissues. The purification was then scaled up, and a total of 1 kg of normal human kidney tissue was obtained and pooled from autopsy specimens from eight individuals. Autopsy reports were reviewed to insure that none of the patients died from renal disease or from diseases affecting kidney function. The majority of the material by weight was obtained from a 38-year-old woman who died from medulloblastoma. The tissue was washed and homogenized in a Waring blender, and GSLs were then prepared by a series of standard purification steps. First, an organic extraction with isopropanol-hexane-water was performed (10), followed by a modified Folch extraction (3) to produce lower and upper phases. No further purification of the lower phase was performed for these studies. The upper phase was then subjected to anion-exchange chromatography (41). Neutral GSL fractions were collected in the flowthrough, and acidic fractions were eluted with 0.05, 0.15, and 0.45 M ammonium acetate washes. The neutral fraction was then further purified by reverse-phase chromatography followed by acetylation and deacetylation to remove phospholipids and cholesterol (40, 41). The acidic fractions were then subjected to normal-phase silica gel high-performance liquid chromatography (HPLC) (13). SGG and DSGG were then identified and purified from the HPLC fractions by stepwise combinations of HPTLC immunostaining (12, 24), bacterial overlay assays (36), HPTLC in multiple solvent systems, and preparative HPTLC (28). The purification of SGG and DSGG as well as the structural characterization of SGG will be described more fully elsewhere (37a).
HPTLC immunostaining and bacterial overlay assays.
GSLs isolated and purified from the kidney tissues and then separated on HPTLC were immunostained according to the procedure of Magnani et al. (24), as modified by Kannagi et al. (12). Briefly, after HPTLC, the plates were blocked for 2 h in 5% bovine serum albumin in phosphate-buffered saline, washed, and incubated with the primary MAb in phosphate-buffered saline. After an incubation with the secondary antibody, the plates were washed, incubated with 125I-labeled protein A solution, washed, dried, and subjected to autoradiography. MAbs ID4 and RM-1, directed against SGG (31, 36), were used to monitor the purification of both SGG and DSGG. Since DSGG differs in structure from SGG by only one sialic acid residue, DSGG was identified by subjecting the compound to a timed, limited desialylation procedure to produce SGG (27). Briefly, aliquots of the purified putative DSGG compound to be tested were incubated in 1% acetic acid for 1, 3, and 7 min at 100°C and the reactions were terminated by plunging the tubes in ice and adding ice-cold ethanol. The samples were then dried and subjected to HPTLC, and immunostaining with MAb ID4 was performed. The presence of globoseries GSL moieties, particularly SGG and DSGG, was also monitored in the various fractions obtained during the lengthy purification steps with HPTLC bacterial overlay assays. Assays were performed as previously described (36) with metabolically 35S-labeled E. coli isolate R45, a wild-type cystitis isolate (35) which expresses P fimbriae carrying the class II pap-encoded adhesin (9) and binds globoseries GSLs (36). At every step, the results of HPTLC immunostaining and bacterial overlay experiments were compared, and relevant fractions and individual bands visualized by HPTLC were then subjected to further purification, as described above.
Bacterial binding curves. (i) GSL standards.
Globoseries GSL standards were isolated and purified in our laboratory from the following sources, using methods similar to those described above for purifying SGG from human kidney tissue (29): (i) ceramide trihexoside (CTH; globotriaosylceramide [Gb3]), from human erythrocytes; (ii) globoside (globotetraosylceramide [Gb4]), from human erythrocytes; and (iii) SGG and DSGG, purified from human kidney tissue as described above. Ceramide monohexoside (CMH) was purified from colonic adenocarcinoma and was used as a negative control for the binding of E. coli expressing P fimbriae carrying pap-encoded adhesins (36, 37). GSL standards were quantitated by a combination of the resorcinol and sphingosine assays (25) and densitometry. Relative quantities of GSLs were standardized by HPTLC by the comparative dilution method, using an appropriate reference GSL having a carbohydrate chain of equal length and charge and of similar molarity to that of the GSL being standardized. (34). The structures of the compounds utilized are shown in Table 1.
TABLE 1.
Structures of GSL standards used in this study
| GSLa | Structureb | Source |
|---|---|---|
| CMH | Glcβ1-1cer | Colonic adenocarcinoma |
| CTH | Galα1-4 Galβ1-4 Glcβ1-1cer | Human erythrocytes |
| Globoside | GalNAcβ1-3 Galα1-4 Galβ1-4 Glcβ1-1cer | Human erythrocytes |
| Gal globoside | Galβ1-3 GalNAcβ1-3 Galα1-4 Galβ1-4 Glcβ1-cer | Human kidney |
| SGG | NeuAcα2-3 Galβ1-3 GalNAcβ1-3 Galα1-4 Galβ1-4 Glcβ1-1cer | Human kidney |
| DSGG | NeuAcα2-3 Galβ1-3 (NeuAcα2-6) GalNAcβ1-3 Galα1-4 Galβ1-4 Glcβ1-1cer | Human kidney |
| Forssman | GalNAcα1-3 GalNAcβ1-3 Galα1-4 Galβ1-4 Glcβ1-1cer | Goat erythrocytes |
| ASGM1 | Galβ1-3 GalNAcβ1-4 Galβ1-4 Glcβ1-1cer | Desialylated GM1 from bovine brain |
Globoside, Gb4 (globotetraosylceramide); Gal globoside, galactosyl globoside; ASGM1, asialo-GM1.
Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine; NeuAc, N-acetylneuraminic (sialic) acid; cer, ceramide.
(ii) Bacterial binding assays.
To construct binding curves, GSL standards were serially diluted on HPTLC plates from 300 to 18.25 ng and overlaid with metabolically 35S-labeled E. coli isolates in bacterial overlay assays, as previously described (36). This range of GSL concentrations was chosen on the basis of preliminary experiments with two of the E. coli isolates described below that showed saturation of bacterial binding for SGG at higher concentration ranges of these GSL standards. After bacterial overlay, the HPTLC plates were subjected to autoradiography, and densitometry of the autoradiographs was performed to assess the quantity of bacterial binding to each GSL relative to the others. A second method of assessing bacterial binding, using the same plates, was performed by scraping the silica band corresponding to bacterial binding to each GSL standard, followed by counting the radioactivity in a scintillation counter.
E. coli isolates.
The E. coli isolates that were tested included the following: (i) R45, a wild-type cystitis isolate from a woman with acute cystitis which expresses P fimbriae carrying a class II pap-encoded adhesin (8, 35); (ii) IA2, a second wild-type isolate, from which HB101/pDC1 (called pDC1 in this paper) was cloned and which expresses P fimbriae carrying a class II pap-encoded adhesin (2); (iii) JJ122, which expresses P fimbriae carrying a class I papG-encoded adhesin (PapG from J96) (HB101/pJJ48); (iv) pDC1, which expresses P fimbriae carrying a class II pap-encoded adhesin (PapG from IA2) (2); (v) P678-54/pJFK102 (called pJFK102 in this paper), which expresses P fimbriae carrying a class III adhesin (PrsG from J96) (15); and (vi) the negative control isolates HB101 and P678-54. Wild-type organisms were grown on sheep’s blood agar, and recombinant isolates were grown on Luria broth agar plates containing the appropriate antibiotics. Under the growth conditions utilized for these studies, type 1 fimbriae were not expressed by any of the isolates (data not shown).
RESULTS
Purification of SGG and DSGG from human kidney tissue.
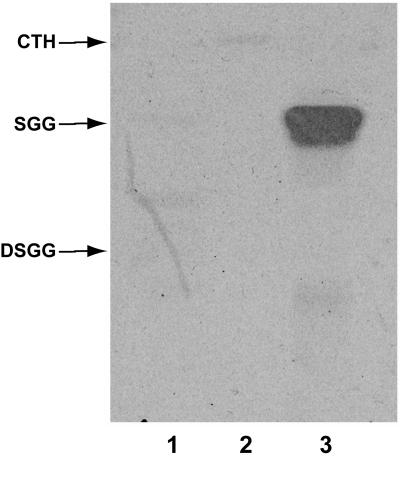
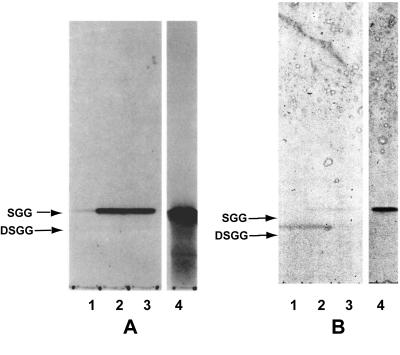
As described above, the purification of SGG and DSGG was monitored by HPTLC immunostaining and bacterial overlay assays on fractions putatively containing the compounds of interest. The results of performing HPTLC immunostaining on samples of purified SGG and DSGG, using MAb RM-1 directed against SGG (31), are shown in Fig. 1. The MAb stained only the band corresponding to SGG and did not stain DSGG or the negative control GSL, ceramide trihexoside (Gb3). The results of experiments to identify DSGG are shown in Fig. 2. In these experiments, the fraction putatively containing DSGG was subjected to a timed, limited desialylation procedure to produce SGG, followed by HPTLC and immunostaining with MAb ID4, directed against SGG (36). A comparison of lanes 1 to 3 in Fig. 2 shows that increasing amounts of SGG are produced over time through desialylation of DSGG, resulting in increasing staining of a band corresponding to SGG on the autoradiograph of MAb ID4 staining shown in Fig. 2A. In the replicate HPTLC plate stained with orcinol (Fig. 2B), this is reflected by a reduction in orcinol staining of the band corresponding to DSGG, along with an increase in staining of the band corresponding to SGG. At the 7-min desialylation time point, a portion of the sample has likely also been converted to galactosyl globoside, seen as a faint band migrating more rapidly than SGG in lane 3.
FIG. 1.
Identification of SGG purified from human kidney tissue by HPTLC immunostaining. GSL standards, including SGG and DSGG purified from human kidney tissue, were chromatographed and immunostained with MAb RM-1, directed against SGG, as described in Materials and Methods. Lane 1, ceramide trihexoside (Gb3; negative control); lane 2, DSGG; lane 3, SGG.
FIG. 2.
Identification of DSGG purified from human kidney tissue by timed, limited desialylation of DSGG to SGG, followed by HPTLC immunostaining. DSGG purified from human kidney tissue was identified through desialylation to form SGG, followed by immunostaining. A putative DSGG fraction was subjected to a limited desialylation procedure by incubating aliquots of the sample for 1, 3, and 7 min in 1% acetic acid and then drying the samples, subjecting them to HPTLC, and performing TLC immunostaining with MAb ID4, directed against SGG. (A) Autoradiograph of immunostained HPTLC plate; (B) same HPTLC plate stained with orcinol reagent after the immunostaining procedure. Lanes 1, DSGG fraction after 1 min of desialylation of DSGG; lanes 2, DSGG fraction after 3 min of desialylation; lanes 3, DSGG fraction after 7 min of desialylation; lanes 4, SGG standard.
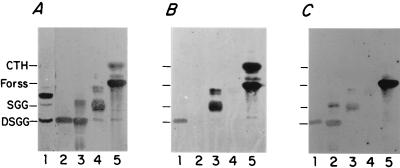
Figure 3 shows an example of the multiple bacterial overlay experiments used to purify SGG and DSGG as well as the final result of these purification steps. These experiments demonstrate that SGG and DSGG purified from human kidney tissue bind metabolically 35S-labeled representative E. coli isolates R45, JJ122, and pJFK102, expressing P fimbriae carrying pap-encoded adhesins of classes II, I, and III, respectively. Nineteen bacterial overlay experiments were performed during the course of purifying SGG and DSGG (12 experiments using E. coli R45 and 7 experiments using one or more of the other four E. coli isolates described above). We repeatedly observed qualitative differences between the avidities of bacterial binding to SGG and to CTH and other GSL standards with shorter-chain oligosaccharide moieties, based on comparing orcinol staining of known quantities of these purified GSL standards with the amount of SGG in samples still in the process of purification. For example, bacterial binding to 5 to 10 μg of CTH GSL standard was approximately equivalent to the amount of binding to SGG that was at the limits of staining with orcinol, estimated at 20 ng or less. These observations led to the bacterial binding quantitation experiments described below, using purified SGG and DSGG.
FIG. 3.
Binding of representative E. coli isolates expressing pap-encoded adhesins to SGG and DSGG purified from human kidney tissue. GSLs were purified and separated on HPTLC plates and then overlaid with metabolically [35S]methionine-labeled E. coli R45 organisms, which bind globoseries GSLs. Autoradiographs are shown. (A) E. coli R45; GSLs: ganglioside fraction (lane 1), DSGG standard (lane 2), DSGG standard isolated from a different preparation and subjected to formal carbohydrate structural analysis (31) (lane 3), SGG standard (note traces of DSGG and galactosyl globoside) (lane 4), and CTH (Gb3) and Forssman (Forss) standards (lane 5). (B) E. coli JJ122; GSLs DSGG standard (lane 1), blank (no GSLs) (lane 2), SGG standard (lane 3), asialo-GM1 (ASGM1) standard (lane 4), and CTH and Forssman standards (lane 5). (C) E. coli pJFK102; GSLs: DSGG standard (lane 1), DSGG standard isolated from a different preparation and subjected to formal carbohydrate structural analysis (lane 2), SGG (lane 3), ASGM1 standard (lane 4), and CTH and Forssman standards (lane 5).
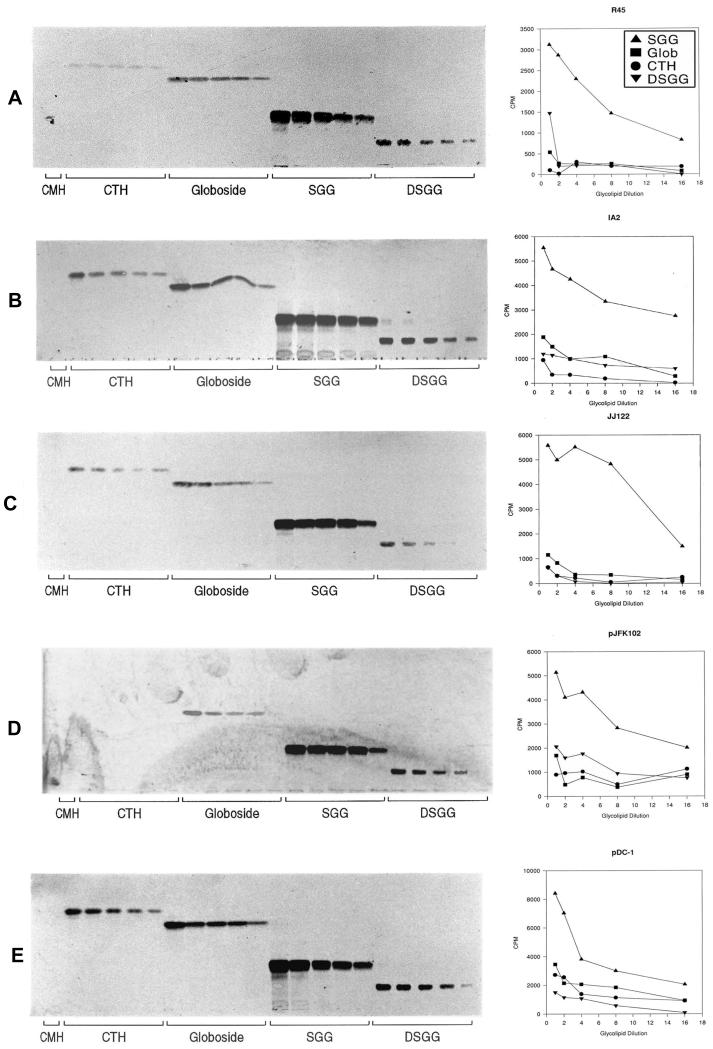
Bacterial binding curves.
The results of quantitating bacterial binding to serially diluted CTH, globoside, SGG, and DSGG standards both by means of scraping and counting radioactive bands from the silica plates and by performing densitometry of the autoradiographs were essentially identical. Figure 4 shows the autoradiographs from these experiments (left panels) as well as the results of counting radioactivity scraped from bands on silica plates corresponding to binding of metabolically 35S-labeled E. coli isolates R45, IA2, pDC1, JJ122, and pJFK102 (right panels). Results of representative experiments are shown for each strain. For each isolate, the relative binding to SGG was greater than the binding to other globoseries GSLs tested. No binding of GSLs by HB101 or in bacterial overlay assays was observed, even when the plates were exposed to film for 7 days (data not shown).
FIG. 4.
Binding of representative E. coli isolates to serial dilutions of globoseries GSLs in bacterial overlay assays. Autoradiographs of binding are shown in the lefthand panels, and the righthand panels show the quantification of the binding demonstrated in the adjoining autoradiographs. GSLs were serially diluted from 300 to 18.25 ng, chromatographed by HPTLC, and overlaid with representative E. coli isolates, as described in the text. The lanes containing standards for CMH (5 μg; negative control), CTH (Gb3), globoside, SGG, and DSGG are labeled. (A) E. coli R45; (B) E. coli IA2; (C) E. coli JJ122; (D) E. coli pJFK102; (E) E. coli pDC1. Glob, globoside.
DISCUSSION
In a previous study, we demonstrated that vaginal epithelial cells from nonsecretors selectively express SGG and DSGG and that these compounds bind a wild-type uropathogenic E. coli strain, R45, expressing a pap-encoded adhesin (36). Binding did not occur under conditions where pap-encoded adhesins were not expressed (36). We reasoned that the presence of these E. coli-binding GSLs on the vaginal epithelial cells of nonsecretors but not secretors might explain the increased propensity of nonsecretors for developing recurrent UTIs (5, 17, 32). In the studies reported here, we have now shown that SGG and DSGG are also expressed in human kidney tissues and that these compounds, purified from this source, bind cloned and wild-type uropathogenic E. coli isolates expressing pap-encoded adhesins. These strains represent the three known classes of P fimbrial adhesins. Using a PCR method that distinguishes the three classes of adhesins (7), we previously determined that E. coli R45 expresses P fimbriae carrying a class II adhesin (8, 9). In addition, we demonstrated the binding of SGG and DSGG by IA2, another wild-type isolate expressing P fimbriae carrying a class II pap-encoded adhesin, as well as by a cloned isolate expressing this adhesin (PapG from IA2), pDC1 (2). The class I papG-encoded adhesin was represented by an isolate expressing P fimbriae carrying PapG from J96 (HB101/pJJ48), expressing the pap operon from pHU845 (26), and the class III papG-encoded adhesin was represented by pJFK102, which expresses P fimbriae carrying PrsG from J96 (15). Thus, we have demonstrated that SGG and DSGG are relevant bacterial binding moieties for uropathogenic E. coli isolates expressing P fimbriae carrying all three known members of the family of pap-encoded adhesins.
To investigate the possible biological implications of this finding, we designed experiments to assess the relative binding of these E. coli isolates to the GSLs SGG and DSGG (nonsecretor-restricted in the vaginal epithelium [36]) as well as to other relevant globoseries GSLs that we previously identified on both secretors’ and nonsecretors’ vaginal epithelial cells (36). Before the various classes of pap-encoded adhesins were genetically defined, the binding of various wild-type and cloned uropathogenic E. coli isolates expressing pap-encoded adhesins to globoseries GSLs other than SGG and DSGG was investigated (21, 37). These studies demonstrated relatively little difference between GSL binding to globoside and binding to Gb3 for those E. coli isolates expressing P fimbriae carrying pap-encoded adhesins of classes I or II. Isolates expressing P fimbriae carrying a class III pap-encoded adhesin demonstrated a preference for binding to extended globoseries GSLs (37). In preliminary experiments, we found that binding of E. coli to SGG and DSGG was completely saturated in the GSL concentration range (0 to 1.0 μg) reported in one of these two previous studies, in which a similar technique was used (21, 37). Thus, we constructed GSL binding curves in lower concentration ranges (18 to 300 ng). Although we confirmed most of the previously reported data regarding the relative efficiency of binding of E. coli expressing P fimbriae carrying pap-encoded adhesin(s) to globoseries GSLs such as Gb3 and Gb4, we found that all five E. coli isolates bound more strongly to SGG than to the other globoseries GSLs tested, including DSGG. These data demonstrate that, at least in the urogenital epithelia of nonsecretors, SGG may be a preferred ligand for uropathogenic E. coli isolates.
In the studies reported here, we have isolated and purified SGG and DSGG from normal human kidney tissue for the first time. Further structural analysis of the SGG sample we obtained from this tissue source by proton nuclear magnetic resonance spectroscopy, mass spectroscopy, and linkage analysis has been completed and will be reported elsewhere (37a), while similar chemical characterization of DSGG from human kidney tissue is ongoing. SGG has been previously isolated, purified, and definitively characterized as to structure only from a human teratocarcinoma cell line, 2102Ep (11). DSGG has been purified from chicken muscle, human erythrocytes, and kidney tumor tissue, and its structure has been definitively proven to be that shown in Table 1 (1, 18, 20, 31). Previous studies by Karr et al. suggested that histological sections of human kidneys could be stained by a MAb directed against stage-specific embryonic antigen 4 (SSEA-4) and that E. coli pJFK102 also bound these kidney sections in the same areas stained by the antibodies (15, 16). SSEA-4 is defined as an epitope staining with a MAb raised against 4- and 8-cell-stage mouse embryos and a human teratocarcinoma cell line (33). Based on MAb MC813-70 staining, SSEA-4 has been identified in undifferentiated human embryonic carcinoma cells and seminomas (30). Agglutination of papain-treated human erythrocytes also occurs with MAb MC813-70, identifying the Luke antigen (38, 39), but the molecule on which the Luke antigen is carried on erythrocytes has not been isolated and structurally characterized. Thus, the antibody staining data previously reported by Karr et al. suggested, but did not prove, that SGG was expressed in human kidney tissue. Our data unequivocally demonstrate the presence of both SGG and DSGG in human kidney tissue.
In conclusion, our studies demonstrate the presence of SGG and DSGG in the human kidney and define SGG as a GSL to which each of the three classes of pap-encoded adhesins binds avidly. The biological significance of these findings requires further study, but since E. coli isolates bearing P fimbrial adhesins are very strongly associated with renal infection (6), SGG may well play a role in the pathogenesis of acute pyelonephritis. Svanborg and associates have also reported an association between nonsecretor status and an increased likelihood of clinically defined inflammatory responses suggestive of pyelonephritis, such as fever, leukocytosis, and elevated C-reactive protein (23). The presence of SGG in the kidneys of nonsecretors could play a role in predisposing these patients to renal inflammation. Further studies are needed to more extensively define the expression of SGG and DSGG in epithelial tissues throughout the urogenital tract. Our data demonstrate the presence of these compounds in the vagina (36) and kidney; we are currently studying the GSL composition of normal human bladder epithelium, including assaying for the presence of SGG and DSGG. Data derived from these studies will increase our knowledge of bladder glycobiology and may eventually lead to novel preventive strategies for UTI through the use of carbohydrate-based compounds that competitively inhibit bacterial attachment.
ACKNOWLEDGMENTS
This work was supported in part by grants AI01115 and DK-40045 from the National Institutes of Health, by a grant from the Edwin Beer Foundation of the New York Academy of Medicine, and by USAMRAA grant no. DAMD17-96-1-6301.
We gratefully acknowledge the technical assistance of Cynthia Fennell and Vivian de la Rosa and the gift of strain JJ122 from James Johnson.
REFERENCES
- 1.Chien J-W, Hogan E L. Novel pentahexosyl ganglioside of the globo series purified from chicken muscle. J Biol Chem. 1983;258:10727–10730. [PubMed] [Google Scholar]
- 2.Clegg C. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli. Infect Immun. 1982;38:739–744. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folch J, Lees M, Sloane Stanley G H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 4.Hakomori S-I. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- 5.Hooton T M, Roberts P L, Stamm W E. Effects of recent sexual activity and use of a diaphragm on the vaginal microflora. Clin Infect Dis. 1994;19:274–278. doi: 10.1093/clinids/19.2.274. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson J R, Brown J J. A novel multiply primed polymerase chain reaction assay for identification of variant papG genes encoding the gal(α1-4)gal-binding PapG adhesins of Escherichia coli. J Infect Dis. 1996;173:920–926. doi: 10.1093/infdis/173.4.920. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J R, Russo T A, Brown J J, Stapleton A E. papG alleles of Escherichia coli strains causing first-episode or recurrent acute cystitis in adult women. Clin Infect Dis. 1996;23:920. doi: 10.1086/513824. [DOI] [PubMed] [Google Scholar]
- 9.Johnson J R, Stapleton A E, Russo T A, Scheutz F, Brown J J, Maslow J N. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the class I and class III alleles of papG. Infect Immun. 1997;65:2153–2159. doi: 10.1128/iai.65.6.2153-2159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannagi R, Fukuda M N, Hakomori S. A new glycolipid antigen isolated from human erythrocyte membranes reacting with antibodies directed to globo-N-tetraosylceramide (globoside) J Biol Chem. 1982;257:4438–4442. [PubMed] [Google Scholar]
- 11.Kannagi R, Levery S B, Ishigami F, Hakomori S-I, Shevinsky L H, Knowles B B, Solter D. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983;258:8934–8942. [PubMed] [Google Scholar]
- 12.Kannagi R, Nudelman E, Levery S B, Hakomori S. A series of human erythrocyte glycosphingolipids reacting to the monoclonal antibody directed to a developmentally regulated antigen, SSEA-1. J Biol Chem. 1982;257:14865–14872. [PubMed] [Google Scholar]
- 13.Kannagi R, Watanabe K, Hakomori S. Isolation and purification of glycosphingolipids by high-performance liquid chromatography. Methods Enzymol. 1987;138:3–12. doi: 10.1016/0076-6879(87)38003-6. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson K-A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- 15.Karr J F, Nowicki B, Truong L D, Hull R A, Hull S I. Purified P fimbriae from two cloned gene clusters of a simple pyelonephritogenic strain adhere to unique structures in the human kidney. Infect Immun. 1989;57:3594–3600. doi: 10.1128/iai.57.11.3594-3600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karr J F, Nowicki B J, Truong L D, Hull R A, Moulds J J, Hull S I. pap-2-encoded fimbriae adhere to the P blood group-related glycosphingolipid stage-specific embryonic antigen 4 in the human kidney. Infect Immun. 1990;58:4055–4062. doi: 10.1128/iai.58.12.4055-4062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinane D F, Blackwell C C, Brettle R P, Weir D M, Winstanley F P, Elton R A. ABO blood group, secretor state and susceptibility to recurrent urinary tract infection in women. Br Med J. 1982;28:7–9. doi: 10.1136/bmj.285.6334.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundu S K, Samuelsson B E, Pascher I, Marcus D M. New gangliosides from human erythrocytes. J Biol Chem. 1983;258:13857–13866. [PubMed] [Google Scholar]
- 19.Leffler H, Svanborg-Eden C. Chemical identification of a glycosphingolipid receptor for Escherichia coli attaching to human urinary tract cells and agglutinating human erythrocytes. FEMS Microbiol Lett. 1980;8:127–134. [Google Scholar]
- 20.Levery S B, Salyan M E, Steele S J, Kannagi R, Dasgupta S, Chien J L, Hogan E L, van Halbeck H, Hakomori S. A revised structure for disialosyl globo-series gangliosides of human erythrocytes and chicken skeletal muscle. Arch Biochem Biophys. 1994;312:125–134. doi: 10.1006/abbi.1994.1290. [DOI] [PubMed] [Google Scholar]
- 21.Lindstedt R, Baker N, Falk P, Hull R, Hull S, Karr J, Leffler H, Svanborg-Eden C, Larson G. Binding specificities of wild-type and cloned Escherichia coli strains that recognize globo-A. Infect Immun. 1989;57:3389–3394. doi: 10.1128/iai.57.11.3389-3394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomberg H, Cedergren B, Leffler H, Nilsson B, Carlstrom A-S, Svanborg-Eden C. Influence of blood group on the availability of receptors for attachment of uropathogenic Escherichia coli. Infect Immun. 1986;51:919–926. doi: 10.1128/iai.51.3.919-926.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomberg H, Jodal U, Leffler H, De Man P, Svanborg C. Blood group non-secretors have an increased inflammatory response to urinary tract infection. Scand J Infect Dis. 1992;24:77–83. doi: 10.3109/00365549209048404. [DOI] [PubMed] [Google Scholar]
- 24.Magnani J L, Smith D, Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980;109:399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- 25.Naoi M, Lee Y C, Roseman S. Rapid and sensitive determination of sphingosine bases and sphingolipids with fluorescamine. Anal Biochem. 1974;58:571–577. doi: 10.1016/0003-2697(74)90226-7. [DOI] [PubMed] [Google Scholar]
- 26.Normark S, Lark D, Hull R, Norgren M, Båga M, O’Hanley P, Schoolnik G, Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983;41:942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nudelman E, Fukush Y, Levery S B, Higuchi T, Hakomori S-I. Novel fucolipids of human adenocarcinomas: disialosyl Lea antigen (III4FucIII6NeuAcIV3NeuAcLc4) of human colonic adenocarcinoma and the monoclonal antibody (FH7) defining this structure. J Biol Chem. 1986;261:5487–5495. [PubMed] [Google Scholar]
- 28.Nudelman E, Kannagi R, Hakomori S, Parsons M, Lipinski M, Wiels J, Fellous M, Tursz T. A glycolipid antigen associated with Burkitt lymphoma defined by a monoclonal antibody. Science. 1983;220:509–511. doi: 10.1126/science.6836295. [DOI] [PubMed] [Google Scholar]
- 29.Nudelman E D, Mandel U, Levery S B, Kaizu T, Hakomori S. A series of disialogangliosides with binary 2-3 sialosyllactosamine structure, defined by monoclonal antibody NUH2, are oncodevelopmentally regulated antigens. J Biol Chem. 1989;264:18719–18725. [PubMed] [Google Scholar]
- 30.Olie R A, Fenderson B, Daley K, Oosterhuis J W, Murphy J, Looijenga L H J. Glycolipids of human primary testicular germ cell tumours. Br J Cancer. 1996;74:133–140. doi: 10.1038/bjc.1996.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitoh S, Levery S B, Salyan M E K, Goldberg R I, Hakomori S. Common tetrasaccharide epitope NeuAca2-3Galβ1-3(NeuAca2-6)GalNAc, presented by different carrier glycosylceramides or O-linked peptides, is recognized by different antibodies and ligands having distinct specificities. J Biol Chem. 1994;269:5644–5652. [PubMed] [Google Scholar]
- 32.Sheinfeld J, Schaeffer A J, Cordon-Cardo C, Rogatko A, Fair W R. Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773–777. doi: 10.1056/NEJM198903233201205. [DOI] [PubMed] [Google Scholar]
- 33.Shevinsky L H, Knowles B B, Damjanov I, Dolter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell. 1982;30:697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui B, Hakomori S. Change of glycolipid pattern in Morris hepatomas 5123 and 7800. Cancer Res. 1970;30:2930–2936. [PubMed] [Google Scholar]
- 35.Stapleton A, Moseley S, Stamm W E. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991;163:773–779. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- 36a.Stapleton A E, Stroud M R, Hakomori S I, Stamm W E. Uropathogenic Escherichia coli bind with highest affinity to the globo-series glycosphingolipid sialosyl galactosyl globoside. Clin Infect Dis. 1995;21:727. . (Abstract.) [Google Scholar]
- 36.Stapleton A, Nudelman E, Clausen H, Hakomori S-I, Stamm W E. Binding of uropathogenic Escherichia coli R45 to glycolipids extracted from vaginal epithelial cells is dependent on the histo-blood group secretor status. J Clin Invest. 1992;90:965–972. doi: 10.1172/JCI115973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stromberg N, Marklund B-I, Lund B, Ilver D, Hamers A, Gaastra W, Karlsson K-A, Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to galα1-4 gal-containing isoreceptors. EMBO J. 1990;9:2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Stroud, M. R., A. E. Stapleton, and S. B. Levery. Submitted for publication.
- 38.Tippett P, Andrews P W, Knowles B B, Solter D, Goodfellow P N. Red cell antigens P (globoside) and Luke: identification by monoclonal antibodies defining the murine stage-specific embryonic antigens -3 and -4 (SSEA-3 and SSEA-4) Vox Sang. 1986;51:53–56. doi: 10.1111/j.1423-0410.1986.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 39.Tippett P, Sanger R, Race R R, Swanson J, Busch S. An agglutinin associated with the P and the ABO blood group systems. Vox Sang. 1965;10:269–280. doi: 10.1111/j.1423-0410.1965.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe K, Arao Y. A new solvent system for the separation of neutral glycosphingolipids. J Lipid Res. 1981;22:1020–1024. [PubMed] [Google Scholar]
- 41.Yu R K, Ledeen R W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972;13:680–686. [PubMed] [Google Scholar]